Abstract
OBJECTIVES
The purpose of this study was to investigate real world safety and efficacy of hypertonic saline therapy in cases of refractory acute decompensated heart failure (ADHF) at a large U.S. academic medical center.
BACKGROUND
Hypertonic saline therapy has been described as a potential management strategy for refractory ADHF, but experience in the United States is limited.
METHODS
A retrospective analysis was performed in all patients receiving hypertonic saline for diuretic therapy-resistant ADHF at the authors’ institution since March 2013. The primary analytic approach was a comparison of the trajectory of clinical variables prior to and after administration of hypertonic saline, with secondary focus on predictors of treatment response.
RESULTS
A total of 58 hypertonic saline administration episodes were identified across 40 patients with diuretic-therapy refractory ADHF. Prior to hypertonic saline administration, serum sodium, chloride, and creatinine concentrations were worsening but improved after hypertonic saline administration (p < 0.001, all). Both total urine output and weight loss significantly improved with hypertonic saline (p = 0.01 and <0.001, respectively). Diuretic efficiency, defined as change in urine output per doubling of diuretic dose, also improved over this period (p < 0.01). There were no significant changes in respiratory status or overcorrection of serum sodium with the intervention.
CONCLUSIONS
In a cohort of patients who were refractory to ADHF, hypertonic saline administration was associated with increased diuretic efficiency, fluid and weight loss, and improvement of metabolic derangements, and no adverse respiratory or neurological signals were identified. Additional study of hypertonic saline as a diuretic adjuvant is warranted. (J Am Coll Cardiol HF 2020;8:199–208) © 2020 by the American College of Cardiology Foundation.
Keywords: cardiorenal, diuretic resistance, sodium regulation
A cute decompensated heart failure (ADHF) accounts for more than 1 million hospitalizations annually and poses a high burden of health care-related expenditures (1). The primary driver of symptoms leading to hospitalization in ADHF is congestion with volume overload, for which diuretic therapy is the mainstay of treatment (2, 3). However, a subset of patients with ADHF develop diuretic resistance and progressive cardiorenal dysfunction despite escalating doses of loop diuretics. This resistance is associated with a high risk of mortality and presents a significant clinical challenge, as therapies to relieve congestive symptoms are sometimes limited by a further decline in renal function (4). Several therapies have been tested as adjuncts to loop diuretic agents with no demonstrable clinical improvement (5–9). A potential common pitfall of those therapies is the failure to address the underlying sodium-avid state that the kidney is defending in the setting of aggressive diuresis. The conventional paradigm has held that salt is universally deleterious, whereby excessive sodium may exacerbate acute heart failure and congestion; however, it is also known that restriction of salt intake leads to a heightened sodium avidity signal resulting in neurohormonal activation and questionable differences in congestive symptoms (10–12). The use of supplemental sodium chloride to disrupt the sodium avidity signal in conjunction with high-dose natriuretic agents may therefore have theoretical utility.
As an extension of this concept, several studies in patients with ADHF have shown that administration of intravenous (IV) hypertonic saline (HS) can improve diuresis, renal function, and clinical outcomes when they are given concomitantly with high doses of diuretic agents (13–15). However, the use of HS in ADHF has been studied only at a limited number of centers outside United States, with some results receiving scrutiny by the scientific community, including a retraction and subsequent exclusion from a meta-analysis based on concerns regarding data validity (16–18). This retraction, in conjunction with the counterintuitive nature of administering salt in an effort to remove salt, has led to a slow adoption of HS for ADHF in the United States. The present study reports the authors’ experience with HS therapy and describes the safety and efficacy of this treatment in a real-world setting at a large US center.
METHODS
STUDY POPULATION, CLINICAL CARE, AND MONITORING.
Patients included in this report received HS for the treatment of ADHF between March 5, 2013, and December 14, 2017. All patients were treated at a single hospital in the cardiac intensive care unit or the cardiac step-down unit and were identified by the presence of a 3% NaCl order placed in the heart and vascular center at our institution. Charts were subsequently reviewed, and patients not being managed for ADHF or who received HS for an indication other than for the management of ADHF (e.g., for cerebral edema as documented in a neurology consultant note) were excluded. Providers followed a clinical protocol that was developed and approved at our institution, which was implemented exclusively for advanced heart failure physicians for use in patients with ADHF with clinical signs of diuretic resistance. The protocol suggested 150 ml of 3% NaCl to be given over 30 min (300 ml/h), administered simultaneously with high doses of loop diuretic agents (Online Figure 1, Online Table 1). The preferred method of administration was through a central line, using an infusion pump, but large-bore peripheral IV lines were acceptable if central lines were not feasible. As all patients were located in the cardiac intensive care unit or step-down unit, monitoring during and after HS infusion consisted of frequent monitoring of vital signs, continuous monitoring of peripheral capillary oxygen saturation, and serial cardiopulmonary and neurologic examinations. Stopping parameters included respiratory decompensation, in addition to changes in mental status or neurological examinations.
DATA COLLECTION.
Chart data regarding diuretic agents administered, urine output, and laboratory values were extracted for 24-h intervals with the administration of the first dose of HS used as the reference time point. Patients who received multiple doses of HS during admission were split into separate observation groups when time between HS doses was >7 days to ensure no residual effects. Doses of loop diuretic agents were extracted and converted to IV furosemide equivalents, with 40 mg of IV furosemide equivalent to 80 mg of PO furosemide, 1 mg of IV or PO bumetanide, and 20 mg of PO torsemide (19). Similarly, doses of thiazide diuretic agents were extracted and converted to metolazone equivalents, where 100 mg of chlorothiazide was equal to 10 mg of hydrochlorothiazide, which was equal to 1 mg of metolazone (20). Net urine output in milliliters was determined based on the difference between the documented fluid intake (IV or PO) and documented urine output. Daily weights were extracted from the chart. For days in which multiple weights were recorded, priority was given to standing weight over bed weight and then to the first measurement of the day. Oxygenation data were extracted in 4-h intervals for the 72-h periods before and after HS administration. Oxygenation data collected included peripheral oxygen saturation, oxygen delivery device, oxygen flow rate for nasal cannula, and fraction of inspired oxygen (FiO2) for high-flow nasal cannula and ventilators. To assess trends in oxygen requirements, available data were used to extrapolate FiO2 where they were not available, with FiO2 at room air considered 21% with 4% increase per liter flow through standard nasal cannula. Missing oxygenation data were handled by last observation carried forward. The formal radiology reports of chest radiographs and qualitative reports of pulmonary examinations were extracted manually from the patient’s chart and coded according to relative improvement or deterioration, comparing the pre-HS to post-HS period.
STATISTICAL ANALYSIS.
Descriptive analysis and statistical tests were performed using SAS version 9.4 software (SAS Institute Inc., Cary, North Carolina), and Stata version 13.1 software (Statacorp, College Station, Texas). Baseline characteristics are presented as median (interquartile range [IQR]: quartile 1, quartile 3) or mean ± SD. The outcomes of weight, fluid, respiratory, and serum chemistries were collected at 24, 48, and 72 h, both before and after HS administration. Changes in the outcomes during post-intervention (between 24 and 72 h post-HS administration) were examined statistically to determine whether there were changes during pre-intervention (between 24 and 72 h pre-HS administration). The observations at 24 h pre-HS administration were considered the baseline values for evaluating the post-intervention changes. To account for correlations of repeated measures and multiple episodes from the same individual, general linear mixed models (GLMM) with random intercepts were used, and the 2 estimated slopes of changes per 24 h were compared between pre-and post-HS periods by using piecewise linear regression. The proportion of patients requiring supplemental oxygen use also was examined, which was measured every 12 h between 48 h prior to and after the intervention. Logistic regression with random intercepts was used to compare the change per 12 h between pre- and post-intervention periods. In addition, post hoc analysis was performed to examine whether changes in urine output were associated with changes in serum chemistries after administration of HS. The longitudinal weight, net fluid loss, and total urine output were predicted by Δ values for each serum chemistry value (i.e., changed amount from 24 h prior to the intervention) by using GLMM adjusted for weight or urine output at 24 h prior to the intervention. All statistical tests were performed at a 5% level of significance.
RESULTS
A total of 40 patients received HS for treatment of ADHF over 50 distinct admissions. Within these 50 admissions, 5 patients received HS for more than 1 distinct episode for a total of 58 distinct episodes of HS administration. Patients received a median of 3 doses (IQR: 2 to 7 doses) during a treatment episode. On average, patients receiving HS had high disease severity with a high incidence of hyponatremia, hypochloremia, and renal dysfunction (Table 1). The median dose of diuretic agents prior to HS administration was 400 mg (IQR: 200 to 875 mg) of furosemide equivalents per 24 h. At the initiation of HS treatment, 64% of patients were receiving inotropes/vasopressors, and the median length of stay was 29 days (IQR: 17 to 77 days).
TABLE 1.
Baseline Characteristics of the Cohort (N = 58)
| Age, yrs | 60 ± 11 |
| Females | 45 |
| Medical history, % | |
| Hypertension | 55 |
| Diabetes mellitus | 36 |
| Coronary artery disease | 45 |
| Implantable cardioverter-defibrillator | 60 |
| Moderate to severe valvular disease | 62 |
| Left ventricular assist device | 25 |
| Ejection fraction | 35 ± 22 |
| Ejection fraction ≤40% | 65 |
| Vital signs | |
| Heart rate, beats/min | 85 ± 17 |
| Systolic blood pressure, mm Hg | 103 ± 14 |
| Diastolic blood pressure, mm Hg | 60 ± 13 |
| Mean Arterial Pressure, mm Hg | 72 ± 11 |
| Estimated FiO2, % | 28 (21–33) |
| Laboratory values | |
| Sodium, mmol/l | 131 (125–134) |
| Chloride, mmol/l | 88 (83–93) |
| BUN, mg/dl | 64 (40–83) |
| Creatinine, mg/dl | 1.8 (1.5–2.8) |
| eGFR, ml/min/m2 | 36 ± 20 |
| Hemoglobin, g/dl | 9.9 ± 1.9 |
| Inotropes/vasopressors, % | 64 |
| Milrinone | 36 |
| Dopamine | 33 |
| Dobutamine | 10 |
| Norepinephrine | 2 |
| Multiple | 17 |
| Length of stay and outcomes | |
| Length of stay, days | 29 (17–76) |
| Rehospitalized within 30 days of discharge, % | 17 (10/58) |
| Deaths within 30 days of discharge, % | 33 (13/40) |
| Discharged to hospice, % | 21 (12/58) |
| Deaths, discharge to hospice, or readmissions within 30 days, % | 47 (27/58) |
| Baseline diuretics | |
| Loop diuretic dose, mg of furosemide equivalents | 400 (200–875) |
| Thiazide diuretic | 35 (59)* |
| Thiazide diuretic dose, mg of metolazone equivalents | 10 (10–20) |
| Acetazolamide, % | 3 (5) |
| Acetazolamide dose, mg | 500 (500–2,000) |
| Tolvaptan | 5 (8) |
Values are mean ± SD, n, %, median (interquartile range), % (n/N), or n (%).
46 patients (79%) received a thiazide at some point prior to hypertonic saline administration during that admission.
BUN = blood urea nitrogen; FiO2 = fraction of inspired oxygen; HS = hypertonic saline
SAFETY. Respiratory status.
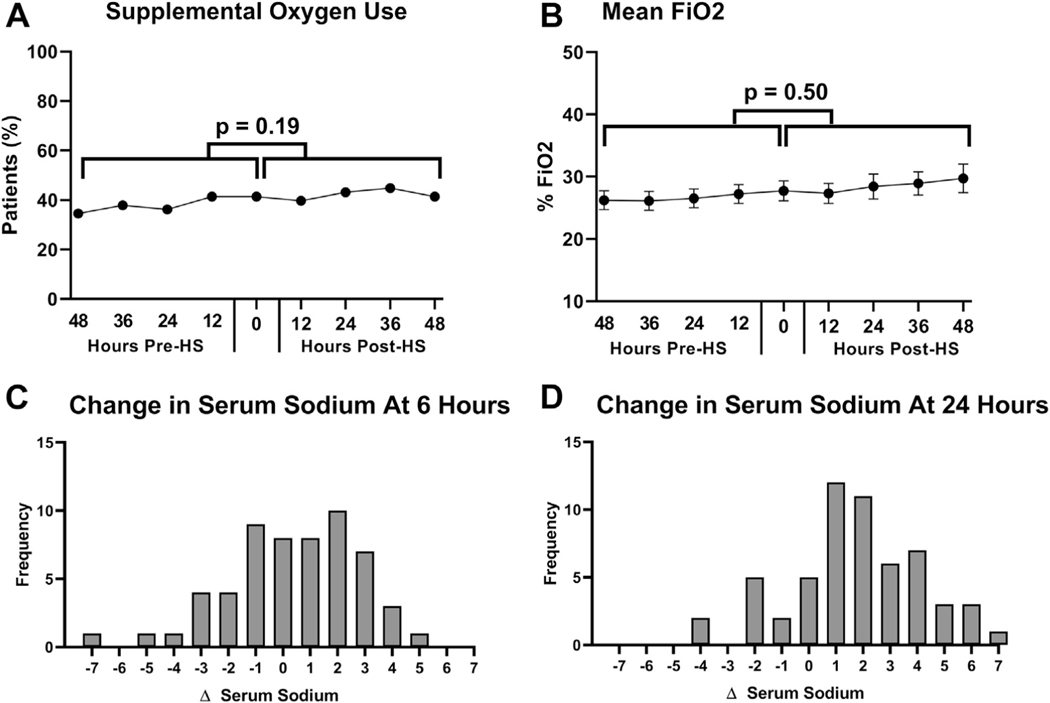
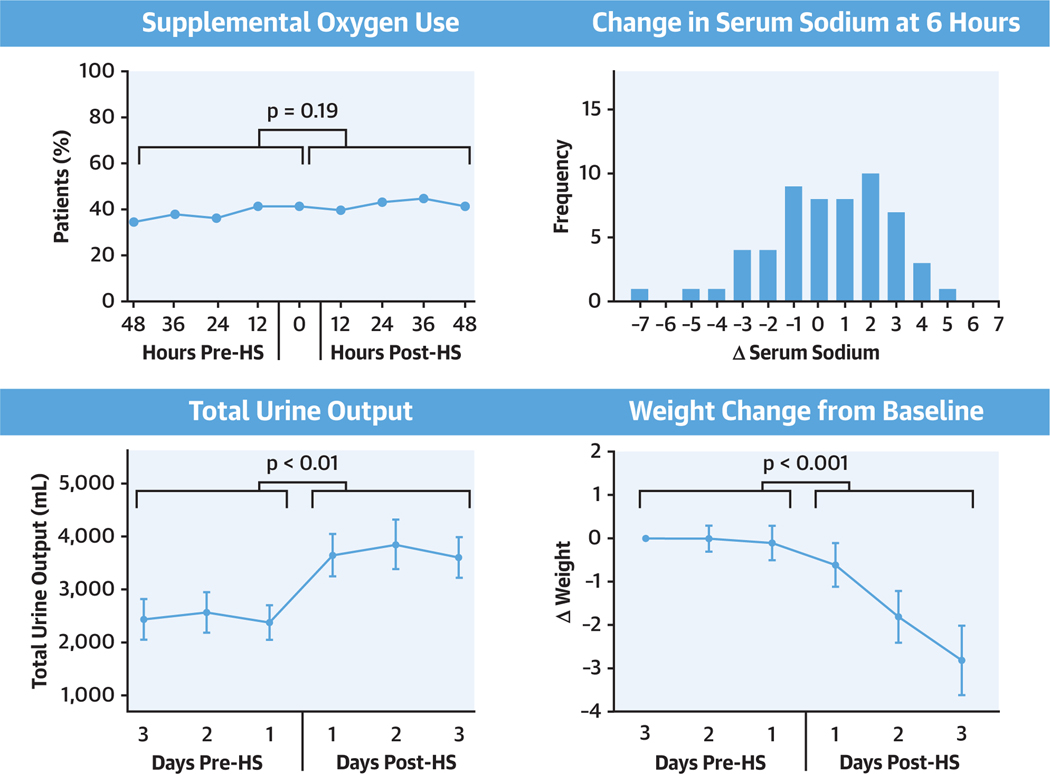
At the initiation of HS treatment, 43% of patients were receiving supplemental oxygen, which remained unchanged after the initiation of HS (Figure 1A). Average estimated FiO2 also did not change from pre- to post-HS administration (Figure 1B). Overall, there were no differences in the trend of the use of supplemental oxygen or FiO2 before and after initiation of HS (p = 0.18 and p = 0.49, respectively) (Table 2). Pre- and post-HS chest radiographs were performed for 14 patients (24%). Of those patients, 12 showed no change, 1 showed mild improvement in pulmonary edema, and 1 showed worsened pulmonary edema. Subjective data regarding post-intervention serial pulmonary auscultation was available for 53 patients (91%), and the majority had no change in their examination values (62%), 19 had improvement in crackles (36%), and only 1 had worsening crackles (2%; p < 0.001 for improving examination values).
FIGURE 1. Safety Profile of Hypertonic Saline.
(A, B) Trends in respiratory measurements before and after initiation of treatment with HS. There were no changes in the percentage of patients who required supplemental oxygen (p = 0.19). The mean FiO2 (p = 0.50) in the 48 h before HS administration, compared with the 48 h after administration. Histograms of changes in serum sodium at (C) 6 h and (D) 24 h after initiation of treatment with HS. FiO2 = fraction of inspired oxygen; HS = hypertonic saline.
TABLE 2.
Estimated Changes Per Day of Outcome Variables in Pre- and Post-Intervention Periods
| Pre-Intervention Change per Day |
Post-Intervention Change per Day |
Difference in Pre- Versus Post-Intervention Trends |
|||
|---|---|---|---|---|---|
| Estimate ± SE | p Value | Estimate ± SE | p Value | p Value | |
| Fluid loss | |||||
| Δ Weight, kg | −0.02 ± 0.26 | 0.93 | −1.1 ± 0.17 | <0.001 | <0.001 |
| Net fluid loss, ml | 30 ± 12 | 0.80 | 337 ± 73 | <0.001 | 0.03 |
| Δ Total urine output, ml | −23 ± 122 | 0.85 | 379 ± 77 | <0.001 | 0.01 |
| Respiratory measurements | |||||
| % Δ Number of patients on supplemental oxygen | 0.2 ± 0.2 | 0.22 | −0.2 ± 0.3 | 0.44 | 0.19 |
| Δ FiO2 | 0.5 ± 0.5 | 0.31 | 0.8 ± 0.3 | <0.01 | 0.50 |
| Laboratory measurements | |||||
| Δ Sodium, mEq/l | −1.0 ± 0.2 | <0.001 | 0.9 ± 0.2 | <0.001 | <0.001 |
| Δ Chloride, mEq/l | −1.1 ± 0.3 | <0.001 | 0.5 ± 0.2 | <0.01 | <0.001 |
| Δ BUN, mg/dl | 2.7 ± 0.90 | <0.01 | 1.2 ± 0.57 | 0.04 | 0.15 |
| Δ Creatinine, mg/dl | 0.1 ± 0.03 | <0.01 | −0.1 ± 0.02 | <0.001 | <0.001 |
Values are mean estimate ± SE.
BUN = blood urea nitrogen; FiO2 = fraction of inspired oxygen.
Serum sodium levels.
Median serum sodium change after treatment was +1.5 mmol/l (IQR: 0.0 to 3.0 mmol/l) at 6 h; +2 mmol/l (IQR: 0.5 to 3.5 mmol/l) at 24 h; and +3 mmol/l (IQR: −1.0 to 5.0 mmol/l) at 72 h. The largest change in serum sodium was +5 mmol/l at 6 h and +7 mmol/l at 24 h, which occurred in 2 separate patients after both had received the first dose of HS. Neither patient experienced an adverse neurologic outcome (Figures 1C and 1D).
PARAMETERS OF EFFICACY. Changes in weight and fluid output.
Prior to treatment with HS, both the weight and the daily total urine output demonstrated little variation but improved significantly post-HS (Central Illustration). Daily net urine output improved by 489 ± 241 ml during day 1 (p = 0.04), 1,019 ± 241 ml during day 2 (p < 0.001), and 921 ± 244 ml during day 3 (p < 0.01) compared to the values at 24 h prior to treatment (Central Illustration). Compared to weight immediately prior to treatment, average weight decreased by 0.6 ± 0.5 kg at 24 h (p = 0.23), 2.0 ± 0.5 kg at 48 h (p < 0.001), and 3.1 ± 0.5 kg at 72 h (p < 0.001) after HS administration (Central Illustration). Similarly, diuretic efficiency was stable prior to HS administration but increased significantly following treatment (Figure 2). There was a significant decrease in weight (p < 0.001) and an increase in net urine output (p = 0.03) during the 3 days after initiation of HS administration compared to no significant changes over the 3 days prior to initiation of HS therapy (Table 2).
CENTRAL ILLUSTRATION. Patients Receiving HS Had Improved Urine Output and Weight Loss.
Patients receiving hypertonic saline (HS) had improved urine output and weight loss, without dangerous fluctuations in serum sodium or respiratory decompensation. There were no changes in the percentage of patients requiring supplemental oxygen, and fluctuations in serum sodium were within the acceptable range following administration of hypersonic saline. Both total urine output and weight loss improved.
FIGURE 2. Trends of Diuretic Efficiency Before and After Initiation of Treatment With Hypertonic Saline.
There was a significant improvement in diuretic efficiency in the 72 h prior to hypertonic saline (HS) administration compared that in the 72 h after administration (p = 0.01). Diuretic efficiency is defined as the increase in urine output per doubling of loop diuretic dose.
Change in laboratory parameters.
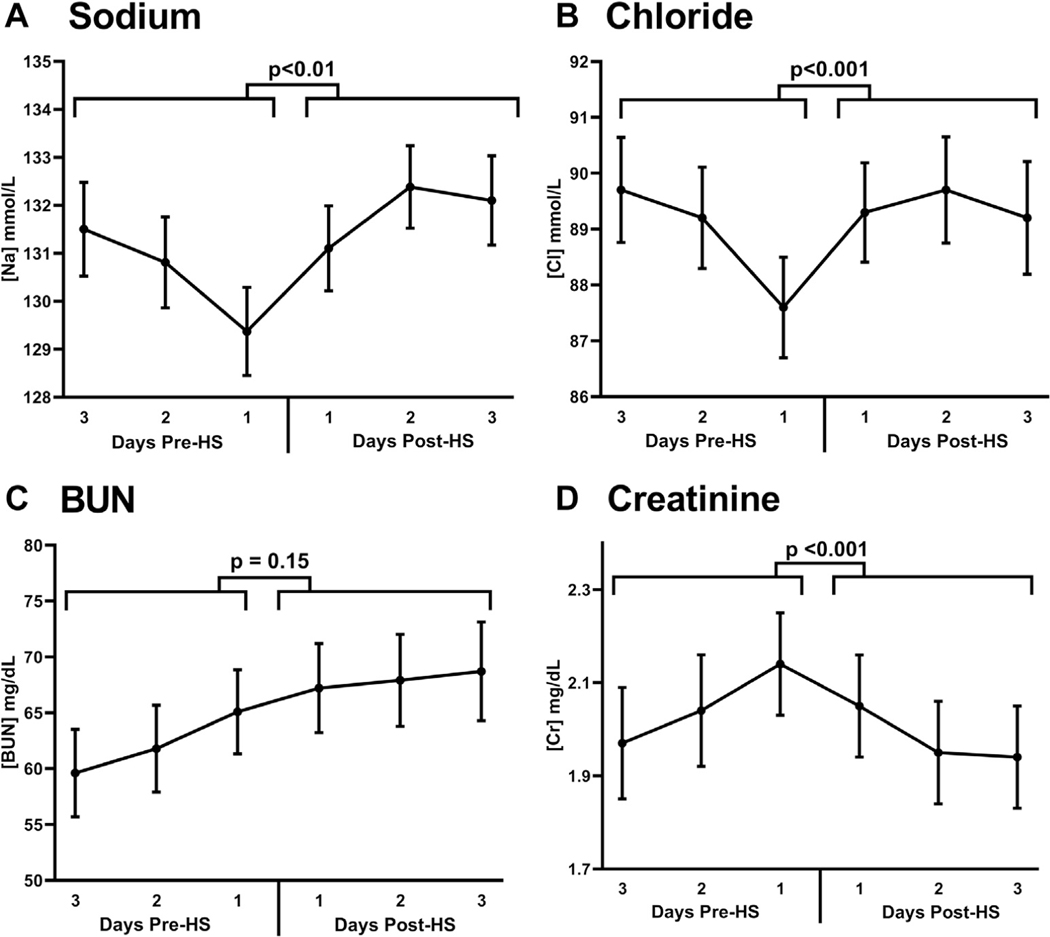
Prior to HS administration, serum sodium, chloride, and creatinine concentrations were deteriorating but improved after HS administration (pre- to post-treatment trend comparison: p < 0.001 for all) (Figure 3, Central Illustration, Table 2). Blood urea nitrogen was significantly increased both before and after HS, without a significant difference in pre- to post-treatment rate of change (p = 0.15) (Table 2).
FIGURE 3. Trends of Blood Chemistries Before and After Initiation of Treatment With HS.
Improvements were seen in the trends of serum sodium (A, p < 0.01), serum chloride (B, p < 0.001), blood urea nitrogen [BUN] (C, p = 0.15), and serum creatinine (D, p < 0.001) after hypertonic saline (HS) administration, whereas there was no statistically significant change in the trend of BUN before and after administration (p = 0.15).
DISCUSSION
This retrospective analysis provides the first description of the real-world use of HS in refractory ADHF at a large US center. Hypertonic saline as a loop diuretic adjuvant appeared to be safe and well tolerated in a cohort of severely ill patients with rising serum creatinine, hyponatremia, and stagnant urine output in the 72 h preceding therapy. There was no discernible deterioration in respiratory status, overcorrection of hyponatremia, or progressive salt and water retention after the administration of HS. There were also trends demonstrating that HS administration was associated with statistically significant improvements in urine output, weight loss, diuretic efficiency, renal function, and electrolyte abnormalities, raising the possibility that HS may be effective for the treatment of refractory volume overload for select patients. In sum, these observations suggest that there may be value to this therapy and that rigorous study of HS for refractory ADHF is warranted.
A large barrier to the widespread adoption of HS, despite published studies of the topic indicating impressive efficacy (21–25), relates to physicians’ concerns that administering salt to a patient with total body sodium/fluid overload will lead to worsening heart failure. The most compelling observation in this series of extremely sick patients was that signals for adverse effects of HS on oxygenation or electrolyte balance were not detected. More importantly, there was no change in the trend of oxygen use after the administration of HS, nor were there any significant changes in chest radiograph findings. There was also improvement in respiratory status based upon serial pulmonary auscultatory examinations. These findings suggest that, with appropriate patient selection, administration of HS to an ADHF patient does not obligatorily result in worsening pulmonary edema and hypoxemia. Additionally, an excessive correction of serum sodium in this series was not seen, as the median change in serum sodium 24 h after HS administration was +1.5 mmol/l with a maximum of +7 mmol/l over 24 h in a single patient. This is within the recommended correction of >6 to 8 mmol/l per 24 h in hyponatremic patients (26). Thus, this series of HS use would not support a high risk for osmotic demyelination, nor was this complication observed in any of these patients.
A fundamental physiological principle of edematous states is that positive sodium balance is the primary driver of water retention and ultimately volume overload in ADHF (27). As a direct result, sodium restriction has been assumed to be a critical component of the treatment of patients with both chronic-stable and acute-decompensated heart failure, with the logic that less sodium intake would help facilitate an even or negative sodium balance. However, an accumulating body of research has begun to challenge the completeness of this conceptual paradigm. Although studies have shown that sodium intake leads to worsening of congestive symptoms, contradictory data have revealed that sodium restriction may not confer a clear benefit with respect to congestive symptoms and clinical outcomes, and leads to heightened neurohormonal activity (10–12). As an extension of that concept, several studies in patients with ADHF have shown that administration of HS to diuretic drug-resistant patients may improve diuresis, renal function, and clinical outcomes when given concomitantly with high doses of loop diuretics. It was hypothesized that bolus dosing of HS mobilizes fluid from the interstitial space to the intravascular compartment through osmotic forces, thereby restoring effective intravascular volume, enhancing renal blood flow, and improving the delivery of diuretic agents to the loop of Henle (28). This hypothesis was conceptually challenged by a group in Japan who showed that slow continuous infusion of HS at 0.35 ml/min of 1.7% NaCl, which is so slow the hypothesized osmotic improvement in effective circulating volume should not occur, resulted in a benefit similar to that of bolus dosing of HS (29). As such, a definitive mechanism explaining the reported benefits of HS has remained elusive, but there is evidence to suggest that the method of saline administration may not be crucial to its effects.
It has been known for decades that renal salt sensing appears primarily driven by the chloride anion rather than the sodium cation, with salt-sensitive renal responses such as tubuloglomerular feedback and renin release determined by chloride (30,31). Recently, a family of serine-threonine kinases (with-no-lysine [K] [WNK]) have been identified as the molecular sensor for salt and have shown they play a key role in the regulation of electrolyte homeostasis, in the actions of the reninangiotensin-aldosterone system, and in the regulation of the transporters upon which loop and thiazide diuretic agents work (32–35). These observations led to the hypothesis that chloride may play a significant role in human heart failure. Over a series of observational studies, it has been shown that low chloride rather than low sodium is strongly and independently associated with the following: 1) diuretic responsiveness; 2) neurohormonal activation; and 3) prognosis. Furthermore, in a pilot study of supplementation of sodium-free chloride for 3 days (in the form of lysine chloride), signals for improvement were observed in intravascular volume (hemoconcentration) and reduction in N-terminal pro–B-type natriuretic peptide levels. As such, it follows that a potential mechanism for the benefit of HS is in fact the supplemental chloride the patients receive. Notably, the patients in the present cohort experienced a progressive worsening of serum chloride prior to HS administration, which improved after they received HS. The present authors are currently formally testing the hypothesis that chloride may have positive cardiorenal effects in a trial of sodium-free chloride supplementation in patients with ADHF (Mechanism and Effects of Manipulating Chloride Homeostasis in Stable Heart Failure; NCT03440970).
STUDY LIMITATIONS.
As a retrospective cohort study, the patients were not randomized or compared to a control group, thus trends were only assessed by patients treated to HS compared to their own baseline values. As a result, causality should not be assumed, and these observations should be considered hypothesis-generating only. Dosages of diuretic agents and treatment duration of HS were carried out at the discretion of the treating physician, making conclusions regarding dose-dependent effects and diuretic responsiveness difficult to determine. In addition, the data were subject to selection bias. Although patients who received HS generally had high disease severity, with diuretic resistance and dysfunction, this was the subjective determination of the treating physician, and no formal criteria were applied. Notably, most cases in this series were referred from a single physician (J.M.T.). It is likely that physicians used HS selectively in patients for whom it was thought be safe and potentially effective. As such, it remains unclear whether a more rigorously defined population would have demonstrated the same safety and efficacy. Finally, although this study did not identify acute respiratory, renal, or electrolyte safety concerns, the sample size is too small to identify lesser severity or rare safety issues with HS administration.
CONCLUSIONS
In a cohort of severely ill patients with ADHF, treatment with HS did not appear to trigger respiratory decompensation or electrolyte abnormalities. Treatment with HS was associated with increased fluid and weight loss, as well as improvement in renal function and diuretic efficiency. These findings suggest additional rigorous study of HS as a therapeutic agent for ADHF is warranted.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In select patients with diuretic-resistant ADHF, HS was both safe and possibly effective. Specifically, there were no episodes of respiratory decompensation or rapid overcorrection of hyponatremia. Patients also lost more weight and fluid, with improvement in renal function in the days following treatment compared with the days before, suggesting potential efficacy.
TRANSLATIONAL OUTLOOK:
Hypertonic saline may be a viable rescue therapy for patients with diuretic therapy-resistant ADHF. Further investigation with randomized trials is needed to better characterize potential risks, benefits, and mechanism of action.
Acknowledgments
*Drs. Griffin and Soufer are joint first authors. Supported by U.S. National Institutes of Health grants K23HL114868, L30HL115790, R01HL139629, R21HL143092, R01HL128973 (to J.M.T.); R01DK113191 and P30DK079210 (to Dr. Wilson); and 5T32HL007950 (to Dr. Griffin). Dr. Riello is a consultant for Janssen, Johnson & Johnson, Portola, Medicure, and AstraZeneca. Dr. Coca is a consultant for RenalytixAI, CHF Solutions, Takeda, and Bayer; and has equity in RenalyitxAI. Dr. Testani has received research funding from Sequana Medical, 3ive Labs, Cardionomic, Bayer, Boehringer Ingelheim, MagentaMed, Otsuka, Sanofi, FIRE1, and Abbott; and is a consultant for Bristol-Myers Squibb AstraZeneca, Novartis, 3ive Labs, Cardionomic, Bayer, Boehringer Ingelheim, MagentaMed, Reprieve Medical, Sanofi, FIRE1, and W.L. Gore. Dr. Tang has received consulting fees for Sequana. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ADHF
acute decompensated heart failure
- FiO2
fraction of inspired oxygen
- HS
hypertonic saline
- IQR
interquartile range
- IV
intravenous
- NaCl
sodium chloride
- PO
taken by mouth
Footnotes
APPENDIX For a supplemental table and figure, please see the online version of this paper.
REFERENCES
- 1.Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:447–54. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119:S3–10. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010;12:423–33. [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015;36:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367: 2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365: 32–43. [DOI] [PubMed] [Google Scholar]
- 10.Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med 2013;173:1058–64. [DOI] [PubMed] [Google Scholar]
- 11.Doukky R, Avery E, Mangla A, et al. Impact of dietary sodium restriction on heart failure outcomes. J Am Coll Cardiol HF 2016;4:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WL, Borgeson DD, Grantham JA, Luchner A, Redfield MM, Burnett JC Jr. Dietary sodium modulation of aldosterone activation and renal function during the progression of experimental heart failure. Eur J Heart Fail 2015;17: 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: a meta-analysis of the literature. Herz 2015;40: 423–35. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi S, Mosleh W, Myers RBH. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol 2014;173:139–45. [DOI] [PubMed] [Google Scholar]
- 15.Licata G, Di Pasquale P, Parrinello G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J 2003;145:459–66. [DOI] [PubMed] [Google Scholar]
- 16.Retraction. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta-analysis. Heart. Published online first: August 21, 2012. Heart 2013;99:820. [DOI] [PubMed] [Google Scholar]
- 17.Francis GS. Notice of concern. J Card Fail 2013; 19:523. [DOI] [PubMed] [Google Scholar]
- 18.Mahtani KR, Heneghan C, Onakpoya I, et al. Reduced salt intake for heart failure: a systematic review. JAMA Intern Med 2018;178:1693–700. [DOI] [PubMed] [Google Scholar]
- 19.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int 1984;26:183–9. [DOI] [PubMed] [Google Scholar]
- 20.Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011;13:639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafreniere G, Beliveau P, Begin JY, et al. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J Cardiol 2017;9:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yayla C, Akyel A, Canpolat U, et al. Comparison of three diuretic treatment strategies for patients with acute decompensated heart failure. Herz 2015;40:1115–20. [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, Li L, Niu H, et al. Impact of compound hypertonic saline solution on decompensated heart failure. Int Heart J 2017;58:601–7. [DOI] [PubMed] [Google Scholar]
- 24.Okuhara Y, Hirotani S, Ando T, et al. Comparison of salt with low-dose furosemide and carperitide for treating acute decompensated heart failure: a single-center retrospective cohort study. Heart Vessels 2017;32:419–27. [DOI] [PubMed] [Google Scholar]
- 25.Paterna S, Di Gaudio F, La Rocca V, et al. Hypertonic saline in conjunction with high-dose furosemide improves dose-response curves in worsening refractory congestive heart failure. Adv Ther 2015;32:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adrogue HJ, Madias NE. The challenge of hyponatremia. J Am Soc Nephrol 2012;23:1140–8. [DOI] [PubMed] [Google Scholar]
- 27.Hall JE, Guyton AC. Textbook of Medical Physiology. Philadelphia: WB Saunders; 2011. [Google Scholar]
- 28.Tuttolomondo A, Pinto A, Parrinello G, Licata G. Intravenous high-dose furosemide and hypertonic saline solutions for refractory heart failure and ascites. Semin Nephrol 2011;31:513–22. [DOI] [PubMed] [Google Scholar]
- 29.Okuhara Y, Hirotani S, Naito Y, et al. Intravenous salt supplementation with low-dose furosemide for treatment of acute decompensated heart failure. J Card Fail 2014;20:295–301. [DOI] [PubMed] [Google Scholar]
- 30.Kotchen TA, Galla JH, Luke RG. Contribution of chloride to the inhibition of plasma renin by sodium chloride in the rat. Kidney Int 1978;13: 201–7. [DOI] [PubMed] [Google Scholar]
- 31.Schnermann J, Ploth DW, Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch 1976;362:229–40. [DOI] [PubMed] [Google Scholar]
- 32.Gamba G. WNK lies upstream of kinases involved in regulation of ion transporters. Biochem J 2005;391:e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naray-Fejes-Toth A, Snyder PM, Fejes-Toth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Naþ transport. Proc Natl Acad Sci USA 2004;101:17434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinehart J, Kahle KT, de Los Heros P, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 2005;102: 16777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 2003;111:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.