Abstract
The discovery of actionable oncogenic driver alterations has significantly improved treatment options for patients with advanced non-small cell lung cancer (NSCLC). In lung adenocarcinoma (LUAD), approved drugs or drugs in clinical development can target more than half of these altered oncogenic driver genes. In particular, several gene fusions have been discovered in LUAD, including ALK, ROS1, NTRK, RET, NRG1 and FGFR. All these fusions involve tyrosine kinases (TK), which are activated due to structural rearrangements on the DNA level. Although the overall prevalence of these fusions in LUAD is rare, their detection is extremely important, as they are linked to an excellent response to TK inhibitors. Therefore, reliable screening methods applicable to small tumor samples (biopsies and cytology specimens) are required in the diagnostic workup of advanced NSCLC. Several methods are at disposal in a routine laboratory to demonstrate, directly or indirectly, the presence of a gene fusion. These methods include immunohistochemistry (IHC), fluorescence in-situ hybridization (FISH), reverse transcriptase-polymerase chain reaction (RT-PCR), multiplex digital color-coded barcode technology or next-generation sequencing (NGS) either on DNA or RNA level. In our review, we will summarize the increasing number of relevant fusion genes in NSCLC, point out their underlining molecular mechanisms and discuss different methods for the detection of fusion genes.
Keywords: Non-small cell lung cancer (NSCLC), tyrosine kinase fusion (TK fusion), predictive testing
Introduction
Worldwide, lung cancer is the most common malignancy and by far the leading cause of cancer deaths, as most patients are diagnosed at an advanced, inoperable stage of disease (1). Only with the discovery of actionable oncogenic driver alterations, the outcome of advanced-stage non-small cell lung cancer (NSCLC) patients with biomarker-driven treatment has significantly improved. Particularly lung adenocarcinoma (LUAD) is not a single disease but a cluster of distinct molecular subtypes defined by a single oncogenic driver alteration, comprising gene mutations, rearrangements, and amplifications (2). Importantly, about 70% of these oncogenic driver alterations can be targeted by approved or investigational drugs. So far, EGFR, ALK, ROS1, BRAF and in some countries NTRK inhibitors are approved by health care regulatory authorities in advanced-stage NSCLC harboring the respective oncogenic alteration (ESMO and NCCN guidelines) (3). Recurrent gene rearrangements act as strong oncogenic drivers, leading to a state of oncogene addiction and, therefore, their fusion proteins are ideal targets for anticancer drugs. With the availability of improved detection strategies, there has been an exponential discovery of gene fusions across various malignancies, which is paralleled by the development of new targeted drug compounds (4,5).
In this review, we will summarize the growing number of relevant fusion genes in NSCLC, highlight their underlining molecular mechanism, and discuss different methods for the detection of fusion genes. In addition, we will provide examples of how the detection of gene fusions can be integrated into a predictive testing algorithm to guide treatment selection of NSCLC patients. We present the following article in accordance with the NARRATIVE REVIEW reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-676).
Tyrosine kinase (TK) fusions in lung cancer and molecular mechanisms
In NSCLC several targetable gene fusions involving ALK, ROS1, NTRK, RET, NRG1, and FGFR have been discovered (3,6). Overall these are rare events with ALK rearrangements being the most prevalent in 3–5% of all advanced-stage LUAD, followed by ROS1 with a prevalence of 1–2% (7,8). NTRK fusions are extremely rare and only present in 0.2% of NSCLC and can involve all of the three NTRK genes (9). However, it should be emphasized that the detection of even these rare NSCLC patients is important as they show an excellent response to TRK inhibitors (3). RET fusions are currently investigated in clinical trials and found in 2% of NSCLC. First studies with selective RET inhibitors show remarkable response and RET testing should be offered, to allow patients access to either clinical trials or off-label use of the drug (10). Several other rearrangements are known in lung cancer but have not yet proven to be of clinical benefit in terms of treatment. These include NRG1, especially in invasive mucinous LUAD, FGFR1/2/3 (mostly in squamous cell carcinomas) and PDGFRA, MET and BRAF rearrangements (5,11).
All of these oncogenic gene fusions involve TK and result from structural rearrangements on the DNA level, such as interchromosomal translocations or intrachromosomal inversions, deletions and insertions (12). Oncogenic rearrangements put the TK domain of the involved gene under the promotor control of a partner gene, which leads to overexpression of chimeric fusion proteins. Otherwise, constitutive activation can be driven by oligomerization mediated by the fusion partner. Importantly, the fusion preserves the TK function with a conservative breakpoint region in the TK domain and numerous fusion partners, resulting in many gene fusion variants (5). For example, the breakpoint of ALK is highly conservative and located at exon 20, with various breakpoints in the amino-terminal part of EML4, leading to different EML4-ALK fusion variants. Over 20 different ALK fusion variants have been described in NSCLC, mostly comprising different EML4-ALK isoforms and rarely involving other fusion partners (13). The same principle applies also to ROS1 and NTRK rearrangements (14). Importantly all variants result in fusion proteins, which are optimized for constitutive ligand-independent kinase activation and downstream signaling and are therefore predictive for response to the respective TK inhibitor.
Therefore, screening methods should be able to detect all possible rearrangements irrespective of the fusion partner. In this review, we will discuss different methods for the detection of fusion genes and how they can be integrated into predictive testing algorithms to guide treatment selection of NSCLC patients for drugs targeting gene fusions.
Clinicopathological features of NSCLC with gene rearrangements
Gene rearrangements in NSCLC have been associated with several clinicopathological features. ALK, ROS1 and RET rearrangement are enriched in younger patients, female sex and never-smokers (15-17). Data on NTRK fusions in NSCLC are still limited due to low numbers, but NTRK fusions appear to occur across gender, age, and smoking history (18). Several histological features are associated with rearrangements. ALK, ROS1 and RET rearrangements are more frequent in LUAD with a solid-predominant pattern, and solid signet ring or mucinous cribriform patterns. Additionally, club cell (Clara cell)-like cells were found to be typical for ALK rearranged NSCLC and nuclei with macronucleoli for ROS1 rearranged NSCLC, respectively (16,19-21). Psammoma bodies and lymphangitic spread have been described as characteristic for a subset of RET rearranged LUAD (22). The uncommon NRG1 rearrangements are enriched in but not restricted to KRAS wild-type invasive mucinous LUAD (23,24). Despite of these associations, neither clinical nor histological features are reliable enough to predict or exclude predictive gene rearrangements in non-squamous NSCLC. Moreover, although these rearrangements are mostly found in LUAD or non-small cell cancer, not otherwise specified (NSCC-NOS), they have rarely been reported in lung squamous cell carcinoma (25).
Diagnostic approaches
Several methods are at disposal to demonstrate, directly or indirectly, the presence of a gene fusion in tumor samples. Fusions can be detected on the protein level by immunohistochemistry (IHC), on the DNA level by fluorescence in-situ hybridization (FISH) or DNA-based targeted next-generation sequencing (NGS) and on the RNA level by reverse transcriptase-polymerase chain reaction (RT-PCR), multiplex digital color-coded barcode technology or RNA-based targeted NGS (26). Each of these methods has its advantages and disadvantages, which should be taken into account when performing and evaluating an analysis for gene fusions. Notably, all techniques discussed below are not only applicable to histological but also to cytological specimens as long as the required quality parameters are fulfilled and continuous quality assurance is in place (27-29).
IHC
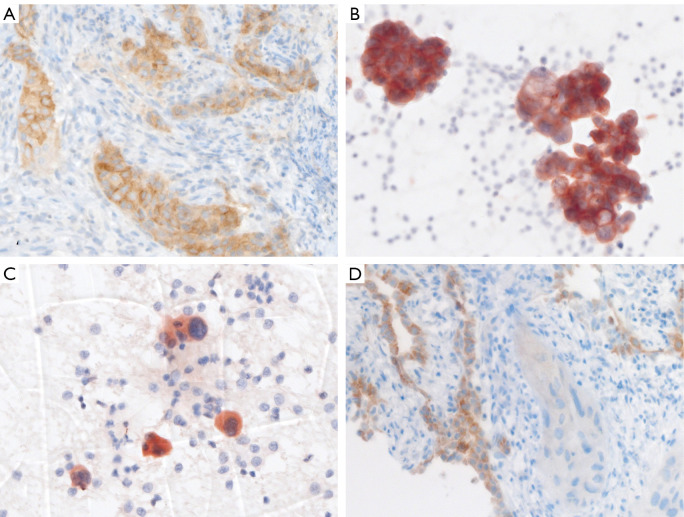
In LUAD or NSCC-NOS, predictive IHC for ALK and ROS1 is common practice and has recently been complemented by pan-Trk IHC for prescreening of NTRK rearrangements (30,31). ALK, ROS1 and pan-Trk expression detected by IHC is a surrogate for the respective rearrangements as protein levels of these genes are mostly below detection limit by IHC in their native form. IHC is cost-effective, has a fast turn-around time, is way simpler to evaluate compared to molecular methods, and can easily be integrated into a diagnostic laboratory. Moreover, IHC only needs a small amount of tumor cells on one unstained formalin-fixed and paraffin-embedded (FFPE) slide or one cytological specimen. Examples of ALK and ROS1 IHC are shown in Figure 1.
Figure 1.
IHC for detection of ALK- and ROS1-rearrangements in lung cancer. (A,B) Homogenous cytoplasmic staining of ALK protein of a LUAD, corresponding to Figure 2A. (A) Bronchial biopsy (5A4 clone on Ventana Benchmark Ultra) and (B) matched bronchial cytology (5A4 clone on Leica Bond). (C) Pulmonary cytology with rare LUAD cells showing diffuse cytoplasmic ROS1 positivity (D4D6 clone on Leica Bond). (D) Solid infiltrates of ROS1 negative LUAD (center and lower right). Adjacent alveolar structures with reactive type 2 pneumocyte hyperplasia showing physiological ROS1 expression (left side) serving as an internal positive staining control (D4D6 clone on Ventana Benchmark Ultra). Magnification 400×. IHC, immunohistochemistry; LUAD, lung adenocarcinoma.
Because preanalytic procedures, antibody clones, and detection platforms vary between laboratories, a proper validation of IHC protocols is crucial to ensure accurate and reproducible IHC staining. General recommendations for analytic validation of predictive laboratory-developed tests (LDT) have been published (32). These guideline statements propose that for initial analytical validation of a new predictive LDT protocol, a minimum of 20 positive and 20 negative controls, fixed and processed in the same manner as the clinical cases, should be tested. The LDT result should ideally be compared with the result of a validated assay on the same validation set and achieve an overall concordance of at least 90%. Benign tissue controls (for example ganglion cells of the appendix for ALK and brain or testis for pan-Trk) or cell lines harboring the respective fusions can serve as positive controls. However, clinical tumor specimens with a known rearrangement are the optimal positive control. The high number of proposed positive controls is unrealistic in this setting, as the prevalence of oncogenic rearrangements in NSCLC is low (NTRK only 0.2%). Internal and external quality control measures are therefore crucial to ensure accurate IHC results.
ALK
For ALK IHC two highly sensitive antibody clones are available, 5A4 (NovocastraTM, Leica Biosystems) and D5F3 (Cell Signaling, Ventana). Many studies have demonstrated that the performance of both antibodies using well-validated IHC protocols is very good compared to FISH. The pooled sensitivity and specificity for both antibodies are 97% and 100%, respectively (30). In addition to laboratory developed tests there is a commercially available and FDA approved, highly standardized, fully automated ALK kit assay for BenchMark immunostainers using D5F3 (Ventana). Discordant ALK IHC and FISH results are rare and have been reported in 1% of NSCLC (33). It has been suggested that these are mainly driven by false-positive FISH results, especially in borderline FISH positive NSCLC with ALK-positive cells ranging from 15% to 20% (34). Aberrant ALK staining has rarely been reported in high-grade neuroendocrine carcinomas (35).
Based on published evidence and according to current predictive testing guidelines, ALK IHC, using 5A4 or D5F3, is an equivalent alternative to FISH, which used to be the gold standard for ALK testing (30). However, evaluation of ALK IHC is not standardized and criteria for an ALK-positive result vary significantly across different studies (36). ALK staining in ALK-rearranged NSCLC is usually cytoplasmic and diffuse across the tumor with moderate to strong intensity (Figure 1A,B). In NSCLC with moderate to strong and diffuse ALK staining, confirmation by a molecular method can be omitted. Nonetheless, the threshold to perform confirmation by a molecular method should be low and should be performed in case of heterogeneous, focal, or weak staining. As a matter of fact, we still confirm every ALK IHC positive case by FISH for quality assurance purposes at our institute.
ROS1
For ROS1 IHC there are two commercially available antibody clones, the D4D6 (Cell Signaling Technology) and the recently introduced SP384 (Ventana). In contrast to ALK, no commercial ROS1 assay is available, and ROS1 IHC has to rely on LDTs. Additionally, compared to ALK, significantly fewer studies have investigated the performance of ROS1 IHC. However, these studies demonstrated a high sensitivity and specificity using D4D6 ranging from 94–100% and 87–100%, respectively (30). The first study investigating SP384 showed a similar performance (37). ROS1 rearranged NSCLC typically reveals finely granular cytoplasmic IHC staining, though the EZR-ROS1 fusion can result in a membranous staining (8). The staining can be more heterogeneous compared to ALK-positive carcinomas with variable staining intensities within the same tumor (8). Although ROS1 protein is essentially absent in normal human lung tissue, a non-specific IHC staining may be observed in reactive alveolar type II pneumocytes and macrophages and should not be misinterpreted as a positive result. According to current predictive testing guidelines, ROS1 IHC may be used as a screening test, but positive ROS1 IHC results should be confirmed by a molecular method (30).
NTRK
NTRK includes three genes, NTRK1, 2, and 3, which encode transmembrane receptor TK TrkA, B, and C, respectively (38). These three proteins have a high level of homology between the kinase domains and can all be detected by pan-Trk antibodies. Commercially available pan-Trk clones are EPR17341 (Abcam) and A7H6R (Cell Signaling Technologies). Trk expression by IHC can be variable in intensity and subcellular localization (cytoplasmic, nuclear or membranous), which might depend on the 5' fusion gene partner (31). The largest study on the performance of pan-Trk IHC across different tumor types, including lung cancer, has been performed using the EPR17341 antibody (9). The sensitivity for detecting NTRK fusions seems higher for NTRK1 and 2 (96% and 100%, respectively) and lower for NTRK3 (79%). Additionally, specificity differs between different tumor types. Lung cancer showed a sensitivity of 88% and a specificity of 100%. Again, there is no standardized pan-Trk IHC interpretation. Any positive staining, cytoplasmic, nuclear or membranous, even if only present in 1% of tumor cells, should be evaluated by a molecular method (9,31). Pan-Trk IHC can be used for screening in NSCLC with confirmation of positive results by a molecular method, preferentially by RNA-sequencing (31). However, in the absence of other driver alterations (EGFR, KRAS, ALK, and ROS1), molecular confirmation of a negative pan-Trk staining might also be considered as pan-Trk IHC can miss up to 20% of NTRK3 rearrangements.
In summary, since ALK, ROS1 and NTRK fusions are rare, IHC greatly simplifies large-scale screening of NSCLC and reduces costs, since IHC-negative NSCLC do not normally require molecular testing for fusions already screened by IHC.
Other fusion proteins
No established antibody exists for the detection of RET fusions (39). Similarly, there is no reliable antibody to detect NRG1 fusions. However, studies have reported that phospho-HER3 (p-ERBB3) IHC has a high sensitivity of 100% and a specificity of 97.5% for the detection of NRG1 fusions in LUAD (23,40). Although several studies have included IHC for FGFR1, 2 or 3, none of these antibodies has proven reliable sensitivity and specificity, in particular for FGFR3 fusions, the most frequent fusion (41,42). For other rare fusions involving genes such as MET, BRAF, EGFR, and PIK3CA, there are also no antibodies available for accurate detection of the respective fusion proteins (39).
FISH
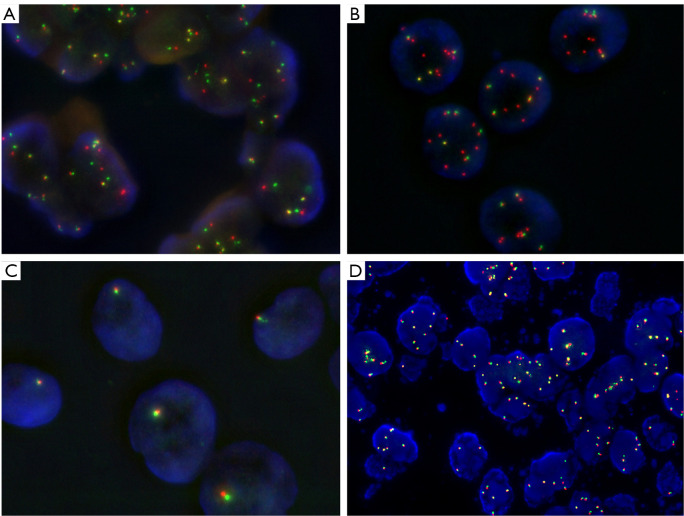
FISH can detect large structural variants at the DNA level and is widely used in clinical laboratories to test for oncogenic fusions (Figure 2). Its advantage is the little amount of tissue needed and that fusions can be detected within the cells of interest. However, besides the expertise required and its labor-intensive nature, FISH analysis also has some technical shortcomings one has to keep in mind. When using fusion probes, the fusion partner has to be known and only one partner at a time can be detected. Otherwise, break-apart probes are necessary, which cover all possible partners, with the downside that the fusion partner remains unknown. In theory, break-apart probes can detect large structural variants with sufficient sensitivity and specificity, however, short inversions and intrachromosomal translocations may be missed by FISH due to an insufficient splitting signal as has been shown, e.g., for ALK (43). This notion may also be relevant for NTRK1 fusions, which are often intrachromosomal events (44). Likewise, translocations can be complicated by deletions or atypical fusion signals, leading to false-negative results (45). Moreover, while a break-apart probe with a split positive signal might show a structural variant involving the gene probed, it cannot be determined whether the abnormal signal actually results in the generation of a fusion transcript or protein (46). Indeed, it has been shown that patients whose tumors were ALK-negative by IHC but positive for FISH, did not respond to ALK inhibitor therapy, except when IHC was negative due to poor sample quality (47,48). Conversely, ALK IHC positive but FISH negative NSCLC have been associated with impaired survival after targeted treatment (49). Finally, FISH testing for NTRK1/2/3 fusions is laborious and expensive in routine practice since it requires three different break-apart probes for coverage.
Figure 2.
FISH for detection of predictive gene rearrangements using break-apart FISH probes. (A,B) LUADs with ALK rearrangements and increased number (polysomy) of chromosome 2. (A) Break-apart with one or two split green and red signals and 2–3 non-rearranged gene copies. The distance between the split signal must be at least twice the size of one signal by definition. (B) Deletion FISH pattern: 6–7 single red signals without corresponding green signals and 4 non-rearranged gene copies. (C) ROS1 FISH: LUAD cells with only one gene copy, probably due to monosomy chromosome 6, but no rearrangement. (D) RET FISH: LUAD with increased copy RET copy number but no rearrangement. Magnification A,C: 1,000×; B,D: 630×. FISH, fluorescence in-situ hybridization; LUAD, lung adenocarcinoma.
RT-PCR
Extracted RNA can be reverse-transcribed and RT-PCR can be performed to either qualitatively or quantitatively detect the presence of a single oncogenic fusion for which both fusion partners are known. The advantage of this method is the low cost per assay and the high sensitivity and specificity. However, because of the large number of different fusion partners and break-points involved, the utility of RT-PCR for individual fusion transcripts is limited, because each fusion would need to be detected separately. However, differences in expression of the 5' versus the 3' end of a gene can provide indirect evidence of a fusion, a phenomenon which is also being used in NGS technology using the ratio of imbalance (50).
Multiplex digital color-coded barcode technology
Among the non-NGS fusion detection methods, multiplex digital color code technology has recently gained attention, in particular the nCounter platform (NanoString, Seattle, WA). This method enables the detection of fusions by direct counting of specific mRNA molecules without any retro-transcription of amplification steps. Thus, it is particularly suitable for degraded RNA and samples with low amount of RNA (51). Because it detects known fusion transcripts but also measures the 3' and 5' gene region imbalance, this technique can detect previously unknown fusions. Moreover, it provides the possibility to test several fusions at the same time such as ALK, ROS1, RET and NTRK1. nCounter fusion gene assays have been successfully used in both histological and cytological samples with high concordance to FISH results and represents a valuable technique (52-54).
NGS
NGS provides a precise method to detect fusions in lung cancer. One main advantage is that multiple fusions and their corresponding partners can be tested at the same time from one single tumor sample. In contrast, turn-around time and costs are higher in comparison to IHC and FISH and more tumor material is necessary. Several NGS approaches to detect fusions exist, which can be applied on either DNA or RNA level.
DNA based NGS
The main application of NGS analysis on tumor samples is the assessment of the somatic mutational status. This is performed in most diagnostic laboratories by a targeted NGS panel, which covers the most frequently and clinically relevant mutated genes for a given entity. Besides, some of the respective panels also allow identifying a large series of different fusions (6). One of the main challenges in detecting fusions by DNA-based NGS is that most genomic breakpoints leading to fusion genes occur in introns. These introns cannot always be fully covered by targeted NGS panels because they are either too long or contain repetitive elements (6,55,56). Consequently, inadequate coverage and difficulty in assessing highly repetitive regions can lead to false-negative results. Besides, and analogous to FISH, some fusions discovered by DNA-based NGS panels might not have a functional consequence, representing a non-functional event.
RNA based NGS
In the case of RNA-based NGS, the mature mRNA is sequenced, which has several advantages. Mature mRNA is devoid of long introns, which facilitates sequencing and data analysis. The detection of fusions at the RNA level also provides direct evidence that these fusions are indeed transcribed, which increases confidence in the results (46). Fusion transcripts can also be detected at low tumor cell content because they are often highly expressed and unique in the tissue. Finally, RNA-based NGS can test for multiple fusions simultaneously, which justifies to some extent its higher costs and longer turnaround time. For these reasons, an RNA-based NGS would be the method of choice. However, the most important disadvantage is that RNA is less stable than DNA and the quality of the RNA does not always meet the requirements of sequencing. Especially with RNA from FFPE samples this can lead to a higher dropout rate and thus possibly to false negative results.
Many NGS platforms allow for the detection of fusions on RNA based NGS, either using an amplicon-based or hybrid capture methodology. Amplicon based methods enrich for target genes by PCR amplification of a distinct set of genes but can only detect fusion partners that are already known and included in the panel. Alternatively, fusion detection by an imbalance of 5' to 3' gene expression can be used to detect fusions with unknown partners, however, this needs further confirmation. Capture based approaches for RNA fusion analysis work by transcribing RNA first in cDNA and subsequent sequencing similar to DNA based sequencing. With this method, only one fusion partner needs to be known. Finally, an anchored multiplex PCR (AMP), for example with the Archer FusionPlex platform, can be used which also allows for the detection of novel fusion partners. Thanks to the initial adapter ligation step that facilitates priming without a priori knowledge of the gene fusion partner, the AMP method has been shown to have high technical sensitivity and specificity even in FFPE-derived RNA samples (31). Several studies have therefore used the AMP technology to select for patients with, e.g., NTRK fusions followed by FISH for confirmation (57).
These different methods and techniques need different amounts of RNA input. Therefore, when choosing RNA-based sequencing technology, the amount of available tissue must also be considered. Finally, because of the reduced RNA quality, methods have to be established to interrogate RNA quality such as measuring RNA fragment size distribution and examining amplification of a housekeeping gene in a quantitative PCR based assay (58). This will allow greater confidence in the fusion results obtained.
Testing algorithms considerations
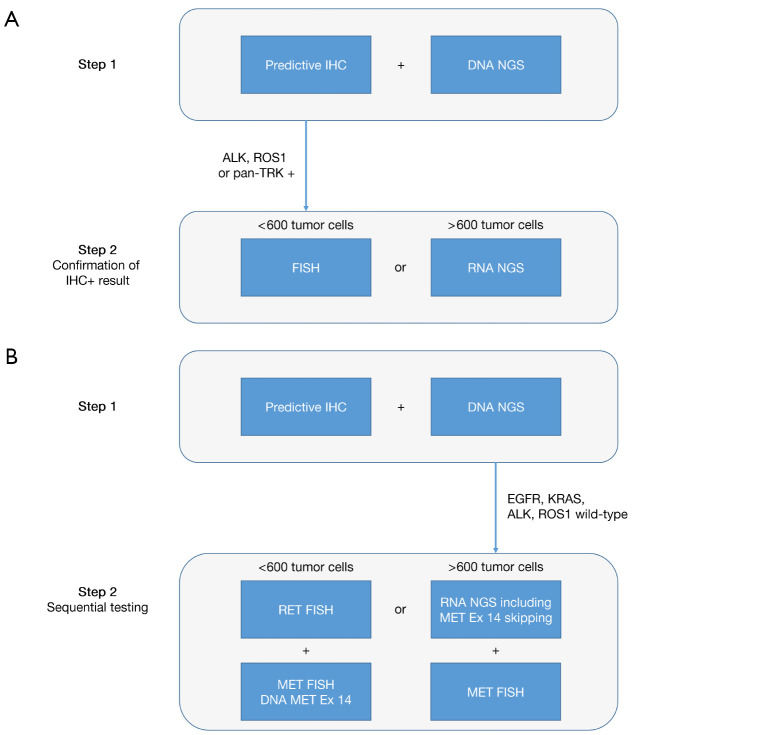
Despite the scarcity of most targeted rearrangements in NSCLC, they should eventually be tested in every patient, at least in the case of wild-type status of other established driver genes (esp., EGFR and KRAS). While the importance of identifying patients that could benefit from targeted therapy is unquestioned, feasibility in terms of tumor cell quantity, locally available testing methods, access to drugs, and economic aspects have to be taken into consideration when creating testing algorithms and guidelines. Methods might need to be combined and algorithms flexibly adapted. Simultaneous frontline DNA- and RNA-based NGS for mutation and rearrangement testing appears to be the most comprehensive and preferable approach. In contrast, sequential testing by restricting RNA-based rearrangement testing to the 50% of patients without exclusive driver mutations is more economical but can lead to treatment delay. For NTRK, which is particularly rare and challenging, several guidelines have been published (26,31,46). In our practice, we initiate IHC to prescreen for ALK, ROS1, and NTRK rearrangements simultaneously with mutational testing by NGS in all qualifying NSCLC (Figure 3). This allows the rapid identification of the rare 5–7% of patients with predictive rearrangements within one day. Positive or ambiguous results are further evaluated using an additional method such as FISH or RNA-based NGS. RNA-based NGS with a panel that covers fusions for RET, NRG1, FGFR2/3, and MET exon 14 skipping mutation is the preferred method for NSCLC which are wild-type for EGFR, KRAS, ALK, ROS1 and pan-Trk negative. RET FISH is a valid final analysis if the material is insufficient for RNA-NGS panel tests.
Figure 3.
Sequential predictive testing algorithm for NSCLC at the University Hospital Basel. Predictive IHC includes ALK (clone 5A4), ROS1 (clone D4D6), pan-Trk (EPR17341) and PD-L1 (Ventana SP263 assay). Predictive IHC and DNA NGS are performed in parallel. (A) In case of ALK, ROS1 or pan-Trk expression by IHC, the respective rearrangement is confirmed by a molecular method. The method used depends on the amount of tumor cells present in the specimen. FISH requires only 50–100 tumor cells. Of note, to cover all NTRK genes (NTRK1, 2 and 3), three FISH tests are necessary. For good quality RNA usually at least around 600 tumor cells need to be extracted. (B) In EGFR and KRAS wild-type NSCLC negative for ALK and ROS1 by IHC, sequential testing for further driver alterations is performed. Again the method used depends on the amount of tumor cells, and covers at least the detection of RET rearrangements and MET alterations (amplification and MET exon 14 skipping mutations). IHC, immunohistochemistry; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Silvia Novello, Francesco Passiglia) for the series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” published in Translational Lung Cancer Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the NARRATIVE REVIEW reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-676
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-676
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-676). The series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” was commissioned by the editorial office without any funding or sponsorship. Dr. MSM reports personal fees from Thermo Fisher, outside the submitted work. Dr. SS reports personal fees from MSD, personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from Roche, personal fees from Pfizer, personal fees from Thermo Fisher Scientific, outside the submitted work. Dr. LB reports grants from Sanofi, grants and personal fees from Roche, grants and personal fees from MSD, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from Takeda, outside the submitted work. The other author has no other conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Tsao AS, Scagliotti GV, Bunn PA, Jr, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613-38. 10.1016/j.jtho.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 3.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens F, Johansson B, Fioretos T, et al. The emerging complexity of gene fusions in cancer. Nat Rev Cancer 2015;15:371-81. 10.1038/nrc3947 [DOI] [PubMed] [Google Scholar]
- 5.Schram AM, Chang MT, Jonsson P, et al. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 2017;14:735-48. 10.1038/nrclinonc.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benayed R, Offin M, Mullaney K, et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin Cancer Res 2019;25:4712-22. 10.1158/1078-0432.CCR-19-0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thai AA, Solomon BJ. Treatment of ALK-positive nonsmall cell lung cancer: recent advances. Curr Opin Oncol 2018;30:84-91. 10.1097/CCO.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 8.Bubendorf L, Buttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch 2016;469:489-503. 10.1007/s00428-016-2000-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon JP, Linkov I, Rosado A, et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol 2020;33:38-46. 10.1038/s41379-019-0324-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer Discov 2020;10:498-505. 10.1158/2159-8290.CD-19-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 2017;2017:PO.17.00011. [DOI] [PMC free article] [PubMed]
- 12.Latysheva NS, Babu MM. Discovering and understanding oncogenic gene fusions through data intensive computational approaches. Nucleic Acids Res 2016;44:4487-503. 10.1093/nar/gkw282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012;17:1351-75. 10.1634/theoncologist.2012-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JJ, Shaw AT. Recent Advances in Targeting ROS1 in Lung Cancer. J Thorac Oncol 2017;12:1611-25. 10.1016/j.jtho.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan L, Feng Y, Wan H, et al. Clinicopathological and demographical characteristics of non-small cell lung cancer patients with ALK rearrangements: a systematic review and meta-analysis. PLoS One 2014;9:e100866. 10.1371/journal.pone.0100866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park E, Choi YL, Ahn MJ, et al. Histopathologic characteristics of advanced-stage ROS1-rearranged non-small cell lung cancers. Pathol Res Pract 2019;215:152441. 10.1016/j.prp.2019.152441 [DOI] [PubMed] [Google Scholar]
- 17.Stinchcombe TE. Current management of RET rearranged non-small cell lung cancer. Ther Adv Med Oncol 2020;12:1758835920928634. 10.1177/1758835920928634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farago AF, Taylor MS, Doebele RC, et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis Oncol 2018;2018:PO.18.00037. [DOI] [PMC free article] [PubMed]
- 19.Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 2014;84:121-6. 10.1016/j.lungcan.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Miyata-Morita K, Morita S, Matsutani N, et al. Frequent appearance of club cell (Clara cell)-like cells as a histological marker for ALK-positive lung adenocarcinoma. Pathol Int 2019;69:688-96. 10.1111/pin.12864 [DOI] [PubMed] [Google Scholar]
- 21.Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer 2014;110:1571-8. 10.1038/bjc.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S, Pennell NA, Ali SM, et al. RET-rearranged lung adenocarcinomas with lymphangitic spread, psammoma bodies, and clinical responses to cabozantinib. J Thorac Oncol 2014;9:1714-9. 10.1097/JTO.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 2014;4:415-22. 10.1158/2159-8290.CD-13-0633 [DOI] [PubMed] [Google Scholar]
- 24.Cadranel J, Liu SV, Duruisseaux M, et al. Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series. Oncologist 2020. [Epub ahead of print]. doi: . 10.1634/theoncologist.2020-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe J, Togo S, Sumiyoshi I, et al. Clinical features of squamous cell lung cancer with anaplastic lymphoma kinase (ALK)-rearrangement: a retrospective analysis and review. Oncotarget 2018;9:24000-13. 10.18632/oncotarget.25257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penault-Llorca F, Rudzinski ER, Sepulveda AR. Testing algorithm for identification of patients with TRK fusion cancer. J Clin Pathol 2019;72:460-7. 10.1136/jclinpath-2018-205679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain D, Roy-Chowdhuri S. Molecular Pathology of Lung Cancer Cytology Specimens: A Concise Review. Arch Pathol Lab Med 2018;142:1127-33. 10.5858/arpa.2017-0444-RA [DOI] [PubMed] [Google Scholar]
- 28.Jain D, Nambirajan A, Borczuk A, et al. Immunocytochemistry for predictive biomarker testing in lung cancer cytology. Cancer Cytopathol 2019;127:325-39. 10.1002/cncy.22137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savic S, Bubendorf L. Role of fluorescence in situ hybridization in lung cancer cytology. Acta Cytol 2012;56:611-21. 10.1159/000339792 [DOI] [PubMed] [Google Scholar]
- 30.Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323-58. 10.1016/j.jtho.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 31.Marchio C, Scaltriti M, Ladanyi M, et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol 2019;30:1417-27. 10.1093/annonc/mdz204 [DOI] [PubMed] [Google Scholar]
- 32.Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2014;138:1432-43. 10.5858/arpa.2013-0610-CP [DOI] [PubMed] [Google Scholar]
- 33.Marchetti A, Di Lorito A, Pace MV, et al. ALK Protein Analysis by IHC Staining after Recent Regulatory Changes: A Comparison of Two Widely Used Approaches, Revision of the Literature, and a New Testing Algorithm. J Thorac Oncol 2016;11:487-95. 10.1016/j.jtho.2015.12.111 [DOI] [PubMed] [Google Scholar]
- 34.Savic S, Diebold J, Zimmermann AK, et al. Screening for ALK in non-small cell lung carcinomas: 5A4 and D5F3 antibodies perform equally well, but combined use with FISH is recommended. Lung Cancer 2015;89:104-9. 10.1016/j.lungcan.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H, Tsuta K, Yoshida A, et al. Aberrant anaplastic lymphoma kinase expression in high-grade pulmonary neuroendocrine carcinoma. J Clin Pathol 2013;66:705-7. 10.1136/jclinpath-2012-201329 [DOI] [PubMed] [Google Scholar]
- 36.Tsao MS KK, Hirsch FR, Yatabe Y, et al. editors. IASLC Atlas of ALK and ROS1 Testing in Lung Cancer. 1nd Ed. Aurora, CO: International Association for the Study of Lung Cancer. 2016. [Google Scholar]
- 37.Conde E, Hernandez S, Martinez R, et al. Assessment of a New ROS1 Immunohistochemistry Clone (SP384) for the Identification of ROS1 Rearrangements in Patients with Non-Small Cell Lung Carcinoma: the ROSING Study. J Thorac Oncol 2019;14:2120-32. 10.1016/j.jtho.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 38.Kheder ES, Hong DS. Emerging Targeted Therapy for Tumors with NTRK Fusion Proteins. Clin Cancer Res 2018;24:5807-14. 10.1158/1078-0432.CCR-18-1156 [DOI] [PubMed] [Google Scholar]
- 39.Ferrara R, Auger N, Auclin E, et al. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27-45. 10.1016/j.jtho.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 40.Trombetta D, Graziano P, Scarpa A, et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by Phospho-ErbB3 expression. Oncotarget 2018;9:9661-71. 10.18632/oncotarget.23800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin Cancer Res 2014;20:4107-14. 10.1158/1078-0432.CCR-14-0284 [DOI] [PubMed] [Google Scholar]
- 42.Theelen WS, Mittempergher L, Willems SM, et al. FGFR1, 2 and 3 protein overexpression and molecular aberrations of FGFR3 in early stage non-small cell lung cancer. J Pathol Clin Res 2016;2:223-33. 10.1002/cjp2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin V, Bernasconi B, Merlo E, et al. ALK testing in lung adenocarcinoma: technical aspects to improve FISH evaluation in daily practice. J Thorac Oncol 2015;10:595-602. 10.1097/JTO.0000000000000444 [DOI] [PubMed] [Google Scholar]
- 44.Hsiao SJ, Zehir A, Sireci AN, et al. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J Mol Diagn 2019;21:553-71. 10.1016/j.jmoldx.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies KD, Le AT, Sheren J, et al. Comparison of Molecular Testing Modalities for Detection of ROS1 Rearrangements in a Cohort of Positive Patient Samples. J Thorac Oncol 2018;13:1474-82. 10.1016/j.jtho.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon JP, Benayed R, Hechtman JF, et al. Identifying patients with NTRK fusion cancer. Ann Oncol 2019;30:viii16-viii22. [DOI] [PMC free article] [PubMed]
- 47.van der Wekken AJ, Pelgrim R, t Hart N, et al. Dichotomous ALK-IHC Is a Better Predictor for ALK Inhibition Outcome than Traditional ALK-FISH in Advanced Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4251-8. 10.1158/1078-0432.CCR-16-1631 [DOI] [PubMed] [Google Scholar]
- 48.Tsao MS, Yatabe Y. Old Soldiers Never Die: Is There Still a Role for Immunohistochemistry in the Era of Next-Generation Sequencing Panel Testing? J Thorac Oncol 2019;14:2035-8. 10.1016/j.jtho.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 49.Thunnissen E, Lissenberg-Witte BI, van den Heuvel MM, et al. ALK immunohistochemistry positive, FISH negative NSCLC is infrequent, but associated with impaired survival following treatment with crizotinib. Lung Cancer 2019;138:13-8. 10.1016/j.lungcan.2019.09.023 [DOI] [PubMed] [Google Scholar]
- 50.Beadling C, Wald AI, Warrick A, et al. A Multiplexed Amplicon Approach for Detecting Gene Fusions by Next-Generation Sequencing. J Mol Diagn 2016;18:165-75. 10.1016/j.jmoldx.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 51.Ali G, Bruno R, Savino M, et al. Analysis of Fusion Genes by NanoString System: A Role in Lung Cytology? Arch Pathol Lab Med 2018;142:480-9. 10.5858/arpa.2017-0135-RA [DOI] [PubMed] [Google Scholar]
- 52.Sgariglia R, Pisapia P, Nacchio M, et al. Multiplex digital colour-coded barcode technology on RNA extracted from routine cytological samples of patients with non-small cell lung cancer: pilot study. J Clin Pathol 2017;70:803-6. 10.1136/jclinpath-2017-204373 [DOI] [PubMed] [Google Scholar]
- 53.Reguart N, Teixido C, Gimenez-Capitan A, et al. Identification of ALK, ROS1, and RET Fusions by a Multiplexed mRNA-Based Assay in Formalin-Fixed, Paraffin-Embedded Samples from Advanced Non-Small-Cell Lung Cancer Patients. Clin Chem 2017;63:751-60. 10.1373/clinchem.2016.265314 [DOI] [PubMed] [Google Scholar]
- 54.Lira ME, Choi YL, Lim SM, et al. A single-tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J Mol Diagn 2014;16:229-43. 10.1016/j.jmoldx.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 55.Alkan C, Coe BP, Eichler EE. Genome structural variation discovery and genotyping. Nat Rev Genet 2011;12:363-76. 10.1038/nrg2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 2011;13:36-46. 10.1038/nrg3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2015;10:1670-4. 10.1097/01.JTO.0000473485.38553.f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy DA, Ely HA, Shoemaker R, et al. Detecting Gene Rearrangements in Patient Populations Through a 2-Step Diagnostic Test Comprised of Rapid IHC Enrichment Followed by Sensitive Next-Generation Sequencing. Appl Immunohistochem Mol Morphol 2017;25:513-23. 10.1097/PAI.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as