Abstract
Mechanical loading is an important factor in musculoskeletal health and disease. Tendons and ligaments require physiological levels of mechanical loading to develop and maintain their tissue architecture, a process that is achieved at the cellular level through mechanotransduction-mediated fine tuning of the extracellular matrix by tendon and ligament stromal cells. Pathological levels of force represent a biological (mechanical) stress that elicits an immune system-mediated tissue repair pathway in tendons and ligaments. The biomechanics and mechanobiology of tendons and ligaments form the basis for understanding how such tissues sense and respond to mechanical force, and several mechanical stress-related tendon and ligament disorders overlap anatomically with joints affected by chronic inflammatory arthritis. The role of mechanical stress in ‘overuse’ injuries, such as tendinopathy, has long been known, but mechanical stress is now also emerging as a possible trigger for some forms of chronic inflammatory arthritis, including spondyloarthritis and rheumatoid arthritis. Thus, seemingly diverse diseases of the musculoskeletal system might have similar mechanisms of immunopathogenesis owing to conserved responses to mechanical stress.
Introduction
The tissues that connect muscle to bone (tendons) and bone to bone (ligaments) have evolved specialized biochemical properties that enable the transmission of mechanical force [G] between different parts of the musculoskeletal system. Tendons and ligaments have a fibrous composition and little cellular heterogeneity; characteristics that are relatively consistent throughout the body. By contrast, biochemical and cellular differences occur longitudinally along individual tendons and ligaments, producing microenvironments at the myotendinous junctions (MTJs; the points at which muscle and tendon join) and at the entheses (the points at which tendons and ligaments connect to bone; Figure 1a) that differ from that at the midportion. Biomechanically, tissue failure that results in injury is prone to occur at the point at which tissues of differing physical properties meet1. Such a structural mismatch in the presence of high levels of strain [G] is particularly evident at the enthesis, where a fifty-fold difference in tissue resistance to tensile load [G] occurs between the elastic tendon or ligament and the stiff bone they attach to2. Once tissue failure takes place in adult tendons and ligaments, the pre-injury tissue architecture is never regained, making these structures prone to re-injury3,4. Indeed, in the USA, tendon and ligament injuries result in a high number of work place injuries5 and potentially account for the majority of musculoskeletal disorders globally through sprains and strains of the lower back6,7.
Figure 1. The anatomy of tendons and ligaments in the peripheral and axial skeletons.
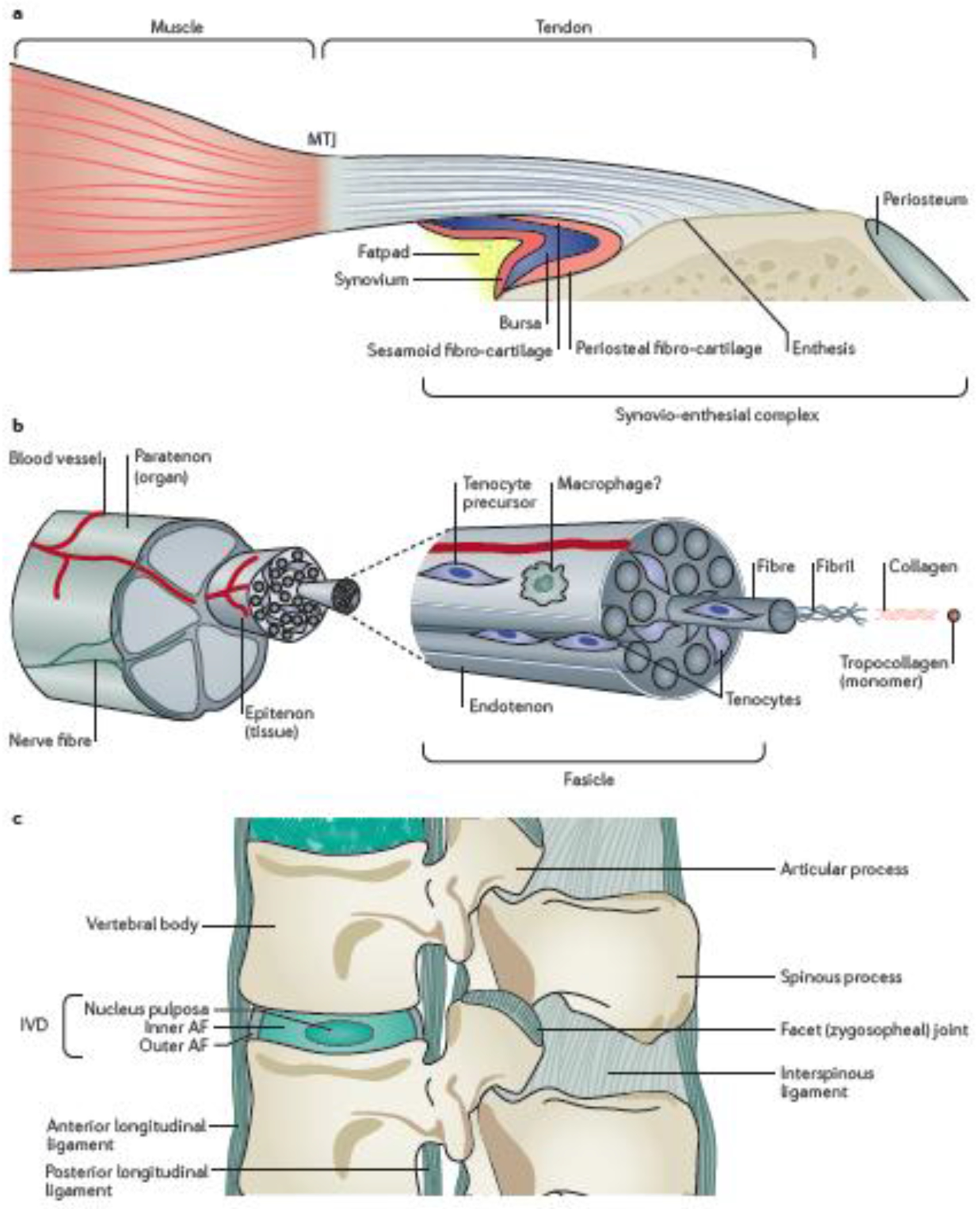
a| A macroscopic overview of tendon structure and the fibrocartilaginous synovio-entheseal complex at the bone insertion. b| A cross-sectional view of a tendon showing the hierarchal structure of the organ at tissue and molecular levels. c| The anatomy of spinal ligaments and the intervertebral disc (IVD). AF, annulus fibrosus.
From the perspective of the rheumatologist, tendons and ligaments are usually thought to belong to the remit of other medical specialists (such as orthopaedic surgeons), and are often ignored when considering the pathophysiology of joint diseases such as chronic inflammatory arthritis (encompassing rheumatoid arthritis (RA) and spondyloarthritis (SpA)). RA is characterized by the presence of defined autoantibodies (in seropositive individuals) and arthritis that is generally restricted to the peripheral joints8. By contrast, a range of seronegative inflammatory arthritides are covered by the term SpA, the main forms of which are ankylosing spondylitis (AS) and psoriatic arthritis (PsA), which predominantly affect the axial and peripheral joints, respectively9,10. Owing to the symptomatic overlap between AS and PsA, SpA is often simply defined as axial or peripheral, depending on the principal location of inflammation11,12. These forms of chronic inflammatory arthritis have prodromal periods with measurable extra-articular features, such as autoantibodies in RA and psoriasis or inflammatory bowel disease in SpA, but it has been unclear how systemic inflammation results in localized arthritis of the axial and/or peripheral skeleton in these diseases. However, there is now growing evidence that micro-trauma to tendons and ligaments might function as a joint-focusing trigger in the progression of chronic inflammatory arthritis13.
In this Review, we introduce the cellular and molecular mechanisms whereby tendons and ligaments sense mechanical force. This knowledge serves as a base for understanding how tendons and ligaments respond to mechanical stress [G] to maintain homeostasis under healthy conditions; a process that is heavily dependent on the immune system. The field of orthopaedics has recognized that mechanical stress-induced immune responses can go awry, resulting in primary tendinopathy14,15. We therefore draw parallels between the immunological events that occur in tendinopathy and in chronic inflammatory arthritis (specifically RA and SpA) to demonstrate that tendons and ligaments are relevant tissues to consider in the pathophysiology of arthritis. As such, musculoskeletal diseases that initially seem diverse are potentially more similar than expected at the cellular and molecular levels owing to a shared anatomical basis in their pathophysiology.
Anatomy of tendons and ligaments
Generally speaking, tendons and ligaments have a hierarchal anatomical structure14,16–18 (Figure 1b). At a molecular scale, the building blocks of tendons and ligaments are tropocollagen monomers, which polymerize into chains of type I collagen. Three of these chains twist to form a triple helix, which cross-links with adjacent chains to form fibrils. Fibrils coil to form fibres, at which point the structure moves from a molecular to a cellular scale. The fibres are bundled together by a sheath called the endotenon to define the unit known as the fascicle for either tendons or ligaments; the endotenon contains nerves and blood vessels to support the fascicle. Multiple fascicles bundle together to form a macroscopic tissue-level unit surrounded by a secondary sheath called the epitenon. Multiple epitenon-surrounded units bundle to form the complete organ that is itself enveloped by a tertiary sheath, which can be either a synovial sheath or a paratenon. Paratenon sheaths function as elastic sleeves to assist the free movement of the tendon against surrounding tissues, whereas synovial sheaths serve as tunnels for tendons and ligaments at locations where they wrap around bony or fibrous processes. These synovial sheaths enable the tendon or ligament to withstand high levels of stress [G].
Although the main extracellular matrix (ECM) component of tendons and ligaments is collagen, other ECM components are also required for their function19. The non-collagenous ECM of tendons and ligaments consists of two main groups of molecules: proteoglycans and glycoproteins, which have protein and sugar cores, respectively20,21. Small proteoglycans such as fibromodulin are essential in regulating collagen assembly, whereas large proteoglycans, such as aggrecan, have hydrophilic properties and function to dampen compressive forces21,22. Glycoproteins can provide lubrication (such as lubricin), elasticity (such as elastin) or bind to inactive growth factors (such as tenascin-C, which binds to latent transforming growth factor-β (TGFβ))20,23.
A range of cell types are found in healthy tendons and ligaments. Fibroblast-like stromal cells, commonly referred to as tenocytes, constitute 90-95% of the cells in a healthy tendon18. These cells are also commonly found in ligaments24–26, where they can be referred to as ligamentocytes, however they are less well characterized than their tendon counterparts. Tenocytes are interspersed among collagen fibres within fascicles and can be phenotypically identified by the transcription factors scleraxis (Scx) or mohawk (Mkx)27,28 (Box 1). However, although highly expressed by tenocytes, these markers are not tenocyte-specific and can also be found in other tissues, including the heart and testis29,30. The primary function of tenocytes is to build tendons and ligaments by producing collagen, or to break them down via protease release14,31. Populations of tenocyte-like progenitor cells reside in the supportive tissue (the endotenon and epitenon) and can replace tenocytes as they die32–34. The endotenon might also contain resident immune cells such as M2-like macrophages35–38; however, it is unclear as to whether these cells are present under healthy conditions, as most studies have been performed in diseased tissue.
Box 1. The developmental biology of tenocytes.
A number of molecules have essential and non-redundant roles in tenocyte development, including transforming growth factor-β (TGFβ), mothers against decapentaplegic homolog 3 (SMAD3), the transcription factor Egr1 and the transmembrane glycoprotein tenomodulin43; however, the transcription factors scleraxis (Scx) and mohawk (Mkx) are the best characterized. Scx is thought to be upregulated at an early stage in tenocyte development during limb bud formation, in which it initiates tenocyte differentiation and tendon development208,209. Scx is also essential for the development of the enthesis210. By contrast, Mkx is upregulated at a late stage during tenocyte development and is responsible for modifying the strength of a tendon once it has been formed211. Tenocytes are developmentally linked to other joint stromal cells, particularly chondrocytes and osteoblasts. Whereas Scx-expressing progenitor cells maintain some flexibility and can differentiate into chondrocytic cells given the right cues212, Mkx strongly prevents the expression of the transcription factors Sox9 and Runx2, which define chondrocytes and osteoblasts, respectively213,214. As both Mkx and Scx are induced and/or activated by loading78,79, tenocyte development and survival depends on exposure to mechanical stress.
The basic anatomical and cellular information about tendons and ligaments outlined above enables the comparison of these two classes of load-transferring tissue. In general, ligaments are thought to have slightly less collagen content and a greater density of tenocytes than tendons39. For example, in the ligaments and tendons in the knee, the cruciate ligaments have a greater cell density, more glycosaminoglycans and a higher amount of type III collagen than the patellar and Achilles tendons40. However, the dominant mechanical forces exerted on a tendon or ligament are actually thought to be a more important promoter of tissue phenotype than the anatomical distinction of connecting muscle to bone or bone to bone. For example, a detailed ultrastructural and biochemical analysis of knee tendons and ligaments revealed that the properties of these structures differed depending on their intra-articular or extra-articular location: the anterior cruciate ligament (ACL), which is intra-articular, had less compact collagen and more elastin, aggrecan and versican than extra-articular knee tendons and ligaments, an outcome attributed to the high compressive forces exerted on the ACL41.
Notably, non-classical tendons and ligaments also exist at specific locations in the body. As discussed above, the body of a tendon can wrap around bones to function as a pulley. At the point of contact with bone, such tendons lose their typical structure, widen to spread the force and become fibrocartilagenous, with woven rather than parallel collagen fibres22. Atypical ligaments also exist, such as the outer annulus fibrosis (AF) of the intervertebral disc (IVD) (Figure 1c). Although the outer AF contains tenocytes that express Scx and Mkx24 and has organized type I collagen fibres and a sheath-like structure that contains progenitor cells with proliferative capacity, it does not have the hierarchal compartments that are typically found in ligaments25,42.
Anatomy of entheses and MTJs
Distinct cellular and molecular transitions are present at the MTJs and the entheses. At the MTJ, these changes are abrupt and tenocytes directly interact with myocytes43. Although the transition zone between tissues is short at the MTJ, it is worth noting that ECM changes are present, such as an increase in type VI collagen and other components, including laminin, relative to the tendon and the muscle44.
The changes at the enthesis are more substantial than at the MTJ; the complexity of these changes depends on whether the enthesis inserts directly into the bone, or indirectly, via the periosteum [G]. These attachments can be distinguished by the amount of fibrocartilage present; indirect insertions have little fibrocartilage and are termed ‘fibrous entheses’, whereas direct insertions have more fibrocartilage and are thus termed ‘fibrocartilaginous entheses’4. Anatomically, tendons and ligaments that attach to the metaphyses [G] or diaphyses [G] have fibrous entheses. By contrast, those that attach to the epiphyses [G] of long bones typically have fibrocartilaginous entheses4. Fibrous entheses, such as the deltoid, have straight insertions into the humerus that experience limited compressive or shear forces and are thus structurally less complex than curved fibrocartilaginous entheses, such as the Achilles tendon39,40.
At the microscopic level, the fibrocartilaginous enthesis has defined strata and a collagen gradient (Box 2). Entheses are considered to be avascular at the point of attachment to bone because the presence of blood vessels conflicts with the requirements of load bearing and smooth movement at these locations45. The bulk of cells in the enthesis are tenocytes, although chondrocytes constitute up to 10% of entheseal cells46. Enthesis-resident immune cells are relatively rare. Resident lymphocytes have been reported in mouse and human tissues, including γδ T cells and type 3 innate lymphoid cells47–49, and IL-23-producing CD14+ myeloid cells have been observed in human spinal ligament entheses50.
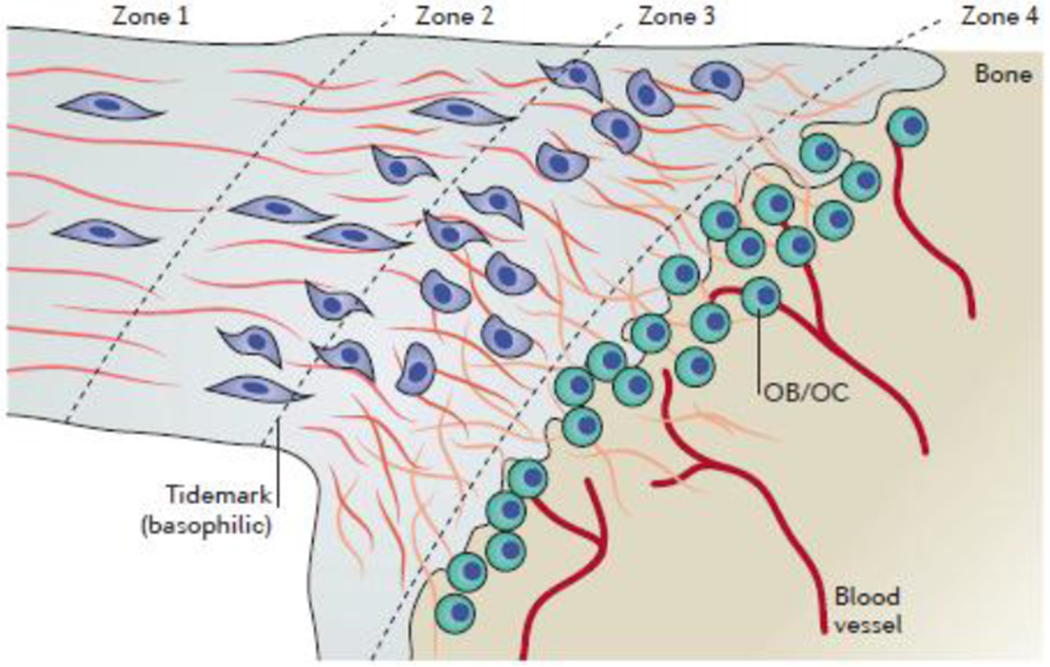
Box 2. The structural zones of the enthesis.
When examined at a histological level, the fibrocartilaginous enthesis comprises four distinct zones (see the figure). Zone 1 contains a pure tendinous or ligamentous tissue with aligned type I collagen fibres and sparse tenocytes. Zone 2 is a region of uncalcified fibrocartilage with an increased tenocyte density and an increased amount of aggrecan compared with zone 1. A transition from type I collagen fibres to type II and type III collagen fibres occurs in zone 2. Zone 3 is a region of calcified fibrocartilage in which type II collagen is dominant. In this zone, type II collagen forms a mesh to anchor the tendons and ligaments to the bone215. Morphologically, tenocytes transition from an elongated morphology in zone 1 to rounded ‘chondroid’ cells in zones 2 and 3. Finally, in zone 4, the bone is highly vascularized to provide nutrients to the avascular enthesis. The entire transition from tendon to bone occurs over ~0.5mm of tissue215.
Biomechanics of tendons and ligaments
In the field of biomechanics, the fundamental principles of mechanics are applied to biological problems through the examination of the forces acting upon and within biological structures51. The amount of force exerted on tendons and ligaments is substantial: it is estimated that regular activity can induce forces of up to 3 times the weight of a body on the human Achilles tendon, whereas intense activities induce forces of 10 times body weight52,53. Yet despite anatomy-imposed variations that occur between tendons and ligaments, all tendon and ligament tissues are remarkably resistant to mechanical force (mechano-resistant). Although a detailed cellular and molecular discussion of the biomechanics of tendons and ligaments is beyond the scope of this Review, the basic types of force and the response of tendons and ligaments to these forces are outlined in the following sections. Readers are referred to several excellent resources for further information16,31,54–57.
Types of mechanical force
The different types of mechanical force that act on tendons and ligaments are not equivalent16 (Figure 2a and b). Tensile force, the mechanical force generated as a result of stretching an object along its primary axis, is the dominant force exerted on tendons and ligaments, and as such, these tissues have a high degree of resistance to it58. Compressive force, which is exerted on tendons and ligaments in a direction that is perpendicular to their primary axis, is high at the entheses and in wrap-around tendons59,60. Compressive force can also be exerted by the tertiary (paratenon or synovial) sheath in pathological conditions such as trigger finger61. Shear force occurs between adjacent objects that are moving at different speeds or in different directions, such as when two fascicles slide past one another or when a tendon or ligament slides in its synovial sheath62. Additional types of mechanical force are unique to different anatomical locations. For example, the AF is resistant to axial rotation in addition to longitudinal compressive and tensile forces22. The AF can also withstand hydrostatic force that occurs as a result of volumetric changes in the IVD, the water content of which can vary by 20% throughout the day63. Finally, it is important to note that these forces do not act alone, and it has been suggested that a combination of tensile and compressive forces can function as a trigger for the onset of tendon pathology60.
Figure 2. Mechanical forces exerted on tendons and ligaments.
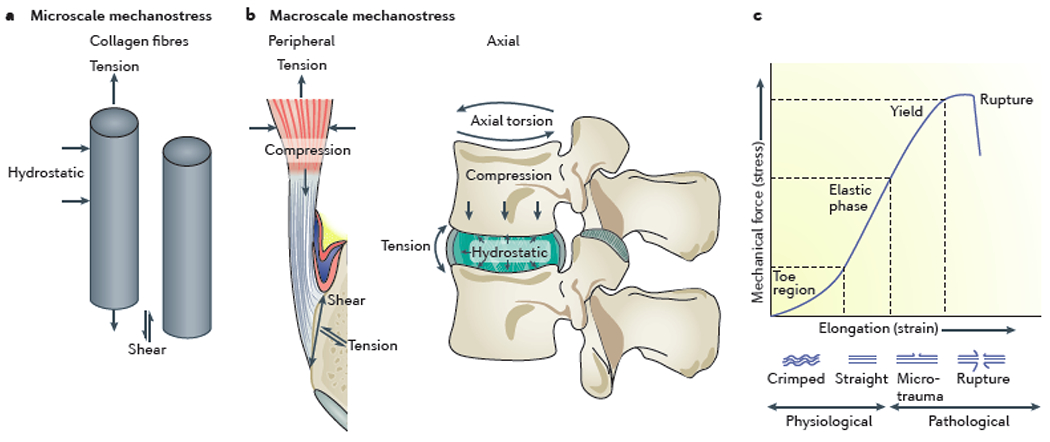
The types of mechanical forces that are exerted on tendons and ligaments can be examined at the microscale, in which forces act on and between collagen fibres (a) and at the macroscale, in which forces act on the complete tendon or ligament (b). Whereas microscale mechanical forces are common to all tendons and ligaments, macroscale mechanical forces differ depending on the anatomical location (axial skeleton or peripheral skeleton) of the tendon or ligament. The relationship between normalized force placed on a tendon or ligament, and the subsequent tissue elongation, can be represented by a stress–strain curve (c). Each phase of tendon or ligament stretch is defined by physical changes to the structure of collagen fibres as they go from a resting (crimped) state to an undamaged stretched state, on to a microdamaged stretched state and finally to complete rupture. The slope of the linear elastic region represents the modulus of the tissue.
Resistance to mechanical stress
The ability of tendons and ligaments to resist mechanical force lies in the biochemical properties of the ECM. In biomechanical studies, the strength [G] of a tendon or ligament is often represented as a stress–strain curve (Figure 2c), in which the stress is the force normalized to the cross-sectional area of the tissue it is being applied to, whereas the strain can be thought of as the ability of the tissue to stretch57. The slope of the stress–strain curve in the elastic region is the modulus [G], which can be used to describe the stiffness [G] of the tissue. The shape of the stress–strain curve in tendons and ligaments is largely a result of the physical properties of collagen. In a resting state, collagen fibres assume a crimped orientation. Tension straightens out the collagen fibres, enabling the tissue to rapidly stretch from its crimped state, followed by a slower stretch as straightened fibres pull away from one another. Removal of this tension allows tendons and ligaments to return to their pre-stretched length, whereas an increase in tension leads to microdamage of the tissue (such as tearing of the collagen fibres)64. Multiple factors enable the return of tendons and ligaments to their pre-stretched length; for example, proteoglycan-mediated accumulation of water enables hydrostatic resistance to compression, and elastin molecules, which are highly organized around tenocytes65, can resist tensile stress66. However, this ‘elasticity’ is not limitless: tendons can only stretch up to 8% of their length before reaching their breaking point67, after which rupture occurs. By contrast, ligaments are able to resist greater stress than tendons owing to a higher elastin content and can subsequently stretch up to 30% of their length before rupture68.
The response of a particular tendon or ligament to a specific mechanical load depends on many factors, including the type of mechanical stress, the anatomical function of the tendon or ligament and age. The type of mechanical stress involved can cause changes in the ECM; tensile stress induces the expression of decorin and versican, whereas biglycan and aggrecan are induced in response to compressive stress21,69,70. Such changes in the ECM composition subsequently affect the ability of the tissue to withstand mechanical stress. For example, biglycan and aggrecan protect the tendon against compression, but reduce its resistance to tension, injury and rupture71,72. The anatomical function of the tendon or ligament also has a direct effect on its biomechanical properties. Flexor tendons in the hand have a ‘grasping’ function, and thus have a high stiffness, are resistant to failure and have a high energy storage capacity compared with extensor tendons in the hand, which function to straighten digits73. Similarly, ligaments in the knee that stabilize the tibiofemoral joint have a higher stiffness and resistance to failure than ligaments in the ankle that stabilize the tibiotalar joint owing to the high mechanical forces exerted on the knee ligaments during tibiofemoral joint stabilization74,75. Finally, resistance to failure reduces as the tendon increases in age as a result of a reduction in cellularity and ECM component expression73,76.
Mechanotransduction
Mechanical stress, like any biological stress, has a physiological range in which a tissue can mount a response to return it to its homeostatic set point. Tendons and ligaments adapt to mechanical stress by modifying their ECM to adjust their stiffness, as previously discussed16,31,77. However, it is the cells within tendons and ligaments that sense and orchestrate the response to mechanical stress. The tenocyte is thus the master regulator in charge of transforming mechanical signals into adaptive responses in tendons and ligaments.
The tenocyte is particularly adept at mechanotransduction [G], a process that utilizes a variety of cytokines, adhesion molecules and small molecules31 (Figure 3). The transcription factors Scx and Mkx orchestrate mechanotransduction in tenocytes both in vitro and in vivo78,79 by stimulating the expression of mechanical stress-activated genes, including those encoding ECM molecules such as collagen and adhesion molecules such as integrins80. The molecular mechanisms by which Scx and Mkx are activated are poorly characterized. Scx is known to shuttle between the cytoplasm and nucleus in cell lines81, and can be phosphorylated at serine residues82, thereby implicating cytoplasmic kinases as activators of Scx. Once activated, Scx binds to the co-transcriptional factors E12 and E47, which enables Scx to bind to specific motifs in the DNA of genes that define tenocyte function, such as COL1A126,83. Less is known about the control of Mkx activity, although one study has shown that mechanical force can induce the activity of cytoplasmic GTF2IRD1, a transcription factor that can translocate to the nucleus and induce Mkx expression78.
Figure 3. Proposed tenocyte response mechanisms to mechanical stress.
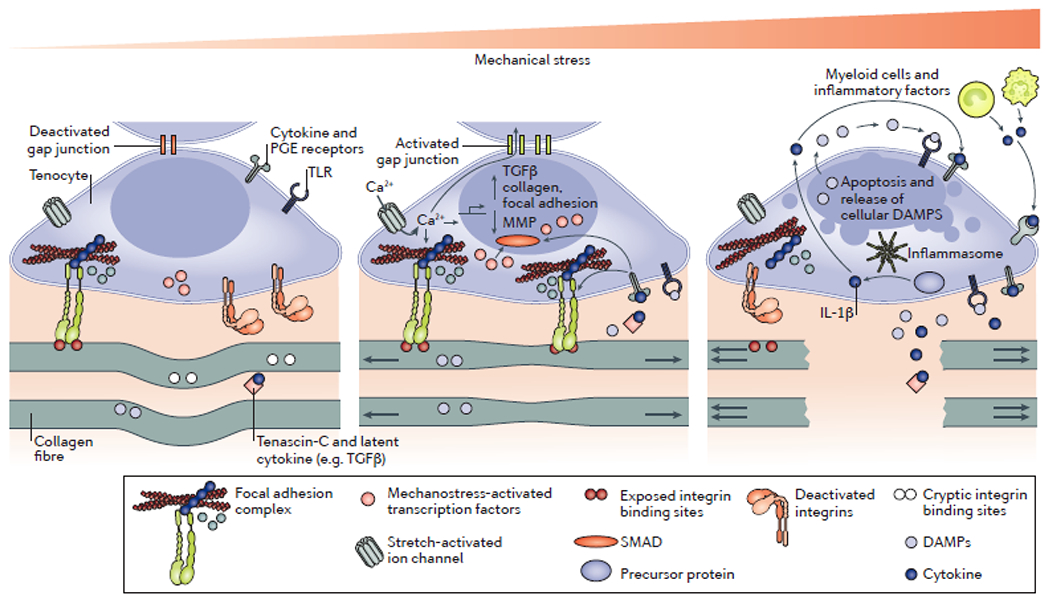
The mechanical loading of collagen fibres in tendons and ligaments is sensed by tenocytes, which mount a biological response to alter the extracellular matrix (ECM) composition of the surrounding tissue. In the unloaded state (left-hand side), collagen fibres are crimped and tenocytes receive minimal internal and external mechanical stress signals. Inactive integrins do not form focal adhesion complexes, and active integrins do not initiate mechanotransduction. As a result, mechanical stress-related transcription factors are either destroyed, such as YAP and TAZ, or fail to enter the nucleus, such as Scx. The result is a catabolic programme to reduce tenocyte and tendon strength in an effort to maintain homeostatic tension. At physiological levels of mechanical stress (centre), internal and external mechanisms of mechanostransduction are engaged, resulting in increased formation and activation of focal adhesions and the buffering of internal mechanical stress by talin. Actin tension is converted to the activation of YAP and TAZ, whereas Scx is activated via post-translational modification to induce the expression of a tenocyte-defining transcriptional programme. Transforming growth factor-β (TGFβ) promotes a tenocyte phenotype and integrin activation, potentially through the proximity of integrins and TGFβ receptor, mediated by tenascin-C. Calcium might also enter the cells through stress-activated ion channels such as Piezo and transient receptor potential vanilloid 4, and might subsequently be transmitted to adjacent cells via gap junctions. Excessive mechanical stress (right-hand side) results in ECM damage and a rapid loss of mechanotransduction in the tenocyte. The result is cell death and the release of danger-associated molecular patterns (DAMPs) and inflammasome-activated cytokines such as IL-1β. These molecules can propagate cell death in adjacent tenocytes and can promote the recruitment of pro-inflammatory cells, which perpetuate the inflammatory cycle by releasing pro-inflammatory molecules such as TNF, IL-6, IL-17 and prostaglandin E2 (PGE2). MMPs, matrix metalloproteinases; TLRs, Toll-like receptors.
But how then is mechanical force translated into chemical signals that induce a cellular response via such transcription factors? Research in the field of mechanobiology has identified a number of mechanisms to achieve this translation of the physical into the chemical, either directly through cellular mechanosensors, or indirectly through mechanical stress-induced release of molecules from the ECM. The following section introduces several mechanisms of mechanotransduction. A detailed discussion is beyond the scope of this Review, but readers are directed towards excellent in depth reviews for further information31,84–87.
Focal adhesions.
A dominant mechanism of direct mechanotransduction in tenocytes is through focal adhesions, which consist of collagen-binding, membrane-embedded integrins and their intracellular adaptor proteins88 (Figure 3). The intracellular domains of integrins are connected to the actin cytoskeleton via an intermediate protein called talin89. Through tensile force-induced rearranging of protein domains, talin can function as both a shock absorber90 and a molecular switch via tension-revealed binding sites for focal adhesion kinase (FAK)91 and focal adhesion stabilizing proteins such as vinculin92. Although not extensively studied in the context of tendon and ligament biology, parts of focal adhesion complexes are known to mediate mechanotransduction. Mechanical force applied to tendon-derived tenocytes in vitro causes an upregulation of focal adhesion components, particularly integrins80,93. Furthermore, in ligament-derived tenocytes, FAK is recruitment to integrin clusters at the cell surface is associated with increased collagen expression94. Interestingly, in vitro stretching of mesenchymal stem cells induces the expression of a pattern of genes found in tenocytes, an upregulation that can be inhibited if parts of focal adhesions complexes, such as FAK, are specifically blocked95.
FAK can also activate the transcription factors YAP and TAZ96. These ubiquitous mechanical stress-sensing transcription factors can also be activated by changes to the nucleus-associated cytoskeleton that occur during nuclear deformation97. The activation of YAP and TAZ induces the expression of a range of genes, including those encoding focal adhesion complex components98 and those that regulate apoptosis and cell proliferation99. The roles of YAP and TAZ in tendons and ligaments are not well defined; however, these transcription factors are known to be important in regulating muscle fibre size100 and to protect against experimental osteoarthritis in mice by interfering with the transcription factor NF-κB101. Furthermore, nuclear deformation in tenocytes can occur during mechanical loading of tendons102, suggesting that YAP and TAZ might be activated under such circumstances.
Mechanical stress-activated ion channels.
Cells are able to directly sense mechanical stress through stretch-activated ion channels such as Piezo molecules, which are activated upon cell membrane deformation103. Mechanistically, Piezo molecules selectively allow cations such as calcium into the cytoplasm when activated. Although Piezo activity in tenocytes has yet to be studied, it is worth noting that Piezo molecules have an important role in chondrocyte mechanosensing104. Interestingly, individuals with PIEZO2 mutations develop joint contractures and scoliosis105; however, it should be noted that this phenotype is probably caused by altered proprioceptor function106. Transient receptor potential vanilloid 4 (TRPV4) is another stretch-activated ion channel that has a role in mechanical sensing via calcium flux107; however, TRPV4 has also yet to be studied in tenocytes.
Calcium signalling itself, such as that facilitated by stretch-activated ion channels, probably has an important role in tenocyte mechanotransduction108,109 (Figure 3). A transient increase in intracellular calcium can be translated into molecular signals through calcium-binding molecules such as calmodulin110 and the calpain enzymes111, which can cleave molecules associated with focal adhesions112. Intracellular calcium flux can also be passively transmitted to adjacent cells through gap junction-mediated cytoplasm sharing113. In tenocytes, physiological levels of mechanical stress promotes gap junction permeability, whereas excessive mechanical stress is inhibitory114,115. The functional relevance of gap junctions in tenocytes is not yet clear, but the longitudinal arrangement of inter-fascicle tenocytes suggests that cell–cell contact is important116.
Mechanical stress-induced cytokines.
Another method of relaying mechanical stress signals between adjacent cells is through the release of cytokines and signalling molecules, such as prostaglandin E2 (PGE2), TNF and TGFβ117. For example, the tenocyte stem cell phenotype is reinforced by low concentrations of PGE2, whereas high concentrations promote transition to an osteoblast phenotype118. In vitro, TNF reduces collagen protein expression in human tenocytes while increasing the expression of genes encoding MMPs119. Furthermore, TNF induces the expression of integrins and pro-inflammatory cytokines (such as IL-6 and IL-8) in cultured human tenocytes120. In tenocytes, Mkx and Scx control the expression of TGFβ, which is incorporated into the ECM in an inactive form43 (Figure 3). TGFβ can be released and activated by exposure to proteases, interaction of integrins with the ECM or by mechanical shearing of the ECM19,121. Physiological concentrations of TGFβ reduce tenocyte proliferation and promote collagen expression at the gene and protein levels and SCX expression in rabbit and rat tenocytes in vitro122,123. TGFβ receptor signalling can also activate integrins to promote their adhesion to the ECM121. By contrast, high concentrations of TGFβ promote tenocyte death79, which occurs within hours of tendon transection in vivo and within one day of tendon microdamage (induced by low level loading ex vivo)79,124. Mechanical stress-induced tenocyte cell death causes the release of a range of potent cell activators, including IL-1β, probably through inflammasome activation125 (Figure 3). Indeed, both tenocytes exposed to mechanical stress and tendinopathic tissues express inflammasome components and active IL-1β126–128. Interestingly, IL-1β can function in an paracrine manner to trigger an anabolic response in adjacent tenocytes129–131. Cell death can also release Toll-like receptor (TLR)-activating danger-associated molecular patterns (DAMPs). The DAMP, HMGB1, is found in damaged tendons and can induce the expression of inflammatory molecules including IL-1β, IL-6 and CCL223. Relevant to chronic inflammatory arthritis, monosodium urate (MSU) crystals are another class of DAMP that can be found at the enthesis in patients with gout132 and can induce new bone formation in mice133. MSU crystals rupture cell membranes, causing cell death, inflammasome activation and the subsequent release of IL-1β134.
ECM-mediated tenocyte activation.
Mechanical stress can also indirectly activate tenocytes through alterations in the ECM. Fibronectin is a collagen-associated, mechano-sensitive ECM component that is found in tendons and ligaments and is upregulated with injury135–137. DAMPs can be released from fibronectin via enzyme-mediated degradation and mechanical stress can expose cryptic integrin binding sites on collagen fibres that promote cell adhesion to the ECM19 (Figure 3). Tenascin-C is less well-characterized than fibronectin but is highly expressed in healthy and injured tendons and ligaments138. Tenascin-C contains integrin-binding fibronectin domains and TGFβ binding motifs, and fragments of tenascin-C are potent activators of TLR4138,139. Hypothetically, ECM molecules such as tenascin-C function in part to concentrate cytokines and DAMPs at the tenocyte cell surface140.
Such indirect responses of tenocytes to mechanical stress, similar to direct responses, function to elicit a homeostatic response. If mechanical stress exceeds the physiological upper or lower thresholds in tendons and ligaments, tenocyte death can occur within hours32, which will subsequently initiate tissue repair processes.
Tendon and ligament repair processes
Generic tissue repair follows a conserved process141,142: trauma-associated cell death and tissue damage causes the rapid recruitment of immune cells, particularly neutrophils and monocytes, to maintain sterility and clear debris. After the initial insult, macrophages coordinate the deposition of a temporary matrix with the resident stromal cells, which in turn enables a return of tissue to its pre-injury state through stromal cell differentiation. It is worth highlighting the prominent role of the macrophage in the healing process and its capacity to transition through a range of phenotypes depending on the inflammatory milieu143. The tissue healing process is dependent on immune cell polarization; excessive inflammation mediated by T helper 1 (TH1) cells and/or TH17 cells causes necrosis and excessive TH2 cell-mediated inflammation causes fibrosis141. Damage to the tendons and ligaments can effectively be considered sterile inflammation, a process characterized by DAMP-induced IL-1β release144.
As with generic tissue repair, injured tendons and ligaments follow a prescribed tissue repair process16,145 (Figure 4). Initially, 1 to 5 days post-injury, an inflammatory response occurs in which haematoma and neutrophil infiltrates are common. Following this stage, fibroblastic proliferation occurs between 6 days and 6 months post-injury, during which time new collagen (predominantly type III collagen) is synthesized. Finally, collagen remodelling takes place 6 to 12 months post-injury. This repair pathway is potentially perturbed in systemic inflammatory conditions such as RA or SpA; however, no evidence currently exists to support or reject this hypothesis.
Figure 4. The tendon and ligament tissue repair process.
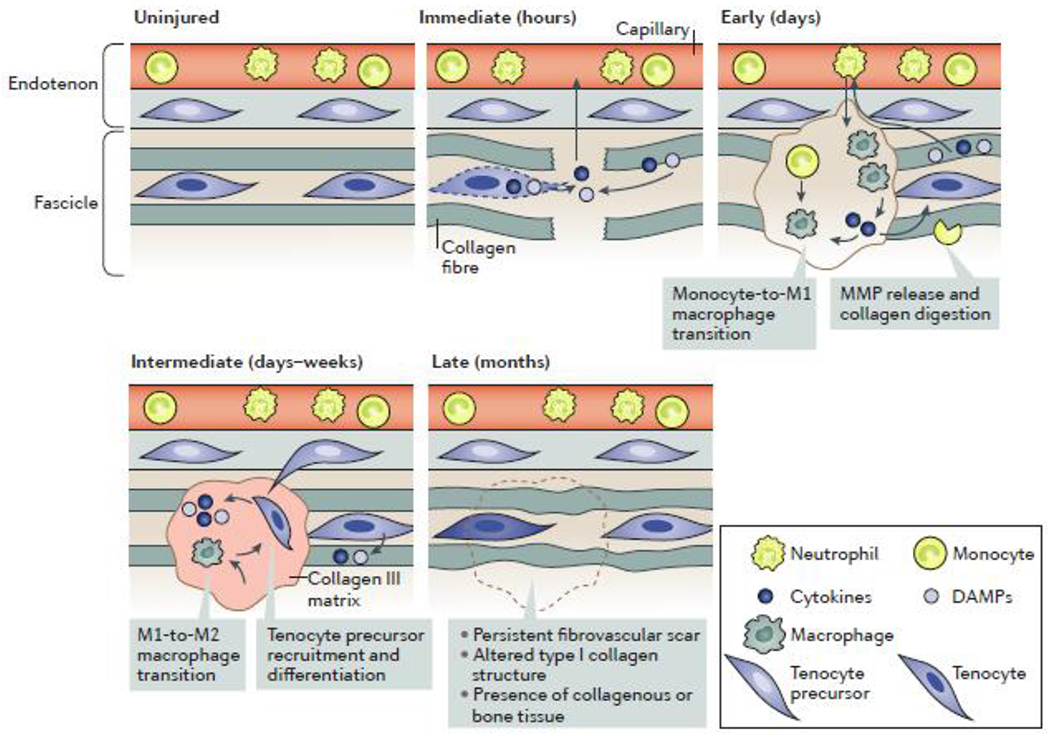
Tenocyte death occurs following unloading of tendons, which happens after rupture and microdamage, causing the release of cytokines and damage-associated molecular patterns (DAMPs). This death results in the rapid activation of adjacent stromal cells and, potentially, tissue-resident immune cells, propelling the injured tissue into a state of repair. The early phase of tendon and ligament repair is an inflammatory phase, characterized by myeloid cell recruitment, the transition of monocytes to pro-inflammatory (M1) macrophages and the removal of apoptotic tenocytes and extracellular matrix (ECM) debris by phagocytosis. Tenocytes and myeloid cells work together to ensure an adequate catabolic response. The intermediate phase of tendon and ligament repair is characterized by a resolution of inflammation and an increase in pro-resolving (M2) macrophages. During this stage, tenocyte precursor cells are recruited into the lesion to assist with ECM repair through the deposition of a temporary type III collagen matrix. In the late phase, this temporary matrix is slowly remodelled to a permanent, type I collagen-dominated matrix. The recovered tissue in adult tendons and ligaments never regains its pre-injury architecture and is frequently scar-like in appearance.
Tendon and ligament healing is initiated by fascicle damage. Acute or chronic tendon injuries cause the unloading of tenocytes through rupture or microdamage, respectively16. Tenocyte death occurs within hours of unloading32,79,146. As discussed above, cell death is associated with the release of pro-inflammatory factors such as PGE2, IL-1β, IL-6 and TNF, which can in turn activate a pro-inflammatory and phagocytic response in myeloid cells14,37,147. In wound healing, resident myeloid cells function as sentinels of tissue damage and are rapidly activated by such pro-inflammatory molecules148. However, whether resident myeloid cells truly exist in healthy tendons and ligaments is not yet clear owing to a scarcity of studies on healthy tissue.
The recruitment of immune cells and aqueous elements of blood creates a haematoma, thereby marking the early stage of wound healing. This stage is characterized by monocyte and neutrophil influx, mediated in part by CCL2. Indeed, running-exacerbated peripheral arthritis is mediated by CCL213 and IVD expression of CCL2 strongly correlates with oedema and fatty lesions in patients with degenerated discs149. These recruited myeloid cells are activated by DAMPs and cytokines in the damaged tissue, triggering the removal of debris via phagocytosis147. Activated myeloid cells further promote the degradation of damaged ECM through MMP release and stimulate the deposition of a temporary matrix of type III collagen32 and fibronectin135 to facilitate tissue healing. During this stage, a positive feedback cycle probably exists between tenocytes and immune cells, which promotes the conversion of monocytes and/or macrophages into a pro-inflammatory, M1-like phenotype120.
Compared with myeloid cells, the role of lymphocytes in tendon and ligament healing is poorly defined. Enthesis-resident populations of lymphocytes exist in mice and humans47,48,150, and lymphocytes are also recruited during injury to tendons and ligaments151. One possibility is that resident or recruited lymphocytes fine-tune the immune response in tendon and ligament repair through their known role in macrophage activation152. It is also important to highlight the role that tissue-resident lymphocytes have in tissue repair and homeostasis, specifically through their promotion of stromal cell function153–155. One possibility is that such a function could be carried out by entheseal-resident lymphocytes; however, this hypothesis has yet to be explored.
During the intermediate stage of tendon and ligament healing, days-to-weeks post-injury, tenocyte progenitor cells differentiate and migrate from the sheath into the damaged tissue following acute rupture32 or chronic overloading156. TGFβ and Scx are crucial for the promotion of tenocyte phenotype during this phase79. Failure of Scx induction in tenocyte progenitor cells promotes a chondrogenic or osteoblastic phenotype43 and, ultimately, ossification at tendon wound sites32. Immunologically, the intermediate phase is characterized by a transition of macrophages from a pro-inflammatory, M1-like phenotype to a pro-resolving, M2-like phenotype35. Pro-resolving macrophages have an increased phagocytic capacity, which enables them to remove collagen, potentially through mannose receptors such as CD206157. These macrophages also have an increased capacity for promoting ECM production compared with pro-inflammatory macrophages143. Interestingly, CD206, a marker of pro-resolving macrophages is highly expressed in patients who are free of pain following tendon surgery, supporting a role for these macrophages in tendon and ligament repair37.
Finally, the tendon and ligament tissue repair process ends with the late remodelling stage, which is characterized by the replacement of the temporary type III collagen matrix with a long-term type I collagen matrix, as well as a reduced cellularity of repaired tissue. In adults, this repaired tissue never regains its pre-damage architecture despite regaining some function16,158,159.
Mechanical stress in disease
The field of musculoskeletal research has used knowledge of the mechanobiology and of the healing process of tendons and ligaments to make advances in understanding the basic biology of tendon and ligament disorders. These disorders cover a spectrum from those of an acute, self-resolving nature, such as strains, to chronic conditions involving tendon and ligament failure (Box 3). The pathophysiology of chronic tendon or ligament inflammation, however, such as occurs in tendinopathy, is less well-understood, in part owing to limitations of current in vivo models16,145.
Box 3. Types of tendinopathy.
Tendinopathy is a broad term to describe pain and decline of tendon and ligament function associated with overuse. Classic examples of tendinopathy include those of moderate severity, including tennis elbow and Achilles tendon inflammation, and more serious injuries, such as tears or ruptures of the rotator cuff. On the basis of available evidence, it can be assumed that the repair process in tendinopathy is similar to acute tendon injury healing, as pro-inflammatory cell types, fibroblast proliferation and type III collagen turnover are present, but for reasons that are poorly understood, the repair process fails to enter the resolution phase, resulting in long-term inflammation and fibrosis151,216.
The two main categories of tendinopathy (or ligament injury) are classified according to their anatomical location52: insertional tendinopathies occur at the entheses, whereas non-insertional tendinopathies occur at the midportion of tendons (for example, 2 to 6 cm proximal to the enthesis of the Achilles tendon)217. In an epidemiological investigation of 1,394 non-athletes presenting at an orthopaedic clinic, Achilles tendinopathy was found in 5.6% of individuals with a roughly equal presentation of insertional and non-insertional types218. Insertional tendinopathy tends to occur more frequently in active individuals, whereas, when controlling for confounding factors, non-insertional tendon injury tends to occur in older, less active and overweight individuals219. By contrast, the majority of ligament injuries seem to occur in the midportion220,221; however, certain ligaments, such as the medial collateral ligament, more commonly fail at the insertion222. These differences in location are seldom addressed in studies, and it is unknown whether mechanisms of pathogenesis differ between insertional and non-insertional tendinopathy or ligament injury.
In this section, we focus on mechanical stress and immune aspects of tendon and ligament failure of the peripheral and axial skeletons, namely tendinopathy and IVD degeneration, respectively. Lessons from these examples are juxtaposed to forms of chronic inflammatory arthritis, in which evidence is emerging for mechanical stress as an environmental trigger for the onset of arthritis, thereby demonstrating the relevance of tendons and ligaments in the pathophysiology of arthritis. Before diving into the discussion, it is prudent to note that although mechanical stress is an important factor for triggering tendinopathy and inflammatory arthritis, it is not the only factor. Inflammatory arthritis has clear genetic and microbial associations160, whereas factors such as inappropriate innervation and vascularization have been proposed as triggers for tendinopathy161.
Tendon and ligament disorders
Tendinopathy versus inflammatory arthritis.
The role of mechanical stress in tendinopathies has been comprehensively characterized by basic research. Broadly speaking, trauma causes a shift in collagen isotype expression and tertiary structure in tendons, as well as the rounding of stromal cells162. In animal models of tendinopathy, adult tendons fail to regain full function and do not regain their pre-injury architecture158. The failure of tendons in humans to completely heal after injury potentially explains why patients with tendinopathy are at risk of re-injury, and why surgical intervention for tendinopathy often fails4,159. Abnormal cartilage and bone formation occurs in response to injury or excessive stress in the axial and peripheral skeletons of mice deficient in Mkx or Scx24,163, highlighting the important role of the tenocyte in tendon and ligament homeostasis. Likewise, in human tendinopathy, hardening of the tendons and ligaments often occurs and can vary in presentation from cartilaginous metaplasia164 to ossification165.
By contrast, less is known about the role of mechanical stress in chronic inflammatory arthritis. Mechanical loading can induce measurable signs of tendon and ligament inflammation, albeit often at a subclinical level; assessments of healthy individuals undergoing intense physical activity, such as athletes or military recruits, often reveal sacro-iliac joint lesions on MRI similar to those seen in patients with SpA166,167. Epidemiologically, physical trauma is associated with the onset of PsA168, and physical workload increases the risk of developing RA169. Indeed, enthesitis is a well-established prodromal symptom in patients with SpA170 and tenosynovitis can be predictive of the development of RA171. Importantly, although both RA and SpA involve bone erosion localized to points of enthesis insertion, only SpA presents with coincident bone formation at these locations. In RA, bone erosions of the small joints of the peripheral skeleton have traditionally been described as ‘peri-articular’172, although a study from the past year has linked the site of erosions to entheseal insertions13. In SpA, new bone formation occurs at peripheral entheses, such as at the Achilles tendon and metacarpophalangeal joint insertions, and at axial entheses, such as the sacro-iliac joint, vertebral facet joints and between vertebral bodies173,174. Peripheral and axial entheses are considered to be a principal site of clinical symptoms and pathological changes in SpA, despite the presence of systemic perturbations to the immune system175,176.
Genetic studies, evidence from animal models and current therapeutic strategies all suggest an important role for systemic inflammation in SpA117 and RA141. Although some of inflammatory pathways (such as TNF signalling) seem to be involved in both RA and SpA, other inflammatory pathways seem to be specific for one disease or the other. For example, the IL-17–IL-23 pathway has a role in active joint inflammation in SpA, whereas it seems to only be involved in early stages of RA, prior to the onset of arthritic symptoms178. This discrepancy is reflected in the efficacy of IL-17 inhibitors in treating SpA179,180 but not RA181. Notably, IL-23 seems to be important in peripheral SpA182 but its involvement in axial SpA is uncertain183. In RA, IL-6, IL-1β and B cells have well-defined roles, as reflected by the success of therapeutics blocking these cytokines and cells184. However, these systemic inflammatory components do not explain why these diseases affect particular anatomical locations in the axial and peripheral joints.
Although a few studies have explored the genetics of tendinopathy, little progress has been made in comparison to the genetic understanding of chronic inflammatory arthritis. Large genetic studies have yet to be performed in patients with tendinopathy to connect common genetic variants to disease. Small-scale studies have linked a few genetic variants to tendinopathy, particularly in genes encoding ECM-related molecules such as collagen, and pro-inflammatory or tissue remodelling molecules such as IL-1β and MMPs185. However, the functional relevance of such variants are unknown. At the transcriptomic level, microarray analyses of tendinopathy tissue samples have revealed changes in the expression of genes related to ECM molecules and ECM-interacting molecules such as integrins, as well as TGFβ and components of IL-1β signalling pathways186.
Despite the suggestions of immune involvement in these genomic studies149,150, and the known role of the immune system in tendon healing (outlined in the previous section), the contribution of the immune system to tendinopathy is not well-understood. A 2018 meta-analysis of tendinopathy reviews concluded that over the previous 5 years, a paradigm shift has occurred in the field of basic tendinopathy research regarding ideas about a bona fide immune contribution to the disease, rather than tendinopathy being viewed purely as a degenerative disorder15. The studies driving this paradigm shift examined haematopoietic cell subsets using general cell markers such as CD68 for macrophages or CD3 for T cells187, which revealed the presence of immune cells in damaged tendons, but few studies have reported a deeper level of immunological analysis. Persistent activation of NF-κB in tenocytes is thought to promote tendinopathy37,188,189; however, it is unclear exactly what is activating this transcription factor, as it can be activated by many pro-inflammatory stimuli, including IL-1β, TNF and IL-17. In early rotator cuff injury, evidence suggests a role for IL-17, which is present in macrophages and mast cells and causes tenocytes to produce IL-6, IL-8 and CCL2151. In addition, a shift in tenocyte phenotype has been identified in tendinopathy, whereby integrins and angiogenic markers are upregulated and the cells become hypersensitive to IL-1β stimulation38,188. In summary, immunological studies of chronic inflammatory arthritis and tendinopathy display remarkable similarities in terms of their molecular mediators. Despite this similarity, research in the two fields has been relatively independent.
Lessons from IVD degeneration.
Notably, the pathology of spinal ligament injury is biologically comparable to that of tendinopathy. Mechanical stress can be a trigger for IVD degeneration through damage to the outer AF25,190, resulting in severe back pain191. As with tendinopathy, injury to the IVD involves ECM degradation and an appreciable immune contribution, with IL-1β and TNF being strongly implicated191. AF stromal cells, like classical tenocytes, also die in response to mechanical stress in the IVD192. In support of a role for inflammation in IVD degeneration, mice that express human TNF and mice that cannot negatively regulate IL-1β both develop spontaneous inflammatory disc degeneration193,194. Mice that lack Mkx also have spontaneous disc degeneration and ectopic bone formation between vertebrae, which can be expedited by exerting mechanical stress on the axial skeleton through prolonged bending of the tail24. Interestingly, new bone growth between vertebral bodies in patients with AS seems to occur in areas associated with the outer AF, and not with longitudinal ligaments195–197. In fact, studies estimate that over 40% of patients with axial SpA have signs of IVD degeneration198,199, and end-stage disease in SpA is characterized by complete fusion of the axial skeleton, making a discussion of IVD degeneration and the outer AF particularly relevant for this disease.
Tendons and ligaments in arthritis
Evidence from mouse models of inflammatory arthritis is generally supportive of tendon and ligament involvement. Although some models have mild tendon and ligament inflammation, such as in aged DBA1 mice200, LysM-cre.A20 mice (which have a conditional knockout of A20 in myeloid cells)201, TNFΔARE mice202 (which have a dysregulation of TNF mRNA, resulting in systemic TNF overexpression) or mice with collagen antibody induced arthritis (CAIA)150, other models have severe entheseal inflammation, such as in SKG mice203 or B10.RIII mice with plasmid-induced overexpression of IL-23150. In addition to revealing the important roles of cytokines such as TNF and IL-23 in tendon and ligament-associated arthritis, studies in mouse models of disease also led to the discovery of a population of enthesis-resident γδ T cells47. Studies from the past few years have also demonstrated that mechanical stress can exacerbate inflammatory arthritis in some of these mouse models. Hind limb unloading to relieve mechanical stress in TNFΔARE mice and in mice with collagen-induced arthritis (CIA) limited enthesitis in these animals13,204. By contrast, increasing mechanical stress by allowing mice to undergo voluntary running accelerated the onset and worsened the severity of arthritis in mice with CIA, mice with CAIA and in TNFΔARE mice13. Notably, these studies do not report an effect of mechanical stress on systemic immunity, as reflected by equivalent autoantibody titres with or without mechanical loading in mice13. However, surprisingly, RAG knockout mice, which are deficient in T cells and B cells, still developed mechanical stress-exacerbated arthritis13,204, ruling out a role for adaptive immune cells in the initiating phases of disease.
Given the importance of tenocytes in responses to mechanical stress, questions arise as to the extent of tendon and ligament stromal cell involvement in inflammatory arthritis. Currently, little data is available to address this question. An elegant study involving TNFRI deficiency in non-haematopoetic cells revealed an essential role for TNF in stromal cells in the onset of arthritis in TNFΔARE mice202. However, this study did not specifically address tenocytes, as the authors used a type VI collagen-Cre system to delete TNFRI in a range of stromal cells, including those in the bone, cartilage and muscle205. Further evidence of altered tendon and ligament stromal cells in inflammatory arthritis comes from the observation that CCL2 is induced in mechanically stressed tendons13. CCL2 is also induced by mechanical stress of tenocytes in vitro13. Finally, mice with CAIA undergoing voluntary running developed arthritis in the absence of an LPS boost, which is usually required after anti-collagen antibody administration13. This result suggests that mechanical stress-induced activation of complement206, or release of DAMPs, such as HMGB1, from injured tendons23,126 might contribute to the focusing of systemic inflammation at the tendons and ligaments.
To fully address the question of how mechanical stress-induced inflammation is diverted from resolution of injury to joint destruction in inflammatory arthritis (Figure 5), it is important to highlight that the dominant location of articular inflammation differs by disease: in SpA, inflammation is predominantly entheseal, whereas in RA it is synovial207. This difference in location suggests that the underlying immune perturbations in SpA and RA promote different mechanisms of pathogenesis in each disease. In SpA, mechanical stress-induced microdamage might fail to completely heal owing to systemic increases in IL-17–IL-23-mediated immunity, resulting in ectopic bone formation as the tissue fails to adapt to subsequent mechanical loading. In RA, tendon and ligament damage could trigger the deposition of autoantibodies in the synovioentheseal complex, after which epitope spreading could facilitate the transition of inflammation to the synovium. Such a provocative hypothesis requires mechanistic studies that interface immunology and biomechanics to fully understand the relationship between mechanical load and chronic inflammatory arthritis.
Figure 5. Proposed model of the relationship between mechanical stress and inflammatory arthritis.
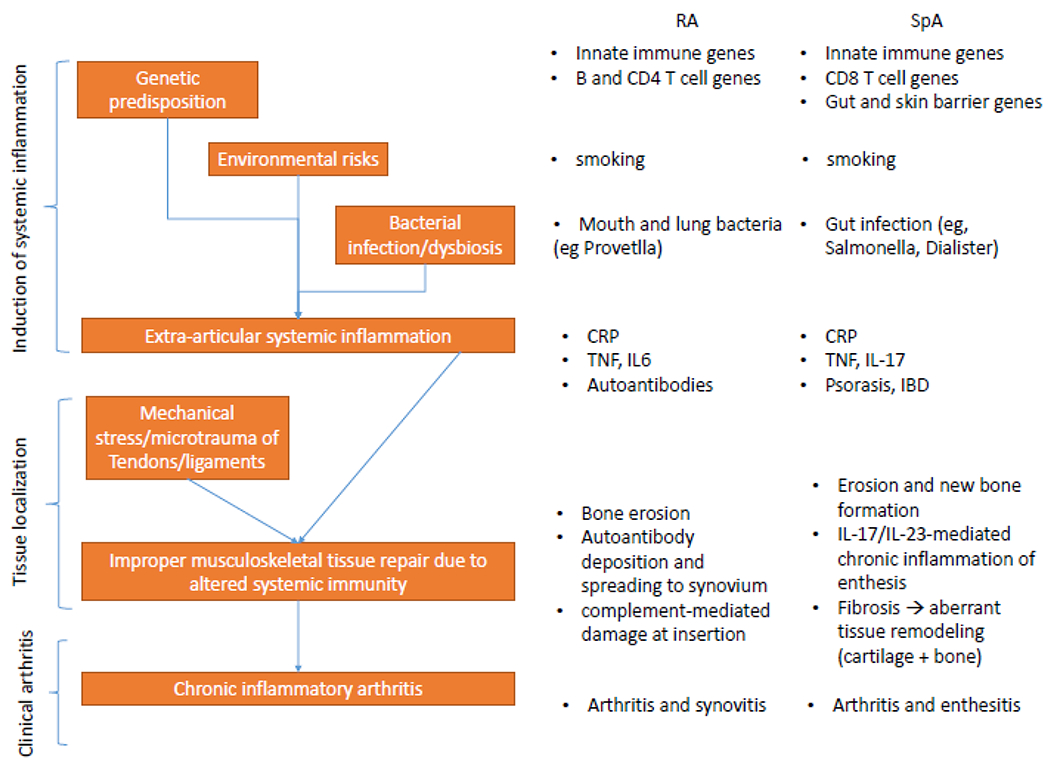
Chronic inflammatory arthritis, including rheumatoid arthritis (RA) and spondyloarthritis (SpA), has defined genetic and environmental risk factors, but the relative contribution of these factors to the onset of systemic inflammation differs between diseases. Smoking is a well-recognized environmental risk factor for both SpA and RA, as are bacterial infections of barrier surfaces, albeit at different anatomical locations in RA and SpA. Genetic and environmental risk factors synergize to promote systemic inflammation that has both shared and distinct features in RA and SpA. The normal healing process that occurs after mechanical stress-induced microdamage at entheseal sites is hijacked by perturbed systemic immunity resulting in sustained entheseal inflammation and aberrant tissue healing. In RA, the result is bone erosion, probably as a result of protracted osteoclast activation in the subchondral bone adjacent to the enthesis. In SpA, subchondral bone erosion occurs coincident to entheseal fibrosis and subsequent ossification, possibly owing to persistent tenocyte activation through the IL-17–NF-κB axis. IBD, inflammatory bowel disease.
Conclusions
Musculoskeletal disorders are a substantial burden to society5–7 therefore it is paramount that the mechanobiology of tendons and ligaments be understood. Tools and knowledge are being developed in the fields of biomechanics, cellular biology and immunology that, when combined, will provide great insight into musculoskeletal diseases of seemingly diverse origins, such as tendinopathy and chronic inflammatory arthritis. Homeostatic responses to mechanical stress is a fundamental concept in biology: a concept that involves elements of the immune system. Although an appreciation for immune involvement in mechanical stress-associated disorders of tendons and ligaments of the peripheral and axial joints is emerging, the underlying immunopathogenesis is poorly understood in comparison to inflammatory arthritis, which could serve as inspiration for future tendinopathy research. Similarly, mechanical stress is an important co-factor that should be considered in future studies of inflammatory arthritis, especially during the initiating phases. In vitro and in vivo models of mechanical stress that are used routinely in tendinopathy research can be leveraged to properly understand the interaction between perturbed immunity and mechanical stress in the onset of arthritis. Undoubtedly, future research will shed new light on responses to mechanical stress in musculoskeletal diseases, which will uncover novel therapeutic opportunities.
Key points.
Mechanical loading is a biological stressor that elicits a homeostatic response to ensure the health and survival of the cells and/or tissues it is applied to.
Tissues that encounter high amounts of mechanical stress are prone to damage, especially the tendon and ligament entheses.
The immune system is crucial in responding to and orchestrating the repair of damaged tendons and ligaments.
Mechanical loading is a well-defined factor in the immunopathology of tendon and ligament disorders such as tendinopathy.
Mechanical loading is associated with the onset of chronic inflammatory arthritis, including spondyloarthritis (SpA) and rheumatoid arthritis (RA).
Microdamage associated with mechanical loading potentially focuses systemic autoimmune disease on the joint in the initiating phases of SpA and RA.
Acknowledgements
The work of I.M. is supported by the Versus Arthritis Centre of Excellence for Rheumatoid Arthritis Pathogenesis. The work of H.A. is supported by the Japan Agency for Medical Research and Development (Core Research for Evolutional Science and Technology grants) and National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR050631 and AR065379). The work of D.E. is supported by FWO-VI, Research Council of Ghent University and Interuniversity Attraction Pole grant Devrepair from Belspo Agency (project P7/07) and an FWO Excellence of Science (EOS) Grant.
Competing interests
G.S. declares he has received funding from BMS, Celgene, Janssen, Novartis and UCB in the area of psoriatic arthritis research. R.L. declares he has received consultancy, speaker’s fees or research support from Celgene, Eli-Lilly, Janssen, Novartis and UCB in the areas of psoriatic arthritis and spondyloarthritis research. I.M. declares he has received funding from BMS, Celgene, Janssen, Novartis and UCB in the area of psoriatic arthritis research. D.E. declares he has received funding from Boehringer Ingelheim, Janssen, Novartis and Pfizer in the areas of psoriatic arthritis and spondyloarthritis research. The other authors declare no competing interests.
Glossary terms
- Force
A vector quantity that describes the action of one structure on another; measured in Newtons.
- Strain
Deformation that occurs at a point in a structure under loading; measured as the percentage change in length from the resting state.
- Load
The sum of all force components acting on an object or body.
- Mechanical stress
Mechanical load acting on cells or tissues as a physical stressor that elicits a biological response to ensure homeostasis.
- Stress
The force per unit area that develops within a structure in response to externally applied loads; measured in Newtons per m2 or Megapascals.
- Periosteum
A fibrous tissue that envelops non-joint surfaces of bone and contains supportive tissue for the cortical bone, such as blood vessels and nerves.
- Metaphyses
The flare (or cone-shaped) portion of long bones that connects the diaphysis to the growth plate.
- Diaphyses
The conical shaft of long bones.
- Epiphyses
The portion of bones above the growth plate that interfaces with other bones to form the joint.
- Strength
The maximum amount of force that a material can absorb before failure.
- Modulus
The ratio of stress to strain in the elastic region of a stress–strain curve.
- Stiffness
The ratio of load to elongation in the elastic region of a stress–strain curve.
- Mechanotransduction
The processes through which cells sense mechanical force and translate it into biological responses.
- ToC blurb
Mechanical load is an important factor in the development of tendon and ligament disorders. In this Review, the authors discuss the evidence for the known role of mechanical load in tendinopathy and its potential role in inflammatory arthritis.
Footnotes
Peer review information
Nature Reviews Rheumatology thanks A. Traweger and the other anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Thomopoulos S, Genin GM & Galatz LM The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing -. J. Musculoskelet. Neuronal Interact. 10, 35–45 (2010). [PMC free article] [PubMed] [Google Scholar]
- 2.Genin GM et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J 97, 976–985 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu HH & Thomopoulos S Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng 15, 201–226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolakos J et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 4, 333–342 (2014). [PMC free article] [PubMed] [Google Scholar]
- 5.United States Department of Labor. 2018 Survey of occupational injuries and illness. US Bureau of Labor Statistics; https://www.bls.gov/iif/soii-charts-2018.pdf (2019). [Google Scholar]
- 6.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute of Neurological Disorders and Stroke. Low back pain fact sheet. National Institute of Neurological Disorders and Stroke https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Low-Back-Pain-Fact-Sheet (2019).
- 8.Aletaha D et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Taurog JD, Chhabra A & Colbert RA Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med 374, 2563–2574 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Ritchlin CT, Colbert RA & Gladman DD Psoriatic arthritis. N. Engl. J. Med 376, 957–970 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis 68, 770–776 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Rudwaleit M et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis 70, 25–31 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Cambré I et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun 9, 4613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar NL, Murrell GAC & McInnes IB Inflammatory mechanisms in tendinopathy – towards translation. Nat. Rev. Rheumatol 13, 110–122 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Mosca MJ et al. Trends in the theory that inflammation plays a causal role in tendinopathy: a systematic review and quantitative analysis of published reviews. BMJ Open Sport Exerc. Med 4, e000332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snedeker JG & Foolen J Tendon injury and repair – A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 63, 18–36 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Duthon VB et al. Anatomy of the anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc 14, 204–213 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Kannus P Structure of the tendon connective tissue. J Med Sci Sport 10, 312–320 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Vogel V Unraveling the mechanobiology of extracellular matrix. Annu. Rev. Physiol 80, 353–387 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Screen HRC, Berk DE, Kadler KE, Ramirez F & Young MF Tendon functional extracellular matrix. J. Orthop. Res 33, 793–799 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon JH & Halper J Tendon proteoglycans: biochemistry and function. J. Musculoskelet. Neuronal Interact 5, 22–34 (2005). [PubMed] [Google Scholar]
- 22.Benjamin M & Ralphs JR Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J. Anat 193 (Pt 4), 481–494 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbar M et al. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open 3, e000456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamichi R et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat. Commun 7, 12503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torre OM, Mroz V, Bartelstein MK, Huang AH & Iatridis JC Annulus fibrosus cell phenotypes in homeostasis and injury: implications for regenerative strategies. Ann. N. Y. Acad. Sci 1442, 61–78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukunami C et al. Scleraxis is a transcriptional activator that regulates the expression of Tenomodulin, a marker of mature tenocytes and ligamentocytes. Sci. Rep 8, 3155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweitzer R et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Ito Y et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. U. S. A 107, 10538–10542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levay AK et al. Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circ. Res 103, 948–956 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muir T, Sadler-Riggleman I & Skinner MK Role of the basic helix-loop-helix transcription factor, scleraxis, in the regulation of sertoli cell function and differentiation. Mol. Endocrinol 19, 2164–2174 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Lavagnino M et al. Tendon mechanobiology: Current knowledge and future research opportunities. J. Orthop. Res 33, 813–822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakabe T et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J. Biol. Chem 293, 5766–5780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walia B & Huang AH Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J. Orthop. Res 37, 1270–1280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider M, Angele P, Järvinen TAH & Docheva D Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Advanced Drug Delivery Reviews 129, 352–375 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Sugg KB, Lubardic J, Gumucio JP & Mendias CL Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J. Orthop. Res 32, 944–951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dakin SG et al. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One 7, e32333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dakin SG et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med 7, 311ra173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dakin SG et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med 52, 359–367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowin SC & Doty SB Tissue Mechanics. 559–594 (Springer; New York, 2007). [Google Scholar]
- 40.Amiel D, Frank C, Harwood F, Fronek J & Akeson W Tendons and ligaments: A morphological and biochemical comparison. J. Orthop. Res 1, 257–265 (1983). [DOI] [PubMed] [Google Scholar]
- 41.Kharaz YA, Canty-Laird EG, Tew SR & Comerford EJ Variations in internal structure, composition and protein distribution between intra- and extra-articular knee ligaments and tendons. J. Anat 232, 943–955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakai T et al. CD146 defines commitment of cultured annulus fibrosus cells to express a contractile phenotype. J. Orthop. Res 34, 1361–1372 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Subramanian A & Schilling TF Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development 142, 4191–204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Can T et al. Proteomic analysis of laser capture microscopy purified myotendinous junction regions from muscle sections. Proteome Sci 12, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benjamin M & McGonagle D The enthesis organ concept and its relevance to the spondyloarthropathies. Adv. Exp. Med. Biol 649, 57–70 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Sharma P & Maffulli N Basic biology of tendon injury and healing. Surg. 3, 309–316 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Reinhardt A et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 68, 2476–2486 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Cuthbert RJ et al. Brief report: Group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 69, 1816–1822 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Cuthbert RJ et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann. Rheum. Dis 78, 1559–1565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bridgewood C et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann. Rheum. Dis 78, 929–933 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nigg BM & Herzog W Biomechanics of the musculo-skeletal system, 3rd edn (Wiley, 2007). [Google Scholar]
- 52.Scott SH & Winter DA Internal forces at chronic running injury sites. Med. Sci. Sports Exerc 22, 357–369 (1990). [PubMed] [Google Scholar]
- 53.Thompson J & Baravarian B Acute and chronic Achilles tendon ruptures in athletes. Clinics in Podiatric Medicine and Surgery 28, 117–135 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Zitnay JL & Weiss JA Load transfer, damage, and failure in ligaments and tendons. J. Orthop. Res 36, 3093–3104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wall ME et al. in Metabolic influences on risk for tendon disorders (eds. Ackermann PW & Hart DA) 79–95 (Springer, 2016). [Series eds. Crusio WE, Lambris JD & Rezaei N Advances in experimental medicine and biology] [Google Scholar]
- 56.Wall M et al. Key developments that impacted the field of mechanobiology and mechanotransduction. J. Orthop. Res 36, 605–619 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Jung H-J, Fisher MB & Woo SL-Y Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med. Arthrosc. Rehabil. Ther. Technol 1, 9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JH-C, Guo Q & Li B Tendon biomechanics and mechanobiology—a minireview of basic concepts and recent advancements. J. Hand Ther 25, 133–141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelzer E, Blitz E, Killian ML & Thomopoulos S Tendon-to-bone attachment: From development to maturity. Birth Defects Res. Part C Embryo Today Rev. 102, 101–112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook J & Purdam C Is compressive load a factor in the development of tendinopathy? Br. J. Sports Med 46, 163–168 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Ryzewicz M & Wolf JM Trigger digits: Principles, management, and complications. J. Hand Surg. Am 31, 135–146 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Docking S, Samiric T, Scase E, Purdam C & Cook J Relationship between compressive loading and ECM changes in tendons. Muscles. Ligaments Tendons J. 3, 7–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marras WS, Walter BA, Purmessur D, Mageswaran P & Wiet MG The contribution of biomechanical-biological interactions of the spine to low back pain. Hum. Factors J. Hum. Factors Ergon. Soc 58, 965–975 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Wang JH-C Mechanobiology of tendon. J. Biomech 39, 1563–1582 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Grant TM, Thompson MS, Urban J & Yu J Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J. Anat 222, 573–579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grant TM, Yapp C, Chen Q, Czernuszka JT & Thompson MS The mechanical, structural, and compositional changes of tendon exposed to elastase. Ann. Biomed. Eng 43, 2477–2486 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Alexander RM Elastic energy stores in running vertebrates. Am. Zool 24, 85–94 (1984). [Google Scholar]
- 68.Martin RB, Burr DB, Sharkey NA & Fyhrie DP Skeletal Tissue Mechanics 175–225 (Springer; New York, 2015). [Google Scholar]
- 69.Thomopoulos S, Williams GR, Gimbel JA, Favata M & Soslowsky LJ Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res 21, 413–419 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Xu Y & Murrell GAC The basic science of tendinopathy. Clin. Orthop. Relat. Res 466, 1528–1538 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burssens A et al. Arguments for an increasing differentiation towards fibrocartilaginous components in midportion Achilles tendinopathy. Knee Surgery, Sport. Traumatol. Arthrosc 21, 1459–1467 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Maffulli N, Barrass V & Ewen SWB Light microscopic histology of achilles tendon ruptures. Am. J. Sports Med 28, 857–863 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Svensson RB, Heinemeier KM, Couppé C, Kjaer M & Magnusson SP Effect of aging and exercise on the tendon. J. Appl. Physiol 121, 1353–1362 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Momersteeg TJA et al. The effect of variable relative insertion orientation of human knee bone-ligament-bone complexes on the tensile stiffness. J. Biomech 28, 745–752 (1995). [DOI] [PubMed] [Google Scholar]
- 75.Attarian DE, McCrackin HJ, DeVito DP, McElhaney JH & Garrett WE Biomechanical characteristics of human ankle ligaments. Foot Ankle 6, 54–8 (1985). [DOI] [PubMed] [Google Scholar]
- 76.Corps AN et al. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology 45, 291–294 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Galloway MT, Lalley AL & Shearn JT The role of mechanical loading in tendon development, maintenance, injury, and repair. J. Bone Joint Surg. Am 95, 1620–1628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayama T et al. Gtf2ird1-dependent mohawk expression regulates mechanosensing properties of the tendon. 36, 1297–1309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeda T et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol 21, 933–941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nichols AEC, Settlage RE, Werre SR & Dahlgren LA Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol. 19, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abe H et al. Scleraxis modulates bone morphogenetic protein 4 (BMP4)-Smad1 protein-smooth muscle α-actin (SMA) signal transduction in diabetic nephropathy. J. Biol. Chem 287, 20430–20442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bagchi RA, Wang R, Jahan F, Wigle JT & Czubryt MP Regulation of scleraxis transcriptional activity by serine phosphorylation. J. Mol. Cell. Cardiol 92, 140–148 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Léjard V et al. Scleraxis and NFATc regulate the expression of the pro-α1(I) collagen gene in tendon fibroblasts. J. Biol. Chem 282, 17665–17675 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Kechagia JZ, Ivaska J & Roca-Cusachs P Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol 20, 457–473 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Douguet D & Honoré E Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell 179, 340–354 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Eyckmans J, Boudou T, Yu X & Chen CS A hitchhiker’s guide to mechanobiology. Dev. Cell 21, 35–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martino F, Perestrelo AR, Vinarský V, Pagliari S & Forte G Cellular mechanotransduction: from tension to function. Front. Physiol 9, 824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jokinen J et al. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem 279, 31956–31963 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Goult BT, Yan J & Schwartz MA Talin as a mechanosensitive signaling hub. J. Cell Biol 217, 3776–3784 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao M et al. The mechanical response of talin. Nat. Commun 7, 11966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das M, Ithychanda S, Qin J & Plow EF Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim. Biophys. Acta 1838, 579–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bays JL & DeMali KA Vinculin in cell-cell and cell-matrix adhesions. Cell. Mol. Life Sci 74, 2999–3009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popov C et al. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol 16, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tetsunaga T et al. Mechanical stretch stimulates integrin αVβ3-mediated collagen expression in human anterior cruciate ligament cells. J. Biomech 42, 2097–2103 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Xu B et al. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol 227, 2722–2729 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Totaro A, Panciera T & Piccolo S YAP/TAZ upstream signals and downstream responses. Nature Cell Biology 20, 888–899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elosegui-Artola A et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Nardone G et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun 8, 15321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aragona M et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Watt KI et al. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat. Commun 6, 6048 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Deng Y et al. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun 9, 4564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freedman BR et al. Dynamic loading and tendon healing affect multiscale tendon properties and ECM stress transmission. Sci Rep 8, 10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murthy SE, Dubin AE & Patapoutian A Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol 18, 771–783 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Rocio Servin-Vences M, Moroni M, Lewin GR & Poole K Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rolfe RA et al. Abnormal fetal muscle forces result in defects in spinal curvature and alterations in vertebral segmentation and shape. J. Orthop. Res 35, 2135–2144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woo S-H et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci 18, 1756–1762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilchrist CL et al. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci 116, 1992–1997 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wall ME & Banes AJ Early responses to mechanical load in tendon: Role for calcium signaling, gap junctions and intercellular communication. Journal of Musculoskeletal Neuronal Interactions 5, 70–84 (2005). [PubMed] [Google Scholar]
- 109.Arnoczky SP et al. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: The effects of strain frequency, strain magnitude, and cytosolic calcium. J. Orthop. Res 20, 947–952 (2002). [DOI] [PubMed] [Google Scholar]
- 110.O’Day DH CaMBOT: profiling and characterizing calmodulin-binding proteins. Cell. Signal 15, 347–354 (2003). [DOI] [PubMed] [Google Scholar]
- 111.Ono Y & Sorimachi H Calpains — An elaborate proteolytic system. Biochim. Biophys. Acta - Proteins Proteomics 1824, 224–236 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Li J et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evans WH & Martin PEM Gap junctions: structure and function (Review). Mol. Membr. Biol 19, 121–136 (2002). [DOI] [PubMed] [Google Scholar]
- 114.Maeda E et al. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol 11, 439–447 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Maeda E & Ohashi T Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem. Biophys. Res. Commun 465, 281–286 (2015). [DOI] [PubMed] [Google Scholar]
- 116.McNeilly CM, Banes AJ, Benjamin M & Ralphs JR Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat 189 (Pt 3), 593–600 (1996). [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J & H-C Wang J Production of PGE 2 increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res 28, 198–203 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Zhang J & Wang JH-C Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PLoS One 9, e87706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.John T et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J. Orthop. Res 28, 1071–1077 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Stolk M et al. New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Sci. Rep 7, 9801 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Munger JS & Sheppard D Cross talk among TGF- signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol 3, a005017–a005017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klein MB, Yalamanchi N, Pham H, Longaker MT & Chan J Flexor tendon healing in vitro: Effects of TGF-β on tendon cell collagen production. J. Hand Surg. Am 27, 615–620 (2002). [DOI] [PubMed] [Google Scholar]
- 123.Mendias CL, Gumucio JP & Lynch EB Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fibroblasts. J. Appl. Physiol 113, 56–62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stauber T, Blache U & Snedeker JG Tendon tissue microdamage and the limits of intrinsic repair. Matrix Biol 10.1016/j.matbio.2019.07.008 (2019). [DOI] [PubMed] [Google Scholar]
- 125.Bergsbaken T, Fink SL & Cookson BT Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol 7, 99–109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thankam FG et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci. Rep 8, 8918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berthet E et al. Smad3 binds scleraxis and mohawk and regulates tendon matrix organization. J. Orthop. Res 31, 1475–1483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Q et al. Cyclic stretching exacerbates tendinitis by enhancing NLRP3 inflammasome activity via F-actin depolymerization. Inflammation 41, 1731–1743 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Zhang K, Asai S, Yu B & Enomoto-Iwamoto M IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun 463, 667–672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Archambault J, Tsuzaki M, Herzog W & Banes AJ Stretch and interleukin-1β induce matrix metalloproteinases in rabbit tendon cells in vitro. J. Orthop. Res 20, 36–39 (2002). [DOI] [PubMed] [Google Scholar]
- 131.Tsuzaki M et al. IL-1β induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J. Orthop. Res 21, 256–264 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Dalbeth N et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann. Rheum. Dis 72, 1545–1548 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Czegley C et al. A model of chronic enthesitis and new bone formation characterized by multimodal imaging. Dis. Model. Mech 11, dmm034041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He Y, Hara H & Núñez G Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences 41, 1012–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jozsa L et al. Fibronectin and laminin in Achilles tendon. Acta Orthop. Scand 60, 469–71 (1989). [DOI] [PubMed] [Google Scholar]
- 136.McKee TJ, Perlman G, Morris M & Komarova SV Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci. Rep 9, 10542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kubow KE et al. Mechanical forces regulate the interactions of fibronectin and collagen i in extracellular matrix. Nat. Commun 6, 8026 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Midwood KS, Chiquet M, Tucker RP & Orend G Tenascin-C at a glance. J. Cell Sci 129, 4321–4327 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Zuliani-Alvarez L et al. Mapping tenascin-C interaction with toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat. Commun 8, 1595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Midwood KS & Orend G The role of tenascin-C in tissue injury and tumorigenesis. Journal of Cell Communication and Signaling 3, 287–310 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eming SA, Wynn TA & Martin P Inflammation and metabolism in tissue repair and regeneration. Science 356, 1026–1030 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Eming SA et al. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med 6, 265sr6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wynn TA & Vannella KM Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen GY & Nuñez G Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol 10, 826–837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomopoulos S, Parks WC, Rifkin DB & Derwin KA Mechanisms of tendon injury and repair. J. Orthop. Res 33, 832–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scott A et al. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br. J. Sports Med 39, e25 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Manning CN et al. The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J. Orthop. Res 32, 645–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Minutti CM, Knipper JA, Allen JE & Zaiss DMW Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol 61, 3–11 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Dudli S et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur. Spine J 26, 1362–1373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sherlock JP et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+ CD4− CD8− entheseal resident T cells. Nat. Med 18, 1069–1076 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Millar NL et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep 6, 27149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mosser DM & Edwards JP Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 8, 958–969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fan X & Rudensky AY Hallmarks of tissue-resident lymphocytes. Cell 164, 1198–1211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Constantinides MG et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jameson J & Havran WL Skin γδ T-cell functions in homeostasis and wound healing. Immunological Reviews 215, 114–122 (2007). [DOI] [PubMed] [Google Scholar]
- 156.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB & Brooks SV Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J. Orthop. Res 30, 606–612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Madsen DH et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 202, 951–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Howell K et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci. Rep 7, 45238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Voleti PB, Buckley MR & Soslowsky LJ Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng 14, 47–71 (2012). [DOI] [PubMed] [Google Scholar]
- 160.Ranganathan V, Gracey E, Brown MA, Inman RD & Haroon N Pathogenesis of ankylosing spondylitis-recent advances and future directions. Nat. Rev. Rheumatol 13, 359–367 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Rees JD, Wilson AM & Wolman RL Current concepts in the management of tendon disorders. Rheumatology 45, 508–521 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Dean BJF, Franklin SL & Carr AJ A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res. 1, 158–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Agarwal S et al. Scleraxis-lineage cells contribute to ectopic bone formation in muscle and tendon. Stem Cells 35, 705–710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khan KM, Cook JL, Bonar F, Harcourt P & Åstrom M Histopathology of common tendinopathies. Update and implications for clinical management. Sport. Med 27, 393–408 (1999). [DOI] [PubMed] [Google Scholar]
- 165.O’Brien EJO, Frank CB, Shrive NG, Hallgrímsson B & Hart DA Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol 93, 319–331 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Varkas G et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study. Rheumatology 57, 508–513 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Weber U et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes. Arthritis Rheumatol. 70, 736–745 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Thorarensen SM et al. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis 76, 521–525 (2017). [DOI] [PubMed] [Google Scholar]
- 169.Zeng P, Klareskog L, Alfredsson L & Bengtsson C Physical workload is associated with increased risk of rheumatoid arthritis: results from a Swedish population-based case–control study. RMD Open 3, e000324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Watad A et al. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front. Immunol 9, 2668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mankia K et al. Identification of a distinct imaging phenotype may improve the management of palindromic rheumatism. Ann. Rheum. Dis 78, 43–50 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Deal C Bone loss in rheumatoid arthritis: systemic, periarticular, and focal. Curr. Rheumatol. Rep 14, 231–237 (2012). [DOI] [PubMed] [Google Scholar]
- 173.Lories RJU & Schett G Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum. Dis. Clin. NA 38, 555–567 (2012). [DOI] [PubMed] [Google Scholar]
- 174.Finzel S et al. Inflammatory bone spur formation in psoriatic arthritis is different from bone spur formation in hand osteoarthritis. Arthritis Rheumatol. 66, 2968–2975 (2014). [DOI] [PubMed] [Google Scholar]
- 175.Sieper J & Poddubnyy D Axial spondyloarthritis. The Lancet 390, 73–84 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Veale DJ & Fearon U The pathogenesis of psoriatic arthritis. The Lancet 391, 2273–2284 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Malmström V, Catrina AI & Klareskog L The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol 17, 60–75 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Pfeifle R et al. Regulation of autoantibody activity by the IL-23–TH17 axis determines the onset of autoimmune disease. Nat. Immunol 18, 104–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McInnes IB et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Baeten D et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013). [DOI] [PubMed] [Google Scholar]
- 181.Genovese MC et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann. Rheum. Dis 72, 863–869 (2013). [DOI] [PubMed] [Google Scholar]
- 182.Deodhar A et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 391, 2213–2224 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Baeten D et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis 77, 1295–1302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burmester GR, Bijlsma JWJ, Cutolo M & McInnes IB Managing rheumatic and musculoskeletal diseases — past, present and future. Nat. Rev. Rheumatol 13, 443–448 (2017). [DOI] [PubMed] [Google Scholar]
- 185.September A, Rahim M & Collins M in Metabolic influences on risk for tendon disorders (eds. Ackermann, P.W. & Hart, D.A.) 109–116 (Springer, 2016). [Series eds. Crusio WE, Lambris JD & Rezaei N Advances in experimental medicine and biology] [Google Scholar]
- 186.Jelinsky SA et al. Regulation of gene expression in human tendinopathy. BMC Musculoskelet. Disord 12, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dean BJF, Dakin SG, Millar NL & Carr AJ Review: Emerging concepts in the pathogenesis of tendinopathy. Surg. 15, 349–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dakin SG et al. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res. Ther. 19, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abraham AC et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Sci. Transl. Med 11, eaav4319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Iatridis JC, Michalek AJ, Purmessur D & Korecki CL Localized intervertebral disc injury leads to organ level changes in structure, cellularity, and biosynthesis. Cell. Mol. Bioeng 2, 437–447 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Risbud MV & Shapiro IM Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol 10, 44–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Walter Yz BA et al. Complex loading affects intervertebral disc mechanics and biology. Osteoarthr. Cartil 19, 1011–1018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gorth DJ, Shapiro IM & Risbud MV Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 10, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Phillips KLE, Jordan-Mahy N, Nicklin MJH & Le Maitre CL Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann. Rheum. Dis 72, 1860–1867 (2013). [DOI] [PubMed] [Google Scholar]
- 195.Tan S & Ward MM Computed tomography in axial spondyloarthritis. Current Opinion in Rheumatology 30, 334–339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tan S, Yao J, Flynn JA, Yao L & Ward MM Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann. Rheum. Dis 74, 437–443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tan S, Dasgupta A, Flynn JA & Ward MM Aortic-vertebral interaction in ankylosing spondylitis: syndesmophyte development at the juxta-aortic vertebral rim. Ann. Rheum. Dis 78, 922–928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.de Bruin F et al. Prevalence of degenerative changes of the spine on magnetic resonance images and radiographs in patients aged 16–45 years with chronic back pain of short duration in the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology 55, 56–65 (2016). [DOI] [PubMed] [Google Scholar]
- 199.de Bruin F et al. Prevalence of degenerative changes and overlap with spondyloarthritis-associated lesions in the spine of patients from the DESIR cohort. RMD Open 4, e000657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lories RJU, Matthys P, de Vlam K, Derese I & Luyten FP Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann. Rheum. Dis 63, 595–598 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Wilde K et al. A20 inhibition of STAT1 expression in myeloid cells: A novel endogenous regulatory mechanism preventing development of enthesitis. Ann. Rheum. Dis 76, 585–592 (2017). [DOI] [PubMed] [Google Scholar]
- 202.Armaka M et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med 205, 331–337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ruutu M et al. β-Glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum. 64, 2211–2222 (2012). [DOI] [PubMed] [Google Scholar]
- 204.Jacques P et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis 73, 437–445 (2014). [DOI] [PubMed] [Google Scholar]
- 205.Cescon M, Gattazzo F, Chen P & Bonaldo P Collagen VI at a glance. J. Cell Sci 128, 3525–3531 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Cambré I et al. Running promotes chronicity of arthritis by local modulation of complement activators and impairing T regulatory feedback loops. Ann. Rheum. Dis 78, 787–795 (2019). [DOI] [PubMed] [Google Scholar]
- 207.Schett G et al. Enthesitis: From pathophysiology to treatment. Nature Reviews Rheumatology 13, 731–741 (2017). [DOI] [PubMed] [Google Scholar]
- 208.Murchison ND et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708 (2007). [DOI] [PubMed] [Google Scholar]
- 209.Pryce BA et al. Recruitment and maintenance of tendon progenitors by TGF signaling are essential for tendon formation. Development 136, 1351–1361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Killian ML & Thomopoulos S Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 30, 301–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu W et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell. Biol 30, 4797–4807 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Blitz E, Sharir A, Akiyama H & Zelzer E Tendon-bone attachment unit is formed modularly by a distinct pool of Scx - and Sox9 -positive progenitors. Development 140, 2680–2690 (2013). [DOI] [PubMed] [Google Scholar]
- 213.Nakahara H et al. Transcription factor Mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 65, 2081–2089 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suzuki H et al. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci. USA 113, 7840–7845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rossetti L et al. The microstructure and micromechanics of the tendon–bone insertion. Nat. Mater 16, 664–670 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Fredberg U & Stengaard-Pedersen K Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand. J. Med. Sci. Sports 18, 3–15 (2008). [DOI] [PubMed] [Google Scholar]
- 217.van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J & Maffulli N Terminology for Achilles tendon related disorders. Knee Surgery, Sport. Traumatol. Arthrosc 19, 835–841 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Waldecker U, Hofmann G & Drewitz S Epidemiologic investigation of 1394 feet: Coincidence of hindfoot malalignment and Achilles tendon disorders. Foot Ankle Surg. 18, 119–123 (2012). [DOI] [PubMed] [Google Scholar]
- 219.Strocchi R et al. Human Achilles tendon: morphological and morphometric variations as a function of age. Foot Ankle 12, 100–104 (1991). [DOI] [PubMed] [Google Scholar]
- 220.Osti OL, Vernon-Roberts B, Moore R & Fraser RD Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J. Bone Joint Surg. Br. 74, 678–682 (1992). [DOI] [PubMed] [Google Scholar]
- 221.van der List JP, Mintz DN & DiFelice GS The location of anterior cruciate ligament tears: a prevalence study using magnetic resonance imaging. Orthop. J. Sport. Med 5, 2325967117709966 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen L, Kim PD, Ahmad CS & Levine WN Medial collateral ligament injuries of the knee: current treatment concepts. Curr. Rev. Musculoskelet. Med 1, 108–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


