Summary
Serotonin receptor 6 (5-HT6R), a typical G protein-coupled receptor (GPCR) mainly expressed in the neurogenic area with constitutive activity, is of particular interest as a promising target for emotional impairment. Here, we found that 5-HT6R was highly expressed in human NSCs and activation of the receptor promoted self-renewal of human NSCs, and thus induced the expansion and folding of human cerebral organoids; dysfunction of receptor or inhibition of its constitutive activity resulted in the premature differentiation of NSCs, which ultimately depleted the NSC pool. The following mechanistic study revealed that EPAC-CREB signaling was involved in 5-HT6R regulation. Furthermore, we showed that mice with genetic deletion of 5-HT6R or knockin A268R mutant presented depression-like behaviors and impaired hippocampal neurogenesis for progressive decrease of the NSC pool. Thus, this study indicates that the modulation of 5-HT6R and its constitutive activity may provide a therapeutic alternative to alleviate depression.
Keywords: 5-HT6R, constitutive activity, depression, neurogenesis, organoids, human neural stem cell, self-renewal, CRISPR-Cas9, iPSC
Graphical Abstract
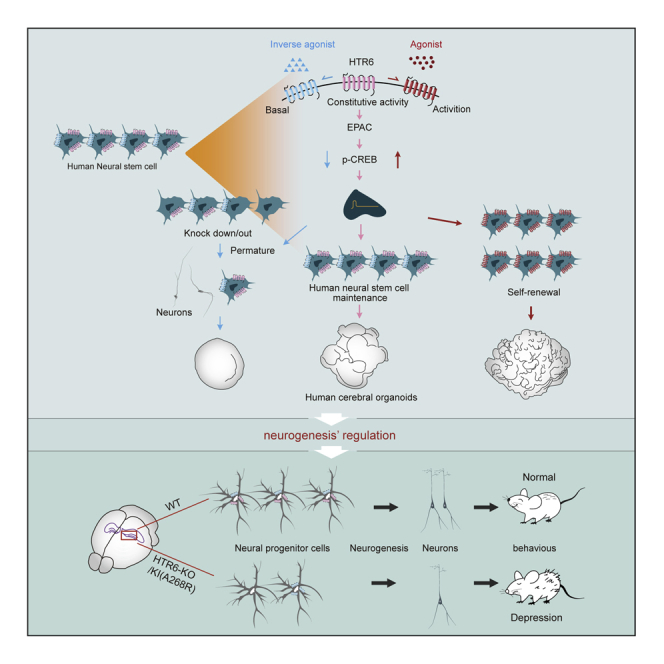
Highlights
-
•
5-HT6R regulates human neural stem cell proliferation
-
•
The constitutive activity of 5-HT6R is essential for human neural stem cell's multipotency
-
•
5-HT6R modulates neurogenesis of human cerebral organoids
-
•
Mice with reduced constitutive activity of 5-HT6R show depression-like behaviors
In this study, Dr. Pei and colleagues demonstrate a potential role of serotonin receptor 5-HT6R constitutive activity in the regulation of neurogenesis and depression-like behaviors in mice. Furthermore, the group found that hNSC-specific activation and inhibition of 5-HT6R bidirectionally regulates hNSC proliferation and expansion and folding of cerebral organoids, leading to changes in neurogenesis.
Introduction
Depression is the most prevalent psychiatric disorder, affecting more than 250 million people worldwide according to a recent report produced by the World Health Organization. However, a significant proportion of depressed patients do not achieve remission with current therapy (Kondo et al., 2018; Shen et al., 2019). Due to the elusive nature of this disorder with complex genetic and environmental interactions and the lack of better understanding of its pathophysiology, finding an effective management is still challenging (Kim et al., 2019; Pitsillou et al., 2020). Notable hallmarks of depression are the structural and functional deficits apparent in the hippocampus, a brain region linked to mood regulation and memory. Neurogenesis is well accepted to occur in the hippocampus. People with depression had decreased hippocampal volume with a tendency toward reduced neurogenesis in the dentate gyrus (DG) (Eisch and Petrik, 2012; Kronmuller et al., 2008). Stimulation of chronic stress reduced neurogenesis and also induced depression (Coe et al., 2003; Ming and Song, 2011). Therefore, neurogenesis deficiency is still one of the working hypotheses for depression (Eisch and Petrik, 2012).
Adult neurogenesis in the hippocampus is evolutionarily conserved across mammals and relies on the maintenance of the neural stem cell (NSC) pool, which supports neuron generation (Goncalves et al., 2016; Kempermann et al., 2018). As one of the most widespread neurotransmitters of the nervous system, serotonin (5-HT) is crucial for normal brain development and also for NSC maintenance (Cheng et al., 2010; Lv and Liu, 2017). Several studies demonstrated the involvement of the serotonergic system in neurogenesis to influence the progress of depression (Kiyasova and Gaspar, 2011). Brezun and Daszuta (1999) showed that serotonin deficit in adult rats was accompanied with decreased adult neurogenesis in the DG and subventricular zone (SVZ). Benninghoff et al. (2010) found that serotonin depletion hindered the survival and proliferation of NSC neurospheres derived from adult mouse hippocampus, which could contribute to depression (Djavadian, 2004). Antidepressant drugs, such as selective serotonin reuptake inhibitors that can specifically increase 5-HT levels in the synaptic cleft, are widely used to treat depression (Boldrini et al., 2012; David and Gardier, 2016), implying the beneficial effects of 5-HT receptor (5-HTR) activation. There are 13 established subtypes of 5-HTRs and a 1-ion channel in mammals. Behavioral and neuropharmacology studies have attempted to identify the association of 5-HTR subtypes with the therapeutic effects of SSRIs (Carr and Lucki, 2011; Zmudzka et al., 2018), and 5-HT1AR and 5-HT4R have been implicated in the modulation of depression-like behaviors or in the response to antidepressant treatments (Alenina and Klempin, 2015; Carr and Lucki, 2011). However, the effects of 5-HT6R on depression are ambiguous owing to the absence of genetic evidence. Although the pharmacological activation of 5-HT6R showed antidepressive activity in animal studies (Jastrzebska-Wiesek et al., 2015; Nikiforuk et al., 2011), the underlying mechanism remains unclear.
The ligand-independent spontaneous activation, or constitutive activity of receptors, has been well demonstrated for various G protein-coupled receptors (GPCRs) (Fukami et al., 2018; Rosenbaum et al., 2009). Mounting evidence clearly indicates the importance of receptor constitutive activity in the maintenance of diverse physiological functions. Receptor polymorphisms of single-nucleotide mutations modulating constitutive activity can lead to the occurrence of various diseases (Harrod et al., 2017; Seifert and Wenzel-Seifert, 2002; Yasuda et al., 2012). Inverse agonists defined to reduce the constitutive activity of a receptor and to produce the opposite effect as an agonist have been developed for disease treatment (Berg and Clarke, 2018; Sato et al., 2016). For 5-HT6R, its constitutive activity has been established at Gα signaling by overexpression of wild-type (WT) or mutant receptors, and the results showed that the constitutive activity was related to neuronal development or neocortical radial migration (Deraredj Nadim et al., 2016; Duhr et al., 2014; Jacobshagen et al., 2014; Kohen et al., 2001; Purohit et al., 2003). However, the precise role of the constitutive activity of 5-HT6R in the regulation of NSC function and depression behavior in mice remains unknown.
Results
5-HT6R Activation Promotes Self-Renewal of Human NSCs and Their Constitutive Activity Is Essential for Maintaining Human NSC Multipotency
To determine the role of 5-HT6R in human NSCs, which were induced from pluripotent stem cells (iPSCs), we first tested the expression pattern of each 5-HTR subtype by qRT-PCR. The 5-HT1DR and 5-HT6R showed relatively high expressions in human NSCs compared with other subtypes (Figure S1A). In 2D adherent culture systems, cells were cultured in either the presence or absence of respective ligands for 24 h followed by subsequent determination of ATP levels using the Cell Titer-Glo assay as an index of cell proliferation. We found that ST1936, a selective agonist of 5-HT6R, highly promoted the cell number compared with the controls, indicating increased proliferative activity upon ST1936 stimulation (Figure S1B). By contrast, the 5-HT1DR agonist GR46611 did not obviously influence NSC proliferation. Notably, the number of NSCs cultured with 5-HT, a pan agonist for 5-HTR, was numerically but not significantly higher than that of the control (Figure S1B). We also found that the 5-HT6R selective agonist ST1936 significantly promoted human NSC proliferation in a dosage- and time-dependent manner when it was cultured in low growth factor medium (Figures 1A and 1B). The promotion of cell proliferation by ST1936 was further confirmed by EdU incorporation (Figures 1C and 1D). The effect of ST1936 was not observed in a neuroblastoma cell line, SK-N-SH, which is also enriched in 5-HT6R (Li et al., 2017a), indicating its cell-specific effect (Figure S1C). In addition, the activation of 5-HT6R by other agonists, EMD386088 and WAY181187, could also promote human NSC proliferation (Figure S1D).
Figure 1.
5-HT6R Activation Promotes Human NSC Self-Renewal, and Its Constitutive Activity Is Essential for Maintaining Human NSC Multipotency
(A) Dosage-dependent response of cell growth after treatment with ST1936 (n = 4, N = 1). Data are means ± SEM. ∗∗∗p < 0.001, one-way analysis of variance (ANOVA) with Dunnett’s post-test.
(B) Time lapses of cell growth in response to ST1936 at 10 μM (n = 4, N = 1). Data are means ± SEM. ∗∗∗p < 0.001, one-way ANOVA with Dunnett’s post-test.
(C and D) The percentage of EdU-positive cells after being treated with/without ST1936 at 10 μM and its representative images (n = 3–6, N = 1). Data are means ± SEM. ∗∗∗p < 0.001, unpaired Student's t test. Scale bar, 200 μm.
(E and F) The number of secondary neurospheres formed from human NSCs, at 1,000/well cell density, treated with/without ST1936 at 10 μM and its representative images (n = 3–5, N = 4). Data are means ± SEM. ∗∗p < 0.01, unpaired Student's t test. Scale bars, 200 μm.
(G) Time lapses of cell growth in response to SB271046 at 10 μM (n = 4, N = 1). Data are means ± SEM. ∗∗∗p < 0.001, one-way ANOVA with Dunnett’s post-test.
(H and I) The number of secondary neurospheres formed from human NSCs treated with/without SB271046 at 10 μM and its representative images (n = 4, N = 4). Data are means ± SEM. ∗∗∗p < 0.001, unpaired Student's t test. Scale bars, 200 μm.
(J–L) Representative images of immunofluorescence staining for SOX2+, NESTIN+, and KI67+ in human NSCs treated with ST1936/SB271046 at 10 μM and subsequent quantitation (n = 3, N = 3). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(M–N) Representative images of immunofluorescence staining for SOX2+, PAX6+, and DCX+ in human NSCs treated with ST1936/SB271046 at 10 μM and subsequent quantitation (n = 3, N = 3). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm. n = 3.
(O) ATP assay on 2D adherent human NSC culture after knockdown of 5-HT6R (n = 3, N = 1). Data are means ± SEM. ∗∗p < 0.01, unpaired Student's t test.
(P and Q) The number of secondary neurospheres formed from human NSCs after knockdown of 5-HT6R and their representative images (n = 4, N = 4). Data are means ± SEM. ∗∗p < 0.01, unpaired Student's t test. Scale bars, 200 μm.
(R and S) The shNC- or sh5-HT6R-1-treated human NSCs were infected with empty plasmid (mock), 5-HT6R-WT, or mutant A266R and both the number of secondary neurosphere formed and their representative images were shown (n = 4, N = 4). Data are means ± SEM. ∗p < 0.05, ∗∗∗p < 0.001 compared with the control of each group, one-way ANOVA with Dunnett’s post-test. Scale bars, 200 μm.
(T and U) The number of secondary neurosphere formed in human NSCs without or with A266R mutant knockin by CRISPR-Cas9 and sequences of the 5-HT6R-KI (A266R) NSC clone (n = 3–5, N = 4). Data are means ± SEM. ∗∗p < 0.01, unpaired Student's t test. Scale bars, 200 μm.
To assess whether ST1936 could promote human NSC self-renewal, we treated neurosphere (“primary neurosphere”) with DMSO (control) and ST1936. Then, 7-day-old primary neurospheres were trypsinized to single cells and replated again at the same density, the overall number of secondary neurospheres formed after 7 days of cultivation was counted (Figures 1E and 1F). However, treatment of human NSCs with SB271046 and SB742457, which behave as inverse agonists and inhibit the receptor's constitutive activity, significantly reduced the cell number at 10 μΜ (Figures 1G and S1E). Compared with the controls, SB271046-treated cells showed a distinct reduction in neurosphere frequency and clone diameter (Figures 1H and 1I). The effects of ST1936 and SB271046 were weakened by the antagonist Ro046790 (Figures S1F–S1G) and the knockdown of 5-HT6R (Figure S1H), indicating its 5-HT6R-dependent effect.
We next analyzed whether ST1936-induced proliferation is coupled to the maintenance of the stem cell state. The number of KI67+ cells was largely increased by the treatment of human NSCs with ST1936 in the low factor defined medium for 3 days (Figures 1J and 1K). In addition, compared with the control, ST1936-expanded human NSCs were more primitive progeny, expressing the NSC markers SOX2 and NESTIN with only a few immature and neuron-like cells positive for neuron marker DCX. However, SB271046 treatment dramatically enhanced neuronal differentiation and cell apoptosis of human NSCs, which were characterized by the expression of DCX with few NSC markers detected (Figures 1L–1N and S1I) and proportion/trend of an apoptosis marker (S1J). These datas indicate that activation of 5-HT6R promotes the symmetrical divisions of human NSCs and the maintenance of multipotency, and that the inhibition of 5-HT6R constitutive activity could result in the loss of human NSC stemness with premature phenotypes.
To further verify the effect of 5-HT6R and its constitutive activity on human NSC self-renewal, human NSCs were then infected with lentivirus of small hairpin RNA (shRNA) against 5-HT6R (sh5-HT6R-1), and the knockdown of 5-HT6R markedly decreased cell growth (Figure 1O). We observed a massive reduction in the frequency of colony-forming cells under neurosphere-generating conditions after the reduction of 5-HT6R levels (Figures 1P and 1Q). The secondary shRNA independently designed against 5-HT6R (sh5-HT6R-2) resulted in similar phenotypes in the neurosphere assay (Figure S1K), suggesting that the RNAi-mediated knockdown is specific and that 5-HT6R function in human NSCs was related to the effect of the receptors on proliferation or self-renewal (Figure S1L). Moreover, in the cells with 5-HT6R knockdown, we noted a striking change in cell morphology, showing elongated processes arranged in parallel fashion (Figure S1M), which indicates that cells may lose their characteristic phenotype and become differentiated.
As a further verification, we attempted to rescue the 5-HT6R RNAi phenotype by exogenous expression of 5-HT6R. Human NSCs were co-infected with construct expressing the 5-HT6R (WT) or 5-HT6R mutant (A266R) with reduced constitutive activity (Figure S1N) and a 5-HT6R shRNA. Results showed that the inhibitory effect of 5-HT6R knockdown on neurosphere formation was complemented by exogenous expression of 5-HT6R. However, exogenous expression of 5-HT6R (A266R) generated a reduced promotion on the number of secondary neurospheres compared with the WT and failed to efficiently rescue 5-HT6R knockdown-mediated reduction of neurospheres (Figures 1R, 1S, and S1O). To confirm that the observed effect of 5-HT6R is not through the potential binding of endogenous serotonin in the culture medium, we investigated the effect of a 5-HT6R-D106A mutant, which harbors absent serotonin binding affinity but consistent constitutive activity as the WT (Zhang et al., 2006). Introduction of the 5-HT6R-D106A mutant promoted human NSC self-renewal to a level similar to the WT (Figures S1P and S1Q). At the endogenous level, 5-HT6R was mutagenized using the CRISPR-Cas9 genome-editing system, using guide RNAs directed to the 5-HT6R DNA loci to mutate the amino acids alanine 266 (A266) into arginine 266 (R266). Sequence analysis validated the correct mutation of the 5-HT6R gene (Figure 1T), and the cAMP levels were reduced in the 5-HT6R-knockin (KI) (A266R) cell line (Figure S1R). In these cells, the numbers of secondary neurospheres decreased compared with those in the WT NSCs (Figure 1U). All these data strongly suggest that 5-HT6R and its constitutive activity are involved in the regulation of human NSC self-renewal.
5-HT6R Regulates Human NSC Function by Modulating EPAC-Rab1-CREB Signaling
To investigate the possible underlying mechanism by which 5-HT6R regulates human NSC function, we performed RNA sequencing (RNA-seq) to analyze the transcriptome of human NSCs with vehicle (DMSO) or ST1936/SB271046 treatment for 24 h, and the results showed that ST1936/SB271046 treatment dramatically altered the transcriptome of human NSCs (Figures 2A and 2B). Compared with the control, there were 400/70 upregulated and 148/10 downregulated genes in ST1936/SB271046-treated cells (Figures 2C and 2D). We then subjected the differentially expressed genes to gene ontology analysis of biological processes. The gene ontology biological process term analysis indicated a strong enrichment for genes involved in the regulation of neuron differentiation and organismal development, such as the actin filament-based process and cell morphogenesis (Figure 2E). Detailed analysis showed that, compared with controls, the genes upregulated by ST1936 treatment were involved in regulation of cytoskeleton organization related to cell migration, such as focal adhesion, cell-substrate adhesion, organization of cell junction, organization of the extracellular matrix, regulation of protein serine/threonine kinase activity, and the transmembrane receptor protein and tyrosine kinase signaling pathways, underlining the determination of cell proliferation and cell fate (Figure S2A). The downregulated genes in ST1936-treated samples were enriched for processes relating to neuronal development, such as neuron differentiation and pattern specification processes (Figure S2B). Compared with controls, the upregulated gene population was also enriched, in response to SB271046 treatment, for processes relating to neuronal development, such as neuron differentiation and chemical synaptic transmission, which was reflective of observed changes in phenotypes (Figure S2C). Furthermore, the Kyoto Encyclopedia of Genes and Genomes (http://www.kegg.jp/) pathway-based gene set enrichment analysis revealed that the MAPK/PI3K-AKT//Notch/Gas-cAMP/Rab1 signaling pathway was involved in expressed gene sets (Figures 2F and 2G). Then, we performed a small screening of a kinase inhibitor with higher related signaling to distinguish the mediator. This did not dismiss the effect of ST1936/SB271046 under pretreatment with the MAPK/AKT/Notch pathway inhibitors Doramapimod, MK2206, or L685,458 (Figure 2H). These results suggest that MAPK/PIK3-AKT/Notch-mediated signaling is not involved in the effect of ST1936/SB271046 on human NSC proliferation. Our preliminary results revealed that the 5-HT6R agonist ST1936 promoted extracellular signal-regulated kinase (ERK) phosphorylation, but we found that the inhibitor of ERK also did not change the effect of ST1936 (Figure S2D). However, we found that the exchange protein directly activated by the cAMP (EPAC) inhibitor ESI-05 could block the effect of ST1936/SB271046. Also, GGT1298, the inhibitor of rab1 that was downstream of EPAC could also block the effect of ST1936/SB271046; but we noticed that GGT1298 itself causes growth inhibition of NSCs, which may be because it is a geranyl-geranyl-transferase I inhibitor that could induce apoptosis of many cell lines or reduce its proliferation by upregulation of senescence-related molecules (Dou et al., 2015; Ghavami et al., 2012). This indicated that EPAC-Rab1 signaling may contribute to the effect of ST1936/SB271046 on human NSCs, while this could be independent of classical cAMP-PKA signaling since the PKA inhibitor H89 did not abolish the effect of ST1936/SB271046 (Figure 2H). The results were confirmed by exogenous expression of a 5-HT6R-Gas-dead mutant, which abolished agonist-induced cAMP response (Harris et al., 2010). The expression of the mutant did not result in increased human self-renewal of NSCs (Figure S2E), suggesting that the effect of 5-HT6R on human NSCs is dependent on Gas-mediated cAMP signaling. The potential influence of 5-HT6R on the cAMP response element-binding protein (CREB) was then investigated. CREB is a known transcription factor and a common phosphorylation substrate for many kinase-mediated signaling pathways. The phosphorylated CREB could recruit the coactivators CBP and P300 to modulate histone acetylation for regulating the expression of genes related to proliferation and differentiation (Mantamadiotis et al., 2012; Teo and Kahn, 2010). We found that treatment with ST1936 or heterogeneous expression of 5-HT6R increased the phosphorylation of CREB (p-CREB), which was reduced by treatment with SB271046 or knockdown of 5-HT6R (Figures S2F and S2G). Furthermore, the inhibition of p-CREB levels by KG-501 or the knockdown of CREB could attenuate the effect of ST1936 and SB271046 on growth of human NSCs (Figures S2H–S2J). Furthermore, the cyclic adenosine monophosphate response element-binding protein (CBP), and the E1A-binding protein of P300 (EP300) inhibitors, PF-CBP1 and SGC-CBP300, could block the effect of ST1936 and SB271046 on growth of human NSCs (Figures S2K and S2L). Collectively, these results indicate that the constitutive activity of 5-HT6R and its activation mediate self-renewal of human NSCs through 5-HT6R coupling to Gas protein and modulate p-CREB to integrate higher signals. The CREB coactivators, CBP and P300, were recruited to generate a coordinated transcriptional response. For example, the enhanced expression of HES1, a key regulator of self-renewal and a downstream element of CREB signaling, was detected in the cells challenged by ST1936 or infected with 5-HT6R. HES1 expression was reduced by SB271046 or knockdown of 5-HT6R (Figure S2M). In summary, these data indicate that 5-HT6R mediates the effects on human NSC functions at least partially by modulating EPAC-Rab1-CREB signaling.
Figure 2.
5-HT6R Regulates Human NSC Proliferation through Modulating EPAC-CREB Signaling
(A) Heatmap and hierarchical clustering of genes with significance from RNA-seq analysis data. Samples of control, ST1936, and SB271046 at 10 μM were compared. In the heatmap, red indicates increased expression, whereas green stands for decreased expression as compared with that in the vehicle sample.
(B) A Venn diagram illustrating the overlaps among the expression changes identified in ST1936 and SB271046 compared with control.
(C and D) The volcano plots showing the gene expression changes for control versus ST1936 or control versus SB271046. The unregulated, unaltered, and downregulated genes are highlighted in light red, gray, and green, respectively. The numbers in the upper left corner indicate the number of genes with indicated expression changes.
(E) Gene ontology (GO) analysis of the unregulated and downregulated genes.
(F) The normalized enrichment scores (NES) for selected pathways that are significantly enriched following control versus ST1936 treatment.
(G) The NES for selected pathways that are significantly enriched following control versus SB271046 treatment.
(H) Exploring the potential target of ST1936/SB271046 on human NSC proliferation (n = 4, N = 1) (Doramapimod, 10 μM; L685.685, 5 μM; MK2206, 5 μM; H89, 5 μM; ESI-05, 10 μM; GGTI298, 5 μM). Data are means ± SEM. n.s, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared with the control of each group, two-way ANOVA, followed by Tukey's multiple comparisons test.
5-HT6R and Its Constitutive Activity Regulate the NSC Pool and Neurogenesis in Human Cerebral Organoids
To investigate the effect of 5-HT6R on human brain function, we adopted a three-dimensional (3D) culture system to generate organoids derived from human iPSCs (13A). We treated developing human cerebral organoids with ST1936 or SB271046 on day 7 before stem cell expansion. This coincided with the emergence of the neuroepithelial layer and transition from embryoid body to cerebral organoids. The ST1936-treated organoids were larger in size compared with vehicle-treated organoids over time. On the contrary, SB271046 treatment resulted in particularly poor growth in suspension over time (Figures 3A and S3A–S3D). Then we imaged the organoids under a light sheet fluorescence microscope to get a more stereoscopic outline of the organoids over 3 weeks. ST1936-treated organoids displayed markedly increased surface area and overall volume, and reduced sphericity compared with control-treated ones. However, the SB271046-treated organoids were smaller and smoother than the controls (Figures 3B–3E). Because the increased surface area was often organized in continuous folds, we further demonstrated a significant increase in fold density in ST1936-treated organoids, while the ventricular zone structure was barely observed in SB271046-treated organoids (Figures 3F, 3G, and S3E).
Figure 3.
5-HT6R and It Constitutive Activity Regulates the NSC Pool and Neurogenesis in Human Cerebral Organoids
(A) Representative bright-field images of control and ST1936/SB271046-treated human cerebral organoids at 21 days (n = 4, N = 4–8). Scale bars, 200 μm.
(B–E) Representative light sheet images of control and ST1936/SB271046-treated human cerebral organoids with corresponding reconstructed models and quantitation of the volume, surface area, and sphericity at 21 days (n = 3, N = 4–8). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 300 μm.
(F and G) Representative images (higher-magnification view) of human cerebral organoids with/without ST1936/SB271046 treatment using the stereological method, and quantitation of surface folds via canny edge detection (n = 3, N = 4–8). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 200 μm.
(H–J) Representative images of immunostaining for the SOX2+ NESTIN+ and KI67+ cells in control and ST1936/SB271046-treated human cerebral organoids at 21 days and the corresponding quantitation (n = 3, N = 4–8). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(K–M) Representative images of immunostaining for the SOX2+, PAX6+ and DCX+ cells in control and ST1936/SB271046-treated human cerebral organoids at 21 days and the corresponding quantitation (n = 3, N = 4–8). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(N) Representative images of immunostaining for the SOX2+/TUJ1+ of control and ST1936-treated human cerebral organoids at 35 days (n = 3, N = 4–8). Scale bars, 250 μm.
(O) Representative bright-field images of WT/5-HT6R-KO/KI (A266R) human cerebral organoids at 21 days (n = 3, N = 4–8). Scale bars, 200 μm.
The proliferation of NSCs has been reported to induce expansion and folding of cerebral organoids (Li et al., 2017b). We performed molecular anatomical staining on the cerebral organoids to determine the cellular organizations. Compared with controls, ST1936-treated human organoids harbored significantly more SOX2+ and NESTIN+ cells. In ST1936-treated human organoids, the increased proliferation coincided with an expansion of the NSC pool, as shown by a higher proportion of progenitors labeled with KI67+ with fewer DCX+-labeled cells (Figures 3H–3J). However, we found a significant reduction of SOX2+-, NESTIN+-, PAX6+-, and KI67+-labeled NSCs accompanied with a corresponding increase in DCX+ cortical neurons in SB271046-treated organoids (Figures 3K–3M and S3F). This premature phenotype was consistent with the results from the 2D culture system. In addition, ST1936 promoted NSCs proliferation and consequently produced more neurons (Figure 3N), which indicates that ST1936 may have potential to promote neurogenesis in vivo and could be used to treat the symptoms of depression. Furthermore, we treated the developing human organoids derived from the other iPSC cell line (3L) with ST1936 or SB2771046. The growth of cerebral organoids was tracked over 6 days after treatment. The growth of ST1936-treated cerebral organoids increased and the levels of KI67+ and SOX2+ cells were enhanced compared with the vehicle-treated ones. On the contrary, SB271046 treatment exhibited particularly poor growth in suspension and even abrogated growth in organoids that were invariably disintegrated before they could be analyzed (Figures S3G–S3I). These were therefore omitted from the subsequent assays.
To determine whether aberrant 5-HT6R function could lead to human brain defects in 3D culture, we performed RNAi using lentivirus with shRNAs to knock down endogenous 5-HT6R. We observed that sh 5-HT6R-infected organoids exhibited significantly attenuated growth relative to the control after infection (Figure S3J). Knockdown of 5-HT6R led to striking loss of SOX2+ progenitors and increase in TUJ1+ neurons (Figure S3K). These results were also reminiscent of the aforementioned observation of human NSCs in 2D culture, supporting the conclusion that loss of 5-HT6R leads to premature neural differentiation at the expense of progenitors. As a further independent approach, we generated 5-HT6R-knockout (KO)/KI (A266R) hiPSC clones using the CRISPR-Cas9 genome-editing system. The successful knockout or mutation of the 5-HT6R gene was validated by sequence analysis (Figures S3L and S3M). The 5-HT6R-KO clone resulted in a frameshift and led to premature generation of the stop codon. qRT-PCR confirmed the absence of 5-HT6R mRNA in mutant hiPSC clones (Figure S3N). Although 5-HT6R-KO/KI organoids displayed distinct brain-like regions, they were smaller in size and displayed a large number of neural axon structures outside the organoids (Figure 3O). To confirm phenotype specificity, we also generated cerebral organoids using mutant hiPSC clones that independently derived from another cell line (5-HT6R-KO-2) (Figure S3O). After 10 days of culture, the 5-HT6R-KO also resulted in smaller sizes with neural axon structures outside the organoids compared with the controls (Figures S3P and S3Q).
5-HT6R-KO/KI (A268R) Mice Shows Depression-like Behaviors and Impairs Adult Neurogenesis at the DG
To investigate the function of 5-HT6R in mice, we constructed a 5-HT6R-KO mouse line using the CRISPR-Cas9 technology to specifically delete exons 1–3 of 5-HT6R, which generated a disrupted 5-HT6R allele (5-HT6R-KO) (Figure S4A). The genotype of mice was detected by PCR (Figure S4B), and 5-HT6R deficiency was confirmed by measuring the 5-Ht6r transcript levels in the hippocampus (Figure S4C). The complete elimination of the 5-HT6R protein in the hippocampus was further verified (Figure S4D). In addition, we also generated a 5-HT6R-KI [5-HT6R-KI (A268R)] transgenic mouse line using the CRISPR-Cas9 genome-editing system. The guide RNAs directed to the 5-HT6R DNA loci were designed to mutate the amino acid alanine 268 (A268) into arginine 268 (R268), generating a previously reported mutation (Kohen et al., 2001) with reduced constitutive activity [5-HT6R-KI (A268R)] (Figure S4E). Although 5-HT6R deficiency did not cause embryo lethality, it led to numerically but not significantly reduced body weight of the mice (Figure S4F).
We measured the population of proliferating neural progenitor cells (NPCs) by KI67 and SOX2 staining. The number of SOX2+ KI67+ cells decreased in the DG of 5-HT6R-KO/KI (A268R) mice (Figures 4A and 4B). The GFAP-expressing radial glia-like progenitors are known to be the principal source of constitutive neurogenesis in postnatal adult mice and the depletion of the NPC pool in the brain can lead to adult neurogenesis defects (Wang et al., 2014). We found that, in the DG of 2-month-old mouse brain, the numbers of SOX2+ GFAP+ cells were also reduced in the DG of 5-HT6R-KO/KI (A268R) mice (Figures 4C and 4D). Furthermore, DCX staining was performed to label the immature granule neurons at the DG. Consistently, the number of DCX+ cells at the DG were slightly attenuated in the 2-month-old 5-HT6R-KO/KI (A268R) mice compared with their WT littermates (Figures 4E and 4F). Ultimately, the number of mature neurons represented by BrdU+ NeuN+ cells was also significantly reduced in the 5-HT6R-KO/KI (A268R) mice (Figures 4G and 4H). This indicates defective adult neurogenesis in mice with 5-HT6R dysfunction.
Figure 4.
5-HT6R-KO/KI (A268R) Mice Show Depression-like Behaviors and Impaired Adult NSC Neurogenesis at DG
(A and B) Representative images of immunostaining for SOX2+ and KI67+ cells at DG of WT and 5-HT6R-KO/KI (A268R) mice and subsequent quantitation (n = 3, N = 3–6). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 200 μm.
(C and D) Representative images of immunostaining for GFAP+ and SOX2+ cells at DG of WT and 5-HT6R-KO/KI (A268R) mice and subsequent quantitation (n = 3, N = 3–6). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(E and F) Representative images of immunostaining for DCX+ cells to examine immature neurons at DG of WT and 5-HT6R-KO/KI mice and subsequent quantitation (n = 3, N = 3–6). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(G and H) Representative images of immunostaining for BrdU+ and NeuN+ at DG of WT and 5-HT6R-KO/KI mice and subsequent quantitation (n = 3, N = 3–6). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test. Scale bars, 250 μm.
(I and J) Depression-like behavior measured by forced swimming of the 5-HT6R-KO/KI (A268R) mice (n = 2, N = 10–13 mice). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test.
(K and L) Depression-like behavior measured by tail suspension test of the 5-HT6R-KO/KI (A268R) mice (n = 2, N = 10–14 mice). Data are means ± SEM. ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett’s post-test.
In addition, we cultured NPCs derived from 5-HT6R-KO/KI (A268R) and WT mice and found that the 5-HT6R-deficient NSCs formed neurospheres with decreased number and diameter compared with the WT (Figure S4G). When the neurospheres were cultured in the medium containing low basic fibroblast growth factor, compared with the WT, the percentage of GFAP+ SOX2+ NPCs was significantly lower in the 5-HT6R-KO/KI (A268R) NPC, whereas the percentage of DCX+ increased concomitantly (Figure S4H). We also observed that the proliferation of NPCs derived from WT was disrupted when infected with the shRNA of 5-HT6R mice (Figures S4I and S 4J). All these data indicate that loss of 5-HT6R and its constitutive activity results in the premature differentiation of NPCs in vitro. However, we did not observe the obvious effects of ST1936/SB271046 in mouse NPCs, suggesting that it is specific to humans (Figure S4K).
Decreased DG neurogenesis has been implicated as a potential mechanism in the etiology of depression, anxiety, or learning memory (Yun et al., 2016). To test whether the 5-HT6R-KO/KI (A268R) mice had the aforementioned behavioral changes that were related to neurogenesis defects, we subjected 5-HT6R-KO mice to a battery of behavioral assessments. In the open-field test, 5-HT6R-KO mice showed no differences in the total distance traveled compared with WT mice during the test session (Figures S4L and S4M). These behavioral phenotypes are indications of a null status of anxiety and did not alter motor abilities. Moreover, loss of 5-HT6R also does not affect spatial recognition behaviors in these mice using the Morris water maze test (Figure S4N). We then examined the depression symptoms of the mice using the forced swimming test (FST) and tail suspension test (TST), which are two common tests used for evaluating depression-like behaviors in mice. In the FST, the total immobility time and the latency to immobility in the 5-HT6R-KO/KI (A268R) mice and their WT littermates were recorded. Results showed that the latency to immobility largely decreased in 5-HT6R-KO/KI (A268R) mice (Figure 4I), while the total immobility time was markedly increased in 5-HT6R-KO/KI (A268R) mice compared with their WT littermates (Figure 4J). These phenomena in 5-HT6R-KO mice were more apparent than those in 5-HT6R-KI (A268R) mice. These results indicate that the adult 5-HT6R-KO/KI (A268R) mice showed a lack of escape-related behaviors under normal conditions. To further confirm the results from the FST, the immobility time and the latency to immobility were compared between the 5-HT6R-KO/KI (A268R) mice and their WT littermates in the TST. The results consistently showed the 5-HT6R-KO/KI (A268R) mice had decreased latency to immobility and increased immobility time compared with their WT littermates (Figures 4K and 4L). Altogether, observations from behavioral studies indicate that 5-HT6R deficiency could cause depression-like behaviors in adulthood mice.
Discussion
To date, evidence has been accumulated toward understanding the significance of serotonin and its targeting receptors in the modulation of depression (Nautiyal and Hen, 2017; Zmudzka et al., 2018), whereas the role of each individual 5-HTR subtype on depression is ambiguous owing to the distinct models, the receptor distribution in different brain regions, receptor polymorphism, and the antagonistic effects of different receptors because of their distinct attributes (Banasr et al., 2004; Klempin et al., 2010; Tong et al., 2014). This study clearly shows that genetic and chemical modulation of one of the 5-HTRs with neurogenic significance, namely 5-HT6R, led to depression-like behaviors. Furthermore, these behaviors are likely correlated to the impaired neurogenesis, as shown in in vivo results from mice and in vitro results from human NSCs. This was strengthened by the abnormal formation of cerebral organoids by disrupting 5-HT6R or its constitutive activity. The results of ST1936 promoting proliferation of human NSCs is inspiring.
The expression of 5-HT6R in the human hippocampus during development was analyzed in the Allen BrainSpan Atlas, showing that 5-HT6R levels are increased with development, especially in adults with approximate incidence of depression. This indicates that modulating 5-HT6R could be valuable to combat depression in humans. However, we failed to observe the accordant effects of ST1936/SB271046 in mouse NPCs, which indicated that the effect of ST1936 might be specific for humans. Interspecies differences have been noted in the pharmacological profiles of the 5-HT6R ligands using radioligand binding, site-directed mutagenesis, and molecular modeling between rat and human species (Hirst et al., 2003). Also, a series of antagonists present different affinities toward the rat and mouse receptors due to differences in four critical amino acids located in the receptor-binding pocket (Karila et al., 2015). Moreover, several research groups have highlighted species differences between human and rodent dopamine D1 receptors (DRD1). For instance, the ligands were found to be active at the human DRD1 in HEK cells, but inactive at the rat DRD1 in both HEK cells and rat primary neurons. Under these circumstances, many pharmaceutical companies have developed a mouse line with the endogenous DRD1 replaced by the human receptor to assess the efficacy of the compounds (Felsing et al., 2019). So, the specific effect of ST1936 on human NSCs and neurogenesis in human cerebral organoids may indicate its potential therapeutic effects for depression or neurodegenerative disease in humans.
Receptor constitutive activity is an important characteristic and partially explains the role of each receptor under physiological and pathological conditions. Here several lines of evidence support a role of 5-HT6R constitutive activity in NSC functions: (1) 5-HT6R-KI mice with the same mutant displayed reduced NSC populations causally related to depression-like behaviors; (2) human NSCs with the 5-HT6R mutant (A266R) showed inefficient self-renewal; (3) 5-HT6R inverse agonists SB271046/SB742457 decreased the self-renewal of human NSCs thereby leading to a defect in cerebral organoid formation. Many works have found that SB271046/SB742457 has procognitive and antidepressant-like properties for cholinesterase inhibition/increased brain monoamine levels/regulating cilia function (Zajdel et al., 2016). In fact, we detected SB742457 on mice NPCs, and found that SB742457 shows slightly stronger inhibition effects (data not shown), which may not be a desirable effect (as noted in the aforementioned study). In that case, 5-HT6R inverse agonists originally designed to treat Alzheimer disease could have undesired effects on human NSC function, which might be one of the reasons for the recent failure of SB271046/SB742457 in a phase I/III clinical trial (Khoury et al., 2018). It has been reported that the activation of other 5-HTRs, such as 5-HT1AR, 5-HT2AR, 5-HT4R, and 5-HT7R, could promote NSCs in the DG (Samuels et al., 2016). Also, modulation of some dopamine receptors, such as DRD2, DRD3, and DRD4, also regulates NSCs in the SVZ (Dolma et al., 2016; Lao et al., 2013). Interestingly, all of those receptors harbor different degrees of constitutive activity (Cotecchia, 2007; Seifert and Wenzel-Seifert, 2002; Zhang et al., 2014), and their potential function in neurogenesis is worthy of further study.
A better understanding of receptor and receptor signaling responsible for the effect of serotonin on neurogenesis may help in the development of novel and more targeted drugs (Kishi et al., 2010; Marazziti et al., 2013a, 2013b). In particular, studies that were performed using human cell models might be more conducive to the clinical transformation under some circumstances. Several studies using patient-derived iPSCs have uncovered some disease- and gene-associated cellular phenotypes, such as progenitor cell proliferation, morphological maturation, cell differentiation, and neuronal activity. For instance, schizophrenia (SZ) patient-derived iPSCs/NPCs show abnormal gene and protein expression that are related to cytoskeletal remodeling and oxidative stress (Brennand et al., 2015). The SZ patient-derived hiPSCs showed altered expressions of some cellular adhesion genes when differentiated into forebrain NPCs, which presented reduced WNT signaling and aberrant cellular migration (Breen et al., 2020). The hiPSCs derived from patients with treatment-resistant depression have altered expression of serotonergic receptors in serotonergic neurons. Also, pharmacological blockade of 5-HT2 and 5-HT7 receptors rescues 5-HT-induced hyperactivity in non-remitter patient neurons (Vadodaria et al., 2019). These all indicate that the detailed study of each serotonin receptor on human cell models may unlock some of the elusive answers, which would be beneficial for precise, individualized therapy.
CREB is a key transcriptional factor with diverse functions and is a central regulator of cellular growth and development (Kim et al., 2010; Kumar and Singh, 2017). This study shows that modulation of 5-HT6R could change the p-CREB levels and in turn alter CREB-dependent transcription, such as HES1 and so on. These observations show that knocking down the CREB or inhibiting CREB activation weakened the effect of 5-HT6R ligands. All these data support the notion that the EPAC-CREB pathway is one of the major underlying mechanisms for 5-HT6R effects. In addition to CREB, other nuclear protein components may also contribute to the recruitment of CBP and P300 or other modifications to target promoter regions, which could provide a molecular basis for gene-specific transcriptional regulation by 5-HT6R. On the other hand, there may be other collaborative pathways involved. 5-HT4R is one of the Gas subfamily of serotonin receptors, and we noted it also has slightly higher expression than others, but we found that it has no effect on human NSC proliferation when we treated human NSCs with the agonist (data not shown). This suggests that other synergistic and elaborate signaling pathways mediated by 5-HT6R need to be further elucidated.
Experimental Procedures
Animals
The original 5-HT6R-KO/KI (A268R) mice and their WT mates were generated using a CRISPR-Cas9 genome-editing system in AG-haESCs to generate semi-cloned embryos. ICAHCI and embryo transferring were performed as described previously (Zhong et al., 2015). They were then crossed to generate the heterozygous mice. Then, heterozygous male and female mice were mated to generate the homozygous mice, and PCR screening was performed for genotyping. Littermates were used as controls. For behavioral studies, at least 10 pairs of gender-matched mice were tested. All animal procedures were performed under the ethical guidelines of the Shanghai Institute of Biochemistry and Cell Biology. A detailed description is provided in the Supplemental Experimental Procedures.
Statistical Analysis
All experiments were repeated at least three times. Data are representative or means ± SEM. All data were analyzed using Prism 6.0 (GraphPad Software, San Diego, CA). Concentration-response curves were analyzed using a three-parameter non-linear regression analysis. Unpaired Student's t test was applied for comparisons of two datasets. One-way or two-way analysis of variance with Tukey's multiple comparisons test for multiple comparisons, or Dennett's post-test to compare each group with a single control group, were used where more than two groups were compared. N refers to the number of individual samples and n refers to the number of individual experiments. Statistical significance was accepted at p < 0.05.
Data and Code Availability
The Gene Expression Omnibus accession number for the RNA-seq dataset in this study is GSE158248. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
Q.W. designed and performed most of the experiments and data analysis. C.Q. prepared the 5-HT6R-KO/KI (A268R) mice generation. X.D. and T.H. were responsible for identification and staining of mice. Q.W. and T.H. were responsible for the TST and FST of mice. Q.W. and Y.Z. performed the open field and Morris water maze tests of mice. G.P. designed and supervised the project. Q.W., Jing L., Jinsong L., and G.P. wrote the manuscript. All the authors reviewed and commented on the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Dr. Jian Zhao (ShanghaiTech University) for the cell lines. We are grateful to Fengming Liu, Yao Li, Yanke Wang, and Yang Yu of the Integrated Laser Microscopy System and Dr. Chao Peng and Zhihong Li of the Mass Spectrometry System at the National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab, SARI, China for data collection and analysis. We thank Dr. Yujie Chen and NianSu Cheng from Uli Schwarz public laboratory platform in PICB for light sheet microscope technical support. We appreciate all the lab members for sharing reagents and advice. This research was supported by the Program of the "Strategic Priority Research Program" of the Chinese Academy of Sciences (XDA16010309), the National Key Research and Development Program of China (2018YFA0108003), the National Science Foundation for Young Scientists of China (31701240), the National Key Research and Development Program of China Stem Cell and Translational Research (2016YFA0101200, 2016YFA0101202).
Published: December 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.11.015.
Supplemental Information
References
- Alenina N., Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav. Brain Res. 2015;277:49–57. doi: 10.1016/j.bbr.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Banasr M., Hery M., Printemps R., Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Benninghoff J., Gritti A., Rizzi M., Lamorte G., Schloesser R.J., Schmitt A., Robel S., Genius J., Moessner R., Riederer P. Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology. 2010;35:893–903. doi: 10.1038/npp.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K.A., Clarke W.P. Making sense of pharmacology: inverse agonism and functional selectivity. Int. J. Neuropsychopharmacol. 2018;21:962–977. doi: 10.1093/ijnp/pyy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Hen R., Underwood M.D., Rosoklija G.B., Dwork A.J., Mann J.J., Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol. Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M.S., Browne A., Hoffman G.E., Stathopoulos S., Brennand K., Buxbaum J.D., Drapeau E. Transcriptional signatures of participant-derived neural progenitor cells and neurons implicate altered Wnt signaling in Phelan-McDermid syndrome and autism. Mol. Autism. 2020;11:53. doi: 10.1186/s13229-020-00355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K., Savas J.N., Kim Y., Tran N., Simone A., Hashimoto-Torii K., Beaumont K.G., Kim H.J., Topol A., Ladran I. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol. Psychiatry. 2015;20:361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun J.M., Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Carr G.V., Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl) 2011;213:265–287. doi: 10.1007/s00213-010-2097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Scott A.L., Ladenheim B., Chen K., Ouyang X., Lathia J.D., Mughal M., Cadet J.L., Mattson M.P., Shih J.C. Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development. J. Neurosci. 2010;30:10752–10762. doi: 10.1523/JNEUROSCI.2037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe C.L., Kramer M., Czeh B., Gould E., Reeves A.J., Kirschbaum C., Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Cotecchia S. Constitutive activity and inverse agonism at the alpha1 adrenoceptors. Biochem. Pharmacol. 2007;73:1076–1083. doi: 10.1016/j.bcp.2006.10.024. [DOI] [PubMed] [Google Scholar]
- David D.J., Gardier A.M. [The pharmacological basis of the serotonin system: application to antidepressant response] Encephale. 2016;42:255–263. doi: 10.1016/j.encep.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Deraredj Nadim W., Chaumont-Dubel S., Madouri F., Cobret L., De Tauzia M.L., Zajdel P., Benedetti H., Marin P., Morisset-Lopez S. Physical interaction between neurofibromin and serotonin 5-HT6 receptor promotes receptor constitutive activity. Proc. Natl. Acad. Sci. U S A. 2016;113:12310–12315. doi: 10.1073/pnas.1600914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavadian R.L. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol. Exp. (Wars) 2004;64:189–200. doi: 10.55782/ane-2004-1505. [DOI] [PubMed] [Google Scholar]
- Dolma S., Selvadurai H.J., Lan X., Lee L., Kushida M., Voisin V., Whetstone H., So M., Aviv T., Park N. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29:859–873. doi: 10.1016/j.ccell.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X., Wei J., Sun A., Shao G., Childress C., Yang W., Lin Q. PBK/TOPK mediates geranylgeranylation signaling for breast cancer cell proliferation. Cancer Cell Int. 2015;15:27. doi: 10.1186/s12935-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F., Deleris P., Raynaud F., Seveno M., Morisset-Lopez S., Mannoury la Cour C., Millan M.J., Bockaert J., Marin P., Chaumont-Dubel S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat. Chem. Biol. 2014;10:590–597. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- Eisch A.J., Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsing D.E., Jain M.K., Allen J.A. Advances in dopamine D1 receptor ligands for neurotherapeutics. Curr. Top. Med. Chem. 2019;19:1365–1380. doi: 10.2174/1568026619666190712210903. [DOI] [PubMed] [Google Scholar]
- Fukami M., Suzuki E., Igarashi M., Miyado M., Ogata T. Gain-of-function mutations in G-protein-coupled receptor genes associated with human endocrine disorders. Clin. Endocrinol. (Oxf) 2018;88:351–359. doi: 10.1111/cen.13496. [DOI] [PubMed] [Google Scholar]
- Ghavami S., Mutawe M.M., Schaafsma D., Yeganeh B., Unruh H., Klonisch T., Halayko A.J. Geranylgeranyl transferase 1 modulates autophagy and apoptosis in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L420–L428. doi: 10.1152/ajplung.00312.2011. [DOI] [PubMed] [Google Scholar]
- Goncalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Harris R.N.,, 3rd, Stabler R.S., Repke D.B., Kress J.M., Walker K.A., Martin R.S., Brothers J.M., Ilnicka M., Lee S.W., Mirzadegan T. Highly potent, non-basic 5-HT6 ligands. Site mutagenesis evidence for a second binding mode at 5-HT6 for antagonism. Bioorg. Med. Chem. Lett. 2010;20:3436–3440. doi: 10.1016/j.bmcl.2010.03.110. [DOI] [PubMed] [Google Scholar]
- Harrod A., Fulton J., Nguyen V.T.M., Periyasamy M., Ramos-Garcia L., Lai C.F., Metodieva G., de Giorgio A., Williams R.L., Santos D.B. Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene. 2017;36:2286–2296. doi: 10.1038/onc.2016.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst W.D., Abrahamsen B., Blaney F.E., Calver A.R., Aloj L., Price G.W., Medhurst A.D. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol. Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Jacobshagen M., Niquille M., Chaumont-Dubel S., Marin P., Dayer A. The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development. 2014;141:3370–3377. doi: 10.1242/dev.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska-Wiesek M., Siwek A., Partyka A., Szewczyk B., Sowa-Kucma M., Wasik A., Kolaczkowski M., Wesolowska A. Antidepressant-like activity of EMD 386088, a 5-HT6 receptor partial agonist, following systemic acute and chronic administration to rats. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388:1079–1088. doi: 10.1007/s00210-015-1141-2. [DOI] [PubMed] [Google Scholar]
- Karila D., Freret T., Bouet V., Boulouard M., Dallemagne P., Rochais C. Therapeutic potential of 5-HT6 receptor agonists. J. Med. Chem. 2015;58:7901–7912. doi: 10.1021/acs.jmedchem.5b00179. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury R., Grysman N., Gold J., Patel K., Grossberg G.T. The role of 5 HT6-receptor antagonists in Alzheimer's disease: an update. Expert Opin. Investig. Drugs. 2018;27:523–533. doi: 10.1080/13543784.2018.1483334. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Yang M., Cho J., Kim S.H., Kim J.C., Shin T., Moon C. Promotion of cAMP responsive element-binding protein activity ameliorates radiation-induced suppression of hippocampal neurogenesis in adult mice. Toxicol. Res. 2010;26:177–183. doi: 10.5487/TR.2010.26.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.K., Ham B.J., Han K.M. Interactive effects of genetic polymorphisms and childhood adversity on brain morphologic changes in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;91:4–13. doi: 10.1016/j.pnpbp.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Kishi T., Fukuo Y., Yoshimura R., Okochi T., Kitajima T., Naitoh H., Umene-Nakano W., Nakamura J., Ozaki N., Iwata N. Pharmacogenetic study of serotonin 6 receptor gene with antidepressant response in major depressive disorder in the Japanese population. Hum. Psychopharmacol. 2010;25:481–486. doi: 10.1002/hup.1142. [DOI] [PubMed] [Google Scholar]
- Kiyasova V., Gaspar P. Development of raphe serotonin neurons from specification to guidance. Eur. J. Neurosci. 2011;34:1553–1562. doi: 10.1111/j.1460-9568.2011.07910.x. [DOI] [PubMed] [Google Scholar]
- Klempin F., Babu H., De Pietri Tonelli D., Alarcon E., Fabel K., Kempermann G. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front. Mol. Neurosci. 2010;3:14. doi: 10.3389/fnmol.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R., Fashingbauer L.A., Heidmann D.E., Guthrie C.R., Hamblin M.W. Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Brain Res. Mol. Brain Res. 2001;90:110–117. doi: 10.1016/s0169-328x(01)00090-0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Koyama Y., Nakamura Y., Shimada S. A novel 5HT3 receptor-IGF1 mechanism distinct from SSRI-induced antidepressant effects. Mol. Psychiatry. 2018;23:833–842. doi: 10.1038/mp.2017.87. [DOI] [PubMed] [Google Scholar]
- Kronmuller K.T., Pantel J., Kohler S., Victor D., Giesel F., Magnotta V.A., Mundt C., Essig M., Schroder J. Hippocampal volume and 2-year outcome in depression. Br. J. Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh N. Inhibitor of phosphodiesterase-4 improves memory deficits, oxidative stress, neuroinflammation and neuropathological alterations in mouse models of dementia of Alzheimer's type. Biomed. Pharmacother. 2017;88:698–707. doi: 10.1016/j.biopha.2017.01.059. [DOI] [PubMed] [Google Scholar]
- Lao C.L., Lu C.S., Chen J.C. Dopamine D3 receptor activation promotes neural stem/progenitor cell proliferation through AKT and ERK1/2 pathways and expands type-B and -C cells in adult subventricular zone. Glia. 2013;61:475–489. doi: 10.1002/glia.22449. [DOI] [PubMed] [Google Scholar]
- Li X., Wang Q., Hu T., Wang Y., Zhao J., Lu J., Pei G. A tricyclic antidepressant, amoxapine, reduces amyloid-beta generation through multiple serotonin receptor 6-mediated targets. Sci. Rep. 2017;7:4983. doi: 10.1038/s41598-017-04144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Muffat J., Omer A., Bosch I., Lancaster M.A., Sur M., Gehrke L., Knoblich J.A., Jaenisch R. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell. 2017;20:385–396 e383. doi: 10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Liu F. The role of serotonin beyond the central nervous system during embryogenesis. Front. Cell. Neurosci. 2017;11:74. doi: 10.3389/fncel.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T., Papalexis N., Dworkin S. CREB signalling in neural stem/progenitor cells: recent developments and the implications for brain tumour biology. Bioessays. 2012;34:293–300. doi: 10.1002/bies.201100133. [DOI] [PubMed] [Google Scholar]
- Marazziti D., Baroni S., Borsini F., Picchetti M., Vatteroni E., Falaschi V., Catena-Dell'Osso M. Serotonin receptors of type 6 (5-HT6): from neuroscience to clinical pharmacology. Curr. Med. Chem. 2013;20:371–377. [PubMed] [Google Scholar]
- Marazziti D., Baroni S., Pirone A., Giannaccini G., Betti L., Testa G., Schmid L., Palego L., Borsini F., Bordi F. Serotonin receptor of type 6 (5-HT6) in human prefrontal cortex and hippocampus post-mortem: an immunohistochemical and immunofluorescence study. Neurochem. Int. 2013;62:182–188. doi: 10.1016/j.neuint.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal K.M., Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi: 10.12688/f1000research.9736.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A., Kos T., Wesolowska A. The 5-HT6 receptor agonist EMD 386088 produces antidepressant and anxiolytic effects in rats after intrahippocampal administration. Psychopharmacology (Berl) 2011;217:411–418. doi: 10.1007/s00213-011-2297-1. [DOI] [PubMed] [Google Scholar]
- Pitsillou E., Bresnehan S.M., Kagarakis E.A., Wijoyo S.J., Liang J., Hung A., Karagiannis T.C. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020;47:753–770. doi: 10.1007/s11033-019-05129-3. [DOI] [PubMed] [Google Scholar]
- Purohit A., Herrick-Davis K., Teitler M. Creation, expression, and characterization of a constitutively active mutant of the human serotonin 5-HT6 receptor. Synapse. 2003;47:218–224. doi: 10.1002/syn.10157. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B.A., Mendez-David I., Faye C., David S.A., Pierz K.A., Gardier A.M., Hen R., David D.J. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist. 2016;22:26–45. doi: 10.1177/1073858414561303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J., Makita N., Iiri T. Inverse agonism: the classic concept of GPCRs revisited [Review] Endocr. J. 2016;63:507–514. doi: 10.1507/endocrj.EJ16-0084. [DOI] [PubMed] [Google Scholar]
- Seifert R., Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Shen C.J., Zheng D., Li K.X., Yang J.M., Pan H.Q., Yu X.D., Fu J.Y., Zhu Y., Sun Q.X., Tang M.Y. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 2019;25:337–349. doi: 10.1038/s41591-018-0299-9. [DOI] [PubMed] [Google Scholar]
- Teo J.L., Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Adv. Drug Deliv. Rev. 2010;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Tong C.K., Chen J., Cebrian-Silla A., Mirzadeh Z., Obernier K., Guinto C.D., Tecott L.H., Garcia-Verdugo J.M., Kriegstein A., Alvarez-Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14:500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadodaria K.C., Ji Y., Skime M., Paquola A., Nelson T., Hall-Flavin D., Fredlender C., Heard K.J., Deng Y., Le A.T. Serotonin-induced hyperactivity in SSRI-resistant major depressive disorder patient-derived neurons. Mol. Psychiatry. 2019;24:795–807. doi: 10.1038/s41380-019-0363-y. [DOI] [PubMed] [Google Scholar]
- Wang J., Cheng A., Wakade C., Yu R.K. Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J. Neurosci. 2014;34:13790–13800. doi: 10.1523/JNEUROSCI.2275-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda N., Akazawa H., Ito K., Shimizu I., Kudo-Sakamoto Y., Yabumoto C., Yano M., Yamamoto R., Ozasa Y., Minamino T. Agonist-independent constitutive activity of angiotensin II receptor promotes cardiac remodeling in mice. Hypertension. 2012;59:627–633. doi: 10.1161/HYPERTENSIONAHA.111.175208. [DOI] [PubMed] [Google Scholar]
- Yun S., Reynolds R.P., Masiulis I., Eisch A.J. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 2016;22:1239–1247. doi: 10.1038/nm.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel P., Marciniec K., Satala G., Canale V., Kos T., Partyka A., Jastrzebska-Wiesek M., Wesolowska A., Basinska-Ziobron A., Wojcikowski J. N1-Azinylsulfonyl-1H-indoles: 5-HT6 receptor antagonists with procognitive and antidepressant-like properties. ACS Med. Chem. Lett. 2016;7:618–622. doi: 10.1021/acsmedchemlett.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Albaker A., Plouffe B., Lefebvre C., Tiberi M. Constitutive activities and inverse agonism in dopamine receptors. Adv. Pharmacol. 2014;70:175–214. doi: 10.1016/B978-0-12-417197-8.00007-9. [DOI] [PubMed] [Google Scholar]
- Zhang J., Shen C.P., Xiao J.C., Lanza T.J., Lin L.S., Francis B.E., Fong T.M., Chen R.Z. Effects of mutations at conserved TM II residues on ligand binding and activation of mouse 5-HT6 receptor. Eur. J. Pharmacol. 2006;534:77–82. doi: 10.1016/j.ejphar.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhong C., Yin Q., Xie Z., Bai M., Dong R., Tang W., Xing Y.H., Zhang H., Yang S., Chen L.L. CRISPR-Cas9-Mediated genetic screening in mice with haploid embryonic stem cells carrying a guide RNA library. Cell Stem Cell. 2015;17:221–232. doi: 10.1016/j.stem.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Zmudzka E., Salaciak K., Sapa J., Pytka K. Serotonin receptors in depression and anxiety: insights from animal studies. Life Sci. 2018;210:106–124. doi: 10.1016/j.lfs.2018.08.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Gene Expression Omnibus accession number for the RNA-seq dataset in this study is GSE158248. The data that support the findings of this study are available from the corresponding author upon reasonable request.






