Abstract
Piezo2 channels are expressed in Merkel cells and somatosensory neurons to mediate mechanotransduction leading to the sense of touch. Components of the cytoskeleton including microtubules are key intracellular structures that maintain cellular membrane mechanics and thereby may be important in mechanotransduction. In the present study, we have explored, with microtubule-targeting agents, the potential role of microtubules in Piezo2-mediated mechanotransduction in Merkel cells of mouse whisker hair follicles. Applying patch-clamp recordings to Merkel cells in situ in whisker hair follicles, we show that Piezo2-mediated mechanically activated (MA) currents in Merkel cells are significantly potentiated by the microtubule stabilizer paclitaxel but reduced by the microtubule destabilizer vincristine. Furthermore, electrophysiological recordings made from whisker hair follicle afferent nerves show that mechanically evoked whisker afferent impulses are significantly enhanced by paclitaxel and its analog docetaxel but significantly suppressed by vincristine and its analog vinblastine. Our findings suggest that microtubules play an essential role in Piezo2 mechanotransduction in Merkel cells.
NEW & NOTEWORTHY Piezo2 channels are expressed in Merkel cells to mediate mechanotransduction leading to the sense of touch. Here we determined the role of microtubules in regulating Piezo2-mediated mechanotransduction in Merkel cells. Piezo2-mediated currents in Merkel cells are potentiated by microtubule stabilizer paclitaxel but reduced by microtubule destabilizer vincristine. Mechanically evoked afferent impulses are also enhanced by microtubule stabilizers and suppressed by microtubule destabilizers. Microtubules may play an essential role in Piezo2 mechanotransduction in Merkel cells.
Keywords: mechanoreceptors, Merkel cells, microtubule, Piezo2, touch
INTRODUCTION
The Merkel disk, also known as the Merkel cell-neurite complex, is a main type of tactile end organs highly abundant in human fingertips, touch domes in the skin, and whisker hair follicles (Halata et al. 2003; Merkel 1875). They are essential in transducing the sense of touch to enable sensory tasks such as environmental explorations, social interactions, and tactile discrimination (Johnson 2001). Merkel disks are composed of Merkel cells and their associated Aβ-afferent nerve endings to form a synaptic-like structure (Halata et al. 2003; Iggo and Muir 1969). They have high tactile acuity and are very sensitive to skin indentation, pressure, hair movement, and other tactile stimuli. Tactile stimuli to Merkel disks in the touch domes of the skin and whisker hair follicles result in slowly adapting type 1 (SA1) afferent impulses, the characteristic Aβ-afferent responses for tactile encoding (Iggo and Muir 1969; Johnson 2001). Recent studies have demonstrated that Merkel cells and their associated Aβ-afferent nerve endings use Piezo2 channels as mechanoreceptors (Ikeda et al. 2014; Maksimovic et al. 2014; Woo et al. 2014). More recent studies have reported that tactile signals are transmitted from Merkel cells to their associated Aβ-afferent nerve endings by serotonin and norepinephrine (Chang et al. 2016; Hoffman et al. 2018). The serotonergic tactile transmission is shown to be regulated by serotonin transporters (Chang and Gu 2020). It is currently unknown whether Piezo2-mediated mechanotransduction, a molecular event upstream to the tactile transmission, may be also regulated at Merkel disks to affect the tactile sensitivity.
Several lines of evidence from studies on somatosensory neurons have suggested that functions of Piezo2 channels can be regulated by cellular signaling pathways. For example, a previous study has shown that inflammatory mediators including bradykinin enhance Piezo2-mediated mechanosensitive currents via protein kinase A and protein kinase C in somatosensory neurons (Dubin et al. 2012). Epac1, a cyclic AMP sensor, has been found to potentiate Piezo2-mediated mechanotransduction in somatosensory neuron, which contributes to inflammatory mechanical allodynia (Eijkelkamp et al. 2013; Singhmar et al. 2016). We have previous shown that Piezo2-medaited mechanically activated (MA) currents became potentiated in a GTP-dependent manner in rat dorsal root ganglion neurons (Jia et al. 2013). Piezo2 channel activity has been found to require the presence of phosphoinositides (Borbiro et al. 2015), and activation of Gi-coupled receptors potentiate Piezo2 currents via Gβγ (Del Rosario et al. 2020).
While most studies focused on the regulation of Piezo2 functions by intracellular signaling pathways, a study has shown that changes of membrane mechanics by depleting membrane cholesterol or its interacting protein STOML3 modulate Piezo2-mediated mechanotransduction in somatosensory neurons (Qi et al. 2015). Consistent with this finding, we have shown that Piezo2-mediated mechanotransduction is regulated by the cytoskeleton actin filaments via their effects on static plasma membrane tension in primary afferent neurons (Jia et al. 2016). In addition to actin filaments, microtubules are also a major type of cytoskeleton that is essential for maintaining membrane mechanics of cells. It has been shown that microtubule acetylation is essential for mechanosensation in drosophila (Yan et al. 2018) and mice (Morley et al. 2016). In the present study, we set out to determine whether Piezo2-medaited mechanotransduction may be regulated by microtubules in Merkel cells of mouse whisker hair follicles.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from Harlan Laboratories. At the age of 2–3 wk, mice were used for patch-clamp recordings from Merkel cells of whisker hair follicles, and at the age of 8- to 12-wk-old mice were used for in vitro whisker hair follicle afferent fiber recordings. In our patch-clamp recording experiments we used younger mice (age of 2–3 wk) because performing in situ patch-clamp recordings on Merkel cells was technically less challenging in younger animals than in more matured adult animals (age of 8–12 wk). Animal care and use conformed to National Institutes of Health guidelines for the care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Patch-clamp recordings from Merkel cells in situ in whisker hair follicles.
Whisker hair follicles were harvested from normal animals for patch-clamp recordings from Merkel cells in situ in whisker hair follicles. In brief, mice were anesthetized with isoflurane and euthanized by decapitation. Whisker hair follicles were dissected out from whisker pads, and the capsule of each hair follicle was removed under a dissection microscope. Whisker hair follicles (without capsules) were then affixed in a recording chamber with a tissue anchor, and the recording chamber was mounted on the stage of an Olympus IX50 microscope that was equipped with the IR-DIC and fluorescent imaging systems. Whisker hair follicles were perfused with oxygenated Krebs solutions that contained the following (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose, bubbled with 95% O2-5% CO2, had pH of 7.3 and osmolarity of 325 mosM, and were maintained at 24°C. The whisker hair follicles were then exposed to 0.05% dispase II and 0.01% collagenase in the Krebs solution for 8∼11 min, and then, the enzymes were washed off with the Krebs solution. The ring sinus and glassy membranes were removed using a glass electrode controlled by a micromanipulator. The whisker hair follicles were then incubated with 0.3 µM quinacrine in the Krebs solution for 15 min to vital-stain Merkel cells. The whisker hair follicles were continuously perfused with the oxygenated Krebs solution at a flow rate of 1.5 ml/min. Quinacrine-labeled Merkel cells were identified using the fluorescent imaging system.
Patch-clamp recordings were made at the room temperature of 23°C from quinacrine-stained cells. Recording electrodes were filled with an internal solution containing the following (in mM): 135 K-gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 HEPES, 5 Na2ATP, and 0.5 GTP-Tris salt; the pH of the solution was adjusted to 7.3 with KOH. Signals were amplified and filtered at 2 kHz using the Multiclamp 700A amplifier and sampled at 4 kHz using pCLAMP10 software (Molecular Devices, San Jose). Membrane and action potential properties of Merkel cells were determined under the whole cell current-clamp mode. Step current pulses were injected into cells through patch-clamp recording electrodes from −60 pA to 220 pA in increments of 20 pA with a pulse duration of 200 ms.
Mechanically evoked currents of Merkel cells.
Mechanically activated currents in Merkel cells were recorded with Merkel cells voltage-clamped at −75 mV using a method described in our previous study (Chang et al. 2016; Ikeda et al. 2014). In brief, a fire-polished blunted glass probe was used for the mechanical stimulation. It was connected to a computer-programmable piezo device (E-625 LVPZT; Physik Instrumente). The tip of the glass probe was ∼3 μm in diameter. It was positioned at an angle of 30° to the surface (the outer root sheath layer) of the hair follicle. The distance from the probe tip to the surface of the hair follicle tissue was set in such a way that the tip would contact the surface if the probe had one step (0.5 μm) forward movement. The stepwise forward movement of the probe was delivered by the piezo device. Merkel cells were mechanically stimulated indirectly by the probe. This was achieved by displacing adjacent nonrecorded cells so that mechanical force was transmitted across two adjacent cells (∼15 µm) to the recorded Merkel cells (Chang et al. 2016; Ikeda et al. 2014).
Whisker afferent fiber recordings of mechanical responses.
Whisker hair follicles were harvested from animals. Whisker hair follicle preparations and whisker hair follicle afferent fiber recordings were performed using our previously described method (Chang et al. 2016; Ikeda et al. 2014). In brief, mice were anesthetized with isoflurane and euthanized by decapitation. Whisker hair follicles with attached afferent fiber bundles were dissected out and anchored in a recording chamber. The whisker hair follicles were submerged and perfused in the oxygenated Krebs solution. Unless otherwise indicated, the end of the follicle capsule was cut open to facilitate bath diffusion into whisker hair follicles. To record whisker hair follicle afferent nerve impulses elicited by whisker deflections, action potentials conducted on whisker hair follicle afferent fibers were recorded using a suction electrode. Signals of nerve impulses were amplified using a Multiclamp 700A amplifier and sampled 10 KHz with low pass filter set at 1 KHz.
To record mechanical responses, hair deflection was used as a tactile stimulus to elicit whisker afferent impulses. In the present study, we anchored the whisker hair follicles in a recording chamber by affixing the whisker hair shaft onto the bottom of the recording chamber and perfused them with the Krebs solution. A fire-polished blunted glass probe was used for delivering mechanical stimuli. The probe was attached onto the capsule surface at the whisker hair follicle center and controlled by a piezo device. When the mechanical probe displaced the whisker hair follicle, it generated a whisker hair shaft deflection. This modified tactile stimulation method improved consistency of tactile responses. Unless otherwise indicated, hair deflection was induced by a 38-µm forward step to push the hair follicle for a duration of 2.62 s; the step had a 56-ms ramp at the speed of 0.68 µm/ms (dynamic phase) before reaching the 38-µm step (static phase).
Data analysis.
Electrophysiological data were analyzed using Clampfit software. Data are presented as means ± SE. Statistical significance was evaluated using Student’s t test or two-way ANOVA with Bonferroni post hoc tests for multiple groups: *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
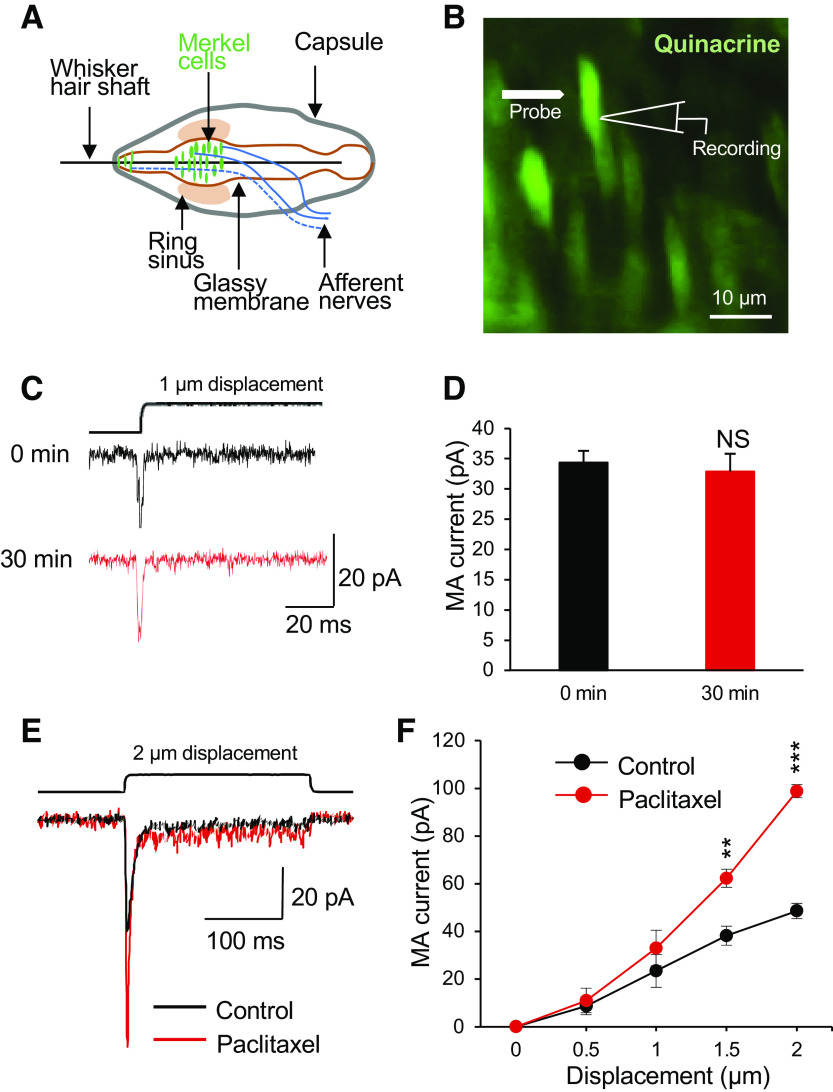
We first determined whether in vitro acute treatment of Merkel cells with paclitaxel, a microtubule stabilizer, may affect mechanically activated currents (MA) in Merkel cells of mouse whisker hair follicles. In this set of experiments, whole cell patch-clamp recordings were performed from Merkel cells preidentified in whisker hair follicles following the vital staining with the fluorescent dye quinacrine (Fig. 1, A and B). MA currents were elicited by the displacement of Merkel cell membranes with a mechanical probe. MA currents were stable during 30 min of recording time (n = 5, Fig. 1, C and D). MA currents mediated by Piezo2 channels in Merkel cells (Ikeda et al. 2014; Maksimovic et al. 2014; Woo et al. 2014) were shown to be evoked in a displacement-dependent manner (Fig. 1, C and D). At the displacements of 1.5 and 2 µm, the amplitudes of MA currents recorded from Merkel cells were significantly larger following the bath applications of 1 μM paclitaxel for 30 min than in control before paclitaxel applications (Fig. 1, C and D). For example, at a 2-µm displacement, the amplitudes of MA currents were 48.5 ± 3.2 pA (n = 6) in the control before paclitaxel application and were significantly increased to 98.9 ± 2.7 pA (n = 6) following the bath application of 1 μM paclitaxel for 30 min.
Fig. 1.
Potentiation of mechanically activated currents in Merkel cells by the microtubule stabilizer paclitaxel. A: schematic diagram illustrates main components of a mouse whisker hair follicle. Merkel cells are covered by the glassy members. B: fluorescent image shows Merkel cells preidentified by vital quinacrine staining in a whisker hair follicle preparation. In the preparation, the capsule, the ring sinus, and the glassy membranes were removed so that Merkel cells could be accessed by patch-clamp recording electrodes. C: mechanically activated (MA) currents recorded from a Merkel cell at the time points of 0 and 30 min. D: summary data (n = 5) showed that MA currents were stable for 30 min. E: overlay sample traces show MA currents recorded from a Merkel cell before (control, black trace) and following the bath application of 1 µM paclitaxel for 30 min (red trace). The mechanical stimulation was a 2-µm membrane displacement as indicated on the top of the MA currents. F: summary data of MA currents recorded from Merkel cells before (control, n = 6) and following the bath application of 1 µM paclitaxel for 30 min (n = 6). The MA currents were evoked by membrane displacements at 0.5, 1, 1.5, and 2 µm. Data represent means ± SE. NS, not significantly different; **P < 0.01, ***P < 0.001, two-way ANOVA with Bonferroni post hoc tests.
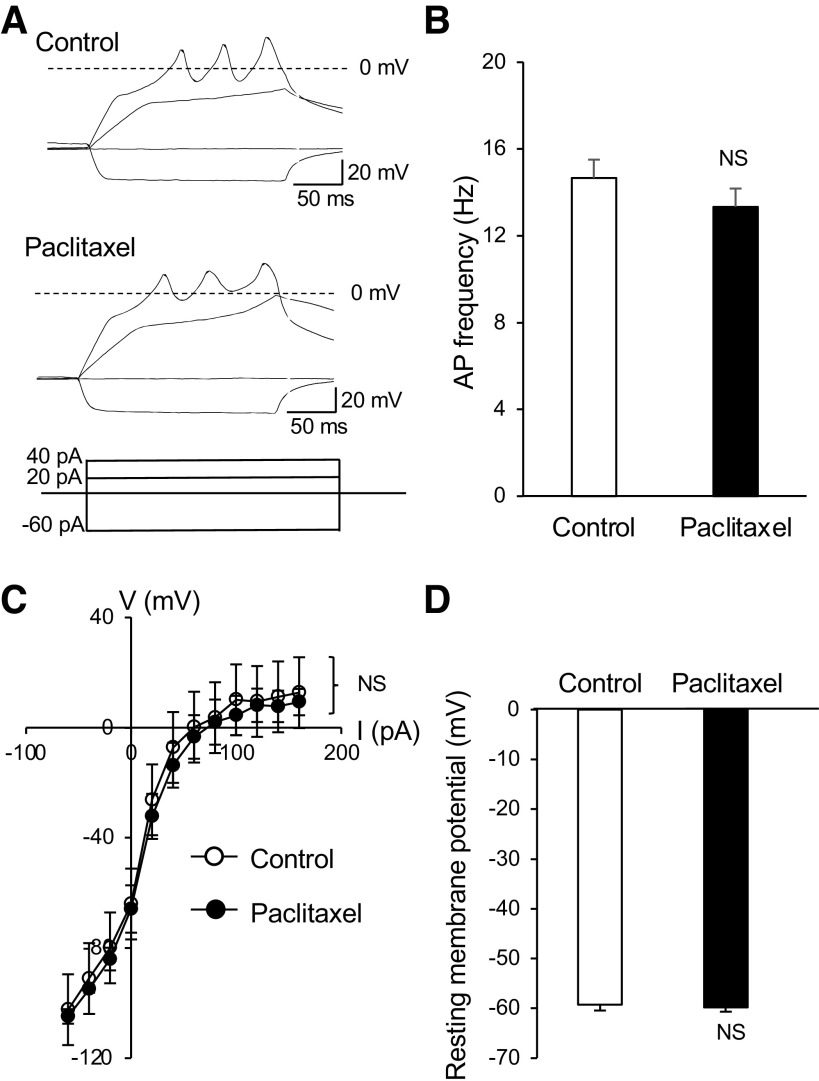
We next determined whether other electrophysiological properties of Merkel cells may be affected by the acute paclitaxel treatment. This was done by examining membrane and action potential properties of Merkel cells in whisker hair follicles to determine if these properties may be affected by the in vitro acute paclitaxel treatment. Patch-clamp recordings were performed in quinacrine-labeled Merkel cells in whisker hair follicles under the whole cell current-clamp configuration. Injections of depolarizing currents elicited membrane depolarization and action potential firing in Merkel cells under both control condition (n = 6) and following the bath applications of 1 μM paclitaxel for 30 min (n = 6, Fig. 2, A and B). Action potential firing frequencies were not significantly different between the control and following the bath application of 1 μM paclitaxel for 30 min (Fig. 2B). A plot of voltage-current relationship (Fig. 2C) showed that membrane responses to depolarizing currents were similar in the control (n = 6) and following the acute paclitaxel treatment (n = 6). Resting membrane potentials were −59.2 ± 1.25 mV (n = 6) in the control and −59.8 ± 0.89 mV (n = 6) following the acute paclitaxel treatment and were not significantly different (Fig. 2D).
Fig. 2.
Lack of effects by paclitaxel on membrane electrophysiological properties of Merkel cells. A: sample traces show membrane responses and action potentials recorded from a Merkel cell. Shown are recordings before (control; top) and following the bath application of 1 µM paclitaxel for 30 min (bottom). Membrane responses were elicited by injections of current steps each at 20 pA. B: summary data of action potential (AP) frequency in Merkel cells of control (n = 6) and following the paclitaxel application (n = 6). C: voltage-current relationship of membrane responses in Merkel cells of the control (n = 6) and following the bath application of paclitaxel for 30 min (n = 6). D: summary data of resting membrane potentials of Merkel cells in the control (open bar, n = 6) and following the bath application of paclitaxel for 30 min (n = 6). Data represent means ± SE. NS, not significantly different, two-way ANOVA with Bonferroni post hoc tests or paired Student’s t test.
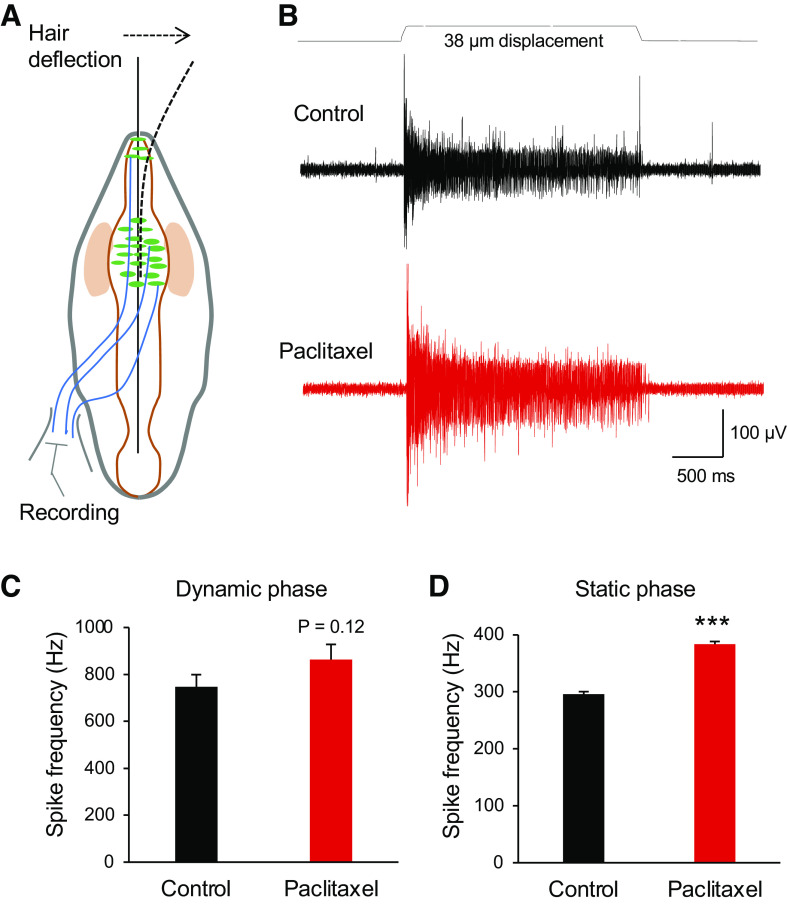
We further determined if, consistent with its potentiation of MA currents in Merkel cells, the in vitro acute paclitaxel treatment may affect mechanically evoked impulses recorded from whisker afferent nerves. In this set of experiments, whisker hair follicles were harvested from normal animals, and impulses of whisker afferent nerves in responses to a 38-µm whisker hair deflection were recorded (Fig. 3, A and B). As shown in Fig. 3, B and D, impulses in the static phase had higher frequency following the bath application of 1 μM paclitaxel for 30 min than the control before the paclitaxel application (Fig. 3D, n = 6). The impulse frequencies in the static phase were 295.5 ± 6.2 Hz (n = 6) in the control and significantly increased to 383.7 ± 7.4 Hz (n = 6, P < 0.001) following the bath application of 1 μM paclitaxel for 30 min. Impulse frequencies in the dynamic phase were 746.6 ± 52.1 Hz (n = 6) in control and were increased to 863.3 ± 82.4 (n = 6) following 1 μM paclitaxel applications for 30 min (Fig. 3C), but the change did not reach the statistically significant difference (P = 0.12).
Fig. 3.
Potentiation by paclitaxel of tactile-elicited whisker afferent impulses. A: schematic diagram shows experimental setting for recordings of whisker afferent impulses while applying tactile stimulation. Whisker afferent impulses were recorded using a suction electrode. Tactile stimuli were delivered by deflecting the whisker hair. B: sample traces show whisker afferent nerve impulses evoked by a 38-µm whisker hair displacement in a whisker hair follicle before (control; top) and following the bath application of 1 µM paclitaxel for 30 min (bottom). C: summary data show impulse frequencies of the dynamic phase in the control (n = 6) and following the bath application of 1 µM paclitaxel for 30 min (n = 6). D: summary data show impulse frequencies of the static phase in the control (n = 6) and following the bath application of 1 µM paclitaxel for 30 min (n = 6). Data represent means ± SE. ***P < 0.001, paired Student’s t test.
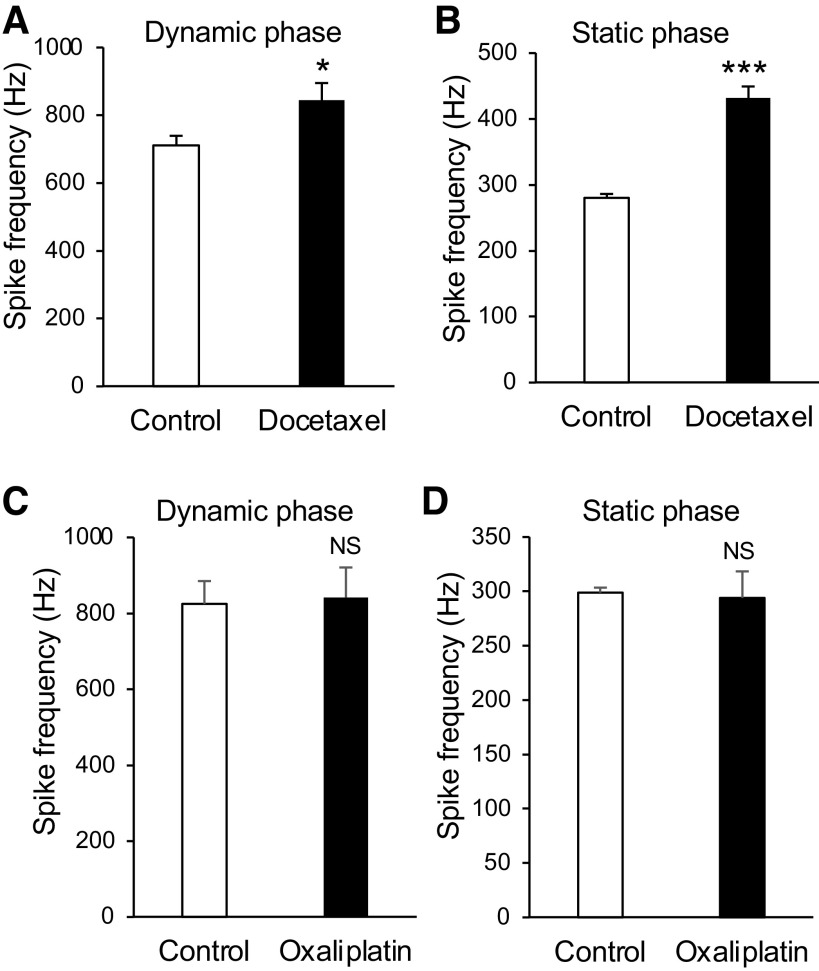
We determined if docetaxel, a paclitaxel analog that also is a microtubule stabilizer, may increase mechanically evoked impulses recorded from whisker afferent nerves. As shown in Fig. 4, A and B, impulse frequency of both dynamic and static phases showed significant increases following the bath application of 2 μM docetaxel for 30 min. In the dynamic phase, the impulse frequencies were 711.4 ± 43.3 Hz in the control (n = 6) and increased to 844.4 ± 72.5 Hz (n = 6) following the bath application of 2 μM docetaxel for 30 min (P < 0.05). In the static phase, the impulse frequencies were 280.2 ± 18.3 Hz in the control (n = 6) and increased to 431.8 ± 21.5 Hz (n = 6) following the bath application of 2 μM docetaxel for 30 min (n = 6, P < 0.001). In contrast to the microtubule stabilizers tested above, oxaliplatin, a chemotherapy drug that had no effects on microtubules, showed no effect on mechanically evoked impulses (Fig. 4, C and D). Impulse frequencies of both dynamic and static phases were not significantly different between the control (n = 6) and following the bath application of 10 μM oxaliplatin for 30 min (n = 6, Fig. 4, C and D).
Fig. 4.
Effects of docetaxel and oxaliplatin on tactile-induced whisker afferent impulses. A: summary data show impulse frequencies of the dynamic phase in the control (n = 6) and following the bath application of 2 µM docetaxel for 30 min (n = 6). B: summary data show impulse frequencies of the static phase in the control (n = 6) and following the application of 2 µM docetaxel for 30 min (n = 6). C: summary data show impulse frequencies of the dynamic phase in the control (n = 6) and following the bath application of 10 µM oxaliplatin for 30 min (n = 6). D: summary data show impulse frequencies of the static phase in the control (n = 6) and following the application of 10 µM oxaliplatin for 30 min (n = 6). Data represent the means ± SE. NS, not significantly different; *P < 0.05, ***P < 0.001, paired Student’s t test.
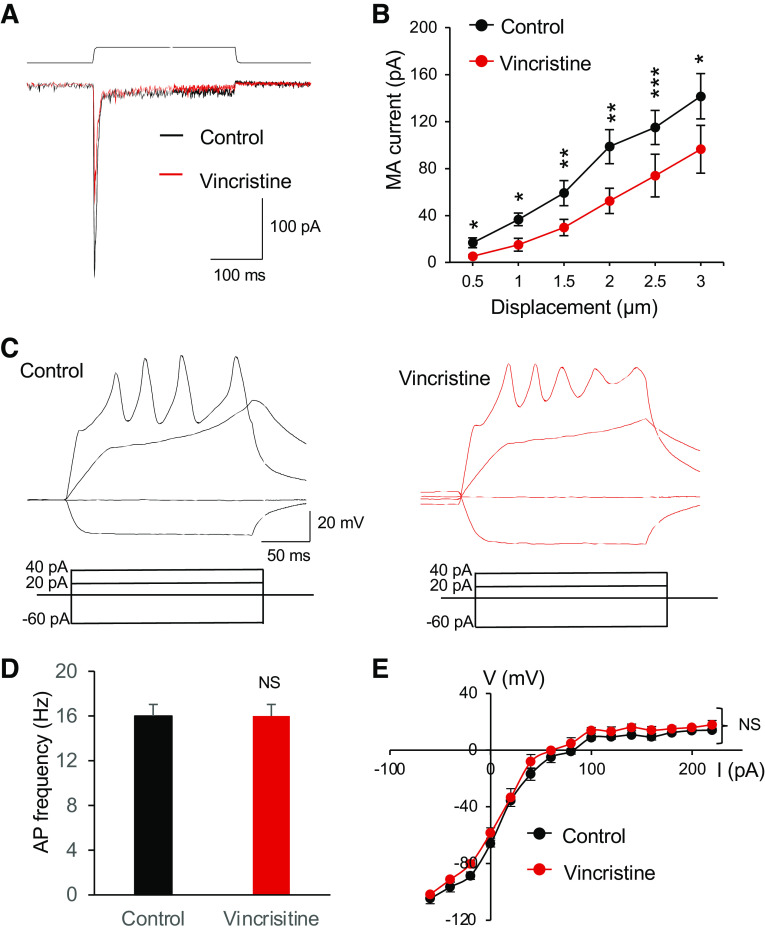
To further elucidate that microtubules are involved in the regulation of mechanotransduction in Merkel cells, we determined whether microtubule destabilizers may have effects, opposite to microtubule stabilizers, on MA currents in Merkel cells. As shown in Fig. 5, A and B, vincristine, a microtubule destabilizer, produced inhibitory effects on MA currents in Merkel cells. MA currents, which showed displacement-dependent increases in their amplitudes, became significantly smaller following the bath application of 100 µM vincristine for 30 min in comparison with the control before vincristine application (n = 6, Fig. 5B). For example, MA current amplitudes in response to a 2-µm displacement were 98.5 ± 14.5 pA (n = 6) in the control and decreased to 52.3 ± 10.8 pA (n = 6) following the bath application of 100 µM vincristine (Fig. 5B). The significant differences in MA current amplitudes between the control and following the vincristine application were observed in all the tested displacements from 0.5 to 3 µm (Fig. 5B).
Fig. 5.
Effects of the microtubule destabilizer vincristine on mechanically activated currents and membrane electrophysiological properties of Merkel cells. A: overlay sample traces show mechanically activated (MA) currents from a Merkel cell before (control, black) and following the bath application of 100 µM vincristine for 30 min. The mechanical stimulation was a 3-µm membrane displacement indicated on the top of MA currents. B: summary data of MA currents recorded from Merkel cells before (control, n = 6) and following the bath application of 100 µM vincristine for 30 min (n = 6). The MA currents were evoked by membrane displacements at 0.5, 1, 1.5, 2, 2.5, and 3 µm. C: sample traces show membrane responses and action potentials recorded from a Merkel cell in the control (left) and following the bath application of 100 µM vincristine for 30 min (right). Membrane responses were elicited by injections of current steps each at 20 pA. D: summary data of action potential (AP) frequencies in Merkel cells in the control (n = 6) and following the bath application of 100 µM vincristine for 30 min (n = 6). E: voltage-current relationship of membrane responses in Merkel cells of the control (n = 6) and following the bath application of 100 µM vincristine for 30 min (n = 6). Data represent means ± SE. NS, not significantly different; *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA with Bonferroni post hoc tests or paired Student’s t test.
We examined membrane and action potential properties of Merkel cells in whisker hair follicles to determine if these electrophysiological properties may be affected by the in vitro acute vincristine treatment. Action potential firing frequencies were not significantly different between the control and following the bath application of 100 µM vincristine for 30 min (n = 6, Fig. 5, C and D). A plot of the voltage-current relationship (Fig. 5E) showed that membrane responses to depolarizing currents were not significantly different between the control (n = 6) and following the application of vincristine for 30 min (n = 6). Resting membrane potentials were −64.1 ± 2.4 mV (n = 6) in the control and −59.4 ± 3.6 mV (n = 6) following the application of vincristine for 30 min, and were not significantly different (Fig. 5E).
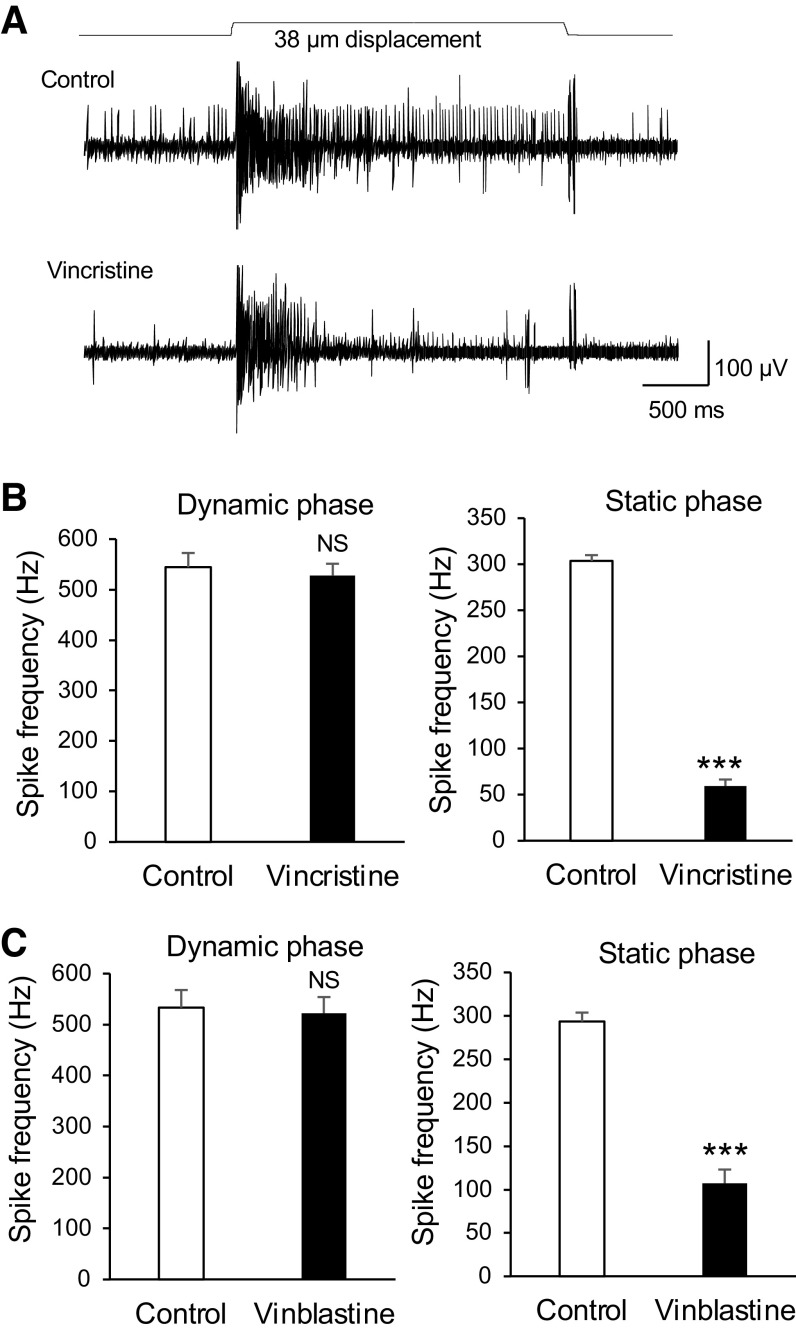
We finally determined whether microtubule destabilizers may have effects, opposite to microtubule stabilizers, on mechanically evoked impulses recorded from whisker afferent nerves. While the impulse frequency in the dynamic phase was not significantly affected by vincristine, the impulse frequency in the static phase was significantly reduced following the bath application of 100 µM vincristine for 30 min (Fig. 6, A and B). The impulse frequency in the static phase was 303.6 ± 6.3 Hz (n = 6) in the control and significantly decreased to 59.3 ± 7.1 Hz (n = 6) following the bath applications of 100 µM vincristine for 30 min. Similar to vincristine, vinblastine, a vincristine analog that also is a microtubule destabilizer, significantly suppressed impulses in the static phase but had no effect on impulses in the dynamic phase (Fig. 6C). The impulse frequency in the static phase was 293.5 ± 10.2 Hz in the control (n = 6) and decreased to 107.1 ± 15.8 Hz (n = 6) following the bath applications of 100 µM vinblastine for 30 min (Fig. 6C).
Fig. 6.
Suppression of tactile-elicited whisker afferent impulses by vincristine and vinblastine. A: sample traces show whisker afferent impulses evoked by a 38-µm whisker hair displacement in a whisker hair follicle before (control, top) and following the bath application of 100 µM vincristine for 30 min (bottom). B: summary data show whicker afferent impulse frequencies of the dynamic phase (left) and the static phase (right) in the control (open bars, n = 6) and following the bath application of 100 µM vincristine (closed bars, n = 6). C: summary data show whisker afferent impulse frequencies of the dynamic phase (left) and the static phase (right) in the control (open bars, n = 6) and following the bath application of 100 µM vinblastine (closed bars, n = 6). Data represent the means ± SE. NS, not significantly different; ***P < 0.001, paired Student’s t test.
DISCUSSION
In the present study, we have shown that in vitro acute treatment of whisker hair follicles with microtubule stabilizing agents potentiates Piezo2-mediated MA currents in Merkel cells and increases mechanically evoked whisker afferent impulses. In contrast, in vitro acute treatment of whisker hair follicles with microtubule destabilizing agents suppresses Piezo2-mediated MA currents in Merkel cells and reduces mechanically evoked whisker afferent impulses. These findings for the first time provide the evidence showing that mechanotransduction mediated by Piezo2 channels is highly regulated by microtubules in Merkel cells.
We observed a large variation of spike frequencies in the dynamic phase but not in the static phase. The large variation in the dynamic phase may be due to a lower number of spikes in a very short time of the dynamic phase. We also observed a large variation in the amplitudes of MA currents recorded from Merkel cells. This could be due to that MA currents were evoked by the indirect mechanic stimulation applied to adjacent tissues in the present study. In addition, mechanical probes used in different experiments were not exactly the same in their sizes, which could also contribute to the variation of recorded MA currents in response to a given displacement. However, since our experiments were designed for paired comparison before and following the application of microtubule targeting agents, the aforementioned variation should not significantly affect our observations of the effects of the microtubule targeting agents tested in the present study.
We have previously shown that in vivo chronic treatment of animals with vincristine also resulted in a reduction of MA currents in Merkel cells. MA currents in Merkel cells have been shown to be mediated by Piezo2 channels (Ikeda et al. 2014; Maksimovic et al. 2014; Woo et al. 2014). It is currently not determined whether a change in the expression of Piezo2 channels or other mechanisms may underlie the reduction of MA currents in Merkel cells in the in vivo chronic treatment of vincristine, For the present study, it is unlikely that the acute treatment of isolated whisker hair follicles with vincristine or paclitaxel can result in an immediate change of Piezo2 channel expression in Merkel cells to contribute to the changes of MA currents. On the other hand, functional regulations of Piezo2 channels have been reported to result in a rapid change of MA currents following the activation of intracellular signaling pathways via protein kinase A, protein kinase C (Dubin et al. 2012), and Epac1 (Eijkelkamp et al. 2013; Singhmar et al. 2016). However, vincristine and paclitaxel, two microtubule targeting agents, are not known to be directly related to the aforementioned intracellular signaling pathways. Therefore, it is unlikely that the intracellular signaling pathways were involved in the regulation of Piezo2 channel functions by the microtubule targeting agents. Alternatively, microtubules may have interactions with Piezo2 channels or with protein tethers (Hu et al. 2010) that link to Piezo2 channels to participate in the gating of Piezo2 channels. However, previous studies have suggested that Piezo channels can open without protein tethers (Murthy et al. 2017), which discount the idea of microtubules being essential for the direct gating of Piezo2 channels.
Microtubules are cytoskeleton essential for maintaining membrane mechanics of cells. We have previous shown that Piezo2-mediated MA currents in somatosensory neurons depend on the states of membrane mechanics of these sensory neurons (Jia et al. 2016). We have shown that actin filaments, also parts of cytoskeleton, play a role in regulating Piezo2 channel functions through their actions on membrane mechanics in somatosensory neurons. Disruption of actin cytoskeleton with cytochalasin D resulted in changes of membrane mechanics with a significant reduction of membrane tension, which was accompanied by a reduction of Piezo2-mediated MA currents (Jia et al. 2016). Consistently, a recent study also has shown that changes of membrane mechanics by depleting membrane cholesterol or its interacting protein STOML3 affected Piezo2 mediated mechanotransduction in somatosensory neurons (Qi et al. 2015). Since vincristine is a microtubule destabilizer and paclitaxel is a microtubule stabilizer, treating Merkel cells with these agents would affect Merkel cell cytoskeleton to alter their membrane mechanics. This would in turn result in the changes of Piezo2-mediated MA currents in Merkel cells. This interpretation is consistent with the recent evidence suggesting that microtubule stability is essential for mechanosensation (Morley et al. 2016; Yan et al. 2018).
In the present study with whisker afferent nerve recordings we have shown that the numbers of mechanically elicited impulses were significantly reduced following the in vitro acute treatment with vincristine and its analog vinblastine and increased following the in vitro acute treatment with paclitaxel and its analog docetaxel. These results are consistent with the inhibition of Piezo2-mediated MA currents by vincristine and the potentiation of Piezo2-mediated MA currents by paclitaxel in Merkel cells. On the other hand, oxaliplatin, which is not a microtubule targeting agent, has no effect on mechanically elicited impulses recorded from whisker afferent nerves. We have shown that both vincristine and paclitaxel had no significant effects on the intrinsic electrophysiological properties of Merkel cells. This supports the idea that the two types of microtubule targeting agents specifically affect Piezo2-mediated mechanotransduction leading to the changes of tactile responses as measured with the whisker afferent nerve recordings. Three types of mechanoreceptors, including rapidly adapting (RA), slowly adapting type 1 (SA1), and slowly adapting type 2, are present in whisker hair follicles. By using the pressured-clamped single-fiber recording technique we have previously recorded all the three types of mechanoreceptor responses in mouse whisker hair follicles (Sonekatsu and Gu 2019; Sonekatsu et al. 2020). In the present study, we did not try to differentiate different types of mechanoreceptor responses by using the single-fiber recording technique. However, it has been known that Merkel cells and its associated whisker afferent endings, i.e., Merkel disks, generate SA1 impulses (Halata and Munger 1980; Iggo and Muir 1969). Since the microtubule targeting agents tested in the present study significantly altered Piezo2-mediated MA currents in Merkel cells, it is conceivable that SA1 should be significantly affected by the microtubule targeting agents.
The present study has provided strong evidence suggesting that Piezo2-mediated mechanotransduction are regulated by microtubules in Merkel cells. Piezo2 channels are also expressed in somatosensory afferent nerves innervating the skin, other hair follicles, and muscle spindles, as well as in autonomic nerves innervating the lung and the heart (Murthy et al. 2017; Zeng et al. 2018). It would be interesting to examine whether microtubules also play roles in regulating Piezo2 channel functions in these cells. Microtubule targeting agents including vincristine and paclitaxel are clinically used as important chemotherapy drugs for treating cancers. The role of microtubules in Piezo2 mechanotransduction shown in the present study may thus have broad clinical implications.
GRANTS
This work was supported by National Institute of Dental and Craniofacial Research Grants DE-018661 and DE-023090 (to J.G.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G.G. conceived and designed research; W.C. performed experiments; W.C. analyzed data; J.G.G. interpreted results of experiments; W.C. and J.G..G prepared figures; W.C. and J.G.G. drafted manuscript; J.G.G. edited and revised manuscript; J.G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Janet McDaniel for proofreading the earlier version of this manuscript.
REFERENCES
- Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 8: ra15, 2015. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Gu JG. Effects on tactile transmission by serotonin transporter inhibitors at Merkel discs of mouse whisker hair follicles. Mol Pain 16: 1744806920938237, 2020. doi: 10.1177/1744806920938237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA 113: E5491–E5500, 2016. doi: 10.1073/pnas.1610176113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosario JS, Yudin Y, Su S, Hartle CM, Mirshahi T, Rohacs T. Gi-coupled receptor activation potentiates Piezo2 currents via Gβγ. EMBO Rep 21: e49124, 2020. doi: 10.15252/embr.201949124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Reports 2: 511–517, 2012. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 4: 1682, 2013. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271: 225–239, 2003. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- Halata Z, Munger BL. Sensory nerve endings in rhesus monkey sinus hairs. J Comp Neurol 192: 645–663, 1980. doi: 10.1002/cne.901920403. [DOI] [PubMed] [Google Scholar]
- Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100: 1401–1413.e6, 2018. doi: 10.1016/j.neuron.2018.10.034. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Chiang LY, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. EMBO J 29: 855–867, 2010. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200: 763–796, 1969. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157: 664–675, 2014. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Ikeda R, Ling J, Gu JG. GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Mol Brain 6: 57, 2013. doi: 10.1186/1756-6606-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Ikeda R, Ling J, Viatchenko-Karpinski V, Gu JG. Regulation of Piezo2 mechanotransduction by static plasma membrane tension in primary afferent neurons. J Biol Chem 291: 9087–9104, 2016. doi: 10.1074/jbc.M115.692384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. doi: 10.1016/S0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509: 617–621, 2014. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel F Tastzellen and Tastkoerperchen bei den Hausthieren und beim Menschen. Arch Mikrosc Anat 11, S1: 636–652, 1875. doi: 10.1007/BF02933819. [DOI] [Google Scholar]
- Morley SJ, Qi Y, Iovino L, Andolfi L, Guo D, Kalebic N, Castaldi L, Tischer C, Portulano C, Bolasco G, Shirlekar K, Fusco CM, Asaro A, Fermani F, Sundukova M, Matti U, Reymond L, De Ninno A, Businaro L, Johnsson K, Lazzarino M, Ries J, Schwab Y, Hu J, Heppenstall PA. Acetylated tubulin is essential for touch sensation in mice. eLife 5: e20813, 2016. doi: 10.7554/eLife.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18: 771–783, 2017. doi: 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun 6: 8512, 2015. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr, Murga C, Heijnen CJ, Kavelaars A. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA 113: 3036–3041, 2016. doi: 10.1073/pnas.1516036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonekatsu M, Gu JG. Functional properties of mechanoreceptors in mouse whisker hair follicles determined by the pressure-clamped single-fiber recording technique. Neurosci Lett 707: 134321, 2019. doi: 10.1016/j.neulet.2019.134321. [DOI] [PubMed] [Google Scholar]
- Sonekatsu M, Yamada H, Gu JG. Pressure-clamped single-fiber recording technique: A new recording method for studying sensory receptors. Mol Pain 16: 1744806920927852, 2020. doi: 10.1177/1744806920927852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang F, Peng Y, Williams CR, Jenkins B, Wildonger J, Kim HJ, Perr JB, Vaughan JC, Kern ME, Falvo MR, O'Brien ET, 3rd, Superfine R, Tuthill JC, Xiang Y, Rogers SL, Parrish JZ. Microtubule acetylation is required for mechanosensation in Drosophila. Cell Rep 25: 1051–1065.e6, 2018. doi: 10.1016/j.celrep.2018.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362: 464–467, 2018. doi: 10.1126/science.aau6324. [DOI] [PMC free article] [PubMed] [Google Scholar]