Abstract
Background
Lung cancer (LC) is the most common cancer worldwide. The prevalence of LC and rate of associated mortality are high and increasing faster in China than in Western countries. Non-small cell lung cancer (NSCLC) accounts for most LCs. This study aims to be the first large, multi-center, non-interventional retrospective study of treatment patterns (type/duration, number of lines, completion rate), real-world outcomes, and medical costs among Chinese patients with advanced/metastatic NSCLC (IIIb/IV) or extensive-stage small cell LC (ES-SCLC).
Methods
This study will enroll 8,800 patients (≥18 years, with a diagnosis of advanced/metastatic NSCLC made between 1 December 2013 to 30 November 2014) from 35 to 50 Chinese sites. Hospital information systems (HIS) and electronic medical records will be retrospectively reviewed, in adherence with regulatory and ethical requirements. Early-stage treatment (starting from 1 December 2010) of patients with recurrent disease or early disease progression will be examined. Data will be collected at baseline (diagnosis) and 6 and 12 months after this. Observation will end after 3 years or death. Data will be stratified by histology, staging, age, region, health insurance, and epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) mutation status. Treatment duration and overall survival will be estimated using Kaplan-Meier curves. Descriptive statistics will be used for disease characteristics and patient demographics. Cox-proportional hazards models will be used to examine the impact of demographics/treatment on survival. Treatment patterns and outcome predictors will be explored using multivariate logistic regression.
Discussion
This protocol describes the methodology for collecting real-world data to guide evidence-based clinical practice and inform unmet needs in NSCLC treatment, with potential to identify gaps between guidelines and current practice.
Trial registration
NCT03505515; data registered on ClinicalTrials.gov: 12h Apr., 2018.
Keywords: China, lung neoplasms, clinical protocol
Introduction
Lung cancer (LC) is the most common cancer worldwide. In China, LC claims more lives than any other malignancy, and its prevalence and mortality have rapidly increased in recent decades (1). The incidence of LC in China is high and the reported rate has risen faster in China than in Western countries (1,2). Key risk factors that contribute to the disease burden of LC in China include smoking and air pollution (1). The National Central Cancer Registry estimated that 651,053 new patients were diagnosed with LC in 2011, representing 19.3% of all new cancer cases in China (2). The distribution of these newly diagnosed patients was similar between rural and urban areas (2). The incidence and mortality rates of LC are relatively low in individuals under 45 years of age, but increase with age (1). In China, LC has a higher prevalence in men than in women, with crude incidence rates of 61.9/100,000 and 29.5/100,000, respectively (1). A Shanghai study estimated that the crude incidences of non-small cell lung cancer (NSCLC) in men and women were 55.9/100,000 and 52.4/100,000, respectively; this would suggest that NSCLC accounts for ~90.3% of LCs reported in Chinese men (3). Historically (2011 and 2014 data), LC mortality rates have been higher in men than in women (2,4).
Data from the US Surveillance, Epidemiology, and End Results (SEER) database (2014 data), show that 22% of LC patients had advanced disease that had spread to regional lymph nodes, while 57% had metastasized cancer (5). Similarly, in China, most cases of LC are diagnosed at an advanced stage (6). A Chinese study of patients with NSCLC, indicated that stage IIIa and IIIb/IV accounted for 12.9% and 63.5% of patients, respectively, for whom the tumor stage was known (1,734/5,099 patients) (3). The majority of Chinese patients diagnosed with LC receive treatment according to the China Expert Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 version), a framework developed in parallel to international guidelines (7). The guidelines for the treatment of NSCLC recommend molecular pathology methods for the detection of epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) gene mutations (7,8). Systemic therapy, including molecular targeted treatment and chemotherapy, is the recommended first-line treatment for advanced NSCLC (8).
Small cell LC (SCLC), which accounts for approximately 15% of LCs in China (9). is a fast-developing, rapidly invasive cancer. At the time of diagnosis, >70% of SCLC patients have metastasis, and the survival rate is low (9). Chemotherapy, including cisplatin-etoposide (EP), carboplatin-etoposide (EC), irinotecan-cisplatin (IP) and irinotecan-carboplatin IC, is the standard therapy for patients with extensive-stage (ES) SCLC (5).
Few large-scale national surveys have explored the treatment patterns, real-world outcomes, and direct economic burden of LC in China. A retrospective study using data from the Guangdong Lung Cancer Institute revealed treatment disparities among advanced NSCLC patients, most notably in the second-line setting (10). Shi et al. investigated SCLC treatment patterns in clinical practice across 12 medical centers, in 5 major Chinese cities (11). They concluded that the treatment options used in these hospitals were consistent with those of international recommendations (11). Site-based retrospective studies have examined real-world outcomes and the use of healthcare resource in China. In these studies, median overall survival (OS) was estimated to range from 13–17 months for patients with stage IIIb LC and from 8.4–24 months for patients with stage IV LC (12-17). Several studies that investigated the direct economic burden of LC in China report an estimated length of hospital stay in the range of 10.6–45.6 days, as well as inpatient expenses ranging from 8,816–58,512 RMB ($1,280.3–8,495.5 USD), which accounted for 81.5–85.2% of the direct economic burden (18-28).
Due to small sample sizes and a lack of geographical representation, current research in this area is limited in its application for evidence-based decision-making. This study aims to be the first large, multi-center, non-interventional retrospective study of treatment patterns, real-world outcomes, and direct medical costs among Chinese patients with advanced/metastatic NSCLC or ES-SCLC. This study protocol highlights the methodology to be used and the resulting study data will provide a baseline for comparison of treatments introduced since the 2017 data collection point.
Methods
Design
This study will enroll 8,800 patients from 35–50 sites in China. Inpatient and outpatient hospital information systems (HIS) and electronic medical records will be retrospectively reviewed, with a focus on patients diagnosed with advanced/metastatic NSCLC (IIIb/IV) or ES-SCLC between 1 December 2013 and 30 November 2014. The index date is the date of diagnosis of IIIb/IV NSCLC or ES-SCLC, and each patient will be retrospectively observed for a period of 3 years from this date (from 1 December 2013 to 30 November 2017). For patients with recurrent disease or progression from early-stage disease, early-stage treatment patterns will be examined from 1 December 2010.
Study objectives (data variables: Table 1)
Table 1. Outcome/endpoint variables.
| Patterns of chemotherapy and biologic therapy |
| • Treatment patterns will be analyzed based on histology and lines of treatment, as well as for predictive factors |
| • The frequency and duration of treatment will be determined for each line of treatment |
| • A list of the agents and regimens used will be compiled, with an investigation into the most common chemotherapy and biologic therapy regimens |
| Line of therapy |
| • First-line therapy: therapy received during the first 28 days after the initiation of treatment, with the number of cycles per regimen counted as intravenous (IV) administrations or prescription fills |
| • Maintenance therapy: therapy delivered after ≥4 cycles of first-line treatment without disease progression and subsequent treatment being initiated within 6 weeks of first-line therapy |
| • Second-line therapy: therapy received after ≥4 cycles of first-line treatment with a time gap of >6 weeks without chemotherapy/biologic treatment between 2 consecutive cycles of > 6 weeks; or if after <4 weeks of first-line therapy, a new treatment (that was not included in the first-line regimen) was administered, regardless of the length of time since the end of the first-line therapy. If one product from a combination regimen was discontinued, this did not constitute a change in the line of therapy |
| • Third-line therapy: a new line of therapy received after ≥4 cycles of second-line treatment with a time gap of > 6 weeks without chemotherapy/biologic treatment; or if after <4 cycles of second-line therapy, a new treatment (that was not included in the second-line regimen) was administered regardless of the length of time since the end of the second-line therapy. If one product from a combination regimen was discontinued, this did not constitute a change in the line of therapy |
| • Fourth-line and beyond: A gap of > 6 weeks in third-line therapy, or evidence of the administration of systemic therapy (excluding any agents used in the third-line regimen), regardless of the length of time since the end of the third-line therapy. |
| Duration of systemic therapy |
| • Determined by calculating the difference between the initial and last date at which the first drug of a regimen was administered |
| Treatment modification |
| • Defined as; dose reductions, treatment interruptions (temporary pause in treatment with intent to resume), and treatment discontinuation. Where available, the reason for the modification will be noted |
| Overall survival |
| • Interval between the date of diagnosis and the date of death (stratified by histology at diagnosis and from time of initiation of systemic therapy) |
| • Deaths will be verified using death certificate searches at sites, reports from caregivers, relatives, or other healthcare providers |
| • The in-hospital mortality rate will be calculated first, followed by analysis of overall survival |
| • Analyzed for each line of therapy and key treatment regimens |
| • Use of other cancer-directed therapies |
| • Surgery |
| • Radiation therapy |
| • Traditional Chinese medicine |
| Use of BSC and palliative care |
| • Best supportive and palliative care are intended to alleviate pain, relieve symptoms, improve quality of life, and enhance the compliance of anti-cancer treatment |
| Ancillary procedures |
| • The frequency of ancillary procedures, such as biopsies and biomarker tests, will be documented and analyzed, overall and for each line of therapy |
| Total direct health care costs |
| • Post-index direct health care costs will be calculated for each patient, overall, and for each line of therapy |
| • Total direct cancer-related costs will be stratified by inpatient and outpatient status |
Primary objectives
To ascertain the treatment patterns of subgroups of patients with advanced/metastatic LC [advanced NSCLC (IIIb/IV)] in China.
To determine which patients receive systemic anti-cancer treatment (chemotherapy or biologic/targeted therapy), as well as the proportion of patients that receive second-, third-, and fourth-line systemic therapy.
To describe the agents/regimens received, treatment duration, and completion rate for each line of therapy.
Secondary objectives
To describe patient demographics and disease characteristics at baseline and at the beginning of each line of therapy, with stratification by histology, staging, age, geographic region, and type of health insurance.
To describe OS by histology and line of therapy, the use of cancer-directed treatments (including surgery, radiation, and supportive care), the interval between each line of therapy, and LC-related healthcare costs.
Exploratory objectives
To explore the overall predictors of treatment patterns and outcomes of patients with advanced/metastatic LC and ES-SCLC, and early-stage treatment patterns prior to the enrolment period for patients with recurrent disease or progression from early-stage disease.
Inclusion criteria
Patients will be selected for inclusion based on the following criteria:
Histology-confirmed advanced NSCLC (IIIb/IV) and ES-SCLC with pathology/cytology recorded between 1 December 2013 and 30 November 2014;
-
Receiving treatment as described here as (i) a hospital inpatient, (ii) a hospital outpatient or (iii) an outpatient at a chemotherapy center:
Received systemic LC treatment as an inpatient, visiting a selected site more than twice (e.g., for diagnosis and treatment).
Alternatively, for hospitals with outpatient records, patients who received oral tyrosine kinase inhibitor (TKI) therapy, and routinely followed up (more than twice a year) outpatients will be included.
For hospitals with an outpatient chemotherapy center, patients will be included if they were prescribed chemotherapy and routinely (more than twice a year, inclusive) followed up in the hospital as an outpatient.
≥18 years of age at initial diagnosis of IIIb/IV NSCLC and ES-SCLC.
Exclusion criteria
Patients will be excluded from the study if they meet any of the following criteria:
Patients who have participated in or are attending clinical trials of LC therapies;
Unknown initial diagnosis time or initial treatment time;
For hospitals without an outpatient chemotherapy center, patients who were prescribed chemotherapy but have no inpatient records;
Patients who received therapy as an inpatient on one occasion but were not routinely followed up as an outpatient, having fewer than 2 visits to receive inpatient care per year.
Data collection
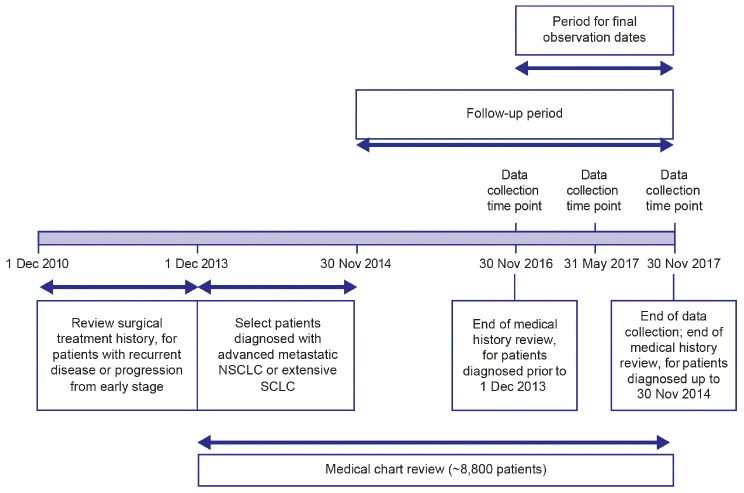
The selection of hospitals (~35–50 hospitals) will be geographically representative, by including sites located in developed and developing cities in different regions of China. Following feasibility assessment, sites will be selected from the National Centre of Cancer Registry Data will be sourced from inpatient and outpatient records, with the lead site being responsible for checking the quality of the data and ensuring completeness of outpatient and inpatient records from surgical and internal medicine oncology departments. Oral therapies, such as TKIs, may be prescribed in both inpatient and outpatient settings in China; usually intravenous chemotherapy is received by inpatients, but some large hospitals have outpatient chemotherapy centers to increase efficiency. Figure 1 illustrates the study timeline for patients with an initial diagnosis of late-stage cancer.
Figure 1.
Study timeline for patients with an initial diagnosis of late-stage cancer.
For patients with recurrent or progressive late-stage disease, the study will review the surgical treatment history from 1 December 2010 to the time of late-stage diagnosis (Figure 1; guidelines recommend surgery for patients with advanced NSCLC who show a good response to targeted therapy or chemotherapy as well as for patients with a single metastatic site) (8). As the sites of internal oncological care and where surgery is performed may differ, treatment histories for multiple sites may be obtained. Data will be collected at three specific time points: at baseline (diagnosis), and at 6 months and 1 year after diagnosis/initiation. Figure 1 shows the timeline for data collection. Depending on the time of diagnosis, the initiation, observational, and data collection periods may differ among patients. Observation will end 3 years after study initiation or on the death of the patient.
Data source and management
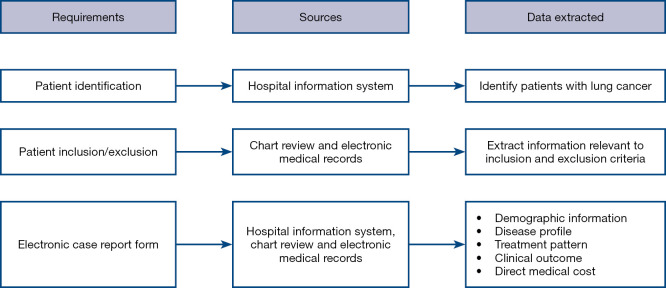
The China HIS will serve as a data source. HIS is an information management system that is widely used in Chinese hospitals, which was designed to manage administrative and clinical functions. Eligible patients will be identified using HIS, electronic medical records (EMRs), and/or chart review. Figure 2 illustrates the patient screening and data extraction process.
Figure 2.
Depiction of the patient screening and data extraction process.
Study variables
Primary outcomes and endpoints of interest include: patterns of chemotherapy and biologic therapy use; OS; the use of other cancer-directed therapies, best supportive care (BSC) and palliative care; ancillary procedures; and direct healthcare costs. The patient outcomes and endpoints will be assessed at initial diagnosis and during the post-diagnostic follow-up (including during treatment with first-line-, maintenance-, and all subsequent lines of -therapy, where applicable). Patients will be censored at the end of a data stream. Further details on these variables are shown in Table 1, with additional information detailed in Table S1.
Independent variables of interest include patient demographics and risk factors, disease profile (including time of diagnosis, histology, staging, and mutation status), admission/discharge status, information relating to the hospital type and size, and the treating department. These data will be collected where available, and any missing data and the resulting potential impact will be documented.
Statistical analysis
Statistical analyses will be performed using SAS version 9.3 statistical software (SAS Institute Inc., Cary, North Carolina, USA) with an a priori significance level set at 0.05. For primary objectives, treatment patterns will be analyzed according to histology, stage, line of therapy (first-line, maintenance, second-line, and beyond), age, region, and type of health insurance. Categorical variables will be presented as percentages, while continuous variables will be reported as mean, standard deviation (SD), median, and interquartile ranges. To analyze the treatment duration of each line of therapy, Kaplan-Meier (KM) curves will be estimated, using the date of the first dose administered as the initiation date and the date of the last infusion or projected final oral dose as the end date (whichever comes first).
For secondary objectives, patient demographics, disease characteristics, adjusted and unadjusted hospital charges, and other cancer-directed therapies will be presented using descriptive statistics. KM survival curves will be used to estimate survival probability for each patient, and Cox-proportional hazards models will be used to examine whether differences in patient OS between regimens or treatments can be accounted for by controlling baseline demographics and clinical variables.
Exploratory objectives will be analyzed using multivariate logistic regression models to identify which observed patient and clinical factors are predictive of specific events associated with treatment patterns. Separate models will be used to analyze impacts of chemotherapy on treatment pattern associated events (versus any other cancer-directed treatment, among patients who received cancer-directed treatment). Multivariate generalized linear models (GLMs) will be generated to assess cost drivers for patients who received some form of cancer-directed therapy.
Mean and 95% confidence intervals (CIs) for proportions of patients receiving a particular treatment pattern (e.g., second-, third- and fourth-line) will be calculated for a sample size of 8,800 patients. In a descriptive setting, the sample size is related to the level of precision in estimating the primary outcomes. For this study, the intended analyses for the primary objectives are only descriptive; therefore, the precision of any estimates (i.e., the size of the CIs) will be affected by the sample size. Several descriptive objectives explore the treatment pattern of second-line therapy. To ensure sufficient power for the smallest histologic group, the proportion of patients receiving a certain regimen in second-line SCLC therapy will be used to calculate the subgroup sample size and back-calculate the total sample size based on the subgroup percentage.
Ethics approval
This observational study will be conducted in accordance with International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Pharmacoepidemiology Practices and the applicable regulatory requirements. Prior to the initiation of the study, Ethics Committee/Institutional review board approval will be obtained, as will the informed consent of patients, in compliance with the relevant laws and regulations.
Discussion
In Western countries, the incidence of LC is declining; in contrast, over the past few decades, China has experienced an increase in the incidence of LC, which has been attributed to tobacco use and air pollution (29). Consistent with Western countries, adenocarcinoma has replaced squamous cell carcinoma as the predominant type of LC in China (29-32). The incidence of EGFR mutations in Chinese LC patients is in the range of 40.3–64.5% for those with adenocarcinoma and 28.4% for NSCLC, which is far higher than the incidence of 3.0–42.0% seen in European and North American patients with NSCLC (29,33). Current treatment practices focus on recommendations from the China Expert Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 version) (8); however, there is still a need for reports on real-world outcomes that can guide evidence-based clinical practice and provide information on unmet clinical needs. These real-world data have the potential to contribute towards a strategy that will improve the quality of care for patients with LC in China.
Although some previous studies have focused on LC treatment patterns, real-world outcomes, and health care utilization in China, none of the literature, to date, has successfully captured all parameters on a large scale, in a geographically representative manner (11,18-28). With the aim of addressing this knowledge gap, this study is appropriately powered according to the descriptive objectives and the optimal sample size determined. It has been designed to include data collection from a wide range of geographical sites, ensuring the inclusion of undeveloped and developed areas. The selection of a geographically representative cohort of patients and hospitals will minimize the potential selection bias, and by its design, this protocol is likely to capture a relatively complete snapshot of treatment patterns and outcomes among LC patients in China.
This study will reflect real-world treatment patterns and outcomes and, importantly, the direct medical costs that potentially have a substantial impact on the national healthcare system. Given the retrospective nature of the study and the difficulty in collecting comparable accurate and meaningful safety data for different treatments, comparisons of drug safety will not be made in this study. It is expected that this study will provide a deeper understanding of any unmet medical needs, through its identification of potential treatment gaps between current practice and guideline recommendations. This may allow areas for improvement to be identified in clinical practice and resource allocation for LC treatment. Furthermore, the robust, large-scale methodology of this protocol will enable the analysis and evaluation of pre-immuno-oncology (IO) treatment patterns and the real-world implications in NSCLC and SCLC. By doing this, we anticipate that this study will set a benchmark for future studies and could even pave the way for post-IO comparisons in China.
This study is not without limitations. The use of HIS as a primary data source limits access to additional information such as socioeconomic status, health behaviors, and quality-of-life. Differences in inter- and intra-hospital practices may contribute to variations in data, and in large hospitals, where the enrolment population may contain a higher proportion of patients with severe disease, there is also potential for OS bias.
Conclusions
In conclusion, here, we have presented the protocol for the first large-scale, geographically representative study to examine the treatment patterns, real-world outcomes, and direct medical costs of patients diagnosed with advanced/metastatic NSCLC or ES-SCLC in China.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The study was funded by Bristol-Myers Squibb Pharmaceuticals Ltd. Editorial assistance in manuscript preparation was provided by Anita Abeygooneseskera and Rachael Profit of MediTech Media, Asia Pacific, and funded by Bristol-Myers Squibb Pharmaceuticals Ltd., China.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This observational study will be conducted in accordance with International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Pharmacoepidemiology Practices and the applicable regulatory requirements. Prior to the initiation of the study, Ethics Committee/Institutional review board approval will be obtained, as will the informed consent of patients, in compliance with the relevant laws and regulations.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1269). ZT was employed by Bristol-Myers Squibb at the time the manuscript was prepared, and SY is employed by Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Zeng H, et al. Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209-15. 10.1111/1759-7714.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng R, Zeng H, Zuo T, et al. Lung cancer incidence and mortality in China, 2011. Thorac Cancer 2016;7:94-9. 10.1111/1759-7714.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan H, Shao ZY, Xiao YY, et al. Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open 2015;5:e009419. 10.1136/bmjopen-2015-009419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng XQ, Huang JF, Lin JL, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res 2019;8:367-79. 10.21037/tlcr.2019.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute NC. Surveillance, Epidemiology, and End Results (SEER) Program. 2014. Available online: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 14 January 2020 2020.
- 6.Ulrich BC, Guibert N. Immunotherapy efficacy and gender: discovery in precision medicine. Transl Lung Cancer Res 2018;7:S211-3. 10.21037/tlcr.2018.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. 10.6004/jnccn.2018.0062 [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Sun Y, Yu J, et al. China Experts Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 Version). Zhongguo Fei Ai Za Zhi 2016;19:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Cheng Y, Li H, et al. Current status of small cell lung cancer in China. Journal of Cancer Biology & Research 2014;2:1032-36. [Google Scholar]
- 10.Yang LL, Zhang XC, Yang XN, et al. Lung cancer treatment disparities in China: a question in need of an answer. Oncologist 2014;19:1084-90. 10.1634/theoncologist.2014-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Xing P, Fan Y, et al. Current small cell lung cancer treatment in China. Thorac Cancer 2015;6:233-8. 10.1111/1759-7714.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Wang Y, An T, et al. Analysis of prognostic factors in 541 female patients with advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2011;14:245-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wang L, Li W, et al. Surgical outcomes of stage IV non-small cell lung cancer: a single-center experience. J Thorac Dis 2019;11:5463-73. 10.21037/jtd.2019.11.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng H, Han HB, Li XQ, et al. Analysis of clinical characteristics and survival of 1279 patients with lung cancer. Chinese Journal of Lung Cancer 2011;21:354-8. [Google Scholar]
- 15.Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res 2019;8:575-83. 10.21037/tlcr.2019.09.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Wu C, Zhang C, et al. A retrospective study of low-dose apatinib combined with S-1 in patients with advanced non-small cell lung cancer. J Thorac Dis 2019;11:1831-7. 10.21037/jtd.2019.05.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong K, Liang W, Zhao S, et al. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl Lung Cancer Res 2019;8:268-79. 10.21037/tlcr.2019.06.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu YY. Epidemiology characteristics and disease burden of lung cancer patients with Shanghai identity [Dissertation]: Fu Dan University; 2013. [Google Scholar]
- 19.Fan XH, Feng Y, Shao W, et al. Application and outcome of clinical pathway in radio- and chemo-therapy in lung cancer patients. Chinese Hospital Management 2011;2:25-7. [Google Scholar]
- 20.Chen Z. Inpatient surgery cost structure and trend analysis of nearly 10,000 lung cancer patients. Chinese Health Research 2013;3:3. [Google Scholar]
- 21.Du W, Zhang X, Gao C, et al. New grey correlation analysis of hospitalization expenses of 600 patients with non-small cell lung cancer. Journal of Shanghai Jiaotong University (Medical Science) 2012;10:1356-9. [Google Scholar]
- 22.Gao YX, Xiao J, Wu XM, et al. Factor analysis of direct inpatient cost of 1666 lung cancer patients. Chinese Journal of Health Statistics 2011;3:278-80. [Google Scholar]
- 23.Ding TT, Zhang X, Gao C, et al. Impact analysis and cost control research on inpatient medical cost of patients with non-small cell lung cancer. Chinese Journal of Health Statistics 2012;2:240-2. [Google Scholar]
- 24.Gao YX, Yang M, Liu G, et al. Path analysis of impact factors of inpatient medical cost of patients with lung cancer. Chinese Journal of Public Health 2012:253-4. [Google Scholar]
- 25.Shang M. Economic burden and impact factors of inpatient lung cancer patients [Dissertation]: Shandong University 2013. [Google Scholar]
- 26.Gao QQ, Li SX, Wang Y, et al. Impact analysis of inpatient cost in lung cancer patients who underwent surgery using Grey relational method. Health Economics Research 2013;12:56-8. [Google Scholar]
- 27.Lv HL, Zhao SF, Xie XP, et al. Impact analysis of inpatient cost of 16,866 lung cancer patients in Si Chuan Province. Chinese Journal of Evidence-Based Medicine 2013;11:1283-7. [Google Scholar]
- 28.Gong H, Liu Y, Ma L, et al. Inpatient cost analysis of patients with lung cancer and liver cancer from 1996 to 2006 in Lanzhou City. Modern Preventive Medicine 2012;39:270-2. [Google Scholar]
- 29.Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res 2014;3:270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoda Y, Nakayama T, Ioka A, et al. Trends in lung cancer incidence by histological type in Osaka, Japan. Jpn J Clin Oncol 2008;38:534-9. 10.1093/jjco/hyn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thun MJ, Lally CA, Flannery JT, et al. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst 1997;89:1580-6. 10.1093/jnci/89.21.1580 [DOI] [PubMed] [Google Scholar]
- 32.Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294-9. 10.1002/ijc.21183 [DOI] [PubMed] [Google Scholar]
- 33.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015;5:2892-911. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as