Abstract
Aims: Arterial stiffness increases with both advancing age and chronic kidney disease (CKD) and may contribute to kidney function decline, but evidence is inconsistent. We hypothesized that greater baseline arterial stiffness (assessed as pulse pressure (PP) and carotid-femoral pulse-wave velocity CFPWV)) was independently associated with kidney disease progression over the follow-up period (3.8 years) in the Systolic Blood Pressure Intervention Trial (SPRINT). Materials and methods: 8,815 SPRINT participants were included in the analysis of PP. 592 adults who participated in a SPRINT ancillary study that measured CFPWV were included in subgroup analyses. Cox proportional hazards analysis was used to examine the association between PP and time to kidney disease progression endpoints: (A) incident estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 in non-CKD participants at baseline; (B) 50% decline in eGFR, initiation of dialysis, or transplant in those with baseline CKD. Mixed model analyses examined the association of baseline PP/CFPWV with follow-up eGFR. Results and conclusion: Mean ± SD age was 68 ± 10 years, baseline PP was 62 ± 14 mmHg, and CFPWV was 10.8 ± 2.7 m/s. In the fully adjusted model, PP ≥ median was associated with an increased hazard of kidney disease progression endpoints (HR: 1.93 (1.43 – 2.61)). The association remained significant in individuals without (2.05 (1.47 – 2.87)) but not with baseline CKD (1.28 (0.55 – 2.65)). In fully adjusted models, higher baseline PP associated with eGFR decline (p < 0.0001 (all, CKD, non-CKD)), but baseline CFPWV did not. Among older adults at high risk for cardiovascular events, baseline PP was associated with kidney disease progression.
Keywords: aging, chronic kidney disease, clinical epidemiology, renal function decline, pulse wave velocity
Introduction
Arterial stiffness increases both with advancing age [1, 2] and as kidney function declines (chronic kidney disease (CKD)) [3, 4, 5] and is an important independent predictor of incident cardiovascular events and mortality [6, 7, 8, 9]. Aortic stiffness may also contribute to reduced kidney function (lower estimated glomerular filtration rate (eGFR)) by transferring excessive flow pulsatility to a susceptible kidney microvasculature, leading to dynamic constriction and/or vessel loss [10].
Evidence whether increased arterial stiffness is associated either cross-sectionally with kidney function [10, 11] or longitudinally with decline in kidney function is inconsistent. Higher baseline arterial stiffness was independently associated with incident CKD (eGFR < 60 mL/min/1.73m2) [12, 13] and eGFR decline [14, 15] in several cohorts of community-dwelling adults; however, evidence is not consistent across studies [13, 16] and analyses have included individuals with diabetes mellitus, who likely have greater baseline arterial stiffness [17]. Evidence regarding the association of arterial stiffness with kidney function decline in individuals with prevalent CKD (reduced eGFR) is also inconsistent [18, 19, 20, 21, 22].
Accordingly, the purpose of the present study was to test the hypothesis that greater baseline arterial stiffness was independently associated with decline in kidney function over the follow-up period in older adults without baseline diabetes mellitus who participated in the recently completed Systolic Blood Pressure Intervention Trial (SPRINT). We hypothesized that greater baseline arterial stiffness, as measured in the entire cohort by pulse pressure (PP) (a surrogate index of arterial stiffness) [23] and by carotid-femoral pulse-wave velocity (CFPWV) in a subgroup who participated in an ancillary study [24], would be associated with kidney disease progression over the follow-up period. We also explored any differences in these associations in individuals with and without baseline CKD.
Materials and methods
Study design
SPRINT was a multi-center, randomized, controlled trial in adults at high risk for cardiovascular events comparing standard (target systolic blood pressure (SBP) of < 140 mmHg) to intensive (target SBP of < 120 mmHg) blood pressure control, with a primary composite endpoint of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes, as described previously [25, 26]. The protocol for the trial is publically available [27]. Briefly, 9,361 adults ≥ 50 years of age with SBP of 130 – 180 mmHg and increased risk of cardiovascular events (but free from diabetes mellitus and prior stroke) were recruited from 102 clinical sites between November 2010 and March 2013. Detailed inclusion and exclusion criteria have been described previously [25].
For the present analysis, participants were classified based on the presence or absence of baseline CKD, defined in SPRINT as a baseline eGFR < 60 mL/min/1.73m2 using the four-variable Modification of Diet in Renal Disease (MDRD) Study equation [25]. Of the 9,361 participants with baseline data, 38 were missing information on kidney disease progression endpoints (defined below), and 493 were missing one or more included covariates, leaving a total cohort of 8,815 for analysis of the association of PP with kidney disease endpoints. The most frequently missed covariate was urinary albumin-to-creatinine ratio (ACR) (n = 422).
652 SPRINT participants from 11 clinical sites enrolled in an ancillary study that measured CFPWV, as described in detail previously [24]. Due to the limited number of kidney disease progression endpoints in this ancillary study (n = 18), the dependent variable for this analysis was instead defined as change in eGFR over the follow-up period. Of the 652 ancillary study participants, 61 were missing one or more covariates, leaving a total cohort of 591 for analysis of the association of CFPWV with change in eGFR. The most frequently missing covariate was urinary ACR (n = 33).
All participants provided written informed consent. This study was approved by the investigational review boards at the participating centers and was conducted in adherence with the Declaration of Helsinki.
Study variables
Exposure variables
PP, a surrogate of arterial stiffness [23], was calculated as SBP – diastolic blood pressure (DBP). There is a significant correlation between PP and CFPWV in the SPRINT CFPWV ancillary study (R = 0.25, p < 0.0001, n = 652) [24]. Blood pressure was measured during the baseline randomization study visit as the mean of three office blood pressure measurements obtained in the seated position using an automated device (Omron Healthcare, Lake Forest, IL, USA) after a 5-minute rest period, as described in detail previously [26, 27, 28].
CFPWV was measured using the SphygmoCor CPV system device with software version 9.0 (AtCor Medical, Itasca, IL, USA) following a standard protocol, as described in detail previously [24].
Outcome variables
In analyses where PP was the predictor, the primary outcome was time to achieve a kidney disease progression endpoint. For participants with baseline eGFR < 60 mL/min/1.73m2 (baseline CKD), the kidney disease progression endpoint was a composite of decrease in the eGFR of 50% or more (confirmed by a subsequent laboratory test at least 90 days apart) or the development of end-stage renal disease (ESRD) requiring long-term dialysis or kidney transplantation, as defined previously in the SPRINT study [25]. The non-CKD group included individuals with a baseline eGFR ≥ 60 mL/min/1.73m2 (as well as individuals with unknown CKD status at baseline) [25]. The kidney disease progression endpoint in this group was incident CKD, defined as a decrease in eGFR of ≥ 30% to a value of < 60 mL/min/1.73m2 [25].
In analyses where CFPWV was the predictor, eGFR over time was the outcome variable, using a random intercept, random slope mixed model analysis using all available eGFR measurements. This analysis was also performed as a secondary endpoint with PP as the predictor variable.
Covariates and stratification variable
Baseline characteristics potentially related to arterial stiffness and kidney function decline, all measured at baseline, were selected a priori as covariates for this analysis. Baseline questionnaires and interviews were administered by trained clinical staff. Race and smoking status were determined by self-report. History of cardiovascular disease (CVD) and heart failure were determined by a detailed medical history collected at screening [25, 26].
Body-mass index (BMI) was calculated as weight in kilograms divided by height in m2. Urinary ACR was calculated as urinary albumin/urinary creatinine (mg/g), using a spot urine. Number of anti-hypertensive agents at baseline (prior to randomization) was determined as described previously [25].
Statistical analyses
The association of baseline PP and kidney disease progression endpoints was analyzed using Cox proportional hazards analysis. PP was considered as both a continuous variable as well as dichotomized as above and below the median PP. The association between CFPWV (dichotomized by median baseline CFPWV) and change in eGFR over time (interaction of baseline CFPWV × time as a predictor) was analyzed using a mixed model with random intercept and random slopes incorporating all available measurements of eGFR. Natural log-transformed eGFR values were used in these analyses.
In each analysis, the initial model was unadjusted, with subsequent multivariable models adjusting for age, sex, race, and randomized treatment arm (model 1), model 1 plus CVD, heart failure, smoking, BMI, baseline eGFR (except for mixed model), and urinary ACR (model 2), and model 2 plus number of antihypertensive agents at baseline (model 3). Finally, mean arterial pressure (MAP) and heart rate were added (model 4). Of note, PP and MAP and were weakly correlated, thus unlikely to be collinear. We also evaluated the interaction of PP with sex and race. It was decided a priori to perform stratified analyses according to CKD and non-CKD groups regardless of the interaction term, as kidney disease progression may differ in individuals with and without baseline CKD.
As a secondary analysis, the association between PP (dichotomized by median baseline PP) and change in eGFR over time (interaction of baseline PP × time as a predictor) was analyzed using a mixed model with random intercept and random slopes incorporating all available measurements of eGFR. Natural log-transformed eGFR values were used in these analyses.
Indices of kidney function decline and covariates at baseline were summarized above and below the median PP/CFPWV, and are presented as mean (standard deviation) or median (interquartile range) for continuous variables and n (%) for categorical variables. Comparisons between PP/CFPWV groups were made using a χ2-test for categorical data and an independent samples t-test for continuous variables. Non-normally distributed variables were log-transformed (urinary ACR) or compared between groups using the Wilcoxon rank sum nonparametric test (eGFR slope). Two-tailed values of p < 0.05 were considered statistically significant for all analyses. All statistical analyses were performed using SAS version 9.4.
Results
Pulse pressure
Among 8,815 SPRINT participants with complete data for PP analyses, the mean ± SD age was 68 ± 10 years and 61% (n = 5,049) were White. The mean PP was 62 ± 14 mmHg, and mean baseline eGFR was 72 ± 21 mL/min/1.73m2. Individuals with a higher PP were more likely to be older, female, White, have prevalent CVD, heart failure, and CKD, have higher MAP and urinary ACR, have a lower baseline BMI, eGFR and heart rate, use more antihypertensive agents, and less likely to smoke (Table 1). The baseline participant characteristics broken down into subgroups with and without baseline CKD are shown in Online Supplemental Table 1 and 2. There were 243 (2.6%) kidney disease progression endpoints over a median follow-up of 3.8 years. Both the number of kidney disease progression endpoints and the annual decline in eGFR were greater in individuals with a higher PP.
Table 1. Baseline characteristics of study participants in the entire cohort by baseline pulse pressure.
| Variable | Baseline PP below the median (< 60 mmHg) (n = 4,249) |
Baseline PP above the median (≥ 60 mmHg) (n = 4,566) |
p-value |
|---|---|---|---|
| Age, y | 64 ± 8 | 72 ± 9 | < 0.0001 |
| Sex, n (%) male | 2,940 (69%) | 2,765 (61%) | < 0.0001 |
| Race, n (%) white | 2,245 (53%) | 2,804 (61%) | < 0.0001 |
| Study randomization, n (%) intensive treatment | 2,121 (50%) | 2,300 (50%) | 0.67 |
| Prevalent CVD, n (%) | 751 (18%) | 1,040 (23%) | < 0.0001 |
| Prevalent heart failure, n (%) | 129 (3%) | 189 (4%) | 0.006 |
| Prevalent CKD, n (%) | 1,050 (25%) | 1,481 (32%) | < 0.0001 |
| Smoking status, n (%) | < 0.0001 | ||
| Never smoked | 1,848 (43%) | 2,027 (44%) | |
| Former smoker | 1,675 (39%) | 2,084 (46%) | |
| Current smoker | 730 (17%) | 455 (10%) | |
| MAP, mmHg | 98.0 ± 10.9 | 99.3 ± 11.9 | < 0.0001 |
| Body mass index, kg/m2 | 30.8 ± 5.9 | 29.0 ± 5.5 | < 0.0001 |
| eGFR, mL/min/1.73m2 | 74 ± 21 | 70 ± 21 | < 0.0001 |
| Urinary albumin to creatinine ratio | 7.9 (5.0, 16.6) | 11.4 (6.5, 26.9) | < 0.0001 |
| Heart rate, beats per minute | 71 ± 12 | 66 ± 11 | < 0.001 |
| Antihypertensive agents, no./patient | < 0.0001 | ||
| 0 | 431 (10%) | 434 (9%) | |
| 1 | 1,503 (33%) | 1,426 (30%) | |
| 2 | 1,545 (34%) | 1,631 (34%) | |
| 3 | 793 (18%) | 1,061 (22%) | |
| 4 | 222 (5%) | 261 (5%) | |
| Pulse pressure, mmHg | 50 ± 7 | 73 ± 11 | < 0.0001 |
| Kidney disease progression endpoints, n (%) | 75 (1.8%) | 159 (3.5%) | < 0.0001 |
| eGFR slope, mL/min/1.73m2 per year | –0.3 (–2.2, 1.6) | –0.8 (–3.1, 1.1) | < 0.0001 |
Data are mean ± SD, median (IQR), or n (%). PP = pulse pressure; CVD = cardiovascular disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate (Modification of Diet in Renal Disease equation); MAP = mean arterial pressure. Kidney disease progression endpoints are defined as incident CKD (a decrease in eGFR of > 30% to a value of < 60 mL/min/1.73m2) in non-CKD participants at baseline and a) 50% decline in eGFR, b) initiation of dialysis, or c) transplant in those participants with CKD at baseline.
Pulse pressure and kidney disease progression endpoints
In both unadjusted and adjusted analyses, higher PP (above the median; ≥ 60 mmHg) was associated with an increased hazard of a kidney disease progression endpoint compared to the reference group (PP below the median; < 60 mmHg) in all participants (Table 2) (Figure 1). This association was only slightly attenuated in the final model (model 4) adjusted for baseline MAP and heart rate. Results were similar when PP was considered as a continuous variable. The interaction between baseline CKD and PP was significant in model 4 (p = 0.004). The association remained significant in individuals without baseline CKD, but not individuals with baseline CKD; however, the total sample size, as well as number of events (n = 35, 1.4%), were smaller in the latter group. The interaction terms for PP with sex and PP with race were not statistically significant (p ≥ 0.13).
Table 2. Associations (hazard ratio (95% CI)) of baseline arterial stiffness (pulse pressure) with kidney disease progression.
| All participants | Baseline PP below the median (< 60 mmHg) (n = 4,249) | Baseline PP above the median (≥ 60 mmHg) (n = 4,566) | Continuous (per mmHg higher baseline PP) (n = 8,815) |
|---|---|---|---|
| Unadjusted | Ref | 2.03 (1.54, 2.67) | 1.03 (1.02, 1.04) |
| Model 1 | Ref | 2.05 (1.53, 2.75) | 1.03 (1.02, 1.04) |
| Model 2 | Ref | 2.09 (1.56, 2.81) | 1.03 (1.02, 1.04) |
| Model 3 | Ref | 2.08 (1.55, 2.80) | 1.03 (1.02, 1.04) |
| Model 4 | Ref | 1.93 (1.43, 2.61) | 1.03 (1.02, 1.04) |
| Prevalent CKD | Baseline PP below the median (< 63 mmHg) (n = 1,050) | Baseline PP above the median (≥ 63 mmHg) (n = 1,481) | Continuous (per mmHg higher baseline PP) (n = 2,531) |
| Unadjusted | Ref | 0.97 (0.50, 1.89) | 1.00 (0.98, 1.03) |
| Model 1 | Ref | 1.45 (0.70, 3.01) | 1.02 (1.00, 1.04) |
| Model 2 | Ref | 1.21 (0.55, 2.65) | 1.01 (0.99, 1.04) |
| Model 3 | Ref | 1.21 (0.55, 2.65) | 1.01 (0.99, 1.04) |
| Model 4 | Ref | 1.28 (0.55, 2.65) | 1.01 (0.99, 1.04) |
| Non-CKD | Baseline PP below the median (< 59 mmHg) (n = 3,199) | Baseline PP above the median (≥ 59 mmHg) (n = 3,085) | Continuous (per mmHg higher baseline in PP) (n = 6,284) |
| Unadjusted | Ref | 2.48 (1.83, 3.35) | 1.04 (1.03, 1.05) |
| Model 1 | Ref | 2.15 (1.56, 2.97) | 1.04 (1.03, 1.05) |
| Model 2 | Ref | 2.25 (1.62, 3.11) | 1.04 (1.03, 1.05) |
| Model 3 | Ref | 2.24 (1.62, 3.11) | 1.04 (1.03, 1.05) |
| Model 4 | Ref | 2.05 (1.47, 2.87) | 1.04 (1.03, 1.05) |
PP = pulse-pressure; CKD = chronic kidney disease; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; ACR = albumin to creatinine ratio; MAP = mean arterial pressure. Kidney disease progression endpoints are defined as incident CKD (a decrease in eGFR of > 30% to a value of < 60 mL/min/1.73m2) in non-CKD participants at baseline and a) 50% decline in eGFR, b) initiation of dialysis, or c) transplant in those participants with CKD at baseline. Model 1: adjusted for age, sex, race, and randomized treatment arm; Model 2: adjusted for covariates in model 1 plus CVD, heart failure, smoking, body mass index, eGFR, urine ACR; Model 3: adjusted for covariates in model 2 plus number of antihypertensive medications at baseline; Model 4: adjusted for covariates in model 3 plus MAP and heart rate.
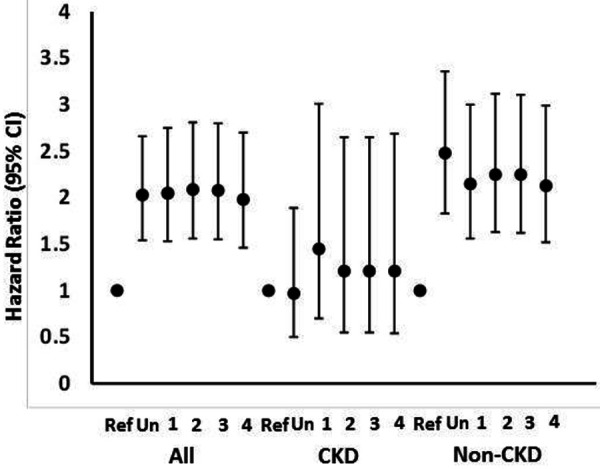
Figure 1. Hazard ratios (95% confidence intervals) for the association of pulse pressure above the median (≥ 60 mmHg vs. Ref (< 60 mmHg)) with kidney disease progression endpoints. Kidney disease progression endpoints are defined as incident chronic kidney disease (CKD) (a decrease in estimated glomerular filtration rate (eGFR) of > 30% to a value of < 60 mL/min/1.73m2) in non-CKD participants at baseline and a) 50% decline in eGFR, b) initiation of dialysis, or c) transplant in those participants with CKD at baseline. Models are unadjusted (Un), adjusted for age, sex, race, and randomized treatment arm [1], covariates in model 1 plus cardiovascular disease, heart failure, smoking, body mass index, eGFR, urine albumin-to-creatinine ratio [2]; covariates in model 2 plus number of antihypertensive medications at baseline [3], and covariates in model 3 plus mean arterial pressure and heart rate [4].
Carotid-femoral pulse-wave velocity
591 SPRINT PWV ancillary study participants were included in the cross-sectional analysis with CFPWV as the predictor variable. Among these participants, the mean ± SD age was 72 ± 10 years, and 67% were White. Individuals with a higher CFPWV were more likely to be older and have a higher MAP (Table 3). The mean CFPWV was 10.8 ± 2.7 m/s. Baseline participant characteristics by CKD status are shown in Online Supplemental Table 3 and 4. There were 18 kidney disease progression endpoints in this ancillary study, too few to evaluate as the dependent variable.
Table 3. Baseline characteristics of study participants in the pulse-wave velocity ancillary study by baseline aortic pulse-wave velocity.
| Variable | Baseline CFPWV below the median (< 10.6 m/sec) (n = 276) |
Baseline CFPWV above the median (≥ 10.6 m/sec) (n = 315) |
p-value |
|---|---|---|---|
| Age, y | 70 ± 9 | 74 ± 9 | < 0.0001 |
| Sex, n (%) male | 192 (61%) | 1657 (60%) | 0.77 |
| Race, n (%) white | 191 (69%) | 204 (65%) | 0.25 |
| Study randomization, n (%) intensive treatment | 137 (50%) | 158 (50%) | 0.90 |
| Prevalent CVD, n (%) | 32 (12%) | 48 (15%) | 0.20 |
| Prevalent CHF, n (%) | 5 (2%) | 6 (2%) | 0.93 |
| Prevalent CKD, n (%) | 106 (34%) | 105 (38%) | 0.27 |
| Smoking status, n (%) | 0.26 | ||
| Never smoked | 131 (48%) | 141 (45%) | |
| Former smoker | 130 (47%) | 146 (46%) | |
| Current smoker | 15 (5%) | 28 (9%) | |
| MAP, mm Hg | 95.8 ± 11.5 | 98.3 ± 11.3 | 0.008 |
| Body mass index, kg/m2 | 28.3 ± 5.1 | 27.7 ± 5.1 | 0.21 |
| eGFR, mL/min/1.73m2 | 69 ± 21 | 66 ± 20 | 0.23 |
| Urinary albumin to creatinine ratio | 9.9 (6.0, 25.2) | 12.3 (7.1, 28.3) | 0.05 |
| Heart rate, beats per minute | 66 ± 12 | 67 ± 20 | 0.83 |
| Antihypertensive agents, no./patient | 0.19 | ||
| 0 | 20 (7%) | 28 (10%) | |
| 1 | 111 (39%) | 104 (36%) | |
| 2 | 84 (30%) | 99 (34%) | |
| 3 | 45 (16%) | 40 (14%) | |
| 4 | 24 (9%) | 21 (7%) | |
| CFPWV, m/s | 8.8 ± 1.3 | 13.1 ± 2.0 | < 0.0001 |
| Pulse pressure, mm Hg | 63 ± 14 | 69 ±15 | < 0.0001 |
| eGFR slope, mL/min/1.73m2 per year | –0.3 (–2.6, 1.3) | –0.5 (–2.6, 1.4) | 0.82 |
Data are mean ± SD, median (IQR), or n (%). CFPWV = carotid-femoral pulse-wave velocity; CVD = cardiovascular disease; CKD = chronic kidney disease; eGFR= estimated glomerular filtration rate (Modification of Diet in Renal Disease equation); MAP = mean arterial pressure.
Carotid-femoral pulse-wave velocity and decline in estimated glomerular filtration rate
In fully adjusted analyses (model 4) including all participants, higher CFPWV was not associated with decline in eGFR, incorporating all available time points where eGFR was measured (baseline CFPWV × time interaction p = 0.34). Compared to baseline CFPWV above the median, annual change in lneGFR in individuals with baseline CFPWV below the median was 0.005 (95% confidence interval: –0.005 to 0.015) (model 4). Stratified analyses were performed, as decided a priori, according to CKD and non-CKD groups. The association remained non-significant in individuals with and without baseline CKD. Compared to baseline CFPWV above the median, annual change in lneGFR in individuals with baseline CFPWV below the median in individuals with baseline CKD was 0.0020 (–0.009 to 0.013) (baseline CFPWV × time interaction p = 0.72) (model 4). Compared to baseline CFPWV above the median, annual change in lneGFR in individuals without baseline CFPWV below the median in individuals without baseline CKD was 0.011 (–0.009 to 0.030) (baseline CFPWV × time interaction p = 0.28) (model 4).
Relation between pulse pressure and decline in estimated glomerular filtration rate
As a secondary endpoint, we also considered the association of PP with change in eGFR over time. PP was associated with a greater decline in eGFR, in the entire cohort in the fully adjusted model (model 4; baseline PP × time interaction p < 0.0001). Compared to baseline PP below the median, annual change in lneGFR in individuals with baseline PP above the median was –0.011 (–0.014 to –0.009). Again, stratified analyses were performed, as decided a priori, according to CKD and non-CKD groups. In individuals with baseline CKD, compared to baseline PP below the median, annual change in lneGFR in individuals with baseline PP above the median was –0.014 (–0.020 to –0.009) (baseline PP × time interaction p < 0.0001) (model 4). In individuals without baseline CKD, compared to baseline PP below the median, annual change in lneGFR in individuals with baseline PP above the median was –0.010 (–0.013 to –0.008) (baseline PP × time interaction p < 0.0001) (model 4).
Discussion
In older individuals with hypertension and at high risk for cardiovascular events, higher arterial stiffness, as measured by PP, was associated with an increased hazard of a kidney disease progression over a median follow-up of 3.8 years. This association was significant in individuals without baseline CKD, but not in those with baseline CKD. Of note, the sample size and number of events was smaller for the CKD group. However, both individuals with and without baseline CKD demonstrated an association of higher baseline PP with decline in eGFR over time. In contrast, we failed to demonstrate an association using the gold-standard measurement of CFPWV as the index of arterial stiffness; notably, the sample size was much smaller in this ancillary study.
Evidence to date regarding the association of arterial stiffness with kidney function and subsequent decline in kidney function has been inconsistent. In cohorts of community-based adults, arterial stiffness (measured by CFPWV or PP), has been both independently associated [10] and not associated with eGFR cross-sectionally [11, 16]. Longitudinally, greater baseline CFPWV has predicted eGFR decline or incident CKD in some, but not other populations of community-based adults [12, 13, 14, 16]. Previous analyses with PP as the predictor variable have been similarly inconsistent [12, 13, 14, 16]. Notably, these previous cohorts have all included individuals with diabetes mellitus, while SPRINT participants were free from diabetes at baseline. In relatively small studies of individuals with prevalent CKD, greater baseline CFPWV has been associated with kidney disease progression in some [20, 29], but not other [18] cohorts. Recently, baseline CFPWV was associated with kidney disease progression in a large number of participants (n = 2,795) with prevalent CKD in the Chronic Renal Insufficiency Cohort [22]. Several previous analyses have also found no independent association between baseline PP and decline in kidney function in individuals with prevalent CKD [18, 20].
In participants in the SPRINT study, we found an independent association of greater baseline PP with kidney disease progression endpoints, defined as incident CKD (a decrease in eGFR of > 30% to a value of < 60 mL/min/1.73m2) in non-CKD participants at baseline, and either a 50% decline in eGFR, initiation of dialysis, or transplant in participants with CKD at baseline. There was a significant interaction term between PP and baseline CKD status in this analysis, such that an association remained for individuals without but not with baseline CKD. However, when change in eGFR over time was considered as a secondary endpoint, the association of PP with this endpoint was significant in both individuals with and without baseline CKD.
Mechanistically, with stiffening of the large elastic arteries, the microvasculature is exposed to highly pulsatile pressure and flow, promoting microvascular damage [30]. Excessive pulsatile pressure in glomerular capillaries can promote reductions in kidney function, as the kidney is a high-flow, low-impedance organ that is particularly susceptible to pulsatile damage [31]. Both dynamic constriction and vessel loss may contribute to reductions in eGFR [10]. This is supported by a mediation analysis from the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES), which demonstrated that the cross-sectional association of higher CFPWV with lower eGFR was mediated in part by increased pulsatility index, lower arterial volume in the cortex, and higher kidney vascular resistance, suggesting an increase transmission of pulsatile energy to the kidneys [10].
Notable strengths of this study include a large sample size available for the PP analyses and including endpoints and a large number of important covariates in the setting of a clinical trial. Additionally, we separately considered progression in individuals with and without baseline CKD, as well as two predictor variables representing arterial stiffness. Our findings are notable as they represent the largest study to date examining the association of markers of arterial stiffness with kidney function decline in a population both with and without baseline CKD.
There are also important limitations of this analysis. The results are associative, and residual confounding may exist, including variables that were not assessed, such as level of inflammation. There were not enough kidney disease events to consider this endpoint in the analyses with CFPWV as the predictor variable because of smaller sample size. The SPRINT cohort did not include younger adults with less CVD burden, nor did it include individuals with stroke or prevalent diabetes; thus, these results may not apply to these populations. Overall, participants who were included in SPRINT may not resemble the broader population of older adults with or without CKD, thus limiting the external validity of the results. Additionally, we were not able to consider individual classes of antihypertensive medications as covariates.
In conclusion, among adults at high risk for cardiovascular events without history of diabetes or stroke, PP was associated with kidney disease progression endpoints as well as change in eGFR over time. The latter association remained significant in those with and without baseline CKD, while the former association was observed in the baseline non-CKD subgroup. In contrast, CFPWV was not associated with decline in eGFR; however, power was limited for this analysis. Overall, these results are consistent with the hypothesis that arterial stiffness may contribute to kidney disease progression.
Acknowledgment
The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm.
Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134 & UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1TR000002, University of Florida: UL1TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
The PWV ancillary study was supported by NHLBI (Mark Supiano: R01HL107241). Kristen Nowak is supported by NIDDK (K01DK103678). Anna Jovanovich is supported by Veterans Administration CDA 5IK2CX001030-03.
Conflict of interest
DEW has participated in advisory boards for Janssen and Akebia and has represented Dialysis Clinic Inc. in advisory boards for Keryx and Relypsa. AJ receives study drug from Shire.
Supplemental material
References
- 1. AlGhatrif M Strait JB Morrell CH Canepa M Wright J Elango P Scuteri A Najjar SS Ferrucci L Lakatta EG Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013; 62: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavalcante JL Lima JA Redheuil A Al-Mallah MH Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011; 57: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 3. Wang MC Tsai WC Chen JY Huang JJ Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005; 45: 494–501. [DOI] [PubMed] [Google Scholar]
- 4. Shinohara K Shoji T Tsujimoto Y Kimoto E Tahara H Koyama H Emoto M Ishimura E Miki T Tabata T Nishizawa Y Arterial stiffness in predialysis patients with uremia. Kidney Int. 2004; 65: 936–943. [DOI] [PubMed] [Google Scholar]
- 5. Briet M Bozec E Laurent S Fassot C London GM Jacquot C Froissart M Houillier P Boutouyrie P Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006; 69: 350–357. [DOI] [PubMed] [Google Scholar]
- 6. Karras A Haymann JP Bozec E Metzger M Jacquot C Maruani G Houillier P Froissart M Stengel B Guardiola P Laurent S Boutouyrie P Briet M Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012; 60: 1451–1457. [DOI] [PubMed] [Google Scholar]
- 7. Pannier B Guérin AP Marchais SJ Safar ME London GM Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005; 45: 592–596. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell GF Hwang SJ Vasan RS Larson MG Pencina MJ Hamburg NM Vita JA Levy D Benjamin EJ Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010; 121: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben-Shlomo Y Spears M Boustred C May M Anderson SG Benjamin EJ Boutouyrie P Cameron J Chen CH Cruickshank JK Hwang SJ Lakatta EG Laurent S Maldonado J Mitchell GF Najjar SS Newman AB Ohishi M Pannier B Pereira T Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014; 63: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woodard T Sigurdsson S Gotal JD Torjesen AA Inker LA Aspelund T Eiriksdottir G Gudnason V Harris TB Launer LJ Levey AS Mitchell GF Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol. 2015; 26: 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michener KH Mitchell GF Noubary F Huang N Harris T Andresdottir MB Palsson R Gudnason V Levey AS Aortic stiffness and kidney disease in an elderly population. Am J Nephrol. 2015; 41: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedaghat S Mattace-Raso FU Hoorn EJ Uitterlinden AG Hofman A Ikram MA Franco OH Dehghan A Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015; 10: 2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madero M Peralta C Katz R Canada R Fried L Najjar S Shlipak M Simonsick E Lakatta E Patel K Rifkin D Hawkins M Newman A Sarnak M Health ABCS Association of arterial rigidity with incident kidney disease and kidney function decline: the Health ABC study. Clin J Am Soc Nephrol. 2013; 8: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang N Foster MC Mitchell GF Andresdottir MB Eiriksdottir G Gudmundsdottir H Harris TB Launer LJ Palsson R Gudnason V Levey AS Inker LA Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol Dial Transplant. 2017; 32: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomiyama H Tanaka H Hashimoto H Matsumoto C Odaira M Yamada J Yoshida M Shiina K Nagata M Yamashina A Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis. 2010; 212: 345–350. [DOI] [PubMed] [Google Scholar]
- 16. Upadhyay A Hwang SJ Mitchell GF Vasan RS Vita JA Stantchev PI Meigs JB Larson MG Levy D Benjamin EJ Fox CS Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol. 2009; 20: 2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prenner SB Chirinos JA Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015; 238: 370–379. [DOI] [PubMed] [Google Scholar]
- 18. Eriksen BO Stefansson VTN Jenssen TG Mathisen UD Schei J Solbu MD Wilsgaard T Melsom T High ambulatory arterial stiffness index is an independent risk factor for rapid age-related glomerular filtration rate decline in the general middle-aged population. Hypertension. 2017; 69: 651–659. [DOI] [PubMed] [Google Scholar]
- 19. Chen SC Chang JM Liu WC Tsai YC Tsai JC Hsu PC Lin TH Lin MY Su HM Hwang SJ Chen HC Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6: 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford ML Tomlinson LA Chapman TP Rajkumar C Holt SG Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010; 55: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 21. Baumann M Wassertheurer S Suttmann Y Burkhardt K Heemann U Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2-4. J Hypertens. 2014; 32: 899–903. [DOI] [PubMed] [Google Scholar]
- 22. Townsend RR Anderson AH Chirinos JA Feldman HI Grunwald JE Nessel L Roy J Weir MR Wright JT Bansal N Hsu CY Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension. 2018; 71: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dart AM Kingwell BA Pulse pressure – a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001; 37: 975–984. [DOI] [PubMed] [Google Scholar]
- 24. Supiano M Lovato L Ambrosius W Bates J Beddhu S Drawz P Dwyer J Hamburg N Kitzman D Lash J Lustigova E Miracle C Oparil S Dominic R Weiner D Taylor A Vita J Yunis R Chonchol M. Pulse wave velocity and central aortic pressure in systolic blood pressure intervention trial participants. PLoS One. 2018; 13: e0203305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright JT Williamson JD Whelton PK Snyder JK Sink KM Rocco MV Reboussin DM Rahman M Oparil S Lewis CE Kimmel PL Johnson KC Goff DC Fine LJ Cutler JA Cushman WC Cheung AK Ambrosius WT A Randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015; 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ambrosius WT Sink KM Foy CG Berlowitz DR Cheung AK Cushman WC Fine LJ Goff DC Johnson KC Killeen AA Lewis CE Oparil S Reboussin DM Rocco MV Snyder JK Williamson JD Wright JT Whelton PK The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014; 11: 532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Systolic Blood Pressure Intervention Trial (SPRINT) protocol. November 1, 2012. . https://www.sprinttrial.org/public/Protocol_Current.pdf
- 28. Johnson KC Whelton PK Cushman WC Cutler JA Evans GW Snyder JK Ambrosius WT Beddhu S Cheung AK Fine LJ Lewis CE Rahman M Reboussin DM Rocco MV Oparil S Wright JT Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018; 71: 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taal MW Sigrist MK Fakis A Fluck RJ McIntyre CW Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract. 2007; 107: c177–c181. [DOI] [PubMed] [Google Scholar]
- 30. O’Rourke MF Safar ME Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005; 46: 200–204. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell GF Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008; 105: 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


