Abstract
BACKGROUND AND PURPOSE:
Detecting intracranial distal arterial occlusions on CTA is challenging but increasingly relevant to clinical decision-making. Our purpose was to determine whether the use of CTP-derived time-to-maximum of the tissue residue function maps improves diagnostic performance for detecting these occlusions.
MATERIALS AND METHODS:
Seventy consecutive patients with a distal arterial occlusion and 70 randomly selected controls who underwent multimodal CT with CTA and CTP for a suspected acute ischemic stroke were included in this retrospective study. Four readers with different levels of experience independently read the CTAs in 2 separate sessions, with and without time-to-maximum of the tissue residue function maps, recording the presence or absence of an occlusion, diagnostic confidence, and interpretation time. Accuracy for detecting distal occlusions was assessed using receiver operating characteristic analysis, and areas under curves were compared to assess whether accuracy improved with use of time-to-maximum of the tissue residue function. Changes in diagnostic confidence and interpretation time were assessed using the Wilcoxon signed rank test.
RESULTS:
Mean sensitivity for detecting occlusions on CTA increased from 70.7% to 90.4% with use of time-to-maximum of the tissue residue function maps. Diagnostic accuracy improved significantly for the 4 readers (P < .001), with areas under the receiver operating characteristic curves increasing by 0.186, 0.136, 0.114, and 0.121, respectively. Diagnostic confidence and speed also significantly increased.
CONCLUSIONS:
All assessed metrics of diagnostic performance for detecting distal arterial occlusions improved with the use of time-to-maximum of the tissue residue function maps, encouraging their use to aid in interpretation of CTA by both experienced and inexperienced readers. These findings show the added diagnostic value of including CTP in the acute stroke imaging protocol.
Intravenous thrombolysis is the mainstay for treatment of arterial occlusions distal to the internal carotid artery, M1 segment of the MCA, and the vertebral and basilar arteries.1 These occlusions are referred to as distal vessel occlusions (DVOs), to distinguish them from proximal large-vessel occlusions.2 While demonstration of DVOs is not a requirement for thrombolysis,1 their detection is becoming increasingly relevant to clinical decision-making. The main reason is that endovascular thrombectomy (EVT) can be used to treat occlusions involving large- and medium-sized distal arteries in carefully selected patients.2 There is evidence of improved functional outcomes with EVT compared with standard medical management in patients with occlusion of the M2 segment of the MCA.2-4 M2 occlusions are, therefore, increasingly considered for EVT, which is also safe and technically feasible for occlusions involving the M3 segment of the MCA, the anterior cerebral artery (ACA), or the posterior cerebral artery (PCA).1,3,5,6
Advances in endovascular device technology have led to the development of smaller and more navigable stent retrievers and thromboaspiration devices that can reach smaller distal arteries, including the M4 segment of the MCA and the A4 segment of the ACA.2 Because these DVOs can cause severe neurologic deficits when eloquent brain regions are supplied, EVT may be justified to achieve rapid reperfusion.4,7 It is also the only option for reperfusion in patients who are ineligible for thrombolysis. Thus, distal-vessel EVT is considered a “promising next potential frontier” for stroke therapy and is the subject of current research.2 Because demonstration of a target arterial occlusion is required for triage to EVT, fast and accurate detection of DVOs is important to ensure timely treatment.
Detecting DVOs also allows the correct diagnosis to be made. This, in turn, is important for prognostication and ongoing management such as work-up for an embolic source and secondary prevention. It is also possible that detection of a target DVO may become a requirement for thrombolysis if the treatment window is extended beyond 4.5 hours, to avoid futile treatment and justify the increased risk of thrombolysis.8
CTA has become a routine part of the acute stroke imaging protocol.9,10 Its main purpose is to identify patients with proximal large-vessel occlusions for triage to EVT. DVOs are more difficult to detect on CTA than these proximal occlusions, due to the smaller caliber, larger number, and poorer opacification of distal arteries. Reported sensitivity is as low as 33%, with 35% of M2-segment MCA occlusions missed at the time of initial CTA evaluation in 1 recent study.11-13
CTP is now widely included in acute stroke CT protocols.14 The time-to-maximum of the tissue residue function (Tmax) is a parameter that is routinely obtained from CTP when deconvolution-based postprocessing is used.15 Tmax is well-established for identifying salvageable ischemic penumbra in patients with proximal vessel occlusions.16,17 We have observed, in our clinical practice, that Tmax delay within a vascular territory indicates severe stenosis or occlusion of the supplying artery. This information can, in turn, be used to detect and localize distal arterial occlusions on CTA. These occlusions may otherwise be missed or difficult to find. Despite its real-world value in routine clinical practice, no previous studies have assessed and quantified the diagnostic utility of Tmax for detecting intracranial arterial occlusions.
The purpose of this study was to assess the added value of Tmax maps and verify our clinical impression that they facilitate detection of distal occlusions on CTA. We hypothesized that diagnostic accuracy, speed, and confidence for detecting DVOs on CTA would increase with the use of Tmax for readers with different levels of experience.
MATERIALS AND METHODS
Patient Selection
Five hundred one consecutive patients who presented to our institution (Barwon Health), a primary stroke center, between January 1, 2017, and December 31, 2018, and underwent multimodal CT for a suspected stroke were screened using our PACS and electronic medical records. Raw and postprocessed images were assessed for technical adequacy by a neuroradiologist with 9 years’ postfellowship experience. We retrospectively identified patients who met the following inclusion criteria: 1) 18 years of age or older, 2) having undergone multimodal CT with CTA and CTP, and 3) within 24 hours of symptom onset or last known well. Exclusion criteria were the following: 1) technically inadequate CTP or CTA (poor contrast bolus or substantial motion), 2) thin-section CTA images not available, and 3) occlusion of the internal carotid artery, M1 segment of the MCA, vertebral artery, or basilar artery (excluded to allow specific assessment of diagnostic performance for detection of more distal occlusions). We excluded 128 patients: 84 with a large-vessel occlusion and 44 with a technically inadequate CTA or CTP (the patient selection flow chart is shown in Online Fig 1).
The multimodal stroke CTs of all consecutive patients who met the inclusion criteria were reviewed by the neuroradiologist, who had access to all clinical records and imaging. All consecutive patients with a DVO were identified and included in the study. The same number of patients without any vessel occlusion was randomly selected from the remaining patients (see Online Appendix, Part 1) and included in the study. Data processing, scan anonymization, and randomization were performed by this neuroradiologist.
A DVO was defined as an arterial occlusion involving the following: A2 to A5 segments of the ACA; M2 to M4 segments of the MCA; P2 to P4 segments of the PCA; or the PICA, AICA, or SCA. Proximal M2 occlusions are challenging to classify due to large interpatient anatomic variability in size and dominance.2 While some may be considered proximal or large-vessel occlusions, they are not recognized as such by the American Heart Association guidelines, are more difficult to detect than M1 occlusions on CTA, and have, therefore, been included as DVOs in the study.
The study was approved by the local institutional review board, which granted a waiver of written consent based on the retrospective study design and anonymization of all data. This investigator-initiated study received no financial support.
CT Image Acquisition, Reconstruction, and Postprocessing
All patients were scanned on a 256-section multidetector CT scanner (iCT 256; Philips Healthcare). Our routine multimodal stroke CT protocol consisted of nonenhanced CT (NECT) followed by CTP and then CTA. Scan techniques and parameters are detailed in the Online Appendix (Part 2).
For CTP, images were acquired axially, reconstructed at 10-mm section thickness, and processed using a commercially available software platform (RAPID 4.9; iSchemaView) that uses a delay-insensitive deconvolution algorithm with automated arterial input function selection.18 The software calculates Tmax values for each image voxel, ranging from 0 to 12 seconds in 2-second increments, and displays them on a color-scale map.
Helically acquired CTA images were reconstructed axially at 0.8-mm sections. Three-plane (axial, coronal, and sagittal) 4-mm-thick MPRs and 10-mm-thick MIPs were also reconstructed.
Reference Standard
Two neuroradiologists (with 9 and 20 years’ postfellowship experience, respectively) interpreted the CTAs in consensus using a systematic approach in conjunction with NECT and all available clinical and imaging data, including all CTP parametric maps (CBF, CBV, MTT, and Tmax) as well any available follow-up scans. These expert reads served as the reference standard.
Image Review
The CTAs were interpreted independently by 4 readers with different levels of experience: a second-year radiology resident, a neuroradiology fellow, an attending radiologist (2 years’ post-cardiovascular fellowship), and an imaging scientist. These readers had 18 months, 6 years, 8 years, and 20 years of experience, respectively, in interpreting acute stroke imaging. All had pre-existing knowledge of the major cerebral arteries, their segments, and their supply territories, acquired through routine radiology training and clinical work. No additional training was provided for this study. Details of the patients’ presenting neurologic deficits were provided (to reflect clinical practice); however, the readers were blinded to all other clinical and follow-up imaging data. There were no cases in which the CTP acquisition did not cover the territory supplied by an occlusion detected by the neuroradiologists.
Reads were performed in 2 separate sittings, 2 months apart, to negate the effects of memory and learning. CTA, and NECT raw data and reconstructions were made available at each sitting and were viewed using a public domain DICOM viewer (Horos, Version 3.3.5; www.horosproject.org). Scans were anonymized and presented in random order. Readers were permitted to manipulate the provided NECT and CTA data (eg, windowing and performing MIPS) as they would in routine clinical practice. In the first sitting, Tmax maps were provided for the first half of the patient cohort but not the second. This was reversed in the second sitting.
The readers were asked to perform the following:
Review Tmax maps, when available, prior to interpreting the CTA, to determine whether there was any territorial Tmax delay (Fig 1).
-
Assess the CTA, recording the presence and location of a DVO/DVOs, and marking the location on thin-slice CTA images. When Tmax maps were available, the following approach was suggested to localize an occlusion on CTA:
If present, use the distribution Tmax delay conforming to an arterial territory to narrow down the side, major vascular territory, and likely occluded segment. Perform a focused search.
If this fails, progressively broaden the search because there is considerable anatomic variability in the areas supplied by the major intracranial arteries and their segments.
Rate diagnostic confidence using a 5-point Likert scale: 1 (occlusion very unlikely), 2 (occlusion unlikely), 3 (uncertain), 4 (occlusion likely), and 5 (occlusion very likely).
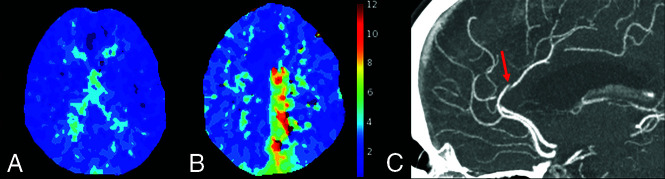
FIG 1.
A, Selected section of a normal Tmax map, with no areas of delay, in a patient without a DVO. B, Tmax map (selected section) shows marked delay in the left distal ACA territory. C, Axial CTA MIP shows the culprit A-segment ACA occlusion (red arrow) in this 72- year-old woman who presented with right-leg weakness.
Statistical Analysis
All statistical analyses were performed using MedCalc (MedCalc Software, Version 17.2, 64 bit).
Each reader’s diagnostic performance for detecting a DVO on CTA was assessed against the reference standard (expert reads) using receiver operating characteristic (ROC) analysis. A true-positive required both the presence and site of a DVO to be correctly identified. Change in accuracy with the addition of Tmax was assessed by pair-wise comparison of the areas under the ROC curves (AUCs) using the DeLong algorithm.
The added value of Tmax on diagnostic confidence was assessed with shift analysis, using the Wilcoxon signed rank test to assess the significance of any change. Interreader agreement was assessed using the Fleiss κ statistic (κF). The Cohen κ statistics were used to determine agreement between each pair of readers.
The Wilcoxon signed rank test was used to determine whether there was a significant difference in the time taken to interpret CTA when Tmax was added.
Confidence intervals were calculated using a bootstrap procedure with 10,000 samples with replacement. An α level of .001 was taken to indicate significance for all tests except the shift analysis of confidence in which a level of .05 was applied.
RESULTS
CTAs were analyzed from 140 patients (median age, 73 years; interquartile range, 64–83 years), of which 77 were men and 70 had a DVO (including 22 with proximal M2 occlusions). Patients’ baseline characteristics and the details of vessel occlusion are provided in Table 1.
Table 1:
Patient demographics and location of vessel occlusions
| No. | Age (Median) (Interquartile Range) | NIHSSa (Median) (Interquartile Range) | Male (%) | |
|---|---|---|---|---|
| All patients | 140 | 73 (64–83) | 5 (1–7) | 77 (55.0%) |
| Patients without a DVO | 70 | 68 (55–82) | 4 (1–7) | 34 (48.6%) |
| Patients with a DVO | 70 | 76 (69–84) | 5 (1–8) | 43 (61.1%) |
| Location of DVO | ||||
| MCA: M2 segmentb | 38 | |||
| Proximal | 22 | |||
| Distal | 16 | |||
| MCA: M3 segment | 5 | |||
| MCA: M4 segment | 8 | |||
| ACA: A3 or A4 segment | 3 | |||
| PCA: P2 segment | 5 | |||
| PCA: P3 or P4 segment | 8 | |||
| PICA | 2 | |||
| SCA | 1 |
Fourteen patients had an occlusion at 2 sites (Online Table 1) and were categorized here according to the most proximal occlusion.
The midpoint of the Sylvian fissure on coronal imaging was used to divide M2 occlusions into proximal (if inferior) and distal (if superior). Occlusions of the superior and inferior division trunks as well as their ascending branches were included under M2-segment occlusions.
Diagnostic Accuracy
The results of ROC analyses for detecting a DVO on CTA are given in Online Table 2. For all readers, sensitivity and specificity increased with the addition of Tmax, and accuracy (as measured by the AUC) increased significantly (P < .001). The mean sensitivity for detecting a DVO increased from 70.7% to 90.4% with the addition of Tmax, while mean specificity increased from 87.5% to 95.7%.
Analysis was repeated following exclusion of the 22 patients with proximal M2 MCA occlusions, including 10 with occlusion of the proximal trunk of a dominant or codominant M2 division, which may be considered proximal vessels (Online Tables 4 and 5). The mean sensitivity for detecting a DVO on CTA increased from 61.0% without Tmax to 86.5% when Tmax was used. The gain in sensitivity was, therefore, larger than when proximal M2 occlusions were included. Following exclusion of the 43 patients with either an M2 MCA or P2 PCA occlusion (Online Table 6), which allowed diagnostic sensitivity for detecting more distal occlusions to be isolated, mean sensitivity increased from 42.6% without Tmax to 81.5% with Tmax. The sensitivity for detecting DVOs on CTA alone was, therefore, much lower than when M2 and P2 occlusions were included. However, the gain in sensitivity with the addition of Tmax and, therefore, an increase in the AUC was larger. On all 3 analyses, the AUC increased significantly (P < .001) for all readers when Tmax was used, with the largest improvement occurring when only the most distal DVOs were considered (ie, following exclusion of M2 or P2 occlusions).
Interreader agreement on CTA improved with the addition of Tmax, from κF = 0.61 (95% CI, 0.54–0.68) to κF = 0.79 (95% CI = 0.72–0.86). There was also greater agreement between pairs of readers (Online Fig 2) when CTA was interpreted with Tmax than without it.
Diagnostic Confidence
The gain in confidence with the addition of Tmax is shown in Online Fig 3 and Online Table 7. In patients deemed to have a DVO on the reference standard, diagnostic confidence that an occlusion was present increased significantly (P < .05) for all 4 readers. Each reader had fewer false-negatives and more patients in whom an occlusion was deemed very likely.
All readers were more confident in dismissing a DVO on CTA when Tmax was used. The increase in confidence reached significance (P < .05) for all readers except the resident, who had a larger number of false-positives (n = 8). The number of patients in whom an occlusion was considered very unlikely increased for all readers.
Time to Interpret CTA
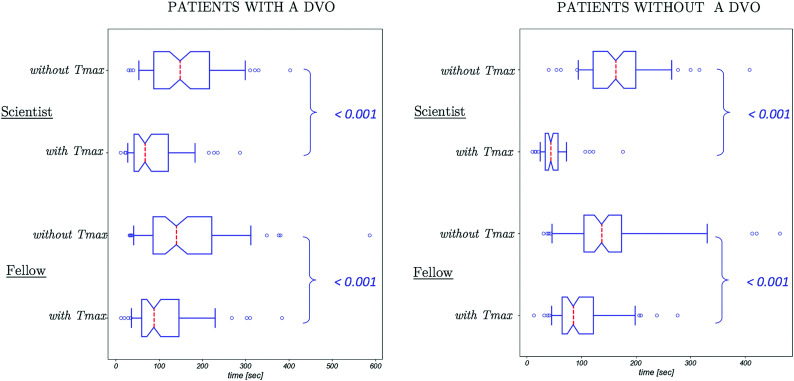
CTA was interpreted significantly faster (P < .001) with use of Tmax (Online Table 8): The median interpretation time was 1.6 times faster for the fellow and 3.3 times faster for the scientist with Tmax. Box-and-whisker plots of CTA interpretation time are shown for the 2 readers who were timed (Fig 2). Patients were dichotomized into those with a DVO and those without a DVO on the reference standard. Interpretation time was significantly (P < .001) shorter with Tmax than without it for both groups. This result indicates that DVOs were both detected and dismissed faster. The median time to detect M2 occlusions was less than that for M3 and M4 occlusions, but this did not reach significance (Online Table 9).
FIG 2.
Box-and-whisker plots of time taken to interpret CTA in patients with and without a DVO on the reference standard. The dashed red line indicates median time; the upper and lower edges of the boxes indicate the first and third quartiles, respectively; the notches represent the 95% confidence intervals of the median; and the whiskers extend to the fifth and 95th centiles. Outliers are shown in the circles. For both readers, interpretation was significantly faster (P < .001) with Tmax than without it. The spread of times also decreased with Tmax.
Post Hoc Analysis
False-negatives and false-positives are detailed in Online Tables 10 and 11, respectively.
The number of false-negatives decreased for all readers with the addition of Tmax. Four proximal M2 occlusions were missed by ≥1 of the readers on CTA without Tmax (Fig 3A, -B). Only one was missed by 1 reader with Tmax. Fewer distal DVOs were missed on CTA when Tmax was used (Fig 3C, -D). M4-segment MCA occlusions remained a challenge, however, with the fellow and radiologist each missing 5 even with Tmax. There were too few distal ACA occlusions in the cohort for meaningful analysis. All 8 PCA occlusions distal to the P2 segment were detected on CTA with Tmax.
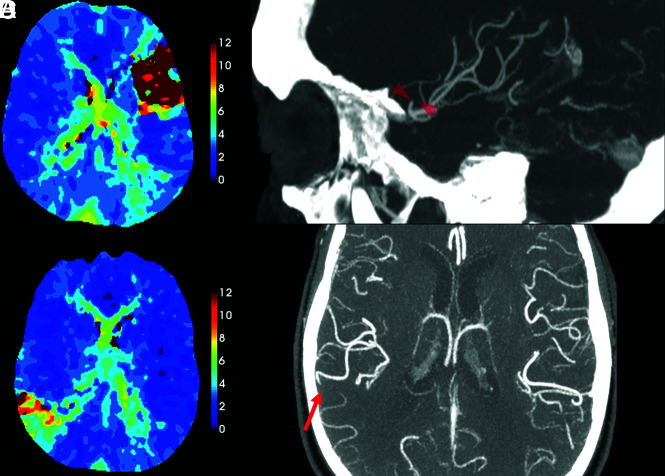
FIG 3.
Examples in which Tmax aided detection of a DVO on CTA. A, A Tmax delay of >10 seconds is evident in the left MCA, superior M2-division territory in a 54-year-old woman who presented with sudden-onset aphasia. B, Sagittal CTA MIP (selected section) shows the occlusion (red arrowheads). This was detected by only 2 readers without Tmax, but by all 4 readers with Tmax. C, Wedge-shaped, territorial Tmax delay is seen in a right parietal lobe in an 83-year-old man. D, The right parietal M4 occlusion (red arrow), shown on an axial CTA MIP, was detected by only 2 readers without Tmax and all 4 readers with Tmax.
There were a number of false-positives for a DVO on CTA without Tmax. All were related to small-caliber distal vessels, especially branch and turning points. The few false-positives on CTA with Tmax were also related to small-caliber, poorly opacified distal vessels. Tmax delay was present in all except one of these cases, but it did not conform to the territory of an intracranial artery. A recurrent cause of false-positives for the 2 less experienced readers was Tmax delay in the deep white matter and external watershed (borderzone pattern) (Online Fig 4E).
Fourteen patients had 2 DVOs (Online Table 1). The benefit of Tmax was greater for the 2 experienced readers; both occlusions were detected and correctly identified in 3 additional patients by the radiologist and in 7 additional patients by the scientist.
DISCUSSION
The added value of Tmax maps on diagnostic performance for detecting DVOs on CTA was assessed in this study. Diagnostic accuracy, confidence, and speed were shown to improve significantly with addition of Tmax for readers with different levels of experience in interpreting stroke imaging. The beneficial effect of Tmax in aiding detection of DVOs on CTA was greater for more distal occlusions.
CTP is now widely included in the acute stroke CT protocol.14 Its primary purpose is to identify patients with proximal arterial occlusions with salvageable brain tissue who may, therefore, benefit from EVT. It also provides information about the macrovasculature that can be leveraged to improve detection of vessel occlusions.11,19 Perfusion maps were shown to improve detection of intracranial arterial occlusions in 1 previous study that was not specifically designed to evaluate DVOs and included only a small number of these distal occlusions.11 Another important point of distinction between this previous study and ours is that Tmax was not used in theirs.
Tmax was used in some EVT trials to identify salvageable ischemic penumbra and is now routinely available on most CTP-postprocessing software platforms.16,17 It can also be used to assess early reperfusion with treatment.17 Tmax reflects the delay in contrast arrival in tissue relative to a proximal arterial reference point.18,19 This arterial reference point is called the arterial input function and is an essential part of deconvolution-based perfusion analysis. An intracranial arterial occlusion prolongs arterial transit time and, therefore, Tmax within the territory that is supplied.19 TTP, another time-based parameter that has been used to assess penumbra, is also prolonged when there is delayed arterial transit.18 However, unlike Tmax, it is not obtained through deconvolution and is, therefore, not corrected for the shape of the contrast bolus. Thus, Tmax is less sensitive than TTP to bolus delay proximal to the arterial input function and is more specific for delays in arterial transit between the arterial input function and tissue caused by an occlusion.18 The distribution of Tmax delay can be used to narrow down the laterality, major territory, probable segment (eg, M2 versus M3), and likely location of an arterial occlusion. A more focused search can then be performed. The alternative of systematically interrogating all cerebral arteries to the most distal discernible level is very time-consuming and therefore not feasible under clinical time pressures. Clinical information regarding neurologic deficits narrows the search field but is not always available or reliable. Tmax maps are objective and consistently available when CTP is performed. To our knowledge, this is the first study evaluating the utility of Tmax for detecting intracranial arterial occlusions.
As hypothesized, diagnostic performance for detecting DVOs on CTA improved significantly with the use of Tmax. The effect size was sufficiently large to show significant improvement using a P value cutoff of .001, even with a sample size of 140, including 70 patients with a DVO. Because information on neurologic deficit was provided, the beneficial effect of Tmax was additive to any gain provided by clinical notes. Sensitivity for the detection of DVOs on CTA alone is likely lower, and improvement in performance with Tmax may, therefore, be even greater when reliable clinical information is unavailable.
Most important, fewer proximal M2 occlusions were missed. Patients with M2 occlusions are increasingly considered for EVT because it may improve their functional outcomes.3,4 M2 occlusions are easily detected by experienced neuroradiologists but can be missed by trainees and general radiologists as shown in this study; 35% of M2 occlusions were missed on CTA evaluation in a previous study performed at a primary stroke center.13 Trainees interpret the bulk of CTAs performed at stroke referral centers, while these scans are typically interpreted by general radiologists at primary stroke centers. Improving detection of M2 occlusions by these less experienced readers is, therefore, of high clinical relevance, to ensure that patients do not miss out on potentially beneficial treatment.
The gain in sensitivity with the addition of Tmax and, therefore, the benefit were greater for more distal occlusions. Poor sensitivity for detecting M4 and distal ACA occlusions on CTA alone can be explained by the small caliber and large number of these vessels, making detection akin to finding a needle in a haystack, even with accurate clinical notes. Tmax maps narrowed the search field, increasing the chances of finding the culprit occlusion on CTA. Despite improvement in sensitivity, some distal anterior circulation occlusions were still missed by the readers. An occlusion is sometimes not clearly visible on CTA (due to the small caliber and poor opacification of the occluded artery), despite unequivocal territorial Tmax delay suggestive of a DVO. If an alternative cause cannot be found, it may be reasonable to diagnose a likely distal occlusion in these cases. The readers fared better in detecting distal PCA than MCA occlusions. This result may be due to the smaller spatial extent and less arborization of the PCA, compared with the MCA. Because sensitivity on CTA with Tmax was imperfect, it cannot be used to definitively exclude a DVO. Of note, sensitivity was not related to reader experience level. The scientist and the resident had the highest sensitivity, both with and without Tmax. A possible explanation is that these readers had a more methodical approach to reading the CTAs.
Specificity for detecting a DVO on CTA exceeded 95% with the addition of Tmax for all readers except the resident, which is important if the findings are used to guide treatment, to ensure that futile and potentially harmful reperfusion is avoided in patients without a DVO. Specificity was related to the level of experience in interpreting CTA and CTP; greater experience enabled the senior readers to differentiate between occlusions and poorly opacified distal vessels and to recognize and dismiss Tmax delay that did not conform to a vascular territory.
Interpretation of CTAs was significantly faster with the addition of Tmax. As expected, DVOs were detected faster due to the search field being narrowed, but occlusions were also dismissed more quickly. Faster diagnosis not only expedites treatment, it also improves workflow efficiency. The latter is important in clinical practice, particularly in the setting of busy comprehensive stroke centers. An important caveat in using Tmax to expedite interpretation is that readers must continue to methodically scrutinize CTAs for other, incidental findings and avoid succumbing to “streetlight effect.”
Diagnostic confidence in the presence or absence of a DVO on CTA increased for all readers with Tmax. One likely reason is that the Tmax maps provide additional evidence to corroborate or reject the findings on CTA alone; diagnostic confidence is greater when 2 separate tests indicate that an occlusion is either present or absent. Another factor that likely contributed to improvement in all metrics of diagnostic performance is that Tmax maps are easy to interpret. They have a high contrast-to-noise ratio. While CBF and CBV have substantial gray-white matter contrast, Tmax values do not vary with tissue type20 and have rather flat contrast in the normal brain. Even small or subtle areas of Tmax delay, therefore, appear conspicuous and can be detected and characterized reliably and confidently. This feature, in turn, contributes to increased sensitivity when the findings on Tmax are used to guide the search for a DVO on CTA. Conversely, maps with no Tmax delay appear monochromatic, making it easy to interpret the absence of an abnormality and dismiss an occlusion with high certainty. While the findings of a DVO on CTA can be subtle due to the small caliber and poor opacification of distal vessels, the findings on Tmax are more apparent and therefore less prone to variable interpretation. Accordingly, the use of Tmax led to greater consistency of CTA interpretation, with higher interreader agreement.
An alternative method that has been reported to improve detection of DVOs is wavelet-transformed angiography (waveletCTA), which is also obtained by postprocessing of CTP data.21 Its clinical uptake has been limited by the requirement for thin-section CTP and specialist postprocessing software that is not widely available. In contrast, most CTP-postprocessing software packages are able to produce Tmax maps and can do so from thick-section CTP, making the use of Tmax feasible in routine clinical practice at even small peripheral centers.
An important limitation is that CTP is only recommended by the AHA guidelines in the late (6- to 24-hour) time window. Not all stroke centers routinely perform CTP in the early window because it incurs an additional radiation dose and its role in patients within 6 hours of stroke onset has not been established. However, because CTP has diagnostic utility beyond tissue classification, aiding the diagnosis of stroke and some stroke mimics such as migraine, and is therefore performed even in the early window at some centers, including ours.
A limitation of using Tmax maps to aid detection of DVOs on CTA is that nonterritorial Tmax delay can lead to false-positives and lower specificity. The experienced readers dismissed DVOs in these cases, suggesting that such errors can be avoided with training in the interpretation of Tmax maps, specifically to differentiate Tmax delay in a vascular territory from delays that either cross territories (eg, due to migraine) or are artifactual or “borderzone” in distribution. Borderzone Tmax delay occurs with contrast bolus dispersion, for example, due to a poor injection, proximal arterial steno-occlusive disease, and impaired cardiac output. It is manifested as Tmax delay in arterial watershed areas.18,19 CTP acquisition with limited brain coverage is a potential cause of false-negatives if the territory supplied by the occluded vessel is not included. This situation is avoided with a whole-brain CTP acquisition on modern multidetector array CT scanners.11 When only limited coverage is feasible, targeting the CTP acquisition to the clinical presentation (eg, posterior circulation) can avoid false-negatives.
A limitation of the study is that only 1 CT scanner and CTP postprocessing software package were used. This may limit the generalizability of the findings. The algorithms used by different postprocessing software packages are variable and most, but not all, use deconvolution-based postprocessing, a requirement for obtaining Tmax.22 There are also substantial differences in the derived perfusion parameters, even between packages that use deconvolution-based postprocessing, resulting in variability in quantification of the infarct core and penumbra.22,23 The variability is less likely to affect qualitative assessment; however, differences in the display of parametric maps (eg, color scale) may impact visual assessment. In turn, these may affect diagnostic performance for the detection of territorial Tmax delay, which warrants further evaluation in a future study.
A potential limitation of the study is that the prevalence of DVOs in the study cohort (50%) was higher than in the population of patients with stroke who undergo multimodal CT. The true prevalence of DVOs is much lower and likely closer to the 14% observed in the cohort screened for the study. Use of a balanced sample may bias the absolute sensitivity and specificity for detecting a DVOs on CTA, both with and without Tmax. These values should, therefore, be interpreted with caution. However, the primary purpose of this study was to assess the relative change in diagnostic performance for detecting DVOs on CTA when Tmax was added, rather than absolute diagnostic performance.
Another potential limitation of the study is the choice of the reference standard: an expert read of the CTAs instead of DSA, which is the reference standard for detecting intracranial vascular pathology. Using CTA was justified because DVOs can recanalize or migrate between angiographic modalities, especially when thrombolysis is administered, which would render DSA inaccurate as a reference standard. The authors recognize that some very distal DVOs may have been missed by all readers, including the expert reader. This possibility would affect the determination of absolute accuracy. It is, however, less relevant for comparative assessment of the diagnostic performance on CTA with and without Tmax, which was the purpose of this study.
CONCLUSIONS
DVOs were detected with greater accuracy, confidence, and speed on CTA when Tmax maps were used to focus the search for a vessel occlusion. While the beneficial effect was greater for more distal occlusions, Tmax also helped detect M2 occlusions, which is important clinically because they are considered a target for EVT. Our findings demonstrate significant added value of CTP beyond tissue classification, with the potential to benefit management of more patients than simply those with proximal arterial occlusions. By showing that Tmax can be leveraged to improve detection of vessel occlusions by trainees and a generalist, our findings encourage the inclusion of CTP in the acute stoke imaging protocol at both comprehensive and primary stroke centers.
ABBREVIATIONS:
- ACA
anterior cerebral artery
- AUC
area under the curve
- DVO
distal vessel occlusion
- EVT
endovascular thrombectomy
- NECT
nonenhanced CT
- PCA
posterior cerebral artery
- ROC
receiver operating characteristic
- Tmax
time to maximum of the tissue residue function
- SCA
Superior Cerebellar Artery
Footnotes
Disclosures: Shalini A. Amukotuwa—UNRELATED: Employment: Monash Health. Angel Wu—UNRELATED: Employment: Monash Health. Roland Bammer—UNRELATED: Board Membership: iSchemaView Inc; Payment for Lectures Including Service on Speakers Bureaus: American Society of Neuroradiology; Stock/Stock Options: iSchemaView Inc.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46–110 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Chapot R, Agid R, et al. ; Distal Thrombectomy Summit Group. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020;51:2872–84 10.1161/STROKEAHA.120.028956 [DOI] [PubMed] [Google Scholar]
- 3.Sarraj A, Sangha N, Hussain MS, et al. . Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol 2016;73:1291–96 10.1001/jamaneurol.2016.2773 [DOI] [PubMed] [Google Scholar]
- 4.Sheth SA, Yoo B, Saver JL, et al. ; UCLA Comprehensive Stroke Center. M2 occlusions as targets for endovascular therapy: comprehensive analysis of diffusion/perfusion MRI, angiography, and clinical outcomes. J Neurointerv Surg 2015;7:478–83 10.1136/neurintsurg-2014-011232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossberg JA, Rebello LC, Haussen DC, et al. . Beyond large vessel occlusion strokes: distal occlusion thrombectomy. Stroke 2018;49:1662–68 10.1161/STROKEAHA.118.020567 [DOI] [PubMed] [Google Scholar]
- 6.Almekhlafi M, Ospel JM, Saposnik G, et al. . Endovascular treatment decisions in patients with M2 segment MCA occlusions. AJNR Am J Neuroradiol 2020;41:280–85 10.3174/ajnr.A6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurre W, Vorlaender K, Aguilar-Perez M, et al. . Frequency and relevance of anterior cerebral artery embolism caused by mechanical thrombectomy of middle cerebral artery occlusion. AJNR Am J Neuroradiol 2013;34:1606–11 10.3174/ajnr.A3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark WM, Albers GW, Madden KP, et al. . The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study: Thromblytic Therapy in Acute Ischemic Stroke Study Investigators. Stroke 2000;31:811–16 10.1161/01.STR.31.4.811 [DOI] [PubMed] [Google Scholar]
- 9.Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke 2016;47:273–81 10.1161/STROKEAHA.115.009171 [DOI] [PubMed] [Google Scholar]
- 10.Wintermark M, Luby M, Bornstein NM, et al. . International survey of acute stroke imaging used to make revascularization treatment decisions. Int J Stroke 2015;10:759–62 10.1111/ijs.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becks MJ, Manniesing R, Vister J, et al. . Brain CT perfusion improves intracranial vessel occlusion detection on CT angiography. J Neuroradiol 2019;46:124–29 10.1016/j.neurad.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 12.Lev MH, Farkas J, Rodriguez VR, et al. . CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001;25:520–28 10.1097/00004728-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 13.Fasen B, Heijboer RJJ, Hulsmans FH, et al. . CT angiography in evaluating large-vessel occlusion in acute anterior circulation ischemic stroke: factors associated with diagnostic error in clinical practice. AJNR Am J Neuroradiol 2020;41:607–11 10.3174/ajnr.A6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kansagra AP, Goyal MS, Hamilton S, et al. . Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med 2020;383:400–01 10.1056/NEJMc2014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell BC, Christensen S, Levi CR, et al. . Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012;43:2648–53 10.1161/STROKEAHA.112.660548 [DOI] [PubMed] [Google Scholar]
- 16.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 17.Lansberg MG, Straka M, Kemp S, et al. ; DEFUSE 2 Study Investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860–67 10.1016/S1474-4422(12)70203-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010;32:1024–37 10.1002/jmri.22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calamante F, Christensen S, Desmond PM, et al. . The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke 2010;41:1169–74 10.1161/STROKEAHA.110.580670 [DOI] [PubMed] [Google Scholar]
- 20.Parkes LM, Rashid W, Chard DT, et al. . Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004;51:736–43 10.1002/mrm.20023 [DOI] [PubMed] [Google Scholar]
- 21.Kunz WG, Sommer WH, Havla L, et al. . Detection of single-phase CTA occult vessel occlusions in acute ischemic stroke using CT perfusion-based wavelet-transformed angiography. Eur Radiol 2017;27:2657–64 10.1007/s00330-016-4613-y [DOI] [PubMed] [Google Scholar]
- 22.Kudo K, Sasaki M, Yamada K, et al. . Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 2010;254:200–09 10.1148/radiol.254082000 [DOI] [PubMed] [Google Scholar]
- 23.Austein F, Riedel C, Kerby T, et al. . Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke 2016;47:2311–17 10.1161/STROKEAHA.116.013147 [DOI] [PubMed] [Google Scholar]