Abstract
Autoimmune pancreatitis is a chronic fibroinflammatory autoimmune mediated disease of the pancreas. Clinically, obstructive painless jaundice and upper abdominal pain are the main symptoms. Focal AIP is characterized by segmental involvement of pancreatic parenchyma and it is often radiologically represented by a pancreatic mass. In these cases, the diagnosis can be very challenging, since it may be easily confused with pancreatic cancer. Therefore, we suggest a combined approach of imaging tests as the diagnostic workup. EUS study combined with CEUS and elastography, if available, increases the accuracy of the method to rule out cancer. Moreover, the lesion should always be sampled under EUS guidance to obtain a cyto/histological diagnosis. The diagnostic workup should also include the use of diagnostic clinical criteria (extrapancreatic lesions, steroid response) and laboratory findings (CA 19.9 and IgG4 evaluations).
Key words: pancreas, areas, structures & systems, abdomen, areas, structures & systems, endoscopy, methods & techniques, ultrasound
Introduction
Autoimmune pancreatitis (AIP) is a chronic pancreatic fibroinflammatory autoimmune mediated disease. Obstructive painless jaundice and upper abdominal pain are the main symptoms. Many different classifications of AIP have been proposed: JPS (2002, 2006) 1 , HISORt (2006, 2009) 2 , Korean (2007) 3 , Asian (2008) 4 , Mannheim (2009) 5 , Italian (2003, 2009) 6 .
A review of these criteria led to the formulation of international consensus diagnostic criteria (ICDC) in 2011 7 , where two main AIP subtypes have been described ( Table 1 ).
Table 1 ICDC criteria for AIP diagnosis.
| TYPE 1 AIP | ||
|---|---|---|
| CRITERION | LEVEL 1 | LEVEL 2 |
| Parenchymal imaging [P] | Typical Diffuse enlargement with delayed enhancement (with or without rim-like enhancement) | Indeterminate (and atypical) Segmental/focal enlargement with delayed enhancement |
| Ductal imaging (ERP) [D] | Long (>1/3 of the total length of MD) or multiple strictures without marked upstream dilation | Segmental/focal narrowing without marked upstream dilation (duct size <5 mm) |
| Serology [S] Other organ involvement [OOI] | IgG4 increased > 2x upper normal value A or B A: histology of extrapancreatic organs (3 or more) 1-marked lymphoplasmacytic infiltration with fibrosis and without granulocytic infiltration 2-storiform fibrosis 3-obliterative phlebitis 4-IgG4-positive cells >10/HPF B: Typical radiological evidence (one of) 1-segmental/multiple proximal or proximal and distal bile duct stricture 2-retroperitoneal fibrosis | IgG4 increased < 2x upper normal value A or B A: histology of extrapancreatic organs+endoscopic biopsies of bile duct (1+2) 1-marked lymphoplasmacytic infiltration without granulocytic infiltration 2- IgG4-positive cells >10/HPF B: physical or radiological evidence (one of) 1-symmetrically enlarged salivary or lachrymal glands 2-radiological evidence of renal involvement |
| Histology of pancreas [H] | LPSP (biopsy/resection) (3 or more) 1-periductal lymphoplasmacytic infiltrate without granulocytic infiltration 2-obliterative phlebitis 3-storiform fibrosis 4-IgG4-positive cells >10/HPF | LPSP (biopsy) (2 of) 1-periductal lymphoplasmacytic infiltrate without granulocytic infiltration 2-obliterative phlebitis 3-storiform fibrosis 4-IgG4-positive cells >10/HPF |
| Response to steroid [Rt] | Diagnostic steroid trial : < 2 weeks treatment with radiological resolution or marked improvement in pancreatic or extrapancreatic manifestations | |
| TYPE 2 AIP | ||
| LEVEL 1 | LEVEL 2 | |
| Parenchymal imaging [P] | Typical Diffuse enlargement with delayed enhancement (with or without rim-like enhancement) | Indeterminate (and atypical) Segmental/focal enlargement with delayed enhancement |
| Ductal imaging (ERP) [D] | Long (>1/3 of the total length of MD) or multiple strictures without marked upstream dilation | Segmental/focal narrowing without marked upstream dilation (duct size <5 mm) |
| Serology [S] Other organ involvement [OOI] | Inflammatory bowel disease | |
| Histology of pancreas [H] | IDCP (resection (biopsy)) (1+2) 1-granulocytic infiltration of duct wall (GEL) with or without granulocytic acinar inflammation 2-IgG4 positive cells 0–10/HPF | IDCP (resection (biopsy)) (1+2) 1-granulocytic and lymphoplasmacytic acinar infiltrate 2-IgG4-positive cells 0–10/HPF |
| Response to steroid [Rt] | Diagnostic steroid trial : < 2 weeks treatment with radiological resolution or marked improvement in pancreatic or extrapancreatic manifestations |
AIP: autoimmune pancreatitis; OOI: Other Organ Involvement; LPSP: lymphoplasmacytic sclerosing pancreatitis; IDCP: idiopathic duct-centric pancreatitis; MD=main pancreatic duct
Type 1 AIP: lymphoplasmacytic sclerosing pancreatitis, with dense periductal infiltration of plasma cells and lymphocytes, peculiar storiform fibrosis, venulitis with lymphocytes and plasma cells, obliteration of veins. Serologically, it shows abundant immunoglobulin (Ig)G4-positive plasma cells, as a pancreatic manifestation of IgG4-related systemic disease, with extrapancreatic lesions with infiltration of IgG4-positive plasma cells 8 . Some possible associations with AIP are sclerosing cholangitis, retroperitoneal fibrosis, lachrymal/salivary gland lesions, pulmonary hilar lymphadenopathy, tubulointerstitial nephritis, hypophysitis, chronic thyroiditis, prostatitis. Moreover, the response to steroid therapy is excellent (97–98%) 9 .
Type 2: Specific IgG4-negative pancreatic disease, with idiopathic duct-centric pancreatitis (IDCP) or AIP with granulocyte epithelial lesions (GELs) and lumen obliteration of medium, intraepithelial neutrophils, periductal lymphoplasmacytic infiltrate fibrosis. The prevalence of inflammatory bowel disease in patients with AIP is 30% 10 .
Despite formulation of the ICDC with clinical/histological criteria, the diagnosis of AIP remains challenging: ICDC criteria are not internationally applied, impossibility of sampling, or technical difficulties in meeting histological criteria. Moreover, the spectrum of clinical presentation is very broad ( Table 2 ) including the presence of a pancreatic mass.
Table 2 AIP clinical presentations.
| Clinical findings |
|---|
| Jaundice |
| Mild abdominal pain |
| Endocrine insufficiency (diabetes) |
| Weight loss |
| Persistent hyperamylasemia |
| Recurrent episodes of acute pancreatitis of unknown origin |
| Pancreatic mass or pancreatic enlargement incidentally found at imaging |
| One of the criteria above and concomitant other organ involvement |
Indeed, the radiological features of AIP range from normal pancreas to diffuse parenchymal enlargement with a “sausage-like” appearance, to a focal mass-like image. The presence of the latter radiological appearance is an indication of focal AIP (f-AIP) 11 12 . Mainly, the focal type appears on imaging as a focal mass with blurred outlines. Dilation of the main pancreatic duct can be evident and the image can be easily confused with a neoplastic pancreatic lesion 11 . Conversely, the other imaging presentations are suggestive of diffuse forms of AIP.
This review aims to describe the available tests to better diagnose focal AIP, ruling out pancreatic cancer (PC) and giving a possible effective diagnostic approach.
Focal autoimmune pancreatitis
F-AIP is characterized by segmental involvement of the pancreatic parenchyma and it is radiologically represented by a pancreatic mass. The literature does not report precise data on the prevalence of the focal form in Type I or II pancreatitis. However, Type I f-AIP seems to definitely be more frequent than Type II 12 . Diagnosis can be very challenging, as it may be easily confused with PC. Since f-AIP is a benign condition that is dramatically responsive to steroid therapy within one month in 90% of cases 7 , it is mandatory to histologically rule out cancer, thereby avoiding pancreatic surgery. Currently, the only reference standard for diagnosis of F-AIP is the surgical specimen. Moreover, the prevalence of PC in the general population is much higher than that of AIP. It is the fourth leading cause of cancer-related fatalities in Western countries. Therefore, early treatment is crucial for achieving cure. Unfortunately, the clinical incidence of f-AIP among all AIP cases is unknown. The only available data relate to f-AIP cases diagnosed as cancer.
Several studies reported large series of misdiagnosed f-AIP that was surgically treated. In 2003, Abraham et al. 13 reported that 10.6% of Whipple resections among 442 pancreaticoduodenectomies were negative for neoplasia, with 12.8% being chronic pancreatitis (CP) of unknown etiology, and 23.4% being AIP 1. In a different surgical series 14 the incidence of CP was 13% (21/162 specimens). Another two series reported benign pathology in 7% 15 and 23% 16 of cases among patients who underwent surgery for cancer. The lack of accurate markers to differentiate between PC and non-malignant pancreatic lesions, such as blood test or other noninvasive tools with a high positive predictive value, is the main reason behind diagnostic failures. Thus, the first goal regarding pancreatic masses is to definitively rule out the presence of cancer, even if clinical and serological findings (high level of IgG4, presence of autoantibodies, low serum levels of Ca 19–9) are suggestive for AIP 17 .
Diagnosis of focal AIP
Serum markers
Serum IgG4 levels may rise to twice the normal value in AIP 1. However, IgG4 elevation may also be present in PC. When IgG4 serum levels were examined in 115 patients with cancer, plasmatic IgG4 levels were higher than normal in 14 patients and double in 2 patients. One case had an overlap diagnosis between f-AIP and PC. No larger quantity of data about overlap between f-AIP and PC is available. Serum IgG4, CEA, and CA19–9 levels were measured in 188 patients 18 . A combined use of serum IgG4 (over 280 mg/dL) and CA19–9 9 (below 85.0 U/ml) was suggested to increase the diagnostic accuracy to distinguish AIP from PC. When using an IgG cutoff value of 175 mg/dL, the sensitivity and specificity for differential diagnosis were 67.5 and 90.4%, respectively 19 . However, these data have weak evidence, and the diagnosis of PC versus AIP cannot be made only using serological parameters.
Imaging
Transabdominal Ultrasound (US)
US is usually the first diagnostic method performed in patients with jaundice or abdominal pain, because of its low cost and wide availability. However, the ability of US to detect pancreatic masses is related to operator experience and is reduced by the possible presence of bowel gas or obesity, due to the retroperitoneal pancreas location. Despite compression to displace bowel gas, asking for inspiration/expiration, changing the patient’s position, US sensitivity, specificity, and accuracy range from 48–95, 40–91 and 46–64%, respectively 20 . In a multicenter retrospective study, US detected the tumor in 52.6% of 135 cases of early-stage PC. Data about cancer screening in Japan showed that US detected less than 0.01% of cases of PC. 21 . Thus, US cannot be considered the reference standard for the study of PC and its limitations contraindicate US sampling. Differentiation between f-AIP and PC is even more difficult. The use of contrast-enhanced US (CEUS) and elastography (EG) may help. In a meta-analyses, the pooled sensitivity and specificity in the differential diagnosis between pancreatic adenocarcinoma (ADK) and other pancreatic masses with CEUS were 86–90 and 75–88%, respectively 22 . Among 123 pancreatic lesions, the difference in stiffness between ADK and the normal pancreas was statistically significant (p 0.05) 23 . However, another study performed ARFI elastography in 27 solid pancreatic lesions: 8 benign (focal pancreatitis and AIP) and 19 malignant. No statistical difference was found. Therefore, US can be considered as a first-line test. CEUS can help in studying pancreatic masses, but it cannot be considered a good test for discriminating PC from f-AIP 24 .
Computed Tomography (CT scan)
Regarding the role of multiphase contrast-enhanced (CE) CT for differentiating f-AIP from carcinoma, 22 f-AIP lesions and 61 malignant lesions were examined 25 . The frequencies of radiological findings between f-AIP and cancer were compared. At multivariate analysis dotted enhancement, the duct-penetrating sign and capsule-like rim were statistically significant for the diagnosis of AIP versus PC. The combination of these findings permitted AIP diagnosis with 82% sensitivity and 98% specificity 26 . However, in another study 27 (32 pancreatic lesions), CT scan showed an accuracy of only 68% in the diagnosis of AIP. In addition, the agreement between radiologists with respect to distinguishing between benign and malignant masses seemed fair (κ, 0.58; p<0.0001). Hence, based on the minimal available evidence, CT scan is a fair method for distinguishing between PC and f-AIP. To date, no large cohorts have been investigated.
Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)
The role of 18F-FDG PET/CT in distinguishing between f-AIP and PC was examined in 26 AIP and 40 PC patients. All 26 patients with AIP had increased pancreatic FDG uptake. The standardized uptake values (SUV) max in AIP patients were higher compared with those in PC patients (p<0.05). However, the diagnostic sensitivity of SUV in the PC group was only 70%. Furthermore, a quite remarkable metabolism was detected in some patients with AIP, leading to false positivity 28 . In a Chinese study 29 , the sensitivity, specificity, and accuracy of 18F-FDG PET/CT in differentiating PC from f-AIP were 95, 60, and 83.3%, respectively. Another retrospective analysis of 232 patients 30 showed that FDG-PET was not effective in detecting early stage PC or in differentiating f-AIP from PC. Therefore, FDG-PET is considered a poor method for distinguishing between f-AIP and PC.
Magnetic Resonance Imaging (MRI)
Regarding MRI, a study 31 examined 36 patients with f-AIP and 72 patients with PC who underwent CE-MRI with triple phases. Quantitative analysis of the lesion contrast using CE-MRI was helpful to differentiate f-AIP from PC. For AIP, the sensitivity and specificity of the contrast arterial phase were 94.4 and 87.5%, respectively, (LR+ 7.55, LR- 0.06) and were comparable or significantly higher than those of all key imaging features. For PC, the sensitivity (87.5%) and specificity (94.4%) of the contrast arterial phase were comparable or significantly higher than those of all key imaging features, except for the discrete mass. Moreover, one study 32 retrospectively evaluated the combination of triple-phase CT scan of 79 patients (19 with f-AIP, 30 with PC, and 30 with a normal pancreas) with MRI findings. The diagnostic performance of CT attenuation changes from the arterial phase to the hepatic phase was significantly higher in f-AIP than in PC (p<0.05), with a sensitivity, specificity, and area under the ROC curve of 87.5%, 100% and 0.974 (95% CI:0.928–1.021), respectively. Analysis of the combination of focal pancreatic enlargement with a capsule-like rim, irregular narrowing of the MPD, and stricture of the CBD in patients with lesions (not located in the pancreatic head) helped to improve the diagnostic accuracy for f-AIP. Conversely, the retrospective analysis of 22 patients 33 found when analyzing CT scans and MRI images that the diagnostic performance of combined unenhanced and CE-MR images was significantly better than that of CT (p<0.01). These data were confirmed also in another retrospective cohort of 187 patients 34 . However, no studies prospectively evaluated in large cohorts the accuracy of either MRI alone or combined with CT, to consistently exclude PC in the case of a pancreatic mass. Hence, the sensitivity and specificity of MRI are good. However, since the available studies are all retrospective with small samples, it is difficult to give external validity of their results and use MRI as the reference standard in differential diagnosis between PC and f-AIP. However, MRI should be used to confirm the final diagnosis, and it could be considered in the follow-up of patients.
Endoscopic Ultrasound (EUS)
Endoscopic ultrasound (EUS) is nowadays widely available and provides high-resolution images of the pancreas without interference from bowel gas. It is an invasive technique, but it does not expose the patient to radiation, allows the use of a contrast medium almost free of side effects, and enables direct guided sampling of pancreatic masses. No EUS imaging features are described as pathognomonic of f-AIP or PC. However, some signs could be helpful in the differential diagnosis ( Fig. 1 ): presence or absence of macroscopic vascular invasion, extrapancreatic local spread of the mass, and presence of pancreatic duct dilation. When vascular or extrapancreatic invasion is clear, the diagnosis of PC can be quite easy. However, EUS can also detect the apparent involvement of the portal and/or superior mesenteric vein in AIP when the inflammatory infiltrate transmurally involves the vessel wall 35 . In the case of well-differentiated ADK appearing as a small lesion with no clear vascular invasion, the differentiation between benign and malignant lesion can be hard. It is exactly in these cases that, if f-AIP is present, ruling out PC with a high level of certainty is mandatory in order to avoid surgery. The application of the Rosemont criteria ( Table 3 ) for the surrounding pancreatic parenchyma around the focal mass could be useful. However, EUS alone has shown slightly disappointing accuracy for differentiating PC from CP ( i. e ., 76% for malignancy and 46% for focal inflammation) 36 .
Fig. 1.
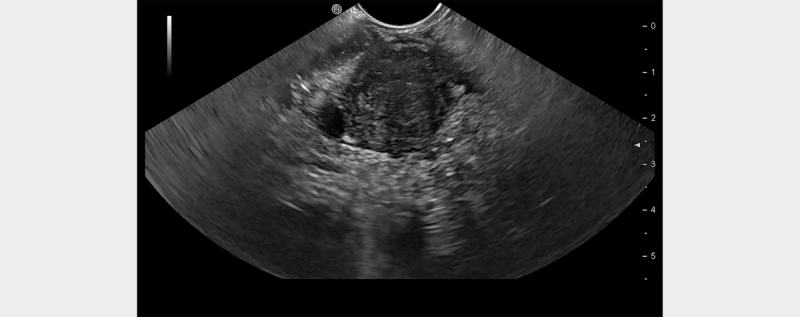
EUS image of a focal pancreatic lesion.
Table 3 Rosemont criteria for the diagnosis of chronic pancreatitis.
| Parenchymal features | Ductal features |
|---|---|
| Hyperechoic foci with shadowing | MPD calculi |
| Lobularity with honeycombing | Irregular MPD |
| Lobularity without honeycombing | Dilated side branches |
| Hyperechoic foci without shadowing | MPD dilation |
| Cysts | Hyperechoic MPD margin |
| Stranding |
FNA/FNB sampling
EUS-guided sampling should always be performed in the case of pancreatic solid lesions. FNA has a diagnostic accuracy ranging from 77–95% 37 38 , especially if coupled with on-site pathological evaluation (ROSE) 39 40 . It is a safe technique, with morbidity and mortality rates <1%. However, FNA is often unable to obtain core tissue with a preserved architecture and ROSE is mainly unavailable. Indeed, the current European Society of Gastrointestinal Endoscopy guidelines suggest, if ROSE is unavailable, to perform three to four needle passes with an FNA needle or two to three passes with an FNB needle. AIP may mimic malignancy presenting the following cytological features: occasional atypical cells, large nuclei, degenerative vacuoles, sparse mitosis. Conversely, cells in AIP tend to lack hyperchromasia, display only minimal architectural disorders, and have only modestly increased nuclear-to-cytoplasmic ratios 40 . Therefore, cytology can be inconclusive. Hence, theoretically, a core biopsy with an FNB needle yields larger specimens, providing better samples with intact histological architecture, to rule out PC. The available needles range from a diameter of 19 G to 25 G. Strong evidence is still lacking, but the literature shows some encouraging results. 25 G FNB seems to guarantee a higher amount of diagnostic cellular material and better preservation of the tissue architecture than 22 G FNA (p=0.030 and 0.010, respectively), with a better diagnostic yield for specific tumor discrimination (p=0.018). In the absence of ROSE, the 20 G FNB needle outperforms the 25 G FNA needle in terms of histological yield (77 vs 44%; P <0.001) and diagnostic accuracy (87 vs. 78%; P =0.002), with a 99% technical success rate for the FNB needle. 40 . Again, it was reported that using EUS trucut biopsy for acquiring core specimens and preserving tissue architecture could enhance the diagnostic accuracy for f-AIP 41 . Moreover, in a retrospective study 42 , FNB reached higher diagnostic accuracy than FNA in distinguishing between inflammatory masses and PC (93 vs. 83.6%, p=0.03). F-AIPs were also included in this cohort. Therefore, FNB should be considered the preferred sampling technique to rule out cancer in patients with underlying CP, including f-AIP.
Contrast-Enhanced Ultrasound (CEUS)
SonoVue is a second-generation microbubble contrast agent used for the characterization of the microvascularization of a lesion to make a differential diagnosis between benign and malignant diseases (SonoVue, 4.8 ml intravenous administration). EUS-US mode for CEUS allows dynamic observation. This clarifies the behavior of the lesion in the arterial and venous phases. Indeed, ADK has a typical hypoenhancement in all phases. Conversely, neuroendocrine tumors show strong arterial hyperenhancement. Mass-forming CP and f-AIP have an isovascular or weak hypervascular appearance, similar to the surrounding pancreatic parenchyma ( Fig. 2 ). These features may help in excluding PC. Indeed, in a retrospective data collection 39 including 60 cases of f-AIP and 16 cases of PC, 86.6% of AIP lesions displayed focal or diffuse isoenhancement in the arterial phase, while 93.7% of PC lesions were hypoenhancing (P<0.01). During the late phase, 65% of AIP lesions were hyperenhancing and 35% were isoenhancing , while 93.7% of PC cases were hypoenhancing. A retrospective study 43 investigated 80 patients diagnosed with f-AIP (27 patients) or PC (53 patients). Hyperenhancement to isoenhancement in the arterial phase (f-AIP 89 vs. PC 13%; p<0.05), homogeneous contrast agent distribution (f-AIP 81 vs. PC 17%; p<0.05), and absent irregular internal vessels (f-AIP 85 vs. PC 30%; p<0.05) were observed more frequently in the f-AIP group. The combination of these features improved the specificity (94%) for differentiating f-AIP from PC. Moreover, the overall diagnostic accuracy for CEUS was 83.33 vs. 44.4% for EUS only. (p<0.001). Importantly, the interobserver agreement for CEUS was significantly higher than that for US alone 44 . Interestingly, although only in a small series (3 AIP versus 17 PC), CEUS perfusion parameters were quantitatively analyzed with VueBox ® quantification software. Significant differences between PC and parenchyma could be found in terms of peak enhancement (PE), wash-in and wash-out AUC, and wash-in perfusion index. The PE of AIP was comparable to that of a normal pancreatic parenchyma. The PE of PC was significantly lower than that of AIP or normal parenchyma (p<0.01) 45 . In conclusion, although investigated in small cohorts, CEUS seems to increase EUS accuracy in the differential diagnosis between f-AIP and PC.
Fig. 2.
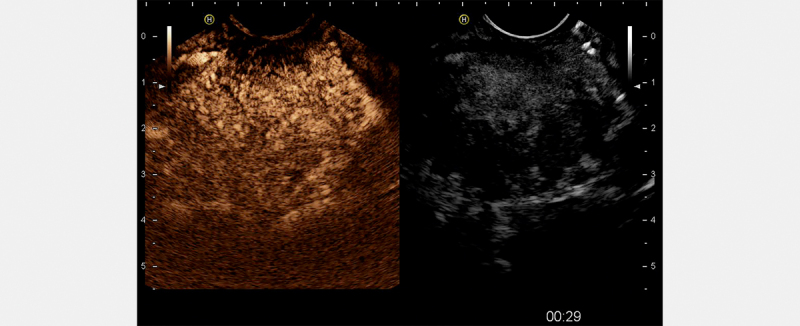
CEUS study of focal autoimmune pancreatitis: iso-hyperenhancement of the mass.
Elastography
Some studies investigated the role of EG in diagnosing f-AIP or other focal pancreatitis versus PC. One of the most popular commercially available EUS-EG techniques is real-time EG ( Fig. 3 ): a strain method with a color scale. The operator evaluates this scale qualitatively during a routine EUS session. Blue and green colors indicate a stiffer tissue, while a red color indicates a less stiff tissue. However, the software can also measure the ratio between the target zone (lesion) and normal surrounding parenchyma. It therefore also provides a semiquantitative result. Cancers often present a higher stiffness value versus normal tissue or inflammation. Values can be expressed in kPa or in velocity of the wave 46 . A study measured the stiffness of 123 lesions (78 PC cases and 45 f-AIP cases). The strain ratio lesion/surrounding parenchyma correlated significantly with malignancies 47 . Similarly, a prospective study (325 patients) investigated the role of real-time EG in the differential diagnosis between benign (CP and AIP) versus malignant nodules. For the strain ratio lesion/parenchyma, a cut-off value of 4.2 versus 10.9 had a sensitivity, specificity, PPV, NPV, accuracy of 95, 63, 89, 81, and 87%, respectively, versus 75, 88, 95, 54, and 79%, respectively 48 . In another study with 9 cases of AIP, 40 cases of CP, and 130 cases of PC 49 , EG had a sensitivity of 99%, a specificity of 63%, and an accuracy of 88%. The best cut-off level of strain ratio to obtain the maximal ROC curve was 7.8 (accuracy of 88%). Notably, in a meta-analysis that included 17 studies (1544 lesions), the pooled sensitivity and specificity for qualitative EG were 0.97 (95% CI, 0.95–0.99) and 0.67 (95% CI, 0.59–0.74), respectively; the pooled sensitivity and specificity for strain ratio were 0.98 (95%CI, 0.96–0.99) and 0.62 (95% CI, 0.56–0.68), respectively; the pooled sensitivity and specificity for CEUS were 0.90 (95% CI, 0.83–0.95) and 0.76 (95% CI, 0.67–0.84), respectively; and the pooled sensitivity and specificity for EUS-FNA were 0.84 (95% CI, 0.77–0.90) and 0.96 (95% CI, 0.88–1.00), respectively. These results suggest a very similar sensitivity and specificity for EUS-EG and CEUS and they may be complementary studies for EUS-FNA 50 .
Fig. 3.
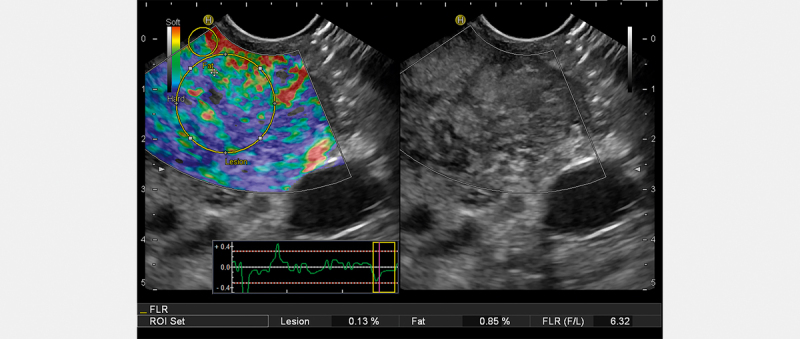
Real-time elastography study of focal autoimmune pancreatitis.
Comparison of the techniques
Based on previous observations regarding the accuracy of imaging in distinguishing between f-AIP and PC, the advantage of the CT scan is the availability of combining more elements in the study of the lesion, but some studies reported very low accuracy of CT and the lack of a good agreement between radiologists. The advantage of MRI is the high sensitivity and specificity of the contrast arterial phase study in the differential diagnosis of f-AIP versus PC. However, the lack of prospective studies is a relevant bias with regard to trusting the accuracy of the method as a reference standard. FGD-PET has the lowest sensitivity, specificity, and accuracy among the imaging techniques. Therefore, neither advantages of using FDG-PET alone in the diagnosis of f-AIP versus PC were described, nor are studies combining MRI and/or CT with FDG-PET available. US can be used as the first screening modality but cannot be considered the reference test to study pancreatic masses or to differentiate PC from f-AIP, due to its low accuracy in detecting cancer and characterizing masses. EUS has the advantage of good evaluation of the pancreas in all patients, with the best sensitivity, specificity, and accuracy among the imaging tests with respect to detecting pancreatic masses and distinguishing between PC and f-AIP. The main advantage of EUS over US is the possibility of ruling out cancer combining B-mode, CEUS, elastography and sampling the mass. The best sensitivity, specificity, and accuracy reported in the studies for the diagnosis of PC for each technique are: 48–95, 40–91, and 46–64%, respectively, for US; 77–80, 89–100, and 70–73%, respectively, for CT scan; 98, 97, and 90%, respectively, for EUS; 85–90, 96–98, and 85–100%, respectively, for EUS-FNA; 83–92, 63–89%, respectively, for MRI 20 21 22 23 24 . Table 4 summarizes the advantages and disadvantages of the imaging methods.
Table 4 Advantages and disadvantages of the imaging techniques to rule out cancer and diagnose f-AIP
| Imaging technique | Advantages | Disadvantages |
|---|---|---|
| Transabdominal US |
|
|
| CT scan |
|
|
| MRI |
|
|
| FDG-PET |
|
|
| EUS |
|
|
Discussion: A Practical Flowchart to Diagnose F-AIP
The diagnosis of f-AIP can be very challenging. Currently, the only reference standard for the differential diagnosis between f-AIP and cancer is histological examination after surgery. Among the imaging tests, a reference standard for a definitive diagnosis is lacking. A combined approach with imaging and sampling of the lesion increases the diagnostic accuracy, helping to rule out cancer. Based on these observations, we propose the following diagnostic approach.
If a focal pancreatic mass is detected by means of CT scan or MRI, EUS should always be performed and combined with CEUS study and EG if available. When sampling a suspected pancreatic cancer, EUS-FNA sampling is recommended as the first-line procedure 37 . The lesion should be sampled with FNA needles if ROSE is available or with FNB in the absence of ROSE to obtain a core histology. This method seems to reduce the inconclusive diagnosis and better rule out cancer 39 40 . The diagnostic workup should also include: clinical examination, detection of possible extrapancreatic lesions, and CA 19.9 and IgG4 evaluation, in order to meet the clinical ICDC criteria if possible.
If f-AIP is diagnosed, steroid treatment can be started. If f-AIP cannot be diagnosed but cancer can be confidently ruled out, steroid treatment should be started and the patient should be strictly followed up. EUS can be performed again after 3–4 weeks, since this is the time estimated to obtain response after steroid treatment. In the case of AIP, the radiological features should change and if the diagnosis of AIP is correct, the second EUS examination should find an impressive reduction of the “lesion”. We recommend using EUS for the reported good accuracy with respect to distinguishing between f-AIP and PC and, importantly, for the possibility of a second sampling 37 (if the first histology was inconclusive/negative for cancer, but the mass does not reduce). However, MRI can be also considered for follow-up. The diagnostic flowchart for f-AIP diagnosis is presented in Fig. 4 .
Fig. 4.
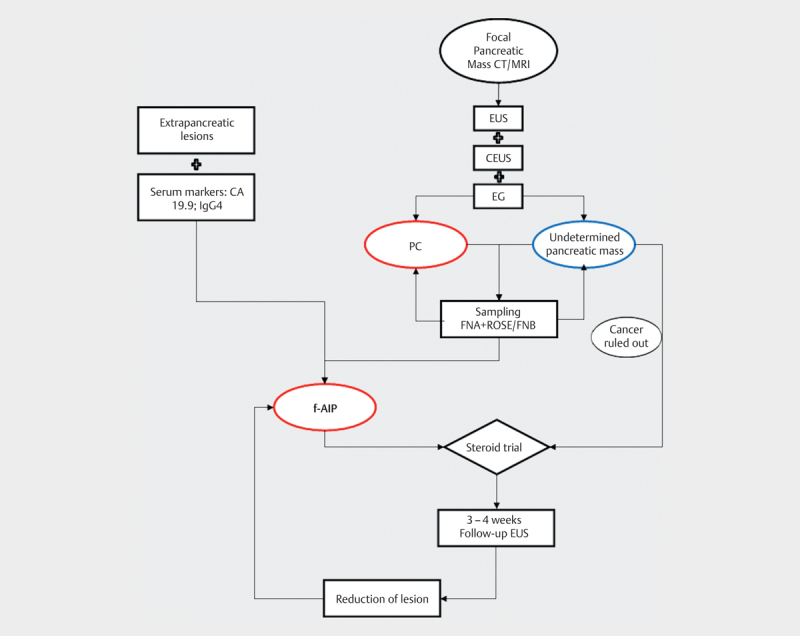
Diagnostic flowchart for focal autoimmune pancreatitis.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Members of the Criteria Committee for Autoimmune Pancreatitis of the Japan Pancreas SocietyDiagnostic criteria for autoimmune pancreatitis by the Japan Pancreas Society (2002) J Jpn Pancreas Soc (Suizou) 200217585–587. [Google Scholar]
- 2.Chari S T, Takahashi N, Levy M J.A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer Clin Gastroenterol Hepatol 200971097–3.PMID:19410017 [DOI] [PubMed] [Google Scholar]
- 3.Kwon S, Kim M H, Choi E K.The diagnostic criteria for autoimmune chronic pancreatitis: it is time to make a consensus Pancreas 200734279–286.PMID: 17414049 [DOI] [PubMed] [Google Scholar]
- 4.Otsuki M, Chung J B, Okazaki K.Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis J Gastroenterol 200843403–408.PMID: 18600383 [DOI] [PubMed] [Google Scholar]
- 5.Schneider A, Lohr J M.[Autoimmune pancreatitis] Internist (Berl) 200950318–330.PMID: 19212732 [DOI] [PubMed] [Google Scholar]
- 6.Pearson R K, Longnecker D S, Chari S T.Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003271–13.PMID:12826899 [DOI] [PubMed] [Google Scholar]
- 7.Shimosegawa T, Chari S T, Frulloni L.“International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the international association of pancreatology,” Pancreas 20113352–358.PMID: 21412117 [DOI] [PubMed] [Google Scholar]
- 8.Okazaki K, Uchida K.Current concept of autoimmune pancreatitis and IgG4-related disease Am J Gastroenterol 20181131412–1416.PMID: 30002467 [DOI] [PubMed] [Google Scholar]
- 9.Kamisawa T, Zen Y, Pillai S.IgG4-related disease The Lancet 20153851460–1471.PMID: 25481618 [DOI] [PubMed] [Google Scholar]
- 10.Okazaki K, Kawa S, Kamisawa T.Japanese consensus guidelines for management of autoimmune pancreatitis: I. Concept and diagnosis of autoimmune pancreatitis J Gastroenterol 201045249–26.PMID: 20084528 [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi T, Kawaura Y, Satomura Y.Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma Am J Gastroenterol 2003982679–2687.PMID: 14687817 [DOI] [PubMed] [Google Scholar]
- 12.Weber S M, Cubukcu-Dimopulo O, Palesty J A.Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma J Gastrointest Surg 20037129–137.PMID: 12559194 [DOI] [PubMed] [Google Scholar]
- 13.Abraham S C, Wilentz R E, Yeo C J.Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: Are they all ‘chronic pancreatitis’? Am J Surg Pathol 200327110–120.PMID: 12502933 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy T, Preczewski L, Stocker S J.Bell et al. Incidence of benign inflammatory disease in patients undergoing Whipple procedure for clinically suspected carcinoma: a single-institution experience Am J Surg 2006191437–441.PMID: 16490563 [DOI] [PubMed] [Google Scholar]
- 15.Hurtuk M G, Shoup M, Oshima K.Pancreaticoduodenectomies in patients without periampullary neoplasms: lesions that masquerade as cancer Am J Surg 2010199372–376.PMID: 20226913 [DOI] [PubMed] [Google Scholar]
- 16.De la Fuente S G, Ceppa E P, Reddy S K.Incidence of benign disease in patients that underwent resection for presumed pancreatic cancer diagnosed by endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) J Gastrointest Surg 2010141139–1142.PMID: 20424928 [DOI] [PubMed] [Google Scholar]
- 17.Frulloni L, Amodio A, Katsotourchi A M.A practical approach to the diagnosis of autoimmune pancreatitis World J Gastroenterol 2011172076–2079.PMID: 21547125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dite P, Novotny I, Dvorackova J.Pancreatic solid focal lesions: differential diagnosis between autoimmune pancreatitis and pancreatic cancer Dig Dis 201937416–442.PMID: 31079114 [DOI] [PubMed] [Google Scholar]
- 19.Chang M C, Liang P C, Jan S.Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels Pancreatology 201414366–372.PMID: 25278306 [DOI] [PubMed] [Google Scholar]
- 20.Sharma C, Eltawil K M, Renfrew P D et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanno A, Masamune A, Hanada K et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61–67. doi: 10.1016/j.pan.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Ashida R, Tanaka S, Yamanaka H et al. The role of transabdominal ultrasound in the diagnosis of early stage pancreatic cancer: Review and Single-Center Experience. Diagnostics (Basel) 2018;9:2. doi: 10.3390/diagnostics9010002.PMID:30587766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Onofrio M, De Robertis R, Crosara S et al. Acoustic radiation force impulse with shear wave speed quantification of pancreatic masses: A prospective study. Pancreatology. 2016;16:106–109. doi: 10.1016/j.pan.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Park M K, Jo J, Kwon H et al. Usefulness of acoustic radiation force impulse elastography in the differential diagnosis of benign and malignant solid pancreatic lesions. Ultrasonography. 2014;33:26–33. doi: 10.14366/usg.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuhashi N, Suzuki K, Sakurai Y.Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT Eur Radiol 2015251366–1374.PMID: 25433412 [DOI] [PubMed] [Google Scholar]
- 26.Held L.A nomogram for P values BMC Med Res Methodol 20101021PMID: 20233437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaheer A, Singh V K, Akshintala V S.Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography J Comput Assist Tomogr 201438146–155.PMID: 24424563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Jia G, Zuo C.18F- FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer BMC Cancer 201717695PMID: 29061130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto I, Shirakawa S, Shinzeki M.18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma Clin Gastroenterol Hepatol 201311712–718.PMID: 23353642 [DOI] [PubMed] [Google Scholar]
- 30.Gu X, Liu R.Application of 18F-FDG PET/CT combined with carbohydrate antigen 19-9 for differentiating pancreatic carcinoma from chronic mass-forming pancreatitis in Chinese elderly Clin Interv Aging 2016111365–1370.PMID: 27729779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon J H, Kim J H, Kim S Y.Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase Eur Radiol 2019295763–5771.PMID: 31028441 [DOI] [PubMed] [Google Scholar]
- 32.Sun G F, Zuo C J, Shao C W.Focal autoimmune pancreatitis: radiological characteristics help to distinguish from pancreatic cancer World J Gastroenterol 2013193634–3641.PMID: 23801866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang K M, Kim S H, Kim Y K.Missed pancreatic ductal adenocarcinoma: Assessment of early imaging findings on prediagnostic magnetic resonance imaging Eur J Radiol 2015841473–1479.PMID: 26032128 [DOI] [PubMed] [Google Scholar]
- 34.Lee J H, Min J H, Kim Y K.Usefulness of non-contrast MR imaging in distinguishing pancreatic ductal adenocarcinoma from focal pancreatitis Clin Imaging 201955132–139.PMID: 30818163 [DOI] [PubMed] [Google Scholar]
- 35.De Lisi S, Buscarini E, Arcidiacono P G.Endoscopic ultrasonography findings in autoimmune pancreatitis: be aware of the ambiguous features and look for the pivotal ones JOP 20101178–84.PMID: 20065561 [PubMed] [Google Scholar]
- 36.Rösch T, Lorenz R, Braig C.Endoscopic ultrasound in pancreatic tumor diagnosis Gastrointest Endosc 199137347–352.PMID: 2070987 [DOI] [PubMed] [Google Scholar]
- 37.Dumonceau J M, Deprez P H, Jenssen C.Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017 Endoscopy 201749695–714.PMID: 28511234 [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Liu S, Zhao Y.Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer: a meta-analysis Pancreatology 201313298–304.PMID: 23719604 [DOI] [PubMed] [Google Scholar]
- 39.Klapman J B, Logrono R, Dye C E.Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration Am J Gastroenterol 2003981289–1294.PMID: 12818271 [DOI] [PubMed] [Google Scholar]
- 40.Conti C B, Cereatti F, Grassia R.Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc 201911454–471.PMID: 31523377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy M J, Wiersema M J, Chari S T.Chronic pancreatitis: focal pancreatitis or cancer? Is there a role for FNA/biopsy? Autoimmune pancreatitis Endoscopy. 200638S30–S35.PMID: 16802220 [DOI] [PubMed] [Google Scholar]
- 42.Grassia R, Imperatore N, Capone P et al. EUS-guided tissue acquisition in chronic pancreatitis: Differential diagnosis between pancreatic cancer and pseudotumoral masses using EUS-FNA or core biopsy. Endoscopic. Ultrasound. 2020;9:122–129. doi: 10.4103/eus.eus_75_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Y, D’Onofrio M, Hocke M.Autoimmune pancreatitis: Imaging features Endosc Ultrasound 20187196–203.PMID: 28836516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho M K, Moon S H, Song T J.Contrast-Enhanced Endoscopic Ultrasound for Differentially Diagnosing Autoimmune Pancreatitis and Pancreatic Cancer Gut Liver 201812591–596.PMID: 29699060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitali F, Pfeifer L, Janson C.Quantitative perfusion analysis in pancreatic contrast enhanced ultrasound (DCE-US): A promising tool for the differentiation between autoimmune pancreatitis and pancreatic cancer Z Gastroenterol 2015531175–1181.PMID 26480053 [DOI] [PubMed] [Google Scholar]
- 46.Dietrich C F, Hocke M.Elastography of the pancreas Current viewClin Endosc 201952533–540.Doi: 10.5946/ce.2018.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyrla P, Gil J, Niemczyk S.Elastography in the Diagnosis of Pancreatic Malignancies Adv Exp Med Biol 2019113341–48.PMID:30443726 [DOI] [PubMed] [Google Scholar]
- 48.Okasha H H, Mahdy R E, Elkholy S.Endoscopic ultrasound (EUS) elastography and strain ratio, could it help in differentiating malignant from benign pancreatic lesions? Medicine (Baltimore) 201897e11689PMID: 30200064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okasha H, Elkholy S, El-Sayed R.Real time endoscopic ultrasound elastography an strain ratio in the diagnosis of solid pancreatic lesions World J Gastroenterol 2017235962–5968.PMID: 28932088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Y, Chen L, Li C.Diagnostic utility of endoscopic ultrasonography-elastography in the evaluation of solid pancreatic masses: A meta-analysis and systematic review Med Ultrason 201719150–158.PMID: 28440348 [DOI] [PubMed] [Google Scholar]


