Abstract
Background
The treatment landscape of metastatic clear cell renal cell carcinoma (mccRCC) has been transformed by targeted therapies with tyrosine kinase inhibitors (TKI) and more recently by the incorporation of immune checkpoint inhibitors (ICI). Today, a spectrum of single agent TKI to TKI/ICI and ICI/ICI combinations can be considered and the choice of the best regimen is complex.
Materials and methods
We performed an updated decision-making analysis among 11 international kidney cancer experts. Each expert provided their treatment strategy and relevant decision criteria in the first line treatment of mccRCC. After the collection of all input a list of unified decision criteria was determined and compatible decision trees were created. We used a methodology based on diagnostic nodes, which allows for an automated cross-comparison of decision trees, to determine the most common treatment recommendations as well as deviations.
Results
Diverse parameters were considered relevant for treatment selection, various drugs and drug combinations were recommended by the experts. The parameters, chosen by the experts, were performance status, International Metastatic renal cell carcinoma Database Consortium (IMDC) risk group, PD-L1 status, zugzwang and contraindication to immunotherapy. The systemic therapies selected for first line treatment were sunitinib, pazopanib, tivozanib, cabozantinib, ipilimumab/nivolumab or pembrolizumab/axitinib.
Conclusion
A wide spectrum of treatment recommendations based on multiple decision criteria was demonstrated. Significant inter-expert variations were observed. This demonstrates how data from randomized trials are implemented differently when transferred into daily practice.
Key words: decision-making, immune checkpoint inhibitor, clear cell renal cell carcinoma, systemic treatment, tyrosine kinase inhibitor
Highlights
-
•
Treatment options for metastatic clear cell renal cell carcinoma have become diverse.
-
•
We performed a decision-making analysis among 11 international kidney cancer experts.
-
•
Significant inter-expert variations for systemic first line treatments were observed.
-
•
Influencing factors were performance status, IMDC risk group, PD-L1 status, zugzwang and contraindication to immunotherapy.
-
•
The treatments selected were: sunitinib, pazopanib, tivozanib, cabozantinib, ipilimumab/nivolumab or pembrolizumab/axitinib.
Introduction
The treatment of metastatic clear cell renal-cell carcinoma (mccRCC) has changed dramatically over the past decades. The disease is characterized by a susceptibility to both immunotherapeutic and targeted agents, mainly antiangiogenics, while being typically resistant to cytotoxic chemotherapy.1,2
Starting in 2007, multiple tyrosine kinase inhibitors (TKI), targeting either the vascular endothelial growth factor (VEGF) and VEGR-receptors, or the mammalian target of rapamycin (mTOR) pathways showed significant improvements in progression free survival (PFS) and even overall survival (OS).3, 4, 5, 6 These agents were subsequently approved by entities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Recently, several trials changed this treatment landscape. They investigated either the combination of two immune checkpoint inhibitors (ICI) or an ICI/TKI combination.
In the CheckMate 214 trial, OS was significantly higher for the combination of ipilimumab and nivolumab compared to the standard treatment with sunitinib in intermediate- and poor-risk patients based on the International Metastatic renal cell carcinoma (RCC) Database (IMDC) risk classification.7 Sunitinib was again outperformed in the KEYNOTE-426 trial, which tested the combination of pembrolizumab and axitinib, demonstrating a benefit in overall response rate (ORR), PFS and OS across all IMDC-risk groups.8
Ipilimumab/nivolumab and pembrolizumab/axitinib were quickly incorporated into internationally accepted treatment guidelines, including the National Comprehensive Cancer Network (NCCN)9 and European Society for Medical Oncology (ESMO) guidelines, and are listed as options with equal levels of evidence.10,11
Signs of superior activity over sunitinib were also recorded with the combinations of atezolizumab/bevacizumab (IMmotion151),12 and avelumab/axitinib (JAVELIN Renal 101).13 Both achieved their pre-defined PFS co-primary endpoint, but neither a significant OS advantage over sunitinib.
Very recently, a fifth combination of newer agents with benefit over sunitinib was presented at ESMO Virtual Congress 2020, namely the CheckMate 9ER trial. The combination of nivolumab and cabozantinib showed significant improvement in ORR, PFS and OS across all subgroups.14
Due to strict in- and exclusion criteria in clinical trials, the real-world patient population usually does not completely reflect the study population and requires a patient-individual approach to treatment.15 A major driver for the clinical decision-making process are comorbidities, for example hepatic insufficiency, autoimmune disease or a solid-organ transplant. In addition, age and performance status often prohibit patients from receiving guideline-recommended treatments. In these cases, expert opinion and personal experience are needed for a treatment decision.
This is an updated decision-making analysis. Our first analysis was published in 2015, before data on ICI-combinations was available.16
Materials and methods
Medical oncology experts in the field of RCC, representing 11 centres in Austria, France, Germany, UK, Italy, Norway, Switzerland and the United States, were selected according to their track record in RCC and previous decision-making analyses.16,17
The experts provided their algorithm for the first-line treatment of patients with mccRCC outside of clinical trials in a real-life setting. For the purposes of the present analysis, nephrectomy, active surveillance, local treatment strategies and best supportive care were not considered. We focused on the choice of initial systemic therapy and criteria for this selection. Importantly, parameters included in the decision tree were not suggested but chosen by the experts themselves. Hence, not all decision trees include the same parameters.
Each expert was asked to describe their treatment strategy in any preferred format (diagram, text, phone call). This initial input was collected and unified criteria were proposed to enable cross compatibility.18 The initial input was converted into decision trees consisting of decision criteria (branches) and specific recommendations associated with each parameter combination. These trees were discussed bilaterally between the coordinators and the individual experts to ensure they represented the initially described treatment strategy. The final versions of the decision trees were confirmed by the individual experts in April 2020. The decision trees were analysed for consensus and discrepancies by comparing every combination of decision criteria.19
Results
Eleven decision trees were analysed and compared. Parameters, considered relevant for treatment choice were diverse. Criteria chosen by the experts were performance status [PS, fit (PS 0-1) versus unfit (PS 2)], IMDC risk group (favourable versus intermediate versus poor), anti-programmed death ligand 1 (PD-L1) status [positive (pos) versus negative (neg)], zugzwang (ZZ, compulsion to obtain a rapid response), and contraindication to immune checkpoint inhibitor therapy (CI to ICI versus No CI to ICI).
The panel's recommended first line treatments were sunitinib, pazopanib, tivozanib, cabozantinib, ipilimumab/nivolumab or pembrolizumab/axitinib.
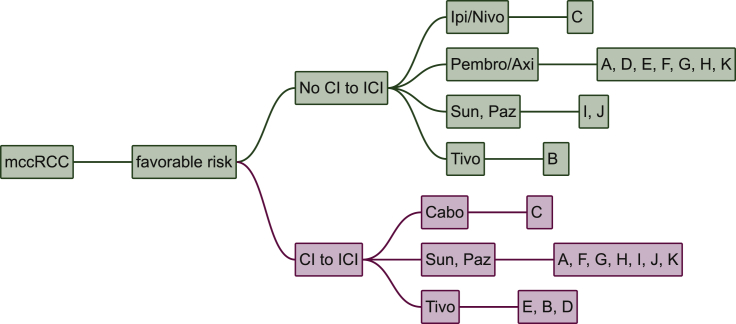
In IMDC favourable risk patients, the capability to receive ICI-containing regimes is the single most important factor. Only three experts give a TKI-monotherapy to all patients. For patients without CI to ICI, most experts (seven of 11 experts) chose pembrolizumab/axitinib as first line treatment. Two experts treat with sunitinib or pazopanib and one expert each gives ipilimumab/nivolumab or tivozanib. In case of CI to ICI seven experts give sunitinib or pazopanib; three experts prescribe tivozanib and one cabozantinib (Figure 1).
Figure 1.
Favourable risk patients.
Cabo, cabozantinib; CI, contraindication; ICI, immune checkpoint inhibitors; Ipi/Nivo, ipilimumab/nivolumab; mccRCC, metastatic renal cell carcinoma; Paz, pazopanib; Pembro/Axi, pembrolizumab/axitinib; Sun, sunitinib; Tivo, tivozanib. A, Paris, France; B, Vienna, Austria; C, Boston, USA; D, London, UK; E, Essen, Germany; F, Nashville, USA; G, New York, USA; H, Oslo, Norway; I, Cambridge, UK; J, Bari, Italy; K, St. Gallen, Switzerland.
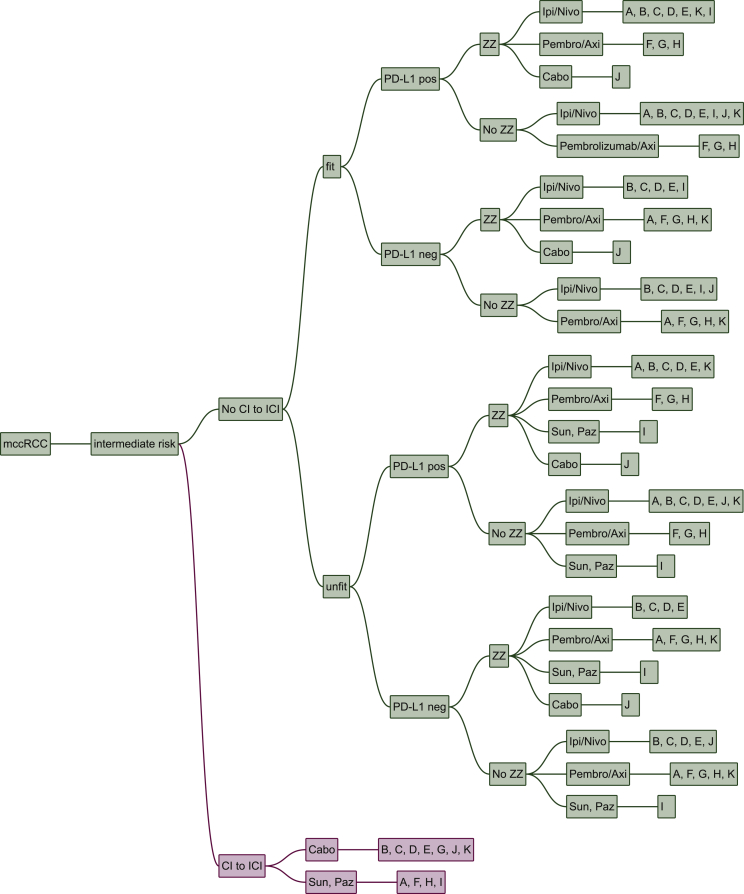
In intermediate risk patients, many factors influence treatment decision, namely CI to ICI, fitness, PD-L1 status and zugzwang. However, their weighting differs substantially: the most important condition for treatment choice is a CI to ICI. Fitness and zugzwang, the compulsion to obtain a rapid response, only influences one expert each. Zugzwang led to a switch from ipilimumab/nivolumab to cabozantinib, while poor fitness led to a switch from ipilimumab/nivolumab to sunitinib or pazopanib.
PD-L1 status is relevant for two experts, where PD-L1 positivity led to a switch from cabozantinib to ipilimumab/nivolumab for both experts in the intermediate risk setting (and for one expert a switch from pembrolizumab/axitinib to ipilimumab/nivolumab in the poor risk setting).
In patients with CI to ICI, seven centres prescribe cabozantinib, while four give sunitinib or pazopanib, respectively. In case of no CI to ICI, the majority of centres prescribe either ipilimumab/nivolumab or pembrolizumab/axitinib. In the two centres, in which PD-L1 status is part of their treatment algorithms, it determines whether patients will receive ipilimumab/nivolumab or pembrolizumab/axitinib (Figure 2).
Figure 2.
Intermediate risk patients.
Cabo, cabozantinib; CI, contraindication; ICI, immune checkpoint inhibitors; Ipi/Nivo, ipilimumab/nivolumab; mccRCC, metastatic renal cell carcinoma; Paz, pazopanib; Pembro/Axi, pembrolizumab/axitinib; Sun, sunitinib; Tivo, tivozanib; ZZ, zugzwang. A, Paris, France; B, Vienna, Austria; C, Boston, USA; D, London, UK; E, Essen, Germany; F, Nashville, USA; G, New York, USA; H, Oslo, Norway; I, Cambridge, UK; J, Bari, Italy; K, St. Gallen, Switzerland.
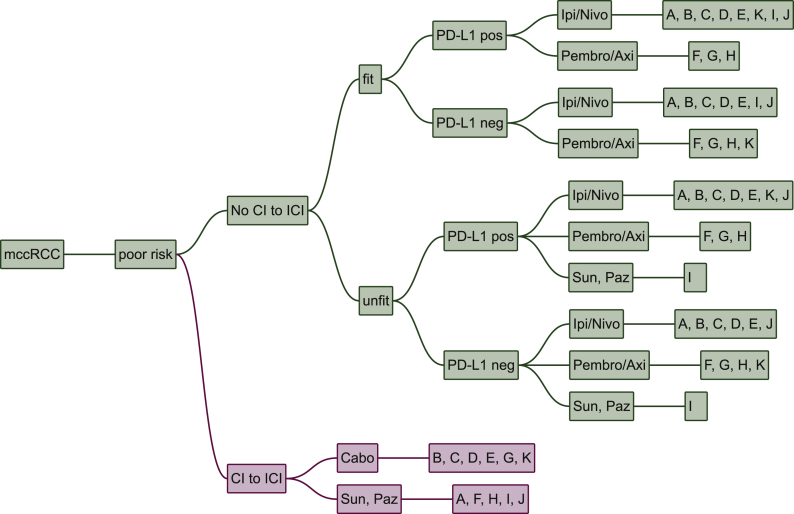
The treatment of choice in IMDC poor risk patients is similar to intermediate risk patients. However, zugzwang no longer influences treatment choice and ipilimumab/nivolumab is chosen more often.
In patients with CI to ICI, cabozantinib is selected by six experts and sunitinib or pazopanib by five. In poor risk patients with no CI to ICI PD-L1 status is a relevant decision criterion for one centre: ipilimumab/nivolumab being the treatment of choice in PD-L1 positive and pembrolizumab/axitinib in PD-L1 negative patients, respectively. One centre treats with sunitinib or pazopanib in unfit patients with no CI to ICI. Seven centres prescribe ipilimumab/nivolumab to poor risk patients with no CI to ICI independent of PD-L1 status, whereas three centres chose pembrolizumab/axitinib in this situation (Figure 3).
Figure 3.
Poor risk patients.
Cabo, cabozantinib; CI, contraindication; ICI, immune checkpoint inhibitors; Ipi/Nivo, ipilimumab/nivolumab; mccRCC, metastatic renal cell carcinoma; Paz, pazopanib; Pembro/Axi, pembrolizumab/axitinib; Sun, sunitinib; Tivo, tivozanib. A, Paris, France; B, Vienna, Austria; C, Boston, USA; D, London, UK; E, Essen, Germany; F, Nashville, USA; G, New York, USA; H, Oslo, Norway; I, Cambridge, UK; J, Bari, Italy; K, St. Gallen, Switzerland.
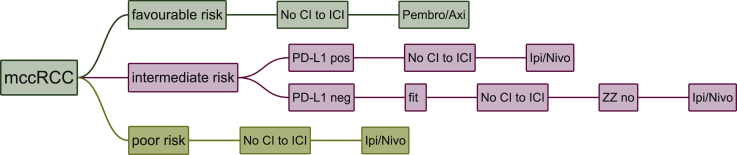
The following treatment strategies where chosen by the majority (>50%) of centres in these specific situations: pembrolizumab/axitinib in favourable risk patients with no CI to ICI, ipilimumab/nivolumab in intermediate risk patients, who are PD-L1 positive with no CI to ICI, ipilimumab/nivolumab in intermediate risk patients who are PD-L1 negative, fit, with No CI to ICI and no zugzwang, ipilimumab/nivolumab in poor risk patients with No CI to ICI (Figure 4).
Figure 4.
Decision tree representing only combinations of parameters where a majority was achieved.
Cabo, cabozantinib; CI, contraindication; ICI, immune checkpoint inhibitors; Ipi/Nivo, ipilimumab/nivolumab; mccRCC, metastatic renal cell carcinoma; Paz, pazopanib; Pembro/Axi, pembrolizumab/axitinib; Sun, sunitinib; Tivo, tivozanib. A, Paris, France; B, Vienna, Austria; C, Boston, USA; D, London, UK; E, Essen, Germany; F, Nashville, USA; G, New York, USA; H, Oslo, Norway; I, Cambridge, UK; J, Bari, Italy; K, St. Gallen, Switzerland.
Discussion
This is an updated analysis of the first line treatment algorithms in mccRCC. As expected, treatment choices were diverse and represent a subjective and potentially biased opinion of the participating experts. This underlines the complexity of this issue: Having multiple treatment options with similar levels of evidence complicates individual treatment decision in a real-life setting. Guidelines from medical societies are intended to provide recommendations for the best standard of cancer care. However, they may not be transferable to patients seen in daily practice due to different reasons, i.e. comorbidities, patient preference, access, and route of administration. This analysis provides insight into treatment strategies in tertiary cancer centres across many countries with different interpretation of data, as well as rules concerning drug reimbursement and overall availability.
Pazopanib and/or sunitinib, which were the favoured treatment choices in our last analysis16 have taken a back seat and are only chosen occasionally by three experts, usually in situations with CI to ICI or unfit patients. They are still listed as alternative treatment options in the ESMO-guidelines across all subgroups.
With tivozanib and cabozantinib, two new multi-kinase inhibitors are now represented in this algorithm. Cabozantinib has shown a better response rate (ORR, 46 versus 18%), a PFS-benefit (8.2 versus 5.6 months), and a 34% reduction in rate of progression or death (HR 0.66, 95%-CI 0.46-0.95, P = 0.012) compared to sunitinib for first line treatment in intermediate and poor risk patients, albeit in a small, randomized phase II study.20 Of note, sunitinib was previously explored mainly in favourable and intermediate risk patients, making its efficacy in poor risk patients unclear. This could explain the inferior results in the standard of care arm in CABOSUN. Cabozantinib is mentioned as treatment of choice by five experts in poor and intermediate risk patients with CI to ICI. One centre, based in the United States, uses cabozantinib for first line treatment for favourable risk patients as well. Although any data in favourable risk patients are lacking, the NCCN Guidelines for Kidney Cancer has listed cabozantinib as an option (category 2B) for first-line treatment in the favourable risk group extrapolating the data from poor and intermediate risk patients.21 The FDA has approved cabozantinib for the treatment of patients with advanced RCC irrespective of risk group,22 whereas EMA restricted reimbursement to treatment-naïve patients with intermediate or poor risk.23 In the ESMO-guidelines cabozantinib is mentioned as a treatment alternative in intermediate- and poor risk patients.
Tivozanib is named as a treatment option by three centres for favourable risk patients in different scenarios. The available data are conflicting. The phase III TIVO-1 trial compared tivozanib to sorafenib in patients who were either untreated or had received cytokines. The study met its primary endpoint by showing a significant improvement of PFS. However, the secondary endpoint OS was detrimental for tivozanib.24 As a result, the FDA rejected approval in May 2013.25 TIVO-3 was then designed to address those inconsistencies. It compared tivozanib to sorafenib in highly refractory metastatic RCC and was able to show improvement in PFS. In the final OS analysis no difference between treatment with tivozanib and sorafenib was observed (HR 0.97) however, frequency of grade 3/4 toxicities was lower with tivozanib.26 These studies leave some open questions, for example the unusual choice of sorafenib as the standard treatment arm and why TIVO-3 included patients with highly refractory disease and not untreated patients like TIVO-1. The role of tivozanib in first line treatment metastatic RCC remains unclear. Tivozanib is not included in the NCCN Guidelines, but has an ESMO [II, A; ESMO-Magnitude of Clinical Benefit Scale (MCBS) 1] recommendation for favourable risk and a ESMO (II, B; MCBS 1) recommendation for intermediate risk patients.27
Two trials, investigating either the combination of ICI/ICI (CheckMate 214)7 or an ICI/TKI combination (KEYNOTE-426)8 changed the treatment landscape in recent years. In CheckMate 214, ipilimumab/nivolumab showed superior ORR and OS (47.0 versus 26.6 months, HR 0.66, P < 0.0001) to sunitinib in intermediate and poor risk patients, with a complete response (CR) rate of 11% in the intention to treat (ITT) population. It failed, however, to show benefit in favourable risk patients, where sunitinib outperformed ipilimumab/nivolumab. The high CR-rate in favourable risk patients with ipilimumab/nivolumab and promising data from a phase I trial may nevertheless support the use of ipilimumab/nivolumab in favourable risk patients.28 This is reflected in the NCCN-Guidelines, which list ipilimumab/nivolumab as a preferred regimen for first-line treatment in intermediate- and poor risk patients and a treatment option for favourable risk patients, respectively.21 The ESMO-treatment guidelines for renal cell carcinoma recommends ipilimumab/nivolumab for first line treatment only in intermediate and poor risk patients (recommendation I, A).11
The combination of pembrolizumab and axitinib showed superiority over sunitinib in the intention to treat analysis including all IMDC risk groups, where ORR (60.2 versus 39.9%), PFS (HR 0.71, 95%-CI 0.60-0.84, P < 0.001), and OS (HR 0.68; 95%-CI 0.55-0.85, P < 0.001) were significantly improved. Pembrolizumab/axitinib is listed as a preferred regimen for all risk groups in the American and European guidelines. Of note, in a subgroup analysis favourable risk patients have no OS benefit as of yet.
Both trials, CheckMate 214 and KEYNOTE-426, used monotherapy with a TKI (sunitinib) as the standard treatment arm, leaving the question open, whether ICI/ICI or ICI/TKI is the preferred regimen. Subgroup analysis from CheckMate 214 suggests that patients with high PD-L1 expression (≥1%) perform better than patients with no PD-L1 expression (<1%). In KEYNOTE-426 no such difference was observed. Different PD-L1 scoring systems were used [CheckMate 214: Dako PD-L1 IHC 28-8 pharDx test (tumour proportion score, TPS) and KEYNOTE-426: combined positive score (CPS)], which makes comparison difficult. Furthermore, these trials were not powered for difference in PD-L1 status and thus this subgroup analyses have to be interpreted with caution. In our analysis, only two centres consider PD-L1 status in their treatment algorithm, with PD-L1 positivity favouring ipilimumab/nivolumab and PD-L1 negativity favouring pembrolizumab/axitinib. Pembrolizumab/axitinib is the recommended treatment choice in the ESMO-guidelines irrespective of IMDC-risk classification, whereas ipilimumab/nivolumab is recommended only in intermediate- and poor risk patients.
Interestingly, no expert mentioned and chose the combination treatment of the PD-L1 antibody avelumab and axitinib. The primary objective of the phase III trial JAVELIN Renal 101 was to show the superiority of avelumab and axitinib over sunitinib with respect to either PFS or OS among patients with PD-L1–positive tumours.13 Until today, only a PFS benefit has been demonstrated. Nevertheless, FDA and EMA approved avelumab in combination with axitinib for the treatment of renal cell carcinoma. The combination is not mentioned in the ESMO-guidelines, but is listed as an ‘other recommended regimen’ in all risk groups in the NCCN-guidelines.
The combination of nivolumab/cabozantinib is not part of our decision-making analysis, since the results of CheckMate 9ER were first presented after the collection of our data. The results are comparable to the other combination therapies (ipilimumab/nivolumab, pembrolizumab/axitinib), by showing benefit in ORR, PFS and OS over sunitinib. This combination is already approved by the FDA and is a recommended (recommendation I, A) treatment choice among all IMDC-subgroups in the ESMO guidelines.11
Sunitinib and pazopanib are still mentioned by some experts across all risk groups, even though sunitinib has been shown to be inferior to ipilimumab/nivolumab, pembrolizumab/axitinib and cabozantinib. A reason for its continued use could be reimbursement issues for newer agents in some countries.
Owing to the lack of predictive markers, it is unknown, which patient will respond best to VEGF inhibition and which one to ICI. In the IMmotion150 phase II study of atezolizumab/bevacizumab versus sunitinib, investigators conducted an exploratory biomarker analysis, looking for predictive markers. A heatmap, with prespecified genes, showed three distinct subgroups: high expression of angiogenesis gene signature (Angio, high vascular density), high expression of T-effector gene signature (Immune, high PD-L1 expression) and myeloid inflammation-associated genes (resistance to ICI). It was shown that sunitinib was more efficacious in highly angiogenic tumours, the combination of atezolizumab and bevacizumab improved clinical benefit compared with sunitinib in ‘immune high’ tumours and atezolizumab monotherapy was less effective in immunogenic tumours with high myeloid inflammation.29 More data is needed to confirm this concept, but validation of biologic subgroups predictive of clinical outcome to certain regimens would greatly facilitate decisions in first line treatment.
Among others, a relevant limitation of this updated analysis is lack of information on the reasons why certain treatments were considered in some centres and not in others. The disparity may reflect the availability of drugs in the different participating countries. However, it also mirrors experience, convenience, and personal preference. In addition, the terms CI to ICI and patient fitness were used as generic terms and were deliberately not well defined. Experts interpret these criteria differently, e.g. for one centre, only patients with an organ transplant on immunosuppressive treatment or patients with uncontrolled active autoimmune disease have a clear CI to ICI, while for others centres inflammatory bowel syndrome might be a CI to ICI.
Even though zugzwang is not a classical medical term, we use it as a composite criterion. It describes the perceived necessity to obtain a rapid treatment response due to symptoms, extent of disease, present or imminent organ dysfunction.
Another limitation of this analysis is the volatility in the treatment of mccRCC due to evolving trial results and access to new drugs. Namely, regulators in the United Kingdom (UK) allowed more flexibility during the intervening period from April 2020 until publication of this manuscript, e.g. the combination axitinib/avelumab is currently available in the UK within an access programme.
The ongoing coronavirus pandemic has changed cancer care around the world.30,31 Subject to the severity of the impact on the health care system, adaptation of therapeutic pathways differs substantially. Even though most experts in this decision-making analysis have modified their mccRCC treatment approach in response to the pandemic,32 this was not the main purpose of this manuscript.
While this analysis provides insight into the decision-making process of individual experts, it does not supersede clinical judgment or established guidelines. However, for individual cases, these variations in treatment recommendations may provide additional space for flexibility in the context of shared decision-making.
Conclusion
This analysis of treatment algorithms for first line treatment in mccRCC demonstrated the following treatment choices: Ipilimumab/nivolumab, pembrolizumab/axitinib, cabozantinib, tivozanib, sunitinib or pazopanib. It also revealed several decision criteria, namely performance status, IMDC risk group, PD-L1 status, zugzwang, and contraindication to immunotherapy. Significant inter-expert variation was observed. This shows how data from randomized trials is interpreted differently when transferred into daily practice.
Acknowledgments
Funding
None declared.
Disclosure
SA: MSD (C/A), Sanofi-Genzyme (C/A) recipient: my institution. MSchmaus: none. TE: AstraZeneca Personal: (RF, E, OI), Bayer (RF), Pfizer (RF), Roche (E, OI); Institution: AstraZeneca (RF), Roche (RF). BE: Pfizer (C/A), BMS (C/A), Ipsen (C/A), Roche (C/A), Oncorena (C/A), Aveo (C/A). VG: Astra Zeneca (CA, H, OI; RF), Bristol-Myers Squibb (C/A, H, OI; RF), Roche Pharma AG (C/A, H), MSD Oncology (C/A, H, OI; RF), Ipsen (C/A, H; RF), Bayer (H; RF), Merck Serono (C/A, H), Janssen Cliag (C/A, H), Pfizer (C/A, H), Lilly (C/A, H), PharmaMar (H), EUSAPharm (C/A, H), Novartis (C/A, H, RF), EISAI (H), Onkowissen (C/A). JL: Achilles Therapeutics (C/A, grant support), Bristol-Myers Squibb (C/A, grant support), Merck Sharp & Dohme (C/A, grant support), Nektar (C/A, grant support), Novartis (C/A, grant support), Pfizer (C/A, grant support), Roche–Genentech (C/A, grant support), Immunocore (C/A, grant support), AstraZeneca (C/A), Boston Biomedical (C/A), Eisai (C/A), EUSA Pharma (C/A), GlaxoSmithKline (C/A), Ipsen (C/A), Imugen (C/A), Incyte (C/A), iOnctura (C/A), Kymab (C/A), Merck Serono (C/A), Pierre Fabre (C/A), Secama (C/A), Vitaccess (C/A), Covance (C/A), Aveo (C/A), Pharmacyclics (C/A). DMcD: BMS (H, C/A), Pfizer (H, C/A), Merck (H, C/A), Alkermes, Inc. (H, C/A). JO: none. CP: Bristol-Myers Squibb (personal fees), Merck Sharpe & Dohme (personal fees), Novartis (personal fees), Ipsen (personal fees), EUSA (personal fees), Eisai (personal fees), Janssen (personal fees), AstraZeneca (personal fees), General Electric (personal fees), Pfizer (grants and personal fees). BIR: Merck (C/A), BMS (C/A), AVEO (C/A), Pfizer (C/A), Roche (C/A), Pfizer (RF), Merck (RF), BMS (RF), AVEO (RF), Astra-Zeneca (RF), Roche (RF). MSchmidinger: Pfizer, BMS, Ipsen, MSD, Merck, Exelixis, EISAI, EUSA, Roche, Novartis, Alkermes. CNS: Pfizer (C/A), MSD (C/A), Merck (C/A), AstraZeneca (C/A), Astellas (C/A), Sanofi-Genzyme (C/A), Roche-Genentech (C/A), Incyte (C/A). CR: Pfizer (C/A), Bristol-Myers Squibb (C/A), Roche Pharma AG (C/A), MSD Oncology (C/A), Merck (Schweiz) AG (C/A) recipient for all: my institution. Astellas Pharma (RF) recipient: my institution. PMP: AstraZeneca (RF), Celgene (RF), Takeda (RF): Educational grants to the institution. Legend: (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 2.Motzer R.J., Mazumdar M., Bacik J., Russo P., Berg W.J., Metz E.M. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18(9):1928–1935. doi: 10.1200/JCO.2000.18.9.1928. [DOI] [PubMed] [Google Scholar]
- 3.Motzer R.J., Hutson T.E., Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg C.N., Davis I.D., Mardiak J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G., Carducci M., Tomczak P. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B., Pluzanska A., Koralewski P. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Motzer R.J., Tannir N.M., McDermott D.F. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini B.I., Plimack E.R., Stus V. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 9.Network NCC Kidney cancer (version 2.2020) https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf Available at.
- 10.Escudier B., Porta C., Schmidinger M. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 11.ESMO eUpdate – renal cell carcinoma treatment recommendations. https://www.esmo.org/guidelines/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations-3 Available at: Accessed November 30, 2020.
- 12.Rini B.I., Powles T., Atkins M.B. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 13.Motzer R.J., Penkov K., Haanen J. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ESMO CheckMate 9ER. https://www.esmo.org/newsroom/press-office/esmo2020-metastatic-kidney-cancer-nivolumab-cabozantinib-checkmate9er Available at: Accessed September 19, 2020.
- 15.Heng D.Y., Choueiri T.K., Rini B.I. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25(1):149–154. doi: 10.1093/annonc/mdt492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothermundt C., Bailey A., Cerbone L. Algorithms in the first-line treatment of metastatic clear cell renal cell carcinoma – analysis using diagnostic nodes. Oncologist. 2015;20(9):1028–1035. doi: 10.1634/theoncologist.2015-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothermundt C., von Rappard J., Eisen T. Second-line treatment for metastatic clear cell renal cell cancer: experts' consensus algorithms. World J Urol. 2017;35(4):641–648. doi: 10.1007/s00345-016-1903-6. [DOI] [PubMed] [Google Scholar]
- 18.Panje C.M., Glatzer M., von Rappard J. Applied swarm-based medicine: collecting decision trees for patterns of algorithms analysis. BMC Med Res Methodol. 2017;17(1):123. doi: 10.1186/s12874-017-0400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putora P.M., Panje C.M., Papachristofilou A., Dal Pra A., Hundsberger T., Plasswilm L. Objective consensus from decision trees. Radiat Oncol. 2014;9:270. doi: 10.1186/s13014-014-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choueiri T.K., Halabi S., Sanford B.L. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network Kidney cancer (version 2.2020 – August 5, 2019) https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf Available at: [DOI] [PubMed]
- 22.Administration USFD FDA grants regular approval to Cabometyx for first-line treatment of advanced renal cell carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-cabometyx-first-line-treatment-advanced-renal-cell-carcinoma Available at:
- 23.Agency EM Cabometyx (Cabozantinib) https://www.ema.europa.eu/en/medicines/human/EPAR/cabometyx#authorisation-details-section Available at:
- 24.Mehta A., Sonpavde G., Escudier B. Tivozanib for the treatment of renal cell carcinoma: results and implications of the TIVO-1 trial. Future Oncol. 2014;10(11):1819–1826. doi: 10.2217/fon.14.120. [DOI] [PubMed] [Google Scholar]
- 25.Drugs.com AVEO and Astellas Report FDA Oncologic Drug Advisory Committee votes tivozanib application did not demonstrate favorable benefit-to-risk evaluation in treatment of advanced renal cell carcinoma. https://www.drugs.com/nda/tivozanib_130502.html Available at:
- 26.Rini B.I., Pal S.K., Escudier B.J. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95–104. doi: 10.1016/S1470-2045(19)30735-1. [DOI] [PubMed] [Google Scholar]
- 27.Cherny N.I., Dafni U., Bogaerts J. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 28.Hammers H.J., Plimack E.R., Infante J.R. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott D.F., Huseni M.A., Atkins M.B. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ASCO COVID-19 provider & practice information. https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19 Available at:
- 31.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4):e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aeppli S., Eboulet E.I., Eisen T. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. ESMO open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000852. [DOI] [PMC free article] [PubMed] [Google Scholar]