Abstract
Alternaria solani, a plant pathogenic fungus causes significant economical losses of potato crop. The disease is controlled primarily through some traditional methods and most commonly via the application of chemical fungicides. Fungicides treatment is not protected as chemicals pollute environment, effect health vulnerability in humans and when these harmful chemicals enter into the food chain become hazardous to all living entities. Recent efforts have focused on developing environmentally safe, long-lasting, and effective biocontrol methods for the management of plant diseases. Present research focus on screening of crude and partially purified leaf extract of Thevetia peruviana for the presence of antifungal efficacy against Alternarai solani. It was observed that 100% alcoholic crude and alcoholic fraction of partially purified extract showed maximum inhibitory activity which is due to the presence of different secondary metabolites, revealed by phytochemical screening. Active column fraction (possess best antifungal activity against Alternaria solani) was subjected to Gas Chromatography-Mass Spectrometry (GS-MS) analysis. On the basis of peaks matching of GC-MS chromatogram with available data base showed the presence of benzoic acid and oxo-benzoate in active fraction of Thevetia peruviana leaf extract which is already known chemical among the phytochemicals described for antimicrobial activity. Further research on development of herbal formulation from the same would be very helpful environment friendly approach to manage concern crop disease.
Keywords: Alternaria solani, Plant extract, GC-MS, Phytochemicals
Highlights
-
•
Isolation of active principle compound was found maximum in Alcohol extract of Thevetia peruviana leaf extract.
-
•
Phytochemical tests suggest that Alkaloids, steroids, volatile oils, flavonoids, and tannins were found to be present in alcohol extract of Thevetia peruviana leaf extract.
-
•
In vitro assay of antifungal activity of all column fractions fraction no. F9 which exhibited most significant antifungal activity against the test fungus.
-
•
GC–MS analysis of column fraction showed the occurrence of total 1 constituent.
1. Introduction
Plants have capability to produce a great number of various bioactive compounds like essential oils, gums and resins etc. that have been revealed to possess biological activity against plant fungal pathogens in vitro and in vivo and can be used as bio-fungicidal products [1]. These plants derived bioactive compounds are produced as secondary metabolites and remain accumulated in different plant parts. The presence of such phytochemicals is a marker that the plant can be a prospective store of these precursors in the development of herbal formulation [2]. These phytochemicals are usually supposed to be more satisfactory and less unsafe for the ecosystems and could be used as substitute remedies for treatment of plant diseases [3]. In the recent years, research work on decoction of biologically active compounds from plant parts and their utilization for therapeutic purposes has increased intensively (4). Screening of plant parts for the presence of antimicrobial activity is usually done by first preparing plant extract using cold and hot extraction methods. Primary evaluation of antifungal activity commonly done with 100% alcohol, 50% alcohol and 100% aqueous extracts. The secondary metabolites which could be present in the extracts can be isolated in various solvents on the basis of their solubility in the solvents used. Various organic solvents are used on the basis of their polarity in hot extraction using soxhlet assembly for successive extraction.
Early blight is one of the most important diseases which are caused by the fungus Alternaria solani. Jones and Grout, that occurs in most potato growing regions worldwide [5]. The most common and effective method for the control of early blight is through the application of foliar fungicides, but the fungicides treatment is not protected as chemicals pollute environment, effect health vulnerability in humans and when these harmful chemicals enter into the food chain become hazardous to all living entities.
Natural plant products have a narrow target range with specific mode of action, therefore are suitable for a specific target, mostly nontoxic for antagonistic microorganisms, show limited field persistence and have a shorter shelf life and no residual threats. They are generally safe to humans and environment in comparison to conventional synthetic chemical pesticides. They can easily be adopted by farmers in developing countries who traditionally use plant extracts for the treatment of human diseases [6]. Botanical derivatives are environmentally safe and may be used as an alternative to commercial fungicides for controlling pathogenic fungi [7]. Furthermore, biocides of plant origin are nonphytotoxic, systemic and easily biodegradable [8]. It is now known that various natural plant products can reduce populations of foliar pathogens and control the disease development, and then these plant extracts have a potential as environmentally safe alternatives and as components in integrated pest management. Hence present research study based on evaluation of antifungal efficacy of crude and partially purified faction of extract prepared from T. peruviana leaves and subsequent identification and structure determination of active molecule by Gas chromatography/mass spectrometry (GC-MS).
2. Materials and methods
2.1. Crude and partially purified extracts preparations
Fresh leaves of Thevetia peruviana were collected from Botanical garden of Mohanlal Sukhadia University, Udaipur Rajasthan, India. The plant was submitted for identification at Herbarium of Department of Botany, University of Rajasthan, Jaipur, India. Thevetia peruviana Linn. Vide voucher specimen number RUBL 211651. The collected plant material was shade dried at room temperature. It was then ground in an electrical grinder. The ground material was passed through a sieve of mesh with size 60 mm to obtain a fine powder. It was then used to prepare the extract. Crude extract was prepared according to the cold extraction method Cold extraction was prepared in water, 50% hydro alcohol and absolute alcohol. 20 gm of dried and powdered plant material was suspended in 100 ml water, a mixture of 50 ml water and 50 alcohols and 100 ml absolute alcohol separately, and kept for 48 h. After 48 h each mixture was filtered through Whatman filter paper no.1. And filtered material was vacuum dried using rotary vacuum evaporator (Remi, India). The dried residue was used as extract and solvent was recycled [9].
2.2. Antifungal activity of crude and partially purified fraction of Thevetia peruviana (pers.) schum. leaf extract
Poison food technique [10] was used for the examining the antifungal activity of crude and partially purified fractions of Thevetia peruviana leaf extract. In this respect 100 mg of extract was dissolved in 10 ml acetone to prepare stock solution of 10 mg/ml concentration. The Petri-plates inoculated with tested fungi containing extract were incubated at 28 ± 2 °C for seven days. Mancozeb, Bavistin, and only PDA culture media were used as control series along with test samples. Antifungal activities of extracts were measured as a function of increasing or decreasing the growth of 6 mm disc of each inoculum. After seven day of incubation the average diameter of the fungal colonies was measured and mycelial growth in percentage was calculated by the following formula:
Where, Gc, growth of mycelia of control; Gt, growth of mycelia of treatment.
2.3. Effect of extracts on morphology of test fungi
Effect of extract on a variety of morphological and cytological parameters of test pathogen was studied. Test fungi were treated with rising concentrations from 1.25 mg/ml to 10 mg/ml of the extract up till MIC. A small fungal biomass containing mycelium, vesicle and conidia/spores were separated from each tube and microscopic assessment was made after staining with cotton blue and mounting in lacto-phenol. Alteration in mycelium width, conidia size morphology were recorded with the help of Olympus trinocular research microscope BX- 51 and analyzed by Image analysis software Olysia Bioreport 3.2 of Olympus. Conidia counting were done by haemocytometer.
2.4. Phytochemical analysis of leaf extracts of Thevetia peruviana Linn
Qualitative methods were used for the identification of different secondary metabolites or phytochemicals present in the plant extracts. Various fractions of leaf obtained by successive extraction were then subjected to qualitative test suggested by Kokate et al. [11]. The extract was analyzed for the presence and absence of alkaloids, steroids, volatile oils, carbohydrates, tannins, flavonoids and saponins.
2.5. Tests for detection of secondary metabolites
Phytochemistry of plant extracts was also studied by rapid qualitative tests [12].
2.6. Alkaloids
Alkaloids are heterocyclic nitrogenous compounds which are found naturally in plants. Structurally have one or more nitrogen which hold heterocyclic ring. Mayer's test or Wagner's test or Hager's test used to find out the occurrence of alkaloids in the partially purified fractions. A cream coloured precipitate is produced in the reaction with Mayor's reagent; Wagner's reagent results in formation of reddish brown precipitate whereas Hager's reagent gives yellow precipitate. After adding few drops of diluted HCL in the little quantity of extract it was stirred and filtered. Various alkaloid reagents were tested on the filtrate and development of coloured precipitate was observed.
2.7. Volatile oils
Volatile or essential oils are the aromatic volatile chemical constituents of plants. Sudan III test can be employed to identify occurrence of volatile oils. Red colour was observed, on mixing with Sudan III which reveals the presence of volatile oils. Little quantity of extract and Sudan III dye are mixed for the examination of the development of red colour.
2.8. Tannins
Chemically, tannins have a combination of complex organic substances. Polyphenols are found in these substances. The occurrence of condensed tannins is showed by the appearance of green colour and the existence of hydrolysable tannins is shown by blue colour. Small quantity of extract was used and treated with alcohol FeCl3 solution and observed for colour development.
2.9. Saponin
Saponins are complex glycoside compounds. In these compounds aglycone is steroidal in nature. Foam test can be employed to identify existence of saponins. Small quantity of extract diluted with 20 ml of distilled water was shaken in graduated cylinder for 15 min. Development of a layer of foam at surface indicates presence of saponins.
2.10. Carbohydrates
Carbohydrates are extensively distributed in plants. Presence of carbohydrate can be detected by Molisch's test or Fehling's test. 5 ml of distilled water was mixed in a small quantity of extract and filtered after dissolution. A few drops of naphthol and H2SO4 were mixed to the filtrate and the colour of the filtrate changed to purple. It shows the presence of sugar. Likewise, when small amount of filtrate was heated with equal quantity of Fehling A and Fehling B solution, the colour changed to brick red colour. It indicates presence of carbohydrates.
2.11. Flavonoids
Flavonoids are frequently found in plants as glycosides. Structurally, one or more of phenolic hydroxyl groups are combined with sugar residues. Alkaline reagent test is employed to identify flavonoids. Standard test for detection is, mixing of aqueous NaOH with a small quantity of extract. Development of red brown colour shows presence of flavonoids.
2.12. Sterols
Sterols are triterpenes Structurally sterols are based on cyclopentaneperhydroxyphenanthrene (sterane) ring system. They are also recognized as phytosterols, Liebermann's Burchard test is used for revealing the presence of phytosterols in extract. Small quantity of extract was mixed with 1 ml of acetic anhydride and 2 ml CHCl3 followed by gradual addition of concentrated H2SO4 through the side of the test tube. Development of a ring of brown colour at junction of two layers is the indication of the presence of sterols.
2.13. Isolation and characterization of active principle from selected leaf extract by using various chromatography techniques
Isolation of active principle from acetone extract of T. peruviana leaf was done by thin layer chromatographic fingerprinting, column chromatography and Gas chromatography. Characterization of the active compound was done by Mass spectrometry analysis of the extracted phytochemicals.
2.14. TLC finger printing of alcohol fraction of Thevetia peruviana Linn
For TLC Finger printing of Alcohol Fraction of Thevetia peruviana Linn., 10 cm long TLC plate was cut and marked carefully. 10 μl of plant extract was spotted onto the marked plate with the help of a capillary tube or pipette.
n-Hexane: Ethyl acted (5 ml: 5 ml) was used as mobile phase. The TLC plate was kept in a chromatographic chamber containing the respective solvent system and the chamber was covered with glass plate to prevent the evaporation of the solvent. The plate was allowed to remain in the chamber till the solvent reached up to 9 cm distance. The plate was then observed in UV-fluorescence analysis cabinet at short and long wavelengths.
TLC finger printing plate was derivatized with anisaldehyde sulphuric acid reagent followed by heating at 100 °C till coloured bands of various secondary metabolites appeared. The observations were taken before and after derivatization in visible as well as ultraviolet light.
Rf (Retention factor) values were calculated as follows:
2.15. Column chromatography of partially purified alcohol fraction of Thevetia peruviana leaf extract
10 gm of dried and partially purified alcohol fraction of Thevetia peruviana leaf was dissolved in the mobile phase i.e. 5 ml n-Hexane and 5 ml Ethyl Acetate and this solution were subjected to column chromatography. Glass column (Merck: 100–200 mm) filled with 650 gm of silica gel was used for column chromatography. According to the colour band developed in column different fractions of extract containing various secondary metabolites were collected. All fractions obtained from column were dried in rotary vacuum evaporator under reduced pressure. These fractions were screened for their antifungal activity against A. solani. The fraction showing best antifungal activity against A. solani was subjected to further purification and characterization for active molecule via gas chromatography and mass spectrometry analysis [13,14].
2.16. Identification and structure determination of active molecule by gas chromatography/mass spectrometry (GC-MS)
The GC MS analysis was performed on a GC (PerkinElmer) system coupled to Perkin- Elmer Turbo Mass MS. HP1-MS capillary column (30 m × 0.25 μm × 0.25 μm) was used under the following conditions: oven temperature programmed from 70 °C for 10 min, then gradually increased at 290 °C at 3 min; injector temperature, 250 °C, carrier gas Helium, flow rate 1 ml/min; the volume of injected sample was 0.4 μl; split ratio 1:60; ionization energy 70eV: Run time 40 min. The relative amount of each component was calculated by comparing its average peak area to the total area. The identification of the separated volatile compounds was done through retention indices and mass spectrometry by comparing mass spectra of the unknown peaks with those stored in the Nist 98/Nbs 75 K GC-MS library.
3. Results and observations
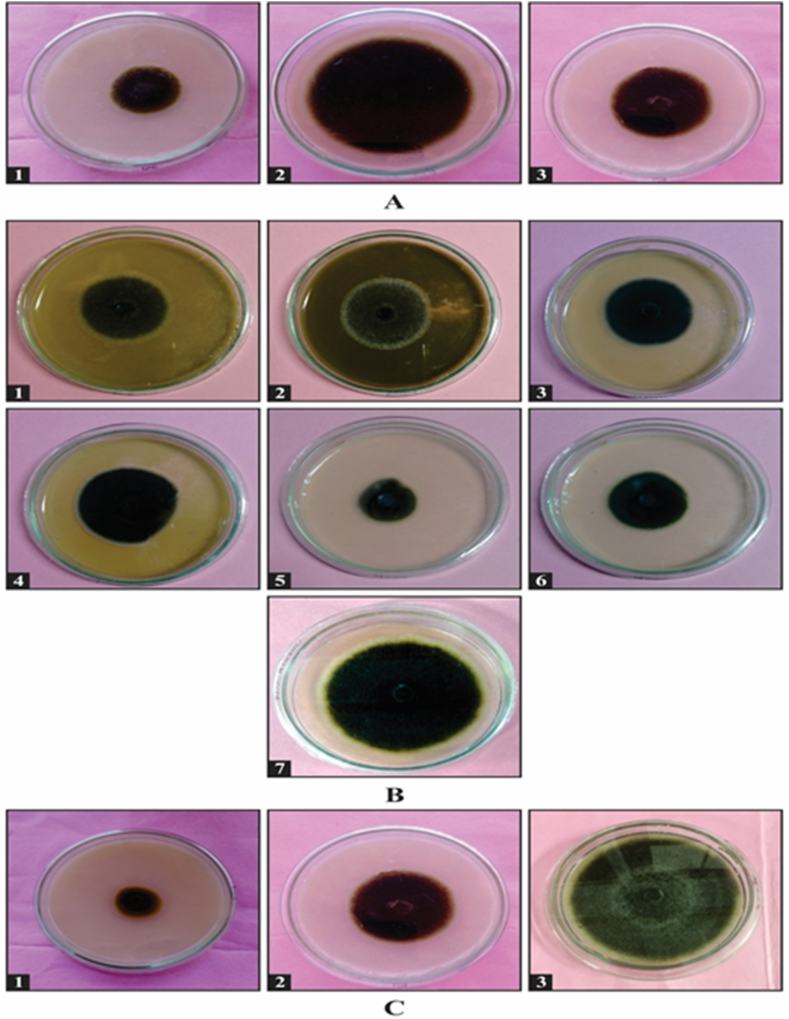
In order to check antifungal activity of crude and partially purified extract, extracts were dissolved in inert solvent i.e. acetone. It was observed to be neutral for growth of test fungus. Results of antifungal activity of crude as well as partially purified extracts were given in Table 1, Table 2 respectively (Fig. 1 A, B). Inhibitory activity of standards and control tabulated in Table 3 (Fig. 1C). In case of crude extract, 100% alcoholic extract was observed to be best which showed 71.48% inhibition followed by aqueous and 50% hydroalcoholic extract which showed inhibition percent of 56.60% and 20.24% respectively.
Table 1.
Antifungal activity of crude extract of Thevetia peruviana leaf extract against Alternaria solani.
| S. No. | Type of extract | Growth Diameter after 7 days (mm) | % Mycelial growth inhibition |
|---|---|---|---|
| 1. | 100% alcoholic | 23.00 ± 1.00 | 71.48 |
| 2. | 50% alcoholic | 64.33 ± 0.57 | 20.24 |
| 3. | Aqueous | 35.00 ± 1.00 | 56.60 |
Table 2.
Antifungal activity of various purified fractions of Thevetia peruviana leaf extract against Alternaria solani.
| S.NO. | Type of extract | Growth Diameter after 7 days (mm) | % Mycelial growth inhibition |
|---|---|---|---|
| 1 | Petroleum ether | 36.33 ± 0.57 | 54.95 |
| 2 | Benzene | 40.33 ± 0.57 | 50.00 |
| 3 | Chloroform | 44.33 ± 0.57 | 45.04 |
| 4 | Acetone | 35.00 ± 1.00 | 56.60 |
| 5 | Alcohol | 20.66 ± 0.57 | 74.38 |
| 6 | Methanol | 31.33 ± 0.57 | 61.15 |
| 7 | Aqueous | 60.33 ± 0.57 | 25.20 |
Fig. 1.
Antifungal activity of Thevetia peruviana against Alternaria solaniA. Crude extracts [1] 100% alcoholic [2] 50% alcoholic [3] 100% aqueous, B. Partially purified fractions [1] Petroleum ether fraction [2] Benzene fraction [3] Chloroform fraction [4] Acetone fraction [5] Alcohol fraction [6] Methanol fraction [7] Aqueous fraction C. Antifungal activity of standard fungicides with water control against Alternaria solani [1] Mancozeb [2] Bavistin [3] Control (only PDA media).
Table 3.
Antifungal activity of standard fungicides with water control Alternaria solani.
| S.No. | Standard fungicides andwater control | Growth Diameter after 7 days (mm) | % Mycelial growth inhibition |
|---|---|---|---|
| 1. | Mancozeb | 16.00 ± 1.00 | 80.16 |
| 2. | Bavistin | 33.66 ± 0.57 | 58.26 |
| 3. | Water (control) | 80.66 ± 0.57 | No inhibition |
Among the partially purified fractions obtained, maximum mycelial growth inhibition of 74.38% was observed with partially purified alcohol fraction of T. peruviana leaf extract. The inhibitory effect of leaf extract of Thevetia peruviana on morphology and reproduction of test fungi are depicted in Table 4, Table 5 and Fig. 2 A, B. There was normal growth pattern i.e. abundant mycelial growth and sporulation of A. solani in control tube. Sequential decrease in conidial size and raise in mycelial width was observed with the increase in concentration of leaf extract. A. solani also showed decreased sporulation with rising concentrations of the extract. Average mycelium width in control was 1.35 μm, which increased up to 5.40 μm at 1.25 mg/ml 74.57% reduction in conidial size was observed at 1.25 mg/ml concentration.
Table 4.
Effect of Different Concentrations of Alcohol Extract of Thevetia peruviana leaf extract on Mycelium width of A. solani.
| S.No. | Extract Concentration (mg/ml) | Mycelium width (μm) ± SD | % Increase in mycelium width |
|---|---|---|---|
| 1. | Control | 1.35 ± 0.03 | – |
| 2. | 2.5 | NF | – |
| 3. | 1.25 | 5.40 ± 0.05 | 75.00 |
| 4. | 0.625 | 5.10 ± 0.04 | 73.52 |
| 5. | 0.312 | 4.09 ± 0.03 | 66.99 |
| 6. | 0.156 | 3.30 ± 0.02 | 59.09 |
| 7. | 0.078 | 2.75 ± 0.02 | 50.90 |
| 8. | 0.039 | 2.30 ± 0.05 | 41.30 |
| 9. | 0.019 | 1.58 ± 0.04 | 14.55 |
Table 5.
Effect of Different Concentrations of alcohol extract of Thevetia peruviana leaf extract on Conidia size A. solani.
| S.No. | Extract Concentration (mg/ml) | Conidial size (μm) (LXW) | Conidial size (μm) ± SD (Area) | % Reduction in conidial size |
|---|---|---|---|---|
| 1. | Control | 58.91 X 33.05 | 1946.97 ± 0.016 | – |
| 2. | 2.5 | NF | NF | – |
| 3. | 1.25 | 30.90 X 16.02 | 495.018 ± 0.19 | 74.57 |
| 4. | 0.625 | 33.18 X 16.06 | 532.87 ± 0.15 | 72.63 |
| 5. | 0.312 | 35.58 X 18.85 | 670.680 ± 0.28 | 65.55 |
| 6. | 0.156 | 38.17 X 21.89 | 835.540 ± 0.37 | 57.08 |
| 7. | 0.078 | 41.19 X 24.57 | 1012.030 ± 0.76 | 48.02 |
| 8. | 0.039 | 50.05 X 28.90 | 1446.440 ± 0.39 | 25.70 |
| 9. | 0.019 | 57.67 X 30.95 | 1784.88 ± 0.18 | 8.3 |
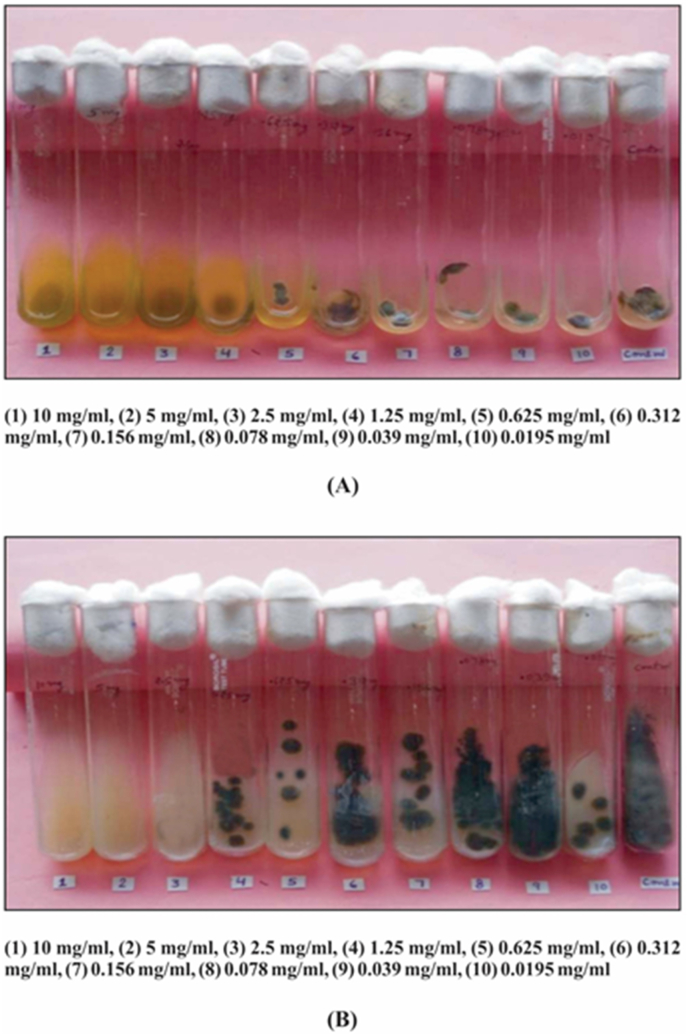
Fig. 2.
MIC and MFC of alcohol fraction of Thevetia peruviana against Alternaria solaniA. Minimum Inhibitory concentration (MIC) [1] 10 mg/ml; [2] 5 mg/ml; [3] 2.5 mg/ml; [4] 1.25 mg/ml; [5] 0.625 mg/ml; [6] 0.312 mg/ml; [7] 0.156 mg/ml; [8] 0.078 mg/ml; [9] 0.039 mg/ml; [10] 0.0195 mg/ml, B. Minimum Fungicidal Concentration (MFC) [1] 10 mg/ml; [2] 5 mg/ml; [3] 2.5 mg/ml; [4] 1.25 mg/ml; [5] 0.625 mg/ml; [6] 0.312 mg/ml; [7] 0.156 mg/ml; [8] 0.078 mg/ml; [9] 0.039 mg/ml; [10] 0.0195 mg/ml.
Table 6 showed the results of phytochemical screening of T. peruviana leaf extract prepared in different polarity of solvents. In petroleum ether fraction, steroids and tannins were found. Benzene fraction of leaf extract contained tannins only. All flavonoids, steroids, carbohydrates, tannins and saponins were observed in methanol fraction. Saponins were found both in aqueous as well as choloroform extract and acetone fraction contained flavonoids and saponins.
Table 6.
Phytochemical screening of various fractions of Thevetia peruviana leaf extract.
| S. No | Fractions | Alkaloids | Steroids | Vol. oil | Fat | Tannins | Carbohydrate | Saponins | Flavonoids |
|---|---|---|---|---|---|---|---|---|---|
| 1. | PE | - ve | -ve | + ve | -ve | -ve | + ve | + ve | - ve |
| 2. | Benzene | - ve | - ve | - ve | - ve | - ve | +ve | + ve | - ve |
| 3. | Chloroform | - ve | - ve | - ve | - ve | - ve | -ve | - ve | + ve |
| 4. | Acetone | - ve | + ve | - ve | - ve | - ve | - ve | -ve | + ve |
| 5. | Alcohol | + ve | + ve | - ve | - ve | +ve | + ve | +ve | + ve |
| 6. | Aqueous | - ve | - ve | - ve | - ve | - ve | - ve | - ve | + ve |
+ve = Presence, -ve = Absence.
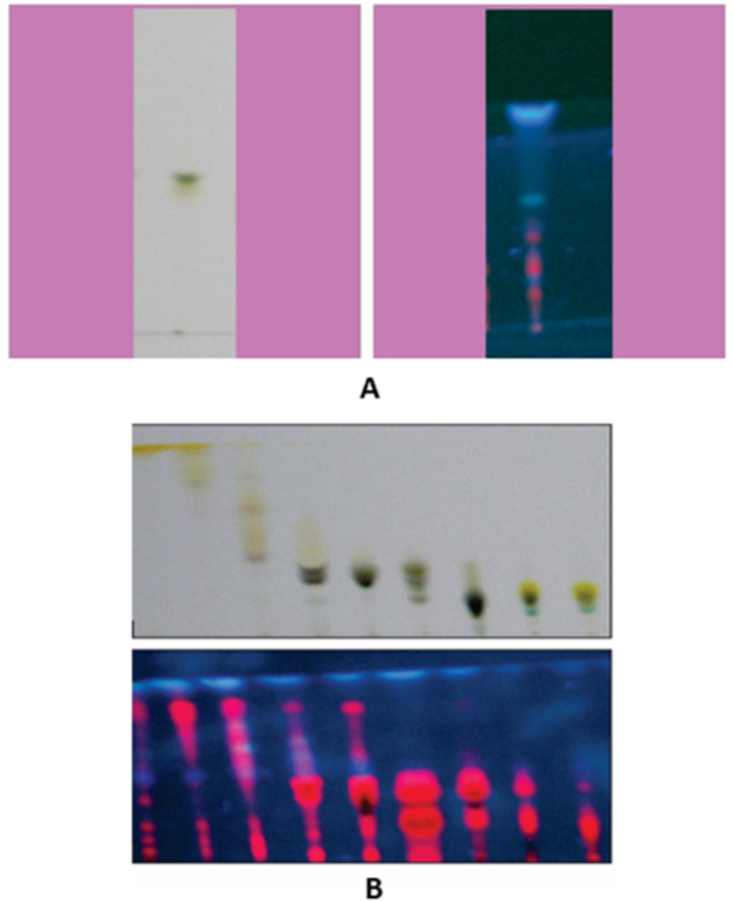
Rf values and different colours of bands observed after TLC of active fraction are seen in Table 7 and Fig. 3A, total 7 bands were observed after TLC of active fraction of T. peruviana leaf extract. Every band showed its characteristics Rf value calculated using the formulae. Active fraction (alcoholic fraction) of leaf extract of T. peruviana was also subjected to column chromatography. In column fractionation of alcoholic leaf extract of T. peruviana, total 9 fractions were collected. Each of 9 fractions was further fractionized via TLC. Different colour bands from one to three were observed for different column fractions. The TLC plate was observed under UV light at 254 nm before and after derivatization (Fig. 3B). Table 7 shows the results of TLC of different column fractions. Different bands showed characteristics Rf value and colour after derivatization using anis-aldehyde reagent on the TLC plates. Rf value of different bands observed were ranges from 0.62 to 0.94.
Table 7.
Rf of TLC finger printing of alcohol fraction of Thevetia peruviana leaf extraction.
| Band Number | Colour of Bands | RfValue |
|---|---|---|
| 1. | Blue green | 0.10 |
| 2. | Dark blue | 0.16 |
| 3. | Yellow | 0.33 |
| 4. | Yellow-blue | 0.44 |
| 5. | Purple | 0.56 |
| 6. | Purple | 0.70 |
| 7. | Yellow | 0.80 |
Fig. 3.
Thin Layer Chromatography (TLC) of various fractions of Thevetia peruviana leaf A. (i) TLC of alcohol fraction of leaf under visible light (ii) TLC of alcohol fraction of leaf under UV light (after derivatization), B. (i) TLC of column fractions [[1], [2], [3], [4], [5], [6], [7], [8], [9]] of alcohol fraction of leaf under visible light (ii) TLC of column fractions of alcohol fraction of leaf under visible light (After derivatization).
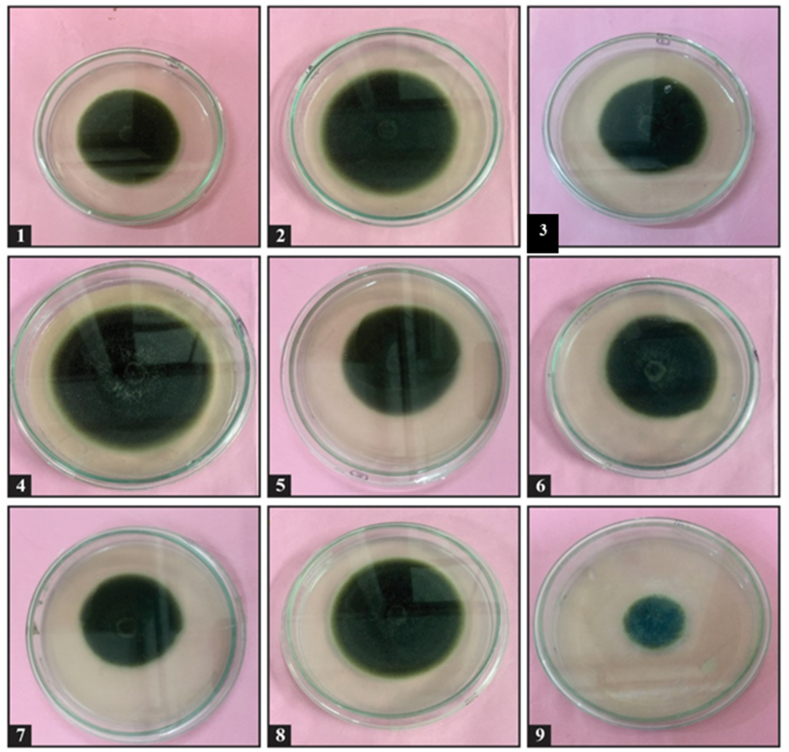
GC-MS Analysis Fractions obtained from column chromatography was subjected to determine antifungal activity against plant pathogenic fungus A. Solani. Table 8 and Fig. 4 depicted the results of antifungal activity of column fractions. As compared to control, maximum inhibition in growth of test fungus was observed by fraction F9 i. e followed by F7, F3, F1, F5, F6, F8, F2 and F4. All column fractions were checked for antifungal activity against test pathogens. Among the fractions most significant inhibitory activity against A. solani was shown by fraction F9. This fraction F9 was subjected to GC-MS analysis for further refining and identification of active compound it contained. Fig. 5 showed the chromatogram obtained for fraction F9. There was total number of one compound seen on GC-MS chromatogram.
Table 8.
Antifungal activity of column fraction (1–10 numbers) of alcohol extract of Thevetia peruviana leaf extraction against Alternaria solani.
| S. No | Column fraction | Growth Diameter after 7 days (mm) ± SD | % Mycelial growth inhibition |
|---|---|---|---|
| 1. | F1 | 40.66 ± 0.577 | 49.59 |
| 2. | F2 | 53.66 ± 0.577 | 33.47 |
| 3. | F3 | 39.33 ± 0.577 | 51.23 |
| 4. | F4 | 56.00 ± 0.00 | 30.57 |
| 5. | F5 | 41.66 ± 0.577 | 48.35 |
| 6. | F6 | 45.66 ± 0.577 | 43.39 |
| 7. | F7 | 34.66 ± 0.577 | 57.02 |
| 8. | F8 | 50.66 ± 0.577 | 37.19 |
| 9. | F9 | 20.66 ± 0.577 | 74.38 |
| 10. | Control (Water) | 80.66 ± 0.577 | NI |
Fig. 4.
Antifungal activity of column fractions (F1–F9) of alcohol extract of Thevetia peruviana leaf against Alternaria solani.
Fig. 5.
Mass spectrum of Benzoic acids.
4. Discussion
Herbal renaissance ‘is happening all over the globe and people returning to the naturals with hope of safety and security. Generally, the public is progressively moving towards acceptance and practice of herbal preparations [14]. In recent years, medicinal plants has started to increase its appeal to pharmaceutical companies and the scientific research community. This was due to evidences that these plant-derived compounds have a potential for many biological activities which include antimicrobial activity [15]. Scientist's aim is to extract and characterize active phytocompounds found in plants, since they have provided high activity profile drugs [16].
Shade dried plant material is used for crude extraction as well as partial purification of extract. Most of the time, dried sample is favoured in view of the time required for experimental design. Sulaiman et al. [17] Suggested that there should be a 3 h of time duration gap between harvest and experimental work in order to sustain freshness of samples, as fresh samples are delicate and be likely to deteriorate quicker than dried samples. According to Sulaiman et al. [18] maceration or percolation of fresh green plants or dried powdered plant material in water and organic solvent systems is best for extraction of plant material.
Results suggested that in the presence of leaf extract of Thevetia peruviana, reduction in conidial dimension and raise in mycelia width of A. solani was recorded. The manner of antifungal activity of leaf extract of Thevetia peruviana might be mainly due to cell wall attack and withdrawal of cytoplasm in the hyphae and eventually death of the mycelium [19]. Such modifications induced by the exposure to plant extract components may interfere with enzymatic reactions of wall synthesis and affect fungal morphogenesis and development.
Preliminary phytochemical studies and literature reveal that the leaves of T. peruviana contain iridoid glycosides, flavonoids [20], triterpenes [21], monoterpenes [22] and cardiac glycosides [23]. These compounds are known to cause structural and functional damage of cellular membrane. Impairment in membrane results in dissipation of two components of proton motive force, the pH gradients (ΔpH) and electrical potential (Δψ) [24,25]. Detrimental effects on proton motive force are strongly correlated with leakage of specific ions [26,27].
The medicinal plants are rich in secondary metabolites, a diverse group of chemicals, which include alkaloids, glycosides, amines, insecticides, steroids, flavonoids, and related metabolites, which have been extensively used in drug and pharmaceutical industry [28]. Flavonoids, tannins and phenolic acids belong to phenolic compound group of secondary metabolites. Phenolic compounds have been shown to exhibit antioxidant and antimicrobial activity [29]. In terms of antimicrobial properties, not the quantity of phenolic compounds and flavonoids is often important but the type of substances present in each extract [30]. Each fraction of plant extract prepared by successive extraction carries a specific set of secondary metabolites because solubility of secondary metabolites differs with different solvent. Qualitative phytochemical tests are used to detect phyto-constituents present in individual fraction. Several workers have studied phytochemical properties of plant extracts by qualitative phytochemical tests [[31], [32], [33]].
Active fraction with best inhibitory activity was incorporated to TLC and subsequently into column fractionation and GC-MS analysis to determine active ingredients present in extract. TLC is an analytical procedure employed in this case first to identify precise secondary metabolites extracts, and second to separate the components of these metabolites. Calculating the front ratio (Rf) or retention factor of a compound; ratio of the migration distance of substance on the migration distance of solvent-front, showed significant diversity of compounds separated from the different researched secondary metabolites. Prepared chromatograms have revealed the existence of numerous types of tannins, flavonoids, coumarins, quinones, carotenoids, saponins, alkaloids and terpenes relative to phytochemicals colouring reactions in various organs of the plants tested.
TLC of active fraction showed different colour bands representing the different compound found in these extract. Bands clearly indicate the presence of secondary metabolites which showed characteristics colour of band i.e. light blue, dark blue, colours characteristics of triterpenoids and pink and red colour bands indicates the presence of flavonoids and tannins respectively. Bouyahya et al. [35] also described that chromatogram preparation via TLC is best for initial understanding of secondary metabolites, Banu & Nagarajan [36] also confirmed that obtained bands after TLC fingerprinting corresponds to specific class of secondary metabolites and are accountable for antimicrobial activity. This was confirmed for leaf extracts of Wedelia chinensis (Osbeck) Merrill. Subsequently elution via column chromatography of active fraction i.e. alcoholic fraction of leaf was completed utilizing solvent system develops during TLC chromatogram study. After column chromatography 9 fractions were obtained from active alcoholic fraction of leaf. Each fraction comprised of different set of active compounds with antimicrobial characteristics. Presence of different active metabolites confirmed by the TLC chromatogram prepared from all the fractions obtained which showed characteristics Rf value and colour bands corresponding to active secondary metabolites or compound [[36], [37], [38]]. Antimicrobial activity of plants extract may be attributed instead of existence of active ingredients in prepared infusions. Extraction processes usually used to extract active ingredients are crude extraction and partially purified extraction process [17,39,40]. The principle of series of extraction process is to isolate the soluble plant metabolites, and left behind the insoluble cellular mass (residue). Initial crude extraction procedure i.e. cold extraction of plant parts, able to extract compound mix of many plant metabolites, such as alkaloids, glycosides, phenolics, terpenoids and flavonoids.
GC-MS is the best technique to identify the bioactive constituents of long chain hydrocarbons, alcohols, acids, esters, alkaloids, steroids, amino and nitro compounds etc. Hence, Gas chromatography (GC) and Mass spectroscopy (MS) associated with particular detection techniques have become a sophisticated means for analysis of various active compounds from plant extract [41]. Priya et al. [42] Identified eighteen phytochemical constituents from the ethanolic extract of the leaves of Desmodium gyrans by Gas chromatogram Mass spectrometry (GC-MS). Nanadagopalan et al. [43] reported the presence of Phytol in the leaves of Kirganelia reticulata aerial parts, which was found to be effective in different stages of arthritis. Aja et al. [44] GC/MS analysis of Moringa oleifera leaf and seed which revealed that 9-octadecenoic acid (20.89%) constitutes the major constituent of the leaf extract while oleic acid (84%) is the major component of the seed extract.
Active compound, active column fraction (having best antifungal activity against A. solani) was subjected to GC-MS analysis. Only one active compounds observed on GC-MS chromatogram. According the peaks matching of GC-MS chromatogram with available data base showed the presence of benzoic acid in active fraction of Thevetia peruviana leaf extract which is already known chemical among the phytochemicals described for antimicrobial activity.
Benzoic acids are C6–C1aromatic carboxylic acids that serve as precursors for a wide variety of essential compounds and natural products [45]. Benzoic acid also provide skeleton for numerous specialized metabolites. Non-volatile, benzyl benzoyl, and anthraniloyl compounds can also act as defense compounds [46]. Phytochemicals like benzoic acid have also been reported in extract prepared from plant parts of Caryophyllus aromaticus and Syzygyum joabolanum by Ref. [47].
5. Conclusion
Plant-based formulations can successfully be used in agriculture to treat plant diseases and can limit the use of chemical control agents to safer levels. Thus use of plant extract against plant pathogenic fungus is an important field of study and will be a best alternative of existing chemical antifungal in the near future. Antifungal effect of plant extract is mainly attributed to existence of secondary metabolites. These active constituents binds to membrane receptors and alter the cascade action generated thus leading to cyto-morphological alterations in test pathogenic fungus and directly or indirectly effect the growth and reproduction of the pathogen, thus bringing about its inhibition. Phytochemicals responsible for antimicrobial activity can be extracted from plant parts by using solvents of different polarity. Partial purification leads partial fractionization of phyto-constituents (i.e. much in alcoholic fraction of Thevetia leaf extract) which can be further refined by column fractionation. This further refinement of phyto-constituents via GC-MS analysis is much sensitive and sophisticated technique of fractionation. In this study benzoic acid and its derivatives were observed in active column fraction which is already reported for antimicrobial activity in different plant.
Author statement
Bhanu Raj: Conceptualization, Methodology, Execute experiments, Software, Data curation, Writing- Original draft preparation. Sanjeev Meena: Methodology, Execute some experiments, Reviewing and Editing. Deepali Chittora: Writing- Reviewing and Editing Kanika Sharma: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors gratefully acknowledge the SICART Gujarat for GC-MS analysis. Authors also thankful to Mr. Shani Raj for his help in analysis, figure preparations and editing and drafting the manuscript.
References
- 1.Romanazzi G., Lichter A., Gabler F.M., Smilanick J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012;63(1):141–147. [Google Scholar]
- 2.Ayoola G.A., Coker H.A., Adesegun S.A., Adepoju-Bello A.A., Obaweya K., Ezennia E.C., Atangbayila T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharmaceut. Res. 2008;7(3):1019–1024. [Google Scholar]
- 3.Chuang P.H., Lee C.W., Chou J.Y., Murugan M., Shieh B.J., Chen H.M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007;98(1):232–236. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Pan S.Y., Zhou S.F., Gao S.H., Yu Z.L., Zhang S.F., Tang M.K., Ko K.M. New perspectives on how to discover drugs from herbal medicines: CAM's outstanding contribution to modern therapeutics. Evid. base Compl. Alternative Med. 2013;2013 doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Waals J.E., Korsten L., Aveling T.A.S. A review of early blight of potato. Afr. Plant Protect. 2001;7(2):91–102. [Google Scholar]
- 6.Hadizadeh I., Peivastegan B., Hamzehzarghani H. Antifungal activity of essential oils from some medicinal plants of Iran against Alternaria alternate. Am. J. Appl. Sci. 2009;6(5):857–861. [Google Scholar]
- 7.Kagale S., Marimuthu T., Thayumanavan B. Nanda kumar R, Samiyappan R. Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonasoryzae pv. oryzae. Physiol. Mol. Plant Pathol, 2004;65(2):91–100. [Google Scholar]
- 8.Qasem J.R., Aau‐Blan H.A. Fungicidal activity of some common weed extracts against different plant pathogenic fungi. J. Phytopathol. 1996;144(3):157–161. [Google Scholar]
- 9.Barupal T., Sharma K. Effect of leaf extracts of lawsonia inermis Linn. On curvularia lunata, caused leaf spot disease of maize. Int. J. Innov. Res. Adv. Stud. 2017;4(2):64–67. [Google Scholar]
- 10.Grove R.K., Moore J.D. Toximetric studies of fungicides against brown rot organism Sclerotina fruticola. Phytopathology. 1962;52:876–880. [Google Scholar]
- 11.Kokate C.K., Purohit A.P., Gokhale S.B. Pharmacognosy. first ed. Nirali Prakashan; Pune: 1990. [Google Scholar]
- 12.Banerji A., Luthria D.L., Kokate S.D. Toxicity of capillin, the insecticidal principle of Artemisia nilagirica Clarke. Indian J. Exp. Biol. 1990;28(6):588–589. [PubMed] [Google Scholar]
- 13.Harborne J.B. Phytochemical Methods. Springer; Dordrecht: 1984. Methods of plant analysis; pp. 1–36. [Google Scholar]
- 14.Tewtrakul S., Nakamura N., Hattori M., Fujiwara T., Supavita T. Flavanone and flavonol glycosides from the leaves of Thevetia peruviana and their HIV-1 reverse transcriptase and HIV-1 integrase inhibitory activities. Chem. Pharm. Bull. 2002;50(5):630–635. doi: 10.1248/cpb.50.630. [DOI] [PubMed] [Google Scholar]
- 15.Musa A.Y., Chidiogor O.B., Nazif A.M., Esther I. Phytochemical constituents, thin layer chromatography and antimicrobial activity of methanol extract of the stem and leave of citrus limon (L) Int. J. Biochem. Biophys. Mol. Biol. 2017;2(4):31–35. [Google Scholar]
- 16.Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012;7(8):979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 17.Vaghasiya Y., Dave R., Chanda S. Phytochemical analysis of some medicinal plants from western region of India. Res. J. Med. Plant. 2011;5(5):567–576. [Google Scholar]
- 18.Sulaiman S.F., Sajak A.A.B., Ooi K.L., Seow E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011;24(4–5):506–515. [Google Scholar]
- 19.Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Latha L.Y. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr. J. Tradit., Complementary Altern. Med. 2011;8(1) [PMC free article] [PubMed] [Google Scholar]
- 20.Ghani S.B.A., Weaver L., Zidan Z.H., Ali H.M., Keevil C.W., Brown R.C. Microwave-assisted synthesis and antimicrobial activities of flavonoid derivatives. Bioorg. Med. Chem. Lett. 2008;18(2):518–522. doi: 10.1016/j.bmcl.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 21.Ali M., Ravinder E., Ramachandram R. New ursane-type triterpenic esters from the stem bark of Thevetia peruviana. Pharmazie. 2000;55(5):385. [PubMed] [Google Scholar]
- 22.Abe F., Chen R.F., Yamauchi T. Dinormonoterpenoids and their apiosylglucosides from Thevetia peruviana. Phytochemistry. 1996;43(1):161–163. doi: 10.1016/0031-9422(96)00200-2. [DOI] [PubMed] [Google Scholar]
- 23.Abe F., Chen R.F., Yamauchi T. Dinormonoterpenoids and their apiosylglucosides from Thevetia peruviana. Phytochemistry. 1996;43(1):161–163. doi: 10.1016/0031-9422(96)00200-2. [DOI] [PubMed] [Google Scholar]
- 24.Abe F., Yamauchi T., Yahara S., Nohara T. Glycosides of 19-formylthevetiogenin and 5α-thevetiogenin from Thevetia neriifolia. Phytochemistry. 1994;37(5):1429–1432. doi: 10.1016/s0031-9422(00)90426-6. [DOI] [PubMed] [Google Scholar]
- 25.Sikkema J., de Bont J.A., Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995;59(2):201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson P.M., Taylor T.M., Schmidt S.E. Chemical preservatives and natural antimicrobial compounds. Food Microbiol.: Fundamentals Front. 2012:765–801. [Google Scholar]
- 27.Kroll R.G., Booth I.R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem. J. 1981;198(3):691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakker E.P., Mangerich W.E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 1981;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharanabasappa G.K., Santosh M.K., Shaila D., Seetharam Y.N., Sanjeevarao I. Phytochemical studies on Bauhinia racemosa lam. Bauhinia purpurea Linn. And hardwickia binata roxb. E-J. Chem. 2007;4 [Google Scholar]
- 30.Martins N., Barros L., Henriques M., Silva S., Ferreira I.C. Activity of phenolic compounds from plant origin against Candida species. Ind. Crop. Prod. 2015;74:648–670. [Google Scholar]
- 31.Šernaitė L. Plant extracts: antimicrobial and antifungal activity and appliance in plant protection. Sodininkystė ir daržininkystė. 2017;36(3/4):58–68. [Google Scholar]
- 32.Khadilkar M.S., Mengi S.A., Deshpande S.G. Phytochemical and pharmacological investigation of water extracts of Boerhaavia diffusa (Punarnava) and Azadirachta indica (Neem) in experimentally induced conjunctivitis. Indian Drugs Bombay. 2001;38(1):40–45. [Google Scholar]
- 33.Patil M.B., Jalalpure S.S., Ashraf A. Preliminary phytochemical investigation and wound healing activity of the leaves of Argemone mexicana Linn.(Papaveraceae) Indian Drugs Bombay. 2001;38(6):288–293. [Google Scholar]
- 35.Bouyahya A., Bakri Y., Et-Touys A., Khouchlaa A., El Idrissi A.E.Y., Abrini J., Dakka N. In vitro screening of antibacterial and antioxidant activities of essential oils from four Moroccan medicinal plants. Microbiol. Res. J. Int. 2017:1–10. [Google Scholar]
- 36.Banu R., Nagarajan N. TLC and HPTLC fingerprinting of leaf extracts of Wedelia chinensis (Osbeck) Merrill. J. Pharmacogn. Phytochem. 2014;2(6) [Google Scholar]
- 37.Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Latha L.Y. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr. J. Tradit., Complementary Altern. Med. 2011;8(1) [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatesh U., Javarasetty C., Murari S.K. Purification and fractional analysis of methanolic extract of Wedelia trilobata possessing apoptotic and anti-leukemic activity. Afr. J. Tradit., Complementary Altern. Med. 2017;14(3):167–174. doi: 10.21010/ajtcam.v14i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gini T.G., Jothi G.J. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt. J. Basic Appl. Sci. 2018;5(3):197–203. [Google Scholar]
- 40.Balakrishnan B.R., Sangameswaran B., Arul B., Bhaskar V.H. Antibacterial activity of aerial part extracts of Achyranthes bidentata Blume. Indian J. Pharmaceut. Sci. 2003;65(2):186. [Google Scholar]
- 41.Das K., Tiwari R.K.S., Shrivastava D.K. Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. J. Med. Plants Res. 2010;4(2):104–111. [Google Scholar]
- 42.Vinodh K.S., Natarajan A., Devi K., Senthilkumar B. Chemical composition of aqueous leaf extract of Murraya Koenigii. Int. J. Pharm. Biol. Archiv. 2013;4:493–497. [Google Scholar]
- 43.Priya S., Nethaji S., Sindhuja B. GC-MS Analysis of some bioactive constituents of Diospyros virginiana. Res. J. Pharm. Technol. 2014;7(4):429–432. [Google Scholar]
- 44.Nanadagopalan V., Johnson Gritto M., Doss A. GC-MS analysis of biomolecules on the leaves extract of Sterculia urens Roxb. J. Pharmacogn. Phytochem. 2015;3(6):193–196. [Google Scholar]
- 45.Aja P.M., Nwachukwu N., Ibiam U.A., Igwenyi I.O., Offor C.E., Orji U.O. Chemical constituents of Moringa oleifera leaves and seeds from Abakaliki, Nigeria. Am. J. Phytomed. Clin. Ther. 2014;2(3):310–321. [Google Scholar]
- 46.Lee S., Kaminaga Y., Cooper B., Pichersky E., Dudareva N., Chapple C. Benzoylation and sinapoylation of glucosinolate R‐groups in Arabidopsis. Plant J. 2012;72(3):411–422. doi: 10.1111/j.1365-313X.2012.05096.x. [DOI] [PubMed] [Google Scholar]
- 47.Okazaki Yozo, Isobe Taishi, Iwata Yoichi, Matsukawa Tetsuya, Matsuda Fumio, Miyagawa Hisashi, Ishihara Atsushi, Nishioka Takaaki, Iwamura Hajime. Metabolism of avenanthramide phytoalexins in oats. Plant J. 2004;39(4):560–572. doi: 10.1111/j.1365-313X.2004.02163.x. [DOI] [PubMed] [Google Scholar]