All cells encode specific DNA binding proteins that ensure that genetic material is appropriately expressed, replicated, and transmitted from one generation to the next. Mother Nature solved the DNA recognition problem by inventing a handful of protein motifs, including the zinc finger, the helix-turn-helix, and the leucine zipper. As is the case with all good solutions to a problem, these motifs are used over and over again in biological systems; for example, DNA binding proteins containing the helix-turn-helix motif are found in both prokaryotes and eukaryotes, and zinc finger–containing proteins are the most abundant protein class encoded by the human genome. It is surprising, therefore, to learn from studies by Boch et al. (1) on page 1509 and Moscou and Bogdanove (2) on page 1501 of this issue, about a new DNA binding motif that has heretofore escaped description.
The new motif plays a central role in the function of transcription activator–like (TAL) effectors, proteins expressed by bacterial plant pathogens of the genus Xanthomonas. TAL effectors are an important weapon in a battle waged between Xanthomonas species and their plant hosts. They are translated in bacteria and deposited into plant cells by the bacteria’s type III secretion system. Once in a plant, TAL effectors activate the transcription of plant genes that enable pathogen spread. For example, PthXo1, a TAL effector of a Xanthomonas rice pathogen, activates expression of the rice gene Os8N3, allowing Xanthomonas to colonize rice plants (3). Because Os8N3 is also necessary for normal plant development, the gene cannot simply be discarded to avoid infection. Rather, there is strong selective pressure for the plant to accumulate mutations that prevent PthXo1 binding, and, consequently, for compensatory changes in the TAL effector’s DNA specificity. In the end, DNA recognition determines the outcome of the pathogen-plant war, and so TAL effectors would benefit from a simple, malleable mechanism for DNA recognition. Such a mechanism is revealed by Boch et al. and Moscou and Bogdanove in complementary studies that have deciphered the TAL effectors’ DNA recognition code.
Both groups focused on the TAL effector’s central domain, which contains a variable number of tandem, 34–amino acid repeats (see the figure). The repeat domain was previously shown to bind specific DNA sequences in promoter regions of target genes (4). Amino acid sequences of the repeats are conserved, except for two adjacent highly variable residues (at positions 12 and 13) that were obvious candidates for specificity determinants. Both groups deduced a simple code relating specific diamino acids in the repeat unit to specific nucleotides in the DNA target. Remarkably, there appears to be a one-to-one correspondence between sequential amino acid repeats in the array and sequential nucleotides in the target DNA.
Code is broken.
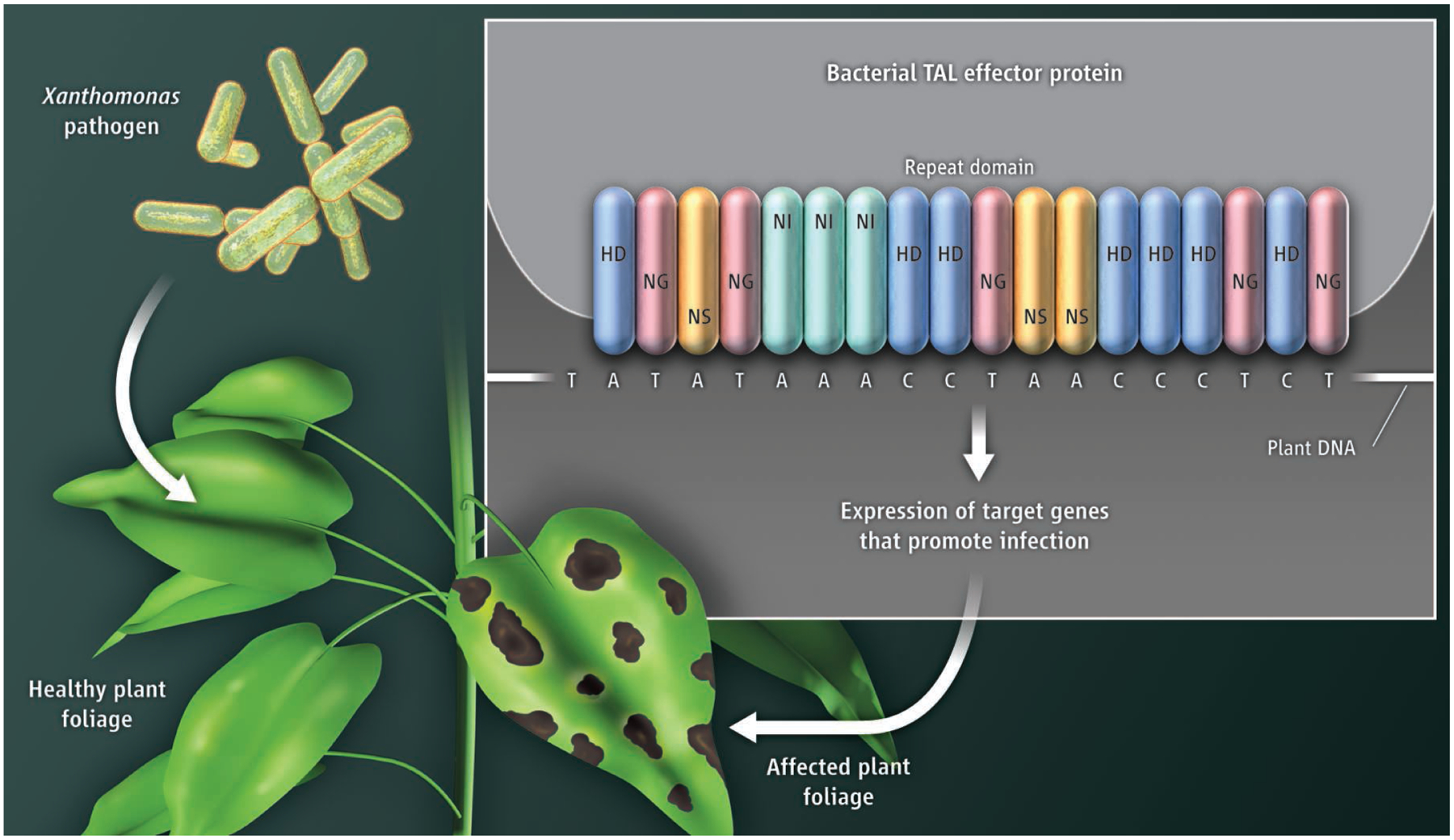
The DNA binding domain of bacterial TAL effectors contains tandem repeats (about 17; shown in blue, pink, yellow, and green for AvrBs3) of amino acids (34 or 35). The repeat is highly conserved, except for residues at positions 12 and 13 (shown within each repeat; H, histidine; D, aspartic acid; N, asparagine; G, glycine; I, isoleucine; S, serine). The code relates diamino acids in the repeat unit to nucleotides (T, thymine; A, adenine; C, cytosine) in the DNA target.
Moscou and Bogdanove broke the DNA recognition code computationally, by searching for nonrandom alignments between the variable diamino acids in the TAL effector and DNA sequences of target promoters. For a handful of well-characterized TAL effectors, DNA sequences identified from the alignments were important for transcriptional activation, thus providing evidence that the alignments and the resulting code were biologically meaningful. Boch et al., on the other hand, deduced the code through molecular genetic analyses of the interaction between AvrBs3 (the TAL effector of a Xanthomonas pathogen of pepper) and its target DNA sequence in the promoter of an AvrBs3-regulated pepper gene. The deduced code was experimentally validated by several approaches: Target DNA sites were predicted for uncharacterized TAL effectors and shown to confer TAL effector–dependent expression of reporter genes; target DNA sequences were mutated to demonstrate the specificity of TAL effector repeats for predicted nucleotide signatures; TAL effectors expressed in plant cells (heterologous hosts) were shown to activate expression of genes with the predicted target sequences in their promoters; and, quite remarkably, artificial transcription factors with novel DNA binding specificities were created by shuffling the repeat domains and demonstrating their ability to promote the transcription of target genes.
Although both studies establish a central role for the diamino acid sequences in the TAL effectors for recognizing DNA, many important additional questions remain. For example, a three-dimensional structure of a TAL effector complexed to its target DNA may reveal how the diamino acids recognize individual nucleotides within the context of the larger protein domain. Structural information may also clarify whether DNA recognition is influenced by other residues in the effector protein that cannot be discerned from the primary amino acid sequence of the TAL repeat unit. Apparently, TAL effectors function as dimers (5), making it further difficult to envision how DNA recognition is achieved.
An additional surprising aspect is the modularity of the TAL effector repeat. Boch et al. could create novel DNA binding proteins with remarkable ease for a small number of target DNA sites. Similarly, Moscou and Bogdanove’s computational analyses suggest that nucleotide specificity is independent of the association of a repeat unit with neighboring repeat units in the TAL central domain. Nucleotide specificity also has some interesting limitations. It is curious that guanine is not recognized by TAL effectors, particularly because guanines are bound with high affinity by other DNA binding motifs (zinc fingers) (6).
Deciphering the TAL effector DNA recognition code suggests several obvious potential biotechnological applications. Most evident is the parallel to artificial zinc finger transcription factors, one of which is currently being evaluated in human clinical trials as a therapeutic agent for diabetic neuropathy (7). Boch et al. show that artificial TAL effectors activate transcription in plants; moreover, native TAL effectors activate transcription in yeast (8), suggesting that they may be broadly useful. The latest rage in genome engineering is to use nucleases with novel DNA binding specificities to create targeted chromosome breaks that stimulate gene targeting (9, 10). The extent to which the TAL effector DNA binding domains can be used for such purposes remains to be determined. It is curious, however, that Xanthomonas’ simple solution to DNA binding is not more widely used in biology, and this may be a hint that there are limitations to broader applicability. Then again, necessity is the mother of invention, and the host-pathogen conflict has proven time and again to be a rich source of biological innovation.
A mechanism by which proteins of bacterial plant pathogens recognize and control the expression of host plant genes to promote infection is identified.
References
- 1.Boch J et al. , Science 326, 1509 (2009); published online 29 October 2009 ( 10.1126/science.1178811). [DOI] [PubMed] [Google Scholar]
- 2.Moscou MJ, Bogdanove AJ, Science 326, 1501 (2009); published online 17 November 2009 ( 10.1126/science.1178817). [DOI] [PubMed] [Google Scholar]
- 3.Yang B, Sugio A, White FF, Proc. Natl. Acad. Sci. U.S.A 103, 10503 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu K et al. , Nature 435, 1122 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Gurlebeck D, Szurek B, Bonas U, Plant J. 42, 175 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Pavletich NP, Pabo CO, Science 252, 809 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Pearson H, Nature 455, 160 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Nissan G et al. , Mol. Microbiol 61, 1118 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Cathomen T, Joung JK, Mol. Ther 16, 1200 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Paques F, Duchateau P, Curr. Gene Ther 7, 49 (2007). [DOI] [PubMed] [Google Scholar]


