Abstract
Introduction:
In the last two decades, the evidence related to using vaccine patches with multiple short projections (≤ 1 mm) to deliver vaccines through the skin increased significantly and demonstrated their potential as an innovative delivery platform.
Areas covered:
We review the vaccine patch literature published in English as of March 1, 2019, as well as available information from key stakeholders related to vaccine patches as a platform. We identify key research topics related to basic and translational science on skin physical properties and immunobiology, patch development, and vaccine manufacturing.
Expert opinion:
Currently, vaccine patch developers continue to address some basic science and other platform issues in the context of developing a potential vaccine patch presentation for an existing or new vaccine. Additional clinical data and manufacturing experience could shift the balance toward incentivizing existing vaccine manufactures to further explore the use vaccine patches to deliver their products. Incentives for innovation of vaccine patches differ for developed and developing countries, which will necessitate different strategies (e.g., public-private partnerships, push or pull mechanisms) to support the basic and applied research needed to ensure a strong evidence base and to overcome translational barriers for vaccine patches as a delivery platform.
Keywords: vaccine, human skin, microneedle, microarray patch
1. Introduction
The history of vaccine development includes exploration of vaccine delivery to humans through all possible routes of entry into the body using a wide range of strategies [1]. The earliest vaccination technique involved applying virus particles directly to disrupted skin (i.e., variolation with smallpox). Currently, although a limited number of licensed oral vaccines (e.g., oral poliovirus vaccine, oral rotavirus vaccine) and aerosol vaccines (e.g., FluMist™) exist [2], the use of syringe and needle that carries the vaccine through the skin barrier represents the dominant current vaccine delivery strategy. Delivery of vaccines by syringe and needle is generally well-accepted by vaccine recipients, even though they may experience some fear (i.e., needle phobia), pain associated with receiving injections, and/or in rare instances of injuries, such as shoulder injuries related to syringe and needle vaccine administration [3] or adverse events from lyophilized vaccine reconstitution errors. Health systems also broadly accept syringe and needle delivery of vaccines and benefit from the interchangeability and stability of a supply chain supported by multiple suppliers. However, syringe and needle vaccine delivery require the use of trained, skilled healthcare workers to administer the vaccines, and even with sufficient training these workers face risks of needle-related occupational injuries. In addition, the disposal of used syringes and needles leads to system costs and risks.
The evaluation of delivering vaccines into different skin strata and underlying tissues dates back at least to the 1930s [4]. Vaccine administration can occur via injection into a muscle (i.e., intramuscular [IM]), the dermis (i.e., intradermal [ID]), the hypodermis (i.e., subcutaneous [SC]), or onto the epidermis (i.e., topical or transcutaneous) [1]. Given the size of the molecules in vaccines and the need to deliver them past immunological skin defenses in the epidermis, most vaccine injection occurs either into the IM or SC layer, with the recommended needle size varying by vaccine recipient age and body mass, injection site, and target. Some ID vaccine delivery into the relatively shallow dermis use needles and require training for proper vaccine administration (e.g., BCG, and historically, smallpox, which used a bifurcated needle that left a signature scar). A comprehensive review of the literature shows mixed success with the use of jet injector devices for ID delivery, with multiple studies suggesting effective use, but some reporting issues with cross-contamination [1]. More recent disposable-syringe jet injectors with sophisticated applicators avoid some of risks of cross-contamination and offer a needle-free immunization opportunity [5–8].
Transcutaneous immunization (TCI) involves the application of vaccine antigen (sometimes in the presence of an adjuvant) directly to the skin [9]. Some TCI methods involve direct application of liquid or dry vaccine to intact skin, and sometimes using a hydrated patch which disrupts the stratum corneum. Other TCI methods apply a vaccine antigen prior to or following disruption of the stratum corneum by scraping, electroporation, nonablative fractional laser (NAFL), or another mechanism that allows the antigens in a vaccine applied topically to the skin to pass into the epidermis. Some TCI methods include covering the skin coated with vaccine with a patch that occludes the area for some time. Despite extensive investment in the development of TCI dating back to 2000 [10], the technique showed inferiority in human clinical trials, including a phase 3 trial for an Enterotoxigenic Escherichia coli traveler’s diarrhea vaccine [11], a phase 1/2 trial for measles vaccine [12], and a graded phase 1 trial for influenza vaccine [13]. The history of successes and failures of alternative vaccine delivery systems generally offer some important lessons for the development of vaccine patches, which fall beyond the scope of this review.
In the last two decades, new materials and technologies explored the use of micron-sized (≤1 mm) projections arranged as an array or matrix that can deliver vaccine past the stratum corneum, but not typically beyond the dermis. As the evidence base for this new vaccine patch technology continues to expand, we appreciate the need to review the literature to understand the current status of and challenges to development of vaccine patches as a delivery platform. For this review, we define a vaccine patch as a product that applies a vaccine antigen (with or without adjuvants) using a single array or matrix of projections less than 1 mm in height applied directly to the skin. Thus, our definition of a vaccine patch requires skin penetration by a submillimeter projection array (i.e., it goes beyond a singled, bored ID hollow needle). The focus of our systematic review differs from other reviews of related topics that focused specifically on TCI [9], ID vaccine delivery [14], microneedles [15–17], dissolvable microneedles [18], clinical trials for non-invasive vaccine delivery [2], low- and middle-income country markets [19,20], or other topics. We note that parallel development of patches to deliver other therapeutic agents also impacts this technology (e.g., by potentially establishing design, fabrication, manufacturing, and/or regulatory precedents), but for purposes of this review we focus on the platform issues related to human vaccines. We evaluated the literature to date and identified unresolved platform research issues that would benefit from coordinated research funding and activities, including basic research related to immunology and skin characteristics, and applied research related to production, licensing, and acceptability.
2. Conceptual Framework
The pathway to develop a successful vaccine patch, like other therapeutic agents, involves multiple stakeholders and includes many stages to get from the concept to a licensed product. Figure 1 depicts key stages for vaccine patch development in the US regulatory context [21] for an existing licensed vaccine product, and it shows the anticipated intensity of efforts for three key workstreams by: (1) immunobiologists and other basic scientists, (2) patch developers, and (3) vaccine manufacturers required to converge to develop a single vaccine patch product. Figure 1 highlights the unique challenge in vaccine patch commercialization in that a technology handoff is currently required between patch developers and vaccine manufacturers mid-cycle of the commercialization path. This differs from the development of incremental improvements of existing vaccines (e.g., dose sparing using adjuvants, reformulation, etc.) or improvements in patch fabrication technologies in which the development cycle is largely driven by one or the other industry. Furthermore, recognizing vaccine patches as a generalizable pharmaceutical platform leads to appreciation that some of the early research and development in the process of developing one vaccine patch (i.e., for a specific antigen) will likely influence the entire platform. For vaccine patch development, the following sections provide a systematic review of the literature to date and then discusses key platform concepts across the three streams of work in Figure 1 that need to converge to see the realization of vaccine patches in the market.
Figure 1:

Vaccine patch development pathway and intensity of efforts (indicated by triangles) required from stakeholders that need to resolve key issues and converge to commercialize a clinical vaccine patch, including successful handoff from patch developers to vaccine manufacturers.
3. Systematic Literature Review
We performed a systematic literature review by searching Web of Science and PubMed for papers published in English prior to March 1, 2019 that included a combination of the terms: “vaccine” and “patch” or “microneedle.” The review process included screening the titles and abstracts of the search results to create a database of all studies that explored the development of vaccine patches for one or more specific vaccines. The review process included the extraction of information about the patch developer, the vaccine(s), and characteristics of the vaccine patch. We reviewed the full text for any papers that lacked enough information in the abstract. The search included the identification of prior reviews, and the review process included any relevant studies not captured in our search that we identified in the references of these reviews or in the references of the papers identified by our search. We included papers that reported the results of pre-clinical or clinical testing of vaccine patches for identified pathogens. We excluded studies that described experiments for non-specific genetic materials (DNA, RNA, plasmids), proteins (ovalbumin [OVA]), or other materials (non-specific laboratory bacterium or virus strains or components). Although these studies may provide generic information relevant to vaccine patches, we wanted to characterize the state of the literature specific to vaccines that target identified human pathogens. We excluded all studies not relevant to the review, and we report the numbers of excluded studies according to broad exclusion categories. We recognize that synergistic innovation in the use of patch technology for general pharmaceutical product delivery (non-vaccine products) may help to stimulate vaccine patch development and adoption over time [15], but we do not include consideration of this in our review. For the included studies, we extracted the specific vaccine used and its status as licensed or experimental, the group that conducted the study, the nature of the vaccine patch (i.e., height, number, and arrangement of projections, and whether the projections were coated with the vaccine, dissolving (and containing the vaccine), or hollow (such that the vaccine flowed through them), the species used for the tests, and the method of patch application and time applied to the skin (i.e., wear times).
Figure 2 provides a summary of the systematic literature review search process that led to the extraction of information from 116 studies [22–137]. Our review overlapped some with prior reviews (e.g. compared to the 2016 review by Marshall et al. [17], our 116 studies included 46 of the same studies ([22–26,29–37,42–49,51,53,54,56,62–65,83,85,86,99,100,103,108,109,113,115,116,121,123,126,127]) but we excluded TCI studies that it included (e.g., [138]).
Figure 2:
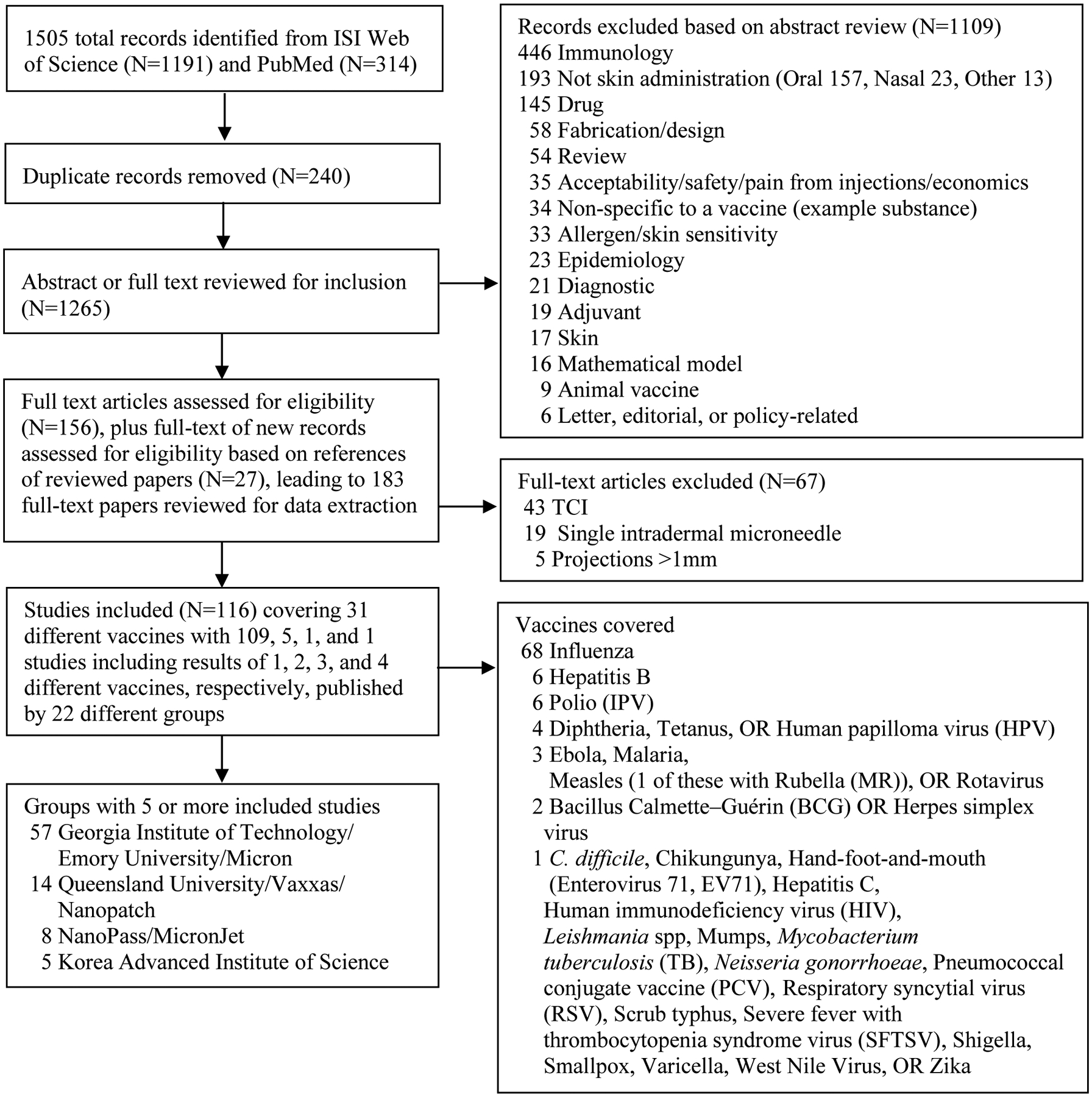
Process used to identify 116 relevant peer-reviewed vaccine patch papers published in English that met the inclusion criteria.
Table 1 summarizes some key characteristics of included studies. Studies involving coated (N=69) and dissolving (N=39) projections dominate. The literature continues to increase with time, with half of the studies (N=64) published in the last 5 years. Multiple patch developers contributed to the literature, but 2 groups contributed the majority of the studies (i.e., Georgia Institute of Technology/Emory University/Micron (N=57) and Queensland University/Vaxxas/Nanopatch (N=14)). We identified 31 human vaccines with studies by at least one vaccine patch developer, more than 4 papers for some vaccines, and the same relatively small number of patch developer groups reporting on the use of essentially the same (albeit evolving) technology for more than one vaccine. With nearly half of the studies (N=68) focused on influenza, the use of a vaccine patch presentation for the delivery of influenza vaccine appears likely to lead the vaccine platform as the first product to progress through late-stage development and potential licensure as a product. Most studies reported pre-clinical results of tests using experimental animals. The potential opportunity to save on antigen costs using vaccine patches to offset other costs of their development represents a key consideration for some studies. As shown in Table 1, 44 of the 116 studies (38%) demonstrated or suggested the potential for dose sparing with the use of vaccine patches compared to syringe and needle, while 6 studies looked for but did not find evidence of dose sparing.
Table 1:
Summary characteristics of 116 included vaccine patch studies
| Patch projection type* |
| Coated (N=69) [22–61,81,83–94,103–107,109–112,119,121,125,128,132,133] [62,108]* |
| Hollow (N=10) [108]*[95–102,134] |
| Dissolving (N=39) [63–80,82,113–118,120,122–124,126,127,129–131,135–137] [62]* |
| Publication Year |
| 2005–2009 (N=8) [22–26,95,108,125] |
| 2010–2014(N=44) [27–53,63,81–88,96–98,113,114,116,119,121] |
| 2015–2019 (N=64) [54–62,64–80,89–94,99–107,109–112,115,117,118,120,122–124,126–137] |
| Patch Developer |
| Georgia Institute of Technology/Emory University/Micron (N=57) (2009–2019) [22–80] |
| Queensland University/Vaxxas/Nanopatch (N=14) (2010–2018) [81–94] |
| NanoPass MicronJet (N=8) (2009–2016) [95–102] |
| Korea Advanced Institute of Science and Technology (N=5) (2015–2017) [103–107] |
| Leiden University (N=4) (2009–2019) [108–111] |
| Osaka University/MicroHyala (N=4) (2012–2017) [112–115] |
| Queen’s University (N=3) (2012–2018) [116–118] |
| Cork/ImmuPatch (N=2) (2012–2016) [119,120] |
| Novartis (N=2) (2013–2015) [121,122] |
| Wellman Laboratory (N=2) (2015–2017) [123,124] |
| Anhui Medical University (N=1) (2015) [126] |
| Chinese Academy of Sciences (N=1) (2016) [127] |
| China State Institute of Pharmaceutical Industry (N=1) (2016) [129] |
| GSK Fujifilm (N=1) (2017) [130] |
| Hamamatsu University School of Medicine, ASTI (N=1) (2018) [134] |
| Hokkaido University/Fujifilm (N=1) (2017) [131] |
| Mercer (N=1) (2018) [135] |
| Tufts University/Massachusetts Institute of Technology/Vaxxes (N=1) (2017) [132] |
| USAMRID Easy Vax™ (N=1) (2007) [125] |
| University of Pittsburgh (N=1) (2016) [128] |
| University of Navarra/Cardiff University (N=1) (2017) [133] |
| Yonsei (N=1) (2018) [136] |
| Zhejiang University of Technology (N=1) (2018) [137] |
| Vaccine** |
| Influenza (N=68) |
| H1N1 (N=29) [24–26,28–33,39,41,43–46,49–52,55,57,63,66,69,70,91,94,105,106] |
| H1N1,H3N2,B (N=18) [62,68,73,81,82,87,92,95,96,98,101,104,112,113,115,120–122] |
| H1N1,H3N2,B,B (N=2) [112,132] |
| H1N1 Pandemic (N=5) [40,97,103,111,123] |
| H3N2 (N=4) [23,38,78,108] |
| H5N1 (N=3) [34,35,42] |
| H1N1,H5N1 (N=2) [27,131] |
| H1N1,H3N2 (N=1) [53] |
| H1N1 Pandemic,H3N2,A other (N=1) [54] |
| H1N1,H3N2,H5N1,H7N9 (N=1) [79] |
| H1N1,H3N2,H1N1 Pandemic (N=1) [72] |
| H1N1,H3N2,H1N1 Pandemic,A other (N=1) [61] |
| H1N1-like (A other) (N=1) [77] |
| Hepatitis B surface antigen (HepBsAg) (N=6) [22,75,80,126,127,130] |
| Polio (N=6) [65,90,93,99,100,109] |
| Diphtheria toxoid (N=4) [108,110,113,114] |
| Tetanus toxoid (N=4) [67,110,113,114] |
| Human Papilloma virus (HPV) (N=4) [56,85,117,118] |
| Ebola (N=3) [58,59,71] |
| Malaria (N=3) [88,113,119] |
| Rota (N=3) [48,76,102] |
| Bacillus Calmette–Guerin (BCG) (N=2) [37,124] |
| Herpes simplex virus (N=2) [83,84] |
| Measles (N=2) [47,64] |
| C. difficile (N=1) [132] |
| Chikungunya (N=1) [86] |
| Gonorrhoeae (N=1) [135] |
| Hand-foot-and-mouth disease (Enterovirus 71, EV 71) (N=1) [129] |
| Hepatitis C (N=1) [36] |
| Human immunodeficiency virus (HIV) (N=1) [116] |
| Leishmania spp (N=1) [133] |
| Measles rubella (MR) (N=1) [74] |
| Mumps (N=1) [134] |
| Pneumococcal conjugate vaccine (PCV) (N=1) [89] |
| Respiratory syncytial virus (RSV) (N=1) [60] |
| Scrub typhus (N=1) [136] |
| Severe fever with thrombocytopenia syndrome virus (SFTSV) (N=1) [107] |
| Shigella (N=1) [132] |
| Smallpox (N=1) [125] |
| Tuberculosis (non-BCG) (N=1) [137] |
| Varicella (N=1) [134] |
| West Nile Virus (N=1) [86] |
| Zika (N=1) [128] |
| Species |
| Mice (N=87) [23–36,38–46,48–63,66,67,69–73,76–89,91,92,103–108,110,111,116–120,124–129,131–133,135–137] |
| Human (N=10) [68,94–101,115] |
| Rats (N=7) [47,90,93,109,112,114,134] |
| Rhesus macaques (N=3) [64,65,74] |
| Pigs (N=3) [22,102,130] |
| Guinea pigs (N=3) [37,121,122] |
| Mice, rats*** (N=1) [113] |
| Mice, pigs*** (N=1) [123] |
| Mice, rhesus macaques*** (N=1) [75] |
| Dose sparing (N=50) |
| Demonstrated (N=22) [23,32–35,37,43,44,69,76,81,83,90,91,93,96,98,101,103,108,129,134] |
| Suggestive (N=22) [25,27,36,38,49,53–55,62,70,75,77,84,85,95,97,111,115,120–122,132] |
| Looked for but not found (N=6) [48,64,65,99,100,133] |
Studies with multiple vaccines (diphtheria and tetanus toxoids, influenza, and malaria [113], C. difficile, influenza, and Shigella [132], diphtheria and tetanus toxoids [110,114], diphtheria toxoid and influenza [108], mumps and varicella [134], Chikungunya and West Nile Virus [86], and 2 types of influenza [112])
Studies with more than one species
Table 2 provides some of the details extracted from the 116 included studies organized by patch developer groups with the most-to-least studies. As reflected in the number of studies identified, the review shows 2 predominant strategies emerging: dissolving microneedles of approximately 0.5–0.7 mm in height, and coated microneedles of approximately 0.1–0.3 mm height. The review also shows a wide range of administration approaches, applicators (if used), and times of application. Some patch designs without an applicator include a built-in force feedback indicator to give audible or visible cues for the vaccine administrator. The design of applicators (if used) reflects different factors considered by patch developers to date related to the expected immunological performance of the projections and/or other factors (e.g., requirements for consistent delivery, requirements of the products to prevent choking for small objects). The use of different species in part reflects the appropriateness of different animal models for various vaccines; ideally the optimal animal model for each antigen is used that allows for a measurable immune response as a proxy indicator for the induction of immunity in humans using vaccine patches. As noted by others, the relatively small number of human studies for vaccine patches limits the opportunities to advance vaccine patch technology and get it into clinical practice [17,139].
Table 2:
Details extracted from 116 included studies organized by group with the most to least studies
| Year | Species | Vaccine | Type: Projection shape (array density), height, SA (dosing details for studies using more than 1 patch) | Administration time and applicator (if used) |
|---|---|---|---|---|
| Georgia Institute of Technology/Emory University/Micron | ||||
| 2009 [22] | Pigs | HepBsAg | C: Square (5×10), 0.6 mm, SA=0.5 cm2 | 30 min |
| 2009 [23] | Mice | Influenza (H3N2) | C: Line (5×1), 0.7 mm, SA=NR | 2 min |
| 2009 [24] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2009 [25] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2009 [26] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 5 min |
| 2010 [27] | Mice | Influenza (H1N1,H5N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [28] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [29] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [30] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [31] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [32] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [33] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [34] | Mice | Influenza (H5N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [35] | Mice | Influenza (H5N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2010 [36] | Mice | HepC | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2011 [37] | Guinea pigs | BCG * | C: Line (5×1), 0.7 mm, SA=NR (x10 patches) | 10 min |
| 2011 [38] | Mice | Influenza (H3N2) | C: Line (5×1), 0.75 mm, SA=NR | 5 min |
| 2011 [39] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2011 [40] | Mice | Influenza (H1N1 Pandemic) | C: Line (5×1), 0.7 mm, SA=NR | 5 min |
| 2012 [41] | Mice | Influenza (H1N1) | C: Line (5×1), 0.75 mm, SA=NR | 10 min |
| 2012 [42] | Mice | Influenza (H5N1) | C: Line (5×1), 0.7 mm, SA=NR | 20 min |
| 2012 [43] | Mice | Influenza (H1N1) | C: Line (5×1), 0.75 mm, SA=NR | 20 min |
| 2012 [44] | Mice | Influenza (H1N1)** (Novartis) | C: Line (5×1), 0.75 mm, SA=NR | 5 min |
| 2012 [45] | Mice | Influenza (H1N1)** (Novartis) | C: Line (5×1), 0.7 mm, SA=NR | 5 min |
| 2012 [46] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 5 min |
| 2013 [47] | Cotton rats | Measles | C: Line (5×1), 0.75 mm, SA=NR | 10 min |
| 2013 [48] | Mice | Rotavirus | C: Line (5×1), 0.75 mm, SA=NR (x5 patches) | 10 min |
| 2013 [49] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2013 [50] | Mice | Influenza (H1N1) | C: Line (5×1), 0.75 mm, SA=NR | NR |
| 2013 [51] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 20 min |
| 2014 [52] | Mice | Influenza (H1N1)** (Novartis) | C: Line (5×1), 0.7 mm, SA=NR | 5 min |
| 2014 [53] | Mice | Influenza (H1N1,H3N2) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2015 [54] | Mice | Influenza (H1N1 Pandemic H3N2,A other) | C: Line (5×1), 0.7 mm, SA=NR | NR |
| 2015 [55] | Mice | Influenza (H1N1)** (Novartis) | C: Line (5×1), 0.7 mm, SA=NR | NR |
| 2015 [56] | Mice | HPV | C: Line (5×1), 0.7–0.75 mm, SA=NR | 2 min |
| 2015 [57] | Mice | Influenza (H1N1) | C: Line (5×1), 0.75 mm, SA=NR (x5 patches) | NR |
| 2018 [58] | Mice | Ebola | C: Line (5×1), 0.7 mm, SA=NR (2 doses, 4-week interval) | 2 min |
| 2018 [59] | Mice | Ebola | C: Line (5×1), 0.7 mm, SA=NR (2 doses, 4-week interval) | 2 min |
| 2018 [60] | Mice | RSV | C: Line (5×1), 0.7 mm, SA=NR (x2 patches) | 10 min |
| 2019 [61] | Mice | Influenza (H1N1,H3N2, H1N1 Pandemic, A other) | C: Line (5×1), 0.65 mm, SA=NR | 10 min |
| 2015 [62] | Mice | Influenza (H1N1,H3N2,B) | C: Line (5×1), 0.7 mm, SA=NR D: Square (10×10), 0.65 mm, SA=1 cm2 |
C; 5 min D; 10 min |
| 2010 [63] | Mice | Influenza (H1N1) | D: Square (10×10), 0.65 mm, SA=0.5 cm2 | 15 min |
| 2015 [64] | Rhesus macaques | Measles | D: Square (10×10), 0.6 mm, SA=0.29 cm2 | 10 min |
| 2015 [65] | Rhesus macaques | IPV** (GSK) | D: Square (10×10), 0.6 mm SA=1.29 cm2 (x2 patches for IPV serotype 1, 1 patch each for serotypes 2 and 3) | 15 min |
| 2016 [66] | Mice | Influenza (H1N1) | D: Square (10×10), 0.6 mm, SA=NR | NR |
| 2016 [67] | Mice | Tetanus** (Serum Institute of India) | D: Square (10×10), 0.65 mm, SA=NR (x0.5, half of patch used) | 2 min held in, left 20 min |
| 2017 [68] | Human (Phase 1) | Influenza (H1N1,H3N2,B) | D: Square (10×10), 0.65 mm, SA=NR | 20 min, force feedback indicator |
| 2017 [69] | Mice | Influenza (H1N1) | D: Square (10×10), 0.65 mm, SA=0.56 cm2 (x0.5, half of patch used) | 1 min held in, 10 min |
| 2017 [70] | Mice | Influenza (H1N1) | D: Square (10×10), 0.7 mm, SA=NR | 10 min, force feedback indicator |
| 2017 [71] | Mice | Ebola | D: Square (10×10), 0.7 mm, SA=0.56 cm2 (4 doses, 4-week intervals) | 5 min |
| 2017 [72] | Mice | Influenza (H1N1,H3N2, H1N1 Pandemic) | D: Square (10×10), 0.65 mm, SA=NR (booster dose only) | 1 min held in, 21 min |
| 2017 [73] | Mice | Influenza (H1N1,H3N2,B) | D: Square (NR), NR, SA=NR | 1 min held in, 21 min |
| 2018 [74] | Rhesus macaques | MR | D: Square (10×10), 0.7 mm, SA=1 cm2 | 30 sec held in, 15 min |
| 2018 [75] | Rhesus macaques and Mice | HepBsAg | C: Line (5×1), 0.75 mm, SA=NR (x2 patches) D: Square (10×10), 0.65 mm, SA=1 cm2 |
C: 10 min D: 20 min, held 30 sec, explored different levels |
| 2018 [76] | Mice | Rotavirus | D: Square (10×10), 0.85 mm, SA=1 cm2 | 30 sec held in, 15 min |
| 2018 [77] | Mice | Influenza (A other (H1N1-like)) | D: Square (NR), NR, SA=NR | 1 min held in, 20 min |
| 2018 [78] | Mice | Influenza (H3N2) | D: Square (10×10), 0.65 mm, SA=NR | 20 min |
| 2018 [79] | Mice | Influenza (H1N1,H3N2, H5N1,H7N9) | D: Square (10×10), 0.65 mm, SA=NR | 1 min held in, 30 min |
| 2019 [80] | Mice | HepBsAg | D: Square (10×10), 0.6 mm (x2 patches) | NR |
| Queensland University/Vaxxas/Nanopatch | ||||
| 2010 [81] | Mice | Influenza (H1N1,H3N2, B)** (Seqirus Pty Ltd) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) | 2 min, spring applicator (1.96 m/s) |
| 2010 [82] | Mice | Influenza (H1N1,H3N2, B)** (CSL Limited) | D: Square (58×58), 0.116 mm, SA=0.16 cm2 (x2 patches, except x1 for low dose group) | 5 min, spring applicator (1.96 m/s) |
| 2010 [83] | Mice | Herpes simplex virus | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) (3 doses, 3-week intervals) | 5 min, spring applicator (2 m/s) |
| 2010 [84] | Mice | Herpes simplex virus | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) (3 doses, 3-week intervals) | 5 min, spring applicator (2 m/s) |
| 2010 [85] | Mice | HPV** (Merck) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) | 5 min, spring applicator (2 m/s) |
| 2010 [86] | Mice | West Nile, Chikungunya |
C: Square (58×58), 0.065, 0.11 mm, SA=0.16 cm2 | 5 min, spring applicator (2 m/s) |
| 2012 [87] | Mice | Influenza (H1N1,H3N2, B)** (CSL Limited) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) | 2 min, spring applicator (2 m/s) |
| 2013 [88] | Mice | Malaria | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) (2 doses, 2- or 8-week interval) | 5 min, spring applicator (1.96 m/s) |
| 2015 [89] | Mice | PCV** (Pfizer) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (2 doses, 2-week interval) | 2 min, spring applicator (3.1 m/s) |
| 2016 [90] | Rats | IPV** (Bilthoven Biologicals) | C: Square (58×58), 0.23 mm, SA=0.16 cm2 (x2 patches) (3 doses, 3-week intervals) | 2 min, spring applicator (3.1 m/s) |
| 2016 [91] | Mice | Influenza (H1N1) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) | 2 min, spring applicator (NR m/s) |
| 2016 [92] | Mice | Influenza (H1N1,H3N2, B)** (bioCSL) | C: Square (58×58), 0.11 mm, SA=0.16 cm2 (x2 patches) | 2 min, spring applicator (2.3 m/s) |
| 2017 [93] | Rats | IPV** (Bilthoven Biologicals) | C: Square (58×58), 0.23 mm, SA=0.16 cm2 (x1 patch for each serotype) (3 doses, 3-week intervals) | 2 min, spring applicator (3.1 m/s) |
| 2018 [94] | Human (Phase 1) | Influenza (H1N1)** (Seqirus Pty Ltd) | C: Square (100×100), 0.25 mm, SA=1 cm2 (x2 patches) | 2 min, spring applicator (20 m/s) |
| NanoPass MicronJet | ||||
| 2009 [95] | Human (Phase 1) | Influenza (H1N1,H3N2, B)** (GSK) | H: Line (4×1), 0.45 mm, SA=NR | NR |
| 2012 [96] | Human (RCT) | Influenza (H1N1,H3N2, B)** (Sanofi Pasteur) | H: Line (3×1), 0.6 mm, SA=NR | NR |
| 2012 [97] | Human (RCT) | Influenza (H1N1 Pandemic)** (Sanofi Pasteur) | H: Line (4×1), 0.45 mm, SA=NR | NR |
| 2014 [98] | Human (Phase 2) | Influenza (H1N1, H3N2,B)** (Crucell) | H: Line (4×1), 0.45 mm, SA=NR | NR |
| 2015 [99] | Human (RCT) | IPV** (Sanofi Pasteur) | H: Line (3×1), 0.6 mm, SA=NR | NR |
| 2015 [100] | Human (RCT) | IPV** (Netherlands Vaccine Institute) | H: Line (3×1), 0.6 mm, SA=NR (2 doses, 8-week interval) | NR |
| 2016 [101] | Human (Phase 1) | Influenza (H1N1,H3N2,B) | H: Line (3×1), 0.6 mm, SA=NR | NR |
| 2016 [102] | Pigs | Rotavirus | H: Line (3×1), 0.6 mm, SA=NR (3 doses, days 0, 10, 21) | NR |
| Korea Advanced Institute of Science and Technology | ||||
| 2015 [103] | Mice | Influenza (H1N1 Pandemic) | C: Line (5×1), 0.7 mm, SA=NR | 10 min |
| 2016 [104] | Mice | Influenza (H1N1,H3N2,B) | C: Line (5×1), 0.7 mm, SA=NR (2 doses, 4-week interval) | 10 min |
| 2017 [105] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR (3 doses, 4-week intervals) | 15 min |
| 2017 [106] | Mice | Influenza (H1N1) | C: Line (5×1), 0.7 mm, SA=NR | 10–15 min |
| 2017 [107] | Mice | SFTSV | C: Line (5×1), 0.7 mm, SA=NR (2 doses, 4-week interval) | 15 min |
| Leiden University | ||||
| 2009 [108] | Mice | Diphtheria, Influenza (H3N2) | C: Square (4×4), 0.3 mm, SA=0.5 cm2 H: Square (4×4, 9×9), 0.245 mm, SA=0.5 cm2 |
1 s, electronic applicator (3 m/s) |
| 2015 [109] | Rats | IPV | C: Square (24×24), 0.2 mm, SA=0.25 cm2 (x3 patches) (2 doses, 3-week interval) | 1 min, 3lectronic applicator (3 m/s) |
| 2017 [110] | Mice | Diphtheria, Tetanus |
C: Circular (105 total projections), 0.475 mm, SA=0.75 cm2 (x3 patches) (2 doses, 3-week interval) | 30 min, handheld applicator (4N) |
| 2019 [111] | Mice | Influenza (H1N1 Pan) | C: Circular (105 total projections), 0.475 mm, SA=0.75 cm2 (x3 patches) (2 doses, 3-week interval) | 30 min, handheld applicator (4N) |
| Osaka University/MicroHyala | ||||
| 2017 [112] | Rats | Influenza (H1N1,H3N2,B or H1N1,H3N2,B,B)** (Biken) | C: Circular (481 total projections), 0.27 or 0.3 mm, SA=0.785 cm2 (2 doses, 3- or 4-week interval) | 30 or 60 min, spring applicator |
| 2012 [113] | Rats and Mice | Diphtheria, Tetanus, Influenza (H1N1,H3N2,B), Malaria | D: Square (40×40), 0.2, 0.3, or 0.8 mm, SA=0.8 cm2 (number, height, dosing, and interval depends on vaccine) | 6 hr, handheld applicator (12.8N/20 projections) |
| 2013 [114] | Rats | Diphtheria, Tetanus | D: Square (200 total projections), 0.2, 0.3, or 0.8 mm, SA=0.8 cm2 (5 doses, 2-week intervals) | 1 or 6 hr, spring applicator |
| 2015 [115] | Human (Pre-clinical) | Influenza (H1N1,H3N2,B) | D: Square (40×40), 0.2, 0.3, or 0.8 mm, SA=0.8 cm2 (2 doses, 3-week intervals) | 6 hr, handheld applicator (12.8N/20 projections) |
| Queen’s University | ||||
| 2012 [116] | Mice | HIV | D: Square (19×19), 0.6 mm, SA=1 cm2 (4 doses, days 0, 14, 28, and 42) | Overnight |
| 2017 [117] | Mice | HPV | D: Square (19×19), 0.6 mm, SA=1 cm2 (x2 patches) (3 doses, 2-week interval) | 5 mins held in, 24 hours |
| 2018 [118] | Mice | HPV | D: Square (19×19), 0.6 mm, SA=1 cm2 (3 doses, 2-week interval) | 5 mins held in, 24 hours |
| Cork University | ||||
| 2012 [119] | Mice | Malaria | C: Square (5×5), 0.2 mm, SA=NR | 4 hr |
| 2016 [120] | Mice | Influenza (H1N1,H3N2,B) | D: Square (5×5 or 12×12), 0.5 or 0.28 mm, SA=1 cm2 | 10–20N, 18 hr |
| Novartis | ||||
| 2013 [121] | Guinea pigs | Influenza (H1N1,H3N2, B)** (Novartis) | C: Hexagonal (320 total projections), 0.5 mm, SA=1.29 cm2 (2 doses, 3-week interval) | 15 min, spring applicator (8 m/s) |
| 2015 [122] | Guinea pigs | Influenza (H1N1,H3N2,B) ** (Novartis) | D: Circular (low dose 2520 projections, SA=0.9 cm2 or high dose 5600 projections, SA=2 cm2), 0.2 mm (2 doses, 3-week interval) | 5 min, spring applicator (NR m/s) |
| Wellman Laboratories | ||||
| 2015 [123] | Pigs and Mice | Influenza (H1N1 Pandemic) | D: Rectangular (6×9), 0.6 mm, SA=1 cm2 (x2 patches for pigs, x1 for mice) | 15 min (some with nonablative fractional laser (NAFL)) |
| 2017 [124] | Mice | BCG* | D: Rectangular (6×9), 0.6 mm, SA=1 cm2 (x2 patches) | 15 min |
| All others | ||||
| 2007 [125] | Mice | Smallpox | C: Rectangular (8×10), 0.45 mm, SA=NR (x4 patches) | 1 sec, skin electroporation device (6 pulses of 100 Volts, 100 S pulse duration and 125mS pulse interval) |
| 2015 [126] | Mice | HepBsAg | D: Square (6×6), 0.66 mm, SA=0.36 cm2 | NR |
| 2016 [127] | Mice | HepBsAg | D: Square (6×6), 0.65 mm, SA=0.36 cm2 (2 doses, 3-week interval) | 3 min, 2N |
| 2016 [128] | Mice | Zika | C: Square (10×10), 0.75 mm, SA=NR (2 doses, 2-week interval) | NR |
| 2016 [129] | Mice | EV 71 | D: Square (15×15), 0.55 mm, SA=NR (3 doses, 2-week interval) | spring-driven applicator, ~10N/ patch |
| 2017 [130] | Pigs | HepBsAg ** (GSK) | D: Square (10×10), 0.6 mm, SA=1 cm2 (2 doses, 4-week interval) | 10 min, handheld applicator (0.5J), 5N |
| 2017 [131] | Mice | Influenza (H1N1,H5N1) | D: Square (3×3), 0.43 mm, SA=1 cm2 (H1N1 1 dose, H5N1 2 doses, 4-week interval) | 5 min |
| 2017 [132] | Mice | Influenza (H1N1,H3N2,B,B) | C: Square (20×20), 0.7 mm, SA=2.25 cm2 (2 doses, 2-week interval) | 24 hr |
| 2017 [133] | Mice | Leishmania spp | C: Rectangular (3×10), 0.4 mm (0.15 mm penetrating), SA=NR (3 doses, 3-week intervals) | 10 min |
| 2018 [134] | Rats | Mumps** (Takeda Pharmaceutical), Varicella (Biken) | H: Line (6×1), 0.41 mm, SA=NR (2 doses, 1-week interval) | NR |
| 2018 [135] | Mice | Gonorrhoeae | D: Square (10×10), 0.6 mm, SA=1 cm2 (3 doses, weeks 0, 4, 6) | 20 min |
| 2018 [136] | Mice | Typhus | D: Square (5×5), 0.44 mm, SA=1 cm2 (3 doses, 2-week intervals) | Microlancer implantation system |
| 2018 [137] | Mice | Tuberculosis (non-BCG) | D: Square (8×8), 0.25 mm, SA=1 cm2 (x1(low), x3(high, (2 doses, 4-week interval) | NR |
Abbreviations: BCG, Bacille Calmette–Guerin; C, Coated; D, Dissolving; EV 71, Enterovirus 71; H: Hollow, HepBsAg, Hepatitis B surface antigen; HIV, Human immunodeficiency virus; HPV, Human Papilloma virus; HepC, Hepatitis C; IPV, inactivated poliovirus vaccine; MR, measles rubella; N, newtons; NAFL, nonablative fractional laser; NR, not reported; PCV, pneumococcal conjugate vaccine; RSV, respiratory syncytial virus; SA, surface area; SFTSV, severe fever with thrombocytopenia syndrome virus
Notes:
Pre-clinical reference standard
Study used a licensed vaccine (vaccine manufacturer)
Our review of the vaccine patch literature summarized the published information regarding the characteristics of human skin and strategies to induce immunity in the human skin needed to support the development of vaccine patches. The next section provides a brief review of the key concepts of the microscopic anatomy of normal human skin, which provides context relevant to the depth of vaccine patch projection penetration and the state of the evidence of skin immunobiology, and then discusses key platform concepts related to inducing immunity through the skin.
4. Key platform concepts and issues related to skin characteristics and inducing immunity
As the largest and most accessible of all body organs, extensive studies of skin date back centuries. Physical properties of skin vary as a function of body site, gender, race/ethnicity, environmental exposure (e.g., the sun) and age, and selective studies are required to address the pathobiology of skin in various systemic conditions that specifically affect the skin such as systemic sclerosis, sun damage, and inflammatory dermatoses. Changes in physical properties of skin as a function of other environmental parameters such as hydration and temperature, physical conditioning (e.g., shaving or alcohol sterilization), or injury remain less well-studied. Although discussion of the vast literature on the cellular, molecular, and tissue biology of the human skin falls beyond the scope of this review, we provide a high-level discussion of microanatomy and antigen presentation in the human skin to introduce key cellular constituents of the skin immune system and their anatomy relevant to the development of vaccine patches. We go into sufficient detail in this section to address key gaps in basic sciences that have emerged as a consequence of recent advances in vaccine patch development. A recent review highlights an urgent need for technical consistency across models and platforms used by vaccine patch developers [140]. Meaningful comparisons will help to promote successful development, testing, and commercialization of vaccine patches in human skin.
Skin Variability as a Function of Age and Body Site
The available evidence provides limited information about the nature of physiological and structural changes in skin as a function of age. Some studies show measurable differences in human skin morphology as a function of age [141,142], but an in vivo study reported no significant functional or physiological differences in the skin barrier function [143]. Uncertainty remains about the extent to which observed morphological changes result in equivalent changes in physical properties and/or physiological functions of the skin in various sites. On average, most body sites studies show an epidermal thickness of approximately 60 microns, except for the forehead with an epidermal thickness between 75 and 80 microns.
Several studies that focused on the dermal thickness as a function of age, gender, body site and/or body mass index (BMI) showed some general differences across multiple categories, including subcutaneous fat as a function of gender and body site [144–147]. A study of the epidermal thickness in multiple sites in the body using Reflectance Confocal Microscopy showed variation between sites in the thickness of stratum corneum, granular layer, and papillary length, but they also noted the presence of a “striking variation at any one body site for a single individual” [148], representing 50–74% of total morphological variation measured at any given body site. Despite these studies, insufficient evidence exists for the pediatric and elderly (i.e., ≥65 years) age groups to characterize skin structure and physiology as a function of age across the full CDC-recommended vaccination schedule to make definitive conclusions about individual vaccines, and the existing studies do not provide sufficient cellular, functional, or physiological data.
Microscopic Anatomy of Normal Human Skin
The microscopic anatomy of normal human skin is commonly described in the form of several flat structural layers that in combination form an “integument” at the interface of the body and the outside environment. Starting from the outside, these include the keratin layer (also known as stratum corneum), epidermis, papillary dermis, reticular dermis, and subcutis (also known as hypodermis). At the physical scale and biological scope of vaccine patch development, the epidermis, papillary dermis, and reticular dermis represent the three most relevant subsystems of the human skin.
Figure 3 shows a histological preparation of excised human skin with micron-level resolution that reveal specific cells and compartments by general morphology and selective immunostaining. The staining technique in Panel A shows keratinocytes as the majority of cells visible, but intraepithelial lymphocytes and other cell types (e.g., Langerhans cells [LCs]) are not specifically identifiable. The dense collagen bundles seen as broad eosinophilic clumps at the bottom of the image characterize the reticular dermis, and the more delicate region of connective tissue between the reticular dermis and the epidermis is the papillary dermis, which shows multiple vascular structures, including capillaries (arrows) and lymphatics (arrowheads). The image does not provide sufficient information or detail to identify specific other cell types or structures (e.g., small nerves). Panel B shows immunostaining of an adjacent section, in which brown pigment shows on the surface of cells that express CD1a. Stained cells include intraepithelial LCs (arrowheads). CD1a-positive cells in the dermis (arrows) are also antigen presenting cells, but CD1a does not have sufficient specificity in the dermis to distinguish circulating LCs from other dermal antigen presenting cells (APCs). Morphological studies combined with selective immunohistochemistry and related techniques have the ability to characterize skin constituents with increasing sophistication. Although detailed catalogues of normal human skin as a function of age, gender and other variable are not generally available, various compositive pictures can be developed by combining data across multiple studies and techniques, some of which are described in more detail below.
Figure 3:

Histological preparation of the excised human skin for which the solid horizontal bar represents 100 microns in both panels. Panel A shows a hematoxylin and eosin stained section of paraffin-embedded human skin (original magnification 200x), with capillaries noted by arrows and lymphatics by arrowheads. Panel B shows immunostaining of an adjacent section using antibodies to CD1a with intraepithelial Langerhans Cells noted by arrowheads and CD1a-positive cells in the dermis noted by arrows.
Immunobiology of the Normal Human Skin
As suggested in Figure 3, normal human skin harbors a variety of APCs, the majority of which belong to the general category of conventional dendritic cells (DCs). Skin DCs show extensive immunophenotypic diversity relevant to both health and disease, but their subclassification into epidermal DCs, also known as LCs, and dermal DCs (DDC) serves as a useful high-level subdivision [149]. LCs, reviewed in detail by others [150,151], are resident epidermal APCs responsible for T-cell priming to antigens encountered in the epidermis. The ability of LCs to migrate to regional lymph nodes where classical T-cell priming takes place facilitates their significant immunological function. Although LCs and DDCs share many morphological and functional similarities, LCs also share many similarities with macrophages, and may behave as resident tissue macrophages with the ability for local self-renewal, as opposed to conventional DCs that derive from hematopoietic stem cells [150]. Immunophenotypically, human LCs express high levels of CD1a, which is an MHC-related membrane protein involved in the presentation of lipid antigens [151]. An endosomal protein called CD207/Langerin also expresses at high levels in humans and is a constituent of Birbeck granules, which may play a role in the internalization of viruses [152].
Kashem et al. [151] offer a catalogue of human LCs immunophenotypes, although detailed pathobiology remains an active area of investigation. Dermal APCs include a highly heterogenous population of cells [149–151,153] with at least 4 different subgroups recognized in humans [151]. First and the most prevalent of the DDCs in human skin, CD1c+ conventional DCs are classically involved in Th2 cell differentiation and immunity against parasites and helminths. CD1c+ DDCs migrate to regional lymph nodes and generally co-localize with resident lymph node DCs in proximity to the sinus endothelium of paracortex. Second, CD141+ DDCs are a minor population of conventional DCs thought to induce Th1 and cytotoxic T lymphocytes in response to a variety of antigens, including fungi, intracellular pathogens, and tumor antigens. CD207/Langerin is expressed in mouse homologs of CD141+ DDCs, creating a complex picture involving CD1c+ DDCs that may also express Langerin, and migrating LCs also express Langerin. CD141+ DDCs turn over at a high rate and migrate rapidly into the deep T cell zone of regional lymph nodes. Third, plasmacytoid DCs (pDCs) circulate throughout the body and can be found in human skin under inflammatory conditions. Morphologically, pDCs resemble plasma cells and lack dendritic extensions typical of LCs and other DDCs. Finally, monocyte-derived macrophages represent the last known category of dermal APCs in human skin. Dermal macrophages are well adapted for phagocytosis but are inferior to DDCs in antigen presentation to T cells. Despite the wealth of evidence, as summarized in Table 3, uncertainty (or possibility biological variability) remains about some of the markers of human immunophenotypes for some APCs in some parts of the skin relevant to vaccine patches. In addition, testing of vaccine patches in animals must consider the different sets of immunophenotypes relevant to those species and locations (e.g., comparison of mice to humans [9], and choose appropriate animal models for human vaccine-preventable diseases [81,113,154]).
Table 3:
Summary of evidence about the location, Antigen Presenting Cell type, and typical immunophenotype characteristics
| Langerhans Cell | CD1c+ Conventional Dermal Dendritic Cell | CD141+ Conventional Dermal Dendritic Cell | Plasmacytoid Dendritic Cell | Macrophage | |
|---|---|---|---|---|---|
| Primary Skin Location | Epidermis | Dermis | Dermis | Dermis | Dermis |
| Mouse Homolog | LC | cDC2 | cDC1 | pDC | Macrophage |
| CD1a | ++ | + | −/+ | −/+ | − |
| CD1c | + | + | − | −/+ | + |
| CD11b | +/− | + | − | − | +/− |
| CD11c | +/− | + | +/− | − | + |
| CD14 | − | − | − | + | − |
| CD141 | − | −/+ | ++ | −/+ | − |
| CD207/Langerin | ++ | +/− | −/+ | − | − |
| Other Unique Characteristic | Birbeck Granule | CD163+ CD68+ |
Grayed out boxes indicate markers that positively identify each category. Variability in expression and/or lack of consensus about human immunophenotype is indicated using +/− (likely positive) and −/+ (likely negative).
In addition to normal APCs in the human skin, the role of the disruption of the physical barrier in triggering of an immune response represents a key consideration in vaccine patch immunobiology. As immune sentinels, keratinocytes can sense physical injury (e.g., abrasion, puncture wounds) induced by micron-sized projections and produce pro-inflammatory cytokines that in turn activate DDCs [153,155]. Similarly, dermal fibroblasts and other dermal components can activate various components of the immune system in response to puncture or other forms of physical trauma. Review of the existing literature demonstrates that the application of vaccine patches can induce an inflammatory response, as evidenced by skin erythema and other macroscopic inflammatory changes [68,156]. However, the extent and pattern of local immune system activation secondary to vaccine patch application and its role in inducing immunity remains a topic in need of further study. Of note, one of the 2 leading vaccine patch development groups (i.e., Queensland/Vaxxas/Nanopatch) uses very dense arrays of coated and relatively short projections intended to kill epidermal cells while depositing the vaccine, because the immunogenic signals that are released from impacted cells may help with dose sparing [96,157].
Non-Invasive Skin Imaging
As shown above, ex vivo microscopy in the form of conventional histology, or in various other forms such as immunofluorescence or electron microscopy, represents the gold standard for cellular and subcellular study of the human skin. However, ex vivo microscopy is an invasive procedure requiring tissue removal, often in the form of a skin punch or excisional biopsy. Although skin biopsy represents a minor medical procedure, healthy human volunteers, especially in the pediatric age group, do not commonly undergo biopsy for purely investigational purposes. Post-mortem studies at the time of an autopsy offer a more practical option for baseline human studies, but post-mortem changes add complications, which depend on the typically not-controlled post-mortem time interval and co-morbid conditions that often served as the primary reason for medical autopsies. Forensic autopsies for accidental death can potentially overcome some of these limitations, but baseline studies in forensic autopsies are rarely performed. Because of these limitations, many recent dermal vaccine and vaccine patch studies rely on non-invasive imaging by high resolution ultrasound or in vivo optical technologies. Many studies used high-frequency ultrasound scanners of 10–50 MHz to image skin because they offer a reasonable tradeoff between axial resolution and penetration depth [144,145,158]. However, ultrasound devices generally prove inferior to optical methods in measuring submillimeter structures that may be necessary for detailed characterization of cellular and/or microvascular morphology in the submillimeter penetration depth, which is the relevant scale for vaccine patch applications. Ultrahigh frequency ultrasounds (70–100 MHz) can counter this limitation, but they are rarely used in skin measurements. In addition, ultrahigh frequency ultrasound methods typically lose some of the benefits of general ultrasound techniques, such as deeper penetration depth. In one comparative study involving ultrahigh frequency ultrasound and optical coherence tomography (OCT), the optical method showed better axial resolution than 100 MHz ultrasound (5.5 μm and 16 μm, respectively), while both had limited penetration depth in the range of approximately 1 mm [158]. More generally, OCT gave better lateral resolution, while ultrasound provided better contrast of the lesion to surrounding tissue [158].
The inability to include multiplex investigations such as immunophenotyping for cellular or subcellular studies represents a limitation of in vivo imaging technologies. However, in vivo imaging methods provide a significant opportunity for live cell and/or dynamic studies, which is difficult in animal models and virtually impossible in humans with invasive skin biopsies. Bachy et al. present a highly informative animal study of the dynamics of immune system activation by live adenovirus microneedle arrays that sheds light on the immunobiology of the system and provides a nice animal model for in vivo imaging of microneedle dissolution dynamics and skin repair [159]. In a separate study, Liu et al. used OCT to successfully characterize dynamics of the controlled release of dissolving silk microneedles in mice [160]. Unfortunately, the various methodologies developed in animal studies do not immediately apply in human subjects without limitation or further development. Bal et al. used an in vivo confocal microscopy technique to study the dynamics of a fluorescent dye tracer through conduits produced by microneedles of similar length but with various shapes in a small group of adult volunteers [161]. They observed that microneedle geometry, but not the manner of application affected the shape and depth of the conduits and the penetration of the fluorescent dye. Using a comparable imaging strategy of multiphoton microscopy, Wei et al. characterized the diffusion of rhodamine-conjugated dextrans applied to excised human skin through microneedle arrays [162]. As such, they successfully characterized the rate of dissipation of dextran macromolecules as a function of molecular weight at the imaged skin layers. Although more limited in nature than the animal imaging studies, human subject studies that combine in vivo imaging with dynamic biochemical or cellular studies are increasingly needed to better characterize the interaction between various vaccine patches and the human subjects.
Multiple research groups used other imaging modalities such as scanning electron microscopy (SEM) and fluorescence microscopy in a variety of circumstances [31,92,159,161–166]. These invasive techniques offer super-high resolution, generally for static and morphologically well-defined objects such as the projections of the vaccine patch, but they appear to provide limited value in dynamic, large scale or in vivo studies.
Figure 4 provides a high-level view of some of the physical issues and imaging constraints described here. Panels A-D show shows the representative performance of four imaging technologies in the context of typical vaccine patch projections, which are depicted in Panel E. Panel A shows a hematoxylin and eosin stained section of paraffin-embedded human skin showing the relative proportions of keratin, epidermis, papillary dermis, reticular dermis and subcutaneous adipose tissue (hypodermis), respectively. (For ease of reference, these layers are highlighted in the right-hand side of Panel A using pseudocolors of red, pink, blue, no color, and yellow, respectively.) The scale bar represents the 1 mm scale across all panels, corresponding to the cut off used for needle size in this review. Panel B (adapted from [145] and redrawn to scale) represents the high frequency ultrasound (40 MHz) methodology commonly used to assess skin thickness in various body sites. Although the epidermis, dermis, and subcutaneous layers are generally discernable, the image shows insufficient resolution to assess structural details within each layer, particularly at the scale relevant to vaccine patches. Panel C (adapted from supplementary data in [167] and redrawn to scale) shows increased morphological detail in the dermis and superficial subcutaneous tissue by ultrasound imaging at ultrahigh frequencies (70 MHz shown). The increased resolution at higher frequencies generally comes at the expense of shallower penetration depth for comparable probes, as seen by the relative loss of signal below the dermis compared to Panel B. At frequencies approaching 100 MHz (not shown), ultrasound and optical imaging technologies reach comparable resolution at the superficial layers of the skin [158]. Panel D (adapted from [168] and redrawn to scale) shows simultaneous dual-band line-field confocal OCT. Unlike typical ultrasound images, this high-resolution OCT image provides significant cellular detail at the level of epidermis and superficial papillary dermis (inset), but the penetration depth is not sufficient for full thickness imaging of the entire dermis. Panel E facilitates comparison of the images in Panels A-D by redrawing to scale a single dissolving microneedle (adapted from [64] on the left) and a single projection of a coated nanopatch (adapted from [92] on the right). The black rectangle drawn at the base of the needle corresponds to the position of the corresponding patch inner surface.
Figure 4.

Scale drawing (bar represents 1 mm) with Panel A showing a hematoxylin and eosin stained section of paraffin-embedded human skin showing the relative proportions of keratin, epidermis, papillary dermis, reticular dermis, and subcutaneous adipose tissue, respectively, highlighted in the right-hand side by pseudocolors of red, pink, blue, no color, and yellow, respectively. Panel B shows an image from high frequency ultrasound (40 MHz) (adapted with permission from [145]), Panel C shows detail in the dermis and superficial subcutaneous tissue by ultrasound imaging at ultrahigh frequencies (70 MHz shown) (adapted with permission under Creative Common license http://creativecommons.org/licenses/by/4.0/ from supplementary data in [167]). Panel D shows simultaneous dual-band line-field confocal optical coherence tomography (OCT) (adapted with permission from [168] © The Optical Society) that provides very high resolution (note the significant cellular detail in the inset below at the level of the epidermis and superficial papillary dermis not visible in Panels B and C), which comes at the expense of relatively short penetration depth. Panel E shows a single dissolving projection (adapted with permission from [64]) on the left, and a single coated nanopatch projection (adapted with permission under Creative Common license http://creativecommons.org/licenses/by/4.0/ from [92]). Finally, the black rectangle drawn at the base of the needle corresponds to the position of the corresponding vaccine patch inner surface.
5. Key platform concepts and issues for patch developers
Over the course of the last 15 years, vaccine patch developers explored and addressed numerous concepts related to the fabrication and design of the vaccine patches, which supported the 116 published studies identified in section 3 of our review (with multiple published reviews of different fabrication and design approaches, including [15,169,170]). Based on our review of the literature and discussions with key stakeholders, this section discusses key platform issues that we identified for patch developers.
To become viable commercial products, innovative vaccine patches will need to prove safe and effective, and meet non-inferiority criteria when compared to any existing vaccines. Several recent reviews and studies [18,20,139,171] identified key issues that influence design choices made by patch developers. These reviews highlight opportunities to identify desirable product attributes and to develop target product profiles as a means for potential users to provide guidance about their preferences for different product attributes. The ideal site placement and duration of wear for vaccine patches will influence their initial design and guide their subsequent redesign ahead of clinical deployment. Research that identifies the optimal delivery sites for each product with respect to the induction of immunity and acceptability by vaccine recipients could reduce the need for testing each product by guiding development and potentially offering some standardization across the platform. In the absence of platform-related guidance, vaccine patch developers and other researchers continue to demonstrate safety and acceptability of their designs [68,94,156,172–175], with published literature reviews of acceptability studies now available [20,176]. In addition to recommendations related to ideal vaccine patch site placement, guidance related to vaccine patch delivery that would help across the platform includes recommendations related to wear time duration, characterization of recipient tolerance of vaccine patch application force by age and body site, skin site preparation needs (e.g., hair removal, cleaning, etc., which must not neutralize live vaccines or react with any components and should be consistent with the design of any clinical trials performed), post-application site care (if needed), and requirements related to the ability to confirm successful delivery (i.e., at the time of injection and/or long-term). The innovation of potential long-term recording of vaccine delivery [177] could add costs and affect acceptability (e.g., positively by helping health systems and individuals track their immunity status and/or negatively by leaving permanent markers that individuals may not want). Additional guidance, including development of international consensus guidelines and standards as appropriate, necessary, and/or useful [20,140], would also help individual vaccine patch developers prioritize their designs to achieve required or desirable attributes, and allow for trade-offs on product attributes that matter less. For example, vaccine patches could be designed to offer increased efficacy, increased thermostability, eliminate the need for reconstitution, make vaccine delivery easier, require less training, reduce or eliminate sharps, decrease medical waste, offer single-use and single-dose administration, reduce vaccine wastage, reduce or eliminate the pain and risks of injections, save costs, etc., if any such requirements were known a priori and included in the initial design and fabrication. The ideal target product profile may vary for developed and developing countries and/or for different vaccines [20,139], and any such differences could also be recognized and incorporated early in the development stage. The World Health Organization (WHO) recently published an example of a target product profile for measles-rubella (MR) combination vaccine [178]. Current research on patch delivery for other pharmaceutical products (i.e., non-vaccine) may provide information relevant to the vaccine patch platform, particularly for products that target healthy children and adults.
Vaccine patches will require high-quality, cost-effective, and reliable processing under good manufacturing practice (GMP) conditions. Vaccines are highly-regulated given their use in healthy children, which will imply significant investments of regulatory compliance costs. In addition, significant uncertainty remains about the need for sterile vs. low bioburden production, and the processes regulatory authorities will find acceptable [20,139,140,179]. Low bioburden production means not requiring sterility for vaccine patches (due to their administration of vaccine to non-sterile skin) but would limit any organisms in the final product to very low levels. Low bioburden production would save significantly on production costs, because sterile production processes require isolators and other costly equipment. Production and design choices will determine the cost of vaccine patches, and thus the cost premium relative to existing syringe and needle or other presentations for existing vaccines.
Developing low-cost, scalable, and reliable designs for manufacturing of vaccine patches currently represents a translational hurdle [20]. Developing mass production processes typically occurs in the context of proprietary activities by companies who will need to make significant financial investments that they will need to be convinced that they can recover [139]. Lessons learned and technology transfer from the commercial development and large-scale production of non-vaccine patch products may help to support some vaccine patch development. For effective and timely clinical translation, vaccine patch developers will need to partner with vaccine manufacturers or decide to become vaccine manufacturers themselves, for example by purchasing a vaccine manufacturer to ultimately deliver a licensed product. We emphasize that the process for patch developers to become a vaccine manufacturer de novo would take many years under current regulatory pathways.
6. Key platform concepts and issues for vaccine manufacturers
The research laboratory processes used by vaccine patch developers to create the patches they use for clinical trials will influence the starting point for vaccine manufacturers as they evaluate the potential for mass production. Mass production of vaccine patches will also require overcoming significant design challenges related to operating at scale, including the need to perform inline inspection and quality control (QC) testing, establish and maintain environmental controls (temperature and humidity), and manage the logistics of chemistry, manufacturing, and control (CMC) processes. A recent review highlighted the absence of standardized techniques and equipment used by patch developers to demonstrate mechanical properties of vaccine patches, and suggested an “urgent need” for standards that would support consistent comparisons to promote innovation and successful commercialization of vaccine patches [140].
For all vaccine manufacturers, commercializing a new product will likely require the payment of significant costs for any late-stage (e.g., phase 2 or 3) clinical trials to demonstrate efficacy, non-inferiority, and durability of immunity required to support licensing the new vaccine patch product, and they will need to perceive a reasonable expectation of recovering these costs. In contrast to new vaccines, the development of vaccine patches for existing vaccines may only require bridging studies, but it could also lead to changes in required formulation or concentrations (e.g., excipients, stabilizers), which may ultimately require extensive and expensive regulatory changes for the vaccine itself [20]. Few financial incentives for innovation seem apparent for current low-cost, well-established vaccines, in some cases licensed many years ago, suggesting potential high costs and risks to reopening safety profiles or changing production processes to meet current regulatory requirements [20]. In addition, for vaccines already produced at large scale, vaccine manufacturers will not see the need for dose sparing, which would only serve to make some of their existing vaccine production capacity redundant. However, if one manufacturer dominates a particular market, then incentives may exist for alternative new products offered by other manufacturers that allow them to compete for market share.
The stability of the supply chain will represent a critical consideration for vaccine manufactures. Manufacturers will most likely prefer to co-locate the patch and vaccine production, but they will need to evaluate the cost-effectiveness of performing some production elsewhere. As with all vaccine manufacturing processes, evaluation of the supply chain will include consideration of raw material sourcing (e.g., particularly those derived from animals like bovine and porcine components), which may raise issues related to the acceptability of the ultimate vaccine patch product. For example, the use of a porcine-based component in a rubella-containing vaccine led to acceptability issues in Indonesia that significantly affected coverage [180], whereas vaccines containing bovine components may not be acceptable in other countries (e.g., India).
In addition to any specific issues related to the vaccine patch presentation, vaccine manufacturers will need to address the issues that typically come with the production and licensing of vaccine products. These include managing the nature of the batch processing, QC, packaging, labeling, and storage of vaccines, and the time delays related to regulatory processing, licensing, compliance, and WHO pre-qualification (for those seeking to sell to markets that require it), all of which will require resource investments. All the details related to labeling and packaging requirements (e.g., required language or codes, temperature deviation monitors similar in purpose to vaccine vial monitors, desiccants, etc.) represent areas that will need development. Vaccine manufacturers will also face decisions related to pursuing single or multiple antigens in the vaccine patch formulation and on making trade-offs associated with product attributes based on their perceptions and expectations about the future market. For large multi-national companies that work with multiple national regulatory authorities, seeking and obtaining approval for process changes can represent a time-consuming and expensive undertaking, which may also impact the willingness of vaccine manufacturers to adopt unfamiliar technologies. In addition, the innovative nature of vaccine patches implies the need for a new regulatory pathway, and currently no consensus exists across functioning national regulatory authorities (NRAs) on such a pathway, which will mean extensive discussions with multiple NRAs and imply associated costs and time delays.
The nature of the vaccine market, which includes relatively few manufacturers and a relatively small number of large buyers, influences incentives and leads to segmentation. Specifically, segmentation translates into lower-priced multi-dose vaccines for developing countries, and relatively higher-priced vaccines targeted at developed countries (e.g., single dose, combination vaccines), often produced by only one or two manufacturers. In developed countries, the existing market incentives are relatively favorable, with some incentives for innovation coming from the opportunity of gaining significant market share for superior products (i.e., more effective, safer, easier-to-deliver, and/or more cost-effective options than any currently available). As discussed above, influenza appears likely to be the first potential vaccine patch product to complete phase 3 clinical trials. In addition, with potential demand for the entire population for annual administration of seasonal influenza vaccine that changes in formulation annually, a flu vaccine patch could prove to be a potentially viable vaccine patch product with significant consumer appeal. The development and licensure of FluMist™ as a nasal delivery method suggests a willingness by vaccine manufacturers in developed countries to pursue alternatives to the traditional needle injections despite the estimated $1 billion cost (i.e., $340 million to license FluMist™ and an estimated total costs of 2–3 times higher by the time patients started receiving it [181]). However, innovation in vaccine delivery for some products targeted for developed countries may require some support from push incentives, which subsidize development costs, and/or pull incentives, which reward manufactures for offering the desired product, or a public-private partnership (e.g., for pandemic vaccines), particularly in the context of highly uncertain demand and expected relatively low margins for vaccine products compared to other therapeutic agents.
Based on the current status of vaccine patch development and discussions with key stakeholders, the business case for vaccine patches does not currently support the required investments by vaccine manufacturers that supply the developing country markets to expect licensed vaccine patches to come to the market soon [20]. For developing countries, prior experience with new vaccine products will influence manufacturer willingness to engage in development activities. For example, the rotavirus vaccine market currently includes multiple products now pre-qualified by WHO with different attributes, and it does not show a willingness of vaccine purchases to pay a price premium for a thermostable vaccine (i.e., to prefer thermostable ROTASIIL®[182] to less thermostable but cheaper alternatives [183]) to date. Public-private partnerships can bring investments that share the costs and risks of the vaccine development process, particularly for products with thin profit margins or relatively smaller markets. For example, public-private partnerships were required to support the development of vaccine products needed in developing countries, including a meningitis vaccine for central Africa (i.e., for MenAfriVac™ [184])), oral cholera vaccine [185], and other vaccines [186,187] However, such partnerships do not always lead to commercial success. For example, the partnership that sought to develop an aerosol formulation for MR vaccine invested significant resources in the conduct of several clinical trials, including a trial that ultimately showed the aerosol formulation did not meet the non-inferiority criteria compared to syringe and needle presentation [188].
The licensure and successful marketing of the first vaccine patch product will establish the platform and set precedents that create opportunities and/or challenges for future vaccine patch products. Consumer demand for multiple vaccine patches could follow the establishment of a licensed vaccine patch product that receives wide public acceptance and establishes the platform. In this regard, health system and consumer experience with the first vaccine patch product will likely influence acceptance and demand for other vaccine patch products, as may any experience with other (i.e., non-vaccine) patch-delivered pharmaceutical products. Notably, if the first vaccine patch product leads to greater acceptance of and demand for vaccination and thus higher coverage, then this could increase population immunity and demonstrate the potential for significant benefits. In addition, the first product to market will establish the first regulatory path for the platform, packaging requirements (e.g., protection of projection integrity and the vaccine antigen, the need for desiccants, waste disposal), acceptability of the product within the health system (e.g., costs, ease of use, administration times and skills needed, storage, potential sharps, residual active ingredients after delivery, confirmation of delivery signals), acceptability by individual vaccine recipients, and other firsts. In addition, widespread use of the first vaccine patch will present the first opportunity to observe potential new adverse events (e.g., skin reactions, unintended use or ingestion of a vaccine patch, packaging, etc.), as well as any potential injury due to application/misapplication. With seasonal influenza as the apparent front runner for vaccine patch development, we note the potential for issues unrelated to the vaccine patch itself to impact the product. For example, following the recommendation and adoption of FluMist™ by the US, choices related to the strains used in the formulation led to the temporary suspension of its recommended use in the US market [189–191], which had nothing to do with the delivery mechanism.
7. Conclusion
Over the past two decades, significant advances in engineering supported the development of vaccine patch technologies with the potential to deliver vaccines through the skin while taking advantage of other favorable properties (e.g., increased thermostability, no reconstitution or field preparation, etc.) [15,18,192,193]. At the same time, we now have a detailed understanding of antigen presentation in the human skin [149–151], and development of non-invasive imaging modalities have enabled in vivo studies of the human skin with submicron resolution [145,158,160,168]. A coordinated convergence of the three disciplines discussed here could help to develop the baseline basic science necessary to characterize and model the immunobiology of various vaccine patch technologies and accelerate progress in vaccine patch platform development. For instance, further research could help develop broad reference standards for physical characteristics of skin across multiple categories of age, gender, site, BMI, as well as other potential variables such as nutritional status and ethnicity, or physical conditions such as humidity and temperature. Specific vaccine patch delivery methodologies could be refined to specific human skin characteristics relevant to the given technique. For instance, BMI and gender may be relevant to longer projections that potentially penetrate through the dermis at a given site, while age and body site may be more relevant to shorter projections that either stay within the epidermis or superficially penetrate the papillary dermis. Several studies also demonstrate that the design of the projections can impact pain [194–196].
This review highlights tremendous progress to date in the development of vaccine patches, and provides a glimpse into the many future opportunities to deploy vaccine patches in broad use. Innovative vaccine delivery technologies offer many promises for increasing immunization coverage. Given the apparent lack of incentives that exist for vaccine manufacturers to pursue the development of vaccine patches for developing country markets, public-private partnerships will likely be needed if any key stakeholder wants to realize (or accelerate) the licensure of vaccine patches for widespread use, particularly for existing, low-margin vaccines. Although developed countries continue to explore the use of vaccine patches to support disease eradication efforts that require reaching difficult-to-access populations in developing countries, the willingness-to-pay a premium for vaccine patches remains uncertain, even in an eradication context. The opportunity for new vaccines, and for expensive antigens that would benefit from dose-sparing, might improve the value proposition for vaccine patches and make investments in their development attractive to vaccine manufacturers in some cases. Overall, the incentives that vaccine manufacturers perceive and realize will depend on the value that consumers and health systems ultimately place on the set of attributes that each product brings to the system.
8. Expert Opinion
Despite substantial advances in microneedle technology and basic science, progress towards clinical deployment of microneedle skin patches for vaccination remains relatively slow, which reflects current financial incentives. The lack of a perceived sufficient return on investment for vaccine manufacturers to make incremental improvements in vaccine delivery technology broadly, and for low-cost, legacy vaccines specifically, represents a critical barrier or translational valley of death [197,198]. The lack of competitive forces that would create financial incentives for patch developers to become first movers and disrupt the market by also taking on the vaccine manufacturing aspects of the products (i.e., becoming vaccine manufacturers themselves) similarly means vaccine patch development will likely depend on external financing or financial incentives.
Similar to other situations discussed above, the development and adoption of vaccine patch products for developing country markets will likely require the support from public-private partnerships and/or other incentives, such as targeted national or international investments, to overcome the early economic barriers. In this context, financial partners may influence the overall timing of availability of vaccine patches to the market. This could accelerate progress (e.g., by supporting the establishment of large-scale GMP manufacturing processes and facilities in parallel with phase 2 clinical testing [20]) and/or slow it down (e.g., by requiring multiple patch developers to all reach each specific stage before proceeding such that the trials generate comparable results by using the same settings and criteria for advancement to the next stage). Thus, while some patch developers may complete initial development with respect to clinical formulation, processing, and assessing prototype stability, they may experience delays in the clinical testing timeline at multiple points if they need to wait for others to finish the prior phase. In addition, all patch developers will need to wait for as any financial partners evaluate results and makes stage-gate decisions about continuing to the next step. The establishment of a public-private partnership to develop the general platform would further allow the public health and donor partners to negotiate some controls on the prices of the final products, particularly if the financial support that they provide shares the costs and risks of product development.
For developed country markets, sufficient private financing (e.g., venture capital) may already exist and the competitive nature of the market may alleviate the need for a public-private partnership. Specifically, vaccine patches may provide an opportunity to differentiate a product from other competitors, which could offer a significant market advantage. Nevertheless, national research funding mechanisms (e.g., a National Institutes of Health study section, and/or a targeted funding program, and/or support from Biomedical Advanced Research and Development Authority in the US) could significantly stimulate basic science and translational research for vaccine patches and accelerate their use. The creation of broad funding mechanisms for the patch platform (including non-vaccine applications) could help to resolve some shared questions, while specific funding to support vaccine patches would likely better support the basic science immunobiological questions relevant to specific vaccines.
Although we discuss a number of current issues, these represent surmountable hurdles that can be overcome with sufficient resources, and they should not be viewed as barriers to innovation or clinical deployment. Vaccine patches could support efforts to significantly increase immunization coverage [20]. Future reviews will likely show a continued acceleration of vaccine patch research and development activity and insights related to many of the topics discussed in this review. Innovation in the use of patch technology for general pharmaceutical product delivery (non-vaccine products) may further patches as a delivery platform broadly for pharmaceutical products, which may help to accelerate vaccine patch development [15]. Health systems and consumers could easily adapt to the platform and could prefer immunization delivered by vaccine patches, although perceptions about efficacy and actual efficacy will impact uptake [175]. We anticipate that within the next 10 years, the commercial viability of a vaccine patch for influenza vaccine will become clear, and that efforts to develop vaccine patches as a delivery platform will mature. Progress will depend on the investments made to support multiple streams of work by numerous stakeholders.
9. Article highlights.
Systematic review of the literature reveals substantial progress in the development of vaccine patches
Efforts to translate research and development into commercial products will depend on success convergence of multiple streams of work
Different incentives exist for commercialization of vaccine patches in developed and developing countries
Financial incentives for the platform will impact vaccine patch design
Standardizing models and techniques would help to promote successful commercialization of vaccine patches in human skin
Acknowledgments
The initial draft of this article was prepared when Kamran Badizadegan was serving as the Chair of Pathology at Nationwide Children’s Hospital and professor of pathology at The Ohio State University Wexner College of Medicine. We thank Shanda Boyle, Gitte Giersing, David Hoey, Suresh Jadhav, Ravi Menon, Michael Royals, and multiple anonymous individuals for helpful comments and discussions.
Funding
This paper was supported by Cooperative Agreement Number 5NU2RGH001913-03-00 funded by the Centers for Disease Control and Prevention (first and last authors). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Appendix
Histological preparations shown in Figures 3 and 4 represent normal portions of skin in pediatric patients who underwent surgical excision for a pigmented skin lesion located at least 2 mm away from the normal skin represented in the images. All tissues were fixed in neutral-buffered formalin for at least 24 hours before being processed routinely for paraffin embedding and sectioning at 4–5 microns followed by hematoxylin and eosin staining. For immunohistochemistry, paraffin sections were deparaffinized with xylene and hydrated through a graded series of alcohol. After antigen retrieval in 0.1M citrate buffer (pH 6.0) in a microwave oven for 10 min, inhibition of endogenous peroxidase activity was performed by immersion in 3% hydrogen peroxidase in methanol. The sections were then incubated with the primary antibodies, followed by thorough washing in phosphate-buffered solution (PBS), incubation with the biotinylated secondary antibody, followed by the avidin-biotinylated horseradish peroxidase complex, and finally developed using 3,3’-Diaminobenzidine as chromogen. The nuclear counterstaining was accomplished using Mayer’s hematoxylin. All reagents, antibodies and instruments used to process and stain the tissue were from Leica Microsystems Inc. (Buffalo Grove, IL). Photographs were taken on a BX43 microscope (Olympus America, Center Valley, PA) coupled to an Infinity2–5 camera (Lumenera, Ottawa, Ontario). Images were captured in Photoshop (Adobe Systems, San Jose, California), optimized for brightness and color to generally maximize the width of the image histogram. Final images were cropped to assemble the composite images shown here.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Papania M, Zehrung D, Jarrahian C. Technologies to Improve Immunization In: Plotkin’s Vaccines. Plotkin S, Orenstein W, Offit P, Edwards K (Ed.^(Eds) (Elsevier, Philadelphia, PA, 2017) 1320–1353. [Google Scholar]
- 2.Zheng Z, Diaz-Arevalo D, Guan H, Zeng M. Noninvasive vaccination against infectious diseases. Hum Vaccin Immunother, 14(7), 1717–1733 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine, 28(51), 8049–8052 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Tuft L, Yagle EM, Rogers S. Comparative Study of the Antibody Response After Various Methods of Administration of Mixed Typhoid Vaccine: With Particular Reference to the Intradermal and Oral Methods*. The Journal of Infectious Diseases, 50(2), 98–110 (1932). [Google Scholar]
- 5.Bavdekar A, Oswal J, Ramanan PV et al. Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in India: A randomized, parallel group, non-inferiority trial. Vaccine, 36(9), 1220–1226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudinski MR, Houser KV, Morabito KM et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. The Lancet, 391(10120), 552–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bavdekar A, Malshe N, Ravichandran L et al. Clinical study of safety and immunogenicity of pentavalent DTP-HB-Hib vaccine administered by disposable-syringe jet injector in India. Contemporary clinical trials communications, 14, 100321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro JR, Hodgins B, Hendin HE et al. Needle-free delivery of influenza vaccine using the Med-Jet(R) H4 is efficient and elicits the same humoral and cellular responses as standard IM injection: A randomized trial. Vaccine, 37(10), 1332–1339 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Matsuo K, Hirobe S, Okada N, Nakagawa S. Frontiers of transcutaneous vaccination systems: novel technologies and devices for vaccine delivery. Vaccine, 31(19), 2403–2415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nature Medicine, 6(12), 1403–1406 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Behrens RH, Cramer JP, Jelinek T et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis, 14(3), 197–204 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Etchart N, Hennino A, Friede M et al. Safety and efficacy of transcutaneous vaccination using a patch with the live-attenuated measles vaccine in humans. Vaccine, 25(39–40), 6891–6899 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Van Kampen KR, Shi Z, Gao P et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine, 23(8), 1029–1036 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Saitoh A, Aizawa Y. Intradermal vaccination for infants and children. Hum Vaccin Immunother, 12(9), 2447–2455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev, 64(14), 1547–1568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides an extensive review of using microneedle patches to deliver drugs and vaccines
- 16.Suh H, Shin J, Kim Y-C. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res, 3(1), 42–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother, 12(11), 2975–2983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leone M, Mönkäre J, Bouwstra JA, Kersten G. Dissolving Microneedle Patches for Dermal Vaccination. Pharmaceutical Research, 34(11), 2223–2240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 240, 135–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyraud N, Zehrung D, Jarrahian C, Frivold C, Orubu T, Giersing B. Potential use of microarray patches for vaccine delivery in low- and middle- income countries. Vaccine, 37(32), 4427–4434 (2019). [DOI] [PubMed] [Google Scholar]; ** Provides the perspective of the World Health Organization Initiative for Vaccine Research on vaccine patches for developing countries
- 21.Gruber M, Marshall V. Regulation and Testing of Vaccines In: Plotkin’s Vaccines. Plotkin S, Orenstein W, Offit P, Edwards K (Ed.^(Eds) (Elsevier, Philadelphia, PA, 2017) 1547–1565. [Google Scholar]
- 22.Andrianov AK, DeCollibus DP, Gillis HA et al. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proceedings Of The National Academy Of Sciences Of The United States Of America, 106(45), 18936–18941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One, 4(3), e4773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y-C, Quan F-S, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine, 27(49), 6932–6938 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan F-S, Kim Y-C, Yoo D-G, Compans RW, Prausnitz MR, Kang S-M. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. Plos One, 4(9), e7152–e7152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Q, Zarnitsyn VG, Ye L et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A, 106(19), 7968–7973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearton M, Kang SM, Song JM et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine, 28(37), 6104–6113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y-C, Quan F-S, Song J-M et al. Influenza immunization with trehalose-stabilized virus-like particle vaccine using microneedles. Procedia Vaccinol, 2(1), 15–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS Pharmscitech, 11(3), 1193–1201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 142(2), 187–195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y-C, Quan F-S, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. The Journal Of Infectious Diseases, 201(2), 190–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan F-S, Kim Y-C, Compans RW, Prausnitz MR, Kang S-M. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 147(3), 326–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan F-S, Kim Y-C, Vunnava A et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. Journal Of Virology, 84(15), 7760–7769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JM, Kim YC, Barlow PG et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res, 88(2), 244–247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song J-M, Kim Y-C, Lipatov AS et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clinical And Vaccine Immunology: CVI, 17(9), 1381–1389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther, 17(6), 811–814 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiraishi Y, Nandakumar S, Choi S-O et al. Bacillus Calmette-Guérin vaccination using a microneedle patch. Vaccine, 29(14), 2626–2636 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weldon WC, Martin MP, Zarnitsyn V et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol, 18(4), 647–654 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res, 28(1), 135–144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. The Journal of infectious diseases, 204(4), 582–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi H-J, Yoo D-G, Bondy BJ et al. Stability of influenza vaccine coated onto microneedles. Biomaterials, 33(14), 3756–3769 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y-C, Song J-M, Lipatov AS et al. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. European Journal Of Pharmaceutics And Biopharmaceutics: Official Journal Of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik E.V, 81(2), 239–247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song J-M, Kim Y-C, O E, Compans RW, Prausnitz MR, Kang S-M. DNA vaccination in the skin using microneedles improves protection against influenza. Molecular Therapy: The Journal Of The American Society Of Gene Therapy, 20(7), 1472–1480 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weldon WC, Zarnitsyn VG, Esser ES et al. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. Plos One, 7(7), e41501–e41501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsonanos DG, Vassilieva EV, Stavropoulou A et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific Reports, 2, 357–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.del Pilar Martin M, Weldon WC, Zarnitsyn VG et al. Local response to microneedle-based influenza immunization in the skin. Mbio, 3(2), e00012–e00012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine, 31(34), 3403–3409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine, 31(34), 3396–3402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan F-S, Kim Y-C, Song J-M et al. Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch. Clinical And Vaccine Immunology: CVI, 20(9), 1433–1439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi H-J, Bondy BJ, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 166(2), 159–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y-C, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 172(2), 579–588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pulit-Penaloza JA, Esser ES, Vassilieva EV et al. A protective role of murine langerin⁺ cells in immune responses to cutaneous vaccination with microneedle patches. Scientific Reports, 4, 6094–6094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang BZ, Gill HS, He C et al. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release, 178, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim M-C, Lee JW, Choi H-J et al. Microneedle patch delivery to the skin of virus-like particles containing heterologous M2e extracellular domains of influenza virus induces broad heterosubtypic cross-protection. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 210, 208–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koutsonanos DG, Esser ES, McMaster SR et al. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine, 33(37), 4675–4682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kines RC, Zarnitsyn V, Johnson TR et al. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS One, 10(3), e0120797 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi H-J, Song J-M, Bondy BJ, Compans RW, Kang S-M, Prausnitz MR. Effect of Osmotic Pressure on the Stability of Whole Inactivated Influenza Vaccine for Coating on Microneedles. Plos One, 10(7), e0134431–e0134431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Ye L, Lin F et al. Intradermal Vaccination With Adjuvanted Ebola Virus Soluble Glycoprotein Subunit Vaccine by Microneedle Patches Protects Mice Against Lethal Ebola Virus Challenge. The Journal Of Infectious Diseases, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Ye L, Lin F et al. Intradermal immunization by Ebola virus GP subunit vaccines using microneedle patches protects mice against lethal EBOV challenge. Scientific Reports, 8(1), 11193–11193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Lee Y, Kwon YM et al. Vaccination by microneedle patch with inactivated respiratory syncytial virus and monophosphoryl lipid A enhances the protective efficacy and diminishes inflammatory disease after challenge. PLoS One, 13(10), e0205071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MC, Kim KH, Lee JW et al. Co-Delivery of M2e Virus-Like Particles with Influenza Split Vaccine to the Skin Using Microneedles Enhances the Efficacy of Cross Protection. Pharmaceutics, 11(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vassilieva EV, Kalluri H, McAllister D et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Delivery And Translational Research, 5(4), 360–371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan SP, Koutsonanos DG, Del Pilar Martin M et al. Dissolving polymer microneedle patches for influenza vaccination. Nature Medicine, 16(8), 915–920 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine, 33(37), 4712–4718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine, 33(37), 4683–4690 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu LY, Ye L, Dong K, Compans RW, Yang C, Prausnitz MR. Enhanced Stability of Inactivated Influenza Vaccine Encapsulated in Dissolving Microneedle Patches. Pharmaceutical Research, 33(4), 868–878 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esser ES, Romanyuk A, Vassilieva EV et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 236, 47–56 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Rouphael NG, Paine M, Mosley R et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet (London, England), 390(10095), 649–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esser ES, Pulit-Penaloza JA, Kalluri H et al. Microneedle patch delivery of influenza vaccine during pregnancy enhances maternal immune responses promoting survival and long-lasting passive immunity to offspring. Scientific Reports, 7(1), 5705–5705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vassilieva EV, Wang S, Li S, Prausnitz MR, Compans RW. Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine. Scientific Reports, 7(1), 17855–17855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang H-W, Ye L, Guo XD, Yang C, Compans RW, Prausnitz MR. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γPGA Nanoparticles Administered Using a Microneedle Patch. Advanced Healthcare Materials, 6(1) (2017). [DOI] [PubMed] [Google Scholar]
- 72.Zhu W, Pewin W, Wang C et al. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 261, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mistilis MJ, Joyce JC, Esser ES et al. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Delivery And Translational Research, 7(2), 195–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joyce JC, Carroll TD, Collins ML et al. A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. The Journal Of Infectious Diseases, 218(1), 124–132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.B. Perez Cuevas M, Kodani M, Choi Y et al. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng Transl Med, 3, 186–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Resch TK, Wang Y, Moon S-S et al. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. Scientific Reports, 8(1), 561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Littauer EQ, Mills LK, Brock N et al. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 276, 1–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng L, Chang TZ, Wang Y et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proceedings Of The National Academy Of Sciences Of The United States Of America, 115(33), E7758–E7767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu W, Li S, Wang C, Yu G, Prausnitz MR, Wang B-Z. Enhanced Immune Responses Conferring Cross-Protection by Skin Vaccination With a Tri-Component Influenza Vaccine Using a Microneedle Patch. Frontiers In Immunology, 9, 1705–1705 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzara JM, Ochyl LJ, Hong JKY, Moon JJ, Prausnitz MR, Schwendeman SP. Self-healing encapsulation and controlled release of vaccine antigens from PLGA microparticles delivered by microneedle patches. Bioeng Transl Med, 4(1), 116–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernando GJ, Chen X, Prow TW et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One, 5(4), e10266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJP, Kendall MAF. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small (Weinheim An Der Bergstrasse, Germany), 6(16), 1785–1793 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Chen X, Kask AS, Crichton ML et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 148(3), 327–333 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Kask AS, Chen X, Marshak JO et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine, 28(47), 7483–7491 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Corbett HJ, Fernando GJP, Chen X, Frazer IH, Kendall MAF. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. Plos One, 5(10), e13460–e13460 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prow TW, Chen X, Prow NA et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small, 6(16), 1776–1784 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Chen X, Fernando GJ, Raphael AP et al. Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 158(1), 78–84 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Pearson FE, McNeilly CL, Crichton ML et al. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. Plos One, 8(7), e67888–e67888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearson FE, Muller DA, Roalfe L, Zancolli M, Goldblatt D, Kendall MAF. Functional anti-polysaccharide IgG titres induced by unadjuvanted pneumococcal-conjugate vaccine when delivered by microprojection-based skin patch. Vaccine, 33(48), 6675–6683 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Muller DA, Pearson FE, Fernando GJP et al. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Scientific Reports, 6, 22094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernando GJ, Zhang J, Ng HI, Haigh OL, Yukiko SR, Kendall MA. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J Control Release, 237, 35–41 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Crichton ML, Muller DA, Depelsenaire ACI et al. The changing shape of vaccination: improving immune responses through geometrical variations of a microdevice for immunization. Scientific Reports, 6, 27217–27217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller DA, Fernando GJP, Owens NS et al. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Scientific Reports, 7(1), 12644–12644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernando GJP, Hickling J, Jayashi Flores CM et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine, 36(26), 3779–3788 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine, 27(3), 454–459 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Hung IF, Levin Y, To KK et al. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine, 30(45), 6427–6435 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Hung IF, Levin Y, To KK. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine, 30(17), 2707–2708 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine, 32(34), 4249–4252 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Troy SB, Kouiavskaia D, Siik J et al. Comparison of the Immunogenicity of Various Booster Doses of Inactivated Polio Vaccine Delivered Intradermally Versus Intramuscularly to HIV-Infected Adults. J Infect Dis, 211(12), 1969–1976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anand A, Zaman K, Estivariz CF et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine, 33(48), 6816–6822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levin Y, Kochba E, Shukarev G, Rusch S, Herrera-Taracena G, van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine, 34(44), 5262–5272 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Vlasova A, Velasquez DE et al. Skin Vaccination against Rotavirus Using Microneedles: Proof of Concept in Gnotobiotic Piglets. Plos One, 11(11), e0166038–e0166038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin JH, Park JK, Lee DH, Quan FS, Song CS, Kim YC. Microneedle Vaccination Elicits Superior Protection and Antibody Response over Intranasal Vaccination against Swine-Origin Influenza A (H1N1) in Mice. PLoS One, 10(6), e0130684 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim Y-C, Lee S-H, Choi W-H et al. Microneedle delivery of trivalent influenza vaccine to the skin induces long-term cross-protection. Journal Of Drug Targeting, 24(10), 943–951 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Seok H, Noh JY, Lee DY, Kim SJ, Song CS, Kim YC. Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J Control Release, 265, 66–74 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Shin JH, Noh JY, Kim KH et al. Effect of zymosan and poly (I:C) adjuvants on responses to microneedle immunization coated with whole inactivated influenza vaccine. J Control Release, 265, 83–92 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Jung D, Rejinold NS, Kwak JE, Park SH, Kim YC. Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf B Biointerfaces, 159, 54–61 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Ding Z, Verbaan FJ, Bivas-Benita M et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release, 136(1), 71–78 (2009). [DOI] [PubMed] [Google Scholar]
- 109.van der Maaden K, Sekerdag E, Schipper P, Kersten G, Jiskoot W, Bouwstra J. Layer-by-Layer Assembly of Inactivated Poliovirus and N-Trimethyl Chitosan on pH-Sensitive Microneedles for Dermal Vaccination. Langmuir : the ACS journal of surfaces and colloids, 31(31), 8654–8660 (2015). [DOI] [PubMed] [Google Scholar]
- 110.de Groot AM, Platteel ACM, Kuijt N et al. Nanoporous Microneedle Arrays Effectively Induce Antibody Responses against Diphtheria and Tetanus Toxoid. Front Immunol, 8, 1789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schepens B, Vos PJ, Saelens X, van der Maaden K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 136, 259–266 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Ono A, Azukizawa H, Ito S et al. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. International Journal Of Pharmaceutics, 532(1), 374–383 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Matsuo K, Hirobe S, Yokota Y et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release, 160(3), 495–501 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Hirobe S, Azukizawa H, Matsuo K et al. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm Res, 30(10), 2664–2674 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Hirobe S, Azukizawa H, Hanafusa T et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials, 57, 50–58 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Pattani A, McKay PF, Garland MJ et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release, 162(3), 529–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ali AA, McCrudden CM, McCaffrey J et al. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomedicine : nanotechnology, biology, and medicine, 13(3), 921–932 (2017). [DOI] [PubMed] [Google Scholar]
- 118.Cole G, Ali AA, McCrudden CM et al. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 127, 288–297 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Vrdoljak A, McGrath MG, Carey JB et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release, 159(1), 34–42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vrdoljak A, Allen EA, Ferrara F, Temperton NJ, Crean AM, Moore AC. Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods. Journal Of Controlled Release: Official Journal Of The Controlled Release Society, 225, 192–204 (2016). [DOI] [PubMed] [Google Scholar]
- 121.Kommareddy S, Baudner BC, Bonificio A et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine, 31(34), 3435–3441 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Bonificio A, Ghartey-Tagoe E, Gallorini S et al. Fabrication of cell culture-derived influenza vaccine dissolvable microstructures and evaluation of immunogenicity in guinea pigs. Vaccine, 33(25), 2930–2938 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Wang J, Li B, Wu MX. Effective and lesion-free cutaneous influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America, 112(16), 5005–5010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen F, Yan Q, Yu Y, Wu MX. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J Control Release, 255, 36–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine, 25(10), 1814–1823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T, Zhen Y, Ma X, Wei B, Li S, Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf B Biointerfaces, 126, 520–530 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Qiu Y, Guo L, Zhang S et al. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv, 23(7), 2391–2398 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Kim E, Erdos G, Huang S, Kenniston T, Falo LD, Gambotto A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine, 13, 315–320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Z, Ye X, Ku Z et al. Transcutaneous immunization via rapidly dissolvable microneedles protects against hand-foot-and-mouth disease caused by enterovirus 71. J Control Release, 243, 291–302 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Poirier D, Renaud F, Dewar V et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials, 145, 256–265 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Nakatsukasa A, Kuruma K, Okamatsu M et al. Potency of whole virus particle and split virion vaccines using dissolving microneedle against challenges of H1N1 and H5N1 influenza viruses in mice. Vaccine, 35(21), 2855–2861 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Stinson JA, Raja WK, Lee S et al. Silk Fibroin Microneedles for Transdermal Vaccine Delivery. ACS Biomaterials Science & Engineering, 3(3), 360–369 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Moreno E, Schwartz J, Calvo A et al. Skin vaccination using microneedles coated with a plasmid DNA cocktail encoding nucleosomal histones of Leishmania spp. Int J Pharm, 533(1), 236–244 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Ogai N, Nonaka I, Toda Y et al. Enhanced immunity in intradermal vaccination by novel hollow microneedles. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 24(4), 630–635 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Gala RP, Zaman RU, D’Souza MJ, Zughaier SM. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines, 6(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee C, Kim H, Kim S et al. Comparative Study of Two Droplet-Based Dissolving Microneedle Fabrication Methods for Skin Vaccination. Adv Healthc Mater, 7(11), e1701381 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Yan Q, Cheng Z, Liu H et al. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice. Vaccine, 36(30), 4471–4476 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Dean CH, Alarcon JB, Waterston AM et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Human vaccines, 1(3), 106–111 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Prausnitz MR. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annual Review Of Chemical And Biomolecular Engineering, 8, 177–200 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Lutton R, Moore J, Larrañeta E, Ligett S, Woolfson A, Donnelly R. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug delivery and translational research, 5 (2015). [DOI] [PubMed] [Google Scholar]; ** Discusses the need for universal acceptance criteria and GMP specifications to support commercialization
- 141.Sauermann K, Clemann S, Jaspers S et al. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 8(1), 52–56 (2002). [DOI] [PubMed] [Google Scholar]
- 142.Bonta M, Daina L, Mutiu G. The process of ageing reflected by histological changes in the skin. Rom J Morphol Embryol, 54(3 Suppl), 797–804 (2013). [PubMed] [Google Scholar]
- 143.Michel M, L’Heureux N, Auger FA, Germain L. From newborn to adult: phenotypic and functional properties of skin equivalent and human skin as a function of donor age. J Cell Physiol, 171(2), 179–189 (1997). [DOI] [PubMed] [Google Scholar]
- 144.Laurent A, Mistretta F, Bottigioli D et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine, 25(34), 6423–6430 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Van Mulder TJ, de Koeijer M, Theeten H et al. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine, 35(14), 1810–1815 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Derraik JG, Rademaker M, Cutfield WS et al. Effects of age, gender, BMI, and anatomical site on skin thickness in children and adults with diabetes. PLoS One, 9(1), e86637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ploin D, Schwarzenbach F, Dubray C et al. Echographic measurement of skin thickness in sites suitable for intradermal vaccine injection in infants and children. Vaccine, 29(46), 8438–8442 (2011). [DOI] [PubMed] [Google Scholar]
- 148.Robertson K, Rees JL. Variation in epidermal morphology in human skin at different body sites as measured by reflectance confocal microscopy. Acta Derm Venereol, 90(4), 368–373 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front Immunol, 5, 131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deckers J, Hammad H, Hoste E. Langerhans Cells: Sensing the Environment in Health and Disease. Front Immunol, 9, 93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol, 35, 469–499 (2017). [DOI] [PubMed] [Google Scholar]; ** Comprehensive review of antigen presentation in the skin.
- 152.de Witte L, Nabatov A, Pion M et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med, 13(3), 367–371 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol, 9(10), 679–691 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei JCJ, Edwards GA, Martin DJ, Huang H, Crichton ML, Kendall MAF. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: from mice, rats, rabbits, pigs to humans. Scientific Reports, 7(1), 15885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol, 126(5), 976–984, 984 e971–975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arya J, Henry S, Kalluri H, McAllister DV, Pewin WP, Prausnitz MR. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials, 128, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Depelsenaire ACI, Meliga SC, McNeilly CL et al. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol, 134(9), 2361–2370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Varkentin A, Mazurenka M, Blumenrother E et al. Comparative study of presurgical skin infiltration depth measurements of melanocytic lesions with OCT and high frequency ultrasound. J Biophotonics, 10(6–7), 854–861 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Bachy V, Hervouet C, Becker PD et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A, 110(8), 3041–3046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu R, Zhang M, Jin C. In vivo and in situ imaging of controlled-release dissolving silk microneedles into the skin by optical coherence tomography. J Biophotonics, 10(6–7), 870–877 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Bal SM, Kruithof AC, Zwier R et al. Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo. J Control Release, 147(2), 218–224 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Wei JCJ, Haridass IN, Crichton ML et al. Space- and time-resolved investigation on diffusion kinetics of human skin following macromolecule delivery by microneedle arrays. Sci Rep, 8(1), 17759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crichton ML, Ansaldo A, Chen X, Prow TW, Fernando GJ, Kendall MA. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials, 31(16), 4562–4572 (2010). [DOI] [PubMed] [Google Scholar]
- 164.Crichton ML, Donose BC, Chen X, Raphael AP, Huang H, Kendall MA. The viscoelastic, hyperelastic and scale dependent behaviour of freshly excised individual skin layers. Biomaterials, 32(20), 4670–4681 (2011). [DOI] [PubMed] [Google Scholar]
- 165.Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm Res, 30(4), 1099–1109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gill HS, Andrews SN, Sakthivel SK et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur J Pharm Sci, 38(2), 95–103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Adabi S, Hosseinzadeh M, Noei S et al. Universal in vivo Textural Model for Human Skin based on Optical Coherence Tomograms. Sci Rep, 7(1), 17912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides comprehensive in vivo analysis of microanatomy of human skin as a function of body site.
- 168.Davis A, Levecq O, Azimani H, Siret D, Dubois A. Simultaneous dual-band line-field confocal optical coherence tomography: application to skin imaging. Biomed Opt Express, 10(2), 694–706 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waghule T, Singhvi G, Dubey SK et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomedicine & Pharmacotherapy, 109, 1249–1258 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Yang J, Liu X, Fu Y, Song Y. Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharmaceutica Sinica B, 9(3), 469–483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jacoby E, Jarrahian C, Hull HF, Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine, 33(37), 4699–4704 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine, 32(16), 1856–1862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Griffin P, Elliott S, Krauer K et al. Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine, 35(48 Pt B), 6676–6684 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Marshall S, Fleming A, Moore AC, Sahm LJ. Acceptability of microneedle-patch vaccines: A qualitative analysis of the opinions of parents. Vaccine, 35(37), 4896–4904 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Guillermet E, Alfa DA, Phuong Mai LT et al. End-user acceptability study of the nanopatch™; a microarray patch (MAP) for child immunization in low and middle-income countries. Vaccine, 37(32), 4435–4443 (2019). [DOI] [PubMed] [Google Scholar]
- 176.Marshall S, Sahm LJ, Moore AC. Microneedle technology for immunisation: Perception, acceptability and suitability for paediatric use. Vaccine, 34(6), 723–734 (2016). [DOI] [PubMed] [Google Scholar]
- 177.McHugh KJ, Jing L, Severt SY et al. Biocompatible near-infrared quantum dots delivered to the skin by microneedle patches record vaccination. Science Translational Medicine, 11(523), eaay7162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.World Health Organization. Measles-rubella microarray patch (MR–MAP) target product profile June 2019. (2019) (Available at: https://www.who.int/immunization/research/ppc-tpp/WHO_MR_MAP_TPP.pdf, Accessed: September 1, 2019).
- 179.McCrudden MT, Alkilani AZ, Courtenay AJ et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv Transl Res, 5(1), 3–14 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Rochmyaningsih D. Indonesian ‘vaccine fatwa’ sends measles immunization rates plummeting (2018). [DOI] [PubMed]
- 181.Francis DP. Successes and failures: Worldwide vaccine development and application. Biologicals, 38(5), 523–528 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Naik SP, Zade JK, Sabale RN et al. Stability of heat stable, live attenuated Rotavirus vaccine (ROTASIIL(R)). Vaccine, 35(22), 2962–2969 (2017). [DOI] [PubMed] [Google Scholar]
- 183.Pecenka C, Debellut F, Bar-Zeev N et al. Re-evaluating the cost and cost-effectiveness of rotavirus vaccination in Bangladesh, Ghana, and Malawi: A comparison of three rotavirus vaccines. Vaccine, 36(49), 7472–7478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Butler D Vaccine offers meningitis hope. Nature, 468(7321), 143 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Desai SN, Pezzoli L, Martin S et al. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. The Lancet Global Health, 4(4), e223–e224 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Jadhav S, Datla M, Kreeftenberg H, Hendriks J. The Developing Countries Vaccine Manufacturers’ Network (DCVMN) is a critical constituency to ensure access to vaccines in developing countries. Vaccine, 26(13), 1611–1615 (2008). [DOI] [PubMed] [Google Scholar]
- 187.Jadhav S, Gautam M, Gairola S. Role of vaccine manufacturers in developing countries towards global healthcare by providing quality vaccines at affordable prices. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 20 Suppl 5, 37–44 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Low N, Bavdekar A, Jeyaseelan L et al. A Randomized, Controlled Trial of an Aerosolized Vaccine against Measles. New England Journal of Medicine, 372(16), 1519–1529 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Harper SA, Fukuda K, Cox NJ, Bridges CB. Using live, attenuated influenza vaccine for prevention and control of influenza: Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports, 52(RR13), 1–8 (2003). [PubMed] [Google Scholar]
- 190.Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016–2017 flu season. (2016) (Available at: https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html, Accessed: February 12, 2017).
- 191.Grohskopf L, Sokolow L, Fry A, Walter E, Jernigan D. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR, 67(22), 643–645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Materials Science and Engineering: R: Reports, 104, 1–32 (2016). [Google Scholar]
- 193.Sanjay ST, Dou M, Fu G, Xu F, Li X. Controlled Drug Delivery Using Microdevices. Curr Pharm Biotechnol, 17(9), 772–787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci, 35(3), 193–202 (2008). [DOI] [PubMed] [Google Scholar]
- 195.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain, 24(7), 585–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haq MI, Smith E, John DN et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices, 11(1), 35–47 (2009). [DOI] [PubMed] [Google Scholar]
- 197.Butler D Translational research: crossing the valley of death. Nature, 453(7197), 840–842 (2008). [DOI] [PubMed] [Google Scholar]
- 198.O’Brien KL, Binka F, Marsh K, Abramson JS. Mind the gap: jumping from vaccine licensure to routine use. Lancet, 387(10031), 1887–1889 (2016). [DOI] [PubMed] [Google Scholar]


