Abstract
Objective
Inflammation has been considered as an important factor in cardiovascular diseases (CVD). Curcumin has been well known for its anti-inflammatory effects. In current research, protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide (LPS) was investigated in rats.
Material and methods
The animals were divided into five groups and received the treatments during two weeks [1]: Control in which vehicle was administered instead of curcumin and saline was injected instead of LPS [2], LPS group in which vehicle of curcumin plus LPS (1 mg/kg) was administered [3-5], curcumin groups in them three doses of curcumin (5, 10 and 15 mg/kg) before LPS were administered.
Results
Administration of LPS was followed by an inflammation status presented by an increased level of white blood cells (WBC) (p < 0.001). An oxidative stress status was also occurred after LPS injection which was presented by an increased level of malondialdehyde (MDA) while, a decrease in thiols, superoxide dismutase (SOD) and catalase(CAT) in all heart, aorta and serum (p < 0.001). The results also showed that curcumin decreased WBC (doses: 10 and 15 mg/kg) (p < 0.001) accompanying with a decrease in MDA (P < 0.01 and P < 0.001). Curcumin also improved the thiols and the activities of SOD and catalase (P < 0.05, P < 0.01 and P < 0.001).
Conclusion
Based on our findings, curcumin can ameliorates oxidative stress and inflammation induced by LPS in rats to protect the cardiovascular system.
Keywords: Curcumin, Inflammation, Oxidative stress, Cardiovascular diseases, Lipopolysaccharide
Highlights
-
•
The aim of the present study was to investigate the cardiovascular protective effects of curcumin in lipopolysaccharide (LPS) challenged rats
-
•
Lipopolysaccharide (LPS) induced inflammation model in rats
-
•
LPS injection was followed by inflammation and induced oxidative stress status in the serum, aorta and heart.
-
•
Administration of curcumin attenuated oxidative stress and inflammation in the serum, aorta and heart tissues induced by LPS.
1. Introduction
Cardiovascular diseases (CVD) are consisting of disorders affecting the heart and the blood vessels. It represents one third of all deaths, and incidence is still growing [1]. Studies show that inflammation plays a crucial role in beginning and progression of CVD [2]. Chronic inflammatory situations also decrease endogenous antioxidant abilities because of constant production of high levels of reactive oxygen species (ROS). Patients with CVD are often with low blood levels of antioxidants and increased levels of oxidative stress indicators [3]. Some researches have shown that inflammation is related with change in signaling pathways, which results in increased levels of free radicals, inflammatory markers and lipid peroxides [4,5]. Therefore, the key mechanisms of arterial damage and endothelial dysfunction are including oxidative stress and inflammation which both of them are associated with arterial stiffness and aging related vascular damage [6]. Released cytokines during inflammation are also play a key role in inducing atherosclerosis by attracting monocytes/macrophages to the vessel wall, which facilitates atherosclerotic lesions and plaque susceptibility. In addition, circulating levels of these proinflammatory cytokines rise in patients with acute myocardial infarction and unstable angina [7].
Lipopolysaccharide (LPS) is a endotoxin from a gram-negative bacteria and a major factor that is partly responsible for multiple organ failure, for example heart injury [8]. It is widely used to generate immune system responses [9]. While several mechanisms of LPS-induced myocardial injury have been hypothesized, the overproduction of auto-oxidation-induced cytotoxic free radicals is the most widely accepted [10]. LPS systemic administration can induce several organ disorder including heart damage [11]. Also it was shown that single dose of LPS induced a systemic inflammation and cardiovascular toxicity [12]. LPS exposure is thought to cause severe stress in cardiomyocytes, resulting in a loss of myocardial integrity due to a combination of oxygen deficiency, calcium overload, and the overproduction of free radicals [13]. The heart is one of the highest tissue oxygen consumption levels, and also poor antioxidant enzyme production and is thus highly vulnerable to ROS [14]. Increased production of ROS may result in myocyte hypertrophy, apoptosis, and interstitial fibrosis that may contribute to the development of depressed heart function and heart failure progression. It has been well known that in health and disease, endothelial function is a main factor of vessel wall and myocardial function [15]. For preventing the progression of heart diseases, modulation of oxidative stress-induced signaling pathways is effective [16].
Beneficial effects of dietary plants rich in antioxidants is proven to prevent free radical formation, remove free radicals before damage, healing oxidative damage, remove damaged molecules; studies showed their beneficial role in cardiovascular disease, and we can put them in our diet [17].
Although significant progress has been made in managing synthetic drugs for cardiovascular diseases, the search for indigenous cardioprotective agents is continuing. It has been shown that at least 30 food spices and herbs possess antioxidant properties such as Boswellia serrata, Nigella sativa, saffron and their major components such as thymoquinone, and safranol, [[18], [19], [20], [21]]. Curcumin, the major yellow bioactive component of turmeric (Curcuma longa L., Zingiberaceae), is a more recent discovery [22,23]. Based on some investigations, curcumin supplement has indicated to be a safe and potential therapeutic agent against diseases, such as arthritis, pancreatitis, inflammatory bowel disease, as well as certain kinds of cancers [24]. It has many pharmacological effects for example: antioxidant, anti-inflammation, removing free radicals, anti-tumor, lipid regulation and anti-coagulation [25].
Curcumin plays a key role against oxidative stress mediated pathological conditions and it has anti-inflammatory activities when used as a therapy for treatment and prevention of chronic diseases [26,27]. The clinical trials did not indicated severe toxic or side effect [28,29]. It was shown that myocardial tissue from diabetic rats exhibited increased levels of eNOS and iNOS mRNA, and curcumin treatment prevented eNOS and iNOS mRNA upregulation showing a decrease in the oxidative DNA damage [30].
In current research, protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide (LPS) was investigated in rats.
2. Material and methods
2.1. Animals and treatments
The present study consisted of 35 male Wistar rats from the Central Animal House of Mashhad University of Medical Sciences which weighed 250 ± 10g. During the study, the animals were kept in a room with a cycle of 12h light/dark and standard temperature (22 °C ± 2 °C).
The animals were randomly categorized into five groups:
-
1.
Control: the rats in this group were intraperitoneally (IP injected with 2 ml/kg vehicle instead of Cur and 1 ml/kg saline instead of LPS.
-
2.
LPS: the animals in this group received 2 mL/kg saline diluted Dimethyl sulfoxide (DMSO) as a vehicle instead of curcumin and 1 ml/kg LPS.
-
3.
LPS-Cur 5: the animals in this group received 5 mg/kg Curcumin dissolved in saline diluted DMSO as vehicle and 1 mg/kg LPS.
-
4.
LPS-Curcumin10: the rats in this group were injected with 10 mg/kg Curcumin dissolved in DMSO as vehicle and 1 mg/kg LPS.
-
5.
LPS-Cur 15: the rats in the group were injected with 15 mg/kg Curcumin dissolved in DMSO as vehicle and 1 mg/kg LPS.
The daily IP injections took place in a period of two weeks. LPS was purchased from Sigma Aldrich Co. (purities ≥ 97). Also, curcumin powder (C1386) was purchased from sigma-aldrich Co. and we injected LPS 1 mg/ml. At the end of injections, the rats were injected with urethane to induce deep anesthesia. The blood samples were collected to be used for white blood cell (WBC) count and to be used for the oxidative stress indicators measurements in the serum. The heart and aorta of the rats were also collected to be used for oxidative stress criteria.
Malondialdehyde (MDA), thiol, catalase (CAT) and superoxide dismutase (SOD) were determined in the serum, aorta and heart.
The Animal Care and Use Committee of Mashhad University of Mashhad Sciences (IR.MUMS.MEDICAL.REC.1399.162) and also the National Institute of Health guidelines for the Care and Use of Laboratory animals approved this experimental protocol.
2.2. Biochemical measurements
2.2.1. WBC count
Total WBC count was done in duplicate using a hemocytometer (in a Burker chamber).
2.2.2. MDA and total thiol concentration
The TBA-trichloroacetic acid-HCL reagent was initially applied to the tissue which we have already homogenized. The solution was then put in water and heated for 30 min. MDA reacts with thiobarbituric Acid [31] to form a red complex [32].
Besides, total thiol concentration was determined in the aorta, heart and serum. In this procedure, the reaction between DTNB (2,2′-dinitro-5,5′-dithiol benzoic acid) and thiol groups form a yellow complex. Absorbance was read at 412 nm [32].
2.3. SOD and CAT activities
SOD activity was measured by the procedure described by Madesh and Balasubramanian [33]. It was measured at 570 nm according to a colorimetric technique. One unit of SOD was equal to the amount of enzyme that should be inhibited by 50% of the MTT reduction rate. Aebi method was used to measure CAT activity using hydrogen peroxide (30 mM) as a substrate [32].
2.4. Statistical analysis
All the data is presented as mean ± standard error of the mean. Data was evaluated by analysis of variance(one-way ANOVA) followed by Tukey post hoc test using Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA). The differences were considered statistically significant when P < 0.05.
3. Results
3.1. Total WBC count
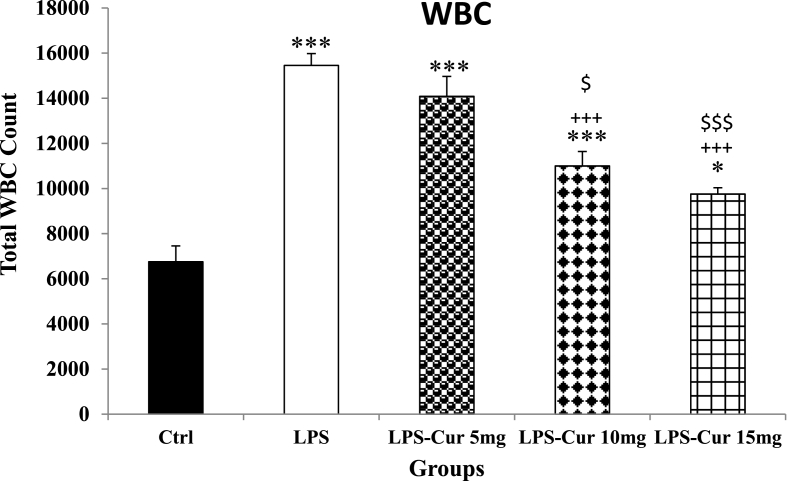
The results showed that the number of total WBCs were significantly increased in LPS group compared with Control group (p < 0.001). Curcumin with doses 10 and 15 mg induced a significant reduction in total blood WBC count (p < 0.001; Fig. 1) but 5 mg/kg of curcumin was not effective. In the rats treated by all doses of curcumin WBC count was still higher than the Control group (p < 0.001, p < 0.001 and p < 0.05 for 5, 10 and 15 mg/kg doses respectively). In addition, WBC count in the rats treated by 10 mg/kg and 15 mg/kg curcumin was lower than that in the group treated by 5 mg/kg(p < 0.05 and p < 0.001 respectively). There was no significant difference between the effects of the medium and the highest dose of curcumin(Fig. 1).
Fig. 1.
WBC count. Data are shown as mean ± SEM of 10 animals per group. *p < 0.05 and ***p < 0.001 vs. Control group, +++ p < 0.001 vs. LPS group and $ p < 0.05 and $$$ p < 0.001 vs. LPS-Cur 5 mg group.
3.2. Total thiol and MDA concentration in the heart
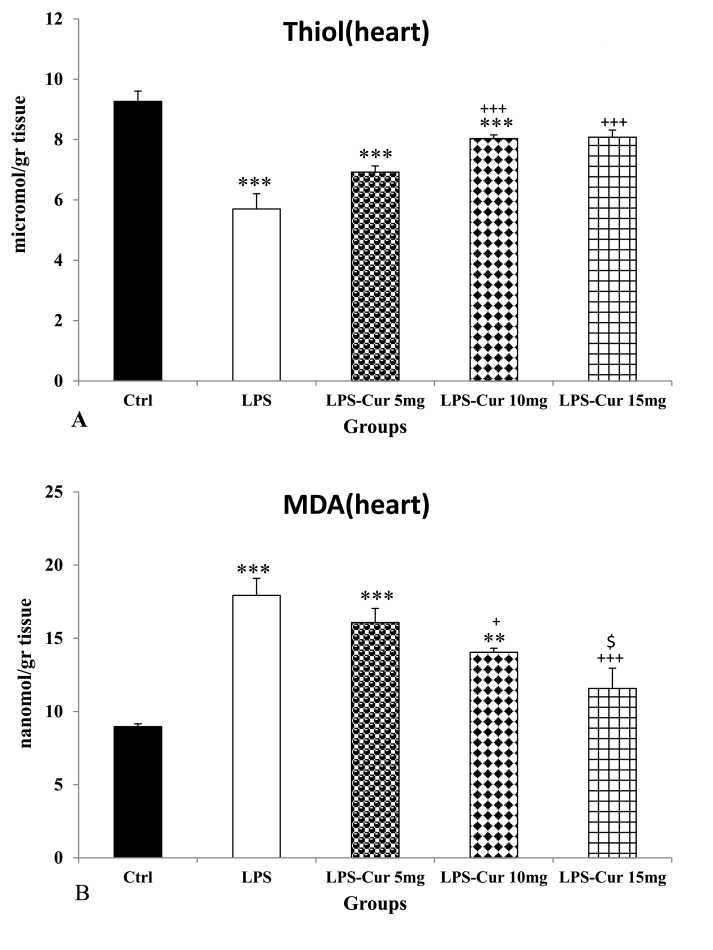
Injection of LPS showed a significant decrease of thiol level in the heart of the LPS group compared to the Control group (p < 0.001). Also we observed a significant increase in 10 and 15 mg/kg curcumin treated groups compared to LPS group (p < 0.001 for both) but 5 mg/kg curcumin was not effective(Fig. 2A). The results also showed that thiol content in the heart tissue of treated groups by 5 and 10 mg/kg doses of curcumin was still lower than that in the Control group (p < 0.001 for both) but there was no significant difference between LPS-Cur 15 mg and Control groups. There was no significant difference between three doses of curcumin.
Fig. 2.
Thiol (A) and malondialdehyde (MDA) (B) of heart tissues. Data are shown as mean ± SEM of 10 animals per group. **p < 0.01 and ***p < 0.001 vs. Control group, + p < 0.05 and +++ p < 0.001 vs. LPS group and $ p < 0.05 vs. LPS-Cur 5 mg group.
Consequence of LPS injection was also seen as a significant increase in MDA level in the heart tissue of LPS group compared to the Control group (p < 0.001). Another result was seen as a significant decrease of MDA in the heart of the groups treated with 10 and 15 mg/kg doses of curcumin compared to LPS group (p < 0.05 and p < 0.001 respectively) but 5 mg/kg curcumin was not effective (Fig. 2B). It was also observed that there was a more significant decrease in the group treated with dose of 15 mg/kg curcumin compared to 5 mg/kg treated group (p < 0.05) but there was no significant difference between LPS-Cur 5 mg and LPS-Cur 10 mg groups and also between LPS-Cur 15 mg and LPS-Cur 10 mg groups(Fig. 2B). Finally, treatment by the highest dose of curcumin was able to attenuate MDA in the heart to the level of Control group and there was no significant difference between LPS-Cur 15 mg and the Control groups. In the heart of both LPS-Cur 5 mg and LPS-Cur 10 mg groups, MDA level was higher than in the Control group(p < 0.001 and p < 0.01 respectively).
3.3. Total thiol and MDA concentration in the aorta
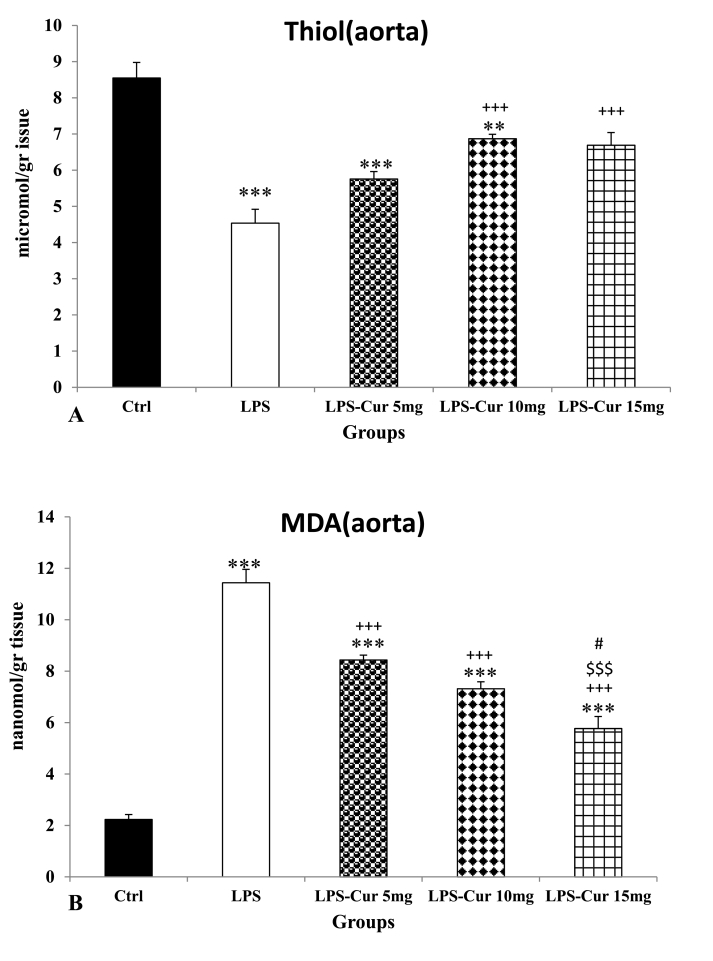
Injection of LPS showed a considerable reduction of thiol concentration in the aorta of LPS group compared to the Control group (p < 0.001). Administration of the medium and highest doses of curcumin showed a considerable increase in treatment groups compared to LPS group (p < 0.001 for both) but the lowest dose was not effective. There was no significant difference between the three doses of curcumin. The results also showed that thiol content in the aorta of treated groups by 5 and 10 mg/kg doses of curcumin was still lower than that in the Control group (p < 0.001 and p < 0.001 respectively) but there was no significant difference between LPS-Cur 15 mg and Control groups(Fig. 3A).
Fig. 3.
Thiol (A) and malondialdehyde (MDA) (B) of aorta tissues. Data are shown as mean ± SEM of 10 animals per group. **p < 0.01 and ***p < 0.001 vs. Control group, +++ p < 0.001 vs. LPS group, $$$ p < 0.001 vs. LPS-Cur 5 mg group, #p < 0.05 vs. LPS-Cur 10 mg group.
Outcome of LPS injection was also seen as a considerable increase in MDA in the aorta of LPS group compared to the Control group (p < 0.001). A considerable reduction of MDA in the aorta of all curcumin treated groups was seen compared to LPS group (p < 0.001 for all) but it was still higher than that in the Control group(p < 0.001 for all). MDA level in aorta tissues of the rats treated by 15 mg/kg curcumin was lower than ones treated by 5 mg/kg and 10 mg/kg (p < 0.001 and p < 0.05 respectively) but there was no significant difference between the effects 5 and10 mg/kg doses of curcumin(Fig. 3B). MDA level in the aorta tissues of all LPS- Cur 5 mg, LPS- Cur 10 mg and LPS- Cur 15 mg was still higher than Control group(p < 0.001 for all).
3.4. Total thiol and MDA concentration in the serum
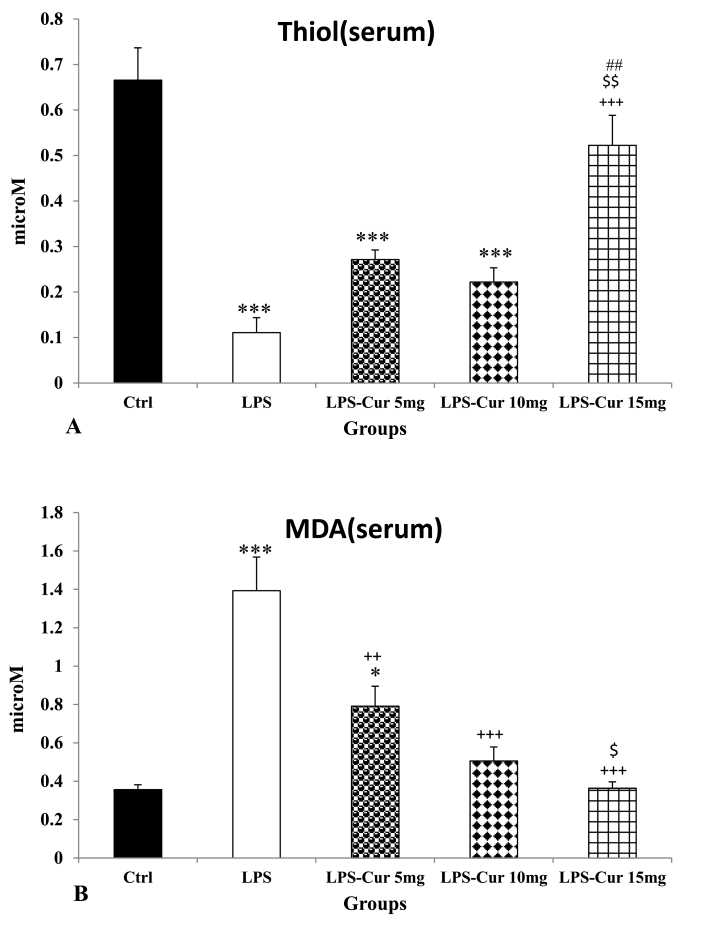
Injection of LPS was accompanied with a reduction of thiol content in the serum of LPS group compared to the Control group (p < 0.001). Administration of curcumin showed a noticeable increase in treated group with 15 mg/kg dose compared to LPS group (p < 0.001) but there was no significant difference between LPS- Cur 5 mg, LPS- Cur 10 mg and the Control groups (Fig. 4A). Also it was indicated a more increase in group treated with 15 mg/kg dose of curcumin compared to other treatment groups (p < 0.01). There was no significant difference between the effects of the medium and the lowest doses of curcumin. In addition, serum thiol content in the all groups treated by 5 mg/kg and 10 mg/kg curcumin was still lower than that in the Control group(p < 0.001 for both) but there was no significant difference between LPS-Cur 15 mg and the Control group(Fig. 4A).
Fig. 4.
Thiol (A) and malondialdehyde (MDA) (B) of serum. Data are shown as mean ± SEM of 10 animals per group. *p < 0.05 and ***p < 0.001 vs. Control group, ++ p < 0.01and +++ p < 0.001 vs. LPS group and $ p < 0.05 and $$ p < 0.01 vs. LPS-Cur 5 mg group, ##p < 0.01 vs. LPS-Cur 10 mg group.
Result of LPS injection was seen as a noticeable increase in serum MDA level in LPS group Compared to the control group (p < 0.001). The result was also seen as a noticeable reduction of MDA in all curcumin treated groups compared to LPS group (p < 0.01, p < 0.001 and p < 0.001for lowest, medium and highest doses respectively). In addition, MDA level in the serum of LPS-Cur 15 mg group was lower than that in LPS-Cur 5 mg group(p < 0.05) but there was no significant difference between LPS-Cur 5 mg and LPS-Cur 10 mg groups and also between LPS-Cur 10 mg and LPS-Cur 15 mg groups. In addition, serum MDA in LPS-Cur 5 mg group was still higher than that in the Control group(p < 0.05) but there was no significant difference when MDA level in the serum of LPS-Cur 10 mg and LPS-Cur 15 mg groups was compared to the Control group(Fig. 4B).
3.5. CAT and SOD activities in the heart
Administration of LPS caused a significant decrease in CAT activity in heart tissues of LPS group compared to control group (p < 0.001). Also we found a significant increase in the CAT activity in the heart tissue of the rats treated groups 10 and 15 mg/kg curcumin compared to LPS group (p < 0.001 for both) but 5 mg/kg curcumin was not effective. In the heart of the groups treated with both10 and 15 mg/kg doses curcumin, CAT was higher than that in the group treated by 5 mg/kg group (p < 0.05 for both) but there was no significant difference between 10 and 15 mg/kg curcumin. CAT activity in the heart of all LPS- Cur 5 mg, LPS- Cur 10 mg and LPS- Cur 15 mg groups was still lower than that in the Control group(p < 0.001, p < 0.01 and p < 0.01 respectively) (Table 1).
Table 1.
The CAT (A) and SOD (B) activity of all tissues. Data are shown as mean ± SEM of 10 animals per group. *p < 0.01 and ***p < 0.001 vs. Control group, + p < 0.05, ++ p < 0.01 and +++ p < 0.001 vs. LPS group, $ p < 0.05, $$ p < 0.01 and $$$ p < 0.001 vs. LPS-Cur 5 mg group, #p < 0.05 and ##p < 0.01 vs. LPS-Cur 10 mg group.
| Tissues | Enzyme activity | Ctrl | LPS | LPS-Cur5mg | LPS-Cur10mg | LPS-Cur15mg |
|---|---|---|---|---|---|---|
| Heart | CAT(U/g tissue) | 0.45 ± 0.03 | *** 0.17 ± 0.02 |
*** 0.24 ± 0.02 |
**+++$ 0.33 ± 0.02 |
**+++$ 0.32 ± 0.03 |
| SOD(U/g tissue) | 20.80 ± 2.65 | *** 2.29 ± 0.99 |
*** 2.69 ± 0.94 |
*** 7.12 ± 1.62 |
***+$ 9.46 ± 1.41 |
|
| Aorta | CAT(U/g tissue) | 0.49 ± 0.03 | *** 0.19 ± 0.01 |
***+ 0.27 ± 0.02 |
***+++$ 0.36 ± 0.02 |
**+++$$ 0.38 ± 0.02 |
| SOD(U/g tissue) | 9.92 ± 0.70 | ** 4.82 ± 0.70 |
** 5.51 ± 1.12 |
** 5.27 ± 0.90 |
++$# 8.89 ± 0.33 |
|
| Serum | CAT(U/ml) | 66.21 ± 5.28 | *** 21.21 ± 2.32 |
44.84 ± 4.88 |
++ 58.76 ± 11.24 |
+ 49.68 ± 5.83 |
| SOD(U/ml) | 0.61 ± 0.07 | *** 0.08 ± 0.02 |
*** 0.13 ± 0.02 |
*** 0.22 ± 0.07 |
+++$$$## 0.51 ± 0.03 |
A significant decrease in SOD activity was observed following injection of LPS in LPS group compared to the Control group (p < 0.001). In treated group by15 mg/kg dose of curcumin we had asignificant increase in SOD of LPS-Cur 15 mg compared to LPS group (p < 0.05) but there was no significant effect for 5 and 10 mg/kg of curcumin on SOD activity. In the heart of the groups treated by 15 mg/kg curcumin, SOD activity was higher than that in the group treated by 5 mg/kg(p < 0.05) but there was no significant difference between LPS- Cur 15 mg and LPS- Cur 10 mg groups and between LPS- Cur 5 mg and LPS- Cur 10 mg groups. In the heart of all LPS- Cur 5 mg, LPS- Cur 10 mg and LPS- Cur 15 mg groups, SOD activity was still lower than that in the Control group(p < 0.001 for all) (Table 1).
3.6. CAT and SOD activities in the aorta
Administration of LPS caused a considerable reduction in CAT activity in the aorta tissues of LPS group compared to the Control group (p < 0.001). Also we had a considerable increase in all treatment groups (curcumin doses: 5, 10 and 15 mg/kg) compared to LPS group (p < 0.05, p < 0.001 and p < 0.001 respectively). In curcumin treated groups with 5, 10 and 15 mg/kg doses a considerable increase in CAT in the aorta tissue was observed compared to the LPS group (p < 0.01, p < 0.001 and p < 0.001 respectively; Table 1). In addition, CAT activity in the aorta tissue of LPS- Cur 10 mg and LPS- Cur 15 mg groups was higher than in LPS- Cur 5 mg group(p < 0.05, p < 0.01) but there was no significant difference between LPS- Cur 15 mg and LPS- Cur 10 mg groups. CAT activity in the aorta tissue of all LPS- Cur 5 mg, LPS- Cur 10 mg and LPS- Cur 15 mg groups was still lower than that in the Control group(p < 0.001, p < 0.001 and p < 0.01for all) (Table 1).
A considerable reduction in SOD activity was obtained from injection of LPS in LPS group compared to Control group (p < 0.01). In the aorta of the group treated with 15 mg/kg dose of curcumin we observed a considerable increase in SOD activity compared to LPS group (p < 0.01) but 5 and 10 mg/kg of curcumin was not effective. Besides we observed another considerable increase with this group (dose: 15mg/kg) compared to other treatment groups (p < 0.05 for both). There was no significant difference between LPS-Cur 15 mg and the Control groups but in the aorta of both LPS-Cur 10 mg and LPS-Cur 5 mg groups, SOD activity was lower than that in the Control group(p < 0.01) (Table 1).
3.7. CAT and SOD activities in the serum
Administration of LPS caused a noticeable reduction in CAT activity in serum of LPS group compared to control group (p < 0.001). Also we had a noticeable increase in CAT activity in the serum of the treated groups by both 10 and 15 mg/kg curcumin compared to LPS group (p < 0.01 and p < 0.01 respectively) but there was no significant difference between LPS-Cur 5 mg and LPS groups. There was no noticeable difference between treatment groups. There was also no significant difference between curcumin treated groups and control group(Table 1).
A noticeable reduction in SOD activity resulted from injection of LPS in LPS group compared to Control group (P < 0.001). In the group treated by 15 mg/kg dose of curcumin, SOD activity was significantly higher than LPS group (p < 0.001). In the serum of LPS-Cur 15 mg, SOD activity was higher than that in both LPS-Cur 10 mg and LPS-Cur 5 mg groups(p < 0.001 and p < 0.01). In both LPS-Cur 10 mg and LPS-Cur 5 mg groups, the serum SOD activity was lower than Control group(p < 0.001) but there was no significant difference between LPS-Cur 15 mg and Control group (Table 1).
4. Discussion
In the present study we showed that LPS injection was followed by an inflammation status and oxidative stress damage in aorta, heart and serum which was reversed by Cur. Using a bacterial endotoxin LPS, an extensive inflammatory response is occurred in the body which can be used to study the effects of inflammation in the experiments [34]. Inflammation status has been sometimes confirmed by WBC count [35] and it has been shown that WBC count can be a marker of inflammation [36]. The strong relation between WBC count, hypertension, and cardiovascular disease has been documented in several researches [37]. The association between WBC count and inflammatory factors in individuals with hypertension or complicating cardiovascular diseases has definitely been of crucial importance [37]. In spite of all these researches, the complete WBC count has not been studied and exploited for its usefulness in cardiovascular risk prediction.
In the current study injection of LPS induced an inflammation status which confirmed by increased number of WBC in LPS group (Fig. 1). Previously it was shown that the normal range of WBC count in normal rat age 45–60 was 4000–10000 [38]. The WBCs count in LPS group was 15,400 and it showed inflammation status in this group.
In the current research, LPS induced inflammation was accompanied with an oxidative stress status in all heart, aorta and serum. Both inflammation and oxidative stress have been reported to be involved in the pathophysiology of CVD like congestive heart failure (CHF) [39].
In addition, oxidative stress can activate some factors, which can cause expression of several genes of inflammatory pathways [40]. On the other hand oxidative stress is sometimes occurred as a consequence of inflammation [41].
LPS as particle of gram negative bacteria has been reported to generate free radicals to cause lipid peroxidation of the membrane-bound polyunsaturated fatty acids, resulting in structural and functional integrity degradation of the membranes [8]. We assessed the effect of LPS on lipid peroxidation, which was measured in terms of MDA, a stable metabolite of the free radical-mediated lipid peroxidation cascade [42]. The MDA levels increased significantly following LPS treatment in rat serum (Fig. 4B) and heart (Fig. 2B) and aorta (Fig. 3B) tissues The results also showed that total thiol concentration and CAT and SOD activity decreased in serum, heart and aorta tissues due to LPS injection (Table 1). These findings are consistent with previous study, which showed that LPS induced lipid peroxidation [43]. In agreement with these results, it was previously shown that the antioxidant capacity of the heart was decreased in myocardial injury induced by LPS in rats [8]. The data presented here clearly indicated how biochemical markers of the heart and aorta may be affected by the alteration in oxidative and anti-oxidative balance in the body following LPS administration. Additionally, LPS has been reported to have important roles in heart failure [44]. Researchers showed that LPS increased serum MDA and NO levels and decreased antioxidant enzymes activity [45].
It has been well known that the antioxidant and anti-inflammatory agents are useful for CVD. So polyphenols like curcumin are suggested [17]. Curcumin has been reported to have therapeutic potential for several chronic inflammatory diseases, essentially its anti-oxidative and anti-inflammatory properties against a huge of molecular targets [27]. Our results showed that the medium and the highest doses of curcumin decreased the WBC count to reach to the normal range but the lowest dose was not effective. Considering these results it seems that effect of curcumin on WBC was dose dependent and WBC count in the rats treated by 10 and 15 mg/kg was lower than that in ones treated by 5 mg/kg. This makes us realize that curcumin reduces inflammation in the body. In confirm with our results, another study showed that curcumin decreased WBC count elevation in collagen-induced arthritis and decreased the inflammation in this study [46].
Another mechanism studied for the effects of curcumin on the cardiovascular system is its effects on oxidative stress damage. The capacity of curcumin to prevent lipid peroxidation has been shown in many studies, a crucial factor in the development and progression of many diseases [47]. Curcumin's ability to stabilize the membrane was also documented [48]. In vitro, through activated macrophages, which play an important role in inflammation, curcumin significantly inhibited ROS generation such as superoxide anions, H2O2, and radical nitrite generation [49]. In addition, Venkatesan et al. observed a protective effect of curcumin against adriamycin-produced cardiotoxicity in rats, showing a reduction in parameters indicating lipid peroxidation [50]. Also, the results of our study showed that treatment with curcumin reduced the subsequent LPS damage in rat serum, heart and aorta tissues, demonstrated by a decreased level in lipid peroxidation (reduced MDA), an increased level in total thiol concentration and improvement in antioxidant enzyme status (increased activity of SOD and CAT). According to the results, the highest dose of curcumin had the best effect and it might be suggested that the effect of curcumin on oxidative stress indicators was dose dependent. In disease associated with increased oxidative stress, pharmacological increase of endogenous antioxidants has been established as a promising therapeutic strategy [51]. In the event of increased free radical generation, an increase in SOD activity has been reported to be beneficial [31]. Nonetheless, an increase in catalase activity at the same time is important for an overall beneficial effect of an increase in SOD activity [52]. Protection may have been mediated by an increase in basal myocardial endogenous antioxidants induced by curcumin. Rao et al. [53] argued that the presence of phenolic groups in curcumin structure is important in understanding its ability to remove oxygen-derived free radicals from the medium that is largely responsible for cell lipid peroxidation. Ruby et al. reported it in 1995 while studying the antitumor and antioxidant activity of natural curcuminoids that inhibits curcumin in the production of superoxide radicals [54]. Our findings also corroborate the above findings. Other effect of curcumin on heart is modulation of vascular dysfunction and oxidative stress.
The results of the graphs show that in general, the level of MDA in heart and aortic tissues as well as in serum in curcumin-treated groups was decreased. The graphs also showed that the amount of total thiol groups as well as the activity of SOD and CAT enzymes in curcumin-treated groups was increased. However, due to the difference in the unit of measurement in tissues and serum, it is possible to make an accurate comparison to determine in which tissue curcumin has acted more effectively.
In this study, functional changes in cardiovascular tissue were not investigated. However, considering the oxidative damage and inflammation, it can be concluded that these can lead to CVD. Finally, it is proposed that other precise experiments at cellular and molecular levels should be carried out to explain the exact mechanism(s). Also it is suggested to examine inflammatory cytokines and the related signaling pathways.
Chronic inflammation has been shown to cause cardiovascular disease, and curcumin can prevent these diseases [55]. The results of this study can be examined in the people who suffer from cardiovascular dysfunction as a result of systemic inflammation. Curcumin with its antioxidant and anti-inflammatory properties can prevent these side effects however; it needs to be more investigated.
5. Conclusion
The results of current study showed that curcumin had protective effect against cardiovascular oxidative stress in a rat model of systemic inflammation induced by LPS. Considering these results, the beneficial effects of curcumin on cardiovascular disorders which are accompanied with oxidative stress and inflammatory conditions is suggested however, it needs to be more investigated.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the Vice Presidency of Research of Mashhad University of Medical Sciences for their financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100908.
Author statement
Somaieh Ahmadabady: Analysis and/or interpretation of data.
Farimah Beheshti: Drafting the manuscript, revising the manuscript critically for important intellectual content.
Mahmoud Hosseini: Conception and design of study.
Elnaz Khordad: Acquisition of data.
Fatemeh Shahidpour: Drafting the manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Duvall W.L., Vorchheimer D.A. Multi-bed vascular disease and atherothrombosis: scope of the problem. J. Thromb. Thrombolysis. 2004;17(1):51–61. doi: 10.1023/B:THRO.0000036029.56317.d1. [DOI] [PubMed] [Google Scholar]
- 2.Sin D.D., Man S.P. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 3.Ge Y., Van Eyk J. Cardiovascular disease: the leap towards translational and clinical proteomics. Proteonomics Clin. Appl. 2014;8(7–8):473–475. doi: 10.1002/prca.201470044. [DOI] [PubMed] [Google Scholar]
- 4.Schraufstatter I., Hyslop P.A., Jackson J.H., Cochrane C.G. Oxidant-induced DNA damage of target cells. J. Clin. Investig. 1988;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Canc. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 6.Guzik T.J., Touyz R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 7.Ito T., Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr. Drug Targets - Inflamm. Allergy. 2003;2(3):257–265. doi: 10.2174/1568010033484106. [DOI] [PubMed] [Google Scholar]
- 8.Goraca A., Piechota A., Huk-Kolega H. Effect of alpha--Lipoic acid on LPS-induced oxidative stress in the heart. Acta Physiol. Pol. 2009;60(1):61. [PubMed] [Google Scholar]
- 9.Ronco C. Lipopolysaccharide (LPS) from the cellular wall of Gram-negative bacteria, also known as endotoxin, is a key molecule in the pathogenesis of sepsis and septic shock. Preface. Blood purification. 2014;37:1. doi: 10.1159/000357412. [DOI] [PubMed] [Google Scholar]
- 10.Suliman H.B., Welty-Wolf K.E., Carraway M., Tatro L., Piantadosi C.A. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc. Res. 2004;64(2):279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X.-P., Zhang J., Ma M.-L., Cai Y., Xu R.-J., Xie Q. Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis. Hepatobiliary Pancreat. Dis. Int.: HBPD INT. 2010;9(1):83–87. [PubMed] [Google Scholar]
- 12.Asci H., Ozmen O., Erzurumlu Y., Sofu A., Icten P., Kaynak M. Agomelatine protects heart and aorta against lipopolysaccharide-induced cardiovascular toxicity via inhibition of NF-kβ phosphorylation. Drug Chem. Toxicol. 2019:1–10. doi: 10.1080/01480545.2019.1663209. [DOI] [PubMed] [Google Scholar]
- 13.Su Q., Yao J., Sheng C. Geniposide attenuates LPS-induced injury via up-regulation of miR-145 in H9c2 cells. Inflammation. 2018;41(4):1229–1237. doi: 10.1007/s10753-018-0769-8. [DOI] [PubMed] [Google Scholar]
- 14.Tanguy S., de Leiris J., Besse S., Boucher F. Ageing exacerbates the cardiotoxicity of hydrogen peroxide through the Fenton reaction in rats. Mech. Age. Dev. 2003;124(2):229–235. doi: 10.1016/s0047-6374(02)00185-9. [DOI] [PubMed] [Google Scholar]
- 15.Sudano I., Spieker L.E., Hermann F., Flammer A., Corti R., Noll G. Protection of endothelial function: targets for nutritional and pharmacological interventions. J. Cardiovasc. Pharmacol. 2006;47(2):S136–S150. doi: 10.1097/00005344-200606001-00008. discussion S72-6. [DOI] [PubMed] [Google Scholar]
- 16.Takano H., Zou Y., Hasegawa H., Akazawa H., Nagai T., Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxidants Redox Signal. 2003;5(6):789–794. doi: 10.1089/152308603770380098. [DOI] [PubMed] [Google Scholar]
- 17.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr. Opin. Lipidol. 2005;16(1):47–54. doi: 10.1097/00041433-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Alkhalaf M.I., Hussein R.H., Hamza A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J. Biol. Sci. 2020;27(9):2410–2419. doi: 10.1016/j.sjbs.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yildiz S., Turan S., Kiralan M., Ramadan M.F. Antioxidant properties of thymol, carvacrol, and thymoquinone and its efficiencies on the stabilization of refined and stripped corn oils. J. Food Meas. Char. 2020:1–12. [Google Scholar]
- 20.Forouzanfar F., Asadpour E., Hosseinzadeh H., Boroushaki M.T., Adab A., Dastpeiman S.H. Safranal protects against ischemia-induced PC12 cell injury through inhibiting oxidative stress and apoptosis. N. Schmied. Arch. Pharmacol. 2020:1–10. doi: 10.1007/s00210-020-01999-8. [DOI] [PubMed] [Google Scholar]
- 21.Yang F., Cho W.-Y., Lee N., Kim D.-H., Lee J., Lee H.-J. Effects of Boswellia serrata and whey protein powders on physicochemical properties of pork patties. Foods. 2020;9(3):334. doi: 10.3390/foods9030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teiten M.H., Dicato M., Diederich M. Hybrid curcumin compounds: a new strategy for cancer treatment. Molecules. 2014;19(12):20839–20863. doi: 10.3390/molecules191220839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaikert R., Paisooksantivatana Y. Variation of total curcuminoids content, antioxidant activity and genetic diversity in turmeric (Curcuma longa L.) collections. Kasetsart J./Nat. Sci. 2009;43(3):507–518. [Google Scholar]
- 24.Xiao L., Ding M., Fernandez A., Zhao P., Jin L., Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur. Cell. Mater. 2017;33:279–293. doi: 10.22203/eCM.v033a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal R., Sandhu S.K., Sharma I., Kaur I.P. Development and evaluation of curcumin-loaded elastic vesicles as an effective topical anti-inflammatory formulation. AAPS PharmSciTech. 2015;16(2):364–374. doi: 10.1208/s12249-014-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 27.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman M.M., Nassan M.A., Ismail T.A. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Compl. Alternative Med. 2014;14(1):457. doi: 10.1186/1472-6882-14-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz K., Dobiasch S., Nguyen L., Schilling D., Combs S.E. Modification of radiosensitivity by Curcumin in human pancreatic cancer cell lines. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-60765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farhangkhoee H., Khan Z.A., Chen S., Chakrabarti S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr. Metabol. 2006;3(1):27. doi: 10.1186/1743-7075-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen H.-C., Oberley T.D., Vichitbandha S., Ho Y.-S., St Clair D. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J. Clin. Investig. 1996;98(5):1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eftekhar N., Moghimi A., Hossein Boskabady M., Kaveh M., Shakeri F. Ocimum basilicum affects tracheal responsiveness, lung inflammatory cells and oxidant–antioxidant biomarkers in sensitized rats. Drug Chem. Toxicol. 2019;42(3):286–294. doi: 10.1080/01480545.2018.1459672. [DOI] [PubMed] [Google Scholar]
- 33.Madesh M., Balasubramanian K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J. Biochem. Biophys. 1998;35(3):184–188. [PubMed] [Google Scholar]
- 34.Auvin S., Shin D., Mazarati A., Sankar R. Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia. 2010;51:34–38. doi: 10.1111/j.1528-1167.2010.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boskabadi J., Mokhtari-Zaer A., Abareshi A., Khazdair M.R., Emami B., Mohammadian Roshan N. The effect of captopril on lipopolysaccharide-induced lung inflammation. Exp. Lung Res. 2018;44(4–5):191–200. doi: 10.1080/01902148.2018.1473530. [DOI] [PubMed] [Google Scholar]
- 36.Bhat T., Teli S., Rijal J., Bhat H., Raza M., Khoueiry G. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expet Rev. Cardiovasc. Ther. 2013;11(1):55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 37.Karthikeyan V., Lip G. White blood cell count and hypertension. J. Hum. Hypertens. 2006;20(5):310–312. doi: 10.1038/sj.jhh.1001980. [DOI] [PubMed] [Google Scholar]
- 38.Thewlis E.W., Meyer O.O. The blood count of normal white rats. Anat. Rec. 1942;82(1):115–125. [Google Scholar]
- 39.Toyoda S., Haruyama A., Inami S., Arikawa T., Saito F., Watanabe R. Effects of carvedilol vs bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J. Cardiol. 2020;75(2):140–147. doi: 10.1016/j.jjcc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. 2016. Oxidative Stress and Inflammation: what Polyphenols Can Do for Us? Oxidative Medicine and Cellular Longevity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds A., Laurie C., Mosley R.L., Gendelman H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 42.Ambrosio G., Flaherty J.T., Duilio C., Tritto I., Santoro G., Elia P. Oxygen radicals generated at reflow induce peroxidation of membrane lipids in reperfused hearts. J. Clin. Investig. 1991;87(6):2056–2066. doi: 10.1172/JCI115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sewerynek E., Melchiorri D., Chen L., Reiter R.J. Melatonin reduces both basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free Radic. Biol. Med. 1995;19(6):903–909. doi: 10.1016/0891-5849(95)00101-3. [DOI] [PubMed] [Google Scholar]
- 44.Sandek A., Anker S.D., Sv Haehling. The gut and intestinal bacteria in chronic heart failure. Curr. Drug Metabol. 2009;10(1):22–28. doi: 10.2174/138920009787048374. [DOI] [PubMed] [Google Scholar]
- 45.Ogetman Z., Dirlik M., Caglikulekci M., Canbaz H., Karabacak T., Yaylak F. The effect of aminoguanidine on blood and tissue lipid peroxidation in jaundiced rats with endotoxemia induced with LPS. J. Invest. Surg. 2006;19(1):19–30. doi: 10.1080/08941930500444396. [DOI] [PubMed] [Google Scholar]
- 46.Kamarudin T.A., Othman F., Ramli E.S.M., Isa N.M., Das S. Protective effect of curcumin on experimentally induced arthritic rats: detailed histopathological study of the joints and white blood cell count. EXCLI J. 2012;11:226. [PMC free article] [PubMed] [Google Scholar]
- 47.Farombi E., Ekor M. Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food Chem. Toxicol. 2006;44(9):1443–1448. doi: 10.1016/j.fct.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 1999;66(2):PL21–P28. doi: 10.1016/s0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 49.Joe B., Lokesh B. Role of capsaicin, curcumin and dietary n—3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta Mol. Cell Res. 1994;1224(2):255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 50.Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br. J. Pharmacol. 1998;124(3):425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siveski-Iliskovic N., Hill M., Chow D., Singal P. Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation. 1995;91(1):10–15. doi: 10.1161/01.cir.91.1.10. [DOI] [PubMed] [Google Scholar]
- 52.Engelman D.T., Watanabe M., Engelman R.M., Rousou J.A., Kisin E., Kagan V.E. Hypoxic preconditioning preserves antioxidant reserve in the working rat heart. Cardiovasc. Res. 1995;29(1):133–140. [PubMed] [Google Scholar]
- 53.Rao M. Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharm. Pharmacol. 1994;46(12):1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 54.Ruby A.J., Kuttan G., Babu K.D., Rajasekharan K., Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Canc. Lett. 1995;94(1):79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 55.White C.M., Pasupuleti V., Roman Y.M., Li Y., Hernandez A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.