Abstract
Background
The wheat dwarfing gene increases lodging resistance, the grain number per spike and harvest index. Dwarf Polish wheat (Triticum polonicum L., 2n = 4x = 28, AABB, DPW), initially collected from Tulufan, Xinjiang, China, carries a semi-dwarfing gene Rht-dp on chromosome 4BS. However, Rht-dp and its dwarfing mechanism are unknown.
Results
Homologous cloning and mapping revealed that Rht-dp is the ‘Green Revolution’ gene Rht-B1b. A haplotype analysis in 59 tetraploid wheat accessions showed that Rht-B1b was only present in T. polonicum. Transcriptomic analysis of two pairs of near-isogenic lines (NILs) of DPW × Tall Polish wheat (Triticum polonicum L., 2n = 4x = 28, AABB, TPW) revealed 41 differentially expressed genes (DEGs) as potential dwarfism-related genes. Among them, 28 functionally annotated DEGs were classed into five sub-groups: hormone-related signalling transduction genes, transcription factor genes, cell wall structure-related genes, reactive oxygen-related genes, and nitrogen regulation-related genes.
Conclusions
These results indicated that Rht-dp is Rht-B1b, which regulates pathways related to hormones, reactive oxygen species, and nitrogen assimilation to modify the cell wall structure, and then limits cell wall loosening and inhibits cell elongation, thereby causing dwarfism in DPW.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-021-07367-x.
Keywords: Dwarf polish wheat, Homologous cloning, Molecular mapping, Rht-B1b, RNA-seq
Background
Plant height is an important agronomic trait of crops. The discovery and utilization of semi-dwarfing genes in rice (Oryza sativa) and wheat (Triticum aestivum) triggered the “Green Revolution”, as dwarfism not only improves lodging resistance [1], but also increases the grain number per spike and harvest index [2, 3]. Increasing numbers of dwarf varieties of crops are being bred for production [4], and the dwarfing mechanisms in many crops are clearly revealed [5–7].
In wheat, 27 dwarfing genes including 32 alleles are present on chromosomes 2A, 2B, 2D, 3B, 4B, 4D, 5A, 5D, 6A, 7A, and 7B [8–16]. Twenty-two of those genes were discovered from hexaploid wheat, including Rht1 (Rht-B1b), Rht2 (Rht-D1b), Rht8, and Rht12. Those genes are widely utilized to breed new cultivars while only Rht1 and Rht2 have been cloned [6, 17, 18]. As the parent of hexaploid wheat, tetraploid wheat owns many dwarfing genes, for example, Rht14, Rht15, Rht16, Rht18, Rht19, and Rht-R107 in Triticum durum [19, 20], Rht22 in T. turgidum [21], contains Rht-B1f in T. aethiopicum [15], and Rht-B1IC12196 and Rht-dp in T. polonicum [10, 14]. Due to T. polonicum has a high 1000-grain weight and accumulates high concentrations of zinc and iron in grains, it is recommended to be a valuable material for wheat genetic improvement [22]. However, the details of its dwarfing genes, Rht-dp and Rht-B1IC12196, are still unknown.
As a gibberellin (GA)-insensitive semi-dwarfing gene, Rht-dp was identified from dwarf Polish wheat (DPW, T. polonicum) originally collected from Tulufan, Xinjiang province, China [10, 23]. Transcriptomic and proteomic analyses suggested that Rht-dp is probably involved in the phenylpropanoid pathway. It was found to reduce the contents of lignin, cellulose, and S-adenosyl-methionine, and increase the contents of flavonoids, which ultimately limits cell expansion and causes dwarfism [24]. Although those results indicated the potential mechanism of Rht-dp, the candidate gene of Rht-dp remained unknown. Genetic analysis of F2 population derived from the cross of DPW and tall Polish wheat (TPW) indicated that Rht-dp should be a recessive gene [10]. However, the separated threshold of plant height was significant larger than the plant height of DPW [10, 23], which implied that the effect of Rht-dp on reducing plant height might be partially covered by one or more non-allelic loci. Further study mapped Rht-dp onto chromosome 4BS between the SSR markers Xgpw3017 and Xwmc511, and suggested that Rht-dp may be an alternative allele at the Rht-B1 locus [10]. However, due to the limited numbers of F2 plants and molecular markers used in the analysis, a genomic alignment against the genome of Triticum aestivum ‘Chinese Spring’ (IWGSC RefSeq v1.0) (International Wheat Genome Sequencing Consortium, 2018) indicated that the region between Xgpw3017 and Xwmc511 did not include the Rht-B1 locus. Additionally, Rht-B1b and its alleles are semi-dominant genes [6, 25, 26]. Thus, we can’t confirm whether Rht-dp is Rht-B1b or its allele, or a new gene.
Rht-B1b encodes a premature DELLA protein, which prevents GID1 from binding to its target [12]. The premature DELLA protein truncates the GA response, resulting in dwarfism. Rht-B1b originates from the native Japanese dwarf variety ‘Norin 10’ [27]. However, it was successfully transferred from ‘Norin 10’ to ‘Cando’ in the 1960s and widely used in durum wheat breeding [28]. Meanwhile, three alleles of Rht-B1b, Rht-B1f, Rht-R107, and Rht19, were also discovered from T. aethiopicum and T. durum, respectively [15, 19]. Although DPW is originally collected from Tulufan, Xingjiang, China [23] and the progenitor of T. polonicum is neither ‘Norin 10’, T. aethiopicum nor T. durum [10, 29], we still hypothesized that the candidate gene of Rht-dp may be Rht-B1b or its one of alleles, because only Rht-B1b and its alleles as dwarfing genes have been found on 4BS to date [10, 23, 28, 30].
To test this hypothesis and to understand the dwarfing mechanism of Rht-dp in DPW, we firstly cloned Rht-B1 to investigate sequence differences in Rht-B1 between DPW and TPW. Secondly, we developed and applied a specific molecular marker of Rht-B1 and SSR markers on 4BS to genetically confirm the candidate region using three recombinant inbred lines (RILs). Thirdly, two pairs of near-isogenic line (NIL) obtained from the F7 population of DPW × TPW were conducted transcript analyses to reveal the molecular mechanism of Rht-dp; meanwhile, F1 plants and a F2 population derived from the cross of a pair of NIL were developed for further genetic analysis. Finally, we conducted a haplotype analysis of Rht-dp to reveal the natural distribution among 59 tetraploid wheat accessions.
Methods
Plant materials and growth conditions
The DPW and TPW lines were originally collected from Tulufan, Xinjiang province, China, by Prof. Chi Yen and Junliang Yang (Sichuan Agricultural University, China) in the 1980s. The F1 population of DPW × TPW and the F2 population (401 plants) derived from DPW × TPW were individually developed for trait investigation. Two RIL populations (F7 including 330 lines and F8 including 300 lines) derived from DPW × TPW, and a RIL population (F6 including 194 lines) derived from DPW × Jianyangailanmai (AABB, 2n = 4x = 28, T. turgidum L., Ailanmai), were developed for gene mapping. Two pairs of NILs (D_60/T_58, and D_33/T_35, D and T represent dwarf and tall phenotype, respectively) derived from two heterozygous F7 lines were selected for transcript analyses. Meanwhile, F1 plants and a F2 population (244 plants) derived from the cross of D_60 and T_58 were developed for trait investigation. The haplotype analysis was conducted using 59 tetraploid wheat accessions (Table S1).
DPW, TPW and their F1 plants and F2 population were grown at the Wenjiang experimental field of Sichuan Agricultural University, Chengdu, China, in the 2011–2012 (from October 2011 to June 2012) and 2012–2013 (from October 2012 to June 2013) wheat growing seasons. The F7 and F8 RIL populations of DPW × TPW were grown at two experimental fields (Wenjiang and Chongzhou) of Sichuan Agricultural University (Chengdu, China) in the 2017–2018 (from October 2017 to June 2018) and 2018–2019 (from October 2018 to June 2019) wheat growing seasons, respectively. The F6 RIL population, the F1 plants of D_60 × T_58, two pairs of NILs, and 59 tetraploid wheat accessions were grown at the Wenjiang experimental field in the 2018–2019 (from October 2018 to June 2019) wheat growing season. The F2 population of D_60 × T_58 was grown at the Wenjiang experimental field in the 2019–2020 (from October 2019 to June 2020) wheat growing season. Each line was planted with 20 plants per row. The rows were 2 m long and the spacing between rows was 30 cm.
Phenotypic measurements and analysis
Plant height, spike length, and stem length were measured at maturity. We selected three individual plants per line and calculated the average value. Data was analysed using SPSS software (version 18.0; SPSS, Chicago, IL, USA) Figures were drawn using SigmaPlot software (version 12.0; Systat, Point Richmond, CA, USA).
Homologous cloning of Rht-B1
According to the genomic sequence of T. aestivum cv. ‘Chinese Spring’ (IWGSC RefSeq v1.0), a pair of Rht-B1-specific primers (forward: 5′-CGATGCCGTC TACAACTACT-3′; reverse: 5′-CAACTCCTAGATCGGGAAACTT-3′) was designed using Beacon designer software (version 7.0; Premier Biosoft International, Palo Alto, CA, USA). These primers were used to amplify the full-length Rht-B1 sequence from DPW and TPW. Each PCR reaction mixture contained 2 μl DNA, 2 μl mixture of forward and reverse primers (4 pmol/μl), 2 μl dNTP (2.5 mM/μl), 1 μl Ex-Taq polymerase (5 U/μl), 2 μl MgCl2 (2.5 mM/μl), 2.5 μl 10× PCR buffer, and 13.5 μl ddH2O. The PCR amplification conditions were 95 °C for 5 min, 40 cycles (95 °C for 30 s, 58 °C for 30 s, and 72 °C for 2 min), and final extension at 72 °C for 10 min. Each amplified fragment was cloned into the pMD19-T vector for sequencing. Differences in Rht-B1 sequences between DPW and TPW were detected in an alignment analysis using Vector NTI software (version 11.5.1; Invitrogen, Carlsbad, CA, USA).
Exploitation of indel marker of Rht-B1 for mapping
According to the sequence differences in Rht-B1 between DPW and TPW, a pair of Rht-B1-specific primers (Rht-B1 Indel-F: 5′-GGCGGGAGATCGAAGTAC-3′, Rht-B1 Indel-R: 5′-GACACCGTGCACTACAAC-3′) was designed using Beacon designer software.
Exploitation of SSR markers on 4BS for mapping
According to the genomic sequence of 4BS of T. aestivum cv. ‘Chinese Spring’ (IWGSC RefSeq v1.0) (http://plants.ensembl.org/), microsatellites were predicted using the MIcroSAtellite identification tool (https://webblast.ipk-gatersleben.de/misa/) [31, 32]. Beacon designer software was used to design SSR markers (Table S2).
Genotyping and genetic mapping
Genomic DNA was extracted from DPW, TPW, Ailanmai and the mapping populations RIL6 (DPW × Ailanmai), RIL7 and RIL8 (DPW × TPW) using a plant genomic DNA kit (TIANGEN BIOTECH, Beijing, China). Each PCR reaction mixture contained 1 μl DNA, 2 μl mixture of forward and reverse primers (4 pmol/μl), 1.5 μl dNTP (2.5 mM/μl), 0.5 μl Taq polymerase (5 U/μl), 1.5 μl MgCl2 (2.5 mM/μl), 2 μl 10× PCR buffer, and 11.5 μl ddH2O. The PCR amplification conditions were 95 °C for 5 min, 35 cycles (95 °C for 45 s, 58 °C for 45 s, and 72 °C for 45 s), and final extension at 72 °C for 7 min. The PCR products were separated on 8% polyacrylamide gels. The polymorphic bands between the parents were used to genotype individual lines of the mapping populations.
The Rht-B1 Indel marker and 15 polymorphic SSR markers were first used for genetic mapping of Rht-dp in the F7 RIL population. Then, Rht-B1Indel and its four flanking SSR markers (Xgpw2994.1, Xgpw3128.1, Xgpw3427.1, and Xgpw4800.1) were further used to confirm the candidate region in the F8 RIL and F6 RIL populations. The F7 RIL population was hybridized on the wheat 55 K SNP array by CapitalBio Technology (Beijing, China) (unpublished data).
Linkage analysis was performed using the JoinMap software (version 4.0; Kyazma BV, Wageningen, Netherlands) with a logarithm of odds (LOD) threshold of 3.0. The Kosambi mapping function was used to convert the recombination frequencies into genetic distances (cM) [33].
Haplotype analysis of Rht-B1 in 59 tetraploid wheat accessions
Genomic DNA was extracted from each tetraploid wheat accession using a plant genomic DNA kit (TIANGEN BIOTECH, Beijing, China), and PCR amplification was performed as described in the section “Homologous cloning of Rht-B1”. The amino acid sequence was deduced using ExPASy software (http://web.expasy.org/ translate/). All sequences were aligned using Vector NTI software (Invitrogen). A phylogenetic tree was constructed using the neighbour-joining algorithm in MEGA5 (https://www.megasoftware.net/).
Expression analysis of Rht-B1b
Tissues at the three growth stages (jointing, booting, and grain filling stages) were collected, including roots, basal stems, leaf sheaths, leaf blades, young leaves, lower leaf blades, first and second internodes, flag leafs, and spikes. The collected tissues were snap-frozen in liquid nitrogen and stored at − 80 °C until RNA extraction. Total RNA was extracted using a Plant RNA Kit (Omega Bio-Tek, American). cDNA was synthesized using the M-MLV First Strand cDNA Synthesis kit (Invitrogen).
Quantitative real-time PCR (qPCR) was performed on the CFX-96 system as described by Wang et al. using a pair of Rht-B1b-specific primers (forward: 5′-GGCGGGAGATCGAAGTAC-3′; reverse: 5′-GACACCGTGCACTACAAC-3′) [34]. To normalize gene expression levels, the Actin gene was used as the reference gene [34]. Relative expression levels were calculated according to the 2ΔΔCt method using the CFX Manager (version 3.1; Bio-Rad, Hercules, CA, USA).
Transcript analysis of two pairs of NILs
Sample collection
At the booting stage, the first internode was collected individually from two pairs of NILs, and then snap-frozen in liquid nitrogen and stored at − 80 °C until RNA extraction.
RNA extraction, library preparation and sequencing
Total RNA was isolated as described above, and RNA degradation and contamination were monitored on 1% agarose gels. A NanoPhotometer® spectrophotometer (Implen GmbH, Munich, Germany) RNA purity was used to check RNA purity. The mRNA was purified from total RNA using poly-T oligo-attached magnetic beads and divided into short fragments using NEBNext First Strand Synthesis Reaction Buffer (5×) (New England Biolabs, Ipswich, MA, USA). The cDNA was synthesized using the fragments as templates and then purified and resolved with EB buffer for the end-repair step and addition of a single adenine (A) nucleotide. To select cDNA fragments 250 ~ 300 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, CA, USA), and suitable fragments were chosen for a PCR amplification. The PCR products were purified (AMPure XP system) and the library quality was assessed using the Agilent Bioanalyzer 2100 system. The prepared libraries were sequenced on the Illumina Hiseq platform.
RNA-seq data analysis
Raw data (raw reads) of in fastq format were first processed using in-house perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapters, reads containing poly-N, and low-quality reads from the raw data. All the downstream analyses were conducted using clean, high-quality data.
The Chinese Spring (IWGSC RefSeq v1.0) reference genome and gene model annotation files were downloaded from the genome website (https://urgi.versailles. inra.fr/download/iwgsc/IWGSC_RefSeq_Assemblies/v1.0). The D genome sequences were excluded from the reference before mapping the processed reads of the tetraploid lines (A and B genomes). An index of the Chinese Spring reference genome was built using Bowtie v2.2.3 and paired-end clean reads were aligned to the reference genome using TopHat v2.0.12. HTSeq v0.6.1 was used to count the number of reads mapped to each gene. The mean fragments per kilobase of transcript per million mapped reads (FPKM) value for each gene was calculated based on the length of the gene and the number of reads mapped to it [35].
Differential expression analysis
Read counts were adjusted by the edgeR program package through one scaling normalized factor. Analysis of differential gene expression between two pairs of NILs (D33/T35 and D60/T58) was performed using the DEGSeq R package. The P values were adjusted using the Benjamini and Hochberg method. A corrected P-value of 0.005 and log2 (fold change) of 1 were set as the thresholds for significantly different gene expression.
QPCR for validation
Two differentially expressed genes Auxin-repressed protein (ARP) and L-ascorbate oxidase homolog (ASCO) from RNA-Seq were verified by qPCR, and their gene-specific primers sequences were APR (forward: 5′-ATTAAGCAGTCGCCG TCGAT-3′; reverse: 5′-TCGCTGTAAAGCCAG TCGTA − 3′) and ASCO (forward: 5′-AATGGCAATAGGTTCACAGTAGA-3′; reverse: 5′-CTTCACGAGGAACGAGT AGG-3′), respectively.
Results
Phenotype of plants harbouring Rht-dp
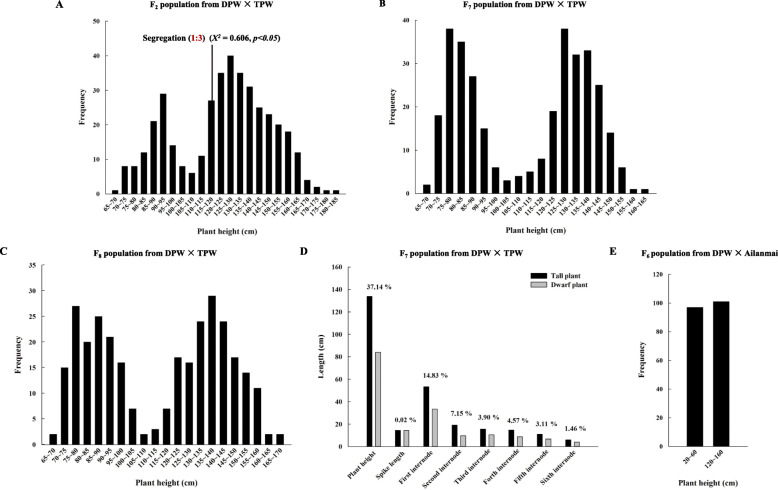
The average heights of DPW and TPW were 91.52 ± 2.97 cm and 189.88 ± 1.72 cm, respectively. No significant difference in plant heights between F1 plants (179.12 ± 3.65 cm) and TPW was observed (Fig. S1). The plant heights of F2 plants ranged from 65 to 185 cm. According to the frequency distribution of plant height, F2 plants were separated into two groups of dwarf and tall phenotypes at 110 cm (Fig. 1a). The dwarf and tall phenotype groups included 107 and 294 plants, respectively, consistent with the expected Mendelian segregation ratio of 1:3 (X2 = 0.606, p < 0.05). These results validate that Rht-dp should be a major recessive gene. However, the separated threshold of plant height with 110 cm was significantly larger than the plant height of DPW with 91.52 ± 2.97 cm, which implied that the effect of Rht-dp on reducing plant height might be partially covered by one or more non-allelic loci.
Fig. 1.
Phenotypic characterization. a frequency distribution of plant heights in the F2 population from DPW × TPW; b frequency distribution of plant heights in the DPW × TPW F7 population; c frequency distribution of plant heights in the DPW × Ailanmai F6 population; d plant height, the lengths of spike and each internode of DPW × TPW NILs F7 at the maturate stage; e frequency distribution of plant heights in the DPW × TPW F8 population
To fine-map Rht-dp, two RIL populations including 330 F7 and 300 F8 plants were constructed. The plant heights of F7 and F8 plants ranged from 65 to 165 cm (Fig. 1b) and from 65 to 170 cm (Fig. 1c), respectively. For the F7 population, the average heights of dwarf and tall phenotypes were 84.07 ± 1.97 cm and 133.75 ± 2.01 cm, respectively. Compared with the tall phenotype, the lines harbouring Rht-dp showed a reduction in plant height of up to 37.14%. The reduced plant height was because of the shortened first internode (by 14.83%), second internode (by 7.15%), and basal internode (by 1.46%), but the length of the spike was not affected (Fig. 1d). These results indicate that Rht-dp reduces plant height mainly by restricting elongation of the first and second internodes at the booting stage.
To validate the candidate region of Rht-dp in a different genetic background, an F6 RIL population including 194 lines derived from DPW × Ailanmai was constructed. The average height of Ailanmai was 100.98 ± 0.37 cm. Ailanmai has a recessive dwarfing gene Rht22, which has an additive effect with Rht-dp. The RIL population was grouped into dwarf and tall phenotypes with heights ranging from 20 to 60 cm and from 120 to 160 cm, respectively (Fig. 1e).
Characterization of Rht-dp in F1 plants and F2 population derived from the cross of a pair of NIL
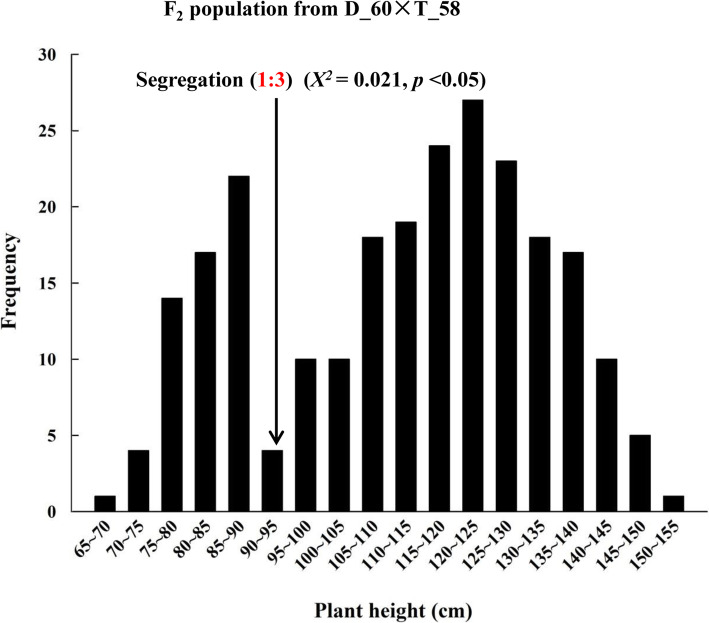
Since genetic analysis suggested that the effect of Rht-dp on reducing plant height was probably influenced by one or more non-allelic loci derived from TPW, a QTL analysis was performed on the F7 RIL population using the wheat 55 K SNP array. Beside of a major-locus on 4BS (Rht-dp) derived from DPW caused dwarfism, a micro-locus on 5A derived from TPW heightened plant was detected (unpublished data). To further confirm the information of Rht-dp, we measured the plant height of F1 plants and F2 population derived from the cross of a pair of NIL (D_60 and T_58). The average heights of D_60 and T_58 were 93.52 ± 1.83 cm and 159.67 ± 2.72 cm, respectively; the average plant height of F1 was 123.23 ± 2.55 cm. Compared with T_58, F1 plants harbouring Rht-dp showed a reduction in plant height up to 22.82%. The plant heights of F2 plants ranged from 65 to 155 cm. According to the frequency distribution of plant height, F2 plants were separated into two groups of dwarf and tall phenotypes at 95 cm (Fig. 2). The dwarf and tall phenotype groups included 62 and 182 lines, respectively, consistent with the expected Mendelian segregation ratio of 1:3 (X2 = 0.021, p < 0.05). Meanwhile, the separated threshold of plant height with 95 cm was similar to the plant height of D_60 with 93.52 ± 1.83 cm. These results indicate that the dwarfing gene of Rht-dp should be a single semi-dominant gene, and further imply that the candidate gene is Rht-B1b.
Fig. 2.
Frequency distribution of plant heights in the F2 population from D_60 × T_58
Differences in sequence of Rht-B1 between DPW and TPW
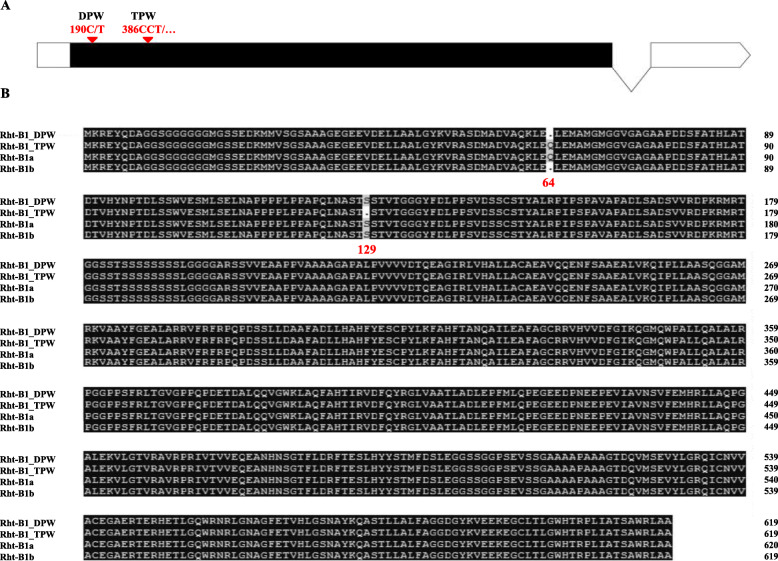
To test the implication that the candidate gene of Rht-dp is Rht-B1b or one of its alleles, the sequences of Rht-B1 were cloned from DPW and TPW. Sequence analysis showed that Rht-B1 of DPW is Rht-B1b, with a single nucleotide change from C to T at the nucleotide position 190 when compared with Rht-B1a (Fig. 3a) that results in a premature termination codon at amino acid position 64 (Fig. 3b). Although Rht-B1 of TPW did not have this single nucleotide change from C to T at nucleotide position 190, it had a three-nucleotide deletion at nucleotide position 386–388 when compared with Rht-B1a (Fig. 3a), resulting in a serine (S) deletion at amino acid position 129 (Fig. 3b). These results imply that the candidate gene of Rht-dp might be Rht-B1b. An Rht-B1 Indel marker was developed from the three-nucleotide deletion of Rht-B1 in TPW for further analysis.
Fig. 3.
Sequences of Rht-B1 in DPW and TPW. a: nucleotide mutations of Rht-B1 in DPW and TPW; b: amino acid mutations of Rht-B1 in DPW and TPW
Mapping of Rht-dp
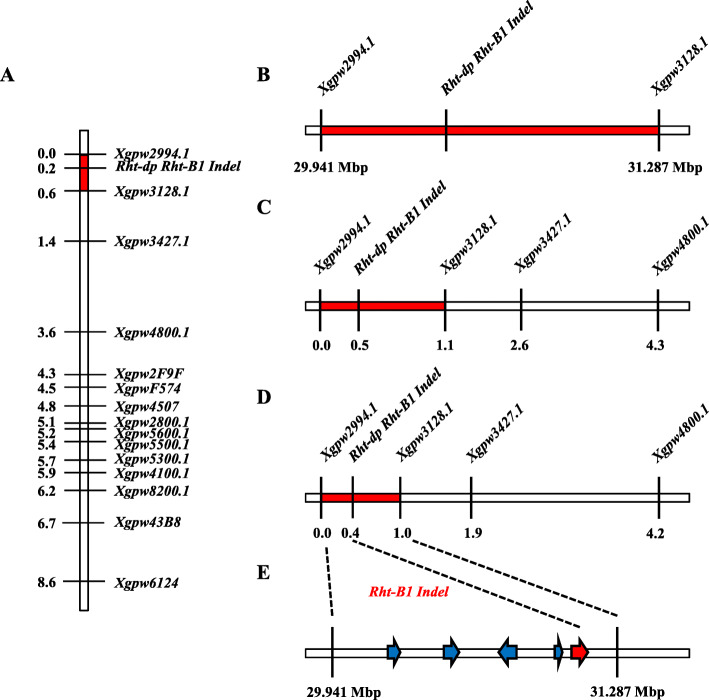
To confirm that the candidate gene of Rht-dp is Rht-B1b, the Rht-B1Indel marker was first used to determine whether Rht-B1 was tightly linked with Rht-dp. Genetic mapping analyses confirmed that the Rht-B1Indel marker completely co-segregated with Rht-dp in three RIL populations and a F2 population derived from a pair of NIL (Fig. 4).
Fig. 4.
Mapping of Rht-dp. a: mapping of Rht-dp in the DPW × TPW RILs F7; b: mapping of Rht-dp in the F2 population from D_60 × T_58; c: mapping of Rht-dp in DPW × Ailanmai RILs F6; d: mapping of Rht-dp in DPW × TPW RILs F8; e: candidate genes between SSR markers Xgpw2994.1 and Xgpw3128.1
To further confirm that Rht-B1b is located in the candidate region of Rht-dp, 190 pairs of SSR markers were exploited according to the genome reference of 4BS (Table S2). Fifteen pairs of SSR markers exhibited polymorphism between DPW and TPW, and were linked with Rht-dp in the F7 RIL population. Of them, two SSR markers, Xgpw2994.1 and Xgpw3128.1, were tightly linked with Rht-dp with a genetic distance of 0.6 cM (Fig. 4a; Table S3). Xgpw2994.1 and Xgpw3128.1 were further confirmed as tightly linked markers flanking Rht-dp in the F2 population derived from NIL (Fig. 4b), and the F6 (Fig. 4c) and F8 (Fig. 4d) RIL populations (Table S3).
Based on the gene annotation of wheat 4BS from 29.94 to 31.29 Mbp, flanked by Xgpw2994.1 and Xgpw3128.1, there were five potential genes: TraesCS4B01G042700 (encodes a teosinte branched 1 protein), TraesCS4B01G042800 (encodes an uncharacterized protein), TraesCS4B01G042900 (a RING finger protein), TraesCS4B01G043000 (EamA-like transporter family), and TraesCS4B01G043100 (Rht-B1 encodes a DELLA protein) (Fig. 4e). Apart from Rht-B1, sequence difference of other four genes (primers listed in Table S4) between DPW and TPW was not found. These results indicate that the candidate gene of Rht-dp should be Rht-B1b.
Expression patterns of Rht-B1b in DPW
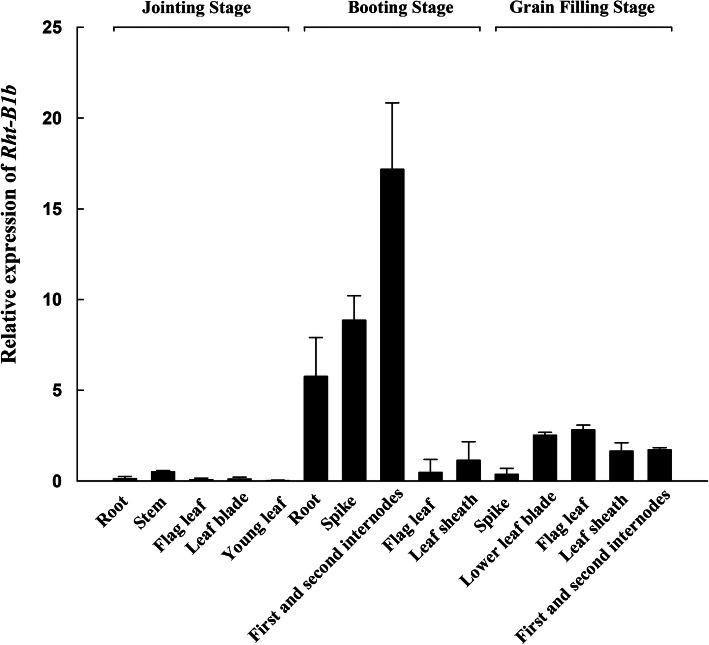
To confirm that Rht-B1b reduces plant height via its effects on elongation of the first and second internodes at the booting stage, the transcriptional patterns of Rht-B1b were investigated in different DPW tissues at the jointing, booting, and grain-filling stages. Rht-B1b was mainly expressed in the first and second internodes at the booting stage, and at dramatically higher levels in those tissues than in other tissues at the jointing, booting, and grain-filling stages (Fig. 5).
Fig. 5.
Expression patterns of Rht-dp in various wheat tissues at jointing, booting, and grain filling stages
Allelic variations of Rht-B1 in tetraploid wheat accessions
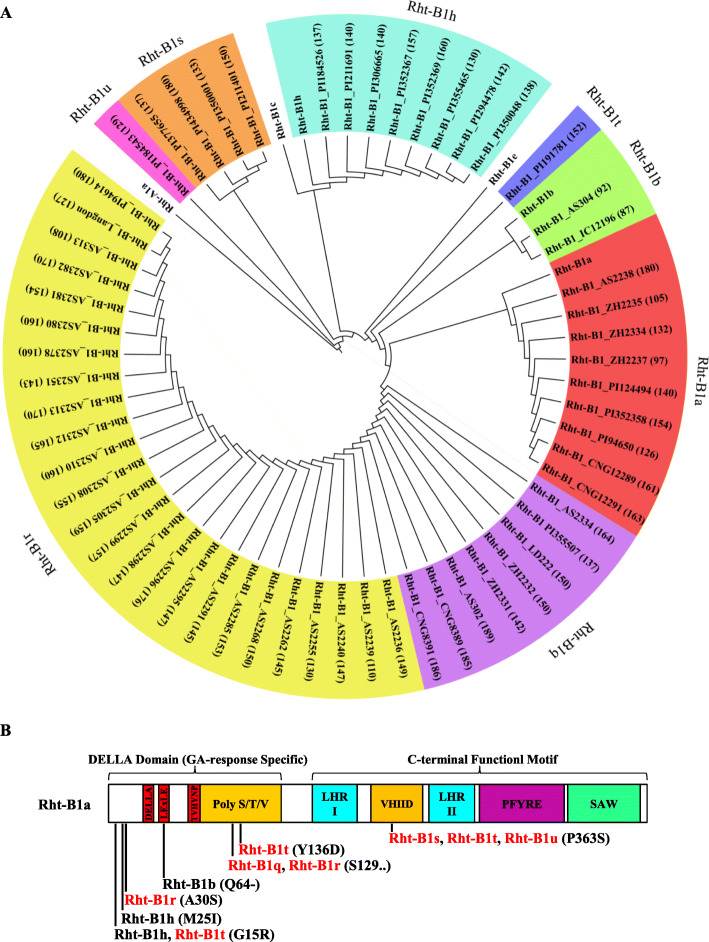
Rht-B1b has never been found in spontaneous tetrapolid accession. Since Rht-B1b is the candidate gene of Rht-dp in DPW, the haplotypes of Rht-B1b in 59 tetraploid wheat accessions were analysed. Among them, five accessions were dwarf phenotypes including two T. turgidum (AS313 and AS2239), two T. polonicum [AS304 (DPW) and IC12196], and one T. durum (ZH2237). The 59 sequences cloned from the 59 tetraploid wheat accessions were grouped into eight types. Rht-B1b was only obtained from two T. polonicum (DPW and IC12196) accessions; and Rht-B1t and Rht-B1u were only obtained from T. turgidum. Subsp. dicoccon (PI191781) and T. turgidum. Subsp. Turanicum (PI184543), respectively. Of them, five novel types (named Rht-B1q–B1u, respectively) were identified by comparison with Rht-B1a (Fig. 6b). Rht-B1q contained an S deletion at position 129 (S129); Rht-B1r carried a mutation at position 30 (A30S) and an S deletion at position 129 (S129); Rht-B1s contained a mutation at position 363 (P363S). Rht-B1t had two mutations at positions 15 (G15R) and 363 (P363S). Rht-B1u also had two mutations at positions 136 (Y136D) and 363 (P363S) (Fig. 6b).
Fig. 6.
Haplotype analysis of Rht-B1 in 59 tetraploid wheat accessions. a phylogenetic relationship of Rht-B1 alleles; b nonsynonymous mutations in Rht-B1
Among these variations, Rht-B1q had the highest frequency (43.9%). The frequencies of Rht-B1a, Rht-B1b, Rht-B1h, Rht-B1r, Rht-B1s, Rht-B1t, and Rht-B1u were 15.3, 3.4, 13.6, 13.6, 6.8, 1.7, and 1.7%, respectively.
Dwarfism-related DEGs induced by DELLA mutant Rht-B1b
To understand the molecular networks of Rht-B1b, the DEGs induced by the DELLA mutation Rht-B1b in the first internode of two pairs of NILs were investigated. A total of 41 DEGs was obtained, 30 of which were successfully functionally annotated (Table S5). Twenty-eight DEGs were further classed into five sub-groups; hormone-related signalling transduction genes, transcription factor genes, cell wall structure-related genes, reactive oxygen-related genes, and nitrogen regulation-related genes (Table 1). Among the hormone-related signal transduction genes, two brassinolide (BR) signal-related genes serine carboxypeptidase II-3 (SCP) and cytochrome P450 710A1 (CYP450) were down-regulated; and genes encoding salicylic acid (SA)-binding protein 2 and ARP were up-regulated in the dwarf phenotype. The only down-regulated transcription factor gene was MybAS2. Fifteen DEGs were grouped into cell wall structure-related genes (seven pectin-related genes and eight xylan acetylation-related genes). In the dwarf phenotype, five pectin-related genes [encoding a pectate lyase 15 (PEL15), three subtilisin-like protease (SBT1.7), and an alpha-galactosidase (α-Gal)] involved in pectin modification were down-regulated; while all eight xylan acetylation-related genes, including three GDSL esterase/lipase genes, two ESKIMO genes, IRX15-L, ALTERED XYLOGLUCAN 4-like (AXY-L), and an uncharacterized acetyltransferase gene were up-regulated. For the reactive oxygen-related genes, plant cysteine oxidase 2 (PCO2) and ASCO were down-regulated; and genes encoding germin-like protein 5–1 (GLP) and blue copper protein (BCP) were up-regulated in the dwarf phenotype. For nitrogen assimilation-related genes, two phosphoenolpyruvate carboxylase kinase 2 (PPCK2) genes and early nodulin (ENOD) were down-regulated; and asparagine synthetase (APS) was up-regulated in the dwarf phenotype. We verified the expression of ARP and ASCO in the first and second internodes at the booting stage (Fig. S2).
Table 1.
Dwarfism-related DEGs induced by DELLA mutant Rht-dp
| Gene ID | Description | Fold change of transcript | |
|---|---|---|---|
| D_60/T_58 | D_33/T_35 | ||
| Hormone-related signaling transduction genes | |||
| TraesCS2B01G157100 | Serine carboxypeptidase II-3 | −32 | −20 |
| TraesCS3B01G167400 | Cytochrome P450 710A1 | −25 | −18 |
| TraesCS2B01G471800 | Salicylic acid-binding protein 2 | 28 | 39 |
| TraesCS4B01G070300 | Auxin-repressed 125 kDa protein | 12 | 15 |
| Transcription factor | |||
| TraesCS1B01G055200 | Myb-related protein MYBAS2 | −13 | −26 |
| Cell wall structure-related genes | |||
| Pectin-related genes | |||
| TraesCS2A01G016500 | Pectate lyase 15 | −17 | −29 |
| TraesCS4A01G237500 | Subtilisin-like protease SBT17 | −20 | −22 |
| TraesCS4B01G077600 | Subtilisin-like protease SBT17 | −29 | −17 |
| TraesCS6A01G339400 | Subtilisin-like protease SBT17 | −14 | −12 |
| TraesCS6B01G332900 | Alpha-galactosidase | −16 | −11 |
| TraesCS1B01G249000 | (1–3,1–4)-beta-D-glucanase | 21 | 28 |
| TraesCS2A01G341400 | Sugar transport protein 5 | 11 | 14 |
| Xylan acetylation-related genes | |||
| TraesCS3A01G258100 | GDSL esterase/lipase | 15 | 11 |
| TraesCS3B01G290800 | GDSL esterase/lipase | 13 | 10 |
| TraesCS7B01G250700 | GDSL esterase/lipase | 13 | 23 |
| TraesCS4A01G110000 | ESKIMO 1 | 14 | 10 |
| TraesCS4B01G194100 | ESKIMO 1 | 17 | 10 |
| TraesCS6A01G131900 | IRX15-like | 13 | 11 |
| TraesCS7A01G191700 | ALTERED XYLOGLUCAN 4-like | 17 | 11 |
| TraesCSU01G204900 | Uncharacterized acetyltransferase | 28 | 16 |
| Reactive oxygen-related genes | |||
| TraesCS5A01G025200 | Plant cysteine oxidase 2 | −11 | −15 |
| TraesCS7A01G459400 | L-ascorbate oxidase homolog | −34 | −51 |
| TraesCS3A01G165500 | Germin-like protein 5–1 | 16 | 22 |
| TraesCS6A01G315800 | Blue copper protein | 13 | 12 |
| Nitrogen regulation-related genes | |||
| TraesCS6A01G375800 | Phosphoenolpyruvate carboxylase kinase 2 | −12 | −20 |
| TraesCS6B01G413500 | Phosphoenolpyruvate carboxylase kinase 2 | −12 | −15 |
| TraesCS7A01G091800 | Early nodulin-93 | −40 | −12 |
| TraesCS3B01G385400 | Asparagine synthetase | 11 | 12 |
Discussion
The GA-insensitive dwarfing gene Rht-B1b is the predominant source of the semi-dwarf growth habit of wheat plants grown in parts of Northern Europe [36], the Mid and Lower Yangtze Valley Autumn-sown Spring Wheat Region in China [37], and the Great Plains Hard Winter Wheat Region in the USA [38]. Because Rht-B1b significantly decreases plant height to reduce plant lodging and increase wheat yield [37, 39], it has been introduced into tetraploid wheat T. durum for dwarf breeding [28]. However, it is well known that the progenitor of T. polonicum is not Norin 10 or T. durum. Additionally, DPW was originally collected from Tulufan, Xingjiang, China [23]. Thus, the dwarfing gene Rht-dp of T. polonicum cannot be derived from Norin 10 or T. durum. However, our results show that the candidate gene Rht-dp of DPW is Rht-B1b. This conclusion is supported by the following evidences: (1) Rht-dp is a single semi-dominant dwarfing gene, as is Rht-B1b [6]. (2) Rht-dp and Rht-B1b reduce plant height mainly via reducing the length of the first and second internodes (Fig. 1d), and their effects on reducing plant height are similar with 22% [18, 39]. (3) The sequence of Rht-B1 of DPW is the same as that of Rht-B1b (Fig. 3). (4) Mapping work revealed that the candidate region of Rht-dp was between SSR markers Xgpw2994.1 and Xgpw3128.1 (Fig. 4b-d). This region contains five potential genes including Rht-B1 (Fig. 4e); except of Rht-B1, other four genes have no sequence difference between DPW and TPW. (5) The Rht-B1 Indel marker developed based on the sequence difference of Rht-B1 between DPW and TPW is completely co-segregated with Rht-dp in a F2 population derived from NIL and three RIL populations (Fig. 4). In the haplotype analysis, Rht-B1b was only obtained from T. polonicum (Fig. 6a), implying that it might originate from this species, or might be introduced into T. polonicum from other unknown species but not T. aethiopicum and T. durum.
In wheat, Rht-B1b encodes a DELLA mutant protein resembling the SLRL1 protein. Its accumulation represses GA-regulated growth and developmental responses and causes the typical semi-dwarf phenotype [6, 40]. DELLA not only regulates the expression of downstream genes but also interacts with DNA-binding transcription factors. Our transcript analysis identified 28 DEGs regulated by the DELLA mutant Rht-B1b involved in the processes of nitrogen assimilation, oxidation-reduction, modification of the cell wall components and structures, and hormone-related signal transduction (Table 1). However, this list of DEGs only slightly overlaps with those identified in previous studies, suggesting that the effects of DELLA on transcription depend on the species, organ, and developmental context [41–44]. Since Rht-B1b is mainly expressed in the first and second internodes (Fig. 5) to dramatically reduce their lengths at the booting stage in DPW (Fig. 1d), we explored the molecular network of Rht-dp by conducting a transcript analysis of the first and second internodes at the booting stage.
The control of plant growth and development by DELLA is dependent on GA-regulated growth and developmental responses [44–46]. However, we did not find genes involved in GA metabolism among the DEGs in this study. Instead, the DEGs identified in this study included auxin-, SA- and BR-related genes (Table 1). These results suggested that GA interacts with these hormones [46]. DELLA can directly trigger the expression of auxin- and BR-related genes to affect plant growth [47, 48]. For example, the expressions of SCP and CYP450 (both grouped into BR-related genes) were dramatically down-regulated by the DELLA mutation Rht-B1b to potentially cause dwarfism in DPW (Table 1), because the expression of SCP positively affects plant growth [49]. Auxin represses the expression of ARP genes [50, 51]. In a previous study, overexpression of an ARP of Brassica rapa caused a reduction in vegetative growth [50]. Auxin also modulates the expression of ASCO, which encodes a crucial enzyme that produces oxidative molecules, including H2O2 [52]. Overexpression of an ASCO in cotton enhanced the accumulation of H2O2 and promoted cell elongation, whereas suppression of an ASCO in tobacco and Arabidopsis inhibited stem cell growth [53]. Our results show that the DELLA mutation Rht-B1b resulted in dramatically up-regulated ARP and down-regulated ASCO in DPW (Table 1). Auxin-induced growth inhibition is accompanied by decreased levels of reactive oxygen species [54]. Thus, the accumulation of the DELLA mutant protein regulated via auxin-mediated signal transduction may reduce the contents of reactive oxygen species such as H2O2 [41], thereby limiting cell expansion to cause dwarfism in DPW.
In rice, over-expression of an early nodulin gene resulted in improved nitrogen-use efficiency and increased nitrogen assimilation [55]. In C3 plants, nitrogen assimilation is positively correlated with phosphoenolpyruvate carboxylase (PEPC) phosphorylation [56, 57], which is catalysed by phosphoenolpyruvate carboxylase kinase (PPCK). The extent of phosphorylation is largely determined by PPCK activity, which is controlled by the level of PPCK transcripts [56, 58, 59]. A reduction in PEPC activity leads to serious stunting of growth [60]. Our results showed that the DELLA mutation Rht-B1b led to significant down-regulation of early nodulin and two PPCKs in DPW (Table 1). Thus, decreased nitrogen assimilation and PPCK activity may decrease the activity of PEPC [43, 59] to cause dwarfism in DPW.
The hemicellulose xylan and pectins are two abundant polysaccharides in the plant cell wall [61]. Their modifications, such as methylesterification and acetylation, have been proposed to influence cell wall architecture and function, causing various plant growth phenotypes [61–64]. Our results showed that the DELLA mutation Rht-B1b led to significant down-regulation of the expression of several pectin-related genes, including PEL, three SBTs, and α-Gal (Table 1). Decreases in the transcript levels of these genes may lead to the repression of pectin degradation and the accumulation of de-esterified pectin [63], enhanced pectin methylesterase activity to stiffen the cell wall [65], and reduced adherence of pectin to the cell wall [66]. Thus, the DELLA mutation Rht-B1b may result in modifications of pectin that limit cell wall loosening and inhibit cell elongation, thereby causing dwarfism in DPW.
Many studies have reported that either excess or inadequate acetylation of xylan disrupts the cell wall structure, thereby causing dwarfism in plants [67, 68]. Our results show that the DELLA mutation Rht-B1b up-regulated eight xylan acetylation-related genes, including three GDSL esterase/lipase genes, two ESKIMO genes, IRX15-L, AXY-L, and an uncharacterized acetyltransferase gene (Table 1). ESKIMO and AXY-L are xylan acetyltransferases, and IRX-L is involved in synthesis of the xylan backbone [61, 67–70]. A specific interaction between acetyltransferases and xylan backbone biosynthetic enzymes may repress acetylation of adjacent residues [68, 70]. Therefore, even though the transcript levels of ESKIMO, IRX15-L, AXY-L and IRX-L were up-regulated (Table 1), the acetylation of xylan might be decreased. GDSL esterase/lipase is a xylan deacetylation enzyme [64]. The DELLA mutation Rht-B1b resulted in up-regulated expression of GDSL esterase/lipase, leading to enhance xylan deacetylation. Therefore, the DELLA mutation Rht-B1b may reduce acetylation of xylan to limit cell wall loosening and inhibit cell elongation, causing dwarfism in DPW.
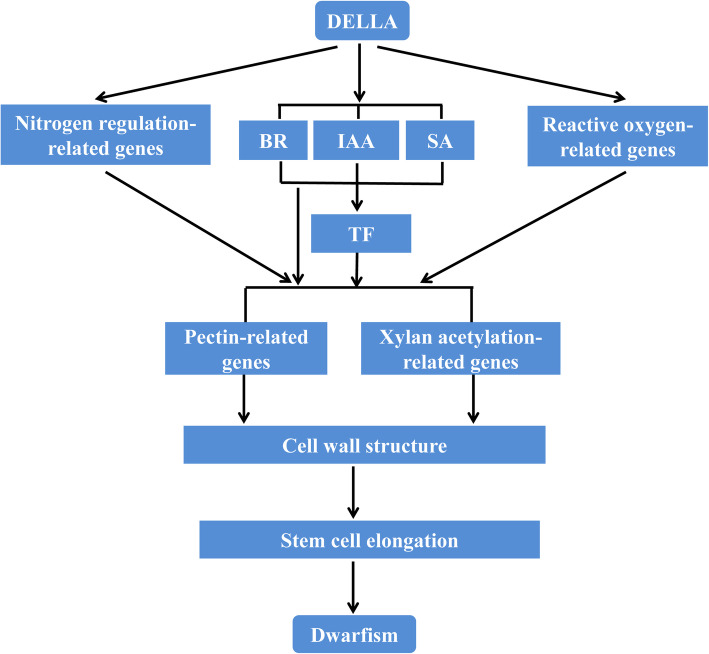
A model summarizing how the DELLA mutation Rht-dp causes dwarfism in DPW is proposed (Fig. 7). Whether the DELLA mutation Rht-B1b regulates the pathway of hormones, reactive oxygen species, and nitrogen assimilation, it ultimately affects the cell wall structure to limit cell wall loosening and inhibit cell elongation, thereby causing dwarfism in DPW.
Fig. 7.
Molecular network model of the DELLA mutation Rht-dp in DPW
Conclusion
In summary, our results indicated that the semi-dwarfing gene Rht-dp is the“Green Revolution” gene Rht-B1b. It regulates pathways related to hormones, reactive oxygen species, and nitrogen assimilation to modify the cell wall structure, and then limits cell wall loosening and inhibits cell elongation, thereby causing dwarfism in DPW.
Supplementary Information
Additional file 1: Fig. S1. The plant height of DPW, TPW, and DPW × TPW F1.
Additional file 2: Fig. S2. Relative expression of ARP and ASCO in the first and second internodes at the booting stage.
Additional file 3: Table S1. The information of 59 tetraploid wheat accessions.
Additional file 4: Table S2. The information of SSR primers on 4BS chromosome.
Additional file 5: Table S3. Genotype data of RIL populations and the F2 population from D_60 × T_58.
Additional file 6: Table S4. Gene-specific primers for Rht-dp candidate genes in DPW and TPW.
Additional file 7: Table S5. The information of Dwarfism-related DEGs induced by DELLA mutant Rht-dp.
Acknowledgements
We thank the professor Lianquan Zhang (Sichuan Agricultural University, China) who provided the grain of tetraploid wheat accessions for haplotype analysis.
Abbreviations
- BR
Brassinolide
- cM
centimorgan
- DEGs
Differentially expressed genes
- DPW
Dwarf polish wheat
- FPKM
Fragments per kilobase of transcript per million mapped reads
- GA
Gibberellin
- Jianyangailanmai
Ailanmai
- LOD
Logarithm of odds
- NIL
Near-isogenic line
- qPCR
Quantitative real-time PCR
- RIL
Recombinant inbred line
- RNA-seq
RNA sequencing
- SA
Salicylic acid
- TPW
Tall polish wheat
Authors’ contributions
SC, XX, JL, YW and YZ designed the research and wrote the manuscript; SC, QY, XZ and XX performed the experiments; SC, XF, JZ, LS, HK, HZ and YW performed the data analysis and revised the manuscript. Thel author(s) read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No 31671688), and the Bureau of Science and Technology of Sichuan Province, China.
Availability of data and materials
All data generated or analyzed during this study were included in this article and the supplementary files.
Ethics approval and consent to participate
The DPW and TPW lines were originally collected from Tulufan, Xinjiang province, China, by Prof. Chi Yen and Junliang Yang (Sichuan Agricultural University, China) in the 1980s. No permission was necessary to collect this sample. Professor Chi Yen undertook the formal identification of the sample. The voucher specimen and the seed are deposited in the Triticeae Research Institute, Sichuan Agricultural University, Chengdu, Sichuan, China. Collection of the dwarf Polish wheat complied with the institutional, national and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Songyue Chai, Email: chaisy@stu.sicau.edu.cn.
Qin Yao, Email: 18280836779@163.com.
Xu Zhang, Email: zhangxu8534@163.com.
Xue Xiao, Email: demi821214@126.com.
Xing Fan, Email: fanxing9988@163.com.
Jian Zeng, Email: zengjian@sicau.edu.cn.
Lina Sha, Email: rice_shazhi@163.com.
Houyang Kang, Email: xiaokang_69@126.com.
Haiqin Zhang, Email: haiqinzhang@163.com.
Jun Li, Email: lijunchd@126.com.
Yonghong Zhou, Email: zhouyh@sicau.edu.cn.
Yi Wang, Email: wangyi@sicau.edu.cn.
References
- 1.Gale MD, Youssefian S, Russell GE. Dwarfing genes in wheat. Progress in. Plant Breed. 1985;1:1–35. [Google Scholar]
- 2.Flintham JE, Börner A, Worland AJ, Gale MD. Optimizing wheat grain yield: effects of Rht (gibberellin-insensitive) dwarfing genes. J Agri Sci. 1997;128(1):11–25. doi: 10.1017/S0021859696003942. [DOI] [Google Scholar]
- 3.Youssefian S, Kirby EJM, Gale MD. Pleiotropic effects of the GA- insensitive Rht dwarfing genes in wheat. 2. Effects on leaf, stem, ear and floret growth. Field Crop Res. 1992;28(3):191–210. doi: 10.1016/0378-4290(92)90040-G. [DOI] [Google Scholar]
- 4.Hedden P. The genes of the green revolution. Trends Genet. 2003;19(1):5–9. doi: 10.1016/S0168-9525(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 5.Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S. Loss- of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 2002;32(4):495–508. doi: 10.1046/j.1365-313X.2002.01438.x. [DOI] [PubMed] [Google Scholar]
- 6.Peng JR, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP. ‘Green Revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400(6741):256. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M. Green revolution: a mutant gibberellin- synthesis gene in rice. Nature. 2002;416(6882):701–702. doi: 10.1038/416701a. [DOI] [PubMed] [Google Scholar]
- 8.Chen SL, Gao RH, Wang HY, Wen MX, Xiao J, Bian NF, Zhang RQ, Hu WJ, Cheng SH, Bie TD, Wang XU. Characterization of a novel reduced height gene (Rht23) regulating panicle morphology and plant architecture in bread wheat. Euphytica. 2015;203(3):583–594. doi: 10.1007/s10681-014-1275-1. [DOI] [Google Scholar]
- 9.Ellis MH, Rebetzke GJ, Azanza F, Richards RA, Spielmeyer W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor Appl Genet. 2005;111(3):423–430. doi: 10.1007/s00122-005-2008-6. [DOI] [PubMed] [Google Scholar]
- 10.Kang HY, Lin LJ, Song ZJ, Yuan JY, Zhong MY, Zhang HQ, Fan X, Sha LN, Wang Y, Xu LL, Zeng J, Zhou YH. Identification, fine mapping and characterization of Rht-dp, a recessive wheat dwarfing (reduced height) gene derived from Triticum polonicum. Genes Genom. 2012;34(5):509–515. doi: 10.1007/s13258-012-0022-z. [DOI] [Google Scholar]
- 11.Mo Y, Vanzetti LS, Hale I, Spagnolo EJ, Guidobaldi F, Al-Oboudi J, Dubcovsky J. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor Appl Genet. 2018;131(10):2021–2035. doi: 10.1007/s00122-018-3130-6. [DOI] [PubMed] [Google Scholar]
- 12.Pearce S, Saville R, Vaughan SP, Chandler PM, Wilhelm EP, Sparks CA, Hedden P. Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiol. 2011;157(4):1820–1831. doi: 10.1104/pp.111.183657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vikhe P, Patil RM, Chavan A, Oak MD, Tamhankar SA. Mapping gibberellin- sensitive dwarfing locus Rht18 in durum wheat and development of SSR and SNP markers for selection in breeding. Mol Breeding. 2017;37(3):28. doi: 10.1007/s11032-017-0641-9. [DOI] [Google Scholar]
- 14.Watanabe N. Triticum polonicum IC12196: a possible alternative source of GA3-insensitive semi-dwarfism. Cereal Res Commun. 2004;32(4):429–434. doi: 10.1007/BF03543331. [DOI] [Google Scholar]
- 15.Watanabe N. Genetic mapping of the genes and development of near-isogenic lines in durum wheat. EWAC Newsletters. 2008. pp. 27–28. [Google Scholar]
- 16.Würschum T, Langer SM, Longin CFH, Tucker MR, Leiser WL. A modern green revolution gene for reduced height in wheat. Plant J. 2017;92(5):892–903. doi: 10.1111/tpj.13726. [DOI] [PubMed] [Google Scholar]
- 17.Gasperini D, Greenland A, Hedden P, Dreos R, Harwood W, Griffiths S. Genetic and physiological analysis of Rht8 in bread wheat: an alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J Exp Bot. 2012;63(12):6760. doi: 10.1093/jxb/ers138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebetzke GJ, Ellis MH, Bonnett DG, Mickelson B, Condon AG, Richards RA. Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L) Field Crop Res. 2012;126:87–96. doi: 10.1016/j.fcr.2011.09.022. [DOI] [Google Scholar]
- 19.Haque MA, Martinek P, Kobayashi S, Kita I, Ohwaku K, Watanabe N, Kuboyama T. Microsatellite mapping of genes for semi-dwarfism and branched spike in Triticum durum Desf var ramosoobscurum Jakubz “Vetvistokoloskaya”. Genet Resour Crop Ev. 2012;59(5):831–837. doi: 10.1007/s10722-011-9722-5. [DOI] [Google Scholar]
- 20.Konzak CF. Mutations and mutation breeding. Wisconsin, American. In: Heyne EG, editor. wheat and wheat improvement 2nd Edition American Society of Agronomy. 1987. pp. 428–443. [Google Scholar]
- 21.Peng ZS, Li X, Yang ZJ, Liao ML. A new reduced height gene found in the tetraploid semi-dwarf wheat landrace Aiganfanmai. Genet Mol Res. 2011;10(4):2349–2357. doi: 10.4238/2011.October.5.5. [DOI] [PubMed] [Google Scholar]
- 22.Wiwart M, Suchowilska E, Kandler W, Sulyok M, Groenwald P, Krska R. Can polish wheat (Triticum polonicum L) be an interesting gene source for breeding wheat cultivars with increased resistance to Fusarium head blight. Genet Resour Crop Ev. 2013;60(8):2359–2373. doi: 10.1007/s10722-013-0004-2. [DOI] [Google Scholar]
- 23.Liu GX, Zhou YH, Zheng YL, Yang RW, Ding CB. The reaction of hormone gibberellic acid in dwarfing polish wheat (Triticum polonicum) from Tulufan, Xinjiang. J Sichuan Agric Univ. 2002;20:81–83. [Google Scholar]
- 24.Wang Y, Xiao X, Wang XL, Zeng J, Kang HY, Fan X, Sha LN, Zhang HQ, Zhou YH. RNA-Seq and iTRAQ reveal the dwarfing mechanism of dwarf polish wheat (Triticum polonicum L) Int J Bio Sci. 2016;12(6):653. doi: 10.7150/ijbs.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazhenov MS, Divashuk MG, Amagai Y, Watanabe N, Karlov GI. Isolation of the dwarfing Rht-B1p (Rht17) gene from wheat and the development of an allele-specific PCR marker. Mol Breeding. 2015;35(11):1–8. doi: 10.1007/s11032-015-0407-1. [DOI] [Google Scholar]
- 26.Wen W, Deng QY, Jia HY, Wei LZ, Wei JB, Wan HS, Yang LM, Cao WJ, Ma ZQ. Sequence variations of the partially dominant DELLA gene Rht-B1c in wheat and their functional impacts. J Exp Bot. 2013;64(11):3299–3312. doi: 10.1093/jxb/ert183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reitz LP, Salmon SC. Origin, history, and use of Norin 10 wheat 1. Crop Sci. 1968;8:686–689. doi: 10.2135/cropsci1968.0011183X000800060014x. [DOI] [Google Scholar]
- 28.Quick JS, Miller JD, Donnelly BJ. Cando North Dakota's first Semidwarf durum. North Dakota Farm Res. 1976;33:15–18. [Google Scholar]
- 29.Börner A, Plaschke J, Korzun V, Worland AJ. The relationships between the dwarfing genes of wheat and rye. Euphytica. 1996;89(1):69–75. doi: 10.1007/BF00015721. [DOI] [Google Scholar]
- 30.Haque MA, Martinek P, Watanabe N, Kuboyama T. Genetic mapping of gibberellic acid-sensitive genes for semi-dwarfism in durum wheat. Cereal Res Commun. 2011;39(2):171–178. doi: 10.1556/CRC.39.2011.2.1. [DOI] [Google Scholar]
- 31.Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33(16):2583–2585. doi: 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiel T, Michalek W, Varshney RK, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L) Theor Appl Genet. 2003;106(3):411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 33.Kosambi DD. The estimation of map distances from recombination values. Ann Hum Genet. 1943;12(1):172–175. [Google Scholar]
- 34.Wang Y, Wang XL, Gu MX, Kang HY, Zeng J, Fan X, Sha LN, Yu KF, Zhou YH. Cloning and characterization of four novel SnRK2 genes from Triticum polonicum. Biol Plantarum. 2015;59(2):211–219. doi: 10.1007/s10535-015-0501-6. [DOI] [Google Scholar]
- 35.Trapnell C, Roberts A, Goff L, Pertea KGD, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knopf C, Becker HC, Ebmeyer E, Korzun V. Occurrence of three dwarfing Rht genes in German winter wheat varieties. Cereal Res Commun. 2008;36(4):553–560. doi: 10.1556/CRC.36.2008.4.4. [DOI] [Google Scholar]
- 37.Zhang XK, Yang SJ, Zhou Y, He ZH, Xia XC. Distribution of the Rht-B1b, Rht-D1b and Rht8 reduced height genes in autumn-sown Chinese wheats detected by molecular markers. Euphytica. 2006;152(1):109–116. doi: 10.1007/s10681-006-9184-6. [DOI] [Google Scholar]
- 38.Guedira M, Brown-Guedira G, Van Sanford DA, Sneller C, Souza E, Marshall D. Distribution of Rht genes in modern and historic winter wheat cultivars from the eastern and Central USA. Crop Sci. 2010;50(5):1811–1822. doi: 10.2135/cropsci2009.10.0626. [DOI] [Google Scholar]
- 39.Liu Y, Zhang J, Hu YG, Chen J. Dwarfing genes Rht4 and Rht-B1b affect plant height and key agronomic traits in common wheat under two water regimes. Field Crop Res. 2017;204(204):242–248. doi: 10.1016/j.fcr.2017.01.020. [DOI] [Google Scholar]
- 40.Van De Velde K, Ruelens P, Geuten K, Rohde A, Van Der Straeten D. Exploiting DELLA signaling in cereals. Trends Plant Sci. 2017;22(10):880–893. doi: 10.1016/j.tplants.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Achard P, Renou JP. BerthoméR, Harberd NP, Genschik P. plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol. 2008;18(9):656–660. doi: 10.1016/j.cub.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Carrera E, Ruiz-Rivero O, Peres LE, Atares A, Garcia-Martinez JL. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012;160(3):1581–1596. doi: 10.1104/pp.112.204552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Tian YH, Wu K, Ye YF, Yu JP, Zhang JQ, Liu Q, Hu MY, Li H, Tong YP, Harberd NP, Fu XD. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature. 2018;560(7720):595–600. doi: 10.1038/s41586-018-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locascio A, Blázquez MA, Alabadí D. Genomic analysis of DELLA protein activity. Plant Cell Physiol. 2013;54(8):1229–1237. doi: 10.1093/pcp/pct082. [DOI] [PubMed] [Google Scholar]
- 45.Cao DN, Cheng H, Wu W, Soo HM, Peng JR. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 2006;142(2):509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claeys H, De Bodt S. InzéD. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends Plant Sci. 2014;19(4):231–239. doi: 10.1016/j.tplants.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Bai MY, Shang JXOE, Fan M, Bai Y, Zentella R, Suin TP, Wang ZY. Brassinosteroid gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol. 2012;14(8):810–817. doi: 10.1038/ncb2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willige BC, Losno E, Richter R, Zourelidou M, Schwechheimer C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell. 2011;23(6):2184–2195. doi: 10.1105/tpc.111.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou AF, Li J. Arabidopsis BRS1 is a secreted and active serine carboxypeptidase. J Biol Chem. 2005;280(42):35554–35561. doi: 10.1074/jbc.M503299200. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Han CT, Hur Y. Molecular characterization of the Brassica rapa auxin-repressed, superfamily genes, BrARP1 and BrDRM1. Mol Biol Rep. 2013;40(1):197–209. doi: 10.1007/s11033-012-2050-9. [DOI] [PubMed] [Google Scholar]
- 51.Park S, Han KH. An auxin-repressed gene (RpARP) from black locust (Robinia pseudoacacia) is posttranscriptionally regulated and negatively associated with shoot elongation. Tree Physiol. 2003;23(12):815–823. doi: 10.1093/treephys/23.12.815. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Xin S, Tao CC, Jin X, Li HB. Cotton ascorbate oxidase promotes cell growth in cultured tobacco bright Yellow-2 cell through generation of apoplast oxidation. Int J Mol Sci. 2017;18(7):1346. doi: 10.3390/ijms18071346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto A, Bhuiyan MN, Waditee R, Tanaka Y, Esaka M, Oba K, Jagendorf AT, Takabe T. Supressed expression of the appoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J Exp Bot. 2005;56(417):1785–1796. doi: 10.1093/jxb/eri167. [DOI] [PubMed] [Google Scholar]
- 54.Liszkay A, Der Zalm EV, Schopfer P. Production of reactive oxygen intermediates (O2−, H2O2 and OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136(2):3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi YM, Kant S, Clark J, Gidda S, Ming F, Xu JY, Rochon A, Shelp BJ, Hao LX, Zhao R, Mullen RT, Zhu T, Rothstein SJ. Increased nitrogen-use efficiency in transgenic rice plants over-expressing a nitrogen-responsive early nodulin gene identified from rice expression profiling. Plant Cell Environ. 2009;32(12):1749–1760. doi: 10.1111/j.1365-3040.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 56.Fukayama H, Tamai T, Taniguchi Y, Sullivan S, Miyao M, Nimmo HG. Characterization and functional analysis of phosphoenolpyruvate carboxylase kinase genes in rice. Plant J. 2006;47(2):258–268. doi: 10.1111/j.1365-313X.2006.02779.x. [DOI] [PubMed] [Google Scholar]
- 57.Van Quy L, Foyper C, Champigny ML. Effect of light and NO3− on wheat leaf phosphoenolpyruvate carboxylase activity: evidence for covalent modification of the C3 enzyme. Plant Physiol. 1991;97(4):1476–1482. doi: 10.1104/pp.97.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chollet R, Vidal J, O’Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants, annual review of plant physiology and plant. Mol Biol. 1996;47(1):273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- 59.Nimmo HG. Control of the phosphorylation of phosphoenolpyruvate carboxylase in higher plants. Arch Bioche Biophysisc. 2003;414(2):189–196. doi: 10.1016/S0003-9861(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 60.Chen L, Hao L, Condon AG, Hu YG. Exogenous GA3 application can compensate the morphogenetic effects of the GA-responsive dwarfing gene Rht12 in bread wheat. PLoS One. 2014;9(1):e86431. doi: 10.1371/journal.pone.0086431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultink A, Naylor D, Dama M, Pauly M. The role of the plant-specific ALTERED XYLOGLUCAN19 protein in Arabidopsis cell wall polysaccharide O-acetylation. Plant Physiol. 2015;167(4):1271–1283. doi: 10.1104/pp.114.256479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sénéchal F, Graff L, Surcout O, Marcelo P, Rayon C, Bouton S, Mareck A, Mouille G, Stintzi A, Höfte H, Lerouge P, Schaller A, Pelloux J. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT35, a subtilisin-like serine protease. Ann Bot. 2014;114(6):1161–1175. doi: 10.1093/aob/mcu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang HH, Guo Y, Lv F, Zhu HY, Wu SJ, Jiang YJ, Li FF, Zhou BL, Guo WZ, Zhang TZ. The essential role of GhPEL gene, encoding a pectate lyase, in cell wall loosening by depolymerization of the de-esterified pectin during fiber elongation in cotton. Plant Mol Biol. 2010;72(s4–5):397–406. doi: 10.1007/s11103-009-9578-7. [DOI] [PubMed] [Google Scholar]
- 64.Zhang BC, Zhang LJ, Li F, Zhang DM, Liu XL, Wang H, Xu ZP, Chu CC, Zhou YH. Control of secondary cell wall patterning involves xylan deaceylation by a GDSL esterase. Nat Plants. 2017;3:17017. doi: 10.1038/nplants.2017.17. [DOI] [PubMed] [Google Scholar]
- 65.Schaller A, Stintzi A, Graff L. Subtilases-versatile tools for protein turnover, plant development, and interactions with the environment. Plant Plantarum. 2012;145(1):52–66. doi: 10.1111/j.1399-3054.2011.01529.x. [DOI] [PubMed] [Google Scholar]
- 66.Voiniciuc C, Heinrich-Wilhelm Schmidt M, Berger A, Yang B, Ebert B, Scheller HV, North HM, Usadel B, Günl M. MUCILAGE-RELATED10 produces galactoglucomannan that maintains pectin and cellulose architecture in Arabidopsis seed mucilage. Plant Physiol. 2015;169(1):403–420. doi: 10.1104/pp.15.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Busse-Wicher M, Gomes TCF, Tryfona T, Nikolovski N, Stott K, Grantham NJ, Bolam DN, Skaf MS, Dupree P. The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J. 2014;79(3):492–506. doi: 10.1111/tpj.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen JK, Kim H, Cocuron JC, Orler R, Ralph J, Wilkerson CG. The DUF579 domain containing proteins IRX15 and IRX15-L affect xylan synthesis in Arabidopsis. Plant J. 2011;66(3):387–400. doi: 10.1111/j.1365-313X.2010.04475.x. [DOI] [PubMed] [Google Scholar]
- 69.Lefebvre V, Fortabat MN, Ducamp A, North HM, Maia-Grondard A, Trouverie J, Boursiac Y, Mouille G, Durand-Tardif M. ESKIMO1 disruption in Arabidopsis alters vascular tissue and impairs water transport. PLoS One. 2011;6(2):e16645. doi: 10.1371/journal.pone.0016645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manabe Y, Verhertbruggen Y, Gille S, Harholt J, Chong SL, Pawar PMA, Mellerowicz EJ, Tenkanen M, Cheng K, Pauly M, Scheller HV. Reduced wall acetylation proteins play vital and distinct roles in cell wall O-acetylaton in Arabidopsis thaliana. Plant Physiol. 2013;163(3):1107–1117. doi: 10.1104/pp.113.225193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The plant height of DPW, TPW, and DPW × TPW F1.
Additional file 2: Fig. S2. Relative expression of ARP and ASCO in the first and second internodes at the booting stage.
Additional file 3: Table S1. The information of 59 tetraploid wheat accessions.
Additional file 4: Table S2. The information of SSR primers on 4BS chromosome.
Additional file 5: Table S3. Genotype data of RIL populations and the F2 population from D_60 × T_58.
Additional file 6: Table S4. Gene-specific primers for Rht-dp candidate genes in DPW and TPW.
Additional file 7: Table S5. The information of Dwarfism-related DEGs induced by DELLA mutant Rht-dp.
Data Availability Statement
All data generated or analyzed during this study were included in this article and the supplementary files.