Key Points
Question
Among patients with ischemic stroke secondary to large vessel occlusion and eligible for thrombolysis, is endovascular treatment alone noninferior to intravenous alteplase plus endovascular treatment with regard to functional independence?
Findings
In this randomized clinical trial that included 234 patients with acute ischemic stroke, the proportion who achieved functional independence at 90 days was 54.3% in the endovascular treatment alone group vs 46.6% in the intravenous alteplase plus endovascular treatment group, a difference that met the prespecified noninferiority margin of 10%.
Meaning
Among patients with acute ischemic stroke due to large vessel occlusion and eligible for thrombolysis, endovascular treatment alone, compared with intravenous alteplase plus endovascular treatment, met the prespecified statistical threshold for noninferiority for the outcome of 90-day functional independence, although the clinical acceptability of the threshold for noninferiority should be considered when interpreting the results.
Abstract
Importance
For patients with large vessel occlusion strokes, it is unknown whether endovascular treatment alone compared with intravenous thrombolysis plus endovascular treatment (standard treatment) can achieve similar functional outcomes.
Objective
To investigate whether endovascular thrombectomy alone is noninferior to intravenous alteplase followed by endovascular thrombectomy for achieving functional independence at 90 days among patients with large vessel occlusion stroke.
Design, Setting, and Participants
Multicenter, randomized, noninferiority trial conducted at 33 stroke centers in China. Patients (n = 234) were 18 years or older with proximal anterior circulation intracranial occlusion strokes within 4.5 hours from symptoms onset and eligible for intravenous thrombolysis. Enrollment took place from May 20, 2018, to May 2, 2020. Patients were enrolled and followed up for 90 days (final follow-up was July 22, 2020).
Interventions
A total of 116 patients were randomized to the endovascular thrombectomy alone group and 118 patients to combined intravenous thrombolysis and endovascular thrombectomy group.
Main Outcomes and Measures
The primary end point was the proportion of patients achieving functional independence at 90 days (defined as score 0-2 on the modified Rankin Scale; range, 0 [no symptoms] to 6 [death]). The noninferiority margin was −10%. Safety outcomes included the incidence of symptomatic intracerebral hemorrhage within 48 hours and 90-day mortality.
Results
The trial was stopped early because of efficacy when 234 of a planned 970 patients had undergone randomization. All 234 patients who were randomized (mean age, 68 years; 102 women [43.6%]) completed the trial. At the 90-day follow-up, 63 patients (54.3%) in the endovascular thrombectomy alone group vs 55 (46.6%) in the combined treatment group achieved functional independence at the 90-day follow-up (difference, 7.7%, 1-sided 97.5% CI, −5.1% to ∞)P for noninferiority = .003). No significant between-group differences were detected in symptomatic intracerebral hemorrhage (6.1% vs 6.8%; difference, −0.8%; 95% CI, −7.1% to 5.6%) and 90-day mortality (17.2% vs 17.8%; difference, −0.5%; 95% CI, −10.3% to 9.2%).
Conclusions and Relevance
Among patients with ischemic stroke due to proximal anterior circulation occlusion within 4.5 hours from onset, endovascular treatment alone, compared with intravenous alteplase plus endovascular treatment, met the prespecified statistical threshold for noninferiority for the outcome of 90-day functional independence. These findings should be interpreted in the context of the clinical acceptability of the selected noninferiority threshold.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR-IOR-17013568
This noninferiority trial compares the effects of endovascular treatment with vs without intravenous alteplase on 90-day functional independence among patients with acute ischemic stroke.
Introduction
Seven randomized clinical trials have consistently demonstrated that patients with a large vessel occlusion in the anterior circulation benefit from endovascular treatment following intravenous thrombolysis (IVT).1,2,3,4,5,6,7 However, IVT prior to endovascular treatment has potential benefits and risks. Intravenous thrombolysis may sometimes contribute to reperfusion, averting the need for endovascular treatment.5 Intravenous thrombolysis may facilitate endovascular reperfusion and promote dissolution of downstream microemboli improving distal perfusion.8,9 Conversely, IVT may increase the risk of intracranial or systemic hemorrhage, lead to thrombus fragmentation and worsening distal perfusion, and delay the start of the endovascular treatment.10,11 Furthermore, IVT limits the use of antithrombotic therapy within 24-hour, and increases health care costs.12
The recently reported randomized clinical trial of Endovascular Thrombectomy With or without Intravenous Alteplase in Acute Stroke (DIRECT-MT) showed that endovascular thrombectomy alone was noninferior to thrombectomy after IVT.13 Similar effect directions were seen in the SKIP trial,14 although this study was underpowered and, as such, was unable to demonstrate significance for noninferiority. The aim of the Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation (DEVT) trial was to test the hypothesis that endovascular thrombectomy alone was noninferior to combined IVT and endovascular thrombectomy in patients with proximal anterior circulation occlusions treated within 4.5 hours of onset.
Methods
Trial Design and Oversight
This trial was a multicenter, randomized, open-label, clinical trial with blinded central evaluation of outcomes in patients with stroke. The trial protocol was approved by medical ethics committee of the Second Affiliated Hospital of the Army Medical University and all participating centers. Written informed consent was obtained from all enrolled patients or their legal representatives before randomization. The trial protocol is available in Supplement 2, the statistical analysis plan, in Supplement 3.
The trial was monitored by an independent data and safety monitoring board. An independent clinical events committee validated adverse events, procedural-related complications, and serious adverse events. All images were assessed in a blinded manner by a core laboratory. The methods of this trial have been published previously.15 eFigure 1 in Supplement 4 shows planned trial assessments and interventions.
Participating Centers and Patients
The study was conducted at 33 stroke centers in China. To qualify for participation, each center had to perform more than 50 mechanical thrombectomies annually and each neurointerventionist, at least 10 thrombectomies per year. To ensure the quality of the trial, training of study protocol and endovascular treatment technique was held every 6 months in Chongqing (eMethods 4 in Supplement 4).
Study candidates were 18 years or older with acute ischemic stroke, eligible for IV alteplase treatment within 4.5 hours of onset, and had occlusion of the intracranial internal carotid artery or the first segment of the middle cerebral artery confirmed by computed tomographic (CT) angiography or magnetic resonance (MR) angiography. Time of stroke onset was defined as when the patient was last known to be well. Randomization was performed no later than 4 hours 15 minutes from onset (Figure 1). The main exclusion criteria were imaging evidence of intracranial hemorrhage and premorbidity with a modified Rankin Scale score of 2 or more (mRS; range, 0 [no symptoms] to 6 [death]). Additional detailed study criteria are provided in eMethods 1 in Supplement 4. Central blinded readers assigned a collateral score using the 5-point American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) collateral flow grading scale (range, 0 [no collaterals visible] to 4 [complete or rapid collaterals to entire ischemic territory]).
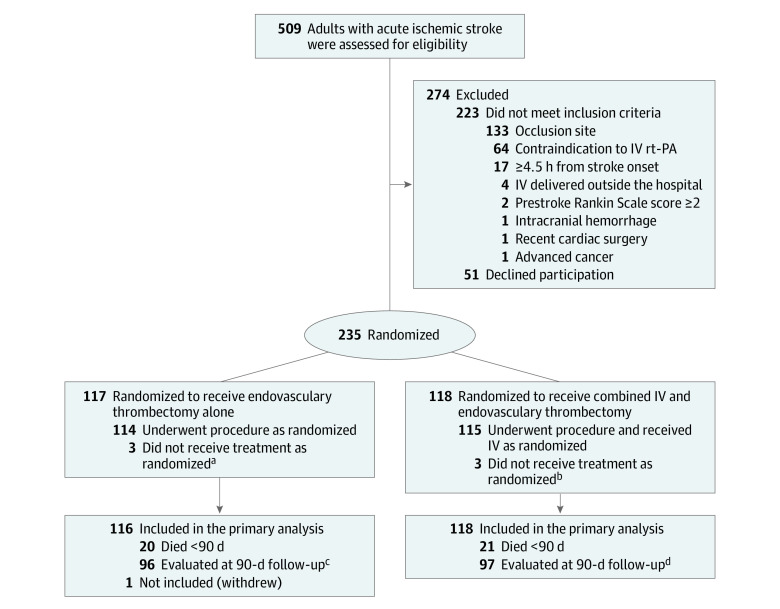
Figure 1. Eligibility, Randomization, and Follow-up of Patients Through the DEVT Randomized Clinical Trial of Intravenous Alteplase in Patients With Acute Ischemic Stroke.
aOne patient had expanded Thrombolysis in Cerebral Infarction (eTICI) 3 on the first intracranial angiography; the other, eTICI 2c on first intracranial angiography. One patient withdrew immediately after randomization.
bOne patient had eTICI 2c on first intracranial angiography; 2 had eTICI 2b on first intracranial angiography.
cOf the survivors, 91 had video recording, 4 had voice recording, 1 outcome determined by local investigators blinded to the treatment assignments because video or voice recording was unavailable.
dOf the survivors, 95 had video recording, 2 had voice recording, 0 outcomes determined by local investigators blinded to the treatment assignments.
DEVT indicates Direct Endovascular Thrombectomy vs Combined IV Thrombolysis and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation Trial; IV indicates intravenous; rt-PA, recombinant tissue type plasminogen activator.
Randomization and Masking
Patients were randomized in a 1:1 ratio to either the endovascular thrombectomy alone group or combined IV thrombolysis and endovascular thrombectomy group. The randomization procedure was web-based, and competitive recruitment was applied in this trial. Randomization allocations were created by an independent statistician with the use of SAS version 9.3 (SAS Institute Inc) to keep the study group blind. Four-block randomization was used. Personnel conducting clinical examinations were qualified assessors who had not participated in the study procedures.
Intervention
Patients in both groups received rapid endovascular treatment. In the endovascular thrombectomy alone group, patients underwent endovascular treatment only, which included thrombectomy with stent retrievers, thromboaspiration, intraarterial thrombolysis, balloon angioplasty, stenting, or a combination of these approaches at the discretion of the treatment team.
Patients in the combined treatment group underwent the endovascular procedure after receiving a standard dose of IV alteplase (0.9 mg/kg of body weight; 10% administered as a bolus followed by 1-hour infusion of the remaining dose). Endovascular treatment was initiated as soon as possible without waiting for clinical response from IV alteplase. The full alteplase dose was infused even if successful reperfusion had already been established.
Outcome and Safety Measures
The primary outcome was the proportion of patients achieving functional independence (mRS score, 0-2) at 90 days (+/− 14 days), with central evaluation by 2 blinded independent mRS-certified neurologists based on video or voice recordings taken at the outpatient clinic, during a telephone or video call, or by the patient’s family. If video or voice recordings were unavailable, the outcomes were determined in person by local investigators blinded to the treatment assignments. Disagreements were resolved through consensus (eMethods 3 in Supplement 4).
Secondary outcomes included the distribution of 90-day mRS scores and the rates of excellent functional outcome (mRS score, 0-1), successful reperfusion on postprocedural angiography ( grade 2b-3 on the expanded Thrombolysis in Cerebral Infarction [eTICI] scale , indicating reperfusion of ≥50% of the affected territory),16 vessel reperfusion on CT or MR angiography within 48 hours after treatment (successful recanalization was defined by a core laboratory–adjudicated modified Arterial Occlusive Lesion score of 2 or 3 on CT or MR angiography, indicating partial or complete recanalization of the occluded artery), the change of the National Institutes of Health Stroke Scale (NIHSS) score (range, 0 to 42, with higher values indicating more severe deficit) from baseline to 24 hours and 5 to 7 days (or discharge if earlier), and score of European Quality of Life 5-Dimension 5-level scale (EQ-5D-5L; range, −0.39 to 1, higher scores indicate a better quality of life) at 90 days. The NIHSS assessment was based on central evaluation through video recording or, if recording was unavailable, by blinded local investigators. A post hoc analysis evaluated the rates of successful reperfusion before endovascular treatment (defined as an eTICI score of 2b, 2c, or 3 on the initial intracranial angiogram).
Adverse events, including incidence of symptomatic intracranial hemorrhage within 48 hours, 90-day mortality, procedure-associated complications, and serious adverse events, were adjudicated by an independent clinical events committee unaware of treatment assignment. Assessment of intracranial hemorrhage was based on the Heidelberg classification.17 A post hoc analysis investigated the rates of asymptomatic and symptomatic intracranial hemorrhage using the European Cooperative Acute Stroke Study III (ECASS III), ECASS II, the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST), and the National Institute of Neurological Disorders and Stroke (NINDS) definitions.
Statistical Analysis
The primary hypothesis was that the rate of 90-day functional independence with endovascular treatment alone would be noninferior to IV alteplase plus endovascular treatment, the primary aim of the statistical analysis. Sample size calculation was made assuming that 43% of patients in both groups would achieve functional independence at the 90-day follow-up according to previous studies.18,19,20 Among the recent high-quality randomized clinical trials, the endovascular group in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)6 trial had the lowest proportion (about 33%) of 90-day functional independence. We assumed that the proportion of patients achieving 90-day functional independence of the endovascular thrombectomy alone group could not be lower than 33%. Therefore, the noninferiority margin was set at −10.0% as the clinically relevant limit for the outside bound of the CI.
A sample size of 918 patients (459 patients per group) would provide 80% statistical power to show noninferiority of endovascular thrombectomy alone group compared with combined treatment group including a 5-interim group-sequential analysis plan, using a 1-sided significance level of .025 and a noninferiority margin of 10% of the functional independence proportion in the combined treatment group. To adjust for a 5% attrition rate, the total sample size increased to 970. The evaluable sample size was 194 at each interim analysis (97 patients in each group). The termination rule for efficacy was defined by using the Pocock analog boundaries on the binary outcome of functional independence.21
The primary outcome was analyzed using a noninferiority test for the difference of 2 proportions. The adjusted odds ratio was estimated using a logistic regression model by taking the following variables into account: age, baseline NIHSS score, baseline Alberta Stroke Program Early Computed Tomography Score (ASPECTS), onset to randomization time, and occlusion site. Secondary outcomes were analyzed using a logistic, ordinal logistic, or linear regression model as appropriate. The Kaplan-Meier method was used to assess the mortality. The log-rank test was applied to compare the 2 treatment groups. Moreover, the death data of each treatment group were reported as 90-day binary deaths. The post hoc hierarchical modeling and sensitivity analyses were performed to assess site effects.
The missing values of baseline variables were imputed with multiple imputation. Subgroup analyses were conducted for the primary effect variable, and the Wald χ2 test was used to test the interaction. All enrolled patients were analyzed according to their randomization group. Patients who actually received the assigned treatment and did not have major protocol violations were included in the per-protocol analysis. The rates of successful reperfusion before endovascular treatment was analyzed post hoc. All statistical tests were 2-sided with a significance level at .05 unless otherwise noted. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were performed using SAS version 9.4. Figures were drawn using Excel software 2019 (Microsoft).
Trial Termination
The first interim analysis was performed as planned after 194 patients (20% of the maximum sample size) had completed their 90-day follow-up on May 12, 2020. The proportion of patients achieving functional independence for the endovascular thrombectomy alone group (54.64%) exceeded that of the combined treatment group (47.42%) by 7.2% (1-sided 97.5% CI, −6.8% to ∞). The noninferiority test demonstrated that endovascular thrombectomy alone was noninferior to combined treatment (z = 2.4042, P for noninferiority = .008). Because this crossed the prespecified efficacy boundary (z = 2.35826, P for noninferiority = .009) that was prespecified for early termination, the steering committee accepted the recommendation from the data and safety monitoring board of concluding the trial on May 14, 2020 (eMethods 2 and eFigure 2 in Supplement 4).
Results
Patients and Baseline Characteristics
From May 20, 2018, to May 2, 2020, a total of 509 patients across 33 stroke centers in China were screened. Among them, 235 qualified and were randomly enrolled in the trial. One patient enrolled into the endovascular thrombectomy alone group could not be included in the analysis because the legal representative withdrew consent immediately after randomization. The median age of the 234 patients was 70 years (interquartile range [IQR], 60-78 years); 132 patients (56.4%) were men. Of 234 patients, 116 were randomized to the endovascular thrombectomy alone group and 118 to combined treatment group. No crossovers occurred, and no one was lost to the 90-day follow-up. Baseline characteristics were similar in both groups (Table 1; eTable 1 in Supplement 4). The distribution of participating centers is illustrated on the map of China (eFigure 3), another map shows the number of patients recruited at each center (eFigure 4), and the third map shows the enrollment and follow-up of patients (eFigure 5 in Supplement 4).
Table 1. Baseline Characteristics and Workflow Measures.
| No. (%) of patients | ||
|---|---|---|
| Endovascular thrombectomy alone (n = 116) | Combined IV thrombolysis and endovascular thrombectomy (n = 118) | |
| Demographic characteristics | ||
| Age, median (IQR), y | 70 (60-77) | 70 (60-78) |
| Sex | ||
| Men | 66 (56.9) | 66 (55.9) |
| Women | 50 (43.1) | 52 (44.1) |
| Medical historya | ||
| Hypertension | 69 (59.5) | 74 (62.7) |
| Atrial fibrillation | 62 (53.5) | 62 (52.5) |
| Coronary heart diseaseb | 30 (25.9) | 19 (16.1) |
| Smokingc | 28 (24.1) | 29 (24.6) |
| Diabetes | 25 (21.6) | 20 (17.0) |
| Hyperlipidemia | 18 (15.5) | 22 (18.6) |
| Ischemic stroke | 14 (12.1) | 19 (16.1) |
| Prestroke score on the modified Rankin Scale of 1d | 6 (5.2) | 11 (9.3) |
| Stroke etiology | ||
| Cardioembolism | 65 (56.0) | 69 (58.5) |
| Large artery atherosclerosis | 32 (27.6) | 28 (23.7) |
| Intracranial atherosclerosis | 28 (24.1) | 23 (19.5) |
| Unknown | 15 (12.9) | 20 (16.9) |
| Othere | 4 (3.4) | 1 (0.8) |
| Imaging characteristicsf | ||
| Baseline ASPECTS, No.g | 115 | 117 |
| Median (IQR) | 8 (7-9) | 8 (7-9) |
| Occlusion site on CT or MR angiography | ||
| Intracranial internal carotid artery | 18 (15.5) | 17 (14.4) |
| M1 middle cerebral artery segment | 95 (81.9) | 99 (83.9) |
| M2 middle cerebral artery segment | 3 (2.6) | 2 (1.7) |
| Clinical examination at arrival, median (IQR) | ||
| NIHSS scoreh | 16 (12-20) | 16 (13-20) |
| Systolic blood pressure, mm Hgi | 146 (129-165) | 145 (128-168) |
| Glucose level, mmol/L | 6.7 (5.7-8.1) | 6.9 (5.9-8.9) |
| No. of patients | 114 | 115 |
| Workflow times, median (IQR), min | ||
| Onset to randomizationj | 170 (129-204) | 168 (144-216) |
| Arrival to intravenous alteplase | NA | 61 (49-81) |
| Arrival to arterial puncture | 101 (80-135) | 105 (80-132) |
| Onset to puncturej | 200 (155-247) | 210 (179-255) |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CT, computed tomography; IQR, interquartile range; IV, intravenous; MR, magnetic resonance; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
SI conversion factor: To convert glucose from mmol/L to mg/dL, divide by 0.0555.
Patient self-report or family report.
Included self-reported coronary artery bypass grafting surgery, angina pectoris (confirmed by angiogram), angioplasty, or coronary artery stents, besides myocardial infarction.
Current or within the prior 5 years.
See the Methods section for functional disability ranges. A score of 2 or less indicates functional independence. The score before onset was evaluated with patients or families through the specified Rankin Structured interview. Only patients with scores of 0 or 1 were enrolled.
The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification assessed stroke etiology; other causes included small vessel occlusion, nonatherosclerotic vasculopathies, hypercoagulable states, and hematologic disorders.
Imaging characteristics were assessed by the imaging core laboratory.
Imaging measures the extent of ischemic stroke (range, 0-10, higher scores indicate smaller infarct core). Listed are values for the core laboratory assessment.
National Institutes of Health Stroke Scale (NIHSS) score (range, 0-42, lower scores indicate less severe neurological deficits).
Assessed at enrollment; antihypertensives were not permitted prior to determination of inclusion.
Onset from patient or witness. Unknown times were the last known time well as reported by a proxy.
Primary Outcome
Among the patients who survived and were followed up to 90 days, video materials were acquired from 186 patients and voice recordings from 6 patients. One patient’s assessment was determined by local investigators blinded to the treatment assignments because video or voice recording technology was unavailable. Sixty-three patients (54.3%) in the endovascular thrombectomy alone group achieved functional independence vs 55 patients (46.6%) in the combined treatment group (difference, 7.7%; 1-sided 97.5% CI, −5.1% to ∞; Table 2). The lower boundary of the CI of −5.1% and was greater than the prespecified noninferiority margin of −10%. In addition, the noninferiority test demonstrated that the endovascular thrombectomy alone was noninferior to the combined IV thrombolysis and endovascular thrombectomy group (z = 2.7157, P for noninferiority = .003) since it crossed the efficacy boundary (z = 2.35826, Pfor noninferiority = .009) that was prespecified for early termination. The per-protocol analysis indicated that results were consistent with the primary analysis (eTable 3 and eTable 4 in Supplement 4). Prespecified subgroup analyses are shown in eFigures 6 and 7 (Supplement 4), and the post hoc hierarchical modeling and sensitivity analyses indicate that the site effects were not significant (eTable 5 in Supplement 4).
Table 2. Modified Rankin Scale Score at 90 Days and Secondary Outcomes.
| No. (%) | Unadjusted difference (95% CI) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)a | ||
|---|---|---|---|---|---|
| Endovascular thrombectomy alone (n = 116) | Combined IV thrombolysis and endovascular thrombectomy (n = 118) | ||||
| Primary efficacy outcomeb | |||||
| Functional independencec | 63 (54.3) | 55 (46.6) | 7.7 (−5.1 to ∞)d | 1.36 (0.82 to 2.28) | 1.48 (0.81 to 2.74) |
| Secondary efficacy outcomes | |||||
| Excellent outcomec | 44 (37.9) | 37 (31.4) | 6.6 (−5.6 to 18.7) | 1.34 (0.78 to 2.30) | 1.38 (0.75 to 2.56) |
| Disability level, median (IQR), mRS scorec | 2 (1 to 4) | 3 (1 to 4) | 0 (−1 to 0) | 1.17 (0.75 to 1.84)e | 1.13 (0.71 to 1.79)e |
| Successful reperfusion (eTICI 2b-3) at final angiogramf | 100 (88.5) | 102 (87.2) | 1.3 (−7.1 to 9.8) | 1.13 (0.51 to 2.53) | 1.14 (0.50 to 2.61) |
| No. | 113 | 117 | |||
| Reperfusion on follow-up CT or MR angiography ≤48 hg | 96 (97.0) | 94 (93.1) | 3.9 (−2.1 to 9.9) | 2.38 (0.64 to 11.31) | 2.37 (0.63 to 11.34) |
| No. | 99 | 101 | |||
| NIHSS score, median (IQR), change from baseline | β Coefficient (95% CI)h | ||||
| 24 hi | −4 (−8 to 0) | −3 (−6 to −1) | −1 (−2 to 1) | −0.14 (−1.97 to 1.69) | −0.26 (−2.06 to 1.54) |
| 5 to ≈7 d or early dischargei | −7 (−11 to −1) | −6 (−10 to −2) | 0 (−2 to 2) | 1.02 (−1.76 to 3.80) | 0.77 (−1.96 to 3.50) |
| Health–related qualify of life, EQ-5D-5L score, median (IQR)j | 0.89 (0.22 to 1.00) | 0.74 (0.26 to 0.96) | 0.00 (0.00 to 0.05) | 0.04 (−0.06 to 0.14) | 0.04 (−0.04 to 0.13) |
Abbreviations: CT, computed tomography; EQ-5D-5L, European Quality of Life 5-Dimensions 5-Levels questionnaire; eTICI, Expanded Thrombolysis In Cerebral Infarction, IQR, interquartile range; IV, intravenous; MR magnetic resonance; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Values were adjusted for age, baseline NIHSS and Alberta Stroke Program Early Computed Tomography Score, occlusion site, and time from onset to randomization.
Noninferiority test for the difference of 2 proportions.
See the Methods section for mRS score ranges and evaluation criteria.
Indicates 1-sided 97.5% CI.
The common odds ratio was estimated from an ordinal logistic regression model and indicates the odds of improvement of 1 point on the mRS, with a common odds ratio greater than 1 favoring the endovascular thrombectomy treatment alone group.
The eTICI grade was determined at the final angiogram (range, 0 [no reperfusion] to 3 [completed reperfusion]). A score of 2b to 3 indicates successful reperfusion. Data were missing from 3 patients in the endovascular thrombectomy alone group and 1 in the combined treatment group. A complete list of eTICI grades is provided in eTable 2 in Supplement 3.
Data were not available for 23 patients (12 in the endovascular thrombectomy alone group; 11 in the combined treatment group). Reperfusion failed in 11 patients (5 in the endovascular thrombectomy alone group and 6 in the combined treatment group). Vessel patency was adjudicated by 2 blinded independent neuroradiologists. Disagreements were resolved by consensus.
The β coefficient was estimated from a multivariable linear regression model.
Lower scores (range, 0-42), indicate less severe neurological deficits.
Higher scores (range, −0.39 to 1) indicate better quality of life; 0 is the value of a health state equivalent to death; negative values, worse than death; 1, full health.
Secondary Outcomes
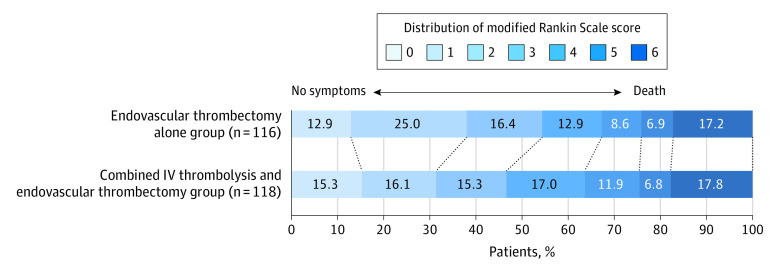
The median of 90-day mRS scores for endovascular thrombectomy alone was 2 (IQR, 1-4) and for the combined treatment, 3 (IQR, 1-4). The unadjusted difference was 0 (95% CI, −1 to 0). The adjusted common odds ratio was 1.13 (95% CI, 0.71-1.79). There were no significant differences in other secondary efficacy outcomes (Table 2). In a post hoc analysis, successful reperfusion before endovascular treatment assessed on initial angiography was observed for 2 patients in the endovascular thrombectomy alone and 3 in the combined IV thrombolysis and endovascular thrombectomy group (1.7% vs 2.6%). Further details are provided in the Table 2 and Figure 2.
Figure 2. Distribution of the Modified Rankin Scale Score at 90 Days.
Shown are scores on the modified Rankin Scale for patients in each group who were evaluated by means of video (186 patients) and voice (6 patients) recordings and by local investigators (1 patient). Forty-one patients died before 90 days. IV indicates intravenous.
Safety Outcomes
The proportion of patients who died within 90 days was 17.2% among those in the endovascular thrombectomy alone group vs 17.8% among those in the combined treatment group (difference, −0.5%; 95% CI, −10.3% to 9.2%). According to the Heidelberg bleeding classification, the symptomatic intracerebral hemorrhage in the 2 groups was 6.1% vs 6.8% (difference, −0.8%; 95% CI, −7.1% to 5.6%), respectively. Furthermore, in post hoc analyses, based on other definitions, the rate of symptomatic intracranial hemorrhage did not differ significantly between the 2 groups (Table 3). In a post hoc analysis, asymptomatic intracerebral hemorrhage occurred in 15.7% vs 25.6% (difference, −10.0%; 95% CI, −20.3% to 0.3%), respectively. Clot migration occurred in 20 patients (17.7%) of 113 in the endovascular thrombectomy alone group vs 28 of 117 (23.9%) in combined treatment group. There were no significant differences in proportions of procedures associated complications or serious adverse events (Table 3). Kaplan-Meier estimates of the probability of death are provided in eFigure 5 in Supplement 4.
Table 3. Reported Severe Adverse Events and Procedure Associated Complications.
| No./total (%) | ||
|---|---|---|
| Endovascular thrombectomy alone group (n = 116) | Combined IV thrombolysis and endovascular thrombectomy group (n = 118) | |
| Patients with at least 1 serious adverse event | 30 (25.9) | 26 (22.0) |
| Any intracranial hemorrhagea | 25/115 (21.7) | 38/117 (32.5) |
| Symptomatic intracerebral hemorrhage definitions | ||
| NINDSb | 10/115 (8.7) | 12/115 (10.3) |
| ECASS IIc | 9/115 (7.8) | 10/117 (8.5) |
| HBCd | 7/115 (6.1) | 8/117 (6.8) |
| ECASS IIIe | 5/115 (4.3) | 7/117 (6.0) |
| SITS-MOSTf | 4/115 (3.5) | 5/117 (4.3) |
| Mortality at 90 d | 20 (17.2) | 21 (17.8) |
| Acute respiratory failureg | 14 (12.1) | 12 (10.2) |
| Large or malignant middle cerebral artery strokeh | 13 (11.2) | 9 (7.6) |
| Acute heart failurei | 12 (10.3) | 9 (7.6) |
| Hemicraniectomyj | 3 (2.6) | 5 (4.2) |
| Patients with at least 1 procedure-associated complicationk | 34/113 (30.1) | 48/117 (41.0) |
| Clot migrationl | 20/113 (17.7) | 28/117 (23.9) |
| Distal occlusion present at procedure endm | 19/113 (16.8) | 21/117 (18.0) |
| Contrast extravasationn | 16/115 (13.9) | 17/117 (14.5) |
| Arterial perforation | 2 (1.7) | 6 (5.1) |
| Puncture access complications | 1 (0.9) | 6 (5.1) |
| Arterial dissection | 0/113 | 1/117 (0.8) |
Abbreviations: ECASS, European Cooperative Acute Stroke Study; HBC, Heidelberg bleeding classification; NINDS, National Institute of Neurological Disorders and Stroke; SITS-MOST, Safe Implementation of Thrombolysis in Stroke–Monitoring Study.
A complete list of intracranial hemorrhage classifications is provided in eTable 7 and definitions of symptomatic intracerebral hemorrhage in eTable 8 in Supplement 4. Data were not available for 1 patient in the endovascular thrombectomy alone group and 1 in the combined treatment group.
Symptomatic if hemorrhage had not been seen on a previous computed tomographic (CT) scan but either subsequent suspicion of hemorrhage or decline in neurological status existed.
The ECASS II was the same as ECASS III, except that establishment of a causal relationship between the hemorrhage and clinical deterioration or death was not a requirement.
Symptomatic intracerebral hemorrhage detected by brain imaging as a relevant change in neurological status; absence of another explanation for deterioration; an event leading to intubation, hemicraniectomy, or external ventricular draining placement; or other major medical or surgical intervention.
Any hemorrhage with neurological deterioration identified as the predominant cause.
Local or remote parenchymal hematoma type 2 on the imaging scan obtained 22 to 36 hours after treatment, plus neurological deterioration.
Need for mechanical ventilation or oxygen through a reservoir mask or a Pulmonary Severity Index greater than 130.
Any large infarction with mass effect observed on imaging that required medical or surgical treatment of mass effect.
Brain natriuretic peptide (BNP) concentration, changes in N-terminal pro-BNP (NT-pro-BNP) or Killip class II or higher.
Surgical procedure to decompress the swollen hemisphere caused by large or malignant middle cerebral artery stroke.
All procedure-associated complications were reported by the clinical events committee.
Clot observed to have moved before and or during the intervention at the occlusion site. Data were not available for 3 patients in the endovascular thrombectomy alone group and 1 in the combined treatment group.
Occurred after reperfusion of the primary occlusion site, any vessel occlusions distal from the primary occlusion site were considered emboli due to periprocedural thrombus fragmentation.
Contrast extravasation is a hyperdense lesion with a maximum Hounsfield unit greater than 90 that persists on a follow-up CT scan.22 Data were not available for 1 patient in the endovascular thrombectomy alone group and 1 in the combined treatment group.
Discussion
In this randomized clinical trial involving patients with ischemic stroke who presented within 4.5 hours from onset, had proximal anterior circulation occlusion, and were eligible for intravenous alteplase, endovascular thrombectomy alone, compared with combined IV alteplase and endovascular thrombectomy, met the prespecified statistical threshold for noninferiority for achieving 90-day functional independence. Different from the prior trials, this present trial excluded participants with middle cerebral artery M2 segment occlusion and used a full dose of alteplase.
The rate of 90-day functional independence with combined IV thrombolysis and endovascular thrombectomy in this trial was 46.6%, which is similar to the 46.4% observed in the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) meta-analysis.23 However, the overall frequency of 90-day functional independence in this study was higher (50.4%) than that observed in the Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals (DIRECT-MT) trial (36.6%).13 The potential explanations for these differences include that the intracranial internal carotid artery occlusions occurred more frequently in the DIRECT-MT trial (35%) than in either this trial (15%) or the HERMES trial (21%), whereas the patients with favorable collateral status (defined as grade 2-3 on the ASITN/SIR scale) had fewer in the DIRECT-MT trial (24%) than patients in either this trial (68.4%) or the HERMES trial (85%).13,24
More patients participating in both groups in this study achieved 90-day functional independence than patients in the DIRECT-MT trial. This provided an important supplement of noninferiority despite the early termination and consequent smaller sample size in the current study. That middle cerebral artery M2 occlusions were excluded from this trial, which are known to have a better response to IVT,25 may have contributed to the differences across the 2 trials. Indeed, higher rates of successful reperfusion prior to thrombectomy were seen with combined IVT and endovascular thrombectomy in the DIRECT-MT trial but not in this trial. Some other factors that may have theoretically favored endovascular thrombectomy alone in this trial include the lower proportions of both clot migration (17.7% vs 23.9%) and asymptomatic intracranial hemorrhage (15.7% vs 25.6%) over combined IV thrombolysis and endovascular thrombectomy because both these factors appear to be associated with worse functional outcomes after endovascular stroke treatment.10,26
A cohort study that used data from 2017 to 2019 and included 1526 patients showed that patients with asymptomatic intracranial hemorrhage have a worse functional outcome at 3 months than do those without it after endovascular treatment for acute ischemic stroke.27 This observation may be attributed to the cytotoxic effect of the contrast agent and the mass effect caused by the destruction of the blood-brain barrier, compressing the adjacent healthy parenchyma with subsequent impairment of the functional recovery.27
In comparing the results of this trial with those of the SKIP and DIRECT-MT trials, the rate of intracranial hemorrhage was similarly higher among patients in the combined treatment group this trial and in the SKIP trial (34% vs 50%, P = .02), even though patients in the SKIP trial received a lower dose of alteplase (0.6 mg/km) than did the patients in this study. Conversely, patients in the DIRECT-MT trial had similar rates of asymptomatic intracranial hemorrhage between treatment groups (33.3% vs 36.2%). This could be partially because in the DIRECT-MT trial’s combined IV thrombolysis and endovascular thrombectomy group, 30 patients (9.1%) did not receive either the full dose (n = 20) or any dose (n = 10) of alteplase, and 37 patients (11.2%) did not undergo thrombectomy. Moreover, eTable 6 lists other important factors—including trial design, inclusion criteria, primary end point—used in the DIRECT-MT and SKIP trials alongside those used in this trial (Supplement 4).
Limitations
This study has several limitations. First, the noninferiority margin used in this trial was not selected using the minimal clinically important difference or fixed margin methods. The noninferior margin of 10% is broad, reaching no consensus in the clinical community, which may lead to concerns about the robustness of study results. Second, the early termination of the trial does create potential for overestimation of the effect size. However, it followed a highly conservative prespecified statistical stopping rule.
Third, the median time from hospital arrival to the start of IV alteplase of 61 minutes (IQR, 49-81 minutes) in this trial was similar to the 59 minutes (IQR, 45-78 minutes) in the DIRECT-MT trial but was longer than what has been reported in the other recent thrombectomy trials.2,3,4,5,6,7 The main reason for these differences is that alteplase could not be administrated immediately after standard clinical and neuroimaging screening as they were in the previous trials because additional time was required for study screening, obtaining patient consent, and randomization in this and the DIRECT-MT trials. This explanation is supported by the findings of a recent multinational neuroprotection randomized trial. Despite having one of most efficient workflows for IVT and thrombectomy reported to date, the median time from hospital arrival to the initiation of the experimental drug in the Safety and Efficacy of Nerinetide in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE NA-1) trial was 64 minutes (IQR, 48-86 minutes).28 This highlights that achieving hospital arrival to initiate IV alteplase times as short as those in the previous trials with the absence of waiver of informed consent is unlikely.
In China, informed consent is required even before the standard administration of IVT because patients or their families often have to pay in advance before receiving alteplase. Relatedly, the median door-to-needle time was 62 minutes (IQR, 40-94 minutes) among 24 542 patients treated with IV alteplase in a recent analysis of the Chinese Stroke Center Alliance Program,29 a finding that is fully consistent with the times seen in this and the DIRECT-MT trials. Nevertheless, given the relatively long times from hospital arrival to alteplase initiation in this trial, it remains possible that IV alteplase might still be beneficial in the presence of faster delivery times. Moreover, as bridging with tenecteplase vs alteplase was associated with higher rates of prethrombectomy reperfusion and better 90-day functional outcomes in the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA-TNK) trial,30 it is possible that tenecteplase bridging might yield better results than thrombectomy alone.
Fourth, because this, the DIRECT-MT, and the SKIP trials only included Asian patients, the generalizability of their findings is somewhat limited due to the significantly higher incidence of intracranial atherosclerotic disease in Asian than in Western populations.
Conclusions
Among patients with ischemic stroke due to proximal anterior circulation occlusion within 4.5 hours from onset, endovascular treatment alone, compared with IV alteplase plus endovascular treatment, met the prespecified statistical threshold for noninferiority for the outcome of 90-day functional independence. These findings should be interpreted in the context of the clinical acceptability of the selected noninferiority threshold.
Collaborators List
Trial Protocol
Statistical Analysis Plan
CONSORT checklist
eMethods
eFigure 1. Overview of the DEVT trial
eFigure 2. Analysis of the primary outcome in the first interim analysis (n=194) used by the DSMB to take the decision to stop the trial
eFigure 3. Distribution of participating centers on the map of China
eFigure 4. Number of patients recruited by each center
eFigure 5. Kaplan-Meier estimates of the probability of death in patients
eFigure 6. Analysis of functional independence at 90 days in prespecified subgroups
eFigure 7. Distribution of modified Rankin Scale scores at 90 days in prespecified subgroups
eTable 1. Additional baseline characteristics
eTable 2. Additional workflow metrics and procedural characteristics
eTable 3. Primary and secondary outcomes in per-protocol analysis
eTable 4. Safety outcomes in per-protocol analysis
eTable 5. The hierarchical modeling and sensitivity analyses for assessment of site effects (post hoc analysis)
eTable 6. Differences between DEVT, DIRECT-MT, and SKIP trial
eTable 7. Assessment of intracranial hemorrhage based on Heidelberg classification
eTable 8. Definitions of Symptomatic Intracerebral Hemorrhage
eReferences
Data Sharing Statement
References
- 1.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 8.Behme D, Kabbasch C, Kowoll A, et al. Intravenous thrombolysis facilitates successful recanalization with stent-retriever mechanical thrombectomy in middle cerebral artery occlusions. J Stroke Cerebrovasc Dis. 2016;25(4):954-959. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Desilles J-P, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46(11):3241-3248. doi: 10.1161/STROKEAHA.115.010721 [DOI] [PubMed] [Google Scholar]
- 10.Ren Y, Churilov L, Mitchell P, Dowling R, Bush S, Yan B. Clot migration is associated with intravenous thrombolysis in the setting of acute ischemic stroke. Stroke. 2018;49(12):3060-3062. doi: 10.1161/STROKEAHA.118.022751 [DOI] [PubMed] [Google Scholar]
- 11.Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. 2014;71(9):1181-1185. doi: 10.1001/jamaneurol.2014.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai AT, Boo S, Buseman C, et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs without improving outcomes. J Neurointerv Surg. 2018;10(1):17-21. doi: 10.1136/neurintsurg-2016-012830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Kimura K, Takeuchi M, et al. Randomized study of endovascular therapy with versus without intravenous tissue plasminogen activator in acute stroke with ICA and M1 occlusion—a prospective multicenter randomized trial. American Heart Association Professional Heart Daily Accessed December 1, 2020. https://professional.heart.org/en/science-news/skip-study-clinical-trial-details
- 15.Qiu Z, Liu H, Li F, et al. ; DEVT study investigators . DEVT: A randomized, controlled, multicenter trial of direct endovascular treatment versus standard bridging therapy for acute stroke patients with large vessel occlusion in the anterior circulation—protocol. Int J Stroke. 2020;1747493020925349. doi: 10.1177/1747493020925349 [DOI] [PubMed] [Google Scholar]
- 16.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Zi W, Hao Y, et al. ; ACTUAL Investigators . Direct endovascular treatment: an alternative for bridging therapy in anterior circulation large-vessel occlusion stroke. Eur J Neurol. 2017;24(7):935-943. doi: 10.1111/ene.13311 [DOI] [PubMed] [Google Scholar]
- 19.Broeg-Morvay A, Mordasini P, Bernasconi C, et al. Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke. 2016;47(4):1037-1044. doi: 10.1161/STROKEAHA.115.011134 [DOI] [PubMed] [Google Scholar]
- 20.Bellwald S, Weber R, Dobrocky T, et al. Direct mechanical intervention versus bridging therapy in stroke patients eligible for intravenous thrombolysis: a pooled analysis of 2 registries. Stroke. 2017;48(12):3282-3288. doi: 10.1161/STROKEAHA.117.018459 [DOI] [PubMed] [Google Scholar]
- 21.Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med. 2011;30(28):3267-3284. doi: 10.1002/sim.4102 [DOI] [PubMed] [Google Scholar]
- 22.Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35(4):876-881. doi: 10.1161/01.STR.0000120726.69501.74 [DOI] [PubMed] [Google Scholar]
- 23.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 24.Román LS, Menon BK, Blasco J, et al. ; HERMES collaborators . Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895-904. doi: 10.1016/S1474-4422(18)30242-4 [DOI] [PubMed] [Google Scholar]
- 25.Seners P, Turc G, Maïer B, Mas J-L, Oppenheim C, Baron J-C. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47(9):2409-2412. doi: 10.1161/STROKEAHA.116.014181 [DOI] [PubMed] [Google Scholar]
- 26.Jiang F, Zhao W, Wu C, et al. Asymptomatic intracerebral hemorrhage may worsen clinical outcomes in acute ischemic stroke patients undergoing thrombectomy. J Stroke Cerebrovasc Dis. 2019;28(6):1752-1758. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 27.Constant Dit Beaufils P, Preterre C, De Gaalon S, et al. ; Endovascular Treatment in Ischemic Stroke (ETIS) Research Investigators . Prognosis and risk factors associated with asymptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke: a prospective multicenter cohort study. Eur J Neurol. 2020;0(0):1-9. doi: 10.1111/ene.14539 [DOI] [PubMed] [Google Scholar]
- 28.Hill MD, Goyal M, Menon BK, et al. ; ESCAPE-NA1 Investigators . Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878-887. doi: 10.1016/S0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 29.Gu H-Q, Rao Z-Z, Yang X, et al. ; Chinese Stroke Center Alliance Investigators . Use of Emergency Medical Services and Timely Treatment Among Ischemic Stroke. Stroke. 2019;50(4):1013-1016. doi: 10.1161/STROKEAHA.118.024232 [DOI] [PubMed] [Google Scholar]
- 30.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators . Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378(17):1573-1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collaborators List
Trial Protocol
Statistical Analysis Plan
CONSORT checklist
eMethods
eFigure 1. Overview of the DEVT trial
eFigure 2. Analysis of the primary outcome in the first interim analysis (n=194) used by the DSMB to take the decision to stop the trial
eFigure 3. Distribution of participating centers on the map of China
eFigure 4. Number of patients recruited by each center
eFigure 5. Kaplan-Meier estimates of the probability of death in patients
eFigure 6. Analysis of functional independence at 90 days in prespecified subgroups
eFigure 7. Distribution of modified Rankin Scale scores at 90 days in prespecified subgroups
eTable 1. Additional baseline characteristics
eTable 2. Additional workflow metrics and procedural characteristics
eTable 3. Primary and secondary outcomes in per-protocol analysis
eTable 4. Safety outcomes in per-protocol analysis
eTable 5. The hierarchical modeling and sensitivity analyses for assessment of site effects (post hoc analysis)
eTable 6. Differences between DEVT, DIRECT-MT, and SKIP trial
eTable 7. Assessment of intracranial hemorrhage based on Heidelberg classification
eTable 8. Definitions of Symptomatic Intracerebral Hemorrhage
eReferences
Data Sharing Statement