Abstract
Background
Keratitis due to by filamentous fungi are not easy to diagnose thus causing a delay in correct therapy. There are many descriptions of keratitis due to Candida, Fusarium and Aspergillus genera. Subramaniula genus has only recently been reported to cause human infections and there are few descriptions of eye infections due to this filamentous fungus. Diagnosis of fungal keratitis is usually based on microscopic and cultural techniques of samples obtained by corneal swabbing or scraping. Considering the amount of time required to obtain culture results it is wise to use other diagnostic methods, such as molecular analyses. Therapeutic options against these fungi are limited by low tissue penetration in the eye due to ocular barriers. We describe the first case of S. asteroides human keratitis treated with isavuconazole.
Case presentation
We describe a rare case of fungal keratitis unresponsive to antimicrobial treatment in a 65-year-old male patient without a history of diabetes or immunological diseases. He reported that the onset of symptoms occurred during a long holiday in Cape Verde Island. Initial treatment with topical antibiotics associated to steroids were ineffective, allowing a slow clinical progression of disease to corneal perforation. On admission in our Hospital, slit-lamp examination of the left eye showed conjunctival congestion and hyperemia, a large inferior corneal ulceration with brown pigment, corneal edema, about 3 mm of hypopyon and irido-lenticular synechiae. The slow clinical progression of the disease to corneal perforation and the aspect of the ulcer were consistent with a mycotic etiology. Molecular methods used on fungal colonies isolated by Sabouraud’s dextrose agar cultures allowed the identification of Subramaniula asteroids from corneal scraping. Antimicrobial test showed a good susceptibility of this filamentous fungus to voriconazole and isavuconazole. Moreover, this fungal keratitis was successfully treated with isavuconazole, without side effects, observing a progressive clinical improvement.
Conclusions
Molecular methods may be useful for the identification of filamentous fungal keratitis on scraping samples thus shortening the time of diagnosis. Systemic therapy by isavuconazole could be useful to treat the filamentous fungal keratitis, reducing the possible adverse effects due to the use of voriconazole by systemic administration.
Keywords: Fungal keratitis, Subramaniula asteroides, Molecular identification, β-Tubulin gene, Isavuconazole, Case report
Background
Fungal keratitis (FK) is considered responsible for 30–60% of infectious keratitis in humans with serious damage to vision that often require corneal restroom surgeries, and sometimes, corneal transplantation, enucleation or evisceration [1–3].. They have become more frequent over the last four decades favoured by conditions such as diabetes, chronic ocular diseases, immuno-compromised patients, increased use of contact lens, topical steroids and antibacterial drugs, as well as an increase in surgical procedures [1, 2, 4–6].
FKs can be mainly caused by Candida, Fusarium and Aspergillus species [1, 3, 7]. Usually, FK is due to fungal access into the corneal stroma through a defect in the epithelium and trauma represents a frequent event. Diagnosis of fungal keratitis is commonly based on standard methods such as microscopic and cultural techniques of samples obtained by corneal swabbing. Identification of filamentous moulds is based mostly on microscopic examination of sporulating colonies. These methods could result inadequate in confirming a fungal agent, because fungi penetrate deeper layers of the cornea. Moreover, corneal culture is scarcely sensitive and requires long growth times, usually taking 1 to 35 days, especially when an antibiotic resistance test should be performed. In vivo confocal microscopy may be a helpful clinical adjunct but is still not available everywhere and lacks sufficient resolution to identify fungal hyphae [8, 9].
Molecular methods have been developed, demonstrating their usefulness as a rapid, highly sensitive and accurate diagnostic tool [10], though the latter are not easy and limited to only a few laboratories that are able to use these techniques. To avoid negative results due to deep infiltrates, corneal scrapings are recommended, and these samples should be analysed by traditional and molecular techniques. Direct-PCR on biological sample is highly sensitive and could be considered a good method for FK diagnosis as it reduces the time needed, taking 2 to 3 h. The obstacle to using PCR is that it requires very specialized equipment that may not always be available. Therefore, Kuo et al. [11] suggested implementing a dot hybridizaton assay, which is highly sensitive and can detect a wide variety of fungi. This assay used PCR to first amplify the highly conserved fungal 5.8S rRNA gene before adding it to immobilized oligonucleotide probes specific for fungi fixed to a nylon membrane. Detection by this dot assay, which could be seen with the naked eye, was reported to have been 100% sensitive and 96.7% specific for fungi identification.
FKs treatment is difficult because the diagnosis is often delayed and no antifungal drugs for topical eye use are commercially available; most used topical galenic antifungals often fail to achieve an adequate control of keratitis. Once fungi reach the deep stroma, they can penetrate into the anterior chamber and there is a high risk of endophthalmitis that can lead to ocular enucleation. One study suggested early deep anterior lamellar keratoplasty as a possible safe therapeutic approach to effectively eradicate fungal keratitis affecting the optic zone and poorly responsive to medical treatment, thus avoiding corneal transplant [12]. Therapeutic options against these fungi are limited by low tissue penetration in the eye due to ocular barriers. Most of the inside of the eye lacks blood vessels and the outer and inner blood-retinal barrier, which limits the influx of drugs into the retina and vitreous regions, requires the systemic administration of high doses to achieve therapeutic concentrations within the eye. Moreover, blinking and tear film turnover limits the residence time of a drug and the access to the deeper structures of the eye is hindered by corneal epithelium and stroma with varying lipophilicity.
We report a rare case of keratitis due to Subramaniula asteroides [13] in a patient without co-morbidities and with no reported eye trauma, identified by molecular methods and successfully treated with systemic isavuconazole associated to topic voriconazole.
Case presentation
A 65-year-old male patient without a history of diabetes or immunological diseases presented to the Emergency Unit of University Hospital S. Anna of Ferrara, Italy, with photophobia and ocular pain in the left eye. The patient had just returned from a long holiday in Cape Verde and he reported that the onset of symptoms had occurred about 20 days earlier with the sensation of a foreign body in the eye, without any previous ocular trauma. In a Medical Centre of Cape Verde Island, he was diagnosed with a keratitis in the left eye, initially treated with tobramycin 0.3% and dexamethasone 0.1% eye ointment for a week, then discontinued because of the worsening of his condition. Then oral amoxicillin 875 mg and clavulanic acid 125 mg tid and topical ofloxacin 0.3% eye drops qid were administered for the following 10 days. Since the symptoms were more severe and the visual acuity reduced, the patient decided to return to Italy.
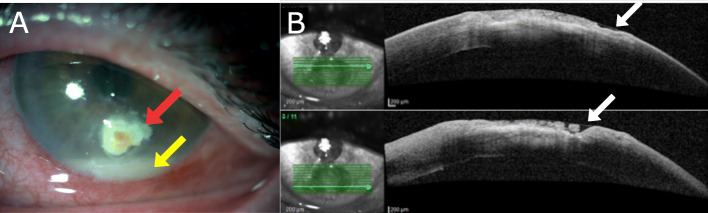
On admission to our Hospital the best correct visual acuity was 20/200 at distance in the left eye. Slit-lamp examination of the left eye showed conjunctival congestion and hyperemia, a large inferior corneal ulceration with brown pigment, corneal edema, about 3 mm of hypopyon and irido-lenticular synechiae (Fig. 1). The posterior segment was not visible, ultrasonography did not show any sign of intraocular infection. Corneal scrapings were obtained for microbiological analyses. Sample obtained by scraping was directly inoculated onto chocolate agar plate (Vacutest® KIMA) and sent to the Clinical Microbiology Laboratory of University Hospital of Ferrara and incubated at 37 °C with 5% of CO2 atmosphere.
Fig. 1.
a Slit lamp picture taken on initial presentation in our Hospital showing an inferior corneal ulceration with brown pigment (red arrow) and about 3 mm of hypopyon (yellow arrow) in the left eye. b Anterior segment optical coherence tomography (AS-OCT) showing the depth of the ulcer (white arrow)
Since the clinical picture was suggestive of a fungal keratitis, pending the outcome of microbiological tests, the patient immediately started treatment with fortified topical tobramycin 14 mg/ml qid, moxifloxacin 0.5% eye drops qid, cyclopentolate 1% tid and voriconazole 1% every 2 hours [14]. In addition, oral voriconazole was administered at the loading dose of 400 mg bid on days 1 and 2 and subsequently at the dose of 200 mg bid from day 3. Blood cell count and liver and kidney function blood tests were monitored every 15 days.
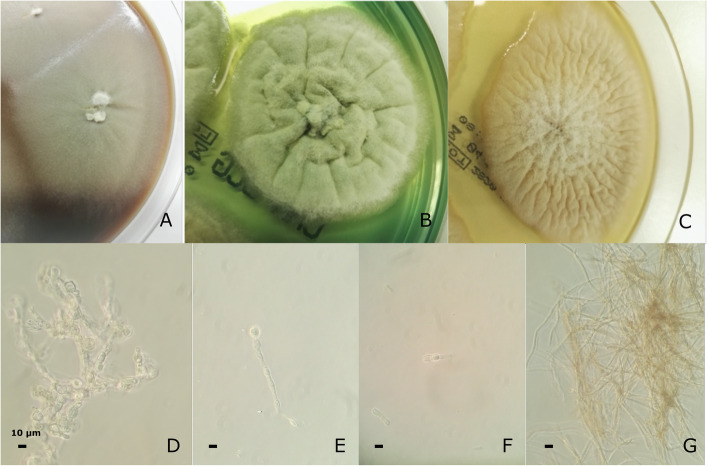
Forty-eight hours after corneal scraping fungi colonies were observed without any bacterial growth. Microscopic examination evidenced hyaline filamentous forms with clear mycelium. Growth was shown at 30 °C and 37 °C after 1 week of incubation on Chocolate Agar plate (CHOC), BCG agar plate, and Sabouraud Dextrose Agar plate (Fig. 2a,b,c). Microscopic observation showed hyphae broad, septate, hyaline, turning dark brown with age. Conidiophores phialidic, terminal or intercalary, short, hyaline, obclavate or cylindrical. Conidia hyaline, unicellular, obovoidal or ellipsoidal (Fig. 2d, e, f, g).
Fig. 2.
Macroscopic view colonies after one week of incubation on: a Chocolate Agar plate (CHOC); b BCG agar plate; c Sabouraud Dextrose Agar plate; d-g Microscopic view 0,25% Lugol stain. Microscopic description: hyphae broad, septate, hyaline, turning dark brown with age. Conidiophores phialidic, terminal or intercalary, short, hyaline, obclavate or cylindrical. Conidia hyaline, unicellular, obovoidal or ellipsoidal
Cultured samples on Sabouraud’s agar plates were sent to the Laboratory of Mycology at Policlinico “A. Gemelli” of the Catholic University of Rome for biomolecular analyses to identify the filamentous fungus obtained by cultures [15, 16].
Molecular methods allowed the identification of Subramaniula asteroides by fungal DNA extraction (using Plant extraction kit, Qiagen®), PCR amplification of β-tubulin gene (βtub) by specific primers and Sanger sequencing method of fungal gene [13, 17, 18]. PCR products were sequenced and compared in GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The βtub sequence shared 99% identity with the reference sequence (KP900696.1) for Subramaniula asteroides (Strain CBS 128679).
Antifungal susceptibility testing on the case strain was performed by broth micro-dilution according to CLSI methods for filamentous fungi (Sensititre™ YeastOne ITAMYUCC, Thermo Scientific) [10]. This test showed a low minimal inhibitory concentration (MIC) of S. asteroides for isavuconazole and the other triazoles, echinocandins and amphotericin B (Table 1).
Table 1.
Susceptibility of Subramaniula asteroides to antifungal drugs tested
| Antifungal drugs | MIC |
|---|---|
| Amphotericin B | 0.25 |
| Anidulafungin | 0.125 |
| Caspofungin | 0.5 |
| Micafungin | 0.125 |
| Voriconazole | 0.06 |
| Posaconazole | 0.06 |
| Itraconazole | 0.06 |
| Isavuconazole | 0.06 |
Antifungal susceptibility testing on the case strain was performed in triplicate by broth microdilution according to CLSI methods for filamentous fungi (Sensititre™ YeastOne ITAMYUCC, a modified panel YO10 with isavuconazole instead of 5-fluorocytosine, Thermo Scientific). Sensititre YeastOne was tested for efficacy in Aspergillus spp. and non-Aspergillus molds MIC valuation as previously described [19–21]. MIC values of the triazoles for Candida krusei ATCC 6258, A. fumigatus ATCC MYA-3626, and A. flavus ATCC 204304, which were used as quality control isolates, were all within the expected ranges (data not shown). The MICs of the antifungal drugs amphotericin B, anidulafungin, caspofungin, micafungin, voriconazole, posaconazole, itraconazole, isavuconazole were 0.25, 0.125, 0.5, 0.125, 0.06, 0.06, 0.06, 0.06 μg/mL, respectively (Table 1). Unfortunately, there are no clinical breakpoints available for Subramaniula asteroides. However, CLSI epidemiological cutoff values for antifungal susceptibility testing for Aspergillus spp. have recently been released [22]. Regarding these molds, the document shows ECV (μg/mL) of amphotericin B, caspofungin, isavuconazole, itraconazole, posaconazole, voriconazole.
Due to the scarce improvement of the keratitis and considering the inability to determine the ocular tissue concentration levels of the drugs in the patient, voriconazole was stopped and isavuconazole was started at the loading oral dose of 200 mg tid for the first 48 h, continuing with 200 mg per day. Ten days later, hypopyon had been reduced and symptoms were improved. Over the next 4 weeks the cornea had epithelized, and the inflammation was solved. Oral isavuconazole, topical antimicrobials and cyclopentolate were discontinued after 6 weeks.
The fungal corneal ulcer due to S. asteroides was successfully treated; the best correct visual acuity 2 months after treatment was 20/50 with a residual corneal scar (Fig. 3). Periodic eye tests showed a progressive improvement.
Fig. 3.
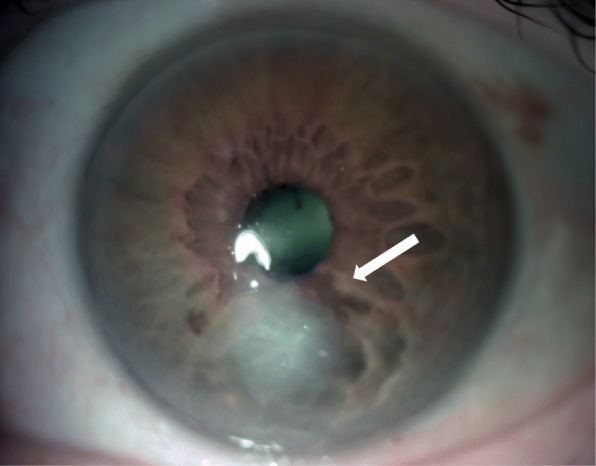
Two months after treatment non signs of active infection. A residual para-central corneal scar (white arrow) limits the visual acuity to 20/50
Discussion and conclusion
Filamentous fungi may be implicated in fungal keratitis in humans, which is particularly widespread in tropical countries. It is very important that a specific diagnosis be made as quickly as possible to ensure a prompt institution of adequate antifungal therapy.
Aspergillus spp. and Fusarium spp. are the most common species responsible for human keratitis. We report a first case of keratitis due to S. asteroides treated with isavuconazole. S. asteroides is a filamentous fungus present in the natural environment and strongly associated with traumatic eye injury or skin infections [13, 18]. Subramaniula asteroides belongs to the phylum Ascomycota, family Chaetomiaceae and genus Subramaniula. The members of this family are ascosporulating fungi. The Chaetomiaceae (Subramaniula asteroids, Subramaniula obscura and mainly Chaetomium anamorphosum) were considered opportunistic pathogens rarely causing skin infections and keratitis due to their ability to grow at high temperatures [18]. Apart from these reports, the role of this fungus as a human pathogen is largely ignored.
In our case, the slow clinical progression of disease to corneal perforation and the aspect of the ulcer were consistent with a mycotic etiology. The patient was initially treated with a topical antibiotic associated to a steroid. Because of the poor response, further treatments were prescribed with prolonged cycles of topical antibiotics and steroids without any benefits and consequent loss of the visual acuity. Corneal scraping performed at the Ophthalmologic Clinic allowed the identification of filamentous moulds by preliminary microscopic examination. There are findings that the delay of diagnosis could require surgical treatment up to the point of an eventual ocular evisceration, as described in Fusarium spp. keratitis [7].
Only molecular techniques allowed the exact identification of S. asteroides as responsible for keratitis. Previous reports of S. asteroids human infections are described in a case of endophthalmitis in a patient with non-insulin dependent diabetes mellitus after an eye trauma treated with topical amphotericin B deoxycholate in combination with fluconazole for 42 days [14], in a male patient with sinusitis without other disorders [14], and in a case of keratitis by S. asteroides after a corneal trauma [23].
This is to our knowledge the first case of S. asteroides human keratitis treated with isavuconazole. This drug was able to eradicate the infection of S. asteroides keratitis after a poor response to a first course of voriconazole. Prolonged topical voriconazole treatment in fungal keratitis can induce ocular surface dysplasia [24]. Moreover, patients who received voriconazole had a corneal perforation or required therapeutic penetrating keratoplasty [25]. Isavuconazole was well tolerated, confirming the data of less hepatobiliary, eye and skin disorders. The favourable outcome allows us to hypothesize that the ocular concentration of isavuconazole is sufficient to eradicate the fungal infection, as suggested by Schmitt-Hoffmann A. in a study about the tissue concentrations of isavuconazole in the eye and in the lacrimal glands of rats [26]. Unlike the other azoles, to our knowledge there are no data regarding the concentration of isavuconazole in the human eye.
Acknowledgements
The authors would like to thank the patient and all members of the study team, with a particular reference to the Clinical Pharmacy, the Laboratory of Clinical Microbiology of University ‘S. Anna’ Hospital of Ferrara and the Laboratory of Mycology of Policlinico Universitario “A. Gemelli” IRCCS.
Abbreviations
- tid
Ter in die
- qid
Quarter in die
- S. asteroides
Subramaniula asteroides
- MIC
Minimal inhibitory concentration
- Btub
β-tubulin gene
- CLSI
Clnical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, PA 19087, USA
Authors’ contributions
All authors contributed to the study conception and design. RC and MS performed the methodology. PP and CS performed the collection of ocular samples. AM followed the ophthalmological treatment. DS performed material preparation. GC performed microbiological cultures and microscopic identification and RT performed molecular analyses and antifungal susceptibility testing. RC was a major contributor in writing the manuscript and all authors commented on previous versions of the manuscript. Writing - review and editing were performed by RC and MS. All authors read and approved the final manuscript. This manuscript has not been published and is not under consideration for publication elsewhere.
Funding
All authors state, that for this work, there was no funding.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and additional files. The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable. This type of study does not require approval by our Ethics Committee.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rosario Cultrera, Email: ctr@unife.it.
Riccardo Torelli, Email: riccardo.torelli@policlinicogemelli.it.
Caterina Sarnicola, Email: c.sarnicola@hotmail.it.
Daniela Segala, Email: sgldnl@unife.it.
Andrea Mengoli, Email: a.mengoli@ospfe.it.
Giuseppina Chiaretto, Email: g.chiaretto@ospfe.it.
Paolo Perri, Email: paolo.perri@unife.it.
Maurizio Sanguinetti, Email: Maurizio.Sanguinetti@unicatt.it.
References
- 1.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19:210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira Dos Santos C, Kolwijck E, van Rooij J, Stoutenbeek R, Visser N, Cheng YY, et al. Epidemiology and Clinical Management of Fusarium keratitis in the Netherlands, 2005–2016. Front Cell Infect Microbiol. 2020;10:133. doi: 10.3389/fcimb.2020.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kredics L, Narendran V, Shobana CS, Vágvölgyi C, Manikandan P. Indo-Hungarian fungal keratitis working group. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses. 2015;58(4):243–260. doi: 10.1111/myc.12306. [DOI] [PubMed] [Google Scholar]
- 4.Peggy Chang HY, Chodosh J. Diagnostic and therapeutic considerations in fungal keratitis. Int Ophthalmol Clin. 2011;51(4):33–42. doi: 10.1097/IIO.0b013e31822d64dc. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz EH, Haresh A, Hammersmith KM, Eagle RC, Rapuano CJ, Cohen EJ. Alternaria and paecilomyces keratitis associated with soft contact lens wear. Cornea. 2010;29:564. doi: 10.1097/ICO.0b013e3181ba0cde. [DOI] [PubMed] [Google Scholar]
- 6.Hung N, Yeh LK, Ma DH, Lin HC, Tan HY, Chen HC, et al. Filamentous fungal keratitis in Taiwan: based on molecular diagnosis. Transl Vis Sci Technol. 2020;9(8):32. doi: 10.1167/tvst.9.8.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun S, Lui Q, Han L, Ma Q, He S, Li X, et al. Identification and characterization of Fusarium proliferatum, a new species of Fungi that cause fungal keratitis. Sci Rep. 2018;8:4859–4867. doi: 10.1038/s41598-018-23255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chidambaram JD, Prajna NV, Larke N, Macleod D, Srikanthi P, Lanjewar S, et al. In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br J Ophthalmol. 2017;101:1119–1123. doi: 10.1136/bjophthalmol-2016-309656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takezawa Y, Shiraishi A, Noda E, Hara Y, Yamaguchi M, Uno T, Ohashi Y. Effectiveness of in vivo confocal microscopy in detecting filamentous fungi during clinical course of fungal keratitis. Cornea. 2010;29:1346. doi: 10.1097/ICO.0b013e3181cd3c84. [DOI] [PubMed] [Google Scholar]
- 10.Embong Z, Hitam WHW, Yean CY, Rashid NHA, Kamarudin B, Abidin SKZ, et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008;8(1):7. doi: 10.1186/1471-2415-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo MT, Chang HC, Cheng CK, Chien CC, Fang PC, Chang TC. A highly sensitive method for molecular diagnosis of fungal keratitis: a dot hybridization assay. Ophthalmology. 2012;119:2434–2442. doi: 10.1016/j.ophtha.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Sabatino F, Sarnicola E, Sarnicola C, Tosi GM, Perri P, Sarnicola V. Early deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment. Eye. 2017;31:1639–1646. doi: 10.1038/eye.2017.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XW, Houbraken J, Groenewald JZ, Meijer M, Andersen B, Nielsen KF, et al. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud Mycol. 2016;84:145–224. doi: 10.1016/j.simyco.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun CQ, Lalitha P, Prajna NV, Karpagam R, Geetha M, O'Brien KS, et al. Mycotic ulcer treatment trial group. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology. 2014;121:1495–1500. doi: 10.1016/j.ophtha.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanguinetti M, Posteraro B. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2017;55:369–379. doi: 10.1128/JCM.01640-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Zhai H, Yuan Q, Sun S, Liu T, Xie L. Rapid and sensitive diagnosis of fungal keratitis with direct PCR without template DNA extraction. Clin Microbiol Infect. 2014;20:O776–O782. doi: 10.1111/1469-0691.12571. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Liu H, Luo Y, Zhou S, An L, Wang C, et al. Molecular evolution and functional divergence of tubulin superfamily in the fungal tree of life. Sci Rep. 2014;4:6746. doi: 10.1038/srep06746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed SA, Khan Z, Wang X, Moussa TAA, Al-Zahrani HS, Almaghrabi OA, et al. Chaetomium-like fungi causing opportunistic infections in humans: a possible role for extremotolerance. Fungal Divers. 2016;76:11–26. doi: 10.1007/s13225-015-0338-5. [DOI] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Wayne, PA: CLSI Document M38, Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 20.Mello E, Posteraro B, Vella A, De Carolis E, Torelli R, D’Inzeo T, Verweij PE, Sanguinetti M. Susceptibility testing of common and uncommon Aspergillus species against posaconazole and other mold-active antifungal azoles using the Sensititre method. Antimicrob Agents Chemother. 2017;61(6):e00168–e00117. doi: 10.1128/AAC.00168-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Wang H, Hou X, Huang JJ, Wang PC, Xu YC. Identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry and antifungal susceptibility testing of non-Aspergillus molds. Front Microbiol. 2020;11:922. doi: 10.3389/fmicb.2020.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute (CLSI) Epidemiological Cutoff Values for antifungal susceptibility testing of yeasts. Wayne, PA: CLSI Document M59; 2018. [Google Scholar]
- 23.Vinod Mootha V, Shahinpoor P, Sutton DA, Xin L, Najafzadeh MJ, de Hoog GS. Identification problems with sterile fungi, illustrated by a keratitis due to a non-sporulating chaetomium-like species. Med Mycol. 2012;50:361–367. doi: 10.3109/13693786.2011.611179. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal M, Gayatri S, Kumar SK, Rajagopal R. Voriconazole Induced Ocular Surface Dysplasia - Report of Two Cases. Ocul Immunol Inflamm. 2020;1-5. 10.1080/09273948.2020.1781903. [DOI] [PubMed]
- 25.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt-Hoffmann AH, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, Spickermann J, Schneidkraut MJ. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother. 2017;61:e01292–e01217. doi: 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and additional files. The datasets used during the current study are available from the corresponding author on reasonable request.