Summary
Adult hematopoietic stem cell (HSC) self-renewal requires precise control of protein synthesis, but fetal and adult HSCs have distinct self-renewal mechanisms and lineage outputs. This raises the question of whether protein synthesis rates change with age. Here, we show that protein synthesis rates decline during HSC ontogeny, yet erythroid protein synthesis rates increase. A ribosomal mutation that impairs ribosome biogenesis (Rpl24Bst/+) disrupts both fetal and adult HSC self-renewal. However, the Rpl24Bst/+ mutation selectively impairs fetal erythropoiesis at differentiation stages that exhibit fetal-specific attenuation of protein synthesis. Developmental changes in protein synthesis thus differentially sensitize hematopoietic stem and progenitor cells to impaired ribosome biogenesis.
Keywords: hematopoietic stem cell, protein synthesis, translation, erythropoiesis, erythroid, progenitor, ribosome, proteostasis, ribosomopathy, hematopoiesis
Graphical Abstract
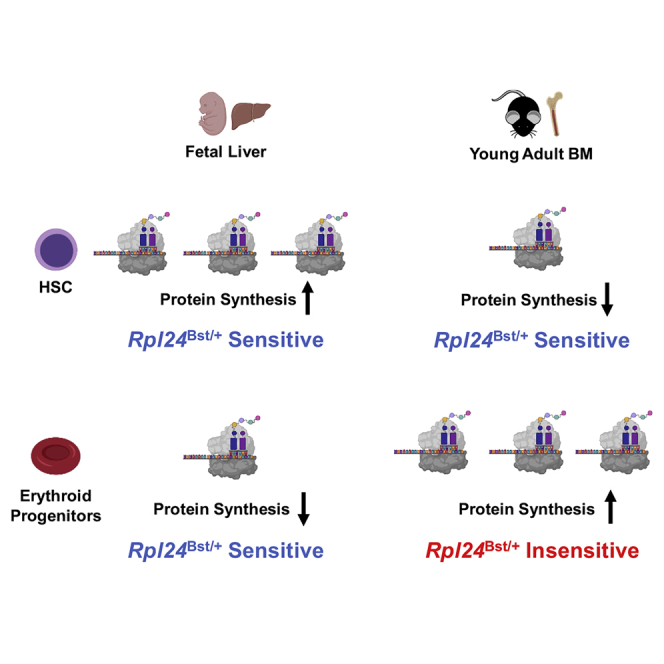
Highlights
-
•
Fetal HSCs synthesize much more protein per hour than young adult HSCs in vivo
-
•
Fetal erythroid progenitors synthesize less protein than adult erythroid progenitors
-
•
Differences in protein synthesis distinguish fetal and adult erythroid differentiation
-
•
Rpl24Bst/+ impairs fetal and adult HSCs, but only impairs fetal erythroid progenitors
In this report, Magee and Signer show that protein synthesis rates decline during HSC ontogeny, but erythroid protein synthesis rates increase. A mutation that impairs ribosome biogenesis (Rpl24Bst/+) disrupts both fetal and adult HSC self-renewal. However, the Rpl24Bst/+ mutation selectively impairs fetal erythropoiesis. Developmental changes in protein synthesis differentially sensitize hematopoietic stem and progenitor cells to impaired ribosome biogenesis.
Introduction
Adult hematopoietic stem cells (HSCs) maintain exquisite control of protein synthesis (Signer et al., 2014). Inappropriately high or low protein synthesis rates impair HSC self-renewal and can lead to leukemia or bone marrow (BM) failure (Cai et al., 2015; Signer et al., 2014). Adult HSCs exhibit very low protein synthesis compared with other hematopoietic cells (Signer et al., 2014, 2016), which is required to protect HSCs from stress associated with protein misfolding (Hidalgo San Jose et al., 2020). Most adult HSCs are quiescent, but, when forced into cycle, their protein synthesis remains low (Signer et al., 2014, 2016). This raises the question of whether HSCs maintain low protein synthesis when they cycle in other contexts, such as fetal development (Morrison et al., 1995). Ontogeny-driven changes in protein synthesis carry potential implications for human disease. Ribosomopathies, such as Diamond-Blackfan anemia and Shwachman-Diamond syndrome, cause congenital anemia and BM failure, and present early in life (Armistead and Triggs-Raine, 2014; Mills and Green, 2017; Narla and Ebert, 2010; Sakamoto et al., 2010). This could reflect the germline nature of the mutations, but it could also reflect exquisite sensitivity to altered protein synthesis in fetal HSCs or erythroid progenitors. We therefore investigated whether hematopoietic stem and progenitor cells undergo cell-type- and developmental stage-specific changes in protein synthesis that can alter the susceptibility to the deleterious effects of a ribosomal mutation in vivo.
Results and Discussion
Fetal HSCs Do Not Have Low Protein Synthesis
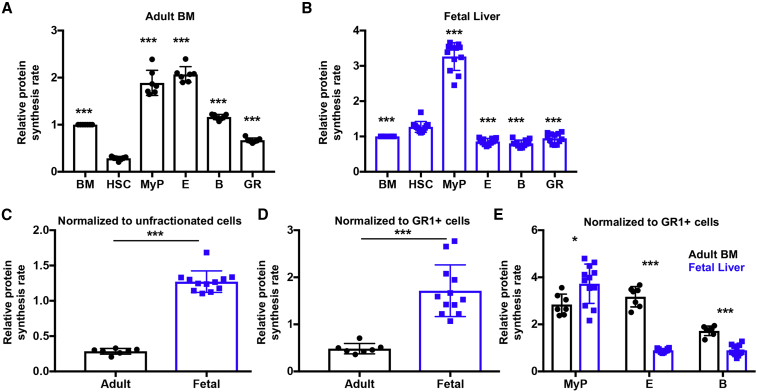
To investigate protein synthesis in adult and fetal hematopoietic stem and progenitor cells, we administered O-propargyl-puromycin (OP-Puro) (Hidalgo San Jose and Signer, 2019; Liu et al., 2012; Signer et al., 2014) to young adult wild-type (8–12 weeks old) or timed pregnant mice. One hour later, we quantified OP-Puro incorporation in BM and E15.5 fetal liver hematopoietic stem and progenitor cells by flow cytometry. Consistent with previous reports (Signer et al., 2014, 2016), adult HSCs exhibited significantly lower protein synthesis than restricted hematopoietic progenitors (Figure 1A). Bulk protein synthesized per hour in adult CD150+CD48−Lineage−SCA1+CKIT+ (CD150+CD48−LSK) HSCs (Kiel et al., 2005) was ∼3.5-fold lower than unfractionated BM cells, ∼6.6-fold lower than CD127−Lineage−ckit+Sca1− myeloid progenitors (Akashi et al., 2000), ∼7.3-fold lower than CD71+TER119+ erythroid progenitors, ∼4.1-fold lower than IgM−B220+ B cell progenitors, and ∼2.4-fold lower than GR1+ granulocytes (Figure 1A; p < 0.001). In contrast, fetal liver HSCs did not exhibit low protein synthesis compared with most fetal hematopoietic progenitors (Figure 1B). Bulk protein synthesis in fetal HSCs was ∼2.6-fold lower than myeloid progenitors (p < 0.001), but was ∼1.3-fold higher than unfractionated liver cells (p < 0.001), ∼1.5-fold higher than erythroid progenitors (p < 0.001), ∼1.6-fold higher than B cell progenitors (p < 0.001), and ∼1.4-fold higher than granulocytes (p < 0.01). These data indicate that fetal HSCs do not exhibit low protein synthesis compared with most other fetal hematopoietic cells.
Figure 1.
Distinct Protein Synthesis Rates in Fetal and Adult Hematopoietic Stem and Progenitor Cells
(A and B) Protein synthesis in hematopoietic stem and progenitor cells relative to unfractionated cells in (A) young adult BM and (B) E15.5 fetal liver. Data are shown for unfractionated BM cells, liver cells, CD150+CD48−LSK HSCs, CD127−Lineage−SCA1−CD117+ myeloid progenitors (MyP), CD71+TER119+ erythroid progenitors (E), IgM−B220+ B lineage progenitors (B), GR1+ cells (GR).
(C and D) Relative protein synthesis in young adult BM and fetal liver HSCs normalized to (C) unfractionated cells or (D) GR1+ cells.
(E) Relative protein synthesis in young adult BM and fetal liver restricted hematopoietic progenitor cells normalized to GR1+ cells.
All data represent mean ± SD. N = 7 adult mice and 11 embryos. Statistical significance was assessed relative to HSCs using a repeated-measures one-way ANOVA followed by Dunnett's multiple comparisons test (A and B), or using a two-tailed Student's t test to compare differences between fetal and adult cells (C–E); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Because of differences in OP-Puro perfusion/uptake in distinct tissues (Hidalgo San Jose and Signer, 2019), we could not directly compare OP-Puro fluorescence between adult BM and fetal liver cells. To circumvent this roadblock, we compared adult and fetal HSC protein synthesis by normalizing OP-Puro fluorescence to unfractionated hematopoietic cells in the BM and fetal liver, respectively. On this basis, fetal HSCs exhibited ∼4.5-fold higher protein synthesis than adult HSCs in vivo (Figure 1C; p < 0.001). Since the hematopoietic cell compositions of adult BM and fetal liver are quite distinct, we sought a secondary benchmark for normalization of adult and fetal HSC protein synthesis rates. Therefore, we also assessed differences in adult and fetal HSC protein synthesis by normalizing OP-Puro fluorescence to adult and fetal GR1+ cells, respectively. Using this standard, we found that fetal HSCs exhibited ∼3.5-fold higher protein synthesis than adult HSCs (Figure 1D; p < 0.001). Despite limitations in the ability to directly compare protein synthesis between fetal and adult cell populations in vivo, data obtained using these distinct benchmarks strongly suggest that fetal HSCs synthesize substantially more protein per hour than adult HSCs in vivo.
Protein synthesis differences between adult and fetal HSCs cannot be fully explained by differences in proliferation, as adult HSCs driven to undergo rapid proliferation exhibit only modestly elevated protein synthesis (Signer et al., 2014, 2016). Thus, protein synthesis rates change during HSC ontogeny, and they decline between fetal development and adulthood.
Next, we wondered if elevated protein synthesis in fetal compared with adult HSCs was a general feature that distinguishes fetal and adult hematopoietic progenitors. When normalized to age-matched GR1+ cells, fetal myeloid progenitors also exhibited significantly increased protein synthesis compared with their adult counterparts, but the effect was much smaller than in HSCs (Figure 1E; ∼1.3-fold; p < 0.05). However, in contrast to the situation in HSCs, fetal erythroid and B cell progenitors exhibited significantly lower rates of protein synthesis than their adult counterparts (Figure 1E; p < 0.001). Overall, these data indicate that there are significant developmental and hematopoietic cell-type-specific differences in protein synthesis, including a substantial reduction in protein synthesis within HSCs as they progress from the fetal to adult stage of development.
Developmental Differences in Protein Synthesis Dynamics Distinguish Fetal and Adult Erythroid Differentiation
While investigating developmental stage-specific differences in HSC protein synthesis, we found that adult CD71+TER119+ erythroid progenitors synthesized ∼3.5-fold more protein than their fetal counterparts (Figure 1E; p < 0.001). The magnitude of this change was similar to HSCs, but in the inverse direction. Intrigued by this difference, we more closely investigated protein synthesis in adult and fetal erythroid progenitors.
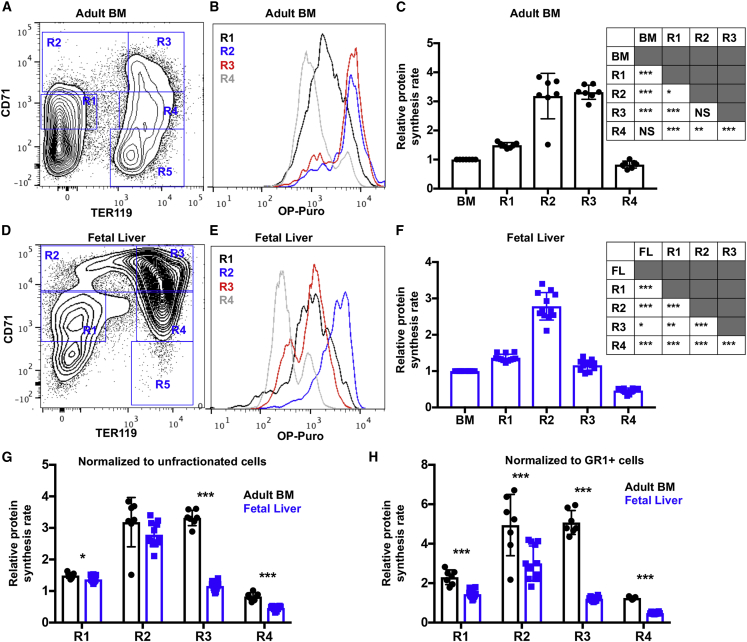
The differentiation trajectory of erythroid lineage progenitors can be broken down into at least five distinct cell populations in adult BM and fetal liver based on surface expression of CD71 and TER119 as follows (Zhang et al., 2003): CD71lowTER119− (R1), CD71highTER119−/low (R2), CD71highTER119+ (R3), CD71lowTER119+ (R4), and CD71−TER119+ (R5) (Figures 2A and 2D). We examined protein synthesis by assessing OP-Puro incorporation within adult and fetal R1-R4 erythroid populations in vivo (Figures 2B and 2E). R5 cells were reproducibly lost during the fixation and permeabilization procedure that is required to assess OP-Puro incorporation and were thus not included in the analyses.
Figure 2.
Dynamic Changes in Protein Synthesis during Erythroid Differentiation Are Distinct in Adult Bone Marrow and Fetal Liver
(A) Representative flow cytometry plot showing gating strategy for R1-R5 erythroid lineage cells in young adult BM.
(B) Representative histograms showing OP-Puro incorporation in R1-R4 erythroid progenitors in young adult BM.
(C) Relative protein synthesis based on OP-Puro incorporation in young adult R1-R4 erythroid progenitors relative to unfractionated BM cells in vivo. Statistical differences are summarized in the adjacent table.
(D) Representative flow cytometry plot showing gating strategy for R1-R5 erythroid lineage cells in E15.5 fetal liver.
(E) Representative histograms showing OP-Puro incorporation in R1-R4 erythroid progenitors in E15.5 fetal liver.
(F) Relative protein synthesis based on OP-Puro incorporation in E15.5 fetal liver (FL) R1-R4 erythroid progenitors relative to unfractionated liver cells in vivo. Statistical differences are summarized in the adjacent table.
(G and H) Relative protein synthesis in young adult BM and E15.5 fetal liver R1-R4 erythroid progenitors normalized to (G) unfractionated cells or (H) GR1+ cells. All data represent mean ± SD. N = 7 adult mice and 11 embryos. Statistical significance was assessed using a repeated-measures one-way ANOVA followed by Tukey's multiple comparisons test (C and F), or using a two-tailed Student's t test to compare differences between fetal and adult cells (G and H); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Erythroid differentiation was associated with significant and dynamic changes in protein synthesis, but the timing was distinct between adult and fetal stages. In young adult BM, differentiation of R1 to R2 cells was associated with a ∼2-fold increase in protein synthesis (Figure 2C; p < 0.05). Protein synthesis remained elevated through the R3 stage before a 4-fold reduction at the R4 stage (Figure 2C; p < 0.001). Similar to adult BM, fetal liver erythroid differentiation from the R1 to R2 stage was accompanied by a ∼2-fold spike in protein synthesis (Figure 2F; p < 0.001). However, in contrast to adult BM, protein synthesis declined at the R3 stage of differentiation. Protein synthesis within fetal R3 cells was reduced ∼2.5-fold compared with R2 cells (Figure 2F; p < 0.001). Protein synthesis continued to decline as fetal R3 erythroid cells further differentiated. Fetal R4 cells exhibited ∼6-fold less protein synthesis than R2 cells and ∼2.5-fold less protein synthesis than R3 cells (Figure 2F; p < 0.001). These data reveal cell-type and developmental stage-specific differences in protein synthesis among erythroid progenitors. Specifically, fetal erythroid progenitors attenuate protein synthesis earlier in their differentiation program than their adult counterparts.
We next sought to directly compare protein synthesis rates between adult and fetal erythroid progenitors. Similar to HSCs, we did this by normalizing protein synthesis rates to both unfractionated cells (Figure 2G) and GR1+ cells (Figure 2H). Fetal R1, R3, and R4 cells exhibited significantly reduced protein synthesis compared with their adult counterparts when normalized to unfractionated cells (Figure 2G). When normalized to GR1+ cells, all fetal erythroid progenitor subsets exhibited significantly lower protein synthesis rates than their adult counterparts. Fetal R1 and R2 cells exhibited ∼40% lower protein synthesis compared with their adult counterparts (Figure 2H; p < 0.001). Fetal R3 and R4 cells exhibited ∼75% and 60% lower protein synthesis than their respective adult counterparts (Figure 2H; p < 0.001). These data suggest that fetal erythroid progenitors generally exhibit lower protein synthesis than adult erythroid progenitors, with particularly pronounced differences at the R3 and R4 stage of differentiation. Thus, fetal erythroid progenitors exhibit lower protein synthesis than their adult counterparts, and they attenuate protein synthesis earlier in the differentiation program.
Fetal and Adult HSCs Are Both Sensitive to Impaired Ribosome Biogenesis
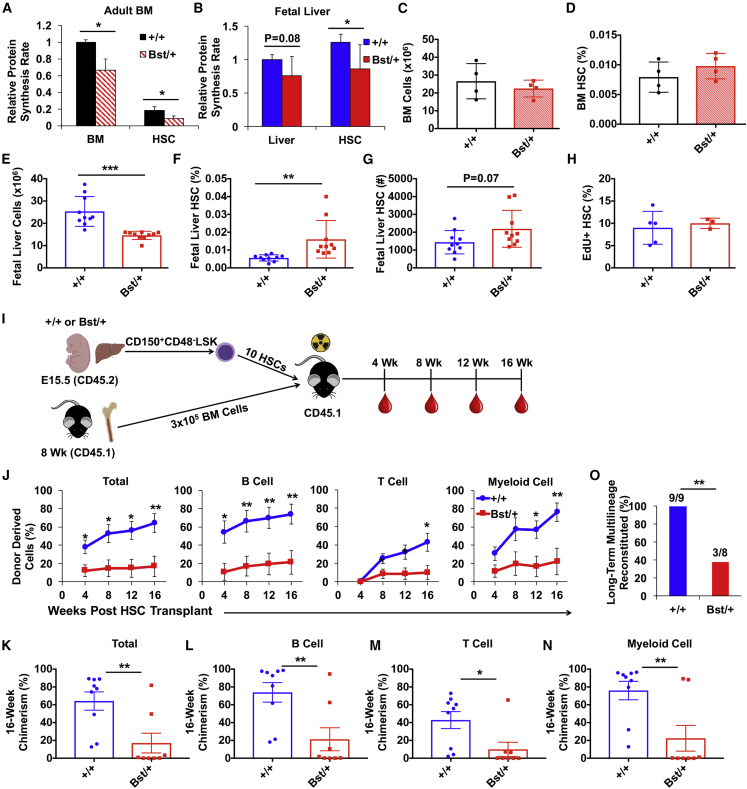
Our findings raised the question of whether ontogeny-dependent changes in protein synthesis correlate with changes in sensitivity to ribosomal mutations. To test this, we utilized mice with a ribosomal protein L24 mutation (Rpl24Bst/+) (Oliver et al., 2004). Fetal and adult Rpl24Bst/+ HSCs exhibited 30%–50% reductions in protein synthesis (Figures 3A and 3B). Reduced protein synthesis did not significantly affect adult BM cellularity, HSC frequency, or HSC number (Figures 3C, 3D, and S1A). In contrast, Rpl24Bst/+ fetal liver cellularity was significantly reduced, and HSC frequency and number were increased compared with controls (Figures 3E–3G). Rpl24Bst/+ fetal HSCs proliferated at normal rates (Figure 3H), consistent with the notion that protein synthesis and proliferation are partially decoupled in HSCs (Signer et al., 2016).
Figure 3.
Fetal Liver Rpl24Bst/+ HSCs Have Impaired Long-Term Multilineage Reconstituting Activity
(A and B) Relative protein synthesis rates in (A) unfractionated BM and HSCs in young adult Rpl24Bst/+ and control mice, and (B) unfractionated fetal liver cells and HSCs in E15.5 Rpl24Bst/+ and littermate control embryos. Values are normalized to control BM (N = 4 mice/genotype) or control liver cells (N = 12–13 embryos/genotype).
(C and D) (C) BM cellularity and (D) HSC frequency in young adult Rpl24Bst/+ and control mice (1 femur +1 tibia/mouse; N = 4 mice/genotype).
(E–G) (E) Fetal liver cellularity, (F) HSC frequency, and (G) HSC number in E15.5 Rpl24Bst/+ and littermate control embryos (N = 10 embryos/genotype).
(H) Frequency of fetal liver HSCs that incorporated EdU after a 1-h pulse in vivo (N = 3–5 embryos/genotype).
(I) Diagram of experimental strategy to test long-term multilineage reconstituting activity of fetal liver Rpl24Bst/+ HSCs.
(J) Donor-cell engraftment when 10 Rpl24Bst/+ (Bst/+) or littermate control (+/+) HSCs were transplanted with 3 × 105 recipient-type young adult BM cells into irradiated mice. Total hematopoietic, B-, T-, and myeloid-cell engraftment is shown 4, 8, 12, and 16 weeks after transplantation (N = 8–9 recipients per genotype).
(K–N) Long-term (16-week) donor (K) hematopoietic, (L) B, (M) T, and (N) myeloid cell engraftment in the peripheral blood of individual recipient mice from (J).
(O) Frequency of recipient mice in (J) that exhibited long-term (16-week) multilineage reconstitution (≥0.5% donor-derived peripheral blood B, T, and myeloid cells).
Data represent mean ± SD (A–H) or SEM (J–N). Statistical significance was assessed using a two-tailed Student's t test or a Fisher's exact test (O); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To test whether fetal HSC function is compromised by the Rpl24Bst/+ mutation, as we previously observed for adult HSCs (Figures S1B and S1C) (Signer et al., 2014), we transplanted 10 E15.5 HSCs from Rpl24Bst/+ or control mice (CD45.2+) with 3 × 105 recipient-type BM cells into irradiated mice (CD45.1+) (Figure 3I). Long-term hematopoietic reconstitution from Rpl24Bst/+ fetal liver HSCs was severely reduced compared with controls (Figures 3J–3N). Overall, only 38% (3 out of 8) of recipients of Rpl24Bst/+ fetal liver HSCs exhibited long-term multilineage reconstitution compared with 100% (9 out of 9) of recipients of wild-type littermate control HSCs (Figure 3O; p < 0.01). These data indicate that Rpl24Bst/+ fetal liver HSCs have impaired reconstituting activity. Reduced protein synthesis thus impairs the function of both adult and fetal HSCs, demonstrating that HSCs are exquisitely sensitive to impaired ribosome biogenesis, irrespective of whether they have high or low baseline protein synthesis.
Rpl24Bst/+ Mutation Impairs Fetal but Not Adult Erythroid Progenitors
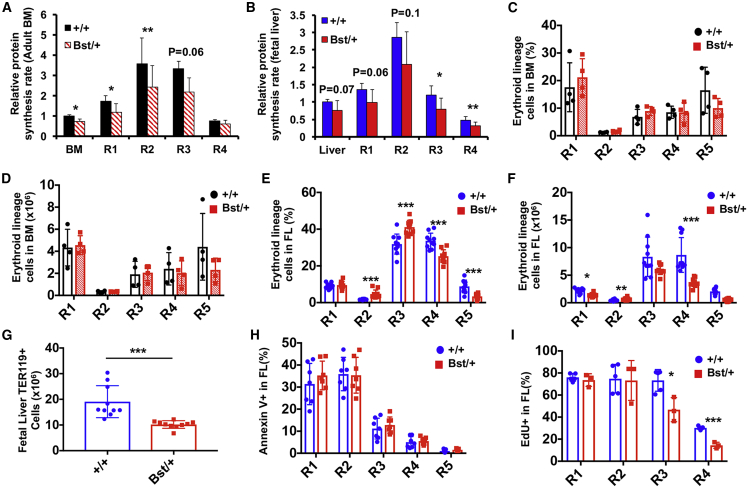
Since both fetal and adult HSC function was disrupted by the Rpl24Bst/+ mutation, we tested if the same was true for erythroid lineage cells given the temporal differences in erythroid progenitor protein synthesis rates. The Rpl24Bst/+ mutation reduced protein synthesis by 25%–55% in fetal and adult erythroid progenitors (Figures 4A and 4B). Adult Rpl24Bst/+ BM contained similar frequencies and numbers of erythroid progenitors to controls (Figures 4C, 4D, S2A, and S2B), and there was no change in erythroid progenitor apoptosis (Figure S2C) or peripheral red blood cells (Signer et al., 2014).
Figure 4.
The Rpl24Bst/+ Mutation Impairs Fetal But Not Adult Erythroid Lineage Cells
(A and B) Relative protein synthesis in Rpl24Bst/+ (Bst/+) or control (+/+) erythroid progenitor cells in (A) young adult BM (N = 4 mice/genotype) and (B) E15.5 fetal liver (N = 12–13 embryos/genotype) in vivo.
(C) Frequency and (D) absolute number of erythroid progenitors in Rpl24Bst/+ (Bst/+) or control (+/+) young adult BM (1 femur +1 tibia/mouse; N = 3–4 mice/genotype).
(E) Frequency and (F) absolute number of erythroid progenitors in Rpl24Bst/+ (Bst/+) or control (+/+) E15.5 fetal liver (FL; N = 10 embryos/genotype).
(G) Number of TER119+ cells in Rpl24Bst/+ (Bst/+) or control (+/+) E15.5 fetal liver (N = 10 embryos/genotype).
(H) Frequency of erythroid progenitors that are Annexin V+ in Rpl24Bst/+ (Bst/+) or control (+/+) E15.5 fetal liver (N = 7 embryos/genotype).
(I) Frequency of Rpl24Bst/+ (Bst/+) or control (+/+) fetal liver erythroid progenitors that incorporated EdU after a 1-h pulse in vivo (N = 3–5 embryos/genotype).
Data represent mean ± SD. Statistical significance was assessed using a two-tailed Student's t test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
In contrast to adult BM, Rpl24Bst/+ fetal livers contained significantly more R2 and R3 erythroid progenitors and significantly fewer R4 and R5 cells compared with controls (Figures 4E and 4F). Overall, Rpl24Bst/+ fetal livers contained ∼45% fewer TER119+ erythroid cells than controls (Figure 4G). The decline in erythroid cells largely accounted for the overall reduction in Rpl24Bst/+ fetal liver cellularity (Figure 3E). The depletion of fetal erythroid progenitors was not attributable to changes in apoptosis, as Annexin V labeling was similar in Rpl24Bst/+ and control R1-R5 cells (Figure 4H). However, Rpl24Bst/+ fetal R3 and R4 cells exhibited significantly reduced proliferation in vivo (Figure 4I), which coincides with the stage at which fetal progenitors specifically exhibit a decline in protein synthesis. Low protein synthesis may thus hypersensitize differentiating fetal erythroid progenitors to ribosomal mutations by restricting proliferation and translation of essential mRNAs (Basak et al., 2020; Chua et al., 2020; Khajuria et al., 2018; Liu et al., 2017; Ludwig et al., 2014).
This study reveals that protein synthesis is dynamically controlled in a cell-type- and developmental stage-specific manner in the hematopoietic system. Adult HSCs depend on maintaining low protein synthesis (Signer et al., 2014, 2016), and modest increases in protein synthesis partially impair adult HSC self-renewal by increasing the biogenesis of misfolded proteins (Hidalgo San Jose et al., 2020). Fetal HSCs maintain relatively high protein synthesis, yet they retain self-renewal capacity, suggesting that they can cope with misfolded proteins more effectively than adult HSCs. Bile acids in the fetal liver protect HSCs by restricting activation of the unfolded protein response (Sigurdsson et al., 2016), but a more complete understanding of how fetal HSCs cope with protein misfolding that accompanies high protein synthesis could yield interventions to improve proteostasis maintenance in adult HSCs (Chua and Signer, 2020).
Unlike HSCs, erythroid progenitors exhibit fetal-specific susceptibility to defects in ribosome biogenesis, suggesting that ribosomal mutations may disrupt erythropoiesis to a greater degree in early stages of life when protein synthesis is low. Indeed, some Diamond-Blackfan anemia patients go into spontaneous remission as they age (Vlachos and Muir, 2010). An intriguing possibility is that this remission is associated with maturation of their erythroid compartment into the adult stage. Since different ribosomal mutations could have distinct effects on translation, further studies should resolve whether temporal changes in protein synthesis convey susceptibility to disease-relevant mutations (Ulirsch et al., 2018).
Experimental Procedures
Mice
Rpl24Bst/+ mice (Oliver et al., 2004; Signer et al., 2014) were obtained from The Jackson Laboratory (000516) and backcrossed for 10+ generations onto a C57BL background. Wild-type littermates or age-matched C57BL/Ka-Thy-1.1 mice were used as controls for experiments with adult mice, and wild-type littermates were used as controls for fetal experiments. C57BL/Ka-Thy1.2 (CD45.1) mice were used as transplant recipients. Young adult mice were 8–12 weeks old. Embryos were utilized at ∼ E15.5. Animals were housed in the Animal Resource Center at UT Southwestern and the vivarium at the UC San Diego Moores Cancer Center. Protocols were approved by the UT Southwestern and UC San Diego Institutional Animal Care and Use Committees.
Cell Isolation and Flow Cytometry
BM cells were isolated by flushing the long bones and fetal liver cells were isolated by crushing the fetal liver between frosted slides in Hank's buffered salt solution (HBSS; Corning) supplemented with 2% heat-inactivated bovine serum (Gibco). Cell number was assessed with a Vi-CELL cell viability analyzer (Beckman Coulter).
For flow cytometric analysis and isolation, cells were incubated with combinations of antibodies (eBiosciences or BioLegend) to the following cell-surface markers, conjugated to FITC, PE, PerCP-Cy5.5, APC, PE-Cy7, Alexa Fluor 700, or APC-eFluor 780: CD3ε (17A2; Cat #100204), CD4 (GK1.5; Cat #100406), CD5 (53–7.3; Cat #100606), CD8α (53–6.7), CD11b (M1/70; Cat #12-0112-83 and 17-0112-82), CD45.1 (A20; Cat #47-0453-82), CD45.2 (104; Cat #109806), CD45R (RA3-6B2; Cat #11-0452-85 and #45-0452-82), CD48 (HM48-1; Cat #103404), CD71 (R17217; Cat #11-0711-85), CD117 (2B8; Cat #17-1171-83), CD127 (A7R34; Cat# 135010), CD150 (TC15-12F12.2; Cat #115904 and #115910), TER119 (TER-119; Cat# 116212), Sca-1 (D7, E13–161.7; Cat# 45-5,981-82), GR1 (RB6-8C5; Cat #108406 and #108416) and IgM (II/41; 17-5,790-82). For HSCs, lineage markers included CD3, CD5, CD8, B220, GR1, and TER119. For myeloid progenitors, lineage markers were supplemented with antibodies against CD4 and CD11b. Incubations were for at least 30 min on ice.
Mouse HSCs were pre-enriched by selecting CKIT+ cells using paramagnetic microbeads and an autoMACS (Miltenyi) before sorting. Non-viable cells were excluded using DAPI. Apoptotic cells were identified using APC-conjugated anti-Annexin V (BD Biosciences). Data acquisition and cell sorting were performed on a FACSAria II, LSR II, or FACSCanto flow cytometer (BD Biosciences). Data were analyzed by FlowJo (TreeStar) software.
Measurement of Protein Synthesis
OP-Puro (Liu et al., 2012) (Medchem Source; 50 mg/kg) was injected intraperitoneally into young adult or pregnant female mice. BM or fetal liver cells were collected 1 h later and 4 × 106 cells were stained with antibodies and processed as previously described (Hidalgo San Jose and Signer, 2019).
Transplantation Assays
Recipient mice were administered two doses of 540 rad at least 3 h apart using an XRAD 320 X-ray irradiator (Precision X-Ray). For fetal HSC transplants, 10 CD150+CD48−LSK HSCs (Kim et al., 2006) and 3 × 105 recipient-type BM cells were transplanted. For adult BM transplants, 5 × 105 donor BM cells were transplanted with 5 × 105 recipient-type BM cells. Blood was obtained from tail veins of recipient mice every 4 weeks for 16 weeks. Red blood cells were lysed with ammonium chloride potassium buffer. Remaining cells were stained with antibodies against CD45.2, CD45.1, CD45R, CD11b, CD3, and GR1 to assess donor-cell engraftment. Mice were considered long-term multilineage reconstituted if they exhibited >0.5% donor-derived peripheral blood B, T, and myeloid cells 16 weeks post transplant.
Cell Proliferation Analysis
Two milligrams of EdU (Thermo) in PBS was injected intraperitoneally into pregnant female mice. Fetal livers were harvested 1 h later, and 3 × 106 cells were stained with antibodies as described above. EdU incorporation was assessed as previously described (Signer et al., 2016).
Authors Contributions
J.A.M. and R.A.J.S. conceived the project, performed HSC analyses, and wrote the manuscript. R.A.J.S. performed protein synthesis and erythroid analyses.
Acknowledgments
Work in the Signer Laboratory is supported by the NIH/NIDDK (R01DK116951; R01DK124775), a Blood Cancer Discoveries grant (8025-20) from the Leukemia & Lymphoma Society, The Mark Foundation for Cancer Research and the Paul G. Allen Frontiers Group, a Scholar Award from the American Society of Hematology, the Sanford Stem Cell Clinical Center, and the UCSD Moores Cancer Center, which is supported by the NIH/NCI Specialized Cancer Center Support Grant (2P30CA023100). The LJI Flow Cytometry core facility is supported by the NIH Shared Instrumentation Grant Program (S10 RR027366). Work in the Magee Laboratory is supported by the NHLBI (R01HL136504 and R01HL152180), Alex’s Lemonade Stand Foundation (‘A’ Award), Hyundai Hope on Wheels, and the Children's Discovery Institute of Washington University and St. Louis Children's Hospital. Some figures were created with BioRender.com. We would like to thank Sean Morrison for support and resources and Kellie Machlus for advice.
Published: January 12, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.11.017.
Supplemental Information
References
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Armistead J., Triggs-Raine B. Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett. 2014;588:1491–1500. doi: 10.1016/j.febslet.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Basak A., Munschauer M., Lareau C.A., Montbleau K.E., Ulirsch J.C., Hartigan C.R., Schenone M., Lian J., Wang Y., Huang Y. Control of human hemoglobin switching by LIN28B-mediated regulation of BCL11A translation. Nat. Genet. 2020;52:138–145. doi: 10.1038/s41588-019-0568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Gao L., Teng L., Ge J., Oo Z.M., Kumar A.R., Gilliland D.G., Mason P.J., Tan K., Speck N.A. Runx1 deficiency decreases ribosome biogenesis and confers stress resistance to hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;17:165–177. doi: 10.1016/j.stem.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B.A., Signer R.A.J. Hematopoietic stem cell regulation by the proteostasis network. Curr. Opin. Hematol. 2020;27:254–263. doi: 10.1097/MOH.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B.A., Van Der Werf I., Jamieson C., Signer R.A.J. Post-transcriptional regulation of homeostatic, stressed, and malignant stem cells. Cell Stem Cell. 2020;26:138–159. doi: 10.1016/j.stem.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo San Jose L., Signer R.A.J. Cell-type-specific quantification of protein synthesis in vivo. Nat. Protoc. 2019;14:441–460. doi: 10.1038/s41596-018-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo San Jose L., Sunshine M.J., Dillingham C.H., Chua B.A., Kruta M., Hong Y., Hatters D.M., Signer R.A.J. Modest declines in proteome quality impair hematopoietic stem cell self-renewal. Cell Rep. 2020;30:69–80 e66. doi: 10.1016/j.celrep.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria R.K., Munschauer M., Ulirsch J.C., Fiorini C., Ludwig L.S., McFarland S.K., Abdulhay N.J., Specht H., Keshishian H., Mani D.R. Ribosome levels selectively regulate translation and lineage commitment in human hematopoiesis. Cell. 2018;173:90–103 e119. doi: 10.1016/j.cell.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz O.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kim I., He S., Yilmaz O.H., Kiel M.J., Morrison S.J. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xu Y., Stoleru D., Salic A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc. Natl. Acad. Sci. U S A. 2012;109:413–418. doi: 10.1073/pnas.1111561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Y., Ni M., Cao H., Signer R.A.J., Li D., Li M., Gu Z., Hu Z., Dickerson K.E. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol. 2017;19:626–638. doi: 10.1038/ncb3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L.S., Gazda H.T., Eng J.C., Eichhorn S.W., Thiru P., Ghazvinian R., George T.I., Gotlib J.R., Beggs A.H., Sieff C.A. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat. Med. 2014;20:748–753. doi: 10.1038/nm.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.W., Green R. Ribosomopathies: there's strength in numbers. Science. 2017;358:eaan2755. doi: 10.1126/science.aan2755. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Hemmati H.D., Wandycz A.M., Weissman I.L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla A., Ebert B.L. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E.R., Saunders T.L., Tarle S.A., Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K.M., Shimamura A., Davies S.M. Congenital disorders of ribosome biogenesis and bone marrow failure. Biol. Blood Marrow Transplant. 2010;16:S12–S17. doi: 10.1016/j.bbmt.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer R.A., Magee J.A., Salic A., Morrison S.J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509:49–54. doi: 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer R.A., Qi L., Zhao Z., Thompson D., Sigova A.A., Fan Z.P., DeMartino G.N., Young R.A., Sonenberg N., Morrison S.J. The rate of protein synthesis in hematopoietic stem cells is limited partly by 4E-BPs. Genes Dev. 2016;30:1698–1703. doi: 10.1101/gad.282756.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson V., Takei H., Soboleva S., Radulovic V., Galeev R., Siva K., Leeb-Lundberg L.M., Iida T., Nittono H., Miharada K. Bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver. Cell Stem Cell. 2016;18:522–532. doi: 10.1016/j.stem.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ulirsch J.C., Verboon J.M., Kazerounian S., Guo M.H., Yuan D., Ludwig L.S., Handsaker R.E., Abdulhay N.J., Fiorini C., Genovese G. The genetic landscape of Diamond-Blackfan anemia. Am. J. Hum. Genet. 2018;103:930–947. doi: 10.1016/j.ajhg.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A., Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116:3715–3723. doi: 10.1182/blood-2010-02-251090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Socolovsky M., Gross A.W., Lodish H.F. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.