Abstract
Objective
We aimed to investigate the influence of income level on guideline-directed medical therapy (GDMT) prescription rates and prognosis of patients with heart failure (HF) following implementation of a nationwide health insurance programme.
Methods
A total of 633 098 hospitalised patients with HF from 1996 to 2013 were identified from Taiwan National Health Insurance Research Database. Participants were classified into low-income, median-income and high-income groups. GDMT utilisation, in-hospital mortality and postdischarge HF readmission, and mortality rates were compared.
Results
The low-income group had a higher comorbidity burden and was less likely to receive GDMT than the other two groups. The in-hospital mortality rate in the low-income group (5.07%) was higher than in the median-income (2.47%) and high-income (2.51%) groups. Compared with the high-income group, the low-income group had a significantly higher risk of postdischarge HF readmission (adjusted HR (aHR): 1.29, 95% CI 1.27 to 1.31), all-cause mortality (aHR: 1.98, 95% CI 1.95 to 2.02) and composite HF readmission/all-cause mortality (aHR: 1.54, 95% CI 1.52 to 1.56). These results were generally consistent among the population after propensity matching (low vs high: HR=2.08 for mortality and 1.36 for HF readmission; median vs high: HR=1.23 for mortality and 1.12 for HF readmission; all p<0.001) and after inverse probability of treatment weighting (low-income vs high-income group: HR: 2.19 for mortality and 1.16 for HF readmission; median-income vs high-income group: HR: 1.53 for mortality and 1.09 for HF readmission; all p<0.001). Lower utilisation of GDMT and poorer prognosis in lower-income hospitalised patients with HF appeared to mitigate over time.
Conclusions
Low-income patients with HF had nearly a twofold increase in the risk of in-hospital mortality and postdischarge events compared with the high-income group, partly due to lower GDMT utilisation. The differences between postdischarge HF outcomes among various income groups appeared to mitigate over time following the implementation of nationwide universal health coverage.
Keywords: heart failure, medication adherence, health care economics
Introduction
Heart failure (HF) emerges as a global threat in all cardiovascular diseases,1 especially in hospitalised patients, leading to high morbidity and mortality.2 HF inflicts a considerable economic burden on the healthcare system worldwide, not merely in Western nations but also in the Asia-Pacific regions, particularly in low-income and middle-income countries.3 As the final pathway of most cardiovascular disorders4 and the leading cause of hospitalisation among adults and the elderly population, the prevalence and burden of HF will continue to rise (up to 25%) in the next two decades in both developing and developed countries.1
It is generally believed that individuals with lower socioeconomic status are much more likely to develop heart disease than those who are wealthier.5 The poorer prognosis of patients with HF with lower income may be due to the misallocation of medical resources and differences in education level, degree of urbanisation, ability for self-care, wealth, environment and family support. Shorter life expectancy in HF was observed regardless of gender or ethnicity in developing countries, such as some Asian countries,6 partly attributable to highly diverse quality and performance of healthcare (ie, evidence-based therapies) across different socioeconomic regions. A healthcare system with universal coverage of health (UCH) insurance, for example, implementation of nationwide healthcare system, would be expected to eliminate gaps and variations of healthcare quality among subjects with different income levels and sociodemographic backgrounds within the same society, and therefore might theoretically improve clinical endpoints.7
Taiwan as a unique country paved the way for UCH by establishing a unique healthcare system for universal health coverage (National Health Insurance (NHI)) for more than two decades by assuring equal access to healthcare resources for all citizens (ie, guideline-directed medical therapy (GDMT)) regardless of socioeconomic level.8 By reviewing data regarding the temporal transitions of several key outcome measures, we may find evidence reflecting efficacy following enforcement of the nationwide healthcare insurance from the country level. In the present study, we aimed to investigate the impact of income level on the prognosis of patients with HF at the nationwide level.
Methods
Database
This study used data from the National Health Insurance Research Database (NHIRD), released by the Taiwan National Health Research Institutes. The NHI system is a universal, government-endorsed health insurance programme passed in 1994 and launched in 1995 that offers comprehensive medical care coverage to nearly all (>99.99%) Taiwanese population, with the NHI Administration overseeing the plan and controlling the global expenditure.9 The NHIRD collects detailed healthcare data from more than 23 million NHI enrollees in Taiwan. More information regarding the NHI system in Taiwan and categorisation of patients’ income level (as low: <20 000; median: 20 000–39 999; and high: ≥40 000 new Taiwan dollars) are further detailed in online supplemental materials. In this cohort data set, patients’ original identification numbers were encrypted to protect their privacy; however, the encrypting procedure was consistent so the claims belonging to the same patient could be linked within the NHI database and patients could be followed up.8 10
heartjnl-2020-316793supp001.pdf (5.6MB, pdf)
Study population
From 1 January 1996 to 31 December 2013, a total of 633 098 subjects aged 20 or older with a diagnosis of HF hospitalisation, according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 428.0–428.4, 428.9, without coexistence of a main diagnosis of acute coronary syndrome, were identified from the NHIRD. Information on important comorbid conditions for each individual was also retrieved from the NHIRD based on the ICD-9-CM codes. The diagnostic accuracy of important comorbidities in the NHIRD, including hypertension, diabetes mellitus, myocardial infarction, hyperlipidaemia and chronic obstructive pulmonary disease, has been previously validated,10 11 with Charlson Comorbidity Index (CCI) used to represent the comorbidity burden of the patients.12
Clinical outcomes
The clinical outcomes of the present study included in-hospital mortality and postdischarge HF readmission and composite outcome of all-cause mortality and HF readmission assessed following the index date of adjudicated HF discharge in survivors. Validity of the main outcome measures in the current study is detailed in the online supplemental materials. The temporal trends of events (HF readmission or all-cause mortality) were investigated. The risk of events was compared between the different income groups.
Propensity matching analysis
We performed propensity score-matched analyses for two kinds of comparisons: low-income versus high-income, and median-income versus high-income, conditional on all key baseline covariates listed in table 1. Online supplemental figures 1 and 2 show the distributions of propensity scores of study subjects for being as low-income and median-income groups before and after the propensity match, respectively. To show consistency of the estimates after matching, alternative matching methods were conducted using inverse probability of treatment weighting (IPTW). Methods on these matching processes are detailed in online supplemental materials.
Table 1.
Baseline characteristics of patients with heart failure
| Income groups | All | Low-income | Median-income | High-income | P value |
| n | 633 098 | 401 639 | 190 167 | 41 292 | |
| Baseline demographics | |||||
| Age, years, mean (SD) | 71.7 (13.4) | 74.6 (11.7) | 68.3 (14.5) | 58.9 (12.6) | <0.001 |
| ≥75, n (%) | 308 705 (48.8) | 231 539 (57.6) | 72 761 (38.3) | 4405 (10.7) | <0.001 |
| 65–74, n (%) | 165 987 (26.2) | 107 431 (26.7) | 49 760 (26.2) | 8796 (21.3) | <0.001 |
| <65, n (%) | 158 406 (25.0) | 62 669 (15.6) | 67 646 (35.6) | 28 091 (68.0) | <0.001 |
| Male gender, n (%) | 323 573 (51.1) | 194 733 (48.5) | 96 457 (50.7) | 32 383 (78.4) | <0.001 |
| Charlson Comorbidity Index, mean (SD) | 6.49 (2.98) | 6.40 (2.97) | 6.78 (2.98) | 6.11 (3.06) | <0.001 |
| Comorbidities, n (%) | |||||
| Hypertension | 482 638 (76.2) | 300 199 (74.7) | 151 610 (79.7) | 30 829 (74.7) | <0.001 |
| Diabetes mellitus | 258 863 (40.9) | 160 734 (40.0) | 80 407 (42.3) | 17 722 (42.9) | <0.001 |
| Stroke/TIA | 181 724 (28.7) | 119 711 (29.8) | 53 108 (27.9) | 8905 (21.6) | <0.001 |
| Vascular diseases | 368 897 (58.3) | 226 478 (56.4) | 117 955 (62.0) | 24 464 (59.2) | <0.001 |
| ESRD | 88 555 (14.0) | 54 582 (13.6) | 27 684 (14.6) | 6289 (15.2) | <0.001 |
| COPD | 251 642 (39.7) | 165 592 (41.2) | 74 750 (39.3) | 11 300 (27.4) | <0.001 |
| Malignancy | 96 215 (15.2) | 60 958 (15.2) | 28 955 (15.2) | 6302 (15.3) | 0.827 |
| Autoimmune diseases | 41 480 (6.6) | 23 854 (5.9) | 15 108 (7.9) | 2518 (6.1) | <0.001 |
| Liver cirrhosis | 29 717 (4.7) | 18 246 (4.5) | 9576 (5.0) | 1895 (4.6) | <0.001 |
| Dyslipidaemia | 195 356 (30.9) | 103 933 (25.9) | 73 023 (38.4) | 18 400 (44.6) | <0.001 |
| CKD | 125 624 (19.8) | 75 543 (18.8) | 40 527 (21.3) | 9554 (23.1) | <0.001 |
| VHD | 40 031 (6.3) | 23 816 (5.9) | 13 499 (7.1) | 2716 (6.6) | <0.001 |
| Anaemia | 158 116 (25.0) | 101 442 (25.3) | 48 959 (25.7) | 7715 (18.7) | 0.001 |
| Valvular heart surgery | 4053 (0.6) | 1565 (0.4) | 1717 (0.9) | 771 (1.9) | <0.001 |
| CABG | 12 349 (2.0) | 6337 (1.6) | 4202 (2.2) | 1810 (4.4) | <0.001 |
| AF | 118 744 (18.8) | 74 111 (18.5) | 37 075 (19.5) | 7558 (18.3) | <0.001 |
| Degree of urbanisation, n (%) | <0.001 | ||||
| Urban | 309 424 (48.9) | 209 448 (52.1) | 71 285 (37.5) | 28 691 (69.5) | |
| Suburban | 203 913 (32.2) | 127 761 (31.8) | 64 879 (34.1) | 11 273 (27.3) | |
| Rural | 119 761 (18.9) | 64 430 (16.0) | 54 003 (28.4) | 1328 (3.2) | |
| Medications, n (%) | |||||
| ACEIs | 85 014 (13.4) | 51 916 (12.9) | 26 851 (14.1) | 6247 (15.1) | <0.001 |
| ARBs | 117 728 (18.6) | 61 179 (15.2) | 45 576 (24.0) | 10 973 (26.6) | <0.001 |
| Amiodarone | 57 169 (9.0) | 33 268 (8.3) | 19 144 (10.1) | 4757 (11.5) | <0.001 |
| Digoxin | 167 864 (26.5) | 114 885 (28.6) | 43 387 (22.8) | 9592 (23.2) | <0.001 |
| Beta-blockers | 145 048 (24.3) | 83 617 (20.8) | 54 795 (28.8) | 15 636 (37.9) | <0.001 |
| Diuretics | 336 887 (53.2) | 219 954 (54.8) | 97 190 (51.1) | 19 743 (47.8) | <0.001 |
| MRA* | 106 170 (16.8) | 61 396 (15.3) | 36 407 (19.1) | 8367 (20.3) | <0.001 |
*MRA excluded.
ACEIs, ACE inhibitors; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass graft; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; MRA, mineralocorticoid receptor antagonist (eplerenone/spironolactone); TIA, transient ischaemic attack; VHD, valvular heart disease.
Statistical analysis
Data were summarised using mean and SD for continuous variables and proportions for categorical variables. Group differences for continuous values were assessed using unpaired two-tailed t-tests or one-way analysis of variance. Group differences for nominal variables were compared using χ2. An interaction analysis was performed by adding an interaction term to a regression model between income strata and three major time intervals (1996–2001, 2002–2007 and 2008–2013) as a continuous linear predictor with respect to CCI (age-adjusted and sex-adjusted). A linear regression analysis was used to test the linear trends of CCI (age-adjusted and sex-adjusted), HF pharmacological prescription patterns and in-hospital mortality (expressed as adjusted ORs for median-income/high-income groups, and low-income as reference) across three major time intervals as ordinal category. The survival function estimating the risk of HF readmission, all-cause mortality and composite outcome of HF readmission/mortality postdischarge was assessed using Cox regression analysis. The risk of in-hospital mortality was assessed using logistic regression analysis. The cumulative incidence curve of all-cause mortality was plotted using the Kaplan-Meier method, with statistical significance examined with the log-rank test. Subgroup analyses for HF outcomes using Cox regression models among income strata (median-income/low-income vs high-income group) were conducted according to key baseline characteristics (including age, gender, degree of urbanisation, comorbidities and HF-related medications). Statistical significance was set at p<0.05. All analyses were performed using IBM SPSS Statistics for Windows V.20.0 and SAS software V.9.4.
Patient and public involvement
Participants were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
Baseline demographics
Baseline characteristics are displayed in table 1. Among 633 098 patients hospitalised with HF from 1996 to 2013, 401 639 (63.4%) were categorised as low income, 190 167 (30.0%) as median income and 41 292 (6.5%) as high income. The mean age of HF diagnosis was 71.7 (SD=13.4) years, and gender was nearly equally distributed (51.1% men). There was a significant difference (p<0.001) in mean age between the income groups: 58.9 (12.6) years in the high-income, 68.3 (14.5) in the median-income and 74.6 (11.7) in the low-income group. In our study cohort, patients with HF with low income were older, more likely to be female, more likely to have a history of stroke/transient ischaemic attack and chronic obstructive pulmonary disease, less likely to have vascular diseases (including coronary artery disease), chronic kidney disease and hyperlipidaemia, and more likely to live in rural regions, compared with median-income and high-income groups. CCI was higher in low-income and median-income groups than in high-income patients with HF (6.4 and 6.78 vs 6.11, p<0.001; table 1).
Association between income level, comorbidity burden and pharmacological use
Comorbidity burden as measured by CCI increased in a graded fashion from 1996 to 2013 (classified into 1996–2001, 2002–2007 and 2008–2013) for all patients with HF postdischarge irrespective of income strata (all ptrend <0.001; figure 1). The age-adjusted and sex-adjusted CCI increment over time was 1.01 (95% CI 0.99 to 1.02 per decade, p<0.001) and was most pronounced in the low-income group, followed by the median-income and high-income groups (1.49 (95% CI 1.47 to 1.51), 0.2 (95% CI 0.17 to 0.23), 0.36 (95% CI 0.29 to 0.42) per decade for low-income, median-income and high-income HF groups, respectively; pinteraction <0.001), indicating a temporal trend of increasing comorbidity burden in discharged patients with HF over time particularly in the low-income group.
Figure 1.
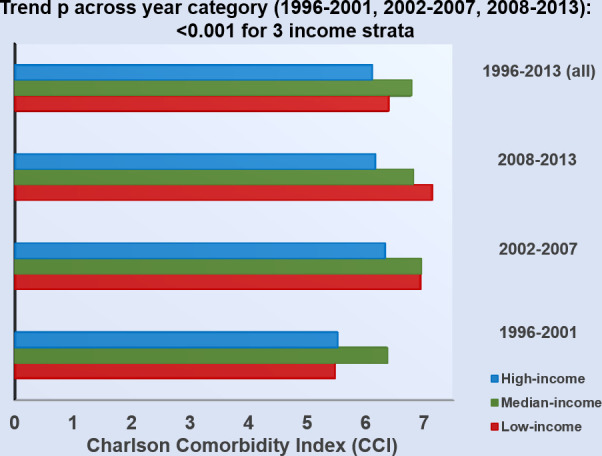
Charlson Comorbidity Index (CCI) stratified by three income groups. CCI increased in a graded fashion for all postdischarge patients with HF over time (classified into 1996–2001, 2002–2007 and 2008–2013) irrespective of income strata (all ptrend <0.001).
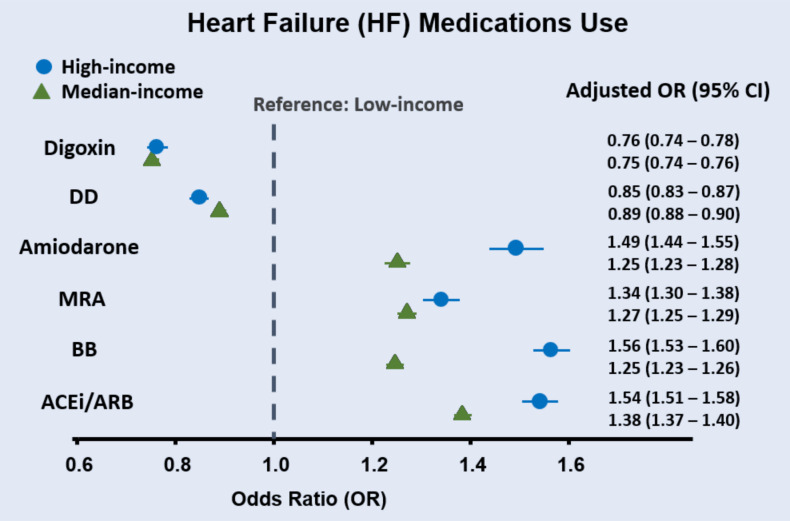
We also observed different prescription patterns for several HF-related medications across the income groups. Low-income patients with HF were less frequently prescribed GDMT for reduced ejection fraction HF (ie, ACE inhibitors/angiotensin receptor blockers (ACEIs/ARBs), beta-blockers (BBs) and mineralocorticoid receptor antagonists (MRAs)) and amiodarone, although they were more likely to receive digoxin and diuretics when compared with middle-income and high-income HF groups (all p<0.001; figure 2). These findings indicate a different pharmacological prescription pattern of HF medications across different income strata. Overall, the differences in HF pharmacological prescription patterns among income groups decreased in fully adjusted models (as adjusted ORs, with low-income as reference) across time intervals (1996–2001, 2002–2007 and 2008–2013) (all ptrend <0.001) (online supplemental table 1).
Figure 2.
Heart failure medications stratified by three income groups. Different patterns of medication use across income groups were observed, with the median-income and high-income groups being more likely to receive GDMT (including ACEi/ARB, BB and MRA) and amiodarone, and less commonly prescribed DD (MRA excluded) and digoxin in fully adjusted models. ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; DD, diuretic drugs; GDMT, guideline-directed medical therapy; MRA, mineralocorticoid receptor antagonist (eplerenone/spironolactone).
Association between income level and in-hospital mortality
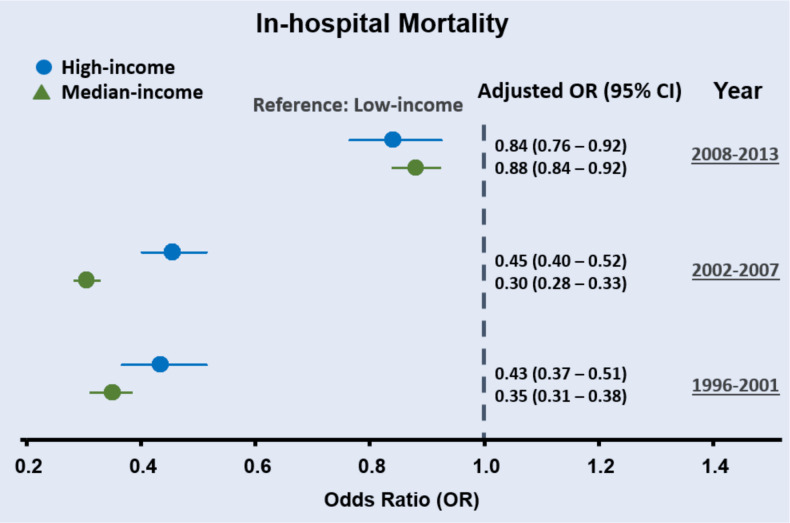
Among 633 098 patients aged 20 or older between 1996 and 2013 with HF hospitalisation, 26 093 (4.1%) died during admission. A significantly higher in-hospital mortality rate was observed in the low-income (5.07%) compared with the median-income (2.47%) and high-income (2.51%) HF groups (table 1). The risk of in-hospital mortality was significantly higher for the low-income HF population (crude OR: 2.07 (95% CI 1.94 to 2.21), p<0.05; table 2) and remained significant in the fully adjusted model (adjusted OR: 1.53 (95% CI 1.43 to 1.64), p<0.05; table 2). Differences in in-hospital mortality for median-income and high-income groups compared with low-income group also decreased (as adjusted ORs) across time intervals (1996–2001, 2002–2007 and 2008–2013) (both ptrend <0.001) in fully adjusted models (figure 3).
Table 2.
Incidence of all-cause mortality, HF readmission and composite endpoint in patients with heart failure
| Income groups | Total | High-income | Median-income | Low-income |
| Patients, n | 633 098 | 41 292 | 190 167 | 401 639 |
| In-hospital mortality, n | 26 093 | 1038 | 4703 | 20 352 |
| Events rate, % | 4.12 | 2.51 | 2.47 | 5.07 |
| Unadjusted OR (95% CI) | – | – | 0.98 (0.92 to 1.05)† | 2.07 (1.94 to 2.21)† |
| Model 1: OR (95% CI) | – | – | 0.95 (0.89 to 1.02)† | 1.92 (1.80 to 2.05)† |
| Model 2: OR (95% CI) | – | – | 0.96 (0.90 to 1.03)† | 1.53 (1.43 to 1.64)† |
| All-cause mortality, n | 391 337 | 12 872 | 85 797 | 292 668 |
| Person-years | 2 454 689 | 201 095 | 898 631 | 1 354 963 |
| Incidence* | 15.94 (15.89 to 15.99) | 6.40 (6.29 to 6.51) | 9.55 (9.48 to 9.61) | 21.60 (21.52 to 21.68) |
| Unadjusted HR (95% CI) | – | – | 1.48 (1.46 to 1.51)† | 3.16 (3.10 to 3.21)† |
| Model 1: HR (95% CI) | – | – | 1.13 (1.11 to 1.15)† | 1.99 (1.96 to 2.03)† |
| Model 2: HR (95% CI) | – | – | 1.16 (1.14 to 1.18)† | 1.98 (1.95 to 2.02)† |
| HF readmission, n | 287 226 | 16 255 | 85 954 | 185 017 |
| Person-years | 1 593 620 | 140 944 | 596 458 | 856 217 |
| Incidence* | 18.02 (17.96 to 18.09) | 11.53 (11.36 to 11.71) | 14.41 (14.31 to 14.51) | 21.61 (21.51 to 21.71) |
| Unadjusted HR (95% CI) | – | – | 1.20 (1.18 to 1.22)† | 1.55 (1.53 to 1.58)† |
| Model 1: HR (95% CI) | – | – | 1.08 (1.06 to 1.10)† | 1.28 (1.26 to 1.30)† |
| Model 2: HR (95% CI) | – | – | 1.08 (1.06 to 1.09)† | 1.29 (1.27 to 1.31)† |
| All-cause mortality/HF readmission, n | 476 425 | 22 425 | 124 745 | 329 255 |
| Person-years | 1 593 618 | 140 944 | 596 458 | 856 216 |
| Incidence* | 29.90 (29.81 to 29.98) | 15.91 (15.70 to 16.12) | 20.91 (20.80 to 21.03) | 38.45 (38.32 to 38.59) |
| Unadjusted HR (95% CI) | – | – | 1.27 (1.25 to 1.29)† | 2.04 (2.02 to 2.07)† |
| Model 1: HR (95% CI) | – | – | 1.08 (1.07 to 1.10)† | 1.55 (1.53 to 1.57)† |
| Model 2: HR (95% CI) | – | – | 1.09 (1.07 to 1.11)† | 1.54 (1.52 to 1.56)† |
Model 1: adjusted for age and gender.
Model 2: adjusted for age, gender, hypertension, diabetes mellitus, previous stroke/TIA, vascular diseases, ESRD, COPD, autoimmune diseases, liver cirrhosis, dyslipidaemia, anaemia, CABG, AF, Charlson Comorbidity Index, ACEIs, ARBs, amiodarone, digoxin, beta-blockers and MRA.
*Number of events presented per 100 person-years of follow-up.
†Compared with high-income group.
ACEIs, ACE inhibitors; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HF, heart failure; MRA, mineralocorticoid receptor antagonist (eplerenone/spironolactone); TIA, transient ischaemic attack.
Figure 3.
Temporal trend of in-hospital mortality stratified by three income groups. For in-hospital mortality, differences in in-hospital mortality for median-income and high-income groups compared with low-income heart failure group decreased over time (classified as 1996–2001, 2002–2007, 2008–2013).
Association between income level and HF outcomes
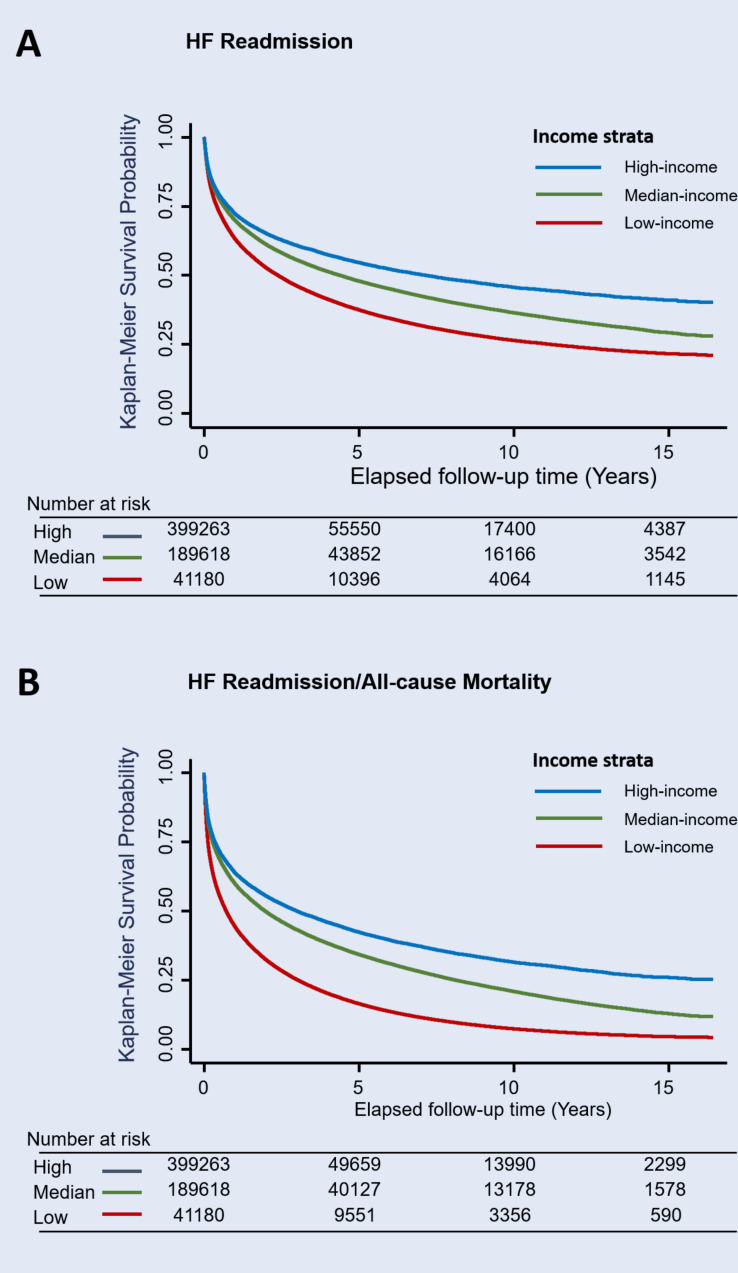
Among the total 607 005 discharged HF survivors, all-cause mortality, HF readmission, and composite all-cause mortality and HF readmission were observed in 391 337 (64.5%), 287 226 (47.3%), and 476 425 (78.5%) patients, respectively, during the study observation period. The cumulative incidence curves of postdischarge HF readmission and HF readmission/mortality are shown in figure 4A and B, respectively. Overall, 16.8%, 15.6% and 17.4% of mortality/HF readmission cases occurred within the first month (30 days) postdischarge across the three income groups (for low-income, median-income and high-income HF groups, respectively). Similar trends in HF readmission or mortality were also observed (table 2). Notably, the temporal trends of risk of HF readmission or mortality in the low-income group diminished markedly after nearly one decade from the initiation of the NHI programme (HR of HF readmission and composite HF readmission/mortality: 2.64 and 4.94 in 1996 vs 1.46 and 2.65 in 2008 for the low-income group, using high-income group as reference; ptrend <0.001; figure 5A and B). Findings from subgroup analyses are shown in online supplemental figure 3, with details provided in online supplemental materials.
Figure 4.
Heart failure (HF) readmission (A) and composite HF readmission/all-cause mortality (B) using Kaplan-Meier survival-free curves stratified by three income groups. Patients with HF of low income consistently demonstrated higher risk for postdischarge HF readmission or composite HF readmission/all-cause mortality compared with patients of higher-income strata.
Figure 5.
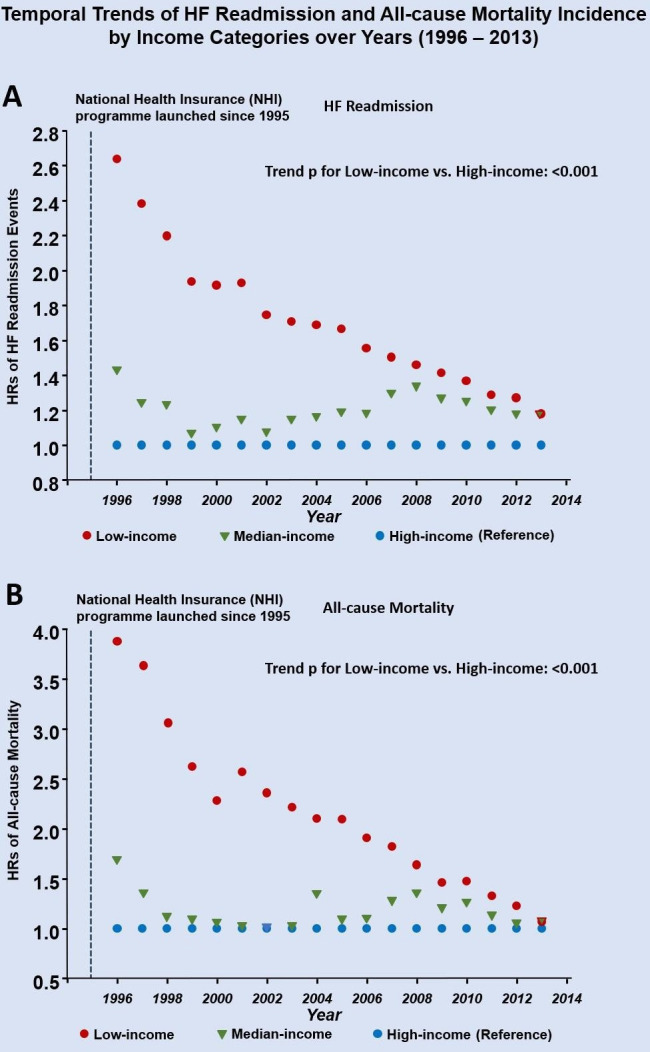
Temporal trends of heart failure (HF) readmission (A) and all-cause mortality (B) by three income groups over time (1996–2013). A marked decrease in the incidence of HF readmission and all-cause mortality was observed over time for the low-income group (expressed as HR, reference: high-income group). A linear trend analysis was used for adjusted HR for low-income versus high-income HF group (as reference) across observation time (per year as ordinal category).
Propensity analysis
The baseline characteristics of patients after matching are shown in online supplemental table 2. The propensity scores did not differ significantly for low-income versus high-income group, and median-income versus high-income group. Comparisons of in-hospital mortality after propensity matching are shown in online supplemental table 2. Postdischarge HF readmission and mortality remained the lowest in the high-income group compared with the median-income group (median-income vs high-income: HR=1.23 (1.20–1.25) for mortality, HR: 1.12 (1.10–1.15) for HF readmission) and low-income group (low-income vs high-income: HR: 2.08 (2.04–2.13) for mortality, HR: 1.36 (1.33–1.39) for HF readmission; all p<0.001) after matching (online supplemental table 3). The results of various subgroup analyses of outcomes by different income strata were broadly consistent after matching (online supplemental figure 3). Temporal changes on main outcome measures show similar trends as shown in online supplemental figure 4. Subgroup analyses were broadly similar after matching (online supplemental figure 3).
Baseline characteristics of patients after IPTW are shown in online supplemental table 4. After weighting, the three groups were well balanced in most characteristics (absolute standardised mean difference <0.1). The main outcome measures after IPTW remained the lowest in the high-income group compared with the median-income group (median-income vs high-income: HR: 1.53 (1.26–1.75) for mortality, 1.09 (1.05–1.25) for HF readmission) and low-income group (low-income vs high-income: HR: 2.19 (2.07–2.86) for mortality, 1.16 (1.08–1.35) for HF readmission; all p<0.001) (online supplemental table 5).
Discussion
In a nationwide data set with nearly full coverage of healthcare insurance, we investigated the temporal trends of comorbidity burden, GDMT utilisation and prognosis among discharged patients with HF with various sociodemographic backgrounds. The main findings of our study are as follows: Patients with HF with lower income had a markedly higher comorbidity burden, less likely to receive GDMT and showed a twofold increased risk of in-hospital mortality, along with nearly threefold and 1.5-fold increased risk of postdischarge HF readmission and all-cause mortality, even after correction for several key baseline demographic information. These findings were broadly consistent after propensity matching. Second, there appeared to be a temporal trend of mitigated variations of GDMT utilisation and postdischarge HF prognosis across different income strata about one decade following implementation of the nationwide healthcare insurance, despite an overall increase in comorbidity burden among all patients with HF.
Prior reports consistently found that socioeconomically deprived individuals might show a higher incidence of HF.13 The causal relationship between lower socioeconomic status and poorer prognosis has also been confirmed from a longitudinal study exploring income changes with incident cardiovascular events including HF.14 A recent global between-country analysis showed that income inequality, rather than income level alone, may impact on HF outcomes to a similar degree as do major comorbidities.15 The relationship between lower socioeconomic status and worse clinical outcome could be bidirectional due to higher economic burden imposed by HF per se or HF-related comorbidities. Our findings were consistent with prior reports in that poorer prognosis is more likely to occur in lower-income patients with HF, presumptively explained by multiple influences from sociodemographic diversity including healthcare access, quality of practice, barriers to evidence-based care and underlying nutritional status.16–18 Findings of markedly older age, lower rates of valvular heart or coronary artery bypass graft surgery in both low-income and median-income strata compared with the high-income HF group may reflect the fact that lower-income patients with HF may remain poorly recognised or tend to seek medical help only when sicker. Furthermore, lower-income HF populations were more likely to stay in suburban or rural areas, supporting effects of geographical variations and aggregated poverty, resulting in disparities in healthcare utilisation.19 20 Nevertheless, the observed differences in postdischarge HF outcome from socioeconomic disparities appeared to diminish about one decade following NHI programme implementation (figure 5).
Notably, we noticed that patients with HF with lower income showed a lower prescription rate of evidence-based GDMT for heart failure with reduced ejection fraction (HFrEF),21 including ACEIs/ARBs, BBs and MRA.22 Instead, prescription rate of digoxin or diuretics was substantially higher in low-income patients with HF despite their older age, higher clinical disease complexity and yet less prominent variations of prevalent atrial fibrillation compared with higher-status groups (all <20%).23 This finding likely supported the gap in evidence-based HF practice and likely represents variations in prescription habits of healthcare providers, along with lower awareness on GDMT adherence in low-income patients with HF, especially in certain areas among Asian societies. Interestingly, income level has been proposed as an essential component of socioeconomic status influencing medication adherence in HF polypharmacy.24 Based on a more recent study, even suboptimal adherence to GDMT (ie, nearly half of the guideline-recommended dosage) has been shown to substantially improve HF outcomes.25 To the best of our knowledge, this study is the first to delineate the demographics of postdischarge HF survivors in a large-scale, population-based study examining the temporal associations of income level and GDMT use with postdischarge HF outcome following the implementation of the nationwide universal healthcare coverage. The strength of the current study included data extraction from a healthcare system, providing >99% coverage for all citizens less likely to be biased according to geographical variations, subpopulations/strata or degree of urbanisation tightly bound to income status, therefore disclosing real-world HF key features reflecting demographics, managements and outcomes by income strata with a relatively long span of follow-up time at the country level.
Patients of lower socioeconomic status may have lower chance of receiving evidence-based treatments due to financial pressures in a society without global healthcare coverage.26 As such, reform of the nationwide health insurance policies and integrations of multidisciplinary teams working (such as Post-Acute Care (PAC) programme) with optimal discharge planning and referral system27–29 may theoretically improve the adherence of evidence-based HF therapy (ie, GDMT) based on the public health standpoint. Taken together, our findings highlight the potential benefits of implementing nationwide health insurance to overcome barriers to effective therapeutic interventions and thus to improve HF outcomes.
Study limitations
The analysis and findings of the current study were not without limitations. The data extracted from Taiwan’s NHIRD did not contain information on the distinct HF phenotypes (reduced (HFrEF) or preserved ejection fraction HF); nevertheless, accumulating data have suggested that the rate of acute HF may distribute evenly in distinct phenotypes of HF with similar outcomes.30 Notably, although we controlled for several key baseline demographics, comorbidities and GDMT use, the impact of socioeconomic disparities on outcomes remained prominent across different income strata, implying potentially unmeasurable societal and patient-level confounders (eg, cultural backgrounds, health maintenance behaviour or lifestyle factors). Moreover, although we speculated that the observed reduction of the gap in HF outcomes may likely be attributable to the implementation of NHI programme, we could not preclude an influence for an overall improved systemic public health service and diminished gap in economic and social inequities that ultimately affect patients’ prognosis. Furthermore, major advances in new pharmacological or interventional HF therapies (such as implantable cardioverter-defibrillator or cardiac resynchronisation therapy) may have resulted in overall enhanced quality of care.
Conclusions
Lower-income level is associated with lower utilisation of evidence-based pharmacological HF treatments with higher in-hospital death rates and poorer postdischarge outcomes. The observed worse postdischarge outcomes in lower-income patients with HF appeared to mitigate over time following the implementation of the nationwide universal health coverage. However, some caution should be exercised in interpreting these findings due to overall a variety of unmeasurable factors over time. Despite these, understanding these data as a temporal trend may probably provide future directions to improve healthcare policies and financing models regarding public health infrastructure, thereby aiming for better resource reallocation for healthcare policy makers.
Key messages.
What is already known on this subject?
Income level and socioeconomic status have shown to be prognostic factors in cardiovascular diseases, including heart failure (HF).
Epidemiological transitions of guideline-directed medical therapy (GDMT) utilisation and postdischarge outcomes in patients with HF following implementation of universal health coverage remain largely unexplored.
What might this study add?
Based on a nationwide data set, postdischarge patients with HF with lower income were less likely to receive GDMT, had a higher clinical comorbidity burden and significantly higher events when compared with median-income and high-income groups.
Such differences among various income groups of patients with HF appeared to mitigate over time about one decade following initiation of the nationwide universal health coverage policy.
How might this impact on clinical practice?
Our findings likely demonstrated the efficacy of implementing nationwide universal health coverage in HF management by eliminating the gap between barriers to guideline-directed medical resources, access to standardised treatment and improved healthcare quality over time.
Footnotes
Twitter: @CLHung
C-LH and T-FC contributed equally.
Contributors: C-LH, T-FC: coordination and helped draft the manuscript. C-HS, H-IY: conceptual framework and reviewed the manuscript. J-NL, K-TS: performed the statistical analysis. C-EC: conceived of the study and participated in its design.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The present study was approved by the Institutional Review Board at Taipei Veterans General Hospital (2019-04-004AC), Taipei, Taiwan. Informed consent was waived due to anonymous data.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement: This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. Also, the data could not be spread or distributed out of the institution.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conrad N, Judge A, Tran J, et al. . Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–80. 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajadurai J, Tse H-F, Wang C-H, et al. . Understanding the epidemiology of heart failure to improve management practices: an Asia-Pacific perspective. J Card Fail 2017;23:327–39. 10.1016/j.cardfail.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 4. Ceia F, Fonseca C, Mota T, et al. . Prevalence of chronic heart failure in southwestern Europe: the EPICA study. Eur J Heart Fail 2002;4:531–9. 10.1016/S1388-9842(02)00034-X [DOI] [PubMed] [Google Scholar]
- 5. Franks P, Winters PC, Tancredi DJ, et al. . Do changes in traditional coronary heart disease risk factors over time explain the association between socio-economic status and coronary heart disease? BMC Cardiovasc Disord 2011;11:28. 10.1186/1471-2261-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mensah GA. Heart failure in low-income and middle-income countries: rising burden, diverse etiology, and high mortality. J Card Fail 2018;24:833–4. 10.1016/j.cardfail.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 7. Obama B. United States health care reform: progress to date and next steps. JAMA 2016;316:525–32. 10.1001/jama.2016.9797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin L-Y, Warren-Gash C, Smeeth L, et al. . Data resource profile: the National health insurance research database (NHIRD). Epidemiol Health 2018;40:e2018062. 10.4178/epih.e2018062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsiao WC. Taiwan's path to universal health coverage-an essay by William C Hsiao. BMJ 2019;367:l5979. 10.1136/bmj.l5979 [DOI] [PubMed] [Google Scholar]
- 10. Hsieh C-Y, Su C-C, Shao S-C, et al. . Taiwan's National health insurance research database: past and future. Clin Epidemiol 2019;11:349–58. 10.2147/CLEP.S196293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin C-C, Lai M-S, Syu C-Y, et al. . Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005;104:157–63. [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13. Hawkins NM, Scholes S, Bajekal M, et al. . Community care in England: reducing socioeconomic inequalities in heart failure. Circulation 2012;126:1050–7. 10.1161/CIRCULATIONAHA.111.088047 [DOI] [PubMed] [Google Scholar]
- 14. Wang SY, Tan ASL, Claggett B, et al. . Longitudinal associations between income changes and incident cardiovascular disease: the Atherosclerosis risk in Communities study. JAMA Cardiol 2019;4:1203–12. 10.1001/jamacardio.2019.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dewan P, Rørth R, Jhund PS, et al. . Income Inequality and Outcomes in Heart Failure: A Global Between-Country Analysis. JACC Heart Fail 2019;7:336–46. 10.1016/j.jchf.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 16. Dokainish H, Teo K, Zhu J, et al. . Global mortality variations in patients with heart failure: results from the International congestive heart failure (INTER-CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–72. 10.1016/S2214-109X(17)30196-1 [DOI] [PubMed] [Google Scholar]
- 17. Callender T, Woodward M, Roth G, et al. . Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2014;11:e1001699. 10.1371/journal.pmed.1001699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Downing NS, Wang C, Gupta A, et al. . Association of racial and socioeconomic disparities with outcomes among patients hospitalized with acute myocardial infarction, heart failure, and pneumonia: an analysis of within- and between-hospital variation. JAMA Netw Open 2018;1:e182044. 10.1001/jamanetworkopen.2018.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soria-Saucedo R, Xu P, Newsom J, et al. . The role of geography in the assessment of quality: evidence from the Medicare advantage program. PLoS One 2016;11:e0145656. 10.1371/journal.pone.0145656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auchincloss AH, Hadden W. The health effects of rural-urban residence and concentrated poverty. J Rural Health 2002;18:319–36. 10.1111/j.1748-0361.2002.tb00894.x [DOI] [PubMed] [Google Scholar]
- 21. Fonarow GC, Albert NM, Curtis AB, et al. . Associations between outpatient heart failure process-of-care measures and mortality. Circulation 2011;123:1601–10. 10.1161/CIRCULATIONAHA.110.989632 [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, et al. . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 23. Masoudi FA, Baillie CA, Wang Y, et al. . The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998-2001. Arch Intern Med 2005;165:2069–76. 10.1001/archinte.165.18.2069 [DOI] [PubMed] [Google Scholar]
- 24. Allen LA, Fonarow GC, Liang L, et al. . Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation 2015;132:1347–53. 10.1161/CIRCULATIONAHA.115.014281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ouwerkerk W, Voors AA, Anker SD, et al. . Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J 2017;38:1883–90. 10.1093/eurheartj/ehx026 [DOI] [PubMed] [Google Scholar]
- 26. The HJ. Recession and health insurance coverage. Health Aff 2007-09;2011:145–52. [DOI] [PubMed] [Google Scholar]
- 27. Feltner C, Jones CD, Cené CW, et al. . Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med 2014;160:774–84. 10.7326/M14-0083 [DOI] [PubMed] [Google Scholar]
- 28. Albert NM, Barnason S, Deswal A, et al. . Transitions of care in heart failure: a scientific statement from the American heart association. Circ Heart Fail 2015;8:384–409. 10.1161/HHF.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 29. Riley JP, Masters J. Practical multidisciplinary approaches to heart failure management for improved patient outcome. Eur Heart J Suppl 2016;18:G43–52. 10.1093/eurheartj/suw046 [DOI] [Google Scholar]
- 30. Senni M, Gavazzi A, Oliva F, et al. . In-Hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. results from IN-HF outcome registry. Int J Cardiol 2014;173:163–9. 10.1016/j.ijcard.2014.02.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-316793supp001.pdf (5.6MB, pdf)