Abstract
The acidic fraction (P3a) of Pleurotus eous was successfully sulfated by sulphur trioxide-pyridine complex method. The effect of sulfate modification (SP3a) on the structure, physicochemical properties and in vitro biological activity of P3 was studied. The structural characteristics were established by UV absorption, FT-IR, HPGPC and GC-MS. Biological studies were carried out, such as in vitro antioxidant, anticoagulant, anti-tumour and antibacterial activities. The sulfation process changed its physicochemical and biological characteristics. Compared with P3a, the molecular weight of SP3a is reduced. P3a and SP3a are composed of galactose, xylose, arabinose with different molar percentages. Sulfated derivatives have strong antioxidant and anticoagulant properties. Compared with P3a, SP3a showed obvious cytotoxicity to Jurkat and HeLa cells. SP3a showed a higher inhibition zone for Gram-positive and Gram-negative bacteria. This article demonstrates that sulfation is an effective way to enhance biological activity, especially SP3a is a promising candidate for bioactive macromolecules and has great potential for industrial and biomedical applications.
Keywords: Pleurotus eous, sulfation, Polysaccharide, Antioxidant, Anticoagulant, Anti-tumour, Antibacterial activity
Pleurotus eous; sulfation; Polysaccharide; Antioxidant; Anticoagulant; Anti-tumour; Antibacterial activity.
1. Introduction
Polysaccharides are essential polymers distributed in vegetation, animals, fungi and bacteria, and have become one of the hot spots in contemporary medical research. Mushroom-derived polysaccharides are being studied in nutrition and pharmaceutical industry (Xie et al., 2016). Recently, increasing attention has been paid on mushroom derived polysaccharides due to their wide range of biological activities (Li et al., 2016, 2019; Liu et al., 2018; Zhang et al., 2015), which in turn depends on molecular weight, monosaccharide composition and degree of substitution and solubility (Gao et al., 2015). The molecular modification of polysaccharides has aroused considerable interest and offers an approach to enhance the bioactivities by changing the structural and conformational properties (Chen and Huang., 2019a, Chen and Huang., 2019b; Wang et al., 2018b; Liu et al., 2017a, Liu et al., 2017b). Among the various chemical modification methods, sulfated modification is usually selected to enhance their biological properties.
Russula virescens polysaccharide exhibited potential antioxidant, anticoagulant, anti-tumour and antibacterial activities (Li et al., 2019) after sulfated modification. Sulfate modification improved the antioxidant activities of polysaccharides isolated from Ganoderma atrum (Zhang et al., 2015), Crasstogerea gigas (Zhao et al., 2019), Sargassum pallidum (Xiao et al., 2019), Mesona chinensis (Huang et al., 2019), Diospyros kaki (Zhang et al., 2011b), Cyclocarya paliurus (Xie et al., 2016) and Pleurotus eryngii (Jia et al., 2017). Sulfation of marine green algae Enteromorpha linza (Wang et al., 2013), Gracilaria debilis (Sudharsan et al., 2015), Pleurotus sajor caju (Telles et al., 2011) and Catathelasma ventricosum (Liu et al., 2018) was found to be effective anticoagulant and antioxidants. The sulfated polysaccharides of Cyclina sinensis and Tricholoma lobayense demonstrated significant inhibitory activity on human gastric cancer (BGC-823), human cervical cancer (HeLa) and breast cancer (MCF) cell lines respectively (Li et al., 2016; Jiang et al., 2015). After sulfation, the polysaccharides of Pleurotus eryngii and Streptococcus thermophilus displayed higher activity against Gram-positive and Gram-negative bacteria (Li and Shah, 2014). From these studies, it is evident that the sulfated modification elevates the biological properties of polysaccharides.
Pleurotus mushroom belongs to the Basidiomycota family and is one of the most cultivated mushrooms across the globe. Compared with other mushrooms, it has high health benefits (Inácio et al., 2015). Edible variety of mushrooms of the species Pleurotus such as P. florida, P. ostreatus, P. djamor, P. citrinopileatus, P. sajor-caju and P. eous are considered non-toxic. Polysaccharide extraction and its biological properties from Pleurotus sajor-caju (Seedevi et al., 2019; Rout et al., 2008), Pleurotus florida (Pramanik et al., 2007), Pleurotus ostreatus (Silva et al., 2012), Pleurotus eryngii (Xu et al., 2016) and Pleurotus tuber-regium (Wu et al., 2013) have been reported. Pleurotus eous (Berk.), commonly known as the pink oyster mushroom, is a nutritious and valuable edible mushroom. However, till date, there seems to be no investigation on purification and sulfated modification of purified acidic fraction of Pleurotus eous polysaccharide. Therefore, this study inspired us to explore the potential, functional and medicinal value of this mushroom polysaccharide. The present study illustrates the sulfation of acidic fraction (P3a), its structural characterization and biological properties. Besides, the anti-oxidant, anti-coagulant, anti-bacterial and anti-tumour activity P3a was compared with SP3a.
2. Materials and methods
2.1. General
Fresh fruit bodies of Pleurotus eous were sourced from a Mushroom lab, Bio-Centre, Department of Horticulture, Bengaluru, Karnataka, India. The human cervical cancer cell (HeLa) and human lymphocytic leukaemia cell-lines (Jurkat) were procured from the National Centre for Cell Science, India. DMEM medium, fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco/Invitrogen (Grand Island, NY, USA). DEAE cellulose 52, Sephadex G-100, T series dextrans, monosaccharide standards, glucose (Glc), galactose (Gal), arabinose (Ara), mannose (Man), fucose (Fuc), ribose (Rib), rhamnose (Rha), xylose (Xyl), glucuronic acid (GluA), galacturonic acid (GalA), sulfur trioxide-pyridine complex (S03-Py), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Bovine serum albumin (BSA), Fetal Bovine Serum (FBS), dimethylformamide (DMF) Coomassie brilliant blue G250, bovine serum albumin (BSA), Vitamin C (Vc), were obtained from Sigma Aldrich (St. Louis, MO, USA). The DEAE cellulose 52 for the fast flow was obtained from GE Healthcare Life Science (Piscataway, NJ, USA). APTT, PT and TT reagent kit was purchased from Agappe diagnostic Ltd., Cochin. All other chemicals are analytical reagent grade and purchased from Himedia in Mumbai, India and Merck, India.
2.2. Crude P. eous polysaccharide
Hot water extraction (HWE) and alcohol precipitation is the most popular and convenient method and has been widely used in laboratories and industry (He et al., 2014). It has the advantages of simple operation, low equipment requirements and cost-effective. The plasma wall of the cell is separated with hot water, and the contents of vacuole are dissolved by diffusion into an external solvent. It dissolves the non-digestible fiber, which can be removed from the extract when water is removed. The Pleurotus eous (100 g) was ground into a powder and soaked in 90% petroleum ether overnight to remove the lipid substances. Filter and extract three times with hot water (1:25 (w/v), 100 °C, 3 h). The liquid part was reduced to one-tenth of the actual volume using a rotary evaporator and to the resultant liquid, ethyl alcohol (five times) was added, vigorously stirred and stored at 4 °C. Centrifuge at 8000 rpm (20 min), collect the precipitate, re-dissolve in deionized water, then use Sevags reagent (chloroform: n-butyl alcohol = 4:1, v/v) for deproteinization treatment (Tang et al., 2015). The resulting fraction was dialyzed for 72 h, and lyophilized to obtain Pleurotus eous crude polysaccharide (PECP). The whole process was performed in three different time intervals to check the consistency and observed almost the same.
2.3. Purification of P. eous crude polysaccharide
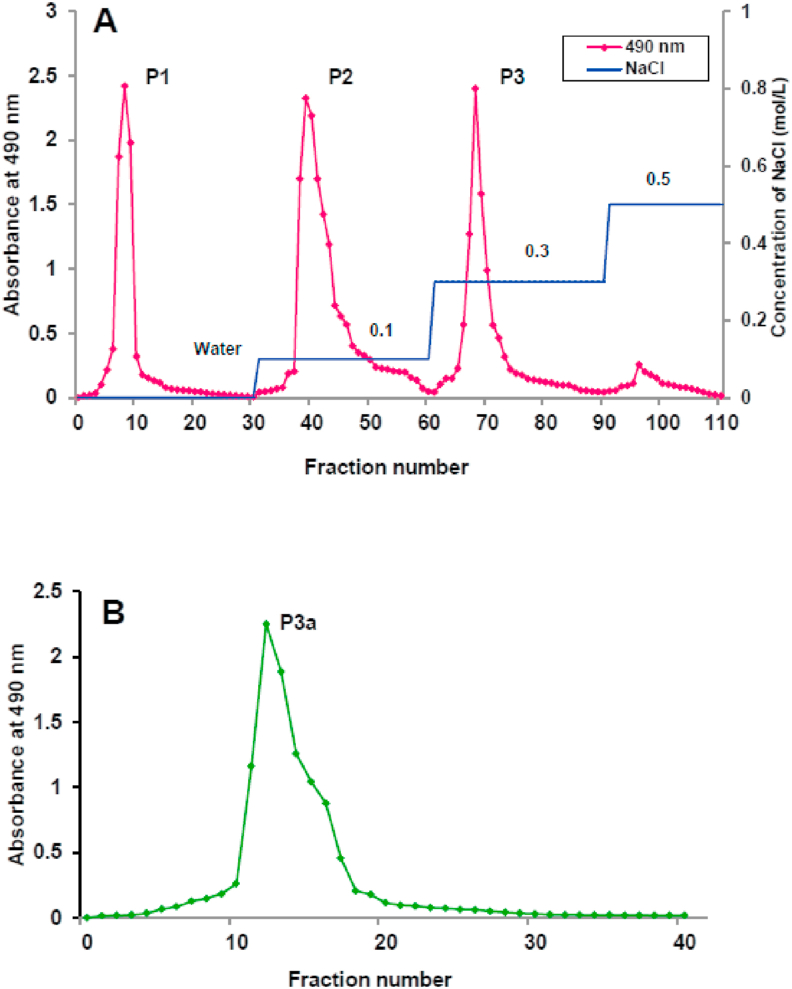
PECP was purified by DEAE-52 and Sephadex G-100 chromatography as per the described protocols by minor alterations (Xiong et al., 2013). After equilibrating the DEAE cellulose-52 column with deionized water, the PECP solution (5 mL, 20 mg/mL) was loaded into it. The polysaccharide was fractionated and eluted with double-distilled water, and then using a saline solution (0.1, 0.3 and 0.5 mol/L) was gradually added at a flow rate of 1 mL/min (5 mL/tube). The first fraction was eluted with double-distilled water (neutral polysaccharide), whereas the second and third fractions were eluted by using 0.1 M and 0.3 M brine solution (acidic polysaccharide) respectively (Hokputsa et al., 2004). The phenol–sulfuric acid process was utilized to determine the carbohydrates in the neutral and acidic polysaccharide fractions by measuring the absorbance at 490 nm. The 0.3 N NaCl fractions showing a single peak was recovered, lyophilized and further purified on Sephadex G-100 gel filtration assembly (1.6 × 50 cm). Purification was completed by using deionized water (run rate: 0.5 mL/min) to obtain pure of P3a fraction, which was dialyzed, lyophilized and used for further study. The reliability of the purification process was checked by performing the process for three times and the results are found to be same.
2.4. Sulfation of P3a
Sulfation was performed according to a procedure reported by Yang et al. (2003). The P3a (200 mg) was mixed with dimethylformamide (40 mL, 25 °C, stirred for 30 min) and about 400 mg of sulfur trioxide pyridine (SO3-Pyr) complex was supplemented, and heated to 80 °C (3 h), and then brought down to 25 °C using a cooling bath and made to neutral pH using sodium hydroxide solution (4 M). Further, it was dialyzed against distilled water at 4 °C for five days by changing the water two times a day. The resulting solution was collected, lyophilized to attain the sulfated derivative (SP3a) and stored at 30 °C for further analysis. The sulfation experiment is reproducible as it was performed multiple times on a small scale.
2.5. Structural analyses
2.5.1. Determination of degree of substitution (DS)
The percentage yield of SP3a is calculated as given in Eq. (1).
| Yield (%) = (W1/ W0) × 100 | (1) |
Where W1 = weight of SP3a and W0 = weight of P3a
The quantification of sulfate was performed by barium chloride-gelatin process (Liang et al., 2018), using potassium sulfate as the standard and DS was calculated as per Eq. (2) given below
| DS = [(1.62 × S%) / (32–1.02 × S%)] | (2) |
The DS represents the average number of O-sulfate groups per sugar residue, and the S (%) is the content of sulfate in the sulfated derivative.
A CHNS (Carbon, Hydrogen, Nitrogen and Sulfur) elemental analyzer (Perkin Elmer 2400 series II) was used to quantify the sulfur content. The calculation of DS was done according to Neto et al. (2011) using the formula given in Eq. (3).
| DS = 2.25 × S% / C% | (3) |
2.5.2. Chemical composition
The overall sugar content was evaluated through the calibration curve of standard glucose by the phenol-sulfuric acid method (Dubois et al., 1956). The carbazole method (Karamanos et al., 1988) is used to determine the uronic acid by galacturonic as the reference compound. The Bradford protein assay measures protein content by using standard BSA (Li and Shah, 2016). Entire measurements were done in three-fold.
2.5.3. Molecular weight (Mw)
High-performance size-exclusion chromatography was used for determining molecular weight (Mw) of P3a and SP3a provided with a refractive index detector (RID) and Shodex SB-804 HQ column (300 × 8.0 mm, Showa Denko Corp., Tokyo, Japan). The temperature of the column was maintained at 45 °C. The mobile phase is ultra-pure water, the injection volume is 10 μL, and the constant flow rate is 0.9 mL/min. By plotting the logarithm of the molecular weight against the retention value (RT) of the standard, a standard curve was established using dextran of different molecular weights, and the molecular weight was estimated based on this.
2.5.4. Monosaccharide composition
GC-MS instrument is utilized to determine the monosaccharide configuration of P3a and SP3a. The sample (10 mg) was hydrolyzed with trifluoroacetic acid (2 mL, 2 M) at 120 °C for 2 h. The hydrolysed sample was then reduced by NaBH4, derivatized with N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA), and acetylated with acetic anhydride at 100 °C (1 h). The resulting sugar alcohol acetate was analyzed on a Perkin-Elmer Clarus 690 AQ8 autosampler GC-MS instrument equipped with a PDA detector and an RTX-5MS fused silica capillary column (30 m, 0.32 mm ID, 0.25 μm thickness). Derivatives were separated at a ratio of 20:1 with 1 μL injection at a flow rate of 1 mL/min of helium. The temperature program starts at 65 °C (hold for 2 min), increases at a rate of 20 °C/min to 180 °C (holds for 2 min), and then increases at a rate of 5 °C/min to 280 °C for 10 min. Derivatize standard monosaccharides (glucose, galactose, arabinose, mannose, fucose, ribose, rhamnose, xylose, glucuronic acid and galacturonic acid) under the same conditions, and compare standard monosaccharides. The retention time and mass spectrometry determine the composition of monosaccharides from the library.
2.5.5. Spectral analysis
In the wavelength of 200–500 nm (UV-1800; Shimadzu, Kyoto, Japan), ultraviolet-visible spectra of P3a and SP3a (1 mg/mL) were recorded. The functional groups present in P3a and SP3a were identified by a Thermo Scientific Nicolet 5700 IR spectrophotometer by mixing 1 mg dry sample with 100 mg KBr and pressing into pellets.
2.5.6. Congo red analysis
The triple-helix properties of polysaccharides are found by the Congo red method (Rout et al., 2008) by the redshift of the Congo red–polysaccharide complex. By observing the maximum absorption wavelength (λmax) of Congo-red polysaccharides at various concentration of sodium hydroxide, a transition from a triple-helix structure to a single-stranded conformation was observed. The sample (6 mg/2 mL) dissolved in distilled water was reacted with Congo red (2.0 mL, 100 μM) in a gradient of sodium hydroxide solution (0.1–0.5 M). At each sodium hydroxide concentration, the absorbance was measured. Distilled water served as the control.
2.6. Antioxidant activity
Through various in vitro tests, such as DPPH radical scavenging activity (Shimada et al., 1992; Subramanian and Ramani, 2020), hydroxyl radical scavenging activity (Smirnoff and Cumbes, 1989) and ferric reducing antioxidant capacity (Oyaizu 1986), the antioxidant studies of samples P3a and SP3a were evaluated. Ascorbic acid (Vc) was used as a positive control in all the assays.
2.7. Anticoagulant activity
The anticoagulant activity of P3a and SP3a (5 and 10 mg/mL dissolved in saline) was evaluated by using a semi-automatic hemagglutinator (Transasia Bio-medicals, Mumbai, India Ltd.). According to the reported protocol, in vitro coagulation determination of activated partial thromboplastin time (APTT), prothrombin time (PT) and thrombin time (TT) was determined using specific reagents and standard human plasma (Liu et al., 2018).
2.8. Anti-proliferative activity on HeLa and Jurkat cell lines
The in vitro antiproliferative activity of P3a and SP3a (10, 20, 40, 80, 160 and 320 μg/mL) against HeLa and Jurkat cell lines were measured by MTT method (Mosmann, 1983). Cisplatin was chosen as a positive control. The cells were grown in DMEM medium supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin (100 IU/mL) in a humid environment (37 °C, 5% CO2). Cell suspensions of HeLa and Jurkat cells were seeded in 96-well microplates at a density of 5 × 103 cells/mL. Add 100 μL of suspension to each well, incubate for one day, and treat with different concentrations of P3a and SP3a. After incubating for one day of at 37 °C, MTT reagent (20 μL, 5 mg/mL) was added to each well, and further incubated for four hours. The supernatant was removed and restored with DMSO (150 μL). The absorbance was recorded (490 nm) using a Bio Tek ELISA microplate reader, Winooski, Vermont, USA. The anti-proliferative activity is calculated using the formula given in Eq. (4).
| Antiproliferative activity (%) = [(Ac–As)/(Ac–Ab)] × 100 | (4) |
where As, Ac and Ab represent the absorbance of sample, reference and blank, respectively.
2.9. Antibacterial analysis
The antibacterial properties of P3a and SP3a were performed against Gram-negative bacteria: Escherichia coli, Klebsiella pneumonia and Gram-positive bacteria: Staphylococcus aureus, Bacillus subtilis. The bacterial cells were grown in nutrient agar medium at 37 °C and sub-cultured before each experiment. As described by previous authors, the antibacterial activity was determined by a good diffusion method of agar (Valgas et al., 2007; Chirayath et al., 2019; Subramanian et al., 2018). Spread 200 μL of 1 × 106 colonies forming unit (CFU)/mL of bacterial cell suspension on Mueller–Hinton broth agar media. A hole with a diameter of 5 mm was punched into the agar medium, and 20 μL of P3a and SP3a (25 and 50 mg/mL) were inoculated, and the plate was incubated at 37 °C for one day. The antibacterial activity was observed by observing the inhibition area (mm) and compared with chloramphenicol (positive control) and distilled water (negative control).
2.10. Statistical analysis
All the protocols are performed thrice and the data represented as mean ± standard deviation. One-way ANOVA was performed to use Duncans Multiple- Range Test (DMRT) (SPSS statistical version 20) to determine the significance of differences between samples. The value of p < 0.05 was used as the significance threshold.
3. Results and discussion
3.1. Extraction and purification
The crude polysaccharide (PECP) resulted after hot water extraction, precipitation with ethanol, Sevag's deproteinization and lyophilization were then purified by ion-exchange chromatography on DEAE-52 cellulose column. PECP is divided into three parts, called P1, P2 and P3 (Figure 1A). P3 was applied to a Sephadex G-100 gel filtration column for further purification. The column was eluted with ultrapure water, and then the subsequent eluent was concentrated, dialyzed and lyophilized. As displayed in Figure 1B, the single elution peak P3a is further sulfated to obtain SP3a.
Figure 1.
(A) Stepwise elution curve of PECP on DEAE-52 chromatography column with gradient of NaCl solution (0, 0.1, and 0.3 M); (B) The elution curve of P3 on Sephadex G-100 gel chromatography column with distilled water.
3.2. Chemical analysis and DS
The yield and DS of P3a and SP3a are summarized in Table 1. The yield of SP3a was 10.58 ± 0.42 mg/(100 mg dry weight P3). Due to degradation at relatively high temperatures during the sulfation process, yields are reduced (Wang et al., 2010; Sandhya et al., 2018). This is consistent with the prior investigation (Liu et al., 2018).
Table 1.
Degree of substitution and yield of P3 and SP3a.a
| Sample | Yield (w/w %) | BaCl2 assay |
Elemental analysis (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| S (%) | DS | C | H | N | S | DS | ||
| P3 | - | 3.02 | 0.169 | 41.08 | 6.25 | 4.99 | 2.79 | 0.152 |
| SP3a | 10.58 | 7.50 | 0.498 | 41.11 | 6.11 | 3.87 | 8.09 | 0.443 |
Values are expressed as mean ± SD (n = 3).
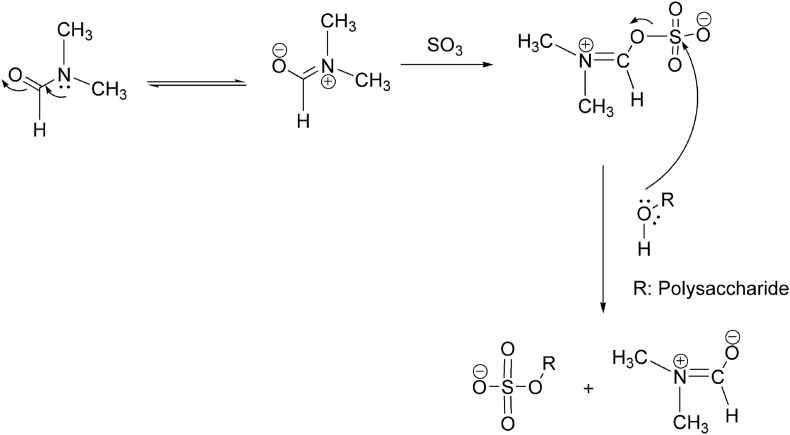
DS is viewed as one of the significant factor influencing the structure and bioactivities of polysaccharide. DS is utilized to quantify the level of hydroxyl substituted by the sulfated group which is evaluated by BaCl2 - gelatin method. The sulfur content in polysaccharides was determined by SO3-Pyr method and the DS method. A general sulfation and reaction mechanism is shown in Figure 2.
Figure 2.
General sulfation and reaction mechanism.
The sulfur content detected in P3a was 3.02 %, but it increased to 7.50 % after sulfation and the DS was 0.498. The elements in P3a and SP3a were analyzed by CHN analyzer, and P3a contained 41.08 % carbon, 6.25 % hydrogen, 4.99 % nitrogen, and 2.79 % sulfur, followed by SP3a containing 41.11 % carbon, 6.11 % hydrogen, 3.87 % nitrogen and 8.09 % sulfur. The sulfur content of SP3a determined by elemental analysis is 2.79 % higher than that of natural polysaccharides, reaching 8.09 %, and the DS of SP3a is 0.443. The increased sulphate content of SP3a as compared to P3a indicates successful sulfation. These results are on par with the findings of Li et al. (2019) and Liu et al. (2018) reveal the sulfated modification in Russula virescens and Catathelasma ventricosum.
Polysaccharides display a wide range of solubility and in particular, water solubility is essential for polysaccharides to employ their functional properties in the medicinal application. PEPA-2 is soluble in water and SPEPA-2 was highly soluble in water. The chemical composition of P3a and SP3a (Table 2) is determined by measuring the content of sugar, protein and uronic acid. P3a contains 80.6% carbohydrate, 2.01 % protein and 7.55 % uronic acid. It was found that the contents of carbohydrate, protein and uronic acid in SP3a were 68.80 %, 0.50 % and 16.34 % respectively. Compared with natural P3a, the chemical composition of sulfated derivatives has changed significantly. The carbohydrate and protein content in SP3a increased significantly. Studies have shown that sulfated modification will change the chemical composition of P3a, which may affect the different bioactivities of polysaccharides. The outcomes of the tests are on par with the report on the sulfate modification of Russula virescens study by Li et al. (2019). The uronic acid content is higher than P3a, which is due to slight degradation that may occur during the sulfation process. The present findings are on par with the study of Huang et al. (2019).
Table 2.
Physicochemical properties, molecular weight and monosaccharide composition analysis of P3 and SP3a.a
| Physicochemical properties | P3 | SP3a |
|---|---|---|
| Colour | Brown | Dark brown |
| Solubility | Soluble | Easily soluble |
| Carbohydrate (%) | 80.60 ± 5.68 | 68.80 ± 3.95 |
| Protein (%) | 2.01 ± 0.10 | 0.50 ± 0.02 |
| Uronic acid (%) | 7.55 ± 0.30 | 16.34 ± 0.95 |
| Mw (Da) | 5.660 × 103 | 2.513 × 103 |
| Monosaccharide composition (%) | ||
| Glucose | 4.80 | 7.86 |
| Galactose | 24.70 | 78.42 |
| Rhamnose | 9.76 | 1.53 |
| Ribose | 6.54 | - |
| Xylose | 14.33 | 6.18 |
| Arabinose | 29.94 | - |
| Mannose | 9.93 | 6.02 |
Values are expressed as mean ± SD (n = 3).
3.3. Molecular weight and monosaccharide composition
The biological activity of polysaccharides is affected by their molecular weight. As per the calibration curve of dextran standards, the molecular weight of P3a and SP3a was calculated to be 5.660 × 103 and 2.513 × 103 Da respectively as depicted in Table 2. The results show that both P3a and SP3a are both low molecular weight polysaccharides. Low molecular weight polysaccharides have high biological activity and our findings agree with the literature of Yang et al. (2008) and our findings are on par with the literature. It can be inferred from our research that the relative molecular mass of SP3a is less than P3a. Li et al. (2019) reported that the Mw of sulfated derivatives were reduced, compared to the native R. virescens polysaccharide (RVP), which is consistent with the current findings. The decrease in Mw may be owing to the mild degradation of polysaccharides, and these results are in agreement with earlier reports (Zhang et al., 2015; Liu et al., 2009).
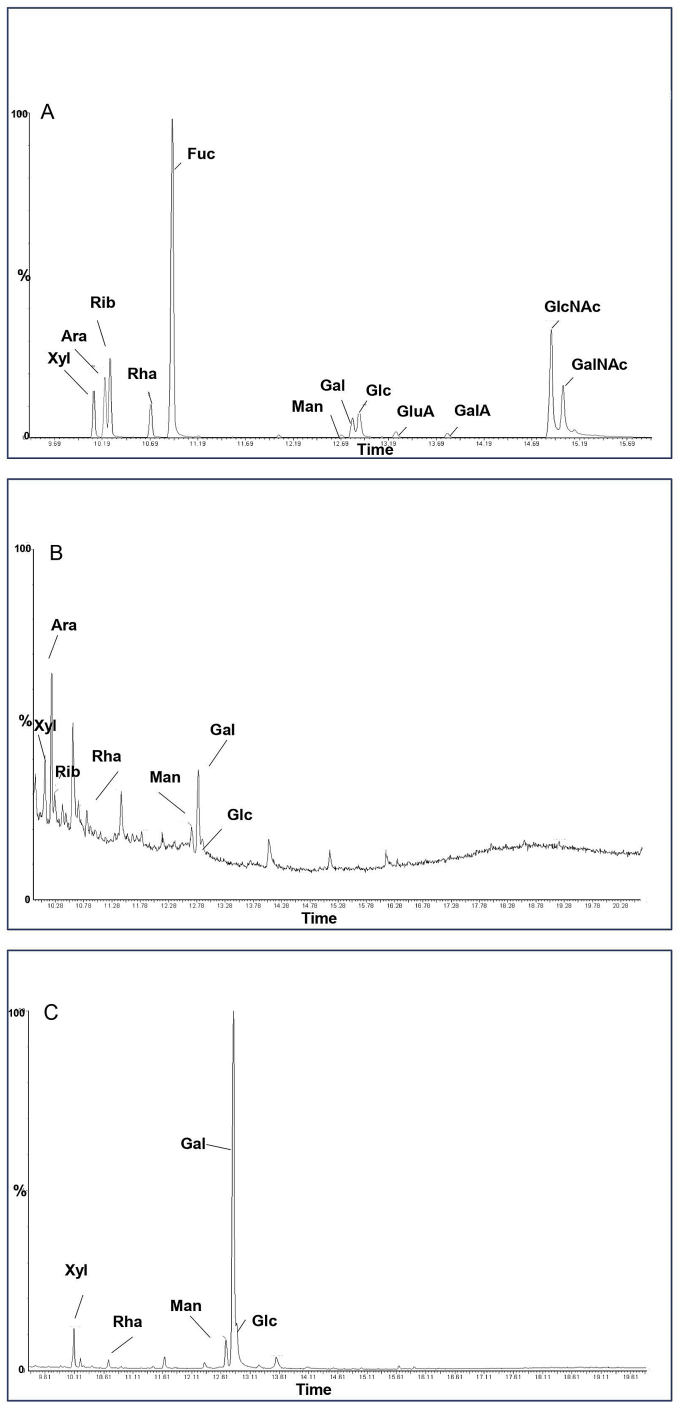
The biological efficacy of polysaccharides is closely associated with its monosaccharide composition and was analyzed using HPGPC. The type of monosaccharide is determined by comparing the retention time among sample and standard. The compositions of standard monosaccharides, P3a and SP3a are presented in Figure 3 (A, B and C) and Table 2 respectively. P3a and SP3a are heteropolysaccharides. P3a, contains xylose, arabinose, ribose, rhamnose, mannose, galactose and glucose, the molar percentage are 14.33, 29.94, 6.54, 9.76, 9.93, 24.70, 4.80, while SP3a consists of xylose, rhamnose, mannose, galactose and glucose, the molar percentages are 6.18, 1.53, 6.02, 78.42 and 7.86. Arabinose and galactose are the predominant sugars in P3a, while SP3a is galactose. Sulfated modification can change the mole percentage of each monosaccharide without changing the type of monosaccharide. In the absence of Rib and Ara, the mole percentage of Gal and Glu in SP3a increased, while the mole percentage of Xyl, Rha, and Man decreased. Our findings revealed that the sulfation modification changes the monosaccharide composition and additionally reduces the sugar content, which may be due to mild degradation of the polysaccharide backbone in the course of sulfation procedure (Wang et al., 2010). Our results are consistent with previous studies (Li et al., 2019).
Figure 3.
GC of monosaccharides: (A) Standards, (B) P3 and (C) SP3a.
3.4. Spectral analysis
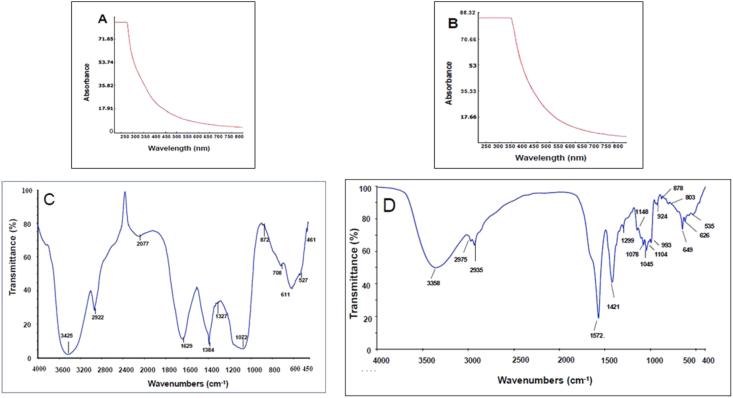
The UV absorption spectra of P3a and SP3a have no absorption peak at 260–280 nm indicating the absence of protein (Figure 4 A and B). Based on the low protein content, this observation is consistent with Table 2.
Figure 4.
UV-visible spectra of P3 (A) and SP3a (B), FT-IR spectra of P3 (C) and SP3a (D).
The characteristic strong broadband near 3425/3358 cm−1 point to the presence of O–H stretching, which indicates that there are strong intermolecular and intramolecular interactions between polysaccharide chains (Figure 4 C and D). The peak around 2922/2935 cm−1 is due to C–H tensile vibration (Chen and Huang., 2019a, Chen and Huang., 2019b; Chen et al., 2011). Due to the presence of more acidic hydroxyl groups (present in SO3H), a decrease in O–H tensile peak strength was observed in SP3a, resulting in a higher dissociation of this group. Compared with P3a, two new absorption peaks at about 1299 cm−1 and 803 cm−1 was found in the spectrum of SP3a which belongs to S–O (asymmetric stretch) and C–O–S (symmetric stretch) respectively. The peaks around 800-850 cm−1 can be used to infer the location of sulfate radicals in sulfated polysaccharides (Chen et al., 2015). FTIR spectroscopy successfully confirms the sulfation process through the presence of the sulfate group in SP3a. Similar results have found in other sulfated polysaccharides (Li et al., 2016; Chen et al., 2015; Zhang et al., 2015).
3.5. Congo red analysis
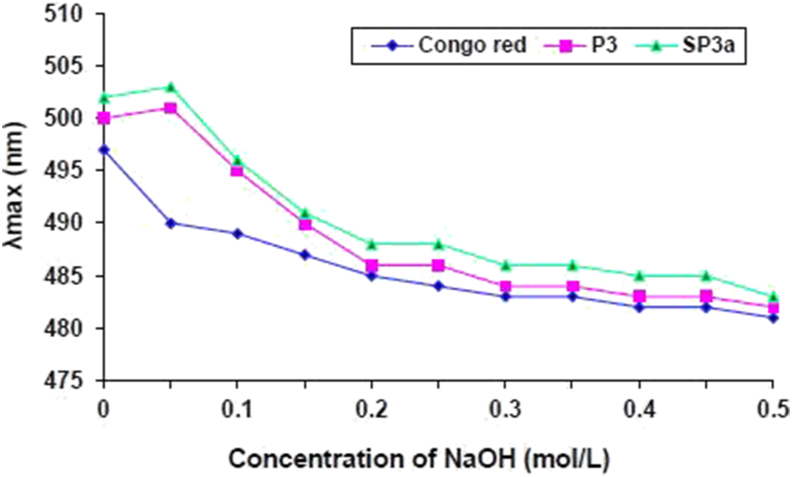
Congo red (an acidic dye) can form a triple-helix configuration complex with polysaccharides and cause a redshift in the maximum absorption wavelength (λmax) (Palacios et al., 2012). Figure 5 depicts the λmax change of Congo red in the existence of P3a and SP3a at different concentrations of sodium hydroxide (0–0.5 M). The results show that as the sodium hydroxide concentration increases, λmax first increases, and then gradually decrease until it reaches a constant. In the presence of P3a and SP3a, the λmax of Congo red has been red-shifted to a large extent, which indicates that they have a triple-helix conformation in low concentration of sodium hydroxide (0–0.2 M). As the NaOH concentration increased above 0.2 M, the λmax of Congo red-polysaccharide complex progressively reduced, demonstrating that the triple-helix structure decomposed into random coils. At alkaline pH, the triple-helix of polysaccharides is confirmed to be broken by the breaking of intramolecular and intermolecular bonds, and the λmax will be significantly reduced. Previous studies have stated that sulfated polysaccharides display diverse intra-molecular hydrogen bonds and show chain extension that expands as sulfate groups increase (Li et al., 2019; Liu et al., 2018).
Figure 5.
Change in the λmax of P3 and SP3a at various NaOH concentrations.
3.6. In vitro antioxidant activity
In the present study, DPPH, hydroxyl radicals and reducing power were chosen for the evaluation of the antioxidant activity. Recent studies show that DPPH and hydroxyl radical scavenging activity can be enhanced by sulfated alteration (Kishk and Al-Sayed, 2007; Liu et al., 2016).
3.6.1. DPPH radical scavenging analysis
This is one of the most frequently used processes to decide the antioxidant behaviour of natural compounds (Fu et al., 2013). By receiving electrons or hydrogen from antioxidants, DPPH radicals are reduced to the corresponding hydrazine, thereby turning purple into yellow, reflecting the free radical scavenging ability of the sample, which can be measured at 517 nm.
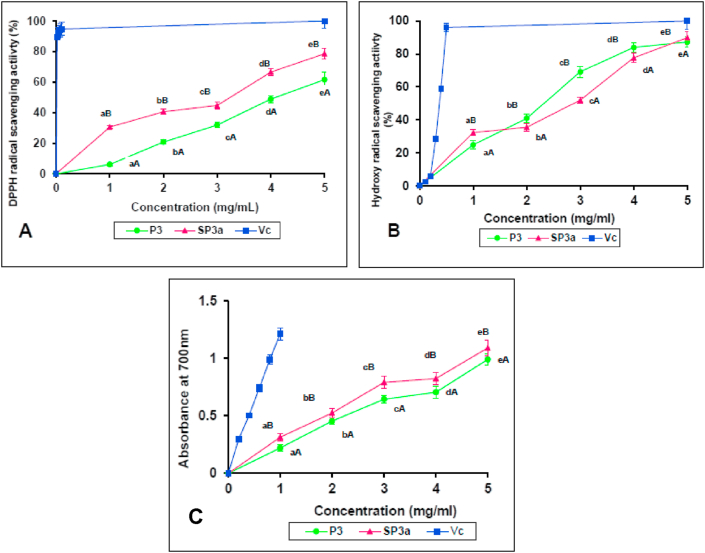
The ability of P3a and SP3a to scavenge DPPH free radicals is represented in Figure 6A. As the concentration of the sample increases, the removal effect increases. At 1–5 mg/mL, the scavenging capacity of P3a and SP3a on DPPH free radicals increased from 6.14 to 61.60% and 30.66–78.58%, respectively, and SP3a was the most effective. The EC50 of P3a and SP3a were 4.16 and 2.81 mg/mL, respectively, but lower than Vc (0.03 mg/mL). At each concentration, the clearance activity between P3a and SP3a varies significantly. The highest scavenging activity of SP3a (1 mg/mL) was 30.66%, which was five folds higher than P3a (6.14% of 1 mg/mL). In this study, SP3a showed 30.66% DPPH clearance activity, while sulfated P. eryngi polysaccharide (PEPS) showed only 14.55% clearance rate (Li and Shah, 2014), which indicates SP3a is more efficient in scavenging DPPH radical. Previous studies focused on sulfated derivatives showed better antioxidant effects than the original polysaccharides (Liu et al., 2018; Li et al., 2016; Chen et al., 2015) which is consistent with this study and proves the significance of the sulfate group in antioxidant capacity. DPPH free radicals are converted into stable DPPH-H molecules through hydrogen donation by antioxidants (Chen et al., 2008). Higher the sulfate group substitution, the stronger will be the scavenging ability. The SO3H group promotes the capture of electrons by DPPH, thereby enhancing the antioxidant capacity of polysaccharides. Compared with P3a, SP3a has a strong proton donor ability by activating the anomeric carbon atoms and can be used as a free radical inhibitor or scavenger. The reason for higher DPPH scavenging activity of SP3a compared to P3a may be the difference in the mass percentage of monosaccharides and their side-chain bonds. Secondly, polysaccharides with lower Mw may have better water solubility, which can make contact between active sites and free radicals easier (Xu et al., 2015).
Figure 6.
Antioxidant activities of P3 and SP3a in vitro: (A): DPPH radical scavenging assay, (B): hydroxy radical scavenging assay, (C): reducing power assay. a-e Different letters indicate a significant difference (p < 0.05) between the concentrations of the same polysaccharide. A−B Different letters indicate a significant difference (p < 0.05) between different polysaccharide in the same concentration (Vitamin C (Vc) was excluded). Results were expressed as mean ± SD (n = 3).
3.6.2. Hydroxy radical scavenging analysis
Hydroxy radicals, the supreme toxic ROS react with various biomolecules in living cells, resulting in peroxidative damage and cell death (Yuan et al., 2008). The scavenging ability of P3a and SP3a for hydroxyl radicals increased from 1.0 to 5.0 mg/mL in a dose-dependent mode (Figure 6B). The scavenging effect of P3a in 5 mg/mL was 87.24%, the EC50 value was 2.52 mg/mL, while the scavenging effect of SP3a in 5 mg/mL was 89.91% and the EC50 value was 2.33 mg/mL. The scavenging activity of vitamin C (Vc) at 0.5 mg/mL was 96.01%, and the EC50 was 0.37 mg/mL. Compared with Vc, the clearance ability of the two samples is weaker, but the clearance ability of P3a is lower than that of SP3a. This result indicates that SP3a with sulfate groups exhibited better scavenging activity towards hydroxyl radicals (•OH), which is consistent with the results of Li et al. (2019). Compared with natural polysaccharides, sulfated Tricholoma lobayense (STLH-3) improves the ability to scavenge hydroxyl radicals (Li et al., 2016), similar to this study. According to the results reported by Li and Shah (2014), Pleurotus eryngii polysaccharide with greater DS was found to be further effective in quenching hydroxyl radicals than non-sulphated polysaccharides, and our results are consistent with this study. Sulfation can improve the water solubility of the polysaccharide, and reduce its chain conformation. Also, the sulfated group can activate the hydrogen atom of the anomeric carbon, thereby providing thee polysaccharide with a strong hydrogen donor ability, changing its biological activity. Wang et al. (2008) proposed two anti-oxidant mechanisms, one is to use metal complexes to inhibit the generation of hydroxyl radicals, and the other is to directly eliminate the generated hydroxyl radicals.
3.6.3. Reducing power
In the determination of reducing power, antioxidants will cause Fe3+ to be reduced to Fe2+, which will change the solution from green to blue according to the reducing power of the sample, which may be an indicator of its possible antioxidant behaviour (Ferreira et al., 2007). This method is commonly utilized to estimate the ability of antioxidants to contribute electrons. Reducing power is an important indicator of antioxidant activity. Figure 6C portrays the reducing power of P3a, SP3a and Vc. The reducing capacity increases with the increasing concentration, displaying a dose-effective relationship. The absorbance of P3a at 5 mg/mL is 0.991, the EC50 is 2.43 mg/mL, and at the same concentration, the absorbance of SP3a is 1.091 and the EC50 is 1.87 mg/mL. The reducing power of SP3a is significantly greater than that of P3a (p < 0.05), but the absorbance of natural and modified derivatives is lower than Vc (1.213, 1.0 mg/mL). The results of reducing ability of P3a and SP3a indicate that sulfonic acid groups associated with the reducing ketones of polysaccharides can function as electron contributors and respond to free radicals and change them into further steady compounds. The reducing properties are typically related to the existence of reducing ketones, which have been presented to exert antioxidant effects by donating hydrogen atoms to destroy free radical chains (Liu et al., 2017a, Liu et al., 2017b). Compared to TLH-3, the reducing power of sulfated T. lobayense polysaccharide (STLH-3) has been improved, which supports our research that the sulfated polysaccharide has higher reducing power than the natural polysaccharides (Liu et al., 2016). The absorbance of sulphated mycelial polysaccharide of Catathelasma ventricosum (sm CVP-1S10) is only 0.75, which is lower than SP3a (0.825) in this study. Besides, SP3a showed a reducing ability of 0.325 at 1 mg/mL, which was lower than the reducing ability of sulphated Artemisia sphaerocephala polysaccharide (SASP-5) (0.114 at 1 mg/mL). By comparing other sulfated polysaccharides, SP3a has substantial advantages in terms of reducing capacity.
In the determination of three antioxidants, SP3a showed significant antioxidant activity, and the results revealed that the action of sulfated derivatives was enhanced due to sulfate group (Li et al., 2016), which indicates that the sulfate group is the importance of the antioxidant activity. Also, the content uronic acid is considered to be an important indicator reflecting the antioxidant activity of the polysaccharides. Generally, polysaccharides have free radical scavenging activity due to their ability to donate electrons or hydrogen. The uronic acid groups present in polysaccharides can interact with hydrogen atoms on the anomeric carbon atoms. Therefore, the higher the uronic acid, the stronger the antioxidant activity. The lower the molecular weight, the higher the antioxidant activity of polysaccharides. In this study, the SP3a has the highest uronic acid content and the lowest Mw, which is consistent with previous reports (Li et al., 2019; Huang et al., 2019). It can be inferred that the antioxidant activity of polysaccharides is influenced by a combination of multiple factors, such as uronic acid content, sulfate content, molecular weight, monosaccharide composition, structure or conformation. Therefore, the sulfation modification of polysaccharides may be an effective method to obtain new antioxidants.
3.7. In vitro anticoagulant activity
Sulfated polysaccharides are gaining contemporary significance and acts as good anticoagulant drugs when compared to traditional treatment methods using heparin. The anticoagulant activity of natural (P3a) and its sulfated derivative (SP3a) was measured in vitro by APTT, TT and PT analysis. Heparin a known commercially available anticoagulant was used as a positive control. Functional tests that can detect clump formation are APTT, PT and TT analysis which can assess blood clot formation and assess the intrinsic, extrinsic and third clotting stages in plasma (Leadley et al., 2000).
A concentration-dependent increase in clotting time was noticed in P3a and SP3a at the tested concentrations (Table 3). Compared with P3a, SP3a prolongs the clotting time with an increase in sample concentration. The prolonged APTT, PT and TT respectively represent the internal pathway to inhibit the coagulation process, the external pathway and inhibit thrombin-mediated fibrin formation. Compared with P3a, SP3a effectively prolongs the time of APTT, TT and PT, because the introduction of sulfate into polysaccharide might form a complex with plasma inhibitors and target proteases (Zhang et al., 2008), and play a significant role in the anticoagulant activity of polysaccharides (Hu et al., 2015). In general, the interactions between amino acid residues on coagulation factors (such as antithrombin and sulfate groups) exhibit the anticoagulant activity of sulfated polysaccharides (Fan et al., 2011). The results show that sulfation effectively improved the anticoagulant behaviour of natural polysaccharides. The coagulation time of sulfated polysaccharides was observed to increase with decreasing molecular weight, which is following former reports (Li et al., 2019; Wang et al., 2018a, Wang et al., 2018b). The longer SP3a coagulation time associated with sulfate content and triple-helix properties of polysaccharides show its importance in anticoagulant activity (Zhang et al., 2011a). The DS of polysaccharides is an important parameter affecting anticoagulant activity. Our results are consistent with earlier reports on the anticoagulant behaviour of sulfated Catathelasma ventricosum and Russula virescens polysaccharide (Li et al., 2019; Liu et al., 2018). Compared with natural polysaccharide, sulphated Catathelasma ventricosum mycelia polysaccharide (smCVP-1s1) prolonged APTT from 45.56s to 50.32s (1.1 fold higher) and PT from 16.96s to 19.36s (1.1 fold higher) at 4 mg/mL. In comparison with 5 mg/mL P3a, SP3a lengthened APTT from 23.6s to 27.8s (1.1 fold higher) and PT from 12.7s to 15.9s (1.3 fold higher). In this study, the anticoagulant activity was enhanced due to sulfation, and the trend of sulfation was similar to Catathelasma ventricosum mycelia polysaccharide (Liu et al., 2018).
Table 3.
Anticoagulant activities of P3 and SP3a.a
| Samples | Concentration (mg/mL) | Clotting time (s) |
||
|---|---|---|---|---|
| APTT | TT | PT | ||
| P3 | 5 | 23.6 ± 1.0 | 12.7 ± 0.2 | 13.3 ± 0.1 |
| 10 | 32.6 ± 1.2 | 16.9 ± 0.3 | 14.2 ± 0.2 | |
| SP3a | 5 | 27.8 ± 0.9 | 15.9 ± 0.3 | 14.6 ± 0.3 |
| 10 | 43.3 ± 1.5 | 19.7 ± 0.5 | 15.8 ± 0.3 | |
| Heparin | 10 μg/mL | 49.3 ± 1.3 | 24.1 ± 1.1 | 14.6 ± 0.4 |
Means with different letters within each column are significantly different (p < 0.05).
Values are expressed as mean ± SD (n = 3).
3.8. Anti-tumor activities of P3 and SP3a
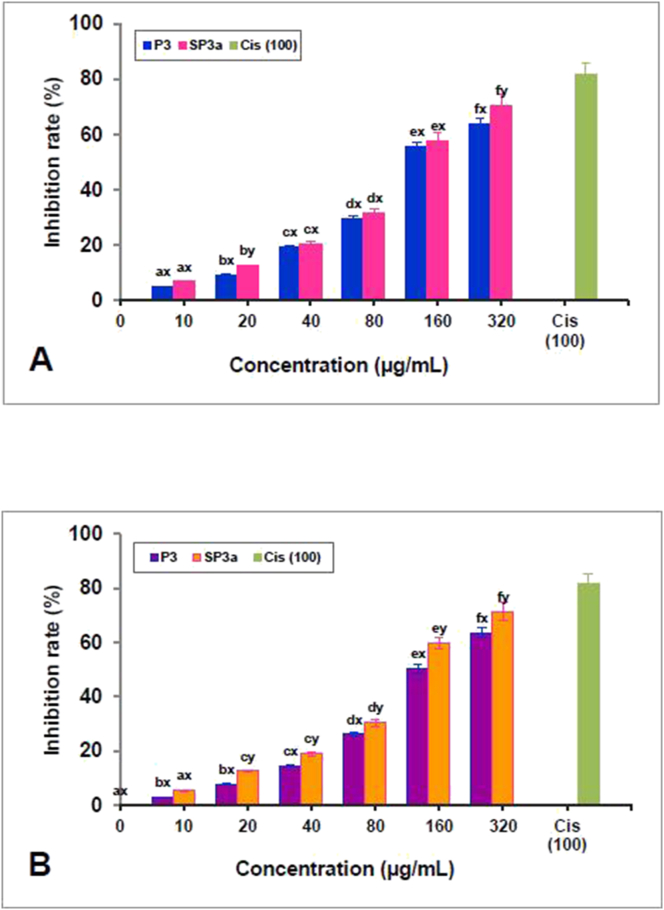
Earlier reports indicate that the introduction of sulphate groups into polysaccharides may alter its physicochemical characteristics and chain conformation, so the sulfation of polysaccharides is beneficial to enhance the antitumor activity (Jiang et al., 2015). In this study, the in vitro antiproliferative activities of P3a and SP3a against HeLa cells and Jurkat cells was examined, and its inhibitory effect was dose-dependent, ranging from 10-320 μg/mL (Fig. 7A and B). The inhibitory effects of P3a and SP3a on HeLa cells were 63.83% and 70.52% at 320 μg/mL respectively. The inhibitory effect at 320 μg/mL on P3a and SP3a of Jurkat cells was 63.83% and 71.34% respectively. The IC50 values of P3a are 141.86 μg/mL and 192.10 μg/mL, while the IC50 values of SP3a on HeLa and Jurkat cells are 136.03 μg/mL and 178.50 μg/mL. The inhibition rates of cisplatin on HeLa and Jurkat cells were 81.77% and 78.42%, respectively at 100 μg/mL, and the IC50 values at were 43.74 and 45.10 μg/mL, respectively. Compared with P3a, SP3a displayed relatively high inhibitory activity on Jurkat and HeLa cells, showing that the anti-tumour nature of P3a can be improved through sulfation modification. In this study, the increase in antiproliferative activity may be due to the decrease in the molecular weight of SP3a after sulfation. Sulfated polysaccharide derivatives of Russula. virescens (Li et al., 2019) Ganoderma lucidum (Tao et al., 2006) and Tricholoma lobayense Heim (Li et al., 2016) exhibited higher anti-tumour activity against Caco-2, HepG2 and HeLa cells, respectively. Besides, compared with these three sulphated derivatives of edible fungal polysaccharides, SP3a has considerable advantages in terms of cytotoxic activity. The sulfated polysaccharide enhances the anti-tumour activity and has potential applications as a natural anti-tumour agent but the anti-tumour mechanism of SP3a remains unclear.
Figure 7.
Anti-proliferative activities of P3 and SP3a against HeLa cells (A) and Jurkat cells (B).
3.9. Antibacterial activity
The inhibition ranges of P3a and SP3a at a concentration of 10 mg/mL for two Gram-negative bacteria (Escherichia coli, Klebsiella pneumonia) and two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) was 8.1–12.3 mm and 10.4–14.2 mm (Table 4). S. aureus is the most sensitive to P3a and SP3a has the largest inhibition zone. The inhibition zones of chloramphenicol against E. coli, K. pneumonia, S. aureus and B. subtilis were 24.6, 28.5, 25.3 and 20.8 mm, respectively. The inhibition zone of P3a and SP3a significantly inhibited the growth of bacteria. Moreover, the antibacterial activity of SP3a against S. aureus is higher than the antibacterial activity against E. coli, indicating that SP3a has a stronger antibacterial effect on Gram-positive bacteria than Gram-negative bacteria, which is in line with the research on sulphated Russula virescens polysaccharide of Li et al. (2019).
Table 4.
Antibacterial activity of P3, SP3a and chloramphenicol.
| Microorganisms | Diameter of inhibition zone (mm)∗ |
||
|---|---|---|---|
| P3 | SP3a | Chloramphenicol | |
|
Gram negative | |||
| Escherichia coli | 11.1 ± 0.7b | 13.3 ± 0.5b | 24.6 ± 0.8b |
| Klebseilla pneumonia | 10.2 ± 0.2b | 12.8 ± 0.3b | 28.5 ± 1.1c |
|
Gram positive | |||
| Staphylococcus aureus | 12.3 ± 0.4c | 14.2 ± 0.4c | 25.3 ± 0.9b |
| Bacillus subtilis | 8.1 ± 0.2a | 10.4 ± 0.2a | 20.8 ± 1.0a |
Values with no letters in common within each column are significantly different (p < 0.05).
Values are expressed as mean ± SD (n = 3).
It can be observed that SP3a shows stronger antibacterial activity than P3a, indicating that sulfation can improve antibacterial activity, which indicates that sulfation is an effective alteration to enhance the biological activity of polysaccharides (Feng et al., 2010). Sulfated modification of polysaccharides can enhance the destruction of the tested bacteria on the cell wall and cytoplasmic membrane of P3a, thus bringing the higher antibacterial activity of SP3a. Both P3a and SP3a can be used as effective antibacterial agents. Our results are in concurrence with earlier reports (Li et al., 2019; Li and Shah, 2014), which revealed that the antibacterial activity against sulfated polysaccharides of Gram-positive and Gram-negative bacteria is stronger than natural polysaccharides.
4. Conclusions
In this study, water-soluble polysaccharides were extracted and purified through ion exchange and gel filtration chromatography and sulfated by SO3-Pyr complex method. Various spectroscopic techniques were used to study the structural characteristics, such as UV, FT-IR, NMR, GCMS, HPGPC and Congo red experiment. These results demonstrate that the molecular modification by sulfation caused changes in chemical composition. The molecular weight decreases during the sulfation process, indicating polysaccharide degradation. The monosaccharide composition of P3a and SP3a is the same, but the mass fraction is different, except that there is no ribose and arabinose. At high alkali concentrations, the triple helix structure becomes random coils. The results of DPPH, hydroxyl radical scavenging and reducing capacity measurements showed that sulfated modification enhanced the antioxidant activity. For Gram-positive bacteria: E. coli, K. pneumonia and Gram-negative bacteria: S. aureus, B. subtilis the bacteriostatic areas of P3a and SP3a were evaluated, and it was found that sulfation was significantly improved antibacterial activity of P3a. Sulfation confers P3a anti-proliferative activity against Jurkat and HeLa cells in vitro. Through various anticoagulant tests, sulfated derivatives show better antioxidant activity. The results of this study indicate that the changes in sulfation enhance the antioxidant, anticoagulant, anti-tumour, and antibacterial activities of P3a, which in turn is related to physical and chemical properties, Mw, and monosaccharide composition. SP3a can be used as a natural antioxidant, anticoagulant, anti-tumour and antibacterial agent for industrial and biomedical applications.
Declarations
Author contribution statement
Sasikala Gunasekaran: Performed the experiments.
Sudha Govindan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Prasanna Ramani: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by a Research Fellowship from Periyar University, India (Ref No. PU/A&A3-URF/2014).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Glycomics and Glycoproteomics facilities at Centre for Cellular and Molecular Platform (C-CAMP) in Bangalore is acknowledged for molecular weight and monosaccharide composition analyses. PR acknowledges the COE in AMGT for providing necessary support for this research.
References
- Chen L., Huang G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019;125:256–261. doi: 10.1016/j.ijbiomac.2018.12.069. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang G. Antioxidant activities of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- Chen Y., Xie M.Y., Nie S.P., Li C., Wang Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107(1):231–241. [Google Scholar]
- Chen R.Z., Liu Z.Q., Zhao J.J., Chen R.P., Meng F.L., Zhang M. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127:434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Wang Y., Nie S., Li C., Xie M. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2015;186:231–238. doi: 10.1016/j.foodchem.2014.10.032. [DOI] [PubMed] [Google Scholar]
- Chirayath R.B., Aparna Viswanathan A., Jayakumar R., Biswas Raja, Vijayachandran Lakshmi Sumitra. Development of Mangifera indica leaf extract incorporated carbopol hydrogel and its antibacterial efficacy against Staphylococcus aureus. Colloids Surf. B Biointerfaces. 2019;178:377–384. doi: 10.1016/j.colsurfb.2019.03.034. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3):350–356. [Google Scholar]
- Fan L.H., Jiang L., Xu Y.M., Zhou Y., Shen Y., Xie W.G. Synthesis and anticoagulant activity of sodium alginate sulfates. Carbohydr. Polym. 2011;83(4):1797–1803. [Google Scholar]
- Feng Y., Li W., Wu X., He L., Ma S. Rapid and efficient microwave assisted sulfate modification of lentinan and its antioxidant and antiproliferative activities in vitro. Carbohydr. Polym. 2010;82:605–612. [Google Scholar]
- Ferreira I.C.F.R., Baptista P., Vilas-Boas M., Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100(4):1511–1516. [Google Scholar]
- Fu L., Wang Y., Wang J., Yang Y., Hao L. Evaluation of the antioxidant activity of extracellular polysaccharides from Morchella esculenta. Food Funct. 2013;4(6):871–879. doi: 10.1039/c3fo60033e. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang T., Jin Z.Y., Xu X.M., Wang J.H., Zha X.Q. Structural characterization, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015;169:430–438. doi: 10.1016/j.foodchem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- He J., Zhang A., Ru Q. Structural characterization of a water-soluble polysaccharide from the fruiting bodies of Agaricus bisporus. Int. J. Mol. Sci. 2014;15(1):787–797. doi: 10.3390/ijms15010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokputsa S., Harding S.E., Inngjerdingen K., Jumel K., Michaelsen T.E., Heinze T. Bioactive polysaccharides from the stems of the Thai medicinal plant Acanthus ebracteatus: their chemical and physical features. Carbohydr. Res. 2004;339:753–762. doi: 10.1016/j.carres.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ye X., Yin X., Chen S. Sulfation of citrus pectin by pyridine- sulfurtrioxide complex and its anticoagulant activity. LWT-Food Sci. Technol. 2015;60:1162–1167. [Google Scholar]
- Huang L., Huang M., Shen M., Wen P., Wu T., Hong Y. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019;136:1000–1006. doi: 10.1016/j.ijbiomac.2019.06.199. [DOI] [PubMed] [Google Scholar]
- Inácio F.D., Ferreira R.O., Araujo C.A.V., Brugnari T., Castoldi R., Peralta R.M. Proteases of wood rot fungi with emphasis on the genus Pleurotus. BioMed Res. Int. 2015;2015:1–10. doi: 10.1155/2015/290161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Wang C., Bai Y., Yu J., Xu C. Sulfation of the extracellular polysaccharide produced by the king oyster culinary-medicinal mushroom, Pleurotus eryngii (Agaricomycetes) and its antioxidant properties in vitro. Int. J. Med. Mushrooms. 2017;19(4):355–362. doi: 10.1615/IntJMedMushrooms.v19.i4.60. [DOI] [PubMed] [Google Scholar]
- Jiang C., Xiong Q., Li S., Zhao X., Zeng X. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 2015;115:200–206. doi: 10.1016/j.carbpol.2014.08.095. [DOI] [PubMed] [Google Scholar]
- Karamanos N.K., Hjerpe A., Tsegenidis T., Engfeldt B., Antonopoulos C.A. Determination of iduronic acid and glucuronic acid in glycosaminoglycans after stoichiometric reduction and depolymerization using high-performance liquid chromatography and ultraviolet detection. Anal. Biochem. 1988;172:410–419. doi: 10.1016/0003-2697(88)90463-0. [DOI] [PubMed] [Google Scholar]
- Kishk Y.F.M., Al-Sayed H.M.A. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT-Food Sci. Technol. 2007;40:270–277. [Google Scholar]
- Leadley R.J., Jr., Chi L., Rebello S.S., Gagnon A. Contribution of in vivo models of thrombosis to the discovery and development of novel antithrombotic agents. J. Pharmacol. Toxicol. Methods. 2000;43:101–116. doi: 10.1016/s1056-8719(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Li S., Shah N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC. 1275. Food Chem. 2014;165:262–270. doi: 10.1016/j.foodchem.2014.05.110. [DOI] [PubMed] [Google Scholar]
- Li S., Shah N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016;197:240–249. doi: 10.1016/j.foodchem.2015.10.113. [DOI] [PubMed] [Google Scholar]
- Li X., Lu Y., Zhang W., Yuan S., Zhou L., Wang L. Antioxidant capacity and cytotoxicity of sulfated polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016;82:913–919. doi: 10.1016/j.ijbiomac.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Li H., Wang X., Xiong Q., Yu Y., Peng l. Sulfated modification, characterization and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2019;154:1438–1447. doi: 10.1016/j.ijbiomac.2019.11.025. [DOI] [PubMed] [Google Scholar]
- Liang L., Ao L., Ma T., Ni Y., Liao X., Hu X. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018;106:447–455. doi: 10.1016/j.ijbiomac.2017.08.035. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu C., Tan H., Zhao T., Cao J., Wang F. Sulfation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulfated derivatives. Carbohydr. Polym. 2009;77:370–375. [Google Scholar]
- Liu C., Chen J., Li E., Fan Q., Wang D., Li X. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharm. 2016;24:299–305. doi: 10.1016/j.intimp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Liu X., Xie J.H., Jia S., Huang L.X., Wang Z.J., Li C. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017;98:576–581. doi: 10.1016/j.ijbiomac.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Liu Y., You Y., Li Y., Zhang L., Tang T., Duan X. Characterization of carboxymethylated polysaccharides from Catathelasma ventricosum and their antioxidant and antibacterial activities. J. Funct. Foods. 2017;38:355–362. [Google Scholar]
- Liu Y., Tang Q., Duan X., Tang T., Ke Y., Li C. Antioxidant and anticoagulant activities of mycelia polysaccharides from Catathelasma ventricosum after sulfated modification. Ind. Crop. Prod. 2018;112:53–60. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Neto Ed.M., Maciel J.S., Cunha P.L.R., de Paula R.C.M., Feitosa J.P.A. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J. Braz. Chem. Soc. 2011;22:1953–1960. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986;44:307–315. [Google Scholar]
- Palacios I., García-Lafuente A., Guillamón E., Villares A. Novel isolation of water-soluble polysaccharides from the fruiting bodies of Pleurotus ostreatus mushrooms. Carbohydr. Res. 2012;358:72–77. doi: 10.1016/j.carres.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Pramanik M., Chakraborty I., Mondal S., Islam S.S. Structural analysis of a water-soluble glucan (Fr.I) of an edible mushroom, Pleurotus sajor-caju. Carbohydr. Res. 2007;342:2670–2675. doi: 10.1016/j.carres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Rout D., Mondal S., Chakraborty I., Islam S.S. The structure and conformation of a water-insoluble (1→3)-,(1→6)-β-D-glucan from the fruiting bodies of Pleurotus florida. Carbohydr. Res. 2008;343:982–987. doi: 10.1016/j.carres.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Sandhya M., Aparna V., Maneesha K.S., Raja B., Jayakumar R., Sathianarayanan S. Amphotericin B loaded sulfonated chitosan nanoparticles for targetingmacrophages to treat intracellular Candida glabrata infections. Int. J. Biol. Macromol. 2018;110:133–139. doi: 10.1016/j.ijbiomac.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Seedevi P., Abirami R.G., Mohan K., Sivakumar V., Sivasankar M., Loganathan P. Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju. RSC Adv. 2019;9:20472–20482. doi: 10.1039/c9ra02977j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40(6):945–948. [Google Scholar]
- Silva S., Martins S., Karmali A., Rosa E. Production, purification and characterisation of polysaccharides from Pleurotus ostreatus with antitumour activity. J. Sci. Food Agric. 2012;92(9):1826–1832. doi: 10.1002/jsfa.5560. [DOI] [PubMed] [Google Scholar]
- Smirnoff N., Cumbes Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28(4):1057–1060. [Google Scholar]
- Subramanian S.K., Ramani P. Antioxidant and cytotoxic activities of Indian caper (Capparis brevispina DC (Capparaceae)) leaf extracts. Eur. J. Integr. Med. 2020;33:101038. [Google Scholar]
- Subramanian S.K., Vaishav Raj K.S., Vignesh S., Ramani P. Invitro anti- inflammatory activity of Kleinia Grandiflora leaves. Mater. Today: Proc. 2018;5:16539–16542. [Google Scholar]
- Sudharsan S., Subhapradhaa N., Seedevi P., Shanmugama V., Madeswaran P., Shanmugama A. Antioxidant and anticoagulant activity of sulfated polysaccharide from Gracilaria debilis (Forsskal) Int. J. Biol. Macromol. 2015;81:1031–1038. doi: 10.1016/j.ijbiomac.2015.09.046. [DOI] [PubMed] [Google Scholar]
- Tang Z., Wang Y., Ma H., Wen S., Qin S. Investigation of deproteinized technology for polysaccharide from Enteromorpha Prolifera. Food Res. Dev. 2015;36:90–92. [Google Scholar]
- Tao Y., Zhang L., Cheung P.C.K. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006;341:2261–2269. doi: 10.1016/j.carres.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Telles C., Sabrya D., Almeida-Lima J., Costa M., Melo-Silveira R., Trindade E. Sulfation of the extracellular polysaccharide produced by the edible mushroom Pleurotus sajor-caju alters its antioxidant, anticoagulant and antiproliferative properties in vitro. Carbohydr. Polym. 2011;85:514–521. [Google Scholar]
- Valgas C., de Souza S.M., Smânia A., Smânia E.F.A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007;38:369–380. [Google Scholar]
- Wang J., Zhang Q.B., Zhang Z.S., Li Z.E. Antioxidant activity of sulfated polysaccharides fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Wang J., Guo H., Zhang J., Wang X., Zhao B., Yao J.Y. Wang Sulfated modification, characterization and structure-antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydr. Polym. 2010;81:897–905. [Google Scholar]
- Wang X., Zhang Z., Yao Q., Zhao M., Qi H. Phosphorylation of low- molecular weight polysaccharide from Enteromorpha linza with antioxidant activity. Carbohydr. Polym. 2013;96:371–375. doi: 10.1016/j.carbpol.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Xie J.H., Shen M.Y., Nie S.P., Xie M.Y. Sulfated modification of polysaccharides: synthesis, characterization and bioactivities. Trends Food Sci. Techol. 2018;74:147–157. [Google Scholar]
- Wang Z., Cai T., He X. Characterization, sulfated modification and bioactivity of a novel polysaccharide from Millettia dielsiana. Int. J. Biol. Macromol. 2018;117:108–115. doi: 10.1016/j.ijbiomac.2018.05.147. [DOI] [PubMed] [Google Scholar]
- Wu G.H., Hu T., Huang Z.L., Jiang J.G. Characterization of water and alkali-soluble polysaccharides from Pleurotus tuber-regium sclerotia. Carbohydr. Polym. 2013;96(1):284–290. doi: 10.1016/j.carbpol.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Xiao H., Fu X., Cao C., Li C., Chen C., Huang Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019;121:407–414. doi: 10.1016/j.ijbiomac.2018.09.197. [DOI] [PubMed] [Google Scholar]
- Xie J.H., Wang Z.J., Shen M.Y., Nie S.P., Gong B., Li H.S. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016;53:7–15. [Google Scholar]
- Xiong Q.P., Jiao Y.P., Zhao X.R., Chen X.M., Zhang Q.H., Jiang C.X. Purification, characterization and immunostimulatory activity of polysaccharide from Cipangopaludina chinensis. Carbohydr. Polym. 2013;98:217–223. doi: 10.1016/j.carbpol.2013.05.075. [DOI] [PubMed] [Google Scholar]
- Xu J., Xu L., Zhou Q., Xie H. Enhanced in vitro antioxidant activity of polysaccharides from Enteromorpha Prolifera by enzymatic degradation. J. Food Biochem. 2015;40:275–283. [Google Scholar]
- Xu D., Wang H., Zheng W., Gao Y., Wang M., Zhang Y. Characterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016;92:30–36. doi: 10.1016/j.ijbiomac.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Yang J., Du Y., Wen Y., Li T., Hu L. Sulfation of Chinese lacquer polysaccharides in different solvents. Carbohydr. Polym. 2003;52:397–403. [Google Scholar]
- Yang C.X., He N., Ling X.P., Ye M.L., Zhang C.X., Shao W.Y. The isolation and characterization of polysaccharides from longan pulp. Separ. Purif. Technol. 2008;63:226–230. [Google Scholar]
- Yuan J.F., Zhang Z.Q., Fan Z.C., Yang J.X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr. Polym. 2008;74:822–827. [Google Scholar]
- Zhang H., Mao W.J., Fang F., Li H.Y., Sun H.H., Chen Y. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissinrom. Carbohydr. Polym. 2008;71:428–434. [Google Scholar]
- Zhang Y., Li S., Wang X., Zhang L., Cheung P.C.K. Advances in lennnan:Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011;251:196–206. [Google Scholar]
- Zhang Y., Lu X., Fu Z., Wang Z., Zhang J. Sulphated modification of a polysaccharide obtained from fresh persimmon (Diospyros kaki L.) fruit and antioxidant activities of the sulphated derivatives. Food Chem. 2011;127:1084–1090. doi: 10.1016/j.foodchem.2011.01.100. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang J.Q., Nie S.P., Wang Y.X., Cui S.W., Xie M.Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015;79:248–255. doi: 10.1016/j.ijbiomac.2015.04.070. [DOI] [PubMed] [Google Scholar]
- Zhao G., Zhai X., Qu M., Tong C., Li W. Sulfated modification of the polysaccharides from Crassostrea gigas and their antioxidant and hepatoprotective activities through metabolomics analysis. Int. J. Biol. Macromol. 2019;129:386–395. doi: 10.1016/j.ijbiomac.2019.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.