Abstract
Glaucoma is considered a chronic disease that requires lifelong management. Chronic diseases are known to be highly associated with psychological disturbances such as depression and anxiety. There have also been many studies on association between anxiety or depression and glaucoma. The majority of these studies explained that the glaucoma diagnosis causes anxiety or depression. However, It is also necessary to evaluate whether the psychological disturbance itself affect glaucoma. Therefore, we investigated the association of anxiety and depression with glaucoma progression, and elucidate mechanisms underlying that. We included 251 eyes with open angle glaucoma who were followed up for at least 2 years in this retrospective case–control study. The Beck Anxiety Inventory (BAI) and Beck Depressive Inventory-II (BDI-II) were used to assess anxiety and depression in glaucoma patients. Patients were classified into groups (high-anxiety group; HA-G, low-anxiety group; LA-G, high-depression group; HD-G, low-depression group; LD-G) according to their score on the BAI or BDI-II (separately). In logistic regression analysis, disc hemorrhage, peak intraocular pressure (IOP) and RNFL thickness loss rate were significantly associated with high anxiety (p = 0.017, p = 0.046, p = 0.026). RNFL thinning rate and disc hemorrhage were significant factors associated with anxiety in multivariate models (p = 0.015, p = 0.019). Multivariate linear regression analysis showed a significant positive correlation between the rate of RNFL thickness loss and BAI score (B = 0.058; 95% confidential interval = 0.020–0.097; p = 0.003), and RNFL loss and IOP fluctuation (B = 0.092; 95% confidential interval = 0.030–0.154; p = 0.004). For the depression scale, visual field mean deviation and heart rate variability were significantly associated with high depression in multivariate logistic regression analysis (p = 0.003, p = 0.006). We suggest that anxiety increase the risk of glaucoma progression and they are also associated with IOP profile and disc hemorrhage.
Subject terms: Diseases, Risk factors
Glaucoma is optic neuropathy characterized by progressive loss of retinal ganglion cells1. There is currently no effective treatment for ganglion cell degeneration, and the treatment of glaucoma is focused on preventing progression2. Therefore, glaucoma is considered a chronic disease that requires lifelong management3.
It is known that chronic diseases are associated with psychological disturbances such as depression and anxiety4,5. In glaucoma, there have been many studies about anxiety or depression, which have reported that the prevalence of anxiety or depression is high in patients with glaucoma6–8. The majority of these studies explained that the high prevalence of anxiety and/or depression is the consequence of being diagnosed with glaucoma, and results from the fear of potential blindness, heavy economic burden and impaired daily activity9,10. Whereas most studies have documented the disease as contributing to anxiety/depression, several lines of evidence now show that negative emotions such as anxiety/depression, are also a risk factor for physical illness11–14. They reported that anxiety or depression may speed the development of disease such as cardiovascular disorders, and worsen diseases such as gastrointestinal or respiratory disorders13–16. Recently, there was a study showing a patient with glaucoma suspect who have history of anxiety or depression developed more glaucoma, suggesting that emotional stress itself may have effect on glaucoma17.
Anxiety and depression are reactions to stress and are thought to arise in the amygdala18. These emotional responses evoke secretion of neurotransmitters and stimulate the autonomic nervous system (ANS), which affects multiple organs19. The ANS, which can be affected by emotions, is also important in the development or progression of glaucoma20–22.
Many studies, including randomized control trials, have investigated risk factors for glaucoma progression23–25. Based on their results, risk factors associated with progression include old age, increased mean or peak intraocular pressure (IOP), greater IOP fluctuation, the presence of disc hemorrhage (DH), myopia, and low or high blood pressure (BP)23–27. These factors largely can be classified into mechanical (IOP) and vascular (DH, BP) caegories27.
The purpose of the present investigation was to evaluate whether anxiety and depression affect glaucoma progression, and to elucidate mechanisms underlying that association through mechanical and vascular factors.
Results
The patients’ baseline characteristics are listed in Table 1. The average follow-up period was 62.8 ± 32.1 (mean ± standard deviation) months. All patients were taking some form of glaucoma medication; 183 (72.9%) had been prescribed prostaglandins. The mean BAI score was 6.0 ± 4.4 (range, 0–22). Among 251 glaucoma patients, 209 (83.3%) were in the LA-G (range, 0–10) and 44 (16.7%) were in the HA-G (range, 11–22). The results of comparisons between the two groups divided according to BAI scale are summarized in Tables 2 and 3. The incidence of DH was higher in the HA-G than LA-G (3.3% vs 11.9%, p = 0.018). Mean IOP (13.76 ± 3.00 vs 14.76 ± 3.02, p = 0.049) and peak IOP (17.43 ± 4.12 vs 18.86 ± 4.32, p = 0.043) were higher in the HA-G than the LA-G (Table 2). The rate of RNFL thinning during follow-up in the HA-G (− 1.96 ± 2.23 µm/year) was faster than in the LA-G (− 0.68 ± 1.39 µm/year, p = 0.021, Table 2). The results of logistic regression are listed in Table 3. RNFL thickness loss rate (OR = 1.69, 95% CI = 1.20–2.38, p = 0.003) and DH (OR = 6.79, 95% CI = 1.48–31.08, p = 0.014) were significant factors associated with anxiety in multivariate model. We investigated which questions were associated with the rate of RNFL loss. Multiple questions (numbness or tingling, feeling hot, wobbliness in legs, dizzy or lightheaded, heart pounding/racing, unsteady, terrified or afraid, face flushed) were shown to be associated with RNFL loss rate (Table 4).
Table 1.
Baseline demographics of patients with glaucoma.
| Variables | Open angle glaucoma, 251 eyes |
|---|---|
| Age, y | 53.23 ± 13.03 |
| Sex, male/female | 107/144 |
| Hypertension, n (%) | 47(18.7%) |
| Diabetics, n (%) | 10(4.0%) |
| CCT, μm | 537.23 ± 46.37 |
| Axial length, mm | 25.31 ± 1.87 |
| PPA/Disc area, per 1 µm larger (%) | 44.18 ± 51.55 |
| Tilt ratio | 1.20 ± 0.21 |
| Torsion ratio | 88.50 ± 9.73 |
| VF MD, dB | − 4.38 ± 5.30 |
| VF PSD, dB | 4.51 ± 3.61 |
| Follow-up duration (month) | 62.78 ± 32.09 |
| Mean IOP | 13.93 ± 3.00 |
| Peak IOP | 17.67 ± 4.18 |
| IOP fluctuation | 5.67 ± 3.37 |
| Mean blood pressure | 94.13 ± 11.68 |
| Disc hemorrhage | 12(4.8%) |
| Heart rate variability | 35.26 ± 21.28 |
| Beck anxiety inventory score | 6.00 ± 5.45 |
| Beck depression inventory-II score | 8.81 ± 6.48 |
CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; IOP, intraocular pressure.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
Table 2.
Comparison of the demographics and test results between two groups divided according to the Beck Anxiety Inventory (BAI) scale.
| Variables | Low anxiety group (LA − G), 209 eyes |
High anxiety group (HA − G), 42 eyes |
P value |
|---|---|---|---|
| Age, y | 53.05 ± 12.16 | 54.14 ± 16.87 | 0.691* |
| Sex, male/female | 89/120 | 18/24 | 0.974† |
| Hypertension, n (%) | 37(17.7%) | 10(23.8%) | 0.355† |
| Diabetics, n (%) | 8(3.8%) | 2(4.8%) | 0.778† |
| CCT, µm | 539.12 ± 48.06 | 527.22 ± 34.98 | 0.184* |
| Axial length, mm | 25.29 ± 1.94 | 25.38 ± 1.47 | 0.764* |
| PPA/Disc area, per 1 µm larger (%) | 46.01 ± 54.13 | 35.27 ± 35.66 | 0.219† |
| Tilt ratio | 1.20 ± 0.22 | 1.20 ± 0.16 | 0.978† |
| Torsion ratio | 88.97 ± 9.69 | 87.33 ± 7.59 | 0.304† |
| VF MD, dB | − 4.55 ± 5.49 | − 3.54 ± 4.18 | 0.206* |
| VF PSD, dB | 4.66 ± 3.70 | 3.78 ± 3.12 | 0.148* |
| VF MD progression rate, dB/year | − 0.55 ± 0.58 | − 0.76 ± 0.73 | 0.310* |
| RNFL thickness loss rate, µm/year | − 0.68 ± 1.39 | − 1.96 ± 2.23 | 0.021* |
| Follow-up duration (month) | 63.85 ± 30.92 | 56.95 ± 38.26 | 0.379* |
| Mean IOP | 13.76 ± 3.00 | 14.76 ± 3.02 | 0.049* |
| Peak IOP | 17.43 ± 4.12 | 18.86 ± 4.32 | 0.043* |
| IOP fluctuation | 5.50 ± 3.41 | 6.50 ± 3.08 | 0.080* |
| Mean blood pressure | 94.48 ± 12.503 | 92.45 ± 6.21 | 0.264* |
| Disc hemorrhage | 7(3.3%) | 5(11.9%) | 0.018† |
| Heart rate variability | 35.09 ± 18.99 | 42.10 ± 29.86 | 0.150* |
CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; IOP, intraocular pressure.
*The comparison was performed using independent samples t-test.
†The comparison was performed using a chi-squared test.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
Statistically significant values appear in boldface.
Table 3.
Factors associated with the Beck Anxiety Inventory (BAI) scores in patients with glaucoma. Logistic Regression Analysis of BAI score.
| Variables | Univariate model | Multivariate model | ||
|---|---|---|---|---|
| Odds ratio, 95% CI | P value | Odds ratio, 95% CI | P value | |
| Age, y | 1.01, 0.98–1.03 | 0.619 | ||
| Sex, male/female | 0.99, 0.51–1.93 | 0.989 | ||
| Hypertension, n (%) | 1.45, 0.66–3.21 | 0.357 | ||
| Diabetics, n (%) | 1.26, 0.26–6.14 | 0.778 | ||
| CCT, µm | 0.99, 0.99–1.00 | 0.185 | ||
| Axial length, mm | 1.03, 0.85–1.24 | 0.802 | ||
| PPA/Disc area, per 1 µm larger (%) | 0.59, 0.25–1.37 | 0.220 | ||
| Tilt ratio | 0.87, 0.17–4.42 | 0.867 | ||
| Torsion ratio | 0.98, 0.95–1.02 | 0.303 | ||
| VF MD, dB | 1.04, 0.97–1.12 | 0.262 | ||
| VF PSD, dB | 0.93, 0.84–1.03 | 0.151 | ||
| VF MD progression rate, dB/year | 1.63, 0.63–4.18 | 0.314 | ||
| RNFL thickness loss rate, µm/year | 1.66, 1.19–2.33 | 0.003 | 1.69, 1.20–2.38 | 0.003 |
| Follow-up duration (month) | 0.99, 0.98–1.01 | 0.377 | ||
| Mean IOP | 1.12, 1.00–1.25 | 0.051 | ||
| Peak IOP | 1.08, 1.00–1.17 | 0.046 | 1.00, 0.87–1.15 | 0.991 |
| IOP fluctuation | 1.08, 0.99–1.19 | 0.084 | ||
| Mean blood pressure | 0.98, 0.94–1.03 | 0.489 | ||
| Disc hemorrhage | 3.90, 1.18–12.95 | 0.026 | 6.79, 1.48–31.08 | 0.014 |
| Heart rate variability | 1.01, 1.00–1.03 | 0.059 | ||
CI, confidence interval; CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; IOP, intraocular pressure.
Variables with p < 0.05 were included in the multivariate analysis.
Statistically significant values are shown in bold.
Table 4.
The questions on the BECK anxiety inventory (BAI) associated with RNFL thickness loss.
| Variables | RNFL thickness progression rate, um/year | P value | |
|---|---|---|---|
| Score 0 | Score 1–3 | ||
| Q1. Numbness or tingling | 0.63 ± 1.47 | 1.49 ± 1.80 | 0.006 |
| Q2. Feeling hot | 0.70 ± 1.56 | 1.34 ± 1.67 | 0.040 |
| Q3. Wobbliness in legs | 0.70 | 2.56 | 0.033 |
| Q4. Unable to relax | 0.75 | 1.20 | 0.158 |
| Q5. Fear of worst happening | 0.89 | 0.83 | 0.851 |
| Q6. Dizzy or lightheaded | 0.62 | 1.19 | 0.042 |
| Q7. Heart pounding/racing | 0.47 | 1.34 | 0.002 |
| Q8. Unsteady | 0.66 | 1.24 | 0.047 |
| Q9. Terrified or afraid | 0.62 | 1.54 | 0.011 |
| Q10. Nervous | 0.82 | 0.94 | 0.661 |
| Q11. Feeling of choking | 0.81 | 1.29 | 0.250 |
| Q12. Hands trembling | 0.75 | 2.25 | 0.121 |
| Q13. Shaky/unsteady | 0.72 | 2.42 | 0.053 |
| Q14. Fear of losing control | 0.75 | 3.51 | 0.114 |
| Q15. Difficulty in breathing | 0.72 | 0.09 | 0.070 |
| Q16. Fear of dying | 0.84 | 1.96 | 0.171 |
| Q17. Scared | 0.78 | 1.04 | 0.400 |
| Q18. Indigestion | 0.73 | 1.08 | 0.234 |
| Q19. Faint/lightheaded | 0.86 | 0.27 | 0.530 |
| Q20. Face flushed | 0.69 | 1.66 | 0.038 |
| Q21. Hot/cold sweats | 0.82 | 1.08 | 0.423 |
The comparison was performed using independent samples t-test.
Statistically significant values are shown in bold.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
The mean BDI-II score was 8.5 ± 6.4 (range, 0–32). Among 251 patients with open angle glaucoma, 211 (84.1%) were in the LD-G (range, 0–14) and 40 (15.9%) in the HD-G (range, 15–32). The results of comparisons between the two groups divided according to BDI-II scores are shown in Table 5. In BDI-II analysis, the HD-G showed worse VF mean deviation (MD) (− 7.30 ± 7.68 dB) than did the LD-G (− 3.81 ± 4.50 dB, p = < 0.001, Table 5). Heart rate variability was significantly higher in the HD-G (44.77 ± 36.27) than the LD-G (34.08 ± 16.28, p = 0.001, Table 5). MD of VF (OR = 0.91, 95% CI = 0.86–0.97, p = 0.003) and Heart rate variability (OR = 1.02, 95% CI = 1.01–1.04, p = 0.006) were significant factors associated with BDI-II in both the univariate and multivariate models (Table 6). We investigated which questions are associated with the worse MD in VF. Patients with punishment feelings (− 4.02 ± 4.94 vs − 5.78 ± 6.35, p = 0.036) or self-dislike (− 3.83 ± 4.19 vs − 5.59 ± 7.06, p = 0.047) showed worse MD than did patients without such feelings (Table 7).
Table 5.
Comparison of the demographics and test results between two groups divided according to the Beck depression inventory-II (BDI-II) scale.
| Variables | Low depression group, (LD-G), 211 eyes |
High depression group, (HD-G), 40 eyes |
P value |
|---|---|---|---|
| Age, y | 52.67 ± 12.02 | 56.20 ± 17.34 | 0.224* |
| Sex, male/female | 93/118 | 14/26 | 0.287† |
| Hypertension, n (%) | 39(18.5%) | 8(20.0%) | 0.822† |
| Diabetics, n (%) | 8(3.8%) | 2(5.0%) | 0.720† |
| CCT, µm | 539.66 | 523.33 | 0.109* |
| Axial length, mm | 25.30 | 25.36 | 0.847* |
| PPA/Disc area, per 1 µm larger (%) | 45.41 | 37.86 | 0.398* |
| Tilt ratio | 1.21 | 1.18 | 0.242* |
| Torsion ratio | 88.93 | 91.04 | 0.699* |
| VF MD, dB | − 3.81 | − 7.30 | < 0.001* |
| VF PSD, dB | 4.31 | 5.54 | 0.053* |
| VF MD progression rate, dB/year | − 0.56 | − 0.67 | 0.539* |
| RNFL thickness loss rate, µm/year | − 0.76 | − 1.45 | 0.074* |
| Follow-up duration (month) | 63.02 31.16 | 61.57 37.36 | 0.851* |
| Mean IOP | 13.93 2.96 | 13.93 3.25 | 0.993* |
| Peak IOP | 17.66 | 17.70 | 0.960* |
| IOP fluctuation | 5.63 | 5.88 | 0.675* |
| Mean blood pressure | 94.89 | 90.42 | 0.102* |
| Disc hemorrhage | 11(5.2%) | 2(2.5%) | 0.461† |
| Heart rate variability | 34.08 ± 16.28 | 47.77 ± 36.27 | 0.001* |
CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; IOP, intraocular pressure.
Statistically significant values appear in boldface.
*The comparison was performed using independent samples t-test.
†The comparison was performed using a chi-squared test.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
Table 6.
Factors associated with the Beck Depression Inventory (BDI-II) scale in patients with glaucoma.
| Variables | Univariate model | multivariate model | ||
|---|---|---|---|---|
| Odds ratio, 95% CI | P value | Odds ratio, 95% CI | P value | |
| Age, y | 1.02, 0.99–1.05 | 0.118 | ||
| Sex, male/female | 1.46, 0.72–2.96 | 0.289 | ||
| Hypertension, n (%) | 1.10, 0.47–2.58 | 0.822 | ||
| Diabetics, n (%) | 1.34, 0.27–6.53 | 0.721 | ||
| CCT, µm | 0.99, 0.98–1.00 | 0.110 | ||
| Axial length, mm | 1.02, 0.84–1.23 | 0.847 | ||
| PPA/Disc area, per 1 µm larger (%) | 0.71, 0.32–1.57 | 0.379 | ||
| Tilt ratio | 0.42, 0.07–2.47 | 0.337 | ||
| Torsion ratio | 1.01, 0.98–1.05 | 0.502 | ||
| VF MD, dB | 0.91, 0.86–0.96 | < 0.001 | 0.91, 0.86–0.97 | 0.003 |
| VF PSD, dB | 1.09, 1.00–1.19 | 0.051 | ||
| VF MD progression rate, dB/year | 1.32, 0.54–3.22 | 0.536 | ||
| RNFL thickness loss rate, µm/year | 1.30, 0.97–1.73 | 0.079 | ||
| Follow-up duration (month) | 0.99, 0.98–1.01 | 0.849 | ||
| Mean IOP | 1.00, 0.89–1.12 | 0.993 | ||
| Peak IOP | 1.00, 0.92–1.09 | 0.960 | ||
| IOP fluctuation | 1.02, 0.93–1.13 | 0.674 | ||
| Mean blood pressure | 0.94, 0.92–1.01 | 0.103 | ||
| Disc hemorrhage | 0.47, 0.06–3.71 | 0.471 | ||
| Heart rate variability | 1.02, 1.01–1.04 | 0.001 | 1.02, 1.01–1.04 | 0.006 |
CI, confidence interval; CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; IOP, intraocular pressure.
Variables with p < 0.05 were included in the multivariate analysis.
Statistically significant values appear in boldface.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
Table 7.
The questions on the BECK Depression Inventory-II (BDI-II) associated with visual field mean deviation.
| Variables | Mean deviation of visual field, dB | P value | |
|---|---|---|---|
| Score 0 | Score 1–3 | ||
| Q1. Sadness | − 4.14 ± 5.02 | − 4.65 ± 5.61 | 0.454 |
| Q2. Pessimism | − 3.93 | − 4.56 | 0.405 |
| Q3. Past Failure | − 4.08 | − 5.30 | 0.206 |
| Q4. Loss of Pleasure | − 4.53 | − 4.14 | 0.568 |
| Q5. Guilty Feelings | − 4.47 | − 4.22 | 0.851 |
| Q6. Punishment Feelings | − 4.02 ± 4.94 | − 5.78 ± 6.35 | 0.036 |
| Q7. Self-Dislike | − 3.83 ± 4.19 | − 5.59 ± 7.06 | 0.047 |
| Q8. Self-Criticalness | − 3.98 | − 4.74 | 0.263 |
| Q9. Suicidal Thoughts or Wishes | − 4.33 | − 4.67 | 0.726 |
| Q10. Crying | − 4.43 | − 3.91 | 0.663 |
| Q11. Agitation | − 4.27 | − 4.60 | 0.642 |
| Q12. Loss of Interest | − 4.22 | − 4.87 | 0.409 |
| Q13. Indecisiveness | − 3.99 | − 5.09 | 0.137 |
| Q14. Worthlessness | − 4.49 | − 4.13 | 0.625 |
| Q15. Loss of Energy | − 4.21 | − 4.52 | 0.647 |
| Q16. Changes in Sleeping Pattern | − 4.43 | − 4.34 | 0.171 |
| Q17. Irritability | − 4.24 | − 4.47 | 0.743 |
| Q18. Changes in Appetite | − 3.96 | − 6.27 | 0.056 |
| Q19. Concentration Difficulty | − 4.35 | − 4.61 | 0.823 |
| Q20. Tiredness or Fatigue | − 4.88 | − 4.01 | 0.201 |
| Q21. Loss of Interest in Sex | − 3.76 | − 4.81 | 0.113 |
The comparison was performed using independent samples t-test.
Statistically significant values are shown in bold.
Continuous data are mean ± mean standard deviation unless otherwise indicated.
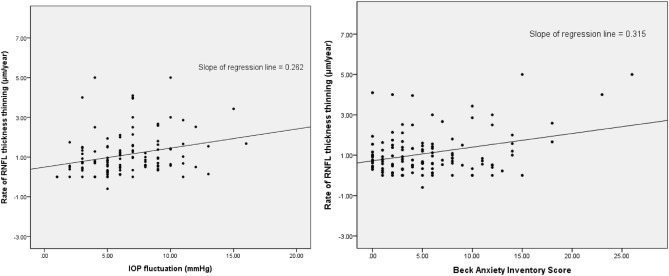
Parameters related to the rate of RNFL thinning were evaluated by linear regression analyses. BAI score (B = 0.058, 95% confidential interval = 0.020–0.097, p = 0.003) and IOP fluctuation (β = 0.092, 95% confidential interval = 0.030–0.154, p = 0.004) were significantly related to the rate of RNFL thinning, based on multivariate analyses (Table 8). The relationships between RNFL thinning rates, and BAI scores and IOP fluctuations are shown in Fig. 1. The slope of the linear fit was positive for the rate of RNFL loss against both BAI score and IOP fluctuation.
Table 8.
Regression analysis of factors associated with the RNFL thickness loss rate.
| RNFL thickness loss rate | Univariate model | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| B | β | 95% CI | P value | B | β | 95% CI | P value | |
| Age, y | − 0.003 | − 0.035 | − 0.022 to 0.015 | 0.710 | ||||
| Sex, male/female | − 0.029 | − 0.013 | − 0.426 to 0.369 | 0.546 | ||||
| Hypertension, n (%) | − 0.288 | − 0.089 | − 0.878 to 0.302 | 0.336 | ||||
| Diabetics, n (%) | − 0.018 | − 0.003 | − 1.109 to 1.073 | 0.998 | ||||
| CCT, µm | 0.001 | 0.057 | − 0.146 to 0.105 | 0.747 | ||||
| Axial length, mm | − 0.020 | − 0.033 | − 0.164 to 0.136 | 0.855 | ||||
| PPA/Disc area (%) | 0.102 | 0.034 | − 0.454 to 0.658 | 0.717 | ||||
| Tilt ratio | 0.453 | 0.075 | − 0.681 to 1.586 | 0.621 | ||||
| Torsion ratio | − 0.015 | − 0.137 | − 0.035 to 0.005 | 0.150 | ||||
| VF MD, dB | 0.025 | 0.127 | − 0.011 to 0.061 | 0.172 | ||||
| VF PSD, dB | − 0.036 | − 0.125 | − 0.089 to 0.017 | 0.177 | ||||
| VF MD rate, dB/year | − 0.008 | − 0.027 | − 0.090 to 0.074 | 0.847 | ||||
| Mean IOP | 0.003 | 0.011 | − 0.050 to 0.056 | 0.907 | ||||
| Peak IOP | 0.047 | 0.116 | − 0.004 to 0.098 | 0.072 | ||||
| IOP fluctuation | 0.096 | 0.262 | 0.031 to 0.161 | 0.015 | 0.092 | 0.251 | 0.030 to 0.154 | 0.004 |
| Mean blood pressure | 0.005 | 0.041 | − 0.080 to 0.090 | 0.902 | ||||
| Disc hemorrhage | 0.075 | 0.015 | − 0.859 to 1.008 | 0.874 | ||||
| Heart rate variability | 0.013 | 0.199 | 0.001 to 0.025 | 0.031 | 0.007 | 0.104 | − 0.005 to 0.019 | 0.255 |
| BAI score | 0.068 | 0.315 | 0.030 to 0.105 | 0.001 | 0.058 | 0.272 | 0.020 to 0.097 | 0.003 |
| BDI-II score | 0.015 | 0.073 | − 0.023 to 0.054 | 0.431 | ||||
B, non-standardized coefficient; β, standardized coefficient; CI, confidence interval; CCT, central corneal thickness; PPA, peripapillary atrophy; VF, visual field; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; IOP, intraocular pressure; BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory-II.
Statistically significant values appear in boldface.
Figure 1.
Scatter plot showing the relationships between the rate of RNFL thinning and IOP fluctuations (left) and Beck Anxiety Inventory scores (right).
Discussion
Depression and anxiety are highly prevalent in individuals with chronic disease4,5.The relationship between the chronic disease and depression/anxiety can be experienced as independent or inter-related (with either one causing the other)12.The majority of papers have reported that anxiety/depression is the consequence of being diagnosed with a chronic disease12. Diagnosis of chronic disease can cause anxiety/depression due to functional limitations, social isolation, loss of relationships, guilty feelings and anxiety about the future9,10.
Meanwhile, some other studies demonstrated that anxiety/depression led to or worsen chronic disease11,12,28,29. For example, high emotions cause high BP, depression causes heart disease, and persistent anxiety causes high blood sugar and diabetes13,28.
As glaucoma is a chronic disease, it has been the focus of many studies about anxiety and depression, and these studies have shown that the prevalence of anxiety and depression are high in glaucoma. The prevalence of anxiety in glaucoma patients has been reported to be in the range of 13.0–30%, and the prevalence of depression has been reported from 10.9 to 24.7%5–9. Most qualitative studies have reported that glaucoma patients interpret their disease as contributing to anxiety and/or depression5–9. However, as in other chronic disease studies, anxiety/depression could affect glaucoma9,10. Recently, Samuel et al.17 reported that a history of anxiety in glaucoma suspects was associated with developing glaucoma. This study did not investigate how anxiety might influence glaucoma progression. So we tried to clarify this mechanism by investigating the association between anxiety and well-known risk factors for glaucoma progression.
In our study, anxiety was significantly associated with the rate of RNFL thickness decline in patients with glaucoma (Tables 2 and 3). Although the statistical significance was borderline (p = 0.074, independent t-test), the rate of RNFL thinning was faster in the HD-G than the LD-G. These results suggest that not only is glaucoma a risk factor for anxiety/depression, but also that anxiety/depression could be a risk factor for glaucoma. The rate of VF progression was not significantly different between the low and high groups for either anxiety or depression. This is probably because the follow-up period was not long (5.23 ± 2.67 years), and the subjects had relatively early glaucoma (− 4.38 ± 5.30 dB). RNFL thinning or structural loss appears before functional VF defects, so OCT is more sensitive than VF testing for the detection of progression in early glaucoma30. Moreover, VF tests are difficult for some patients and are known to have increased variability31. For this reason, although the rate of VF progression did not show statistical significance, the rate of RNFL thickness loss is sufficient to indicate the progression of glaucoma. In linear regression analysis, BAI score (B = 0.058, 95% confidential interval = 0.020–0.097, p = 0.003) were significantly related to the rate of RNFL thinning, based on multivariate analyses. Figure 1 shows a significant positive correlation between the rate of RNFL thinning and BAI score. IOP parameters (mean, peak and fluctuation) were higher in the HA-G than the LA-G and DH occurred more often in the HA-G than the LA-G. Elevated IOP and disc hemorrhage are well-known risk factors for the development and progression of glaucoma. The results of our study indicate that anxiety is probably associated with variation in IOP and the occurrence of DH. IOP is one of the mechanical risk factors and DH is one of the vascular risk factors that indicate blood flow insufficiency. Faster progression in HA-G can probably be explained by these mechanical and vascular risk factors.
The VF MDs were worse in the HD-G (− 7.30 ± 7.68 dB) than the LD-G (− 3.81 ± 4.50 dB). This result suggests that the more severe a patient’s glaucoma is, the more likely they are to be depressed, which is consistent with previous reports9,10. The rate of RNFL thinning in the HD-G was faster than in the LD-G (p = 0.074, independent t-test), but with borderline statistical significance. The relationship with depression is weaker than that with anxiety, but suggests the possibility of an association with progression.
How could emotions such as anxiety and depression change mechanical (IOP) and vascular (DH) factors? There are several studies on the association between stress and iop32,33. They reported that psychological stress elevate IOP and cortisol hormone (known as the HPA axis) mediate this mechanism32,33. But hypothalamus first activates autonomic nervous system before it affects the hypothalamic–pituitary–adrenal (HPA) axis34. Anxiety and/or depression is a reaction to stress. When the body experiences a stressful event, the amygdala, an area of the brain that contributes to emotional processing, sends a signal to the hypothalamus35. The hypothalamus activates the adrenal medulla and causes the ‘fight or flight’ response via the sympathomedullary pathway35. The adrenal medulla, part of the ANS, secretes adrenaline, a hormone of fear. The ANS comprises the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS)36. When the body is stressed, the SNS contributes to coping with the threat35. SNS hormones increase the heart rate and respiration rate, and dilate blood vessels in the arms and legs to deal with the emergency35,36. When this reaction is over, the body usually returns to the pre-emergency, unstressed state35,36. This recovery is facilitated by the PNS, which generally has opposing effects to the SNS35,36. However, excessive PNS activity can also contribute to stress reactions such as bronchoconstriction, exaggerated vasodilation and compromised blood circulation35,36. Repetitive emotional changes and continual anxiety responses can destroy the balance in the ANS36. As the ANS is responsible for biological equilibrium in the body, it functions in regulation of the intraocular pressure and blood flow.
Although the relative importance of the mean, peak and fluctuation remain controversial, IOP is considered the most important modifiable factor in the development or progression of glaucoma23–27. Decreased ocular blood flow is associated with glaucoma progression37. DH, a surrogate for local blood flow disturbance, is also a well-known risk factor for the development and progression of glaucoma. Dysfunction of the ANS may impair all of these functions. In this way, emotional stress, such as anxiety or depression, affects the variation of IOP and the disturbance of blood flow through the unstable ANS.
There are limitations of the present study. First, since the questionnaire on anxiety or depression was measured at the time of study inclusion, it was not possible to confirm whether the scores were the same throughout the follow up period. Second, VF test could have fluctuation of accuracy, and anxiety could affect the reliability of VF. Third, glaucoma progression is slow, the observation period may not have been long enough. The observation period was not constant and the standard deviation was rather large in study. However, when comparing the two groups (high or low in anxiety or depression), these difference were not statistically significant. Finally, the small effects of other variables, confounding variables could have been fully apparent in the present analysis, because this study only included modest sample sizes.
To summarize, patients with anxiety showed faster rates of RNFL decline, as measured by OCT. These finding offer new insights into the care of patients with glaucoma. Therefore, the management of depression or anxiety may be helpful in managing glaucoma.
Materials and methods
Subjects
This study included 251 patients with open angle glaucoma who visited the Seoul St. Mary's Hospital between December 2018 and February 2020. It was approved by the Institutional Review and Ethics Board of Seoul St. Mary's Hospital. We followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all the eligible subjects.
Only those patients with at least 2 years of follow-up were eligible for the study. One eye per patient was enrolled. If both eyes are eligible, only the right eye has been enrolled. All patients enrolled underwent ophthalmologic examination consisting of slit lamp biomicroscopy, IOP measurement, by Goldmann applanation tonometry; anterior chamber angle measurement, by gonioscopy; dilated stereoscopic examination of the optic disc; red-free fundus photography (Kowa nonmyd WX; Kowa Company Ltd., Tokyo, Japan); central corneal thickness, measured with ultrasound pachymetry (Tomey Corp, Nagoya, Japan); and axial length measurement, by ocular biometry (IOLMaster, Carl Zeiss Meditec, Dublin, CA). Retinal nerve fiber layer (RNFL) thickness was measured by the Cirrus OCT (Carl Zeiss Meditec). It calculates the global RNFL thickness automatically. Humphrey visual fields (VFs) were tested using Swedish Interactive Threshold Algorithm standard 24-2 perimetry (Carl Zeiss Meditec) at each visit. History of DH was investigated through review of medical records.
Open angle glaucoma (primary open angle glaucoma or normal tension glaucoma) was defined as the open angle, glaucomatous optic nerve damage, and associated, repeatable VF damage. Diagnosis of glaucomatous optic nerve damage was based on the presence of focal or diffuse thinning of the RNFL. Glaucomatous VFs were defined as a cluster of 3 or more non-edge points on the pattern deviation map with a probability < 5% of the healthy population, including at least 1 of those points with the probability of < 1% of the healthy population (reliable tests; fixation losses < 20%, false negative < 15% and false positives < 15%)38.
Optic disc tilt, torsion, and peripapillary atrophy (PPA)-to-disc ratio were measured on photographs, by two independent examiners (DYS and HYP) using image-analysis software (ImageJ version 1.40; http://rsb.info.nih.gov/ij/index.html; National Institutes of Health, Bethesda).
Optic disc tilt was defined as the ratio between the longest and shortest diameters of the optic disc20,39. Optic disc torsion was defined as the deviation of the long axis of the optic disc from the vertical meridian20,40. PPA-to-disc ratio was defined as the ratio between the PPA area and disc area (PPA to disc ratio = PPA area ÷ disc area)20,41. The areas of the PPA and disc were calculated using the imageJ software. The techniques for assessing the disc tilt, torsion and PPA-to-disc ratio have been described and applied in previous investigations20.
Mean IOP is the average of all measurements obtained during the follow up period. Peak IOP was the maximum IOP of all measurements obtained during follow-up period. The fluctuation of IOP was calculated by subtracting the lowest value from the largest value of the IOPs of all measurements obtained during follow-up period.
BP measurements included systolic and diastolic BP at the height of the heart, measured with an Omron Automatic BP instrument (model BP791IT; Omron Hearlthcare, Inc., Lake Forest, IL). Mean arterial BP was calculated as 1/3 systolic BP + 2/3 diastolic BP.
Heart rate variability was measured with a Medicore Heart rate Analyzer, Model SA-3000P (Medicore, Seoul, Korea). The standard deviation value of the qualified normal to normal intervals(SDNN) was used as a representative indicator of heart rate variability. It is believed to primarily be a measure of autonomic influence on heart rate variability.
Beck’s Anxiety Inventory (BAI) and Beck’s Depression Inventory-II (BDI-II)
We used the BAI and BDI-II to evaluate psychological status42,43. The BAI and BDI-II are commonly used self-report questionnaires, used to determine the presence of anxiety disorder or depression disorder. We used these questionnaires measure common somatic and to evaluate the degree of anxiety or depression. The BAI questionnaire measures common somatic and cognitive symptoms of anxiety. Both the BAI and BDI-II include 21 items scored from 0 to 3, to generate a total score ranging from 0 to 63. Higher scores indicate greater anxiety/depression. In the BAI, total scores of 0–9 indicate normal levels of anxiety, and scores higher than 9 indicate clinically significant anxiety symptoms, based on published guidelines42. In the BDI-II, total scores higher than 13 indicate clinically significant depressive symptoms, based on guidelines and previous studies44. The questions on the BAI and BDI-II are listed in Tables 4 and 7, respectively.
Statistical analysis
Sample size calculations were performed using a statistical power analysis program (G*Power 3.1 software). The minimum sample size was calculated as 244 total, 41 in group 1 and 203 un group 2 after setting the effect size at 0.05 (minimum size), the alpha error at 0.04 and the power at 0.80 (5 times difference between the two groups for t-test statistic).
To explore the hypothesis that in glaucoma patients, the group with high anxiety or depression will show different characteristics of glaucoma, patients were grouped and compared according to their BAI or BDI-II scores (separately). The independent t-test and chi-square test for independent samples were used to assess the differences between high and low (anxiety or depression) group. The RNFL loss rate was calculated from serial OCT measurements and observation times. Logistic regression analyses were used to identify parameters of the glaucoma that were associated with anxiety and depression. Factors with a P-value of < 0.05 in the univariate model were included in the multivariate model. P-values < 0.05 indicated statistical significance. All statistical analyses were performed with SPSS for Windows statistical software (ver.24.0; SPSS Inc., Chicago, IL). Data are presented as mean standard deviation except where stated otherwise. Linear regression analysis was used to search for correlations between the RNFL loss rate or MD of VF and the ocular parameters, including age, sex, and axial length, BAI score, BDI-II score, HRV and so on.
Acknowledgements
We thank Claire Barnes, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Author contributions
D.Y.S. and C.K.P. wrote main manuscript text. D.Y.S. and C.K.P. conceived of the study, and participated in its design. K.I.J. and H.Y.L.P. performed the statistical analysis. All authors discussed the results, and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatib TZ, Martin KR. Protecting retinal ganglion cells. Eye (London, England) 2017;31:218–224. doi: 10.1038/eye.2016.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jindal V. Glaucoma: an extension of various chronic neurodegenerative disorders. Mol. Neurobiol. 2013;48:186–189. doi: 10.1007/s12035-013-8416-8. [DOI] [PubMed] [Google Scholar]
- 4.Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med. J. Aust. 2009;190:S54–60. doi: 10.5694/j.1326-5377.2009.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 5.Moussavi S, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet (London, England) 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, et al. The association between glaucoma, anxiety, and depression in a large population. Am. J. Ophthalmol. 2017;183:37–41. doi: 10.1016/j.ajo.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MR, et al. Depression in patients with glaucoma as measured by self-report surveys. Ophthalmology. 2002;109:1018–1022. doi: 10.1016/S0161-6420(02)00993-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang SY, Singh K, Lin SC. Prevalence and predictors of depression among participants with glaucoma in a nationally representative population sample. Am. J. Ophthalmol. 2012;154:436–444.e432. doi: 10.1016/j.ajo.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezapour J, et al. Prevalence of depression and anxiety among participants with glaucoma in a population-based cohort study: The Gutenberg Health Study. BMC Ophthalmol. 2018;18:157. doi: 10.1186/s12886-018-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelder M, Gath D, Mayou R. Oxford Medical Publications. Oxford Textbook of Psychiatry. 2. Oxford: Oxford University Press; 1989. [Google Scholar]
- 11.Bailey PH. The dyspnea-anxiety-dyspnea cycle–COPD patients' stories of breathlessness: "It's scary/when you can't breathe". Qual. Health Res. 2004;14:760–778. doi: 10.1177/1049732304265973. [DOI] [PubMed] [Google Scholar]
- 12.DeJean D, Giacomini M, Vanstone M, Brundisini F. Patient experiences of depression and anxiety with chronic disease: a systematic review and qualitative meta-synthesis. Ont. Health Technol. Assess. Ser. 2013;13:1–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol. Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 14.Goldney RD, Ruffin R, Fisher LJ, Wilson DH. Asthma symptoms associated with depression and lower quality of life: a population survey. Med. J. Aust. 2003;178:437–441. doi: 10.5694/j.1326-5377.2003.tb05285.x. [DOI] [PubMed] [Google Scholar]
- 15.Midenfjord I, Polster A, Sjövall H, Törnblom H, Simrén M. Anxiety and depression in irritable bowel syndrome: exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019;31:e13619. doi: 10.1111/nmo.13619. [DOI] [PubMed] [Google Scholar]
- 16.Sibelli A, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 2016;46:3065–3080. doi: 10.1017/S0033291716001987. [DOI] [PubMed] [Google Scholar]
- 17.Berchuck S, Jammal A, Mukherjee S, Somers T, Medeiros FA. Impact of anxiety and depression on progression to glaucoma among glaucoma suspects. Br. J. Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-316617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin. Lab. Med. 2010;30:865–891. doi: 10.1016/j.cll.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Hoehn-Saric, R., McLeod, D. R., Funderburk, F. & Kowalski, P Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch. Gen. Psychiatry. 2004;61:913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- 20.Shin DY, Jeon SJ, Park HYL, Park CK. Posterior scleral deformation and autonomic dysfunction in normal tension glaucoma. Sci. Rep. 2020;10:8203. doi: 10.1038/s41598-020-65037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HL, Jung SH, Park SH, Park CK. Detecting autonomic dysfunction in patients with glaucoma using dynamic pupillometry. Medicine. 2019;98:e14658. doi: 10.1097/MD.0000000000014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr. Opin. Ophthalmol. 2016;27:94–101. doi: 10.1097/ICU.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998;126:498–505. doi: 10.1016/S0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 24.The Advanced Glaucoma Intervention Study (AGIS) 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/S0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 25.Lichter PR, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/S0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 26.Rezapour J, Hoffmann EM. The role of intraocular pressure fluctuation in the development and progression of glaucoma. Klin. Monatsbl. Augenheilkd. 2019;236:667–671. doi: 10.1055/s-0043-106299. [DOI] [PubMed] [Google Scholar]
- 27.Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. BioMed Res. Int. 2015;2015:141905. doi: 10.1155/2015/141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manderson L, Kokanovic R. "Worried all the time'': distress and the circumstances of everyday life among immigrant Australians with type 2 diabetes. Chronic Illn. 2009;5:21–32. doi: 10.1177/1742395309102243. [DOI] [PubMed] [Google Scholar]
- 29.Bogner HR, Dahlberg B, de Vries HF, Cahill E, Barg FK. Older patients' views on the relationship between depression and heart disease. Fam. Med. 2008;40:652–657. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, et al. Comparison of glaucoma progression detection by optical coherence tomography and visual field. Am. J. Ophthalmol. 2017;184:63–74. doi: 10.1016/j.ajo.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artes PH, et al. Properties of the statpac visual field index. Investig. Ophthalmol. Vis. Sci. 2011;52:4030–4038. doi: 10.1167/iovs.10-6905. [DOI] [PubMed] [Google Scholar]
- 32.Vera J, Redondo B, Álvarez-Rodríguez M, Molina R, Jiménez R. The intraocular pressure responses to oral academic examination: the influence of perceived levels of public speaking anxiety. Appl. Ergon. 2020;88:103158. doi: 10.1016/j.apergo.2020.103158. [DOI] [PubMed] [Google Scholar]
- 33.Abe RY, et al. Can psychologic stress elevate intraocular pressure in healthy individuals? Ophthalmol. Glaucoma. 2020;3:426–433. doi: 10.1016/j.ogla.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Currie AR, Symington T. The pathology of the pituitary and adrenal glands in systemic disease in man. Proc. R. Soc. Med. 1955;48:908–909. [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira-Silva I, Silva VA, Cunha RM, Foster C. Autonomic changes induced by pre-competitive stress in cyclists in relation to physical fitness and anxiety. PLoS ONE. 2018;13:e0209834. doi: 10.1371/journal.pone.0209834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki Y, Drance SM. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am. J. Ophthalmol. 1997;124:287–295. doi: 10.1016/S0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- 38.Jeong JH, Park KH, Jeoung JW, Kim DM. Preperimetric normal tension glaucoma study: long-term clinical course and effect of therapeutic lowering of intraocular pressure. Acta Ophthalmol. 2014;92:e185–193. doi: 10.1111/aos.12277. [DOI] [PubMed] [Google Scholar]
- 39.Vongphanit J, Mitchell P, Wang JJ. Population prevalence of tilted optic disks and the relationship of this sign to refractive error. Am. J. Ophthalmol. 2002;133:679–685. doi: 10.1016/S0002-9394(02)01339-9. [DOI] [PubMed] [Google Scholar]
- 40.Park HY, Lee K, Park CK. Optic disc torsion direction predicts the location of glaucomatous damage in normal-tension glaucoma patients with myopia. Ophthalmology. 2012;119:1844–1851. doi: 10.1016/j.ophtha.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Shin DY, et al. Association between peripapillary scleral deformation and choroidal microvascular circulation in glaucoma. Sci. Rep. 2019;9:18503. doi: 10.1038/s41598-019-54882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory Manual. 2. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 44.Barber TR, et al. Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40:zsx071s. doi: 10.1093/sleep/zsx071. [DOI] [PMC free article] [PubMed] [Google Scholar]