Abstract
Purpose
This study aimed to describe a novel cancer vaccine developed using H2O2-inactivated Salmonella typhimurium RE88 [with deletions of AroA (the first enzyme in the aromatic amino acid biosynthesis pathway) and DNA adenine methylase] as the carrier.
Methods
The pVLT33 plasmid was used to engineer an RE88 strain induced to express ovalbumin (OVA) by isopropylthiogalactoside (RE88-pVLT33-OVA). The immune responses and anticancer effects of H2O2-inactivated RE88-pVLT33-OVA were compared with those of non-inactivated RE88-pVLT33-OVA and OVA (positive control) in mice carrying OVA-expressing tumors (EG7-OVA) cells.
Results
Anti-ovalbumin IgG (immunoglobulin G) titer following vaccination with H2O2-inactivated RE88-pVLT33-OVA was higher for subcutaneous than for intragastric vaccination. When subcutaneous administration was used, H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU (colony forming units)/mouse) achieved an anti-ovalbumin IgG titer higher than that for the same dose of RE88-pVLT33-OVA and comparable to that for 10 µg ovalbumin (positive control). The binding of mouse serum antibodies to EG7-OVA cells was stronger for H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU/mouse) than for 10 µg ovalbumin. Furthermore, subcutaneous vaccination with H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU/mouse) induced greater activation of splenic T cells and more extensive tumor infiltration with CD4+/CD8+ T cells compared with 10 µg ovalbumin (positive control). The mice vaccinated subcutaneously with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 108 or 6 × 108 CFU/mouse had smaller tumors compared with mice in the negative control groups. Tumor weight in mice vaccinated with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse was significantly lower than that in both negative control groups (P < 0.05) and decreased with the increasing dose of H2O2-inactivated RE88-pVLT33-OVA. H2O2-inactivated RE88-pVLT33-OVA was potentially safer than the non-inactivated strain, could carry exogenous antigens, and had specific epitopes that could be exploited as natural adjuvants to facilitate the induction of cellular and humoral immune responses.
Conclusion
It was anticipated that H2O2-inactivated RE88-pVLT33-OVA could be used as a novel delivery system for new cancer vaccines.
Keywords: cancer vaccine, H2O2 inactivation, RE88-pVLT33-OVA, whole-cell bacterial vaccine
Introduction
Cancer vaccines are immunotherapies used to prevent the development of cancer in high-risk populations (prophylactic vaccines) or treat existing cancer (therapeutic vaccines)1 Therapeutic cancer vaccines generally work by enhancing the ability of the immune system to recognize tumor antigens, thereby activating antigen-specific cytotoxic effects that target and kill cancer cells.2 Various bacterial species have been shown to enter and replicate within tumors, exhibiting “tumor-finding” behavior.3 A study found that attenuated S. typhimurium after intravenous injection would accumulate specifically in a tumor and then trigger tumor thrombosis due to the release of TNF-α (tumor necrosis factor-alpha, TNF-α) and proinflammatory factors.4 In another report, an engineered nonpathogenic Escherichia coli strain could lyse specifically within the tumor microenvironment and release an anti-CD47 antagonist nanobody.5
Several studies investigated the use of checkpoint inhibitors to target neoantigens (somatic tumor mutations) in nonsmall cell lung cancer,6 melanoma,7 and colorectal cancer,8,9 and the combination of an immune-enhancing therapeutic cancer vaccine with immune checkpoint blockade might serve as a novel treatment for cancer.10
Several factors need to be considered in the design of vector-based vaccines that generate long-term immunological memory, including the balance between safety and immunogenicity, site of antigen delivery, role of adjuvants, and dose and route of administration.11–14 Important advantages of bacteria-mediated cancer therapies are that they can potentially overcome issues related to pharmacokinetics, tumor microenvironment, and drug resistance that impair conventional treatment methods.15 Bioengineered Gram-negative bacteria such as Salmonella typhimurium (S. typhimurium) have received considerable attention as vectors to deliver antigens to elicit host immunity against a tumor.16 For example, the combination of tumor-targeting S. typhimurium A1-R and chemotherapy was highly effective against melanoma in a patient-derived orthotopic xenograft model,17 illustrating the potential of this technique. Furthermore, subcutaneous administration of an attenuated S. typhimurium lacking the ZnuABC transporter inhibited the growth of mammary adenocarcinoma in mice and increased animal life expectancy.18 Additionally, the oral administration of attenuated S. typhimurium containing pcDNA3.1.H5 could elicit immune responses against the avian influenza virus in chickens.19 Various other studies also reported T cell responses and anticancer effects of vaccines based on attenuated S. typhimurium.20–23 An advantage of using attenuated or inactivated S. typhimurium as a therapeutic cancer vaccine is that it can stimulate humoral and cellular systemic immune responses as well as mucosal immune responses.
Numerous studies reported that strong immune responses could be elicited by the doubly attenuated RE88 strain of S. typhimurium, which lacks DNA adenine methylase (ΔDam) and the first enzyme in the aromatic amino acid biosynthesis pathway (ΔaroA). The oral delivery of an RE88-based DNA vaccine encoding secretory chemokine CCL21 (which chemoattracts activated antigen-presenting dendritic cells and naive T cells) and survivin (an inhibitor of apoptosis expressed by tumor cells and tumor vasculature) elicited the activation of antigen-presenting dendritic cells and a CD8+ T cell immune response, resulting in prophylactic and therapeutic suppression of pulmonary metastases in a mouse model of non-small cell lung carcinoma.24. Another RE88-based vaccine encoding endoglin, which was overexpressed in breast cancer vasculature, was found to induce a CD8+ T cell–mediated immune response and inhibit the metastasis of D2F2 breast carcinoma cells to the lungs.25 Furthermore, an RE88-based oral vaccine encoding legumain, which was overexpressed by stromal tumor-associated macrophages, was shown to protect mice against challenge with D2F2 breast carcinoma cells through the induction of a specific CD8+ T cell response against legumain-positive tumor-associated macrophages.26
Ovalbumin (OVA)-expressing tumor (EG7-OVA) cells are mouse thymoma (EL4) cells that express chicken OVA as a unique antigen. Since OVA has strong immunogenicity, it has been used as a model antigen in constructing tumor vaccine systems targeting EG7-OVA cancer cells. For example, a recombinant baculovirus-based vaccine encoding fragment C of OVA was found to exert prophylactic and therapeutic anti-tumor effects against tumors grown from EG7-OVA cells.27 Moreover, synthetic vaccines based on nanomicelles,28 hydrogels,29 and nanoparticles30 have also been tested on EG7-OVA cells using OVA as the model antigen.
The present research group investigated the use of hydrogen peroxide (H2O2) for the inactivation of bacterial carriers and established that H2O2 deactivation of bacterial vaccines retained more complete epitopes and exhibited lower toxicity, compared with formaldehyde, a conventional agent associated with vaccine production. Furthermore, vaccination with H2O2-deactivated whole-cell bacteria in mice could elicit whole-cell specific antibodies and balance the IgG2a and IgG1 response.31 These findings demonstrated that the deactivation of whole-cell bacteria with H2O2 was potentially advantageous for immune responses. However, no previous studies evaluated the safety and efficacy of an anticancer vaccine based on H2O2-inactivated, attenuated S. typhimurium. Therefore, this study aimed to make a preliminary assessment of safety, immunogenicity, and anti-tumor effects of the RE88 strain of S. typhimurium inactivated by H2O2. To achieve this aim, OVA was used as the model antigen and a mouse model of cancer based on EG7-OVA cells. Since the RE88 vector can be administered via various routes, the present investigation compared intragastric, subcutaneous, and intraperitoneal administrations.
Materials and Methods
Mice, Bacterial Strains, and Cell Lines
Female C57BL/6 mice (6–8 weeks old) were provided by Huafukang Biological Technology Company (Beijing, China). All mice were maintained under specific pathogen-free conditions in accordance with the protocols approved by the Ethics Review Committee for Animal Experimentation of Sichuan University. Each mouse was euthanized by cervical dislocation under intraperitoneal injection of 0.2 mL of 4% sodium pentobarbital anesthesia solution. EG7-OVA cells [mouse lymphoma cell line EL4, American Type Culture Collection (ATCC) TIB-39] were purchased from ATCC (VA, USA) and cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum at 37°C in a 5% CO2 atmosphere. RE88, an attenuated Salmonella strain engineered to have a deletion of Dam and AroA, was a kind gift from Rong Xiang and colleagues (Scripps Research Institute, CA, USA; Nankai University School of Medicine, Tianjin, China). BL21(DE3), a strain of E. coli suitable for the expression of nontoxic proteins, was purchased from ATCC.
Generation of an Inactivated RE88-pVLT33-OVA Strain Expressing OVA
Construction of a Recombinant pVLT33-OVA Expression Vector
Using the cDNA sequence of OVA in GenBank (Gene ID: 396058) as a template, the target sequence of OVA was amplified by polymerase chain reaction (PCR) using the following primers: forward, 5ʹ-CCGGAATTCGAAGGAGATATACAATGGGCTCCATCGGCGCAGC-3ʹ; reverse, 5ʹ-CCCAAGCTTTTAAGGGGAAACACATCTGCC-3ʹ. After digestion with EcoR and HindIII restriction enzymes (New England Biolabs, MA, USA), OVA was ligated to the pVLT33 vector (Invitrogen, CA, USA) by T4 ligase (New England Biolabs). The constructed clone was identified by sequencing.
Preparation of RE88 Competent Cells
The RE88 strain was transferred to an LB (Luria-Bertani) plate and incubated overnight at 37°C. The colonies were cultured in 5 mL of LB medium overnight at 37°C and 225 rpm. Then, 1 mL of bacterial solution was inoculated into 100 mL of LB medium at 37°C for 4 h. Once the optical density at 600 nm (OD600) had reached 0.6–0.8, the bacterial solution was cooled for 30 min in an ice bath and then centrifuged at 4000 rpm for 10 min at 4°C. After discarding the supernatant, the pellet was washed twice with 10% glycerol in an ice bath. Then, the bacteria were resuspended so that each 10 mL of original culture material was suspended in 50 µL of 10% glycerol. The resuspended bacteria were preserved at –80°C.
Electrotransformation of RE88 and BL21(DE3) Cells with the pVLT33-OVA Expression Vector
A total of 40 µL bacterial suspension was fully mixed with 0.5 µg plasmid. The mixed bacterial solution was added to a precooled cuvette and then electroporated at 2.0 kV/cm for 5 ms. Following the addition of 1 mL of LB medium, the bacterial solution was aspirated and incubated for 1 h at 37°C and 225 rpm. The clone growth was monitored after coating on a kanamycin-resistant plate overnight. The pVLT33-OVA plasmids were also electrotransformed into BL21(DE3) cells.
Induction of OVA Expression by RE88-pVLT33-OVA and BL21(DE3) Cells
The bacteria were collected, shaken overnight in kanamycin-resistant LB, and then incubated for 1.5–2 h at 37°C and 225 rpm until the OD600 reached 0.6–0.8. After the induction of protein expression by 1mM isopropyl β-d-1-thiogalactopyranoside (IPTG), the bacterial cells were centrifuged at 13,000 rpm for 1 min, collected, and washed three times with phosphate-buffered saline (PBS).
Inactivation of the RE88-pVLT33-OVA Strain by H2O2
After adjusting the concentration of the bacterial suspension to 108 CFU/mL, the RE88-pVLT33-OVA strain was inactivated by incubation with 3% H2O2 for 60 min at 37°C. In a previous study,31 RE88 strains were all treated with 3% H2O2 at 37°C after 2 h and then cultured on LB plates. The inactivation efficiency of RE88-pVLT33-OVA strains was detected in the same way, except the incubation time (Fig. S1), and the culture had no bacterial growth. Potassium iodide was used for determining residual H2O2 (H2O2 oxidizes potassium iodide to produce iodine that dissolves in water to give a yellow solution; if the solution remains colorless, it is assumed no residual H2O2 is present). After the reaction, the bacterial suspension was centrifuged at 6000 rpm for 5 min, washed twice with sterile PBS, and diluted to the appropriate concentration.
Characterization of OVA Expression by the RE88-pVLT33-OVA Strain
Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay (Double-Antibody Sandwich ELISA)
The RE88-pVLT33-OVA strain was ultrasonically lysed and diluted. The immunosorbent plates (96-well, Nunc, Roskilde, Denmark) were coated with 2 µg/mL rabbit anti-OVA polyclonal antibodies (AB1225, MilliporeSigma, MA, USA) in 20mM sodium bicarbonate (pH 9.6; 100 µL/well) overnight at 4°C. The wells were washed with 0.05% Tween-20 in PBS (PBST) and blocked with 5% bovine serum albumin (BSA) in PBST for 1 h at room temperature. After washing, the diluted bacterial cell lysate and standards were incubated in the wells for 2 h at room temperature. The wells were washed, and 0.5 µg/mL peroxidase-conjugated rabbit anti-OVA polyclonal antibody (a kind gift from Guangping Gao and colleagues, Horae Gene Therapy Center and Department of Microbiology and Physiological Systems, University of Massachusetts Medical School, MA, USA) in PBST containing 5% BSA was added (100 µL/well). After incubation for 1 h at room temperature and subsequent washing, a SureBlue TMB Microwell Peroxidase Substrate (KPL, MD, USA) was added for 10 min in the dark, and the reaction was then stopped with 1M H2SO4. OD450 was measured using a Multiskan spectrophotometer (Thermo Fisher Scientific, Yokohama, Japan).
Western Blot Analysis
RE88-pVLT33-OVA cells were lysed with RIPA buffer, and the protein concentration was determined using the bicinchoninic acid assay. Equal amounts of bacterial cell lysate were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, MA, USA). After blocking with 5% nonfat milk/Tris-buffered saline containing 0.1% Tween-20 (TBST), the blots were probed overnight at 4°C with anti-OVA antibody (1:500). The blots were developed using a Supersignal West Pico chemiluminescent substrate kit (Pierce, IL, USA), following incubation with appropriate horseradish peroxidase-conjugated secondary antibody. The blots were imaged with a ChemiScope 6000 Touch Integrated Chemiluminescence Imaging System (Qinxiang, Shanghai, China).
Immunization of Mice with RE88-pVLT33-OVA
Animal experiments were designed and conducted using a double-blind method. A mouse model was used to evaluate whether the vaccination with the RE88-pVLT33-OVA strain would inhibit the subsequent development of tumors comprising EG7-OVA cells. C57/B6 mice aged 6–8 weeks (n = 12 per group) were randomly allocated to different groups to be vaccinated subcutaneously three times (on days 0, 14, and 28) with RE88-pVLT33-OVA (2 × 108, 6 × 108, or 2×109 CFU/mouse), RE88 (isotype control), and OVA in complete Freund unrefined adjuvant (positive control: 10 µg per mouse, with the dose adjusted to be comparable to that of a high dose of RE88-pVLT33-OVA) or NS (negative control).
One week after the third immunization, the mice were challenged with EG7-OVA cells by subcutaneously injecting 2 × 106 tumor cells into the back. Tumor width and tumor length were measured every 3 days (once a tumor mass had become evident), and the tumor volume was calculated as follows: tumor volume = 0.5 × length (mm) × [width (mm)]2. The survival of the mice was monitored.
Ability of a Specific IgG Antibody in the Serum of Immunized Mice to Bind to EG7-OVA Cells
EG7-OVA cells were cultured overnight in 96-well plates (3–5 × 105 cells/well), washed with PBS, and fixed with 4% polyformaldehyde at room temperature for 30 min. Then, 1 mL of 0.5% Triton X-100 was added for 20 min at room temperature, followed by PBS containing 2% BSA for 20 min at 4°C. The serum from mice after the third immunization was diluted 400-fold and incubated with the treated EG7-OVA cells for 60 min at 4°C. After three washes, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (BD Biosciences, CA, USA) for 30 min at 4°C and analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Histology and Immunofluorescence
Liver, spleen, and small intestine were excised from the mice 48h after RE88-pVLT33-OVA or H2O2-inactivated RE88-pVLT33-OVA strain injection. Tissues were fixed with 4% paraformaldehyde, dehydrated in a series of graded alcohol solutions, embedded in paraffin, and sectioned (5 µm thickness). For histological analysis, the paraffin-embedded sections were stained with hematoxylin and eosin (H&E).31 For immunofluorescence, tumor samples were excised from the mice 20 days after the tumor challenge, the slides of tumor tissue were deparaffinized with xylene and rehydrated with a series of graded alcohol solutions. Antigen retrieval was achieved by steam heat in 10mM sodium citrate buffer, and endogenous peroxidase was quenched with 3% H2O2. The sections were blocked with rabbit serum and then incubated overnight at 4°C with FITC-conjugated anti-CD4 monoclonal antibody or phycoerythrin (PE)-conjugated anti-CD8 monoclonal antibody (1:100 dilution; Abcam, MA, USA). The sections were then incubated with 4ʹ,6-diamidino-2-phenylindole (DAPI). Infiltrating lymphocytes were observed and quantified under a microscope at 200× magnification. For flow cytometry, tumor tissue were digested by mechanical homogenization with collagenase A (1 mg/mL; Roche) and DNase I (0.5 μg/mL; Roche) in isolation buffer (RPMI 1640 supplemented with 5% FBS) for 1 hour at 37°C. The isolated tumor cells were filtered through 100 μm cell strainers, washed with PBS and stained at 4°C for 30min with FITC-CD4 monoclonal antibody or PE-CD8 monoclonal antibody.
Detection of Intracellular Interferon-Gamma
The mice were sacrificed 1 week after the last immunization (n ≥ 3 per group). The splenocytes were isolated from PBS- or vaccine-immunized mice and incubated overnight in six-well plates (2 × 107 cells/well). The splenocytes were challenged with 10 μg/mL OVA at 37°C for 1 h and then incubated with GolgiPlug (BD Biosciences, Beijing, China) for an additional 6 h. After staining with FITC-conjugated anti-mouse CD4 and PE-conjugated anti-mouse CD8α (BD Pharmingen), the splenocytes were fixed and permeabilized using a Cytofix/Cytoperm Kit (BD Pharmingen) following the manufacturer’s protocols. Intracellular interferon-gamma (IFN-γ) was detected using allophycocyanin–conjugated anti-mouse IFN-γ (BD Pharmingen), a FACSCalibur flow cytometer, and CellQuest Pro software (BD Biosciences).
Data Analysis
The data were analyzed using SPSS21.0 (IBM Corp., NY, USA). Comparisons between groups were made using one-way analysis of variance and the Scheffe post hoc test. A P value < 0.05 was considered significant. The LD50 was calculated using the Reed–Muench formula.
Results
Expression of OVA Was Maintained in H2O2-Inactivated RE88-pVLT33-OVA
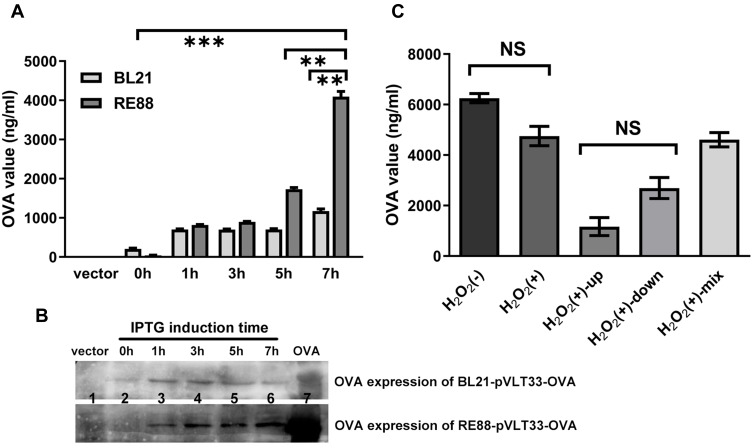
ELISA demonstrated that the expression of OVA by RE88-pVLT33-OVA increased progressively over time after induction with IPTG to reach a peak 7 h before subsequently declining (Fig. S2). OVA expression by BL21-pVLT33-OVA also increased gradually after induction with IPTG (Figure 1A). However, the OVA expression level 7 h after induction was significantly higher in RE88-pVLT33-OVA than in BL21-pVLT33-OVA (P < 0.01; Figure 1A and B). The OVA concentration reached 4000 ng/mL for RE88-pVLT33-OVA at 5 × 108 CFU/mL 7 h after induction, equivalent to 800 ng OVA per 108 CFU (Figure 1A). Additional experiments were carried out using RE88 inactivated by incubation with 3% H2O2 for 60 min at 37°C, since the inactivation of the attenuated strain would be expected to improve safety further. The quantification of OVA expression by ELISA revealed that H2O2-inactivated RE88-pVLT33-OVA was still able to express a substantial quantity of OVA, equivalent to about 500 ng OVA per 108 CFU 7 h after induction (Figure 1C). Notably, the OVA expression level 7 h after induction was not significantly different between H2O2-inactivated RE88-pVLT33-OVA and RE88-pVLT33-OVA not treated with H2O2 (P = 0.59; Figure 1C). For H2O2-inactivated RE88-pVLT33-OVA, the OVA expression level was not significantly different between the supernatant and the pellet after centrifugation of the lysate (P = 0.85; Figure 1C).
Figure 1.
Inducible expression of OVA by RE88-pVLT33-OVA and BL21(DE3)-pVLT33-OVA. OVA was engineered into a bacterial expression plasmid (pVLT33), and RE88 and E. coli BL21 (DE3) strains bearing recombinant pVLT33-OVA were induced to express OVA by IPTG. (A) The strains were treated with 1mM IPTG for 0, 1, 3, 5, or 7 h, and OVA expression in the bacterial lysate was measured by ELISA. (B) Western blot analysis showing OVA expression. Lane 1: BL21/RE88 vector; lane 2: BL21-pVLT33-OVA/RE88-pVLT33-OVA, 0 h; lane 3: BL21-pVLT33-OVA/RE88-pVLT33-OVA, 1 h; lane 4: BL21-pVLT33-OVA/RE88-pVLT33-OVA, 3 h; lane 5: BL21-pVLT33-OVARE88-pVLT33-OVA, 5 h; lane 6: BL21-pVLT33-OVA/RE88-pVLT33-OVA, 7 h, lane 7: OVA standards. (C) OVA expression by RE88-pVLT33-OVA measured by ELISA. H2O2(–): RE88-pVLT33-OVA not inactivated by H2O2; H2O2(+): RE88-pVLT33-OVA inactivated by H2O2; H2O2(+)-up: supernatant of the H2O2-inactivated RE88-pVLT33-OVA lysate after centrifugation; H2O2(+)-down: pellet of the H2O2-inactivated RE88-pVLT33-OVA lysate after centrifugation; H2O2(+)-mix: supernatant and pellet of the H2O2-inactivated RE88-pVLT33-OVA lysate after centrifugation. Data are presented as the mean ± standard error of mean of three independent experiments. **P < 0.01, ***P < 0.001, NS, P > 0.05.
Evaluation of the Safety of Vaccination with RE88-pVLT33-OVA
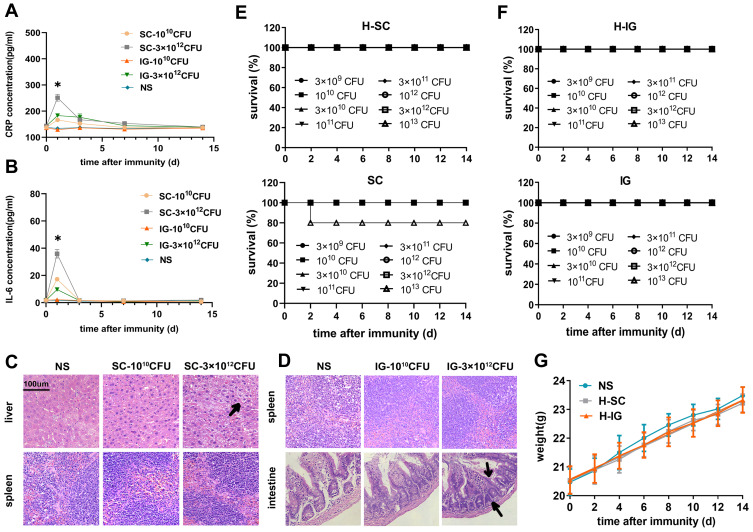
Previous studies reported that the mice could be safely immunized with attenuated typhoid bacilli at doses up to 1012 CFU/mouse for intragastric immunization and 109 CFU/mouse for subcutaneous immunization.14,17,23 Various doses of RE88-pVLT33-OVA strains were administered via different routes to investigate the safety of immunization with RE88-pVLT33-OVA. Survival rates were determined and median lethal dose (LD50) values were calculated 14 days after immunization. All mice that received intragastric immunization with RE88-pVLT33-OVA or H2O2-inactivated RE88-pVLT33-OVA at doses ranging from 3 × 109 to 1013 CFU/mouse survived (Figure 2F). The mice in the high-dose group (3 × 1012 CFU/mouse) exhibited slight changes in tissue histology, such as proliferation of the intestinal mucosa, a small increase in lymphocyte levels, and varying degrees of damage to the villi 48 h after injection, compared with the low-dose group (1010 CFU/mouse) and NS group (Figure 2D). All mice that received subcutaneous immunization with RE88-pVLT33-OVA or H2O2-inactivated RE88-pVLT33-OVA at doses ranging from 1010 to 3 × 1013 CFU/mouse also survived except the highest-dose group injected with RE88-pVLT33-OVA (1013 CFU/mouse) (Figure 2E). However, the histology demonstrated vacuolar degeneration and edema in some liver cells after administering a high dose of H2O2-inactivated RE88-pVLT33-OVA (Figure 2C). In addition, mouse serum was collected (on days 0, 1, 3, 7 and 14 after the first immunization) to detect acute inflammatory response. The expression levels of CRP (C reactive protein, CRP) and IL-6 (Interleukin-6) increased significantly in the serum of the high-dose group (3 × 1012 CFU/mouse) 24 h after subcutaneous immunization (P < 0.05; Figure 2A and B); no statistically significant difference was found in the levels of CRP and IL-6 in the serum of the low-dose group (1010 CFU/mouse) 24h after intragastric or subcutaneous immunization and high-dose group (3 × 1012 CFU/mouse) 24h after intragastric immunization (Figure 2A and B).
Figure 2.
Safety of the H2O2-inactivated RE88-pVLT33-OVA vaccine in mice. (A and B) Serum were collected from mice vaccinated with H2O2-inactivated RE88-pVLT33-OVA on 0, 1, 3, 7, 14 days after immunization, and CRP or IL-6 expression levels were measured by ELISA. (Beyotime Institute of Biotechnology). *P < 0.05 as compared with the SC-3 × 1012 CFU, NS groups. (C) Histological sections of the liver and spleen stained with H&E. Vaccination with H2O2-inactivated RE88-pVLT33-OVA was given via the subcutaneous route. NS: Normal saline; SC-1010 CFU: low dose of H2O2-inactivated RE88-pVLT33-OVA (1010 CFU/mouse); SC-3 × 1012 CFU: high dose of H2O2-inactivated RE88-pVLT33-OVA (3×1012 CFU/mouse). Black arrows: vacuolar degeneration and edema in the liver. The data are representative of n = 3 mice. (D) Histological sections of the spleen and intestine stained with H&E. Vaccination with H2O2-inactivated RE88-pVLT33-OVA was given via the intragastric route. NS: Normal saline; IG-1010 CFU: low dose of H2O2-inactivated RE88-pVLT33-OVA (1010 CFU/mouse); IG-3 × 1012 CFU: high dose of H2O2-inactivated RE88-pVLT33-OVA (3 × 1012 CFU/mouse). Black arrows: Proliferated mucosa and increased lymphocytes in the intestine. The data are representative of n = 3 mice. (E) Survival rates for mice vaccinated with various doses of H2O2-inactivated RE88-pVLT33-OVA (H-SC) or RE88-pVLT33-OVA (SC) via the subcutaneous route (n = 10 mice per group). (F) Survival rates for mice vaccinated with various doses of H2O2-inactivated RE88-pVLT33-OVA (H-IG) or RE88-pVLT33-OVA (IG) via the intragastric route (n = 10 mice per group). (G) Mouse body weight after immunization with H2O2-inactivated RE88-pVLT33-OVA (2 × 108 CFU/per mouse). Data are presented as the mean ± SEM of three independent experiments.
Abbreviations: NS, Normal saline; H-SC, subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA; H-IG, intragastric administration of H2O2-inactivated RE88-pVLT33-OVA.
The LD50 of non-inactivated RE88-pVLT33-OVA for C57 mice after subcutaneous immunization was 3.56 × 1013 CFU/mouse. Notably, all mice were immunized intragastrically or subcutaneously with 108 CFU of H2O2-inactivated RE88-pVLT33-OVA and observed for 14 days. No significant difference was observed in the average body weight of mice given intragastric immunization, subcutaneous immunization, and normal saline (Figure 2G). Although intraperitoneal immunization was also tested in this study, the mortality rate was 86.7%, and therefore no further experiments were conducted using this administration route. In terms of survival rates, histological analyses, and inflammatory response, subsequent experiments were carried out using doses less than 1011 CFU/mouse via the intragastric or subcutaneous routes.
Preliminary Evaluation of the Immune Response to Vaccination with H2O2-Inactivated RE88-pVLT33-OVA
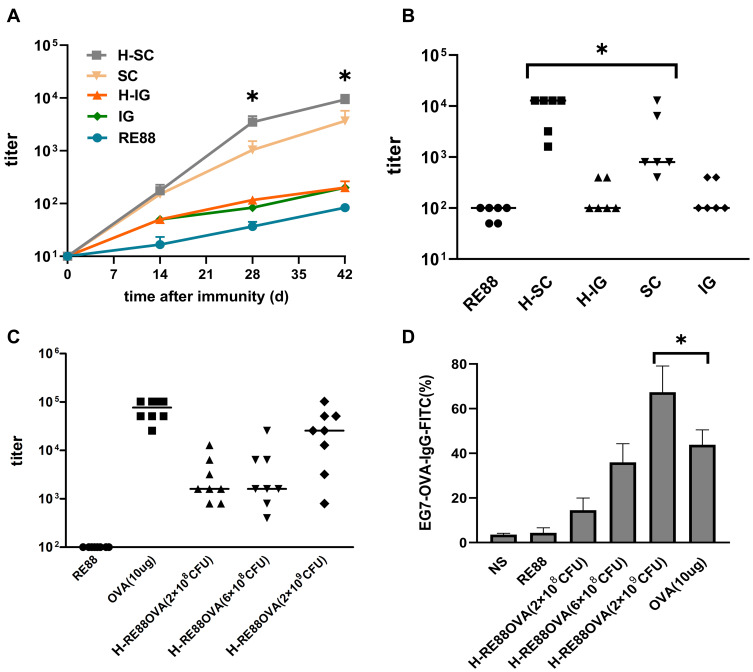
The mice were immunized three times on days 0, 14, and 28, and the mouse serum was collected weekly after the first of these immunizations (day 0) to detect whether OVA-specific antibodies were produced. For both the non-inactivated and H2O2-inactivated RE88-pVLT33-OVA vaccines, the total titer of anti-OVA IgG was higher for subcutaneous immunization than for intragastric immunization as the number of vaccination increased (Figure 3A). Notably, the anti-OVA IgG titer 42 days after subcutaneous immunization with H2O2-inactivated RE88-pVLT33-OVA was significantly higher than that 42 days after subcutaneous immunization with non-inactivated RE88-pVLT33-OVA (P < 0.05; Figure 3B). The antibody response after subcutaneous immunization of mice with inactivated H2O2-RE88-pVLT33-OVA varied with the dose administered: the serum anti-OVA IgG titer was around 103 42 days after immunization with doses of 2 × 108 or 6 × 108 CFU/mouse but reached around 105 after immunization with a dose of 2 × 109 CFU/mouse, which was a similar antibody response to that achieved by injection of 10 µg OVA (Figure 3C). The analysis of IgG subtypes following subcutaneous immunization with RE88-pVLT33-OVA or H2O2-inactivated RE88-pVLT33-OVA revealed that the antibody titer was higher for IgG1 than for IgG2a or IgG3 (Fig. S3).
Figure 3.
Evaluation of humoral immunity induced by prophylactic vaccination in a mouse tumor model. (A) Anti-OVA IgG titer in mouse serum at various times after the first immunization (109 CFU/per mouse). Data are presented as the mean ± SEM of ≥6 independent experiments. *P < 0.05 as compared with the H-SC, H-IG groups. H-SC: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA; SC: subcutaneous administration of non-inactivated RE88-pVLT33-OVA. H-IG: intragastric administration of H2O2-inactivated RE88-pVLT33-OVA; IG: intragastric administration of non-inactivated RE88-pVLT33-OVA; RE88: unmodified strain. (B and C) Anti-OVA IgG titer in mouse serum 42 days after the first immunization. H-RE88OVA: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA (3 doses); OVA-10 µg (positive control), *P < 0.05. (D) Ability of the specific IgG antibody in mouse serum to bind to the target tumor cells, subcutaneous administration with various vaccine types (1:400 serum dilution) of H2O2-inactivated RE88-pVLT33-OVA (n ≥ 3).*P < 0.05.
Abbreviation: NS, normal saline (negative control).
The diluted mouse serum was incubated with EG7-OVA cells to determine whether immunization-induced antibodies could target and bind to tumor cells, and the ability of IgG antibodies to bind to the cells was measured. For a 1:400 dilution of mouse serum, IgG binding to EG7-OVA cells was stronger for the subcutaneous vaccination group than for the intragastric vaccination group (Fig. S4). The average rate of IgG binding to EG7-OVA cells after subcutaneous immunization with H2O2-inactivated RE88-pVLT33-OVA at 2 × 108, 6 × 108, and 2 × 109 CFU/mouse was 15.8%, 39.2%, and 71.6%, respectively, compared with an average binding rate of 43.6% after immunization with OVA (the positive control; Figure 3D). Thus, the subcutaneous immunization with H2O2-inactivated RE88-pVLT33-OVA generated IgG that was able to bind strongly to target tumor cells.
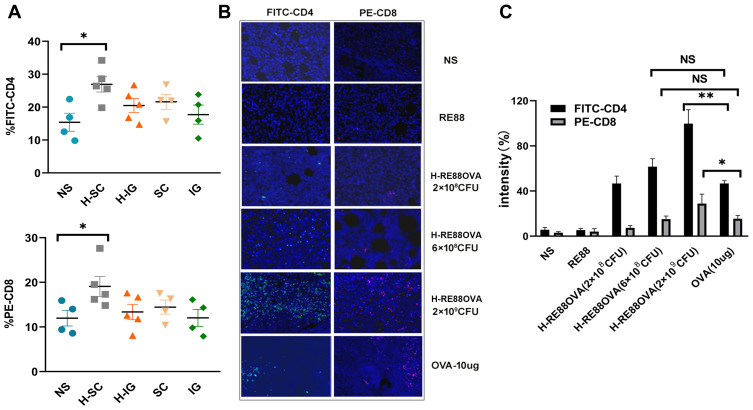
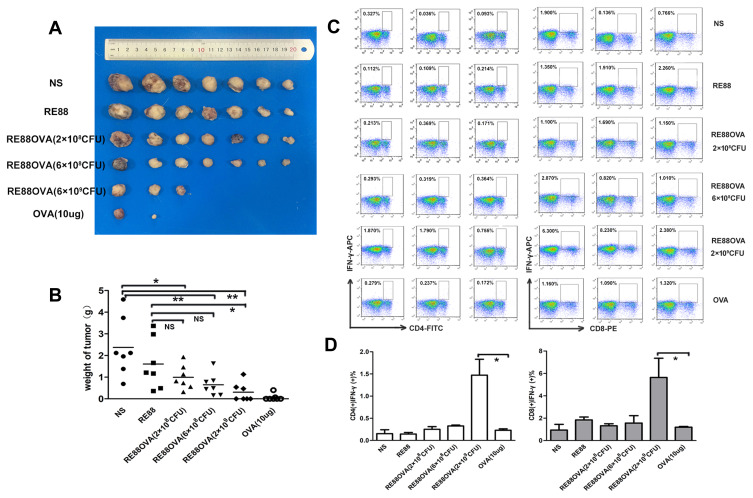
The numbers and types of lymphocytes that infiltrate tumor tissue are important indicators of the cellular immune response. Therefore, flow cytometry and immunofluorescence experiments were carried out to analyze CD4+ and CD8+ lymphocyte infiltration into the tumor tissue of mice immunized with H2O2-inactivated and non-inactivated RE88-pVLT33-OVA. Virtually no fluorescence was detected in tumor tissue from mice in the intragastric immunization group, indicating that very few CD4+ and CD8+ lymphocytes infiltrated the tumor (Figure 4A). However, fluorescence was detected in tumor tissue from mice in the subcutaneous immunization group, indicating the presence of CD4+ and CD8+ lymphocytes; the fluorescence intensity was slightly stronger for H2O2-inactivated RE88-pVLT33-OVA than for non-inactivated RE88-PVLT33-OVA (Figure 4A). Furthermore, the subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse resulted in greater infiltration of CD4+ and CD8+ lymphocytes into tumor tissue compared with subcutaneous administration of 10 µg OVA (positive control), (P < 0.05 for CD8+ lymphocytes and P < 0.01 for CD4+ lymphocytes; Figure 4B and C). The aforementioned results suggested that subcutaneous vaccination with H2O2-inactivated RE88-pVLT33-OVA was effective at inducing the infiltration of CD4+/CD8+ lymphocytes into the target tumor.
Figure 4.
Infiltration of EG7-OVA tumor tissue by lymphocytes. (A) Percentages of intratumoral CD4+/CD8+ T lymphocytes. Lymphocyte infiltration of tumor tissue in mice immunized subcutaneously or intragastrically with 2 × 108 CFU of non-inactivated or H2O2-inactivated RE88-pVLT33-OVA. Data are pooled from two independent experimental replicates. (B) Lymphocyte infiltration of tumor tissue in mice immunized subcutaneously with various types of vaccine. CD4-positive T cells were labeled with FITC (green), CD8-positive T cells were labeled with PE (red), and nuclei were labeled with DAPI (blue). (C) Mean fluorescence intensity indicating the extent of tumor infiltration with CD4-positive and CD8-positive T cells. Data are presented as mean ± SEM (n ≥ 3). *P < 0.05, **P < 0.01, NS, P > 0.05. H-IG: Intragastric administration of H2O2-inactivated RE88-pVLT33-OVA; H-SC: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA; IG: intragastric administration of non-inactivated RE88-pVLT33-OVA; SC: subcutaneous administration of non-inactivated RE88-pVLT33-OVA. NS: Normal saline (negative control); RE88: unmodified strain; H-RE88OVA: subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA (3 doses); OVA-10 µg (positive control).
Anti-Tumor Effects of Vaccination with H2O2-RE88-pVLT33-OVA
A tumor mass first became evident on day 6 after inoculation of EG7-OVA cells, and the tumor size was measured every 3 days thereafter (Fig. S5). Figure 5A shows a photograph of the tumors excised from the mice in various experimental groups on day 20 after EG7-OVA cell inoculation. Tumors were present in all seven mice from each of the negative control groups (normal saline or RE88 administration), and the sizes of the tumors were comparable between these two groups, with volumes exceeding 2000 mm3 in most cases (Figure 5A and S5). The mice vaccinated subcutaneously with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 108 or 6 × 108 CFU/mouse had smaller tumors compared with mice in the negative control groups (Fig. S5). Notably, four of the seven mice immunized subcutaneously with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse and five of the seven mice immunized with 10 µg OVA had no detectable tumors (Figure 5A and S5). The tumor weight decreased with increasing dose of H2O2-inactivated RE88-pVLT33-OVA, and tumor weight in mice vaccinated with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse was significantly lower than that in both negative control groups (P < 0.05; Figure 5B).
Figure 5.
Effects of prophylactic subcutaneous vaccination with H2O2-inactivated RE88-pVLT33-OVA on tumor growth in mice. (A) Photographs of tumors excised from mice in the various experimental groups (n = 7 per group). (B) Tumor weight (n = 7 per group). (C and D) Activation of T cells isolated from the spleen 1 week after the last immunization in mice immunized subcutaneously with various doses of vaccine. The cells were immunostained for CD4, CD8, and IFN-γ. Data are presented as mean ± SEM (n ≥ 3), *P < 0.05, **P < 0.01. NS: Normal saline (negative control); RE88: unmodified strain; RE88OVA: H2O2-inactivated RE88-pVLT33-OVA (3 doses); OVA-10 µg (positive control).
The histological analysis of H&E-stained sections showed no obvious pathological changes in the heart, liver, lungs, or kidneys (data not shown). The activation of CD4+ and CD8+ lymphocytes in the spleen was evaluated by measuring IFN-γ expression (Figure 5C and D). Notably, the expression of IFN-γ in CD4+ and CD8+ T cells was significantly higher in mice immunized subcutaneously with H2O2-inactivated RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse than in mice immunized with 10 µg OVA (positive control) (Figure 5C and D). These findings suggested that the subcutaneous vaccination with H2O2-inactivated RE88-pVLT33-OVA provided an effective cellular immune response that inhibited tumor growth.
Discussion
A notable finding of this study was that the subcutaneous administration of H2O2-inactivated RE88-pVLT33-OVA achieved a higher anti-OVA IgG titer compared with intragastric administration. Importantly, when subcutaneous administration was used, the anti-OVA IgG titer induced by H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU/mouse) was higher than that induced by RE88-pVLT33-OVA and comparable to that induced by 10 µg OVA. Furthermore, the binding of mouse serum antibodies to EG7-OVA cells was stronger for H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU/mouse) than for 10 µg ovalbumin. In addition, the subcutaneous vaccination with H2O2-inactivated RE88-pVLT33-OVA (2 × 109 CFU/mouse) induced greater activation of splenic T cells and more extensive tumor infiltration with CD4+/CD8+ T cells compared with ovalbumin injection. It is anticipated that H2O2-inactivated RE88-pVLT33-OVA could be used as a novel delivery system to develop new cancer vaccines.
Bacterial vaccine carriers have several advantages, including a straightforward production process, low cost, and flexibility. Numerous studies investigated the use of S. typhimurium or its attenuated strain, RE88, as cancer vaccine carriers.16,18–26,32,33 When pathogens are used as vaccine carriers, the challenge is to control the immunogenicity of the carrier itself without inhibiting the immunogenicity of the target antigen. One solution is to use viruses with low seropositivity. Bacteria carry various immune activators and ligands, such as lipopolysaccharide, flagellin, polysaccharides, and double-stranded deoxyribonucleic acid. One approach to using bacteria as cancer vaccines involves the use of a whole-cell bacterial vaccine sensitive to antibiotics so that appropriate antibiotics can be administered if adverse effects arise due to the pathogenicity of the carrier. An alternative method of ensuring safety is to use inactivated bacteria as the vaccine carrier. The inactivation of bacteria with H2O2 has advantages that include low toxicity, sterilization, and short treatment time (compared with traditional agents such as formaldehyde).
H2O2 was used to inactivate a variety of bacteria, including the RE88 strain, and thereby suppress bacterial activity and enhance the safety of the bacterial vector.31 However, whether the target antigens retained sufficient immunogenicity and caused an adequate immune response after the inactivation of the bacteria with H2O2 was not clear. In the present study, Western blot analysis and ELISA demonstrated that H2O2-inactivated RE88-pVLT33-OVA maintained the expression of a model tumor antigen (OVA). Furthermore, the evaluation of anti-OVA IgG titer, antibody binding to EG7-OVA cells, and lymphocyte infiltration into tumor tissue revealed that H2O2-inactivated RE88-pVLT33-OVA retained the immunogenicity of its OVA antigen. In addition, the subcutaneous or intragastric administration of H2O2-inactivated RE88-pVLT33-OVA at a dose of less than 1011 CFU/mouse was not associated with mortality or obvious damage to important organs, suggesting that this technique was safe. The aforementioned findings indicated that H2O2-RE88-pVLT33-OVA retained antigen expression and immunogenicity and appeared to be safe to administer as a vaccine.
The comparisons of two routes of administration revealed that the subcutaneous vaccination elicited stronger cellular and humoral immune responses than intragastric vaccination. At the highest dose tested (2 × 109 CFU/mouse), the subcutaneous injection of H2O2-RE88-pVLT33-OVA achieved an anti-OVA IgG titer 100 times higher than that obtained after intragastric administration, and IgG1 was the predominant antibody subtype. The specific binding to target cells was approximately 70% for serum (1:400 dilution) obtained from mice administered a high dose of H2O2-RE88-pVLT33-OVA subcutaneously, which was higher than that obtained from mice administered an equivalent dose of OVA (10 µg).
The numbers and types of lymphocytes that infiltrate tumor tissue are important indexes for measuring the cellular immune response. With regard to cellular immunity, tumor infiltration by CD4+/CD8+ lymphocytes and the expression of IFN-γ by CD4+/CD8+ lymphocytes were greater for subcutaneous injection of H2O2-RE88-pVLT33-OVA than for vaccination with non-inactivated RE88-pVLT33-OVA or OVA (positive control). Cellular immunity plays an important role in anti-tumor immunity, and poor induction of cellular immunity is a recognized deficiency of traditional whole-cell bacterial vaccines. No previous studies reported stimulation of cellular immunity by a whole-cell bacterial vaccine inactivated by H2O2. Thus, the advantages of the RE88-pVLT33-OVA strain with regard to immunogenicity were maintained after treatment with H2O2. Notably, the subcutaneous vaccination with H2O2-RE88-pVLT33-OVA exerted a dose-dependent effect to suppress tumor growth in the present mouse model. Indeed, only three of the seven mice vaccinated with H2O2-RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse developed tumor masses. The results suggested that it was important to identify the optimal doses of H2O2-RE88-pVLT33-OVA that maximized efficacy while maintaining safety. Nevertheless, compared with the same dose of H2O2-RE88-pVLT33-OVA strains, OVA showed a better anti-tumor effect but weaker immune responses probably due to the following reasons: the size of solid tumor in the immunized mice was firstly affected by activated IgG antibody titer, the anti-OVA IgG average titer 42 days after subcutaneous immunization with OVA (10ug) was slightly higher than that 42 days after subcutaneous immunization with H2O2-RE88-pVLT33-OVA at a dose of 2 × 109 CFU/mouse (Figure 3C), OVA mainly activated the killing effect of OVA-mediated specific antibody which was significant for tumor prevention in a short period, however, it easily caused excessive humoral immunity and disruption of immune balance. The H2O2-inactivated strain had more oxidation-specific epitopes sensed by a variety of cellular pattern recognition receptors on cell surface,34 it has been found the H2O2-inactivated strain released of bacterial DNA to regulate the activated T cells, followed by NF-kappaB (NF-κB) pathway enhancement.31 Therefore, H2O2-inactivated strain might stimulate better cellular immune responses than OVA. In addition to the binding of specific IgG antibody to EG7-OVA cells, anti-tumor cellular immunity activated by H2O2-RE88-pVLT33-OVA strain could maintain the immune microenvironment homeostasis and suppress the tumor recurrence and metastasis.
A large number of recent studies aim to investigate the use of multiple adjuvants in cancer vaccines. The addition of multiple adjuvants not only helps regulate the body’s immune system but also enhances the immunogenicity of the tumor antigen. H2O2-RE88-pVLT33-OVA has great potential as a high-performance cancer vaccine because it is a whole-cell vaccine that carries multiple natural adjuvants and thus can elicit a strong cellular immune response.
Conclusions
H2O2-inactivated RE88-pVLT33-OVA could maintain the expression of a model tumor antigen (OVA) and demonstrated greater immunogenicity and anti-tumor effects than non-inactivated RE88-pVLT33-OVA in a mouse model of cancer. H2O2-inactivated RE88-pVLT33-OVA was potentially safer than a non-inactivated strain, could carry exogenous antigens, and had specific epitopes that could be exploited as natural adjuvants to facilitate the induction of cellular and humoral immune responses. It was anticipated that H2O2-inactivated RE88 could be used as a novel delivery system for new cancer vaccines. Further studies are needed to optimize the stability of H2O2-inactivated RE88 expressing tumor antigens, investigate the potential benefits of expressing multiple tumor antigens, and evaluate the effectiveness of combination therapies (eg, vaccines based on H2O2-inactivated RE88 used together with targeted chemotherapy and/or monoclonal antibodies).
Acknowledgments
Yingzi Fan and Tingting Bai are co-first authors for this study.
Funding Statement
This study was supported by the National Major Scientific and Technological Special Project for “Significant New Drugs Development (2018ZX09733001 and 2018ZX09201018) and 1.3.5 project for disciplines of excellence,” West China Hospital, Sichuan University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
ANOVA, analysis of variance; BSA, bovine serum albumin; DAPI, 4ʹ,6-diamidino-2-phenylindole; H2O2, hydrogen peroxide; IFN-γ, interferon-gamma; IPTG, isopropyl β-d-1-thiogalactopyranoside; OVA, ovalbumin; PBS, phosphate-buffered saline; PBST, Tween-20 in PBS; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Data Sharing Statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Review Committee for Animal Experimentation of Sichuan University. All procedures involving animal treatment were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication Pub No 85-23, revised 1996).
Consent for Publication
Not applicable.
Disclosure
The authors declare no competing interests.
References
- 1.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol. 2007;37(Suppl 1):S148–155. doi: 10.1002/eji.200737820 [DOI] [PubMed] [Google Scholar]
- 2.Allahverdiyev A, Tari G, Bagirova M, Abamor ES. Current approaches in development of immunotherapeutic vaccines for breast cancer. J Breast Cancer. 2018;21(4):343–353. doi: 10.4048/jbc.2018.21.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22(3):313–320. doi: 10.1038/nbt937 [DOI] [PubMed] [Google Scholar]
- 4.Yi X, Zhou H, Chao Y, et al. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci Adv. 2020;6(33):eaba3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury S, Castro S, Cokeret C, et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van AEM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RA, Diamond MS, Rech AJ, et al. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight. 2016;1(14):e88328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mougel A, Terme M, Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92(4):535–545. doi: 10.1016/S0092-8674(00)80946-0 [DOI] [PubMed] [Google Scholar]
- 12.Song YC, Liu SJ. A TLR9 agonist enhances the anti-tumor immunity of peptide and lipopeptide vaccines via different mechanisms. Sci Rep. 2015;5:12578. doi: 10.1038/srep12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carretero-Iglesia L, Couturaud B, Baumgaertner P, et al. High peptide dose vaccination promotes the early selection of tumor antigen-specific CD8 T-cells of enhanced functional competence. Front Immunol. 2019;10:3016. doi: 10.3389/fimmu.2019.03016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao ZQ, Wu B, et al. Subcutaneous vaccination with attenuated Salmonella enterica serovar Choleraesuis C500 expressing recombinant filamentous hemagglutinin and pertactin antigens protects mice against fatal infection with both S. enterica serovar Choleraesuis and Bordetella bronchiseptica. Infect Immun. 2008;76(5):2157–2163. doi: 10.1128/IAI.01495-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(2):727–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin IY, Van TT, Smooker PM. Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery. Vaccines. 2015;3(4):940–972. doi: 10.3390/vaccines3040940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi K, Igarashi K, Murakami T, et al. Salmonella typhimurium A1-R targeting of a chemotherapy-resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozolomide. Cell Cycle. 2017;16(13):1288–1294. doi: 10.1080/15384101.2017.1314420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirullo B, Ammendola S, Leonardi L, et al. Attenuated mutant strain of Salmonella Typhimurium lacking the ZnuABC transporter contrasts tumor growth promoting anti-cancer immune response. Oncotarget. 2015;6(19):17648–17660. doi: 10.18632/oncotarget.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jazayeri SD, Ideris A, Zakaria Z, Yeap SK, Omar AR. Improved immune responses against avian influenza virus following oral vaccination of chickens with HA DNA vaccine using attenuated Salmonella typhimurium as carrier. Comp Immunol Microbiol Infect Dis. 2012;35(5):417–427. doi: 10.1016/j.cimid.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Meng JZ, Dong YJ, Huang H, et al. Oral vaccination with attenuated Salmonella enterica strains encoding T-cell epitopes from tumor antigen NY-ESO-1 induces specific cytotoxic T-lymphocyte responses. Clin Vaccine Immunol. 2010;17(6):889–894. doi: 10.1128/CVI.00044-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YJ, Hou Y, Huang H, Liu GR, White AP, Liu SL. Two oral HBx vaccines delivered by live attenuated Salmonella: both eliciting effective anti-tumor immunity. Cancer Lett. 2008;263(1):67–76. doi: 10.1016/j.canlet.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Guo B, Jia H, et al. Targeted therapy via oral administration of attenuated Salmonella expression plasmid-vectored Stat3-shRNA cures orthotopically transplanted mouse HCC. Cancer Gene Ther. 2012;19(6):393–401. doi: 10.1038/cgt.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentschev I, Fensterle J, Schmidt A, et al. Use of a recombinant Salmonella enterica serovar Typhimurium strain expressing C-Raf for protection against C-Raf induced lung adenoma in mice. BMC Cancer. 2005;5:15. doi: 10.1186/1471-2407-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang R, Mizutani N, Luo Y, et al. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65(2):553–561. [PubMed] [Google Scholar]
- 25.Lee SH, Mizutani N, Mizutani M, et al. Endoglin (CD105) is a target for an oral DNA vaccine against breast cancer. Cancer Immunol Immunother. 2006;55(12):1565–1574. doi: 10.1007/s00262-006-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewen S, Zhou H, Hu HD, et al. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57(4):507–515. doi: 10.1007/s00262-007-0389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondou K, Suzuki T, Chang MO, Takaku H. Recombinant baculovirus expressing the FrC-OVA protein induces protective antitumor immunity in an EG7-OVA mouse model. J Biol Eng. 2019;13:77. doi: 10.1186/s13036-019-0207-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim NW, Kim SY, Lee JE, et al. Enhanced cancer vaccination by in situ nanomicelle-generating dissolving microneedles. ACS Nano. 2018;12(10):9702–9713. doi: 10.1021/acsnano.8b04146 [DOI] [PubMed] [Google Scholar]
- 29.Umeki Y, Saito M, Takahashi Y, Takakura Y, Nishikawa M. Retardation of antigen release from DNA hydrogel using cholesterol-modified DNA for increased antigen-specific immune response. Adv Healthc Mater. 2017;6:20. doi: 10.1002/adhm.201700355 [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim JJ, Kang KY, Paik MJ, Lee G, Yee ST. Enhanced anti-tumor immunotherapy by silica-coated magnetic nanoparticles conjugated with ovalbumin. Int J Nanomedicine. 2019;14:8235–8249. doi: 10.2147/IJN.S194352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y, Mu Y, Lu L, et al. Hydrogen peroxide-inactivated bacteria induces potent humoral and cellular immune responses and releases nucleic acids. Int Immunopharmacol. 2019;69:389–397. doi: 10.1016/j.intimp.2019.01.055 [DOI] [PubMed] [Google Scholar]
- 32.Stark FC, Sad S, Krishnan L. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 2009;69(10):4327. doi: 10.1158/0008-5472.CAN-08-3160 [DOI] [PubMed] [Google Scholar]
- 33.Jazani NH, Sohrabpour M, Mazloomi E, Shahabi S. A novel adjuvant, a mixture of alum and the general opioid antagonist naloxone, elicits both humoral and cellular immune responses for heat-killed Salmonella typhimurium vaccine. FEMS Immunol Med Microbiol. 2011;61(1):54–62. doi: 10.1111/j.1574-695X.2010.00747.x [DOI] [PubMed] [Google Scholar]
- 34.Christoph J, Binder C, Papac-Milicevic N, Joseph L. Witztum, Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]