Abstract
Background/purpose
The use of cavity treatments may help in the reduction of bacteria remaining in dentinal tubules after selective carious tissue removal. This study aimed to investigate the effect of selective carious tissue removal and treatment with either 35% phosphoric acid +0.12% chlorhexidine or dentine conditioner on the residual intratubular bacteria in coronal dentine of deep carious lesions.
Materials and methods
Thirty carious human molars were randomly divided into three groups; group 1: untreated carious teeth (positive control), group 2: carious teeth treated with 35% phosphoric acid and chlorhexidine disinfectant after selective carious tissue removal and group 3: carious teeth treated with dentine conditioner after selective carious tissue removal. Another six non-carious teeth was used as negative control. The presence of bacteria and depth of bacteria remaining in dentinal tubules were determined by scanning electron microscopy (SEM). Chi square test and one-way, repeated-measures analysis of variance were used for statistical analysis.
Results
Using SEM, coronal dentine of group 1, 2 and 3 revealed cocci, rod and filamentous bacteria within dentinal tubules. Positive rates of bacteria detection in coronal dentine of group 1 were significant higher than those of group 2 and 3 (P < 0.05). The distance of bacteria remaining in the dentinal tubules in group 1, 2 and 3 were 1149.14 ± 384.44, 707.98 ± 357.19 and 869.25 ± 470.75 μm, respectively.
Conclusion
Both treatment groups had similar ability to reduce the number of intratubular bacteria in coronal dentine of carious teeth, but not complete elimination.
Keywords: Intratubular bacteria, Selective carious tissue removal, Chlorhexidine, Dentine conditioner, Scanning electron microscopy
Introduction
To retain the longevity of the tooth and the pulpal health, the selective carious tissue removal is the minimal invasive approach in the deep carious lesions in order to maintain the deeper layer of partially infected carious dentine which can be remineralized.1 Previous studies reported that selective carious tissue removal technique has advantage in terms of avoidance of pulp exposure, preservation of dental and pulpal tissues as well as the longevity of the restorations.2,3 The selective carious tissue removal are developed to permit the minimal cavity preparations which involve removing decayed tissues with hand instruments, and restoring the dental cavity with an adhesive material such as glass ionomer cement (GIC), composite resins, resin-modified glass-ionomer cement (RM-GICs) and compomers.4,5 Glass ionomer cements (GIC) are commonly used because of their biocompatibility, fluoride release and adhesive properties.6 It has antibacterial properties, because of its release of fluoride, which also potentiates remineralization, promoting a hardening of the layer of demineralized dentine left after carious tissue excavation, but they have weak mechanical and aesthetic properties.6 The concern about GIC is its possible microleakage and poor physical properties, which result in more wear in stress-bearing occlusal cavities.7,8 For longevity of the glass ionomer restorations, dentine conditioner or 10% polyacrylic acid cavity conditioner is used to remove the dentinal smear layer, thus enhancing the adhesion and the marginal seal of glass ionomer cements.9,10 Besides GIC, composite resins are also frequently used because of high mechanical and good aesthetic properties but limited bioactive properties.11 Infection beneath composite resin restorations should be avoided by removing the superficial smear layer and disinfecting the cavity preparation before inserting the restoration.12,13
The use of antibacterial solutions is an optional to decrease intratubular bacteria from cavity preparation in teeth with deep dental caries.14 Chlorhexidine has been widely used as gold standard for antimicrobial effect before placement of restorations.14,15 It has broad antibacterial activity against Gram-positive bacteria, especially Streptococcus mutans, Gram-negative bacteria, facultative anaerobes and aerobes, and yeasts.13 In controlling caries progression in humans, 0.12% chlorhexdine has a bactericidal effect in reducing Streptococcus mutans,16 and has a significant inhibitory effect on the activity of dentinal proteolytic enzymes of carious coronal and root dentine.17 It was also found that 0.12% chlorhexdine did not show any influence on the microtensile bond strength of the etch-and-rinse adhesive systems.15 In addition, chlorhexdine is able to inhibit the MMPs’ collagenolytic activity, improving the longevity of the bond between adhesives and dentine.15,18
Previous microbiological studies demonstrated that selective carious tissue removal using either bur or hand instruments showed significant reduction of microbial count in cavities of deciduous teeth with deep caries and further significant reduction microbial count after 3 weeks of sealing the cavity with restoration.3,19 In addition, there was no significant difference in percentage reduction of cariogenic bacteria counts when compared to the complete carious tissue removal using bur and dye.3 Therefore, it does seem that there is no need to perform complete carious tissue removal.
However, the microorganisms could be pushed deep inside the dentinal tubules during caries removal and it is possible that these microorganisms can remain viable for a long time.3 If the burden of bacteria invading the pulpo–dentine complex overcomes the defenses, this may lead to infection of the dental pulp, including pulpitis and pulp necrosis.20 Therefore, the use of cavity disinfectants may help in the reduction or elimination of bacteria remaining in dentinal tubules after selective carious tissue removal, and lead to the success of restorative treatment.13 Hence, the objective of this study was to investigate the effect of selective carious tissue removal and treatment with either 35% phosphoric acid +0.12% chlorhexidine or dentine conditioner on the reduction of intratubular bacteria in coronal dentine of carious teeth.
Materials and methods
The experimental protocol was approved by the Ethics Committee of the Faculty of Dentistry and Faculty of Pharmacy at Mahidol University (COE.No.MU-DT/PY-IRB 2019/020.1704). Thirty deep carious molars and six sound non-carious molars were used after recently extraction for reasons not related to the study. The carious teeth with radiograph involving remaining dentine over the pulp of at least 1–1.5 mm were included. The teeth with history of root canal treatment or crown restoration were excluded. The extracted teeth were kept immediately in 0.9% normal saline solution. Thirty deep carious molars were randomly divided into 3 groups as follows: group 1 (n = 10) no treatment (positive control), group 2 (n = 10) treatment with selective carious tissue removal, acid etching and chlorhexidine application, and group 3 (n = 10) treatment with selective carious tissue removal and dentine conditioner application. Six sound non-carious molars were used as group 4 or negative control group to confirm our methodology is sterile technique.
Experimental procedures
Group 1: carious teeth with no treatment.
Group 2: in each carious tooth, selective carious tissue removal was done, or soft carious dentine was pulpally removed using a sharp spoon excavator until reaching firm or leathery dentine (resistance to hand excavator) on the pulpal floor. Thereafter, dentine was etched with 35% Phosphoric acid (3MESPE, St. Paul, MN, USA) for 15 s to remove smear layer, rinsed with distilled water and treated with 0.12% chlorhexidine digluconate solution (M Dent, Nakhon Pathom, Thailand) for 1 min. We decided to use 0.12% chlorhexidine solution because it has both a disinfection effect on cariogenic bacteria16,17 and a beneficial effect on the preservation of dentine-resin bonds.15,18
Group 3: in each carious tooth, selective carious tissue removal was done as described in group 2 until reaching firm dentine on the pulpal floor. Thereafter, dentine was treated with dentine conditioner or 10% polyacrylic acid solution (GC Corporation, Tokyo, Japan) for 20 s and rinsed with distilled water.
Group 4: in each non-carious sound tooth, the occlusal cavity was prepared in box form, 4 mm in diameter and depth with diamond bur (Intensive® No. 204, Viganello-Lugano, Switzerland). The teeth were divided into 2 subgroups. Subgroup A(n = 3): dentine was etched with 35% Phosphoric acid (3MESPE) and treated with 0.12% chlorhexidine solution (M Dent) as described in group 2. Subgroup B (n = 3): dentine was treated with dentine conditioner (GC Corporation) as described in group 3.
All teeth were vertically fractured in 2 halves using a carborundum disc and a sharp chisel. Then, the teeth were immediately immersed in 10% neutral buffer formalin solution for fixation periods of 1 week.
Scanning electron microscopy
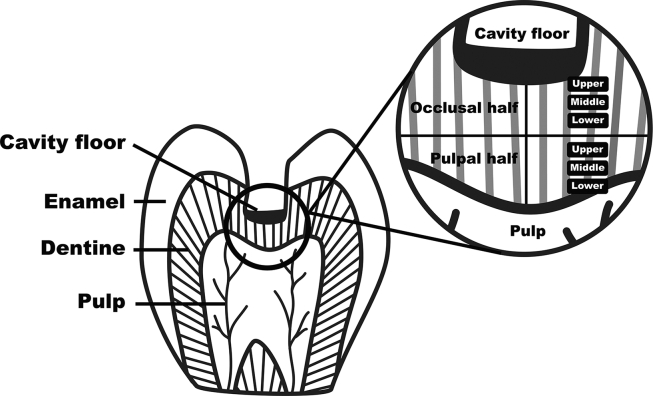
The specimens were gently washed in phosphate buffered saline, dehydrated in increasing concentrations of ethanol, critical-point dried using liquid CO2 replacement, and sputter-coated with gold. The specimens were examined using a scanning electron microscope (JSM-5410 LV; JEOL, Tokyo, Japan) from low to high magnification by three different blinded reviewers. In each specimen, the dentine thickness from pulpal floor of the carious cavity to the roof of pulp chamber was measured and divided equally into two parts, part of beneath cavity floor (occlusal half) and part of above the pulp chamber (pulpal half). Each part was further divided into upper, middle and lower areas (Fig. 1). In each of six areas, the presence of bacteria in five different locations was observed. If we found more than five bacteria or bacterial aggregates, we defined this area as having positive results for bacterial detection.21 The deepest distance of residual bacteria presenting in dentinal tubules of coronal dentine was also measured.
Figure 1.
Schematic diagram of the area of the coronal dentine which be observed under scanning electron microscopy.
Statistical analysis
The presence of bacteria in coronal dentine segments were reported as percentage of positive rates of bacterial detection, and the data were analyzed using Chi square test. The distance of bacteria remaining in the dentinal tubules and dentine thickness including the proportion between them were reported as means ± SD (standard deviation), and the data were analyzed using one-way, repeated-measures analysis of variance (one-way RM ANOVA) and Post Hoc test. P values less than 0.05 were considered to be statistically significant. SPSS software (V. 23; IBM Corp, Armonk, NY, USA) was used for statistical analysis.
Results
Using Scanning electron microscopy, coronal dentine of group 1, 2 and 3 revealed cocci, rod and filamentous bacteria within dentinal tubules as presented in Figure 2, Figure 3, Figure 4.
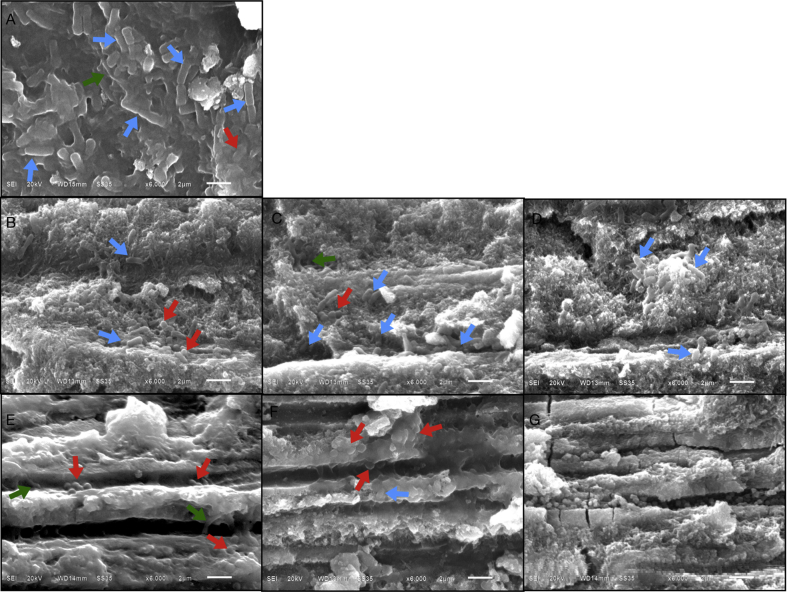
Figure 2.
(A–G) Scanning electron micrographs of coronal dentine of carious teeth without treatment (original magnification X6000). (A) Cavity floor dentine with a great number of intratubular bacteria consisting of cocci (red arrow), rods (blue arrow) and filamentous bacteria (green arrow); (B, C and D) upper, middle and lower portions of occlusal half of coronal dentine with a large number of bacterial aggregates in dentinal tubules; (E, F) upper and middle portions of pulpal half of coronal dentine with a small amount of bacteria in the dentinal tubules; (G) lower portions of pulpal half of coronal dentine with numerous visible cuboid crystals but no bacterial colonization in the dentinal tubules.
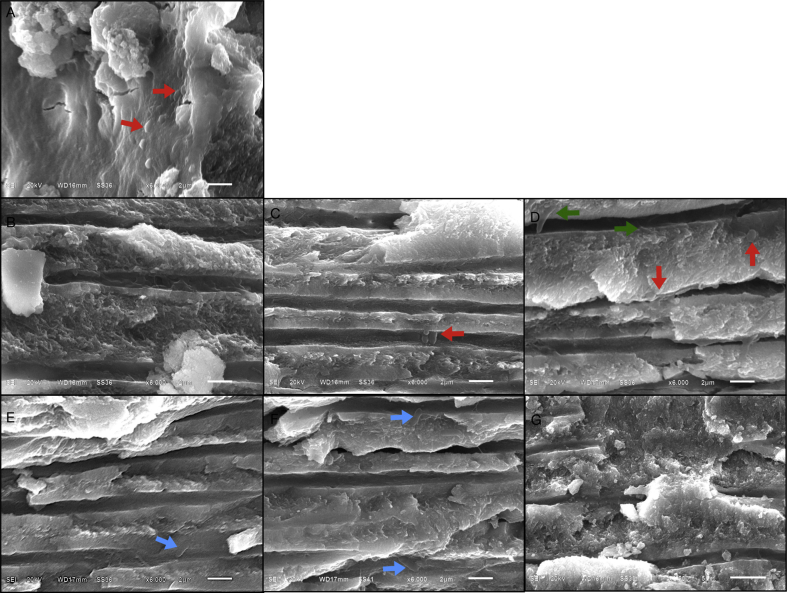
Figure 3.
(A–G) Scanning electron micrographs of carious dentine after selective carious tissue removal and treated with 35% phosphoric acid and 0.12% chlorhexidine solution (original magnification X6000). (A) Cavity floor dentine with open and clear dentinal tubule orifice having a small amount of filamentous bacteria (green arrow); (B, D) upper and lower portions of occlusal half of coronal dentine without bacterial colonization in the dentinal tubules; (C) middle portions of occlusal half of coronal dentine with network aggregated filamentous bacteria on the dentinal walls; (E) upper third of pulpal half of coronal dentine with a small amount of bacilli (blue arrow); (F, G) middle and lower portions of pulpal half of coronal dentine with thickening peritubular dentine, narrow dentinal tubules and no bacterial colonization.
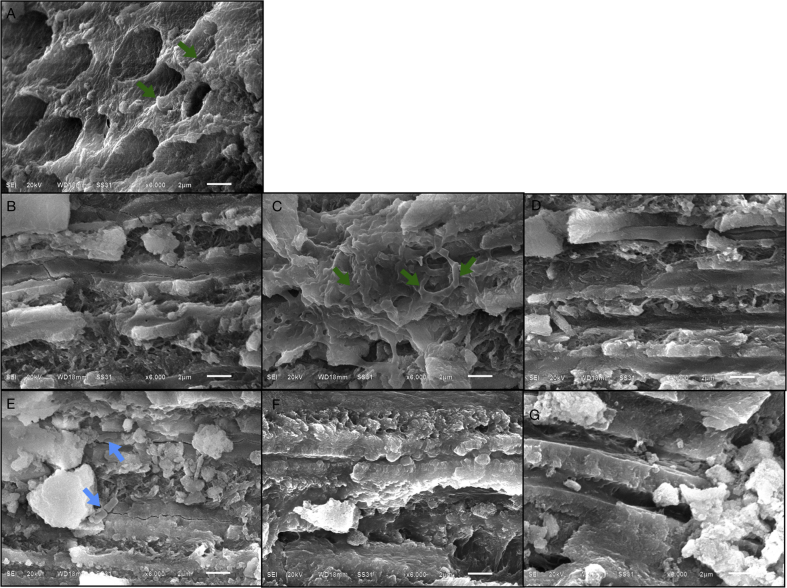
Figure 4.
(A–G) Scanning electron micrographs of coronal dentine after selective carious tissue removal and treated with dentine conditioner (polyacrylic acid) (original magnification X6000). (A) Cavity floor dentine with the smear plugs existed in the orifice of dentinal tubules and a small amount of cocci (red arrow) attached to the walls of the tubules; (B) upper portions of occlusal half of coronal dentine and no bacteria colonization; (C, D) middle and lower portions of occlusal half of coronal dentine consisting of a small amount of cocci and filamentous (green arrow) bacteria; (E, F) upper and middle portions of pulpal half of coronal dentine with a small amount of bacilli (blue arrow); (G) lower portions of pulpal half of coronal dentine and no bacterial invasion into the tubules.
The longitudinal distribution of intratubular bacteria in coronal dentine of each experimental group is presented in Table 1.
Table 1.
Positive rates of bacterial detection in coronal dentine segmentsa.
| Group | Number | Doner age (years) | Positive rates of bacterial detection (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cavity floor | Beneath cavity floor (Occlusal halves) |
Above pulp chamber (Pulpal halves) |
|||||||
| upper | middle | lower | upper | middle | lower | ||||
| 1 | 10 | 33.60 (9.94) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 9 (90) | 4 (40) |
| 2 | 10 | 30.55 (8.71) | 2 (20)∗ | 6 (60)∗ | 9 (90) | 7 (70) | 4 (40)∗ | 4 (40)∗ | 3 (30) |
| 3 | 10 | 29.11 (10.31) | 2 (20)∗ | 6 (60)∗ | 7 (70) | 7 (70) | 4 (40)∗ | 4 (40)∗ | 2 (20) |
| 4A | 3 | 20.66 (4.94) | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0) |
| 4B | 3 | 27.00 (3.00) | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0)∗ | 0 (0) |
Data are presented as n (%).
∗P < 0.05 compared with group 1.
The bacteria or bacterial aggregates for areas of each coronal dentine were observed using scanning electron microscopy under X3,500 magnification. If we found more than five bacteria or bacterial aggregates, we defined this area as having positive results for bacterial colonization.21.
The results of our study revealed that no bacteria were found in group 4 (negative control); whereas, bacteria colonization exhibited 100% on the floor of carious cavity in group 1 (no treatment). However, bacteria detection rate was significant lower in group 2 (20%) and group 3 (20%) (P < 0.05). In occlusal halves, bacteria detection rates of group 2 and group 3 were lower than those found in group 1, but significant differences were found only in upper third area (P < 0.05), when compared to group 1. In pulpal halves, bacteria detection rates of group 2 and group 3 were significant lower than those found in group 1 (P < 0.05). When compared between group 2 and group 3, no significant difference was found in each area of observation.
Bacterial distribution was lighter and less invasive in the treated coronal dentine than in untreated dentine (P < 0.05). Both treatments could reduce the number of intratubular bacteria in coronal dentine of carious teeth, but not complete elimination.
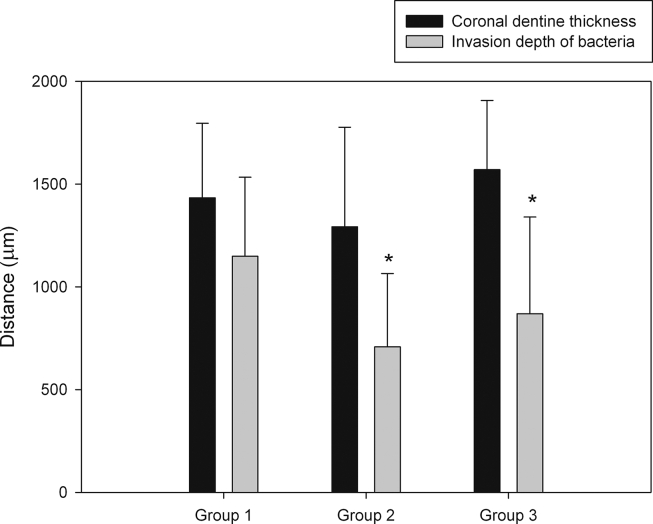
Means and standard deviations of the invasion depth of bacteria (the deepest distance of bacteria remaining in the dentinal tubules) and the thickness of coronal dentine segments are shown in Fig. 5. The remaining dentine thickness of coronal dentine segments in group 1, 2 and 3 were 1433.29 ± 362.90, 1292.49 ± 484.40, 1570.04 ± 336.92 μm, respectively. In group 1, the invasion depth of bacteria was 1149.14 ± 384.44 μm or around 79% of dentine thickness; meanwhile, the invasion depth of bacteria in group 2 was 707.98 ± 357.19 μm or about 58% of dentine thickness. In group 3, the invasion depth of bacteria was 869.25 ± 470.75 μm or approximately 54% of dentine thickness. However, no significant differences were observed between group 2 and group 3 (P > 0.05).
Figure 5.
Bar graph of mean invasion depths of bacteria and dentine thickness of coronal dentine segments. The error bars indicate 1 standard deviation from the mean. ∗P < 0.05 compared with group 1.
Discussion
The result of this study revealed that positive rates of bacteria detection in treated coronal dentine of carious teeth, either treated with 35% phosphoric acid +0.12% chlorhexidine or dentine conditioner (10% polyacrylic acid) after selective carious tissue removal, were significant lower than those in untreated groups. Bacteria were still found in dentinal tubules of both treatment groups, but lighter and less invasive than in untreated group. In untreated group (positive control), the depth of bacteria presenting in the dentinal tubules was approximately 1149 μm or around 79% of dentine thickness, and bacteria colonization exhibited 100% on the floor of carious cavity. In consistent with the previous studies, bacteria from dental plaque invaded into the dentinal tubules, when dentine was exposed in the oral cavity during the carious process.20,22 Lundy and Stanley23 demonstrated that bacteria penetration into the dentinal tubules ranged from 0.04 to 3.00 mm (average 0.52 mm) in 84 days after dentine cavities were prepared by high speed handpiece. The SEM micrographs of our study showed the presence of cocci, bacilli and filamentous bacteria. This confirms the viability of intratubular bacteria in coronal dentine. In agreement with the study of Hamama et al.24 their study found the presence of S. mutans inside the dentinal tubules of infected coronal dentine. Both S. mutans and Enterococcus faecalis could penetrate within dentinal tubules (1.5–2 μm in diameter) because these microorganisms have similar diameters of 0.7–0.9 μm.24
In group 2 treated with 35% phosphoric acid and 0.12% chlorhexidine after selective carious tissue removal with hand instruments, bacterial distribution was lighter and less invasive in the treated coronal dentine when compared to untreated dentine of group 1. Due to the action of phosphoric acid on smear layer removal, the dentine surface was cleared and revealed the opened dentinal tubules which enhance the penetration of irrigants, medicaments and resin adhesives into the dentinal tubules.25 Phosphoric acid used for etch-and-rinse technique also has strong antimicrobial effect which may be related to an increase in external hydrogen ion concentration, leading to interference of the vitality and growth of many microorganisms.26, 27, 28 However, the opened dentinal tubules after phosphoric acid treatment may gain entry for bacteria invasion into the dentinal tubules.22,29 Thus, 0.12% chlorhexidine solution was applied after acid pretreatment in this study. Due to its broad antimicrobial effect and its substantivity to dentine, chlorhexidine has been used to treat acid-etched dentine to arrest caries progression and to maximize stability of resin-dentine bond in etch-and-rinse system.17,30 However, our study showed that the distance of bacteria remaining in the dentinal tubules in group 2 was 707.98 ± 357.19 mm. Thus, chlorhexidine could not penetrate to kill all residual bacteria in acid-etched dentine of carious teeth. In agreement with Vadhana et al.31 who reported that penetration depths of chlorhexidine solution into root dentinal tubules were only between 44 and 138 μm during observation under confocal laser scanning microscope. Unfortunately, no report about penetration depths of chlorhexidine solution into coronal dentinal tubules was found. Another explanation by the confocal laser scanning microscopy study of Hamama et al.24 which determined the effect of 5 min dentine surface treatment with 2% chlorhexidine solution on bacterial viability within dentinal tubules, they found 30% dead/70% live of intratubular bacteria in coronal dentine after treatment.24 However, within the limitation of our study, the SEM technique could not represent the dead/live volume percentage of intratubular bacteria.
In group 3 treated with 10% polyacrylic acid after selective carious tissue removal with hand instruments, polyacrylic acid treatment removed the smear layer and leaved smear plug in the dentinal tubules. The smear layer removal is essential for micromechanical interaction between the glass ionomer cement and underlying dentine, and the smear plug in the dentinal tubules is considered as a barrier to prevent bacterial invasion into the tubules.32 Low pH of polyacrylic acid also has the antimicrobial action against both Streptococcus mutans and Actinomyces viscosus.33 However, either treatment with 35% phosphoric acid +0.12% chlorhexidine or dentine conditioner (10% polyacrylic acid) had similar ability in reducing the number of intratubular bacteria in coronal dentine of carious teeth, but not complete elimination. Therefore, the tooth restorative seal is essential for entombing any residual bacteria3 and preventing ingress of bacteria from oral environment into the dentinal tubules.7 Antibacterial properties of adhesive materials are also beneficial in reducing residual intratubular bacteria in order to prevent pulpal complications.34
In conclusion, selective carious tissue removal and cavity treatment with either 35% phosphoric acid +0.12% chlorhexidine or dentine conditioner (10% polyacrylic acid) were effective in reducing residual intratubular bacteria of coronal dentine in order to minimize the risk of pulp exposure in deep carious lesions. Bacterial distribution was lighter and less invasive in both treatment groups, when compared to untreated carious teeth. Both treatment groups had similar ability to reduce the number of intratubular bacteria in coronal dentine of carious teeth, but not complete elimination.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Faculty of Dentistry, Mahidol University. The authors would like to express sincere gratitude to the Department of Oral Biology and the Department of Pediatric Dentistry, Faculty of Dentistry, Mahidol University for their support and assistance.
References
- 1.Innes N.P.T., Frencken J.E., Bjørndal L. Managing carious lesions: consensus recommendations on terminology. Adv Dent Res. 2016;28:49–57. doi: 10.1177/0022034516639276. [DOI] [PubMed] [Google Scholar]
- 2.Ricketts D.N., Kidd E.A., Innes N., Clarkson J. Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD003808.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Singhal D.K., Acharya S., Thakur A.S. Microbiological analysis after complete or partial removal of carious dentin using two different techniques in primary teeth: a randomized clinical trial. Dent Res J. 2016;13:30–37. doi: 10.4103/1735-3327.174695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorri M., Martinez-Zapata M.J., Walsh T., Marinho V.C.C., Sheiham (deceased) A., Zaror C. Atraumatic restorative treatment versus conventional restorative treatment for the management of dental caries. Cochrane Database Syst Rev. 2017;12 doi: 10.1002/14651858.CD008072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frencken J.E., Holmgren C.J. How effective is ART in the management of dental caries? Community Dent Oral Epidemiol. 1999;27:423–430. doi: 10.1111/j.1600-0528.1999.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 6.Berg J.H., Croll T.P. Glass ionomer restorative cement systems: an update. Pediatr Dent. 2015;37:116–124. [PubMed] [Google Scholar]
- 7.Retief D.H. Are adhesive techniques sufficient to prevent microleakage? Operat Dent. 1987;12:140–145. [PubMed] [Google Scholar]
- 8.Mount G.J. Glass ionomers: a review of their current status. Operat Dent. 1999;24:115–124. [PubMed] [Google Scholar]
- 9.Hajizadeh H., Ghavamnasiri M., Namazikhah M.S., Majidinia S., Bagheri M. Effect of different conditioning protocols on the adhesion of a glass ionomer cement to dentin. J Contemp Dent Pract. 2009;10:9–16. [PubMed] [Google Scholar]
- 10.Unnikrishnan S., Krishnamurthy N.H., Nagarathna C. Marginal microleakage of glass ionomer cement with two different cavity conditioners on primary anterior teeth−An in vitro study. Indian J Dent Res. 2019;30:267–272. doi: 10.4103/ijdr.IJDR_695_17. [DOI] [PubMed] [Google Scholar]
- 11.Donly K.J., García-Godoy F. The use of resin-based composite in children: an update. Pediatr Dent. 2015;37:136–143. [PubMed] [Google Scholar]
- 12.Branström M. Infection beneath composite resin restorations: can it be avoided? Operat Dent. 1987;12:158–163. [PubMed] [Google Scholar]
- 13.Bin-Shuwaish M.S. Effects and effectiveness of cavity disinfectants in operative dentistry: a literature review. J Contemp Dent Pract. 2016;17:867–879. doi: 10.5005/jp-journals-10024-1946. [DOI] [PubMed] [Google Scholar]
- 14.Mobarak E.H. Effect of chlorhexidine pretreatment on bond strength durability of caries−affected dentin over 2-year aging in artificial saliva and under simulated intrapulpal pressure. Operat Dent. 2011;36:649–660. doi: 10.2341/11-018-L. [DOI] [PubMed] [Google Scholar]
- 15.De Campos E.A., Correr G.M., Leonardi D.P., Pizzatto E., Morais E.C. Influence of chlorhexidine concentration on micro-tensile bond strength of contemporary adhesive systems. Braz Oral Res. 2009;23:340–345. doi: 10.1590/s1806-83242009000300019. [DOI] [PubMed] [Google Scholar]
- 16.De Queiroz V.S., Ccahuana-Vásquez R.A., Tedesco A.F., Lyra L., Cury J.A., Schreiber A.Z. Influence of the culture medium in dose-response effect of the chlorhexidine on streptococcus mutans biofilms. Sci Tech Rep. 2016;2016:2816812. doi: 10.1155/2016/2816812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia M.B., Carrilho M.R., Nör J.E. Chlorhexidine inhibits the proteolytic activity of root and coronal carious dentin in vitro. Caries Res. 2009;43:92–96. doi: 10.1159/000209340. [DOI] [PubMed] [Google Scholar]
- 18.Zheng P., Zaruba M., Attin T., Wiegand A. Effect of different matrix metalloproteinase inhibitors on microtensile bond strength of an etch-and-rinse and a self-etching adhesive to dentin. Operat Dent. 2015;40:80–86. doi: 10.2341/13-162-L. [DOI] [PubMed] [Google Scholar]
- 19.Bitello-Firmino L., Soares V.K., Damé-Teixeira N., Parolo C.C.F., Maltz M. Microbial load after selective and complete caries removal in permanent molars: a randomized clinical trial. Braz Dent J. 2018;29:290–295. doi: 10.1590/0103-6440201801816. [DOI] [PubMed] [Google Scholar]
- 20.Love R.M., Jenkinson H.F. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13:171–183. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y.H., Xie S.J., Wang N.N., Ge J.Y. Status of bacterial colonization in teeth associated with different types of pulpal and periradicular disease: a scanning electron microscopy analysis. J Dent Sci. 2015;10:95–101. [Google Scholar]
- 22.Michelich V.J., Schuster G.S., Pashley D.H. Bacterial penetration of human dentin in vitro. J Dent Res. 1980;59:1398–1403. doi: 10.1177/00220345800590080701. [DOI] [PubMed] [Google Scholar]
- 23.Lundy T., Stanley H.R. Correlation of pulpal histopathology and clinical symptoms in human teeth subjected to experimental irritation. Oral Surg. 1969;27:187–201. doi: 10.1016/0030-4220(69)90172-8. [DOI] [PubMed] [Google Scholar]
- 24.Hamama H.H., Yiu C.K., Burrow M.F. Viability of intratubular bacteria after chemomechanical caries removal. J Endod. 2014;40:1972–1976. doi: 10.1016/j.joen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Pashley D., Michelich V., Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent. 1981;46:531–537. doi: 10.1016/0022-3913(81)90243-2. [DOI] [PubMed] [Google Scholar]
- 26.Settembrini L., Boylan R., Strassler H., Scherer W. A comparison of antimicrobial activity of etchants used for a total etch technique. Operat Dent. 1997;22:84–88. [PubMed] [Google Scholar]
- 27.Prado M., Silva E.J.N.L., Duque T.M. Antimicrobial and cytotoxic effects of phosphoric acid solution compared to other root canal irrigants. J Appl Oral Sci. 2015;23:158–163. doi: 10.1590/1678-775720130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arias-Moliz M.T., Ferrer-Luque C.M., Espigares-Rodriguez E., Liébana-Ureña J., Espigares-García M. Bactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e84–e89. doi: 10.1016/j.tripleo.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Brannstrom M., Nordenvall K.J. Bacterial penetration, pulpal reaction and the inner surface of Concise enamel bond. Composite fillings in etched and unetched cavities. J Dent Res. 1978;57:3–10. doi: 10.1177/00220345780570011301. [DOI] [PubMed] [Google Scholar]
- 30.Carrilho M.R., Carvalho R.M., Sousa E.N. Substantivity of chlorhexidine to human dentin. Dent Mater. 2010;26:779–785. doi: 10.1016/j.dental.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadhana S., Latha J., Velmurugan N. Evaluation of penetration depth of 2% chlorhexidine digluconate into root dentinal tubules using confocal laser scanning microscope. Restor Dent Endod. 2014;39:104–108. doi: 10.5395/rde.2015.40.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho R.M., Tjäderhane L., Manso A.P., Carrilho M.R., Carvalho C.A.R. Dentin as a bonding substrate. Endod Top. 2012;21:62–88. [Google Scholar]
- 33.Gökçe K., Benderli Y. Antimicrobial action of various polyacrylic acids on streptococcuss mutans and actinomyces viscosus. OHDMBSC. 2003;2:42–46. [Google Scholar]
- 34.Hafshejani T.M., Zamanian A., Venugopal J.R. Antibacterial glass-ionomer cement restorative materials: a critical review on the current status of extended release formulations. J Contr Release. 2017;262:317–328. doi: 10.1016/j.jconrel.2017.07.041. [DOI] [PubMed] [Google Scholar]