Abstract
The purpose of this investigation was to identify if serum interleukin (IL)-10 and tumor necrosis factor (TNF)-α concentrations and their ratio (IL-10/TNF-α) are altered in subjects predisposed to developing knee osteoarthritis following ligamentous injury and in those with severe knee osteoarthritis. Serum IL-10 and TNF-α concentrations were measured in four groups of subjects (n = 218): (1) reportedly-healthy and non-injured control subjects (CON; n = 92), (2) subjects scheduled to undergo anterior cruciate ligament surgery (ACL; n = 42), (3) non-surgical subjects with knee osteoarthritis (OA; n = 60), and (4) subjects with knee osteoarthritis scheduled to undergo total knee arthroplasty (TKA; n = 24). X-ray images were used to grade the severity of knee osteoarthritis. Serum IL-10 and the serum IL-10/TNF-α ratio were significantly lower while serum TNF-α was not significantly perturbed with severe compared to moderate knee osteoarthritis (i.e., Kellgren-Lawrence grade 4 vs. 3, respectively). Serum IL-10 was significantly lower in the absence of serum TNF-α alterations in the ACL group. We conclude that serum IL-10 concentrations are compromised in subjects predisposed to developing knee osteoarthritis following ligamentous trauma and in subjects with radiographic evidence of severe knee osteoarthritis.
Subject terms: Physiology, Health care
Introduction
Cytokines are pleiotropic and multifunctional signaling molecules that regulate innate and adaptive immunities, inflammation, and hematopoiesis. Of the various cytokines, tumor necrosis factor (TNF)-α is a prototypical pro-inflammatory cytokine and interleukin (IL)-10 is a quintessential anti-inflammatory cytokine that reciprocally down-regulates pro-inflammatory cytokine production. Following one of the most commonly injured ligaments in the knee, the anterior cruciate ligament, reports indicate a local (i.e., synovial fluid) increase in a variety of cytokines1–4, including IL-10 and TNF-α5–10. Although results are inconsistent11, IL-10 and/or TNF-α may be influential on the development of osteoarthritis12,13.
The local increase in IL-10 appears to be transient5 while the increase in TNF-α may persist years after an anterior cruciate ligament injury12, thereby suggesting a temporal shift in the anti- to pro-inflammatory cytokine balance in favor of the latter. The TNF-α increase after an anterior cruciate ligament injury associates with the proteolysis of aggrecan and type II collagen12, and thus, could contribute to trauma-induced joint degradation as found in a porcine model14. With the exception of data regarding serum IL-63, studies identifying if IL-10 and TNF-α concentrations or their concentration balance are perturbed in the circulation following an anterior cruciate ligament injury compared to non-injured control subjects and to those with degenerative knee joint disease are surprisingly sparse12,15 or lacking, respectively.
Knee osteoarthritis is a degenerative joint condition, a leading cause of disability in the United States16, and is characterized by low-grade inflammation17,18. The localized presence of TNF-α could increase the risk of osteophyte progression, joint space narrowing19, cartilage degradation20, and lower IL-10 expression21. IL-10 decreases TNF-α production and TNF-α mediated events associated with osteoarthritis development22–25, thereby suggesting the pathophysiological importance of a regulated anti- and pro-inflammatory cytokine balance on joint integrity. Although an increased production of IL-10 and TNF-α from whole blood associates with the progression in knee osteoarthritis26, and preliminary results indicate that local and circulating cytokine concentrations decrease with severe compared to moderate knee osteoarthritis27,28, it is unknown if the circulating pro- to anti-inflammatory cytokine balance (i.e., ratio) is altered with the radiographic severity in knee osteoarthritis. In septic patients, the IL-10/TNF-α ratio has been previously suggested to serve as an anti-inflammatory index29.
The purpose of this investigation was to identify if serum IL-10 and TNF-α concentrations and their ratio (i.e., IL-10/TNF-α) are altered in subjects predisposed to developing knee osteoarthritis following ligamentous trauma and in those with severe knee osteoarthritis. We hypothesized that serum IL-10 and TNF-α concentrations are higher following an anterior cruciate ligament injury and that the serum IL-10/TNF-α concentration ratio is lower with severe knee osteoarthritis. The anticipated findings could advance our understanding of systemic cytokine alterations following ligamentous trauma in the knee and with severe knee osteoarthritis, both of which, are characterized by inflammatory-driven processes that contribute to localized pathophysiology.
Methods
Serum cytokine concentrations were measured in four groups of subjects (n = 218): (1) reportedly-healthy and non-injured control subjects (CON; n = 92; ≥ 18 years), (2) subjects scheduled to undergo primary, unilateral anterior cruciate ligament surgery (ACL; n = 42; 18–45 years), (3) non-surgical subjects with unilateral knee osteoarthritis (OA; n = 60; 18–60 years), and (4) subjects with end-stage knee osteoarthritis scheduled to undergo a primary, unilateral total knee arthroplasty (TKA; n = 24; ≥ 18 years). Baseline (i.e., prior to intervention in previous studies, where applicable) data from some of the CON30,31, ACL9,32, and OA33,34 subjects have been previously published. All subjects were informed of and provided written and verbal consent to the study protocols and procedures. This study was approved by the Institutional Review Board at Intermountain Healthcare (Salt Lake City, UT USA). All experiments were performed in accordance with relevant guidelines and regulations.
All subjects were recruited from the physician clinics and other clinical departments at The Orthopedic Specialty Hospital (Murray, UT USA). For all study groups, modestly active (i.e., 30 min of continuous physical activity ≥ 3 times per week) subjects were recruited for study participation. To minimize the presence of co-morbidities, potential CON, ACL, OA, and TKA subjects were excluded from participation if they had a history of metabolic bone disease, skeletal muscle pathology, cardiac or peripheral cardiovascular system abnormality, clotting disorder, coronary artery disease, peripheral vascular disease, stroke, cancer, high cholesterol or triglycerides, or high blood pressure. Severely obese [body mass index (BMI) > 40 kg/m2], smokers, or subjects that suffered a leg injury during the previous year that required the use of crutches (with the exception to the ligamentous injury sustained in the ACL group subjects) for more than 1-week in the past year were excluded from study participation as well. Potential subjects were also excluded from participation if they were using warfarin or other anti-coagulants, cholesterol lowering medication, corticosteroid medication, orlistat, phenobarbital, phenytoin, or thiazide, or a daily dietary supplement or vitamin during the previous year, diagnosed with diabetes mellitus, impaired liver or kidney function, or pregnant. Also, no subjects were currently receiving any physician-prescribed or -guided medications based on an underlying condition upon study enrollment, including intra-articular injections.
In addition to those above, other condition specific inclusion and exclusion criteria were implemented. In the OA group, subjects were excluded from participation if they had a recent (within 2 years) surgery on the involved or non-involved leg, planning on undergoing surgery to treat knee osteoarthritis, or received any osteoarthritis specific medication or treatment prior to enrollment. To be included in the OA groups, subjects also had to possess: (1) a Western Ontario and McMaster Universities Osteoarthritis Index pain score ≥ 2 on all of the five questions in its subsection, (2) evidence of muscular weakness (i.e., deficit in peak isokinetic concentric knee extension or flexion torque at 60°/s) in the involved leg compared to the non-involved leg, and (3) a Kellgren–Lawrence (KL) grade ≥ 2 was scored in the involved knee. Therefore, the OA group consisted of subjects with signs and symptoms of unilateral knee osteoarthritis (i.e., pain, muscular weakness, and radiographic evidence of mild-to-severe knee osteoarthritis in the involved leg) that were not scheduled or planning to undergo total knee arthroplasty or any other knee surgery in the foreseeable future. In the TKA group, subjects were excluded from participation if they had a recent (within 2 years) surgery on the involved or non-involved leg, experiencing bilateral symptoms of tibiofemoral osteoarthritis, or planning on undergoing elective surgery on the non-involved leg in the foreseeable future.
Study protocol
Serum cytokine data from a fasting (10–12 h) blood draw sample obtained at baseline, and if applicable, prior to any surgical procedures (i.e., ACL or TKA) or study interventions (e.g., vitamin D or placebo), were aggregated for this investigation. Subjects were instructed to refrain from exercise and using aspirin, ibuprofen, naproxen sodium, acetaminophen, or other anti-inflammatory agents 72-h prior to providing a fasting blood draw sample. After collection and coagulation, samples were centrifuged at 1100 g for 10 min and aliquoted into several small cryovials. Aliquots were stored at − 80° C until analysis.
Analytical procedures
Serum cytokines
Serum cytokine concentrations were determined using the multiplex technology of Luminex (MAGPix, Austin, TX USA) with high sensitivity (EMD Millipore, Billerica, MA USA; catalog #: HSCYMAG60SPMX13) at The Orthopedic Specialty Hospital (Murray, UT USA). Inter-assay precision is < 15% and intra-assay precision is < 10%. The minimum detectable concentration is 0.56 pg/mL and 0.16 pg/mL for IL-10 and TNF-α, respectively. Based on the objectives and hypotheses of this study, we only examined IL-10 and TNF-α from the high sensitivity panel.
Radiographic evidence of knee OA
Weight bearing X-ray images were obtained on each knee in the anterior–posterior view at 45° of knee flexion. Knee joint space was analyzed using ImageJ software (National Institutes of Health). Severity of knee OA was classified according to the KL scoring criteria: 0, no osteophytes or joint-space narrowing; 1, questionable osteophyte indicating possible OA; 2, definite osteophyte, no joint-narrowing (compared to the non-symptomatic knee) indicating mild OA; 3, ≤ 50% joint-space narrowing (compared to the non-symptomatic knee) indicating moderate OA; and 4, > 50% joint-space narrowing (compared to the non-symptomatic knee) indicating severe OA35. X-ray images were part of the previous study protocols or as a standard of care procedure in the OA and TKA groups but not in the CON and ACL groups. Therefore, radiographic images were not available for all the subjects in the CON and ACL groups (see Table 1).
Table 1.
Subject characteristics.
| CON | ACL | OA | TKA | p value | |
|---|---|---|---|---|---|
| n (m/f) | 92 (63/29) | 42 (25/17) | 60 (24/36) | 24 (9/15) | < 0.01 |
| Age (y) | 32 (13) | 33 (12) | 52 (13)a,b | 64 (7)a,b,c | < 0.01 |
| Height (cm) | 174 (14) | 173 (15) | 168 (14)a | 163 (15)a | < 0.01 |
| Body mass (kg) | 78.2 (21.6) | 84.4 (22.9) | 90.8 (32.4)a | 93.1 (35.6)a | < 0.01 |
| BMI (kg/m2) | 25.4 (4.4) | 28.0 (6.5) | 31.6 (8.6)a,b | 32.2 (10.7)a,b | < 0.01 |
| KL grade | < 0.01 | ||||
| 0 (n = 25) | 10 (10.9%) | 15 (35.7%) | 0 | 0 | |
| 1 (n = 18) | 3 (3.3%) | 15 (35.7%) | 0 | 0 | |
| 2 (n = 8) | – | 3 (7.1%) | 5 (8.3%) | 0 | |
| 3 (n = 49) | – | – | 41 (68.3%) | 8 (33.3%) | |
| 4 (n = 30) | – | – | 14 (23.3%) | 16 (66.7%) | |
| n (%) | 13 (14.1%) | 33 (78.6%) | 60 (100%) | 24 (100%) | |
| Days from injury (d) | NA | 17.5 (37.0) | NA | NA |
Data presented as median (interquartile range). ap < 0.05 versus CON; bp < 0.05 versus ACL; cp < 0.05 versus OA. NA not applicable; – not available.
Statistical analysis
Data were checked for normality with a Shapiro–Wilk test prior to statistical analysis. When necessary, rank transformations were performed on data to achieve normality before analysis. Statistical significance of data between groups were assessed with an analysis of variance (ANOVA) and followed by a Tukey test on multiple pairwise comparisons when appropriate. Group associations with categorical variables were assessed with separate Pearson Chi-Square tests. A Pearson Product moment linear correlation was performed to examine the association between variables. Age, gender, and BMI were used as covariates. Significance was set at p < 0.05. All statistical analyses were performed with SYSTAT (version 13.1, Chicago, IL USA).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Intermountain Healthcare (Salt Lake City, UT USA).
Results
Subject characteristics
Subject characteristics were not significantly different between the CON and ACL groups, and except for age (p < 0.05), not significantly different between the OA and TKA groups (Table 1). Age, body mass, and BMI (all p < 0.05) were higher and height (p < 0.05) was lower in the OA and TKA compared to the CON and/or ACL groups. Radiographic images indicate that the severity in knee osteoarthritis was greater (p < 0.05) in the TKA compared to the CON, ACL, and OA groups. Of note, ~ 68% in the OA and ~ 33% in the TKA group possessed moderate knee osteoarthritis (i.e., KL grade of 3), while conversely, ~ 23% in the OA and ~ 67% in the TKA group displayed severe knee osteoarthritis (i.e., KL grade of 4; see Table 1).
Serum cytokine concentrations as a function of concomitant injuries with an anterior cruciate ligament rupture
Additional trauma or damage to the surrounding knee tissue concurrently with a ruptured anterior cruciate ligament increases localized cytokine concentrations8. In the present study, however, serum IL-10 and TNF-α concentrations were not significantly different with compared to without concomitant damage to the localized surrounding tissue found and repaired during arthroscopic surgery in the ACL group (Table 2), a finding consistent with a prior investigation performed in synovial fluid36. Therefore, we subsequently combined those with and without additional knee joint trauma in the ACL group. Additionally, some circulating and localized cytokine concentrations are dependent on the time from anterior cruciate ligament injury occurrence5,6,9. Contrasting with previous data5,6,9, serum IL-10 (r = − 0.01, p = 0.94, data not shown) and TNF-α (r = 0.09, p = 0.57, data not shown) concentrations were not correlated with the days from anterior cruciate ligament injury occurrence (range 1–348 days).
Table 2.
Serum IL-10 and TNF-α concentrations with and without additional surgical procedures performed during ACL reconstructive surgery.
| Without | With | p value | |
|---|---|---|---|
| n | 22 | 20 | 0.76 |
| Additional surgical procedures (n) | 0 | 20 | |
| Lateral meniscus repair (n) | 3 | ||
| Medial meniscus repair (n) | 6 | ||
| Partial lateral meniscectomy (n) | 4 | ||
| Partial medial meniscectomy (n) | 6 | ||
| Posterior oblique ligament repair (n) | 1 | ||
| Serum IL-10 (pg/mL) | 6.14 (9.49) | 7.80 (25.3) | 0.19 |
| Serum TNF-α (pg/mL) | 4.80 (5.14) | 4.76 (9.21) | 0.64 |
Data presented as median (interquartile range).
Serum cytokine concentrations as a function of group (CON, ACL, OA, and TKA)
A primary outcome of this investigation was the difference in serum IL-10 and TNF-α concentrations and the IL-10/TNF-α ratio between the ACL, CON, and OA groups. However, since the investigation also included subjects undergoing knee arthroplasty, we included a TKA group in the comparisons. Serum IL-10 concentrations were not significantly different between the CON and OA groups (Fig. 1A), but concentrations were lower in the ACL (p < 0.05 vs. CON and OA) and TKA (p < 0.05 vs. OA) groups. Serum TNF-α concentrations were lower (p < 0.05) in the OA compared to the CON group (Fig. 1B). The serum IL-10/TNF-α ratio was significantly higher in the OA compared to the CON, ACL, and TKA groups (Fig. 1C).
Figure 1.
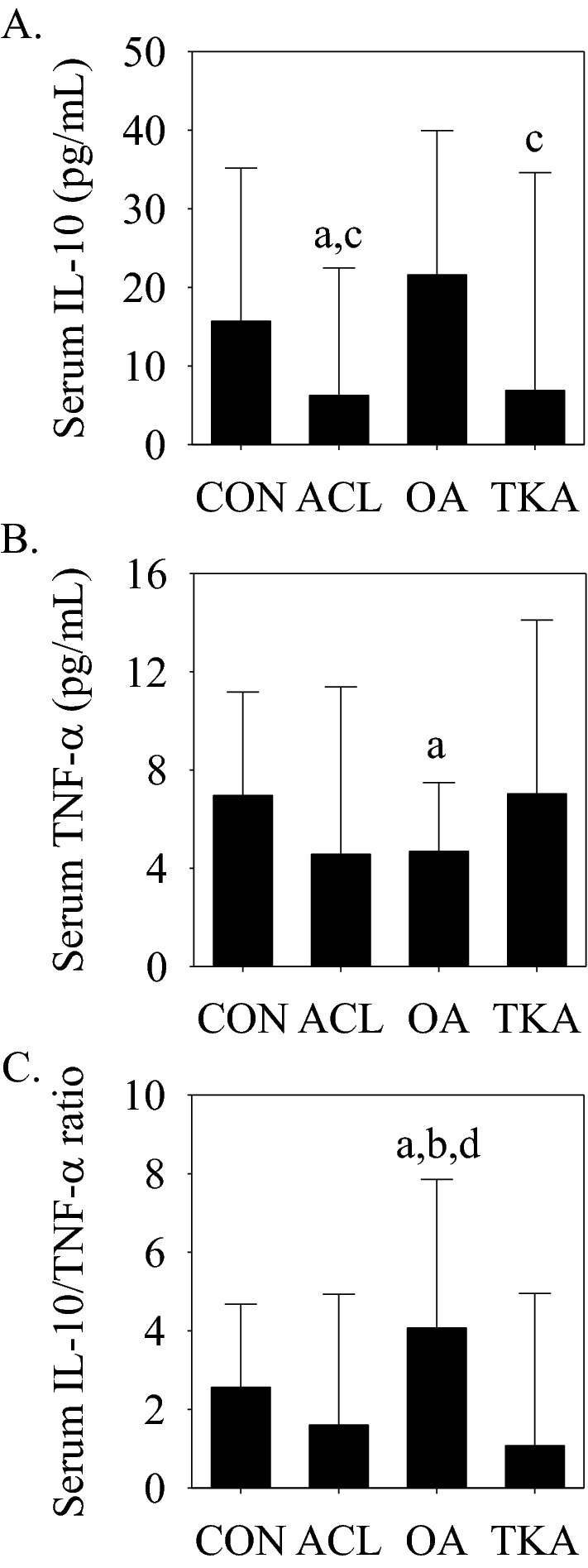
Serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio in the CON, ACL, OA, and TKA groups. (A) Serum IL-10 concentrations were significantly lower in the ACL (ap < 0.05 vs. CON and cp < 0.05 vs. OA) and TKA (cp < 0.05 vs. OA) groups. (B) Serum TNF-α concentrations were significantly (ap < 0.05 vs. CON) lower in the OA group. (C) The serum IL-10/TNF-α ratio was significantly (ap < 0.05 vs. CON, bp < 0.05 vs. ACL, and dp < 0.05 vs. TKA) higher in the OA group. Data presented as median (interquartile range).
Serum cytokine concentrations with (KL ≥ 3) and without (KL < 3) joint space narrowing
To extend those previously26,37–39, serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio were compared between subjects with (i.e., KL grade ≥ 3 n = 79) and without (i.e., KL grade < 3, n = 51) radiographic evidence of joint space narrowing and independent from their initial classifications (i.e., CON, ACL, OA, or TKA). Serum IL-10 (Fig. 2A) and TNF-α (Fig. 2B) concentrations were not significantly different between KL grade groups (KL grade < 3 vs. ≥ 3), but the serum IL-10/TNF-α ratio was significantly higher in subjects with evidence of joint space narrowing (i.e., KL ≥ 3; Fig. 2C).
Figure 2.
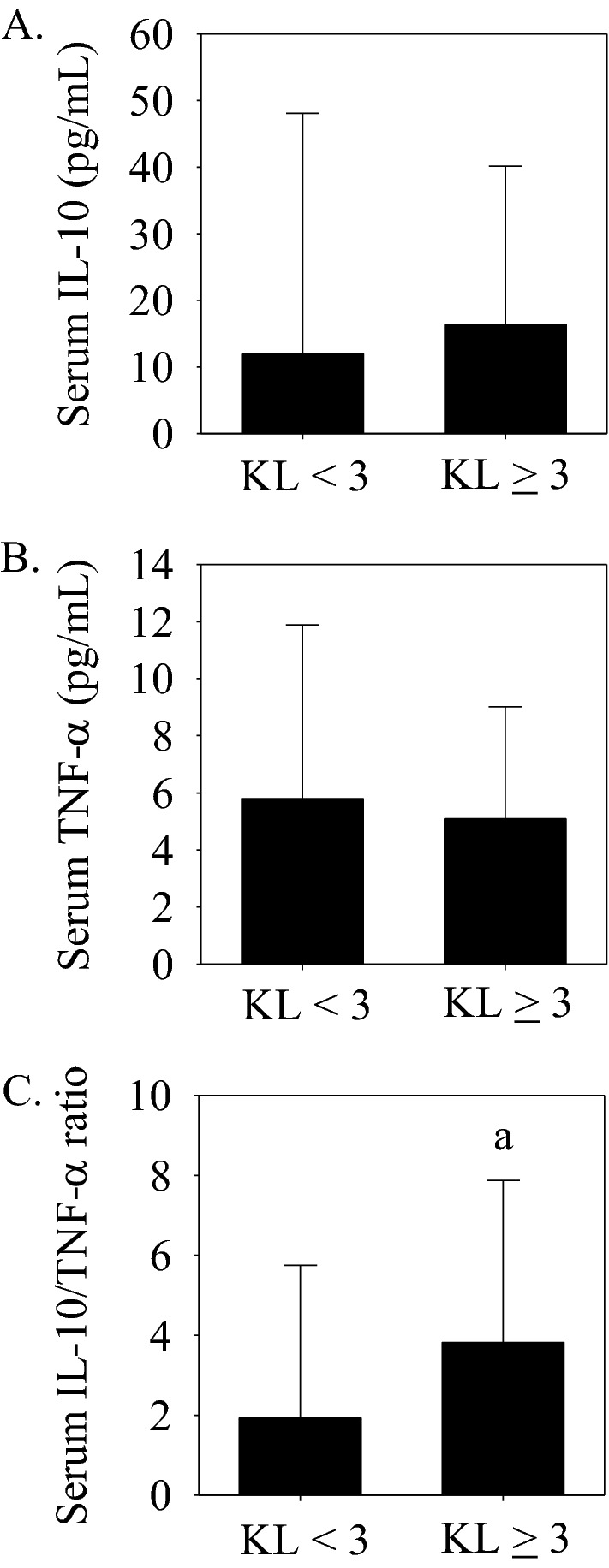
Serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio without (KL grade < 3, n = 51) and with (KL grade ≥ 3, n = 79) radiographic evidence of knee joint space narrowing. (A) Serum IL-10 and (B) Serum TNF-α concentrations were not significantly different between groups. (C) The serum IL-10/TNF-α ratio was significantly (ap < 0.05 vs. KL grade < 3) higher in the KL grade ≥ 3 group. Data presented as median (interquartile range).
Serum cytokine concentrations with moderate (KL = 3) compared to severe (KL = 4) knee joint space narrowing
Another primary outcome of this study was the comparison of serum IL-10 and TNF-α concentrations and the IL-10/TNF-α ratio between moderate (i.e., KL = 3, ≤ 50% joint-space narrowing; n = 49) and severe (i.e., KL = 4, > 50% joint space narrowing; n = 30) knee joint space narrowing. Serum IL-10 concentrations and the serum IL-10/TNF-α ratio were lower and serum TNF-α was not significantly different with a KL grade of 4 compared to a grade 3 (Fig. 3A–C), implying a compromise in the circulating anti-inflammatory status with severe knee osteoarthritis.
Figure 3.
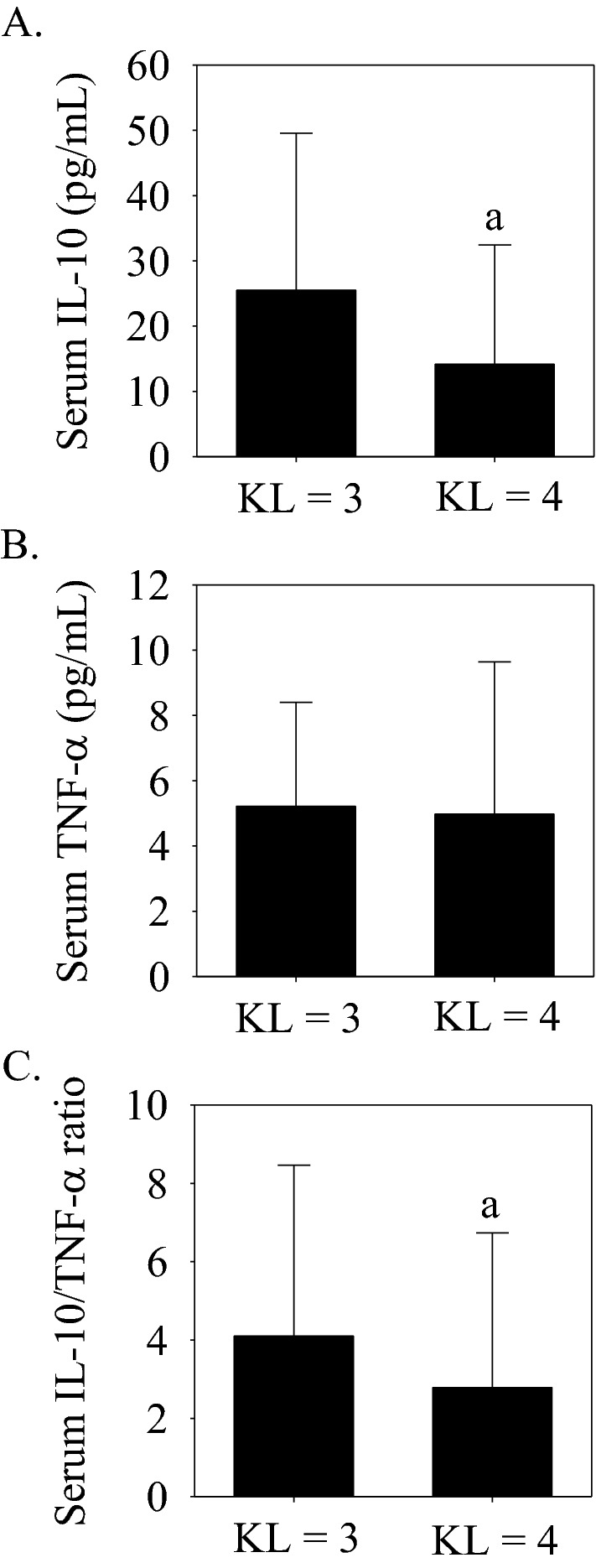
Serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio with a KL grade = 3 (moderate knee osteoarthritis, ≤ 50% joint space narrowing, n = 49) compared to a KL grade = 4 (severe knee osteoarthritis, > 50% joint space narrowing, n = 30). (A) Serum IL-10 was significantly (ap < 0.05 vs. KL grade = 3) lower with a KL grade = 4. (B) Serum TNF-α concentrations were not significantly different between groups. (C) The serum IL-10/TNF-α ratio was significantly (ap < 0.05 vs. KL grade = 3) lower with a KL grade = 4. Data presented as median (interquartile range).
Correlations
As a group (n = 218), age, height, body mass and BMI correlated (Table 3). However, age, height, body mass and BMI were not significantly correlated with serum IL-10 or TNF-α. Thus, although there were discrepancies in subject characteristics (such as gender, age, and BMI) between the CON, ACL, OA, and TKA groups, serum cytokines did not correlate with subject characteristics. TNF-α correlated (p < 0.05) with IL-10 as a group (n = 218; Table 3) and in each group separately (Table 4).
Table 3.
Group correlation coefficients.
| Age | Height | Mass | BMI | IL-10 | |
|---|---|---|---|---|---|
| Height | − 0.25a | ||||
| Mass | 0.32a | 0.43a | |||
| BMI | 0.51a | − 0.08 | 0.84a | ||
| IL-10 | 0.08 | 0.10 | 0.10 | 0.04 | |
| TNF-α | − 0.01 | 0.16 | 0.12 | 0.02 | 0.44a |
ap < 0.05, n = 218; Mass, body mass.
Table 4.
Cytokine correlation coefficients in each group.
| IL-10 | |
|---|---|
| CON (n = 92) | |
| TNF-α | 0.47a |
| ACL (n = 42) | |
| TNF-α | 0.49a |
| OA (n = 60) | |
| TNF-α | 0.53a |
| TKA (n = 24) | |
| TNF-α | 0.52b |
ap < 0.05; bp = 0.05.
Discussion
In this investigation, we provide original evidence that the circulating concentration of an anti-inflammatory cytokine is compromised following an anterior cruciate ligament injury. Despite unperturbed individual concentrations, the circulating pro- to -anti-inflammatory cytokine ratio shifted toward the latter with radiographic evidence of joint space narrowing knee osteoarthritis. However, upon further examination and delineation in osteoarthritic phenotypes (i.e., moderate vs. severe), this report illustrates a lower anti-inflammatory cytokine and IL-10/TNF-α ratio in the absence of a robust pro-inflammatory cytokine deviation in the circulation with severe compared to moderate knee osteoarthritis.
Lower circulating IL-10 corresponds with higher joint effusion of TNF-α, localized and systemic indices of cartilage degeneration, and greater histological evidence of joint osteoarthritis following anterior cruciate ligament transection in experimental rabbits40. In humans, a lower serum IL-10 is arguably mirrored by a decrease in synovial fluid IL-1012,41, which in turn, could compromise joint integrity by exacerbating blood-induced cartilage damage42, and as demonstrated in vitro, minimize the protection against pro-inflammatory cytokine induced damage22–25. Therefore, in the absence of a robust TNF-α alteration, it is plausible that a lower serum IL-10 concentration following an anterior cruciate ligament injury could be detrimental to the joint structure by compromising the anti-inflammatory protection against pro-inflammatory cytokine-mediated cartilage loss and consequentially increase the risk of developing knee osteoarthritis. However, evidence supporting this premise remains elusive11, but if demonstrated, could provide evidence of a systemic biomarker intended to improve prognostic potential and the subsequent rationale necessary for a personalized approach of preventative medicine following an anterior cruciate ligament injury.
In the OA group, the unperturbed serum IL-10 corresponds with a lower serum TNF-α concentration compared to the CON group. Considering the anti-inflammatory property of IL-10 to down-regulate TNF-α production43, these results intuitively imply that a collected anti-inflammatory cytokine status associates with a lower pro-inflammatory cytokine concentration in the circulation with moderate knee osteoarthritis. However, the correlational results are not consistent with this assumption and imply an alternative explanation. Specifically, there is a positive correlation between TNF-α and IL-10 in the OA group, in each group separately, and in all subjects combined, suggesting an increase in IL-10 with increasing TNF-α. This finding, however, is not consistent with previous results as data provided by Koh and colleagues15 failed to demonstrate a correlation between circulating IL-10 and TNF-α in subjects with an anterior cruciate ligament injury, subjects with end-stage knee osteoarthritis, and in anterior cruciate ligament injuried and end-stage knee osteoarthritis subjects combined.
To explore the pro- to anti-inflammatory cytokine balance, we compared the IL-10/TNF-α ratio between groups. The IL-10/TNF-α ratio is considered as an anti-inflammatory index29,44 and is significantly elevated in the OA compared to the CON, ACL, and TKA groups. An elevation in the IL-10/TNF-α ratio (circulating and peripheral blood mononuclear cell messenger RNA) prevents or attenuates joint degeneration in experimental animals with post-traumatic osteoarthritis40 and associates with fewer post-operative complications in cardiac surgery patients45. However, an increase in the ratio is not consistently associated with improved outcomes as transient deviations or sustained elevations in the IL-10/TNF-α ratio could also represent hyper-inflammation or immunosuppression and relate to poor outcomes in pathophysiological conditions, such as sepsis29. The disparate results from the aforementioned studies underscore the importance of a cautious interpretation when examining the IL-10/TNF-α ratio, while herein illustrate an augmentation in circulating IL-10 for a given TNF-α concentration in the OA group predominately characterized by moderate knee osteoarthritis (i.e., KL grade = 3).
Research endeavors and data continue to mount pertaining to the regulatory role of cytokines on osteoarthritis development and progression15,19,21,26,27,33,38,46–49. Here, we performed two separate analyses to further our understanding of the association between the pro- to anti-inflammatory cytokine balance and the severity in knee osteoarthritis. In the first analysis, serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio are compared between those with (i.e., KL ≥ 3, n = 79) to those without (KL < 3, n = 51) radiographic evidence of knee joint space narrowing and independent from initial groupings (i.e., CON, ACL, OA, and TKA). In those with available radiographs (n = 130), the serum IL-10/TNF-α ratio is significantly elevated with gross joint space narrowing (i.e., KL ≥ 3) while serum IL-10 and TNF-α concentrations are not significantly different with compared to without joint space narrowing. This finding implies that the systemic pro- to anti-inflammatory cytokine balance might be more sensitive to gross alterations in joint space narrowing and disease severity than individual TNF-α and IL-10 concentrations.
In the second analysis, we limited the comparison to osteoarthritic subtypes with contrasting evidence in disease severity or joint space narrowing, and again, independent from initial groupings. Specifically, serum IL-10 and TNF-α concentrations and the serum IL-10/TNF-α ratio are compared between moderate (KL = 3, ≤ 50% joint space narrowing) and severe (KL = 4, > 50% joint space narrowing) knee osteoarthritis. Results from this analysis indicate that serum IL-10 concentrations and the serum IL-10/TNF-α ratio are lower with severe knee osteoarthritis while the serum TNF-α concentration is not significantly different between disease severity subgroups. These findings collectively suggest an elevation in the serum IL-10/TNF-α ratio with gross joint space narrowing, but a lower serum IL-10 concentration and IL-10/TNF-α ratio with severe compared to moderate knee osteoarthritis. Considering cartilage loss occurs before the radiographic manifestation of joint space narrowing50,51, future research associating the IL-10/TNF-α ratio with cartilage loss and radiographic evidence of joint space narrowing could reveal a systemic biomarker sensitive to disease development and progression that aids in the clinical decision process. Also, although identification of the potential source(s) responsible for modulating serum IL-10 is beyond the scope of the present investigation, it is plausible that a lower anti-inflammatory cytokine concentration with severe or end-stage knee osteoarthritis—when the majority of the cartilage damage has already occurred—associates with an alteration in the number of infiltrating mononuclear cells (e.g., CD4 + and CD68 + cells)28,52–56, chondrocytes27,57, and other sources that contribute to its production.
In addition to those discussed above, there are other study limitations worthy of discussion. First, more sensitive imaging tools could improve the accuracy in linking disease severity or joint trauma with circulating cytokines, although this could be questionable11. Along these lines, magnetic resonance imaging was not utilized in the ACL group to evaluate additional trauma to the knee joint, but rather, was assessed intraoperatively during arthroscopy. Therefore, it is plausible that additional trauma to knee joint was present in the ACL group that was not captured during surgery. Second, stringent inclusion and exclusion criteria are implemented to minimize the impact of comorbidities on study outcomes, but in turn, could hinder the generalizability of the results to a broader population. Third, we did not delineate primary osteoarthritis from secondary causes of osteoarthritis (e.g., post-traumatic injury) in the OA and TKA groups. Fourth, although subject characteristics were not correlated with circulating cytokines or cytokine ratios, it is plausible that various subject characterstics could be confounding the results. Next, this study did not concurrently examine circulating and localized cytokine concentrations. Finally, although subject characteristics were not significantly different between the osteoarthritis groups and subgroups, including a non-osteoarthritis group with similar demographics to the osteoarthritic groups could reveal a difference in circulating cytokine concentrations with general knee osteoarthritis58.
Conclusion
This investigation provides unique data illustrating an increase in the serum IL-10/TNF-α ratio with robust joint space narrowing. Differentiating moderate from severe knee osteoarthritis, however, revealed a lower serum IL-10 concentration and serum IL-10/TNF-α ratio with severe knee osteoarthritis. This report also provides evidence that serum IL-10 concentrations are lower while serum TNF-α concentrations and the serum IL-10/TNF-α ratio are unperturbed in subjects predisposed to developing knee osteoarthritis following ligamentous trauma. Based on these findings, we conclude that serum IL-10 concentrations are compromised without significant serum TNF-α deviations in subjects predisposed to developing knee osteoarthritis following ligamentous trauma and in subjects with severe radiographic evidence of knee joint space narrowing. Future research assessing the clinical utility of the serum IL-10/TNF-α ratio and other cytokine and cytokine ratios as systemic biomarkers capable of phenotypically differentiating the severity in knee osteoarthritis is warranted.
Abbreviations
- ACL
Anterior cruciate ligament
- BMI
Body mass index
- CON
Control
- IL-10
Interleukin-10
- KL
Kellgren–Lawrence
- OA
Osteoarthritis
- TKA
Total knee arthroplasty
- TNF-α
Tumor necrosis factor-α
Author contributions
T.B., R.T., N.M., and G.R. designed the study. T.B., V.R., and V.H. collected the data. T.B. analyzed the data and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded in part by USANA Health Sciences, Inc. (Salt Lake City, UT USA) and the Intermountain Research and Medical Foundation (Intermountain Healthcare, Salt Lake City, UT USA).
Data availability
The datasets generated and analyzed during this study are not publicly available due data containing potentially identifying or sensitive patient information but are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swärd P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—A cross-sectional analysis. Osteoarthr. Cartil. 2012;20:1302–1308. doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, Williams A, Vincent TL. Acute molecular changes in synovial fluid following human knee injury: Association with early clinical outcomes. Arthritis Rheumatol. 2016;68:2129–2140. doi: 10.1002/art.39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan DJ, Cuellar VG, Jazrawi LM, Strauss EJ. Biomarker changes in anterior cruciate ligament-deficient knees compared with healthy controls. Arthroscopy. 2017;33:1053–1061. doi: 10.1016/j.arthro.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/S0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 6.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am. J. Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 7.Cameron ML, Fu FH, Paessler HH, Schneider M, Evans CH. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol. Arthrosc. 1994;2:38–44. doi: 10.1007/BF01552652. [DOI] [PubMed] [Google Scholar]
- 8.Marks PH, Donaldson MLC. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Barker T, Henriksen VT, Rogers VE, Trawick RH. Improvement in muscle strength after an anterior cruciate ligament injury corresponds with a decrease in serum cytokines. Cytokine. 2015;73:199–202. doi: 10.1016/j.cyto.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Bigoni M, Zanchi N, Omeljaniuk RJ, Zatti G, Locatelli V, Torsello A, Turati M. Role of interleukin-10 in the synovial fluid of the anterior cruciate ligament injured knee. Eur. Rev. Med. Pharmacol. Sci. 2019;23:932–940. doi: 10.26355/eurrev_201902_16979. [DOI] [PubMed] [Google Scholar]
- 11.Roemer FW, Englund M, Turkiewicz A, Struglics A, Guermazi A, Lohmander LS, Larsson S, Frobell R. Molecular and structural biomarkers of inflammation at two years after acute anterior cruciate ligament injury do not predict structural knee osteoarthritis at five years. Arthritis Rheumatol. 2019;71:238–243. doi: 10.1002/art.40687. [DOI] [PubMed] [Google Scholar]
- 12.Struglics A, Larsson S, Kumahashi N, Frobell R, Lohmander LS. Changes in cytokines and aggrecan args neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II Collagen and N-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: An exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheumatol. 2015;67:1816–1825. doi: 10.1002/art.39146. [DOI] [PubMed] [Google Scholar]
- 13.Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, Lane NE, Kraus VB, Ma CB, Li X. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am. J. Sports Med. 2018;46:890–899. doi: 10.1177/0363546517749834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han PF, Wei L, Duan ZQ, Zhang ZL, Chen TY, Lu JG, Zhao RP, Cao XM, Li PC, Lv Z, Wei XC. Contribution of IL-1beta, 6 and TNF-alpha to the form of post-traumatic osteoarthritis induced by "idealized" anterior cruciate ligament reconstruction in a porcine model. Int. Immunopharmacol. 2018;65:212–220. doi: 10.1016/j.intimp.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Koh SM, Chan CK, Teo SH, Singh S, Merican A, Ng WM, Abbas A, Kamarul T. Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee. 2020;27:26–35. doi: 10.1016/j.knee.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 17.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 2020;38:253–257. doi: 10.1002/jor.24457. [DOI] [PubMed] [Google Scholar]
- 19.Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr. Cartil. 2015;23:1906–1914. doi: 10.1016/j.joca.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 21.Moos V, Fickert S, Muller B, Weber U, Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J. Rheumatol. 1999;26:870–879. [PubMed] [Google Scholar]
- 22.Behrendt P, Hafelein K, Preusse-Prange A, Bayer A, Seekamp A, Kurz B. IL-10 ameliorates TNF-alpha induced meniscus degeneration in mature meniscal tissue in vitro. BMC Musculoskelet Disord. 2017;18:197. doi: 10.1186/s12891-017-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alaaeddine N, Di Battista JA, Pelletier JP, Kiansa K, Cloutier JM, Martel-Pelletier J. Inhibition of tumor necrosis factor alpha-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: distinct targeting in the signaling pathways. Arthritis Rheum. 1999;42:710–718. doi: 10.1002/1529-0131(199904)42:4<710::AID-ANR14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–542. [PMC free article] [PubMed] [Google Scholar]
- 25.John T, Muller RD, Oberholzer A, Zreiqat H, Kohl B, Ertel W, Hostmann A, Tschoeke SK, Schulze-Tanzil G. Interleukin-10 modulates pro-apoptotic effects of TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2007;40:226–234. doi: 10.1016/j.cyto.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Botha-Scheepers S, Watt I, Slagboom E, de Craen AJ, Meulenbelt I, Rosendaal FR, Breedveld FC, Huizinga TW, Kloppenburg M. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann. Rheum. Dis. 2008;67:1165–1169. doi: 10.1136/ard.2007.084657. [DOI] [PubMed] [Google Scholar]
- 27.Vangsness CT, Jr, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis–a pilot study. Bull. NYU. Hosp. Jt. Dis. 2011;69:122–127. [PubMed] [Google Scholar]
- 28.Barker T, Rogers VE, Henriksen VT, Aguirre D, Trawick RH, Rasmussen GL, Momberger NG. Serum cytokines are increased and circulating micronutrients are not altered in subjects with early compared to advanced knee osteoarthritis. Cytokine. 2014;68:133–136. doi: 10.1016/j.cyto.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect. Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 30.Barker T, Rogers VE, Levy M, Templeton J, Goldfine H, Schneider ED, Dixon BM, Henriksen VT, Weaver LK. Supplemental vitamin D increases serum cytokines in those with initially low 25-hydroxyvitamin D: A randomized, double blind, placebo-controlled study. Cytokine. 2015;71:132–138. doi: 10.1016/j.cyto.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Barker T, Brown KB, Rogers VE. Soluble TNF receptors are modulated by vitamin D status but not by acute perturbations in 25-hydroxyvitamin D following a bolus of supplemental vitamin D. J. Cytokine Biol. 2017;2:1–9. doi: 10.4172/2576-3881.1000118. [DOI] [Google Scholar]
- 32.Barker T, Henriksen VT, Rogers VE, Trawick RH. Serum cytokines and muscle strength after anterior cruciate ligament surgery are not modulated by high-doses of vitamins E (alpha- and gamma-tocopherol's) and C. Cytokine. 2015;74:279–286. doi: 10.1016/j.cyto.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Barker T, Rogers VE, Henriksen VT, Dixon BM, Momberger NG, Rasmussen GL, Trawick RH. Muscular-based and patient-reported outcomes differentially associate with circulating superoxide dismutases and cytokines in knee osteoarthritis. Cytokine. 2019;115:45–49. doi: 10.1016/j.cyto.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Barker T, Rogers VE, Henriksen VT, Levy M, Schneider ED, Templeton J, Goldfine H, Dixon BM, Rasmussen GL, Trawick RH, Momberger NG. Circulating cytokine concentrations are not altered by supplemental vitamin D in knee osteoarthritis: A pilot study. J. Nutr. Intermed. Metab. 2019;18:1–7. [Google Scholar]
- 35.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, Moretti S, Munegato D, Augusti CA, Bresciani E, Omeljaniuk RJ, Locatelli V, Torsello A. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2013;31:315–321. doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 37.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: A prospective cohort study. Ann. Rheum. Dis. 2013;72:535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 38.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthrit. Cartil. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Mabey T, Honsawek S, Tanavalee A, Yuktanandana P, Wilairatana V, Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers. 2016;21:1–6. doi: 10.3109/1354750X.2016.1171907. [DOI] [PubMed] [Google Scholar]
- 40.Sun ZB, Peng H. Experimental study on the prevention of posttraumatic osteoarthritis in the rabbit knee using a hinged external fixator in combination with exercises. J. Invest. Surg. 2019;32:1–8. doi: 10.1080/08941939.2019.1609141. [DOI] [PubMed] [Google Scholar]
- 41.Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, Fujita K, Lipsky PE. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- 42.Jansen NW, Roosendaal G, Hooiveld MJ, Bijlsma JW, van Roon JA, Theobald M, Lafeber FP. Interleukin-10 protects against blood-induced joint damage. Br. J. Haematol. 2008;142:953–961. doi: 10.1111/j.1365-2141.2008.07278.x. [DOI] [PubMed] [Google Scholar]
- 43.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 44.Molanouri Shamsi M, Chekachak S, Soudi S, Quinn LS, Ranjbar K, Chenari J, Yazdi MH, Mahdavi M. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-alpha ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine. 2017;90:100–108. doi: 10.1016/j.cyto.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Duggan E, Caraher E, Gately K, O'Dwyer M, McGovern E, Kelleher D, McManus R, Ryan T. Tumor necrosis factor-alpha and interleukin-10 gene expression in peripheral blood mononuclear cells after cardiac surgery. Crit. Care Med. 2006;34:2134–2139. doi: 10.1097/01.CCM.0000227647.77356.AB. [DOI] [PubMed] [Google Scholar]
- 46.Attur M, Krasnokutsky S, Statnikov A, Samuels J, Li Z, Friese O, HLG-G MP, Rybak L, Kraus VB, Jordan JM, Aliferis CF, Abramson SB. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015;67:2905–2915. doi: 10.1002/art.39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moradi B, Rosshirt N, Tripel E, Kirsch J, Barie A, Zeifang F, Gotterbarm T, Hagmann S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015;180:143–154. doi: 10.1111/cei.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 49.Radojcic MR, Thudium CS, Henriksen K, Tan K, Karlsten R, Dudley A, Chessell I, Karsdal MA, Bay-Jensen AC, Crema MD, Guermazi A. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain. 2017;158:1254–1263. doi: 10.1097/j.pain.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 50.Jones G, Ding C, Scott F, Glisson M, Cicuttini F. Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthrit. Cartil. 2004;12:169–174. doi: 10.1016/j.joca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Cicuttini FM, Wluka AE, Stuckey SL. Tibial and femoral cartilage changes in knee osteoarthritis. Ann. Rheum. Dis. 2001;60:977–980. doi: 10.1136/ard.60.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito I, Koshino T, Nakashima K, Uesugi M, Saito T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthrit. Cartil. 2002;10:156–162. doi: 10.1053/joca.2001.0494. [DOI] [PubMed] [Google Scholar]
- 54.Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthrit. Cartil. 2002;10:277–281. doi: 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- 55.Rosshirt N, Hagmann S, Tripel E, Gotterbarm T, Kirsch J, Zeifang F, Lorenz HM, Tretter T, Moradi B. A predominant Th1 polarization is present in synovial fluid of end-stage osteoarthritic knee joints: analysis of peripheral blood, synovial fluid and synovial membrane. Clin. Exp. Immunol. 2019;195:395–406. doi: 10.1111/cei.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nees TA, Rosshirt N, Zhang JA, Platzer H, Sorbi R, Tripel E, Reiner T, Walker T, Schiltenwolf M, Lorenz HM, Tretter T, Moradi B, Hagmann S. T helper cell infiltration in osteoarthritis-related knee pain and disability. J. Clin. Med. 2020;9:2423. doi: 10.3390/jcm9082423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, Lord JM, Gordon C, Buckley CD, Salmon M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sachdeva M, Aggarwal A, Sharma R, Randhawa A, Sahni D, Jacob J, Sharma V, Aggarwal A. Chronic inflammation during osteoarthritis is associated with an increased expression of CD161 during advanced stage. Scand. J. Immunol. 2019;90:e12770. doi: 10.1111/sji.12770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are not publicly available due data containing potentially identifying or sensitive patient information but are available from the corresponding author upon reasonable request.


