Highlights
-
•
Splenic abscess (SA) is an uncommon and life-threatening disease.
-
•
Spontaneous rupture of a SA with peritonitis is a rare occurrence.
-
•
Preoperative diagnosis of ruptured SA is a challenge due to non-specific clinical presentation.
-
•
Nowadays there are no guidelines for the management of SA.
-
•
Splenectomy represents the treatment of choice along with antibiotics in ruptured SA.
Keywords: Non-typhoid Salmonella, Splenic abscess, Peritonitis, Ruptured splenic abscess, Splenectomy, Case report
Abstract
Introduction and importance
Splenic abscess (SA) is an uncommon, life-threatening disease with about 600 reported cases in the literature. It is caused by various infective pathogens and generally occurs in immunocompromised patients. SA is a rare complication of non-typhoid Salmonella (NTS) infection. Diagnosis of ruptured SA is a challenge because the absence of specific symptoms and signs. Abdominal computed tomography (CT) scan represents the gold standard in diagnosing of SA. Splenectomy is the treatment of choice of ruptured SA with peritonitis.
Case presentation
A 26-year-old Caucasian female was admitted to the Emergency Department with a three-day history of abdominal pain and fever. Physical examination revealed severe and generalized abdominal pain on superficial and deep palpation with obvious muscle guarding and rebound tenderness. Abdominal CT scan showed ruptured SA. Laboratory tests reported anemia (hemoglobin 10.4 g/dl). The patient was taken emergently to the operating room for splenectomy. The postoperative course was uneventful, the patient was discharged on the 7th post-operative day. Diagnosis of NTS SA was made by pus cultures.
Clinical discussion
SA is a rare complication of NTS infection associated with high morbidity and mortality rates. Although different types of treatment of SA are reported in the literature, splenectomy represents the treatment of choice of ruptured SA.
Conclusion
NTS SA is difficult to diagnose because of its rarity and non-specific clinical presentation, often fatal if left untreated. Although there is no gold standard for treating SA, splenectomy with peritoneal lavage is mandatory in case of ruptured SA with peritonitis.
1. Introduction
Splenic abscess (SA) is an uncommon, life-threatening disease with about 600 reported cases in literature and an incidence rate of 0.14%–0.7% in autopsy series [1]. It is caused by various infective pathogens and generally occurs in males patients with underlying comorbidities (neoplasia, immunodeficiency, trauma, metastatic infection, splenic infarct or diabetes) [2]. Diagnosis of ruptured SA is a challenge because of its rarity and the absence of specific symptoms and signs [3]. Abdominal CT scan represents the gold standard in diagnosing of SA [4]. Although there are no guidelines for the management of SA, splenectomy with peritoneal lavage is mandatory in case of ruptured SA with peritonitis. Mortality rate of SA is high ranging from 0% to 47%, due to the frequent delayed diagnosis, the possible complications (rupture in this case) and the comorbidities affecting patients [1]. A case of a young patient with peritonitis caused by non-traumatic rupture of a unknown non-typhoid Salmonella SA is presented with review of the literature in accordance with SCARE 2020 criteria [5]. The purpose of this case report is to remember that ruptured SA is a rare, life-threatening disease causing acute abdomen that requires urgent diagnosis and treatment.
2. Presentation of case
A 26-year-old Caucasian female was admitted to the Emergency Department with a three-day history of abdominal pain and fever. She was feverish, pale, hypotensive and tachycardic. Vital signs were blood pressure 90/40 mm Hg, pulse 125 beats per minute, respiratory rate 24 per minute, oxygen saturation 96% in ambient air, and fever (39 °C). The patient wasn’t taking any drugs or smoking, her past and familial medical histories were normal. She was employed by profession, unmarried, of low socio-economic status. Physical examination revealed severe and generalized abdominal pain on superficial and deep palpation with obvious muscle guarding and rebound tenderness. The patient was initially managed conservatively, with intravenous broad-spectrum antibiotics, antipyretic drugs, bowel rest, fluids and supportive care. She was promptly evaluated by contrast-enhanced abdominal CT scan that showed splenomegaly with a well defined hypodense peripherally enhancing lesion located in the upper and middle part of the spleen (measuring 9cm × 8cm × 8 cm) with free intra-abdominal fluid, interpreted as a voluminous ruptured splenic abscess (Fig. 1). Laboratory tests reported anemia (hemoglobin 10.4 g/dl), neutrophilic leukocytosis (WBC 15.900 103/μL), platelet count of 125000/mm3 (reference range 150000–450000/mm3), C-reactive protein level of 283.19 mg/L (reference range <7.5 mg/L), prothrombin activity 60% (reference range 80–120%) and INR 1.43 (reference range 0.95–1.15). The patient, after understanding the severity of her medical condition and accepting surgery, was taken emergently to the operating room by experienced general surgeons (the first two authors) for exploratory laparotomy, evacuation of intra-abdominal free fluid and splenectomy under general anesthesia. The patient was placed in the supine position on the operating table: intraoperatively the upper pole of the spleen was found to be ruptured with about 800 mL of frank pus (Fig. 2) in the peritoneal cavity sent for culture and drained. Splenectomy along with peritoneal lavage was performed and left paracolic and pelvis drainage were placed. Widal-Wright test and echocardiogram were negative respectively for Salmonella typhi and infective endocarditis. Pus culture from SA was positive for Salmonella species sensitive to cephalosporins, fluorchinolones and carbapenems. Patient was given an IV injection of Ciproxin 400 mg twice daily for 5 days and Meropenem 1 gm 3 times daily for 5 days. The postoperative course was uneventful: abdominal drains were removed on the 5th postoperative day, postoperative laboratory tests were unremarkable. The patient received pneumococcal, meningococcal and Hemophilus vaccination, was discharged on the 7th postoperative day in a stable condition and referred to Infectious Disease Department. The splenectomy specimen (Fig. 3 a,b), sent for histopathology evaluation, measured 15 × 7 × 5 cm, weighed 220 g and contained a large (9 × 8 cm), abscessed and ruptured cyst. On microscopic examination the splenic parenchyma showed complete effacement of splenic architecture with sinus dilatation, presence of macro and microcysts (Fig. 4), granulocyte inflammatory cells and subcapsular microabscesses (Fig. 5). The patient tolered the advice provided and after a follow-up of six months is asymptomatic.
Fig. 1.
Abdominal CT scan showing a voluminous ruptured splenic abscess.
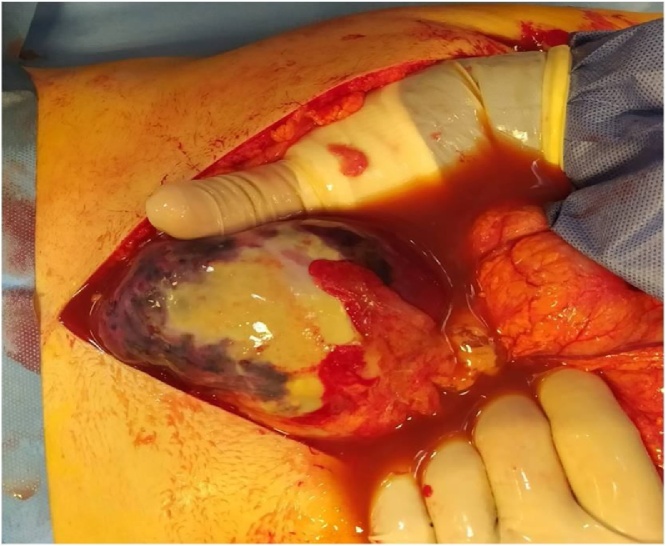
Fig. 2.
Voluminous ruptured splenic abscess with frank pus in the peritoneal cavity: operative findings.
Fig. 3.
(a,b). The ruptured abscess visualized at the upper pole of the surgically removed spleen.
Fig. 4.
Photomicrograph section of abscessed splenic cyst (haematoxylin and eosin, original magnification × 40).
Fig. 5.
Photomicrograph section of subcapsular splenic inflammation (haematoxylin and eosin, original magnification × 20).
3. Discussion
This clinical case describes the spontaneous rupture of a voluminous non-typhoid Salmonella (NTS) SA presenting with peritonitis. SA is an uncommon disease that occurs more often in young, males and immunocompromised patients [6], caused by various infective pathogens ranging from bacteria, protozoa, parasites to fungus [7]. Although the reported incidence of SA in autopsy series varies between 0.14% and 0.7% [1], it appears to be increasing in frequency because of the growing number of immunologically compromised patients [8]. SA may be monomicrobial, polymicrobial or sterile and usually presents as solitary and unilocular lesion with diameters ranging from 1 to 18 cm [9]. SA is usually a complication of bacteremia resulting from metastatic (e.g. endocarditis) or contiguous infection (e.g. intestinal perforation) or represents a superinfection of cysts, pseudocysts or hematomas [10]. In more than 50% of cases Gram-positive cocci and enterobacteriacee (e.g. Salmonella) are the culprits. Anaerobes are also important causes of SA. Fungi, Protozoa, Actinomycetes and Mycobacteria have also been isolated from SA [6,8]. Bacterial pathogens usually cause unilocular abcesses. Mycobacterial, fungal or protozoal infections are most frequently seen in immunocompromised patients. Various studied indicate prior splenic insult in addition to bacteremia as a prerequirement for SA to occur [8]. About half of patients with SA have predisposing factors like as preexisting anatomic abnormalities (splenic hematoma, cysts, pseudocysts, post-traumatic lesion) or immunocompromised status (malignancies, hematologic disorders, drug abuse, cancer, AIDS, chemotherapy, transplantation) [10]. SA is a very rare complication NTS infection that usually gives rise to a self-limited gastroenteritis: approximately 5% of individuals with NTS infection will develop bacteremia with possible suppurative infections of almost any organ [11]. Salmonella can cause endocarditis, pericarditis, arterial infection, abdominal infection, soft tissue infection, urinary tract infection, genital infection, pneumonia, empyema, meningitis, septic arthritis and osteomyelitis [12]. Salmonella has been isolated in 11–15% of patients with SA [13]. Invasive NTS is rare in industrialized parts of the world with an incidence of 1.02/100,000 population [13]. Symptoms and signs of SA are non specific: the most common are high-grade fever, left upper abdominal pain and splenomegaly [14]. Additionally these patients may present with vomiting, nausea, shoulder pain, pleuritic chest pain, left upper quadrant mass and pleural effusiony [2]. In our clinical case the patient presented with high-grade fever, generalized abdominal pain and peritonitis. Although traumatic rupture of spleen is more common [15], spontaneous rupture of SA is uncommon and it must be suspected in case of acute abdomen and signs of haemodynamic compromise in a background of sepsis, malignancy haematological disorders, acute viral infection and immunosuppression. In our clinical case there was no suspicion of splenic rupture due to the lack of known risk factors and non-typhoid Salmonella SA appears to be a sequelae of bacteremic episode in a immunocompetent patient with numerous splenic malformative cysts. CT scan and microbiological study of blood and aspirated components are useful diagnostic modalities to obtain the diagnosis. CT scan represents the diagnostic modality of choice to establish the presence of SA or associated rupture in the peritoneal cavity, with a higher sensitivity (96%) than ultrasonography (75–90%) for the detection of abdominal masses [16]. Blood cultures are positive in 24–80% of cases, however bacterial growth culture of abscess is more efficient (50–80%) [6]. It is important to have cultures from both blood and pus, since the organism only matches in 24% of cases [16]. In our clinical case abdominal CT scan, performed in emergency, diagnosed ruptured SA but only pus cultures were obtained intraoperatively. SA must be differentiated from other splenic lesions such as primitive lymphoma, metastasis, infarction or hematoma [17]. Early diagnosis of SA is valuable to decrease the morbility and mortality. The management of unruptured SA is controversial and based on medical therapy with broad-spectrum antibiotics and splenectomy or percutaneous drainage under imaging with good results. Splenectomy is still the most accepted treatment of a SA with a mortality rates of 0–16.9% and morbidity rates of 28–43% [18] and represents the treatment of choice along with appropriate antibiotics in case of ruptured SA with peritonitis. In our clinical case splenectomy was performed as life-saving treatment for the patient that had no complications after surgery.
4. Conclusion
SA is a rare complication of NTS infection, difficult to diagnose and often fatal if left untreated. It is a uncommon disease that remains mainly a subject of case reports. About 600 cases of SA are reported in the literature and the largest series is that of Chang et al. with 67 cases of SA observed over a period of 19 years. Abdominal CT scan and microbiological study of blood and aspirated components of SA are useful diagnostic modalities to establish the correct diagnosis. Due to non-specific clinical presentation, ruptured SA remains a diagnostic challenge. As there is no gold standard for treatment of SA due to the lack of randomized trials, optimal treatment must be tailored to each patient. Splenectomy with peritoneal lavage is mandatory in case of ruptured SA with peritonitis.
Declaration of Competing Interest
All the authors certify that there is no conflict of interest regarding the material discussed in the manuscript.
Funding
All the authors declare that this research didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval has been exempted by our institution because this is a case report and no new studies or new techniques were carried out.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for the Editor-in-Chief of this journal on request.
Author contribution
Giuseppe Evola: Operated on the patient, drafting the manuscript, literature research.
Enrico Piazzese: Drafting the manuscript and literature research.
Roberto Cantella: Drafting the manuscript and literature research.
Marianna Iudica: Drafting the manuscript and literature research.
Gastone Veroux: Drafting the manuscript and literature research.
Salvatore Sarvà: Drafting the manuscript and literature research.
Registration of research studies
Not Applicable.
Guarantor
The guarantor for this case report is Giuseppe Evola.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.To K.B., Washer L.L., Varban O.A. Splenectomy for splenic abscess. Surg. Infect. 2013;14(May (3)):337–338. doi: 10.1089/sur.2012.073. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N., Sharma A., Garg G. Non-traumatic ruptured splenic abscess presenting with pneumoperitoneum in an immunocompetent patient: a diagnostic dilemma. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-228961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George P., Ahmed A., Maroli R. Peritonitis secondary to ruptured splenic abscess: a grave complication of typhoid fever. Asian Pac. J. Trop. Med. 2012;5(12):1004–1006. doi: 10.1016/s1995-7645(12)60191-6. [DOI] [PubMed] [Google Scholar]
- 4.Khan F.Y. Typhoid splenic abscess: a case report and literature review. Int. J. Infect. 2019;6(1) [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84 doi: 10.1016/j.ijsu.2020.10.034. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Davido B., Dinh A., Rouveix E. Abcès de la rate: du diagnostic au traitement. Rev. Med. Interne. 2017;38(September (9):614–618. doi: 10.1016/j.revmed.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Chang K.C., Chuah S.K., Changchien C.S. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J. Gastroenterol. 2006;12(January (3)):460–464. doi: 10.3748/wjg.v12.i3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duggal S., Mahajan R.K., Biswas N.K. Splenic abscess due to Salmonella enterica Serotype typhi in a young adult. J. Commun. Dis. 2008;40(September (3)):219–222. [PubMed] [Google Scholar]
- 9.Ooi L.L., Leong S.S. Splenic abscesses from 1987 to 1995. Am. J. Surg. 1997;174:87–93. doi: 10.1016/s0002-9610(97)00030-5. [DOI] [PubMed] [Google Scholar]
- 10.Comarmond C., Jauréguiberry S., Vaillant J.C. Giant splenic abscess due to Salmonella enteritidis in a returning traveler. J. Travel Med. 2010;17(July-August (4)):271–273. doi: 10.1111/j.1708-8305.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 11.Galanakis E., Bitsori M., Maraki S. Invasive non-typhoidal salmonellosis in immunocompetent infants and children. Int. J. Infect. Dis. 2007;11(January (1)):36–39. doi: 10.1016/j.ijid.2005.09.004. Epub 2006 Mar 27. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J.I., Bartlett J.A., Corey G.R. Extra-intestinal manifestations of salmonella infections. Medicine (Baltimore) 1987;66(September (5)):349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gordon M.A. Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr. Opin. Infect. Dis. 2011;24:484–489. doi: 10.1097/QCO.0b013e32834a9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang J.T., Lee P.H., Wang S.M. Splenic abscess: a diagnostic pitfall in the ED. Am. J. Emerg. Med. 1995;13(May (3)):337–343. doi: 10.1016/0735-6757(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 15.Evola G., Mazzone G., Corsaro A., Brancato G., Evola F.R., Basile G. Hemorrhagic shock from post-traumatic rupture of microcystic splenic lymphangioma: a case report and review of the literature. Int. J. Surg. Case Rep. 2020;75:376–379. doi: 10.1016/j.ijscr.2020.09.045. Epub 2020 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoff E., Nayeri F. Splenic abscess due to Salmonella schwarzengrund in a previously healthy individual returning from Bali. BMJ Case Rep. 2015;2015(December) doi: 10.1136/bcr-2015-212969. bcr2015212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraioli G., Brunetti E., Gulizia R. Management of splenic abscess: report on 16 cases from a single center. Int. J. Infect. Dis. 2009;13(July (4)):524–530. doi: 10.1016/j.ijid.2008.08.024. Epub 2008 Dec 12. [DOI] [PubMed] [Google Scholar]
- 18.Carbonell A.M., Kercher K.W., Matthews B.D. Laparoscopic splenectomy for splenic abscess. Surg. Laparosc. Endosc. Percutan. Tech. 2004;14(October (5)):289–291. doi: 10.1097/00129689-200410000-00013. [DOI] [PubMed] [Google Scholar]