Abstract
Objective
Vesicourethral anastomotic stenosis (VUAS) is frequently seen after prostate surgery because of various operative and postoperative factors. In this study, we aimed to present our results of perineoscopic bladder neck reconstruction, which is a new technique of the perineal approach in the treatment of patients with VUAS after prostate cancer surgery.
Material and methods
Sixteen consecutive patients who underwent perineoscopic bladder neck reconstruction in our clinic between July 2017 and March 2019 were included in the study. Demographic characteristics, surgical history, postoperative continence status, and additional treatment requirements were recorded. Perineoscopic surgery is defined as the visualization of the surgical site with instruments used in laparoscopy and the surgeon performing the entire operative procedure through the screen.
Results
The mean number of preoperative endoscopic bladder neck resections of the patients was 7±5.1, with a history of suprapubic cystostomy in 7 (43.7%) and radiotherapy in 5 (31.2%) patients before surgery. The mean surgical time was 126.2±13.1 min. The mean follow-up period was 13.2±6.8 months, and the success rate was 81.25%. During follow-up, two (12.5%) patients received perineoscopic re-do reconstruction because of stricture recurrence, and one (6.2%) patient was included in a urethral dilatation program.
Conclusion
Improving visualization and ergonomics with the perineoscopic approach can increase the success rate of bladder neck reconstruction in comparison with the standard approach. In addition, the lack of need for expanded dissection (corporal separation, inferior pubectomy) reduces postoperative complication rates.
Keywords: Bladder neck reconstruction, perineoscopy, radical prostatectomy, vesicourethral stricture
Introduction
Although radical prostatectomy (RP) is safe and standard operative management for the treatment of prostate cancer (Pca), there is a risk of developing vesicourethral anastomotic stenosis (VUAS) or bladder neck contracture during postoperative period.[1] According to Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) data, the incidence of developing VUAS in localized treatment of PCa is revealed as 5.6% (range, 11%–8.4%).[2] With improvements in surgical techniques and growing experience in recent years, this rate has lowered to about 1%–2.5%.[3] There are numerous reasons for the occurrence of VUAS; these, respectively, include surgeon-related influences (experience), patient-related factors (diabetes, hypertension, smoking, coronary artery disease), surgical technique-related causes (retropubic, laparoscopic, robot-assisted, perineal), perioperative aspects (excessive tension, mismatch of the bladder neck to the urethral mucosa, bleeding, narrow anastomosis) and postoprative components (anastomotic leakage, pelvic hematoma, acute urinary retention).[4] In addition to these causes, pressure and inflammatory process produced by urinoma, hematoma, and lymphocele could damage the anastomosis.[5]
Symptoms are usually observed in patients because of the development of VUAS in the first 6 months following surgery. Obstructive symptoms such as incomplete bladder emptying, weak stream, straining, and hesitancy are the main factors. In the literature, information about the management of VUAS consists of case series and expert opinion, and many treatment algorithms are used.[3] Endoscopic interventions or dilatation techniques are the initial treatment of choice for VUAS because of minimal morbidity and the high likelihood of success. However, if these minimally invasive methods have been tried and have failed, performing repeated surgeries afterward may reduce the success rate of future reconstructive therapies.[6] Therefore, reconstructive surgical methods should be used in selected patients after unsuccessful endoscopic treatments.[7] Vesicourethral reconstruction is commonly performed using a transperineal approach, but different methods have also been described in the literature.[1] The transperineal approach is advantageous in patients with a history of pelvic surgery, abdominal hernia repaired using a mesh, a history of radiotherapy, and a history of multiple abdominal surgeries.
Moreover, because robotic and laparoscopic methods are usually used in abdominal reconstructions, complications related to CO2 insufflation may be observed. However, working in a narrow surgical field during the transperineal approach disrupts the surgeon’s vision and surgical ergonomics. Additionally, the narrow surgical view creates an important limitation with regard to surgeon performance and resident training. In narrow surgical areas or in hard-to-reach areas, surgeons in different specialties use endovision video systems as an alternative to the standard open approach.[8] To eliminate the disadvantages of transperineal approach, in addition to the existing techniques, we have defined a perineoscopic reconstruction technique using a robotic optic system as a novel method for treatment of complex, recalcitrant, vesicourethral anastomotic.[9] This study aimed to present the outcomes of the patients with recurrent vesicourethral anastomotic stricture treated by perineoscopic reconstruction at our institution.
Material and methods
After obtaining institutional review board approval, data from patients who were treated with a perineoscopic approach for recurrent VUAS following RP were analyzed retrospectively from July 2017 to March 2019. The diagnosis of VUAS was made in symptomatic patients with clinical suspicion by identifying stenosis using urethrocystoscopic examinations, retrograde urethrography (RUG), and voiding cystographic. Patients with a history of at least one failed endoscopic treatment, a diagnosis of recurrent stenosis, and persistent symptoms after treatment were included in the study. Patients who refused surgery, whose follow-up period was less than 6 months, and those who could not be followed up regularly were excluded from the study. All procedures were performed in accordance with the ethical standards of the respective committees on human experimentation (institutional and national) and the Helsinki Declaration of 1975 as revised in 2013. Informed consent form for the surgery was obtained from all patients participating in the study after the available techniques for vesicourethral reconstruction were explained. Data collection for the study was made using the hospital patient database system. All surgical interventions included in the study were performed by a single reconstructive specialist at a tertiary university hospital.
Surgical technique
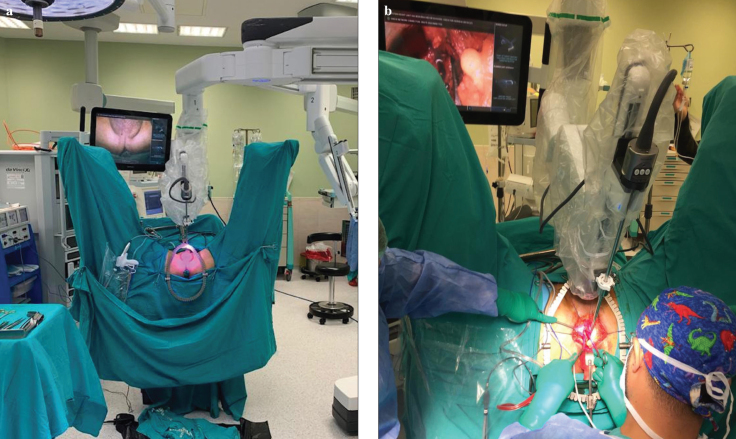
The patient was placed in the exaggerated lithotomy and 15° Trendelenburg position to achieve a more adequate surgical view. The patient was approached from the left side with a Da Vinci Xi-Robotic system (Intuitive Surgical Inc., Sunnyvale, CA, USA), and the first robotic arm was brought to the left side and positioned in the midline of the patient. An overhead monitor was placed on the right side of the patient. All stages of the operation were performed using a 30° up robotic scope with the surgeon coordinating by looking at the screen. All manipulations of the optic system during the surgery were conducted by the surgical registrar or the co-surgeon on the console (Figure 1).
Figure 1. a, b.
The view of the operating room and perinoscopic setup (a). Perineoscopic bladder neck reconstruction being performed (b)
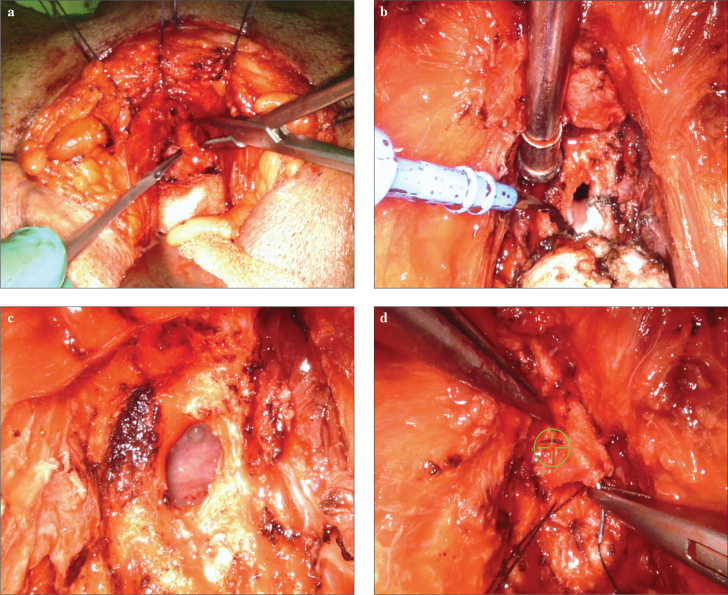
A lambda perineal incision was made, and the bilateral ischiorectal fossa was opened using an index finger after the dissection of the subcutaneous tissue. The anastomotic site was dissected after separating the rectourethral muscles and mobilizing the bulbous urethra. In cases in which insertion of a urethral catheter was not possible, the obliterated bladder neck was identified using a guide wire. The scar tissue at the site of anastomosis was resected to the level of normal tissue structure. Tension-free re-anastomosis between the bulbar urethra and the bladder neck was established using a 3/0 V-Loc ™ (Covidien, Mansfield, MA, USA) continuous suture. After the anastomosis, a 14–16–F urethral and a 16–18–F cystostomy catheters were placed in all patients (Figure 2).
Figure 2.
a–d. Dissection of the dorsel vein (a). Removing of the fibrotic stricture part allowing the exposure of the bladder neck (b). Bladder neck previous to re-anastomosis (c). Running suture with a 3/0 V-Loc to close the bladder neck anastomosis (d)
Operative and follow-up measures
The demographic characteristics of the patients, RP pathology results, cases of receiving adjuvant radiotherapy, number of previous endoscopic treatments, perioperative findings, preoperative and postoperative International Index of Erectile Function-5 questionnaire (IIEF-5) scores, postoperative incontinence status, complications, postsurgery recurrence, and incidents of additional surgical interventions were recorded. Complications were evaluated according to the Satava (intraoperative) and modified Clavien (postoperative) systems.[10,11] Incontinence was defined as the use of >1 pad/day by the patients. Surgical success was monitored by performing anatomic and functional evaluation using a 17-F flexible cystoscope and uroflowmetry. The development of anastomotic stenosis and requirement of new treatment were considered as failure. RUG was obtained between the 2nd and 3rd postoperative weeks in all patients before removal of the catheter, and the status of anastomotic healing was assessed. All patients were followed up for 2–3 weeks postoperative, as well as at the 3rd and 6th months after the surgery.
Statistical analysis
Categorical data that followed a normal distribution were presented as mean±standard deviation (SD). Data that did not follow a normal distribution were presented as median. Some categorical data were presented as percentages. All calculations were performed using the Statistical Package for the Social Sciences version 16.0 (SPSS Inc.; Chicago, IL, USA) software package.
Results
A total of 16 patients with a mean age of 67±7.6 (range, 55–79) years, and mean body mass index of 25.5±4.5 kg/m2 who had undergone perineoscopic reconstruction of VUAS from April 2017 to May 2019 were identified. The approach of RP was open in 12 (75%) patients and robot-assisted in 4 (25%) patients. Before the reconstruction, 5 (31.2%) patients received adjuvant external beam radiation therapy. The mean time period between surgery and diagnosis of VUAS was 39.4±23 (range, 12–84) months. All patients had at least one attempted endoscopic intervention (bladder neck incision) initially to treat the VUAS in different medical centers, with a mean of 7±5.1 (range, 2–14) procedures. Seven (43.7%) patients had a suprapubic catheter before the surgery, and 9 (56.2%) patients were voiding spontaneously.
The mean surgical time was 126.2±13.1 (range, 110–150) minutes, and the mean blood loss was calculated as 169.1±43.3 (range, 90–230) mL. No additional interventions (corporal separation, inferior pubectomy) were required in any patient. The demographic characteristics and perioperative findings of the patients are shown in Table 1.
Table 1.
Demographic characteristics and pre-perioperative findings
| Mean age (years±SD) | 67±7.6 |
|
| |
| Mean BMI (kg/m2±SD) | 25.5±4.5 |
|
| |
| Approach of RP, n (%) | |
| Open | 12 (75) |
| Robot-assisted | 4 (25) |
|
| |
| EBRT history, n (%) | 5 (31.2) |
|
| |
| Number of EI history (n±SD) | 7±5.1 |
|
| |
| Suprapubic catheter, n (%) | 7 (43.7) |
|
| |
| Mean surgical time (min±SD) | 126.2±13.1 |
|
| |
| Mean blood loss (ml±SD) | 169.1±43.3 |
BMI: body mass index; RP: radical prostatectomy; EBRT: external beam radiation therapy; EI: endoscopic intervention
The average length of stay in the hospital was 3.9±1 (range, 3–6) days. Two patients presented with complications at the immediate postoperative follow-up; wound infection developed in one patient who was treated conservatively and a Clavien IIIb complication occurred in the other patient who had a pubovesical fistula. The pubovesical fistula was successfully managed by performing pubic bone debridement, fistula repair, and rectus flap. There were no rectal or ureteral injuries, and no long-term orthopedic issues. With regard to long-term follow-up, the mean follow-up duration after surgery was 13.2±6.8 (range, 6–26) months. The success rate of the reconstruction was 81.25% because two patients received perineoscopic re-do reconstruction because of recurrence of the VUA stricture after failing bladder neck incision interventions, and one patient with anterior urethral stricture was found to have a decline in the maximum flow rate requiring a urethral dilatation program post internal urethrotomy (IU). All patients who underwent reconstruction were completely incontinent during follow-up. Nine (56.2%) patients were managed by placement an artificial urinary sphincter (AUS) and continence was achieved in all cases after this treatment (Table 2). The routine follow-up of all patients, including the those with no problems after additional interventions, were performed in our outpatient clinic.
Table 2.
Postoperative characteristics of patients
| Length of hospitalization (days±SD) | 3.9±1 |
|
| |
| Complications, n (%) | 2 (12.5) |
| Wound infection | 1 (6.75) |
| Pubovesical fistula | 1 (6.75) |
|
| |
| Mean follow-up (months±SD) | 13.2±6.8 |
|
| |
| Success rate of the reconstruction, n (%) | 13 (81.25) |
|
| |
| Additional surgical requirement after operation, n (%) | 3 (18.75) |
| Bladder neck revision | 2 (12.5) |
| Urethrotomy internal | 1 (6.75) |
|
| |
| Placement AUS, n (%) | 9 (56.25) |
AUS: artificial urinary sphincter.
Discussion
VUAS in patients with Pca is a potential complication following RP or radiotherapy.[4] Retropubic, abdomino-perineal or transperineal approaches are described in the open reconstructive treatment of VUAS. The success rate of the transperineal approach is reported as 90%. Compared with the abdominal-perineal approach, the transperineal approach is less invasive and has a lower risk of complications. In addition, the bladder neck and stenotic urethra are easily identified and accessed.[12] However, in this approach, working in a narrow surgical field and having a limited viewing angle may inhibit the surgical skills and ergonomics of the surgeon.
In specialists performing oncologic or reconstructive surgery using the transperineal approach, reports of neck pain, back pain, and chronic fatigue because of continuous overhead work have been increasing in recent years. Endovision systems, flexible cystoscopes, and high-definition) monitors have been developed for this type of surgical approach in different branches der to better define the surgical field and to improve the ergonomics of the surgeon. The use of endovision systems enables the surgeon to work in a neutral position by preventing axial rotation and flexion of the neck and back.[13] In particular, using the magnification setting of robotic technology allows for a wider surgical field, a cleaner definition of tissues, and the ability to work from a sufficient distance. Through the direct view of the transperineal approach, it is difficult for students to determine the anatomy, surgical method, and schematic illustration. The use of a robotic-endovision systems, which provide high resolution magnification, help students and nurses to follow the operation and to complete the learning curve.[8]
The video-assisted perineal approach was first described by Heaton to reduce the obstacles in the transperineal approach and to increase success rates while lowering the incidence of complications.[14] In later years, this method has been demonstrated to be feasible and effective in different studies.[15,16] In our study, the success rate after the first reconstruction was 81.25%, the mean surgical time was 126.2 min, and average estimated blood loss (EBL) was 169.1 mL. In the study of Pfalzgraf et al.[17], the surgical success rate of the open retropubic approach was 60% and the mean surgical time was reported as 140 min. In the study of Schuettfort et al.[18], the success rate of the transperineal approach was 87%, the mean surgical time was 121 minutes, and the average EBL was revealed as 182 mL. In another study where all of abdominal, abdominoperineal, and transperineal approaches were evaluated, the mean success rate was 92%, the mean surgical time was 347 min, and the average EBL was noted as 400 mL.[19] In our study, the success rate was higher, and the surgical time was shorter in comparison with the retropubic approach, and the results were similar to the transperineal approach.
The overall success rates are documented as 60%–80% in the literature. However, in these studies, it is generally stated that additional interventions such as corporeal separation and inferior pubectomy are performed.[20] Notably, an increased need for pubectomy in transabdominal approach causes it to be more invasive than the transperineal approach.[18] The absence of these additional interventions in our study did not increase morbidity, and reduced the surgical time and average EBL. Anatomic dissection of the puboperineal structures, which have a hammock-like arrangement and provide sphincter support by supporting the membranous urethra, and preservation of the bulbar artery during mobilization of the bulbar urethra in the transperineal approach increase postoperative success rates, as well as decreasing morbidity. Reducing blood loss and surgical time also shortens the recovery period of the patient and reduces hospital stay.
In a study that included 35 patients and had the largest number of patients in the literature, the surgical failure rate was reported as 35%.[21] In our study, the surgical failure rate was found as 18.7% after the first operation and no recurrence was observed in any patients during the mean follow-up of 13.2 months after re-do reconstruction and IU. There are studies in the literature where robotic urethroplasty and robotic abdominal VUAS reconstruction have been performed.[1,22] Although not discussed in these studies, having no fee in the perineoscopic approach because of the exclusive use of the robotic system camera does not impose a cost burden on the hospital. In some studies, VUAS surgery and AUS implantation are performed during the same session.[23] In our clinic, we perform AUS implantation in suitable patients in a second session after successful reconstruction surgery because of the risk of infection, stricture recurrence, and the possibility of additional surgical intervention. Continence was achieved in patients undergoing AUS implantation without any complications.
Our study has some limitations that should be taken into consideration. Because of the retrospective nature of our study and the fact that this disease is not very common in the population, the low number of patients and lack of comparison groups may cause bias in our results.
In conclusion, although improving visualization and ergonomics in the perineoscopic approach can increase success rates, not requiring additional surgical procedures reduces morbidity. In this study, the use of an optical imaging system enabled students to learn the procedure more easily and the surgeon to perform the operation more ergonomically. In the future, more accurate results will be obtained with prospective randomized studies comparing our technique with other techniques, as well as evaluating surgical comfort, cost analysis, and students’ learning levels.
Main Points.
In the open reconstructive treatment of VUAS, the transperineal approach has high success rates.
With the help of the endovision systems, perineoscopic approach improves visualization and ergonomics.
Perineoscopy also enables residents and students to be more involved in the cases.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Bakırköy Dr. Sadi Konuk Training and Research Hospital (2019/435)
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.Ş., Y.A., M.G.Y., A.İ.T.; Design - A.Ş., Y.O.D., O.Ö., F.A.A.; Supervision - M.G.Y., F.A.A., A.İ.T.; Resources - Y.O.D.; Materials - M.G.Y.; Data Collection and/or Processing - Y.A., O.Ö.; Analysis and/or Interpretation - Y.O.D., Y.A., M.G.Y.; Literature Search - A.Ş., Y.O.D., Y.A., O.Ö., M.G.Y., F.A.A.; Writing Manuscript - A.Ş., Y.O.D., O.Ö.; Critical Review - Y.O.D., F.A.A., A.İ.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lavollé A, de la Taille A, Chahwan C, Champy CM, Grinholtz D, Hoznek A, et al. Extraperitoneal robot-assisted vesicourethral reconstruction to manage anastomotic stricture following radical prostatectomy. Urology. 2019;133:129–34. doi: 10.1016/j.urology.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Elliott SP, Meng MV, Elkin EP, McAninch JW, Duchane J, Carroll PR, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol. 2007;178:529–34. doi: 10.1016/j.juro.2007.03.126. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Khoury S, Grant A. Evidence-based medicine overview of the main steps for developing and grading guideline recommendations. Prog Urol. 2007;17:681–4. doi: 10.1016/S1166-7087(07)92383-0. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz D, Vicens A, García-Montes F. Vesicourethral anastomotic stricture following radical prostatectomy with or without postoperative radiotherapy. Actas Urol Esp. 2011;35:277–81. doi: 10.1016/j.acuro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe PD, Wang J, Jiao H, Huang Y, Hori K, Moore RB, et al. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;106:1686–94. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 6.LaBossiere JR, Cheung D, Rourke K. Endoscopic treatment of vesicourethral stenosis after radical prostatectomy: outcomes and predictors of success. J Urol. 2016;195:1495–500. doi: 10.1016/j.juro.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Breyer BN, McAninch JW. Management of recalcitrant bladder neck contracture after radical prostatectomy for prostate cancer: endoscopic and open surgery. J Urol. 2011;185:390–1. doi: 10.1016/j.juro.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary DP, Deering-McCarthy E, McGrath D, Walsh D, Coffey JC. Identification of the optimal visual recording system in open abdominal surgery–a prospective observational study. J Vis Commun Med. 2016;39:127–32. doi: 10.1080/17453054.2016.1240584. [DOI] [PubMed] [Google Scholar]
- 9.Taşçı Aİ, Şimşek A, Şam E, Şeker KG, Atar FA, Şahin S. Perineoscopic radical prostatectomy: A novel surgical technique for the treatment of prostate cancer. Turk J Urol. 2020;46:31–6. doi: 10.5152/tud.2019.19052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satava RM. Identification and reduction of surgical error using simulation. Minim Invasive Ther Allied Technol. 2005;14:257–61. doi: 10.1080/13645700500274112. [DOI] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonato A, Gregori A, Lissiani A, Carmignani G. Two-stage transperineal management of posterior urethral strictures or bladder neck contractures associated with urinary incontinence after prostate surgery and endoscopic treatment failures. Eur Urol. 2007;52:1499–504. doi: 10.1016/j.eururo.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 13.Tjiam IM, Goossens RH, Schout BM, Koldewijn EL, Hendrikx AJ, Muijtjens AM, et al. Ergonomics in endourology and laparoscopy: an overview of musculoskeletal problems in urology. J Endourol. 2014;28:605–11. doi: 10.1089/end.2013.0654. [DOI] [PubMed] [Google Scholar]
- 14.Heaton JP. Video-assisted perineal and open surgery. J Urol. 1994;152:923. doi: 10.1016/S0022-5347(17)32611-3. [DOI] [PubMed] [Google Scholar]
- 15.Keller H, Lehmann J, Beier J. Radical perineal prostatectomy and simultaneous extended pelvic lymph node dissection via the same incision. Eur Urol. 2007;52:384–8. doi: 10.1016/j.eururo.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Murakami G. Radical perineal prostatectomy: a novel approach for lymphadenectomy from perineal incision. J Urol. 2003;170:1298–300. doi: 10.1097/01.ju.0000084329.75188.e6. [DOI] [PubMed] [Google Scholar]
- 17.Pfalzgraf D, Beuke M, Isbarn H, Reiss CP, Meyer-Moldenhauer WH, Dahlem R, et al. Open retropubic reanastomosis for highly recurrent and complex bladder neck stenosis. J Urol. 2011;186:1944–7. doi: 10.1016/j.juro.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Schuettfort VM, Dahlem R, Kluth L, Pfalzgraf D, Rosenbaum C, Ludwig T, et al. Transperineal reanastomosis for treatment of highly recurrent anastomotic strictures after radical retropubic prostatectomy: extended follow-up. World J Urol. 2017;35:1885–90. doi: 10.1007/s00345-017-2067-8. [DOI] [PubMed] [Google Scholar]
- 19.Nikolavsky D, Blakely SA, Hadley DA, Knoll P, Windsperger AP, Terlecki RP, et al. Open reconstruction of recurrent vesicourethral anastomotic stricture after radical prostatectomy. Int Urol Nephrol. 2014;46:2147–52. doi: 10.1007/s11255-014-0816-9. [DOI] [PubMed] [Google Scholar]
- 20.Browne BM, Vanni AJ. Management of urethral stricture and bladder neck contracture following primary and salvage treatment of prostate cancer. Curr Urol Rep. 2017;18:76. doi: 10.1007/s11934-017-0729-0. [DOI] [PubMed] [Google Scholar]
- 21.Mundy AR, Andrich DE. Posterior urethral complications of the treatment of prostate cancer. BJU Int. 2012;110:304–25. doi: 10.1111/j.1464-410X.2011.10864.x. [DOI] [PubMed] [Google Scholar]
- 22.Unterberg SH, Patel SH, Fuller TW, Buckley JC. Robotic-assisted proximal perineal urethroplasty: improving visualization and ergonomics. Urology. 2019;125:230–3. doi: 10.1016/j.urology.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Mark S, Pérez LM, Webster GD. Synchronous management of anastomotic contracture and stress urinary incontinence following radical prostatectomy. J Urol. 1994;151:1202–4. doi: 10.1016/S0022-5347(17)35213-8. [DOI] [PubMed] [Google Scholar]