Abstract
The post-translational protein modification known as SUMOylation has conserved roles in the heat stress responses of various species. The functional connection between the global regulation of gene expression and chromatin-associated SUMOylation in plant cells is unknown. Here, we uncovered a genome-wide relationship between chromatin-associated SUMOylation and transcriptional switches in Arabidopsis thaliana grown at room temperature, exposed to heat stress, and exposed to heat stress followed by recovery. The small ubiquitin-like modifier (SUMO)-associated chromatin sites, characterized by whole-genome ChIP-seq, were generally associated with active chromatin markers. In response to heat stress, chromatin-associated SUMO signals increased at promoter-transcriptional start site regions and decreased in gene bodies. RNA-seq analysis supported the role of chromatin-associated SUMOylation in transcriptional activation during rapid responses to high temperature. Changes in SUMO signals on chromatin were associated with the upregulation of heat-responsive genes and the downregulation of growth-related genes. Disruption of the SUMO ligase gene SIZ1 abolished SUMO signals on chromatin and attenuated rapid transcriptional responses to heat stress. The SUMO signal peaks were enriched in DNA elements recognized by distinct groups of transcription factors under different temperature conditions. These observations provide evidence that chromatin-associated SUMOylation regulates the transcriptional switch between development and heat stress response in plant cells.
Keywords: chromatin, development, heat stress, plant cells, SUMOylation, transcription
Whole-genome ChIP-seq in Arabidopsis reveals that the distribution of SUMO-associated signals on chromatin changes in response to heat stress and is associated with the activation and repression of gene transcription. This type of chromatin- associated SUMOylation depends primarily on SUMO ligase SIZ1 and controls the transcriptional switch between plant development and heat stress responses.
Introduction
Organisms must optimize their fitness in response to environmental changes to ensure their survival. Therefore, many sensing and response pathways have evolved to protect various species from environmental stress. For sessile organisms such as plants, the balance between growth and stress responses is crucial for withstanding adverse environmental conditions that are unsuitable for regular development (Zhu, 2016). High temperature is an important stress condition that affects plants. Many transcription factors (TFs) regulate gene expression in plant cells in response to heat stress (HS) (Ohama et al., 2017). However, the roles of protein modifications in this process remain unclear.
HS induces protein modifications by SUMO proteins in a variety of eukaryotes (Miller et al., 2013; Le et al., 2017; Rytz et al., 2018). The covalent attachment of SUMO (SUMOylation) regulates the stability, activity, and subcellular localization of target proteins (Verma et al., 2018). SUMO proteins are thought to be encoded by eight genes in Arabidopsis thaliana, and SUMO1 and SUMO2 are the main proteins involved in SUMO conjugation (Kurepa et al., 2003; Saracco et al., 2007). As in other species, this reaction is catalyzed in Arabidopsis by an enzyme cascade that includes E1 activating enzymes (SAE1a and SAE1b), an E2 conjugating enzyme (SCE1), and—usually—E3 ligases (SIZ1 and HYP2/MMS21) (Augustine and Vierstra, 2018). The isopeptide bonds between SUMO and its target proteins can be cleaved by SUMO proteases, resulting in the removal of this modification (Yates et al., 2016). SUMOylation is a dynamic, reversible process that finely regulates various processes in plant development and stress response (Benlloch and Lois, 2018; Verma et al., 2018).
The levels of SUMO conjugates markedly increase in response to stresses such as acute high temperature; this “SUMO stress response” is conserved in eukaryotes (Lewicki et al., 2015). Proteome-wide studies in human cells have revealed that HS induces SUMOylation, particularly on target proteins in the nucleus (Golebiowski et al., 2009). Several important TFs involved in HS responses, such as HSF1 and HSF2, are functionally regulated by SUMOylation in human cells (Seifert et al., 2015). Genome-wide approaches have revealed that HS-induced SUMOylation restricts the transcriptional activity of its targets by targeting the promoter and enhancer regions of these genes in human cells (Niskanen et al., 2015; Niskanen and Palvimo, 2017). Therefore, HS-induced SUMOylation may be directly associated with transcriptional regulation during the response to HS.
Conjugation by SUMO1 and SUMO2 also dramatically increases in Arabidopsis under heat treatment, and the SUMO conjugates are removed after the plant is returned to normal temperatures (Miller and Vierstra, 2011; Rytz et al., 2018). HS-induced SUMOylation is almost completely lost in the Arabidopsis siz1 mutant. SIZ1 encodes a SUMO ligase that is essential for the regulation of HS responses (Yoo et al., 2006). Many potential SUMOylated substrates, such as protein factors involved in transcription and RNA processing, have been identified in Arabidopsis using large-scale approaches. SUMOylation of the HS TF AtHsfA2 is essential for HS responses and acquired thermotolerance (Cohen-Peer et al., 2010). The attachment of SUMO to BCL-2-ASSOCIATED ATHANOGENE 7 promotes its translocation to the nucleus, where it enhances gene expression during the unfolded protein response in Arabidopsis under HS (Li et al., 2017). However, the global functional connection between transcriptional regulation and chromatin SUMOylation in plant cells remains unknown.
In this study, we characterized SUMO-associated chromatin regions in Arabidopsis by whole-genome chromatin immunoprecipitation sequencing (ChIP-seq) and identified changes in the distribution of SUMO signals on chromatin during HS. Our observations, combined with RNA-seq data, reveal that SUMOylation contributes to the activation of gene transcription during the response to high temperature. We also identified SUMO-associated DNA elements and predicted TFs that may bind to these chromatin regions. Our findings provide novel resources for further study of the role of SUMOylation in the switch between development and stress responses.
Results
The distribution of SUMO-associated signals on chromatin changes in response to HS
SUMO1 and SUMO2 are predominant SUMO moieties that function in the response to HS in Arabidopsis (Kurepa et al., 2003); therefore, we examined the responses of SUMO levels to various temperature treatments using an antibody that recognizes SUMO1/2 (Yoo et al., 2006; Conti et al., 2008; Budhiraja et al., 2009; Lin et al., 2016; Kong et al., 2017). The SUMOylation level markedly increased in plants subjected to 37°C treatment for 30 min and gradually decreased after recovery at 23°C for 90 min (Figure 1A); these results are consistent with previous reports (Saracco et al., 2007; Miller et al., 2013).
Figure 1.
The distribution of SUMO-associated signals on chromatin changes in response to heat stress.
(A) SUMOylation levels in the nuclei of 10-day-old wild-type seedlings under RT (23°C), HS (37°C for 30 min), and RC (recovered at 23°C for 90 min) conditions. Samples prepared from nuclei were used for immunoblotting with an anti-SUMO antibody. Total protein staining with Coomassie blue was used as a loading control.
(B) Overview of SUMO-associated signals on chromosome 1 (chr1) in the wild type under RT, HS, and RC conditions. The signals from two biological replicates of RT (green), HS (red), RC (purple), and input (gray) were visualized using the Integrated Genome Browser.
(C) The line profiles of total SUMO ChIP-seq signals on gene regions under RT, HS, and RC conditions.
(D) Relationship of SUMO-associated peaks with histone markers and Pol II. Line profiles of average ChIP-seq signal intensities of published histone markers or Pol II are shown as ±2 kb areas surrounding the peak centers of the SUMO binding regions. ChIP-seq signals were normalized to 10 million reads.
(E) The numbers of unique SUMO-associated chromatin regions in the RT, HS, and RC samples. The numbers of unique peaks in the RT (green), HS (red), and RC (blue) samples are shown as Venn diagrams comparing HS/RC, RC/HS, and RC/RT.
(F) Distribution of SUMO-associated peaks in different types of genomic regions. The percentages of unique peaks from HS/RT, RC/HS, and RT/RC distributed in the indicated types of genomic regions (promoter-TSS, TTS, intergenic, intron, and exon) are shown. In the HOMER analysis, promoter-TSS was defined from −1 kb to +100 bp by default, and TTS was defined from −100 bp to +1 kb by default.
To investigate the association between chromatin-associated SUMO enrichment and gene expression in response to HS, we identified SUMO-associated chromatin regions in plants grown at room temperature (RT), under HS treatment, and under-recovery conditions (RC) using genome-wide ChIP-seq assays with the anti-SUMO1/2 antibody. We measured SUMO1/2 (abbreviated as SUMO hereafter) peaks based on two independent biological replicates, and correlation plots are shown in Supplemental Figure 1A. The enrichment of SUMO signals on chromatin was visualized using the Integrated Genome Browser (Freese et al., 2016). Signals on chromosome 1 are shown in Figure 1B, and signals on other chromosomes are shown in Supplemental Figure 1B.
We identified 16 192, 15 742, and 18 424 peaks in the RT, HS, and RC samples, respectively (Supplemental Data 1; a Venn diagram of SUMO-associated genes in these samples is shown in Supplemental Figure 2A). Most peaks were in promoter-TSS (transcription start site) regions (Supplemental Figure 2B). Interestingly, further analysis indicated that these SUMO binding sites were predominantly located around TSSs, including promoter-proximal regions and potential 5′ UTR regions (Figure 1C, Supplemental Figure 3A). Because previous work indicated that these regions are critical for TF binding and transcription regulation (Ohler and Wassarman, 2010; Shao and Zeitlinger, 2017; Thomas et al., 2020), our results suggest that SUMOylation may play a role in the modulation of gene transcription. By contrast, SUMO signals were weak in intergenic, transposon, and heterochromatin regions (Supplemental Figure 4).
As histone acetylation and methylation are involved in regulating transcription (He et al., 2011; Chen et al., 2017), we analyzed the distribution of these epigenetic markers in the SUMO-enriched regions (identified under RT conditions) using a publicly available Arabidopsis dataset (Ezer et al., 2017; Liu et al., 2018). The SUMO-enriched sites were bound by RNA polymerase II (Pol II) and contained active histone modifications, such as H3K27ac, H3K14ac, H3K4me3, and H3K36me3, but not the repressive histone modification H3K27me3 (Figure 1D). These observations point to a potential link between chromatin-associated SUMO enrichment and active gene expression in plant cells.
To further explore the mechanism by which chromatin SUMOylation influences plant responses to HS, we compared the ChIP-seq signals and identified SUMO-marked differentially enriched regions (DERs) among plants grown under RT, HS, and RC conditions using DiffBind (Ross-Innes et al., 2012) (|DERs| ≥ 1.5-fold change, false discovery rate [FDR] < 0.05) (Figure 1E; Supplemental Data 2; principal component analysis in Supplemental Figure 5). The analysis of the distribution of unique peaks from HS/RT, RC/HS, and RT/RC in genomic regions indicated that SUMO enrichment signals differed between exon and promoter-TSS regions (Figure 1F). Under normal conditions, the RT-specific peaks were distributed globally in promoter-TSS, exon, intron, transcription termination site (TTS), and intergenic regions. However, the ratios of HS-specific peaks were higher at promoter-TSS regions and lower in exon regions compared with other regions (Figure 1F; Supplemental Figure 3). Under subsequent RC, the distribution of SUMO signals changed back to a pattern similar to that observed under RT conditions, pointing to a potential role for SUMO in the rapid transcriptional regulation of the trade-off between normal development and stress response in Arabidopsis.
SUMOylation on chromatin is associated with gene transcription in response to HS
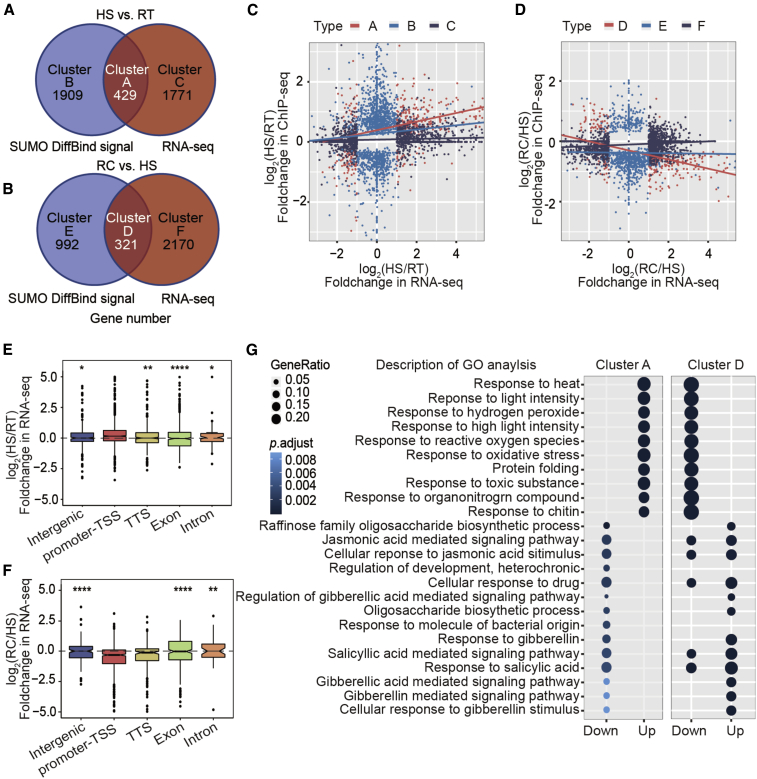
To reveal the potential link between transcription and chromatin-associated SUMOylation during HS, we searched for differentially expressed genes (DEGs) among the RT, HS, and RC samples (|fold change| ≥ 2, p < 0.05) using RNA-seq (Supplemental Data 3). As expected, there was a strong correlation between upregulated genes and SUMO-enriched peaks after HS treatment (Supplemental Figure 6). Therefore, we compared the DEGs with the differentially SUMO-enriched genes identified from our ChIP-seq data (Figure 1E). As shown in Figure 2A and 2B, we identified overlapping genes (429 genes in cluster A for HS/RT; 321 genes in cluster D for RC/HS, Supplemental Data 3), which we considered to be promising candidates because their transcription is directly regulated by SUMOylation. The DEGs and differentially SUMO-enriched genes were positively and negatively correlated in cluster A and cluster D, respectively (Figure 2C and 2D). Perhaps the SUMO signals at promoter-TSS regions increased in the HS samples but decreased in the RT and RC samples. Furthermore, the global expression trend for genes with changes in chromatin-associated SUMO enrichment at promoter-TSS regions was an increase for cluster A but a decrease for cluster D (Figure 2E and 2F) compared with changes in other genomic regions. These results support the association between transcriptional activation and SUMO occupancy at the promoter-TSS regions of genes.
Figure 2.
SUMOylation on chromatin is associated with gene transcription in response to HS.
(A and B) Overlap of differentially SUMO-enriched genes (DSGs) and differentially expressed genes (DEGs). The Venn diagrams show comparisons between DSGs (from ChIP-seq) and DEGs (from RNA-seq) in the wild type under HS/RT (A) and RC/HS (B) conditions, including the numbers of genes in different clusters.
(C and D) Correlations between changes in the transcript levels and SUMO signals of different gene clusters during temperature switches. The correlations between HS and RT (cluster A, red; cluster B, blue; and cluster C, black) are shown in (C); the correlations between RC and HS (cluster D, red; cluster E, blue; and cluster F, black) are shown in (D). Regression coefficients: A = 0.56, B = 0.04, C = 1.08, D = −1.9, E = 1.33, and F = 0.15.
(E and F) Relative expression levels of genes with SUMO peak changes in different types of genomic regions during temperature condition shifts. The data show SUMO binding to different types of regions (intergenic, promoter-TSS, TTS, exon, and intron) in cluster A (E) and cluster D (F). Control group: promoter-TSS. Two-tailed Student’s t-test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
(G) Biological process analysis of genes potentially regulated by chromatin-associated SUMOylation during the HS/RT and RC/HS switches. GO analysis identified the biological processes associated with genes whose transcript levels were upregulated or downregulated in cluster A and cluster D.
We performed gene ontology (GO) analysis of the cluster A and D genes to uncover the potential biological processes mediated by SUMOylation via transcriptional regulation. In cluster A (HS/RT), the upregulated genes were enriched in the GO categories responses to heat, oxidative stress, and high light intensity, whereas the downregulated genes were enriched in the categories control of development and responses to gibberellic acid and other hormones (Figure 2G). The opposite results were obtained for genes in cluster D (RC/HS) (Figure 2G; the results of GO analysis of other clusters are shown in Supplemental Figure 7), supporting the notion that SUMOylation controls a dynamic transcriptional switch between genes involved in development and genes involved in heat responses.
Loss of SUMOylation on chromatin in the siz1 mutant attenuates the rapid regulation of transcription during HS
Because increased heat-induced SUMO conjugation is dependent on the SUMO ligase SIZ1 in Arabidopsis (Yoo et al., 2006; Catala et al., 2007) (Figure 3A), we performed ChIP-seq to profile the SUMO-associated chromatin regions in siz1-2, a null mutant of SIZ1, under normal and HS conditions (correlation plots in Supplemental Figure 8A). As expected, the ChIP-seq data showed that SUMO-associated peaks were nearly absent under both conditions in siz1-2 (Figure 3B; peak information in Supplemental Data 1). Only about 2% of SUMO peaks at RT and about 3% of SUMO peaks at HS were independent of SIZ1; the trace SUMO peaks unique in siz1-2 were preferentially distributed in intergenic regions (Supplemental Figure 8B). These data suggest that SIZ1 is required to maintain the occupancy of most SUMO1/2 on chromatin in plant cells. This result also confirmed the high specificity of the anti-SUMO1/2 antibody used in ChIP-seq. Therefore, the siz1-2 mutant is a good material for further study of transcriptional regulation mediated by chromatin-associated SUMOylation.
Figure 3.
Loss of SUMOylation on chromatin in the siz1 mutant attenuates the rapid regulation of transcription during HS.
(A) The levels of SUMOylation in the nuclei of 10-day-old Col-0 and siz1-2 seedlings under RT (23°C) and HS (37°C for 30 min) conditions. The samples from nuclear isolation were used for immunoblotting with an anti-SUMO antibody. Anti-H3 was used as a control.
(B) Overview of the SUMO-associated signals on chromosome 1 (chr1) of wild type (Col-0) and siz1-2 under RT and HS conditions. The signals from two biological replicates of the RT (green), HS (red), and input (gray) samples were visualized using the Integrated Genome Browser.
(C) The relative expression levels of SUMO-regulated genes in wild type (Col-0) and siz1-2 between HS and RT conditions. The transcript levels of genes in cluster A in Figure 2A were used for analysis. The data for upregulated and downregulated genes in the HS/RT comparison are shown. The y-axis shows relative gene expression levels based on RNA-seq; two-tailed Student’s t-test, p < 0.01.
(D) Comparison of the transcript levels of SUMO-regulated heat-responsive genes in wild type (Col-0) and siz1-2. A heatmap of the relative expression levels of the genes from cluster A in Figure 2A is shown in the left panel. During the shift from RT to HS, the upregulated (log2(fold change) > 1) genes in the wild type (Col-0) or siz1-2 are shown in red and the downregulated (log2(fold change) <−1) genes are shown in green. Several examples of genes related to HS and development are shown in the right panel. Upregulated genes: HSP70-T-2 (AT2G32120), HSP17.6A (AT5G12030), HSP17.6II (AT5G12020), and HSP23.6 (AT4G25200); downregulated genes: RGA2 (AT1G14920), TCP3 (AT1G53230), and HAM3 (AT4G00150). ChIP-seq and RNA-seq data for the genes are shown (the maximum signals are indicated). The y-axis represents the means of normalized reads (1× sequencing depth normalization) per 10-bp non-overlapping bins.
We reasoned that if transcription of the candidate genes in cluster A (Figure 2A) is directly regulated by SUMOylation, then changes in their transcript levels should be attenuated in siz1-2. We therefore used RNA-seq data from siz1-2 under RT and HS conditions (DEGs between RT and HS [(|fold change| ≥ 2, p < 0.05]; principal component analysis in Supplemental Figure 9A; transcript information in Supplemental Data 3) to further analyze the genes in cluster A. Although the expression levels of cluster A genes in siz1-2 still differed from those in wild type, the changes were compromised in siz1-2 (Figure 3C; see also Supplemental Data 3; Supplemental Figure 9B). Similarly, heatmap analysis of cluster A genes revealed that transcriptional changes in SUMO-associated genes in siz1-2 under HS were not as dramatic as those in the wild type (Figure 3D). For instance, many heat-responsive genes, such as HSPs, were dramatically upregulated by high temperature, but this upregulation was smaller in siz1-2 than in the wild type (Figure 3D; Supplemental Figure 10). By contrast, the transcript levels of several genes involved in development, such as RGA2, TCP3, and HAM3, decreased in response to HS, but this decrease was smaller in siz1-2 than in the wild type (Figure 3D, these data were confirmed by ChIP–qPCR and qRT–PCR, as shown in Supplemental Figure 11). These results suggest that the loss of SUMOylation on chromatin attenuates the rapid regulation of transcription during HS in siz1-2.
Analysis of SUMO-associated genomic elements establishes TF networks that potentially regulate gene expression
Our observations indicate that SUMOylation is important for transcriptional regulation and that SUMO signals are concentrated predominantly in narrow chromatin regions around the promoter-TSS. Therefore, we reasoned that SUMOylated TFs likely contribute to these signals. Therefore, we examined the genomic elements in SUMO-associated chromatin regions of interest (peaks uniquely identified in the RT, HS, and RC samples) via HOMER analysis (Heinz et al., 2010; O'Malley et al., 2016) (Figure 4A).
Figure 4.
Analysis of SUMO-associated genomic elements establishes TF networks that potentially regulate gene expression.
(A) Known motif enrichment within the unique peaks from the RT, HS, and RC samples based on HOMER analysis. The top 10 enriched elements potentially recognized by the indicated groups of TFs are shown, sorted in the order of p values. Percentages of SUMO peaks with the indicated elements are indicated by different colors (RT-specific, green; HS-specific, red; RC-specific, blue).
(B) The network of TFs potentially involved in SUMO-associated transcriptional regulation. An interaction and co-expression map of 494 potential SUMO-associated TFs from the STRING database is shown. The NAC, HSF, AP2/EREBP, WRKY, and MYC TF families are highlighted.
(C) A model of the role of chromatin-associated SUMOylation in transcriptional regulation to balance the switch between development and HS responses in Arabidopsis.
Surprisingly, analysis of these SUMO signals indicated that the enriched elements of the different samples were distinct and potentially recognized by different groups of TFs. In the RT samples, SUMO-associated elements were enriched in recognition sites for NAC family TFs, such as CUC subfamily members that are involved in development (Olsen et al., 2005). However, in the HS samples, there was a major switch in the enriched SUMO-associated elements to the binding sites of HSF TFs, which are essential for transcription during the rapid response to HS (Scharf et al., 2012; Li et al., 2019). Recognition elements for AP2/EREBP (APETALA 2/ethylene-responsive element binding protein) TFs (Mizoi et al., 2012), such as ERF members that are functionally associated with both development and stress response, were enriched in the RC-specific peaks. These findings provide hints about how SUMO-related transcriptional regulation switches from genes involved in development to genes involved in HS responses.
We predicted that 449 TFs, including members of the NAC, HSF, AP2/EREBP, WRKY, and MYC TF families, bound DNA elements identified in the SUMO-associated chromatin regions based on our ChIP-seq data (Supplemental Data 4). An interaction map of these proteins generated using the STRING database (Szklarczyk et al., 2019) pointed to complex connections among these potential SUMOylation-regulated TFs (Figure 4B). Notably, several members of these TF groups, such as NAC50, HSFB2B, TCP4, ERF6, and WRKY33, were previously identified as SUMOylation substrates in the proteomic studies of plant cells (Supplemental Data 4, targets previously identified are highlighted with different colors), supporting the notion that these TF families are major targets of SUMOylation on chromatin for the subsequent activation of transcription.
Discussion
HS stimulates SUMOylation in plants, particularly in the nucleus (Niskanen and Palvimo, 2017; Augustine and Vierstra, 2018), suggesting that increased levels of SUMO conjugates are associated with various biological processes in this cellular compartment. Here, we uncovered connections between chromatin-associated SUMOylation and transcriptional regulation in the nucleus during the switch between development and stress response.
Our ChIP-seq data indicated that SUMO-associated chromatin peaks are predominantly located on promoter-TSS gene regions, which harbor active epigenetic markers (Figure 1D and 1F) (He et al., 2011), suggesting that SUMOylation plays a role in transcriptional activation. Interestingly, these SUMO-enriched sites are preferentially localized around the TSS (Figure 1C), similar to the distribution of TFs (Ohler and Wassarman, 2010; Shao and Zeitlinger, 2017; Thomas et al., 2020), such as HSFA1a (Supplemental Figure 12A). Consistently, predicted motifs in the HS SUMO peaks are also highly enriched in these regions (Supplemental Figure 12B). By contrast, there were only weak correlations between chromatin-associated SUMO signals and global nucleosome signals, which are low around the TSS and high in gene bodies (based on published histone H3 ChIP-seq data; Luo et al., 2013) (Supplemental Figure 13). In addition, the distribution patterns of SUMO and Pol II are similar (Figure 1D), implying that SUMOylation may also target the Pol II complex for transcription regulation. During HS, the ratio of HS-specific peaks increased at promoter-TSS regions, possibly contributing to the rapid transcriptional regulation of genes during the response to high temperature. This conclusion is supported by a comparison of RNA-seq and ChIP-seq data from RT and HS samples of wild-type and siz1-2 plants.
Several studies have indicated that SUMOylation is critical for the TF-induced activation of gene expression in plant cells (Augustine and Vierstra, 2018; Benlloch and Lois, 2018; Verma et al., 2018). For instance, SUMOylated ICE1 enhances the transcription of cold-responsive genes (Miura et al., 2007), and SUMOylation is essential for ARF7-mediated LBD16 induction during root development (Orosa-Puente et al., 2018). However, studies of SUMO-associated chromatin suggest that SUMOylation restricts transcription in human cells (Niskanen et al., 2015; Seifert et al., 2015). Several hypotheses have been put forward to explain the roles of SUMOylation in the response of human cells to HS. For example, SUMOylation may stabilize TF complexes, regulate chromatin organization, or minimize the aggregation of misfolded proteins in these cells (Niskanen and Palvimo, 2017). Therefore, SUMOylation may use different mechanisms to regulate gene expression in different species, and it would be interesting to compare these variations.
Given that SUMOylation is involved in plant development and stress responses (Augustine and Vierstra, 2018), we reasoned that chromatin-associated SUMOylation may contribute to the regulation of gene expression during both processes. GO analysis of cluster A and D genes (Figure 2G) indicated that SUMO-associated upregulated genes are enriched in the GO category of stress responses, whereas SUMO-associated downregulated genes are enriched in development-related processes. In addition, the SUMO-associated elements are enriched in recognition sites for TFs related to development in RT samples but in recognition sites for TFs involved in HS responses in HS samples. These results provide evidence that chromatin-associated SUMOylation functions in the transcriptional switch between development and HS responses.
We propose the following model. Under normal conditions, SUMOylation regulates the activity of development-related TFs, such as NAC TFs (Olsen et al., 2005). When plants are exposed to high temperatures, SUMO attachment on HSFs is enhanced to establish a rapid heat response. Following recovery to normal temperatures, SUMOylation is maintained on TFs involved in both development and stress responses (Figure 4C). The HSFA1 family is important for HS responses, and our analysis revealed that more than 50% of HSFA1a binding sites (Cortijo et al., 2017) can be found in the SUMO-enriched peaks (Supplemental Figure 12A), suggesting that HSFA1s are SUMOylation candidates during this process, consistent with our prediction in Figure 4A. The identification of SUMO-targeted TFs will increase our understanding of the precise mechanisms that function during this process.
In the siz1-2 mutant, both the upregulation of HS genes and the downregulation of development-related genes are attenuated (Figure 3D), suggesting that SUMO may be an enhancer of transcriptional switches. However, the change in transcription level is slight in siz1-2. Perhaps TFs in both the SUMOylated and non-SUMOylated states bind to specific chromatin regions to regulate gene transcription. Therefore, SUMOylation may function as an additional regulator of its target TFs. As a result, even in the absence of SIZ1, TFs still function in transcriptional regulation, although their activities may change due to the loss of SUMOylation. These dynamic connections between SUMOylation and transcription may result from SUMOylation or deSUMOylation under different conditions (Augustine and Vierstra, 2018). It would be interesting to analyze the patterns of chromatin-associated SUMO signals from different SUMO proteases.
Collectively, our study uncovered a SUMOylation-mediated transcriptional switch mechanism between development and HS responses in plant cells. Our genome-wide analysis of chromatin-associated SUMOylation provides a foundation for further characterization of novel elements involved in the regulation of gene expression by SUMOylation. Such studies, combined with SUMO-specific proteomics, will help to reveal the connections between SUMOylated TFs and their binding elements, shedding light on the functions of SUMOylation in TF regulation and increasing our understanding of the transcriptional switch between development and stress responses.
Methods
Plant materials and growth conditions
Arabidopsis thaliana Col-0 (wild-type) and siz1-2 (SALK_065397) seeds obtained from the Arabidopsis Stock Center were used in the experiments. The seeds were sterilized for 2 min in 70% ethanol, placed in 2.5% bleach solution containing 0.05% Triton X-100 for 10 min, and rinsed five times with sterile water. The seeds were sown on half-strength Murashige and Skoog (MS) medium with 1.5% sucrose and 0.8% agar, stratified at 4°C in the dark for 2 days, and transferred to a 23°C greenhouse under long day (16 h light/8 h dark) conditions.
HS treatments
Ten-day-old seedlings grown on medium in Petri dishes (90 × 15 mm) were incubated at 37°C for 30 min in a circulating water bath. During recovery from the 37°C treatment, the Petri dishes were removed from the water bath and incubated in a greenhouse at 23°C for 90 min as described previously (Charng et al., 2006). The seedlings were collected at the indicated time points for further analysis.
Nuclear extraction and immunoblotting
Nuclear extraction was performed as described previously (Han et al., 2018). The samples were mixed with SDS loading buffer, boiled for 5 min, and centrifuged at 12 000 g for 3 min. The supernatants were used for immunoblotting with the anti-SUMO1 antibody (Abcam: ab5316), which specifically recognizes Arabidopsis SUMO1 and SUMO2 (Yoo et al., 2006; Conti et al., 2008; Budhiraja et al., 2009; Lin et al., 2016; Kong et al., 2017). The anti-H3 antibody (Abcam: ab1791) was used as a control.
ChIP-seq and ChIP–qPCR
ChIP assays were performed as described previously (Gendrel et al., 2005). In brief, 5-g samples of 10-day-old seedlings grown on half-strength MS medium with the indicated treatment (RT, HS, or RC) were collected, pulverized in liquid nitrogen, and crosslinked using 1% formaldehyde for 15 min under a vacuum. Chromatin was isolated from the samples and fragmented into 200- to 1000-bp segments by sonication. The fragmented chromatin was incubated with 5 μl SUMO1 antibody (Abcam: ab5316) overnight at 4°C. The ChIP DNA was prepared for sequencing or qPCR. Two biological replicates were used for ChIP-seq, and three biological replicates were used for ChIP–qPCR. The numbers of reads of the ChIP-seq samples are shown in Supplemental Table 1, and the primers used for qPCR are listed in Supplemental Table 2. TA3 (At4g07700) was used as a negative control (Schmittgen and Livak, 2008).
ChIP-seq data analysis
At least 10 ng of ChIP DNA was used for library preparation with an Illumina Genomic DNA Sample Prep kit. The ChIP-seq libraries from two biological replicates were used for high-throughput sequencing on an Illumina HiSeq 2500 instrument. Bowtie 2 (Langmead and Salzberg, 2012) was used with default settings to align the raw sequencing reads to the Arabidopsis TAIR10 assembly. The correlation between biological replicates was computed using deepTools2 (Ramírez et al., 2016) on normalized signal intensity (-bs 10 –effectiveGenomeSize 135000000 –normalizeUsing RPGC –ignoreDuplicates -e 300; multiBigwigSummary bins -bs 100; and plotCorrelation –c pearson). To visualize the data, the MACS2 program (Feng et al., 2012) was used to convert the mapped and mixed reads (mixed reads from both replicates; extension size = 200, shift size = 0, FDR ≤ 0.01) to wiggle format using the Integrated Genome Browser. The representative peaks in the genome were examined with pyGenomeTracks to visualize peak changes. The y-axis represents the means of normalized reads (1× sequencing depth normalization) per 10-bp non-overlapping bins. Black boxes indicate the target genes. The SUMO-enriched chromatin regions were converted to a gene list using HOMER (Heinz et al., 2010) with default settings (-annotatePeaks.pl; by default, promoter-TSS was defined from −1 kb to +100 bp and TTS was defined from −100 bp to +1 kb). Differential enrichment of SUMO1/2 was defined as fold change ≥ 2 and FDR < 0.05 based on analysis with the R package DiffBind (Ross-Innes et al., 2012), which uses the DESeq2 tool to identify significantly differentially bound sites between two sample groups. GO analysis was performed using the ChIPseeker R package (Yu et al., 2015). Enrichment of cis-elements in the +250 and −250 promoter sequences of SUMO signals in plants under different conditions was used for motif discovery with HOMER software with default parameters. The sequencing data were screened for the enrichment of known motifs and used to establish a TF network (Szklarczyk et al., 2019). Prediction of regulatory motifs and target genes was performed according to published methods (Cortijo et al., 2017).
RNA-seq and qRT–PCR
Total RNA was extracted from 10-day-old seedlings using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. mRNA was purified from the total RNA and used to construct sequencing libraries with a TruSeq RNA Sample Preparation Kit (Illumina). The libraries from three biological replicate samples were sequenced on an Illumina HiSeq 2500 instrument. Raw sequencing reads were filtered with Trimmomatic and mapped to the Arabidopsis genome (TAIR10) using default parameters. DEGs, which were defined as genes with an expression difference of |log2(fold change)| ≥ 1 with p < 0.05, were identified using CuffDiff (Trapnell et al., 2012). Comparative analyses of the RNA-seq data were conducted using R.
Data deposition
The ChIP-seq and RNA-seq datasets have been deposited in GEO SuperSeries (GEO: GSE140508).
Funding
This work was supported by grants from the Natural Science Foundation of Guangdong (2018B030308002); the National Natural Science Foundation of China (31871222, 31670286, 31771504, and 31970531); the Guangdong YangFan Innovative and Entrepreneurial Research Team Project (2015YT02H032); the Program for Changjiang Scholars; and the Guangdong Special Support Program of Young Top-Notch Talent in Science and Technology Innovation (2019TQ05N651).
Author contributions
C.Y., Y.C., and J. Lai supervised the project. D.H. and C.C. performed the experiments. S.X., J.Liu, J.S., and V.N. provided technological support. D.H., C.C., and J.Lai analyzed the data. D.H., J.Lai, and C.Y. wrote the article.
Acknowledgments
We thank ABRC for the mutant seeds used in this study. No conflicts of interest are declared.
Published: July 2, 2020
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental Information is available at Plant Communications Online.
Contributor Information
Jianbin Lai, Email: 20141062@m.scnu.edu.cn.
Yuhai Cui, Email: yuhai.cui@canada.ca.
Chengwei Yang, Email: yangchw@scnu.edu.cn.
Supplemental information
References
- Augustine R.C., Vierstra R.D. SUMOylation: re-wiring the plant nucleus during stress and development. Curr. Opin. Plant Biol. 2018;45:143–154. doi: 10.1016/j.pbi.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Benlloch R., Lois L.M. Sumoylation in plants: mechanistic insights and its role in drought stress. J. Exp. Bot. 2018;69:4539–4554. doi: 10.1093/jxb/ery233. [DOI] [PubMed] [Google Scholar]
- Budhiraja R., Hermkes R., Muller S., Schmidt J., Colby T., Panigrahi K.C.S., Coupland G., Bachmair A. Substrates related to chromatin and to RNA-dependent processes are modified by Arabidopsis SUMO isoforms that differ in a conserved residue with influence on desumoylation. Plant Physiol. 2009;149:1529–1540. doi: 10.1104/pp.108.135053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R., Ouyang J., Abreu I.A., Hu Y., Seo H., Zhang X., Chua N.H. The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell. 2007;19:2952–2966. doi: 10.1105/tpc.106.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng Y.Y., Liu H.C., Liu N.Y., Hsu F.C., Ko S.S. Arabidopsis Has32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 2006;140:1297–1305. doi: 10.1104/pp.105.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li C., Wang Y., Renaud J., Tian G., Kambhampati S., Saatian B., Nguyen V., Hannoufa A., Marsolais F. Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis. Nat. Plants. 2017;3:814–824. doi: 10.1038/s41477-017-0023-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Peer R., Schuster S., Meiri D., Breiman A., Avni A. Sumoylation of Arabidopsis heat shock factor A2 (HsfA2) modifies its activity during acquired thermotholerance. Plant Mol. Biol. 2010;74:33–45. doi: 10.1007/s11103-010-9652-1. [DOI] [PubMed] [Google Scholar]
- Conti L., Price G., O'Donnell E., Schwessinger B., Dominy P., Sadanandom A. Small ubiquitin-like modifier proteases OVERLY TOLERANT TO SALT1 and-2 regulate salt stress responses in Arabidopsis. Plant Cell. 2008;20:2894–2908. doi: 10.1105/tpc.108.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo S., Charoensawan V., Brestovitsky A., Buning R., Ravarani C., Rhodes D., van Noort J., Jaeger K.E., Wigge P.A. Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol. Plant. 2017;10:1258–1273. doi: 10.1016/j.molp.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer D., Jung J.-H., Lan H., Biswas S., Gregoire L., Box M.S., Charoensawan V., Cortijo S., Lai X., Stöckle D. The evening complex coordinates environmental and endogenous signals in Arabidopsis. Nat. Plants. 2017;3:17087. doi: 10.1038/nplants.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Liu T., Qin B., Zhang Y., Liu X.S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 2012;7:1728. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese N.H., Norris D.C., Loraine A.E. Integrated genome browser: visual analytics platform for genomics. Bioinformatics. 2016;32:2089–2095. doi: 10.1093/bioinformatics/btw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel A.-V., Lippman Z., Martienssen R., Colot V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods. 2005;2:213–218. doi: 10.1038/nmeth0305-213. [DOI] [PubMed] [Google Scholar]
- Golebiowski F., Matic I., Tatham M.H., Cole C., Yin Y., Nakamura A., Cox J., Barton G.J., Mann M., Hay R.T. System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2009;2:ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- Han W., Han D., He Z., Hu H., Wu Q., Zhang J., Jiang J., Qin G., Cui Y., Lai J. The SWI/SNF subunit SWI3B regulates IAMT1 expression via chromatin remodeling in Arabidopsis leaf development. Plant Sci. 2018;271:127–132. doi: 10.1016/j.plantsci.2018.03.021. [DOI] [PubMed] [Google Scholar]
- He G., Elling A.A., Deng X.W. The epigenome and plant development. Annu. Rev. Plant Biol. 2011;62:411–435. doi: 10.1146/annurev-arplant-042110-103806. [DOI] [PubMed] [Google Scholar]
- Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Luo X., Qu G.P., Liu P., Jin J.B. Arabidopsis SUMO protease ASP1 positively regulates flowering time partially through regulating FLC stability. J. Integr. Plant Biol. 2017;59:15–29. doi: 10.1111/jipb.12509. [DOI] [PubMed] [Google Scholar]
- Kurepa J., Walker J.M., Smalle J., Gosink M.M., Davis S.J., Durham T.L., Sung D.Y., Vierstra R.D. The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J. Integr. Plant Biol. 2003;278:6862–6872. doi: 10.1074/jbc.M209694200. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N.T., Martin J.F., Fujiwara K., Abe J.I. Sub-cellular localization specific SUMOylation in the heart. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2041–2055. doi: 10.1016/j.bbadis.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki M.C., Srikumar T., Johnson E., Raught B. The S. cerevisiae SUMO stress response is a conjugation–deconjugation cycle that targets the transcription machinery. J. Proteomics. 2015;118:39–48. doi: 10.1016/j.jprot.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Li Y., Williams B., Dickman M. Arabidopsis B-cell lymphoma2 (Bcl-2)-associated athanogene 7 (BAG7)-mediated heat tolerance requires translocation, sumoylation and binding to WRKY29. New Phytol. 2017;214:695–705. doi: 10.1111/nph.14388. [DOI] [PubMed] [Google Scholar]
- Li B., Gao Z., Liu X., Sun D., Tang W. Transcriptional profiling reveals a time-of-day-specific role of REVEILLE 4/8 in regulating the first wave of heat shock-induced gene expression in Arabidopsis. Plant Cell. 2019;31:2353–2369. doi: 10.1105/tpc.19.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.-L., Niu D., Hu Z.-L., Kim D.H., Jin Y.H., Cai B., Liu P., Miura K., Yun D.-J., Kim W.-Y. An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet. 2016;12:e1006016. doi: 10.1371/journal.pgen.1006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Xin Y., Xu L., Cai Z., Xue Y., Liu Y., Xie D., Liu Y., Qi Y. Arabidopsis ARGONAUTE 1 binds chromatin to promote gene transcription in response to hormones and stresses. Dev. Cell. 2018;44:348–361.e7. doi: 10.1016/j.devcel.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Luo C., Sidote D.J., Zhang Y., Kerstetter R.A., Michael T.P., Lam E. Integrative analysis of chromatin states in Arabidopsis identified potential regulatory mechanisms for natural antisense transcript production. Plant J. 2013;73:77–90. doi: 10.1111/tpj.12017. [DOI] [PubMed] [Google Scholar]
- Miller M.J., Vierstra R.D. Mass spectrometric identification of SUMO substrates provides insights into heat stress-induced SUMOylation in plants. Plant Signal. Behav. 2011;6:130–133. doi: 10.4161/psb.6.1.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Scalf M., Rytz T.C., Hubler S.L., Smith L.M., Vierstra R.D. Quantitative proteomics reveals factors regulating RNA biology as dynamic targets of stress-induced SUMOylation in Arabidopsis. Mol. Cell. Proteomics. 2013;12:449–463. doi: 10.1074/mcp.M112.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.-J., Hasegawa P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Niskanen E.A., Palvimo J.J. Chromatin SUMOylation in heat stress: to protect, pause and organise?: SUMO stress response on chromatin. Bioessays. 2017;39 doi: 10.1002/bies.201600263. [DOI] [PubMed] [Google Scholar]
- Niskanen E.A., Malinen M., Sutinen P., Toropainen S., Paakinaho V., Vihervaara A., Joutsen J., Kaikkonen M.U., Sistonen L., Palvimo J.J. Global SUMOylation on active chromatin is an acute heat stress response restricting transcription. Genome Biol. 2015;16:153. doi: 10.1186/s13059-015-0717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley R.C., Huang S.-s.C., Song L., Lewsey M.G., Bartlett A., Nery J.R., Galli M., Gallavotti A., Ecker J.R. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell. 2016;165:1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N., Sato H., Shinozaki K., Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Ohler U., Wassarman D.A. Promoting developmental transcription. Development. 2010;137:15–26. doi: 10.1242/dev.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A.N., Ernst H.A., Leggio L.L., Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10:79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Orosa-Puente B., Leftley N., von Wangenheim D., Banda J., Srivastava A.K., Hill K., Truskina J., Bhosale R., Morris E., Srivastava M. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science. 2018;362:1407–1410. doi: 10.1126/science.aau3956. [DOI] [PubMed] [Google Scholar]
- Ramírez F., Ryan D.P., Grüning B., Bhardwaj V., Kilpert F., Richter A.S., Heyne S., Dündar F., Manke T. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytz T.C., Miller M.J., McLoughlin F., Augustine R.C., Marshall R.S., Juan Y.T., Charng Y.Y., Scalf M., Smith L.M., Vierstra R.D. SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell. 2018;30:1077–1099. doi: 10.1105/tpc.17.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes C.S., Stark R., Teschendorff A.E., Holmes K.A., Ali H.R., Dunning M.J., Brown G.D., Gojis O., Ellis I.O., Green A.R. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracco S.A., Miller M.J., Kurepa J., Vierstra R.D. Genetic analysis of SUMOylation in Arabidopsis: conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol. 2007;145:119–134. doi: 10.1104/pp.107.102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K.D., Berberich T., Ebersberger I., Nover L. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta. 2012;1819:104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Seifert A., Schofield P., Barton G.J., Hay R.T. Proteotoxic stress reprograms the chromatin landscape of SUMO modification. Sci. Signal. 2015;8:rs7. doi: 10.1126/scisignal.aaa2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W., Zeitlinger J. Paused RNA polymerase II inhibits new transcriptional initiation. Nat. Genet. 2017;49:1045–1051. doi: 10.1038/ng.3867. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Q.A., Ard R., Liu J., Li B., Wang J., Pelechano V., Marquardt S. Transcript isoform sequencing reveals widespread promoter-proximal transcriptional termination in Arabidopsis. Nat. Commun. 2020;11:2589. doi: 10.1038/s41467-020-16390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V., Croley F., Sadanandom A. Fifty shades of SUMO: its role in immunity and at the fulcrum of the growth-defence balance. Mol. Plant Pathol. 2018;19:1537–1544. doi: 10.1111/mpp.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates G., Srivastava A.K., Sadanandom A. SUMO proteases: uncovering the roles of deSUMOylation in plants. J. Exp. Bot. 2016;67:2541–2548. doi: 10.1093/jxb/erw092. [DOI] [PubMed] [Google Scholar]
- Yoo C.Y., Miura K., Jin J.B., Lee J., Park H.C., Salt D.E., Yun D.-J., Bressan R.A., Hasegawa P.M. SIZ1 small ubiquitin-like modifier E3 ligase facilitates basal thermotolerance in Arabidopsis independent of salicylic acid. Plant Physiol. 2006;142:1548–1558. doi: 10.1104/pp.106.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wang L.-G., He Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- Zhu J.-K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.