Abstract
Objective
Chronic pain studies investigating the ability to detect sensory processing differences related to thalamic gating using electroencephalographic (EEG) alpha have yielded conflicting results. Alpha’s basic psychometric properties in pain populations requires further study. The present study reports on the test-retest reliability and internal consistency of EEG alpha power in older adults with chronic knee pain.
Methods
Repeated EEG alpha power measurements were taken of older adults (N=31) with chronic knee pain across two sessions separated by a ten-day period associated with a pilot clinical trial study. Recordings included resting periods (eyes open and eyes closed) as well as periods involving a pain management activity.
Results
Most single alpha-power measures and all within-participant averages of alpha obtained within a session showed high internal consistency (Cronbach’s α > 0.7) and satisfactory-to-excellent re-test reliability (Pearson’s rs > 0.6) of both alpha power and alpha blocking (eyes closed minus eyes open) across repeated conditions.
Conclusions
EEG alpha power seems mostly reliable and consistent, particularly when participants’ eyes are closed, after a period of habituation, and when alpha measures are averaged as within-participant estimates.
Significance
This analysis suggests that within-subject averages of EEG alpha are the most reliable for developing indices of chronic knee pain.
Keywords: EEG alpha, chronic pain, reliability, internal consistency
1. Introduction
Several studies suggest that sustained pain in individuals relates to changes in brain morphology and functioning compared to those of individuals without chronic pain (reviewed in Coppieters et al., 2016; Apkarian et al. 2011, Prichep et al., 2011). While it is unclear how these changes are related to the subjective experience of pain, sensory attention networks are thought to be involved, given a reduction of gray matter in the thalamus (Gwilym et al., 2010) and that pain is characterized as noxious somatosensory stimulation (Loeser, 1991). Importantly for pain research, electroencephalographic (EEG) measures of alpha-band activity (typically defined as oscillatory activity in the frequency range between 8–12 Hz) have previously been linked to sensory reactivity and are thought to be mediated via a thalamic gating mechanism (Adrian and Matthews, 1934; Berger, 1929).
One of the most robust effects observable in human EEG recording is the so-called alpha blocking effect. Alpha blocking refers to the measurable diminution of power within the alpha band of EEG spectra following exposure to sensory stimuli, and it is known to be greater when sensory stimuli are novel or otherwise more salient (Berger, 1929; Durup and Fessard, 1935; Adrian, 1944). In studies in which participants expected a task-relevant stimulus to appear, alpha blocking has also been observed preceding stimulus onset (Klimesch, 2012). Alpha blocking can be quantified as the EEG alpha-band power difference between eyes-closed and eyes-open conditions, readily quantified by spectral analysis of the EEG signal (Könönen and Partanen, 1993). Accordingly, alpha blocking has been used as a metric of healthy electrocortical reactivity in response to changes in visual input and as such has been part of neurological exams for decades (see Niedermeyer, 1997 for a review). In the research laboratory, alpha blocking in response to experimental stimuli has traditionally been interpreted as reflective of attentive stimulus processing, and as indexing states of increased vigilance, arousal, or engagement with the external world (Klimesch, 2012). Complementing these views, a growing body of evidence has emerged in which alpha increase (rather than blocking) is observed during tasks that emphasize internal cognitive processing, such as mental imagery (Bartsch et al., 2015), working memory (Jensen et al., 2002), and sensory suppression (Foxe and Snyder, 2011). Together, these findings highlight the potential of alpha power as a robust marker of sensory versus internal processing, suitable for clinical studies in which patients undergo a sequence of different tasks or interventions. In recent studies of acute pain, the spectral power in the alpha band showed a positive association with pain-severity ratings (Furman et al., 2018; reviewed in Pinheiro et al., 2016). Previously, several chronic pain studies found greater alpha power and slowed peak alpha frequencies (PAFs) observed in more severe chronic pain (Sarnthein et al., 2006), while recent studies have yielded mixed results pertaining to alpha amplitude and blocking (reviewed in Pinheiro et al., 2016).
A better understanding of the relationship between chronic pain and alpha measures may have clinical utility: Current chronic pain assessments offer limited information regarding the underlying mechanisms that contribute to chronic pain experiences. While recent studies suggest that self-report measures of pain may be more reliable than was previously believed (Letzen et al., 2016), patients with disparate etiologies may rate their pain symptoms similarly, impeding the development and prescription of targeted treatments (Davis et al. 2012, de Vries et al. 2013). As such, physiological indices that reflect pain mechanisms are highly desirable in that they may yield mechanism-oriented parameters for diagnosis and treatment (Graversen et al., 2012; de Vries et al. 2013).
Alpha-related indices for self-reported pain symptoms are of particular interest, however, the stability of alpha measures in chronic-pain populations is not well known. To better understand how these measures can be used as biomarkers of pain mechanisms, the inherent variance in alpha-band activity in individuals with chronic pain must be explored further (Davis et al., 2012; Racine and Illes, 2006). Internal consistency and test-retest reliability, two estimates of inherent variance of a given measure, are among the most basic properties that should be understood in order to characterize individual differences within a given population (Thigpen et al., 2017). Indices of alpha activity have previously found satisfactory to excellent stability in healthy adults (younger than 50) as well as other clinical populations (Gasser et al., 1985; Salinsky et al., 1991; Corsi-Cabrera et al., 2007) and in healthy older adults (Pollock et al., 1991). The question arises regarding the reliability in a sample of individuals with chronic pain. It is expected that alpha in this population may be similarly consistent, in spite of the many treatments that pain patients receive. However, investigations of these properties of EEG alpha are lacking in the chronic pain literature. The current investigation aimed to establish the internal consistency and re-test reliability of alpha-band power and blocking in individuals with chronic knee pain, with or at risk for knee osteoarthritis.
2. Methods
2.1. Design
The present investigation was part of an overall pilot study examining the effects of dietary strategies (intermittent fasting and glucose administration) in promoting enhanced neuroplasticity and learning to optimize pain management interventions for chronic knee pain (NCT02681081; Sibille et al., 2016). Because EEG alpha is generally stable across many standard and clinical populations, the analyses in this paper were made under the assumption that neither the pain-management strategies nor the dietary strategies used as interventions during this study would be implemented over a long enough period of time to systematically alter alpha reliability. The extent to which pain and pain management strategies are reflected in EEG data is beyond the scope of this investigation.
2.2. Participants
Thirty-one individuals who experienced persistent pain in one knee for more than three months were recruited from the Gainesville, Florida community via research databases, flyers, and community referrals. Exclusion criteria included concurrent medical conditions that could confound outcome measures or limit the participant’s ability to complete the entire protocol including the following: neurological conditions (Parkinson’s disease, multiple sclerosis, and/or seizures); history of a head injury or stroke; diabetes or taking medications to control blood sugar; mental health issues resulting in hospitalization or outpatient treatment in the past year, and/or psychotropic medication use; current issue or history of treatment for alcohol or other substance abuse; diminished cognitive function (Mini-Mental Status Exam < 22); pregnancy; a high baseline measure of fasting blood sugar (plasma glucose > 7mmol/L); persisting blood pressure greater than 150/95 or a heart condition (e.g., heart attack, heart surgery, frequent chest pain or heart failure); and inability to complete the EEG portion of the study.
Participants with chronic knee pain came into the laboratory for four visits over a two-week period. All participants gave written informed consent prior to participating. The Institutional Review Board of the University of Florida approved all procedures.
2.3. Measures
During the first session, demographic and health history information and chronic pain questionnaires including the Graded Chronic Pain Scale characteristic pain intensity rating (Von Korff et al., 1990) were completed. Additional data were collected as described in the description in clinicaltrials.gov. Methods described are primarily limited to those relevant to the current analysis.
2.4. EEG Procedure
The study protocol included a total of four sessions, two of which involved the EEG recordings included in the present analysis (the first and last sessions). EEG recording sessions were approximately seven-to-ten days apart (mean = 9.33 days, SD = 1.45), and both EEG sessions were identical. The analyses for this paper are limited to measurements of EEG alpha and alpha blocking in each recording session (see Figure 1). However, other EEG-related tasks were implemented using somatosensory stimulation as part of the overarching study. Below are descriptions of both the alpha-related and somatosensory-related procedures in order to provide a full picture of the participants’ experiences that may have influenced their EEG alpha in each session.
Figure 1:
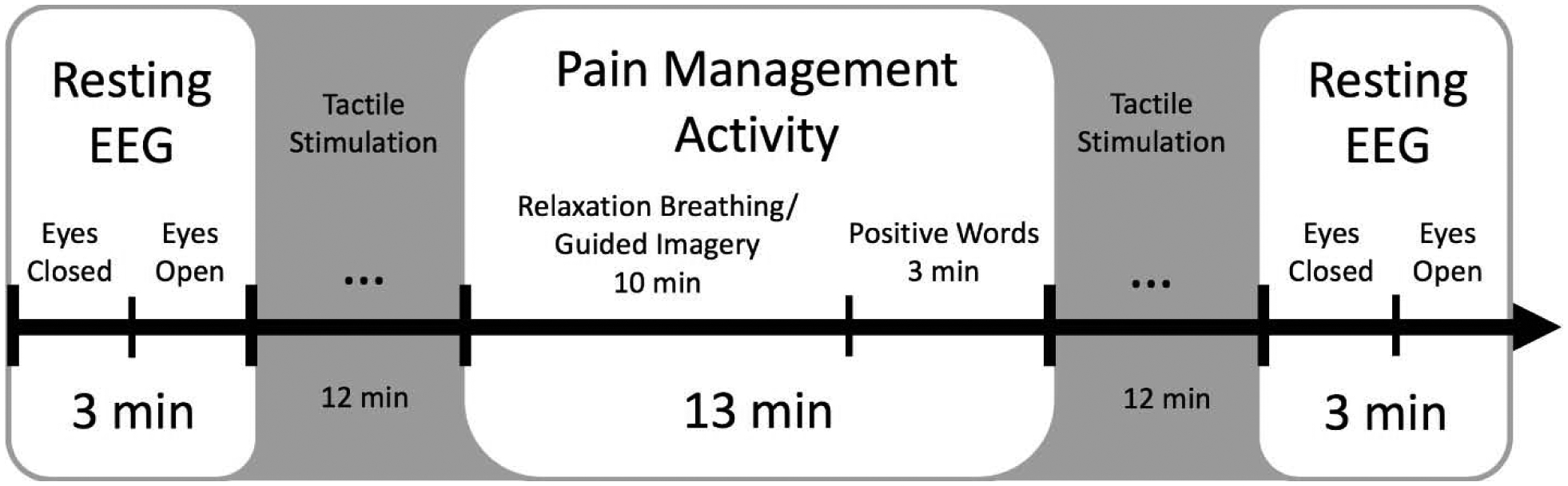
From left to right, each of two experimental sessions started with the recording of a 3-minute electroencephalography (EEG) resting block, followed by a somatosensory stimulation block, a period of relaxation breathing/guided imagery/positive affect activity, another tactile stimulation block, and a final resting block. The resting blocks comprised an initial 90-second eyes-closed condition, followed by 90-second eyes-open condition. Each block from which alpha measures were taken is presented in white. These blocks were used as items for calculating Cronbach’s α and Pearson’s correlation coefficients. Somatosensory stimulation blocks included a 3-minute stimulation for each of four sites: the left wrist, right wrist, left knee, and right knee.
2.5. EEG alpha measurements
During the baseline alpha measurement, participants’ EEG data were collected continuously during a 3-minute resting period. They were instructed to close their eyes throughout the first half of the recording and then open their eyes when prompted, for the remaining minute-and-a-half, while fixating on a marker in front of them and avoiding blinking. Participants were asked to describe the directions they followed after the recording ended and indicated whether they followed instructions as prompted.
Participants were also asked to participate in a 13-minute set of auditory-directed relaxation instructions by listening to an audio recording via earphones, referred to as the pain-management activity block in this report. This block involved relaxation breathing, guided imagery, and positive posturing (10 minutes) followed by a 3-minute segment in which previously validated positively rated words (Affective Norms for English Words, ANEW; Bradley and Lang, 1999) were presented. Participants were encouraged to attend to the auditory content and were permitted to have their eyes open or closed throughout the pain management activity block, while EEG data were recorded and eye movements monitored using horizontal and vertical electrooculogram (EOG). Following the activity block, participants were asked to rate their current affective state in terms of valence, arousal, and dominance using the SAM (Lang, 1980). Participants also repeated as many words as they could remember from the positive-word portion of the pain management activity. At the end of each session, another 3-minute resting period was recorded with the same eyes-closed and eyes-open instructions.
2.6. Somatosensory stimulation
Following the initial baseline recording, four somatosensory stimulation EEG recordings were conducted at each of four different sites (participants’ left and right wrists and left and right knees). For each recording, a non-painful somatosensory stimulator was placed at one of the respective sites on the participant’s body, tapping the respective site for 3 minutes at a rate of 2.77 Hz with a filament accelerated by an 8V pulse that lasted 10 ms between 350 ms inter-trial intervals.
These measurements were repeated following the pain management activity block. Data from the tactile stimulation portions of the study are not discussed in the present paper.
2.7. EEG recording
EEG data were recorded from a 32-channel active electrodes gel sensor array (Ag/Ag-Cl; international 10–20 system; actiCHamp, Brain Products) with a 24-bit battery-supplied actiCHamp active channel amplifier. Impedance threshold was set at 30 kΩ. A common mode sense electrode was located at site FPz, forming a noise suppressing circuit together with a driven right leg electrode located at site CPz. Recordings were digitized online using a 500 Hz sampling rate using BrainVision PyCorder (Brain Vision LLC). An online antialiasing Nyquist filter at 200 Hz was used.
2.8. EEG data processing
Data were re-referenced to averaged reference offline. Data were then filtered in EEGLAB v13.6.5b using a Hamming windowed sinc basic FIR filter with a passband of 1.5 – 20 Hz (−6dB cutoff at 0.75 Hz and 20.75 Hz) and transition bandwidth of 1.5 Hz. Filter order was set to “default” and yielded 1100 points.
2.9. Statistical Analysis
2.9.1. Peak frequencies and topography of alpha and alpha blocking.
Each resting measure was segmented into two 90-second blocks (eyes closed and eyes open). For the pain management activity block, the narrative portion was 610 seconds long, and the positively rated words segment was 160 seconds. For each block, epochs were segmented into 3-second (3000 ms) intervals, and segments whose amplitude variance ranged outside of the 9.5 quantile were rejected using the well-established SCADS procedure (Junghöfer et al., 2000). On average, 3.5 out of 30 segments (11.6%) were excluded for resting blocks (3.4 for eyes closed and 3.6 for eyes open). For the pain management blocks, 27.4 out of 203 segments were excluded for the narrative portion on average (13.5%) and 6.2 out of 53 blocks for the positively rated words (11.6%). One participant was excluded from the resting conditions due to experimenter error while administering instructions for these conditions. Spectral power was extracted from accepted epochs using a fast Fourier transform (FFT), which was then averaged by condition for each participant.
To calculate alpha spectral power, individual EEG spectra were first visually inspected to determine whether alpha peaks fell within the expected range of 8 – 12 Hz. Once this window was determined to contain all alpha peaks, spectra were reduced to signal-to-noise ratios (SNRs) of alpha-band power by averaging spectral power across 8 – 12 Hz frequencies and dividing by the mean of spectral power across the ranges of 6 – 7 Hz and 13 – 14 Hz, after ensuring that no participant showed signal in the frequency bins designated for noise measurement.
This measure is similar to methods extracting relative spectral contributions of a given frequency band to the entire spectrum (i.e., relative power). SNR estimations however have additional benefits; most notably, they address concerns regarding the conflation of offsets of the entire spectrum with true changes at a specific frequency. They also avoid spurious effects that may be due to mere differences in the 1/f shape of the spectrum (see e.g., Voytek et al., 2015). For example, it has been reported that differences in the shape and steepness of the 1/f shape (e.g. in its slope) will lead to differences at a given frequency range, rendering the false impression of frequency-specific differences (Quyang et al., 2020). SNR based analyses minimize both sources of spurious effects. For all analyses, an occipital alpha estimation was derived from combined SNRs from Pz and Oz, in addition to analyses conducted across topographies.
SNRs of eyes-closed alpha-band power were subtracted from corresponding SNRs of eyes-open alpha during the same recording blocks to calculate alpha blocking reactivity (eyes open-minus-closed alpha-band power). Analyses were repeated for raw power to ensure that SNR calculations did not drive the conclusions of the study.
2.9.2. Internal consistency of alpha SNRs
In order to evaluate the internal consistency of alpha measures, Cronbach’s αs were calculated (Cronbach, 1947). Cronbach’s αs were computed for all sensors and plotted as topographies to examine the topographical specificity of the observed effects. They were also computed for occipital alpha using the average alpha-band SNR of Pz and Oz.
Firstly, Cronbach’s α was calculated for each of the two EEG recording sessions, using participants as rows and alpha SNRs as columns from each resting EEG (early and late eyes closed and eyes open) block as well as the two relaxation/guided imagery blocks from each of two sessions, yielding the proportion of variance in alpha-band power attributable to inter- as opposed to intra-individual differences. (Columns: eyes closed pre-activity, eyes open pre-activity, narrative imagery activity, positive words activity, eyes closed post-activity, eyes open post-activity. See Figure 1.) Cronbach’s α was also calculated across both sessions together at each sensor (Columns: first-session eyes closed pre-activity, first-session eyes open pre-activity, first-session narrative imagery activity, first-session positive words activity, first-session eyes closed post-activity, first-session eyes open post-activity, last-session eyes closed pre-activity, last-session eyes open pre-activity, last-session narrative imagery activity, last-session positive words activity, last-session eyes closed post-activity, last-session eyes open post-activity).
Both within- and between-session Cronbach’s α were also tested for occipital alpha by calculating the average SNRs of Pz and Oz alpha and reported separately. Internal consistency was not calculated for corresponding blocks between sessions (e.g., early eyes closed in the first and last session) because a Cronbach’s α requires more than two columns.
2.9.3. Retest reliability of alpha SNRs
Test-retest reliability was also assessed based on Pearson’s correlations of alpha-band SNRs for each condition (first- and last-session eyes closed pre-activity, first- and last-session eyes open pre-activity, first- and last-session alpha blocking pre- activity, first- and last-session narrative imagery activity, first- and last-session positive words activity, first- and last-session eyes closed post-activity, first- and last-session eyes open post-activity, first- and last-session alpha blocking post-activity) calculated between the first and last EEG sessions. Within-session Pearson’s coefficients were calculated for resting blocks before versus after activity (first-session eyes closed pre- and post-activity, first-session eyes open pre- and post-activity). Pearson’s coefficients are reported both as scalp topographies as well as in a table showing the reliability of averaged SNRs of Pz and Oz alpha-band power.
2.9.4. Retest reliability of alpha SNRs averaged across conditions
To determine the effect of signal averaging across EEG measurements on reliability, a final analysis calculated Pearson’s rs of matching conditions between the two sessions of the averaged resting conditions, combining before- and after-activity alpha for the eyes closed and open instructions, as well as for the closed-minus-open alpha blocking. A high Pearson’s r (> 0.6) is typically taken to suggest high test-retest reliability between sessions (Schmidt et al., 2012).
2.9.5. Retest reliability of raw alpha power
An additional Pearson’s correlation was calculated for raw alpha power measures (in addition to the SNRs) to compare like conditions across the two sessions.
3. Results
3.1. Demographics
Our sample included 14 females (45.1%) and 17 males (54.8%) ages 51.1 – 80.7 (mean age = 64.01, SD = 8.48), GCPS characteristic pain intensity ratings ranged from 6.67–83.33 (mean = 50.86, SD = 90.09), duration (in months) of pain reported 12–720 (mean = 214.84, SD = 180.78). Twenty participants reported having pain in their left knee (64.5%), while the other 11 reported pain in their right knee (35.5%). Seventeen participants (55%) reported taking NSAIDs occasionally, as needed. Four individuals reported taking opioids for their chronic pain (13%), which may contribute to differences in EEG data (Smith et al., 1985).
3.2. Peak frequencies and topography of alpha and alpha blocking
The grand-mean EEG spectrum across participants (N = 31) and conditions showed the expected alpha-peak at approximately 10 Hz, with a wide band of amplitude power spanning the 8 – 12 Hz range (Figure 2). As a supplemental check of the selected alpha-band window, a broader-than-expected range of 7 – 14 Hz was used in an analysis of individual peak alpha frequency (PAF) for each participant’s spectra. These peaks were estimated by calculating the alpha center of gravity frequency, which is the weighted sum of spectral estimates divided by their respective alpha power. This is an established approach for characterizing alpha peaks because it can resolve problems such as the appearance of multiple peaks within the alpha band (Klimesch, 1999). Individual PAF estimates were calculated by defining the center of gravity frequency within 7 – 14 Hz of EEG spectra averaged across sensors at Pz and Oz. Individual PAFs were approximately 10.01 Hz on average and likewise fell within the 8 – 12 Hz range (min = 9.44 Hz, max = 10.75 Hz, median = 9.98 Hz, SD = 0.28). The average of grand mean alpha amplitudes across the 8 – 12 Hz range had a resulting SNR of 1.6 when computed against neighboring frequency bands (6 – 7 Hz and 13 – 14 Hz). Grand mean topographic distributions of the difference between eyes-closed and eyes-open alpha demonstrated the expected alpha blocking effect at parieto-occipital electrode sites (Figure 3). Given the pronounced age variability of the present sample, an obvious question is to what extent alpha-band indices examined here vary as a function of age. To examine this, we calculated the Pearson correlation coefficients between age and the dependent variables used in this study. None of these correlations reached the threshold for statistical significance.
Figure 2:
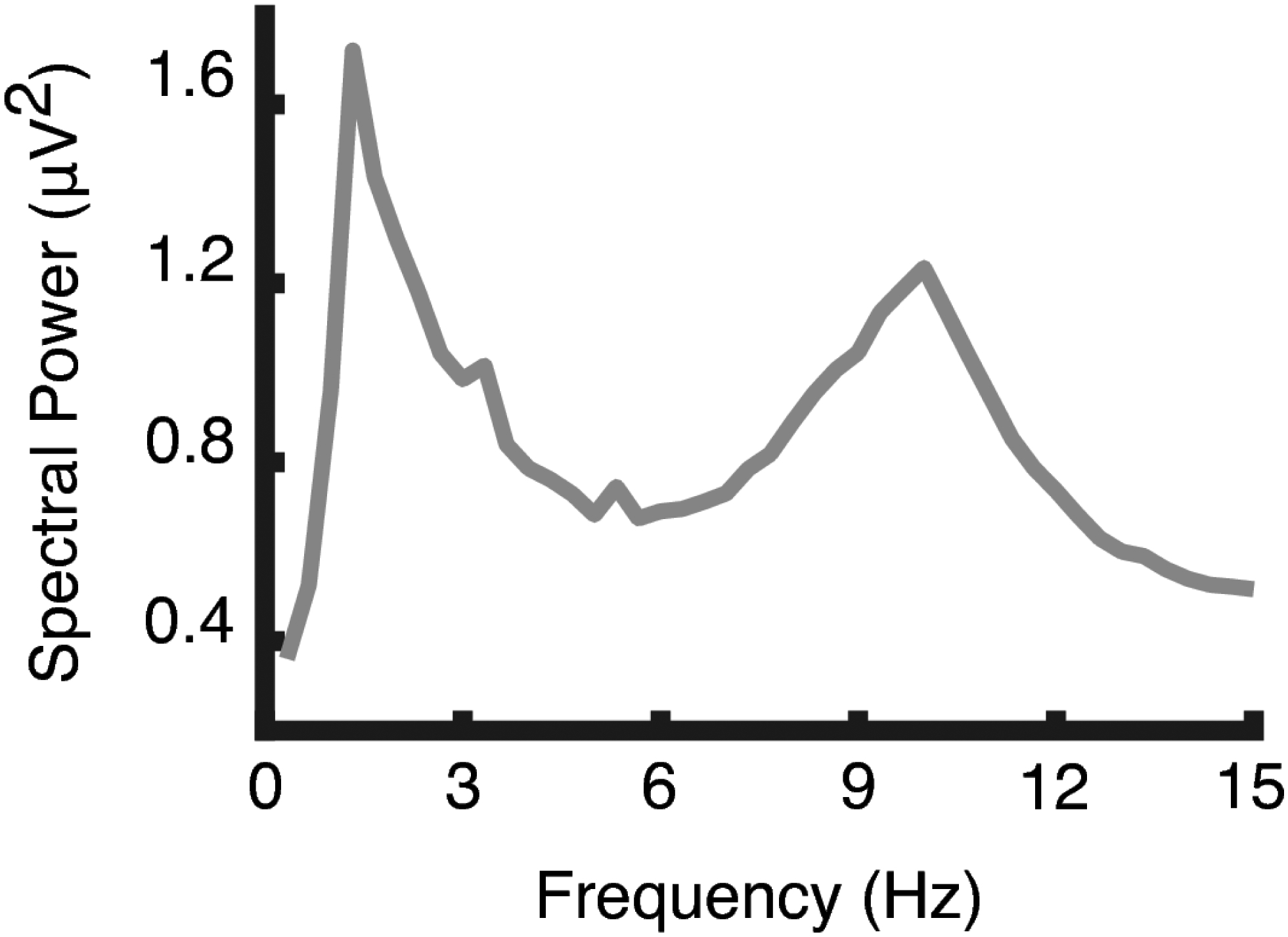
The grand mean spectrum (N=31) across resting conditions computed using a fast Fourier transform (FFT). The graph shows a distinct peak between 8 and 12 Hz, where the alpha band was expected to appear in older adults.
Figure 3:
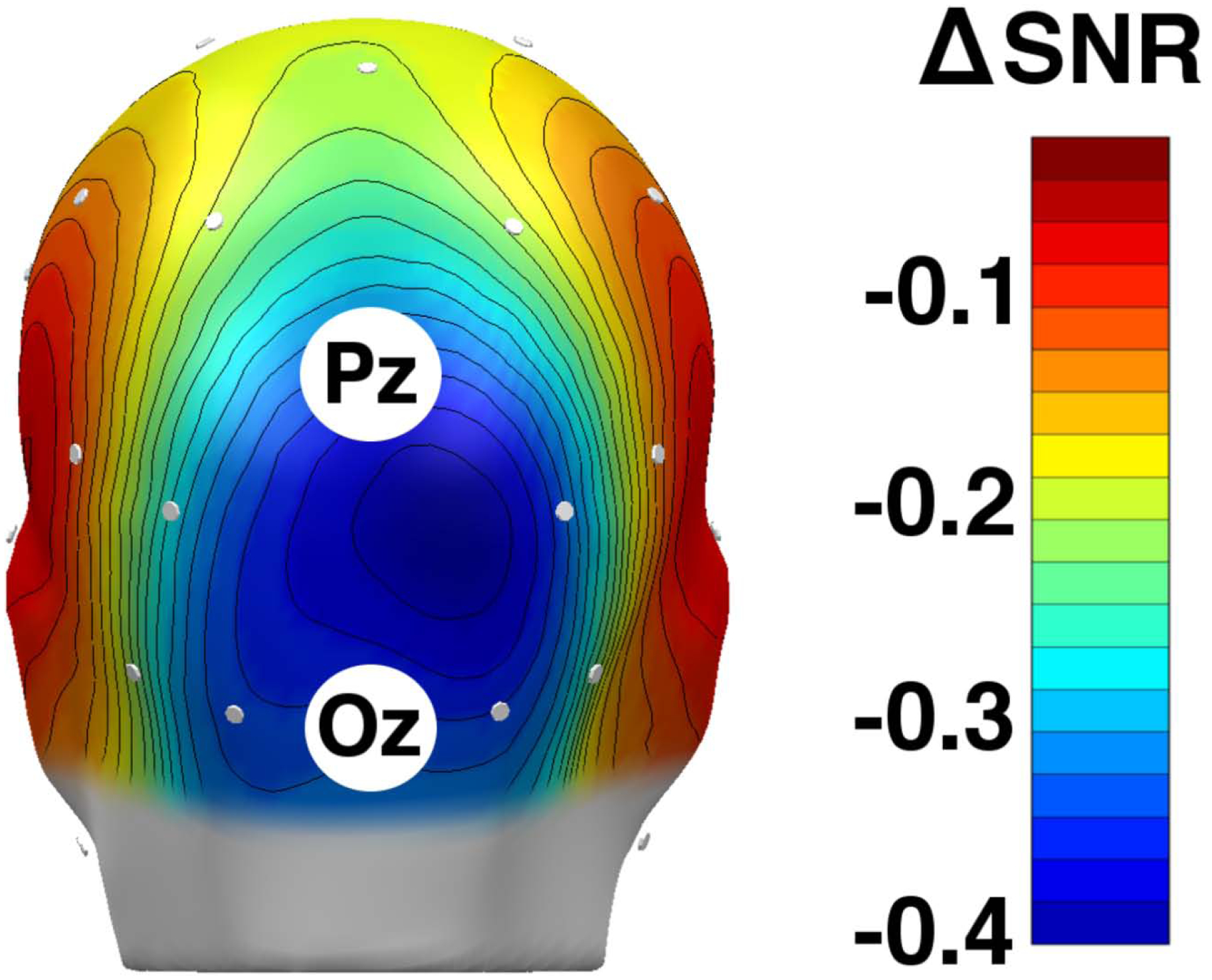
A grand mean (n = 30) spline-interpolated topographical distribution of eyes open-minus-closed difference scores of alpha-band power signal-to-noise ratios (SNR, unitless). One participant was excluded due to lack of eyes open/closed data. The EEG sensors are represented as white dots on the scalp. Blue suggests areas in which eyes-closed alpha power was greater than eyes-open alpha power (alpha blocking). As expected, the alpha blocking effect is observed most strongly at the occipital pole between Pz and Oz.
3.3. Internal consistency of alpha SNRs
The Cronbach’s α across resting EEG measures of occipital (averaged Pz and Oz) alpha was 0.88 in session 1 and 0.86 in session 2. The Cronbach’s α of occipital alpha across all measures was 0.90. This suggests high internal consistency of alpha power for the defined spectral range. (Note: Alpha blocking was not used in calculating Cronbach’s α because it would violate the independence-of-items assumption and because it is a different measure than alpha power.)
Cronbach’s α was also calculated at each sensor across all resting EEG measures of alpha within each session. All Cronbach’s αs were greater than .85 for the first session and greater than .76 for the last session, which suggests high internal consistency in alpha-band power at all sites within participants in multiple sessions. The average Cronbach’s α across sensors are 0.90 and 0.84 for the first and last sessions, respectively, which are comparable to the values calculated for occipital alpha-band reliability. Topographies of Cronbach’s αs are shown in Figure 4.
Figure 4:
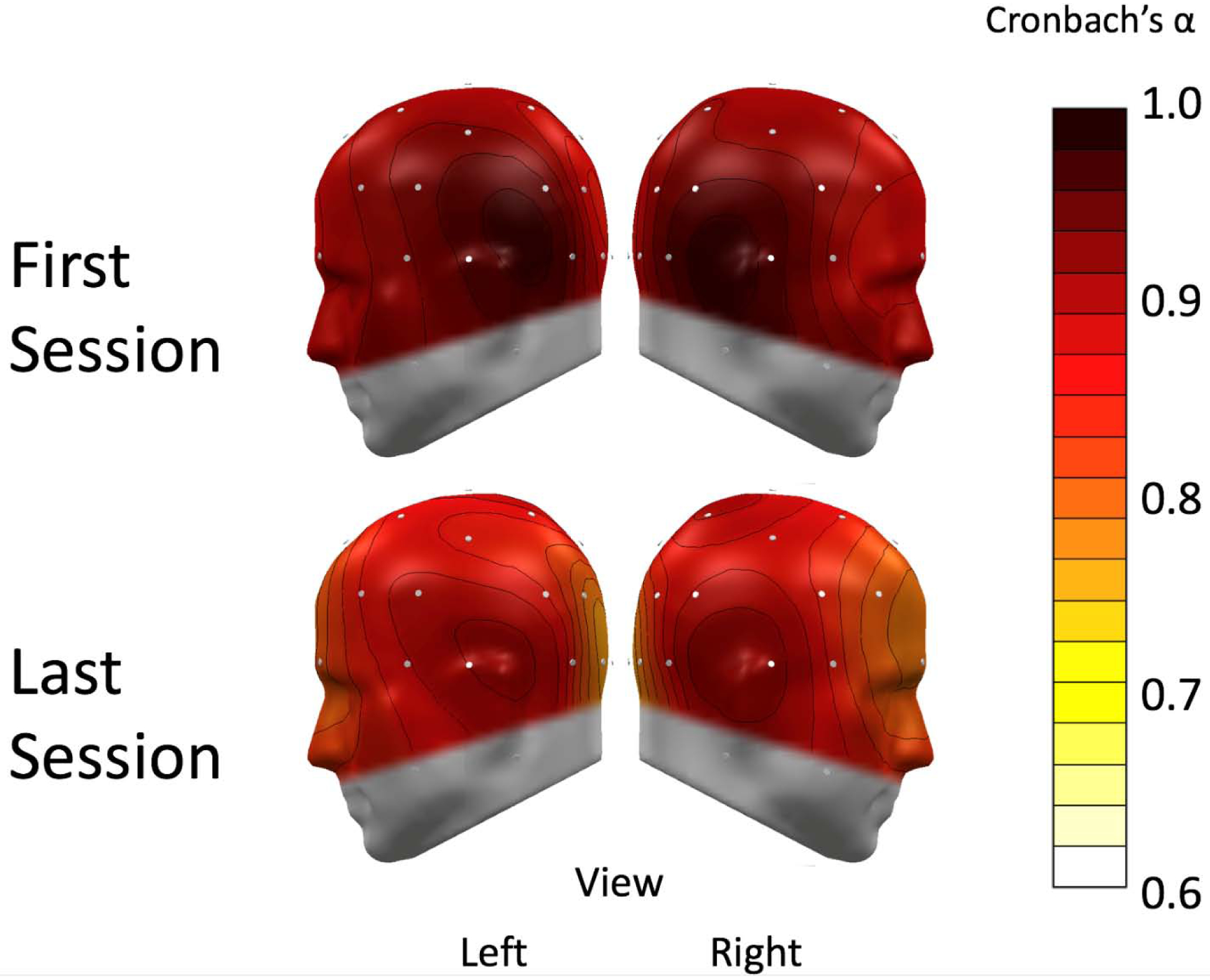
A spline-interpolated topographical distribution of Cronbach’s αs of alpha-band power. The electroencephalography (EEG) sensors are represented as white dots on the scalp. The first session (top) and final session (bottom) internal consistency scores were high across all sensors (>0.7). Left and right hemispheres are both shown for clear viewing.
3.4. Retest reliability of alpha SNRs
Pearson’s correlation coefficients of occipital alpha SNRs between conditions across the two sessions are shown in Table 1 as measures of test-retest reliability. Among the conditions included in this analysis are resting blocks before and after the pain management activity, as well as occipital alpha blocking, here coded as the difference score of eyes open-minus-closed alpha SNRs. Table 2 shows only the diagonal values of Table 1, which are the matching conditions between sessions.
Table 1:
Test-retest correlation coefficients and p-values, calculated for corresponding signal-to-noise (SNR) measurements of alpha-band power (averaged between sensors at Pz and Oz) between the two sessions (diagonal elements shown in bold), and between non-corresponding measures, for reference. Note that the significance level is only given for descriptive purposes, not to imply hypothesis testing.
| Pearson’s r (p-value) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Resting1 (closed) |
Resting 1 (open) |
Resting 1 (block) |
Imagery | Words | Resting 2 (closed) |
Resting 2 (open) |
Resting 2 (block) |
|
| Resting 1 (closed) |
0.567 (0.003) |
0.489 (0.011) |
−0.372 (0.061) |
0.768 (<.001) |
0.653 (<.001) |
0.880 (<.001) |
0.597 (0.004) |
−0.505 (0.020) |
| Resting 1 (open) |
0.076 (0.711) |
0.169 (0.410) |
0.026 (0.901) |
0.096 (0.633) |
0.046 (0.819) |
0.192 (0.404) |
0.424 (0.056) |
0.178 (0.440) |
| Resting 1 (block) |
−0.524 (0.006) |
−0.400 (0.043) |
0.382 (0.054) |
−0.740 (<.001) |
−0.647 (<.001) |
−0.862 (<.001) |
−0.432 (0.051) |
0.645 (0.002) |
| Imagery | 0.450 (0.019) |
0.558 (0.002) |
−0.169 (0.398) |
0.874 (<.001) |
0.842 (<.001) |
0.817 (<.001) |
0.750 (<.001) |
−0.273 (0.220) |
| Words | 0.422 (0.028) |
0.557 (0.003) |
−0.135 (0.503) |
0.750 (<.001) |
0.784 (<.001) |
0.716 (<.001) |
0.809 (<.001) |
−0.089 (0.695) |
| Resting 2 (closed) |
0.532 (0.007) |
0.670 (<.001) |
−0.188 (0.379) |
0.874 (<.001) |
0.834 (<.001) |
0.883 (<.001) |
0.657 (0.001) |
−0.447 (0.037) |
| Resting 2 (open) |
0.437 (0.033) |
0.850 (<.001) |
0.071 (0.743) |
0.555 (0.004) |
0.538 (0.005) |
0.568 (0.006) |
0.877 (<.001) |
0.164 (0.466) |
| Resting 2 (block) |
−0.234 (0.271) |
0.003 (0.988) |
0.306 (0.146) |
−0.580 (<.002) |
−0.544 (0.005) |
−0.667 (0.001) |
0.005 (0.981) |
0.837 (<.001) |
Table 2:
For clarity, test-retest correlation coefficients (in bold) and p-values, calculated for signal-to-noise (SNR) measurements of alpha-band power between only corresponding conditions of the two sessions at occipital sensors (averaged between sensors at Pz and Oz). Note that the significance level is only given for descriptive purposes, not to imply hypothesis testing.
|
0.567 (0.003) |
0.169 (0.410) |
0.382 (0.054) |
0.874 (<.001) |
0.784 (<.001) |
0.883 (<.001) |
0.877 (<.001) |
0.837 (<.001) |
Overall, matched conditions showed moderate-to-high reliability, as shown in Table 2. The first eyes-open at-rest measures of occipital alpha-band power (pre-activity) however correlated poorly between sessions (r = 0.169, p = 0.410). The related measure of initial alpha blocking also correlated relatively poorly between sessions (r = 0.382, p = 0.054).
Figure 5 shows scatterplots of initial eyes-closed (left panel) and eyes-open (right panel) alpha between sessions, respectively. The more compressed distribution and lack of stability in the initial eyes-open recording is visible, compared to the pronounced cross-session stability for the eyes-closed measurement.
Figure 5:
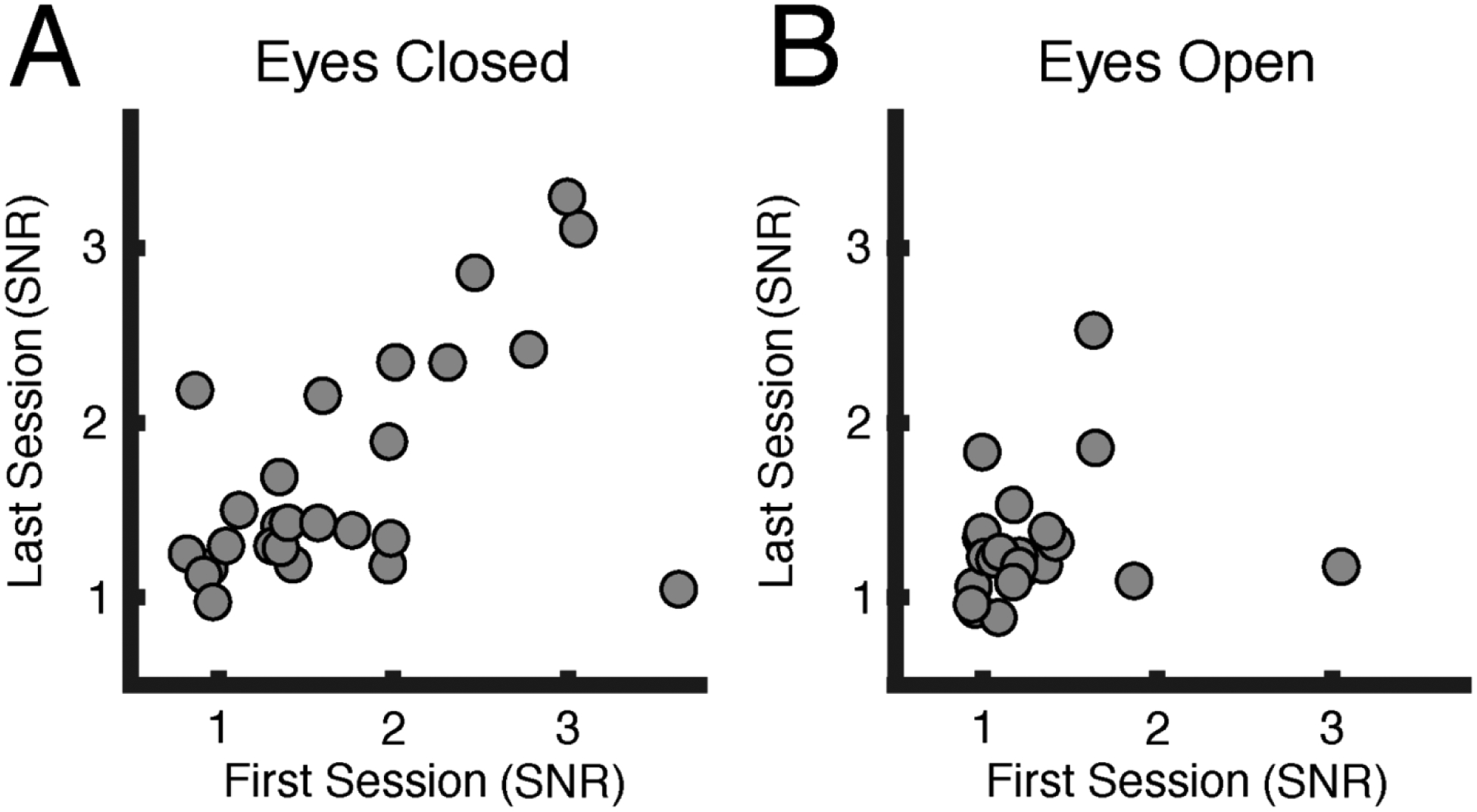
Signal-to-noise ratios (SNRs) of baseline alpha power. These scatterplots show baseline participant alpha signal-to-noise ratios (averaged between sensors at Pz and Oz) for each of two EEG sessions, approximately one week apart. Each dot represents one participant, with the x-axis representing alpha power during the first session and the y-axis representing alpha power during the last session. A. The baseline eyes-closed condition (left) shows a pronounced cross-session stability compared to B. the baseline eyes-open condition (right).
Within-session re-test reliability, shown in Table 3, was also tested for closed, open, and blocking conditions before and after the activity block.
Table 3:
Test-retest correlation coefficients (in bold) and p-values, calculated for signal-to-noise (SNR) measurements of alpha-band power between resting conditions within each session. SNRs were calculated at occipital sensors (averaged between sensors at Pz and Oz). Note that the significance level is only given for descriptive purposes, not to imply hypothesis testing.
| Pearson’s r (p-value) |
Resting (closed) |
Resting (open) |
Resting (block) |
|---|---|---|---|
| First Session |
0.734 (<.001) |
0.927 (<.001) |
0.592 (0.003) |
| Last Session |
0.801 (<.001) |
0.201 (0.346) |
0.721 (<.001) |
Topographies of Pearson’s correlation coefficients of matched conditions across the two EEG sessions are shown in Figures 6 and 7, indexing test-retest reliability. Paralleling occipital alpha analyses above, the initial resting conditions and related alpha blocking before the pain management activity were the only measurements that generally did not display satisfactory reliability.
Figure 6:
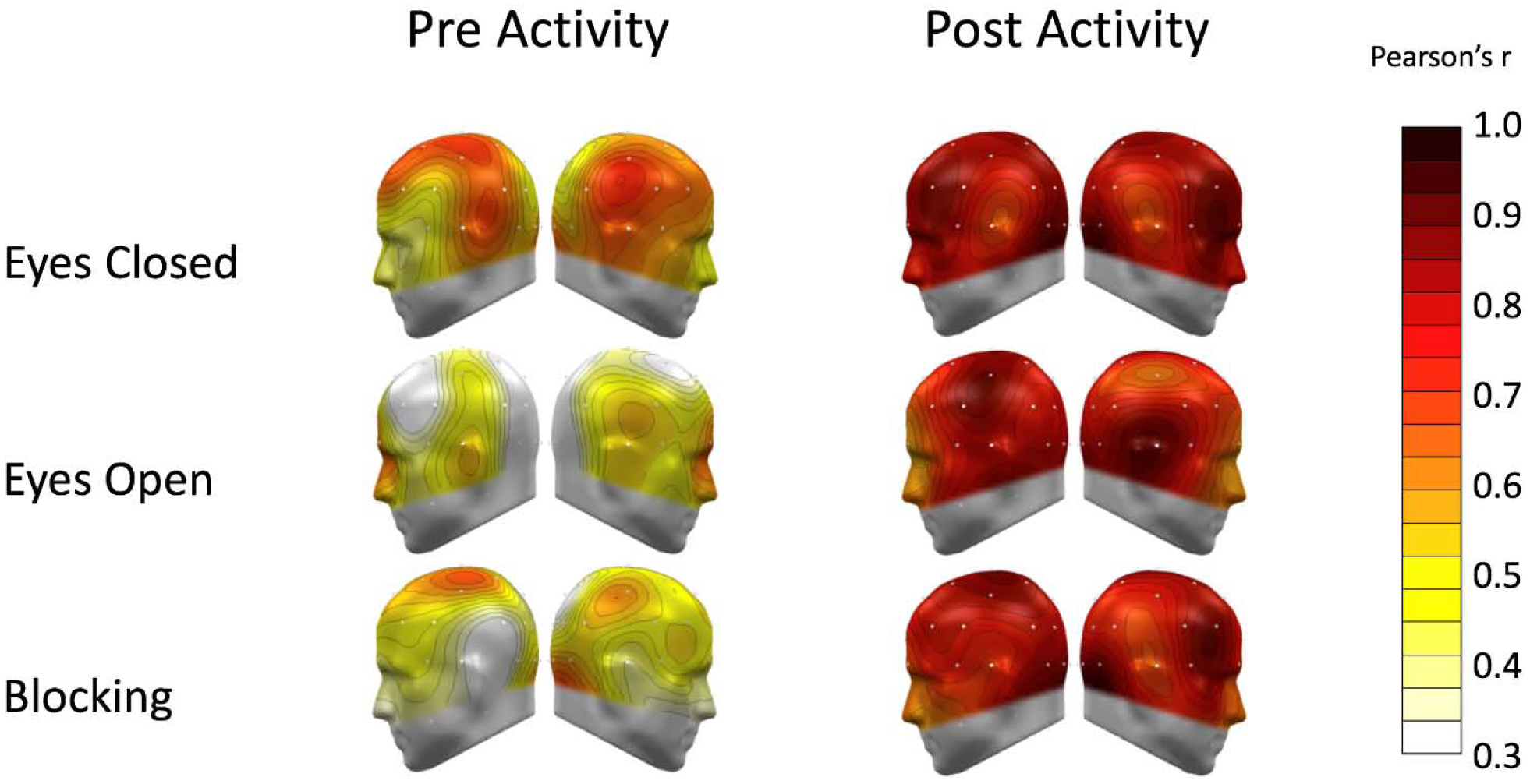
A spline-interpolated topographical distribution of test-retest correlation coefficients of alpha-band power is shown for corresponding conditions across the two sessions. The left panel shows conditions preceding the relaxation breathing/guided imagery activity. White and yellow indicate poor test-retest reliability, particularly for the eyes-open condition. Pale orange-to-red indicate moderate-to-excellent reliability, as in the eyes-closed condition pre-activity. The right panel shows the corresponding topographies for post-activity blocks. Alpha at all sensors had moderate to excellent retest reliability after pain management activity, as shown on the same scale.
Figure 7:
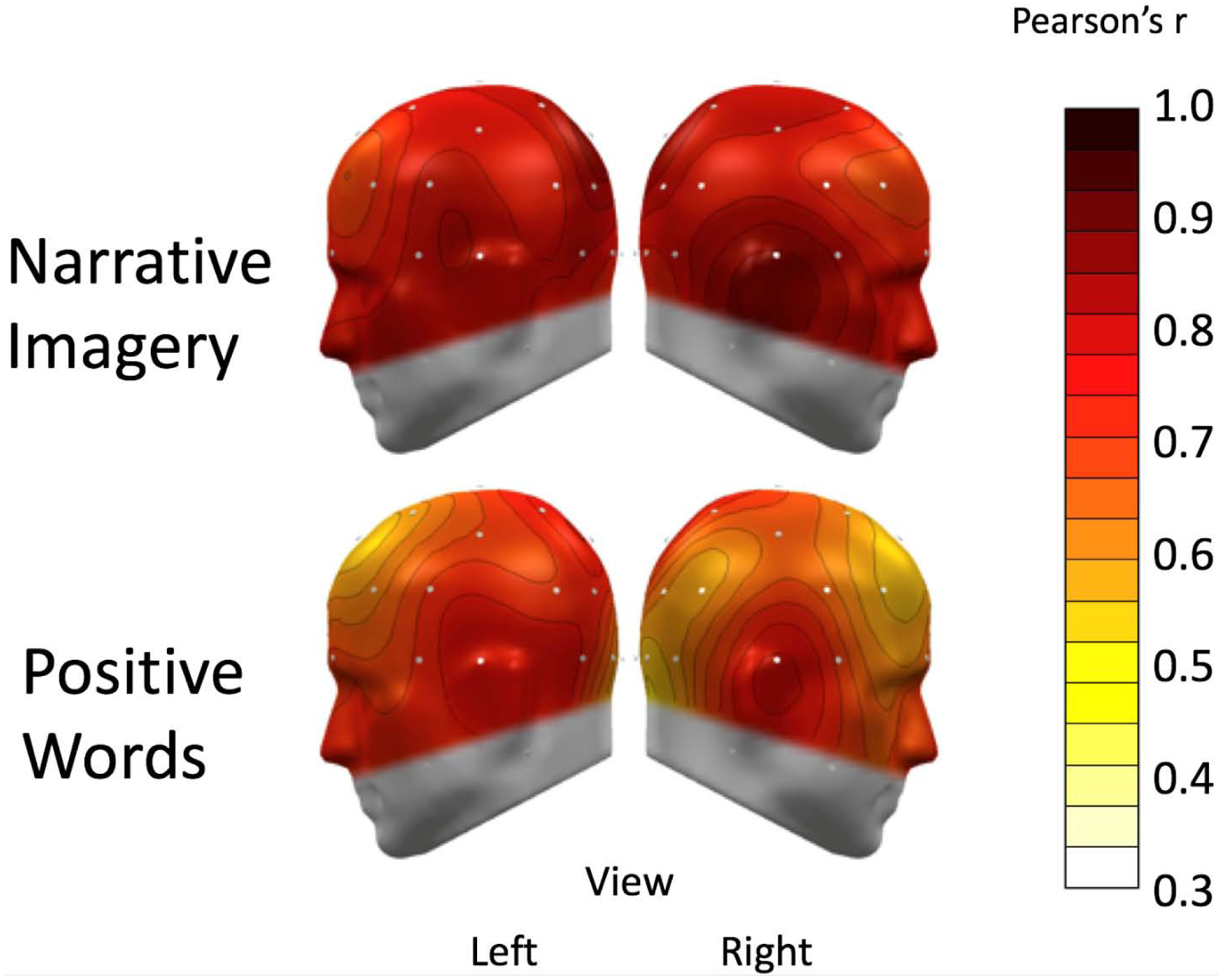
A spline-interpolated topographical distribution of test-retest correlation coefficients of alpha-band power is shown for corresponding conditions across the two sessions. The top panel shows the first block of the pain management activity for the respective sessions, which included relaxation breathing, guided imagery, and positive words (positive words were analyzed separately). White and yellow indicate poor test-retest reliability. Pale orange-to-red indicate moderate-to-excellent reliability, as in the guided-imagery block, which had mostly excellent retest reliability across sensors. The bottom panel shows the topographies for the positive-words block, which showed mostly better-than-moderate reliability across sensors.
3.5. Retest reliability of alpha SNRs averaged across conditions
Table 4 shows Pearson’s correlation coefficients for averaged resting-condition SNRs (averaging resting conditions before and after activity within session), calculated between both sessions. In contrast to individual measurements discussed above, all within-session averages were highly correlated between sessions, and all exceeded reliability for the individual recordings.
Table 4:
Test-retest correlation coefficients and p-values of averaged conditions (pre- and post-activity), calculated for corresponding measurements between the two sessions (diagonal elements shown in bold), and between non-corresponding measures, for reference. Within-session averages of resting alpha signal-to-noise ratios (SNRs) for both eyes closed and eyes open conditions showed moderate-to-excellent retest correlations, while averaged alpha blocking reliability was poor. SNRs were calculated at occipital sensors (averaged between sensors at Pz and Oz). Note that the significance level is only given for descriptive purposes, not to imply hypothesis testing.
| Pearson’s r (p-value) |
First Session | |||
|---|---|---|---|---|
| Resting (closed) |
0.771 <.001 |
0.658 <.001 |
0.440 0.019 |
|
| Resting (open) |
0.483 0.009 |
0.807 <.001 |
−0.099 0.617 |
|
| Resting (block) |
0.618 <.001 |
0.253 0.175 |
0.586 <.001 |
|
3.6. Retest reliability of raw alpha power
As a supplementary analysis, occipital alpha retest reliability was also computed for the raw alpha power of matching conditions for comparison, shown in Table 5.
Table 5:
Test-retest correlation coefficients (in bold) and p-values, calculated for raw alpha-band power measurements (averaged between sensors at Pz and Oz) between only corresponding conditions of the two sessions at occipital sensors. Note that the significance level is only given for descriptive purposes, not to imply hypothesis testing.
| Resting1 (closed) |
Resting 1 (open) |
Resting 1 (block) |
Imagery | Words | Resting 2 (closed) |
Resting 2 (open) |
Resting 2 (block) |
|
|---|---|---|---|---|---|---|---|---|
|
0.636 (<.001) |
0.388 (0.051) |
0.529 (0.005) |
0.862 (<.001) |
0.736 (<.001) |
0.403 (0.063) |
0.877 (<.001) |
−0.054 (0.811) |
4. Discussion
The present study set out to determine the internal consistency and test-retest reliability of oscillatory activity in the alpha-band range, recorded using scalp EEG, in a sample of older adult participants with chronic knee pain. Our results showed that EEG alpha measures were mostly reliable and consistent, particularly when participants’ eyes were closed, toward the end of each session, and when alpha measures were averaged as within-participant estimates.
4.1. Internal consistency of alpha SNRs
Internal consistency of alpha SNRs was strong (Cronbach’s α > 0.7) within each session, with the first EEG session having a higher Cronbach’s α than the last across the whole topography. The overall Cronbach’s α of EEG alpha across both sessions was excellent (> 0.88) at each sensor, with occipital alpha having a Cronbach’s α of 0.9. This suggests that participants with high alpha oscillations amplitudes during one EEG recording tended to also have high alpha during most other recordings as well, relative to other participants. It is notable that the first EEG session had the stronger internal consistency of the two sessions, suggesting that, within a clinical setting, a patient’s data during a single appointment might characterize individual differences in alpha oscillations.
4.2. Retest reliability of alpha SNRs
Test-retest reliability, which quantifies the stability of a measure spread out over the duration of an experimental session, or across multiple sessions, was also assessed (Schmidt et al., 2012). A high Pearson’s r (>0.6) is typically taken to suggest high test-retest reliability between sessions (Schmidt et al., 2012). Retest reliability of alpha SNRs were strongest for eyes closed conditions. Initial eyes open and alpha blocking conditions were the least reliable among the individually matched conditions between sessions. This might suggest that alpha amplitudes after a relaxation/guided imagery activity are more similar between sessions than amplitudes before the activity. These findings were consistent in both EEG alpha calculated at occipital sensors as well as across the whole topography across conditions. This homogeneity of alpha reliability has been observed previously in studies of the general population as well (Gasser et al., 1985).
4.3. Retest reliability of alpha SNRs averaged across conditions
As expected, we found that combining within-session indices further improved re-test reliability across sessions, suggesting that a sequence of two pairs of eyes-open/eyes-closed recordings yields a more stable index of electrocortical reactivity to sensory input.
The same could not be said about averaged open-minus-closed alpha blocking. Although post-activity EEG alpha blocking between the two sessions showed excellent retest reliability (Pearson’s r = 0.837), retest reliability of initial alpha blocking rated poorly, as did within-session reliability in the first session. The difference in alpha power between eyes-open and eyes-closed has been widely regarded as an index of state reactivity (Klimesch et al., 2006; Steriade and Llinás, 1988), stable across the life span (Barry and De Blasio, 2017). The lack of retest reliability for averaged alpha blocking may suggest that participants’ state alertness toward the beginning versus toward the end of a session is substantially variable and not indicative of a stable trait. However, general alertness may become more stable as participants spend more time habituating to the laboratory environment, which may explain the improvement of test-retest reliability of alpha blocking over time: It is possible that the laboratory context and procedures induced a consistent alpha-band oscillatory response over time, which has also previously been observed in the general population in other studies (Papousek and Schulter, 1998). The present study illustrates that having participants engage in a relaxation pain-management activity prior to examining their alpha may provide a more stable and replicable alpha-blocking measure.
4.4. Retest reliability of raw alpha power
An analysis of raw alpha and alpha blocking reliability yielded somewhat poorer results, over all. While alpha and alpha blocking before pain management activity appeared to have marginally better retest reliability, they remained below the threshold of good reliability (Pearson’s r > 0.6) for all but the eyes-closed condition. Interestingly, post-activity alpha measures were generally less reliable for raw power, with alpha blocking being very unstable. It is likely that measurements of alpha that include the overall power of the underlying curve of each frequency spectrum holds very different, and possibly, more variable information, not least of which may be the recording artifacts from movement that are typical in older adult EEG. This population may also be susceptible to greater neural electrophysiological noise (Cremer and Zeef, 1987).
Other studies have found that the variance in power of the overall EEG spectrum may hold information regarding such variables as age-related differences in working memory (Voytek et al., 2015). Computing signal-to-noise ratios for alpha is an approach to investigating reactivity in this population that can be easily implemented in a clinical setting to minimize the contributions of these other factors. The potential utility of quantifying the noise itself in the EEG spectrum should be a topic of future research.
Beyond concerns of the overall power of a spectrum in older versus younger adults, individuals also vary in the relative prominence of alpha in even a low-powered spectrum (Goljahani, 2012). Some individuals do not, as a rule, have a discernable alpha peak to begin with. Therefore, one of the merits of testing the reliability and consistency of alpha SNRs is to quantify to what extent the absence or presence of discernable alpha peaks will persist in the EEG spectra of individuals.
4.5. Peak frequencies and topography of alpha and alpha blocking
Given that the alpha band in older adults tends to be slower than that of the general population (Dustman et al., 1999), we inspected the distribution of peak alpha frequencies (PAFs) in the sample. As a result, we found that all participants showed PAFs within a standard 8 – 12 Hz frequency range. To further evaluate whether this window was appropriate, individual PAF estimates were calculated by defining the center of gravity frequency, which resulted in peaks between 9 and 11 Hz across our sample. However, reliability and consistency of PAFs across participants and conditions is not discussed, given that our frequency resolution (0.33 Hz) was less than the recommended 0.25 Hz resolution for capturing subtle fluctuations in alpha peaks (Klimesch, 1999).
The present report systematically examined alpha suppression, or blocking, following the opening of the eyes. Alpha power is dramatically reduced following sensory events such as the opening of eyes (Adrian and Matthews, 1934; Berger, 1929); the presentation of a visual cue (Cruikshank, 1937; Jasper and Shagass, 1941); or the direction of attention toward a salient visual stimulus (Adrian, 1944; Jasper et al., 1935). These findings have prompted the notion that high-alpha-power states reflect cortical mechanisms of internal processing, or the absence of reactivity to sensory stimulation. By contrast, alpha blocking—the reduction of alpha power—is taken to reflect processes related to active sensory processing, arousal, and effort. In clinical and translational settings, this alpha blocking is most easily induced when participants are instructed to open their eyes after a period of eyes-closed resting. The difference in alpha power between eyes-open and eyes-closed conditions has thus been widely regarded as an index of reactivity, in the case of alpha suppression, or sensory gating in the case of sustained high alpha power/amplitudes when eyes are open (Klimesch et al., 2006). In the present study, at-rest alpha and alpha blocking was deemed most likely to hold information regarding trait reactivity and thus was chosen to investigate the potential for alpha to serve as a biomarker for chronic pain. As expected, alpha and alpha blocking where the most pronounced at Pz and Oz. An average of sensors at these two locations was used as a subset of the analyses.
4.6. Overall implications
Alpha-band oscillation amplitudes tended to be less reliable during eyes-open resting state recordings than during any other condition, which is consistent with other reports of eyes-open retest reliability (John et al., 1983; Burgess and Gruzelier, 1993). However, alpha-band power in all conditions was more reliable after the pain management activity. Of particular interest is that eyes-open alpha before the activity had the poorest retest reliability, which bares the clinical implication that, in future clinical applications, eyes-open alpha may be the least reliable measure during an initial patient visit at a medical facility for this population. Eyes-open alpha after the activity had a Pearson’s r of 0.877 between sessions, which is considered to represent good reliability. In a community sample, it may thus be beneficial for patient visits to include a habituation period before EEG alpha is measured as part of routine clinical assessments.
Chronic pain studies examining EEG alpha have encountered conflicting results with regard to whether alpha-band power correlated with chronic pain diagnosis or severity, and some found that the same participants did not yield the same findings during other EEG recording sessions (reviewed in Pinheiro et al., 2016). There are many reasons why alpha measures may vary, even if most experimental conditions are identical.
Within-subject variation within the alpha range may depend on state reactivity of the individual at a given time. Alpha power tends to increase, for example, as an experimental session progresses, presumably as the participant becomes habituated to the laboratory environment and becomes less attentive to the present task and/or surroundings (Mackworth, 1968). Alpha may also be different for individuals experiencing a current pain episode versus those who are not, due to differences in arousal. Previous research looking at alpha oscillations in conditions of extended periods of capsaicin-induced pain found that peak alpha frequencies (PAFs) during baseline EEG recordings and related change in PAFs during the acute pain condition were predictive of subsequent pain intensity reports (Furman et al., 2018), suggesting that variations in pain detection and alpha may be interrelated. Furthermore, few studies have tested alpha blocking as a measure of chronic pain severity, despite its utility in indexing arousal.
The correlation coefficients for averaged resting eyes-closed and eyes-open alpha were robust. Thus, the greatest potential for using alpha power as a physiological index may lie in utilizing characteristic averaged measures of resting alpha as opposed to individual recordings. It seems that alpha power not only increases over time spent in the lab setting (Benwell et al., 2019), but becomes more consistent within participant.
4.7. Limitations and future directions
The present study would have benefitted from more participants and more, longer EEG trials to investigate other aspects of alpha oscillatory activity, such as peak alpha frequency (PAF), as previously mentioned. An additional limitation of this study is its loose characterization of “chronic-knee-pain patients.” While all participants met criteria for a chronic-knee-pain diagnosis, addressing the frequency, duration, intensity, and total sites of pain symptoms was deemed beyond the scope of this analysis. Therefore, the present findings may not apply to chronic knee pain samples that differ greatly across these dimensions. Further research into this population is necessary to determine whether alpha reliability depends on state or trait pain. For example, a similar study measuring current pain intensity preceding and following EEG would help clarify if alpha could potentially index short-term sensory arousal or long-term sensory adaptation in this population.
However, our findings lend confidence to research seeking to generalize findings regarding alpha measures in pain populations. More generally, physiological markers serve to fill in gaps in our knowledge regarding the phenotypes of different clinical and health psychopathologies (Insel and Cuthbert, 2009; Kotov et al., 2017). In this vein, characterizing physiological data, such as alpha oscillations, within different populations of chronic pain may allow for a more thorough evaluation of these disorders. While some markers may explain underlying mechanisms which these disorders have in common, others may delineate differences between them. For example, cross sectional EEG investigations of children and adults with ADHD have identified systematic differences in EEG alpha power between healthy participants and those diagnosed with ADHD (Koehler et al., 2009). More recent efforts have explored the possibility to personalize and tailor ADHD interventions to individual patients based on their frontal EEG alpha power distribution (Arns, 2012). Future research into similar patient-treatment matching regimens for chronic pain will require robust and reliable measurements of alpha, as demonstrated in the present manuscript.
In the present investigation, we found SNRs derived from alpha power in adults with chronic knee pain to be stable across time. Future studies investigating relationships between averaged EEG alpha power and measures of pain stress and duration in individuals with knee pain may benefit from standardizing alpha-related measures based on our recommendations to maximize the replicability of their results. Such research may develop methods for using EEG in place of more expensive and involved brain imagining techniques to track the neurophysiological changes associated with chronic pain. Alpha measures may also serve as an index of how specific stress factors (i.e., severity, frequency, duration, and prevalence of pain sites; King et al., 2016) contribute to the experience of chronic pain and ultimately inform us as to whether clinical interventions succeed in altering pain-related physiology.
5. Conclusion
The data suggest that alpha-band power in older adults with chronic knee pain is stable when averaged across multiple blocks or sessions. Initially, eyes-open alpha and related eyes closed-minus-open blocking vary greatly. Once participants have habituated to a laboratory environment, their at-rest alpha power is likely to maintain the same levels across multiple conditions and retain the same rank ordering within a data set relative to other participant spectra. These findings are similar to the conclusions reached by studies of alpha in other clinical and healthy populations (Gasser et al., 1985; Salinsky et al., 1991; Corsi-Cabrera et al., 2007). Non-clinical research may find a greater use for state measures of alpha power given experimental conditions to understand basic cognitive processes. However characteristic alpha measures such as the averaged alpha power estimates used in the present study may be most useful in describing individual differences in the development of clinical pain symptoms and in the response to treatment interventions (Davis et al. 2012, de Vries et al. 2013, Graversen et al., 2012). Toward this end, it is recommendable to use averaged spectra of EEG recordings across a session with a habituation period as a potential index for clinical conditions.
Highlights.
We establish the retest reliability and internal consistency of electroencephalographic (EEG) alpha power in chronic knee pain.
Alpha power is reliable and consistent across multiple recordings and EEG sensors in participants with chronic knee pain.
Recommendations are provided for reliable alpha quantification in future investigations of alpha-related measures as pain indices.
Acknowledgements
We thank Felix Bartsch, BS, Steven Garcia, MA, and Carlo Custodero, MD, for assistance with data collection. We also thank Angela Mickle, MS, Ramakrishnan Mani, DAc, BPhty, PGCert, PhD, Maeve Boylan, BA, BS, and Kierstin Riels, BS, for comments that greatly improved the manuscript.
Funding
This work was supported by grants from the National Institute of Mental Health to Dr. A. Keil (R01MH112558) at the University of Florida, and in part by the American Pain Society-Sharon S. Keller Chronic Pain Research Grant, the University of Florida CTSI Pilot Award (NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064), and the University of Florida PRICE-CTSI-IOA Pilot Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflict of interest to report associated with this work.
References
- Adrian ED, & Matthews BH (1934). The Berger rhythm: potential changes from the occipital lobes in man. Brain, 57(4), 355–385. [DOI] [PubMed] [Google Scholar]
- Adrian ED(1944). Brain rhythms Nature, London, 153, 360–362. [Google Scholar]
- Apkarian AV, Hashmi JA, & Baliki MN (2011). Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain, 152(3 Suppl), S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M (2012). EEG-Based Personalized Medicine in ADHD: Individual Alpha Peak Frequency as an Endophenotype Associated with Nonresponse. J Neurother, 16(2), 123–141. 10.1080/10874208.2012.677664 [DOI] [Google Scholar]
- Barry RJ, & De Blasio FM (2017). EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol Psychol, 129, 293–304. 10.1016/j.biopsycho.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Bartsch F, Hamuni G, Miskovic V, Lang PJ, & Keil A (2015). Oscillatory brain activity in the alpha range is modulated by the content of word-prompted mental imagery. Psychophysiology, 52(6), 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CS, London RE, Tagliabue CF, Veniero D, Gross J, Keitel C, & Thut G (2019). Frequency and power of human alpha oscillations drift systematically with time-on-task. NeuroImage, 192, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H (1929). Über das Elektrenkephalogramm des Menschen. Arch Psych Nerv, 87, 527–570. [Google Scholar]
- Bradley MM, & Lang PJ (1999). Affective norms for English words (ANEW): Instruction manual and affective ratings (Vol. 30, No. 1, pp. 25–36). Technical report C-1, the center for research in psychophysiology, University of Florida. [Google Scholar]
- Burgess A, & Gruzelier J (1993). Individual reliability of amplitude distribution in topographical mapping of EEG. Clin Neurophysiol, 86(4), 219–223. 10.1016/0013-4694(93)90101-Z [DOI] [PubMed] [Google Scholar]
- Coppieters I, Meeus M, Kregel J, Caeyenberghs K, De Pauw R, Goubert D, & Cagnie B (2016). Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J Pain Res, 17(9), 949–962. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Galindo-Vilchis L, del-Río-Portilla Y, Arce C, & Ramos-Loyo J (2007). Within-subject reliability and inter-session stability of EEG power and coherent activity in women evaluated monthly over nine months. Clin Neurophysiol, 118, 9–21. [DOI] [PubMed] [Google Scholar]
- Cremer R, & Zeef EJ (1987). What kind of noise increases with age? J Gerontol, 42(5), 515–518. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ (1947). Test “reliability”: Its meaning and determination. Psychometrika, 12(1), 1–16. 10.1007/BF02289289 [DOI] [PubMed] [Google Scholar]
- Cruikshank RM (1937). Human occipital brain potentials as affected by intensity-duration variables of visual stimulation. J Exp Psychol Gen, 21(6), 625. [Google Scholar]
- Davis KD, Racine E, & Collett B Neuroethical issues related to the use of brain imaging: can we and should we use brain imaging as a biomarker to diagnose chronic pain? Pain 2012; 153(8):1555–15559.doi: 10.1016/j.pain.2012.02.037 [DOI] [PubMed] [Google Scholar]
- de Vries M, Wilder-Smith OH, Jongsma ML, van den Broeke EN, Arns M, van Goor H, et al. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J Pain Res 2013; 25(6):815–824. 10.2147/JPR.S50919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durup G, & Fessard A (1935). I. L’électrencéphalogramme de l’homme. Observations psycho-physiologiques relatives à l’action des stimuli visuels et auditifs. Annee Psychol, 36(1), 1–32. [Google Scholar]
- Dustman RE, Shearer DE, & Emmerson RY (1999). Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin Neurophysiol, 110(8), 1399–1409. 10.1016/S1388-2457(99)00102-9 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, & Snyder AC (2011). The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol, 2, 154 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AJ, Meeker TJ, Rietschel JC, Yoo S, Muthulingam J, Prokhorenko M, et al. (2018). Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage, 167, 203–210. [DOI] [PubMed] [Google Scholar]
- Gasser T, Bächer P, & Steinberg H (1985). Test-retest reliability of spectral parameters of the EEG. Clin Neurophysiol, 60, 312–319. [DOI] [PubMed] [Google Scholar]
- Goljahani A, D’Avanzo C, Schiff S, Amodio P, Bisiacchi P, & Sparacino G (2012). A novel method for the determination of the EEG individual alpha frequency. Neuroimage, 60(1), 774–786. [DOI] [PubMed] [Google Scholar]
- Graversen C, Olesen SS, Olesen AE, Steimle K, Farina D, Wilder-Smith OH, et al. (2012). The analgesic effect of pregabalin in patients with chronic pain is reflected by changes in pharmaco‐EEG spectral indices. Br J Clin pharmacol, 73(3), 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilym SE, Filippini N, Douaud G, Carr AJ, & Tracey I (2010). Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: A longitudinal voxel‐based morphometric study. Arthritis Rheum, 62(10), 2930–2940. [DOI] [PubMed] [Google Scholar]
- Insel TR, & Cuthbert BN (2009). Endophenotypes: Bridging genomic complexity and disorder heterogeneity. Biol Psychiatry, 66(11), 988–989. 10.1016/j.biopsych.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Jasper HH, Cruikshank RM, & Howard H (1935). Action currents from the occipital region of the brain in man as affected by variables of attention and external stimulation. Psychol Bull, 32, 565. [Google Scholar]
- Jasper H, & Shagass C (1941). Conscious time judgments related to conditioned time intervals and voluntary control of the alpha rhythm. J Exp Psychol Gen, 28(6), 503 10.1037/h0059201 [DOI] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, & Lisman JE (2002). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex, 12(8), 877–882. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep L, Ahn H, Easton P, Fridman J, & Kaye H (1983). Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog Neurobiol, 21(4), 239–290. 10.1016/0301-0082(83)90014-X [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, & Rockstroh B (2000). Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology, 37(4), 523–532. [PubMed] [Google Scholar]
- King CD, Keil A, & Sibille KT (2016). Chronic pain and perceived stress In: Stress: Concepts, Cognition, Emotion, and Behavior (pp. 413–421). Academic Press. [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NEA, Yonelinas AP, & Doppelmayr M (2006). Oscillatory EEG correlates of episodic trace decay. Cereb Cortex, 16(2), 280–290. [DOI] [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev, 29(2–3), 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci, 16(12), 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S, Lauer P, Schreppel T, Jacob C, Heine M, Boreatti-Hümmer A, et al. (2009). Increased EEG power density in alpha and theta bands in adult ADHD patients. J neural Transm, 116(1), 97–104. [DOI] [PubMed] [Google Scholar]
- Könönen M, & Partanen JV (1993). Blocking of EEG alpha activity during visual performance in healthy adults. A quantitative study. Electroencephalogr Clin Neurophysiol, 87(3), 164–166. 10.1016/0013-4694(93)90122-C [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol, 126(4), 454–477. 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Lang PJ (1980). Self-assessment manikin. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Letzen JE, Boissoneault J, Sevel LS, & Robinson ME (2016). Test-retest reliability of pain-related functional brain connectivity compared to pain self-report. Pain, 157(3), 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser JD (1991). What is chronic pain?. Theor Med, 12(3), 213–225. [DOI] [PubMed] [Google Scholar]
- Mackworth JF (1968). Vigilance, arousal, and habituation. Psychol Rev, 75(4), 308–322. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E (1997). Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol, 26(1), 31–49. 10.1016/S0167-8760(97)00754-X [DOI] [PubMed] [Google Scholar]
- Pollock VE, Schneider LS, & Lyness SA (1991). Reliability of topographic quantitative EEG amplitude in healthy late-middle-aged and elderly subjects. Clin Neurophysiol, 79(1), 20–26. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Howard B, Merkin H, & Hiesiger EM (2011). Evaluation of the pain matrix using EEG source localization: a feasibility study. Pain Med, 12(8), 1241–1248. [DOI] [PubMed] [Google Scholar]
- Papousek I, & Schulter G (1998). Different temporal stability and partial independence of EEG asymmetries from different locations: Implications for laterality research. Int J Neurosci, 93(1–2), 87–100. [DOI] [PubMed] [Google Scholar]
- dos Santos Pinheiro ES, de Queiros FC, Montoya P, Santos CL, do Nascimento MA, Ito CH, et al. (2016). Electroencephalographic patterns in chronic pain: a systematic review of the literature. PloS One, 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyang G, Hildebrandt A, Schmitz F, & Herrmann CS (2020). Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. NeuroImage, 205, 116304. [DOI] [PubMed] [Google Scholar]
- Racine E, & Illes J (2006). Neuroethical Responsibilities. Can J NeurolSci, 33(3), 269–277. doi: 10.1017/S0317167100005138 [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Oken BS, & Morehead L (1991). Test-retest reliability in EEG frequency analysis. Clin Neurophysiol, 79, 382–392. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, & Jeanmonod D Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 2006; 129(Pt1):55–64. doi: 10.1093/brain/awh631 [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Santesso DL, Miskovic V, Mathewson KJ, McCabe RE, Antony MM, & Moscovitch DA (2012). Test–retest reliability of regional electroencephalogram (EEG) and cardiovascular measures in social anxiety disorder (SAD). Int J Psychophysiol, 84(1), 65–73. 10.1016/j.ijpsycho.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Sibille KT, Bartsch F, Reddy D, Fillingim RB, & Keil A (2016). Increasing neuroplasticity to bolster chronic pain treatment: a role for intermittent fasting and glucose administration?. J Pain, 17(3), 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille KT (2019). Optimizing Chronic Pain Treatment with Enhanced Neuroplastic Responsiveness (OPTIMIZE). Clinicaltrials.gov Identification No. NCT02681081. Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT02681081
- Smith NT, Westover CJ, Quinn M, Benthuysen JL, Silver HD, & Sanford TJ (1985). An electroencephalographic comparison of alfentanil with other narcotics and with thiopental. J Clin Monit, 1(4), 236–244. [DOI] [PubMed] [Google Scholar]
- Steriade M, & Llinás RR (1988). The functional states of the thalamus and the associated neuronal interplay. Physiol Rev, 68(3), 649–742. 10.1152/physrev.1988.68.3.649 [DOI] [PubMed] [Google Scholar]
- Thigpen NN, Kappenman ES, & Keil A (2017). Assessing the internal consistency of the event-related potential: An example analysis: Assessing internal consistency of the ERP. Psychophysiology, 54(1), 123–138. 10.1111/psyp.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Dworkin SF, & Le Resche L (1990). Graded chronic pain status: an epidemiologic evaluation. Pain, 40(3), 279–291. [DOI] [PubMed] [Google Scholar]
- Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, & Gazzaley A (2015). Age-related changes in 1/f neural electrophysiological noise. J Neurosci, 35(38), 13257–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]


