Abstract
Functional CsPbI3 perovskite phases are not stable at ambient conditions and spontaneously convert to a non-perovskite δ phase, limiting their applications as solar cell materials. We demonstrate the preservation of a black CsPbI3 perovskite structure to room temperature by subjecting the δ phase to pressures of 0.1 – 0.6 GPa followed by heating and rapid cooling. Synchrotron X-ray diffraction and Raman spectroscopy indicate that this perovskite phase is consistent with orthorhombic γ-CsPbI3. Once formed, γ-CsPbI3 could be then retained after releasing pressure to ambient conditions and shows substantial stability at 35% relative humidity. First-principles density functional theory calculations indicate that compression directs the out-of-phase and in-phase tilt between the [PbI6]4− octahedra which in turn tune the energy difference between δ- and γ-CsPbI3, leading to the preservation of γ-CsPbI3. Here, we present a high-pressure strategy for manipulating the (meta)stability of halide perovskites for the synthesis of desirable phases with enhanced materials functionality.
Subject terms: Physical chemistry, Materials for energy and catalysis
Inorganic lead halide perovskites are structurally unstable, which prevents their application in solar cells. Here the authors synthesize, using high pressure and temperature, a perovskite CsPbI3 phase that is metastably preserved to ambient conditions through a structural deformation induced at high pressure.
Introduction
Lead halide perovskite solar cells based on (MA)PbI3 (MA = CH3NH3+) have achieved power-conversion efficiencies comparable to commercial Si-based solar cells in recent years1–3. However, the volatile organic MA cation is largely responsible for this material’s instability to humidity and heat, a critical issue that hinders its large-scale implementation. Substitution of an inorganic cation such as Cs+ has been explored as a way to improve the robustness of lead halide perovskites4–7. There are four known polymorphs of CsPbI38–13: a room-temperature non-perovskite phase (δ), and three high-temperature perovskite-related phases with cubic (α), tetragonal (β), and orthorhombic (γ) structures. Perovskite-structured CsPbI3 has a bandgap of 1.6–1.8 eV that is favorable for photovoltaic applications4–9. However, a major challenge to realizing CsPbI3-based solar cells is that these black perovskite phases are not thermodynamically stable at ambient conditions and spontaneously convert to the non-functional δ phase10–13. A recent study reported that perovskite solar cells made from surface-treated β-CsPbI3 achieved power-conversion efficiencies >18%, but only at high temperature4. It is crucial to find ways to stabilize CsPbI3 perovskites at room temperature for practical device operation.
Previous studies have offered a few strategies for stabilizing CsPbI3 perovskite phases to room temperature, including thermal engineering9–13, compositional tuning14–17, nanocrystal growth5,18,19, solvent and surface treatments6–8,20–28, and strain engineering29. However, these approaches present several drawbacks and limitations30. For instance, thermal engineering based on a solid-state method required rigorously anhydrous reagents, a moisture-free environment, a melt state at high temperature, and extremely rapid cooling. The as-preserved bulk γ-CsPbI3 also showed severe moisture sensitivity13. The strain-stabilized γ-CsPbI3 film was rendered by the use of a combined substrate clamping and biaxial strain in an inert atmosphere and returned back to the δ phase within minutes in air at 27% relative humidity (RH)29. Compositional tuning introduced undesirable changes in the electronic structure20,21, and nanomaterials showed an increase in grain boundaries which inhibited charge transport and caused considerable recombination loss6,15. These drawbacks motivate us to explore other approaches.
Previous calculations suggested that the [PbI6]4− inter-octahedral tilt had a large influence on the formation energies of the three perovskite phases (α, β, and γ) of CsPbI3, among which the γ phase has the lowest energy owing to its largest octahedral tilt9. Hence, tuning the octahedral tilt of the high-temperature perovskite phase(s) using external stimuli such as pressure will be favorable for preserving the desired structure back to ambient conditions. Many halide perovskite systems have been studied at high pressure. Pressure has proven to be an effective and clean tool for modifying their structures and creating exotic physical properties31–35, such as the bandgap modulation31,32 and closure in compressed (MA)PbI335,36 and emission enhancement in compressed halide double perovskites37. The pressure was also found to markedly tilt the [PbI6]4− octahedra in CsPbI3 perovskite nanocrystals38,39. However, none of the desired high-pressure phases has been preserved back to ambient conditions after releasing pressure.
In this work, we study the solid-to-solid structural evolution of CsPbI3 at high-pressure and high-temperature conditions in a resistive-heated diamond–anvil cell (Fig. 1a) using synchrotron X-ray diffraction (XRD), Raman spectroscopy, photoluminescence (PL) measurements, and first-principles density functional theory (DFT) calculations. We identify viable pressure–temperature (P–T) pathways to access and quench a black CsPbI3 perovskite phase back to ambient conditions. The preserved CsPbI3 phase shows substantial stability to moisture and its PL intensity remains above 80% of the initial intensity for more than 30 days and up to 10 days at 20% and 35% RH, respectively.
Fig. 1. Experimental setup and pressure-induced preservation of a metastable γ-CsPbI3 perovskite to ambient conditions.
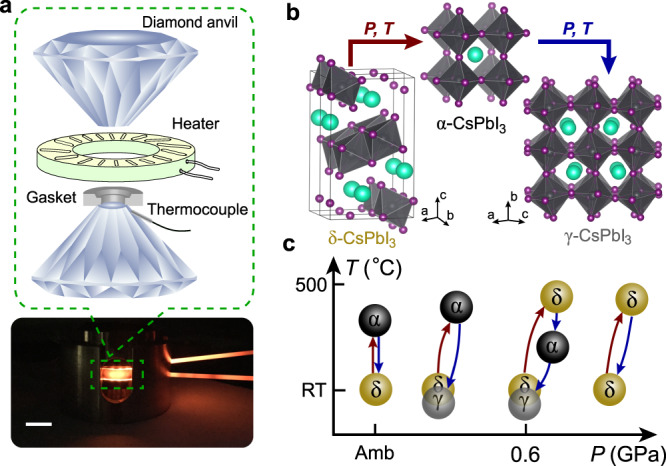
a Illustration of a cross-sectional view of the high-pressure and high-temperature setup. The white scale bar is 1 cm. Inset: Schematic of a diamond–anvil cell and an external resistive heater used in our experiments. b The observed structures of CsPbI3 at varying P–T cycles. The red and blue arrows represent the most viable heating and cooling pathways to preserve a γ-CsPbI3 perovskite phase, where the applied pressure (P) is 0.1–0.6 GPa and temperature (T) is up to 450 °C. Atoms in the structures are shown in gray (Pb), purple (I), and cyan (Cs). c Representative P–T phase transformations observed in the study. RT and Amb indicate room temperature and ambient pressure, respectively. The yellow δ and gray γ circles around RT are in fact both at RT and plotted with a slight offset for clarity. Once γ-CsPbI3 is formed by temperature quenching at high pressure, it can be preserved back to ambient conditions after fully releasing pressure.
Results
P–T phase diagram of CsPbI3
In situ XRD and Raman measurements were conducted to study the structural evolution of CsPbI3 as a function of pressure along heating and cooling routes. All the diamond–anvil–cell preparation, sample loading, and P–T treatments were done in air without special handling. The samples were first compressed to a target pressure at room temperature followed by heating and cooling from high temperature. The pressure may shift slightly as the temperature is varied during the cycle, but the final pressure after cooling back to room temperature is nearly identical to the initial pressure before heating.
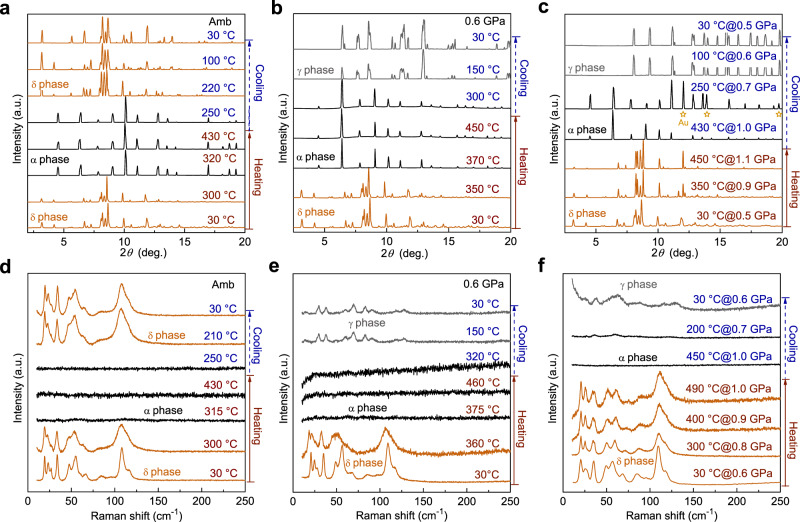
At ambient pressure, XRD indicates that the starting δ-CsPbI3 phase transforms to the cubic α-CsPbI3 phase when heated to 320 °C (Fig. 2a), consistent with previous observations10–12. With increasing pressure, the δ-to-α phase transition occurs at higher temperatures. Specifically, the transition temperature increases to 370 °C at 0.6 GPa (Fig. 2b) and exceeds 500 °C at 1.1 GPa (Fig. 2c). Raman measurements also agree with the XRD results. At ambient pressure and with heating up to 315 °C, the Raman modes of δ-CsPbI3 remain nearly invariant except for subtle frequency shifts, peak broadening, and changes in relative peak intensities (Fig. 2d). As the temperature reaches 315 °C, the color of the sample changes dramatically from yellow to black (Supplementary Fig. 1) and all of the Raman modes suddenly disappear, suggesting that CsPbI3 converts to the cubic α phase which is Raman inactive based on the group theory analysis on a cubic perovskite structure40,41. With increasing pressure, the disappearance of the Raman modes (Fig. 2e, f) and the color change from yellow to black occur at higher temperatures, completely consistent with our XRD results. Following slow cooling, the high-temperature α phase transforms back to the δ phase below the transition temperature. Based on the XRD and Raman results, a P–T phase diagram of CsPbI3 was mapped out as shown in Supplementary Fig. 2.
Fig. 2. X-ray diffraction (XRD) and Raman spectra showing the effect of pressure on the structural transition of CsPbI3 along with thermal treatments.
a–c XRD results (λ = 0.4959 Å) of CsPbI3 along heating and cooling cycles at ambient pressure (a), 0.6 GPa (b), and varying pressures (c). The sample remains in a crystalline state up to the highest temperature studied in each cycle. The intensity differences of XRD patterns for α- and γ-CsPbI3 could be a result of different grain sizes and orientations due to the grain growth to highly textured, coarse grains at high temperatures. d–f Raman spectra of CsPbI3 along heating and cooling cycles at ambient pressure (d), 0.6 GPa (e), and varying pressures (f), confirming the structural transitions observed from XRD measurements.
Pressure effect on preserving γ-CsPbI3 to ambient conditions
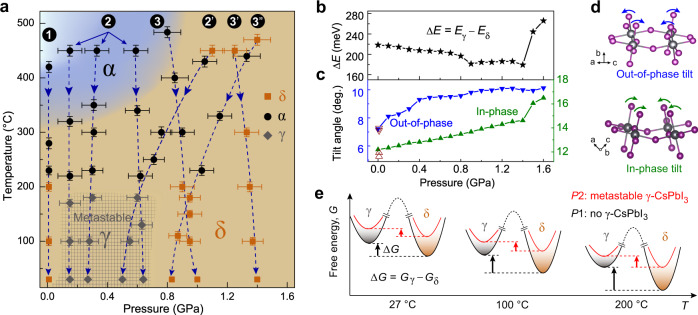
The structural transitions observed upon rapid cooling at a rate of ~90 K/min strongly depend on the pressure applied to the system. XRD and Raman results indicate that a black perovskite phase can be metastably preserved back to room temperature when the initial δ-CsPbI3 is subjected to a pressure between 0.1 and 0.6 GPa followed by rapid cooling from high temperature. Three different sets of experimental routes were identified according to the applied pressure: <0.1 GPa (route 1), 0.1–0.6 GPa (routes 2 and 2’), and >0.6 GPa (routes 3, 3’, and 3”), as shown in Fig. 3a. At ambient pressure (route 1), when rapidly cooling from 430 °C, the high-temperature α-CsPbI3 returns to the thermodynamically preferred δ phase below 220 °C (Fig. 2a).
Fig. 3. Representative cooling routes and the effect of [PbI6]4− octahedral tilt on the energy difference between γ- and δ-CsPbI3 with pressure.
a The experimental cooling routes used to access and preserve γ-CsPbI3 to room temperature. The background color shows the P–T phase diagram of CsPbI3 that includes the δ and α phases. γ-CsPbI3 is not a thermodynamically stable phase and only forms during rapid cooling from high temperature marked as a gray crossed-hatched region. The numbers inside the black circles indicate different cooling routes. The temperature error bars are determined based on the temperature fluctuation before and after measurements, which further causes the pressure uncertainty (see “Methods” for details). b The total energy difference per unit cell between γ- and δ-CsPbI3 (ΔE = Eγ – Eδ) under compression. c The evolution of the calculated out-of-phase tilt along [101] and the in-phase tilt along [010] as a function of pressure. The open triangles are the previously reported tilt angles of γ-CsPbI3 at room temperature and ambient pressure10,13. d Ball-stick models visualizing the out-of-phase and in-phase octahedral tilts. e A schematic Gibbs free energy (G) diagram of γ- and δ-CsPbI3 as a function of temperature at pressure P1 (e.g., 0.8 and 1.6 GPa) without the formation of metastable γ-CsPbI3 (black curve) and at pressure P2 (e.g., 1.2 GPa) with the formation of γ-CsPbI3 (red curve). ΔG (= Gγ – Gδ) at 0.8, 1.2, and 1.6 GPa are 0.806, 0.589, and 0.941 eV at 27 °C, 0.934, 0.614, and 1.011 eV at 100 °C, and 1.128, 0.653, and 1.118 eV at 200 °C, respectively.
Interestingly, when the applied pressure is between 0.1 and 0.6 GPa (routes 2 and 2’), CsPbI3 undergoes different structural transitions upon rapid cooling. Taking the cooling process at ~0.6 GPa as an example (Fig. 2b and route 2 in Fig. 3a), as the sample cools from 450 °C, the diffraction peaks of the high-temperature α-CsPbI3 split obviously below 150 °C, and these changes persist down to room temperature. The preserved diffraction pattern and Raman spectrum differ from those of the non-perovskite δ phase (Fig. 2b, e). The sample also remains opaque black throughout cooling (Supplementary Fig. 1). In route 2’ (Fig. 3a), in which the pressure increases from 0.5 to 1.1 GPa during heating to 450 °C and goes back to 0.5 GPa after cooling to room temperature, XRD and Raman measurements indicate that while CsPbI3 remains in the yellow δ phase during the entire heating cycle (Fig. 2c, f), the sample instantaneously transforms to the α phase at the onset of cooling, followed by the appearance of a similar black phase as observed in route 2 which persists down to room temperature.
The splitting of diffraction peaks indicates that this preserved black phase has a lower symmetry compared to the cubic α-CsPbI3 phase. We indexed the diffraction pattern with the other known phases of CsPbI3 (β, γ, and δ), and found that all of the diffraction peaks of the preserved black phase in Fig. 2b, c could be assigned to the orthorhombic γ-CsPbI3, but not to β- or δ-CsPbI3 (Supplementary Figs. 3 and 4). As shown in Supplementary Fig. 5, the diffraction pattern changes from smooth diffraction rings before heating to spots after CsPbI3 undergoes transitions to the α and γ structures, indicating significant grain growth in the sample to highly textured, coarse grains. The diffraction peak intensities are unreliable as they are mainly determined by the grain size and the orientation of each grain relative to the incident X-ray beam. While all of the peaks in the XRD patterns of the recovered phase can be indexed to γ-CsPbI3, the relative peak intensities look distinct in routes 2 and 2’ (Fig. 2b, c) and are also different from previous studies on powder samples9,10. The apparent differences in the diffraction angle (2θ) values of our diffraction peaks from the previous reports9,10 are due to the different X-ray wavelengths used in the XRD measurements. The Raman spectrum of the preserved black phase also agrees well with that of γ-CsPbI3 synthesized by a solid-state method (Supplementary Fig. 6). No Raman modes or XRD peaks from CsI or PbI2 precursors are observed, indicating no decomposition of CsPbI3.
Our study further indicates that γ-CsPbI3 can only be accessed when the final pressure falls between 0.1 and 0.6 GPa. When the final pressure is above 0.6 GPa, CsPbI3 transforms back to the δ phase after the P–T treatments or remains in the δ phase in the entire P–T range studied, as supported by three independent routes covering varying P–T pathways (routes 3, 3’, and 3”).
Based on the XRD and Raman results, we determined a P–T window marked by a gray cross-hatched area in Fig. 3a for the formation and preservation of γ-CsPbI3. The key ingredient for being able to metastably preserve γ-CsPbI3 to room temperature is the final pressure applied to the system, i.e., 0.1–0.6 GPa. CsPbI3 returns to the non-perovskite δ phase after P–T cycles when the applied pressure falls outside this narrow range. The cubic α-CsPbI3 phase is found to serve as an intermediate for accessing γ-CsPbI3. It is encouraging that once preserved to room temperature, γ-CsPbI3 can be retained even after fully releasing pressure back to ambient conditions.
Relative energetic stability of compressed δ- and γ-CsPbI3
Although γ-CsPbI3 is a kinetically trapped metastable phase and does not appear on the P–T phase diagram, energy calculations can still shed light on the effect of pressure on the relative stability of the competing δ and γ phases (Fig. 3). We first performed first-principles DFT calculations on these two structures as a function of pressure at T = 0 K. At 0 GPa, δ-CsPbI3 has a lower total energy compared to γ-CsPbI3, supporting that δ-CsPbI3 is the thermodynamically stable phase. With the application of pressure, the total energy difference between γ- and δ-CsPbI3 (ΔE = Eγ – Eδ) reduces with pressure (Fig. 3b), indicating an increased stabilization of γ-CsPbI3 with respect to δ-CsPbI3. ΔE reaches its minimum at 0.9 GPa and remains almost invariant up to 1.4 GPa. With further compression, ΔE shows a sharp rise due to a larger rate at which the total energy of γ-CsPbI3 increases relative to that of δ-CsPbI3 (Supplementary Fig. 7). DFT results suggest that 0.9–1.4 GPa is the most suitable pressure range for favoring the formation of γ-CsPbI3, spanning a small pressure window of 0.5 GPa as in our experimental results. To further elucidate the combined effect of pressure and temperature on preserving γ-CsPbI3, the Gibbs free energy (G = E + PV – TS) that includes the vibrational entropy at varying pressures was calculated (Fig. 3e and Supplementary Fig. 8). It is beyond our computational capacity to survey the entire P–T space studied. Representative pressures of 0.8, 1.2, and 1.6 GPa were chosen based on the total energy calculations. The results show that at 1.2 GPa, the Gibbs free energy difference between γ- and δ-CsPbI3 (ΔG = Gγ – Gδ) is much smaller than that at 0.8 and 1.6 GPa, and it keeps reducing as the material cools from 200 °C to room temperature, implying a preferred stabilization of metastable γ-CsPbI3 towards room temperature as pressure falls in the window of 0.9–1.4 GPa.
Discussion
After analyzing the structural details, the tilt of [PbI6]4− octahedra was found to correlate with the stabilization of the γ phase. There are two types of octahedral tilts in γ-CsPbI3: an out-of-phase tilt along the [101] direction with adjacent octahedra turning in opposite directions and an in-phase tilt along the [010] direction with adjacent octahedra rotating towards the same direction (Fig. 3d and Supplementary Fig. 9). The simulated out-of-phase and in-phase tilt angles at 0 GPa are comparable with the values calculated from previously reported structures10,13. Under compression, the out-of-phase tilt increases rapidly below 0.4 GPa and continues the upward trend at a reduced rate with further compression (0.4–0.8 GPa), followed by a pressure-invariant behavior of staying at ~10° between 0.9 and 1.4 GPa (Fig. 3c). On the other hand, the in-phase tilt increases linearly with pressure up to 1.4 GPa. The similar trend between the out-of-phase tilt and ΔE below 1.4 GPa, especially between 0.9 and 1.4 GPa, suggests that the out-of-phase tilt contributes the most to the reduction of ΔE and consequently the preservation of γ-CsPbI3. At above 1.5 GPa, the in-phase tilt rises sharply, concurrent with the dramatic increase of ΔE. This indicates that at higher pressures, the in-phase tilt could be the main contributing factor for the increase of the total energy of γ-CsPbI3 that again favors the formation of δ-CsPbI3.
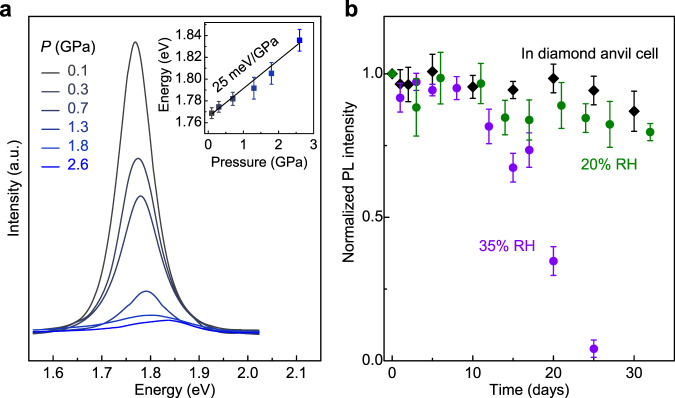
The compression-directed tilt of [PbI6]4− octahedra is also reflected from the PL measurements of re-pressurizing the preserved γ-CsPbI3. The preserved γ-CsPbI3 exhibits a PL peak at ~1.77 eV at ambient conditions (Fig. 4a), which is comparable with the value of the strain-stabilized γ-CsPbI329 and ~ 60 meV smaller than that of γ-CsPbI3 synthesized by a nano crystallization method5. With re-compressing the recovered γ-CsPbI3 at room temperature, the PL peak shows a blue shift at a rate of 25 meV/GPa up to 2.6 GPa beyond which the PL signal disappears (Fig. 4a, inset). The pressure response is different from that observed in CsPbI3 perovskite nanocrystals where the PL at first shifted to lower energies below 0.4 GPa and then to higher energies with further compression38,39. These differences could be caused by the different structures and particle sizes of the samples. However, the PL development of a CsPbI3 perovskite structure as a function of pressure is typically controlled by both the intra-octahedral compression and inter-octahedral tilt, with the former resulting in a red shift and the latter causing a blue shift39. The exclusive blue shift observed in the PL of our preserved γ-CsPbI3 supports the inter-octahedral tilt being significantly modulated at high pressure. The band-structure calculations confirm that the increase of the out-of-phase and in-phase tilt both enlarges the bandgap of γ-CsPbI3 with the in-phase tilt playing a bigger role (Supplementary Fig. 10).
Fig. 4. PL spectra of re-pressurizing the recovered γ-CsPbI3 perovskite and PL intensity over time of the preserved γ-CsPbI3.
a PL spectra collected as a function of pressure at room temperature. Inset: The pressure dependence of the PL energy. A pseudo-Voigt function is used to fit the PL peak that yields the peak position and uncertainty. b Normalized PL intensity of the preserved γ-CsPbI3 perovskite over time in a diamond–anvil cell and in air at 20% and 35% RH, respectively. The error bars are defined as the largest deviation from the average value of the normalized PL intensity collected at different sample positions.
Further study on the functionality of the preserved γ-CsPbI3 shows that this perovskite phase has substantial stability to humidity. The PL intensity of the recovered samples remains above 80% of the initial value for more than 30 days and up to 10 days in air at 20% and 35% RH, respectively (Fig. 4b). After degradation in air over time, γ-CsPbI3 transforms back to the yellow δ-CsPbI3. With reheating to high temperature at ambient pressure, the preserved γ-CsPbI3 transforms to the yellow δ-CsPbI3 phase above 100 ˚C (Supplementary Fig. 11). With applying pressure at room temperature, Raman measurements indicate that the preserved γ-CsPbI3 is metastable to at least 5.0 GPa (Supplementary Fig. 11).
In summary, a metastable γ-CsPbI3 perovskite phase can be synthesized and then preserved back to room temperature via pressure-tuning the tilt of [PbI6]4− octahedra, given a pressure between 0.1 and 0.6 GPa is applied to the yellow δ-CsPbI3 followed by heating and rapid quenching. The occurrence of α-CsPbI3 at high temperatures likely assists the formation of γ-CsPbI3. Once formed, γ-CsPbI3 can be retained after releasing pressure to ambient conditions and shows substantial stability in air at 35% RH for up to 10 days. First-principles DFT calculations suggest that pressure directs the out-of-phase and in-phase octahedral tilt that control the relative energy difference between the competing δ- and γ-CsPbI3 and ultimately stabilizes γ-CsPbI3. Our study provides key insight into manipulating the phase (meta)stability of halide perovskites through structural control and opens effective pathways to synthesizing metastable phases with improved properties.
Methods
Synthesis of δ-CsPbI3 samples
The yellow non-perovskite δ-CsPbI3 samples were synthesized using a solution-based method adapted from literature42. Solid PbI2 (0.46 g, 1.0 mmol) and CsI (0.26 g, 1.0 mmol) were first mixed with aqueous HI (10.0 mL, 7.58 M, stabilized), and then heated to 130 °C with stirring in a closed 20-mL glass vial. The solution was then cooled to room temperature at a rate of 25–35 °C/h by turning off the power of the hot plate. The yellow δ-CsPbI3 solid powders were collected by vacuum filtration, rinsed with large amounts of diethyl ether, and dried under reduced pressure for 12 h.
High-pressure and high-temperature apparatus
High pressure was achieved using a BX-90 type diamond–anvil cell with an anvil culet size of 500 or 600 μm in diameter. A home-made external coiled resistive heater with a resistance of ~2.5 Ω made of KA1 alloy wires (Hyndman Industrial Products) was placed around the T301 stainless-steel gasket and the tip of the diamond anvils to heat up the samples. The exposed KA1 alloy wires were covered with 940 HT fast cure alumina adhesive for electrical insulation. Asbestos thermal insulation layers were used to cover the remaining area of the diamonds, seats, and the resistive heater to ensure temperature uniformity across the sample.
Sample loading and reaching high P–T conditions
Powdered δ-CsPbI3 samples, together with Au powders or a ruby ball as pressure calibrants, were loaded into 250-μm sample chambers in pre-indented T301 stainless-steel gaskets in air. No pressure transmitting medium was used to ensure good contacts between samples and the diamond surface for temperature monitoring. The pressure at the room and the high temperature was calibrated using the equation of state of Au43,44 or the fluorescence shift from a ruby ball45. The temperature was monitored by a K-type thermocouple glued on the diamond pavilion close to the culet. The pressure uncertainty at high temperature was determined based on the temperature fluctuation before and after the XRD and Raman measurements. A higher heating temperature and a longer heating duration favor the conversion to pure γ-CsPbI3.
Synchrotron XRD measurements
High-temperature and high-pressure XRD measurements were performed at beamline 12.2.2 of the advanced light source (ALS), Lawrence Berkeley National Laboratory; and beamline 16-BMD of the Advanced Photon Source (APS), Argonne National Laboratory (ANL). Two-dimensional Debye-Scherrer diffraction rings were collected at a wavelength of λ = 0.4959 Å (12.2.2, ALS) and λ = 0.4133 Å (16-BMD, APS) on a Mar345 image plate detector, and integrated using the Dioptas software package46, yielding intensity versus 2θ patterns. The intensity has been normalized for better comparison. The sample-to-detector distance and other parameters of the detector were calibrated using the CeO2 standard.
Raman spectroscopy measurements
Raman spectra were collected using a Horiba LabRam HR Evolution Raman system at the Stanford Nano Shared Facilities (SNSF). A laser excitation wavelength of 633 nm was utilized. A threshold power of ~0.5 mW was kept throughout the measurements to avoid the potential laser-induced heating on the samples. Three accumulations with an exposure time of 10 s per accumulation were done to obtain a good Raman spectrum. Ultra-low-frequency filter kits (ULFK633-17-LR) were used to obtain Raman signals down to 10 cm−1. Before the measurements, the Raman system was calibrated using the Raman mode of a silicon wafer at 520 cm−1.
PL measurements
The evolution of PL intensity over time was collected on quenched samples using the Horiba system at SNSF (λ = 532 nm) and the Renishaw inVia Raman system in Extreme Environments Laboratory at Stanford University (λ = 514.5 nm). Laser power as low as 0.1 mW and an exposure time of 2 s per measurement were used. The PL peaks were smoothed by using the Savitzking Golay filter to remove the noise and ruby signal. Several positions of the γ-CsPbI3 samples were monitored with time. The PL intensity of each sample position at a different time was first normalized to that measured at day 1, and then the normalized values were averaged to get a normalized PL intensity versus time curve as shown in Fig. 4b. The PL spectra as a function of pressure were obtained by re-compressing the recovered samples to high pressure at room temperature.
Humidity tests
Humidity tests were performed by monitoring the PL intensity of γ-CsPbI3 over time in air at different levels of RH. The preserved γ-CsPbI3, along with a hygrometer for humidity calibration were sealed in a glass bottle. The hygrometer was calibrated using the saturated NaOH (~7% RH) and MgCl2 (~35% RH) solutions at ambient conditions47. A 5% RH uncertainty was observed from the hygrometer readings. A RH of 20% was obtained by sealing the sample, the hygrometer, and a small number of desiccants together in a glass bottle.
First-principles calculations
DFT calculations for the total energies of the δ- and γ-CsPbI3 phases under pressure were performed with Quantum Espresso48. The input structures were from experimental configurations10. The structures at ambient pressure for both phases were calculated under variable cell relaxation. The structure and related total energy at high pressure were then calculated using the relaxed ambient structure as the initial configuration. The pressure was applied by setting the stress to a target value based on the method developed in previous studies49,50, which has been implemented in Quantum Espresso. PBE exchange-correlation functional was chosen for the exchange and correlation terms51. An 8 × 6 × 8 k-grid for the γ phase and a 6 × 12 × 3 k-grid for the δ phase were used. The kinetic energy and charge density cutoff were 75 and 500 Rydberg, respectively. The Gibbs free energy (G = E + PV – TS) was calculated, where V is the optimized unit cell volume of δ- and γ-CsPbI3 at the corresponding pressure and S is the vibrational entropy of each phase. The entropy S was calculated using the following formula:
| 1 |
where is the energy of the jth phonon mode at momentum q. Phonon dispersions were calculated at 0 K using Quantum Espresso48 and Phonopy52. Thermal expansion was neglected in our calculations, and hence the phonon dispersion and phonon density of states at 0 K were used for entropy calculation at high temperatures. The α- and δ-CsPbI3 phases have been found experimentally to have the same volume expansion coefficient of αv = 1.18 × 10−4 K−1 (ref. 53). It is reasonable to assume γ-CsPbI3 has a similar volume expansion coefficient. Within the temperature range of interest from 200 to 27 °C where γ-CsPbI3 is metastably preserved during cooling, the volume change is about ΔV/V = αv × ΔT = 2% at ambient pressure, and this volume reduction will be smaller at high pressures. Further considering that both the δ- and γ-CsPbI3 phases experience similar volume changes, we expect our calculations are valid for predicting the relative phase stability. Previous computational work on CsSnI3 that studied the thermal expansion effect on the Gibbs free energy further validated our assumption54. With the current computing resources, their calculations were done by assuming that high temperature only changes the volume but does not change other degrees of freedom, such as tilt angles. The Gibbs free energies of α- and γ-CsSnI3 showed a similar behavior as a function of temperature with and without considering the thermal expansion effect54. The quasi-harmonic approximation can be taken to include the thermal expansion effect. However, it requires constructing a set of test structures with different expanded unit cell volumes and performing phonon calculations for all the test structures to find out the structure with the lowest Gibbs free energy. In the case of γ-CsPbI3, the structure has many degrees of freedom, including at least three lattice constants, the Pb–I bond length, and the in-phase and out-of-phase octahedral tilt. The [PbI6]4− octahedron also deviates from the ideal geometry in γ-CsPbI3, adding extra degrees of freedom for structural variations. The large number of degrees of freedom in γ-CsPbI3 makes it extremely complex to construct the test structures. It is also beyond the current computational capacity to perform phonon calculations that consider the thermal expansion due to the astronomically increased number of test structures.
Band-structure calculations
The band-structure calculations were performed using Quantum Espresso with GGA exchange-correlation functional, and a 12 × 8 × 12 k-point grid. The evolution of the bandgap as a function of the in-phase/out-of-phase tilt was calculated by changing one of the tilts and fixing the other to the value at ambient conditions. Through analyzing the high-pressure DFT structures, we found that the in-phase tilt was directly related to the ratio of the two in-plane lattice constants, a and c. Therefore, we tuned the in-phase tilt by increasing the lattice constant a by 0.5%, 1.0%, and 2.0% and decreasing c while keeping the unit cell volume and the fractional coordinates of the Pb and I atoms fixed. This way of constructing the structures was found to effectively change the in-phase tilt angle while minimizing the change of the out-of-phase tilt angle by at least an order of magnitude smaller. In the case of tuning the out-of-phase tilt, the apical I atoms were found to always move within the bc plane while changing the out-of-phase tilt. Hence, we tuned the out-of-phase tilt by rotating the octahedron with the internal bond lengths and angles of the [PbI6]4− octahedron being fixed and moving the apical I atoms within the bc plane.
Supplementary information
Acknowledgements
We thank Martin Kunz, Jinyuan Yan, and Andrew Doran of Beamline 12.2.2 at the Advanced Light Source, and Matthew Smith at Stanford University for experimental assistance. This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division (DE-AC02-76SF00515). Beamline 12.2.2 is a DOE Office of Science User Facility under contract No. DE-AC02-05CH11231. Portions of this work were performed at HPCAT (Sector 16), APS, ANL. HPCAT operations are supported by DOE-NNSA’s Office of Experimental Sciences. The APS is a DOE Office of Science User Facility operated for the DOE Office of Science by ANL under Contract No. DE-AC02-06CH11357. Portions of this work were performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation under award ECCS-1542152. First-principles DFT calculations were supported by the resources of the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility operated under Contract No. DE-AC02-05CH11231. N.R.W. was partially supported by a Stanford Interdisciplinary Graduate Fellowship.
Author contributions
F.K. and Y.L. designed the project and wrote the paper. F.K, C.W., J.Y., S.N, W.L.M., and Y.L. conducted the experiments and analyzed the data. N.R.W. synthesized the sample under H.I.K.’s supervision. C.J and T.P.D. performed the calculations. All authors contributed to the discussion and revision of the paper.
Data availability
All data that support the findings of this study are present in the paper and the supplementary information. Additional data related to the study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Feng Ke, Chenxu Wang, Chunjing Jia.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-20745-5.
References
- 1.Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 2.Yang WS, et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science. 2017;356:1376–1379. doi: 10.1126/science.aan2301. [DOI] [PubMed] [Google Scholar]
- 3.Jeon NJ, et al. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy. 2018;3:682–689. doi: 10.1038/s41560-018-0200-6. [DOI] [Google Scholar]
- 4.Wang Y, et al. Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies >18% Science. 2019;365:591–595. doi: 10.1126/science.aav8680. [DOI] [PubMed] [Google Scholar]
- 5.Swarnkar A, et al. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science. 2016;354:92–95. doi: 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- 6.Xiang S, et al. Highly air-stable carbon-based α-CsPbI3 perovskite solar cells with a broadened optical spectrum. ACS Energy Lett. 2018;3:1824–1831. doi: 10.1021/acsenergylett.8b00820. [DOI] [Google Scholar]
- 7.Zhang T, et al. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017;3:1700841–1700846. doi: 10.1126/sciadv.1700841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, et al. Thermodynamically stable orthorhombic γ-CsPbI3 thin films for high-performance photovoltaics. J. Am. Chem. Soc. 2018;140:11716–11725. doi: 10.1021/jacs.8b06050. [DOI] [PubMed] [Google Scholar]
- 9.Sutton RJ, et al. Cubic or orthorhombic? Revealing the crystal structure of metastable black-phase CsPbI3 by theory and experiment. ACS Energy Lett. 2018;3:1787–1794. doi: 10.1021/acsenergylett.8b00672. [DOI] [Google Scholar]
- 10.Marronnier A, et al. Anharmonicity and disorder in the black phases of cesium lead iodide used for stable inorganic perovskite solar cells. ACS Nano. 2018;12:3477–3486. doi: 10.1021/acsnano.8b00267. [DOI] [PubMed] [Google Scholar]
- 11.Dastidar S, et al. Quantitative phase-change thermodynamics and metastability of perovskite-phase cesium lead iodide. J. Phys. Chem. Lett. 2017;8:1278–1282. doi: 10.1021/acs.jpclett.7b00134. [DOI] [PubMed] [Google Scholar]
- 12.Lai M, et al. Structural, optical, and electrical properties of phase-controlled cesium lead iodide nanowires. Nano Res. 2017;10:1107–1114. doi: 10.1007/s12274-016-1415-0. [DOI] [Google Scholar]
- 13.Straus DB, Guo S, Cava RJ. Kinetically stable single crystals of perovskite-phase CsPbI3. J. Am. Chem. Soc. 2019;141:11435–11439. doi: 10.1021/jacs.9b06055. [DOI] [PubMed] [Google Scholar]
- 14.Beal RE, et al. Cesium lead halide perovskites with improved stability for tandem solar cells. J. Phys. Chem. Lett. 2016;7:746–751. doi: 10.1021/acs.jpclett.6b00002. [DOI] [PubMed] [Google Scholar]
- 15.Swarnkar A, Mir WJ, Nag A. Can B-site doping or alloying improve thermal-and phase-stability of all-inorganic CsPbX3 (X = Cl, Br, I) perovskites? ACS Energy Lett. 2018;3:286–289. doi: 10.1021/acsenergylett.7b01197. [DOI] [Google Scholar]
- 16.Wang Y, Zhang T, Kan M, Zhao Y. Bifunctional stabilization of all-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 2018;140:12345–12348. doi: 10.1021/jacs.8b07927. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, et al. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017;2:2219–2227. doi: 10.1021/acsenergylett.7b00508. [DOI] [Google Scholar]
- 18.Protesescu L, et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15:3692–3696. doi: 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman JB, Schleper AL, Kamat PV. Transformation of sintered CsPbBr3 nanocrystals to cubic CsPbI3 and gradient CsPbBrxI3–x through halide exchange. J. Am. Chem. Soc. 2016;138:8603–8611. doi: 10.1021/jacs.6b04661. [DOI] [PubMed] [Google Scholar]
- 20.Luo P, et al. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016;7:3603–3608. doi: 10.1021/acs.jpclett.6b01576. [DOI] [PubMed] [Google Scholar]
- 21.Fu Y, et al. Stabilization of the metastable lead iodide perovskite phase via surface functionalization. Nano Lett. 2017;17:4405–4414. doi: 10.1021/acs.nanolett.7b01500. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, et al. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018;9:2225. doi: 10.1038/s41467-018-04636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eperon GE, et al. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A. 2015;3:19688–19695. doi: 10.1039/C5TA06398A. [DOI] [Google Scholar]
- 24.Li B, et al. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018;9:1076. doi: 10.1038/s41467-018-03169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, T. et al. Efficient and stable CsPbI3 solar cells via regulating lattice distortion with surface organic terminal groups. Adv. Mater. 31, 1900605 (2019). [DOI] [PubMed]
- 26.Wang K, et al. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15% Nat. Commun. 2018;9:4544. doi: 10.1038/s41467-018-06915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J-K, et al. Polar solvent induced lattice distortion of cubic CsPbI3 nanocubes and hierarchical self-assembly into orthorhombic single-crystalline nanowires. J. Am. Chem. Soc. 2018;140:11705–11715. doi: 10.1021/jacs.8b05949. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Efficient α-CsPbI3 photovoltaics with surface terminated organic cations. Joule. 2018;2:2065–2075. doi: 10.1016/j.joule.2018.06.013. [DOI] [Google Scholar]
- 29.Steele JA, et al. Thermal unequilibrium of strained black CsPbI3 thin films. Science. 2019;365:679–684. doi: 10.1126/science.aax3878. [DOI] [PubMed] [Google Scholar]
- 30.Masi A, Gualdron-Reyes AF, Mosa-Sero I. Stabilization of black perovskite phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020;5:1974–1985. doi: 10.1021/acsenergylett.0c00801. [DOI] [Google Scholar]
- 31.Liu G, et al. Isothermal pressure-derived metastable states in 2D hybrid perovskites showing enduring bandgap narrowing. Proc. Natl Acad. Sci. USA. 2018;115:8076–8081. doi: 10.1073/pnas.1809167115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, et al. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite. Angew. Chem. 2017;129:16185–16189. doi: 10.1002/ange.201708684. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, et al. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite. J. Am. Chem. Soc. 2015;137:11144–11149. doi: 10.1021/jacs.5b06346. [DOI] [PubMed] [Google Scholar]
- 34.Jaffe A, et al. High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties. ACS Cent. Sci. 2016;2:201–209. doi: 10.1021/acscentsci.6b00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffe A, Lin Y, Karunadasa HI. Halide perovskites under pressure: accessing new properties through lattice compression. ACS Energy Lett. 2017;2:1549–1555. doi: 10.1021/acsenergylett.7b00284. [DOI] [Google Scholar]
- 36.Jaffe A, Lin Y, Mao WL, Karunadasa HI. Pressure-induced metallization of the halide perovskite (CH3NH3)PbI3. J. Am. Chem. Soc. 2017;139:4330–4333. doi: 10.1021/jacs.7b01162. [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, et al. Pressure-induced emission (PIE) and phase transition of a two-dimensional halide double perovskite (BA)4AgBiBr8 (BA=CH3(CH2)3NH3+) Angew. Chem. Int. Ed. 2019;131:15393. doi: 10.1002/ange.201906311. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, et al. Pressure-tailored band gap engineering and structure evolution of cubic cesium lead iodide perovskite nanocrystals. J. Phys. Chem. C. 2018;122:9332–9338. doi: 10.1021/acs.jpcc.8b01673. [DOI] [Google Scholar]
- 39.Beimborn JC, Hall LM, Tongying P, Dukovic G, Weber JM. Pressure response of photoluminescence in cesium lead iodide perovskite nanocrystals. J. Phys. Chem. C. 2018;122:11024–11030. doi: 10.1021/acs.jpcc.8b03280. [DOI] [Google Scholar]
- 40.Yaffe O, et al. Local polar fluctuations in lead halide perovskite crystals. Phy. Rev. Lett. 2017;118:136001. doi: 10.1103/PhysRevLett.118.136001. [DOI] [PubMed] [Google Scholar]
- 41.Maalej A, et al. Phase transitions and crystal dynamics in the cubic perovskite CH3NH3PbCl3. Solid State Commun. 1997;103:279–284. doi: 10.1016/S0038-1098(97)00199-3. [DOI] [Google Scholar]
- 42.Stoumpos CC, Malliakas CD, Kanatzidis MG. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013;52:9019–9038. doi: 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- 43.Ming L, et al. Gold as a reliable internal pressure calibrant at high temperatures. J. Appl. Phys. 1983;54:4390–4397. doi: 10.1063/1.332685. [DOI] [Google Scholar]
- 44.Heinz DL, Jeanloz R. The equation of state of the gold calibration standard. J. Appl. Phys. 1984;55:885–893. doi: 10.1063/1.333139. [DOI] [Google Scholar]
- 45.Rekhi S, Dubrovinsky LS, Saxena SK. Temperature-induced ruby fluorescence shifts up to a pressure of 15 GPa in an externally heated diamond anvil cell. High. Temp. -High. Press. 1999;31:299–305. doi: 10.1068/htrt161. [DOI] [Google Scholar]
- 46.Prescher C, Prakapenka VB. DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High. Press. Res. 2015;35:223–230. doi: 10.1080/08957959.2015.1059835. [DOI] [Google Scholar]
- 47.Young JF. Humidity control in the laboratory using salt solutions—a review. J. Appl. Chem. 1967;17:241–245. doi: 10.1002/jctb.5010170901. [DOI] [Google Scholar]
- 48.Giannozzi P, et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009;21:395502. doi: 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen OH, Martin RM. First-principles calculation of stress. Phys. Rev. Lett. 1983;50:697. doi: 10.1103/PhysRevLett.50.697. [DOI] [Google Scholar]
- 50.Nielsen OH, Martin RM. Quantum-mechanical theory of stress and force. Phys. Rev. B. 1985;32:3780. doi: 10.1103/PhysRevB.32.3780. [DOI] [PubMed] [Google Scholar]
- 51.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 52.Togo A, Tanaka I. First principles phonon calculations in materials science. Scr. Mater. 2015;108:1–5. doi: 10.1016/j.scriptamat.2015.07.021. [DOI] [Google Scholar]
- 53.Trots DM, Myagkota SV. High-temperature structural evolution of caesium and rubidium triiodoplumbates. J. Phys. Chem. Solids. 2008;69:2520–2526. doi: 10.1016/j.jpcs.2008.05.007. [DOI] [Google Scholar]
- 54.Silva EL, Skelton JM, Parker SC, Walsh A. Phase stability and tranformations in halide perovskite CsSnI3. Phys. Rev. B. 2015;91:144107. doi: 10.1103/PhysRevB.91.144107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are present in the paper and the supplementary information. Additional data related to the study are available from the corresponding author upon request.