Abstract
Citrus originated in Southeast Asia, and it has become one of the most important fruit crops worldwide. Citrus has a long and obscure domestication history due to its clonal propagation, long life cycle, wide sexual compatibility, and complex genetic background. As the genomic information of both wild and cultivated citrus becomes available, their domestication history and underlying traits or genes are becoming clear. This review outlines the genomic features of wild and cultivated species. We propose that the reduction of citric acid is a critical trait for citrus domestication. The genetic model representing the change during domestication may be associated with a regulatory complex known as WD-repeat-MYB-bHLH-WRKY (WMBW), which is involved in acidification and anthocyanin accumulation. The reduction in or loss of anthocyanins may be due to a hitchhiking effect of fruit acidity selection, in which mutation occurs in the common regulator of these two pathways in some domesticated types. Moreover, we have summarized the domestication traits and candidate genes for breeding purposes. This review represents a comprehensive summary of the genes controlling key traits of interest, such as acidity, metabolism, and disease resistance. It also sheds light on recent advances in early flowering from transgenic studies and provides a new perspective for fast breeding of citrus. Our review lays a foundation for future research on fruit acidity, flavor, and disease resistance in citrus.
Keywords: citrus, domestication, fruit acidity, genome, wild germplasm
This review summarizes recent progress in the genome sequencing of different citrus species and highlights the agronomic traits associated with domestication. It proposes that fruit acidity, which determinies fruit taste, is a key trait of citrus domestication, and that the WD-repeat-MYB-bHLH-WRKY complex involved in the regulation of both acidity and anthocyanin metabolism comprises genes targeted during domestication. In addition, this review summarizes genes associated with apomixis (polyembryony), disease resistance, and early flowering.
Introduction
Citrus is widely grown in subtropical and tropical areas in more than 140 countries and regions, among which China, the United States, Mexico, Brazil, India, Spain, and Argentina are the most significant producers (according to FAO statistics, 2016, https://www.fao.org/faostat/en/). Citrus has a high has economic value, it has an annual yield of 150 million tons and covers a cultivation area of more than 14.4 million Ha worldwide (FAO, 2018; http://www.fao.org/faostat/en/#data). Citrus fruit is a good source of nutrition and provides sugars, volatiles, organic acids (citric acid), dietary metabolites, amino acids, fibers, vitamin B6, vitamin C, and macro- and micronutrients (Liu et al., 2012). Citrus fruits are also rich secondary in metabolites (such as limonoids, alkaloids, flavonoids, coumarins, anthocyanins, essential oils, phenol acids, and carotenoids) and therefore have beneficial effects on human health (Rouseff and Nagy, 1994; Economos and Clay, 1999). In addition, citrus fruits are widely used for cosmetics, food, beverage, and pharmaceutical industries for the production of spices, medicines, additives, chemoprophylactic drugs, and others (Krishnaiah et al., 2011; Vun et al., 2015). In addition, some primitive and wild citrus species, such as Citrus medica L., Citrus wilsonii Tanaka, and Atalantia buxifolia, are used for traditional medicine, and others are used as herbal medicine (Gmitter and Hu, 1990; Dave, 2009; Vun et al., 2015).
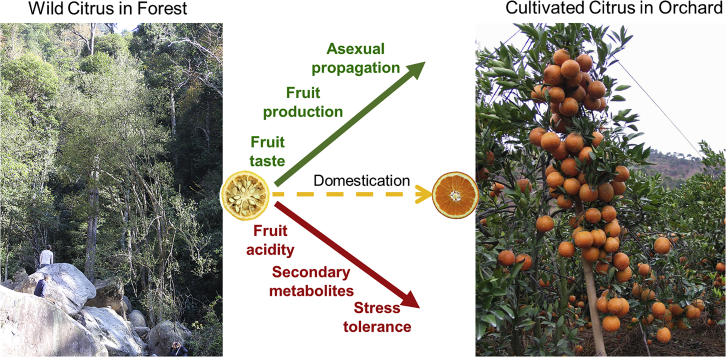
Because the is a long-lived perennial tree species, it is considered to have been domesticated at least 2,000 years ago. An ancient Chinese work titled Records of the Grand Historian recorded the commercial production of citrus and the management of citrus industrial affairs by specific government officials during the Han dynasty. Long juvenile phases, extensive hybridization, widespread outcrossing combined with clonal propagation, cultivar–wild gene flow, and multiple origins have contributed to the domestication of perennials (Mckey et al., 2010; Miller and Gross, 2011). Citrus is one of the most important fruit crops commercially cultivated worldwide. However, how citrus trees were domesticated remains largely unknown (Figure 1). This review focuses on the genomes of wild and cultivated citrus and outlines the progress in the identification of genes associated with key agronomic traits in citrus.
Figure 1.
Morphological changes in cultivated citrus species.
Compared with wild citrus, the cultivars exhibit decreased fruit acidity, secondary metabolite levels (such as bitterness compounds), and tolerance to biotic or abiotic stresses but increased fruit production and taste, which mainly depends on the sugar/acid ratio. Asexual propagation, such as apomixis, is also popular among cultivated citrus species.
Citrus genomes: from cultivars to wild species
The de novo assembly of various citrus genomes is summarized in Table 1. Sweet orange is highly heterozygous, making its assembly by the short-read sequencing technology extremely difficult. Sweet orange is of global interest because it accounts for ∼60% of the total citrus production, and both the fresh fruit and juice are consumed. Using a homozygous dihaploid line derived from anther culture, the genome of Valencia sweet orange was assembled (Xu et al., 2013). The genome of Clementine mandarin was first assembled using a haploid line and the Sanger sequencing technology by an international expert group with members from the United States, France, Italy, Spain, and Brazil (Wu et al., 2014). The above-mentioned studies showed that mandarins have a complex genetic background with intergenic introgressions from “pummelo” (Citrus maxima).
Table 1.
Summary of de novo citrus genome assemblies and their features.
| Serial No. | Species | Common name | Domestication status | Ploidy level | Genome status | Sequencing technology | Total number of scaffolds | Size of assembled scaffolds (Mb) | Longest contig (Mb) | Contig N50 (∗L50) (kb) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fortunella hindsii | Hong Kong kumquat | Wild | Diploid | High quality | PacBio/Illumina | 900 | 373.6 | 12.00 | 2209 | Zhu et al. (2019) |
| 2 | Citrus reticulata | Mangshan wild mandarin | Wild | Diploid | Draft | Illumina | 42 714 | 334 | 0.30 | 24.76 | Wang et al. (2018) |
| 3 | Citrus unshiu | Satsuma mandarin | Cultivar | Diploid | Draft | Illumina/PacBio | 20 876 | 359.7 | 0.54 | 24.26 | Shimizu et al. (2017) |
| 4 | Atalantia buxifolia | Chinese box orange | Wild | Diploid | Draft | Illumina | 25 600 | 316 | 0.20 | 23.89 | Wang et al. (2017a) |
| 5 | Citrus ichangensis | Papeda | Wild | Diploid | Draft | Illumina | 14 915 | 357 | 0.77 | 76.56 | Wang et al. (2017a) |
| 6 | Citrus grandis | Pummelo | Cultivar | Haploid | High quality | PacBio RS II/Illumina | 1612 | 346 | 10.62 | 2183 | Wang et al. (2017a) |
| 7 | Citrus medica | Citron | Wild | Diploid | Draft | Illumina | 32 731 | 405.0 | 0.41 | 46.50 | Wang et al. (2017a) |
| 8 | Citrus × clementina | Clementine | Cultivar | Haploid | High quality | Sanger | 1398 | 301.4 | 1.23 | 118.9∗ | Wu et al. (2014) |
| 9 | Citrus × sinensis | Ridge pineapple | Cultivar | Diploid | Draft | Sanger/454 | 12 574 | 319.2 | 0.119 | 6.6∗ | Wu et al. (2014) |
| 10 | Citrus sinensis | Valencia sweet orange | Cultivar | Dihaploid | Draft | Illumina | 4811 | 327.8 | 0.323 | 49.89 | Xu et al. (2013) |
With the rapid development of sequencing technology, the pummelo and satsuma mandarin reference genomes were assembled using the long-read sequencing technology (Wang et al., 2017a; Shimizu et al., 2017). For the first time, the pummelo (C. maxima) haploid clone was used to produce a de novo sequence assembly using the PacBio RS II single-molecule sequencing system, and 307.3× coverage of Illumina short reads and 56.8× coverage of long reads were used to assemble the pummelo genome.
The genomes of several wild citrus species, such as Citrus ichangensis (Ichang papeda), Mangshan wild mandarin, citron, and A. buxifolia (known as Chinese box orange), have been sequenced and assembled (Wang et al., 2017a, 2018). Comparison of the genomes between wild and cultivated citrus revealed that unique genes in the wild type and diversified genes in the cultivars are enriched in pathways related to defense response, proteolysis, pectate lyase activity, and reproduction (Wang et al., 2017a).
Origin and domestication of citrus
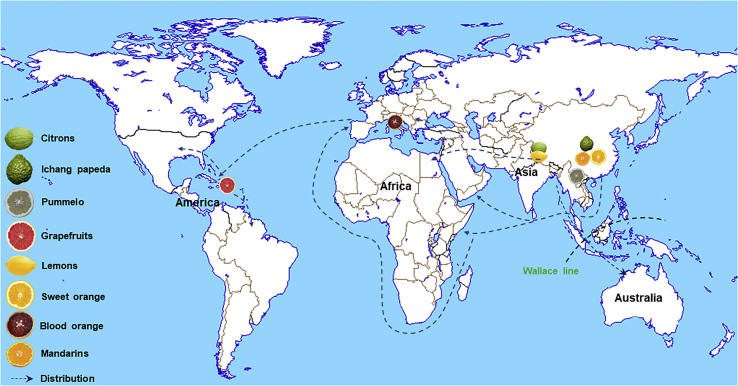
The Citrus genus and its associated genera (Fortunella, Microcitrus, Clymenia, Oxanthera, Eremocitrus, and Poncirus) belong to the Rutaceae family, and they are widely distributed in Southeast Asia, covering the Bismarck Archipelago, the south East Indian Archipelago, New Guinea, New Caledonia, northeastern Australia, the western Polynesian islands, and Malaysia (Reuther, 1967). Despite major morphological and physiological differences, Poncirus, Citrus, Fortunella, Eremocitrus, and Microcitrus are sexually compatible (Krueger and Navarro, 2007; Garcia-Lor et al., 2013, 2015; Wu et al., 2018). The origin, domestication, and taxonomy of Citrus are complex and controversial. Citrus is thought to have originated in Southeast Asia (Figure 2), a biodiversity hotspot region influenced by both south Asian and east Asian monsoons (Jacques et al., 2011, 2014). Some regions in southwestern China, such as the Yunnan province; northeastern India in the Himalayan foothills; and Myanmar are also considered to be places of citrus origin (Reuther, 1967; Gmitter and Hu, 1990). A recent study indicates that the Nanling mountain region in central–south China may also be place of origin for mandarin (Wang et al., 2018).
Figure 2.
The origin and distribution of major citrus species.
The dashed lines indicate possible distribution directions rather than actual dispersal routes.
There are two major taxonomic systems, namely, Swingle's 10 species (Swingle, 1967) and Tanaka's 162 species (Tanaka, 1977). In most studies, citrus is categorized as one of the three basic species, Citrus reticulata (mandarin), C. maxima (pummelo), and C. medica (citron). Most commercial cultivars are either from these three basic species or their hybrids. Nucleotide diversity reflects the degree of intraspecific variation and interspecific divergence, which can reveal the genomic origin of each species (Curk et al., 2016; Wu et al., 2018). Hybrid cultivars (lemon, sour orange, non-Australian limes, and calamondin) are identified from two or more ancestral citrus species with high segmental heterozygosity (∼1.5%–2.4%) and intraspecific diversity (0.1%–0.6%). Bimodal distribution of heterozygosity has been observed in some highly heterozygous citrus accessions, such as grapefruits, sweet orange, and some mandarins, revealing high interspecific heterozygosity and complicated backcross mechanisms. Some pure genotypes without interspecific admixture, such as citrons (a monoembryonic species), display significantly reduced (∼0.1%) intraspecific diversity compared with other species (0.3%–0.6%) (Curk et al., 2016).
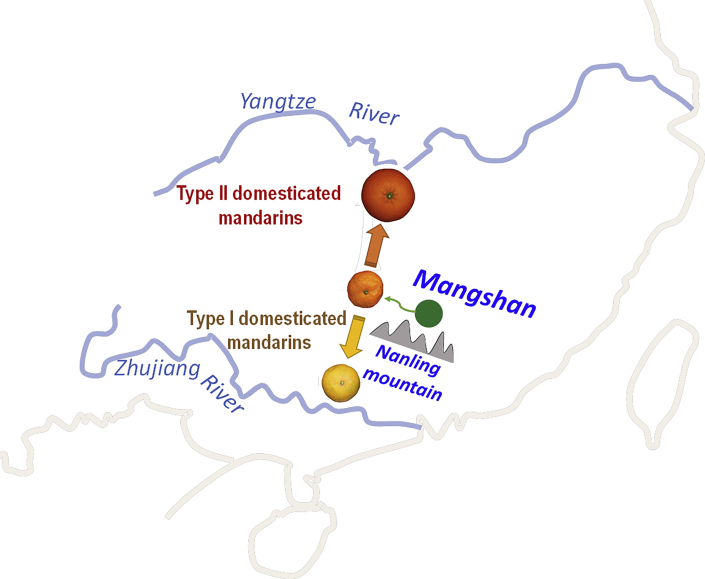
The three basic species (mandarin, pummelo, and citron) probably originated from different places as indicated by updated whole-genome sequencing information on citrus evolution and origins (Xu et al., 2013; Wu et al., 2014, 2018; Wang et al., 2017a). Citron and pummelo are most likely to have originated in a triangle region of northwestern Yunnan (China), northeastern India, and northern Myanmar (Wu et al., 2018), whereas mandarin is most likely to have originated in Mangshan, a branch of the Nanling mountains (central–south China) (Wang et al., 2018). The Mangshan wild mandarin has many primitive characteristics. For example, its fruit is small and the fresh fruit is highly acidic (as acidic as lemon). The Mangshan wild mandarin has a long existence in old-growth forest and a pure genetic background. It has genetic introgressions from a wild citrus variety, whereas Ichang papeda and the cultivated mandarin have wide introgressions from pummelo. Two independent domestication events occurred in south China, resulting in two geographically distinct groups of cultivated mandarins MD1 and MD2 in the north and south of the Nanling mountains, respectively (Figure 3).
Figure 3.
Origin and domestication of mandarin (C. reticulata) in Mangshan of the Nanling mountains in south China.
The model is based on the genomic analysis of Mangshan wild mandarins, Daoxian wild mandarins, semidomesticated mandarins, and domesticated mandarins in the surrounding regions (Wang et al., 2018).
The origin of hybrid citrus species is also getting clearer despite their complex genetic backgrounds that cause wide sexual compatibility between species and even between genera. For example, the origin of sweet orange, as one of the mysteries, is not yet fully understood. Sweet orange is known to be a hybrid between pummelo and mandarin. There are two proposed models for the possible origin of sweet orange. One is a simple model constructed based on the genomes of dihaploid and diploid sweet oranges: sweet orange = (pummelo × mandarin1) × mandarin2, and a comparison between the pummelo and mandarin genomes indicates that sweet orange probably arose from two rounds of hybridization between pummelo and mandarin (Xu et al., 2013). Subsequent genome sequencing data indicate that mandarins yielded from the two rounds of hybridization are different. The other model is complex: sweet orange = (pummelo × mandarin) × pummelo) × mandarin2 (Wu et al., 2014), and it remains to be further verified due to the lack of genotypic data that support (pummelo × mandarin) × pummelo as the female parent of sweet orange. Moreover, whole-genome sequence data have confirmed that lemon is a hybrid between sour orange and citron, grapefruit is a hybrid between pummelo and sweet orange, and sour orange (Citrus aurantium) is a hybrid between pummelo and mandarin (Wu et al., 2018).
The Asian radiation of citrus species is assumed to have originated in the late Miocene (about 6–8 mya), during which the climate changed from wet to dry as the monsoon weakened (Clift et al., 2014), resulting in major variations in biota, including the rapid radiation of several plant lineages (Wen et al., 2014; Favre et al., 2015). A fossil specimen from the late Miocene epoch, Citrus linczangensis, was discovered in the Lincang county of Yunnan province. It has been identified to have the characteristics of the current major citrus family (Xie et al., 2013). A distinct clade of Australian citrus species was formed, which is assumed to relate to citrons (Curk et al., 2016), and Swingle assigned different genus names to these species (Microcitrus and Eremocitrus) in his botanical classification (Batchelor, 1948; Reuther, 1967). However, a whole-genome phylogenetic analysis (Wu et al., 2018) and a molecular dating analysis (Pfeil and Crisp, 2008) suggest that citrus is not of Australian origin (Beattie et al., 2008). Citrus somehow migrated from Southeast Asia to Australasia via intercontinental dispersals, which might be attributed to the elevation advantage of Wallacea and Malesia in the Miocene and Pliocene epochs (Van welzen and Alahuhta, 2005; Hall, 2009). A genomic analysis suggests that the Australian radiation emerged in the Pliocene era (about 4 mya). Phylogenies of the chloroplast and nuclear genomes show clear signs of admixture between two Australian finger limes and round limes (Wu et al., 2018). The diversity among citrus species in northeast Australia is observed in both rainforests and dry environments (Brophy et al., 2001). Citrus tachibana (tachibana mandarin) is naturally found in Japan, Taiwan, and the Ryukyu archipelago (Tanaka, 1931), it separated from Asian mainland mandarins during the early Pleistocene era (about 2 mya). Genomic data suggest that tachibana mandarin does not have a separate taxonomic position but shows close affinity with C. reticulata (Hirai et al., 1990; Shimizu et al., 2016).
Key genes associated with citrus domestication and their important traits
Yield, fruit quality, and taste, including juiciness, texture, acidity, reduction in seed number, and peel color (Goldenberg et al., 2014; Zheng et al., 2019), and some reproductive traits such as apomixis (Wang et al., 2017a) are important traits related to citrus domestication. In addition, disease (especially the citrus canker and Huanglongbing disease) and insect/pest resistance of cultivated citrus generally decreased (Bernet et al., 2005; Bastianel et al., 2009; Asins et al., 2012; Cuenca et al., 2013). Comparative analysis of the wild and cultivated species revealed remarkable changes in reproduction mode from sexual reproduction to apomixis during citrus evolution (Wang et al., 2017a). One recent comparative study of wild species and cultivated mandarins indicated that the fruit acidity of cultivars has reduced significantly (Wang et al., 2018). Fruit acidity is one of the key factors that determine fruit taste, it can be evaluated by the sugar/acid ratio. Based on this, we speculate that fruit taste might be the first selected trait during domestication.
Asexual reproduction through apomixis
Apomixis is a natural phenomenon of asexual reproduction that produces progenies that are genetically identical to the mother plant (Conner et al., 2015). Apomixis is uncommon for most important agricultural crops except for citrus and apple. Apomixis was first reported in citrus In 1719 the same seed was found to produce two plantlets (Batygina and Vinogradova, 2007). Since then, it has been confirmed that some important commercial citrus species, such as sweet orange and grapefruit, develop more than one embryo from somatic nucellar cells and thus share the same genotype (Kepiro and Roose, 2010). Generally, one seed produces 2–10 embryos, but in some cases, it can produce 30 or more embryos (Koltunow, 1993). This phenomenon is known as “polyembryony” (a phenomenon referring to the development of two or more embryos from one fertilized egg). Polyembryony has been used extensively for propagation programs in citrus nurseries to produce uniform rootstocks from seeds. Moreover, the polyembryony trait, as a form of apomixis, has been fixed in citrus cultivars because it allows growers to maintain and disperse an elite line with desirable variations and traits without segregation.
Most commercially grown citrus cultivars are polyembryonic in nature. Relatively fewer citrus cultivars are monoembryonic, these include all citron, some mandarin hybrids, clementine cultivars, and pummelo (Wang et al., 2017a; Zhang et al., 2018). Recently, the citrus CitRWP gene has been shown to associate with polyembryony by both genetic mapping and association analysis (Table 2). Sequence analysis of this gene revealed a 203-bp miniature inverted-repeat transposable element (MITE) insertion in the promoter region of the CitRWP gene in polyembryonic genotypes in the Citrus genus (Wang et al., 2017a). Notably, CitRWP expression is higher in the ovules of polyembryonic cultivars than in those of monoembryonic cultivars, suggesting that it is a key candidate gene that governs polyembryony. A recent gene function study indicates that antisense interference of CitRKD1 (the same gene as CitRWP) expression in sweet orange abolishes nucellar embryogenesis in T1 regenerants (Shimada et al., 2018). Therefore, knockout of the CitRWP gene from polyembryonic citrus cultivars will probably reduce polyembryony in cultivated citrus and provide promising insights into apomixis research in citrus.
Table 2.
Genes controlling important traits in citrus.
| Serial No. | Gene details | Identified/cloned | Function study | Target trait | Reference |
|---|---|---|---|---|---|
| 1 | Papain-like cysteine protease | Sweet orange | – | Citrus Huanglongbing | Clark et al. (2018) |
| 2 | Accelerated cell death 2 | Sweet orange | Duncan grapefruit | Pang et al. (2020) | |
| 3 | 2-oxoglutarate and Fe(II)-dependent oxygenase gene | US-897 (Citrus reticulata × Poncirus trifoliata) | – | Albrecht and Bowman (2011) | |
| 5 | PtCDR2 and PtCDR8 | P. trifoliata | – | Rawat et al. (2017) | |
| 6 | AtNPR1 | Arabidopsis | Duncan grapefruit and Hamlin sweet orange | Robertson et al. (2018) | |
| 7 | CsLOB1 | Sweet orange Duncan grapefruit |
Editing of gene | Citrus canker |
Hu et al. (2014) Jia et al. (2017) |
| 8 | CitRWP | Citrus | – | Polyembryony | Wang et al. (2017a) |
| 9 | CgMYB58 | Pummelo | Expressed in citrus callus | Lignin biosynthesis | Shi et al. (2020) |
| 10 | S-RNase gene | Lemon | – | Self-incompatibility | Zhang et al. (2015) |
| 11 | Sm-RNase | Mandarin | – | Liang et al. (2020) | |
| 12 | FT gene | Citrus | Grapefruit | Early flowering | Sinn et al. (2020) |
| 13 | P. trifoliata | Trifoliate orange and satsuma mandarin |
Endo et al. (2005) Nishikawa et al. (2007) |
||
| 14 | CclSBP7 | Clementine mandarin | Arabidopsis thaliana | Zeng et al. (2019) | |
| 15 | miR3954 | Citrus | Hong Kong kumquat (Fortunella hindsii) | Liu et al. (2017a) | |
| 16 | CitdGlcTs | Citrus | Tobacco | Flavor | Chen et al. (2019) |
| 17 | Cit1,2RhaT | Citrus | Tobacco | Chen et al. (2019) | |
| 18 | Cm1,2RhaT | Pummelo | Hong Kong kumquat (F. hindsii) | Chen et al. (2019) | |
| 19 | CsVPP-1 and CsVPP-2 | Sweet orange | – | Hussain et al. (2020) | |
| 20 | CitAco3–CitIDH1–CitGS2 | Sweet orange | – | Acidity | Chen et al. (2013) |
| 21 | CsAPD2 | Sweet orange | – | Bai et al. (2020) | |
| 22 | CsPH8 | Sweet orange | Pummelo, tomato, and strawberry | Guo et al. (2016); Shi et al. (2019) | |
| 23 | CitPH1 and CitPH5 | Sour lemon, orange, pummelo and rangpur lime fruits | – | Strazzer et al. (2019) | |
| 24 | CWINVs, VINV, SPS2, SUT2, VPP-1, and VPP-2 | Sweet orange | – | Sugar | Hussain et al. (2020) |
| 25 | V-PPase1, 2 | Sweet orange | – | Hussain et al. (2020) | |
| 26 | CitPH1 and CitPH5 | Sour lemon, orange, pummelo, and rangpur lime fruits | – | Anthocyanin and flavor | Strazzer et al. (2019) |
| 27 | CsCYP75B1 | Sweet orange | A. thaliana | Flavonoid biosynthesis | Rao et al. (2020) |
| 28 | CsUDP78D3 | Sweet orange | A. thaliana | Anthocyanin | Rao et al. (2019b) |
| 29 | CgRuby1, CgRuby2, AbRuby2 | Atalantia and pummelo | A. thaliana | Huang et al. (2018) | |
| 30 | Ruby and Noemi (bHLH) | Citrus | – | Catalano et al. (2020) | |
| 31 | MYB3 | Sweet orange | Arabidopsis | Huang et al. (2020) | |
| 32 | CDG1 | Pummelo | Arabidopsis and tobacco | Delayed leaf greening | Yu et al. (2020) |
| 33 | CCD4 | Mandarins and its hybrids | – | Carotenoids | Zheng et al. (2019) |
| 34 | CsDxs and CsPsy | Sweet orange | – | Fanciullino et al. (2008) | |
| 35 | CitDXS1 and three CitPSY1 | Citrus | – | Peng et al. (2013) | |
| 36 | CsMADS5 and CsMADS6 | Sweet orange | – | Lu et al. (2018) | |
| 37 | PSY2, LYCB2, LYCE, and CCD4 | Pummelo | – | Jiang et al. (2019) |
Fruit acidity
Some citrus species, such as lemon, lime, wild mandarin, and sour orange, show an extremely low pH value of 2-3 due to a high level of acidification of vacuoles in juice vesicles. Based on the evidence from a recent population analysis of wild and cultivated mandarins, we speculate that reduced citric acid level is a remarkable trait for citrus domestication (Wang et al., 2018). This is different from the traditional view that increased sugar content is likely the most significant event of the domestication of fruit crops and reduced acidity is a hitchhiking effect.
The two citrus vacuolar P-ATPase homologs, CitPH1 and CitPH5, are strongly induced in highly acidic citrus species such as lime and lemon fruits, by contrast, their expression levels are significantly reduced in acidless mutants (Strazzer et al., 2019). Vacuolar ATPases can regulate the pH gradient across the tonoplast (Müller and Taiz, 2002; Nishi and Forgac, 2002; Shimada et al., 2006; Pittman, 2012; Rienmüller et al., 2012). A steep proton gradient across the vacuolar membrane due to differences in pH can drive the transport of citrate into the vacuole, however, the underlying mechanism remains unclear. The expression levels of CitPH1, CitPH5, and the PH3 (WRKY), PH4 (MYB) (Strazzer et al., 2019), and basic helix-loop-helix (bHLH) (citrus Noemi or CitAN1) transcription factors were reduced in the mutants (Butelli et al., 2019). In petunia, the homologs of these transcription factors are involved in the activation of PH1 and PH5 expression. Homologs of petunia PH4, which is a MYB transcription factor, has been reported to activate the promoter of the proton pumps (PH1 and PH5) in petunia as well as in citrus (Quattrocchio et al., 2006; Faraco et al., 2014; Butelli et al., 2019; Strazzer et al., 2019). These findings provide a guidance for regulating citric acid levels in fruits.
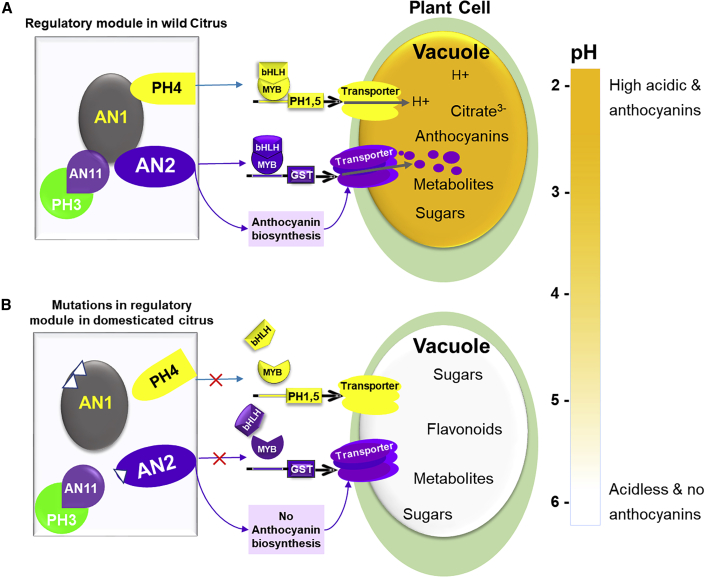
A previous genetic analysis revealed that the insertion of large retrotransposon fragments or deletion in the Noemi (CitAN1) gene results in acidless phenotypes and loss of proanthocyanidin and anthocyanin in sweet lime, citron, sweet orange, lemon, and limetta accessions (Butelli et al., 2019; Figure 4). Moreover, a variation in the core promoter region of the Noemi gene in two limetta accessions reduces its expression and increases the pH of the juice, indicating that Noemi is a key gene that contributes to fruit acidity. Previous studies revealed that a bHLH transcription factor (AN1) is involved in both vacuolar acidification and anthocyanin accumulation in petunia (Spelt et al., 2002; Quattrocchio et al., 2006; Faraco et al., 2014). Recent studies showed that a specific mutation in CitAN1 is responsible for the reduced expression of CitPH1 and CitPH5 in acidless Faris lemon and other acidless citrus fruits (Strazzer et al., 2019). These data suggest that the reduction or loss of anthocyanins may be due to the hitchhiking effect of fruit acidity selection in some domestication types, when mutations occur on the bHLH transcription factor, a common regulator for both citric acid and anthocyanin metabolism.
Figure 4.
Domestication modules of fruit acidity and anthocyanin accumulation in citrus.
The regulatory module is designated WD-repeat-MYB-bHLH-WRKY, it is composed of three anthocyanin pathway-related transcription regulators AN11 (WD-repeat), AN2 (MYB), and AN1 (bHLH) and two pH-specific transcription factors PH3 (WRKY) and PH4 (MYB). These regulators are named after their petunia homologs (Spelt et al., 2002; Quattrocchio et al., 2006; Verweij et al., 2016). (A) Most wild citrus can accumulate high levels of citric acid in fruits. The regulatory module can activate the CitPH1 and CitPH5 genes, which encode the proton-pumping complex and are responsible for generating a discrepancy in proton gradient to drive citrate transport. Anthocyanin accumulation in tender leaves, early flowers, and fruits is mediated by anthocyanin transporter genes. The AN1 transcription factor, also known as Noemi, which encodes a bHLH transcription factor, is involved in the regulation of both pH and anthocyanin accumulation (Butelli et al., 2019). AN1 can interact with both PH4 (a key regulator of fruit pH) and AN2 (also known as Ruby1, a key regulator of anthocyanins), both encode MYB transcription factors (Butelli et al., 2017; Huang et al., 2018). Another bHLH transcription factor named bHLH1 can interact with only AN2 to regulate anthocyanin accumulation in fruits (Huang et al., 2018). (B) Published studies indicating that in domesticated citrus, mutations mostly occurred in the regulatory module containing the AN2 or AN1 transcription factor. The AN2 mutation disrupts anthocyanin biosynthesis, whereas the AN1 mutation affects the accumulation of anthocyanins, proanthocyanins, and citric acid (Butelli et al., 2019).
Fruit color: anthocyanins
Anthocyanins are pigment compounds that are abundant in several wild citrus accessions but absent from most cultivated citrus (Butelli et al., 2017; Huang et al., 2018). Anthocyanins are a subclass of flavonoids, which endow young leaves, flowers, petals, and fruits with different colors. Transcription factor complexes that regulate anthocyanin levels and acidification have long been studied in many species such as Arabidopsis, maize, and petunia (Spelt et al., 2002; Quattrocchio et al., 2006; Verweij et al., 2016). Complexes composed of the WD40-repeat proteins, as well as MYB, bHLH, and WRKY transcription factors are named WD-repeat-MYB-bHLH-WRKY (WMBW for short). Specifically, the regulatory complex is composed of three anthocyanin pathway-related transcriptional regulators, AN1 (bHLH), AN2 (MYB), and AN11 (WD-repeat), as well as two pH-specific transcription factors, PH3 (WRKY) and PH4 (MYB). Ruby1, which is homologous to the AN2 gene, is the first identified MYB transcription factor that plays a critical role in the regulation of anthocyanin biosynthesis in blood orange (Butelli et al., 2012). Ruby2 and Ruby1, two neighboring genes, form a cluster to regulate anthocyanin levels in citrus (Huang et al., 2018). In the distant wild citrus (A. buxifolia), Ruby2 and Ruby1 both function as anthocyanin activators but Ruby2 functions in tender leaves, whereas Ruby1 functions in fruits. Ruby2 mutations are found in both recent wild citrus and domesticated citrus (Huang et al., 2018). Recently, MYB3 has been identified as a balance factor in anthocyanin metabolism because it is subjected to Ruby1 regulation and shows an expression pattern similar to Ruby1, however, MYB3 acts as a repressor of anthocyanin biosynthesis (Huang et al., 2020).
The citrus HLB and canker disease
Huanglongbing (HLB), also known as the greening disease, is the most devastating citrus disease in the world (Wang and Trivedi, 2013; Wang 2019). HLB is caused by three phloem-limited, gram-negative, and fastidious bacteria known as “Candidatus Liberibacter asiaticus” (CLas) (Bové, 2006; Gottwald et al., 2007; Ma et al., 2014), “Candidatus Liberibacter americanus,” and “Candidatus Liberibacter africanus” (Teixeira et al., 2005). HLB can reduce citrus yield by 30%–100% (Bové, 2006) and result in small, greenish, and poor quality fruits, causing huge losses to growers (Bassanezi and Stuchi, 2009; Wang et al., 2017b; Dagulo et al., 2010; Bassanezi et al., 2011). The infected fruits are highly acidic, with reduced sugar contents (Brodersen et al., 2014; Massenti et al., 2016), a metallic, bitter taste, and less juice (Kiefl et al., 2017; Dala Paula et al., 2018).
Recently, some wild citrus germplasms or distantly related citrus species, such as Eremocitrus glauca, Microcitrus australasica (Ramadugu et al., 2016), orange jasmine (Murraya paniculata) (Miles et al., 2017), Poncirus trifoliata (Killiny and Hijaz, 2016), and papeda (C. ichangensis) (Wu et al., 2020) have been identified as highly tolerant to HLB, whereas cultivated citrus such as C. maxima (Ramadugu et al., 2016), Citrus sinensis, and mandarins (C. reticulata) are considered highly susceptible to HLB (Folimonova et al., 2009). The tolerance was speculated to be related to the defense response and/or antimicrobial secondary metabolites (Hammond-Kosack and Jones, 1996; Christeller and Laing, 2005; Liu et al., 2013; Rao et al., 2019a). Interestingly, several transcriptomic studies of HLB-infected citrus revealed that several genes related to the WRKY family, the PR family, and secondary metabolites were stimulated in response to CLas invasion (Rawat et al., 2015; Hu et al., 2017; Yu et al., 2017). Generally, HLB-tolerant citrus species show a fast and resilient response to CLas invasion (before Las stability), whereas susceptible species show no or a delayed response (Hu et al., 2017; Wu et al., 2020). Furthermore, the CLas pathogen releases some virulence proteins and enzymes to degrade salicylic acid and its derivatives, thus damaging the host defense system (Li et al., 2017a) and resulting in severe symptoms (Tolba and Soliman, 2015). Recent studies have advanced the understanding of interactions between CLas effectors and citrus genes, such as the interaction between defense-inducible papain-like cysteine proteases and Sec-delivered effector 1 (SDE1) (Clark et al., 2018) and that between accelerated cell death 2 (ACD2) in susceptible citrus and SDE15 (Pang et al., 2020).
Citrus canker is another devastating bacterial disease caused by Xanthomonas strains, such as Xanthomonas citri subsp. citri and Xanthomonas axonopodis pv. aurantifolii (Schubert et al., 2001; Sun et al., 2004). Citrus canker causes severe necrosis symptoms on leaves and fruits (Stover et al., 2014), resulting in considerable yield losses (Gottwald et al., 2002). Almost all cultivated citrus species are canker susceptible (Gottwald et al., 1993), whereas some wild citrus species show tolerance or resistance to canker. Previous studies have reported that canker bacteria inject transcription activator-like effectors to bind to the promoter elements of susceptible host genes such as the citrus LATERAL ORGAN BOUNDARIES 1 (CsLOB1) gene to stimulate LOB1 expression through type III secretion pathways (Hu et al., 2014). The high expression level of CsLOB1 (a transcription factor) can promote pustule formation and bacterial growth (Xu et al., 2016; Zhang et al., 2017b). A. buxifolia, belongs to primitive citrus, It is tolerant to some abiotic and biotic stresses and is considered canker tolerant (Shi et al., 2014; Yang, et al., 2013). A recent study has suggested that natural variations in LOB1 and TFIIAγ, which encodes a transcription factor that stabilizes the interaction between the effector and LOB1 to confer resistance to the citrus canker disease in A. buxifolia (Tang, et al., 2021).
Recent studies have reported that citrus relatives (M. paniculata) and primitive (A. buxifolia) and wild (E. glauca, M. australasica) citrus show strong tolerance against the devastating HLB and canker diseases. The availability of the bacterial (CLas) and various citrus genomes has facilitated the understanding of pathogenicity, as well as the citrus tolerance and susceptibility mechanisms. We propose that identifying resistant genes from citrus wild relatives and comparing these genes with those in cultivated citrus are important to understand the loss of resistance in cultivated species, and that the the knock down/out of susceptible genes offers an alternative way to improve disease resistance in citrus cultivars.
Juvenility
Normally, citrus seedlings have a very long juvenile phase (about 3–20 years), which hinders the breeding and improvement of citrus. In the past 2 decades, efforts have been made to minimize the juvenile phase (Cervera et al., 2009; Endo et al., 2009; Flachowsky et al., 2009). To promote early flowering in citrus, the Arabidopsis APETALA1 (AP1) and LEAFY (LFY) genes were introduced into citrange (P. trifoliata L. Raf. × hybrid of C. sinensis L. Osbeck) (Peña et al., 2001), and transgenic citrange plants overexpressing the AP1 and LFY genes flowered early and produced fertile and normal flowers. Fruits were obtained from first-year transgenic plants that were only 2–20 months old and bore their first flowers, suggesting that the juvenile phase was significantly reduced to less than 5 years in transgenic citrange compared with control plants (Peña et al., 2001). Zygotic seedlings obtained by crossing AP1 and LFY-transgenic citrange also showed early flowering with normal fruit setting in the first spring. These results confirmed that early flowering can be induced in cultivated citrus species by introducing the AP1 and LFY genes.
In citrus, the use of endogenous genes has also been reported to minimize the juvenile period (Endo et al., 2005; Sinn et al., 2020). The citrus (ortholog) FLOWERING LOCUS T (FT) gene, which is involved in the photoperiodic induction of flowering (Koornneef et al., 1991), was introduced into P. trifoliata (trifoliate orange) under the 35S CaMV promoter. The transgenic trifoliate orange expressing the citrus FT gene showed early flowering just 6–11 months after transformation and produced fertile pollen and normal flowers. Zygotic seedlings obtained by crossing these transgenic plants with the monoembryonic “Kiyomi” tangor (C. sinensis × C. unshiu) also showed a greatly shortened juvenile phase, these seedlings flowered right after germination (Endo et al., 2005). Flowering-related orthologous genes in citrus, such as FT, CsTFL1 (TERMINAL FLOWER 1), CsLFY (LEAFY), and CsAP1 (APETALLA1) (Nishikawa et al., 2007, 2010), were highly expressed during putative induced flowering. These outcomes suggest that FT overexpression might have reduced the juvenile period in cultivated citrus species. Moreover, the overexpression of miR3954 facilitated early flowering in transgenic kumquat (Liu et al., 2017a). One recent study reported that chimeric FT protein successfully reduced flowering time in grapefruit (Citrus paradisi) without negative effects. Therefore, transgenic expression of chimeric FT proteins provides a promising alternative tool for minimizing juvenility in cultivated citrus species (Sinn et al., 2020).
Recently, SQUAMOSA-promoter binding protein (SBP/SPL)-box genes have been shown to play critical roles in plant growth, flowering, and fruit development (Chen et al., 2010; Shalom et al., 2015; Liu et al., 2017b; Long et al., 2018). A total of 16 and 15 SBP-box genes have been identified in Arabidopsis and citrus, respectively. To understand the function of these genes, clementine mandarin SBP-box genes were cloned and overexpressed in Arabidopsis, and transgenic Arabidopsis plants expressing the CclSBP7 gene showed flowered earlier than control plants (Zeng et al., 2019). These results indicate that CclSBP7 from is involved in early flowering in clementine mandarin and can induce early flowering in other citrus species to shorten the juvenile phase.
Other important traits
The appearance of red fruit of some mandarin landraces is the outcome of long-term selection in recent breeding history. Using an integrated genetic approach, we revealed that a change in a cis-regulatory element in CCD4b, which encodes CAROTENOID CLEAVAGE DIOXYGENASE 4b, is a major genetic determinant of natural variation in C30 apocarotenoids responsible for the red color of citrus peel (Zheng et al., 2019). The CsMADS6 transcription factor modulates carotenoid metabolisms by directly binding to a set of carotenogenic genes, including those encoding the rate-limiting enzyme phytoene synthase (PSY), the branch-limiting Lycopene b-cyclases, phytoene desaturase (PDS), and carotenoid cleavage dioxygenase1 (CCD1). The overexpression of CsMADS6 increases carotenoid content (Lu et al., 2018). Recently, Citrus Delayed Greening gene 1 (CDG1) has been reported to contribute to delayed leaf greening by inhibiting the synthesis of chlorophyll (Yu et al., 2020).
Some citrus species show self-incompatibility. A molecular study in lemon indicates that the S-RNase gene is related to self-incompatibility (Zhang et al., 2015). One recent study has reported that a predominant single-nucleotide mutation in Sm-RNase causes the loss of its natural function, in turn producing self-compatible citrus. A large-scale genetic analysis indicates that most wild citrus are self-incompatible, and that the transition from self-incompatibility to self-compatibility is because Sm-RNase initially arose in mandarin, and then passed to its hybrids and became fixed in cultivated cultivars (Liang et al., 2020).
Mini-citrus (Hong Kong kumquat): a wild species as a candidate model for genetic and gene function studies
Fortunella hindsii, commonly known as Hong Kong kumquat, is a wild citrus species (Figure 5). Its fruit is spicy but has an attractive color, it is thus frequently used for gardening and ornamental purposes. Its tree canopy is considered the smallest of all citrus species (Swingle, 1967). In addition, Hong Kong kumquat is also famous among citrus species due to its distinctive features, such as continuous and very early flowering with a very short juvenile period of ∼8 months (Ye, 1985; Zhu et al. 2019). Among all citrus, F. hindsii has the highest callus induction rate and callus can be induced form its roots and seedlings, making it an ideal model species for citrus research (Deng and Zhang, 1988). The F. hindsii transgenic system has been established to unravel the genetic basis of carotenoid biosynthesis (Zhang et al., 2009; Cao et al., 2015). A recent study has reported that F. hindsii exhibits both asexual and sexual reproduction modes (Zhu et al., 2019), and the latter is ideal for both genetic studies and transgenic segregation because most citrus species undergo asexual reproduction.
Figure 5.
Mini-citrus (F. hindsii): a candidate model species for citrus genetics studies and gene functional studies.
Left panel: Fruits on a young F. hindsii seedling; right panel: a mini-citrus plant transformed with the GFP marker.
The successful applications of CRISPR-Cas9 in citrus have been reported recently (Zhang et al., 2017a; Jia et al., 2017; Peng et al., 2017), and this method has also been applied to the mini-citrus (Zhu et al., 2019). The application of CRISPR-Cas9 strategy in the functional genomics studies of citrus species has been restricted due to their heterogeneous genetic backgrounds and their nature as complicated and unstable chimeras (Zhang et al., 2017a; Jia et al., 2017), with different citrus species exhibiting asynchronous growth patterns and distinctive organs (Guo et al., 2012; Zeng et al., 2013; Liu et al., 2016; Lu et al., 2016). Furthermore, a recently published report reveals that CRISPR-Cas9-based targeted mutations successfully reproduced transgenic T1 (heterozygous, biallelic, chimeric, and homozygous) uniformly mutated plants (Ma et al., 2015). Conversely, in citrus, attaining a suitable T1 plant is restricted by polyembryony (asexual reproduction) features and the long juvenility. Hence, it is anticipated that the CRISPR method is applicable in F. hindsii, which has a short juvenile phase, and the application of this method will facilitate the functional study of desired traits and unravel the genomic features of citrus.
Concluding remarks and perspectives
This review summarizes the genomic resources of various citrus species. It reveals current progress in the understanding of citrus origin and domestication with a focus on key agronomic traits and the corresponding genes. We have highlighted that fruit acidity has been targeted during citrus domestication, whereas anthocyanin was very likely selected due to the hitchhiking effect during domestication, considering that the AN1 gene was found to regulate both anthocyanin and acidity metabolism. In addition, we have addressed the latest progress in the identification of genes controlling apomixis (polyembryony), early flowering, and resistance against HLB and citrus canker.
Future research based on more citrus genomes is suggested to comprehensively investigate the genomic changes from the wild to cultivated citrus. The assembly of genomes of wild species and landraces is particularly necessary for fulfilling such purpose. One type of the largely neglected changes during domestication is probably associated with transposable elements. Several studies have revealed that the insertion of MITE into key genes is responsible for apomixis (Wang et al., 2017a), the red color of fruit peel (Zheng et al., 2019), and anthocyanin accumulation (Butelli et al., 2017; Huang et al., 2018). It will be interesting to investigate whether MITE or other types of transposable elements have participated in citrus domestication.
The change in disease resistance/tolerance during citrus domestication is another interesting topic. Citrus HLB and canker are the most devastating diseases when a uniform cultivar is planted in a large farm. Because wild citrus has grown in natural environments for tens or hundreds of years without any artificial protection or management, future studies should focus on revealing the mechanisms of HLB susceptibility, tolerance, and host–pathogen interactions in wild citrus relatives.
The genes related to important agronomic traits provide targets for precise breeding. One way to develop molecular markers is to select ideal genotypes at the young seedling stage. Monoembryonic (sexual reproduction) and polyembryonic (apomixis) species can be selected for different breeding purposes using markers developed from MITE insertions in the CitRWP gene (Wang et al., 2017a). Moreover, using the latest genome editing technology, we can separate linked traits selected during domestication. For example, anthocyanin- and acidity-related genes or their promoters can be manipulated independently to increase anthocyanin content, which is beneficial to human health, while reducing fruit acidity in new cultivars. By tracing the domestication history of thousands of years, we provide a theoretical basis for developing new citrus varieties to meet the consumers' demands in the coming decades.
Funding
This project was supported by the National Natural Science Foundation of China (31925034), the National Key Research and Development Program of China (2018YFD1000101), and Fundamental Research Funds for the Central Universities.
Author contributions
M.J.R., Q.X., and H.Z. wrote the original draft. M.J.R. prepared the figures and tables. Q.X. provided resources and funding and revised/edited the original manuscript.
Acknowledgments
Great gratitude goes to linguistics professor Ping Liu from the Foreign Language College, Huazhong Agriculture University, Wuhan, China, for her work of English editing and language polishing. No conflict of interest declared.
Published: December 30, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
References
- Albrecht U., Bowman K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco× Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience. 2011;46:16–22. [Google Scholar]
- Asins M.J., Fernández-Ribacoba J., Bernet G.P., Gadea J., Cambra M., Gorris M.T., Carbonell E.A. The position of the major QTL for Citrus tristeza virus resistance is conserved among Citrus grandis, C. aurantium and Poncirus trifoliata. Mol. Breed. 2012;29:575–587. [Google Scholar]
- Bai Y.X., Hussain S.B., Wei X., Shi C.Y., Liu D.H., Liu Y.Z. Identification and transcript analysis of CsAPD2 reveal its potential role in citric acid accumulation in citrus fruits. Sci. Hortic. (Amsterdam) 2020;272:109607. [Google Scholar]
- Bassanezi R.B., Stuchi E.S. Effects of huanglongbing on fruit quality of sweet orange cultivars in Brazil. Eur. J. Plant Pathol. 2009;125:565–572. [Google Scholar]
- Bastianel M., Cristofani-Yaly M., de Oliveira A.C., Freitas-Astúa J., Garcia A.A.F., de Resende M.D.V., Rodrigues V., Machado M.A. Quantitative trait loci analysis of citrus leprosis resistance in an interspecific backcross family of (Citrus reticulata Blanco× C. sinensis L. Osbeck)× C. sinensis L. Osb. Euphytica. 2009;169:101–111. [Google Scholar]
- Batchelor L.D. Vol. 19. University of California Press; Berkeley, CA: 1948. History, botany, and Breeding; p. 681. (The Citrus Industry by H. J. Webber and L. D. Batchelor, Vol. II in 1948). [Google Scholar]
- Batygina T.B., Vinogradova G.Y. Phenomenon of polyembryony. Genetic heterogeneity of seeds. Russ. J. Dev. Biol. 2007;38:126–151. [PubMed] [Google Scholar]
- Beattie, G. A. C., Holford, P., Mabberley, D. J., Haigh, A. M., and Broadbent, P. (2008). On the origins of citrus, huanglongbing, Diaphorina citri and Trioza erytreae. In Proceedings of the International Research Conference on Huanglongbing, pp. 23–56. Florida Citrus Mutual Orlando.
- Bernet G.P., Margaix C., Jacas J., Carbonell E.A., Asins M.J. Genetic analysis of citrus leafminer susceptibility. Theor. Appl. Genet. 2005;110:1393–1400. doi: 10.1007/s00122-005-1943-6. [DOI] [PubMed] [Google Scholar]
- Bové J.M. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus [Asia; South Africa; Brazil; Florida] J. Plant Pathol. 2006;88:7–37. [Google Scholar]
- Brodersen C., Narciso C., Reed M., Etxeberria E. Phloem production in Huanglongbing- affected Citrus trees. Hortscience. 2014;49:59–64. [Google Scholar]
- Brophy J.J., Goldsack R.J., Forster P.I. The leaf oils of the Australian species of Citrus (Rutaceae) J. Essent. Oil Res. 2001;13:264–268. [Google Scholar]
- Butelli E., Licciardello C., Zhang Y., Liu J., Mackay S., Bailey P., Reforgiato-Recupero G., Martin C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell. 2012;24:1242–1255. doi: 10.1105/tpc.111.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E., Garcia-Lor A., Licciardello C., Las Casas G., Hill L., Recupero G.R., Keremane M.L., Ramadugu C., Krueger R., Xu Q. Changes in anthocyanin production during domestication of Citrus. Plant Physiol. 2017;173:2225–2242. doi: 10.1104/pp.16.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E., Licciardello C., Ramadugu C., Durand-Hulak M., Celant A., Reforgiato Recupero G., Froelicher Y., Martin C. Noemi controls production of flavonoid pigments and fruit acidity and illustrates the domestication routes of modern citrus varieties. Curr. Biol. 2019;29:158–164 e152. doi: 10.1016/j.cub.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Cao H., Wang J., Dong X., Han Y., Ma Q., Ding Y., Zhao F., Zhang J., Chen H., Xu Q. Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. BMC Plant Biol. 2015;15:1–16. doi: 10.1186/s12870-015-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano C., Ciacciulli A., Salonia F., Russo M.P., Caruso P., Caruso M., Russo G., Distefano G., Licciardello C. Target-genes reveal species and genotypic specificity of anthocyanin pigmentation in citrus and related genera. Genes. 2020;11:807. doi: 10.3390/genes11070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Navarro L., Pena L. Gene stacking in 1-year-cycling APETALA1 citrus plants for a rapid evaluation of transgenic traits in reproductive tissues. J. Biotechnol. 2009;140:278–282. doi: 10.1016/j.jbiotec.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang Z., Liu D., Zhang K., Li A., Mao L. SQUAMOSA promoter-binding protein-like transcription factors: Star players for plant growth and development. J. Integr. Plant Biol. 2010;52:946–951. doi: 10.1111/j.1744-7909.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- Chen M., Xie X., Lin Q., Chen J., Grierson D., Yin X., Sun C., Chen K. Differential expression of organic acid degradation-related genes during fruit development of Navel oranges (Citrus sinensis) in two habitats. Plant Mol. Biol. Rep. 2013;31:1131–1140. [Google Scholar]
- Chen J., Yuan Z., Zhang H., Li W., Shi M., Peng Z., Li M., Tian J., Deng X., Cheng Y. Cit1, 2RhaT and two novel CitdGlcT s participate in flavor-related flavonoid metabolism during citrus fruit development. J. Exp. Bot. 2019;70:2759–2771. doi: 10.1093/jxb/erz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeller J., Laing W. Plant serine proteinase inhibitors. Protein Pept. Lett. 2005;12:439–447. doi: 10.2174/0929866054395329. [DOI] [PubMed] [Google Scholar]
- Clark K., Franco J.Y., Schwizer S., Pang Z., Hawara E., Liebrand T.W.H., Pagliaccia D., Zeng L., Gurung F.B., Wang P. An effector from the Huanglongbing-associated pathogen targets citrus proteases. Nat. Commun. 2018;9:1718. doi: 10.1038/s41467-018-04140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift P.D., Wan S., Blusztajn J. Reconstructing chemical weathering, physical erosion and monsoon intensity since 25 Ma in the northern South China Sea: a review of competing proxies. Earth-science Rev. 2014;130:86–102. [Google Scholar]
- Conner J.A., Mookkan M., Huo H., Chae K., Ozias-Akins P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. U S A. 2015;112:11205–11210. doi: 10.1073/pnas.1505856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca J., Aleza P., Vicent A., Brunel D., Ollitrault P., Navarro L. Genetically based location from triploid populations and gene ontology of a 3.3-Mb genome region linked to Alternaria brown spot resistance in citrus reveal clusters of resistance genes. PLoS One. 2013;8:e76755. doi: 10.1371/journal.pone.0076755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curk F., Ollitrault F., Garcia-Lor A., Luro F., Navarro L., Ollitrault P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016;117:565–583. doi: 10.1093/aob/mcw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagulo L., Danyluk M.D., Spann T.M., Valim M.F., Goodrich-Schneider R., Sims C., Rouseff R. Chemical characterization of orange juice from trees infected with citrus greening (Huanglongbing) J. Food Sci. 2010;75:C199–C207. doi: 10.1111/j.1750-3841.2009.01495.x. [DOI] [PubMed] [Google Scholar]
- Dala Paula B.M., Raithore S., Manthey J.A., Baldwin E.A., Bai J., Zhao W., Glória M.B.A., Plotto A. Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. Food Sci. Technol. 2018;91:518–525. [Google Scholar]
- Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr. J. Microbiol. Res. 2009;3:981–996. [Google Scholar]
- Deng X.X., Zhang W.C. The in vitro induction method of embryonic callus from seedling of Fortunella Hindsii Swingle. South China Fruits. 1988;1988:7–9. [Google Scholar]
- Economos C., Clay W.D. Nutritional and health benefits of citrus fruits. Energy. 1999;62:37. [Google Scholar]
- Endo T., Shimada T., Fujii H., Kobayashi Y., Araki T., Omura M. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.) Transgenic Res. 2005;14:703–712. doi: 10.1007/s11248-005-6632-3. [DOI] [PubMed] [Google Scholar]
- Endo T., Shimada T., Fujii H., Nishikawa F., Sugiyama A., Nakano M., Shimizu T., Kobayashi Y., Araki T., Peña L. Development of a CiFT co-expression system for functional analysis of genes in citrus flowers and fruit. J. Jpn. Soc. Hortic. Sci. 2009;78:74–83. [Google Scholar]
- Fanciullino A.L., Dhuique-Mayer C., Froelicher Y., Talón M., Ollitrault P., Morillon R. Changes in carotenoid content and biosynthetic gene expression in juice sacs of four orange varieties (Citrus sinensis) differing in flesh fruit color. J. Agric. Food Chem. 2008;56:3628–3638. doi: 10.1021/jf0732051. [DOI] [PubMed] [Google Scholar]
- Faraco M., Spelt C., Bliek M., Verweij W., Hoshino A., Espen L., Prinsi B., Jaarsma R., Tarhan E., de Boer A.H. Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep. 2014;6:32–43. doi: 10.1016/j.celrep.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Favre A., Päckert M., Pauls S.U., Jähnig S.C., Uhl D., Michalak I., Muellner-Riehl A.N. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol. Rev. 2015;90:236–253. doi: 10.1111/brv.12107. [DOI] [PubMed] [Google Scholar]
- Flachowsky H., Hanke M., Peil A., Strauss S.H., Fladung M. A review on transgenic approaches to accelerate breeding of woody plants. Plant Breed. 2009;128:217–226. [Google Scholar]
- Folimonova S.Y., Robertson C.J., Garnsey S.M., Gowda S., Dawson W.O. Examination of the responses of different genotypes of citrus to Huanglongbing (citrus greening) under different conditions. Phytopathology. 2009;99:1346–1354. doi: 10.1094/PHYTO-99-12-1346. [DOI] [PubMed] [Google Scholar]
- Garcia-Lor A., Curk F., Snoussi-Trifa H., Morillon R., Ancillo G., Luro F., Navarro L., Ollitrault P. A nuclear phylogenetic analysis: SNPs, indels and SSRs deliver new insights into the relationships in the ‘true citrus fruit trees’ group (Citrinae, Rutaceae) and the origin of cultivated species. Ann. Bot. 2013;111:1–19. doi: 10.1093/aob/mcs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lor A., Luro F., Ollitrault P., Navarro L. Genetic diversity and population structure analysis of Mandarin germplasm by nuclear, chloroplastic and mitochondrial markers. Tree Genet. Genomes. 2015;11:123. [Google Scholar]
- Gmitter F.G., Hu X. The possible role of Yunnan, China, in the origin of contemporary Citrus species (Rutaceae) Econ. Bot. 1990;44:267–277. [Google Scholar]
- Goldenberg L., Yaniv Y., Kaplunov T., Doron-Faigenboim A., Porat R., Carmi N. Genetic diversity among mandarins in fruit-quality traits. J. Agric. Food Chem. 2014;62:4938–4946. doi: 10.1021/jf5002414. [DOI] [PubMed] [Google Scholar]
- Gottwald T.R., Graham J.H., Civerolo E.L., Barrett H.C., Hearn C.J. Differential host range reaction of citrus and citrus relatives to citrus canker and citrus bacterial spot determinated by leaf mesophyll susceptiblity. Plant Dis. 1993;77:1004. [Google Scholar]
- Gottwald T.R., Pierce F., Graham J.H. Citrus canker: the pathogen and its impact plant health progress. Plant Health Prog. 2002;10:1–35. [Google Scholar]
- Gottwald T.R., da Graça J.V., Bassanezi R.B. Citrus huanglongbing: the pathogen and its impact. Plant Heal. Prog. 2007;6:1–37. [Google Scholar]
- Guo F., Zhou W., Zhang J., Xu Q., Deng X. Effect of the citrus lycopene β-cyclase transgene on carotenoid metabolism in transgenic tomato fruits. PLoS ONE. 2012;7:e32221. doi: 10.1371/journal.pone.0032221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L.-X., Shi C.Y., Liu X., Ning D.Y., Jing L.F., Yang H., Liu Y.Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provide a novel insight for an orange mutant. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep29343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. Southeast Asia’s changing palaeogeography. Blumea-Biodiversity. Evol. Biogeogr. Plants. 2009;54:148–161. [Google Scholar]
- Hammond-Kosack K.E., Jones J.D. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Mitsue S., Kita K., Kajiura I. A survey and isozyme analysis of wild Mandarin, tachibana (Citrus tachibana (Mak.) Tanaka) growing in Japan. J. Jpn. Soc. Hortic. Sci. 1990;59:1–7. [Google Scholar]
- Hu Y., Zhang J., Jia H., Sosso D., Li T., Frommer W.B., Yang B., White F.F. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. U S A. 2014;111:521–529. doi: 10.1073/pnas.1313271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhong X., Liu X., Lou B., Zhou C., Wang X. Comparative transcriptome analysis unveils the tolerance mechanisms of Citrus hystrix in response to ‘Candidatus Liberibacter asiaticus’ infection. PLoS One. 2017;12:e0189229. doi: 10.1371/journal.pone.0189229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Wang X., Tang Z., Yuan Y., Xu Y., He J., Jiang X., Peng S.A., Li L., Butelli E. Subfunctionalization of the Ruby2–Ruby1 gene cluster during the domestication of citrus. Nat. Plants. 2018;4:930–941. doi: 10.1038/s41477-018-0287-6. [DOI] [PubMed] [Google Scholar]
- Huang D., Tang Z., Fu J.L., Yuan Y., Deng X.X., Xu Q. CsMYB3 and CsRuby form an ‘Activator-and-repressor’ loop for the regulation of anthocyanin biosynthesis in citrus. Plant Cell Physiol. 2020;61:318–330. doi: 10.1093/pcp/pcz198. [DOI] [PubMed] [Google Scholar]
- Hussain S.B., Guo L.X., Shi C.Y., Khan M.A., Bai Y.X., Du W., Liu Y.Z. Assessment of sugar and sugar accumulation-related gene expression profiles reveal new insight into the formation of low sugar accumulation trait in a sweet orange (Citrus sinensis) bud mutant. Mol. Biol. Rep. 2020;47(4):2781–2791. doi: 10.1007/s11033-020-05387-6. [DOI] [PubMed] [Google Scholar]
- Jacques F.M.B., Guo S.X., Su T., Xing Y.W., Huang Y.J., Liu Y.S.C., Ferguson D.K., Zhou Z.K. Quantitative reconstruction of the Late Miocene monsoon climates of southwest China: a case study of the Lincang flora from Yunnan Province. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011;304:318–327. [Google Scholar]
- Jacques F.M.B., Su T., Spicer R.A., Xing Y.W., Huang Y.J., Zhou Z.K. Late Miocene southwestern Chinese floristic diversity shaped by the southeastern uplift of the Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014;411:208–215. [Google Scholar]
- Jia H., Zhang Y., Orbovi V., Xu J., White F.F., Jones J.B., Wang N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017;15(7):817–823. doi: 10.1111/pbi.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.-C., Zhang Y.-F., Lin Y.-J., Chen Y., Lu X.-K. Illumina® Sequencing reveals candidate genes of carotenoid metabolism in three pummelo Cultivars (Citrus maxima) with different pulp color. Int. J. Mol. Sci. 2019;20:2246. doi: 10.3390/ijms20092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepiro J.L., Roose M.L. AFLP markers closely linked to a major gene essential for nucellar embryony (apomixis) in Citrus maxima× Poncirus trifoliata. Tree Genet. Genomes. 2010;6:1–11. [Google Scholar]
- Kiefl J., Kohlenberg B., Hartmann A., Obst K., Paetz S., Krammer G., Trautzsch S. Investigation on key molecules of Huanglongbing (HLB)-induced orange juice off-flavor. J. Agric. Food Chem. 2017;66:2370–2377. doi: 10.1021/acs.jafc.7b00892. [DOI] [PubMed] [Google Scholar]
- Killiny N., Hijaz F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal. Behav. 2016;11:e1171449. doi: 10.1080/15592324.2016.1171449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A.M. Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell. 1993;5:1425. doi: 10.1105/tpc.5.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C.J., Van der Veen J.H. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. [Google Scholar]
- Krueger R.R., Navarro L. In: Citrus Germplasm Resources. Citrus Genetics, Breeding, and Biotechnology. Khan I.A., editor. CAB International; Wallingford, UK: 2007. pp. 45–140. [Google Scholar]
- Li J., Pang Z., Trivedi P., Zhou X., Ying X., Jia H., Wang N. ‘Candidatus Liberibacter asiaticus’ encodes a functional salicylic acid (SA) hydroxylase that degrades SA to suppress plant defenses. Mol. Plant-microbe Interact. 2017;30:620–630. doi: 10.1094/MPMI-12-16-0257-R. [DOI] [PubMed] [Google Scholar]
- Liang M., Cao Z., Zhu A., Liu Y., Tao M., Yang H., Xu Q., Wang S., Liu J., Li Y. Evolution of self-compatibility by a mutant S m-RNase in citrus. Nat. Plants. 2020;6:131–142. doi: 10.1038/s41477-020-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Heying E., Tanumihardjo S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012;11:530–545. [Google Scholar]
- Liu Y., Wang L., Cai G., Jiang S., Sun L., Li D. Response of tobacco to the Pseudomonas syringae pv. tomato DC3000 is mainly dependent on salicylic acid signaling pathway. FEMS Microbiol. Lett. 2013;344:77–85. doi: 10.1111/1574-6968.12157. [DOI] [PubMed] [Google Scholar]
- Liu C., Long J., Zhu K., Liu L., Yang W., Zhang H., Li L., Xu Q., Deng X. Characterization of a citrus R2R3-MYB transcription factor that regulates the flavonol and hydroxycinnamic acid biosynthesis. Sci. Rep. 2016;6:1–16. doi: 10.1038/srep25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ke L., Wu G., Xu Y., Wu X., Xia R., Deng X., Xu Q. miR3954 is a trigger of phasi RNA s that affects flowering time in citrus. Plant J. 2017;92:263–275. doi: 10.1111/tpj.13650. [DOI] [PubMed] [Google Scholar]
- Liu M.-Y., Wu X.-M., Long J.-M., Guo W.-W. Genomic characterization of miR156 and SQUAMOSA promoter binding protein-like genes in sweet orange (Citrus sinensis) Plant Cell Tissue Organ Cult. 2017;130:103–116. [Google Scholar]
- Long J.M., Liu C.Y., Feng M.Q., Liu Y., Wu X.M., Guo W.W. miR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J. Exp. Bot. 2018;69:2979–2993. doi: 10.1093/jxb/ery132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Zhang Y., Zheng X., Zhu K., Xu Q., Deng X. Molecular characterization, critical amino acid identification, and promoter analysis of a lycopene β-cyclase gene from citrus. Tree Genet. Genomes. 2016;12:106. [Google Scholar]
- Lu S., Zhang Y., Zhu K., Yang W., Ye J., Chai L., Xu Q., Deng X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018;176:2657–2676. doi: 10.1104/pp.17.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Liang M., Guan L., Xu M., Wen X., Deng X., Chen J. Population structures of ‘Candidatus Liberibacter asiaticus’ in southern China. Phytopathology. 2014;104:158–162. doi: 10.1094/PHYTO-04-13-0110-R. [DOI] [PubMed] [Google Scholar]
- Ma X., Chen L., Zhu Q., Chen Y., Liu Y.-G. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol. Plant. 2015;8:1285–1287. doi: 10.1016/j.molp.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Massenti R., Lo Bianco R., Sandhu A.K., Gu L., Sims C. Huanglongbing modifies quality components and flavonoid content of ‘Valencia’oranges. J. Sci. Food Agric. 2016;96:73–78. doi: 10.1002/jsfa.7061. [DOI] [PubMed] [Google Scholar]
- Mckey D., Elias M., Pujol B., Duputié A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010;186:318–332. doi: 10.1111/j.1469-8137.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- Miles G.P., Stover E., Ramadugu C., Keremane M.L., Lee R.F. Apparent tolerance to huanglongbing in citrus and citrus-related germplasm. HortScience. 2017;52:31–39. [Google Scholar]
- Miller A.J., Gross B.L. From forest to field: perennial fruit crop domestication. Am. J. Bot. 2011;98:1389–1414. doi: 10.3732/ajb.1000522. [DOI] [PubMed] [Google Scholar]
- Müller M.L., Taiz L. Regulation of the lemon-fruit V-ATPase by variable stoichiometry and organic acids. J. Membr. Biol. 2002;185:209–220. doi: 10.1007/s00232-001-0124-z. [DOI] [PubMed] [Google Scholar]
- Nishi T., Forgac M. The vacuolar (H+)-ATPases nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- Nishikawa F., Endo T., Shimada T., Fujii H., Shimizu T., Omura M., Ikoma Y. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma Mandarin (Citrus unshiu Marc.) J. Exp. Bot. 2007;58:3915–3927. doi: 10.1093/jxb/erm246. [DOI] [PubMed] [Google Scholar]
- Nishikawa F., Endo T., Shimada T., Fujii H., Shimizu T., Kobayashi Y., Araki T., Omura M. Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.) Tree Physiol. 2010;30:431–439. doi: 10.1093/treephys/tpp122. [DOI] [PubMed] [Google Scholar]
- Pang Z., Zhang Li., Coaker G.L., Ma W., He S.Y., Wang W. Citrus CsACD2 is a target of Candidatus Liberibacter asiaticus in huanglongbing disease. Plant Physiol. 2020 doi: 10.1104/pp.20.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña L., Martín-Trillo M., Juárez J., Pina J.A., Navarro L., Martínez-Zapater J.M. Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat. Biotechnol. 2001;19:263–267. doi: 10.1038/85719. [DOI] [PubMed] [Google Scholar]
- Peng G., Wang C., Song S., Fu X., Azam M., Grierson D., Xu C. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation. Plant Physiol. Biochem. 2013;71:67–76. doi: 10.1016/j.plaphy.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Peng A., Chen S., Lei T., Xu L., He Y., Wu L., Yao L., Zou X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017;15:1509–1519. doi: 10.1111/pbi.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeil B.E., Crisp M.D. The age and biogeography of Citrus and the orange subfamily (Rutaceae: Aurantioideae) in Australasia and New Caledonia. Am. J. Bot. 2008;95:1621–1631. doi: 10.3732/ajb.0800214. [DOI] [PubMed] [Google Scholar]
- Pittman J. Multiple transport pathways for mediating intracellular pH homeostasis: the contribution of H+/ion exchangers. Front. Plant Sci. 2012;3:11. doi: 10.3389/fpls.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F., Verweij W., Kroon A., Spelt C., Mol J., Koes R. PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell. 2006;18:1274–1291. doi: 10.1105/tpc.105.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadugu C., Keremane M.L., Halbert S.E., Duan Y.P., Roose M.L., Stover E., Lee R.F. Long-term field evaluation reveals huanglongbing resistance in Citrus relatives. Plant Dis. 2016;100:1858–1869. doi: 10.1094/PDIS-03-16-0271-RE. [DOI] [PubMed] [Google Scholar]
- Rao M.J., Ding F., Wang N., Deng X., Xu Q. Metabolic mechanisms of host species against citrus Huanglongbing (Greening Disease) Crit. Rev. Plant Sci. 2019;0:1–16. [Google Scholar]
- Rao M.J., Xu Y., Huang Y., Tang X., Deng X., Xu Q. Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 2019;19(1):603. doi: 10.1186/s12870-019-2212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.J., Xu Y., Tang X., Huang Y., Liu J., Deng X. CsCYT75B1, a Citrus CYTOCHROME P450 gene, is involved in accumulation of antioxidant flavonoids and induces drought tolerance in transgenic Arabidopsis. Antioxidants. 2020;9(2):161. doi: 10.3390/antiox9020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat N., Kiran S.P., Du D., Gmitter F.G., Deng Z. Comprehensive meta-analysis, co-expression, and miRNA nested network analysis identifies gene candidates in citrus against Huanglongbing disease. BMC Plant Biol. 2015;15:184. doi: 10.1186/s12870-015-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat N., Kumar B., Albrecht U., Du D., Huang M., Yu Q., Zhang Y., Duan Y.P., Bowman K.D., Gmitter F.G., Jr. Genome resequencing and transcriptome profiling reveal structural diversity and expression patterns of constitutive disease resistance genes in Huanglongbing-tolerant Poncirus trifoliata and its hybrids. Hortic. Res. 2017;4:17064. doi: 10.1038/hortres.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther W. History and development of the Citrus industry. In: Webber H.J., Batchelor D.L., editors. Vol. I. University of California; Berkeley: 1967. pp. 1–39. [Google Scholar]
- Rienmüller F., Dreyer I., Schönknecht G., Schulz A., Schumacher K., Nagy R., Martinoia E., Marten I., Hedrich R. Luminal and cytosolic pH feedback on proton pump activity and ATP affinity of V-type ATPase from Arabidopsis. J. Biol. Chem. 2012;287:8986–8993. doi: 10.1074/jbc.M111.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.J., Zhang X., Gowda S., Orbović V., Dawson W.O., Mou Z. Overexpression of the Arabidopsis NPR1 protein in citrus confers tolerance to Huanglongbing. J. Citrus Pathol. 2018;5:1–8. [Google Scholar]
- Rouseff R.L., Nagy S. Health and nutritional benefits of citrus fruit components. Food Technol. 1994;48(11):125–132. [Google Scholar]
- Schubert T.S., Rizvi S.A., Sun X., Gottwald T.R., Graham J.H., Dixon W.N. Meeting the challenge of eradicating citrus canker in Florida again. Plant Dis. 2001;85:340–356. doi: 10.1094/PDIS.2001.85.4.340. [DOI] [PubMed] [Google Scholar]
- Shalom L., Shlizerman L., Zur N., Doron-Faigenboim A., Blumwald E., Sadka A. Molecular characterization of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) gene family from Citrus and the effect of fruit load on their expression. Front. Plant Sci. 2015;6:389. doi: 10.3389/fpls.2015.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Guo X., Chen Y., Zhou L., Zhang D. Isolation and characterization of 19 polymorphic microsatellite loci for Atalantia buxifolia (Rutaceae), a traditional medicinal plant. Conservation Genet. Res. 2014;6:857–859. [Google Scholar]
- Shi C.Y., Hussain S.B., Yang H., Bai Y.X., Khan M.A., Liu Y.Z. CsPH8, a P-type proton pump gene, plays a key role in the diversity of citric acid accumulation in Citrus fruits. Plant Sci. 2019;289:110288. doi: 10.1016/j.plantsci.2019.110288. [DOI] [PubMed] [Google Scholar]
- Shi M., Liu X., Zhang H., He Z., Yang H., Chen J., Feng J., Yang W., Jiang Y., Yao J.L. The IAA-and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic. Res. 2020;7:1–14. doi: 10.1038/s41438-020-00360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Nakano R., Shulaev V., Sadka A., Blumwald E. Vacuolar citrate/H+ symporter of citrus juice cells. Planta. 2006;224:472–480. doi: 10.1007/s00425-006-0223-2. [DOI] [PubMed] [Google Scholar]
- Shimada T., Endo T., Fujii H., Nakano M., Sugiyama A., Daido G., Ohta S., Yoshioka T., Omura M. MITE insertion-dependent expression of CitRKD1 with a RWP-RK domain regulates somatic embryogenesis in citrus nucellar tissues. BMC Plant Biol. 2018;18:166. doi: 10.1186/s12870-018-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Kitajima A., Nonaka K., Yoshioka T., Ohta S., Goto S., Toyoda A., Fujiyama A., Mochizuki T., Nagasaki H. Hybrid origins of citrus varieties inferred from DNA marker analysis of nuclear and organelle genomes. PLoS ONE. 2016;11:e0166969. doi: 10.1371/journal.pone.0166969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Tanizawa Y., Mochizuki T., Nagasaki H., Yoshioka T., Toyoda A., Fujiyama A., Kaminuma E., Nakamura Y. Draft sequencing of the heterozygous diploid genome of satsuma (Citrus unshiu Marc.) using a hybrid assembly approach. Front. Genet. 2017;8:180. doi: 10.3389/fgene.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn J.P., Held J., Vosburg C., Klee S.M., Orbovic V., Taylor E., Gottwald T., Stover E., Moore G., McNellis T.W. Flowering Locus T chimeric protein induces floral precocity in edible citrus. Plant Biotechnol. J. 2020 doi: 10.1111/pbi.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C., Quattrocchio F., Mol J., Koes R. ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell. 2002;14:2121–2135. doi: 10.1105/tpc.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover E., Driggers R., Richardson M.L., Hall D.G., Duan Y., Lee R.F. Incidence and severity of asiatic citrus canker on diverse citrus and citrus-related germplasm in a Florida field planting. HortScience. 2014;49:4–9. [Google Scholar]
- Strazzer P., Spelt C.E., Li S., Bliek M., Federici C.T., Roose M.L., Koes R., Quattrocchio F.M. Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-08516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Stall R.E., Services C., Indus- D.P., Jones J.B., Pathology P., Cubero J., Alfred L., Tim R., Pierce F. Detection and characterization of a new strain of citrus canker bacteria from key Mexican Lime and Alemow in South Florida. Plant Dis. 2004;88:1179–1188. doi: 10.1094/PDIS.2004.88.11.1179. [DOI] [PubMed] [Google Scholar]
- Swingle W.T., Reece P.C. Vol I. University of California Press; Berkeley, CA: 1967. The botany of Citrus and its wild relatives. (The Citrus Industry). Advance Access published 1967. [Google Scholar]
- Tanaka T. The discovery of Citrus tachibana in Formosa, and its scientific and industrial significance. Stud. Citrol. 1931;5:1–20. [Google Scholar]
- Tanaka T. Fundamental discussion of Citrus classification. Stud. Citrol. 1977;14:1–6. [Google Scholar]
- Tang X.M., Wang X., Huang Y., Ma L., Jiang X.L., Rao M.J., Xu Y.T., Yin P., Yuan M., Deng X.X. Natural variations of TFIIAγ gene and LOB1 promoter contribute to citrus canker disease resistance in Atalantia buxifolia. PLoS Genet. 2021 doi: 10.1371/journal.pgen.1009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D.D.C., Danet J.L., Eveillard S., Martins E.C., De Jesus W.C., Yamamoto P.T., Lopes S.A., Bassanezi R.B., Ayres A.J., Saillard C. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the “Candidatus” Liberibacter species associated with the disease. Mol. Cell Probes. 2005;19:173–179. doi: 10.1016/j.mcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Tolba I.H., Soliman M.A. Citrus Huanglongbing (Greening Disease) in Egypt: symptoms documentation and pathogen detection. Am. Eurasian J. Agric. Environ. Sci. 2015;15:2045–2058. [Google Scholar]
- Verweij W., Spelt C.E., Bliek M., de Vries M., Wit N., Faraco M., Koes R., Quattrocchio F.M. Functionally similar WRKY proteins regulate vacuolar acidification in petunia and hair development in Arabidopsis. Plant Cell. 2016;28:786–803. doi: 10.1105/tpc.15.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vun O., Fn M., Cn I., Ku U. Medicinal & aromatic plants stematic characterization of six citrus species using petiole anatomy. Med. Aromat Plants. 2015;5:1–4. [Google Scholar]
- Wang N. The Citrus Huanglongbing crisis and potential solutions. Mol. Plant. 2019;12:607–609. doi: 10.1016/j.molp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- Wang N., Trivedi P. Citrus huanglongbing: a newly relevant disease presents unprecedented challenges. Phytopathology. 2013;103:652–665. doi: 10.1094/PHYTO-12-12-0331-RVW. [DOI] [PubMed] [Google Scholar]
- Wang X., Xu Y., Zhang S., Cao L., Huang Y., Cheng J., Wu G., Tian S., Chen C., Liu Y. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017;49:765. doi: 10.1038/ng.3839. [DOI] [PubMed] [Google Scholar]
- Wang N., Pierson E.A., Setubal J.C., Xu J., Levy J.G., Zhang Y., Li J., Rangel L.T., Martins J., Jr. The Candidatus Liberibacter host interface: insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017;55:451–482. doi: 10.1146/annurev-phyto-080516-035513. [DOI] [PubMed] [Google Scholar]
- Wang L., He F., Huang Y., He J., Yang S., Zeng J., Deng C., Jiang X., Fang Y., Wen S. Genome of wild Mandarin and domestication history of Mandarin. Mol. Plant. 2018;11:1024–1037. doi: 10.1016/j.molp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Van Welzen P.C., Alahuhta J.W.F.J. Plant Diversity and Complexity Patterns: Local, Regional, and Global Dimensions: Proceedings of an International Symposium Held at the Royal Danish Academy of Sciences and Letters in Copenhagen, Denmark. Kgl. Danske Videnskabernes Selskab; Copenhagen: 2005. Plant distribution patterns and plate tectonics; pp. 25–28. [Google Scholar]
- Wen J., Zhang J., Nie Z.-L., Zhong Y., Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan plateau. Front. Genet. 2014;5:4. doi: 10.3389/fgene.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.A., Prochnik S., Jenkins J., Salse J., Hellsten U., Murat F., Perrier X., Ruiz M., Scalabrin S., Terol J. Sequencing of diverse Mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014;32:656. doi: 10.1038/nbt.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.A., Terol J., Ibanez V., López-garcía A., Pérez-román E., Borredá C., Domingo C., Tadeo F.R., Carbonell-caballero J., Alonso R. Genomics of the origin and evolution of Citrus. Nature. 2018;15:311–316. doi: 10.1038/nature25447. [DOI] [PubMed] [Google Scholar]
- Wu H., Hu Y., Fu S., Zhou C., Wang X. Coordination of multiple regulation pathways contributes to the tolerance of a wild citrus species (Citrus ichangensis ‘2586’) against Huanglongbing. Physiol. Mol. Plant Pathol. 2020;109:101457. [Google Scholar]
- Xie S., Manchester S.R., Liu K., Wang Y., Sun B. Citrus linczangensis sp. n., a leaf fossil of Rutaceae from the late Miocene of Yunnan, China. Int. J. Plant Sci. 2013;174:1201–1207. [Google Scholar]
- Xu Q., Chen L.L., Ruan X., Chen D., Zhu A., Chen C., Bertrand D., Jiao W.B., Hao B.H., Lyon M.P. The draft genome of sweet orange (Citrus sinensis) Nat. Genet. 2013;45:59. doi: 10.1038/ng.2472. [DOI] [PubMed] [Google Scholar]
- Xu C., Luo F., Hochholdinger F. LOB domain proteins: beyond lateral organ boundaries. Trends Plant Sci. 2016;21:159–167. doi: 10.1016/j.tplants.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Yang Y.Y., Yang W., Zuo W.J., Zeng Y.B., Liu S.B., Mei W.L., Dai H.F. Two new acridone alkaloids from the branch of Atalantia buxifolia and their biological activity. J. Asian Nat. Prod. Res. 2013;15:899–904. doi: 10.1080/10286020.2013.803073. [DOI] [PubMed] [Google Scholar]
- Ye Y.M. The status of Fortunella genetic resources in China. Fruit Var. J. 1985;39:17–20. [Google Scholar]
- Yu Q., Chen C., Du D., Huang M., Yao J., Yu F., Brlansky R.H., Gmitter F.G., Jr. Reprogramming of a defense signaling pathway in rough lemon and sweet orange is a critical element of the early response to ‘Candidatus Liberibacter asiaticus. Hortic. Res. 2017;4:17063. doi: 10.1038/hortres.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.W., Lu Z.H., Wang X., Liu D., He J.X., Jiang X.L., Ke L.J., Guo W.W., Deng X.X., Xu Q. Identification of a delayed leaf greening gene from a mutation of pummelo. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1790-0. [DOI] [PubMed] [Google Scholar]
- Zeng W., Huang M., Wang X., Ampomah-Dwamena C., Xu Q., Deng X. Identification and functional characterization of the promoter of a phytoene synthase from sweet orange (Citrus sinensis Osbeck) Plant Mol. Biol. Rep. 2013;31:64–74. [Google Scholar]
- Zeng R.F., Zhou J.J., Liu S.R., Gan Z.M., Zhang J.Z., Hu C.G. Genome-wide identification and characterization of squamosa—promoter-binding protein (sbp) genes involved in the flowering development of Citrus clementina. Biomolecules. 2019;9:66. doi: 10.3390/biom9020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tao N., Xu Q., Zhou W., Cao H., Xu J., Deng X. Functional characterization of citrus PSY gene in Hongkong kumquat (Fortunella hindsii Swingle) Plant Cell Rep. 2009;28:1737. doi: 10.1007/s00299-009-0774-3. [DOI] [PubMed] [Google Scholar]
- Zhang S., Ding F., He X., Luo C., Huang G., Hu Y. Characterization of the ‘Xiangshui’lemon transcriptome by de novo assembly to discover genes associated with self-incompatibility. Mol. Genet. Genomics. 2015;290:365–375. doi: 10.1007/s00438-014-0920-7. [DOI] [PubMed] [Google Scholar]
- Zhang F., LeBlanc C., Irish V.F., Jacob Y. Rapid and efficient CRISPR/Cas9 gene editing in Citrus using the YAO promoter. Plant Cell Rep. 2017;36:1883–1887. doi: 10.1007/s00299-017-2202-4. [DOI] [PubMed] [Google Scholar]
- Zhang J., Huguet-Tapia J.C., Hu Y., Jones J., Wang N., Liu S., White F.F. Homologues of CsLOB1 in citrus function as disease susceptibility genes in citrus canker. Mol. Plant Pathol. 2017;18:798–810. doi: 10.1111/mpp.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liang M., Wang N., Xu Q., Deng X., Chai L. Reproduction in woody perennial Citrus: an update on nucellar embryony and self-incompatibility. Plant Reprod. 2018;31:43–57. doi: 10.1007/s00497-018-0327-4. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhu K., Sun Q., Zhang W., Wang X., Cao H., Tan M., Xie Z., Zeng Y., Ye J. Natural variation in CCD4 promoter underpins species-specific evolution of red coloration in citrus peel. Mol. Plant. 2019;12:1294–1307. doi: 10.1016/j.molp.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Zhu C., Zheng X., Huang Y., Ye J., Chen P., Zhang C., Zhao F., Xie Z. Genome sequencing and CRISPR/Cas9 gene editing of an early flowering mini-Citrus (Fortunella hindsii) Plant Biotechnol. J. 2019;17:2199–2210. doi: 10.1111/pbi.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]