Abstract
Objective.
Barlow’s disease remains challenging to repair given the complex valvular morphology and lack of quantitative data comparing techniques. Though there have been recent strides in ex vivo evaluation of cardiac mechanics, to the best of our knowledge, there is no disease model that accurately simulates the morphology and pathophysiology of Barlow’s disease. The purpose of this study was to design such a model.
Method.
To simulate Barlow’s disease, a cross-species ex vivo model was developed. Bovine mitral valves (n=4) were sewn into a porcine annulus mount to create excess leaflet tissue and elongated chordae. A heart simulator generated physiologic conditions while hemodynamic data, high-speed videography, and chordal force measurements were collected. The regurgitant valves were repaired using nonresectional repair techniques such as neochord placement.
Results
The model successfully imitated the complexities of Barlow’s disease, including redundant, billowing bi-leaflet tissues with notable regurgitation. After repair, hemodynamic data confirmed reduction of mitral leakage volume 25.9±2.9 vs 2.1±1.8 mL, p<0.001) and strain gauge analysis revealed lower primary chordae forces (0.51±0.17 vs 0.10±0.05 N, p<0.001). Additionally, the maximum rate of change of force was significantly lower post-repair for both primary (30.80±11.38 vs 8.59±4.83 N/s, p<0.001) and secondary (33.52±10.59 vs 19.07±7.00 N/s, p=0.006) chordae.
Conclusions
This study provides insight into the biomechanics of Barlow’s disease, including a sharply fluctuating force profile experienced by elongated chordae pre-repair as well as a restoration of primary chordae forces post-repair. Further, the disease model can facilitate an indepth analysis optimizing Barlow’s disease repair.
Introduction
Degenerative mitral valve disease exists on a spectrum of disease severity, ranging to relatively mild disease with chordal rupture or elongation, termed fibroeslastic deficiency, to profound myxomatous degeneration of the valve, with elongated chordae and excessive, thickened, and redundant leaflet tissue, termed Barlow’s Disease. First described by John B Barlow,1 Barlow’s disease is a severe degenerative mitral valve (MV) disease characterized by excess and billowing leaflet tissue, single-leaflet or bi-leaflet prolapse, annular dilation, and elongated chordae.2–4 This complex morphology with significant disparity from a healthy MV can be difficult to repair surgically, and the optimal repair technique for Barlow’s disease remains in question. Current surgical techniques that may be applied in the repair of Barlow’s disease include annuloplasty, leaflet resection, neochord placement, chordal transfer, and nonresectional leaflet remodeling, to name a few.5–10 Ex vivo heart simulators have previously been used with great success to analyze, optimize, and design new surgical techniques for MV repair.11–17 The majority of these studies use porcine mitral valves as a human analog.16,18–22 This is due to the similarities between human and porcine valves, including comparable size, anatomy, tissue microstructures, and anisotropy.16,23,24 Simple modifications to healthy porcine or ovine mitral valves can be used to create mitral regurgitation models via chordae tendineae transection, annular dilatation, or papillary muscle manipulation. However, an explanted healthy porcine or ovine mitral valve is insufficient to model the thickened, redundant leaflets and elongated chordae tendineae characteristic of Barlow’s disease. For this reason, ex vivo studies aimed at comparing surgical techniques to determine the optimal repair strategy for Barlow’s disease have not been performed. The development of a novel disease model that can capture the multifaceted characteristics of Barlow’s disease would enable a full biomechanical analysis of the Barlow’s MV and ultimately provide quantitative insight regarding the surgical repair. In this manuscript, we describe such a model.
Materials and Methods
Model Design
To confirm that there was no previous disease model that accurately simulates the morphology and pathophysiology of Barlow’s disease, a comprehensive search of PubMed was performed for the following keywords: (“Ex vivo” OR “In vitro”) AND ( “Barlow” OR “mitral regurgitation”) AND (“disease model” OR “model”). This yielded 97 results, which we carefully reviewed, finding no disease model for in vitro or ex vivo use replicating Barlow’s disease. To develop an accurate simulation of Barlow’s disease, the model MV must have excess leaflet tissue relative to annulus size, as well as elongated chordae. Bovine mitral valves were selected as the basis for the model as they provide the necessary excess leaflet tissue and chordal length relative to a human or porcine annulus. Fresh bovine hearts were obtained from a local abattoir and the mitral valves were carefully excised to preserve the annulus, leaflets, papillary muscles and chordae.
The valves were sewn to a custom 3D-printed silicone mounting rings (Carbon M2, Carbon 3D Inc., Redwood City, CA) which were positioned between the left atrial and left ventricular chambers. Note that the mounting ring material allows for elasticity of the attachment point to reduce fixation of the annulus. The ring was sized as it would be for a normal porcine valve with an intercommisural distance of 30mm. However, the posterior portion of the ring, where the posterior annulus was affixed, was made to incorporate additional circumferential distance with a geometry simulating that of a Barlow’s MV annulus.2 Due to the reduced size of the mounting ring compared to the bovine annulus, additional care was taken in securing the valve at the level of the mitral anulus onto the mounting ring using at least eight interrupted 2-0 braided polyester sutures for uniform reduction of annular size. A continuous running 2-0 polypropylene suture was then used along the underside of the valve around the left atrial tissue to create a hemostatic suture line.
Papillary muscles were positioned to model elongated chordae typically found in Barlow’s disease. For each valve, the papillary muscles were fixed to silicone holders and instrumented within the simulator as previously described,17 but their positions were raised relative to their native state. The adjustable displacement of the bovine papillary muscles enables our disease model to be actively modulated by researchers to create the desired degree of mitral regurgitation for a given Barlow’s disease model. To produce a lower regurgitation fraction, the papillary muscles can be displaced by a small margin and the annulus mount can be sized closer to the native bovine annulus size; for higher regurgitation, the papillary muscles can be raised more significantly and the annulus mount size discrepancy can be sized larger. For consistency in this study, optimal papillary muscle placement was determined through a combination of methods. First, the gross position of the papillary muscles was estimated based on measurements taken from the valve prior to it being explanted from the porcine heart. Next, we shortened this to a range typically found in human patients.25 We verified appropriate bileaflet prolapse typical of the disease clinically using both visually and with echocardiography. To assure the creation of moderate to severe mitral regurgitation, we ensured that the electromagnetic flow meter readings showed a regurgitant fraction of 35-50%. The regurgitant valve was then repaired by experienced cardiac surgeons using nonresectional mitral repair techniques, primarily via neochord placement using multiple double-armed polytetrafluoroethylene (PTFE) suture (Gore-Tex® Suture, WL Gore & Associates Inc., Flagstaff, Arizona).
Left Heart Simulator
We designed and built a 3D-printed left heart simulator, which has been described previously.16,17,26 The simulator features a custom left ventricular chamber mounted to a programmable pulsatile linear piston pump (ViVitro Superpump, ViVitro Labs, Victoria, BC, Canada). The simulator includes ventricular, aortic, and left atrial pressure transducers (Utah Medical Products Inc., Midvale, Utah) as well as electromagnetic flow probes (Carolina Medical Electronics, East Bend, North Carolina). To ensure proper transduction of the flow meters, 0.9% normal saline was used as the test fluid and held at 37°C. A 29-mm mechanical aortic valve (St. Jude Regent, Abbott Vascular, Lake Bluff, IL, USA) was placed in the aortic position. The pump was programmed to produce a physiologic waveform in compliance with ISO 5840 standards for in vitro valve testing. Peripheral resistance and compliance in the system were initially titrated with a 70 28-mm leakless disc valve (ViVitro) in the mitral position to generate a cardiac output of 5 LPM, a systolic pressure of 120mmHg, and a diastolic pressure of 80mmHg. During testing, the mechanical MV was replaced by the Barlow’s disease model valve, and in each phase 10 cycles of hemodynamic data were collected and averaged. Leakage volume was calculated as the volume of flow leaking across the valve post valve closure during systole. Echocardiographic data were obtained with a Phillips iE33 system with an X7-2T transesophageal probe from a ventricular chamber port so that the ultrasound beam was parallel to the direction of flow.
Chordae Force Measurement
Forces on the chordae tendineae were measured with Fiber Bragg Grating (FBG) strain gauge sensors (DTG-LBL-1550 125μm FBGS International, Belgium), calibrated to correlate measured strains to forces, as previously described.17,26,27 In brief, FBG’s are low-mass optical strain gauges with a thin profile, enabling the instrumentation of multiple chordae without disrupting the hemodynamics and structural integrity of the valve. The sensors were fixed to native primary and secondary chordae using CV-5 to CV-7 PTFE (depending on chordal diameter) flanking each side of the 5mm strain gauge. Once attached, the section of chordae between the sutures was severed so all force on the chordae was imparted to the FBG sensor. Multiple native chordae (n=5-6) were instrumented for each valve, including primary and secondary chordae in both posterior and anterior positions. Maximum chordal forces were calculated as well as the rate of change of force with respect to time (dF/dt).
Statistical Analysis
Statistical significance was defined at P<0.05 for all tests. Hemodynamic and chordal force variables are reported as mean ± standard deviation. The Shapiro–Wilk statistic was used to assess normality, with each group of data assumed to be normally distributed for p<0.05. In all cases, normality was satisfied and thus pre-repair and post-repair continuous variables were compared using a paired-samples t-test. Maximum chordal forces were calculated as the average force during systole and normalized to transmitral mean pressure.
Results
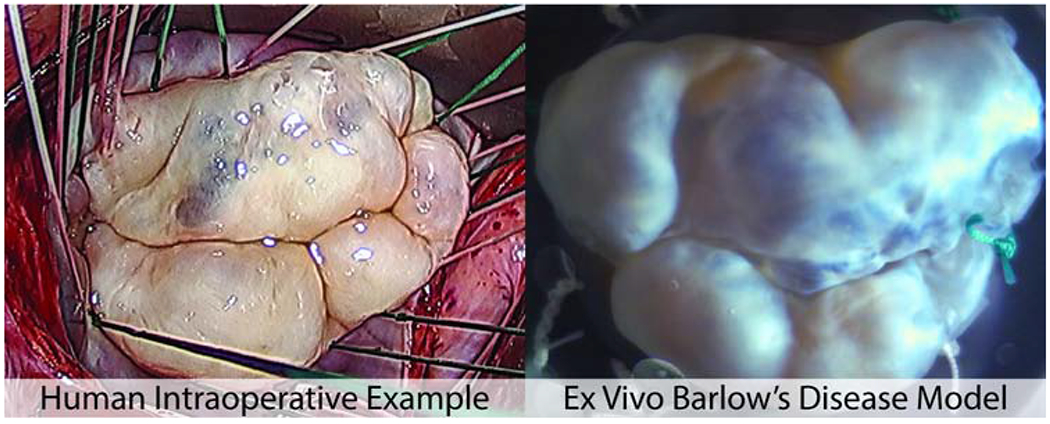
Bovine hearts (n=4) were tested in the porcine mounts, and the resulting disease model closely simulated the human Barlow’s MV with the characteristic prolapsed and billowing bileaflet tissue and elongated chordae that caused significant mitral regurgitation. En face views of the cross-species model are shown in Figure 1 with an intraoperative view of a human Barlow’s MV (Figure 1A) for reference. As a comparison, Figure 1B represents an example of a bovine MV in a pressurized native state prior to explantation—this demonstrates the significant morphological change induced by our model design. Figure 1C shows a Barlow’s disease model valve with a leakage volume of 28.3 mL; this model is also shown in high speed video at 1057 frames per second (Video 1). The redundant leaflet tissue and significant prolapse seen in 1C were observed in all of the disease model valves. Neochordal repair was successfully performed by experienced cardiac surgeons on all valves. Figure 1D shows a Barlow’s disease model valve after neochordal repair with a leakage volume of 3.4 mL. Echocardiography confirmed that in all cases pre-repair, the prolapse was multi-segmental and involved both leaflets; post-repair, the degree of prolapse and billowing was reduced and there coaptation area is increased.
Figure 1.

(A) Intraoperative example of human Barlow’s disease. (B) A bovine valve in its native pressurized state, for reference to compare to the ex vivo Barlow’s disease model. (C) Explanted bovine valve sewn into a porcine-sized mounting ring to simulate Barlow’s disease; valve pictured during systole in a left heart simulator with a leakage volume of 28.3ml. (D) A Barlow’s disease model valve after neochordal repair with a leakage volume of 3.4ml.
Mean mitral flow tracings and pressure tracings of the Barlow’s model pre- and post-repair are shown in Figure 2. Hemodynamic data confirmed that neochordal repair significantly reduced mitral leakage volume in the valves from 25.9±2.9mL to 2.1±1.8mL (p<0.001). Pressure data also confirmed successful repair with significantly higher systolic pressure (p<0.001), diastolic pressure (p<0.001), and left ventricular pressure (p<0.001) post-repair.
Figure 2.

(A) Mean flow confirmed successful repair of the Barlow’s disease model with significantly lower leakage volumes after neochordal repair (25.9±2.9 vs 2.1±1.8ml, p<0.001). Shaded region represents standard deviation. (B) Mean pressure tracings also showed successful repair of the Barlow’s disease model with aortic and left ventricular pressures significantly raised to baseline levels—see Table 1 for hemodynamic values.
By restoring coaptation and reducing the leakage volume, neochordal repair restored coaptation and resulted in significantly lowered forces on primary chordae (0.51±0.17 vs 0.10±0.05N, p<0.001). No significant difference was found for secondary chordae forces preversus post-repair (0.41±0.21 vs 0.35±0.12N, p=0.374). The maximum rate of change of force was also found to be significantly decreased after repair of the valve for both primary chordae (30.80±11.38 vs 8.59±4.83N/s, p<0.001) and secondary chordae (33.52 ±10.59N/s vs 19.07±7.00N/s, p=0.006). Composite force tracings for each class of chordae tendineae also revealed a qualitative difference in the force profiles of the Barlow’s model between the pre-repair (Figure 3A) and the post-repair state (Figure 3B): there appears to be greater fluctuation in the chordal forces during systole in the Barlow’s model, and the fluctuation is consistent across valves such that it remains prominent in the composite tracings.
Figure 3.

Force tracings over the course of one cardiac cycle for composites of each class of chordae at baseline with the Barlow’s model (A) and after neochordal repair (B). Forces were measured using Fiber Bragg Grating strain sensors sewn to native chordae. Primary chordae forces were lower post-repair (0.51±0.17 vs 0.10±0.05N p<0.001), while no significant difference was found for secondary chordae forces (0.41±0.21 vs 0.35±0.12N, p=0.374).
Discussion
A cross-species ex vivo model was successfully developed to reproduce the complexities of Barlow’s disease, including redundant, billowing bi-leaflet tissues with notable regurgitation. After neochordal repair, hemodynamic data confirmed the restoration to physiologic conditions with significant reduction in mitral regurgitation. Strain gauge analysis revealed a sharply fluctuating force profile experienced by the elongated chordae pre-repair as well as significantly lower primary chordae forces post-repair. The graphical abstract (Figure 4) depicts a summary of this study and its implications.
Figure 4.

The development and biomechanical analysis of a novel cross-species model of Barlow’s mitral valve disease. Bovine mitral valves (n=4) were evaluated in a left heart simulator using porcine mounts resulting in an ex vivo simulation of the excess, prolapsing leaflet tissue characteristic of Barlow’s disease. All models were successfully repaired, supporting the use of this model not only as a means of analyzing the biomechanics of the disease state, but also as a tool to optimize surgical repair techniques.
It has been widely theorized that in a healthy MV, primary chordae tendineae aid in positioning the leaflets to ensure proper coaptation while the secondary chordae support the majority of forces during systole; when a valve becomes regurgitant, the optimal coaptation surface is disrupted and the primary chordae are forced to take on additional forces.28 This theory is reinforced by previous findings of low primary chordae forces compared to secondary chordae forces post-repair.27,29,30 In this study, we observed that the primary chordae hold forces on par with secondary chordae for a regurgitant Barlow’s MV. Given that proper coaptation is not achieved during this disease state, it is likely that the primary chordae were required to hold the leaflet free edges during systole, thus raising the primary chordae forces. Once the regurgitation was significantly reduced post-repair, the primary forces were lowered to their expected values.
Additionally, the rate of change of force on the chordae was found to be significantly lower post-repair relative to the baseline Barlow’s disease model. Note that this was the case even for secondary chordae, which had no significant difference in peak force. This difference could be due to the complex mechanics of elongated chordae coupled with a prolapsing valve. We hypothesize that the elongated chordae experience a whipping phenomenon, similar to that observed with long artificial chordae 31,32; this phenomenon was theorized to cause a similar increased rate of change of force in the longer neochords.17 Once the Barlow’s disease model is repaired, the neochords properly position the leaflets to ensure proper coaptation, which then cushions the initial peak in force at the start of systole by distributing the forces across the leaflets and secondary chordae. We also hypothesize that dF/dt for the neochords post-repair is likely less than the dF/dt observed for the native chordae pre-repair due to the fact that the neochords are generally shorter than their adjacent chordae. The rate of change of force on the native chordae and neochords is a crucial metric in analyzing the stresses on the valve and ultimately determining the durability of a repair. Thus, great importance lies in developing an accurate disease model such that the variety of repairs can be compared, and stresses can be optimized for the repaired valve.
Limitations
One area of refinement for this model is more accurately replicating the specific papillary muscle positioning. Though consistency across levels of regurgitation was prioritized for this study, if one wishes to test a specific Barlow’s disease case, the vertical displacement of the bovine papillary muscles must be selected to result in the correct length of excess chordae (i.e. the difference between actual chordal length and a healthy chordal length). Additionally, left ventricular dysfunction often accompanies Barlow’s disease2, which would result in papillary muscle placement that differs from a healthy heart and may require more intricate positioning measures for accuracy.
The morphology and intrinsic tissue properties of the leaflet tissue is also important to consider; though our model successfully replicates the significant excess of leaflet tissue in terms of total leaflet area, it does not fully replicate the myxomatous characteristics or the thickness of human Barlow’s MV. Additionally, some cases of Barlow’s disease can have calcification of the annular or subannular apparatus.2 Further studies are needed to establish a mechanical or chemical treatment to the leaflets that would result in leaflet thickening, annular calcification, or other changes to the material properties of the valve. These modifications to the bovine valve could be subjected to biomechanical testing to compare the material properties to those of human Barlow’s disease. Thus, though the current model can be used to study repair techniques, the ability to include more refined physiologic facets of the disease would enhance our ability to study the biomechanics of the disease itself.
Conclusions
Our novel Barlow’s disease model will ultimately enable a comprehensive biomechanical analysis of the pathophysiology of the disease as well as the many repair techniques currently in use. Ex vivo simulation of valvular diseases represents an important and underutilized method to optimize surgical repair techniques. However, the value of these analyses is determined by the accuracy of the disease model; this study represents an important step in developing a reliable model of Barlow’s disease and enable more accurate analyses of the surgical repair of severe mitral valve disease.
Supplementary Material
Video 1. High-speed video (1057 frames per second) of a bovine mitral valve sewn to a porcine mount in a left heart simulator as a novel disease model for human Barlow’s disease.
Central Picture.
Novel cross-species model simulates Barlow’s disease for ex vivo biomechanical analysis.
Table 1 –
Hemodynamic and Chordae Tendineae Tension Parameters
| Barlow’s Model | Repair | p-value | |
|---|---|---|---|
| HEMODYNAMICS | |||
| Heart rate (bpm) | 70.00 ± 0.00 | 70.00 ± 0.00 | 1.0000 |
| Mean arterial pressure (mmHg) | 54.97 ± 1.49 | 99.85 ± 1.22 | <0.001 |
| Diastolic pressure (mmHg) | 43.29 ± 2.17 | 81.67 ± 1.64 | <0.001 |
| Systolic pressure (mmHg) | 69.98 ± 1.91 | 119.53 ± 1.54 | <0.001 |
| Mean atrial pressure (mmHg) | 7.90 ± 2.80 | 10.55 ± 0.70 | 0.0901 |
| Mean ventricular pressure (mmHg) | 25.82 ± 1.31 | 44.14 ± 0.92 | <0.001 |
| Cardiac output (liters/min) | 3.04 ± 0.28 | 4.23 ± 0.15 | 0.0077 |
| Effective stroke volume (ml) | 43.44 ± 3.99 | 60.50 ± 2.19 | 0.0077 |
| Pump stroke volume (ml) | 109.82 ± 0.04 | 109.81 ± 0.19 | 0.9658 |
| Mitral valve mean gradient (mmHg) | −0.47 ± 1.65 | −3.80 ± 1.59 | 0.0187 |
| Mitral valve mean back pressure (mmHg) | 51.71 ± 3.97 | 98.50 ± 2.23 | <0.001 |
| Mitral forward flow time (sec) | 0.52 ± 0.01 | 0.53 ± 0.01 | 0.2620 |
| Mitral forward volume (ml) | 92.03 ± 5.58 | 76.56 ± 1.58 | 0.0102 |
| Mitral valve RMS forward flow (ml/sec) | 246.86 ± 12.41 | 202.78 ± 5.06 | 0.0026 |
| Mitral regurge fraction (%) | 52.85 ± 1.80 | 20.98 ± 2.30 | <0.001 |
| Mitral leakage rate (ml/sec) | −112.88 ± 12.99 | −8.15 ± 8.45 | <0.001 |
| Mitral leakage volume (ml) | −25.88 ± 2.92 | −2.14 ± 1.79 | <0.001 |
| Mitral closing volume (ml) | −22.70 ± 1.16 | −14.07 ± 1.73 | <0.001 |
| Ventricular energy (mJ) | 673.54 ± 16.64 | 885.22 ± 43.25 | <0.001 |
| TransMitral forward energy loss (mJ) | 16.10 ± 19.23 | 2.18 ± 13.87 | 0.0011 |
| TransMitral closing energy loss (mJ) | 105.54 ± 8.05 | 58.85 ± 12.92 | 0.0197 |
| TransMitral leakage energy loss (mJ) | 187.67 ± 32.00 | 31.28 ± 29.37 | <0.001 |
| TransMitral total energy loss (mJ) | 309.30 ± 15.62 | 92.31 ± 21.53 | <0.001 |
| CHORDAE TENDINEAE FORCES | |||
| Primary (N) | 0.51 ± 0.17 | 0.10 ± 0.05 | <0.001 |
| Secondary (N) | 0.41 ± 0.21 | 0.35 ± 0.12 | 0.3742 |
| Rate of Change of Force - Primary (N/s) | 30.80 ± 11.38 | 8.59 ± 4.83 | <0.001 |
| Rate of Change of Force - Secondary (N/s) | 33.52 ± 10.59 | 19.07 ± 7.00 | 0.0060 |
Data presented as mean ± standard deviation.
Central Message.
A cross-species ex vivo model simulates Barlow’s disease and provides insight into the biomechanics of the disease and surgical repair.
Perspective Statement.
Ex vivo heart simulation can provide vital quantitative biomechanical analyses of valve repair techniques. However, there is a need for disease models that recapitulate the complex morphology of severe mitral valve disease to support this surgical optimization. Our cross-species model successfully simulates Barlow’s disease and will enable a comprehensive analysis of the numerous repair techniques.
Acknowledgements
This work was supported by the National Institutes of Health (NIH R01 HL089315-01, YJW), the American Heart Association (17POST33410497, MJP), the National Science Foundation Graduate Research Fellowship Program (AMI), and a Stanford Graduate Fellowship (AMI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Disclosure and Funding:
The authors have no conflicts of interest or financial relationships with industry to disclose. This work was supported by the National Institutes of Health (NIH R01 HL089315-01, YJW), the American Heart Association (17POST33410497, MJP), the National Science Foundation Graduate Research Fellowship Program (AMI), and a Stanford Graduate Fellowship (AMI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Glossary of Abbreviations
- MV
Mitral Valve
- PTFE
Polytetrafluoroethylene
- N
Newtons
- N/s
Newtons per second
- mJ
Millijoules
- dF/dt
Rate of change of force with respect to time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. Am. Heart J 1966;71(2):166–178. doi: 10.1016/0002-8703(66)90179-7. [DOI] [PubMed] [Google Scholar]
- 2.Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19(2):90–96. doi: 10.1053/j.semtcvs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lawrie GM. Barlow disease: Simple and complex. J. Thorac. Cardiovasc. Surg 2015;150(5):1078–1081. doi: 10.1016/j.jtcvs.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11(4):307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 5.Lawrie GM, Earle EA, Earle NR. Nonresectional repair of the barlow mitral valve: importance of dynamic annular evaluation. Ann. Thorac. Surg 2009;88(4): 1191–1196. doi: 10.1016/j.athoracsur.2009.05.086. [DOI] [PubMed] [Google Scholar]
- 6.Varghese R, Adams DH. Techniques for repairing posterior leaflet prolapse of the mitral valve. Operative Techniques in Thoracic and Cardiovascular Surgery 2011;16(4):293–308. doi: 10.1053/j.optechstcvs.2011.11.001. [DOI] [Google Scholar]
- 7.da Rocha E Silva JG, Spampinato R, Misfeld M, et al. Barlow’s Mitral Valve Disease: A Comparison of Neochordal (Loop) and Edge-To-Edge (Alfieri) Minimally Invasive Repair Techniques. Ann. Thorac. Surg 2015;100(6):2127–33; discussion 2133. doi: 10.1016/j.athoracsur.2015.05.097. [DOI] [PubMed] [Google Scholar]
- 8.Yano M, Nishimura M, Yokota A, Mori K. Mitral valve repair in Barlow’s disease by chordal reconstruction using the adjustable slip-knot technique. Gen Thorac Cardiovasc Surg 2018. doi: 10.1007/s11748-018-1004-0. [DOI] [PubMed] [Google Scholar]
- 9.MacArthur JW, Cohen JE, Goldstone AB, et al. Nonresectional single-suture leaflet remodeling for degenerative mitral regurgitation facilitates minimally invasive mitral valve repair. Ann. Thorac. Surg 2013;96(5):1603–1606. doi: 10.1016/j.athoracsur.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo YJ, MacArthur JW. Posterior ventricular anchoring neochordal repair of degenerative mitral regurgitation efficiently remodels and repositions posterior leaflet prolapse. Eur. J. Cardiothorac. Surg 2013;44(3):485–9; discussion 489. doi: 10.1093/ejcts/ezt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siefert AW, Rabbah J-PM, Pierce EL, Kunzelman KS, Yoganathan AP. Quantitative Evaluation of Annuloplasty on Mitral Valve Chordae Tendineae Forces to Supplement Surgical Planning Model Development. Cardiovasc. Eng. Technol 2014;5(1):35–43. doi: 10.1007/s13239-014-0175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontaine AA, He S, Stadter R, Ellis JT, Levine RA, Yoganathan AP. In vitro assessment of prosthetic valve function in mitral valve replacement with chordal preservation techniques. J Heart Valve Dis 1996;5(2):186–198. [PubMed] [Google Scholar]
- 13.Erek E, Padala M, Pekkan K, et al. Mitral web--a new concept for mitral valve repair: improved engineering design and in-vitro studies. J Heart Valve Dis 2009;18(3):300–306. [PubMed] [Google Scholar]
- 14.Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, Adams DH. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J. Thorac. Cardiovasc. Surg 2009;138(2):309–315. doi: 10.1016/j.jtcvs.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siefert AW, Siskey RL. Bench models for assessing the mechanics of mitral valve repair and percutaneous surgery. Cardiovasc. Eng. Technol 2015;6(2):193–207.doi: 10.1007/s13239-014-0196-4. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen MJ, Kasinpila P, Imbrie-Moore AM, et al. Modeling conduit choice for valve-sparing aortic root replacement on biomechanics with a 3-dimensional-printed heart simulator. J. Thorac. Cardiovasc. Surg 2018. doi: 10.1016/j.jtcvs.2018.10.145. [DOI] [PubMed] [Google Scholar]
- 17.Imbrie-Moore AM, Paulsen MJ, Thakore AD, et al. Ex vivo biomechanical study of apical versus papillary neochord anchoring for mitral regurgitation. Ann. Thorac. Surg 2019. doi: 10.1016/j.athoracsur.2019.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabbah J-P, Saikrishnan N, Yoganathan AP. A novel left heart simulator for the multi-modality characterization of native mitral valve geometry and fluid mechanics. Ann. Biomed. Eng 2013;41(2):305–315. doi: 10.1007/s10439-012-0651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vismara R, Pavesi A, Votta E, Taramasso M, Maisano F, Fiore GB. A pulsatile simulator for the in vitro analysis of the mitral valve with tri-axial papillary muscle displacement. Int J Artif Organs 2011;34(4):383–391. doi: 10.5301/IJAO.2011.7729. [DOI] [PubMed] [Google Scholar]
- 20.Al-Atassi T, Toeg HD, Jafar R, Sohmer B, Labrosse M, Boodhwani M. Impact of aortic annular geometry on aortic valve insufficiency: Insights from a preclinical, ex vivo, porcine model. J. Thorac. Cardiovasc. Surg 2015;150(3):656–64.e1. doi: 10.1016/j.jtcvs.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 21.He Z, Sacks MS, Baijens L, Wanant S, Shah P, Yoganathan AP. Effects of papillary muscle position on in-vitro dynamic strain on the porcine mitral valve. J Heart Valve Dis 2003;12(4):488–494. [PubMed] [Google Scholar]
- 22.Adams J, O’Rourke MJ. In vitro measurement of the coaptation force distribution in normal and functional regurgitant porcine mitral valves. J Biomech Eng 2015;137(7). doi: 10.1115/1.4029746. [DOI] [PubMed] [Google Scholar]
- 23.Pham T, Sun W. Material properties of aged human mitral valve leaflets. J. Biomed. Mater. Res. A 2014;102(8):2692–2703. doi: 10.1002/jbm.a.34939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber JE, Kasper FK, Ratliff NB, Cosgrove DM, Griffin BP, Vesely I. Mechanical properties of myxomatous mitral valves. J. Thorac. Cardiovasc. Surg 2001;122(5):955– 962. doi: 10.1067/mtc.2001.117621. [DOI] [PubMed] [Google Scholar]
- 25.Kavitha S. Histomorphometric and morphological study of papillary muscles in adult human hearts. 2015.
- 26.Paulsen MJ, Imbrie-Moore AM, Wang H, et al. Mitral chordae tendineae force profile characterization using a posterior ventricular anchoring neochordal repair model for mitral regurgitation in a three-dimensional-printed ex vivo left heart simulator. Eur. J. Cardiothorac. Surg 2019. doi: 10.1093/ejcts/ezz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen MJ, Bae JH, Imbrie-Moore A, et al. Development and ex vivo validation of novel force-sensing neochordae for measuring chordae tendineae tension in the mitral valve apparatus using optical fibers with embedded Bragg gratings. J Biomech Eng 2019. doi: 10.1115/1.4044142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazari S, Carli F, Salvi S, et al. Patterns of systolic stress distribution on mitral valve anterior leaflet chordal apparatus. A structural mechanical theoretical analysis. J Cardiovasc Surg (Torino) 2000;41(2):193–202. [PubMed] [Google Scholar]
- 29.He Z, Jowers C. A novel method to measure mitral valve chordal tension. J Biomech Eng 2009;131(1):014501. doi: 10.1115/1.3005160. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen SL, Soerensen DD, Libergren P, Yoganathan AP, Nygaard H. Miniature C-shaped transducers for chordae tendineae force measurements. Ann. Biomed. Eng 2004;32(8):1050–1057. doi: 10.1114/B:ABME.0000036641.69903.62. [DOI] [PubMed] [Google Scholar]
- 31.Gammie JS, Wilson P, Bartus K, et al. Transapical Beating-Heart Mitral Valve Repair With an Expanded Polytetrafluoroethylene Cordal Implantation Device: Initial Clinical Experience. Circulation 2016;134(3):189–197. doi: 10.1161/CIRCULATIONAHA.116.022010. [DOI] [PubMed] [Google Scholar]
- 32.Lancellotti P, Radermecker M, Durieux R, Modine T, Oury C, Fattouch K. Transapical beating-heart chordae implantation in mitral regurgitation: a new horizon for repairing mitral valve prolapse. J Thorac Dis 2016;8(12):E1665–E1671. doi: 10.21037/jtd.2016.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. High-speed video (1057 frames per second) of a bovine mitral valve sewn to a porcine mount in a left heart simulator as a novel disease model for human Barlow’s disease.


