Abstract
Multiple sclerosis (MS), a neuroinflammatory disease that affects millions worldwide, is widely thought to be autoimmune in etiology. Historically, research into MS pathogenesis has focused on autoreactive CD4 T cells, due to their critical role in the animal model, experimental autoimmune encephalomyelitis (EAE), and the association between MS susceptibility and single nucleotide polymorphisms in the MHC II region. However, recent studies have revealed prominent clonal expansions of CD8 T cells within the central nervous system (CNS) during MS. Here we review the literature on CD8 T cells in MS, with an emphasis on their potential effector and regulatory properties. We discuss the impact of disease modifying therapies, currently prescribed to reduce MS relapse rates, on CD8 T cell frequency and function. A deeper understanding of the role of CD8 T cells in MS may lead to the development of more effective and selective immunomodulatory drugs for particular subsets of patients.
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS), characterized by spatiotemporal dissemination of demyelinating lesions that span the optic nerves, brain and spinal cord (1). It is the most common cause of non-traumatic neurological disability among young adults in the Western Hemisphere. As a consequence of the multifocal distribution of lesions, individuals with MS experience diverse neurological symptoms including numbness, weakness, visual loss, double vision, tremor, and gait imbalance (2). MS typically presents with a relapsing-remitting course, although it can also manifest as gradually worsening neurological disability, referred to as progressive disease (1, 3). Clinical relapses correspond with the development of acute inflammatory lesions in neuroanatomically “eloquent” sites. Genome wide association studies (GWAS) implicate multiple adaptive and innate immune system pathways in MS susceptibility, suggesting that MS is likely triggered by a perturbation of peripheral immune responses that is translated to the CNS and leads to a neurodegenerative process (4). Identification of the immune effector cells that mediate the CNS damage, and their mechanisms of action, has been a major goal of MS researchers over the past 50 years.
Despite numerous attempts to prove otherwise, there is a dearth of evidence that a local viral infection, or another extraneous threat, drives the destructive neuroinflammatory response during MS. Rather, a large body of circumstantial data supports an autoimmune etiology. Experimental autoimmune encephalomyelitis (EAE), a multifocal demyelinating disease in laboratory rodents and non-human primates that simulates MS, is commonly induced via vaccination against CNS autoantigens, particularly peptide or protein components of the myelin sheath (5). Interestingly, acute inflammatory demyelinating syndromes, with radiological and/or histopathological features reminiscent of MS, have also occurred in human subjects inadvertently exposed to myelin antigens in an immunogenic context (6–8). The genetic architecture of MS susceptibility implicates a broad range of immune cell subsets in MS risk (9). Analyses of GWAS data using system biology approaches indicate that relapsing MS clusters closely with non-CNS diseases also thought to have an autoimmune basis, such as Type 1 diabetes mellitus, Crohn’s disease, and rheumatoid arthritis, and not with primary neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (9, 10). Pharmaceutical agents that deplete lymphocytes from the circulation, or block their passage across the blood-brain-barrier (BBB), significantly reduce MS relapse rates (11, 12). Collectively these observations provide a strong argument in support of the importance of autoreactive lymphocytes specific for CNS antigens in MS pathogenesis. The relevance of individual T lymphocyte subsets has yet to be definitively clarified.
Historically, CD4, as opposed to CD8, T cells were depicted as the pivotal effector cells in MS lesion development. There is a growing recognition that CD8 T cells play a more important role than widely appreciated (13–15). Histopathological studies of postmortem brain tissue show that CD8 T cells are actually more prevalent in MS infiltrates than CD4 T cells, across different lesion subtypes and clinical subsets (16). Although less commonly reported than encephalitogenic CD4 T cells, myelin-reactive CD8 T-cell lines are capable of inducing EAE (16–18). More recently, single cell transcriptomic analyses have demonstrated preferential expansion of CD8, compared with CD4, T cell clones in the blood and cerebrospinal fluid (CSF) of people with MS, as well as in individuals at a high risk for the future development of MS (19, 20). Together, these findings indicate that the role of CD8 T cells in MS should be revisited. In this review, we discuss evolving perspectives on the role of CD8 T cells during MS and EAE. We describe the characteristics of CD8 T cells in the CNS and periphery of patients with MS, focusing on clonality, as well as potential pathogenic and regulatory properties. Finally, we highlight how disease modifying therapies (DMT) that attenuate MS disease activity modulate CD8 T cells in a manner that might underlie their mechanism of action.
The role of CD4 versus CD8 T cells
The longstanding focus of many laboratories on the role of CD4 T cells in MS immunopathology is largely based on the fact that the majority of EAE models, induced by active immunization against myelin antigens, are CD4 T cell/MHC class II dependent (21–24). In certain inbred mouse strains, the adoptive transfer of highly purified myelin-primed CD4 T cells into naïve syngeneic hosts is sufficient to induce full-blown EAE (25, 26). Furthermore, genes relevant to the differentiation and function of CD4 T cells are overrepresented among those mapping close to MS genetic susceptibility loci (4, 10, 27). Nonetheless, the requirement of CD4 T cells for MS lesion development has been brought into question by the failure of a series of clinical trials in which subjects with relapsing remitting disease were treated with monoclonal antibodies that either specifically deplete CD4 T cells or neutralize factors believed to be critical for the polarization of encephalitogenic CD4 T cells (28–30). In contrast, therapies that globally target lymphocytes, such as alemtuzamab and fingolimod, are highly effective at suppressing MS relapses (11, 31). Drugs that specifically target CD8 T cells have yet to be tested in MS.
Numerous published studies on the histopathology of MS have concluded that CD8 T cells at least equal, and in many cases greatly outnumber, CD4 T cells in perivascular and parenchymal infiltrates, as well as at the edge of active plaques (16). CD8:CD4 ratios have been reported to range between 1:1 to 50:1 (32–38). Sparse perivascular cuffs, detected in normal appearing white matter adjacent to lesions, also primarily consist of CD8 T cells (32). The predominance of CD8 T cells in MS lesions has held true irrespective of patient age, clinical subtype, disease duration, tempo of evolution, lesion stage, or history of immunosuppressive treatment (16, 32–37). In the majority of studies, postmortem tissues were primarily obtained from older individuals in the progressive subset with long disease durations. However, a predominance of CNS CD8 T cells was recently reported in 12 cases of acute or relapsing MS (37). Spatially, CD8 T cells within MS lesions interact with microglia, oligodendrocytes, and transected axons (38). Several laboratories have found that the frequency of circulating CD8 T cells falls during clinical MS exacerbations, which might reflect their recruitment from the bloodstream into the inflamed CNS (39–41).
Evidence of CD8 T cell accumulation and expansion in the CNS of individuals with MS
The majority of CD8 T cells in MS lesions have a cell surface phenotype consistent with tissue resident memory cells (TRM; CD44+CD103+/−CD69+), a recently recognized subset of memory T cells that do not recirculate and have a low threshold for reactivation (19, 36–38, 42, 43). A subset of intralesional CD8 T cells express markers indicative of recent activation and proliferation (36–38). Macrophages and other immune cells within MS infiltrates express high levels of MHC I and costimulatory molecules, equipping them to present antigen to autoreactive CD8 T cells (44). CD8 T cells are in direct communication with myeloid cells in MS lesions and appear to form immunological synapses (45). In addition, MHC class I is upregulated on cerebrovascular endothelium, neurons, astrocytes, and oligodendrocytes within, and surrounding, MS lesions (46). Although the most prominent MS genetic risk loci reside within the MHC II region, MHC I variability has also been implicated in MS. The MHC class I allele, HLA-A3 (A*0301), is associated with increased susceptibility, while MHC class I allele HLA-A*0201 is associated with increased resistance (16, 47).
Compelling evidence that the CD8 T cells in MS lesions are stimulated with cognate antigen and expand in situ comes from T cell receptor gene sequencing analyses of CSF leukocytes and/or white matter lesion specimens. Although several studies have revealed clonal expansions of CNS-infiltrating B cells and CD4 T cells, the majority of expansions have generally been detected within the CD8 T cell compartment (19, 20, 34, 48). Monozygotic twins of individuals with MS are statistically at high risk of developing clinically definite disease (49). Interestingly, clonal expansions of CD8 T cells were observed in the CSF of the healthy monozygotic twins of MS patients, all of whom had evidence of subclinical disease based on MRI (19). Babbe and colleagues isolated individual T cells from white matter lesional tissue of 2 individuals with MS and performed T cell receptor (TCR) β-chain variable gene (TRBV) sequencing (34). In both patients, the majority of CD8 T cells belonged to relatively few clones. Identical expanded CD8 T-cell clones were detected in the CSF, brain, and blood of each patient. In contrast, CD4 T cells exhibited a more diverse TCR repertoire with limited clonal expansion (34). Similarly, in an independent study, TCR Vβ repertoire analysis of paired peripheral blood CD8 T cells, CSF cells, and CNS lesion samples from several subjects with MS consistently revealed a limited number of predominant clones, most of which were common between the 3 locations (50). Identical CD8 T cell clones have been found in distinct lesions, as well as the normal appearing white matter, of individual MS patients (51). Some CD8 T cell clones detected in the cerebrospinal fluid and/ or blood of individuals with MS persisted for over 5 years (52).
A critical unresolved issue regards the antigenic specificity of the oligoclonally expanded CD8 T cells in the CNS of MS patients. It is widely assumed that those cells are reactive against CNS restricted epitopes. Although some studies have detected myelin antigen-specific CD8 T cells at a higher frequency in the circulation of MS patients compared with age and sex matched healthy controls (53), other studies have found no differences between those groups (54). CD8 T cell lines generated from the peripheral blood mononuclear cells of MS patients produced TNFα and IFNγ upon co-culture with antigen presenting cells bearing myelin antigens and lysed target cells pulsed with myelin peptides (53, 55). Conversely, a panel of CD8 T cell lines, derived from CSF or white matter brain tissue of MS patients, showed no reactivity towards a broad selection of candidate human myelin or neuronal antigens (38). As will be discussed in greater detail below, regulatory CD8 T cell subsets in MS may be specific for TCR peptides expressed by encephalitogenic CD4 T cells.
If the CD8 T cells that infiltrate MS lesions are specific for CNS antigens, the question arises as to how they are initially activated in the periphery in order to upregulate adhesion molecules and chemotactic receptors necessary for passage across the intact BBB. One possibility is that the disease initiating CD8 T cells are cross-reactive against structurally similar microbial and CNS epitopes, and gain the capacity to infiltrate the CNS following stimulation in the context of a systemic infection. Once having breached the BBB, they are reactivated in response to the homologous CNS auto-antigen (56). Consistent with that scenario, MBP-reactive CD8 T cells have been isolated from MS patients that are cross-reactive to the EBV latency antigen (EBNA-1) of Epstein Barr Virus (EBV) (57). In an independent study, multiple short-term CD8 T cell lines, derived from MS lesional tissue, upregulated IFNγ and CD137 in response to co-culture with autologous EBV-transformed B cell lines that express the late lytic viral antigen glycoprotein 350 (38). This might explain why exposure to EBV as an adult is a risk factor for the development of MS (58).
CD8 T cell entry into the CNS
Under homeostatic conditions, immune surveillance of the CNS parenchyma by peripheral cells is limited by the inability of naïve lymphocytes to penetrate the intact BBB (59). Activated CD8 T cells could participate in BBB breakdown via perforin-mediated astrocyte activation, tight junction alteration, and VEGF induction (60–62). In the context of active neuroinflammation, CNS homing of immune cells is facilitated by chemokines and cell adhesion molecules. Migration of CD8 T cells across cerebrovascular endothelial monolayers in vitro, or across the BBB in vivo during EAE and mouse hepatitis virus encephalitis, is dependent on very late antigen 4 (VLA-4) (63). Melanoma cell adhesion molecule (MCAM/CD116) has also been implicated in CD8 T cell infiltration, specifically in the context of MS. MCAM is up-regulated by circulating CD8 T cells coincident with MS relapses (64). MCAM+ CD8 T cells express higher levels of pro-inflammatory cytokines and cytotoxicity towards cultured oligodendrocytes than their MCAM− counterparts. MCAM blockade diminishes the severity of EAE induced by the adoptive transfer of encephalitogenic CD8 T cells (64).
Chemokines actively attract lymphocytes to migrate from the blood into the CNS and from perivascular spaces into the parenchyma. CD8 T cell clones that are expanded in the CSF of MS patients strongly upregulate CXCR6 compared with non-expanded CD8 T cells, while intrathecal monocytes and dendritic cells express elevated levels of the CXCR6 ligand, CXCL16 (19). The majority of CD8 T cells isolated from active MS lesions, mixed active/inactive MS lesions, or normal appearing white matter are CXCR6+; CXCL16 is upregulated in the lesion rim (36). CXCR6-CXCL16 interactions are required for the recruitment of pathogenic CD8 T cells in animal models of psoriasis and hepatitis, suggesting that they might play a similar role in MS (65, 66). Therapies that selectively modulate CD8 T cell homing to the CNS might suppress MS relapses with less of an impact on beneficial immunity than currently employed DMT.
Pathogenic properties of CNS-infiltrating CD8 T cells
CNS-infiltrating CD8 T cells could, theoretically, inflict damage to glia and neurons through release of perforin, granzymes and granulysin, or via direct cell-to cell interactions, such as Fas ligand (CD95L)-mediated apoptosis. This is most likely to occur when CD8 T cells are reactivated by MHC I-expressing oligodendrocytes, neurons, and/or microglia in situ (67). Circulating CD8 perforin+ T cells are increased in MS, most strikingly in the progressive disease subsets (68). A higher percentage of CD8 T cells in white matter lesions express CD95L compared with CD8 T cells in paired blood (38). Granzyme B-expressing CD8 T cells have been consistently identified in active MS lesions, in some cases adjacent to caspase-3 expressing cells (38, 46).
CD8 T cells in active MS lesions produce pro-inflammatory cytokines that have been linked to destructive neuroinflammation, in general, and oligodendrocyte apoptosis, in particular (69, 70). A high percentage of these CD8 T cells express IL-17, sometimes in combination with IFNγ, compared with CD8 T cells in adjacent normal-appearing white matter or inactive lesions (71). IFNγ, produced by infiltrating CD8 T cells and/or CD4 T cells, could upregulate MHC I expression on oligodendrocyte precursor cells (OPCs) and microglia, thereby amplifying local autoreactive CD8 responses in a positive feedback loop (72). CD8 T cells may promote CNS pathology in synergy with CD4 T cells via a number of additional mechanisms. For example, in some EAE models, IL-17 secretion by CNS-infiltrating CD8 T cells drives the local accumulation of IL-17 producing, encephalitogenic CD4 T cells (73, 74). Conversely, CD4 T cells can prime microglia and CNS macrophages to activate CD8 T cells via CD40-CD40 ligand interactions (75, 76).
There is circumstantial evidence that CD8 T cells mediate axonal pathology that occurs during MS. Axon transections and spheroids are prominent features of MS lesions from the earliest stages of development and are believed to be a major cause of chronic disability (77). Granzyme B-expressing CD8 T cells have been observed in close proximity to demyelinated axons in MS tissue, with the cytotoxic granules polarized towards axons (78). The extent of axonal damage in active MS lesions correlates with the frequency of infiltrating CD8 T cells (78). In two independent experimental systems, CD8 T cell lines formed stable adhesions with neurites of dissociated neurons, and subsequently induced neuritic spheroids and cytoskeletal breaks in a MHC I/peptide dependent fashion (79, 80). Lytic granules, isolated from antigen-activated murine CD8 T cells, drive microtubule destabilization in axons ex vivo (81). CD8 T cells are critical and selective mediators of axonopathy in encephalomyelitis secondary to Theiler’s Murine Encephalomyelitis Virus (TMEV) infection, an alternative rodent model of inflammatory demyelinating disease. Hence, MHC I deficiency or CD8 T cell blockade protects TMEV-inoculated mice from axonal degeneration and the development of functional and physiological neurological deficits, without impacting the degree of neuroinflammation or demyelination (82). Perforin-deficient mice exhibit a similar phenotype, suggesting that CD8 T cells inflict axonal damage during TMEV infection via a perforin-dependent pathway (83).
CD8 T cells may also play a direct role in promoting demyelination and suppressing remyelination. Mature oligodendrocytes isolated from postmortem MS tissue express MHC I, making them susceptible to CD8 cytotoxicity (78). Alloreactive and MBP-specific CD8 T cell lines have been shown to lyse human oligodendrocytes in co-cultures (84, 85). Interestingly, immature OPCs upregulate MHC I upon stimulation with IFNγ and engulf, process, and present antigen to CD8 T cells in vitro (72). This presentation not only results in the activation of cytotoxic CD8 T cells, but also in the direct death of the presenting OPC. Hence, CD8 mediated apoptosis of OPC could underlie, in part, the failure of remyelination that has been observed in MS lesions.
Regulatory CD8 T cells in MS
Although cytotoxic CD8 T cells exhibit gliotoxic and neuro-toxic properties in in vitro assays and in some animal models of inflammatory demyelination, alternative CD8 T cell subsets have been identified that possess anti-inflammatory functions (Fig. 1). Immunization of C57BL/6 mice with an immunodominant peptide of myelin oligodendrocyte glycoprotein (MOG) elicits the early expansion of encephalitogenic CD4 T cells in the periphery and CNS, followed by the delayed expansion of CD44+ Ly49+ regulatory CD8 T cells in both compartments (20). These expanded CD8 T cells suppress MOG-specific CD4 T cells ex vivo and are reactive against foreign peptides (as opposed to MOG) complexed to classic MHC I. Furthermore, CD8 T cells isolated from the spleens of Lewis rats that had recovered from adoptively transferred EAE selectively lyse myelin-specific CD4 T cell lines ex vivo, and counter their encephalitogenic functions in vivo (86). The presence of an endogenous pool of regulatory CD8 T cells in wildtype mice, that expand during EAE and can suppress neuroinflammatory responses, is reinforced by the observation that CD8 knock-out mice are more prone to EAE relapse than their WT counterparts (87). In a separate EAE model, CD8 T cell depletion facilitated the induction of clinical relapses following an initial episode of inflammatory demyelination (88). An array of regulatory CD8 T cell subsets have been isolated from both human subjects and laboratory animals that suppress myelin reactive CD4 responses ex vivo, but differ in cell surface phenotype (ex. FoxP3+CD25+ versus LAP-1+), antigenic specificity (neuroantigen versus CD4 T cell receptor epitopes), MHC restriction (classical versus non-classical MHC I) and mechanism of action (direct lysis of encephalitogenic CD4 T cells versus bystander suppression via release of soluble factors).
Figure 1: Potential roles of CD8 T cells in MS.
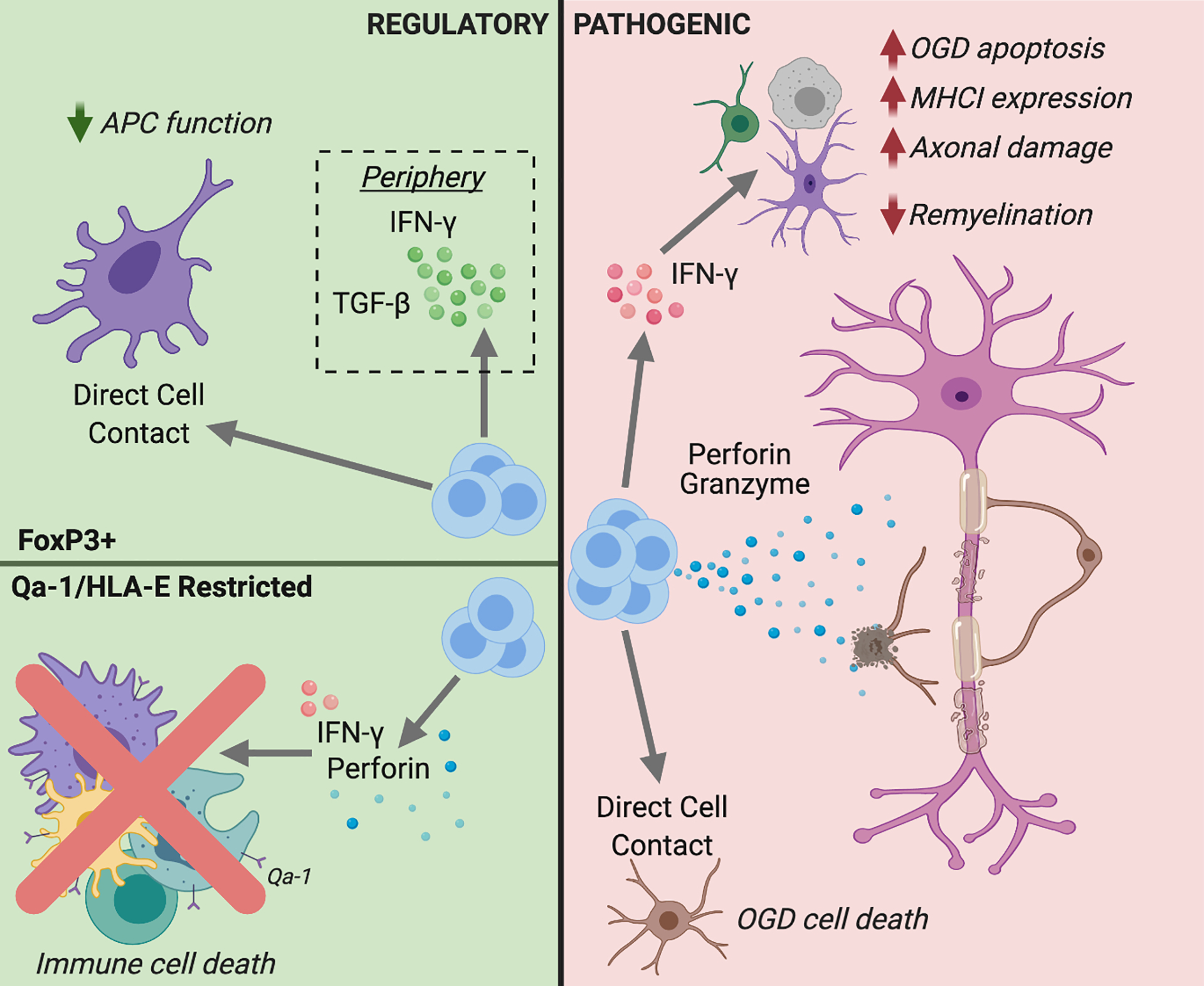
CD8 T cells with regulatory (left panel/ green background) or pathogenic (right panel/ pink background) functions might be deployed during MS. FoxP3+ regulatory CD8 T cells have been shown to disarm antigen presenting cells (APC), as well as suppress encephalitogenic CD4 T cells directly, via cell-to-cell interactions or the release of immunosuppressive cytokines. Qa-1- (mouse) or HLA-E- (human) restricted CD8 T cells could curtail neuroinflammation by killing APC or CD4 effector cells via FasL, IFNγ,and/ or Perforin mediated pathways. The horizontal line in the left panel separates events in the CNS (above) from the periphery (below). Conversely, pathogenic mechanisms of CNS-infiltrating CD8 T cells include lysing oligodendrocytes and oligodendrocyte precursor cells, inflicting axonal damage, enhancing encephalitogenic CD4 T cell responses, and activating glia. Figure created with BioRender.com.
CD25+FoxP3+ CD8 T cell clones (TCC), isolated from the peripheral blood or CSF of MS patients, and expanded in the presence of irradiated myelin-specific CD4 T cells, inhibit the proliferation and cytokine expression of autologous myelin-reactive CD4 Th1 and Th17 cell clones in co-cultures (89). The cloning frequency of these regulatory CD8 T cells is lower during MS exacerbations compared with remissions. In an independent study, the frequency of circulating FoxP3+CD8 T cells was reduced in relapsing remitting patients during relapses, but not remissions, when compared with healthy controls (90). Terminally differentiated CD8 T cells isolated from the blood of MS patients lyse autologous, neuroantigen-specific CD4 T cells in an IFNγ, Granzyme B, and perforin dependent manner (91). The antigenic specificities and CNS homing capacity of human FoxP3+CD8 T cells or terminally differentiated regulatory CD8 T cells have yet to be demonstrated.
Unconventional subpopulations of CD8 T cells that are restricted to non-classical MHC Class Ib molecules (HLA-E in humans and Qa-1b in mice) can also suppress myelin-reactive CD4 T cell responses. Qa1-deficient mice develop exaggerated CD4 T cell responses to myelin peptides and experience an earlier onset of clinical EAE than WT mice (92). Murine CD8 T cell lines and clones reactive against a TCR Vβ8.2 peptide complexed to Qa-1 directly kill activated myelin-specific Vβ8.2+ T cells ex vivo, and are protective when transferred into mice with a form of EAE that is primarily mediated by encephalitogenic Vβ8.2+ CD4 T cells (93). Similarly, Qa-1 restricted CD8 T cells reactive to a non-classical epitope of myelin oligodendrocyte glycoprotein transfer EAE suppression (94). There is circumstantial evidence for a role of HLA-E restricted regulatory CD8 T cells in MS. HLA-E expression is enhanced on T cells, as well as on B cells and myeloid cells, in MS lesions (95, 96). HLA-E restricted CD8 T cell clones, isolated from the CSF or blood of MS patients and healthy controls, and enriched by expansion with irradiated neuroantigen-specific CD4 TCC, lyse autologous myelin-reactive CD4 T cells via a Granzyme B and perforin dependent pathway (97). These regulatory cells are decreased in MS patients during exacerbations, particularly in the CSF compartment. Consistent with these results, intrathecal synthesis of soluble HLA-E is reduced in clinically active versus clinically stable relapsing remitting MS (RRMS) patients (96).
Efficacy of immunomodulatory treatments on CD8 T cells
At present, there are over 15 FDA-approved disease modifying therapies (DMT) that decrease the rate of clinical relapses in individuals with MS (Table I). All of these drugs modulate peripheral immune responses in a manner believed to deplete or inactivate pathogenic lymphocytes, or to block the entry of pathogenic lymphocytes into the CNS. Although none selectively targets CD8 T cells, they all impact the CD8 T cell compartment.
Table I:
Disease modifying therapies and their effects on CD8 T cells.
| Therapy | Proposed Biological Activity | Effect on CD8 T cells |
|---|---|---|
| Plasma exchange | Exchange of pathogenic plasma components |
|
| Beta interferons | unknown |
|
| Glatiramer acetate (Copaxone, Glatopa) | MBP analog | |
| Fingolimod (Gileyna) Siponimod (Mayzent) |
Sphingosine I phosphate receptor modulator | |
| Dimethyl fumarate (Tecfidera) | Nrf2 activation to reduce inflammation and oxidative damage | |
| Ocrelizumab (Ocrevus) | Anti-CD20 monoclonal antibody |
|
| Natalizumab (Tysabri) | Anti-α4 integrin monoclonal antibody | |
| Alemtuzumab (Campath, Lemtrada) | Anti-CD52 monoclonal antibody | |
| Terifllunomide (Aubagio) | Pyrimidine synthesis inhibitor |
|
DMT such as dimethyl fumarate, fingolimod, and alemtuzumab, reduce the absolute number of peripheral blood CD8 T cells by 53%, 70%, and over 80%, respectively (98–100). However, each of these agents has distinctive effects on CD8 T cell subsets. MS patients responsive to dimethyl fumarate treatment showed a reduction in the frequency of circulating IL-17+ or TNFα+ CD8 T cells after treatment as compared to pre-treatment levels, while the frequency of cytokine producing CD8 T cells was not significantly changed in non-responders (101, 102). Fingolimod, a sphingosine-1-phosphate inhibitor that sequesters naïve and central memory T cells in secondary lymphoid tissues, preferentially depletes CCR7+ CD8 T cells from the blood, consistent with its mechanism of action (99). In contrast, senescent CD8 T cells (CD28−CD27−CD57+) were not decreased in number in fingolimod treated patients and, therefore, were significantly increased in frequency within the remaining CD8 T cell pools. Fingolimod has also been shown to preferentially deplete CD8 and CD4 T cells that double produce IFNγ and IL-17 (103). Alemtuzamab significantly reduces the absolute numbers of circulating naïve and memory CD8 T cells, but naïve CD8 T cells are disproportionately impacted (104). Although anti-CD20 antibodies, such as rituximab and ocrelizumab, were initially used in the treatment of autoimmune diseases based on their effects on B cells, these drugs also deplete a subset of CD8 T cells that express CD20 (105). Interestingly, a high percentage of myelin-specific CD8 T cells in MS patients express CD20, and are preferentially reduced following anti-CD20 treatment (105). Administration of alemtuzumab to individuals with MS also results in long term depletion of CD20+ T cells from the blood and CSF (106). In contrast to DMT that reduce the frequency of circulating CD8 T cells, treatment with the anti-α4 integrin antibody, natalizumab, raises their numbers (107–109). This might reflect blockade of CD8 T cell entry into the CNS via α4β1 integrin/VCAM-1 interactions.
Two first line DMT, beta interferon and glatiramer acetate, both curtail the reactivity of CD8 T cells to CNS antigens in vitro (110, 111). Treatment of EAE mice with glatiramer acetate, which is an MBP analog, triggers the priming of CD8 T cells that suppress encephalitogenic CD4 T cells (110). Prophylactic infusion of glatiramer acetate-treated CD8 T cells prevents EAE via a mechanism dependent on MHCI, IFNγ, and perforin (110). In animal models, glatiramer acetate induced Qa-1 restricted regulatory CD* T cells (112).
CONCLUSIONS
Numerous studies demonstrate that CD8 T cells accumulate in active MS lesions, often exceeding the number of CD4 T cells, and preferentially undergo clonal expansion within the CNS during MS. DMTs that suppress MS relapses deplete or modulate CD8 lymphocytes, which might reflect the mechanisms of action of those drugs. Collectively, these observations are highly suggestive of an important role of CD8 T cells in MS. However, we are just beginning to understand their significance. There are conflicting data about the specificity of the expanded CD8 T cells in MS, with different studies implicating neuroantigens, foreign antigens or the TCR hypervariable region of encephalitogenic CD4 T cells. Similarly, their biological function remains to be elucidated. They might be pathogenic effectors that mediate BBB breakdown, promote the activities of encephalitogenic CD4 T cells, lyse oligodendrocytes and OPCs, and/ or directly inflict axonal damage. Conversely, they may limit destructive neuroinflammation by disarming or killing encephalitogenic CD4 T cells. It is likely that the CD8 T cells in MS are heterogeneous, and have different effects that vary depending their location in the periphery versus specific CNS compartments, the stage of lesion evolution, and the clinical phase/ subtype of disease, among a multitude of other factors. However, there is now clear justification to support the investigation of CD8 T cells and related factors as putative biomarkers and therapeutic targets in MS.
Acknowledgments
This work was supported by grants from the NINDS, National Institutes of Health to B.M.S. (R01 NS105385). Dr. Segal holds the Stanley D. and Joan H. Ross Chair in Neuromodulation at the Ohio State University.
REFERENCES
- 1.Dendrou CA, Fugger L, and Friese MA. 2015. Immunopathology of multiple sclerosis. Nat Rev Immunol 15: 545–558. [DOI] [PubMed] [Google Scholar]
- 2.Katz Sand I 2015. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol 28: 193–205. [DOI] [PubMed] [Google Scholar]
- 3.Segal BM. 2014. Stage-specific immune dysregulation in multiple sclerosis. J Interferon Cytokine Res. 34: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consortium IMSG 2019. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal BM 2019. The Diversity of Encephalitogenic CD4+ T Cells in Multiple Sclerosis and Its Animal Models. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FISCH C, and WOOD DE. 1950. Encephalomyelitis due to Pasteur treatment. Report of a fatal case with a review of the literature. J Indiana State Med Assoc 43: 1197–1201. [PubMed] [Google Scholar]
- 7.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, and Martin R. 2000. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 8.Höftberger R, Leisser M, Bauer J, and Lassmann H. 2015. Autoimmune encephalitis in humans: how closely does it reflect multiple sclerosis ? Acta Neuropathol Commun 3: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jager PL, Yang HS, and Bennett DA. 2018. Deconstructing and targeting the genomic architecture of human neurodegeneration. Nat Neurosci 21: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 10.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C, Abecasis GR, Barrett JC, Behrens T, Cho J, De Jager PL, Elder JT, Graham RR, Gregersen P, Klareskog L, Siminovitch KA, van Heel DA, Wijmenga C, Worthington J, Todd JA, Hafler DA, Rich SS, Daly MJ, and Consortia F. N. o.. 2011. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 7: e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cree BAC, Mares J, and Hartung HP. 2019. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol 32: 365–377. [DOI] [PubMed] [Google Scholar]
- 12.Selewski DT, Shah GV, Segal BM, Rajdev PA, and Mukherji SK. 2010. Natalizumab (Tysabri). AJNR Am J Neuroradiol 31: 1588–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassmann H 2019. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front Immunol 9: 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohlfeld R, Dornmair K, Meinl E, and Wekerle H. 2016. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol 15: 317–331. [DOI] [PubMed] [Google Scholar]
- 15.Saxena A, Martin-Blondel G, Mars LT, and Liblau RS. 2011. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett 585: 3758–3763. [DOI] [PubMed] [Google Scholar]
- 16.Goverman J 2009. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 9: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlén C, and Goverman J. 2001. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med 194: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, and Raine CS. 2001. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol 166: 7579–7587. [DOI] [PubMed] [Google Scholar]
- 19.Beltrán E, Gerdes LA, Hansen J, Flierl-Hecht A, Krebs S, Blum H, Ertl-Wagner B, Barkhof F, Kümpfel T, Hohlfeld R, and Dornmair K. 2019. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest 129: 4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saligrama N, Zhao F, Sikora MJ, Serratelli WS, Fernandes RA, Louis DM, Yao W, Ji X, Idoyaga J, Mahajan VB, Steinmetz LM, Chien YH, Hauser SL, Oksenberg JR, Garcia KC, and Davis MM. 2019. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangachari M, and Kuchroo VK. 2013. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun 45: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao P, and Segal BM. 2012. Experimental autoimmune encephalomyelitis. Methods Mol Biol 900: 363–380. [DOI] [PubMed] [Google Scholar]
- 23.Van Lambalgen R, and Jonker M. 1987. Experimental allergic encephalomyelitis in rhesus monkeys: II. Treatment of EAE with anti-T lymphocyte subset monoclonal antibodies. Clin Exp Immunol 68: 305–312. [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman L, Rosenbaum JT, Sriram S, and McDevitt HO. 1981. In vivo effects of antibodies to immune response gene products: prevention of experimental allergic encephalitis. Proc Natl Acad Sci U S A 78: 7111–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal BM, and Shevach EM. 1996. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med 184: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallis RJ, Raine CS, and McFarlin DE. 1989. Chronic relapsing experimental allergic encephalomyelitis in SJL mice following the adoptive transfer of an epitope-specific T cell line. J Neuroimmunol 22: 93–105. [DOI] [PubMed] [Google Scholar]
- 27.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’hooghe MB, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppä V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Rückert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sørensen PS, Søndergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvänen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A, Consortium IMSG, and C. C. C. 2. WT 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Oosten BW, Lai M, Hodgkinson S, Barkhof F, Miller DH, Moseley IF, Thompson AJ, Rudge P, McDougall A, McLeod JG, Adèr HJ, and Polman CH. 1997. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology 49: 351–357. [DOI] [PubMed] [Google Scholar]
- 29.Lindsey JW, Hodgkinson S, Mehta R, Siegel RC, Mitchell DJ, Lim M, Piercy C, Tram T, Dorfman L, and Enzmann D. 1994. Phase 1 clinical trial of chimeric monoclonal anti-CD4 antibody in multiple sclerosis. Neurology 44: 413–419. [DOI] [PubMed] [Google Scholar]
- 30.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, and Investigators UM. 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol 7: 796–804. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletire J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Lappos L, and the TRANSFORMS Study Group. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 32.Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, and Weiner HL. 1986. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol 19: 578–587. [DOI] [PubMed] [Google Scholar]
- 33.Booss J, Esiri MM, Tourtellotte WW, and Mason DY. 1983. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci 62: 219–232. [DOI] [PubMed] [Google Scholar]
- 34.Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S, Ravid R, and Rajewsky K. 2000. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denic A, Wootla B, and Rodriquez M. 2013. CD8(+) T cells in multiple sclerosis. Expert Opin Ther Targets 17: 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fransen NL, Hsiao CC, van der Poel M, Engelenburg HJ, Verdaasdonk K, Vincenten MCJ, Remmerswaal EBM, Kuhlmann T, Mason MRJ, Hamann J, Smolders J, and Huitinga I. 2020. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143: 1714–1730. [DOI] [PubMed] [Google Scholar]
- 37.Machado-Santos J, Saji E, Tröscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J, and Lassmann H. 2018. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141: 2066–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Nierop GP, van Luijn MM, Michels SS, Melief MJ, Janssen M, Langerak AW, Ouwendijk WJD, Hintzen RQ, and Verjans GMGM. 2017. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol 134: 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killestein J, Rep MH, Barkhof F, Roos MT, Adèr HJ, van Lier RA, and Polman CH. 2001. Active MRI lesion appearance in MS patients is preceded by fluctuations in circulating T-helper 1 and 2 cells. J Neuroimmunol 118: 286–294. [DOI] [PubMed] [Google Scholar]
- 40.Bach MA, Phan-Dinh-Tuy F, Tournier E, Chatenoud L, Bach JF, Martin C, and Degos JD. 1980. Deficit of suppressor T cells in active multiple sclerosis. Lancet 2: 1221–1223. [DOI] [PubMed] [Google Scholar]
- 41.Compston A 1983. Lymphocyte subpopulations in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 46: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholler AS, Fonnes M, Nazerai L, Christensen JP, and Thomsen AR. 2019. Local antigen encounter is essentail for establishing persistent CD8(+) T-cell memory in the CNS. Front Immunol. 10: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masopust D, and Soerens AG. 2019. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37: 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gandhi R, Laroni A, and Weiner HL. 2010. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 221: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konjevic Sabolek M, Held K, Beltrán E, Niedl AG, Meinl E, Hohlfeld R, Lassmann H, and Dornmair K. 2019. Communication of CD8+ T cells with mononuclear phagogytes in multiple sclerosis. Ann Clin Transl Neurol 6: 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salou M, Nicol B, Garcia A, and Laplaud DA. 2015. Involvement of CD8(+) T Cells in Multiple Sclerosis. Front Immunol 6: 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brynedal B, Duvefelt K, Jonasdottir G, Roos IM, Akesson E, Palmgren J, and Hillert J. 2007. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS One 2: e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, and Oksenberg JR. 1999. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 163: 5133–5144. [PubMed] [Google Scholar]
- 49.Williams A, Eldridge R, McFarland H, Houff S, Krebs H, and McFarlin D. 1980. Multiple sclerosis in twins. Neurology 30: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 50.Salou M, Garcia A, Michel L, Gainche-Salmon A, Loussouarn D, Nicol B, Guillot F, Hulin P, Nedellec S, Baron D, Ramstein G, Soulillou JP, Brouard S, Nicot AB, Degauque N, and Laplaud DA. 2015. Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol 2: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, Meinl E, Hohlfeld R, and Dornmair K. 2007. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain 130: 2789–2799. [DOI] [PubMed] [Google Scholar]
- 52.Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, and Goebels N. 2004. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A 101: 2428–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zang YC, Li S, Rivera VM, Hong J, Robinson RR, Breitbach WT, Killian J, and Zhang JZ. 2004. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol 172: 5120–5127. [DOI] [PubMed] [Google Scholar]
- 54.Berthelot L, Laplaud DA, Pettré S, Ballet C, Michel L, Hillion S, Braudeau C, Connan F, Lefrère F, Wiertlewski S, Guillet JG, Brouard S, Choppin J, and Soulillou JP. 2008. Blood CD8+ T cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur J Immunol 38: 1889–1899. [DOI] [PubMed] [Google Scholar]
- 55.Tsuchida T, Parker KC, Turner RV, McFarland HF, Coligan JE, and Biddison WE. 1994. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci U S A 91: 10859–10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sospedra M, and Martin R. 2006. Molecular mimicry in multiple sclerosis. Autoimmunity 39: 3–8. [DOI] [PubMed] [Google Scholar]
- 57.Wucherpfennig KW, and Strominger JL. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80: 695–705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ascherio A, and Munger KL. 2016. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin Neurol 36: 103–114. [DOI] [PubMed] [Google Scholar]
- 59.Sospedra M, and Martin R. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol 23: 683–747. [DOI] [PubMed] [Google Scholar]
- 60.Suidan GL, Mcdole JR, Chen Y, Pirko I, and Johnson AJ. 2008. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PloS one 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suidan GL, Dickerson JW, Chen Y, McDole JR, Tripathi P, Pirko I, Seroogy KB, and Johnson AJ. 2010. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. The journal of immunology 184: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larochelle C, Alvarez JI, and Prat A. 2011. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS letters 585: 3770–3780. [DOI] [PubMed] [Google Scholar]
- 63.Ifergan I, Kebir H, Alvarez JI, Marceau G, Bernard M, Bourbonnière L, Poirier J, Duquette P, Talbot PJ, Arbour N, and Prat A. 2011. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on α4 integrin. Brain 134: 3560–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larochelle C, Lécuyer MA, Alvarez JI, Charabati M, Saint-Laurent O, Ghannam S, Kebir H, Flanagan K, Yednock T, Duquette P, Arbour N, and Prat A. 2015. Melanoma cell adhesion molecule-positive CD8 T lymphocytes mediate central nervous system inflammation. Ann Neurol 78: 39–53. [DOI] [PubMed] [Google Scholar]
- 65.Günther C, Carballido-Perrig N, Kaesler S, Carballido JM, and Biedermann T. 2012. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J Invest Dermatol 132: 626–634. [DOI] [PubMed] [Google Scholar]
- 66.Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, and Butcher EC. 2005. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol 174: 277–283. [DOI] [PubMed] [Google Scholar]
- 67.Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, and Lassmann H. 2004. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 14: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giovanni F, Domenico P, Alessandro M, Raffaele I, Viviana N, Katia PA, and Paola BA. 2011. Circulating CD8+CD56-perforin+ T cells are increased in multiple sclerosis patients. J Neuroimmunol 240–241: 137–141. [DOI] [PubMed] [Google Scholar]
- 69.Selmaj KW, and Raine CS. 1988. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 23: 339–346. [DOI] [PubMed] [Google Scholar]
- 70.Vartanian T, Li Y, Zhao M, and Stefansson K. 1995. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med 1: 732–743. [PMC free article] [PubMed] [Google Scholar]
- 71.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, and Fugger L. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J, Davidson T, Dutta R, Goverman J, Bergles D, and Calabresi PA. 2019. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10: 3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C, Wittmann E, Buch T, Prazeres da Costa O, Brüstle A, Brenner D, Mak TW, Mittrücker HW, Tackenberg B, Kamradt T, and Lohoff M. 2013. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest 123: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korn T, Bettelli E, Oukka M, and Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 75.Phares TW, Stohlman SA, Hinton DR, and Bergmann CC. 2012. Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J Neuroinflammation 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walter L, and Albert ML. 2007. Cutting edge: cross-presented intracranial antigen primes CD8+ T cells. J Immunol 178: 6038–6042. [DOI] [PubMed] [Google Scholar]
- 77.Trapp BD, Ransohoff R, and Rudick R. 1999. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol 12: 295–302. [DOI] [PubMed] [Google Scholar]
- 78.Neumann H, Medana IM, Bauer J, and Lassmann H. 2002. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci 25: 313–319. [DOI] [PubMed] [Google Scholar]
- 79.Manning PT, Johnson EM, Wilcox CL, Palmatier MA, and Russell JH. 1987. MHC-specific cytotoxic T lymphocyte killing of dissociated sympathetic neuronal cultures. Am J Pathol 128: 395–409. [PMC free article] [PubMed] [Google Scholar]
- 80.Medana I, Martinic MA, Wekerle H, and Neumann H. 2001. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol 159: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller NM, Shriver LP, Bodiga VL, Ray A, Basu S, Ahuja R, Jana A, Pahan K, and Dittel BN. 2013. Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN Neuro 5: e00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ure DR, and Rodriguez M. 2002. Preservation of neurologic function during inflammatory demyelination correlates with axon sparing in a mouse model of multiple sclerosis. Neuroscience 111: 399–411. [DOI] [PubMed] [Google Scholar]
- 83.Deb C, Lafrance-Corey RG, Zoecklein L, Papke L, Rodriguez M, and Howe CL. 2009. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J Neuropathol Exp Neurol 68: 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jurewicz A, Biddison WE, and Antel JP. 1998. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol 160: 3056–3059. [PubMed] [Google Scholar]
- 85.Ruijs TC, Freedman MS, Grenier YG, Olivier A, and Antel JP. 1990. Human oligodendrocytes are susceptible to cytolysis by major histocompatibility complex class I-restricted lymphocytes. J Neuroimmunol 27: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun D, Qin Y, Chluba J, Epplen JT, and Wekerle H. 1988. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature 332: 843–845. [DOI] [PubMed] [Google Scholar]
- 87.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, and Mak TW. 1992. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science 256: 1210–1213. [DOI] [PubMed] [Google Scholar]
- 88.Jiang H, Zhang SI, and Pernis B. 1992. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 256: 1213–1215. [DOI] [PubMed] [Google Scholar]
- 89.Correale J, and Villa A. 2010. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol 67: 625–638. [DOI] [PubMed] [Google Scholar]
- 90.Frisullo G, Nociti V, Iorio R, Plantone D, Patanella AK, Tonali PA, and Batocchi AP. 2010. CD8(+)Foxp3(+) T cells in peripheral blood of relapsing-remitting multiple sclerosis patients. Hum Immunol 71: 437–441. [DOI] [PubMed] [Google Scholar]
- 91.Cunnusamy K, Baughman EJ, Franco J, Ortega SB, Sinha S, Chaudhary P, Greenberg BM, Frohman EM, and Karandikar NJ. 2014. Disease exacerbation of multiple sclerosis is characterized by loss of terminally differentiated autoregulatory CD8+ T cells. Clin Immunol 152: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, and Cantor H. 2004. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol 5: 516–523. [DOI] [PubMed] [Google Scholar]
- 93.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, Jensen P, and Kumar V. 2006. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol 177: 7645–7655. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Zhang J, Baylink DJ, Li CH, Watts DM, Xu Y, Qin X, Walter MH, and Tang X. 2016. Targeting Non-classical Myelin Epitopes to Treat Experimental Autoimmune Encephalomyelitis. Sci Rep 6: 36064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pannemans K, Broux B, Goris A, Dubois B, Broekmans T, Van Wijmeersch B, Geraghty D, Stinissen P, and Hellings N. 2014. HLA-E restricted CD8+ T cell subsets are phenotypically altered in multiple sclerosis patients. Mult Scler 20: 790–801. [DOI] [PubMed] [Google Scholar]
- 96.Morandi F, Venturi C, Rizzo R, Castellazzi M, Baldi E, Caniatti ML, Tola MR, Granieri E, Fainardi E, Uccelli A, and Pistoia V. 2013. Intrathecal soluble HLA-E correlates with disease activity in patients with multiple sclerosis and may cooperate with soluble HLA-G in the resolution of neuroinflammation. J Neuroimmune Pharmacol 8: 944–955. [DOI] [PubMed] [Google Scholar]
- 97.Correale J, and Villa A. 2008. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol 195: 121–134. [DOI] [PubMed] [Google Scholar]
- 98.Buckle G, Bandari D, Greenstein J, Gudesblatt M, Khatri B, Kita M, Repovic P, Riser E, Weinstock-Guttman B, Thrower B, Loring S, Riester K, Everage N, Prada C, Koulinska I, and Mann M. 2020. Effect of dimethyl fumarate on lymphocyte subsets in patients with relapsing multiple sclerosis. Mult Scler J Exp Transl Clin 6: 2055217320918619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghadiri M, Rezk A, Li R, Evans A, Giacomini PS, Barnett MH, Antel J, and Bar-Or A. 2020. Pre-treatment T-cell subsets associate with fingolimod treatment responsiveness in multiple sclerosis. Sci Rep 10: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, and Schmierer K. 2017. Interpreting Lymphocyte Reconstitution Data From the Pivotal Phase 3 Trials of Alemtuzumab. JAMA Neurol 74: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lückel C, Picard F, Raifer H, Campos Carrascosa L, Guralnik A, Zhang Y, Klein M, Bittner S, Steffen F, Moos S, Marini F, Gloury R, Kurschus FC, Chao YY, Bertrams W, Sexl V, Schmeck B, Bonetti L, Grusdat M, Lohoff M, Zielinski CE, Zipp F, Kallies A, Brenner D, Berger M, Bopp T, Tackenberg B, and Huber M. 2019. IL-17+ CD8+ T cell suppression by diemthylfumarate associates with clinical response in mutliple sclerosis. Nat Commun 10: 5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medina S, Villarrubia N, Sainz S Maza de la, Lifante J, Costa-Frossard L, Roldán E, Picón C, Álvarez-Cermeño JC, and Villar LM. 2018. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Mult Scler 24: 1317–1327. [DOI] [PubMed] [Google Scholar]
- 103.Serpero LD, Filaci G, Parodi A, Battaglia F, Kalli F, Brogi D, Mancardi GL, Uccelli A, and Fenoglio D. 2013. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J Neuroimmune Pharmacol 8: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akgün K, Blankenburg J, Marggraf M, Haase R, and Ziemssen T. 2020. Event-Driven Immunoprofiling Predicts Return of Disease Activity in Alemtuzumab-Treated Multiple Sclerosis. Front Immunol 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabatino JJ, Wilson MR, Calabresi PA, Hauser SL, Schneck JP, and Zamvil SS. 2019. Anti-CD20 therapy depletes activated myelin-specific CD8. Proc Natl Acad Sci U S A 116: 25800–25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Essen MR, Ammitzbøll C, Hansen RH, Petersen ERS, McWilliam O, Marquart HV, Damm P, and Sellebjerg F. 2019. Proinflammatory CD20+ T cells in the pathogenesis of multiple sclerosis. Brain 142: 120–132. [DOI] [PubMed] [Google Scholar]
- 107.Lohmann L, Janoschka C, Schulte-Mecklenbeck A, Klinsing S, Kirstein L, Hanning U, Wirth T, Schneider-Hohendorf T, Schwab N, Gross CC, Eveslage M, Meuth SG, Wiendl H, and Klotz L. 2018. Immune Cell Profiling During Switching from Natalizumab to Fingolimod Reveals Differential Effects on Systemic Immune-Regulatory Networks and on Trafficking of Non-T Cell Populations into the Cerebrospinal Fluid-Results from the ToFingo Successor Study. Front Immunol 9: 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harrer A, Pilz G, Wipfler P, Oppermann K, Sellner J, Hitzl W, Haschke-Becher E, Afazel S, Rispens T, van der Kleij D, Trinka E, and Kraus J. 2015. High interindividual variability in the CD4/CD8 T cell ratio and natalizumab concentration levels in the cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol 180: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaufmann M, Haase R, Proschmann U, Ziemssen T, and Akgün K. 2018. Real-World Lab Data in Natalizumab Treated Multiple Sclerosis Patients Up to 6 Years Long-Term Follow Up. Front Neurol 9: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tyler AF, Mendoza JP, Firan M, and Karandikar NJ. 2013. CD8(+) T Cells Are Required For Glatiramer Acetate Therapy in Autoimmune Demyelinating Disease. PLoS One 8: e66772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zafranskaya M, Oschmann P, Engel R, Weishaupt A, van Noort JM, Jomaa H, and Eberl M. 2007. Interferon-beta therapy reduces CD4+ and CD8+ T-cell reactivity in multiple sclerosis. Immunology 121: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yunliang Y, Han W, Liang J, Ji J, Wang J, Cantor H, and Lu L. 2013. Glatiramer acetate ameliorates inflammatory bowel disease in mice though the induction of Qa-1-restricted CD8+ regulatory cells. Eur J Immunol. 43:125–36. [DOI] [PubMed] [Google Scholar]
- 113.Medina S, Sainz S Maza de la, Villarrubia N, Álvarez-Lafuente R, Costa-Frossard L, Arroyo R, Monreal E, Tejeda-Velarde A, Rodríguez-Martín E, Roldán E, Álvarez-Cermeño JC, and Villar LM. 2019. Teriflunomide induces a tolerogenic bias in blood immune cells of MS patients. Ann Clin Transl Neurol 6: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]


