Abstract
Background:
Frailty is common in cirrhosis and associated with mortality, hospitalization, and reduced quality of life. Interventions aimed at forestalling frailty are limited by a lack of understanding of underlying physiological deficits.
Aims:
This study’s aim was to examine contributions of discrete sensorimotor and neurocognitive capacities to conventional frailty measures of unipedal stance time, chair stands, and grip strength.
Methods:
This cross-sectional study enrolled 119 outpatients with cirrhosis (50% female, aged 62.9±7.3 years). Capacities included sensory (lower limb sensation and visual contrast), neurocognitive (Number Connection Test (NCT) A and B, simple and recognition reaction time), and muscular (hip/core strength determined by lateral plank time (LPT)). Bivariate analyses and linear regression models were performed to identify significant contributors to each frailty measure.
Results:
The average performance was 9.8 ± 3.9 chair stands, 12.7 s ± 9.9 unipedal stance time, and 60.3 ± 25.6 lb grip strength. In multivariate models, factors explained 40% of variance in unipedal stance and 43% of variance in chair stands. The LPT was most strongly associated with unipedal stance and chair stands. Grip strength was associated with LPT, but did not have physiologic predictors.
Conclusions:
Clinically useful measures of frailty in adults with cirrhosis can be explained by disease severity but also deficits in strength and neurocognitive function. Recognition reaction time, a novel measure in cirrhosis, had a significant contribution to frailty. These findings have implications for frailty assessment and suggest that the optimal rehabilitation approach to frailty targets neurocognitive function in addition to strengthening.
Keywords: liver disease, executive function, reaction time, assessment, unipedal stance, chair stands, evaluation
Introduction
The incidence and prevalence of cirrhosis are increasing, as are the disease-related costs which are driven by cirrhotic complications. (Baki and Tapper, 2019; Tapper and Parikh, 2018) The complications of cirrhosis have multi-system consequences that result in decreased physiologic reserve and accelerate the patient’s risk of hospitalization and death. (Bajaj et al., 2011; Lai et al. 2018; Tapper et al., 2015) Frailty is a concept that quantifies physiologic reserve and is associated with poor outcomes, independent of traditional measures of disease severity such as Child Class and MELD. (Lai et al., 2016; Tandon et al., 2012; Tapper et al., 2015) The most widely validated frailty tools among populations with cirrhosis are measures of physical function. (Dunn et al., 2016) An index which combines grip strength, chair stands, and balance – the Liver Frailty Index (LFI) – has been extensively validated in transplant-waitlisted patients. (Lai et al, 2015; Lai et al., 2018; Lai et al., 2019) The LFI effectively operationalizes the concept of physical frailty; however, it does not elucidate the mechanism(s) responsible for frailty in a given patient. Data are currently lacking to support the reversibility of frailty (Gine-Garriga et al., 2014; Lai et al., 2016; Lai et al., 2017; Sayer et al., 2006) but efforts to reverse frailty have lacked specific targets. Knowledge of the underlying physiologic capacities that drive frailty will improve understanding of the frailty phenotype in cirrhosis and guide targeted interventions. In older adults, studies which measure capacities allowing the tailoring of physical interventions for frailty have shown promising results for improving strength. (Dipietro et al., 2019) However, the multi-system deficits responsible for frailty in the context of cirrhosis are more complex.
To address these knowledge gaps, the aim of this study was to identify the specific capacities that independently contribute to frailty in cirrhosis. We hypothesize, based on our previous work (Murphy et al., 2019, Richardson et al., 2017), that deficits in sensory, cognitive, and/or muscle force generating capacities underlie measures of frailty commonly applied to patients with cirrhosis.
Methods
This cross-sectional study was approved by the University of Michigan Medical School Institutional Review Board (Ann Arbor, MI). Participants were recruited through the Hepatology clinic at Michigan Medicine beginning in August 2018 and ending in April 2019. Individuals who were scheduled for an upcoming visit at the clinic were sent a letter informing them of the opportunity to participate in the study. Participants who did not opt out of talking to the study team were called by the research coordinator or were approached in the clinic on the day of their appointment for a 60-minute examination in a clinic room. Inclusion criteria were: age ≥ 50, evidence of portal hypertension (platelet count < 100, ascites, history of hepatic encephalopathy, or varices). Potential participants were excluded based on the following criteria: living in a nursing home, one or more episodes of binge drinking in the 3 months prior to study, use of a wheelchair as a primary method of mobility, hospitalization in the 30 days prior to data collection, or other issues precluding meaningful participation in study procedures (e.g., blindness, lower limb amputation, or inability to understand research procedures in English). All assessments were performed by one experienced team member.
Demographic and Clinical Factors
Demographic variables included age, biological sex, race and ethnicity, level of education completed, and work status. Health status measures of disease severity (MELD-Na, Child Class), history of hepatic encephalopathy, and disease complications were ascertained through medical record review. Depressed mood was assessed by the Patient Reported Outcomes Measurement System (PROMIS) Depression Short Form 4a. (Pilkonis et al., 2011) Overall physical function was measured by the PROMIS Physical Function Short Form 8b. PROMIS measures are scored with reference to population norms on a T scale, where the mean is 50±10. (Cella et al., 2010)
Frailty Assessments
Frailty measures included the 30 second chair stand test, (Jones et al., 1999) unipedal stance time, and grip strength. The outcome for the chair stand test was the number of times participants completed a full stand from a chair 20 inches high without using upper limb support during a 30 second period. The outcome for unipedal stance time was the number of seconds participants could stand on one leg without upper extremity support. Participants were allowed to place their elevated foot in any position except bracing it on the stance leg. This commonly-used balance test is useful in the identification of peripheral neuropathies, as well as the identification of increased fall and injury risk in older adults. (Hurvitz et al., 2000; Richardson et al., 1996; Vellas et al., 1997) Participants performed 3 trials on each leg and the mean of the 6 trials was used for analysis. Grip strength was measured by using a Jamar hand dynamometer (Lafayette Instruments) in pounds of pressure. Three trials were performed for each hand, with the participant sitting upright with their arm flexed 90 degrees at the elbow. (Mathiowetz et al., 1984) The greatest measure of 6 trials was used for the analysis. (Roberts et al., 2011)
Sensory Assessments
Sensory processing was assessed via tests of lower limb sensation and visual contrast severity. To measure lower limb sensation, a vibratory test was performed using a 128-Hz tuning fork applied to the skin immediately proximal to the nail bed of each great toe. The score represents the time from placement of the tuning fork to the time participants reported that the sensation was extinguished.(Oyer et al., 2007) Fewer seconds of vibration perception correlate strongly with less precise ankle proprioceptive precision and worse balance.(Donaghy et al., 2016) The mean time of the right and left sides was used in the analysis.(Donaghy et al., 2016) Visual contrast sensitivity was assessed using the Melbourne Edge Test.(Haymes et al., 2004) This validated test involves a specialized eye chart to assess ability to detect visual contrasts. At a distance of 40 cm, participants are asked to identify the darker shaded orientation of the edge within each circular test patch, beginning at the top of the chart. The test is stopped after two consecutive incorrect responses are made. Contrast sensitivity was recorded as the last correctly identified edge, in decibels (dB). A higher dB score means better ability to detect visual contrast and the maximum score is 24 decibels. A score of ≤ 16 indicates deficits in contrast sensitivity for individuals under the age of 65, and those over age 65 have a deficit when scoring ≤ 15.(Haymes et al., 2004)
Neurocognitive Assessments
Simple reaction time (SRT) and recognition reaction time were measured using the ReactStick, a validated performance-based assessment (Eckner et al., 2012; Van Schooten et al., 2019) designed to be used in clinic settings. This test has good – excellent test-retest reliability at time periods of one and four weeks from initial assessment [intraclass correlation coefficients (ICC) > 0.6]. (Eckner et al., 2009; van Schooten et al., 2019) It is an instrument resembling a yard stick with a small rectangular housing at one end which incorporates accelerometers, circuity, lights, a battery, and digital readout.
Simple Reaction Time:
To assess SRT participants sat with the ulnar aspect of their dominant forearm resting on a desk of standard height (73 to 75 cm). The research staff member held the device at the top of the shaft allowing the housing to approach the participant’s hand. The participant positioned their hand around the ReactStick housing without touching it. The examiner then released the ReactStick at random intervals between 2 and 6 seconds (see Figure 1 Panel A). The participant was instructed to catch the device as quickly as possible after its release. Accelerometers within the housing determine the instants of acceleration and deceleration and the elapsed time between these is presented in milliseconds (ms). Participants performed 2 practice trials and 8 data acquisition trials. The mean of the 8 data acquisition trials was used in the analysis.
Figure 1.
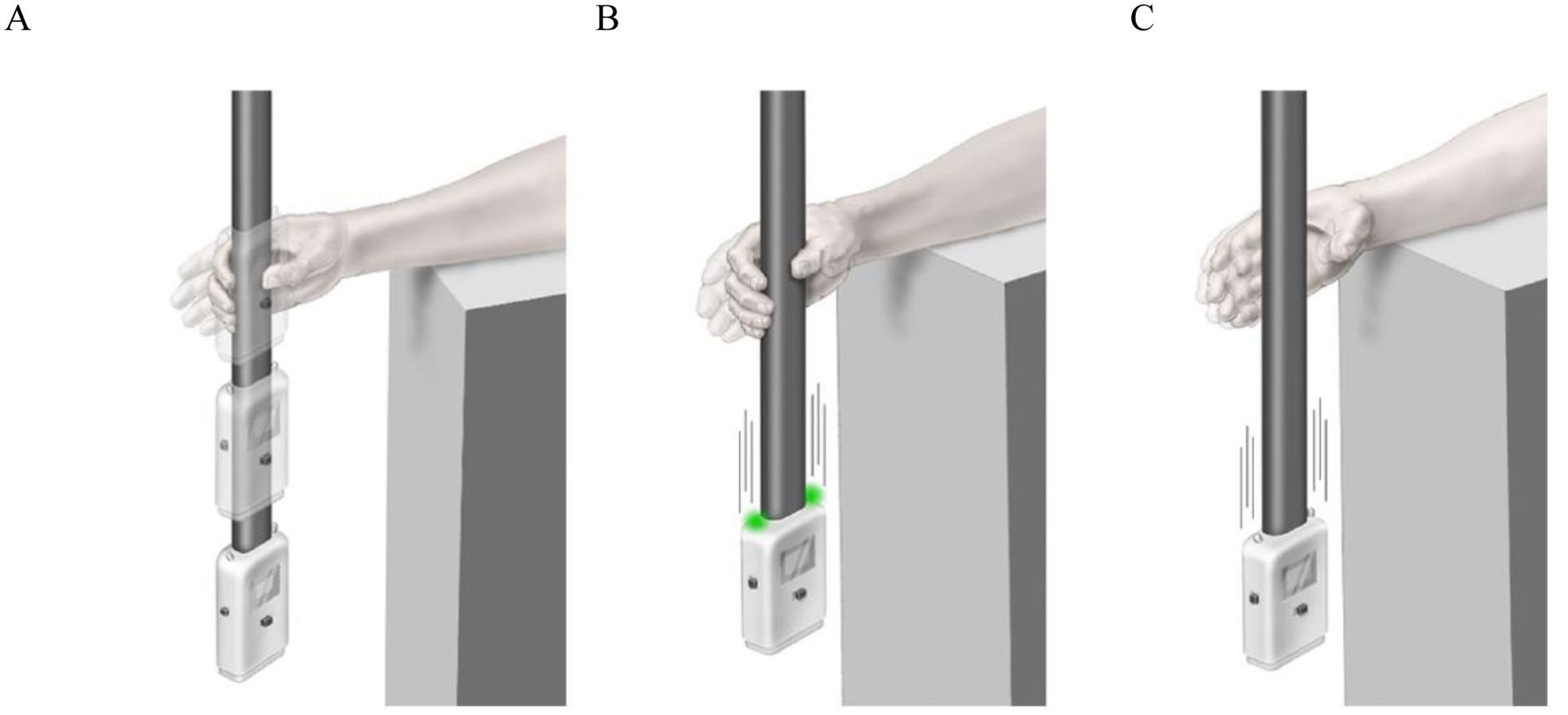
ReactStick Device for Assessment of Reaction Time
Panel A: ReactStick Simple Reaction Time (SRT) Test. The device is dropped and the participant needs to catch it as quickly as possible
Panel B: ReactStick Recognition Reaction Time Test showing the condition where lights illuminate as the device is dropped which is the indicator for the participant to catch the device.
Panel C: ReactStick Recognition Reaction Time Test showing the condition where lights do not illuminate when the device is dropped and the participant must resist the urge to catch the device.
Recognition Reaction Time:
Participants were tested in the same posture as was used for determination of SRT. However, in this test, light-emitting diodes on the top of the ReactStick illuminate at the moment of release during 50% of the trials in a fully random pattern such that neither participant nor examiner is aware (See Figure 1 Panels B and C). Participants were instructed to catch the falling ReactStick only during those trials when the diodes illuminated, and to resist catching it when the diodes did not turn on, consistent with a standard go/no-go testing paradigm (Richardson et al., 2017). Verbal instructions emphasized response accuracy, not speed. Participants performed 4 practice trials, and then 20 data collection trials. Recognition reaction time was recorded as the percentage of correctly performed trials/total number of trials. Of note, given that the ReactStick released from standard desk height strikes the floor within approximately 390 ms, participants were forced to make the decision to grasp the device, or inhibit that impulse, under significant time pressure. This differentiates it from most other tests of inhibitory executive function. We were also interested in examining whether there were differences in accuracy between trials in which the diodes illuminated (ON trials) and did not (OFF trials). ON accuracy was the number of successful ON trials/ number of ON trials. OFF accuracy was the number of successful OFF trials/ number of OFF trials.
Number Connection Tests:
The Number Connection Tests A and B (NCT-A and NCT-B respectively) were also used to measure neurocognitive factors. They are timed paper-and-pencil tests that assess dimensions of executive function such as processing speed and visuospatial orientation. NCT-B also assesses selective attention. NCT-A and NCT-B have been found to be valid and reliable assessment tools used in the detection of cognitive dysfunction with approximate age-adjusted upper-limits of normal of 60 and 120 seconds for NCT-A and NCT-B.(Weissenborn et al., 1998)
Strength Assessments
Hip and trunk strength was assessed using LPT.(Donaghy et al., 2016) Participants performed a lateral plank on each side, with knees either flexed or extended and were timed. The number of seconds in the knees flexed position was multiplied by 0.5, given that task is easier to perform. (Donaghy et al., 2016) The maximum score is 60 seconds. The research team member first demonstrated the LPT with knees bent and straight, and provided verbal and physical cues for positioning when indicated. Time to failure was determined by the participant’s inability to maintain alignment of the trunk and thighs. Trials were conducted on a clinic exam table for those who requested or were unable to get on or off the floor. Participants then performed one trial on each side. The average of the right and left trials was used for analysis.
Analysis
Descriptive statistics were performed to characterize the sample. Bivariate correlations (Pearson’s r) were conducted between and among candidate predictor variables and each of the frailty measures. Three stepwise regression models were performed with chair stand, unipedal stance time, and grip strength as outcomes respectively. We included factors that correlated at .25 or greater with any of the frailty outcomes for inclusion in the models; however, if no variables were associated at that level with the outcome being modeled, we ensured there was at least one factor from each type of capacity (sensory, neurocognitive, muscular) and chose the one with the highest correlation for inclusion in the model. Due to moderate to high inter-correlations between reaction time variables (r ranging from .4 - .9) and between NCT-A and NCT-B tests (r = .55), the one most strongly associated with the outcome was included to reduce the risk of multicollinearity. We used a stepwise procedure with inclusion of p = .05 and exclusion of p = .10. Because of the log transformation of unipedal stance, we calculated the percentage change in the average value of unipedal stance time per unit increase in each predictor to interpret unstandardized beta coefficients.(Vittinghoff et al., 2012) SPSS version 24.0 (Armonk, NY: IBM Corp.) was used for analyses.
Results
Cohort Characteristics
As shown in Table 1, participants were 50% female, had a mean age of 62.9 years, 35% had a 4-year college or graduate degree, and 51% were retired from working. Nineteen percent were classified as Child Pugh B or C, with a mean MELD-Na score of 11. All participants had portal hypertension and 62% had varices. The sample had a mean physical function T score of 42; 64% had a score a half SD or greater below the PROMIS normative sample indicating worse physical function.
Table 1.
Characteristics of Sample (N = 119)
| N | Mean (SD) or % (N) | Range | |
|---|---|---|---|
| Demographics | |||
| Age, years | 119 | 62.9 (7.3) | 50 – 89 |
| Sex, female | 119 | 49.6% (59) | |
| Ethnicity - Hispanic | 119 | 4.2% (5) | |
| Race – White | 113 | 95.0% | |
| Black/Asian | 5 | 5.0% | |
| Education | 118 | ||
| Bachelors or graduate degree | 41 | 34.7% | |
| Work status - Retired | 119 | 51.3% (61) | |
| Body Mass Index, kg/m2 | 119 | 31.71 (7.9) | |
| Clinical Status | |||
| Etiology | 119 | ||
| NAFLD | 41 | 34.5% | |
| Alcohol | 28 | 23.5% | |
| HCV | 18 | 15.1% | |
| Autoimmune | 6 | 5.0% | |
| PBC | 5 | 4.2% | |
| HCV and ETOH | 4 | 3.4% | |
| HCV and NAFLD | 3 | 2.5% | |
| Other Child Pugh Score | 14 | 11.8% | |
| A | 95 | 79.8% | |
| B | 21 | 17.6% | |
| C | 2 | 1.7% | |
| Presence of Varices | 119 | 62.2% (74) | |
| MELD-Na | 118 | 11.0 (5.0) | 6 – 38 |
| Depression (PROMIS t-score) | 97 | 50.1 (9.4) | 41–75.7 |
| Physical Function (PROMIS t-score) | 119 | 42.6 (8.6) | 23.9–60.1 |
Frailty Measures
Table 2 shows the sample’s frailty and capacity characteristics and Figure 2 shows sample means compared to published norms of healthy adults. The unipedal stance time of the sample was 12.7 seconds, which is below the normative values for healthy adults aged 60 – 69 of 26.9 seconds (Springer et al., 2001). A difference in unipedal stance time was found for women and men with women having significantly lower mean time (10.8 ±9.0 vs. 14.5 + 10.5 sec; t (117) = 2.04; p = .044). The number of chair stands completed in 30 seconds was 10.1 for the sample which did not significantly differ between women and men. Chair stands in this sample are below the criterion-referenced standard of the number of chair stands needed to maintain physical independence in later life among adults aged 60 – 69 years -- 15 for women and 17 for men (Rikli et al, 2012). Grip strength of 45 and 76 lbs for women and men respectively for this sample is well below the norms for women and men age 60 – 64 years (55 and 89 lbs/ pressure) (Mathiowetz et al., 1984).
Table 2.
Assessments of Frailty and Physiological Capacities
| Assessment | N | Mean (SD) | Range |
|---|---|---|---|
| Frailty Assessments | |||
| Unipedal stance (sec) | 119 | 12.7 (9.9) | .92 – 30 |
| Number of Chair Stands in 30 seconds | 101 | 10.1 (4.6) | 0 – 28 |
| Hand grip (max of 6 in lbs) | 117 | 60.3 (25.6) | 15 – 117 |
| Male | 59 | 75.5 (23.5) | 24 – 117 |
| Female | 58 | 44.9 (17.1) | 15 – 106 |
| Physiologic Capacities | |||
| Sensory | |||
| Melbourne visual contrast (db) | 108 | 21.7 (3.4) | 5 – 24 |
| Vibratory sense (sec) | 115 | 9.4 (5.8) | 0 – 27.6 |
| Neurocognitive | |||
| NCT-A (sec) | 113 | 40.4 (16.4) | 14.6 – 119.0 |
| NCT-B (sec) | 113 | 103.6 (46.3) | 30.2 – 234.3 |
| Simple Reaction Time (SRT) (ms) | 117 | 227 (50) | 120 – 390 |
| Recognition Reaction Time (%) | 117 | 62% (18%) | 17 – 100 |
| Total Accuracy during ON trials (%) | 117 | 74% (19%) | 22 – 100 |
| Total Accuracy during OFF trials (%) | 117 | 49% (28%) | 0 – 100 |
| Muscular | |||
| Lateral plank test (sec) | 102 | 16.3 (13.7) | 0 – 60 |
db = decibel; vibratory sense is the number of seconds until vibration is no longer detected.
Neurocognitive variables expressed as percent reflect the mean (SD) percent of the sample.
Figure 2.
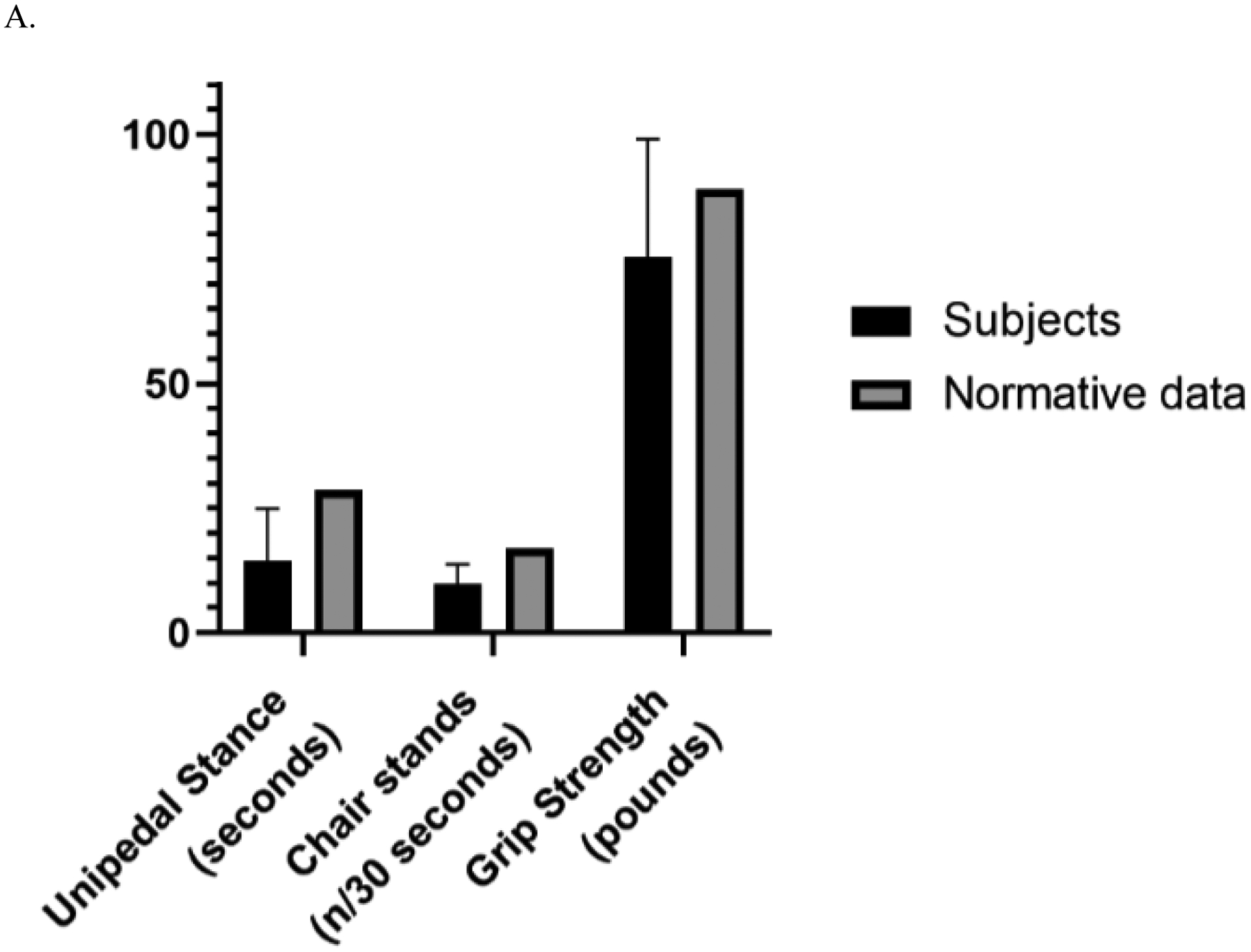
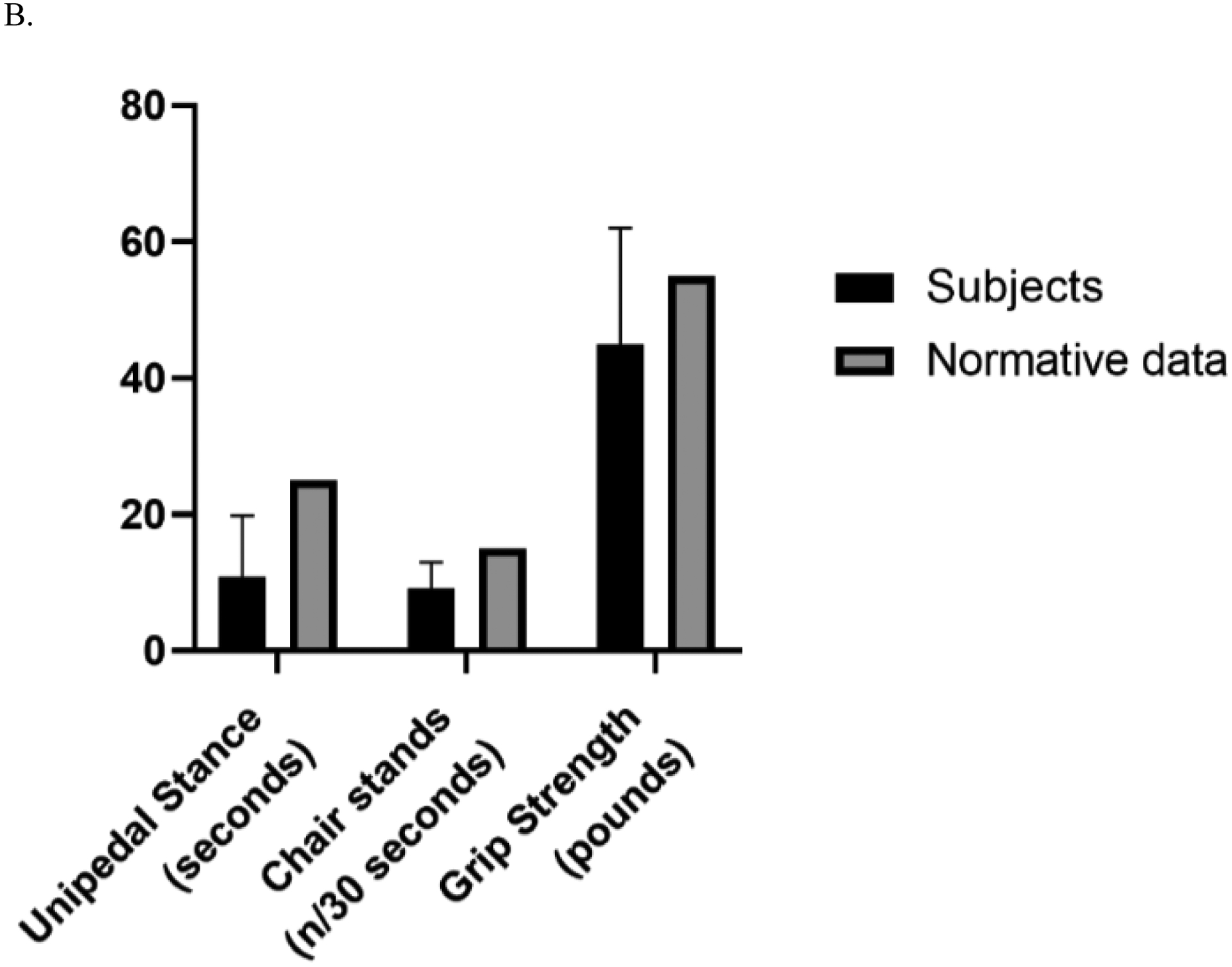
Panel A. Frailty measures for Men and Age- and Sex-Matched Normative Values
Panel B. Frailty Measures for Women and Age- and Sex-Matched Normative Values
Capacity Measures
For sensory measures, visual contrast severity on the Melbourne Edge Test did not indicate impairment for the sample (mean = 22 dB); 8 people in the sample had values of 16 or less that indicated impairment. Vibratory sense (mean = 9s) was diminished relative to reports of healthy adults, akin to a cohort with diabetic neuropathy (33). The numbers connection tests showed 11% of the sample (12/113) had a score of greater than 60 seconds on the NCT A and 33% (37/113) had a score greater than 120 seconds on NCT B. The mean SRT of 227 ± 50 ms in the cirrhosis participants is slower than that of healthy older adults (mean age 72.4 years) who demonstrated mean SRT of 170 ± 25 ms. (unpublished data) Recognition reaction time was 62 ± 18%. Participants were more accurate during the ON trials (74 ± 19%) where they needed to catch the ReactStick when the diode was illuminated than during the OFF trials (49 ± 28%). The latter test has been more challenging in all cohorts evaluated thus far, which highlights the challenge associated with rapid inhibitory function under intense time pressure. The mean LPT (representing muscular capacity) was 16.3 ± 13.7 seconds. Almost 20% (17/102) were not able to perform the plank or maintain it for 2 seconds.
Contributors to Frailty Measures
Unipedal Stance:
From stepwise regression, 40% of the variance in unipedal stance was explained by the factors in the model. In Table 4, the standardized beta coefficients show that lateral plank had the greatest contribution to unipedal stance (β = .43), followed by MELD score (β = −.27) and recognition reaction time (β = .22). Associations between each factor and unipedal stance are depicted by the unstandardized beta coefficients (B). For every one second increase in lateral plank time, there is a 3% increase in unipedal stance time, for every point increase in MELD score, there is a 5% decrease in unipedal stance time. For every 1% increase in recognition reaction time, there is a 1% increase in unipedal stance time.
Table 4 –
Factors Independently Associated with Frailty Measures
| Unipedal Stance† | Chair Stands* | Grip Strength‡ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | t | P value | B | SE B | β | t | P value | B | SE B | β | t | P value | |
| LPT | .03 | .01 | .43 | 4.44 | .0001 | .10 | .03 | .36 | 3.84 | .0001 | .40 | .17 | .21 | 2.38 | .02 |
| MELD-Na Score | −.05 | .02 | −.27 | −.308 | .003 | −.26 | .07 | −.33 | −3.94 | .0001 | |||||
| Recognition reaction time | .01 | .01 | .22 | 2.26 | .03 | .06 | .02 | .26 | 2.83 | .01 | |||||
| Age | −.94 | .34 | −.23 | −2.77 | .007 | ||||||||||
| Sex | −28.4 | 4.5 | −.54 | −6.26 | .0001 | ||||||||||
Note. unstandardized beta (B), the standard error for the unstandardized beta (SE B), the standardized beta (β), the t test statistic (t)
Log transformation of unipedal stance was used. – Adjusted R square = .40; F (3,81) = 19.59; intercept = 1.64 (SE = .36) (variables entered into stepwise model in order – average lateral plank strength, MELD-Na Score, recognition reaction time)
Chair stands –Adjusted R square = .43; F (3,81) = 21.87; p = .0001; Intercept = 7.77 (SE = 1.42) (Variables entered into stepwise model in order - average lateral plank strength, MELD-Na Score, recognition reaction time)
Grip Strength – Adjusted R square = .43; F (3,83) = 21.83; p = .0001; intercept = 127.17 (SE = 21.14); Variables entered into the model in order - biological sex, age, average lateral plank strength
Chair stands:
Similar to factors in the stepwise regression model with unipedal stance as the outcome, lateral plank had the strongest association with number of chair stands performed (β = .36), followed by Meld score (β = −.33) and recognition reaction time (β = .26). These factors explained 43% of the variance in chair stands. For every second increase in lateral plank time, number of chair stands increases by .1. Given that the mean number of chair stands is 10, this equates to a 10% increase in chair stands. For every point increase in MELD score, number of chair stands decreases by .3. For every percent increase in recognition reaction time, there is a .06 increase in number of chair stands.
Grip strength:
In the stepwise model, the factors explained 43% of variance in grip strength. Of the sensory, muscular, and neurocognitive measures assessed, only lateral plank (β = .21). was significantly independently associated with grip strength. Demographic variables of sex and age were most associated with grip strength (β = −.54 and −.23 respectively). For females, there is a 28 lbs/ pressure decrease in grip strength. For each year older, grip strength decreases by .9 lbs /pressure. For every second increase in lateral plank time, there is a .21 increase in lbs/ pressure in grip strength.
Discussion
Frailty has emerged as an important construct in cirrhosis because it enhances prediction of poor outcomes which can be useful in medical decision making.(Lai et al., 2018; Lai et al., 2019; Tapper et al., 2015, Tapper et al., 2019) However, frailty remains poorly characterized. Although frailty results from deficits in multiple body systems, it is typically understood through quantification of physical attributes. In order to craft effective interventions to address frailty and prevent its complications, we must expand our understanding of frailty to include the specific impairments responsible for it. In this prospective study of 119 persons with cirrhosis, our data determines the physiologic capacities responsible for poor performance of three components of the LFI, a standard frailty assessment. We found that, while grip strength was related most strongly to sex and age effects, unipedal stance and chair-stands elucidated deficits in capacities that are amenable to intervention. Specifically, in addition to disease severity, these measures were most associated with muscular and neurocognitive capacities.
Postural control for balance and standing depends on neurocognitive capacities
Our study provides support for the assessment of neurocognitive capacities as part of the frailty phenotype. Previous work from our group has shown that hepatic encephalopathy is strongly associated with frailty and confounds the association between frailty and transplant-free survival.(Tapper et al., 2018) Reduced neurocognitive capacities have been associated with postural control deficits in other studies such as reduced reaction time in people with minimal hepatic encephalopathy versus those without minimal hepatic encephalopathy (Urios et al., 2017) and reduced attentional processing during a dual task paradigm.(Rankin et al., 2000) Our findings extend these data by showing that two of three frailty measures, unipedal stance time and chair rise, are associated with neurocognitive capacities independent of lower limb sensorimotor function, age, and disease severity. In this study in which a number of neurocognitive factors were assessed, recognition reaction time was more strongly associated with frailty measures than commonly used paper and pencil (NCT) tests. Recognition reaction time added 6% to the overall variance in chair stands and 3% to overall variance in unipedal stance time above and beyond other factors in the models. These findings are consistent with prior work finding that total and/or OFF recognition reaction time have predicted unipedal stance time, response to perturbation during gait, severe fall-related injuries, and retrospective falls in cohorts of older adults with and without diabetic neuropathy. (Eckner et al., 2012; Richardson et al., 2017) The ReactStick is a unique test which taps several aspects of executive function such as selective attention, inhibitory control, and processing speed so as to respond with approximately 380 ms. Further, dropping a 3 dimensional device likely activates visuomotor pathways, rather than visuoperceptual, and so better approximates responses that occur in real-life, such as reacting to an unexpected event while driving or walking. In contrast, paper and pencil and computer-based tests are not likely associated with visuomotor pathway activation or intense time pressure. (Murphy et al., 2019; Van Schooten et al., 2019) Simple reaction time was not correlated with any of the frailty measures in this study and was not a strong predictor of age-related cognitive decline in a prior study. (Eckner et al., 2012) The ReactStick measure was designed to be feasible to use in clinic settings, requires minimal training of the assessor, and can be done in 5 – 10 minutes. Future studies should compare performance of the ReactStick to conventional measures like the LFI. Furthermore, there is clearly a role for studies that address the initiation or intensification of therapy directed at neurocognition (e.g. lactulose) in the multimodal treatment of frailty.
Lateral plank time is more associated with physiologic capacities than grip strength
Grip strength is often used as a proxy for whole body strength, and is useful for diagnostic and clinical reasons in cirrhosis such as in measurement of nutritional status (Alvares-da-Silva and Reverbel da Silveira, 2005; Amodio et al., 2013; Peng et al., 2007;) and body cell mass.(Figueiredo et al., 2000) In our study, grip strength was only moderately associated with hip strength as measured by LPT, suggesting that grip strength may not diminish as do other indicators of strength. LPT had strong associations with the performance-based mobility tests of frailty. Although the LPT is not a commonly performed assessment, given its relative importance to frailty measures and the relative ease of performing it in the clinic, this measure may be useful to quickly assess underlying muscular deficits associated with frailty.
Designing interventions for frail persons with cirrhosis
Our findings have potential implications for treatment. Few interventions are available to reduce frailty for people with cirrhosis. While exercise, adequate nutrition, and physical activity are recommended, (Tandon et al., 2018; Trivedi and Tapper, 2018) these interventions do not specifically address hip/trunk strength or underlying neurocognitive deficits that we find to be associated with frailty. However, there is evidence that physical interventions have laudatory cognitive benefits. For instance, resistance training has been shown to improve executive function in some studies (Landrigan et al., 2019, Li et al., 2018) and other work has shown that cognitive training translates to improvement in postural control and gait. (Ng et al., 2015; Smith-Ray et al., 2013; Verghese et al., 2010) Given the little research in this area, interventions that target neurocognitive function will be needed to understand if and how frailty in cirrhosis can be improved. Multiple options are available. These could include prospective trials of lactulose or other hepatic encephalopathy-directed therapy. Finally, to the extent possible, strengthening should focus on hip/trunk musculature as strength in this distribution more strongly influenced frailty measures than did grip strength, which is an accepted measure of generalized strength.
Contextual factors
These data must be interpreted in the context of the study design. First, these data are derived from the general Hepatology clinic of a tertiary referral center in the Midwestern US and may not generalize to other settings. Second, the cross sectional design limits the ability to understand the influence of capacities on frailty status over time. Third, though a sample of 119 subjects is not small, it is unclear whether some covariates would prove significant in larger studies. Further, beyond the cirrhosis diagnosis, other comorbidities such as diabetes or osteoarthritis may attenuate the relationship of physiological attributes and frailty measures and could be measured in future studies. In future assessment of grip strength, larger samples will be needed to tease out how grip strength and other capacities, such as LPT, differ by sex and age. As LPT was a key capacity associated with frailty, future studies to establish norms by age ranges and sex would aid interpretation of deficits by these subgroups within the cirrhosis population. In addition, although validated in other cohorts, many of these assessments have not been validated within the cirrhosis population which would be helpful to characterize the specific psychometric properties of these measures. Lastly, longitudinal studies will also be needed to better understand effects of neurocognitive contributors to frailty as it will be helpful to discriminate acute hepatic encephalopathy versus neurocognitive deficits that are stable over time.
Conclusion
We sought to identify the specific physiologic contributors to frailty in a cohort of patients with cirrhosis. Although frailty spans multiple body systems, muscular and neurocognitive factors were most associated to frailty. Further research is needed to determine if these select muscular and neurocognitive assessments may help improve the frailty phenotype. In addition, interventions should be developed and tested to determine if hip strengthening and neurocognitive benefits can be attained to improve frailty status.
Table 3.
Bivariate Correlations of Frailty Assessments with Demographics, Clinical Status, and Physiologic Capacity Variables
| Unipedal Stance r | Chair Stands r | Hand Grip r | |
|---|---|---|---|
| Demographics and Clinical Status | |||
| Age | −.26* | −.10 | −.29* |
| Meld-Na | −.35* | −.39* | −.06 |
| Total meds | −.31* | −.24* | .04 |
| Depression | −.12 | −.31* | −.09 |
| Sensory | |||
| Melbourne visual contrast | .26* | −.01 | .11 |
| Vibratory sense | .19 | −.20* | .11 |
| Neurocognitive | |||
| NCT-A | −.30* | −.29* | −.08 |
| NCT-B | −.28* | −.39* | −.12 |
| SRT (avg) | −.04 | .06 | .02 |
| Recognition reaction time | .42* | .43* | .25* |
| On accuracy | .27* | .33* | .19 |
| Off accuracy | .29* | .31* | .18 |
| On trial SRT | .05 | .11 | .09 |
| Muscular | |||
| LPT | .57* | .55* | .36* |
Note. N=101; Unipedal stance was log transformed; Chair stands were corrected for outliers
p ≤ .05
Financial support:
This project was supported in part by the Newman Family Foundation awarded to Dr. Richardson. Dr. Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest. Tapper (consultant; Novartis, Allergan. Advisory board; Mallinckrodt, Bausch; Grant support; Valeant, Gilead). Murphy (grant support: Lymphatouch LLC). No author has relevant conflicts of interest.
References
- Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005;21:113–117. [DOI] [PubMed] [Google Scholar]
- Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013;58:325–336. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki JA, Tapper EB. Contemporary epidemiology of cirrhosis. Curr Treat Options Gastroenterol 2019;17:244–253. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro L, Campbell WW, Buchner DM, Erickson KI, Powell KE, Bloodgood B, et al. Physical activity, injurious falls, and physical function in aging: an umbrella review. Med Sci Sports Exerc 2019;51:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy A, DeMott T, Allet L, Kim H, Ashton-Miller J, Richardson JK. Accuracy of clinical techniques for evaluating lower limb sensorimotor functions associated with increased fall risk. PM and R 2016;8:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J of Gastroenterol 2016;111:1768. [DOI] [PubMed] [Google Scholar]
- Eckner JT, Whitacre RD, Kirsch NL, Richardson JK. Evaluating a clinical measure of reaction time: An observational study. Perceptional and Motor Skills 2009; 108: 717–720. [DOI] [PubMed] [Google Scholar]
- Eckner JT, Richardson JK, Kim H, Lipps DB, Ashton-Miller JA. A novel clinical test of recognition reaction time in healthy adults. Psychological Assessment 2012;24(1):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo FA, Dickson ER, Pasha TM, Porayko MK, Therneau TM, Malinchoc M, et al. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transplantation 2000;6:575–581. [DOI] [PubMed] [Google Scholar]
- Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med and Rehabil 2014;95:753–769. e753. [DOI] [PubMed] [Google Scholar]
- Haymes SA, Chen J. Reliability and validity of the Melbourne Edge Test and high/low contrast visual acuity chart. Optom Vis Sci 2004;81:308–316. [DOI] [PubMed] [Google Scholar]
- Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil 2000;81:587–591. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- Lai JC, Covinsky KE, Hayssen H, Lizaola B, Dodge JL, Roberts JP, et al. Clinician assessments of health status predict mortality in patients with end‐stage liver disease awaiting liver transplantation. Liver Int 2015;35:2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end‐stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019;156:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan JF, Bell T, Crowe M, Clay OJ, Mirman D. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol Res 2019. [DOI] [PubMed] [Google Scholar]
- Li Z, Peng X, Xiang W, Han J, Li K. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clin Exp Res 2018;30:1259–1273. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222–226. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Tapper EB, Blackwood J, Richardson JK. Why do individuals with cirrhosis fall? A mechanistic model for fall assessment, treatment, and research. Dig Dis Sci 2019;64:316–323. [DOI] [PubMed] [Google Scholar]
- Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am J Med 2015;128:1225–1236.e1221. [DOI] [PubMed] [Google Scholar]
- Oyer D, Saxon D, Shah A. Quantitative assessment of diabetic peripheral neuropathy with use of the clanging tuning fork test. Endocrine Practice 2007;13:5–10. [DOI] [PubMed] [Google Scholar]
- Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr 2007;85:1257–1266. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 2011;18:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA. Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol Sci Med Sci 2000;55:M112–M119. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil 1996;77:1152–1156. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Eckner JT, Allet L, Kim H, Ashton-Miller JA. Complex and simple clinical reaction times are associated with gait, balance, and major fall injury in older subjects with diabetic peripheral neuropathy. Am J Phys Med Rehabil 2017;96:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2012;53:255–267. [DOI] [PubMed] [Google Scholar]
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429 [DOI] [PubMed] [Google Scholar]
- Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006;35:409–415. [DOI] [PubMed] [Google Scholar]
- Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. J Gerontol B Psychol Sci Soc Sci 2013;70:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther 2007;30:8–15. [DOI] [PubMed] [Google Scholar]
- Takahara M, Fujiwara Y, Sakamoto F, Katakami N, Matsuoka T-A, Kaneto H, et al. Assessment of vibratory sensation with a tuning fork at different sites in Japanese patients with diabetes mellitus. J Diabetes Investig 2014;5:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–1216. [DOI] [PubMed] [Google Scholar]
- Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 2018. 69(5):1164–1177. [DOI] [PubMed] [Google Scholar]
- Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015;62:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant 2018;18:2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper EB, Baki J, Parikh ND, Lok AS. Frailty, psychoactive medications, and cognitive dysfunction are associated with poor patient‐reported outcomes in cirrhosis. Hepatology 2019;69:1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi HD, Tapper EB. Interventions to improve physical function and prevent adverse events in cirrhosis. Gastroenterol Rep 2018;6:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urios A, Mangas-Losada A, Gimenez-Garzo C, Gonzalez-Lopez O, Giner-Duran R, Serra MA, et al. Altered postural control and stability in cirrhotic patients with minimal hepatic encephalopathy correlate with cognitive deficits. Liver Int 2017;37:1013–1022. [DOI] [PubMed] [Google Scholar]
- Van Schooten KS, Duran L, Visschedijk M, Pijnappels M, Lord SR, Richardson J, et al. Catch the ruler: concurrent validity and test–retest reliability of the ReacStick measures of reaction time and inhibitory executive function in older people. Aging Clin Exp Res 2019; 31(8):1147–54. [DOI] [PubMed] [Google Scholar]
- Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc 1997;45:735–738. [DOI] [PubMed] [Google Scholar]
- Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci 2010;65 A:1338–1343. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Glidden D, Shiboski S, McCulloch C. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. 2nd ed. New York: Springer-Verlag; 2012. [Google Scholar]
- Weissenborn K, Rückert N, Hecker H, Manns MP. The Number Connection Tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol 1998;28:646–653. [DOI] [PubMed] [Google Scholar]


