Abstract
Sickle cell trait (SCT) is a risk factor of collapse and sudden death in athletes. We conducted a longitudinal study to determine the hematological responses and hydration status in NCAA Division I American football players with SCT. The study took place over 2 years with 6 SCT and 6 position-matched controls (CON) in year 1; and 4 SCT and 4 CON in year 2. In year 2, three of the four SCT players were recruited and re-enrolled with new position-matched controls (total sample data = 10 SCT and 10 CON). Blood samples were taken at three visits: pre-camp, post-camp, and post-season to examine hemoglobin variants, complete blood counts, and chemistry panel 26. Hydration status was assessed by measuring body weight change, urine specific gravity, and urine and sweat electrolyte concentrations during the pre-season training camp. All SCT players were confirmed to have SCT (HbS = 37.9 ± 2.4%) and had greater red cell distribution width (RDW) compared to CON across all visits. Serum uric acid was higher in SCT (7.3 ± 1.0 mg/dL) compared to CON (6.1 ± 0.6 mg/dL; p = 0.001). Furthermore, serum creatine kinase levels were greater in SCT (1617.0 ± 1034.8 IU/L) at pre-camp compared to CON (1037.4 ± 602.8 IU/L; p = 0.03). SCT players exhibited lower pre- and post-practice urine electrolytes and urine specific gravity (SCT pre: 1.019 ± 0.005 vs. CON pre: 1.026 ± 0.008 p < 0.001; SCT post: 1.020 ± 0.005 vs. CON post: 1.030 ± 0.008 p < 0.01), whereas sweat sodium concentrations were higher in SCT players (55.4 ± 13.6 mmol/L) compared to CON (45.5 ± 10.6 mmol/L; p < 0.001). Given the evidence, greater uric acid and CPK levels in SCT players compared to CON may be an early indicator of altered kidney function and muscle damage, which could be added into NCAA guidelines for surveillance among SCT players. Consistent education and reinforcement of the importance of adequate fluid balance during exercise are critical for both SCT and CON players.
Subject terms: Biomarkers, Diseases, Health care, Risk factors
Introduction
Sickle cell trait (SCT) is a genetic condition characterized by the presence of hemoglobin S (HbS), which makes up approximately 40% of total hemoglobin1. The molecular structure of HbS reduces its oxygen affinity, resulting in less oxygen delivery to active muscle and contributing to local hypoxia, which may impact optimal performance in athletes1,2. SCT is seen in 7–9% of African Americans and up to 40% in Western Africans3. Previous studies have investigated the effects of acute exercise on SCT individuals, suggesting SCT carriers had greater blood viscosity and red blood cell rigidity at rest, during intensive training, and during the subsequent recovery4. In addition, a previous study demonstrated that greater blood viscosity should be considered a risk factor associated with exercise-related sudden death in SCT carriers5. The physiological and environmental stress during exercise including exercise-induced acidosis, dehydration, heat stress, and regional hypoxemia, promote hematological changes that can increase the risk of microcirculatory disorders, cardiovascular complications, and fatality in SCT carriers4.
American football players with SCT have an increased risk of complications due to the exhaustive nature of practice and competitions6–8. In addition, geographical location, specifically hot and humid environmental climates, provides an extra challenge to the players with SCT9. Previous reports showed that NCAA Division I football players with SCT experience a higher percentage of heat-related illness and exertional-related death, up to 16 to 21-fold compared to non-SCT players2,10. From 2000 to 2010, 16 deaths have occurred during sport-related conditioning and games in Division I football players, among which 10 (63%) were SCT carriers10. Hematological alterations, inflammation, and vascular adhesion have been proposed as potential mediators of the microcirculatory changes that induce sickle cell anemia11,12. Although a clear relationship exists between SCT and the risk of exertional-related sudden death in American collegiate football, the pathophysiology of sickling-induced hematological alterations is still unknown8,13. According to the NCAA guidelines, the primary prevention strategies of sickling crisis is includes (1) requiring institutions to confirm SCT status before enrollment in any athletic team and (2) continued education of SCT players to avoid heat stress and dehydration7,8. In SCT, acclimatization should be implemented slowly and gradually, allowing players to have ample rest intervals during exercise due to the nature of the deoxygenated form of HbS, and possibly resulting in greater muscle fatigue4,14. In addition, athletic training staff is trained to identify the signs and symptoms of sickle cell crisis and differentiate it from heat cramps8,9. To our knowledge, no study has investigated the effects of training and competitions across the entire season on SCT players. Therefore, the purpose of this study was to conduct a longitudinal study examining hematological responses and hydration status in NCAA Division I football players with SCT. We hypothesized that SCT players would exhibit altered hematological responses assessed periodically over an entire season. In addition, we hypothesized that, due to the NCAA guidelines, SCT players would have improved hydration status.
Methods
Participants
Twenty NCAA Division I football players were recruited in two consecutive years from a university located in the southeast United States. Players with SCT were approached by athletic training staff and 6 agreed to enroll in year 1. Four SCT players agreed to participate in year 2, of which, three of them were re-enrolled from year 1. In addition, 6 position-matched controls were enrolled during year 1 and 4 new CON players were enrolled in year 2 for a total of 10 SCT and 10 CON participants enrolled in this study. The CON participants were also selected by the athletic training staff to have similar anthropometric characteristics with similar training repetitions and drills compared to their SCT counterparts. SCT was confirmed by the hemoglobin characterized by the presence of HbS, but less than 50%, and a normal percentage of HbA24,7. The physical characteristics of the players are displayed in Table 1. The study was conducted according to relevant guidelines and regulations (Declaration of Helsinki) and the protocol and informed consent were approved by the Louisiana State University Institutional Review Board (IRB #3900). All participants signed informed consents before any assessments were completed.
Table 1.
Physical characteristics of participants by groups.
| SCT (n = 10) | CON (n = 10) | p value | |
|---|---|---|---|
| Age (year) | 19.8 ± 1.6 (18–23) | 21.1 ± 1.2 (19–23) | 0.10 |
| Height (cm) | 186.7 ± 5.4 (177.8–193.1) | 190.9 ± 4.9 (180.3–198.1) | 0.08 |
| Weight (kg) | 110.6 ± 28.4 (80.0–154.7) | 109.9 ± 24.7 (84.5–145.2) | 0.91 |
| Hemoglobin-A (%) | 57.3 ± 2.5 (55.4–64.0)a | 95.2 ± 0.4 (94.8–95.8) | < 0.001 |
| Hemoglobin-A2 (%) | 3.5 ± 0.2 (3.3–3.7)a | 2.9 ± 0.2 (2.5–3.1) | < 0.001 |
| Hemoglobin-F (%) | 0.4 ± 0.2 (0.1–0.8) | 0.5 ± 0.2 (0.2–0.9) | 0.48 |
| Hemoglobin-S (%) | 37.9 ± 2.4 (31.4–40.1)a | 0 | < 0.001 |
| Hemoglobin-C (%) | 0 | 0 | N/A |
Data are presented as mean ± SD (Range).
SCT sickle cell trait, CON position-matched control group.
aSignificant difference between groups.
Study design and visits
Blood samples were taken at three visits: (1) within a week prior to the start of training camp (pre-camp), (2) approximately 48 h after the end of pre-season training camp (post-camp/pre-season), and (3) approximately 48 h after the final competition of the season (post-season). There were 17 consecutive practices in the first year and 16 practices in the second year between pre-camp and post-camp and 13 weekly in-season competitions between post-camp and post-season (Fig. 1). Pre-camp data collection (blood samples) served as the baseline measurements. The follow-up measurements examined the alterations associated with practice and the competitive season. In addition, hydration status (urine and sweat) was examined at each practice in the training camp to determine group differences.
Figure 1.
The time-point of the blood draws throughout the entire football season.
Blood biomarkers
Venous blood was drawn from an antecubital vein in the non-fasted state, ~ 2–3 h post-prandial. Whole blood was collected in 7 mL (K2-EDTA) and 7 mL serum separator tubes, allowed to clot (serum tubes only), centrifuged for 10 min at 2000g, and the resultant plasma and serum transferred to cryovials for future analysis. Serum was analyzed for a comprehensive chemistry panel 26 (CM26). Additional whole blood samples (EDTA) were analyzed by electrophoresis to determine hemoglobin subtyping as well as complete blood cell counts (CBC). Hemoglobin electrophoresis subtypes included the percentage of hemoglobin-A, hemoglobin-A2, hemoglobin-F, hemoglobin-S, and hemoglobin C to diagnose sickle cell. In addition, the estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine15.
Measures of hydration status
Environmental conditions were measured before and after practice using a wet-bulb globe temperature (WBGT8758 Vernon Hills, IL USA). Body weight was assessed using a standard scale (TANITA, Arlington Heights, IL, USA) with a precision of 0.1 kg before and after each practice session. Players were asked to wipe down the body and completely empty their bladder before body weight assessments. In addition, the same amount of clothing was required before and after practice body weight assessments. Hydration status was represented as % body weight change (after toweling dry and urinating), accounting for fluid intake and urine output. Urine samples were collected before and after each practice session before body weight assessments to determine urine specific gravity (USG) and electrolyte concentrations. USG was measured using a spectral refractometer (ATAGO CO., Bellevue, WA, USA) and urine electrolyte concentrations (sodium [Na+], potassium [K+], and chloride [Cl−]) were measured by ion-selected probes (MEDICA EasyLyte; Bedford, MA, USA). In addition, sweat was collected on the lower back region using the technical absorbent patch technique16, which has been showed a valid and simple method to assess large number of players during on-field team sports17. The skin surface was cleaned with 70% ethanol and dried before the sweat patch was attached. Sweat patches were removed 60 min into each practice to avoid patch over-saturation. The local sweat rate was calculated as the weight differences of the absorbent patch pre- and post-practice divided by the product of the sweat patch area and duration of time the patch attached (mg/cm2/min). Sweat was analyzed for electrolyte concentrations (sodium [Na+], potassium [K+], and chloride [Cl−]) similarly to the urine. The sweat electrolyte concentrations and local sweat rate were corrected by the Baker et al. representing the whole body losses because urine volume and fluid intake were not feasible to assess without interrupting players’ training routine18.
Statistical analysis
Data were analyzed using JMPro 14 (SAS Inc., Cary, NC). Given the participants were matched a prior based on their anthropometric, demographic, and on-field characteristics, the SCT and CON groups were analyzed using a within group structure as reported in this manuscript. However, all data were analyzed as if the SCT and CON groups were independent, with no major differences in the overall interpretation of the data. Baseline anthropometric and hematological outcomes were analyzed using a paired t-test. Two-way repeated-measures analysis of variance (RM ANOVA) was used to analyze hemoglobin electrophoresis, CBC, and CM26 between the two groups (SCT and CON) across assessment time (pre-camp, post-camp, post-season). In addition, a paired t-test was used to analyze outcome variables of hydration status between two groups (SCT and CON), including body weight change, sweat rate, sweat and urine electrolyte concentrations, and USG. All players participated in the blood draw across three visits, except 1 SCT and 1 CON missed the post-season visit in year 2. The total sample size for the hydration assessments is displayed in the Fig. 2. A total 18 players participated in training camp practice assessment as researchers did not have reasonable access to another two players from year 2 (1 SCT and 1 CON). They are not the same players who missed blood draw at post-season. Thirty-four samples were missed because of injury during practices. Twenty sweat samples were eliminated due to sweat patch dropped or sample contamination and 8 post-practice urine samples were missed because players left early for treatment. Missing data were eliminated and data were only entered in the final analysis if both the SCT and CON participated in training on any given day. Data are presented as mean ± standard deviation (SD) and statistical significance was accepted at p < 0.05.
Figure 2.
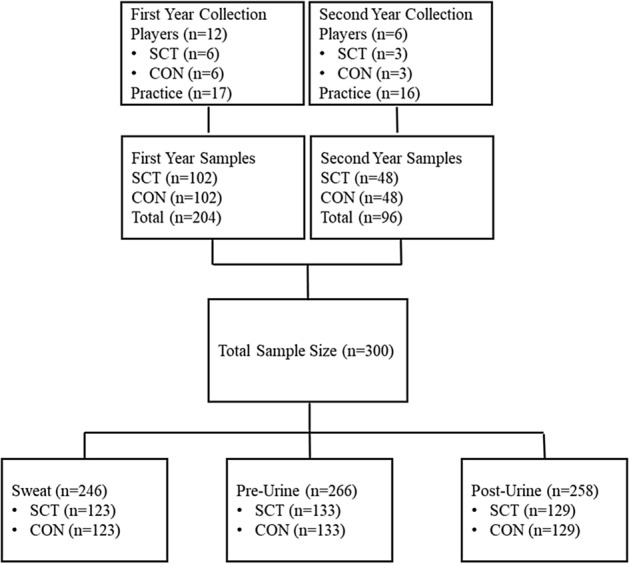
The total hydration samples were collected and pooled across practices by groups.
Results
Table 1 shows the SCT and CON participant characteristics. Age, weight, height, and hemoglobin F were not different between the in the SCT and CON groups. However, the groups differed by hemoglobin S fraction confirming the participants in the SCT were indeed SCT. In addition, the players were matched for race (all African American) and on-field position.
Blood biomarkers
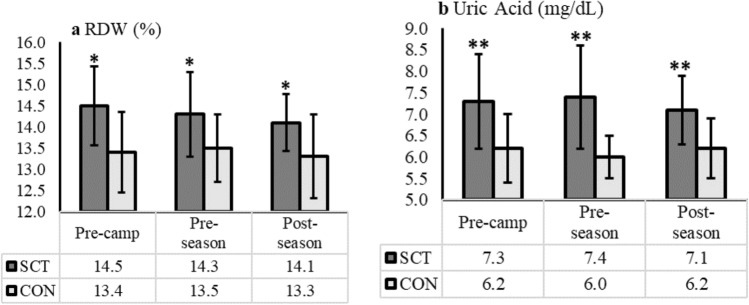
Hemoglobin-electrophoresis showed that SCT players had an average of 57.3 ± 2.5% HbA and 37.9 ± 2.4% HbS, and the percentages of HbA and HbS were consistent throughout the entire season (Table 1). The CBC profile is displayed in Table 2. A lower number of white blood cells (WBC; SCT: 5.6 ± 1.6 vs. CON: 6.9 ± 2.2 × 10^3; p = 0.02) and lymphocytes (SCT: 1.8 ± 0.5 vs. CON: 2.3 ± 0.9 × 10^3; p = 0.01) were observed in SCT compared to CON, but all data were within the normal reference range. In addition, SCT had a greater red cell distribution width (RDW) compared to CON (SCT: 14.3 ± 0.9 vs. CON: 13.4 ± 0.9%; p = 0.02) across all visits (Fig. 3a). Platelet count was significantly lower in SCT (197.9 ± 48.0 × 10^3) compared to CON only at post-season (278.7 ± 78.1 × 10^3; p = 0.03).
Table 2.
The Completed Blood Count file of two groups (SCT vs. CON) across the entire football season (pre-camp, post-camp, and post-season).
| Biomarkers (reference) | SCT (n = 10) | CON (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-camp | Post-camp | Post-season | Average | Pre-camp | Post-camp | Post-season | Average | |
| RBC (4.5–5.9 × 106) | 5.1 ± 0.2 | 5.2 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 | 4.9 ± 0.6 | 5.0 ± 0.6 | 5.1 ± 0.7 | 5.0 ± 0.6 |
| Hb (14–18 g/dL) | 14.1 ± 0.6 | 14.5 ± 0.8 | 14.7 ± 0.4 | 14.4 ± 0.7 | 14.1 ± 0.7 | 14.4 ± 0.7 | 14.8 ± 1.0 | 14.4 ± 0.8 |
| Hematocrit (42–52%) | 41.5 ± 1.8 | 42.3 ± 2.4 | 43.7 ± 1.2 | 42.4 ± 2.0 | 41.4 ± 1.9 | 42.0 ± 1.5 | 43.9 ± 2.5 | 42.4 ± 2.2 |
| MCV (80–94 fL) | 81.8 ± 2.7 | 81.4 ± 3.1 | 82.6 ± 3.1 | 81.9 ± 2.9 | 86.3 ± 8.8 | 85.6 ± 8.4 | 86.7 ± 9.1 | 86.2 ± 8.5 |
| MCH (27–31 pg) | 27.8 ± 1.3 | 27.9 ± 1.3 | 27.7 ± 1.2 | 27.8 ± 1.2 | 29.4 ± 3.4 | 29.5 ± 3.4 | 29.3 ± 3.5 | 29.4 ± 3.3 |
| MCHC (32–36 g/dL) | 34.0 ± 0.8 | 34.3 ± 0.5 | 33.6 ± 0.5 | 34.0 ± 0.7 | 34.1 ± 0.6 | 34.3 ± 1.0 | 33.7 ± 0.8 | 34.1 ± 0.8 |
| RDW (11.5–14.5%) | 14.5 ± 0.9b | 14.3 ± 1.0c | 14.1 ± 0.7d | 14.3 ± 0.9a | 13.5 ± 1.0 | 13.5 ± 0.8 | 13.3 ± 1.0 | 13.4 ± 0.9 |
| Platelet count (150–450 × 103) | 205.9 ± 41.3 | 217.1 ± 60.9 | 197.9 ± 48.0d | 207.2 ± 49.5 | 246.3 ± 58.7 | 251.0 ± 60.5 | 278.7 ± 78.1 | 256.0 ± 65.1 |
| WBC (3.6–8.9 × 103) | 6.1 ± 1.9 | 5.7 ± 1.8 | 5.1 ± 1.0 | 5.6 ± 1.6a | 7.8 ± 2.7 | 5.8 ± 1.0 | 7.0 ± 2.2 | 6.9 ± 2.2 |
| Neutrophil (42.2–75.2%) | 53.7 ± 6.2 | 58.3 ± 14.7 | 48.7 ± 13.5 | 53.7 ± 12.2 | 56.5 ± 13.1 | 55.2 ± 10.8 | 48.6 ± 12.7 | 53.6 ± 12.3 |
| Lymphocyte (20.5–51.1%) | 33.1 ± 6.1 | 30.6 ± 10.9 | 34.4 ± 6.8 | 32.6 ± 8.1 | 32.2 ± 10.9 | 34.4 ± 10.6 | 39.6 ± 11.6 | 35.2 ± 11.0 |
| Monocytes (1.7–9.3%) | 8.9 ± 3.1 | 8.2 ± 2.7 | 10.9 ± 3.1 | 9.3 ± 3.1 | 8.9 ± 3.3 | 7.7 ± 1.0 | 8.7 ± 3.2 | 8.4 ± 2.7 |
| Eosinophils (0–10%) | 5.1 ± 5.0b | 2.3 ± 2.9 | 5.0 ± 5.9 | 4.1 ± 4.8 | 1.7 ± 1.5 | 2.2 ± 1.2 | 2.5 ± 1.8 | 2.1 ± 1.5 |
| Basophils (0–0.8%) | 0.6 ± 0.2 | 0.7 ± 0.5 | 1.0 ± 0.5 | 0.8 ± 0.4 | 0.6 ± 0.1 | 0.6 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| Num of neutrophil (1.5–5.9 × 103) | 3.3 ± 1.1 | 3.5 ± 1.8 | 2.5 ± 0.9 | 3.1 ± 1.3 | 4.7 ± 2.9 | 3.3 ± 1.1 | 3.4 ± 1.4 | 3.8 ± 2.0 |
| Num of lymphocyte (1.2–3.5 × 103) | 2.0 ± 0.7 | 1.6 ± 0.4 | 1.7 ± 0.4d | 1.8 ± 0.5a | 2.3 ± 0.6 | 2.0 ± 0.5 | 2.8 ± 1.4 | 2.3 ± 0.9 |
| Num of monocytes (0.1–0.6 × 103) | 0.6 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.2 | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| Num of eosinophils (0–0.7 × 103) | 0.2 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Num of basophils (0–0.2 × 10^3) | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.03 ± 0.05 | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.03 ± 0.05 | 0.07 ± 0.05 | 0.04 ± 0.05 |
| Reticulocytes (0.9–2.3%) | 1.4 ± 0.6 | 1.3 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.3 ± 0.5 |
Data presented as mean ± SD.
SCT sickle cell trait, RBC red blood cells, Hb hemoglobin, MCV corpuscular volume, MCH corpuscular volume, MCHC corpuscular hemoglobin concentration, RDW red cell distribution width, WBC white blood cell count.
aSignificant difference in SCT compared to CON.
bSignificant difference in SCT compared to CON at pre-camp.
cSignificant differences in SCT compared to CON at pre-season/post-camp.
dSignificant difference in SCT compared to CON at post-season.
Figure 3.
The red blood cell distribution width (RDW; a) and uric acid (b) across the visits by groups (SCT and CON). Dark grey bars are sickle cell trait players (SCT), and light grey bars are position-matched control group (CON). SCT players exhibited significantly greater RDW and uric acid across all visits compared to CON; *Significant differences p < 0.05. **Significant differences p < 0.01.
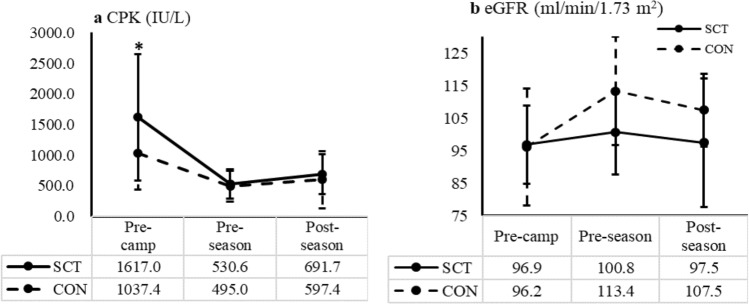
Data from the CM26 can be found in Table 3. The BUN/Creatinine ratio was lower in SCT (12.5 ± 3.7) compared to CON at post-camp (17.0 ± 4.1; p = 0.003). Of note, SCT players had a greater uric acid and near to upper reference range (7.3 ± 1.0 mg/dL) compared to CON throughout the entire season (6.1 ± 0.6 mg/dL; p = 0.001; Fig. 3b). In addition, SCT players had greater CPK levels (955.2 ± 797.2 IU/L) compared to CON (718.1 ± 510.2 IU/L; p = 0.03), which was mainly observed at pre-season (SCT: 1617.0 ± 1034.8 vs. CON: 1037.4 ± 602.8 IU/L; p = 0.03; Fig. 4a). In addition, although no differences in LDH were found between two groups, both groups had elevated LDH that was greater than the upper reference range (SCT: 217.1 ± 34.1 IU/L and CON: 218.5 ± 44.7 IU/L; Reference 82 ~ 195 IU/L). Lastly, SCT players showed a lower eGFR at post-camp (pre-season) and post-season compared to CON, but these differences did not reach the level of statistical significance (all p > 0.1; Fig. 4b).
Table 3.
Chemistry panel 26 profile of the two groups (SCT vs. CON) across the entire football season (pre-camp, post-camp, and post-season).
| Biomarkers (reference) | SCT (n = 10) | CON (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-camp | Post-camp | Post-season | Average | Pre-camp | Post-camp | Post-season | Average | |
| Glucose (70–110 mg/dL) | 88.8 ± 13.4 | 85.3 ± 9.6 | 86.3 ± 14.4 | 86.8 ± 12.2 | 90.8 ± 21.2 | 86.1 ± 13.8 | 97.6 ± 32.2 | 90.7 ± 22.6 |
| BUN (8–23 mg/dL) | 17.5 ± 3.1 | 14.8 ± 3.2 | 14.8 ± 2.1 | 15.6 ± 3.1 | 19.4 ± 3.7 | 18.1 ± 3.0 | 15.0 ± 2.7 | 17.9 ± 3.5 |
| Creatinine (0.9–1.3 mg/dL) | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 |
| BUN/creatinine ratio (6–25) | 14.0 ± 2.1 | 12.5 ± 3.7c | 12.0 ± 2.8 | 12.9 ± 2.9 | 15.5 ± 2.4 | 17.0 ± 4.1 | 13.0 ± 2.5 | 14.9 ± 3.3 |
| Sodium (136–147 mmol/L) | 137.8 ± 1.2 | 137.3 ± 1.6 | 136.9 ± 1.1 | 137.3 ± 1.3 | 138.3 ± 1.2 | 137.1 ± 1.4 | 138.1 ± 1.0 | 137.9 ± 1.3 |
| Chloride (101–111 mmol/L) | 104.1 ± 0.7 | 103.8 ± 0.8 | 103.1 ± 2.2 | 103.7 ± 1.4 | 103.8 ± 1.8 | 103.8 ± 1.3 | 103.6 ± 1.2 | 103.6 ± 1.4 |
| Potassium (3.7–5.0 mmol/L) | 4.1 ± 0.2 | 4.5 ± 0.4 | 4.2 ± 0.5 | 4.3 ± 0.4 | 4.2 ± 0.3 | 4.5 ± 0.2 | 4.1 ± 0.3 | 4.3 ± 0.3 |
| CO2 (25–33 mmol/L) | 24.2 ± 1.4 | 25.1 ± 1.8 | 23.6 ± 2.1 | 24.3 ± 1.8 | 24.4 ± 2.2 | 25.4 ± 1.1 | 21.2 ± 5.3 | 24.1 ± 3.6 |
| Uric Acid (2.6–7.5 mg/dL) | 7.3 ± 1.1b | 7.4 ± 1.2c | 7.1 ± 0.8d | 7.3 ± 1.0a | 6.2 ± 0.8 | 5.9 ± 0.5 | 6.0 ± 0.4 | 6.1 ± 0.6 |
| Total Protein (5.8–7.9 g/dL) | 7.2 ± 0.3 | 7.3 ± 0.3 | 7.5 ± 0.1 | 7.3 ± 0.3 | 7.3 ± 0.4 | 7.4 ± 0.4 | 7.6 ± 0.3 | 7.4 ± 0.4 |
| Albumin (4.1–5.4 g/dL) | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.3 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.3 |
| Calcium (8.9–10.4 mg/dL) | 9.3 ± 0.4 | 9.4 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.2 | 9.4 ± 0.2 | 9.5 ± 0.2 | 9.4 ± 0.2 |
| Phosphorus (2.5–5.1 mg/dL) | 4.2 ± 0.5 | 4.3 ± 0.6 | 4.1 ± 0.6 | 4.2 ± 0.5 | 4.3 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.9 | 4.1 ± 0.6 |
| Magnesium (1.7–2.4 mg/dL) | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 |
| Total Bilirubin (0.2–1.5 mg/dL) | 0.8 ± 0.3 | 0.9 ± 0.4 | 1.0 ± 0.2 | 0.9 ± 0.3 | 1.0 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 |
| CPK (38–333 IU/L) | 1617.0 ± 1034.8b | 530.6 ± 236.5 | 691.6 ± 325.3 | 955.2 ± 797.2a | 1037.4 ± 602.8 | 489.1 ± 268.0 | 451.8 ± 238.0 | 718.1 ± 510.2 |
| LDH (82–195 IU/L) | 207.1 ± 65.5 | 197.5 ± 82.5 | 186.7 ± 73.4 | 197.4 ± 72.1 | 217.1 ± 34.1 | 228.9 ± 37.7 | 225.8 ± 61.4 | 218.5 ± 44.7 |
| AST (13–47 IU/L) | 47.5 ± 21.4 | 31.6 ± 7.8 | 34.1 ± 8.6 | 37.9 ± 15.5 | 36.4 ± 10.4 | 32.2 ± 5.4 | 35.6 ± 11.7 | 35.0 ± 9.4 |
| ALT (10–60 IU/L) | 32.3 ± 12.6 | 26.7 ± 9.3 | 24.7 ± 10.2 | 28.0 ± 10.9 | 31.2 ± 10.9 | 25.6 ± 11.5 | 31.0 ± 14.9 | 29.2 ± 11.6 |
| ALK Phosphate (32–130 IU/L) | 88.5 ± 29.4 | 84.1 ± 22.7 | 83.1 ± 25.8 | 85.3 ± 25.3 | 74.0 ± 24.5 | 72.1 ± 23.2 | 77.5 ± 23.4 | 74.3 ± 21.7 |
| GGT (5–63 IU/L) | 20.9 ± 11.8 | 18.5 ± 11.9 | 16.4 ± 5.0 | 18.7 ± 10.0 | 17.7 ± 8.9 | 14.8 ± 12.3 | 19.0 ± 17.4 | 17.7 ± 12.2 |
| Amylase (28–100 U/L) | 116.9 ± 57.1 | 99.9 ± 37.5 | 99.6 ± 42.3 | 105.9 ± 45.7 | 91.1 ± 45.2 | 64.6 ± 7.7 | 67.9 ± 8.4 | 87.9 ± 48.4 |
| Iron (50–160 μg/dL) | 65.2 ± 24.7 | 86.0 ± 24.0c | 100.6 ± 30.9 | 83.3 ± 29.5 | 73.1 ± 28.5 | 109.2 ± 34.1 | 81.1 ± 23.6 | 92.7 ± 38.3 |
| Cholesterol (122–244 mg/dL) | 166.2 ± 25.3 | 172.4 ± 26.7 | 174.1 ± 26.2 | 170.8 ± 25.3 | 160.7 ± 21.3 | 164.9 ± 10.1 | 166.3 ± 16.0 | 160.8 ± 17.7 |
| Triglycerides (44–201 mg/dL) | 91.0 ± 57.5 | 106.4 ± 63.3 | 67.6 ± 5.7 | 89.8 ± 51.9 | 64.2 ± 30.0 | 117.6 ± 75.4 | 82.6 ± 43.2 | 89.9 ± 55.1 |
| HDL (30–70 mg/dL) | 49.2 ± 12.6 | 54.4 ± 16.3 | 53.7 ± 12.9 | 52.4 ± 13.8 | 61.1 ± 9.5 | 58.7 ± 13.2 | 62.6 ± 12.2 | 61.3 ± 11.0 |
| LDL (66–165 mg/dL) | 98.8 ± 23.5 | 99.8 ± 19.2 | 108.5 ± 19.8 | 102.0 ± 20.7 | 86.7 ± 23.3 | 82.6 ± 9.7 | 87.1 ± 21.9 | 82.8 ± 19.4 |
Data presented as mean ± SD.
BUN blood urine nitrogen, CPK creatine kinase, LDH lactate dehydrogenase, AST aspartate amino transferase, ALT alanine amino transferase, ALK alkaline phosphate, GGT gamma-glutamyl transferase.
aSignificant differences in SCT compared to CON.
bSignificant difference in SCT compared to CON at pre-camp.
cSignificant difference in SCT compared to CON at pre-season/post-camp.
dSignificant difference in SCT compared to CON at post-season.
Figure 4.
The CPK (creatine phosphokinase; a) and eGFR (estimated glomerular filtration rate; b) across the visits by groups (SCT and CON). Solid line indicates sickle cell trait players (SCT), and dash line indicates position-matched control group (CON). CPK levels were found significantly greater in SCT (1617.0 ± 1034.8 IU/L) at pre-camp compared to CON (1037.4 ± 602.8 IU/L; p = 0.04). The eGFR was lower in SCT at pre-season and post-season but not significant compared to CON (all p > 0.1). The eGFR was estimated by the equation from Shlipak et al.15.
Hydration status
Hydration markers derived from urine and sweat samples were collected during practices in the pre-season training camp under averaged 26.8 ± 1.9 °C (WBGT) at a relative humidity of 68.9 ± 11.1%. The hydration markers are presented in Table 4. CON experienced greater body weight loss (− 1.3 ± 1.1 kg; − 1.0 ± 0.9% body weight) and % body weight changes compared to SCT (− 0.9 ± 0.9 kg; − 0.8 ± 0.8% body weight; all p < 0.001). SCT players had lower pre- and post-practice urine electrolyte concentrations ([Na+], [K+], and [Cl−]) compared to CON. In addition, a lower pre- and post-practice USG was observed in SCT compared to CON (SCT pre: 1.019 ± 0.006 vs. CON pre: 1.026 ± 0.008, p < 0.001; SCT post: 1.020 ± 0.005 vs. CON post: 1.030 ± 0.008, p < 0.001). Sweat sodium concentrations (SCT: 55.4 ± 13.6 vs. CON: 45.5 ± 10.6 mmol/L; p < 0.001), potassium concentrations (SCT: 4.7 ± 1.2 vs. CON: 4.3 ± 0.9 mmol/L; p = 0.004), and chloride concentrations (SCT: 52.5 ± 13.0 vs. CON: 43.3 ± 9.9 mmol/L; p < 0.001) were greater in SCT compared to CON. However, sweat rate was not different between the two groups (SCT: 1.22 ± 0.22 vs. CON: 1.25 ± 0.25 mg/cm2/min; p = 0.32).
Table 4.
Football pre-season camp practice hydration markers by groups.
| Variables | SCT (n = 9) | CON (n = 9) | p value |
|---|---|---|---|
| Body weight loss (kg) | − 0.9 ± 0.9 (− 3.6 to − 1.8)a | − 1.3 ± 1.1 (− 4.5 to 1.8) | 0.008 |
| Body weight change (%) | − 0.8 ± 0.8 (− 3.5 to − 1.1)a | − 1.0 ± 0.9 (− 3.2 to 1.3) | 0.04 |
| Pre-urine Na+ (mmol/L) | 98.4 ± 32.1 (19.4–167.6)a | 159.6 ± 62.9 (27.9–284.1) | < 0.001 |
| Pre-urine K+ (mmol/L) | 37.2 ± 18.4 (7.4–115.4)a | 55.8 ± 29.1 (3.8–152.2) | < 0.001 |
| Pre-urine Cl− (mmol/L) | 88.5 ± 34.4 (14.4–177.2)a | 150.2 ± 57.7 (24.2–290.9) | < 0.001 |
| Pre USG | 1.019 ± 0.005 (1.007–1.035)a | 1.026 ± 0.008 (1.005–1.047) | < 0.001 |
| Post-urine Na+ (mmol/L) | 72.0 ± 27.2 (29.7–167.5)a | 116.6 ± 52.5 (23.8–285.6) | < 0.001 |
| Post-urine K+ (mmol/L) | 37.4 ± 11.7 (11.7–80.8)a | 63.8 ± 26.4 (14.5–169.9) | < 0.001 |
| Post-urine Cl− (mmol/L) | 57.1 ± 28.1 (5.4–159.9)a | 93.7 ± 48.6 (6.7–276.0) | < 0.001 |
| Post USG | 1.020 ± 0.005 (1.004–1.030)a | 1.030 ± 0.008 (1.006–1.050) | < 0.001 |
| Sweat Na+ (mmol/L) | 55.4 ± 13.6 (29.6–88.6)a | 45.5 ± 10.6 (25.1–74.5) | < 0.001 |
| Sweat K+ (mmol/L) | 4.7 ± 1.2 (3–7.2)a | 4.3 ± 0.9 (2.9–6.8) | 0.004 |
| Sweat Cl− (mmol/L) | 52.5 ± 13.0 (25.9–83.4)a | 43.3 ± 9.9 (23.3–71.9) | < 0.001 |
| Sweat rate (mg/cm2/min) | 1.22 ± 0.22 (0.74–1.82) | 1.25 ± 0.25 (0.74–1.96) | 0.32 |
Pre pre-practice, Post post-practice, SCT sickle cell players, CON position-matched control.
aSignificant difference in SCT compared to CON, data were presented as mean ± SD (range) of averaged practices in the pre-season training camp.
Discussion
The purpose of this study was to examine longitudinal changes in hematological biomarkers and hydration status in SCT players to better understand the training and seasonal implications and, based on our data, further develop prevention procedures based on NCAA sickling crisis precaution guidelines. Blood biomarkers revealed that SCT players had an average of 37.9% (31.4–40.1%) of HbS and greater RDW values than CON throughout the season. In addition, SCT players had higher serum uric acid throughout the entire season and higher CPK levels at pre-camp compared to CON. Training specific hydration markers showed SCT had an improved fluid balance compared to CON during pre-season training camp practice. In addition, SCT players had significantly lower pre- and post-practice urine electrolyte concentrations and USG, and higher sweat electrolyte concentrations compared to CON. Together, these data suggest that greater uric acid and CPK levels in SCT players compared to CON may be early indicated an altered kidney function and muscle damage. In addition, consistent reinforcement and educating the importance of adequate fluid balance during exercise are critical for both SCT and CON players.
The finding that SCT players had concomitant elevations in HbS and RDW is consistent with previous studies, that suggest HbS is associated with a higher RDW, which is related to the greater degree of red cell anisocytosis and larger variation of red blood cell morphology19,20. This finding further confirms the physiological alterations in red blood cells among asymptomatic SCT players. In the present study, the RDW value of SCT players was near the upper reference limit and previous research showed greater RDW correlated with the degree of anemia19. In addition, the immune cell counts appeared different between the two groups; however, the values remained in the normal reference range in both groups and do not appear to be clinically different. This evidence suggests that long-term conditioning and on-field practice do not affect immune function in SCT players. Given the difference noted in our study, additional studies may be necessary to delineate the clinical and physiological significance in the prevention of sickling crisis.
Surprisingly, serum uric acid remained higher throughout the season in SCT players compared to CON, suggesting either overproduction or insufficient clearance of uric acid. The potential mechanism proposed in the previous research is that serum uric acid is inversely associated with urine clearance rather than to the overproduction of uric acid21. Diamond et al. suggested that uric acid clearance was highly associated with renal excretion approaching the sum of new purine biosynthesis and dietary purine intake22. Previous studies have also reported that renal manifestations of an impaired urinary concentration are commonly observed in SCT carriers23–25. In our data, lower pre- and post-practice urine electrolyte concentrations in SCT players also suggest lower renal excretion to remove waste products through the urine. In addition, even though eGFR was not different between the two groups, SCT players showed a trend for having lower eGFR at post-camp (pre-season) and post-season. The eGFR is considered a good indicator of kidney function representing the ability of the kidneys to excrete fluid and waste product through urine26. We cannot conclude from our data that SCT players had an impaired kidney function or potential risk of kidney disease. Future studies could examine alternate measures of kidney function (e.g. urine creatinine), allowing for the direct assessment of urine clearance in SCT players.
Interestingly, a previous study reported that serum uric acid is an antioxidant that contributes > 50% of the total antioxidant capacity of the blood27. Thus, serum uric acid may play a protective role against exercise-induced oxidative stress28. In addition, previous studies showed that uric acid increased in response to exercise, such as short-distance running29, and ultramarathon racing30. The exercise-induced uric acid elevations may be associated with increased purine oxidation and subsequent formation of uric acid31. In the present study, our participants were long-term, highly trained elite collegiate American football players, and greater serum uric acid may be associated with the chronic exercise effects. To our knowledge, no studies have reported high serum uric acid in SCT players. However, the present study cannot explain whether greater serum uric acid levels observed in SCT players was associated with alterations of renal function or a protective mechanism related to reduced sickling activity. Future research could directly address this question, and thereby alter recommendations to athletic trainers and sport nutritionists to increase antioxidant intake if sickling can be prevented by the increase in serum antioxidant capacity.
Another important consideration is the effect of exercise on muscle damage in SCT players because previous studies frequently suggest SCT player are at a greater risk of exertional rhabdomyolysis7,8,32. Exertional rhabdomyolysis is a syndrome caused by the severe breakdown of skeletal muscle tissue leading to acute and chronic kidney failure33. Exertional rhabdomyolysis should be considered in NCAA football players with SCT, who are frequently exposed to demanding high-intensity exercise in hot and humid environmental conditions. In addition, laboratory tests have shown that serum CPK levels are elevated when rhabdomyolysis occurs34. The present study found that serum CPK levels were 3–5 times higher than the upper reference limits in both groups and at all visits. Furthermore, both groups had a significantly higher serum CPK levels at pre-camp compared to other visits. Moghadam-Kia et al. showed serum CPK levels could increase as much as 30 times of upper limit within 24 h of strenuous exercise35. A previous study also suggests that muscle mass and total body mass were associated with basal serum CPK levels36. In the present study, players’ average body weight was > 100 kg and greater serum CPK levels was possibly associated with this large body weight and muscle mass. In addition, both CON and SCT players had elevated LDH, indicating high degree of metabolic adaptation to the physical training of skeletal muscle37. Of note, serum CPK levels in the present study were greater at pre-camp in both groups and were significantly greater in SCT players. It is possible that the timing of the pre-camp blood draws coincided with heavy resistance training typical of American football players prior to the start of the season. As such, this increase in serum CPK levels prior to camp could be linked to methodological and sampling issues. On the other hand, this may suggest that SCT players had differential effects of exercise on muscle breakdown microvascular alterations, which can be the result of acute ischemia in skeletal muscle38. Future research should include comprehensive assessments of physiological work prior to all blood work to better elucidate the reasons for the elevated muscle damage markers in SCT.
Players with SCT are at greater risk of exercise-associated sickle cell collapse due to dehydration and heat stress7,13. In the present study, SCT players had an improved fluid balance, and both groups were hypohydrated with approximate − 1% body weight change and elevated USG throughout the training. In addition, greater pre-practice USG ≥ 1.020 was observed, regardless of groups, suggesting players were hypohydrated prior to the onset of exercise17,39. Given the data of pre-practice USG and body weight changes, players consumed an insufficient amount of fluid outside of and during practice. On the other hand, pre- and post-practice USG and urine electrolyte concentrations were lower in SCT players compared to CON. Even though the present study did not control for total fluid intake, it could be hypothesized that this result was associated with greater fluid consumed by SCT compared to CON. Recently, the NCAA guidelines required athletic departments to improve SCT players' education on the importance of hydration, heat-related illness prevention, and pre-season conditioning. Our athletic training staff, nutritionists, and coaches are instructed to monitor SCT players during conditioning and on-field practice constantly and verbally encourage them to consume fluids during the practice breaks rather than in response to thirst alone. In addition, our recent research on fluid balance and electrolyte losses in players who frequently complete in hot and humid environments demonstrated clear benefits to the dual consumption of carbohydrate-electrolyte solution and water during practice17. Further, a greater BUN/Creatinine ratio was also observed in CON at post-camp, which suggests CON players were experiencing chronic dehydration during the training camp periods. Therefore, although CON players do not appear at risk for negative health outcomes during competitions and training, the importance of fluid balance and heat-related illness still needs to be reinforced to avoid heat injuries in either group. Furthermore, higher sweat electrolyte and lower urine electrolyte concentrations were found in SCT players. This might suggest SCT players have compensatory sweat and urine mechanisms to normalize total electrolyte losses with training. Given the losses of electrolytes in SCT are likely higher sweat, a recommendation could be made to consume more sodium-rich beverages, as we have recommended in the past17. Future research needs to be conducted to better understand the mechanisms of electrolyte balance in SCT, possibly in controlled, laboratory settings.
A major strength of this study is that it is the first known that addresses the alterations of hematological and hydration status in elite collegiate football players with SCT in a longitudinal study design compared to position-matched controls. A previous study suggested that much of the published information on SCT and exertion injuries consisted of older data or case studies40. The present study provides valuable new information for the NCAA to develop additional guidelines to reduce the possibility of exertion-related injuries during conditioning or on-field practice in SCT athletes by demonstrating significant differences that exist in SCT compared to CON responses to training and competition. In addition, we outlined differences in hematological variables during crucial time points across a season that will allow medical staff to monitor future athletes more accurately for “normal” and “abnormal” SCT responses. A limitation to this study is that fluid intake was not measured during the camp training practices, and we cannot conclude that lower urine electrolytes were associated with more fluid intake or other factors. In addition, three SCT players were recruited in both years, which could be a potential confounding variable that prevents us from knowing the true variability in response in SCT. However, three new CON players were paired with the existing SCT players in year 2 to ensure adequate technical control and data validity. Last, this is an observational, field-based study, thereby increasing the response variability due to a lack of control of confounding variables and, to some extent, when assessments were scheduled. However, while this is a limitation, it is also a relative strength in that the results of this study can be directly generalized to those in active sport.
Conclusions
In the present study, blood biomarkers showed that SCT players exhibited 31–40% HbS of hemoglobin and greater red blood cell distribution width compared to CON. In addition, serum uric acid remained greater in SCT players throughout the entire season. SCT players also had an improved fluid balance compared to CON but had lower urine electrolyte concentrations and greater sweat electrolyte concentrations during training camp. Given the results of the present study, it is of paramount importance to continue educating SCT players on the importance of fluid balance to avoid dehydration and heat exertional injuries. In addition, long-term conditioning and on-field training seems do not appear to affect the immune function in SCT players, however; the differences of uric acid and CPK levels showed SCT players might have a degree of physiological alterations in kidney function and muscle damage. The findings of the present study can significantly add to the screening process and extend the NCAA guidelines for SCT student-athletes. In addition, athletic training staff and physicians can better monitor the blood biomarkers in SCT players during exhaustive training and competitions via repeated blood testing and knowledge of ‘normal” seasonal changes in blood biomarkers in SCT. Future research should explore laboratory-based alterations of hematological biomarkers in SCT players during exhaustive exercise under hot and humid environmental conditions to further verify the results of the present study.
Acknowledgements
We thank the athletes who contributed to this research as well as the graduate students and Athletic Trainers who helped to collect the enormous amount of data. The study was partially funded by the Robert and Patricia Hines Endowed Professorship in Kinesiology (N.J.) and unrestricted gifts to the Tiger Athletics Foundation. In addition, we acknowledge funding from LSUHSC Orthopedics to establish the Scientific Liaison research associate (N.L.).
Author contributions
H.W. wrote the manuscript. H.W., M.M., M.O., N.L., J.M., D.C., and S.M. contributed to the data collection. T.C., J.R., B.H., B.I., and G.S. contributed to data interpretation and manuscript revision. N.J. contributed to the conception and the design of this research and revised the manuscript critically for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Gallais D, Bile A, Mercier J, Paschel M, Tonellot JL, Dauverchain J. Exercise-induced death in sickle cell trait: Role of aging, training, and deconditioning. Med. Sci. Sports Exerc. 1996;28:541–544. doi: 10.1097/00005768-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Eichner ER. Sickle cell considerations in athletes. Clin. Sports Med. 2011;30:537–549. doi: 10.1016/j.csm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Thiriet P, Mbala E, Wouassi D, Gelas H, Geyssant A, Lacour JR. Effect of different modalities of exercise and recovery on exercise performance in subjects with sickle cell trait. Med. Sci. Sports Exerc. 1992;24:1325–1331. doi: 10.1249/00005768-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Connes P, Sara F, Hardy-Dessources MD, Marlin L, Etienne F, Larifla L, Saint-Martin C, Hue O. Effects of short supramaximal exercise on hemorheology in sickle cell trait carriers. Eur. J. Appl. Physiol. 2006;97:143–150. doi: 10.1007/s00421-006-0155-3. [DOI] [PubMed] [Google Scholar]
- 5.Tripette J, Loko G, Samb A, Gogh BD, Sewade E, Seck D, Hue O, Romana M, Diop S, Diaw M, Brudey K, Bogui P, Cisse F, Hardy-Dessources MD, Connes P. Effects of hydration and dehydration on blood rheology in sickle cell trait carriers during exercise. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H908–914. doi: 10.1152/ajpheart.00298.2010. [DOI] [PubMed] [Google Scholar]
- 6.Harmon KG, Drezner JA, Casa DJ. To screen or not to screen for sickle cell trait in American football? Br. J. Sports Med. 2012;46:158. doi: 10.1136/bjsports-2012-090999. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor FG, Bergeron MF, Cantrell J, Connes P, Harmon KG, Ivy E, Kark J, Klossner D, Lisman P, Meyers BK, O'Brien K, Ohene-Frempong K, Thompson AA, Whitehead J, Deuster PA. ACSM and CHAMP summit on sickle cell trait: Mitigating risks for warfighters and athletes. Med. Sci. Sports Exerc. 2012;44:2045–2056. doi: 10.1249/MSS.0b013e31826851c2. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan BK, Siebert DM, Suchsland MLZ, Drezner JA, Asif IM, O'Connor FG, Harmon KG. Sudden death associated with sickle cell trait before and after mandatory screening. Sports Health. 2020;12:241–245. doi: 10.1177/1941738120915690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichner ER. Sickle cell trait. J. Sport Rehabil. 2007;16:197–203. doi: 10.1123/jsr.16.3.197. [DOI] [PubMed] [Google Scholar]
- 10.Eichner ER. Sickle cell trait in sports. Curr. Sports Med. Rep. 2010;9:347–351. doi: 10.1249/JSR.0b013e3181fc73d7. [DOI] [PubMed] [Google Scholar]
- 11.Tripette J, Connes P, Hedreville M, Etienne-Julan M, Marlin L, Hue O, Hardy-Dessources MD. Patterns of exercise-related inflammatory response in sickle cell trait carriers. Br. J. Sports Med. 2010;44:232–237. doi: 10.1136/bjsm.2008.047530. [DOI] [PubMed] [Google Scholar]
- 12.Tripette J, Hardy-Dessources MD, Sara F, Montout-Hedreville M, Saint-Martin C, Hue O, Connes P. Does repeated and heavy exercise impair blood rheology in carriers of sickle cell trait? Clin. J. Sport Med. 2007;17:465–470. doi: 10.1097/JSM.0b013e31815aed23. [DOI] [PubMed] [Google Scholar]
- 13.Loosemore M, Walsh SB, Morris E, Stewart G, Porter JB, Montgomery H. Sudden exertional death in sickle cell trait. Br. J. Sports Med. 2012;46:312–314. doi: 10.1136/bjsports-2011-090521. [DOI] [PubMed] [Google Scholar]
- 14.Vincent L, Oyono-Enguelle S, Feasson L, Banimbek V, Dohbobga M, Martin C, Thiriet P, Francina A, Dubouchaud H, Sanchez H, Chapot R, Denis C, Geyssant A, Messonnier L. Effects of regular physical activity on skeletal muscle structural, energetic, and microvascular properties in carriers of sickle cell trait. J. Appl. Physiol. 2012;1985(113):549–556. doi: 10.1152/japplphysiol.01573.2011. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, Consortium, C. K. D. P. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris NB, Cramer MN, Hodder SG, Havenith G, Jay O. A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate. J. Appl. Physiol. 2013;1985(114):816–823. doi: 10.1152/japplphysiol.01088.2012. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. Effects of field position on fluid balance and electrolyte losses in collegiate women’s soccer players. Medicina. 2020;56(10):502. doi: 10.3390/medicina56100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker LB, De Chavez PJD, Ungaro CT, Sopena BC, Nuccio RP, Reimel AJ, Barnes KA. Exercise intensity effects on total sweat electrolyte losses and regional vs whole-body sweat [Na(+)], [Cl(−)], and [K(+)] Eur. J. Appl. Physiol. 2019;119:361–375. doi: 10.1007/s00421-018-4048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster P, Castro O. Red cell distribution width in sickle cell disease. Ann. Clin. Lab. Sci. 1986;16:274–277. [PubMed] [Google Scholar]
- 20.Olujohungbe A, Garvey B. Red cell distribution width (RDW) in sickle cell disease. Clin. Lab. Haematol. 1993;15:72–73. doi: 10.1111/j.1365-2257.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Nugent CA, Tyler FH. The renal excretion of uric acid in patients with gout and in nongouty subjects. J. Clin. Invest. 1959;38:1890–1898. doi: 10.1172/JCI103966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond HS, Neisel A, Kaplan D. Renal tubular transport of urate in man. Bull. Rheum. Dis. 1976;27:876–881. [PubMed] [Google Scholar]
- 23.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am. J. Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N. Engl. J. Med. 1979;300:1001–1005. doi: 10.1056/NEJM197905033001801. [DOI] [PubMed] [Google Scholar]
- 25.van Eps LWS, Pinedo-Veels C, de Vries GH, de Koning J. Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet. 1970;1:450–452. doi: 10.1016/S0140-6736(70)90836-6. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr. Pharm. Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 28.de Oliveira EP, Burini RC. High plasma uric acid concentration: Causes and consequences. Diabetol. Metab. Syndr. 2012;4:12. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westing YH, Ekblom B, Sjodin B. The metabolic relation between hypoxanthine and uric acid in man following maximal short-distance running. Acta Physiol. Scand. 1989;137:341–345. doi: 10.1111/j.1748-1716.1989.tb08762.x. [DOI] [PubMed] [Google Scholar]
- 30.Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic. Biol. Med. 2001;31:911–922. doi: 10.1016/S0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 31.Tekin A. Xanthine oxidase and uric acid response to a 6-week pre-season training programme in male athletes. Afr. J. Pharm. Pharmacol. 2010;4:511–515. [Google Scholar]
- 32.Harrelson GL, Fincher AL, Robinson JB. Acute exertional rhabdomyolysis and its relationship to sickle cell trait. J. Athl. Train. 1995;30:309–312. [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson DA, Deuster PA, Carter R, 3rd, Hill OT, Wolcott VL, Kurina LM. Sickle cell trait, rhabdomyolysis, and mortality among US Army Soldiers. N. Engl. J. Med. 2016;375:435–442. doi: 10.1056/NEJMoa1516257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang MH. Rhabdomyolosis and its pathogenesis. World J. Emerg. Med. 2012;3:11–15. doi: 10.5847/wjem.j.issn.1920-8642.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghadam-Kia S, Oddis CV, Aggarwal R. Approach to asymptomatic creatine kinase elevation. Cleve Clin. J. Med. 2016;83:37–42. doi: 10.3949/ccjm.83a.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong ET, Cobb C, Umehara MK, Wolff GA, Haywood LJ, Greenberg T, Shaw ST., Jr Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am. J. Clin. Pathol. 1983;79:582–586. doi: 10.1093/ajcp/79.5.582. [DOI] [PubMed] [Google Scholar]
- 37.Brancaccio P, Limongelli FM, Maffulli N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006;40:96–97. doi: 10.1136/bjsm.2005.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connes P. Hemorheology and exercise: Effects of warm environments and potential consequences for sickle cell trait carriers. Scand. J. Med. Sci. Sports. 2010;20(Suppl 3):48–52. doi: 10.1111/j.1600-0838.2010.01208.x. [DOI] [PubMed] [Google Scholar]
- 39.Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BS, Roberts WO, Stone JA. National athletic trainers' association position statement: Fluid replacement for athletes. J. Athl. Train. 2000;35:212–224. [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson AA. Sickle cell trait testing and athletic participation: A solution in search of a problem? Hematol. Am. Soc. Hematol. Educ. Program. 2013;2013:632–637. doi: 10.1182/asheducation-2013.1.632. [DOI] [PubMed] [Google Scholar]