Abstract
Macrophages demonstrate remarkable plasticity that is essential for host-defense and tissue repair. The tissue niche imprints macrophage identity, phenotype, and function. The role of vascular endothelial signals in tailoring the phenotype and function of tissue macrophages remains unknown. The lung is a highly vascularized organ and replete with a large population of resident macrophages. We found that in response to inflammatory injury, lung endothelial cells release the Wnt signaling modulator Rspondin3 which activates β-catenin signaling in lung interstitial macrophages and increases mitochondrial respiration by glutaminolysis. The generated tricarboxylic acid cycle intermediate α-ketoglutarate, in turn, serves as the cofactor for the epigenetic regulator TET2 to catalyze DNA hydroxymethylation. Notably, endothelial-specific deletion of Rspondin3 prevented the formation of anti-inflammatory interstitial macrophages in endotoxemic mice and induced unchecked severe inflammatory injury. Thus, the angiocrine-metabolic-epigenetic signaling axis specified by the endothelium is essential for reprogramming interstitial macrophages and dampening inflammatory injury.
Introduction
Macrophages in all tissues exhibit remarkable phenotypic plasticity, characterized by transitioning into distinct phenotypes with specific functions in response to microenvironmental cues1, 2. Infection and injury drive the generation of pro-inflammatory phenotypes, whereas tissue niche signals can induce the switch of tissue macrophages towards anti-inflammatory and pro-reparative phenotypes to facilitate the resolution of inflammation1, 3. Thus, the orchestration of pro- and anti-inflammatory macrophage phenotypes governs the fate of organs during inflammation and injury1, 3. In the lung, deregulation of macrophages is a leading cause to an unrestrained inflammation to bacterial and viral infection, and is a critical factor in the pathogenesis of acute lung injury (ALI), acute respiratory distress syndrome (ARDS) including its most severe manifestations involving cytokine storms that have been described in COVID-194, 5.
Macrophage reprogramming requires tight regulation of gene expression governed by epigenetic programs and transcriptional regulation6, 7. Also, studies have identified metabolic adaptation is both a critical hallmark and prerequisite for macrophage phenotype switch8, 9. Local microenvironmental cues generated by tissue cells are increasingly recognized as critical determinants of resident macrophage identity, phenotype, and function10, 11, 12. Resident macrophages are highly heterogeneous and unique as they occupy distinct tissue niches and hence exhibit the phenotype and function that is imprinted by niche-derived signals which trigger specific differentiation programs10, 13, 14.
Macrophages represent the most abundant immune cells in the healthy lung, consisting of two types of tissue resident macrophages that are characterized by their localization: alveolar macrophages (AM) which populate alveoli and airways and interstitial macrophages (IM) which reside in lung parenchyma13. Lung IMs are less well understood but findings suggest that they are critical for maintaining lung homeostasis15, 16. The vascular endothelial cells (ECs) lining all blood vessels serve as conduits for blood and tissue nutrient delivery but also constitute a niche for lung macrophages17. How the lung endothelial niche regulates lung macrophage plasticity is not known. In the present study, we found that the vascular endothelial niche was essential for lung IM reprogramming through the activation of a metabolic-epigenetic signaling axis. The ECs release the angiocrine mediator Rspondin3 to induce metabolic and epigenetic reprogramming of IMs towards an anti-inflammatory phenotype via activation of the Wnt signaling pathway and were shown to mitigate inflammatory lung injury.
Results
Endothelial cells instruct macrophage phenotypic transition via angiocrine signaling.
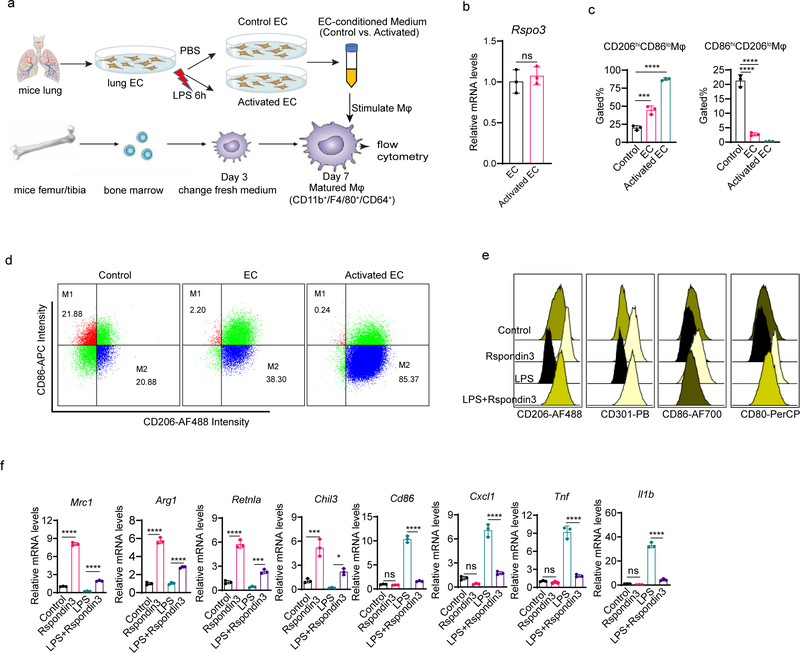
We first analyzed the paracrine factors released by ECs that could regulate macrophage transition (Extended Data Fig. 1a). We isolated lung ECs and collected conditioned medium from either LPS-activated ECs or control ECs, which was added to bone marrow derived macrophages (BMDMs). After 24 hours, BMDMs were collected for flow cytometry analysis. Macrophages were first gated by CD11b+F4/80+CD64+ (gating strategy shown in Supplementary Fig. 1i), and the expression of anti-inflammatory markers (CD206, CD301, Arginase 1, and IL-10) as well as pro-inflammatory macrophage markers (CD86, CD80, TNF, and iNOS) was analyzed. We observed that EC-conditioned medium significantly induced the expression of anti-inflammatory markers while suppressing pro-inflammatory markers, and this trend was markedly augmented in ECs activated by the bacterial endotoxin LPS (Fig. 1a). We also quantified the percentages of “M1-like” (defined as “CD86hiCD206lo”) and “M2-like” (defined as “CD206hiCD86lo”) macrophages18 and found that EC-conditioned medium shifted the balance of macrophage populations towards an M2-like phenotype (Extended Data Fig. 1c–d). We then carried out a secretome analysis to identify candidate proteins mediating the paracrine EC effects on macrophage phenotype transition. We found that several proteins were released by ECs following LPS-activation (Supplementary Table 1), with the Wnt signaling activator Rspondin3 clearly ranked as the top secreted EC protein (Fig. 1b), and the release of Rspondin3 was validated by quantitative ELISA (Fig. 1c) but without change on Rspo3 mRNA levels (Extended Data Fig. 1b). This suggested angiocrine signals like Rspondin3 could be potential regulator for macrophage phenotype transition.
Figure 1. Endothelial cells instruct macrophage phenotype transition via the angiocrine factor Rspondin3.
(a) Levels of anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, TNF, iNOS) in BMDMs incubated with control medium, normal murine lung EC-conditioned medium (EC) or LPS-activated murine lung EC-conditioned medium (Activated EC) as measured by flow cytometry. Left: representative overlaid flow cytometry histograms showing mean fluorescence intensity (MFI), right: quantified data from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Dunnett’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (*P=0.0249, ****P<0.0001), CD301 (****P<0.0001, ****P<0.0001), Arginase 1 (****P<0.0001, ****P<0.0001), IL-10 (****P<0.0001, ****P<0.0001), CD86 (****P<0.0001, ****P<0.0001), CD80 (ns P=0.0889, ****P<0.0001), TNF (****P<0.0001, ****P<0.0001), iNOS (****P<0.0001, ****P<0.0001). (b) Top ranked secreted proteins identified by a proteomics-based secretome assay using conditioned medium collected from LPS-activated murine lung ECs (normalized to total spectra); (c) ELISA measurements for Rspondin3 concentrations in EC supernatants from either LPS activated or control EC from three independent repeats; n=6 samples per group (mean ± sd), two-sided unpaired Student’s t-test was determined using GraphPad Prism. ****P<0.0001. (d) Levels of anti-inflammatory markers (CD206, CD301, Arginase1, IL-10) and pro-inflammatory markers (CD86, CD80, TNF, iNOS) as measured by flow cytometry in BMDMs stimulated with Rspondin3 (40 ng/ml), LPS (100 ng/ml) alone, or combination of both for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (****P<0.0001, ****P<0.0001), CD301 (****P<0.0001, ****P<0.0001), Arginase 1(****P<0.0001, ****P<0.0001), IL-10 (****P<0.0001, ****P<0.0001), CD86 (ns P=0.1457, ****P<0.0001), CD80 (ns P=0.1576, ****P<0.0001), TNF (****P<0.0001, ****P<0.0001), iNOS (****P<0.0001, ****P<0.0001). (e) Heatmap representing fold changes of anti-inflammatory cytokines and pro-inflammatory cytokines measured by ELISA in supernatants from BMDMs treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments; (f) Heatmap representing gene expression levels (mean RQ value) of anti-inflammatory maker genes (Mrc1, Arg1, Retnla, Chil3, Pparg, Il10) and pro-inflammatory marker genes (Cd86, Cxcl1, Il1b, Tnf, Il6, Nos2) as measured by qPCR in BMDMs treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments.
Rspondin3 mediates interstitial macrophage phenotypic transition and prevents inflammatory lung injury.
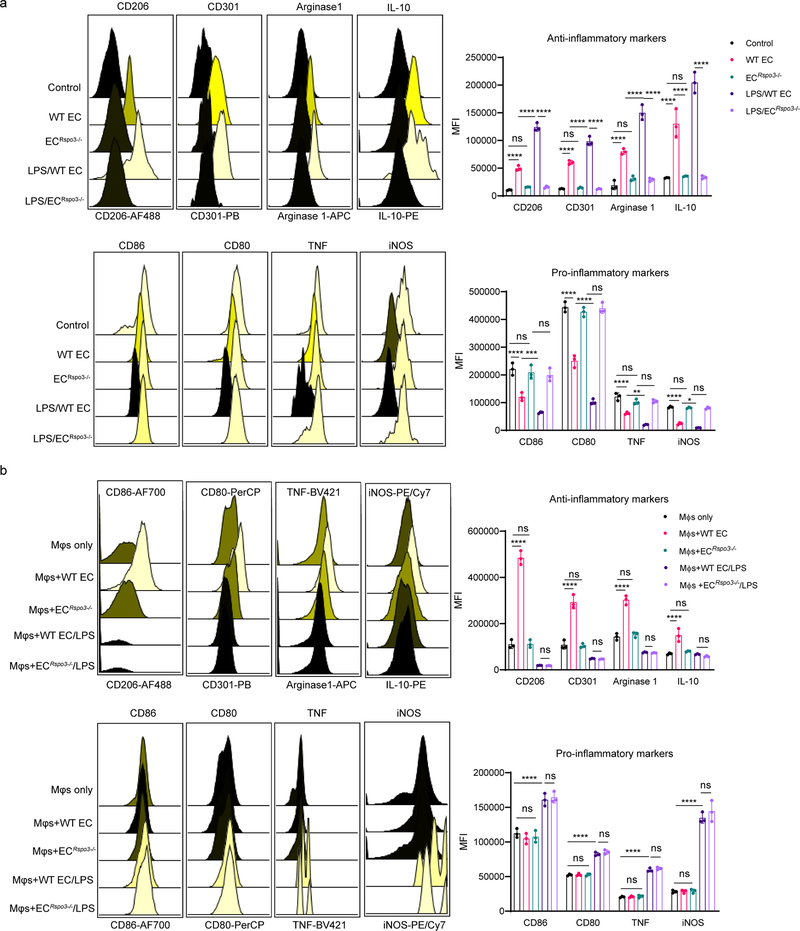
To determine the role of Rspondin3 in regulating macrophage phenotype transition, BMDMs were stimulated with recombinant Rspondin3 protein (40 ng/ml) and LPS (100 ng/ml) alone or in combination. Flow cytometry analysis demonstrated that Rspondin3 increased the expression of anti-inflammatory markers while concomitantly reducing pro-inflammatory markers in macrophages, whereas LPS strongly induced pro-inflammatory and reduced anti-inflammatory markers; crucially, Rspondin3 prevented the generation of LPS-induced pro-inflammatory markers (Fig. 1d, Extended Data Fig. 1e). We also found that Rspondin3 induced the release of anti-inflammatory cytokines such as IL-10 while reducing the release of pro-inflammatory cytokines such as IL-1β in macrophages (Fig. 1e). We observed increased expression of multiple anti-inflammatory marker genes (Mrc1, Arg1, Chil3, Retnla, Pparg and Il10) and decreased expression of pro-inflammatory marker genes (Cd86, Il1b, Tnf, Cxcl1, Il6 and Nos2) induced by Rspondin3 (Fig. 1f, Extended Data Fig. 1f), underscoring the crucial role of Rspondin3 in promoting a macrophage shift towards an anti-inflammatory phenotype. Furthermore, we induced endotoxemia in wild-type (WT) mice and VE-cadherin-CreERT2+;Rspo3fl/fl mice (herein called Rspo3EC−/− mice), and isolated lung ECs from these mice as well as non-endotoxemic control mice (Supplementary Fig. 1). Conditioned medium collected from these ECs showed that Rspo3-deficiency specifically in ECs medium prevented the induction of anti-inflammatory markers and suppression of pro-inflammatory markers in macrophages (Extended Data Fig. 2a), however, EC-Mφ contact is dispensable for these effects (Extended Data Fig. 2b). This demonstrated the critical role of angiocrine Rspondin3 in mediating the effects of lung ECs on macrophage phenotype transition.
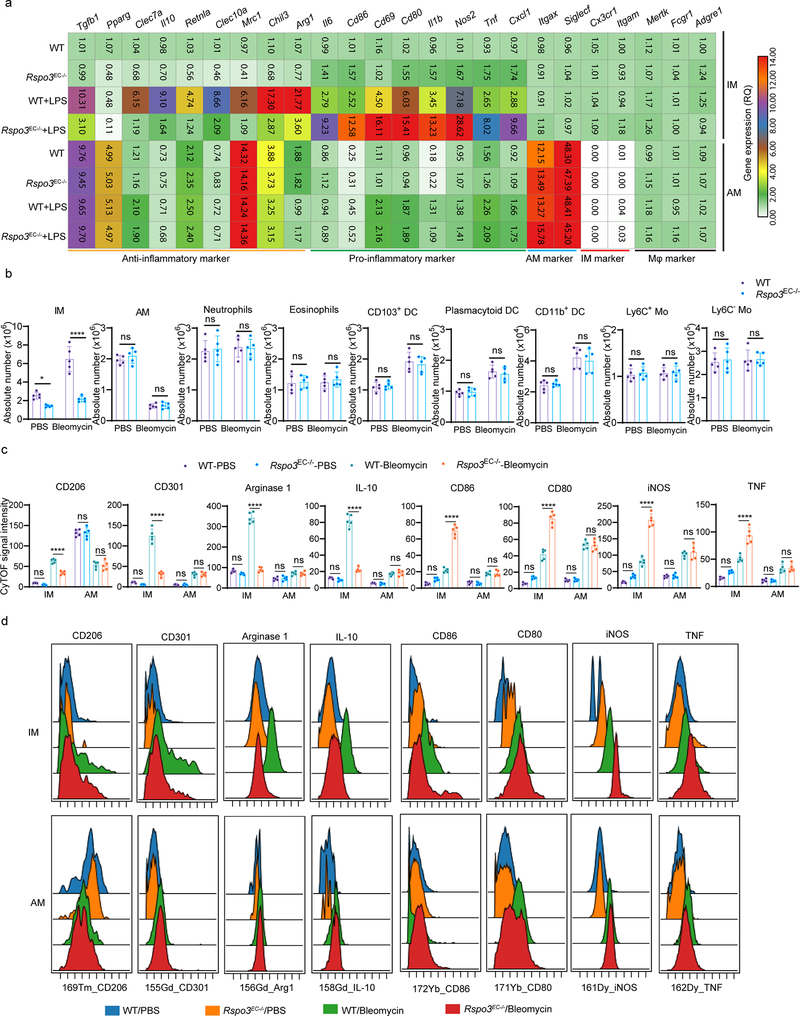
The mouse lung has two distinct resident macrophage populations including AM and IM, which are involved in the regulation of lung homeostasis as well as during lung injury19. In addition, other myeloid cells, such as monocytes (Mo) including Ly6C+ Mo and Ly6C– Mo, neutrophils, dendritic cells (DCs, including CD103+ DC, plasmacytoid DC and CD11b+ DC), and eosinophils also reside in lungs20. We assessed AM, IM and other myeloid lung populations using cytometry by time-of-flight mass spectrometry (CyTOF) which enabled the detection of more than 30 surface markers and intracellular molecules, simultaneously, using metal-labeled antibodies (Supplementary Table 4) with a myeloid cell gating strategy (Fig. 2a). The absolute number of AM, IM, Ly6C+ Mo, Ly6C- Mo, neutrophils, CD103+ DC, plasmacytoid DC, CD11b+ DC, and eosinophils in WT and Rspo3EC−/− mice with or without intravenously administration of rRspondin3 (i.v.) under basal and sublethal LPS challenge conditions (12 mg/kg i.p., for 24h or 48h) are shown in Fig. 2b and Extended Data Fig. 3a. IM expanded 2- to 3- fold in response to endotoxemia at 24h and 48h in WT mice, whereas number of IM did not change in Rspo3EC−/− mice (Fig. 2b). The results thus show that endothelial Rspondin3 is prerequisite for the expansion of IM in response to endotoxemia (Fig. 2b). In addition, rRspondin3 i.v. was able to partially restore endotoxemia-induced IM expansion in Rspo3EC−/− mice (Fig. 2b). However, the number of AM in WT and Rspo3EC−/− mice remained the same during the basal state and post-endotoxemia, and rRspondin3 i.v. did not change AM number (Fig. 2b, right). The other myeloid populations, monocytes, DCs, eosinophils were also not affected by Rspondin3 (Extended Data Fig. 3a). Neutrophils in WT and Rspo3EC−/− mice remained the same in basal condition and 48h post-LPS challenge but there were more neutrophils in Rspo3EC−/− mice 24h post-LPS (Extended Data Fig. 3a), likely reflecting exacerbated inflammatory injury at this time point in the absence of endothelial Rspondin3. Moreover, Rspondin3 activates Wnt signaling in IM but not other lung myeloid cells (Extended Data Fig. 3d).
Figure 2. Rspondin3 mediates lung interstitial macrophage (IM) phenotype transition and prevents inflammatory lung injury.
(a) Identifying lung macrophages as well as other myeloid cells by mass cytometry (CyTOF): resident macrophages were identified by CD45+F4/80+Ly6G−Ly6C−CD64+MerTK+, IM and AM were further identified by CD11b+SiglecF− and CD11b−SiglecF+ respectively; other myeloid populations were identified as: Ly6C+ monocyte (Ly6C+ Mo: CD45+Ly6G−Ly6C+CD11b+CD24−MHCII−SiglecF−CD206−), Ly6C− monocyte (Ly6C− Mo: CD45+Ly6G−Ly6C−CD11b+CD24−MHCII−SiglecF−CD206−), Neutrophils (CD45+Ly6G+CD11b+F4/80−), CD103+ DC (CD45+Ly6G−CD11c+CD11b−CD24+CD64−Ly6C−SiglecF−CD103+BST2−), plasmacytoid DC (CD45+Ly6G−CD11c+CD11b−CD24+CD64−Ly6C+SiglecF−CD103−BST2+), CD11b+ DC (CD45+Ly6G−CD11c+CD11b+CD24+CD64−Ly6C−SiglecF−CD103−BST2−), and Eosinophils (CD45+Ly6G−Ly6C−CD11b+CD24+SiglecF+CD11c−MHCII−CD64−); (b) Absolute cell number for lung IM and AM in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. (0.25 mg/kg) under basal conditions and following post-sublethal LPS challenge (12 mg/kg, i.p.) for 24h or 48h as measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d. with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: IM (ns P=0.3306, ns P=0.5741, ns P=0.2460, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), AM (ns P=0.8041, ns P=0.9768, ns P=0.9902, ns P=0.9835, ns P=0.7590, ns P=0.9409, ns P=0.9998, ns P=0.9862, ns P=0.9844). (c) Heatmap showing levels of the anti-inflammatory markers (upper panel) and pro-inflammatory markers (lower panel) in lung IM in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h or 48h as measured by CyTOF (n = 5 mice per group with three independent repeats, shown as fold changes by the mean CyTOF signal intensity normalized to control group); (d) Lung vascular permeability was measured by using the albumin-Evans blue dye tracer (EBA) in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h or 48h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P=0.9997, ns P=0.9996, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001; (e) Myeloperoxidase (MPO) activity of flushed lung samples from WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h or 48h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P=0.9774, ns P=0.9715, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, *P=0.0250, ****P<0.0001; (f) Survival curves for WT and Rspo3EC−/− mice with or without rRspondin3 i.v. during endotoxemia conditions (n = 16 mice for each group).
The levels of anti-inflammatory markers (CD206, CD301, RELMα, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, CD69, iNOS, TNF) were also analyzed by CyTOF (Fig. 2c, Extended Data Fig. 3b–c). We observed that endotoxemia in WT mice significantly increased the expression of pro-inflammatory markers at 24h in IM (Fig. 2c, Extended Data Fig. 3b), and these inflammatory markers were reduced by 50% at 48h post-LPS (although the values remained greater than basal condition) (Fig. 2c, Extended Data Fig. 3b). These pro-inflammatory markers in IM were 2- to 3-fold greater in Rspo3EC−/− mice than those observed in WT mice at 24h after LPS administration and were maintained at these high levels at 48h (Fig. 2c, Extended Data Fig. 3b). We observed that rRspondin3 i.v. markedly reduced the expression of pro-inflammatory markers in IM in both WT and Rspo3EC−/− mice (Fig. 2c, Extended Data Fig. 3b). The anti-inflammatory markers (CD206, CD301, RELMα, Arginase 1, IL-10) increased to 2 to 4 fold in IM as compared to the basal condition in response to LPS for 24h, and continued to increase 4 to 16 fold in response to LPS at 48h in WT mice (Fig. 2c, Extended Data Fig. 3b. These findings show that the activation of adaptive anti-inflammatory programming of IM is elicited by endotoxemia. However, in Rspo3EC−/− mice, deletion of endothelial Rspondin3 impaired the induction of these anti-inflammatory markers in IM at 24h after LPS challenge, and this impairment became even more evident at 48h post-LPS challenge (Fig. 2c, Extended Data Fig. 3b). Furthermore, we observed that the impairment of anti-inflammatory IM programming in Rspo3EC−/− mice was markedly restored by rRspondin3 i.v. (Fig. 2c, Extended Data Fig. 3b). In contrast to what we observed in IM, the expression of anti-inflammatory and pro-inflammatory markers in AM did not differ between WT and Rspo3EC−/− mice as assessed by CyTOF (Extended Data Fig. 3c). These data suggested angiocrine Rspondin3 specifically regulates IM but not other lung myeloid cells in mice.
We next addressed whether the lung IM phenotype shift induced by endothelial Rspondin3 impacted the extent of inflammatory lung injury. Lung inflammation was assessed by quantifying neutrophil infiltration using the MPO activity assay as well as by quantifying changes in lung vascular permeability using the albumin-Evans blue dye tracer. Deletion of endothelial Rspondin3 (Rspo3EC−/− vs. WT) had no effect on lung inflammation at baseline whereas it markedly enhanced lung inflammatory injury during endotoxemia (Fig. 2d–e). Survival studies also showed that endothelial-specific deletion of Rspo3 significantly increased mortality as compared to control mice (Fig. 2f). rRspondin3 i.v. acted as a therapeutic which attenuated lung inflammatory injury and enhanced survival in endotoxemic Rspo3EC−/− mice (Fig. 2d–f). These data demonstrated Rspondin3-induced IM phenotypic transition prevents inflammatory lung injury.
We also used flow sorting to isolate AM and IM and performed gene profiling by qPCR. We observed that induction of the anti-inflammatory genes (Arg1, Chil3, Retnla, Mrc1, Clec10a, Il10, Clec7a, Pparg, Tgfb1) was significantly reduced in IM obtained from endotoxemic Rspo3EC−/− mice when compared to control mice (Extended Data Fig. 4a). In contrast, activation of pro-inflammatory genes (Cxcl1, Tnf, Nos2, Il1b, Cd80, Cd86, Cd69) was markedly enhanced in IM from endotoxemic Rspo3EC−/− mice (Extended Data Fig. 4a). However, both the expression of anti-inflammatory and pro-inflammatory markers in AM was not affected by the absence of endothelial Rspondin3 during the basal state or during endotoxemia (Extended Data Fig. 4a).
To establish whether the observed role of endothelial Rspondin3 was also applicable to other forms inflammatory lung injury, we used bleomycin model in which bleomycin is administered intratracheally to induce acute lung inflammatory injury15. At day 5 post-bleomycin (acute lung injury phase), WT and Rspo3EC−/− mice were sacrificed and myeloid cells were analyzed by CyTOF. We observed that among the lung myeloid populations, monocytes, neutrophils and eosinophils were not affected by bleomycin-induced acute lung injury; DCs numbers increased after bleomycin but no difference between WT and Rspo3EC−/− mice (Extended Data Fig. 4b). AM decreased by 60% after bleomycin but there was no difference between WT and Rspo3EC−/− mice (Extended Data Fig. 4b). IM expanded 3-fold in WT mice after bleomycin exposure whereas IM in Rspo3EC−/− mice were unable to mount this expansion response to injury (Extended Data Fig. 4b). The anti-inflammatory and pro-inflammatory phenotypes for lung macrophages in bleomycin-induced inflammatory lung injury were also analyzed by CyTOF (Extended Data Fig. 4c–d). Endothelial-specific deletion of Rspondin3 shifted the lung IM population towards a pro-inflammatory phenotype in response to bleomycin-induced lung injury and thus mirrored the effects we had seen in endotoxemia-induced injury. These data suggested that the role of endothelial Rspondin3 as a regulator of an anti-inflammatory lung IM phenotype is a generalizable principle in lung inflammatory injury.
LGR4 is required for Rspondin3-induced macrophage transition.
Rspondin family proteins induce Wnt-β-catenin signaling by binding to members of the Leucine-rich repeat-containing G protein receptors (LGRs) family (Lgr4, Lgr5, and Lgr6)21, 22, 23. We found that only Lgr4 was highly expressed in macrophages (Supplementary Fig. 2a–b). Depletion of Lgr4 in macrophages abrogated Rspondin3-induced expression of the anti-inflammatory marker CD206 and reversed Rspondin3-induced suppression of pro-inflammatory marker CD86 (Fig. 3a). We also observed that Lgr4 depletion dampened Rspondin3-induced gene expression of the anti-inflammatory markers and promoted activation of pro-inflammatory genes in BMDMs in response to LPS (Fig. 3b, Extended Data Fig. 5a). Furthermore, depletion of Lgr4 augmented release of pro-inflammatory cytokine IL-1β in BMDMs in response to LPS and prevented the release of anti-inflammatory cytokine IL-10 induced by Rspondin3 (Fig. 3c). These together indicated the requisite role of LGR4 in mediating Rspondin3-induced macrophage phenotype transition.
Figure 3. LGR4 expressed in macrophages is required for Rspondin3-induced macrophage phenotype transition.
(a) Representative flow cytometry overlay histogram and quantified data for the levels of anti-inflammatory marker CD206 and pro-inflammatory marker CD86 in BMDMs with or without (siNC, nontarget control siRNA) Lgr4 depletion at basal condition, or treated with Rspondin3, LPS alone or combination for both for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA Tukey’s multiple comparisons using GraphPad Prism. P values (left to right) are: CD206 (****P<0.0001, ****P<0.0001, ns P=0.9520, ns P=0.8256), CD86 (*P=0.0286, ****P<0.0001, ns P=0.9252, ns P=0.0619). (b) Heatmap representing the expression levels (mean RQ value) of anti-inflammatory maker genes (Mrc1, Arg1, Retnla, Chil3) and pro-inflammatory marker genes (Cd86, Cxcl1, Il1b, Tnf) measured by qPCR in BMDMs with or without Lgr4 depletion treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments; (c) Concentrations of the anti-inflammatory cytokine IL-10 and pro-inflammatory cytokine IL-1β in supernatants of BMDMs with or without Lgr4 depletion measured by ELISA from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA Sidak’s multiple comparisons using GraphPad Prism. P values (left to right) are IL-10: ns P=0.9995, ****P<0.0001, ns P=0.9643, ****<0.0001; IL-1β: ns P=0.9908, ns P=0.1029, ****<0.0001, ****<0.0001. (d) Wnt signaling activity in BMDMs as measured by TOP-Flash reporter assays from three repeats with three samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA Sidak’s multiple comparisons using GraphPad Prism. P values (left to right) are ****P<0.0001, ns P>0.9999. (e) Absolute cell number of IM in WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under basal and after sublethal LPS challenge for 24h measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P=0.5877, ns P=0.3916, ns P=0.3806, **P=0.0055, ****P<0.0001, ns=0.9983; (f) Levels of the anti-inflammatory and pro-inflammatory markers in IMs in WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (ns P=0.1062, ns P=0.7861, ns P=0.9854, ****P<0.0001, ****P<0.0001, ns P=0.9719), CD301 (ns P=0.9222, ns P=0.7557, ns P=.9865, **P=0.0027, ****P<0.0001, ns P=0.9810), Arginase 1 (ns P=0.1902, ns P=0.5609, ns P=0.9958, ****P<0.0001, ****P<0.0001, ns P=0.9608), IL-10 (ns P=0.1421, ns P=0.6669, ns P=0.9775, ****P<0.0001, ****P<0.0001, ns P=0.8189), CD86 (ns P=0.2238, ns P>0.9999, ns P>0.9999, ****P<0.0001, ***P=0.0009, ns P=0.5211), CD80 (ns P=0.3158, ns P=0.9885, ns P=0.9998, ****P<0.0001, **P=0.0062, ns P=0.9579), iNOS (ns P=0.2172, ns P=0.9996, ns P=0.9930, ****P<0.0001, **P=0.0020, ns P=0.9150), TNF (ns P=0.2758, ns P=0.9947, ns P>0.9999, ****P<0.0001, ****P<0.0001, ns P=0.9986). (g) Active β-catenin (non-phospho) levels in IM in WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h as measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P=0.1588, ****P<0.0001, ns P>0.9999, ***P=0.0001, ****P<0.0001, ns P=0.9971. (h) Lung vascular permeability was measured by using the albumin-Evans blue dye tracer in WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under basal conditions and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P>0.9910, ****P<0.0001, ****P<0.0001, ns P=0.8521. (i) MPO activity of flushed lung samples from WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P>0.9990, ****P<0.0001, ****P<0.0001, ns P=0.5800.
We found that Rspondin3 activated Wnt signaling in BMDMs in a LGR4-dependent manner (Fig. 3d). To investigate the role of LGR4 in lung macrophages in vivo, we first evaluated its expression in IM, AM, and other myeloid cells by CyTOF, and found LGR4 was highly expressed in IM as opposed to AM and other myeloid populations (Extended Data Fig. 5b–c, Supplementary Fig. 2e). In vivo, with macrophage-specific Lgr4 knockout mice (herein called Lgr4Mφ−/− ) (Supplementary Fig. 2f), we found Lgr4Mφ−/− mice showed exacerbated inflammatory lung injury responses as compared to WT mice induced by sublethal LPS challenge, as measured by lung MPO activity and albumin-Evans blue dye tracer assessment of lung vascular permeability (Fig. 3h–i). These changes could not be rescued by rRspondin3 i.v. as was the case in WT mice (Fig. 3h–i). The number of IM in Lgr4Mφ−/− mice also failed to increase in response to endotoxemia by rRspondin3 i.v. (Fig. 3e). Furthermore, expression of anti-inflammatory markers and pro-inflammatory markers in Lgr4Mφ−/− mice and control mice during endotoxemia was analyzed by CyTOF. We found that deletion of Lgr4 prevented the generation of IM and failed to induce expression of anti-inflammatory markers that was coupled to marked increases in pro-inflammatory markers in response to endotoxemia; furthermore, these changes were not reversed by rRspondin3 i.v. (Fig. 3e–f). Also, in Lgr4Mφ−/− mice, Rspondin3 failed to induce Wnt-β-catenin signaling in IM as determined by measuring active β-catenin (non-phosphorylated β-catenin) (Fig. 3g). Taken together, these data showed LGR4 is the receptor in IM required for Rspondin3-mediated reprograming of macrophages and Rspondin3 mediated attenuation of inflammatory lung injury.
β-catenin signals Rspondin3-induced macrophage transition.
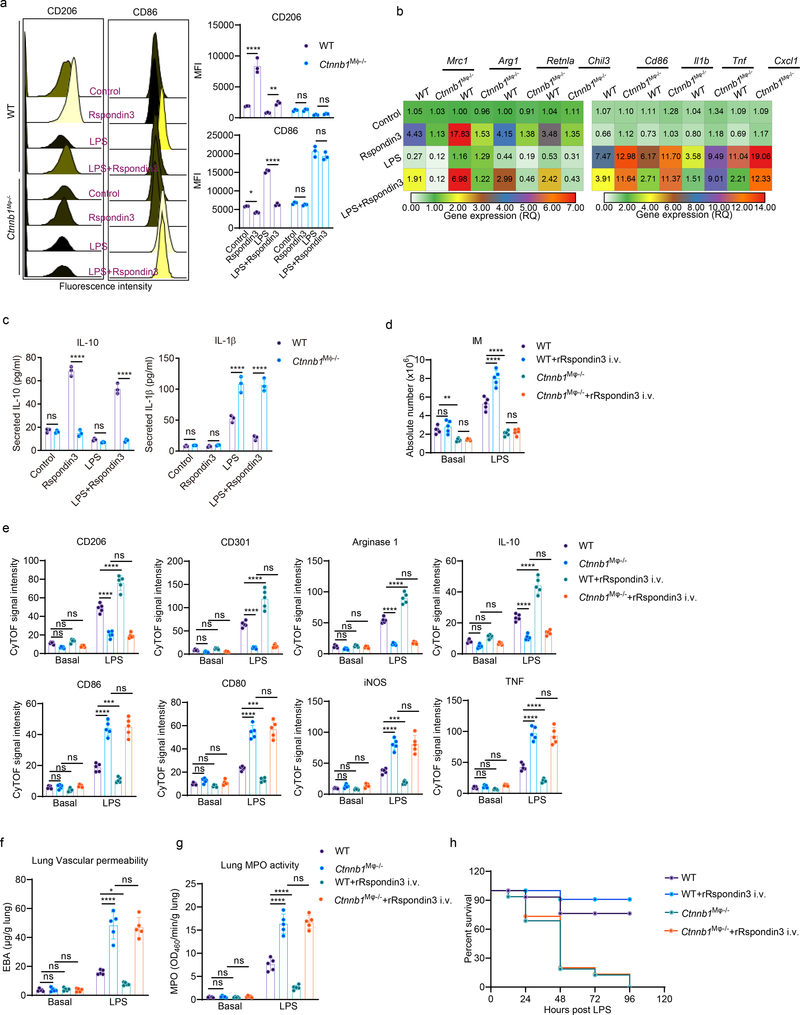
Rspondin family proteins activate both the β-catenin dependent canonical Wnt-β-catenin signaling and β-catenin independent non-canonical Wnt signaling22, 24. We next determined whether β-catenin was essential for Rspondin3 mediated generation of IM and prevention of inflammatory lung injury. Mice with genetic deletion of Ctnnb1 (encodes β-catenin) in macrophages (Lyz2-cre+;Ctnnb1fl/fl, herein called Ctnnb1Mφ−/− ) and Ctnnb1fl/fl (WT) were generated (Supplementary Data Fig. 3a). We measured Ctnnb1 expression in BMDMs prepared from Ctnnb1Mφ−/− mice and WT mice (Supplementary Data Fig. 3b–c) and measured active β-catenin in IM in vivo by CyTOF (Supplementary Data Fig. 3d). We observed that deletion of Ctnnb1 in macrophages abrogated Rspondin3-induced expression of the anti-inflammatory marker CD206 and instead increased the expression of the pro-inflammatory marker CD86 (Fig. 4a). We also found that deletion of Ctnnb1 in macrophages suppressed the expression of the anti-inflammatory genes induced by Rspondin3 and promoted the induction of pro-inflammatory marker genes in BMDMs in response to LPS challenge (Fig. 4a, Extended Data Fig. 6). ELISA confirmed that deletion of Ctnnb1 augmented the release of the pro-inflammatory cytokine IL-1β in BMDMs in response to LPS and prevented the release of anti-inflammatory cytokine IL-10 by Rspondin3 (Fig. 4c). In vivo, the number of IM in Ctnnb1Mφ−/− mice failed to increase in response to endotoxemia, and this failure could not be rescued by rRspondin3 i.v. as it was in the WT mice (Fig. 4d). Furthermore, Ctnnb1Mφ−/− mice showed a failure to induce expression of anti-inflammatory markers in lung IM, while it concomitantly increased the expression of pro-inflammatory markers in response to endotoxemia; this could not be reversed by rRspondin3 i.v. (Fig. 4e). We found that Ctnnb1Mφ−/− mice demonstrated augmented inflammatory lung injury and mortality in response to endotoxemia, which could not be rescued by rRspondin3 i.v (Fig. 4f–h). Thus, these results showed that macrophage β-catenin was required for Rspondin3-induced IM phenotype transition and Rspondin3-induced suppression of prevented lung injury.
Figure 4. β-catenin signals Rspondin3-induced macrophage phenotype transition.
(a) Representative flow cytometry overlay histograms and quantified data for the levels of the anti-inflammatory marker CD206 and pro-inflammatory marker CD86 shown by MFI in BMDMs from WT and Ctnnb1Mφ−/− mice at the baseline condition, or treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA Tukey’s multiple comparisons using GraphPad Prism. P values (left to right) are: CD206 (****P<0.0001, **P=0.0029, ns P=0.9982, ns P=0.9765), CD86 (*P=0.0318, ****P<0.0001, ns P=0.8020, ns P=0.1195). (b) Heatmap representing gene expression levels (shown as mean RQ value) of anti-inflammatory marker genes (Mrc1, Arg1, Retnla, Chil3) and pro-inflammatory marker genes (Cd86, Cxcl1, Il1b, Tnf) measured by qPCR in BMDMs from WT and Ctnnb1Mφ−/− mice in the baseline condition, or treated with Rspondin3, LPS alone or combination of both for 24h from three independent repeats; (c) Concentrations of the anti-inflammatory cytokine IL-10 and pro-inflammatory cytokine IL-1β in supernatants measured by ELISA in BMDMs from WT and Ctnnb1Mφ−/− mice at the baseline condition, or treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA Sidak’s multiple comparisons using GraphPad Prism. P values (left to right) are IL-10: ns P=0.9742, ****P<0.0001, ns P=0.7458, ****<0.0001; IL-1β: ns P=0.9995, ns P=0.9936, ****P<0.0001, ****P<0.0001. (d) Absolute cell number for IM in WT and Ctnnb1Mφ−/− mice with or without rRspondin3 i.v. under basal state and post-sublethal LPS challenge for 24h measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P=0.6725, **P=0.0069, ns P=0.9991, ****P<0.0001, ****P<0.0001, ns P=0.9595; (e) Levels of anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, iNOS, TNF) in lung IM in WT and Ctnnb1Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h as measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (ns P=0.2099, ns P=0.7433, ns P=0.9536, ****P<0.0001, ****P<0.0001, ns P=0.9178), CD301 (ns P=0.8729, ns P=0.9144, ns P=.9998, ****P<0.0001, ****P<0.0001, ns P=0.8828), Arginase 1 (ns P=0.3580, ns P=0.9857, ns P=0.6133, ****P<0.0001, ****P<0.0001, ns P=0.8287), IL-10 (ns P=0.1331, ns P=0.2284, ns P=0.6216, ****P<0.0001, ****P<0.0001, ns P=0.1970), CD86 (ns P=0.9977, ns P=0.7760, ns P=0.9908, ****P<0.0001, ***P=0.0008, ns P=0.9614), CD80 (ns P=0.6493, ns P=0.8283, ns P=0.8780, ****P<0.0001, ***P=0.0008, ns P=0.7463), iNOS (ns P=0.8586, ns P=0.9763, ns P=0.9937, ****P<0.0001, ***P=0.0004, ns P=0.9994), TNF (ns P=0.9677, ns P=0.8926, ns P=0.9740, ****P<0.0001, ****P<0.0001, ns P=0.7855). (f) Lung vascular permeability as measured in WT and Ctnnb1Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P=0.9997, ns P=0.9973, ns P=0.9998, ****P<0.0001, *P=0.0254, ns P=0.9603. (g) MPO activity of flushed lung samples from WT and Ctnnb1Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P=0.9996, ns P=0.9997, ns P>0.9999, ****P<0.0001, ****P<0.0001, ns P=0.9195. (h) Survival curve for WT and Ctnnb1Mφ−/− mice with or without rRspondin3 i.v. following sublethal LPS challenge (n = 16 mice for each group).
Rspondin3 reprograms metabolism in macrophages.
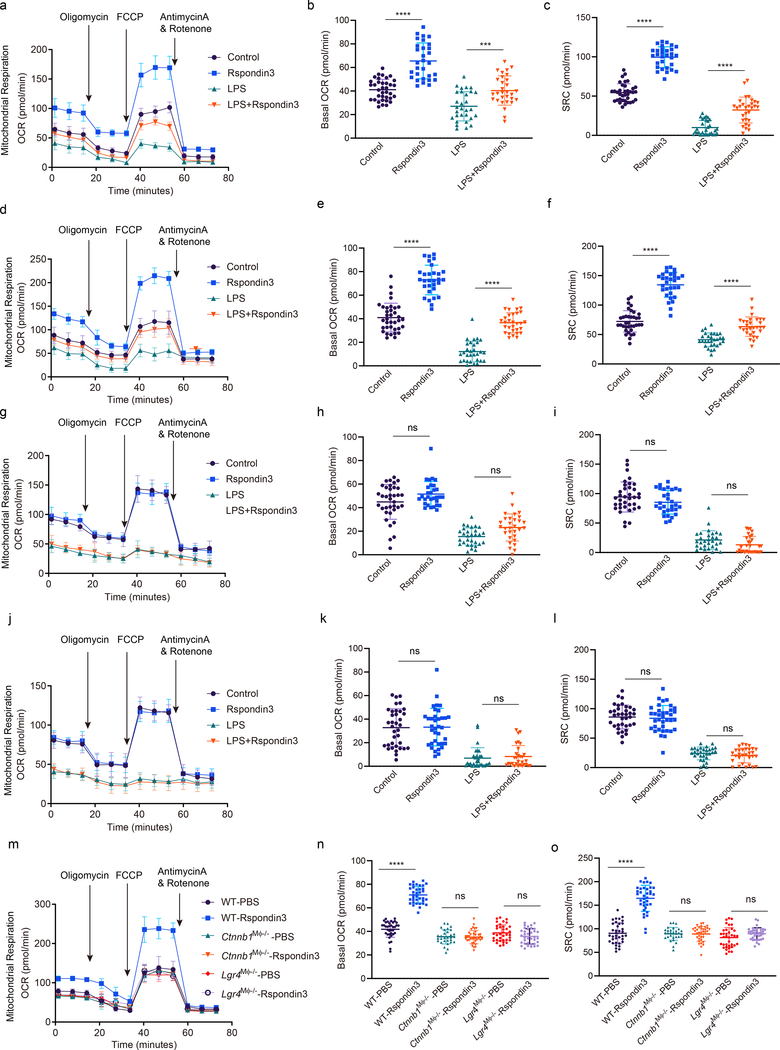
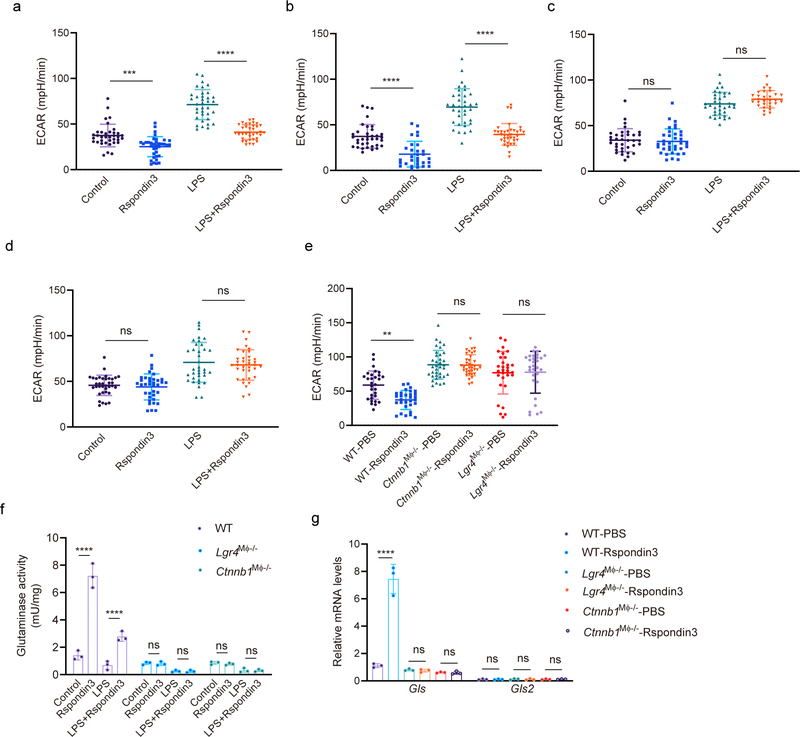
To address whether the Rspondin3-induced macrophage phenotype transition involved a specific metabolic reprogramming, we carried out a metabolic flux assay to detect changes in the mitochondrial oxygen consumption rate (OCR) and rate of extracellular acidification (ECAR), as measures of OXPHOS and glycolysis, respectively. In BMDMs cultured in full DMEM medium containing 10 mM glucose, 2 mM glutamine, and 2 mM sodium pyruvate, we observed that Rspondin3 markedly increased mitochondrial respiration as measured by the basal OCR and the spare respiratory capacity (SRC) which is assessed by mitochondrial uncoupling (Fig. 5a–c). At the same time, Rspondin3 treatment reduced the basal ECAR (Extended Data Fig. 7a). We also found that Rspondin3 treatment of LPS-activated BMDMs was able to reverse the metabolic phenotype of pro-inflammatory macrophages by preventing upregulation of glycolysis and by restoring mitochondrial oxygen consumption (Fig. 5a–c, Extended Data Fig. 7a). To determine the precise carbon source utilized by Rspondin3 increased mitochondrial respiration, we used DMEM medium containing only glucose, glutamine, or free fatty acids as substrates for the metabolic assay, respectively. Here we observed that Rspondin3 failed to increase mitochondrial OCR in macrophages exposed to either glucose or free fatty acids as substrates (Fig. 5g–l), whereas Rspondin3 markedly increased OCR with glutamine as the sole substrate (Fig. 5d–f). We also observed that Rspondin3 increased the activity of glutaminase which is responsible for glutaminolysis (Extended Data Fig. 7f) and induced gene expression of glutaminase (Gls) (Extended Data Fig. 7g). Thus, these results showed that Rspondin3-induced anti-inflammatory switch in macrophages involved increased OXPHOS via glutaminolysis. Next, using macrophages prepared from Lgr4Mφ−/− mice and Ctnnb1Mφ−/− mice, we observed that deletion of Lgr4 or Ctnnb1 abrogated Rspondin3-induced changes in OCR and ECAR (Fig. 5m–o, Extended Data Fig. 7e). Rspondin3-mediated increases in the activity of glutaminase and levels of Gls were prevented in macrophages from Lgr4Mφ−/− mice and Ctnnb1Mφ−/− mice (Extended Data Fig. 7f–g). Thus, Rspondin3-mediated reprogramming of macrophages required the activation of glutaminolysis via LGR4/β-catenin signaling.
Figure 5. Rspondin3 increases mitochondrial respiration through glutamine metabolism.
(a) Mitochondrial oxygen consumption rate (OCR) measured in BMDMs stimulated with Rspondin3, LPS alone or combination of both for 24h with complete DMEM medium containing 10 mM glucose, 2 mM glutamine, 2 mM sodium pyruvate as substrate; Data are representative of three independent experiments, n=10–12 samples per group, graphs show the mean ± s.d. (b) Bar figure for basal OCR in (a); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ***P=0.0003. (c) Bar figure for spare respiratory capacity (SRC) in (a); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ****P<0.0001. (d) OCR measured in BMDMs stimulated with Rspondin3, LPS alone or combination of both for 24h with only 2 mM glutamine as substrate; Data are representative of three independent experiments, n=10–12 samples per group, graphs show the mean ± s.d. (e) Bar figure for basal OCR from (d); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ****P<0.0001. (f) Bar figure for SRC in (d); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ****P<0.0001. (g) OCR measured in BMDMs stimulated with Rspondin3, LPS alone or combination of both for 24h with only 10 nM free fatty acid as palmitate bound to BSA added as substrate; Data are representative of three independent experiments, n=10–12 samples per group, graphs show the mean ± s.d. (h) Bar figure for basal OCR from (g); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample well per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.1000, ns P=0.0528. (i) Bar figure for SRC in (g); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.2595, ns P=0.3785. (j) OCR measured in BMDMs stimulated with Rspondin3, LPS alone or combination of both with only 10 mM glucose as substrate; Data are representative of three independent experiments, n=10–12 samples per group, graphs show the mean ± s.d. (k) Bar figure for basal OCR in (j); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.9992, ns P=0.9773. (l) Bar figure for SRC in (j); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.9556, ns P=0.8668. (m) OCR measurements in WT, Lgr4Mφ−/− and Ctnnb1Mφ−/− BMDMs stimulated with or without Rspondin3 in the medium with only 2 mM glutamine as substrate; Data are representative of three independent experiments, n=10–12 samples per group, graphs show the mean ± s.d. (n, o) Bar figure for basal OCR (n) and SRC (o) for (m); Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual well per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ns P>0.9999, ns P=0.4223, ****P<0.0001, ns P>0.9999, ns P=0.5107.
Rspondin3 induces epigenetic reprogramming through α-ketoglutarate-TET2 to activate anti-inflammatory transition.
Glutaminolysis induces the generation of α-ketoglutarate which serves as the co-factor for DNA hydroxymethylation catalyzed by TET methylcytosine dioxygenase or histone demethylation catalyzed by JMJD3 demethylase25, 26, 27. We thus addressed whether the Rspondin3-mediated increase of α-ketoglutarate generation (Fig. 6a) induced epigenetic reprogramming in DNA hydroxymethylation or histone methylation. Measurements of TET and JMJD3 activities using nuclear extracts from macrophages with or without Rspondin3 stimulation showed that Rspondin3 increased TET activities (Fig. 6b), whereas there was no effect on JMJD3 activities (Supplementary Fig. 4a). As the TET enzymes induce DNA hydroxymethylation and open chromatin and activate gene expression in the associated loci28, we determined whether Rspondin3 induced DNA hydroxymethylation in macrophages. Global 5-hydroxymethylcytosine (5hmC) measurements showed that Rspondin3 markedly increased 5hmC levels and prevented LPS-induced 5hmC downregulation in macrophages (Fig. 6c). Furthermore, 5hmC DNA immunoprecipitation followed by qPCR (hMeDIP-qPCR) used to detect 5hmC enrichment on specific gene loci showed significant enrichment of 5hmC within the proximal promoter regions of the anti-inflammatory genes (Mrc1, Arg1, Chil3, Retnla) (Fig. 6d, Extended Data Fig. 8a). Chromatin immunoprecipitation (ChIP) with an antibody specific for H3K4me3, the active histone marker for open chromatin regions and transcription active genes, followed with qPCR (ChIP-qPCR) targeting the anti-inflammatory gene promoters (Mrc1, Arg1, Chil3, Retnla) also showed that Rspondin3 activated the chromatin state on these gene loci (Fig. 6e, Extended Data Fig. 8b). However, in macrophages obtained from Lgr4Mφ−/− mice or Ctnnb1Mφ−/− mice in which Lgr4 or Ctnnb1 was deleted in macrophages, we observed that Rspondin3 failed to increase α-ketoglutarate levels, TET activity, and 5hmC levels as well as activation of anti-inflammatory genes (Fig. 6a–e, Extended Data Fig. 8a–b). Thus, the Rspondin3-induced metabolic shift enhanced α-ketoglutarate generation in macrophages, which activated the epigenetic program to catalyze DNA hydroxymethylation by TETs that utilize α-ketoglutarate as the cofactor.
Figure 6. Rspondin3 induces TET2-mediated DNA hydroxymethylation of anti-inflammatory genes.
(a) α-ketoglutarate to succinate ratios measured in WT, Lgr4Mφ−/− and Ctnnb1Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are ****P<0.0001, ****P<0.0001, ns P=0.9985, ns P=0.9999, ns P=0.9937, ns P>0.9999. (b) Nuclear extracts from WT, Lgr4Mφ−/−, Ctnnb1Mφ−/− and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both were used for TET activity measurement; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are ****P<0.0001, ****P<0.0001, ns P=0.9988, ns P=0.9953, ns P=0.9760, ns P=0.9998, ns P>0.9999, ns P=0.9989. (c) Genomic DNA prepared from WT, Lgr4Mφ−/− , Ctnnb1Mφ−/− and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both were used for measurement of levels of 5-hydroxymethylcytosine (5hmC); Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are ****P<0.0001, ****P<0.0001, ns P>0.9999, ns P=0.9998, ns P=0.9937, ns P>0.9999, ns P>0.9999, ns P>0.9999. (d) Hydroxymethylated DNA immunoprecipitation (hMeDIP) with an anti-5hmC antibody were performed using genomic DNA prepared from WT, Lgr4Mφ−/− , Ctnnb1Mφ−/− and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both, and qPCR with primers targeting proximal promoters of the indicated genes were used to detect enrichment of 5hmC; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are: Mrc1 (****P<0.0001, ***P=0.0004, ns P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999), Arg1 (****P<0.0001, ****P<0.0001, ns P=0.9996, ns P>0.9999, P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999). (e) Chromatin immunoprecipitation (ChIP) with an anti-H3K4me3 antibody performed on WT, Lgr4Mφ−/− , Ctnnb1Mφ−/−, and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both, and qPCR with primers targeting the proximal promoters of the indicated genes were used to detect the enrichment of H3K4me3; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are: Mrc1 (****P<0.0001, ****P<0.0001, ns P>0.9999, ns P>0.9999, ns P=0.9933, ns P>0.9999, ns P=0.9985, ns P=0.9996), Arg1 (****P<0.0001, ****P<0.0001, ns P=0.9996, ns P>0.9933, ns P=0.9947, ns P>0.9999, ns P>0.9999, ns P>0.9999). (f) Heatmap of levels of anti-inflammatory markers and pro-inflammatory markers in lung IM in WT and Tet2Mφ−/− mice with or without rRspondin3 i.v. under basal conditions and post-sublethal LPS challenge for 24h as measured by CyTOF from three independent repeats (n = 5 mice per group, shown as the mean CyTOF signal intensity); (g) Lung vascular permeability measured by the EBA assay in WT and Tet2Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P>0.9215, ****P<0.0001, **P=0.0044, ns P=0.9554. (h) MPO activity of flushed lung samples from WT and Tet2Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P>0.9872, ****P<0.0001, *P=0.0147, ns P=0.9134.
Using macrophage Tet2 knockout mice (Lyz2-cre+;Tet2fl/fl, herein called Tet2Mφ−/− ) (Supplementary Fig. 4c–e), we observed that the number of IM in Tet2Mφ−/− mice failed to increase in response to endotoxemia, and they could not be rescued by rRspondin3 i.v. (Extended Data Fig. 8d). Tet2Mφ−/− mice also showed a failure of IM to induce expression of anti-inflammatory markers while markedly increasing expression of pro-inflammatory markers in response to endotoxemia, which could not be rescued by rRspondin3 i.v. (Fig. 6f, Extended Data Fig. 8c). Tet2 deletion in macrophages exacerbated inflammatory lung injury induced by endotoxemia, which was not rescued by rRspondin3 i.v. (Fig. 6g–h). We observed that Tet2 deletion prevented Rspondin3-induced DNA hydroxymethylation in macrophages (Fig. 6c–e, Extended Data Fig. 8a–b). We also observed β-catenin translocated to the nucleus of macrophages and markedly increased nuclear TET2/β-catenin interaction as revealed by proximity ligation assays (Extended Data Fig. 6e–f). Therefore, in addition to the role of α-ketoglutarate serving as the co-factor for TET2, direct nuclear β-catenin/TET2 interaction may contribute to epigenetic reprogramming. These data demonstrated Rspondin3 epigenetically reprogrammed macrophages through TET2-catalyzed DNA hydroxymethylation as a consequence of enhanced glutamine metabolism, which provided α-ketoglutarate as the necessary cofactor mediating macrophage phenotype transition.
Taken together, these results show that TET2, the primary enzyme catalyzing DNA hydroxymethylation with α-ketoglutarate as co-factor, was essential for reprogramming macrophages and thereby prevented inflammatory lung injury through the activation of Rspondin3 induced metabolic-epigenetic programs in macrophages (Extended Data Fig. 8g).
Discussion
The tissue niche is emerging as crucial regulator of the identity, phenotype, and function of resident macrophage populations10, 11, 12, 29. Recent studies have identified metabolic shifts, epigenetic modifications and activation of specific transcriptional programs as key mediators of macrophage reprogramming and phenotype switch1, 7, 8, 25, 30, 31. Here, we demonstrated that endothelial cells are essential in driving the generation of a population of lung IM with potent tissue reparative and anti-inflammatory functions during inflammatory injury. The endothelium releases the Wnt signaling activator Rspondin3 that acts as a key angiocrine signal mediating the reprogramming of IM. This transition is the result of metabolic-epigenetic reprogramming fueled by the activation of glutamine metabolism and generation of the metabolite α-ketoglutarate, a requisite co-factor in TET2-mediated DNA hydroxymethylation32. Thus, Rspondin3 generated by endothelial cells orchestrates both metabolic and epigenetic programs to promote lung interstitial macrophage transition towards an anti-inflammatory phenotype and thus promote resolution of inflammation and tissue repair as adaptive mechanisms during inflammatory injury.
The unique lung microenvironment has been demonstrated to be crucial for resident AM development, differentiation and plasticity12, 13, 33, 34, little is known about the properties and generation of the population of IM. Single-cell RNA sequencing recently identified subpopulations of IMs15, 35. However, how the lung microenvironment imprints the phenotype and function of these cells remains unclear. In the present study, using CyTOF, which enabled high-dimensional analysis of macrophage phenotypes and rigorous analysis of IM and AM and other lung myeloid populations, we found a large absolute number of IM that was comparable to AM. These results are consistent with a recent study using design-based stereology showing a relatively large number of IMs in human lungs36. We also found that lung IM number further increased and reached levels significantly greater than AMs post-LPS challenge. This finding is consistent with studies demonstrating high turnover of lung IMs at steady-state and a profound capacity to expand in response to stressors16, 37, 38. Thus, our results are fully congruent with evidence that IM represent an important lung resident macrophage population.
We determined the role of lung ECs in providing the niche signals that tailor lung IM phenotype and function, as IMs are located in close proximity to the lung vascular endothelial niche. We observed that Rspondin3 was constitutively generated by ECs whereas its release increased up to 10-fold in response to LPS. We demonstrated that endothelial derived Rspondin3 bound to its cognate receptor LGR4 in macrophages, and activated β-catenin dependent Wnt signaling. Endothelial-specific genetic deletion of Rspondin3 demonstrated that it was required for the maintenance of the lung IM population during homeostasis and for the expansion of IM following experimental inflammatory injury induced by either endotoxemia or bleomycin-induced injury in mice. The adaptive expansion and phenotypic switch towards anti-inflammatory IMs during endotoxemia was essential for resolution of inflammation. This response was inactivated in the absence of angiocrine Rspondin3. The defective anti-inflammatory switch increased mortality from 15% in control mice to 80% in Rspo3EC−/− mice. Thus, Rspondin3 mediated the phenotypic and functional regulation of IM was required for resolving inflammatory lung injury and reducing mortality. It has been recently reported that deletion of Rspondin receptor LGR4 in myeloid cells enhanced the efficacy of checkpoint blockade therapy by switching the macrophage phenotype in tumors towards a pro-inflammatory state39, and Lgr4-deficient mice were highly susceptible to LPS-induced septic shock due to the increased production of pro-inflammatory cytokines by macrophages40. Our findings complement these reports by demonstrating that LGR4 is the receptor mediating the anti-inflammatory and reparative effects of endothelial Rspondin3 on the macrophage phenotype.
The angiocrine regulation of lung macrophages by Rspondin3 we observed was specific to IM and was not seen in AM. This may be the result several factors: 1) the proximity of IM to vessels which facilitates sensing of endothelial Rspondin3 as opposed to airspace localization of AM5; 2) absence of LGR4 in AM making them irresponsive to endothelial Rspondin3; and 3) the lung mucosal environment in the alveolar space may prevent AM phenotype switches towards the alternatively activated phenotype as recently described12. Other myeloid populations neutrophils, monocytes, DCs and eosinophils were also not affected by Rspondin3, again highlighting the specificity of angiocrine Rspondin3-mediated reprogramming.
We also addressed the metabolic-epigenetic basis underlying the Rspondin3-induced IM reprogramming. Endothelial Rspondin3 enhanced glutamine metabolism, resulting in the generation of α-ketoglutarate, an essential cofactor required for both DNA hydroxymethylation and histone demethylation8, 26. We observed that Rspondin3 induced α-ketoglutarate production in macrophages by augmenting glutamine metabolism, which enhanced TET2 activity to catalyze DNA hydroxymethylation, and thus activated the expression of anti-inflammatory genes. TET2 is an important DNA modifier and epigenetic regulator mediating macrophage functions such as inflammation resolution and pathogen-induced myelopoiesis41, 42. Rspondin3 stimulation also induced direct nuclear interaction of β-catenin with TET2, which thus may also contribute to epigenetic reprogramming through recruiting specific transcription factors to the open chromatin region. Together these findings help to explain the role of TET2-mediated DNA hydroxymethylation as an essential epigenetic program underlying Rspondin3-induced macrophage phenotype transition.
In summary, we demonstrated an orchestrated angiocrine-metabolic-epigenetic signaling axis mediating the maintenance and functional regulation of lung IMs. These studies provide fundamental insights into the endothelial niche in lungs activated by Rspondin3 that reprograms IMs to resolve inflammation and promote tissue repair following inflammatory injury. The role of Rspondin3-β-catenin induced metabolic and epigenetic reprogramming raises the possibility of harnessing angiocrine signaling pathway in macrophages to therapeutically target inflammatory tissue injury.
Methods
Mice
C57BL/6J, Lyz2-cre+ (Jax#019096), Rspo3fl/fl (Jax#027313), Ctnnb1fl/fl (022775), Tet2fl/fl (017573), TCF/Lef:H2B-GFP transgenic mice (Jax#013752), and Rosa26-floxed STOP-Cas9 mice (Jax#024857) were originally purchased from Jackson Laboratory. VE-cadherin-CreERT2 mice (MGI#3848982) were kindly provided by Dr. Ralf Adams. Rspo3fl/fl mice were crossed with VE-cadherin-CreERT2 mice to generate VE-cadherin-CreERT2+;Rspo3fl/fl mice (Rspo3EC−/−), Ctnnb1fl/fl and Tet2fl/fl mice were crossed with Lyz2-cre+ mice to generate Lyz2-cre+;Ctnnb1fl/fl mice (Ctnnb1Mφ−/−) and Lyz2-cre+;Tet2fl/fl mice (Tet2Mφ−/− ), respectively. Genotyping of these mice strains were either done by regular PCR using the recommended primers in Jackson Laboratory website (www.jax.org) followed by DNA gel imaging or by Transnetyx Inc (Cordova, TN, USA) using the TaqMan probe-based qPCR. All mice were housed in a temperature-controlled specific pathogen-free facility under 12-h light/dark cycles in the University of Illinois at Chicago Animal Care Facility. Veterinary care and animal experimental procedures were approved by the University of Illinois Animal Care & Use Committee in accordance with the guidelines of the National Institutes of Health.
Cells
Mouse bone marrow derived macrophages (BMDMs) were isolated and differentiated into mature macrophages as previously described43. Mouse lung microvascular endothelial cells (ECs) were isolated, purified and cultured as previously described44. Mouse lung IMs and AMs were isolated by Fluorescence-activated cell sorting (FACS) on the MoFlo Astrios cell sorter (Beckman Coulter) with the strategy described previously15.
siRNA transfection
Mouse Lgr4 siRNA with four different targeting sites as a pool (Dharmacon, Cat#E-047291–00-0005) and non-targeting pool siRNA (Dharmacon, Cat#D-001910–10) were transfected into BMDMs using the Lipofectamine 3000 reagents (Thermo Fisher Scientific, Cat# L3000015) as described45.
Generation of macrophage specific Lgr4 knockout (Lgr4Mφ−/− ) mice
Lgr4Mφ−/− mice were generated using the CRISPR-Cas9 strategy. Briefly, a transgenic mice strain with Cas9 specific expressed in myeloid cells under control by Lyz2-cre+ was generated by crossing Rosa26-floxed STOP-Cas9 mice with Lyz2-cre+ mice as described25, then sgRNA targeting three different sites (as a pool) for Lgr4 (IDT#Mm.Cas9.LGR4.1.AA, IDT#Mm.Cas9.LGR4.1.AB, IDT#Mm.Cas9.LGR4.1.AC) was packaged in liposome using Invivofectamine 3.0 reagent (ThermoFisher) and delivered into mice via an intravenous tail vein injection (10 nmol per mice), to achieve macrophage uptake by phagocytosis in vivo46.
Recombinant proteins
Recombinant murine Rspondin3 was purchased from RDsystem (Cat#4120-RS-025). Recombinant M-CSF was purchased from PeproTech (Cat#315–02)).
Acute lung injury models
Two acute lung injury mice models were used in this study. The first model is the widely used classical LPS-induced acute lung injury model in which mice are systemically challenged with sub-lethal lipopolysaccharide (LPS, 12 mg/kg i.p.)43, 44, 47. The second inflammatory lung injury model is the bleomycin model that has also been previously described15, 48. Briefly, mice were anesthetized with isoflurane, their lungs were intubated orally with a 20-gauge Angiocath (Franklin Lanes, NJ), and 0.025 IU bleomycin (FisherScientific, Cat#AAJ67560SDL) in 50 μl sterile of PBS were instilled through the catheter. Mice were sacrificed 5 days (acute lung injury phase) after the instillation of bleomycin for further analysis.
RNA isolation and Real-time quantitative PCR (qPCR)
Total RNA from BMDMs or lung ECs was isolated using the PureLink™ RNA kit (Thermo Fisher Scientific, Cat#12183018A) and were reverse-transcribed into cDNA using iScript™ reverse transcription supermix (Bio-rad, Cat#1708840) according to manufacturer’s instructions. The cDNA obtained was mixed with PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Cat#A25742) with specific qPCR primers (Supplementary Table 2) for qPCR on an ABI Prism 7000 system and analyzed with the QuantStudio software v1.3 (Thermo Fisher Scientific). The heatmap for the change folds of gene levels were generated by Morpheus (https://software.broadinstitute.org/morpheus/).
Chromatin immunoprecipitation (ChIP)
ChIP was performed with cross-linked chromatin from mouse BMDMs using a SimpleChIP plus sonication Chromatin IP kit (CST, Cat#56383) with ChIP grade H3K4me3 antibody (CST, Cat#9751) with a dilution ratio of 1:500 according to manufacturer’s instructions. QPCR following ChIP were performed with the primers listed in Supplementary Table 2.
Lung vascular permeability and inflammation measurements
Lung vascular permeability were measured by the albumin-Evans blue dye tracer and lung inflammation were evaluated by myeloperoxidase activity as previously described43, 44.
Preparation of mouse lung single cell suspensions
Mice were anaesthetized with Ketamine/Xylazine and sacrificed, followed by perfusing with 10 ml of PBS via right ventricle. The lungs were removed and cut into tiny pieces with scissors, followed by incubation with digestion buffer (1 mg/ml of Collagenase IV and 0.1 mg/ml DNase I, both from MilliporeSigma) in a shaking water bath (37°C) for 30 min. The digested lung pieces were passed through a 20 G needle for 10 times and filtered by a 40μm nylon mesh to obtain a single-cell suspension. The remaining red blood cells were lysed using RBC lysis buffer (Biolegend, Cat#420301). The acquired lung single cell suspensions were used for the followed applications such as flow cytometry, CyTOF analysis, cell sorting.
Flow cytometry
For BMDMs, flow cytometry was performed with anti-mouse CD11b, F4/80, CD64 to gate matured macrophages. Mouse macrophage anti-inflammatory markers CD206, CD301, IL-10, Arginase 1 and pro-inflammatory markers CD86, CD80, iNOS and TNF were stained with the fluorophore-coupled antibodies listed in Supplementary Table 3. For mouse lung macrophages, flow cytometry was performed to identify lung macrophage subpopulations19. Briefly, cells were stained with anti-mouse CD45, CD11b, CD64, Ly6G, SiglecF, CD86 and CD206 antibodies listed in Supplementary Table 3 using an eBioscience™ flow cytometry staining buffer (Thermo Fisher Scientific, Cat#00–4222-26) according the manual. Samples were run on a CytoFLEX S Flow Cytometer (Beckman) and data were analyzed by the Kaluza Analysis 2.1 software (Beckman).
Cytometry by time-of-flight mass spectrometry (CyTOF)
CyTOF allows for high-dimensional analysis of cell surface markers, cytokines and signaling molecules simultaneously at the single-cell level20. Thus, we used the CyTOF for phenotyping and function assay for the mouse lung myeloid populations including IMs, AMs, monocytes, neutrophils, DCs and eosinophils. A panel of metal-labelled antibodies (Supplementary Table 4) including several markers for macrophages and myeloid cells as well as cytokines and signaling molecules (CD45, CD11b, F4/80, CD64, Ly6C, Ly6G, MerTK, CD24, CD206, SiglecF, CD11c, CD301, Arginase 1, LGR4, β-Catenin, IL-10, iNOS, TNF, CX3CR1, CD80, CD69, CD86, CCR2, CD115, I-A/I-E, BST2, RELMα, CD103, CD3, CD49, TER-119) were used for staining according the FLUIDIGM Inc recommended protocol. Samples were run on the Helios CyTOF Mass cytometer (FLUIDIGM Inc, USA) at the flow cytometry core of Research Resources Center of the University of Illinois at Chicago. Data from the CyTOF were analyzed using Cytobank online analysis tool (www.cytobank.org/, Cytobank Inc, USA).
Metabolism assays
Macrophages (BMDMs) prepared from the indicated mouse strains were uniformly plated in XF96 plates overnight and treated with PBS, Rspondin3, LPS alone or LPS and Rspondin3 together for 24h. Metabolic profiling were performed with the Seahorse XF96 extracellular flux analyzer (Agilent) with the Wave 2.6.1 software (Agilent) as previously described49. Experiments were conducted in XF medium (non-buffered DMEM containing 10 mM glucose, 2 mM glutamine, 2 mM sodium pyruvate), with OCR measured basally and in response to sequential addition of oligomycin, FCCP and rotenone plus antimycin. Fuel preference was measured after 1 h of incubation in Seahorse XF basal medium (Agilent) with no fuel substrates and then immediately before the assay supplemented with either 10 mM glucose, 4 mM glutamine, or 10 nM free fatty acids in the form of palmitate BSA. All seahorse XF data were normalized to an equal cell number of 1 mg total protein.
ELISA
Supernatants were collected from BMDMs under different conditions indicated in Figure legends were collected, and the cytokines IL-10 and IL-1β were measured using mouse IL-10 DuoSet ELISA (RDsystem, Cat#DY417) or mouse IL-1β DuoSet ELISA (RDsystem, Cat#DY401) respectively; Secreted Rspondin3 in cultured lung ECs were measured using mouse Rspondin3 DuoSet ELISA (RDsystem, Cat#DY4120–005) with the supernatants collected. Optical density (OD) data were collected on a Synergy™ HTX multi-mode microplate reader (BioTek) with the Gen 5 software (BioTek). And cell numbers at each well of the cell culture plate were counted using a CyQuant™ kit (Thermo Fisher Scientific, Cat#C7026). Calculated levels of cytokines were adjusted with the cell number.
Immunoblotting
Protein samples for lung ECs and non-ECs were prepared and western blog were performed as previously described44. Anti-RSPO3 polyclonal antibody (Abclonal, A8389) with a dilution of 1:3000 and anti-β-Actin (8H10D10) mouse mAb (CST, Catlog#3700) with a dilution of 1:5000 was used for immunoblotting.
Angiocrine factor profiling
Proteomics-based global secretome analysis were performed by the mass spectrometry core of Research Resources Center in University of Illinois at Chicago using EC-conditioned medium as previously described50.
Immunofluorescence staining and Proximity Ligation Assay (PLA)
An anti-TET2 rabbit monoclonal antibody (CST, Cat#36449) and an anti-β-catenin mouse monoclonal antibody (Thermo Fisher Scientific, Cat#13–8400) with a dilution of 1:500 were used as primary antibodies, and an AF488-conjugated donkey anti-mouse IgG and an AF594-conjugated goat anti-rabbit IgG were used secondary antibody with a dilution of 1:2000 were used for immunofluorescence staining following the CST immunofluorescence general protocol (https://www.cellsignal.com/contents/resources-protocols/immunofluorescence-general-protocol/if). Imaging were taken with the Zeiss LSM880 confocal microscope with Zen software 3.1 (Zeiss)and analyzed with ImageJ software v1.52p. Proximity Ligation Assay for detecting protein interactions with high specificity and sensitivity were performed with Duolink In Situ Red Starter Kit Mouse/Rabbit (Millipore-Sigma, Cat#DUO92101) using the above anti-TET2 rabbit monoclonal antibody and anti-β-catenin mouse monoclonal antibody as validated by immunofluorescence staining according to the standard manual51. Briefly, anti-TET2 rabbit monoclonal antibody and anti-β-catenin mouse monoclonal antibody were first used to detect TET2 and β-catenin on macrophage, then a pair of PLA probes (Anti-rabbit Plus and Anti-mouse Minus) were served as secondary antibodies, next hybridizing connector oligos join the PLA probes only if they are in close proximity to each other and ligase forms closed, circle DNA template for generating rolling-circle amplification that gives red fluorescence.
Assay for specific enrichment of 5-hmC DNA fragments (hMeDIP)
To detect the enrichment of 5-hmC DNA fragments, genomic DNA were isolated from macrophages, and 5-hmC DNA immune precipitation (hMeDIP) were performed using a highly specific purified 5-hmC antibody with a dilution ratio of 1:500 contained by hMeDIP kit purchased from ActiveMotif (Cat#55010) as reported52. Real-time quantitative PCR were performed using the 5-hmC antibody precipitated DNA with specific primers on the proximal promoter regions of genes as listed in Supplementary Table 2.
α-ketoglutarate and succinate measurements and glutaminase activity assay
α-ketoglutarate and succinate levels in macrophages were measured by α-Ketoglutarate Colorimetric/Fluorometric Assay Kit (BioVision, Cat#K677) and Succinate (Succinic Acid) Colorimetric Assay Kit (BioVision, Cat#K649) respectively as previously described25. Glutaminase activity were measured using PicoProbe™ Glutaminase (GLS) Activity Assay Kit (Fluorometric) (BioVision, Cat#K455) according the manual as previously described53.
5mC-Hydroxylase TET activity and JMJD3 Demethylase activity assay
5mC-Hydroxylase TET activity and JMJD3 Demethylase activity were measured using the Epigenase™ 5mC-Hydroxylase TET Activity Assay Kit (Epigentek, Cat#P-3086) and Epigenase™ JMJD3 Demethylase Activity kit (Epigentek, Cat#P-3084) respectively according to the manual as previously reported26, 54. Briefly, nuclear extracts were prepared from macrophages with EpiQuik Nuclear Extraction Kit (Epigentek, Cat#OP-0002), then 5 μg of nuclear protein from each sample were used to incubate with the TET or JMJD3 demethylase substrates respectively, the catalytic activity were measured by the absorbance on a microplate reader (BioTek) with 10 min at 450 nm with a reference wavelength of 655 nm with Gen5 software (BioTek). The TET or JMJD3 activity were represented by OD/min/mg.
Global DNA hydroxymethylation (5-hmC) assay
Global DNA hydroxymethylation (5-hmC) levels were measured using the MethylFlash Global DNA Hydroxymethylation (5-hmC) ELISA Kit (Epigentek, Cat#P-1032) according to the manual as described26. Briefly, genomic DNA were isolated from macrophages from the indicated groups, and 1 μg of DNA were bound to strip-wells that have a high DNA affinity included in the kit, then the hydroxymethylated fraction of DNA is detected using a 5-hmC mAb-based detection complex in a one-step manner and then quantified colorimetrically by reading the absorbance in microplate spectrophotometer (BioTek) at 450 nm with Gen5 software (BioTek). 5hmC% were calculated using the formula generated from an accompanied standard curve.
Statistical analysis
Statistical significance was analyzed using Prism v.8 (GraphPad Software) with tests as described in the figure legends. All the experiments were repeated independently at least three times. Sample size, P values are all cited in the figures and figure captions.
Data Availability Statement
Source data are provided with this paper. Other data that support the findings of this study are available from the corresponding author upon request.
Extended Data
Extended Data Fig. 1: Endothelial cells instruct macrophage phenotype transition via Rspondin3.
(a) Schematic of investigation of the angiocrine effects of ECs on macrophages; (b) mRNA levels of Rspo3 in ECs with or without activated by LPS was measured by qPCR; n=3 samples per group (mean ± sd), two-sided unpaired Student’s t-test was determined using GraphPad Prism. ns P=0.5417. (c) Alternatively activated (M2) and classically activated (M1) macrophages measured by flow cytometry from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ***P=0.0006, ****P<0.0001, ****P<0.0001, ****P<0.0001. (d) Representative flow cytometry plots from three independent repeats showing ratios of M1 (CD86hiCD206lo) and M2 (CD206hiCD86lo) populations in BMDMs treated with control medium, EC-conditioned medium (EC), or activated EC conditioned medium (Activated EC) for 24h, respectively; (e) Represented overlaid flow cytometry histograms from three independent repeats showing the MFI of the anti-inflammatory markers (CD206, CD301) and pro-inflammatory markers (CD86, CD80) in BMDMs stimulated with Rspondin3, LPS alone or in combination for 24h; (f) Gene expression levels of anti-inflammatory maker genes (Mrc1, Arg1, Retnla, Chil3) and pro-inflammatory marker genes (Cd86, Cxcl1, Il1b, Tnf) as measured by qPCR in BMDMs treated with Rspondin3, LPS alone or in combination for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ***P=0.0003, ***P=0.0001, *P=0.0126, ns: P=0.9486, ****P<0.0001, ns P=0.2351, ****P<0.0001, ns P=0.9462, ****P<0.0001, ns P=0.8783, ****P<0.0001.
Extended Data Fig. 2: Rspondin3 regulates macrophage phenotype transition via an angiocrine manner.
(a) Levels of anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, TNF, iNOS) measured by flow cytometry in BMDMs incubated with the EC conditioned medium collected from lung ECs isolated from wildtype mice (WT EC), Rspo3EC−/− mice (ECRspo3−/−) at baseline or endotoxemia conditions (LPS i.p., 12 mg/kg for 24h) (LPS/WT EC, LPS/ ECRspo3−/−), respectively; Data are representative of three independent experiments with n=3 samples per group (mean ± sd), statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (****P<0.0001, ns P=0.9405, ***P=0.0002, ****P<0.0001), CD301 (****P<0.0001, ns P=0.9991, ****P<0.0001, ****P<0.0001), Arginase1 (****P<0.0001, ns P=0.4164, ****P<0.0001, ****P<0.0001), IL-10 (****P<0.0001, ns P=0.9933, ****P<0.0001, ****P<0.0001), CD86 (****P<0.0001, ns P=0.8224, ***P=0.0001, ns P= 0.9075), CD80 (****P<0.0001, ****P<0.0001, ns P=0.7688), TNF (****P<0.0001, ns P=0.4519, **P=0.0061, ns P=0.9997), iNOS (****P<0.0001, ns P=0.9996, ns P=0.6726, ns P>0.9999). (b) Levels of anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, TNF, iNOS) measured by flow cytometry in BMDMs co-cultured in-contact with the ECs from wildtype mice (WT EC), or Rspo3EC−/− mice (ECRspo3−/−) at basal conditions or mice challenged with sublethal LPS (WT EC/LPS, ECRspo3−/−/LPS), respectively; Data are representative of three independent experiments with n=3 samples per group (mean ± s.d), with each dot representing an individual sample. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are CD206 (****P<0.0001, ns P>0.9999, ns P>0.9999), CD301 (****P<0.0001, ns P=0.9977, ns P=0.9998), Arginase1 (****P<0.0001, ns P=0.9328, ns P=0.9998), IL-10 (****P<0.0001, ns P=0.8989, ns P=0.9393), CD86 (ns P=0.8308, ****P<0.0001, ns P=0.9600), CD80 (ns P>0.9999, ****P<0.0001, ns P=0.9679), TNF (ns P=0.9997, ****P<0.0001, ns P=0.9903), iNOS (ns P= 0.9996, ****P<0.0001, ns P= 0.2748).
Extended Data Fig. 3: Rspondin3 regulates lung interstitial macrophage phenotype transition following acute lung injury.
(a) Absolute cell number of lung myeloid cells including neutrophils, eosinophils, Ly6C+ Mo, Ly6C- Mo, CD103+ DC, plasmacytoid DC and CD11b+ DC in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under baseline conditions and post sublethal LPS challenge for 24h or 48h as measured by CyTOF (data are representative of three independent experiments with n=5 mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: neutrophils (ns P>0.9999, ns P=0.9995, ns P=0.9991, ****P<0.0001, ****P<0.0001, ****P<0.0001, ns P=0.9864, ns P=0.2420, ns P=0.6898), eosinophils (ns P>0.9710, ns P>0.9789, ns P>0.9865), Ly6C+ Mo (ns P>0.9933, ****P<0.0001, ns P=0.8936, ns P=0.7754, ns P>0.9878), Ly6C− Mo (ns P>0.9942, ****P<0.0001, ns P=0.9059, ns P=0.7988, ns P>0.9807), CD103+ DC (ns P>0.9676, ns P>0.1488, ns P>0.8806), plasmacytoid DC (ns P>0.9445, ns P>0.2290, ns P>0.9786), CD11b+ DC (ns P>0.9377, ns P>0.2463, ns P>0.9941); (b) Levels of anti-inflammatory markers (CD206, CD301, RELMα, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, iNOS, TNF, CD69) in lung IM in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under basal and post sublethal LPS challenge for 24h or 48h measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (ns P>0.4229, ****P<0.0001, ****P<0.0001, **P=0.0095, ****P<0.0001, ****P<0.0001, ****P<0.0001), CD301 (ns P>0.2041, ***P=0.0002, **P=0.0049, *P=0.0439, ****P<0.0001, ****P<0.0001, ****P<0.0001), RELMα (ns P=0.4547, **P=0.0023, ns P=0.6850, **P=0.0028, ****P<0.0001, ns P=0.1014, ****P<0.0001, ****P<0.0001, ****P<0.0001), Arginase 1 (ns P=0.1315, ns P=0.0640, ns P=0.2260, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), IL-10 (ns P=0.0847, ns P=0.5465, ns P=0.3525, **P=0.0037, *P=0.0448, *P=0.0217, ****P<0.0001, ****P<0.0001, ****P<0.0001), CD86 (ns P>0.7231, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), CD80 (ns P>0.9697, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), iNOS (ns P>0.9568, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), TNF (ns P>0.9977, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001), CD69 (ns P>0.9790, ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001, **P=.0015, ****P<0.0001). (c) Heatmap for the levels of anti-inflammatory markers (CD206, CD301, RELMα, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, iNOS, TNF, CD69) in lung AM in WT and Rspo3EC−/− mice with or without rRspondin3 i.v. under baseline conditions and post sublethal LPS challenge for 24h or 48h as measured by CyTOF (Data are representative of three independent experiments with n = 5 mice per group, shown as fold change by the mean CyTOF signal intensity normalized to Control group); (d) Wnt signaling activities in mice lung myeloid populations determined by CyTOF using Wnt reporter mice (TCF/Lef:H2B-GFP transgenic mice) with or without rRspondin3 i.v. (Data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Sidak’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ns P>0.9999, ns P=0.9301, ns P>0.9999, ****P<0.0001, ns P=0.9992, ns P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999.
Extended Data Fig. 4: Rspondin3 regulates lung interstitial macrophage phenotype transition following acute lung injury.
(a) Gene expression profiling performed by qPCR to evaluate the expression of macrophage marker genes, anti-inflammatory and pro-inflammatory genes in sorted IM and AM from WT and Rspo3EC−/− mice lungs under baseline conditions and endotoxemia. Heatmap shows the fold change of gene levels as normalized to WT IM at basal conditions (Data are representative of three independent experiments, n=3 samples per group); (b) Lung myeloid populations analyzed by CyTOF in WT and Rspo3EC−/− mice subjected to bleomycin induced acute lung injury and basal conditions (Data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Sidak’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: IM (*P=0.0487, ****P<0.0001), AM (ns P<0.3554, ns P<0.9932), neutrophils (ns P=0.9329, ns P=0.9798), eosinophils (ns P=0.9557, ns P=0.7925), Ly6C+ Mo (ns P=0.8486, ns P=0.9777), Ly6C− Mo (ns P=0.9040, ns P=0.9863), CD103+ DC (ns P=0.9526, ns P=0.7602), plasmacytoid DC (ns P=0.9410, ns P=0.7104), CD11b+ DC (ns P=0.9613, ns P=0.7995); (c) Levels of the anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, iNOS, TNF) in IM in WT and Rspo3EC−/− mice under baseline conditions and post bleomycin injury for 5 days as measured by CyTOF (Data are representative of three independent experiments with n=5 mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (ns P=0.7843, ****P<0.0001, ns P>0.9999, ns P=0.9642), CD301 (ns P=0.5538, ****P<0.0001, ns P>0.9999, ns P>0.9999), Arginase 1 (ns P=0.3438, ****P<0.0001, ns P=0.9857, ns P=0.9963), IL-10 (ns P=0.6175, ****P<0.0001, ns P=0.9926, ns P=0.9502), CD86 (ns P=0.0553, ****P<0.0001, ns P=0.9178, ns P=0.9983), CD80 (ns P=0.0882, ****P<0.0001, ns P=0.9978, ns P=0.9970), iNOS (ns P=0.1009, ****P<0.0001, ns P=0.9991, ns P=0.7604), TNF (ns P=0.0736, ****P<0.0001, ns P=0.9997, ns P=0.9979); (d) Representative overlaid CyTOF histograms showing the CyTOF signal intensity of the anti-inflammatory markers (CD206, CD301, Arginase 1, IL-10) and pro-inflammatory markers (CD86, CD80, TNF, iNOS) in IM and AM in WT and Rspo3EC−/− mice at baseline conditions or 5 days after bleomycin i.t. for data in (e).
Extended Data Fig. 5: Macrophage expressed receptor LGR4 is required for Rspondin3-induced macrophage phenotype transition.
(a) Gene expression levels of anti-inflammatory marker genes (Mrc1, Arg1, Retnla, Chil3) and pro-inflammatory marker genes (Cd86, Il1b, Tnf, Cxcl1) measured by qPCR in BMDMs with or without Lgr4 depletion at basal conditions, or treated with Rspondin3, LPS alone or combination of both for 24h from three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Sidak’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: Mrc1 (ns P=0.9997, ****P<0.0001, ns P>0.9999, *P=0.0488), Arg1 (ns P=0.9708, ****P<0.0001, ns P=0.3532, ****P<0.0001), Retnla (ns P=0.7211, ****P<0.0001, ns P=0.9663, ****P<0.0001), Chil3 (ns P=0.6858, ****P<0.0001, ns P=0.6939, ***P=0.0001), Cd86 (ns P=0.3993, *P=0.0419, ****P<0.0001, ****P<0.0001), Il1b (ns P=0.8936, ns P=0.8526, ****P<0.0001, ****P<0.0001), Tnf (ns P>0.9999, ns P=0.3417, ****P<0.0001, ****P<0.0001), Cxcl1 (ns P=0.9976, ns P=0.9584, ****P<0.0001, ****P<0.0001). (b) mRNA levels of Lgr4 in sorted IM and AM measured by qPCR; n=3 samples per group (mean ± sd), two-sided unpaired t-test was determined using GraphPad Prism. **P=0.0012. (c) Levels of LGR4 in myeloid populations in mice lung evaluated by CyTOF (Data are representative of three independent experiments with n=5 mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by ordinary one-way ANOVA with Dunnett’s multiple comparisons test using GraphPad Prism. ****P<0.0001. (d) Levels of LGR4 in IM in WT and Lgr4Mφ−/− mice with or without rRspondin3 i.v. under baseline conditions and post sublethal LPS challenge for 24h measured by CyTOF (Data are representative of three independent experiments with n=5 mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: ****P<0.0001, ****P<0.0001, ****P<0.0001, ****P<0.0001.
Extended Data Fig. 6: β-catenin is required for Rspondin3-induced macrophage phenotype transition.
Levels of anti-inflammatory marker genes (Mrc1, Arg1, Retnla, Chil3) and pro-inflammatory marker genes (Cd86, Il1b, Tnf, Cxcl1) measured by qPCR in BMDMs from Ctnnb1Mφ−/− and WT mice at basal condition, or treated with Rspondin3, LPS alone or combination of both for 24h; Data are representative of three independent experiments with n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: Mrc1 (ns P>0.9999, ****P<0.0001, ns P>0.9999, ****P<0.0001), Arg1 (ns P>0.9999, ****P<0.0001, ns P>0.9999, ****P<0.0001), Retnla (ns P>0.9999, ****P<0.0001, ns P=0.9716, ****P<0.0001), Chil3 (ns P=0.9998, ****P<0.0001, ns P=0.8135, ****P<0.0001), Cd86 (ns P>0.9999, ns P=0.9878, ****P<0.0001, ****P<0.0001), Il1b (ns P=0.9999, ns P=0.9946, ****P<0.0001, ****P<0.0001), Tnf (ns P=0.9854, ns P=0.9426, ****P<0.0001, ****P<0.0001), Cxcl1 (ns P>0.9999, ns P=0.9975, ****P<0.0001, ****P<0.0001).
Extended Data Fig. 7: Rspondin3 mediated increase in mitochondrial respiration through glutamine metabolism.
(a) Basal extracellular acidification rate (ECAR) measured in macrophages stimulated with Rspondin3, LPS alone or in combination for 24h with complete DMEM medium containing 10 mM glucose, 2 mM glutamine, 2 mM sodium pyruvate as substrate; Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ***P=0.0003, ****P<0.0001. (b) Basal ECAR measured in macrophages stimulated with Rspondin3, LPS alone or in combination with only 2 mM glutamine as substrate; Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ****P<0.0001, ****P<0.0001. (c) Basal ECAR measured in BMDMs stimulated with Rspondin3, LPS alone or in combination with only 10 nM free fatty acid as palmitate bound to BSA added as substrate; Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.9681, ns P=0.3389. (d) Basal ECAR measured in BMDMs stimulated with Rspondin3, LPS alone or in combination with only10 mM glucose as substrate; Data are representative of three independent experiments, with 10–12 samples per group. Graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are ns P=0.9694, ns P=0.9000. (e) Basal ECAR measured in WT, Lgr4Mφ−/− and Ctnnb1Mφ−/− macrophages stimulated with or without Rspondin3 with only 2 mM glutamine as substrate; Data are representative of three independent experiments, graphs show the mean ± s.d, with each dot representing an individual sample per time point. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism, P values are **P=0.0041, ns P>0.9999, ns P>0.9999. (f) Glutaminase activity measured in WT, Lgr4Mφ−/− and Ctnnb1Mφ−/− BMDMs under basal, or stimulated with Rspondin3, LPS alone or in combination. Data are representative of three independent experiments (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are ****P<0.0001, ****P<0.0001, ns P=0.9995, ns P>0.9999, ns P=0.9881, ns P>0.9999. (g) Relative gene expression of glutaminase genes – Gls and Gls2 in WT, Lgr4Mφ−/− and Ctnnb1Mφ−/− BMDMs with or without Rspondin3 stimulation measured by qPCR; Data are representative of three independent experiments, n=3 samples from three individual mice per group (mean ± sd), statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values are ns P>0.9999, ****P<0.0001.
Extended Data Fig. 8: Rspondin3 induces TET2-mediated DNA hydroxymethylation of anti-inflammatory genes.
(a) hMeDIP performed on WT, Lgr4Mφ−/−, Ctnnb1Mφ−/− and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or combination of both, and qPCR with primers targeting the proximal promoters of the indicated genes were used to detect the enrichment of 5hmC; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are: Chil3 (****P<0.0001, ****P<0.0001, ns P>0.9999, ns P>0.9999, ns P=0.9998, ns P>0.9999, ns P=0.9983, ns P>0.9999), Retnla (****P<0.0001, ****P<0.0001, ns P>0.9999, ns P>0.9999, P>0.9999, ns P>0.9999, ns P>0.9999, ns P>0.9999). (b) ChIP with an anti-H3K4me3 antibody were performed on WT, Lgr4Mφ−/−, Ctnnb1Mφ−/− and Tet2Mφ−/− BMDMs treated with Rspondin3, LPS alone or in combination, and qPCR with primers targeting the proximal promoters of the indicated genes were used to detect the enrichment of H3K4me3; Data are representative of three independent experiments, n=3 samples per group (mean ± sd); Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. P values (left to right) are: Chil3 (****P<0.0001, ****P<0.0001, ns P=0.9920, ns P>0.9999, ns P=0.9883, ns P=0.9843, ns P=0.6545, ns P>0.9900), Retnla (****P<0.0001, ****P<0.0001, ns P=0.9998, ns P>0.9999, P=0.9992, ns P>0.9999, ns P=0.8940, ns P=0.9994). (c) Bar figure showing levels of anti-inflammatory markers and pro-inflammatory markers in lung IM in WT and Tet2Mφ−/− mice with or without rRspondin3 i.v. under basal and post-sublethal LPS challenge for 24h as measured by CyTOF (data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are: CD206 (****P<0.0001, ****P<0.0001, ns P=0.9618), CD301 (**P=0.0015, ****P<0.0001, ns P=0.9723), Arginase 1 (****P<0.0001, ****P<0.0001, ns P=0.9945), IL-10 (**P=0.0019, ****P<0.0001, ns P=0.8873), CD86 (****P<0.0001, ***P=0.0002, ns P=0.9799), CD80 (****P<0.0001, ns P=0.1255, ns P=0.8631), iNOS (****P<0.0001, ns P=0.6380, ns P=0.8866), TNF (****P<0.0001, **P=0.0012, ns P=0.8863). (d) Absolute cell number for IM in WT and Tet2Mφ−/− mice with or without rRspondin3 i.v. under basal conditions and post sublethal LPS challenge for 24h measured by CyTOF (Data are representative of three independent experiments with five mice per group). Graphs show the mean ± s.d, with each dot representing an individual mouse. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism with individual P values (left to right) are ns P>0.4182, ****P<0.0001, ****P<0.0001, ns P=0.9444; (e) Representative confocal images for immunofluorescent staining with anti-TET2 and anti-β-catenin antibodies performed on BMDMs stimulated with Rspondin3 or PBS from three independent repeats, and quantified data are shown (right) with n=5 samples per group (mean ± sd); Statistical significance was determined by two-tailed unpaired t test using GraphPad Prism. **** P<0.0001. (f) Representative confocal images for in situ proximity ligation assay (PLA) with anti-TET2 and anti-β-catenin antibodies to detect interactions were performed on BMDMs stimulated with Rspondin3 or PBS (three independent repeats), and quantified data are shown (right) with n=5 samples per group (mean ± sd); Statistical significance was determined by two-tailed unpaired t test using GraphPad Prism. **** P<0.0001. (g) Model: The angiocrine-metabolic-epigenetic axis regulates lung IM phenotypic transition. Lung ECs release Rspondin3, which binds to its cell surface receptor-LGR4 in IM and activates β-catenin leading to increased α-ketoglutarate concentration through activation of glutaminolysis, followed by induction of TET2 mediated epigenetic reprograming. TET2 mediated DNA hydroxymethylation increases expression of anti-inflammatory genes in IM and prevents inflammatory lung injury.
Supplementary Material
Acknowledgements
The studies were supported by NIH grants R01-HL45638 (to A.B.M.), P01-HL60678 (to A.B.M. and J.R.), T32-HL007829 (to A.B.M.), R01-HL118068 (to J.R. and A.B.M.), and R01-HL90152 (to J.R. and A.B.M.). The VE-Cadherin-CreERT2 mice were kindly provided by Dr. Ralf Adams at Max-Planck-Institute.
Footnotes
Competing Interests Statement
The authors declare no competing interests.
References
- 1.Ginhoux F, Schultze JL, Murray PJ, Ochando J & Biswas SK New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 17, 34–40 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA & Vannella KM Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ & Wynn TA Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11, 723–737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salome B & Magen A Dysregulation of lung myeloid cells in COVID-19. Nat Rev Immunol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne AJ, Mathie SA, Gregory LG & Lloyd CM Pulmonary macrophages: key players in the innate defence of the airways. Thorax 70, 1189–1196 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Lawrence T & Natoli G Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11, 750–761 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Satoh T et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nature immunology 11, 936–944 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Phan AT, Goldrath AW & Glass CK Metabolic and Epigenetic Coordination of T Cell and Macrophage Immunity. Immunity 46, 714–729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artyomov MN, Sergushichev A & Schilling JD Integrating immunometabolism and macrophage diversity. Semin Immunol 28, 417–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amit I, Winter DR & Jung S The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17, 18–25 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Colegio OR et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svedberg FR et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nature immunology 20, 571–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussell T & Bell TJ Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14, 81–93 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Galli SJ, Borregaard N & Wynn TA Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12, 1035–1044 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakarov S et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Sabatel C et al. Exposure to Bacterial CpG DNA Protects from Airway Allergic Inflammation by Expanding Regulatory Lung Interstitial Macrophages. Immunity 46, 457–473 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Ding BS et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2alpha. Nature communications 9, 559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR & Perlman H Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American journal of respiratory cell and molecular biology 49, 503–510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becher B et al. High-dimensional analysis of the murine myeloid cell system. Nat Immunol 15, 1181–1189 (2014). [DOI] [PubMed] [Google Scholar]
- 21.de Lau W, Peng WC, Gros P & Clevers H The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes & development 28, 305–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glinka A et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO reports 12, 1055–1061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D et al. Structural basis for R-spondin recognition by LGR4/5/6 receptors. Genes & development 27, 1339–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholz B et al. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev Cell 36, 79–93 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Liu PS et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 18, 985–994 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Carey BW, Finley LW, Cross JR, Allis CD & Thompson CB Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell metabolism 24, 542–554 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deplus R et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. The EMBO journal 32, 645–655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramasamy SK, Kusumbe AP, Wang L & Adams RH Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keiran N et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nature immunology 20, 581–592 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Kang S et al. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nature immunology 19, 561–570 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Pan W et al. The DNA Methylcytosine Dioxygenase Tet2 Sustains Immunosuppressive Function of Tumor-Infiltrating Myeloid Cells to Promote Melanoma Progression. Immunity 47, 284–297 e285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goiffon RJ, Martinez SC & Piwnica-Worms D A rapid bioluminescence assay for measuring myeloperoxidase activity in human plasma. Nat Commun 6, 6271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambrecht BN TGF-beta Gives an Air of Exclusivity to Alveolar Macrophages. Immunity 47, 807–809 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Schyns J et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun 10, 3964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hume PS et al. Localization of Macrophages in the Human Lung via Design-based Stereology. Am J Respir Crit Care Med 201, 1209–1217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedoret D et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 119, 3723–3738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamino T & Komuro I Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest 116, 2316–2319 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan B et al. Inhibition of Rspo-Lgr4 Facilitates Checkpoint Blockade Therapy by Switching Macrophage Polarization. Cancer Res 78, 4929–4942 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Du B et al. Lgr4/Gpr48 negatively regulates TLR2/4-associated pattern recognition and innate immunity by targeting CD14 expression. J Biol Chem 288, 15131–15141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Q et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554, 123–127 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only References
- 43.Di A et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity 49, 56–65 e54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu M et al. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nature communications 10, 2126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nepal S et al. STAT6 induces expression of Gas6 in macrophages to clear apoptotic neutrophils and resolve inflammation. Proc Natl Acad Sci U S A 116, 16513–16518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo YL et al. Macrophage-Specific in Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano 12, 994–1005 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Ficz G et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 473, 398–402 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Sorensen I, Adams RH & Gossler A DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680–5688 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Nelson VL et al. PPARgamma is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev 32, 1035–1044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanco MA et al. Global secretome analysis identifies novel mediators of bone metastasis. Cell research 22, 1339–1355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fredriksson S et al. Protein detection using proximity-dependent DNA ligation assays. Nature biotechnology 20, 473–477 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Takayama K et al. TET2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression. Nature communications 6, 8219 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Wang JB et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell 18, 207–219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thienpont B et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537, 63–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided with this paper. Other data that support the findings of this study are available from the corresponding author upon request.