Abstract
Background
Lung function, measured as forced expiratory volume in one second (FEV1), and exacerbations are two endpoints evaluated in chronic obstructive pulmonary disease (COPD) clinical trials. Joint analysis of these endpoints could potentially increase statistical power and enable assessment of efficacy in shorter and smaller clinical trials.
Objective
To evaluate joint modelling as a tool for analyzing treatment effects in COPD clinical trials by quantifying the association between longitudinal improvements in FEV1 and exacerbation risk reduction.
Methods
A joint model of longitudinal FEV1 and exacerbation risk was developed based on patient-level data from a Phase III clinical study in moderate-to-severe COPD (1740 patients), evaluating efficacy of fixed-dose combinations of a long-acting bronchodilator, formoterol, and an inhaled corticosteroid, budesonide. Two additional studies (1604 and 1042 patients) were used for external model validation and parameter re-estimation.
Results
A significant (p<0.0001) association between FEV1 and exacerbation risk was estimated, with an approximate 10% reduction in exacerbation risk per 100 mL improvement in FEV1, consistent across trials and treatment arms. The risk reduction associated with improvements in FEV1 was relatively small compared to the overall exacerbation risk reduction for treatment arms including budesonide (10–15% per 160 µg budesonide). High baseline breathlessness score and previous history of exacerbations also influenced the risk of exacerbation.
Conclusion
Joint modelling can be used to co-analyze longitudinal FEV1 and exacerbation data in COPD clinical trials. The association between the endpoints was consistent and appeared unrelated to treatment mechanism, suggesting that improved lung function is indicative of an exacerbation risk reduction. The risk reduction associated with improved FEV1 was, however, generally small and no major impact on exacerbation trial design can be expected based on FEV1 alone. Further exploration with other longitudinal endpoints should be considered to further evaluate the use of joint modelling in analyzing COPD clinical trials.
Keywords: lung function, bronchodilator, anti-inflammatory
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) are episodes of respiratory symptom worsening requiring additional therapy (eg oral steroids, antibiotics) and/or hospitalization. Prevention of exacerbations is an important goal in COPD treatment and the exacerbation event is recommended by regulatory agencies as an endpoint for assessing efficacy in clinical studies.1 Effects of drug treatment on exacerbation risk are commonly analyzed based on event rates, eg using negative binominal regression, or time-to-first-event, eg using Cox regression. The relatively low frequency of exacerbations in COPD patients means that long and large studies are needed to get desirable precision in treatment effect estimates. Consequently, exacerbations are usually not studied until Phase III, while there is a need to predict efficacy and dosing regimen of novel treatments in earlier phases of development.
Lung function, measured as the change from baseline in forced expiratory volume in one second (FEV1), is another frequently used primary endpoint to assess treatment effects in COPD in Phase III, as well as Phase II dose-finding trials of new treatments. FEV1 is usually measured at baseline and then repeatedly during a study (ie longitudinally), and through analysis of repeated measurement data, using linear or nonlinear mixed-effects (LME, NLME) models, increased precision in treatment effects can be obtained.
It is well established that there is correlation between FEV1 and exacerbation risk; an improved lung function is related to lower risk of exacerbation.2–8 The association between improvements in FEV1 and reduced exacerbation risk was quantified using meta-analysis approaches,4,5 and based on individual patient data.6–9 These results indicate that there may be value in co-analyzing longitudinal FEV1 and exacerbations in clinical trials; ie capturing treatment effects on both endpoints, and the inter-dependencies between endpoints, in the same model could potentially increase statistical power, and enable assessment of exacerbation efficacy in shorter and smaller trials.
The concept of joint modelling of longitudinal biomarker data with time-to-event data provides an approach to adequately handle the influence of an endogenous time-dependent covariate, as it allows the longitudinal biomarker to affect the hazard in a time-dependent manner, while accounting for the measurement error, and can provide more efficient and less biased estimates of treatment effects. This method has received increasing attention, eg in oncology, focusing on association between quality of life10 or tumor size dynamics11 and survival, and treatment of HIV linking longitudinal CD4+ count and survival (eg12). To our knowledge, this method has not been applied in COPD.
The aim of the present work was to evaluate joint modelling as a tool for analyzing treatment effects in COPD clinical trials by (1) quantifying the association between longitudinal improvements in FEV1 and the risk of exacerbation, (2) comparing a joint model of the two endpoints to a Cox proportional hazards model of only exacerbations, and (3) assessing the consistency in parameter estimates across several clinical studies and treatments. To this end, we used a large set of patient-level data from three Phase III clinical studies in moderate-to-severe COPD, evaluating the efficacy of fixed-dose combinations of a bronchodilator (long-acting beta-agonist, LABA) and an anti-inflammatory (inhaled corticosteroid, ICS), two compound classes included in the standard of care treatment for COPD.
Methods
Data Description
Data from three clinical COPD studies, evaluating fixed-dose combinations of formoterol (LABA) and budesonide (ICS) versus the mono-components and placebo, were used in the analysis.13–15 A subset of the original study data was available for analysis, including patients who had provided informed consent for data re-use, as summarized in Table 1. Full details of the original studies have been published elsewhere.13–15 Data from the largest study, Study A (NCT00206167, 1740 patients),13 was used for model development and qualification. Two additional studies: Study B (NCT00206154; 1604 patients)14 and Study C (NCT00419744; 1042 patients)15 were used for external model validation and subsequent re-estimation of model parameters. All three studies were performed across multiple geographical regions and the only country represented in all three studies was the USA. Summaries of baseline characteristics, per study, are shown in Table 2. The studies were selected to provide adequate numbers of exacerbations and included longitudinally measured FEV1, to enable appropriate analysis of the association between these clinical endpoints.
Table 1.
Study Data Details: Duration, Treatment Arms, FEV1 Measurement Schedule
| Study A | Study B | Study C | |
|---|---|---|---|
| Study duration | 12 months | 6 months | 12 months |
| No of patients | 1740 | 1604 | 1042 |
| Treatment arms | Placebo | Placebo | |
| Formoterol DPI 9 μg BID | Formoterol DPI 9 μg BID | Formoterol DPI 9 μg BID | |
| Budesonide/formoterol pMDI 160/9 μg BID | Budesonide/formoterol pMDI 160/9 μg BID | Budesonide/formoterol pMDI 160/9 μg BID | |
| Budesonide/formoterol pMDI 320/9 μg BID | Budesonide/formoterol pMDI 320/9 μg BID | Budesonide/formoterol pMDI 320/9 μg BID | |
| Budesonide pMDI 320 μg BID | |||
| Budesonide pMDI 320 μg BID + formoterol DPI 9 μg BID | |||
| FEV1 measurements | Baseline, 1, 2, 4, 6, 9, 12 months | Baseline, 1, 2, 4, 6 months | Baseline, 1, 2, 4, 6, 9, 12 months |
Abbreviations: BID, twice daily; DPI, dry powder inhaler; no, number; pMDI, pressurized metered dose inhaler.
Table 2.
Summary of Baseline Characteristics, by Study
| Study A | Study B | Study C | ||||
|---|---|---|---|---|---|---|
| Country, n (%) | USA Mexico Germany Hungary Greece Bulgaria Denmark Romania Iceland |
758 (43.6) 82 (4.7) 197 (11.3) 293 (16.8) 37 (2.1) 136 (7.8) 76 (4.4) 139 (8.0) 22 (1.3) |
USA Czech Republic South Africa Poland Netherlands |
659 (41.8) 259 (16.1) 124 (7.7) 473 (29.4) 89 (5.0) |
USA Argentina Brazil South Africa Chile Colombia Mexico Peru Venezuela |
481 (46.2) 183 (17.6) 81 (7.8) 161 (15.4) 43 (4.1) 19 (1.8) 52 (5.0) 17 (1.6) 5 (0.6) |
| Exacerbation historya, median (range) | 1.0 (0–11) | 1.0 (0–10) | 1.0 (1–12) | |||
| Male sex, % | 64.0 | 68.1 | 62.9 | |||
| Race, % | ||||||
| Caucasian | 92.1 | 93.5 | 81.8 | |||
| Black | 2.4 | 3.8 | 3.6 | |||
| Oriental | 0.5 | 0.4 | – | |||
| Asian | – | – | 1.2 | |||
| American Indian or Alaska Native | – | – | 0.2 | |||
| Other | 5.0 | 2.3 | 13.2 | |||
| Season of year at treatment start, % | ||||||
| Autumn | 25.4 | 27.8 | 8.3 | |||
| Spring | 23.9 | 16.6 | 42.4 | |||
| Summer | 34.9 | 35.9 | 22.3 | |||
| Winter | 15.8 | 19.7 | 27.2 | |||
| Breathlessness scoreb, mean (SD) | 2.15 (0.66) | 2.11 (0.67) | 1.84 (0.77) | |||
| Sleep scoreb, mean (SD) | 1.01 (0.88) | 0.98 (0.86) | 1.19 (0.87) | |||
| Sputum scoreb, mean (SD) | 1.44 (0.89) | 1.45 (0.84) | 1.47 (0.85) | |||
| Cough scoreb, mean (SD) | 1.86 (0.85) | 1.81 (0.82) | 1.79 (0.8) | |||
| Eosinophils [x109/L], mean (SD) | 0.24 (0.19) | 0.23 (0.19) | 0.19 (0.3) | |||
| FEV1 [L], mean (SD) | 1.05 (0.40) | 1.05 (0.40) | 1.01 (0.41) | |||
| FVC [L], Mean (SD) | 2.16 (0.72) | 2.25 (0.76) | 2.18 (0.75) | |||
| FEV1/FVC, mean (SD) | 0.49 (0.12) | 0.48 (0.12) | 0.47 (0.12) | |||
Notes: aNumber of exacerbations during previous year. bBaseline scores were averaged for each patient over the 14-day run-in period.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; n, number; SD, standard deviation.
Pre-dose FEV1 was measured repeatedly over time, and the time to first exacerbation was captured in the studies. Exacerbations were defined as in the original study protocols, ie hospitalization and/or oral steroid treatment due to worsening of COPD. The percentage of patients with at least one exacerbation during the study was 34%, 25% and 44% for study A, B and C, respectively.
Analysis
Cox Proportional Hazards Model for Time-to-First Exacerbation
A conventional Cox proportional hazards model for time-to-first exacerbation was used as a reference model to estimate exacerbation treatment effects in Study A. Categorical treatment arm, country and baseline FEV1 were included as covariates.
A Joint Model of FEV1 and Exacerbations
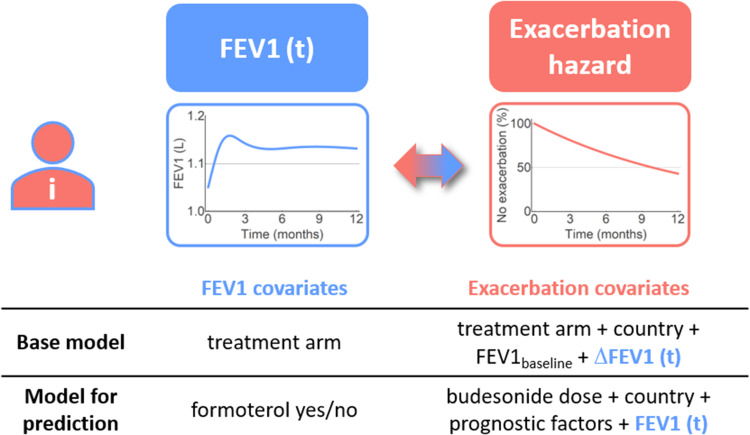
A joint model (referred to as the base model) of exacerbation risk and longitudinal pre-dose FEV1 was constructed. The model consisted of two sub-models, the Cox proportional hazards model for time-to-first exacerbation (above) and an LME model for longitudinal FEV1, linked with an association parameter describing how the estimated individual response in FEV1 affects the exacerbation hazard (Figure 1). For the Cox proportional hazards sub-model, the baseline hazard was defined as a piecewise constant function.
Figure 1.
Overview of the joint models of individual longitudinal FEV1 and exacerbation hazard, indicating the covariates, and longitudinal data, included in the base model and the model for prediction of FEV1 and exacerbation hazard. Prognostic factors refer to additional baseline covariates identified in the covariate search.
The LME sub-model used longitudinal change from baseline FEV1 (∆FEV1) as the independent variable and was built using natural splines. Different placements of the spline knots were investigated, for both the fixed and random effects of the model. Treatment was initially included as a categorical covariate, with one fixed-effect spline estimated for each treatment arm.
This base joint model differed from the reference Cox model (above) only in that the effect of longitudinal ∆FEV1 on the exacerbation hazard was included. Parameter estimates of the model, when applied to Study A, were compared to those of the reference Cox model. For additional details, see the Supplementary Material.
Updated Joint Model for Prediction
After evaluation of the base joint model, it was updated to allow for prediction of outcome in other studies with budesonide and/or formoterol treatments (Figure 1). The categorical treatment arm covariate was removed from the exacerbation hazard and was substituted by separate components to reflect the bronchodilator and anti-inflammatory effects.
Formoterol (bronchodilator) was used as a covariate in the longitudinal FEV1 model and was thereby assumed to only affect the exacerbation hazard via effects on FEV1. Absolute FEV1 was modelled as the independent variable in this model and thus replaced baseline and ∆FEV1. Budesonide (anti-inflammatory) was used as a dose-dependent covariate in the Cox proportional hazards sub-model.
An extensive covariate search was further performed. The following baseline covariates were tested on the exacerbation hazard: country, exacerbation history, breathlessness score, sleep score, sputum score, cough score, eosinophils, sex, age, race, FVC, FEV1/FVC and season when the treatment started. Up to five covariate combinations were tested. The final set of covariates was selected based on the Bayesian Information Criterion (BIC).
Model Validation and Parameter Re-Estimation
The updated model was validated by predicting the outcome of Studies B and C, using a 1-month cutoff for longitudinal FEV1 data, thus including at least one FEV1 measurement post-baseline (see the Supplementary Material for details). Only patients from the USA were included in the validation procedure, since it was the only country represented in all 3 studies (see Table 2 for details). The model was also re-estimated on Studies B and C to investigate the consistency of parameter estimates across studies.
Lastly, the consistency of the association parameter linking FEV1 to the exacerbation hazard across treatment mechanisms was assessed. This was done by re-estimating the association for each treatment arm (vs reference), and for the reference arm alone, in each study separately (for details see the Supplementary Material).
Software
All models were estimated using the JM package version 1.4–716 in R version 3.2.4.17 The joint models were estimated and validated using a joint conditional formulation of the likelihood function, incorporating the impact of FEV1 and hazard function, as described in.16
Results
Comparison of the Cox Proportional Hazards Model and the Base Joint Model
A significant association between longitudinal ∆FEV1 and the risk of exacerbation was estimated (p<0.0001) in the joint model for Study A (Table 3). The estimated association constant for ∆FEV1 was of similar magnitude to that of the baseline FEV1 covariate effect. The hazard ratios (HRs) for each of the treatment arm comparisons estimated with the Cox proportional hazards model and the base joint model are also shown in Table 3 (full model outputs can be found in Supplementary Material - Tables S1 and S2). The estimated HRs are higher for the joint model vs the Cox model (by approximately 5 percentage points) since part of the treatment effect is captured via the longitudinal ∆FEV1 model. The effect of ∆FEV1, however, is small in relation to the overall treatment exacerbation risk reduction (30–35% for treatment arms including budesonide). No improvement in precision was seen in the estimated total treatment effect of the joint model (ie the combined effect of longitudinal FEV1, via the association, and the treatment coefficients in the Cox model) (data not shown). For details on the longitudinal sub-model see the Supplementary Material.
Table 3.
Estimated Hazard Ratiosa for Exacerbation Risk (Cox Model and Base Joint Model, Study A)
| Parameter | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| Cox Proportional Hazards Model | Joint Model | |||
| Formoterol DPI 9 µg | 0.85 | (0.69–1.07) | 0.89 | (0.71–1.12) |
| Budesonide/formoterol pMDI 160/9 µg | 0.71 | (0.56–0.89) | 0.76 | (0.60–0.95) |
| Budesonide/formoterol pMDI 320/9 µg | 0.65 | (0.51–0.81) | 0.69 | (0.55–0.87) |
| Baseline FEV1 association coefficient, increase per 100 mL | 0.92 | (0.90–0.94) | 0.92 | (0.89–0.94) |
| Longitudinal ∆FEV1 association coefficient, increase per 100 mL | 0.91 | (0.86–0.96) | ||
Notes: aDerived from point estimates. Estimates for country not shown. For a full summary of parameters, see the Supplementary Material.
Abbreviations: CI, confidence interval; DPI, dry powder inhaler; FEV1, forced expiratory volume in 1 second; ∆FEV1, change from baseline in forced expiratory volume in 1 second; mL, milliliter; pMDI, pressurized metered dose inhaler.
The Updated Joint Model for Prediction
The updated joint model for prediction (Figure 1), where the categorical treatment effect was substituted by separate components for the bronchodilator (included in the longitudinal sub-model) and anti-inflammatory effects (included in the hazard), resulted in an adequate fit to the data. The estimated HR per 160 µg budesonide was 0.85 (95% CI: 0.76–0.93). The covariate search identified the following baseline covariates: country, breathlessness score (HR 0.84 (95% CI: 0.74–0.95) per decrease by 1 unit) and the number of exacerbations in the previous year (HR 0.88 (95% CI: 0.83–0.94) per decrease by 1 unit).
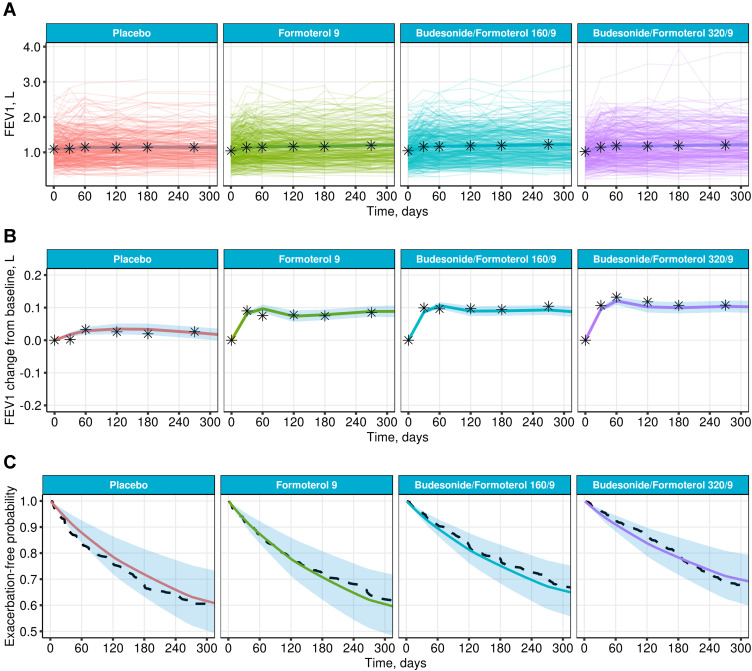
The variability in individual FEV1 profiles was large, as can be seen in Figure 2A. However, the longitudinal behavior of FEV1 was adequately described (Figure 2A and B). The model also adequately described time-to-event data for all four treatment arms in Study A (Figure 2C). Model parameter estimates and additional goodness-of-fit figures can be found in the Supplementary Material (Table S3 and Figure S1).
Figure 2.
Diagnostics of the joint model for prediction fitted to Study A. Mean of individual predictions of FEV1 (A) and change from baseline FEV1 (B) with 95% CI (solid colored lines and shaded areas), superimposed with individual profiles (thin colored lines) and means of the original data (stars) vs time. (C) Predicted mean exacerbation-free probabilities (solid line) with interquartile range (shaded area) and Kaplan–Meier estimates (dashed line).
Model Validation and Parameter Re-Estimation
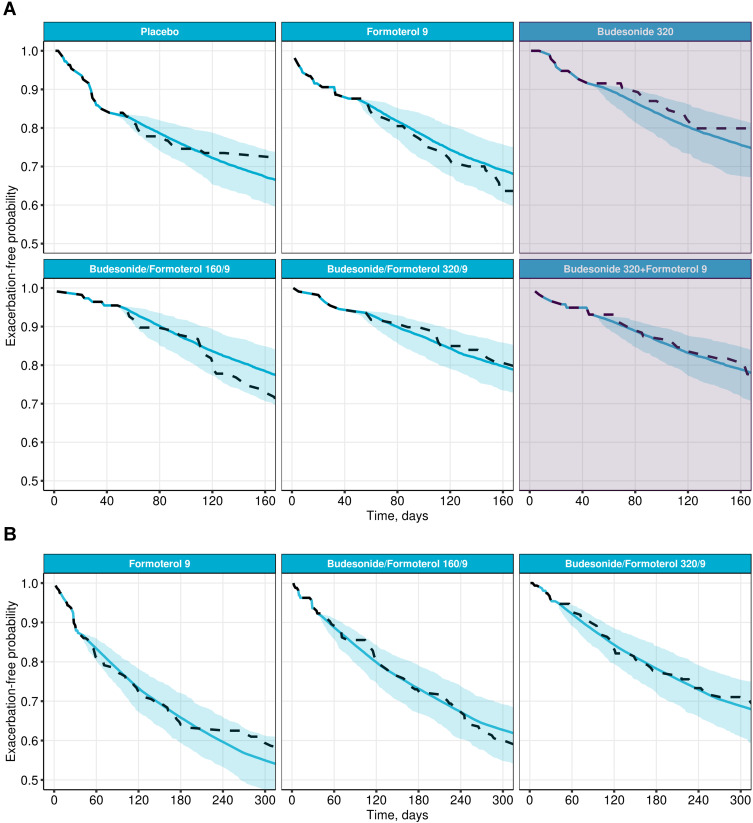
The updated joint model developed based on Study A was assessed in terms of its predictive ability for Studies B and C. As shown in Figure 3, the model successfully predicted 6- and 12-month exacerbation outcomes (for patients from the USA) based on a data cutoff at the second visit (1 month), including the two additional treatment arms in Study B which were not present in Study A: budesonide 320 μg and the combination of budesonide 320 μg and formoterol 9 μg using separate inhalation devices (Figure 3A).
Figure 3.
Prediction of exacerbation outcomes, for patients in the USA, in Study B (A) and Study C (B), using the joint model for prediction developed on Study A, and a 1-month data cut-off. Predicted exacerbation-free probabilities (means and 95% CI, blue) vs Kaplan–Meier estimates (dashed line). The shaded area in (A) denotes additional treatment arms in Study B which was not included in Study A.
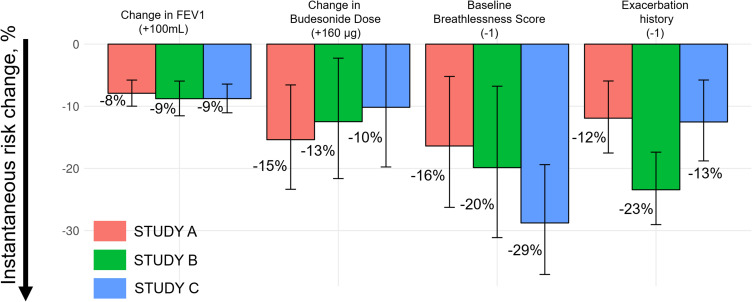
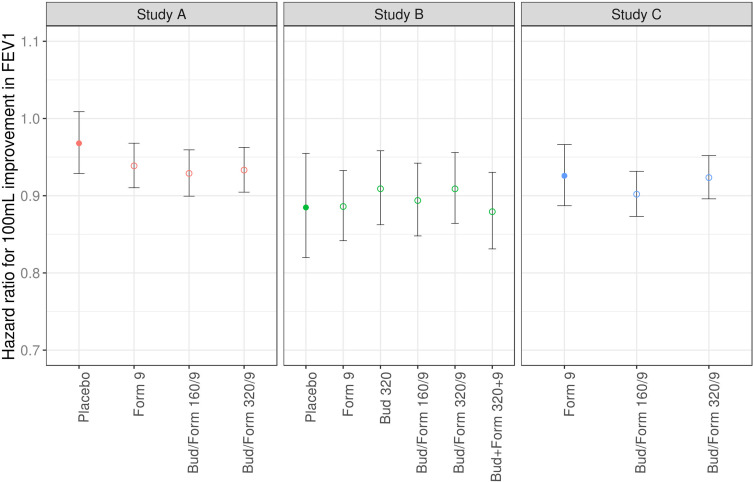
When re-estimating model parameters on Studies B and C, the current longitudinal value of FEV1 was found to be significantly associated with the risk of exacerbation also in these studies (p<0.0001). Moreover, parameter estimates were consistent across all three studies; an increase of 100 mL in FEV1 decreased the risk of exacerbation by 8–9% (Figure 4). Full model outputs can be found in the Supplementary Material (Tables S4 and S5, Figures S2 and S3). Parameter estimates were also similar across treatment arms (Figure 5).
Figure 4.
Parameter estimates of the joint model for prediction with 95% CI per study. Histograms show the instantaneous exacerbation risk change with respect to the relative parameter change.
Figure 5.
Estimated hazard ratio per 100 mL improvement in FEV1 per study arm for each study; for the reference arm alone (filled circles) and each treatment arm vs reference (open circles). Error bars represent 95% CI.
Abbreviations: Form, formoterol; Bud, budesonide.
In addition, a consistent dose-dependent effect of budesonide was estimated; the exacerbation risk decreased by 10–15% per 160 µg of budesonide. A patient’s baseline breathlessness score and previous history of exacerbations influenced the risk of exacerbation, even though the point estimates of the effects varied approximately 2-fold across studies, as shown in Figure 4.
Discussion
The primary aim of this work was to evaluate joint modelling as a tool for analyzing treatment effects in COPD clinical trials. This was achieved by applying joint models to a large set of patient-level data on longitudinal FEV1 and exacerbations, two of the most established endpoints of drug effect in COPD, measured in three phase III studies of budesonide/formoterol. The developed joint models, which included an LME model for longitudinal pre-dose FEV1 and a Cox proportional hazards model for time-to-first exacerbation, estimated statistically significant association between the two endpoints (p<0.0001). Notably, the estimate of the association parameter was consistent across studies and treatments, including placebo, confirming that longitudinal FEV1 contains information about exacerbation risk.
The size of the association parameter implied an approximate 10% reduction in instantaneous exacerbation risk for a 100 mL improvement in pre-dose FEV1. When considering the limited treatment effects on FEV1 in the COPD studies used in our analysis, on average around 50–90 mL, the average exacerbation risk reduction associated with improvements in FEV1 is in the range of 4–7%. The magnitude of the effect is thus relatively small and only a minor part of the exacerbation risk reduction is accounted for by longitudinal changes in FEV1. This can be partly attributed to variability in FEV1 measurements,6 however, it also emphasizes that other factors than lung function improvement are important for exacerbation risk reduction. This is particularly true for anti-inflammatory drugs like ICS, which have limited effects on FEV1 in COPD patients but significantly reduces exacerbation risk.
We found the association between lung function and exacerbation risk to be consistent across three studies of an ICS/LABA combination treatment. Furthermore, we found the FEV1-exacerbation association parameter to be similar across all treatment arms (budesonide, formoterol, budesonide/formoterol and placebo) when estimated separately. Although the studies used in our analysis are of similar design, in similar populations, this suggests that the effect on exacerbation risk, for a specific change in FEV1, is unrelated to the mechanism of action. This is also supported by the meta-analyses done by Zider et al4 and Ribbing et al,5 who identified similar slopes of the relationship for anti-inflammatory and bronchodilator compounds.
The fact that the FEV1-exacerbation association is of a similar magnitude to that previously reported based on other methods7,8 indicates that the joint modelling methodology works and has the potential to be used also with other variables and potentially extended to multivariate models (eg18). It also suggests a strong prior probability can be used for the association between FEV1 and exacerbation risk when applying this type of joint model in a Bayesian setting.19 However, given the modest effect of longitudinal FEV1 on exacerbation risk reduction and large variability in FEV1 data, we see no immediate impact on trial design based on these results. In our updated joint model for prediction, we estimated a significant dose-dependent effect of budesonide (in addition to the longitudinal lung function effect) in the exacerbation hazard model. Ideally, this should be replaced by (longitudinal) biomarkers capturing anti-inflammatory effects. Breathlessness score, at baseline, was found to be a covariate strongly affecting the risk of exacerbations. There may therefore be value in accounting for longitudinally measured breathlessness score in a multivariate joint model. Inclusion of multiple longitudinal variables, such as symptom scores, quality of life assessments (patient-reported outcomes), and biomarkers related to inflammation has the potential to maximize the information from the data and improve statistical inference and predictive ability.
An additional consideration is the link between treatment response in FEV1 and risk of early drop-out. It has been shown that treatment failure of a bronchodilator (ie lower FEV1 response) increased the risk of early drop-out.20 Not accounting for such informative drop-out could lead to both bias and imprecision of parameters,21–23 hence extending the modelling to competing events could be beneficial.
Conclusions
Joint modelling can be used to co-analyze longitudinal FEV1 and exacerbation data in COPD clinical trials. An approximate 10% reduction in exacerbation risk was estimated per 100 mL improvement in FEV1, consistent across three trials and multiple treatment arms, confirming that treatment effects on FEV1 contain information about exacerbation risk reduction. However, due to the relatively small contribution of improved lung function to the overall risk reduction, no major impact on exacerbation trial design can be expected based on FEV1 alone. Further exploration with other longitudinal endpoints should be considered to evaluate the use of joint modelling in analyzing and predicting outcome of COPD clinical trials.
Acknowledgments
We would like to thank all individuals who participated in the primary randomized controlled trials from which the data were derived; the clinical research teams who conducted them; Dr. Dina Chernikova for help in early statistical analyses.
Funding Statement
AstraZeneca funded this research, as well as all the studies included in these analyses. Employees of AstraZeneca participated in the design, data collection, data analysis, and data interpretation of this research. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy, and the protection of intellectual property. The corresponding author had access to all data in the study and had the final responsibility for the decision to submit the manuscript for publication.
Abbreviations
BIC, Bayesian information criterion; BID, twice daily; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; pMDI, pressurized metered dose inhaler; FEV1, forced expiratory volume in 1 second; ∆FEV1, change from baseline FEV1; FVC, forced vital capacity; HIV, human immunodeficiency virus; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LME, linear mixed-effects; NLME, nonlinear mixed-effects; SD, standard deviation.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Consent to Participate
This was a post hoc analysis of three previously conducted, already published, AstraZeneca sponsored studies. The original study protocols received appropriate ethical approval and were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization/Good Clinical Practice, and applicable local regulations. All patients provided written informed consent. Before the analysis, all informed consent forms were reviewed for data re-use in accordance with AstraZeneca data sharing rules, and patient data were anonymized. These re-analyses did not require additional ethics approval, as patients from countries who do not approve data re-use, and patients who had withdrawn consent, were excluded.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
KZ, OS, KP are employees of M&S Decisions LLC, a modelling consultancy contracted by AstraZeneca. RP, AJ, GH, UGE and UWH are all employees of AstraZeneca and may own shares. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD 2016. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed December31, 2020.
- 2.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Molken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zider AD, Wang X, Buhr RG, Sirichana W, Barjaktarevic IZ, Cooper CB. Reduced COPD Exacerbation Risk Correlates With Improved FEV1: A Meta-Regression Analysis. Chest. 2017;152(3):494–501. doi: 10.1016/j.chest.2017.04.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribbing J, Korell J, Cerasoli F, Milligan P, Martin S, O. Karlsson M. Predicting Reductions in Chronic Obstructive Pulmonary Disease (COPD) Exacerbations from FEV1-A Model-Based Meta-Analysis of Literature Data from Controlled Randomized Clinical Trials. J Pharmacokinet Pharmacodyn. 2015;42:S63S63. [Google Scholar]
- 6.Donohue JF, Jones PW, Bartels C, et al. Correlations between FEV1 and patient-reported outcomes: A pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19. doi: 10.1016/j.pupt.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161. doi: 10.1186/1465-9921-12-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facius A, Krause A, Claret L, Bruno R, Lahu G. Modeling and Simulation of Pivotal Clinical Trials Using Linked Models for Multiple Endpoints in Chronic Obstructive Pulmonary Disease With Roflumilast. J Clin Pharmacol. 2017;57(8):1042–1052. doi: 10.1002/jcph.885 [DOI] [PubMed] [Google Scholar]
- 9.Calverley PM, Eriksson G, Jenkins CR, et al. Early efficacy of budesonide/formoterol in patients with moderate-to-very-severe COPD. Int J Chron Obstruct Pulmon Dis. 2016;12:13–25. doi: 10.2147/COPD.S114209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28(16):2796–2801. doi: 10.1200/JCO.2009.25.0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardivon C, Desmée S, Kerioui M, et al. Association between tumor size kinetics and survival in advanced urothelial carcinoma patients treated with atezolizumab: implication for patient’s follow-up. Population Analysis Group Europe (PAGE) 28; 2019. Available from: www.page-meeting.org/?abstract=8824. Accessed December31, 2020. [DOI] [PubMed]
- 12.Lim HJ, Mondal P, Skinner S. Joint modeling of longitudinal and event time data: application to HIV study. J Med Stat Inf. 2013;1(1). doi: 10.7243/2053-7662-1-1 [DOI] [Google Scholar]
- 13.Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi: 10.2165/00003495-200969050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000. doi: 10.2165/00003495-200868140-00004 [DOI] [PubMed] [Google Scholar]
- 15.Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–268. doi: 10.1016/j.rmed.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Rizopoulos D. JM: an R Package for the Joint Modelling of Longitudinal and Time-to-Event Data. J Stat Softw. 2010;35(9):1–33. doi: 10.18637/jss.v035.i0921603108 [DOI] [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, Vienna, Austria; 2016. [Google Scholar]
- 18.Gavrilov S, Zhudenkov K, Peskov K, Helmlinger G, Aksenov S Longitudinal assessment of tumor size and neutrophil count in multivariate joint models are more predictive of survival than their baseline values in patients with non-small cell lung cancer. Population Analysis Group Europe (PAGE) 28; 2019. Available from: www.page-meeting.org/?abstract=9172. Accessed December31, 2020.
- 19.Alsefri M, Sudell M, García-Fiñana M, Kolamunnage-Dona R. Bayesian joint modelling of longitudinal and time to event data: a methodological review. BMC Med Res Methodol. 2020;20(1):94. doi: 10.1186/s12874-020-00976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musuamba FT, Teutonico D, Maas HJ, et al. Prediction of disease progression, treatment response and dropout in chronic obstructive pulmonary disease (COPD). Pharm Res. 2015;32(2):617–627. doi: 10.1007/s11095-014-1490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ. 2013;346:e8668. doi: 10.1136/bmj.e8668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vestbo J, Anderson JA, Calverley PM, et al. Bias due to withdrawal in long-term randomised trials in COPD: evidence from the TORCH study. Clin Respir J. 2011;5(1):44–49. doi: 10.1111/j.1752-699X.2010.00198.x [DOI] [PubMed] [Google Scholar]
- 23.Król A, Palmér R, Rondeau V, Rennard S, Eriksson UG, Jauhiainen A. Improving the evaluation of COPD exacerbation treatment effects by accounting for early treatment discontinuations: a post-hoc analysis of randomized clinical trials. Respir Res. 2020;21(1):158. doi: 10.1186/s12931-020-01419-8 [DOI] [PMC free article] [PubMed] [Google Scholar]