Abstract
Colloquially referred to as “taste,” flavor is in reality a thoroughly multisensory experience. Yet, a mechanistic understanding of the multisensory computations underlying flavor perception and food choice is lacking. Here, we used a multisensory flavor choice task in rats to test specific predictions of the statistically optimal integration framework, which has previously yielded much insight into cue integration in other multisensory systems. Our results confirm three key predictions of this framework in the unique context of flavor choice behavior, providing novel mechanistic insight into multisensory flavor processing.
NEW & NOTEWORTHY The authors demonstrate that rats make choices about which flavor solution (i.e., taste-odor mixture) to consume by weighting the individual taste and odor components according to the reliability of the information they provide about which solution is the preferred one. A similar weighting operation underlies multisensory cue combination in other domains and offers novel insight into the computations underlying multisensory flavor perception and food choice behavior.
Keywords: cross-modal, cue combination, maximum likelihood estimation, multisensory, preference
INTRODUCTION
The ability to combine information from multiple senses allows animals to form a coherent experience of their surroundings and make adaptive decisions (Stein 2012). Flavor perception is considered a prime example of multisensory experience, combining gustatory (i.e., sweet, salty, sour, bitter, umami), olfactory (e.g., vanilla, fruity, smoky), and oral chemesthetic (e.g., spicy) inputs to inform consumption behavior (Shepherd 2006; Small and Green 2012; Spence 2015). Psychophysical studies measuring various aspects of flavor perception—including detection, intensity, and pleasantness ratings—have started to uncover the operations by which flavor components are combined (Fondberg et al. 2018; Murphy and Cain 1980; Veldhuizen et al. 2010b). However, a computational framework for understanding flavor preference judgments in the context of food choice behavior is currently still lacking. One particularly powerful framework for explaining the multisensory computations underlying evaluative perceptual judgments in other systems is Bayesian or statistically optimal integration (Ernst and Banks 2002; Ernst and Bülthoff 2004). This framework predicts that the judgments of multisensory stimuli are a weighted average of the unisensory component judgments, and that the weight carried by the individual component judgments depends on their reliability. This computation effectively reduces the variability of multisensory judgments as compared with the unisensory component judgments, resulting in more robust behavior. Here, we used a multisensory flavor preference task in rats to evaluate the validity of the statistically optimal integration framework for explaining choice behavior in response to taste-odor mixtures and their unisensory components. Critically, we parametrically varied the reliability of gustatory information for task performance, allowing us to test key predictions of the framework: 1) multisensory flavor choices are a weighted average of the choices based on the unisensory taste and odor components; 2) the relative weight placed on the taste component changes with relative reliability of the taste stimulus for choice behavior; and 3) multisensory flavor choices are less variable than choices based on either unisensory component.
METHODS
Animals.
Long–Evans rats (n = 16 total, n = 6 female) were used for this study. Pregnant dams were obtained from https://www.criver.com, and litters were kept under standard conditions until weaning on postnatal day (PND) 21. All procedures took place in animals’ housing facilities and were approved by the Institutional Animal Care and Use Committee of Wake Forest School of Medicine.
Stimuli.
Stimuli consisted of aqueous solutions of taste and/or odor compounds (obtained from www.fischersci.com and https://www.sigmaaldrich.com, >98% purity, dissolved in distilled water) and were acquired by licking solutions from a lick spout, ensuring natural consumption-related multisensory stimulus dynamics, including both orthonasal and retronasal olfactory stimulation. Tastants used were sucrose (2% weight/volume) and citric acid (0.4%). For odorants, we used monomolecular odorants solutions with no known innate palatability and no known gustatory or chemesthetic qualities (n-amyl acetate and 2-hexanone, 0.025%). Maltodextrin, a highly caloric and slightly sweet-tasting polysaccharide, was used as an unconditioned stimulus during conditioning sessions.
Procedures.
The present experiment aimed to test how learned odor preferences interact with innate taste preferences to inform the expression of preferences to taste + odor mixtures. Odor preferences were conditioned during an initial condition phase; preferences for odor, taste, and taste + odor solutions were tested during a subsequent testing phase (Table 1).
Table 1.
Experimental timeline
| Testing |
|||||
|---|---|---|---|---|---|
| Conditioning | Block 1 (odor only, taste + odor) | Block 2 (odor only, taste + odor) | Block 3 (taste only) | Block 4 (taste only) | |
| Number of days | 28 | 9 | 9 | 4 | 4 |
| Age, postnatal days | 21–48 | 51–59 | 60–68 | 69–72 | 73–76 |
Conditioning occurred over a period of 4 wk (from PND 21 to PND 48), during which animals learned to associate one odor with high caloric value (odor H, 250 cal/L) and the other one with low caloric value (odor L, 2.5 cal/L). Each week, animals were exposed to odor solutions mixed with high or low amounts of maltodextrin, for 6 consecutive days. Molecular identity (i.e., n-amyl acetate or 2-hexanone) of odors H and L was counterbalanced between animals. Each conditioning day, animals received a single odor + maltodextrin solution for 16 h overnight, with odor identity alternating, L-H-L-H-L-H, resulting in three exposures per week and 12 exposures in total over the course of training for each odor. Each block of 6 training days was followed by 1 day of ad libitum access to plain water. To ensure equal exposure to solutions containing odors H and L, volume was limited to 20 mL each day and consumption was monitored throughout the training period (amount consumed did not differ between odors H and L, as determined by t test comparing average consumption from odors H and L across animals; t15 = 0.65, P = 0.53). Preference testing occurred over a period of four consecutive blocks, the first one starting 2 days after the last training day (PND 51). Each testing day, animals had access to two bottles, placed side-by-side, containing taste and/or odor solutions for 16 h overnight (200 mL total per bottle, ensuring ad libitum access). One bottle always contained a control solution, consisting of a 50%/50% mixture of citric acid/sucrose and/or odor L; the second bottle contained a test solution, consisting of one of four citric acid/sucrose mixtures of varying palatability relative to control (Table 2) and/or odor H. During the first two blocks, all taste + odor (n = 4) and odor-only (n = 1) conditions were presented (order of conditions randomized within each block for each animal). During the third and fourth blocks, all taste-only conditions (n = 4) were presented (order of conditions randomized within each block for each animal). In between exposures to conditioning or testing solutions (i.e., 8 h during the day time), animals received ad libitum access to water.
Table 2.
Two-bottle testing conditions
| Control Bottle |
Test Bottle |
|||
|---|---|---|---|---|
| Taste | Odor | Taste | Odor | |
| Taste only | 50%/50% | 20%/80% | ||
| 50%/50% | 40%/60% | |||
| 50%/50% | 60%/40% | |||
| 50%/50% | 80%/20% | |||
| Odor only | “Low calorie” (L) | “High calorie” (H) | ||
| Taste + odor | 50%/50% | “Low calorie” (L) | 20%/80% | “High calorie” (H) |
| 50%/50% | “Low calorie” (L) | 40%/60% | “High calorie” (H) | |
| 50%/50% | “Low calorie” (L) | 60%/40% | “High calorie” (H) | |
| 50%/50% | “Low calorie” (L) | 80%/20% | “High calorie” (H) | |
Data analysis.
Consumption was measured by comparing bottle weight before and after testing sessions. Preference for the test bottle was calculated as: ConsumptionTest/(ConsumptionControl + ConsumptionTest). In 19 bottles (3.3% of all bottles), consumption could not be determined due to animals moving the bottles out of reach during testing. Within-condition preferences were consistent between testing blocks (r = 0.57, P < 0.001), and all further analyses were based on the average preference across repetitions of the same condition, unless stated otherwise. All data points obtained in this manner (i.e., average preference in four taste conditions, one odor condition, and four mixture conditions for each animal, n = 144) were treated independently, unless otherwise indicated. Model predictions for mixture preference were obtained for each mixture condition in each animal as follows: PredictionAdditive = PreferenceOdor + PreferenceTaste − 0.5 and PredictionAverage = (PreferenceOdor + PreferenceTaste)/2. Error for each prediction was calculated as the deviation from the observed preference: Error = (PreferenceMixture – Prediction)^2. Component weight was obtained for each condition in each animal by calculating the distance between mixture preference and taste preference, relative to the distance between mixture preference and odor preference: Weight = |PreferenceTaste − PreferenceMixture|/|PreferenceOdor − PreferenceMixture|.
RESULTS
In the present experiment, we asked how the addition of an odorant in mixture with a taste solution affects preference judgments. Whereas taste preferences are innate, odor preferences are heavily dependent on individual experience. Thus, to experimentally control odor preferences, we first subjected animals to a conditioning procedure. During the conditioning phase, animals learned to associate one odorant with high caloric value and the other with low caloric value by consuming the odorants in mixture with high or low amounts of maltodextrin, respectively. Following conditioning of odor preferences, we assessed rats’ flavor preferences in a series of two bottle tests (Table 1). During the preference testing phase, both bottles contained solutions of either taste-only, odor-only, or taste + odor mixture, and preferences were measured as preference for a test solution relative to a control solution (Table 2). Animals were not deprived or trained on the preference task; instead, the two-bottle test harbors an implicit task (“which of these two solutions do you prefer?”), providing a measure of spontaneous, naturalistic choice behavior. Note that the present study differs from previous work on (multisensory) cue combination in that previous studies focused on objective properties of individual stimuli as the relevant cues and determined how estimates of these cues are integrated (Alais and Burr 2004; Ernst and Banks 2002; Knill and Saunders 2003). In contrast, preference judgments are inherently subjective and can only be determined by comparing two stimuli. We, therefore, focus here on relative preference estimates in the taste and odor modalities as the relevant cues and determine how these relative estimates are integrated.
Unisensory taste preferences.
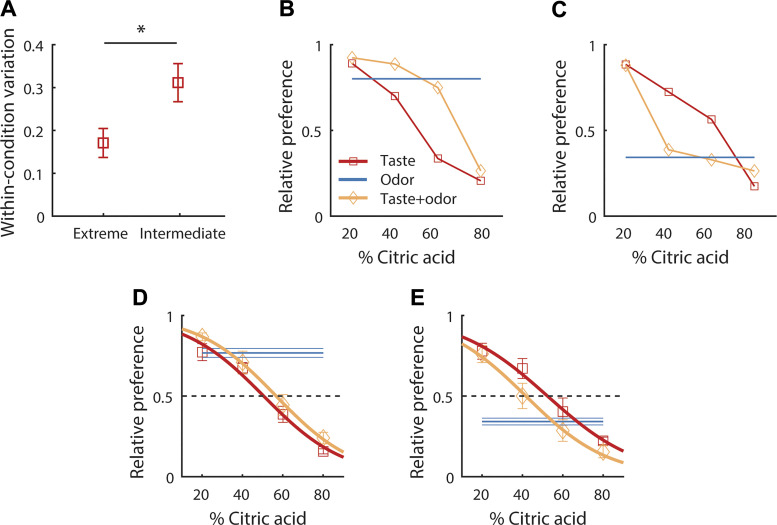
We first characterized preferences for the unisensory component stimuli. Taste components were chosen on the basis of known innate palatability (Maier and Katz 2013) and varied from palatable to unpalatable (relative to the control solution) in four steps by simultaneously increasing citric acid content and decreasing sucrose content. Monotonically decreasing palatability of taste stimuli was confirmed by the results obtained from taste-only conditions (Fig. 1): intermediate stimuli (i.e., 40%/60%, 60%/40%) yielded preferences closer to 0.5 (relative to control: 50%/50%) than extreme stimuli (i.e., 20%/80%, 80%/20%). Testing key predictions of the statistically optimal integration framework critically relies on manipulating the reliability of the sensory estimates, which is inversely proportional to the variance of the estimates. In the context of our experimental design, variance was measured as the absolute difference between two repetitions of the same condition. This analysis revealed that judgments on extreme taste conditions were less variable than judgments on intermediate taste conditions (ANOVA on absolute difference in taste preferences between repetitions of the same condition with factor citric acid content: F3,49 = 2.84, P < 0.05; Fig. 1A). Thus, varying citric acid content effectively modulated mean preference, as well as reliability of gustatory information for task performance.
Fig. 1.
A: means (±SE) deviation between taste-only preferences obtained from two repetitions of the same condition [pooled across intermediate (40%/60%, 60%/40%) and extreme (20%/80%, 80%/20%) taste-only conditions]. B–E: flavor choice behavior in taste (red squares), odor (blue), and mixture (yellow diamonds) conditions. Preferences (relative to control) as a function of citric acid content for one example animal that preferred odor H (B), and one example animal that preferred odor L (C), as well as the means (±SE) preferences over all animals that preferred odor H (n = 12) (D) and odor L (n = 4) (E). Lines in D and E show logistic regression. Results from t test as described in the text is indicated above (A) (*P < 0.05).
Unisensory odor preferences.
To control the palatability of odor components (which have no consistent innate palatability), animals were conditioned to associate one odorant with low caloric value (odor L, control) and the other one with high caloric value (odor H) before testing. As expected, based on the literature (Bolles et al. 1981; Holman 1975), results obtained from the odor-only condition revealed that on average, odor H was preferred over odor L (t test comparing relative preference for odor H to 0.5: t15 = 3.10, P < 0.01). However, there was substantial individual variability ( = 0.66, s = 0.21, minimum = 0.29, maximum = 0.92), and although the majority of individual animals [n = 12/16 (75%)] preferred odor H, some animals [n = 4/16 (25%)] preferred odor L, possibly due to substantial variation in naïve odor preferences (Jagetia et al. 2018). We exploited this individual variation in odor preference in all remaining analyses.
Multisensory preferences.
Next, we asked how estimates of taste preference interact with estimates of odor preference to determine multisensory preference. We predicted that preferences obtained from the taste-only condition would be increased with the addition of a more palatable odor stimulus and decreased with the addition of a less palatable odor stimulus. Figure 1 shows preferences for all conditions in two example animals. In the animal that preferred odor H (Fig. 1B), preferences for multisensory mixtures were mostly increased relative to preferences for taste-only stimuli, whereas the opposite pattern was observed for the animal that preferred odor L (Fig. 1C). The same pattern was observed across the population of animals (Fig. 1, D and E)—on average, more palatable odor stimuli increased preferences; less palatable odor stimuli decreased preferences [two-way ANOVA on preference with factors modality (taste only, taste + odor) and odor preference (prefer odor H, prefer odor L) yielded a significant interaction: F1,62 = 6.35, P < 0.05]. These data demonstrate that more and less palatable odors had opposite effects on taste preferences.
Multisensory judgments reflect component averaging.
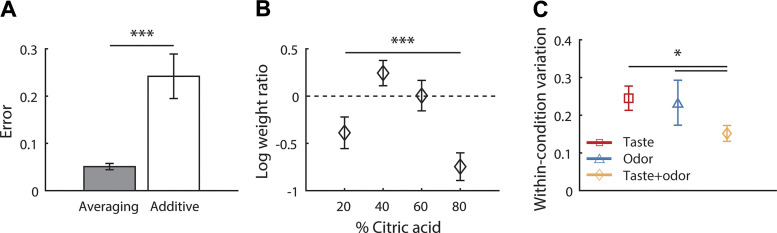
The observed pattern of multisensory preferences is consistent with a linear weighting of the unisensory taste and odor preferences. However, the results may also be consistent with an additive model, which predicts that higher/lower odor preferences (relative to control) are simply added to/subtracted from taste preferences to produce multisensory preferences. An additive operation has previously been suggested to underlie taste + odor mixture perception (Harris and Thein 2005; Murphy and Cain 1980). Explicitly comparing our experimentally obtained multisensory preferences to both averaging and additive predictions revealed a better fit with the averaging model (Fig. 2A; t test comparing error for averaging and additive models: t63 = 4.00, P < 0.001), consistent with the framework of statistically optimal integration.
Fig. 2.
A: deviation between observed multisensory preferences and preferences predicted by averaging (filled bar) and additive (open bar) models. Smaller values indicate better model fit. B: means (±SE) log weight ratio over all multisensory conditions for all animals (n = 64) as a function of citric acid content. Negative values indicate greater weight on taste; positive values indicate greater weight on odor. C: means (±SE) deviation between preferences obtained from two repetitions of the same condition (if available), for taste (red squares, n = 53), odor (blue triangles, n = 14), and mixture (yellow diamonds, n = 61) conditions. Results from t test (A and C) and one-way ANOVA (B), as described in the text are indicated above the graphs (*P < 0.05, ***P < 0.001).
Component weight depends on taste reliability.
The framework of statistically optimal integration further predicts that the relative weight carried by a component of a multisensory stimulus is determined by the reliability of the judgment of that component with respect to task performance. The predicted pattern can be observed in the individual animal data in Fig. 1, B and C: odor had a larger effect on the intermediate taste conditions as compared with the extreme taste conditions. To quantify this effect, we calculated how much of the taste-odor mixture preference judgments is explained by taste preference judgments (relative to odor preference judgments) for each condition in each subject. Figure 2B shows the average log ratio of taste to odor weight, plotted as a function of citric acid content. A log ratio of 0 indicates equal weight on taste and odor, a negative log ratio indicates a higher weight on taste, and a positive log ratio indicates a higher weight on odor. Weight ratios varied significantly as a function of citric acid content (one-way ANOVA on log weight ratio with factor citric acid content: F3,45 = 7.48, P < 0.001), indicating that in conditions where taste information is reliable in signaling the most preferred stimulus (i.e., the extreme conditions), weight ratios are more biased toward taste as compared with conditions where taste information is more ambiguous (i.e., the intermediate conditions).
Multisensory judgments are more reliable than component judgments.
Finally, statistically optimal integration predicts that the reliability of multisensory judgments is increased relative to the component judgments. To test this prediction, we compared the absolute difference between two repetitions of the same condition across modalities (shown in Fig. 2C). Overall, variation within unisensory taste and odor conditions was comparable. Consistent with statistically optimal integration, variation in taste + odor preferences was lower than variation in both taste preferences (t test comparing absolute differences between repetitions of taste conditions to taste + odor conditions: t49 = 2.37, P < 0.05) and odor preferences (t test comparing absolute differences between repetitions of odor conditions to taste + odor conditions: t52 = 2.42, P < 0.05).
DISCUSSION
Our results demonstrate that animals weight taste and odor components of flavor depending on their relative reliability to make more robust multisensory preference judgments. This pattern of results is consistent with the framework of statistically optimal integration and suggests an adaptive mechanism for weighting multisensory information while making flavor preference decisions. Similar operations have previously been proposed to underlie visuohaptic (Ernst and Banks 2002), visuovestibular (Butler et al. 2010), auditory-tactile (Bresciani and Ernst 2007), and auditory-visual (Alais and Burr 2004) perceptual judgments in humans, as well as visuovestibular judgments in monkeys (Fetsch et al. 2009). Compared with these previous studies, the present study is unique in that animals in this study made subjective judgments (palatability of flavor stimuli is individual specific and cannot be assessed using objective means). The fact that behavioral patterns are comparable across species, sensory systems, and types of judgments suggests that multisensory networks in the brain may perform remarkably similar and evolutionarily conserved computations to combine their inputs.
The neural mechanisms underlying multisensory cue integration have been extensively studied in visuovestibular neurons in the brain of rhesus monkeys. These studies demonstrate that populations of neurons in the medial superior temporal area weight their visual and vestibular inputs according to their relative reliability to inform more robust judgments of heading direction (Fetsch et al. 2012; Gu et al. 2008; Morgan et al. 2008; Ohshiro et al. 2011, 2017). Where and how taste and odor inputs are integrated to inform flavor preference decisions is yet to be determined. Physiological and imaging work in rodents, monkeys, and humans identified multiple brain regions where taste and odor inputs converge, including classical “association” areas such as the orbitofrontal and anterior cingulate cortex (de Araujo et al. 2003; Rolls et al. 1996; Small et al. 2004), as well as primary olfactory (piriform) (Avery et al. 2020; Maier et al. 2012, 2015) and gustatory cortices (Samuelsen and Fontanini 2017; Veldhuizen et al. 2010a; Vincis and Fontanini 2016). The present study provides a framework for systematically investigating the computations performed by multisensory neurons in these areas and their role in guiding flavor-related decision-making.
Despite similarities in the multisensory computation performed in flavor and other multisensory domain, taste-odor perception exhibits several unique characteristics that may affect multisensory computations. One major consideration is that the correspondences between visual, vestibular, haptic, and auditory spatial cues in the studies mentioned earlier are fixed and their neural representations are established in early life (Stein et al. 2014). Conversely, flavor perception is shaped by ongoing experience with foods throughout the lifespan. Moreover, even within a given diet, flavor components can have varying relationships. The multisensory computation observed in the present study is consistent with the flexible nature of multisensory flavor correspondences in that it did not appear to depend on experience with specific taste-odor combinations (animals had no prior experience with the particular mixtures used during testing). This is consistent with two recent studies investigating taste-odor mixture consumption (Elliott and Maier 2020; McQueen et al. 2020) and may reflect the imperative to evaluate any flavor stimulus upon consumption (regardless of experience with that particular taste-odor combination). However, previous psychophysical studies measuring perception of objective flavor properties (identity judgments, detection) consistently show that responses to taste-odor mixtures yield responses that are enhanced relative to the unisensory components (Dalton et al. 2000; Seo et al. 2013; Shepard et al. 2015; Veldhuizen et al. 2010b; Welge-Lüssen et al. 2009; White and Prescott 2007) in a manner that cannot simply be explained by summation of independent unisensory processing channels (Veldhuizen et al. 2010b). This superadditivity computation does appear to depend on experience with specific multisensory stimuli: detection of taste-odor mixtures is significantly enhanced relative to its unisensory components, but only when taste and odor are “congruent” (i.e., experienced together as a flavor). Thus, different multisensory computations may underlie different types of flavor judgments that are appropriate for different contexts (i.e., evaluation of suprathreshold flavors via experience-independent statistically optimal integration versus detection of near-threshold flavor components via experience-determined super additivity). Future work using a range of stimulus concentrations will further test the potential role of these different multisensory computations in consumption behavior. Moreover, consistently pairing odorants with a specific tastant is known to result in odorants acquiring that specific taste quality, and thus provides a way to experimentally control congruency of taste-odor pairings (Stevenson and Boakes 2004; Stevenson et al. 1995, 1998). Such an approach will allow for a direct test of how multisensory congruency may affect flavor-related computations.
A second marked difference between flavor perception and perception of spatial cues in other systems involves plasticity in the representation of the unisensory components. It is well known that hedonic evaluation of individual flavor components—in particular odor—is heavily influenced by experience (Blankenship et al. 2019; McQueen et al. 2020; Stevenson et al. 1998). Indeed, in the present study, rats learned the associations between odorants and caloric value (and potentially the sweet taste) of maltodextrin, thereby increasing their palatability. In terms of statistically optimal integration, such experience may influence the prior reliability of the odor component, and as a result, the weight carried by that component during subsequent multisensory decision-making (Adams et al. 2004). For example, prior reliability of the odor component may be influenced by the nutritional value associated with it. Similarly, tastants with high nutritional value may innately carry more weight than tastants with low nutritional value (Green et al. 2012; Lim et al. 2014; Linscott and Lim 2016). The overall extent of the experience with an odor or taste component or the developmental stage during which the experience occurs may also affect prior reliability. In the present study, odor-nutrient conditioning took place during juvenile and adolescent stages. Identical conditioning during adulthood may result in lower prior reliability and an overall smaller weight of the odor component in multisensory decisions. Finally, prior reliability of the odor may be influenced by the route through which it is experienced, with an orally sourced (retronasal) odorant carrying more weight than the same odorant externally sourced (orthonasal; Blankenship et al. 2019).
GRANTS
This work was supported by the National Institute on Deafness and other Communication Disorders of the National Institutes of Health (R01 DC016063 to J. X. Maier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.X.M. and V.E.E. conceived and designed research; V.E.E. performed experiments; J.X.M. and V.E.E. analyzed data; J.X.M. and V.E.E. interpreted results of experiments; J.X.M. prepared figures; J.X.M. drafted manuscript; J.X.M. and V.E.E. edited and revised manuscript; J.X.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Chad Collins for technical assistance and Ben Rowland and Marga Veldhuizen for valuable comments on an earlier version of this manuscript.
REFERENCES
- Adams WJ, Graf EW, Ernst MO. Experience can change the ‘light-from-above’ prior. Nat Neurosci 7: 1057–1058, 2004. doi: 10.1038/nn1312. [DOI] [PubMed] [Google Scholar]
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol 14: 257–262, 2004. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Avery JA, Liu AG, Ingeholm JE, Riddell CD, Gotts SJ, Martin A. Taste quality representation in the human brain. J Neurosci 40: 1042–1052, 2020. doi: 10.1523/JNEUROSCI.1751-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship ML, Grigorova M, Katz DB, Maier JX. Retronasal odor perception requires taste cortex, but orthonasal does not. Curr Biol 29: 62–69.e3, 2019. doi: 10.1016/j.cub.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Hayward L, Crandall C. Conditioned taste preferences based on caloric density. J Exp Psychol Anim Behav Process 7: 59–69, 1981. doi: 10.1037/0097-7403.7.1.59. [DOI] [PubMed] [Google Scholar]
- Bresciani JP, Ernst MO. Signal reliability modulates auditory-tactile integration for event counting. Neuroreport 18: 1157–1161, 2007. doi: 10.1097/WNR.0b013e3281ace0ca. [DOI] [PubMed] [Google Scholar]
- Butler JS, Smith ST, Campos JL, Bülthoff HH. Bayesian integration of visual and vestibular signals for heading. J Vis 10: 23, 2010. doi: 10.1167/10.11.23. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PA. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci 3: 431–432, 2000. doi: 10.1038/74797. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18: 2059–2068, 2003. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Elliott VE, Maier JX. Multisensory interactions underlying flavor consumption in rats: the role of experience and unisensory component liking. Chem Senses 45: 27–35, 2020. doi: 10.1093/chemse/bjz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci 8: 162–169, 2004. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Pouget A, DeAngelis GC, Angelaki DE. Neural correlates of reliability-based cue weighting during multisensory integration. Nat Neurosci 15: 146–154, 2012. doi: 10.1038/nn.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci 29: 15601–15612, 2009. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondberg R, Lundström JN, Blöchl M, Olsson MJ, Seubert J. Multisensory flavor perception: the relationship between congruency, pleasantness, and odor referral to the mouth. Appetite 125: 244–252, 2018. doi: 10.1016/j.appet.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. Enhancement of retronasal odors by taste. Chem Senses 37: 77–86, 2012. doi: 10.1093/chemse/bjr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, Deangelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci 11: 1201–1210, 2008. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Thein T. Interactions between conditioned and unconditioned flavor preferences. J Exp Psychol Anim Behav Process 31: 407–417, 2005. doi: 10.1037/0097-7403.31.4.407. [DOI] [PubMed] [Google Scholar]
- Holman E Immediate and delayed reinforcers for flavor preferences in rats. Learn Motiv 6: 91–100, 1975. doi: 10.1016/0023-9690(75)90037-5. [DOI] [Google Scholar]
- Jagetia S, Milton AJ, Stetzik LA, Liu S, Pai K, Arakawa K, Mandairon N, Wesson DW. Inter- and intra-mouse variability in odor preferences revealed in an olfactory multiple-choice test. Behav Neurosci 132: 88–98, 2018. doi: 10.1037/bne0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, Saunders JA. Do humans optimally integrate stereo and texture information for judgments of surface slant? Vision Res 43: 2539–2558, 2003. doi: 10.1016/S0042-6989(03)00458-9. [DOI] [PubMed] [Google Scholar]
- Lim J, Fujimaru T, Linscott TD. The role of congruency in taste-odor interactions. Food Qual Prefer 34: 5–13, 2014. doi: 10.1016/j.foodqual.2013.12.002. [DOI] [Google Scholar]
- Linscott T, Lim J. Retronasal odor enhancement by salty and umami tastes. Food Qual Prefer 48: 1–10, 2016. doi: 10.1016/j.foodqual.2015.08.004. [DOI] [Google Scholar]
- Maier JX, Blankenship ML, Li JX, Katz DB. A multisensory network for olfactory processing. Curr Biol 25: 2642–2650, 2015. doi: 10.1016/j.cub.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Katz DB. Neural dynamics in response to binary taste mixtures. J Neurophysiol 109: 2108–2117, 2013. doi: 10.1152/jn.00917.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Wachowiak M, Katz DB. Chemosensory convergence on primary olfactory cortex. J Neurosci 32: 17037–17047, 2012. doi: 10.1523/JNEUROSCI.3540-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen KA, Fredericksen KE, Samuelsen CL. Experience informs consummatory choices for congruent and incongruent odor-taste mixtures in rats. Chem Senses 45: 371–382, 2020. doi: 10.1093/chemse/bjaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ML, Deangelis GC, Angelaki DE. Multisensory integration in macaque visual cortex depends on cue reliability. Neuron 59: 662–673, 2008. doi: 10.1016/j.neuron.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Cain WS. Taste and olfaction: independence vs interaction. Physiol Behav 24: 601–605, 1980. doi: 10.1016/0031-9384(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Angelaki DE, DeAngelis GC. A normalization model of multisensory integration. Nat Neurosci 14: 775–782, 2011. doi: 10.1038/nn.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiro T, Angelaki DE, DeAngelis GC. A neural signature of divisive normalization at the level of multisensory integration in primate cortex. Neuron 95: 399–411.e8, 2017. doi: 10.1016/j.neuron.2017.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Treves A. Representation of olfactory information in the primate orbitofrontal cortex. J Neurophysiol 75: 1982–1996, 1996. doi: 10.1152/jn.1996.75.5.1982. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Fontanini A. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci 37: 244–257, 2017. doi: 10.1523/JNEUROSCI.1926-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Iannilli E, Hummel C, Okazaki Y, Buschhüter D, Gerber J, Krammer GE, van Lengerich B, Hummel T. A salty-congruent odor enhances saltiness: functional magnetic resonance imaging study. Hum Brain Mapp 34: 62–76, 2013. doi: 10.1002/hbm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TG, Veldhuizen MG, Marks LE. Response times to gustatory-olfactory flavor mixtures: role of congruence. Chem Senses 40: 565–575, 2015. doi: 10.1093/chemse/bjv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM Smell images and the flavour system in the human brain. Nature 444: 316–321, 2006. doi: 10.1038/nature05405. [DOI] [PubMed] [Google Scholar]
- Small DM, Green BG. A proposed model of a flavor modality In: The Neural Bases of Multisensory Processes, edited by Murray MM, Wallace MT. Boca Raton, FL: CRC Press/Taylor & Francis, 2012. [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol 92: 1892–1903, 2004. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Spence C Multisensory flavor perception. Cell 161: 24–35, 2015. doi: 10.1016/j.cell.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Stein BE (Editor). The New Handbook of Multisensory Processing. Cambridge, MA: MIT Press, 2012. [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci 15: 520–535, 2014. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R, Boakes R. Sweet and sour smells: learned synaesthesia between the senses of taste and smell In: The Handbook of Multisensory Processing, edited by Calvert G, Spence C, Stein B. Cambridge, MA: MIT Press, 2004, p. 69–83. [Google Scholar]
- Stevenson R, Prescott J, Boakes R. The acquisition of taste properties by odors. Learn Motiv 26: 433–455, 1995. doi: 10.1016/S0023-9690(05)80006-2. [DOI] [Google Scholar]
- Stevenson RJ, Boakes RA, Prescott J. Changes in odor sweetness resulting from implicit learning of a simultaneous odor-sweetness association: an example of learned synesthesia. Learn Motiv 29: 113–132, 1998. doi: 10.1006/lmot.1998.0996. [DOI] [Google Scholar]
- Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM. The insular taste cortex contributes to odor quality coding. Front Hum Neurosci 4: 58, 2010a. doi: 10.3389/fnhum.2010.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Shepard TG, Wang MF, Marks LE. Coactivation of gustatory and olfactory signals in flavor perception. Chem Senses 35: 121–133, 2010b. doi: 10.1093/chemse/bjp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincis R, Fontanini A. Associative learning changes cross-modal representations in the gustatory cortex. eLife 5: e16420, 2016. doi: 10.7554/eLife.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lüssen A, Husner A, Wolfensberger M, Hummel T. Influence of simultaneous gustatory stimuli on orthonasal and retronasal olfaction. Neurosci Lett 454: 124–128, 2009. doi: 10.1016/j.neulet.2009.03.002. [DOI] [PubMed] [Google Scholar]
- White TL, Prescott J. Chemosensory cross-modal stroop effects: congruent odors facilitate taste identification. Chem Senses 32: 337–341, 2007. doi: 10.1093/chemse/bjm001. [DOI] [PubMed] [Google Scholar]