Abstract
In December 2019, a new viral respiratory infection known as coronavirus disease 2019 (COVID-19) was first diagnosed in the city of Wuhan, China. COVID-19 quickly spread across the world, leading the World Health Organization to declare it a pandemic on March 11, 2020. The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a similar virus to those involved in other epidemics such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). Epidemiological studies have shown that COVID-19 frequently affects young adults of reproductive age and that the elderly and patients with chronic disease have high mortality rates. Little is known about the impact of COVID-19 on pregnancy and breastfeeding. Most COVID-19 cases present with mild flu-like symptoms and only require treatment with symptomatic relief medications, whereas other cases with COVID-19 require treatment in an intensive care unit. There is currently no specific effective treatment for COVID-19. A large number of drugs are being used to fight infection by SARS-CoV-2. Experience with this therapeutic arsenal has been gained over the years in the treatment of other viral, autoimmune, parasitic, and bacterial diseases. Importantly, the search for an effective treatment for COVID-19 cannot expose pregnant women infected with SARS-CoV-2 to the potential teratogenic risks of these drugs. Therefore, it is necessary to determine and understand the safety of anti-COVID-19 therapies prior to conception and during pregnancy and breastfeeding.
Key words: COVID-19, SARS-CoV-2, antiviral, pregnancy, breastfeeding
Zusammenfassung
Im Dezember 2019 wurde erstmals eine neue virale Atemwegserkrankung, die den Namen Coronavirus-Erkrankung 2019 (COVID-19) bekam, in der chinesischen Stadt Wuhan diagnostiziert. COVID-19 verbreitete sich rasch über die ganze Welt, was die Weltgesundheitsorganisation veranlasste, am 11. März 2020 eine Pandemie auszurufen. Die neuartige Erkrankung wird von einem Virus aus der Familie der Coronaviridae hervorgerufen und wird SARS-CoV-2 ( s evere a cute r espiratory s yndrome co rono v irus type 2 ) genannt. SARS-CoV-2 trägt ähnliche Merkmale wie Viren, die bei anderen Epidemien als Erreger auftraten, z. B. SARS-CoV (severe acute respiratory syndrome coronavirus) und MERS-CoV (Middle East respiratory syndrome coronavirus). Epidemiologische Studien haben gezeigt, dass COVID-19 oft junge Erwachsene im fortpflanzungsfähigen Alter befällt und dass die Sterberaten bei älteren Menschen und Menschen mit chronischen Erkrankungen hoch sind. Über die Auswirkungen von COVID-19 während der Schwangerschaft und in der Stillzeit ist wenig bekannt. Die meisten Fälle mit COVID-19 haben milde grippeähnliche Symptome und benötigen Medikamente nur, um die Symptome zu bekämpfen. Andere Patienten hingegen müssen auf der Intensivstation behandelt werden. Es gibt derzeit keine spezifische, effektive Behandlung gegen COVID-19. Es werden aber eine ganze Reihe von Medikamenten eingesetzt, um gegen die Infektion mit SARS-CoV-2 zu kämpfen. Die Erfahrungen mit diesem therapeutischen Arsenal wurden über Jahrzehnte hinweg durch ihren Einsatz gegen andere virale, autoimmune, parasitäre und bakterielle Erkrankungen gesammelt. Wichtig ist, dass bei der Suche nach einer effektiven Behandlung gegen COVID-19 keine schwangeren Frauen mit SARS-CoV-2 den möglichen teratogenen Risiken dieser Therapeutika ausgesetzt werden. Es ist daher nötig, die Sicherheit von Anti-COVID-19-Therapien präkonzeptionell sowie während der Schwangerschaft und in der Stillzeit zu bestimmen.
Schlüsselwörter: COVID-19, SARS-CoV-2, antiviral, Schwangerschaft, Stillen
Introduction
In December 2019, the city of Wuhan in Hubei province, China, witnessed the first case of a new respiratory infection that evolved into severe respiratory failure. The disease followed a similar clinical course to other past outbreaks such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which occurred in 2002 and 2012, respectively. The new disease was named coronavirus disease 2019 (COVID-19), and was found to be caused by a new coronavirus – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 1 .
The rapid spread of COVID-19 around the world led the World Health Organization (WHO) to declare it a pandemic on March 11, 2020. In early June 2020, more than 6.3 million cases and more than 380 000 deaths have been registered in 216 countries, with the United States of America (USA) the global epicenter of the disease. The WHO has currently estimated an overall mortality rate of 6% 2 .
Studies suggest that COVID-19 mostly affects men (58.1%) and young adults (55.1% of cases occur in individuals aged between 15 and 49 years) 1 . A prevalence study detected the presence of SARS-CoV-2 (positive RT-PCR nasal swab) in 15.4% (33/215) of pregnant women admitted for delivery in a maternity hospital in New York, USA 3 . In the vast majority of cases, the disease takes a benign course. The mortality rate varies according to age group, the presence of comorbidities, and the city/country. Mortality is higher among men over 65 years of age and in people with chronic disease such as hypertension, diabetes, and obesity. More than half of the diagnosed cases occur in people of reproductive age 1 .
Infection with SARS-CoV-2 can manifest as asymptomatic or mild flu-like symptoms to severe respiratory failure. The understanding of the pathophysiology of this disease is still poor. The clinical manifestations of COVID-19 can be divided into three stages 4 . Stage I is characterized by mild flu-like symptoms (symptoms such as malaise, headache, fever, dry cough, and diarrhea). Patients may only present with these symptoms but, in a small proportion of cases, patients progress to a more severe form of the disease. The recommended treatment at this early stage aims only to provide symptom relief, but some authors suggest already using drugs with antiviral and immunomodulatory actions (chloroquine [CQ], ivermectin, azithromycin, or zinc) to reduce the risk of progressing to more severe forms of disease 5 .
Pulmonary impairment is more evident in the second stage of COVID-19. Patients in this stage of disease develop viral pneumonia and present with tachypnea, cough, and fever with absence (stage IIA) or presence (stage IIB) of hypoxia. Patients in this phase should receive therapy for symptom relief and the most severe cases require oxygen therapy. Despite the lack of evidence and lack of consensus, different therapies with antiviral drugs, anticoagulants, corticosteroids, and adjuvant medications are used in a range of protocols 5 , 6 , 7 .
Stage III is the most severe phase and includes systemic manifestations of hyperinflammation (a mechanism known as a cytokine storm), lung injury, and multiple organ involvement. Studies have shown that inflammatory cytokines and biomarkers such as interleukin (IL)-2, IL-6, IL-7, granulocyte colony-stimulating factor, macrophage inflammatory protein 1-α, tumor necrosis factor-α (TNF-α), C-reactive protein, ferritin, and D-dimer are significantly elevated in patients with the more severe form of disease. Different therapies such as the use of antiviral drugs, high doses of corticosteroids, intravenous human immunoglobulin, inflammatory IL inhibitors, and hyperimmune plasma are being evaluated to treat this stage of disease 8 , 9 .
Traditionally, drugs used during pregnancy and breastfeeding are classified by the Food and Drug Administration (FDA) into five groups (A, B, C, D, and X), according to the risk of teratogenicity and maternal, fetal, and neonatal complications. Category A includes drugs for which adequate and well-controlled studies have failed to show a risk to the fetus in the first trimester of pregnancy (and for which there is no evidence of risk in the later trimesters). Category B are drugs for which animal reproduction studies have failed to demonstrate a risk to the fetus, but for which there are no adequate, well-controlled studies in pregnant women. Category C are drugs for which animal reproduction studies have shown an adverse effect on the fetus and for which there are no adequate, well-controlled studies in humans, but whose potential benefits may warrant the use of the drug in pregnant women despite the potential risks. Category D are drugs for which there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but where the potential benefits may warrant the use of the drug in pregnant women despite the potential risks. Category X are drugs where studies in animals or humans have demonstrated fetal abnormalities, and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience. Additionally, the risks involved in use of the drug in pregnant women clearly outweigh the potential benefits 10 , 11 .
In 2015, to provide further clarification, the FDA replaced the old pregnancy risk caregories with a new system – the Pregnancy and Lactation Labeling Rule. The new rule has three main categories: females and males of reproductive potential, pregnancy (includes labor and delivery), and lactation (includes nursing mothers). Despite the recent change, much of the available literature still uses the old methodology 10 , 11 .
The effects of COVID-19 on female fertility and pregnancy outcomes and the severity of SARS-CoV-2 infection in the different trimesters of pregnancy are still unknown. Based on the literature available at the moment, it can be assumed that the clinical course of COVID-19 may be complicated by pregnancy, but conclusions about a high risk of maternal mortality are not yet definitive 12 , 13 . Women of reproductive age and women who are pregnant or breastfeeding represent an important percentage of patients with COVID-19 who are exposed to different drugs, often without clear evidence regarding their safety for use during pregnancy and breastfeeding. Unfortunately, there are only a small number of ongoing studies investigating the safety of these medications during pregnancy and breastfeeding. Smith et al. noted that 388 (0.1%) trials of the 335 447 trials registered on the ClinicalTrials.gov registry were related to COVID-19 and only 5 (1.3%) trials were related to pregnancy 14 .
The purpose of this review is to assess the status of the current literature on the safety of therapies prescribed to treat COVID-19 during the period prior to conception and during pregnancy and breastfeeding. The authors used the following keywords to search the databases of PubMed/MEDLINE and Scopus and identify the main therapies proposed for the treatment of COVID-19: “treatment”, “therapy”, “COVID-19”, “SARS-CoV-2”. The authors then conducted a literature review into the safety of these therapies during preconception, pregnancy and breastfeeding.
Chloroquine/Hydroxychloroquine
Hans Andersag discovered chloroquine (CQ) in 1934. It has been used since 1947 for the treatment and prophylaxis of malaria and is administered orally. The FDA approved the use of CQ in 1949. Because it is well absorbed (67 – 100% absorption), the peak blood concentration is reached in 30 minutes. Binding to plasma proteins is reported at 40 – 60%. Elimination occurs mainly via urine, half of which is non-metabolized CQ 15 .
Hydroxychloroquine (HCQ) is a racemic mixture of R and S enantiomers. It has fewer side effects than CQ. The FDA approved the use of HCQ in 1955. HCQ also shows good absorption, but its plasma peak is only reached at 3.5 hours and it has a half-life of 22 days in blood. Excretion is predominantly urinary, with about 20% non-metabolized HCQ 15 .
The mechanisms of actions of CQ/HCQ are not yet fully understood. An in vitro study revealed that CQ is effective at both the entry and post-entry stages of SARS-COV-2 infection in Vero E6 cells 16 . CQ/HCQ increases the pH of the endosomes and modifies the glycosylation of angiotensin-converting enzyme 2 ([ACE2], SARS-CoV-2 receptor) during the initial stage of infection. These mechanisms justify the use of CQ/HCQ in the treatment of COVID-19. CQ/HCQ also have immunomodulatory and anti-inflammatory effects, which promote downregulation of the Th17 response and decrease the levels of inflammatory ILs (IL-6, IL-17, and IL-22). This action may reduce the cytokine storm occurring with severe forms of COVID-19 ( Fig. 1 ) 15 . The results of the first studies that evaluated the isolated use of CQ/HCQ or in association with azithromycin and/or zinc are being published; however, its effectiveness is still questioned. Recently, Mehra et al. reported that the use of CQ or HCQ with or without a macrolide for the treatment of COVID-19 was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias 17 .
Fig. 1.
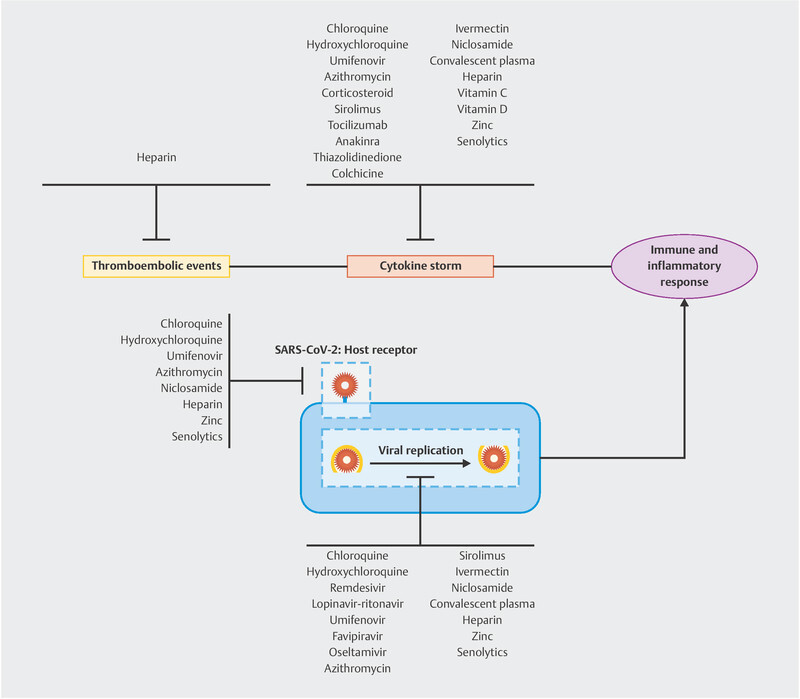
Mechanisms of action of COVID-19 treatment drugs.
Studies on the use of CQ/HCQ in preconception, pregnancy, and breastfeeding are still limited. The drug crosses the placenta and is excreted in small amounts in breast milk. Theoretically, CQ/HCQ presents a risk of damaging the central nervous system via ototoxicity (auditory and vestibular toxicity, congenital deafness), retinal hemorrhages, and abnormal retinal pigmentation to the fetus. Human pregnancies resulting in live births have been reported in the literature, and no increase in the rate of birth defects has been demonstrated. In animal studies, high doses of CQ/HCQ caused embryonic death and eye malformations. CQ/HCQ are excreted in small amounts (approximately 2% of the maternal dose after bodyweight correction) in human breast milk. The use of CQ/HCQ during breastfeeding is accompanied by a potential risk, despite the small amounts excreted in human breast milk 18 .
Observational studies have shown no harmful effects on the fetus when CQ/HCQ are used in the recommended doses for the prophylaxis of malaria and rheumatologic diseases. The use of CQ/HCQ during breastfeeding also appears safe and presents no risk of visual complications or abnormal neurodevelopmental outcome. Some specialists suggest that the use of CQ/HCQ is compatible or presents a low risk during pregnancy and breastfeeding ( Table 1 ) 19 , 20 .
Table 1 Drugs used to treat COVID-19 and recommendations on their use during pregnancy and breastfeeding.
| Drug | FDA category | Transplacental passage | Recommendations | Presence in breast milk | Recommendations |
|---|---|---|---|---|---|
| CM: congenital malformations; MR: miscarriage. FDA pregnancy risk categories: A: well-controlled human studies show no fetal risk; B: animal studies show fetal risk not confirmed by human studies or animal studies do not show fetal risk; C: well-controlled human studies are lacking and animal studies are unavailable or show adverse effects to the fetus; D: human studies or investigational or post-marketing data show fetal risk; benefits may be acceptable despite potential risks; X: animal/human studies or investigational or post-marketing data show fetal risk which clearly outweighs any possible benefit. Notes: a American College of Rheumatology (ACR) strongly recommends as compatible but American College of Obstetrics and Gynecology (ACOG), British Rheumatology Society (BRS), and the European League Against Rheumatism (EULAR) consider low risk is present in pregnancy. b Based on observational studies. c Some authors warn of the risk of cleft lip with or without cleft palate. Systemic corticosteroids are considered category D when used in the first trimester and category C in the second and third trimesters. d ACR considers it compatible for use in pregnancy; ACOG, BRS and EULAR consider low risk is present in pregnancy. e ACR considers it compatible for use in pregnancy. Vitamin C and D, zinc and quercetin are safe when ingested in the doses usually recommended. | |||||
| Chloroquine/ hydroxychloroquine | C | Crosses the placenta | Safe for use in pregnancy a | Present in breast milk No data on infant serum levels |
Compatible with breast feeding |
| Remdesivir | B | Crosses the placenta | No adverse effect on embryofetal development in animals Under investigation in humans |
Present in breast milk in animals No data in humans |
Under investigation |
| Lopinavir-ritonavir | C | Crosses the placenta | No increased rate of CM or MR Safe for use in pregnancy |
Present in breast milk | Compatible with breast feeding |
| Umifenovir | No data | No data | No data | No data | No data |
| Favipiravir | No data | No data | No data | No data | No data |
| Oseltamivir | C | Crosses the placenta | No increased rate of CM or MR Safe for use in pregnancy b |
Present in breast milk | Compatible with breast feeding a |
| Azithromycin | B | Crosses the placenta | Safe for use in pregnancy | Present in breast milk | Compatible with breast feeding |
| Corticosteroids | C/D | Cross the placenta | No increased rate of CM or MR Safe for use in pregnancy c |
Present in breast milk | Compatible with breast feeding a |
| Sirolimus | C | Crosses the placenta | Discontinue during pregnancy | Present in breast milk in animals No data in humans |
Discontinue nursing or discontinue the drug |
| Tocilizumab | C | Crosses the placenta | Discontinue during pregnancy d | Unknown if secreted into breast milk. However, IgG is excreted in human milk. | Discontinue nursing or discontinue the drug |
| Anakinra | B | Crosses the placenta | Use if there are no other options e | No data | Should be avoided |
| Thiazolidinedione | C | Crosses the placenta | Discontinue at pregnancy | Present in breast milk in animals No data in humans |
Discontinue nursing or discontinue the drug |
| Colchicine | C | Crosses the placenta | No increased rate of CM or MR Safe for use in pregnancy |
Present in breast milk | Compatible with breast feeding |
| Ivermectin | C | Crosses the placenta | Discontinue at pregnancy | Present in breast milk in animals No data in human |
Discontinue nursing or discontinue the drug |
| Niclosamide | B | Crosses the placenta | Safe for use in pregnancy | No data | Compatible with breast feeding |
| Hyperimmune plasma | No data | No data | No data | No data | No data |
| Heparin | B | Does not cross the placenta | Safe for use in pregnancy | Absent in breast milk | Compatible with breast feeding |
| Vitamin C | A | Crosses the placenta | Safe for use in pregnancy | Present in breast milk | Compatible with breast feeding |
| Vitamin D | A | Crosses the placenta | Safe for use in pregnancy | Present in breast milk | Compatible with breast feeding |
| Zinc | A | Crosses the placenta | Safe for use in pregnancy | Present in breast milk | Compatible with breast feeding |
| Quercetin | B | Crosses the placenta | Safe for use in pregnancy | Present in breast milk | Compatible with breast feeding |
Remdesivir
Remdesivir is a drug that was developed in 2016 with the aim of fighting the Ebola virus. In 2017, new studies tested the use of remdesivir against SARS-CoV-1 and MERS-CoV in vitro in rats and non-human primates and showed prophylactic and therapeutic action 21 , 22 . In vitro studies revealed that remdesivir is effective at the post-entry stages of SARS-COV-2 infection in Vero E6 cells 16 . This drug is an antiviral analog of adenosine, which is incorporated into RNA polymerase chains when it is metabolized within cells, thereby preventing replication of the virus ( Fig. 1 ) 23 . Its side effects are not yet well known; however, nausea, vomiting, diarrhea, abdominal pain, headache, anemia, and muscle and joint pain were observed as the most common effects, with the same side effects reported for other antivirals in patients with COVID-19 24 .
Grein et al. conducted their study in hospitalized patients with the severe form of COVID-19. The majority of these patients receiving invasive ventilation showed a clinical improvement with the use of remdesivir in relation to their need for oxygen support, especially the group of patients who were receiving non-invasive treatment 23 . In addition, a randomized clinical trial with a larger sample is under development by the National Institute of Allergy and Infectious Diseases, and there have been promising preliminary results regarding the use of the drug, such as reducing the length of hospital stay 25 . Based on this observation, in May 2020, the FDA authorized the emergency use of remdesivir in critically ill hospitalized patients with suspected or confirmed COVID-19 infection in the USA.
The use of remdesivir in pregnant and lactating women has been poorly studied so far. Animal studies demonstrated no adverse effects on embryofetal development. Remdesivir should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and fetus. There is no information regarding the presence of remdesivir in human milk, the effects on the breastfed infant, or the effects on milk production. Some animal studies have detected remdesivir and metabolites in the nursing pups of mothers administered remdesivir ( Table 1 ) 26 . However, it was observed that the drug was shown to be safe in pregnant patients with Ebola, without significant adverse effects 27 .
Lopinavir–ritonavir
The FDA approved the use of lopinavir–ritonavir (LPV/r) in 2000 to assist in the treatment against the HIV virus 28 . Subsequently, some studies have evaluated the use of LPV/r against SARS-CoV and MERS-CoV. Most of these studies did not show satisfactory results with regard to reducing viral replication or any clinical improvement 29 , 30 . However, other studies have shown in vitro and in vivo activity of LPV/r when it was combined with other drugs (ribavirin and interferon [IFN] alfa or beta) in combating these betacoronaviruses 30 , 31 , 32 .
LPV/r are antiretroviral protease inhibitors of the HIV type 1 virus and are known to be effective in treating this infection ( Fig. 1 ) 33 . Lopinavir was observed to have a potentiated effect when it was combined with low doses of ritonavir. It inhibits cytochrome P450 and decreases the hepatic metabolism of lopinavir and increases its half-life in the body 33 . Therefore, these drugs are used only in combination with other drugs. The main side effects of LPV/r are nausea, vomiting, diarrhea, and abdominal pain and, in rarer cases, pancreatitis, hepatotoxicity, and abnormalities in cardiac conduction 9 .
Studies on the use of this medication to treat COVID-19 are still scarce. A randomized controlled trial (RCT) evaluated the action of LPV/r in 199 critically ill patients hospitalized with COVID-19 for 14 days and concluded that this medicine offered no benefits compared to the control group 34 . However, medication was started 13 days after the onset of symptoms, and antivirals are more effective in the first days of disease. The secondary results of this study showed that the group that received LPV/r had a shorter stay in the intensive care unit (ICU) and a lower mortality than the control group 34 . Other clinical trials are evaluating whether LPV/r combined with other drugs has a potentially synergistic effect against SARS-CoV-2 33 , 35 .
The FDA classified the use of LPV/r in pregnancy as category C, thus showing that there are not enough studies in women, and that animal tests had results that justify possible adverse effects during pregnancy 28 . Some studies have also shown that the administration of LPV/r to pregnant women resulted in similar rates of fetal malformations and obstetric complications as those reported for pregnant women who did not use the medication ( Table 1 ) 28 . The use of this drug by pregnant women with respiratory infections has not yet been clarified, but it seems to be safe, given that the few studies on its use in pregnant women with HIV have not associated it with an increased risk of congenital abnormalities or significant perinatal adverse events 36 . Thus, LPV/r is a therapeutic option in the fight against COVID-19 in pregnant women 9 . As for breastfeeding, it was observed that LPV/r is present in human breast milk in much lower concentrations than in the motherʼs plasma. Analysis of plasma concentrations in the baby was unable to detect the substance and indicating that absorption or transport of the drug to the child is insignificant 37 .
Umifenovir
Umifenovir (brand name Arbidol) is an antiviral that was developed in 1988 and used in Russia and China for the prophylaxis and treatment of influenza A or B, oseltamivir-resistant viruses, and also against other viruses that cause flu symptoms. It can be used in the acute stages of infections of the upper respiratory tract areas to attenuate symptoms, reduce lung injury induced by the influenza virus and the duration of disease, and it has a positive impact in reducing mortality. Umifenovir has demonstrated antiviral activity against Ebola, Zika virus, West Nile virus, Chikungunya virus, human herpesvirus 8, hepatitis C virus, Tacaribe arenavirus, SARS-CoV-1, and SARS-CoV-2 35 , 38 , 39 .
Umifenovir works by inhibiting virus-cell membrane and virus-endosome bonds. This action makes this medicine a direct-acting antiviral as it prevents entry of the virus to the target cell, thereby protecting the target cell from infection. In addition, umifenovir has immunomodulatory properties. It stimulates the humoral immune response and modulates the action of cytokines, further suppressing the transcription of cytokines induced by viral infection, preventing the damage caused by macrophages in lung tissue, and containing the inflammatory process ( Fig. 1 ) 38 , 39 .
There have been conflicting results regarding the use of umifenovir in the treatment of COVID-19. In a retrospective study with 81 patients positive for SARS-CoV-2 (45 in the umifenovir group and 36 in the control group), Lian et al. observed that umifenovir did not improve the prognosis or accelerate the disappearance of the virus, and the duration of hospitalization was higher in the treated group (13 vs. 11 days) 40 . In a retrospective cohort, Deng et al. compared the isolated use of LPV/r with the use of umifenovir and observed that viral clearance and pulmonary tomographic aspects showed better results in the LPV/r plus umifenovir group 41 . In contrast, Zhu et al. concluded that umifenovir monotherapy was better than LPV/r monotherapy when assessing the viral load in patients with COVID-19 (patients in the LPV/r group had a higher viral load after 14 days of treatment) 42 .
There are no well-controlled studies on the use of umifenovir during pregnancy. The use of umifenovir during pregnancy is contraindicated. It is not known for certain whether the metabolites generated are able to enter breast milk. If it is necessary to use umifenovir during this period, it is recommended to stop breastfeeding ( Table 1 ) 43 .
Favipiravir
Favipiravir is an antiviral, nucleoside analog that acts by inhibiting the RNA-dependent RNA polymerase of the influenza virus ( Fig. 1 ). This medication first appeared in Japan in 2014 as a new therapeutic option for influenza because viruses were showing increasing resistance to oseltamivir 8 , 44 . It has a broad-spectrum effect against various types of influenza viruses and has been used against other viral families, in addition to being effective against the Ebola virus, where its use was related to a longer survival of patients 45 . Favipiravir can be used in combination with other antiviral medications and exhibits a synergistic effect. Favipiravir is considered a potential drug against COVID-19. However, the available studies do not provide sufficient evidence yet to recommend the routine use of favipiravir for the treatment of SARS-CoV-2 infection 8 .
In a multicenter RCT study involving patients with COVID-19, Chen et al. divided the patients into two groups: 120 patients used favipiravir, whereas the other 120 used umifenovir. Both these groups achieved similar significant recovery rates (between 55 and 61%); however, patients using favipiravir showed improvement in fever and cough earlier compared to the other group 46 . Cai et al. observed an improvement in the radiological aspects of patients with COVID-19 who used favipiravir compared to a group that used LPV/r. Both groups additionally received IFN-α by aerosol inhalation 47 . Although the study is a non-RCT, its findings should not be disregarded because the optimistic results require reproduction and a higher level of scientific evidence.
The data is insufficient to determine whether favipiravir is safe to use during pregnancy. According to the Drugs and Lactation Database, there is still insufficient data on the use of favipiravir during breastfeeding or its excretion in human breast milk. Therefore, serum monitoring of this compound in infants is recommended ( Table 1 ) 48 .
Oseltamivir
The FDA approved the use of oseltamivir in 1999. Since then, it has proven its effectiveness in the treatment and prophylaxis of influenza A and B. It has been shown to be beneficial mainly in patients with uncomplicated influenza virus infections, including pregnant women, and in the first two days of symptoms. However, according to the Centers for Disease Control and Prevention and the WHO, its use is also recommended for critically ill, hospitalized patients 49 . It is a potent antiviral which acts by inhibiting the neuraminidase enzyme, preventing the virus from replicating ( Fig. 1 ) 50 . The most commonly reported side effects of this drug are gastrointestinal, i.e. nausea and vomiting, as well as headache 43 .
The use of this medication against SARS-CoV-2 infection has not been proven effective in in vitro studies and clinical trials 43 , 51 . In some countries, the COVID-19 pandemic began in the peak seasonal influenza period, and some patients were therefore treated with oseltamivir due to the epidemiological context and the delay in diagnosing SARS-CoV-2 infection 52 . A study conducted in Wuhan, China, found that some patients were co-infected with SARS-CoV-2 and the influenza A or B virus, further justifying the use of oseltamivir 53 . After influenza had been ruled out, it was not found that the drug played any role in the management of COVID-19 9 . Some trials on the use of oseltamivir in combination with other drugs (CQ/HCQ and favipiravir) are still being developed, with the aim of showing a possible therapeutic response against SARS-CoV-2 disease 8 .
Animal studies, as well as observational studies in pregnant women, have not shown that teratogenicity is associated with the use of oseltamivir. However, pregnant women are at a higher risk of clinical and obstetric complications due to influenza infection, which can complicate pregnancy outcomes. In lactating rats, oseltamivir is excreted in milk. There are no reliable data on the exposure of nursing infants to oseltamivir ( Table 1 ) 54 , 55 .
Azithromycin
Azithromycin is a macrolide antibiotic that is used to treat respiratory, skin, and soft-tissue infections, along with some sexually transmitted infections. In addition to its antibacterial action, studies have shown that azithromycin has antiviral, immunomodulatory, and anti-inflammatory properties. Azithromycin has already been used to fight viral outbreaks, such as influenza (H1N1), Ebola, dengue, and Zika viruses. Recently, some in vitro studies also found that azithromycin can be effective against SARS-CoV-2 56 , 57 .
There is still a poor understanding of the mechanisms behind the antiviral properties of azithromycin. The drug appears to elevate cellular pH and make it more difficult for the virus to interact with ACE2 receptors, which contributes to blocking the virusʼ entry into the host cell. Another presumed antiviral effect is that it amplifies the production of IFN by host cells. Various mechanisms of action including inhibition of neutrophilic inflammation and macrophage activation, and a reduction in the plasma levels of IL-8, IL-6, IL-1β, and TNF-α contribute to the immunomodulatory and anti-inflammatory properties of macrolides ( Fig. 1 ) 57 , 58 .
Azithromycin, used alone or in combination with other drugs (CQ/HCQ), has been a therapeutic option since the beginning of the COVID-19 pandemic, although the results so far have been conflicting. Azithromycin should be used with caution due to the risk of cardiac complications (e.g., prolongation of the QT interval), an adverse effect also observed for CQ/HCQ 56 , 59 .
The clinical use of azithromycin during pregnancy and breastfeeding appears to be safe (FDA classified it as category B). Among the range of macrolide options, azithromycin appears to be the safest for use in pregnancy. In rats and mice, azithromycin administered at a dose of two to four times the human dose was not associated with any evidence of fetal harm. Observational studies in humans have also not shown a high risk of teratogenicity or obstetric complications associated with the use of azithromycin during pregnancy. Azithromycin is detected in human breast milk and may remain present for 48 hours following the last dose. Although it is generally safe to use while breastfeeding, azithromycin may cause diarrhea, vomiting, or a rash in some babies ( Table 1 ) 60 .
Corticosteroids
The cortisone molecule was isolated in the 1940s. Since then, natural and synthetic corticosteroids have been used to treat numerous diseases because of their anti-inflammatory and immunosuppressive actions. The main objective of using corticosteroids in cases with COVID-19 is to reduce the inflammatory process observed in stages IIB and III of the disease ( Fig. 1 ) 61 , 62 .
To date, the impact of using corticosteroids in COVID-19 has been controversial. Despite the good theoretical foundation, previous studies on the SARS and MERS outbreaks reported an association between corticosteroids and improved survival but demonstrated an association with delayed viral clearance from the respiratory tract and blood, along with high complication rates including hyperglycemia, psychosis, and avascular necrosis 8 , 61 . Other authors have reported a reduction in the mortality rate of patients with the severe form of COVID-19 treated with methylprednisolone 63 . Therefore, it is necessary to understand the role of corticosteroids in COVID-19 therapy (cytokine storm control or comorbidity management), in addition to determining the dose (low dose versus high dose) and the start of treatment (early versus late stage) 8 .Observational studies have reported that in the early stages of COVID-19 corticosteroid therapy did not show any benefits with regard to evolution of the disease. The WHO recommended that systemic corticosteroids should not be routinely administered to patients with COVID-19. There seems to be a benefit from using high doses of corticosteroids in stages II and III of the disease as an attempt to block an exaggerated inflammatory response 64 .
Despite some conflicting results, animal and human studies have found that the use of corticosteroids in the first trimester of pregnancy increased the risk of the newborn having cleft lip with or without a cleft palate (the rate increased from 1.7 to 3 – 5 in 1000 live births). The systemic and prolonged use of corticosteroids during pregnancy appears to be an independent risk factor for preterm birth, low birth weight, or preeclampsia. There are insufficient data to prove the association between systemic use of corticosteroids and gestational diabetes mellitus. Corticosteroids are excreted in small amounts in human breast milk. High doses of corticosteroids can lead to side effects in the newborn ( Table 1 ) 65 .
Sirolimus
Sirolimus, also referred as rapamycin, is a substance extracted from the bacterium Streptomyces hygroscopicus. The European Medicines Agency have authorized the use of this immunosuppressive drug since 2001. Sirolimus blocks the protein known as the mammalian target of rapamycin through intracellular processes. The immunosuppressive effects of sirolimus result from its inhibition of T- and B-cell activities. Sirolimus inhibits several ILs from the T-cell immune response (IL-2, IL-4, IL-12, IL-7, and IL-15) ( Fig. 1 ) 66 . Currently, the drug is used to prevent rejection in transplant patients and treat lymphangioleiomyomatosis 8 .
This drug also has an antiviral effect on viral mechanisms of action; it has been shown to inhibit HIV-1 replication and has an inhibitory effect on MERS-CoV activity, as reported in an in vitro experiment 8 , 67 , 68 . Studies with patients infected with H1N1 and on ventilatory support showed clinical improvement after using sirolimus, a fact suggesting that the drug may be beneficial in the treatment of COVID-19 8 . Sirolimus exerts a senolytic effect while inhibiting the inflammatory process generated by senescent cells (senescence-associated secretory phenotype [SASP]) ( Fig. 1 ). Sirolimus can interact with other drugs also used in the treatment of COVID-19 (clarithromycin, ritonavir, indinavir), thus requiring dose adjustment or drug change 66 , 69 .
Animal studies have shown embryotoxicity in the period of organogenesis and at doses similar to those used in humans. There are no human studies that guarantee safe use of the drug. Therefore, the drug is contraindicated during pregnancy, and women of reproductive age should be advised to use a highly effective contraceptive method before, during, and after treatment with sirolimus. The excretion of the drug in human breast milk and the drugʼs pharmacokinetics in children are not well defined; thus, breastfeeding should be avoided or suspension of the drug is recommended while evaluating the importance of the drug for the mother 70 . The National Transplantation Pregnancy Registry have not reported any structural defects following early pregnancy sirolimus exposure in a limited number of evaluated recipients ( Table 1 ) 71 .
Tocilizumab
Tocilizumab (TCZ) is a monoclonal antibody that inhibits IL-6, which is otherwise responsible for an intense inflammatory and autoimmune response. This drug is currently used to treat rheumatoid arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome (CRS), which can be observed in patients using chimeric antibody receptor-engineered T-cell anticancer therapy 8 , 72 .
CRS mediated by an intense inflammatory state with a large production of cytokines is currently observed in patients with severe SARS-CoV-2 infection; this can evolve into multiple organ dysfunction and death. IL-6 is one of the cytokines most strongly involved in the development of CRS in patients with COVID-19. This suggests that using TCZ could be beneficial because it inhibits IL-6, which could prevent worsening of the inflammatory cascade ( Fig. 1 ) 73 . A study conducted in a hospital in Wuhan, China, reported that the use of TCZ was beneficial in treating and preventing CRS in patients diagnosed with COVID-19 with high rates of IL-6 72 .
There are few studies that have evaluated the safety of TCZ during pregnancy and breastfeeding. A study in monkeys demonstrated a higher number of miscarriage/embryofetal deaths following high doses of TCZ. Data from the Roche Global Safety Database up until December 31, 2014 showed no indications of a substantially increased risk of malformation 74 . Due to the lack of studies and documents on the use of this drug during pregnancy or its repercussions in human breast milk, its use is not recommended during pregnancy and breastfeeding. It is not known whether TCZ is excreted in human breast milk; however, it is believed that it may be present in milk as it is an IgG antibody. Therefore, breastfeeding is contraindicated in users of this medicinal drug ( Table 1 ) 19 , 20 .
Anakinra
Anakinra is an anti-IL receptor antagonist drug belonging to a class of biological disease-modifying anti-rheumatic drugs which have been used since the 1990s to treat autoimmune diseases. Approved in the USA and Europe in 2001 and 2002, respectively, anakinra aims to block the proinflammatory activity of cytokines IL-1α and IL-1β, thereby reducing the inflammatory process and symptoms as well as decreasing the future systemic damage caused by inflammation ( Fig. 1 ) 8 . It is currently used to treat autoimmune conditions, such as adult Stillʼs disease and systemic juvenile idiopathic arthritis, as well as for the off-label treatment of hyperinflammatory conditions, such as macrophage activation syndrome 19 . The use of anakinra was already proposed in other previous viral outbreaks, such as outbreaks of Ebola and Zika viruses 75 , 76 .
In the face of severe inflammation found in some patients with COVID-19, studies have shown that high doses of anakinra can be effective to treat this condition because the drug blocks the action of inflammatory cytokines, in addition to being safe and having a short life, which favors its discontinuation if necessary. This is as an important factor in critically ill patients. A study conducted in Italy reported that the use of high doses of intravenous anakinra (5 mg/kg, twice daily) in patients with COVID-19 and with SARS led to an improvement in respiratory function in patients and a reduced need for orotracheal intubation 77 .
Animal studies conducted with high doses of anakinra (15 – 30-fold greater than the usual dose in humans) found no negative impact on fertility or risk to the fetus. Despite the lack of well-controlled studies in humans, the evidence does not indicate an increase in the rate of congenital deformities; therefore, the use of anakinra before and during pregnancy can be considered safe if there are no other possible options. Its use in the abovementioned conditions is being investigated in further studies. The American College of Rheumatology considers the use of anakinra to be compatible with pregnancy. However, breastfeeding should be discouraged during the use of this drug, as there are no adequate studies to ensure its safety ( Table 1 ) 19 , 20 .
Thiazolidinedione
Thiazolidinedione (TZD) is an oral hypoglycemic agent that is used to treat patients with type 2 diabetes mellitus because of its ability to increase insulin sensitivity. Drugs in this class include rosiglitazone and pioglitazone. A third drug, troglitazone, was also in this class but was withdrawn from the market because of its hepatotoxic effect. Additional studies have demonstrated an association between bone fractures and bladder cancer with the use of pioglitazone, and cardiovascular effects are reported to be related to the administration of rosiglitazone 78 .
TZD is an agonist of peroxisome proliferator-activated receptors (PPARs), which include the subtypes PPARα, PPARβ, and PPARγ. TZD acts specifically on the PPARγ subtype, mainly observed in adipose tissue, by causing adipogenesis and increasing peripheral sensitivity in the skeletal muscle and liver. This action results in a reduction of endogenous glucose production and postprandial gluconeogenesis, an increase in fasting and postprandial glucose clearance, and a reduction in serum glucose and insulin levels. In addition, some studies have also observed changes in B-cell function, cholesterol levels, triglyceride levels, and inflammation levels 79 .
It is important to note that insulin resistance amplifies the inflammatory status. Thus, because of the direct action of TZD on the reduction of insulin resistance, its anti-inflammatory activity was noted in connection with suppression of the production and action of inflammatory cytokines such as TNF-α, IL-1β, IL-2 IL-6, and IL-8. It was found that pioglitazone can inhibit proinflammatory cytokines and increase anti-inflammatory cytokines 80 . Therefore, the possibility of using TZD for the treatment of SARS-CoV-2 disease is being evaluated, as patients with COVID-19 have increased plasma levels of proinflammatory cytokines ( Fig. 1 ). An animal study found that pioglitazone attenuates lung injury by modulating adipocyte inflammation and reported a direct action on pulmonary inflammatory and fibrotic status 80 . However, TZD also appears to increase the expression of ACE2 and may facilitate infection with SARS-CoV-2. Therefore, the adjuvant use of TZD in the treatment of COVID-19 needs better evaluation 81 .
TZD (FDA category C) crosses the placenta. There is no evidence for safe use in pregnancy from well-controlled studies 82 . TZD does not appear to have a teratogenic effect in pregnant rats and rabbits, despite using doses many times higher than the human dose. However, the use of these drugs has been associated with fetal death and growth retardation as the fetus is unable to develop, owing to alterations in placental maturation. TZD is secreted in the milk of lactating rats. It is not known whether TZD is secreted in human breast milk. TZD should not be administered to a breastfeeding woman ( Table 1 ) 83 .
Colchicine
Colchicine is a fat-soluble alkaloid with anti-inflammatory properties that is extracted from Colchicum autumnale plants. It is used to treat gout and familial Mediterranean fever (FMF). Studies have assessed its use to treat other conditions, such as pericarditis, arteriosclerosis, liver cirrhosis, and rheumatic osteoarthritis. Colchicine is administered orally and is absorbed in the jejunum and ileum. It reaches its maximum plasma concentrations between 1 and 2 hours after administration, and has an anti-inflammatory effect over a period of 24 – 48 hours. Its excretion is predominantly hepatobiliary, but 20 – 30% is excreted renally in patients with normal renal function 84 , 85 .
The mechanisms of action of colchicine have not been fully understood yet. Colchicine inhibits cell division (by acting on microtubule tubulins and preventing the formation of the mitotic spindle). It reduces neutrophil chemotaxis, adhesion, and mobilization, decreases mobilization and lysosomal degranulation, and reduces the release of proinflammatory substances. It also restricts the production of superoxide, in addition to inhibiting NACHT-LRRPYD-containing protein 3 inflammasomes and the processing and release of IL-1β 85 .
Therefore, it is believed that colchicine may help in the treatment of COVID-19 because it is an NLRP3 inflammasome inhibitor ( Fig. 1 ). Studies have observed that different SARS-CoV-2 proteins are responsible for activating and triggering the NLRP3 inflammasome, which can subsequently lead to severe adult respiratory distress syndrome. The inhibition of the NLRP3 inflammasome also appears to be associated with the suppression of IL-1β, IL-18, and IL-6 present in the context of lymphocyte myocarditis generated by SARS-CoV-2. Thus, the administration of colchicine could have a mitigating effect on the development of pneumonia and myocardial necrosis caused by SARS-CoV-2 84 .
This medication is classified as category C (FDA classification) for use during pregnancy. A systematic review and meta-analysis by Indraratna et. al revealed that colchicine was not associated with a higher risk of miscarriages and fetal malformations, but there may be a risk of preterm birth or low birth weight. However, it is unclear whether this observation is related to the use of colchicine or to FMF itself 86 . Teratogenicity was observed in animals exposed to high doses, thereby suggesting a dose-dependent effect. It is known that colchicine crosses the placental barrier; however, studies on colchicine during pregnancy basically consisted of observations of women with FMF. In fact, these studies are not well-controlled and scarce. Thus, its use in pregnancy is still controversial. In addition, transmission of colchicine during breastfeeding has been described, but this relationship has not been fully established; therefore, use of this drug during lactation is characterized as risky ( Table 1 ) 87 .
Ivermectin and Niclosamide
Ivermectin is an FDA-approved drug for the treatment of ascariasis, onchocerciasis, gnathostomiasis, cutaneous larva migrans, strongyloidiasis, pediculosis, and scabies. In addition to its antiparasitic property, ivermectin exerts an antiviral effect against HIV-1, dengue virus, West Nile virus, and influenza virus 88 . Recent in vitro studies have shown its antiviral action against SARS-CoV-2, thereby making it a new therapeutic possibility to combat COVID-19 ( Fig. 1 ) 89 .
The mechanism that generates the antiviral action of ivermectin is still poorly understood, but its action is believed to be similar to that observed in the RNA of other viruses, which includes the inhibition of importin (IMP) α/β1. Recent studies have described the anti-inflammatory effects of ivermectin, which include a reduction in the activation, proliferation, and cytokine production of T cells ( Fig. 1 ) 88 .
There are limited studies that have evaluated the use of ivermectin during pregnancy and breastfeeding periods. The FDA classifies its use as category C. Ivermectin has been shown to be teratogenic in mice, rats, and rabbits when it is administered in repeat doses of 0.2, 8.1, and 4.5 times the maximum recommended human dose. Therefore, ivermectin should not be used during pregnancy since its safety in pregnancy has not been established. It is therefore imperative to consider the risks and maternal benefits before using the drug. Ivermectin is found in milk in low concentrations. Nursing women should only use this drug if the risk of delayed treatment to the woman outweighs the risks to the nursing infant ( Table 1 ) 90 .
Niclosamide (NIC) is a FDA-approved anthelmintic drug that is used to treat taeniasis. NIC has been proposed as a potential agent for host defense during viral infections, including infections by SARS-CoV, chikungunya virus, and Zika virus. NIC has also demonstrated anticancer and immunomodulatory activities ( Fig. 1 ) 91 , 92 . The antiviral mechanism of action of NIC is still unknown. Some of the mechanisms that may explain the drugʼs antiviral action include the blocking of SARS-CoV-2 endocytosis by changing the endosome pH, in addition to inhibiting autophagy while minimizing viral replication by blocking S-phase kinase-associated protein 2 93 . There are still no clinical studies evaluating the use of NIC in the treatment of COVID-19. NIC is a category B drug (FDA classification), which means it is safe to use in pregnancy and during breastfeeding ( Table 1 ) 94 .
Hyperimmune Plasma
Passive immunotherapy, which includes the administration of hyperimmune plasma as one of its components, has been used since the 1880s until the era of antibiotic therapy as a therapeutic option for several pathologies of viral and bacterial etiology. During the Spanish flu, the therapeutic potential of hyperimmune plasma was observed for the first time. It was subsequently requested for the management of measles, hemorrhagic fever in Argentina, and, more recently, for the treatment of Ebola, MERS, and avian influenza. Thus, it remains an important therapeutic tool in the absence of effective treatment or immunization 95 . Additionally, it is important to highlight the relevant historical security level 96 .
Hyperimmune plasma (also called convalescent plasma or immune plasma) is obtained by apheresis in patients who survive an infection with the pathology of interest and have developed antibodies against the causative agent of the disease to neutralize it. Anti-inflammatory cytokines, clotting factors, defensins, natural antibodies and other proteins are acquired in addition to neutralizing antibodies (NAbs). Immunomodulation and improvement of the severe inflammatory response can be achieved by transfusing hyperimmune plasma 96 . The use of hyperimmune plasma in the treatment of COVID-19 is being evaluated as a means of suppressing the storm of inflammatory cytokines, comprised mainly of IL-1β, IL-2, IL-6, IL-17, IL-8, and TNF-α, and caused by overactivation of the immune system ( Fig. 1 ) 96 .
Studies on the use of hyperimmune plasma as a therapy for COVID-19 have reported clinical improvement with a normalization of temperature and resolution of SARS, laboratory signs including increased IgG and IgM as well as reduced CPR and viral load, and increased NAbs over time. Treatment had a positive effect on survival times. However, the therapy did not appear to alter the mortality rate of patients who were in the final stage of disease. Thus, hyperimmune plasma would probably have a greater therapeutic value if it is administered during the initial course of disease, i.e., within 14 days of the onset of symptoms 97 , 98 , 99 .
There are no well-controlled studies on the use of hyperimmune plasma in pregnancy and breastfeeding ( Table 1 ). An Italian group from Carlo Poma Hospital (Mantua, Italy) reported the successful use of hyperimmune plasma in a 24-week pregnant woman with severe COVID-19. The patient was transfused with 300 mL of hyperimmune plasma, with no adverse effects. The patient was discharged 13 days after admission. The perinatal outcome was not available in the published manuscript 100 . During the Ebola outbreak, Griensven et al. conducted a study with hyperimmune plasma, which included pregnant women, and found that hyperimmune plasma was not associated with a significant improvement in survival rate. Additionally, there are no additional data on the use of hyperimmune plasma during pregnancy and lactation periods 101 .
Heparin
Originally, heparin was one of a class of drugs used in situations requiring anticoagulation. Among the commonly available heparins, low-molecular weight heparin (LMWH) is the most commonly used form because it has fewer side effects than unfractionated heparin. Studies have demonstrated other beneficial effects of heparin including, but not limited to, antiviral, anti-inflammatory, and antimetastatic effects 102 .
The severe form of COVID-19 has high plasma D-dimer levels that contribute to thromboembolic events and high mortality rates and justify the use of anticoagulant therapy in stages II and III of the disease. Tang et al. observed a significant decrease in mortality in patients with COVID-19 who met the criteria for sepsis-induced coagulopathy and were administered heparin 7 . Heparin has been reported to inhibit the activation of a number of inflammatory cell types and exhibits various immunological effects, including inhibition of TNF-α and IL-6, which may contribute to reducing the risk of a cytokine storm 4 . Mycroft-Wes et al. demonstrated that heparin binds to the Spike (S1) protein receptor-binding domain and induces a conformational change, thereby exerting an in vitro effect against SARS-CoV-2 infection ( Fig. 1 ) 103 .
Currently, the use of oral anticoagulant drugs during pregnancy is not recommended due to the high teratogenic risk. Heparin is therefore a drug that is widely used in reproductive medicine. Heparin is the first choice drug for the prevention and treatment of thromboembolic events in pregnancy. Numerous thrombophilic conditions, especially antiphospholipid syndrome (AFS), have been associated with obstetric complications, such as pregnancy loss, preeclampsia, placental abruption, and intrauterine growth restriction (IUGR). Over the past decades, patients with a history of recurrent miscarriage and AFS, a form of autoimmune thrombophilia described since the 1970s, have been treated with low doses of acetylsalicylic acid and heparin. Studies have also evaluated the use of heparin in cases with implantation failure during in vitro fertilization cycles, in the prevention of preeclampsia, and in IUGR 102 .
The FDA has classified heparin as category B. Due to its large molecular weight, it does not cross the placenta and is not excreted into human breast milk. The use of heparin, preferably LMWH, in pregnancy and during breastfeeding is considered safe. Attention should be paid to the risk of bleeding complications in patients using LMWH in prophylactic doses during pregnancy and postpartum hemorrhage (0.5 and 1%, respectively) or as a therapeutic (1.5 and 2%, respectively). Patients should be evaluated for other complications such as thrombocytopenia and osteoporosis when using heparin ( Table 1 ) 102 .
Vitamin C
Vitamin C ([VC], ascorbic acid) is a water-soluble vitamin that is found in various foods, especially in citrus fruits, which are a main source of VC. VC is a potent antioxidant that is essential for several functions of the body, including immune response. VC contributes to an adequate innate and adaptive immune response. Adequate levels of VC are essential for maintaining the integrity of epithelial barriers, improving phagocytic capacity, improving the differentiation and proliferation of T and B lymphocytes, increasing antibody production, enhancing Treg proliferation, inhibiting the negative immunoregulation of Tregs, and modulating systemic and leukocyte-derived cytokines. In vitro and animal studies have shown that treatment with VC reduces the levels of proinflammatory cytokines (TNF-α, IFN-γ, and IL-6) and increases the levels of anti-inflammatory cytokines (IL-10) ( Fig. 1 ) 104 .
Studies have demonstrated the antiviral effect of VC on bacterial and viral infections, including infections of the respiratory tract. The use of high doses of VC (200 mg/kg body weight/day, divided into 4 doses) in patients admitted to the ICU is associated with shorter hospital stays and reduced mortality rates. Furthermore, these studies have observed an improvement in the respiratory parameters of critically ill patients with COVID-19 after treatment with intravenous doses of VC (doses ranging from 2 to 10 g per day). Therefore, the use of intravenous VC in high doses can assist in the treatment of COVID-19 105 , 106 .
The use of VC in recommended doses during pregnancy and breastfeeding periods appears to be safe. However, there are no studies evaluating the impact of high doses of VC during pregnancy and breastfeeding. Ascorbic acid is excreted into human breast milk. The effects of VC in the nursing infant are still unknown. The manufacturer recommends exercising caution when administering ascorbic acid to nursing women ( Table 1 ) 107 .
Vitamin D
Vitamin D ([VD] calciferol) is a fat-soluble molecule that comes in two forms: vitamin D 2 (ergocalciferol) and vitamin D 3 (cholecalciferol). The main source of VD in the body is endogenous. VD is produced under the skin from its precursor (7-dehydrocholesterol) through exposure to ultraviolet B rays. After the initial stage in the skin, cholecalciferol and ergosterol reach the liver, undergo hydroxylation of carbon 25, and form 25(OH)D or calcidiol. 25(OH)D is the form of VD reserve in the body commonly measured in laboratory tests. A second reaction takes place in the kidney, when 25(OH)D receives another hydroxyl to develop into its active form (1.25[OH]-2D or calcitriol). A small amount of VD is obtained from diet through the ingestion of foods such as fatty cold water fish (tuna and salmon) and plant sources (plants and fungi) 108 .
Classically, VD is responsible for bone metabolism. Over the past few decades, there has been a growing interest in the role played by VD in different organs and tissues. Studies have shown an association between VD deficiency and increased mortality from different causes. VD actively participates in the innate and adaptive immune response. Studies show that VD improves physical barriers and is a potent inducer of antimicrobial peptides in innate immunity. VD recruits immune cells to promote wound healing and fight infections, and it is essential for the activation of T lymphocytes. VD limits tissue damage that occurs during an exaggerated inflammatory response, thereby reducing the excessive production of inflammatory ILs, such as TNF-α and IL-12 ( Fig. 1 ) 108 , 109 , 110 , 111 .
Recently, in a group of patients with suspected COVID-19 whose nasopharyngeal swabs were analysed by PCR, DʼAvolio et al. observed lower 25 (OH)D levels (p = 0.004) in the PCR-positive test results of patients with SARS-CoV-2 (median value: 11.1 ng/mL) compared with those of patients without SARS-CoV-2 (24.6 ng/mL) 112 . Daneshkhah et al. demonstrated a possible relationship between hypovitaminosis D and a higher mortality rate due to COVID-19 in countries such as the USA, France, Iran, and the UK. The authors suggest that an exaggerated inflammatory response (cytokine storm) would be more common in individuals with VD deficiency 113 . Based on these initial studies, the use of VD could reduce the number and severity of COVID-19 cases.
The role of VD in the health of pregnant women and newborns has been widely discussed. Studies have demonstrated associations between VD deficiency and the risk of gestational diabetes, preeclampsia, bacterial vaginosis, and small-for-gestational age infants. Currently, there is no consensus about universal screening for VD deficiency in pregnant women. However, there is a group of pregnant women at risk of obstetric complications who must maintain adequate levels of VD during pregnancy and breastfeeding periods 111 .
Animal studies carried out using high doses of VD have shown teratogenicity; however, there are no controlled data for human pregnancies. The use of VD in prophylactic or therapeutic doses during pregnancy and breastfeeding periods is considered safe. Toxic levels (> 150 ng/ml [375 nmol/L] of 25[OH]D) are rarely found. Early symptoms of VD toxicity are caused by large amounts of calcium in blood (hypercalcemia) and include nausea, vomiting, constipation/diarrhea, weakness, or frequent urination ( Table 1 ) 111 .
Zinc
Zinc is an essential ion that is crucial for the maintenance of normal physiology. Zinc is the most abundant intracellular metal and the second most common metal in the entire body after iron. Zinc modulates the bacterial and viral immune response, and actively participates in local and systemic inflammatory pathways. Historically, zinc deficiency was considered rare. Currently, it is estimated that around two billion people worldwide have zinc deficiency 114 .
In vitro studies demonstrate an antiviral activity of zinc through the inhibition of SARS-CoV RNA polymerase and increased production of IFN-γ, and it may decrease the activity of ACE2, which is known to be the receptor for SARS-CoV-2. Zinc can also contribute to reducing the cytokine storm by exerting an anti-inflammatory activity (inhibiting NF-κB signaling) and modulating Treg lymphocytes 115 . Patients with associated comorbidities (aging, immune deficiency, obesity, diabetes, and atherosclerosis) as well as a high mortality rate for COVID-19 have a higher prevalence of zinc deficiency, thus highlighting the importance of this ion in the prevention and treatment of this disease ( Fig. 1 ) 116 .
Currently, the main recommendation for the use of zinc in the treatment of COVID-19 is in combination with CQ/HCQ. Studies suggest that the association of zinc with CQ/HCQ, a zinc ionophore drug, would enhance the antiviral effect of this therapy, as it achieves greater intracellular concentrations. This effect would be more evident in the initial phase of disease, with no benefits in the hyperinflammation phase (cytokine storm) 116 .
The use of zinc in pregnancy and breastfeeding appears to be safe ( Table 1 ). Zinc deficiency in pregnancy is related to miscarriage, intrauterine growth retardation, premature birth, preeclampsia, altered T-lymphocyte function, congenital abnormalities, and fetal immunological changes. Initial studies demonstrate that zinc supplementation during pregnancy is associated with a reduction in the risk of premature births. There is still a poor understanding of the impact of supplementation at supraphysiological doses. Zinc is excreted in human breast milk, which fulfills the newbornʼs nutritional needs. Zinc toxicity is rare, except in the condition of supplementation or contact with high doses over a long period 117 , 118 .
Senolytic Drugs
Human cells have a limited ability to replicate. Once this limit is reached, these cells no longer have the ability to divide; they lose their normal function and become senescent cells (SCs). SCs acquire a senescence-associated secretory phenotype (SASP) which is responsible for a chronic local and systemic inflammatory response 119 . The process of cellular senescence is associated with chronic diseases such as diabetes, hypertension, and obesity; these conditions also increase the risk of mortality from COVID-19. The accumulation of SCs in the lungs, especially in the elderly, induces a process of pulmonary fibrosis and could contribute to the exaggerated inflammatory response to SARS-CoV-2 (cytokine storm). Thus, senolytic drugs, which induce apoptosis of SCs could assist in the treatment of COVID-19 69 , 120 .
The role of SCs in viral infections is still poorly understood. Some studies reported that SCs help in fighting viral infection, whereas others have observed that the presence of SCs increases viral replication. Kim et al. found greater replication of the influenza virus in SCs compared to non-SCs 121 . Frescas et al. suggested that SCs in the lungs have a higher expression of surface proteins (such as vimentin) that participate, together with ACE2, in the infection of host cells by SARS-CoV-2 ( Fig. 1 ) 122 .
Some drugs used to treat COVID-19 have senolytic properties, either by direct action on the SCs in the lungs (azithromycin and doxycycline) or by reducing SASP mediators (toculizumab, roxilitinib, rapacycin, and melatonin) 57 , 69 . Recently, a study identified quercetin, a dietary supplement with senolytic properties, as a potential therapy against COVID-19, as it reduces the binding of SARS-CoV-2 to host cells 69 . Quercetin present in food is safe during pregnancy and breastfeeding. However, it should not be supplemented in doses above the recommended quantities because there is a lack of information about its safety and efficacy in pregnancy and during lactation. A study conducted in rats exposed to quercetin by oral gavage observed fetal growth retardation ( Table 1 ) 123 , 124 .
Conclusion
Currently, a limited number of drugs are used to treat COVID-19. These drugs are of questionable efficacy, and the evidence for their effectiveness is weak. There are not enough studies in the literature that demonstrate the safety of most drugs used to fight infection by SARS-CoV-2 during pregnancy and lactation. A relevant number of these drugs have been shown in animal studies to be high risk during pregnancy and breastfeeding. Therefore, well-conducted studies that evaluate the safety of anti-COVID-19 drugs during pregnancy and breastfeeding periods are necessary.
Funding
No funding was received for the preparation of this article.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Guan W J, Ni Z Y, Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Coronavirus disease (COVID-19) dashboardAccessed June 04, 2020 at:https://covid19.who.int/
- 3.Sutton D, Fuchs K, DʼAlton M. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N Engl J Med. 2020 doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqi H K, Mehra M R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z, Rochwerg B, Wang Y. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ. 2020 doi: 10.1503/cmaj.200648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z, Wang Y, Colunga-Lozano L E. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020 doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N, Bai H, Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu R, Wang L, Kuo H D. An Update on Current Therapeutic Drugs Treating COVID-19. Curr Pharmacol Rep. 2020 doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders J M, Monogue M L, Jodlowski T Z. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 10.Brucker M C, King T L. The 2015 US Food and Drug Administration Pregnancy and Lactation Labeling Rule. J Midwifery Womens Health. 2017;62:308–316. doi: 10.1111/jmwh.12611. [DOI] [PubMed] [Google Scholar]
- 11.Wilmer E, Chai S, Kroumpouzos G. Drug safety: Pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401–409. doi: 10.1016/j.clindermatol.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Stumpfe F M, Titzmann A, Schneider M O. SARS-CoV-2 Infection in Pregnancy – a Review of the Current Literature and Possible Impact on Maternal and Neonatal Outcome. Geburtshilfe Frauenheilkd. 2020;80:380–390. doi: 10.1055/a-1134-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellington S, Strid P, Tong V T. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status – United States, January 22 – June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith D D, Pippen J L, Adesomo A A. Exclusion of Pregnant Women from Clinical Trials during the Coronavirus Disease 2019 Pandemic: A Review of International Registries. Am J Perinatol. 2020 doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathy S, Dassarma B, Roy S. A review on possible modes of actions of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. doi:10.1016/j.ijantimicag.2020.106028. Int J Antimicrob Agents. 2020;56:106028. doi: 10.1016/j.ijantimicag.2020.106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra M R, Desai S S, Ruschitzka F. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Apotex Product monograph (Hydroxychloroquine Sulfate Tablets)Accessed June 04, 2020 at:https://pdf.hres.ca/dpd_pm/00054599.pdf
- 19.Peterson E A, Lynton J, Bernard A. Rheumatologic Medication Use During Pregnancy. Obstet Gynecol. 2020;135:1161–1176. doi: 10.1097/AOG.0000000000003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birru Talabi M, Clowse M EB. Antirheumatic medications in pregnancy and breastfeeding. Curr Opin Rheumatol. 2020;32:238–246. doi: 10.1097/BOR.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 21.Sheahan T P, Sims A C, Graham R L. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wit E, Feldmann F, Cronin J. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grein J, Ohmagari N, Shin D. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lorenzo G, Di Trolio R, Kozlakidis Z. COVID 19 therapies and anti-cancer drugs: A systematic review of recent literature. doi:10.1016/j.critrevonc.2020.102991. Crit Rev Oncol Hematol. 2020;152:102991. doi: 10.1016/j.critrevonc.2020.102991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigel J H, Tomashek K M, Dodd L E. Remdesivir for the Treatment of Covid-19 – Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 26.Food and Drug Administration (FDA) Fact sheet for health care providers. Emergency use authorization (EUA) of remdesivir (gs-5734 ™ ) Accessed June 04, 2020 at:https://www.fda.gov/media/137566/download
- 27.Dashraath P, Wong J LJ, Lim M XK. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts S S, Martinez M, Covington D L. Lopinavir/ritonavir in pregnancy. J Acquir Immune Defic Syndr. 2009;51:456–461. doi: 10.1097/QAI.0b013e3181a2813f. [DOI] [PubMed] [Google Scholar]
- 29.Chu C M, Cheng V C, Hung I F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung I F, Lung K C, Tso E Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 doi: 10.1016/s0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheahan T P, Sims A C, Leist S R. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zumla A, Chan J F, Azhar E I. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasmi A, Noor S, Tippairote T. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215:108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao B, Wang Y, Wen D. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKee D L, Sternberg A, Stange U. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tookey P A, Thorne C, van Wyk J. Maternal and foetal outcomes among 4118 women with HIV infection treated with lopinavir/ritonavir during pregnancy: analysis of population-based surveillance data from the national study of HIV in pregnancy and childhood in the United Kingdom and Ireland. BMC Infect Dis. 2016;16:65. doi: 10.1186/s12879-016-1400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandhi M, Mwesigwa J, Aweeka F. Hair and plasma data show that lopinavir, ritonavir, and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr. 2013;63:578–584. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaising J, Polyak S J, Pécheur E-I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haviernik J, Štefánik M, Fojtíková M. Arbidol (Umifenovir): A Broad-Spectrum Antiviral Drug That Inhibits Medically Important Arthropod-Borne Flaviviruses. Viruses. 2018;10:184. doi: 10.3390/v10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lian N, Xie H, Lin S. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng L, Li C, Zeng Q. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z, Lu Z, Xu T. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivas P, Sacha G, Koval C. Antivirals for COVID-19. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc030. [DOI] [PubMed] [Google Scholar]
- 44.Takashita E, Ejima M, Ogawa R. Antiviral susceptibility of influenza viruses isolated from patients pre- and post-administration of favipiravir. Antiviral Res. 2016;132:170–177. doi: 10.1016/j.antiviral.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Furuta Y, Gowen B B, Takahashi K. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C, Zhang Y, Huang J. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 47.Cai Q, Yang M, Liu D. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drugs and Lactation Database Bethesda (MD): National Library of Medicine (US); 2006. FavipiravirAccessed June 04, 2020 at:https://www.ncbi.nlm.nih.gov/books/NBK556878/
- 49.Jamieson D J, Honein M A, Rasmussen S A. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 50.Tan Q, Jin Y. Ostavimir is ineffective against COVID-19: in silico assessment, in vitro and retrospective study. medRxiv. 2020 doi: 10.1101/2020.05.15.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng F, Tu L, Yan Y. Management and Treatment of COVID-19: The Chinese Experience. Can J Cardiol. 2020 doi: 10.1016/j.cjca.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phua J, Weng L, Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Q, Lu P, Fan Y. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donner B, Niranjan V, Hoffmann G. Safety of oseltamivir in pregnancy: a review of preclinical and clinical data. Drug Saf. 2010;33:631–642. doi: 10.2165/11536370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Anderson P O. Breastfeeding and Respiratory Antivirals: Coronavirus and Influenza. Breastfeed Med. 2020;15:128. doi: 10.1089/bfm.2020.29149.poa. [DOI] [PubMed] [Google Scholar]
- 56.Damle B, Vourvahis M, Wang E. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parnham M J, Erakovic Haber V, Giamarellos-Bourboulis E J. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann P, Ziesenitz V C, Curtis N. The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gbinigie K, Frie K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020 doi: 10.3399/bjgpopen20X101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bookstaver P B, Bland C M, Griffin B. A Review of Antibiotic Use in Pregnancy. Pharmacotherapy. 2015;35:1052–1062. doi: 10.1002/phar.1649. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Zhao Y, Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coutinho A E, Chapman K E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veronese N, Demurtas J, Yang L. Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Frontiers in Medicine. 2020;7:170. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24:198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bandoli G, Palmsten K, Forbess Smith C J. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum Dis Clin North Am. 2017;43:489–502. doi: 10.1016/j.rdc.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sehgal S N. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7s–14s. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 67.Alfano G, Fontana F, Mori G. Antiviral activity of sirolimus in an HIV-positive kidney transplant recipient. Int J STD AIDS. 2019;30:919–922. doi: 10.1177/0956462419839520. [DOI] [PubMed] [Google Scholar]
- 68.Kindrachuk J, Ork B, Hart B J. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sargiacomo C, Sotgia F, Lisanti M P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12:6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Food and Drug Administration (FDA) Rapamune (sirolimus) oral solution and tablets prescribing informationAccessed June 04, 2020 at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021083s048lbl.pdf
- 71.Armenti V T, Daller J A, Constantinescu S. Report from the National Transplantation Pregnancy Registry: outcomes of pregnancy after transplantation. Clin Transpl. 2006:57–70. [PubMed] [Google Scholar]
- 72.Luo P, Liu Y, Qiu L. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoeltzenbein M, Beck E, Rajwanshi R. Tocilizumab use in pregnancy: Analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. 2016;46:238–245. doi: 10.1016/j.semarthrit.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Lei J, Vermillion M S, Jia B. IL-1 receptor antagonist therapy mitigates placental dysfunction and perinatal injury following Zika virus infection. JCI Insight. 2019;4:e122678. doi: 10.1172/jci.insight.122678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill-Batorski L, Halfmann P, Marzi A. Loss of Interleukin 1 Receptor Antagonist Enhances Susceptibility to Ebola Virus Infection. J Infect Dis. 2015;212 02:S329–S335. doi: 10.1093/infdis/jiv335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavalli G, De Luca G, Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nanjan M J, Mohammed M, Prashantha Kumar B R. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg Chem. 2018;77:548–567. doi: 10.1016/j.bioorg.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 79.Davidson M A, Mattison D R, Azoulay L. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol. 2018;48:52–108. doi: 10.1080/10408444.2017.1351420. [DOI] [PubMed] [Google Scholar]
- 80.Carboni E, Carta A R, Carboni E. Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med Hypotheses. 2020;140:109776. doi: 10.1016/j.mehy.2020.109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan L Y, Yeung J H, Lau T K. Placental transfer of rosiglitazone in the first trimester of human pregnancy. Fertil Steril. 2005;83:955–958. doi: 10.1016/j.fertnstert.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 83.Takeda Canada Inc. Product monograph (pioglitazone hydrochloride)Accessed June 04, 2020 at:https://www.takeda.com/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/actos/actos-pm-18jan2018-clean_en.pdf
- 84.Deftereos S G, Siasos G, Giannopoulos G. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J Cardiol. 2020 doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leung Y Y, Yao Hui L L, Kraus V B. Colchicine–Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Indraratna P L, Virk S, Gurram D. Use of colchicine in pregnancy: a systematic review and meta-analysis. Rheumatology (Oxford) 2018;57:382–387. doi: 10.1093/rheumatology/kex353. [DOI] [PubMed] [Google Scholar]
- 87.Gómez-Meda B C, Bañales-Martínez L R, Zamora-Perez A L. Micronucleated Erythrocytes in Peripheral Blood from Neonate Rats Exposed by Breastfeeding to Cyclophosphamide, Colchicine, or Cytosine-Arabinoside. Biomed Res Int. 2016;2016:9.161648E6. doi: 10.1155/2016/9161648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laing R, Gillan V, Devaney E. Ivermectin – Old Drug, New Tricks? Trends Parasitol. 2017;33:463–472. doi: 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caly L, Druce J D, Catton M G. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merck Sharp & Dohme Corp Product monograph (Ivermectin)Accessed June 04, 2020 at:https://www.merck.com/product/usa/pi_circulars/s/stromectol/stromectol_pi.pdf
- 91.Chen W, Mook R A, Premont R T. Niclosamide: Beyond an antihelminthic drug. Cell Signal. 2018;41:89–96. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu J, Shi P-Y, Li H. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect Dis. 2020;6:909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pindiprolu S KSS, Pindiprolu S H. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Med Hypotheses. 2020;140:109765. doi: 10.1016/j.mehy.2020.109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cook G C. Use of antiprotozoan and anthelmintic drugs during pregnancy: side-effects and contra-indications. J Infect. 1992;25:1–9. doi: 10.1016/0163-4453(92)93369-2. [DOI] [PubMed] [Google Scholar]
- 95.Marano G, Vaglio S, Pupella S. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rojas M, Rodríguez Y, Monsalve D M. Convalescent plasma in Covid-19: Possible mechanisms of action. doi:10.1016/j.autrev.2020.102554. Autoimmun Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rajendran K, Krishnasamy N, Rangarajan J. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown B L, McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. doi:10.1016/j.transci.2020.102790. Transfus Apher Sci. 2020;59:102790. doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng Q L, Yu Z J, Gou J J. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in COVID-19 Patients. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grisolia G, Franchini M, Glingani C. Convalescent plasma for coronavirus disease 2019 in pregnancy: a case report and review. doi:10.1016/j.ajogmf.2020.100174. Am J Obstet Gynecol MFM. 2020;2:100174. doi: 10.1016/j.ajogmf.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Griensven J, Edwards T, de Lamballerie X. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mulloy B, Hogwood J, Gray E. Pharmacology of Heparin and Related Drugs. Pharmacol Rev. 2016;68:76–141. doi: 10.1124/pr.115.011247. [DOI] [PubMed] [Google Scholar]
- 103.Mycroft-West C J, Su D, Pagani I. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv. 2020 doi: 10.1101/2020.04.28.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hemilä H. Vitamin C and Infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fowler A A, Truwit J D, Hite R D. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim W Y, Jo E J, Eom J S. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J Crit Care. 2018;47:211–218. doi: 10.1016/j.jcrc.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Rumbold A, Crowther C A.Vitamin C supplementation in pregnancy Cochrane Database Syst Rev 200502CD004072 10.1002/14651858.CD004072.pub2 [DOI] [PubMed] [Google Scholar]
- 108.Bikle D D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cantorna M T, Snyder L, Lin Y D. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holick M F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 111.Agarwal S, Kovilam O, Agrawal D K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit Rev Food Sci Nutr. 2018;58:755–769. doi: 10.1080/10408398.2016.1220915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.DʼAvolio A, Avataneo V, Manca A. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daneshkhah A, Agrawal V, Eshein A. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv. 2020 doi: 10.1101/2020.04.08.20058578. [DOI] [Google Scholar]
- 114.Baltaci A K, Mogulkoc R, Baltaci S B. Review: The role of zinc in the endocrine system. Pak J Pharm Sci. 2019;32:231–239. [PubMed] [Google Scholar]
- 115.Wessels I, Maywald M, Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skalny A V, Rink L, Ajsuvakova O P. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review) Int J Mol Med. 2020 doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilson R L, Grieger J A, Bianco-Miotto T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients. 2016;8:641. doi: 10.3390/nu8100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ota E, Mori R, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015;2015(02):CD000230. doi: 10.1002/14651858.CD000230.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tchkonia T, Zhu Y, van Deursen J. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chapagain P. Potential Role of Cellular Senescence on Coronavirus Infections. doi:10.20944/preprints202004.0532.v1 Preprints. 2020:2.020040532E9. [Google Scholar]
- 121.Kim J A, Seong R K, Shin O S. Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw. 2016;16:286–295. doi: 10.4110/in.2016.16.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frescas D, Roux C M, Aygun-Sunar S. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci U S A. 2017;114:E1668–E1677. doi: 10.1073/pnas.1614661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson J R, Makaji E, Ho S. Effect of maternal raspberry leaf consumption in rats on pregnancy outcome and the fertility of the female offspring. Reprod Sci. 2009;16:605–609. doi: 10.1177/1933719109332823. [DOI] [PubMed] [Google Scholar]
- 124.Quercetin. IARC Monogr Eval Carcinog Risks Hum. 1999;73:497–515. [PMC free article] [PubMed] [Google Scholar]


