Key Points
Question
Does discontinuation compared with continuation of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) change the number of days alive and out of the hospital through 30 days in patients hospitalized with mild to moderate coronavirus disease 2019 (COVID-19)?
Findings
In this randomized clinical trial that included 659 patients hospitalized with mild to moderate COVID-19 and who were taking ACEIs or ARBs before hospital admission, the mean number of days alive and out of the hospital for those assigned to discontinue vs continue these medications was 21.9 vs 22.9, respectively, a difference that was not statistically significant.
Meaning
These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19.
Abstract
Importance
It is unknown whether angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) have a positive, neutral, or negative effect on clinical outcomes in patients with coronavirus disease 2019 (COVID-19).
Objective
To determine whether discontinuation compared with continuation of ACEIs or ARBs changed the number of days alive and out of the hospital through 30 days.
Design, Setting, and Participants
A randomized clinical trial of 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization (enrolled: April 9-June 26, 2020; final follow-up: July 26, 2020).
Interventions
Discontinuation (n = 334) or continuation (n = 325) of ACEIs or ARBs.
Main Outcomes and Measures
The primary outcome was the number of days alive and out of the hospital through 30 days. Secondary outcomes included death, cardiovascular death, and COVID-19 progression.
Results
Among 659 patients, the median age was 55.1 years (interquartile range [IQR], 46.1-65.0 years), 14.7% were aged 70 years or older, 40.4% were women, and 100% completed the trial. The median time from symptom onset to hospital admission was 6 days (IQR, 4-9 days) and 27.2% of patients had an oxygen saturation of less than 94% of room air at baseline. In terms of clinical severity, 57.1% of patients were considered mild at hospital admission and 42.9% were considered moderate. There was no significant difference in the number of days alive and out of the hospital in patients in the discontinuation group (mean, 21.9 days [SD, 8 days]) vs patients in the continuation group (mean, 22.9 days [SD, 7.1 days]) and the mean ratio was 0.95 (95% CI, 0.90-1.01). There also was no statistically significant difference in death (2.7% for the discontinuation group vs 2.8% for the continuation group; odds ratio [OR], 0.97 [95% CI, 0.38-2.52]), cardiovascular death (0.6% vs 0.3%, respectively; OR, 1.95 [95% CI, 0.19-42.12]), or COVID-19 progression (38.3% vs 32.3%; OR, 1.30 [95% CI, 0.95-1.80]). The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs 7.7% in the continuation group), shock requiring vasopressors (8.4% vs 7.1%, respectively), acute myocardial infarction (7.5% vs 4.6%), new or worsening heart failure (4.2% vs 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs 2.8%).
Conclusions and Relevance
Among patients hospitalized with mild to moderate COVID-19 and who were taking ACEIs or ARBs before hospital admission, there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue vs continue these medications. These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT04364893
This randomized clinical trial compares the effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) on days alive and out of the hospital at 30 days among patients with COVID-19 taking the drugs prior to hospitalization.
Introduction
Membrane-bound angiotensin-converting enzyme 2 (ACE2), an enzyme that physiologically counters renin-angiotensin-aldosterone system (RAAS) activation, is the functional receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic.1 Select preclinical investigations have shown upregulation of ACE2 expression by RAAS inhibitors, such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), raising concerns about their safety in patients with COVID-19.2
Conversely, observational data have demonstrated an association between use of ACEIs or ARBs and better outcomes in patients with COVID-19, leading to speculation that ACEIs or ARBs decrease acute lung damage and prevent angiotensin II–mediated pulmonary permeability, inflammation, and fibrosis.3,4 Therefore, there are conflicting mechanistic hypotheses and observational findings about the effect of ACEIs and ARBs on patients with COVID-19.5 Given the impossibility of obtaining reliable estimates of comparative effectiveness in observational studies, this clinical question needs to be answered in a randomized clinical trial.6,7
A multicenter randomized clinical trial conducted during a pandemic should follow the general standards used in clinical research.8 However, the urgent need for assessing new treatments requires leveraging more efficient research processes to address new challenges in conducting clinical research. Prospective, registry-based randomized clinical trials are a reliable tool for managing cost-effective and efficient research, and might be an attractive approach in critical circumstances like the current COVID-19 pandemic.9
The COVID-19 Registry is a clinical study including patients with suspected or confirmed COVID-19 hospitalized at 35 sites in Brazil.10 Combining the capacity to randomize patients to various therapies within this structure enabled a streamlined process to provide reliable answers to important clinical questions. Thus, the BRACE CORONA (Blockers of Angiotensin Receptor and Angiotensin-Converting Enzyme inhibitors suspension in hospitalized patients with coronavirus infection) trial was conducted to evaluate whether the discontinuation of ACEIs or ARBs has an effect on the number of days alive and out of the hospital through 30 days in patients hospitalized with COVID-19.
Methods
Trial Oversight, Design, and Population
The trial protocol (Supplement 1) and all amendments were approved by the Brazilian Ministry of Health National Commission for Research Ethics and by institutional review boards or ethics committees at participating sites. All patients provided informed consent before enrollment. An independent data and safety monitoring board reviewed safety and efficacy data on an ongoing basis with access to unblinded data. An independent clinical events classification committee (whose members were blinded to the treatment assignment) adjudicated the causes of death and clinical outcomes described as secondary outcomes.
The rationale and design for this trial have been published.10 In brief, this was a multicenter, registry-based, open-label randomized clinical trial with blinded end-point assessment that included patients hospitalized with COVID-19 who were taking ACEIs or ARBs prior to hospital admission to determine whether discontinuation of these drugs compared with continuation of these drugs affects the number of days alive and out of the hospital.
Patients with a suspected diagnosis of COVID-19 hospitalized at 29 centers in Brazil were included in the registry and followed up until the diagnosis of COVID-19 was confirmed. Patients aged 18 years or older with a confirmed diagnosis of COVID-19 who were taking ACEIs or ARBs prior to hospital admission were eligible for the trial. Patients taking more than 3 antihypertensive agents, those taking sacubitril/valsartan for heart failure, and those hospitalized for heart failure within the last 12 months were not eligible. Patients with a clinical indication to stop ACEI or ARB treatment (eg, those with hypotension, acute kidney injury, or shock) also were excluded. The full list of inclusion and exclusion criteria appears in the eMethods in Supplement 2.
Trial Interventions
Eligible patients were randomized using a 1:1 allocation ratio to either discontinue or continue ACEI or ARB therapy for 30 days (Figure 1). Randomization was performed using block sizes of 4. A centralized, web-based automated randomization system was used to conceal allocation. For patients randomized to the ACEI or ARB discontinuation group, other drugs could replace these agents at the discretion of the treating physician. β-Blockers were maintained in patients already taking them for heart failure.
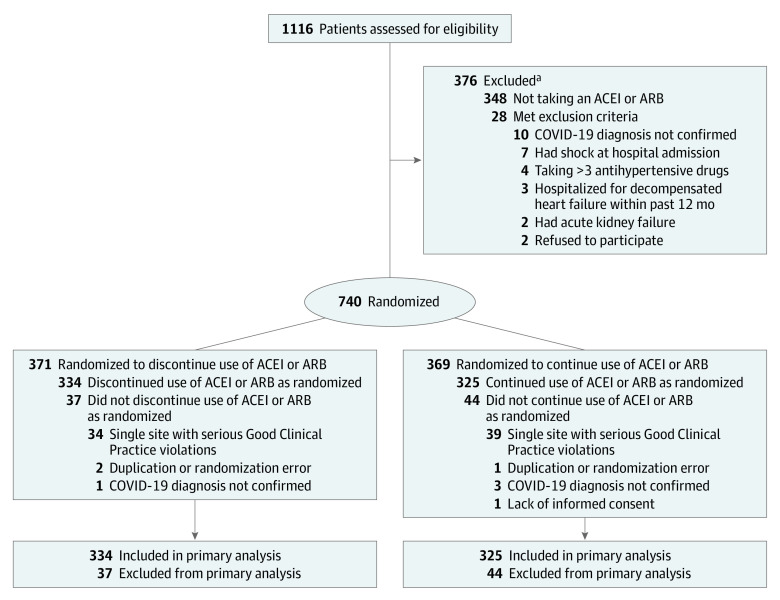
Figure 1. Study Enrollment and Analysis in the BRACE CORONA Trial on the Use of Angiotensin-Converting Enzyme Inhibitors (ACEIs) or Angiotensin II Receptor Blockers (ARBs) Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19).
aMay have met more than 1 exclusion criterion.
The study protocol did not recommend any specific treatment modification beyond discontinuing or continuing use of ACEIs or ARBs. The study team provided oversight on drug replacement, and those decisions were made based on current treatment guidelines. Patients were treated for COVID-19 according to current local standards of supportive care without the systematic use of investigational therapies. Treatment adherence was assessed based on medical prescriptions recorded in the chart throughout hospitalization and in the electronic health record after discharge.
Outcomes
The primary outcome was the number of days alive and out of the hospital from randomization through 30 days. This outcome was calculated for each patient by subtracting the number of days in the hospital and the number of days from death until the end of follow-up from 30 days.11 The secondary outcomes included: length of hospital stay (days), death (during the 30-day follow-up period), in-hospital death, cardiovascular death, COVID-19 progression (worsening of clinical severity during hospitalization in relation to baseline severity), acute myocardial infarction, new heart failure or worsening of preexisting heart failure, hypertensive crisis, transient ischemic attack, stroke, myocarditis, pericarditis, thromboembolic phenomena, arrhythmias requiring treatment, respiratory failure requiring mechanical ventilation, shock requiring vasopressors, kidney failure requiring hemodialysis, troponin level above the upper limit of normal in patients with normal values at baseline, B-type natriuretic peptide level above the upper limit of normal in patients with normal values at baseline, and D-dimer level above the upper limit of normal in patients with normal values at baseline. Detailed definitions of the secondary outcomes appear in the eMethods in Supplement 2.
Sample Size
The initial planned sample size of the trial was 500 patients (additional information appears in Supplement 3). Based on the Coalition Covid-19 Brazil I trial,12 and assuming a mean number of days alive and out of the hospital of 24 days (SD, 4 days), 500 patients would provide 90% power to detect a mean ratio of at least 1.10. This mean ratio of 1.10 represents a difference of approximately 2 days and was considered clinically relevant by the BRACE CORONA investigators and the members of the executive committee. The treatment effect considered relevant in this trial (2 days) represented half of the difference in days of hospitalization between remdesivir and placebo (4 days) in ACTT-1 (Adaptive COVID-19 Treatment Trial).13 Two interim analyses were prespecified after 150 and 250 patients had completed the study follow-up. Because enrollment occurred more quickly than anticipated, and to allow for the interim analyses to be performed, the final sample size exceeded the initial planned number of patients allowed by the trial protocol. The increased sample size by more than 30% led to a final statistical power of 94.5% to detect a minimal between-group difference for days alive and out of the hospital of 2 days.
Statistical Analysis
The primary analysis is expressed as the mean ratio for the number of days alive and out of the hospital at 30 days. The comparison between groups was made using generalized additive models for location, scale, and shape with zero-inflated β binomial distribution (eFigure 1 in Supplement 2).14 The results are presented as mean ratios (95% CIs). A mean ratio greater than 1.0 indicates that patients randomized to discontinuation of ACEIs or ARBs had more days alive and out of the hospital over the 30-day follow-up. A mean ratio less than 1.0 indicates that patients randomized to continuation of ACEIs or ARBs had more days alive and out of the hospital. The primary outcome is also presented as a mean (SD), mean difference (95% CI), median (interquartile range [IQR]), and difference in medians (95% CI).
Subgroup analyses were performed using the same generalized additive models for location, scale, and shape with β binomial distribution inflated at zero that was used for the main outcome, including interaction terms between each subgroup and study treatments. Interaction tests were done for specific subgroups, including age (<65 years or ≥65 years), obesity (yes vs no), previous use of an ACEI (yes vs no), previous use of an ARB (yes vs no), oxygen saturation (<94% of room air vs ≥94% of room air), time from symptom onset to randomization (divided in tertiles), degree of lung involvement assessed by chest computed tomographic scan at hospital admission (≤25%, 26%-50%, >50%), and clinical severity of COVID-19 (mild or moderate).
The primary analysis followed the intention-to-treat principle. A sensitivity analysis was performed in which patients who died received zero days alive and out of the hospital, regardless of when death occurred. Given the large variability in the number of patients randomized among sites (eFigure 2 in Supplement 2), another sensitivity analysis was performed using site as a random effect. An on-treatment analysis for the primary outcome was also performed including only patients who were adherent to the study intervention until time of death or through 30 days.
The secondary end points of death and cardiovascular events at 30 days were compared using log binomial models and are reported as relative risks and odds ratios (ORs) with respective 95% CIs. Continuous variables are reported as median (IQR) or mean (SD) according to normality of the distribution. The medians were compared using the Kruskal-Wallis test and the means were compared using the t test. Categorical variables are reported as absolute and relative frequencies and the proportions were compared using the χ2 test. P < .05 was considered statistically significant and 2-sided tests were performed. Because of the potential for type I error due to multiple comparisons, the findings for the analyses of secondary end points should be interpreted as exploratory. All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing).15
Results
From March 10 to June 26, 2020, 1352 patients from 35 sites were enrolled in the COVID-19 Registry. At the 29 sites participating in this study, 1116 patients were enrolled in the COVID-19 Registry from the start of the trial on April 9, 2020, to the end of enrollment on June 26, 2020. Final follow-up was completed on July 26, 2020.
Of these 1116 patients, 740 were randomized in this trial; however, 81 were excluded from the primary analysis (Figure 1). During routine onsite monitoring, it was discovered that the research coordinator at 1 site randomized 73 patients, but did not obtain informed consent, apply the study intervention, or conduct the protocol-specified follow-up. The site personnel entered falsified information into the clinical trial database regarding the study intervention and follow-up. Because of these Good Clinical Practice violations, the decision was made before unblinding the results to exclude all trial data from this site. Critical variables, including patient identification and informed consent, key eligibility criteria, including COVID-19 status and current use of an ACEI or ARB, randomization and adherence to the study intervention, and the total duration of hospitalization and vital status at 30 days were verified for 100% of the source data to mitigate the risk of similar behavior at other sites.
Therefore, a total of 659 randomized patients from 29 hospitals (28% being academic hospitals) in Brazil were included in the primary analysis, with 334 patients assigned to discontinue use of ACEIs or ARBs and 325 patients assigned to continue use of ACEIs or ARBs. The 30-day follow-up was completed for 100% of patients and no data were missing for the primary outcome. For the baseline variables, there were missing data for less than 10% and the missing data did not affect any of the analyses of the primary or secondary outcomes.
The median number of patients per site was 11 (IQR, 4-24 patients). During the peak of the pandemic (April-June for this study), the median number of beds available at all 29 centers was 166 (IQR, 139-202 beds) for patients with COVID-19 or without COVID-19. During the peak of the pandemic, the median percentage of beds with COVID-19 patients was 32.8% (IQR, 18.7%-41.5%%) (eTable 1 and eFigure 2 in Supplement 2).
Baseline Characteristics
The 2 groups were well matched with respect to baseline characteristics (Table 1 and eTable 2 in Supplement 2). The median age was 55.1 years (IQR, 46.1-65.0 years), 14.7% were aged 70 years or older, 40.4% of patients were women, and 52.2% were obese. Hypertension was present in 100% of the patients and 1.4% had heart failure. A total of 16.7% were taking an ACEI and 83.3% were taking an ARB for a median of 5 years (IQR, 3-8 years) prior to randomization. β-Blockers were taken by 14.6% of patients, diuretics by 31.3%, and calcium channel blockers by 18.4%.
Table 1. Baseline Patient Characteristicsa.
| ACEI or ARB | ||
|---|---|---|
| Discontinue use (n = 334) |
Continue use (n = 325) |
|
| Randomized at academic centers, No. (%) | 131 (39.2) | 127 (39.1) |
| Age, median (IQR), y | 55 (46.1-63.1) | 56 (46.1-66.1) |
| Aged >70 y, No. (%) | 41 (12.3) | 56 (17.2) |
| Sex, No. (%) | ||
| Female | 136 (40.7) | 130 (40) |
| Male | 198 (59.3) | 195 (60) |
| Body mass indexb | ||
| Median (IQR) | 30.5 (27.0-34.0) | 29.8 (26.8-33.6) |
| >30, No./total (%) | 183/331 (55.3) | 158/322 (49.1) |
| Medical history, No. (%) | ||
| Hypertension | 334 (100) | 325 (100) |
| Never smoker, No./total (%) | 249/309 (80.6) | 232/295 (78.6) |
| Diabetes | 111 (33.2) | 99 (30.5) |
| Coronary heart disease | 16 (4.8) | 14 (4.3) |
| Asthma | 15 (4.5) | 11 (3.4) |
| Cancer | 7 (2.1) | 3 (0.9) |
| Kidney disease | 5 (1.5) | 4 (1.2) |
| Heart failure | 2 (0.6) | 7 (2.2) |
| Clinical characteristics at hospital admission | ||
| Symptom duration, median (IQR), d | 6.5 (4.0-9.0) | 6.0 (4.0-9.0) |
| Fever with temperature >37.5°C, No./total (%) | 216/326 (66.3) | 233/320 (72.8) |
| Heart rate | ||
| No. of patients | 333 | 325 |
| Median (IQR), /min | 91.0 (81.0-104.0) | 89.0 (80.0-101.0) |
| Systolic blood pressure, median (IQR), mm Hg | 139.0 (125.0-149.0) | 135.0 (125.0-149.0) |
| Respiratory rate | ||
| No. of patients | 331 | 323 |
| Median (IQR), breaths/min | 19.0 (17.0-20.0) | 19.0 (18.0-20.0) |
| Oxygen saturation <94% on room air, No./total (%) | 88/327 (26.9) | 85/310 (27.4) |
| Cough, No. (%) | 246 (73.7) | 217 (66.8) |
| Dyspnea, No. (%) | 181 (54.2) | 173 (53.2) |
| COVID-19 clinical severity within first 24 h of hospital admission, No. (%)c | ||
| Mild | 193 (57.8) | 183 (56.3) |
| Moderate | 141 (42.2) | 142 (43.7) |
| Lung involvement on initial chest CT scan, No./total (%)d |
||
| ≤25% | 164/317 (51.7) | 153/308 (49.7) |
| 26%-50% | 112/317 (35.3) | 125/308 (40.6) |
| >50% | 41/317 (12.9) | 30/308 (9.7) |
| Medication use at hospital admission, No. (%) |
||
| ARB | 264 (79) | 285 (87.7) |
| ACEI | 70 (21) | 40 (12.3) |
| Diuretice | 105 (31.4) | 101 (31.1) |
| Statin | 75 (22.5) | 64 (19.7) |
| Calcium channel blocker | 59 (17.7) | 62 (19.1) |
| β-Blocker | 44 (13.2) | 52 (16) |
| Antiplateletf | 33 (9.9) | 33 (10.2) |
| Insulin | 14 (4.2) | 13 (4.0) |
| Oral anticoagulantg | 10 (3.0) | 8 (2.5) |
| Duration of ACEI or ARB use | ||
| No. of patients | 324 | 310 |
| Median (IQR), y | 5.0 (2.0-8.0) | 5.0 (3.0-8.0) |
| Laboratory values at hospital admissionh | ||
| Lymphocytes | ||
| No. of patients | 302 | 300 |
| Median (IQR), ×109/L | 1.2 (0.9-1.7) | 1.2 (0.8-1.6) |
| Creatinine | ||
| No. of patients | 325 | 312 |
| Median (IQR), μmol/L | 88.4 (70.7-97.2) | 88.4 (70.7-106.1) |
| C-reactive protein | ||
| No. of patients | 318 | 297 |
| Median (IQR), mg/L | 4.3 (1.6-8.9) | 4.2 (1.4-7.4) |
| Potassium | ||
| No. of patients | 276 | 264 |
| Median (IQR), mmol/L | 4.0 (3.7-4.4) | 4.0 (3.7-4.4) |
| Time from hospital admission to randomization, median (IQR), d | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) |
| Concomitant therapy, No. (%) | ||
| Azithromycin | 301 (90.1) | 296 (91.1) |
| Anticoagulationi | 222 (66.5) | 218 (67.1) |
| Antiviralj | 142 (42.5) | 135 (41.5) |
| Chloroquine or hydroxychloroquine | 72 (21.6) | 58 (17.8) |
| Tocilizumab | 17 (5.1) | 7 (2.2) |
| Corticosteroidk | 169 (50.6) | 157 (48.3) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COVID-19, coronavirus disease 2019; CT, computed tomographic; IQR, interquartile range.
SI conversion factor: To convert creatinine to mg/dL, divide by 88.4.
Additional information appears in eTable 1, eTable 2, and eFigure 2 in Supplement 2.
Calculated as weight in kilograms divided by height in meters squared
Mild defined as blood oxygen saturation of 94% or greater and lung infiltrates less than or equal to 50%; moderate, blood oxygen saturation less than 94%, or lung infiltrates greater than 50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen less than 300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Estimated by visual assessment performed by a radiologist.
Furosemide, hydrochlorothiazide, or spironolactone.
Aspirin, clopidogrel, or ticagrelor.
Warfarin, rivaroxaban, apixaban, dabigatran, or edoxaban.
Reference ranges: 1000 to 5000 × 109/L for lymphocytes; 61.88 to 106.08 μmol/L for creatinine; less than 10 mg/L for C-reactive protein; and 3.5 to 5.0 mmol/L for potassium.
Enoxaparin, unfractioned heparin, warfarin, rivaroxaban, apixaban, dabigatran, or edoxaban. The differentiation between therapeutic and prophylactic anticoagulation was based on the dose.
Oseltamivir, ribavirin, or lopinavir-ritonavir.
Prednisone, dexamethasone, hydrocortisone, or methylprednisolone.
Cough, fever, and shortness of breath were the most common symptoms at hospital admission. The median time from symptom onset to hospital admission was 6 days (IQR, 4-9 days) and 27.2% of patients had an oxygen saturation of less than 94% of room air at baseline. A total of 50.7% of patients had lung involvement of 25% or less on the chest computed tomographic scan, 37.9% had lung involvement of 26% to 50%, and 11.4% had lung involvement greater than 50%. In terms of clinical severity of COVID-19, 57.1% of patients were considered mild at hospital admission and 42.9% were moderate. The median time from hospital admission to randomization was 2 days (IQR, 1-3 days) in both groups.
The in-hospital adherence rate to the study intervention (calculated based on the number of doses of ACEIs or ARBs) was 96.4% in the discontinuation group and 94.8% in the continuation group. At the end of the study, the ACEI or ARB persistence rates, assessed via a phone call, were 71% for the discontinuation group and 92.9% for the continuation group.
Primary Outcome
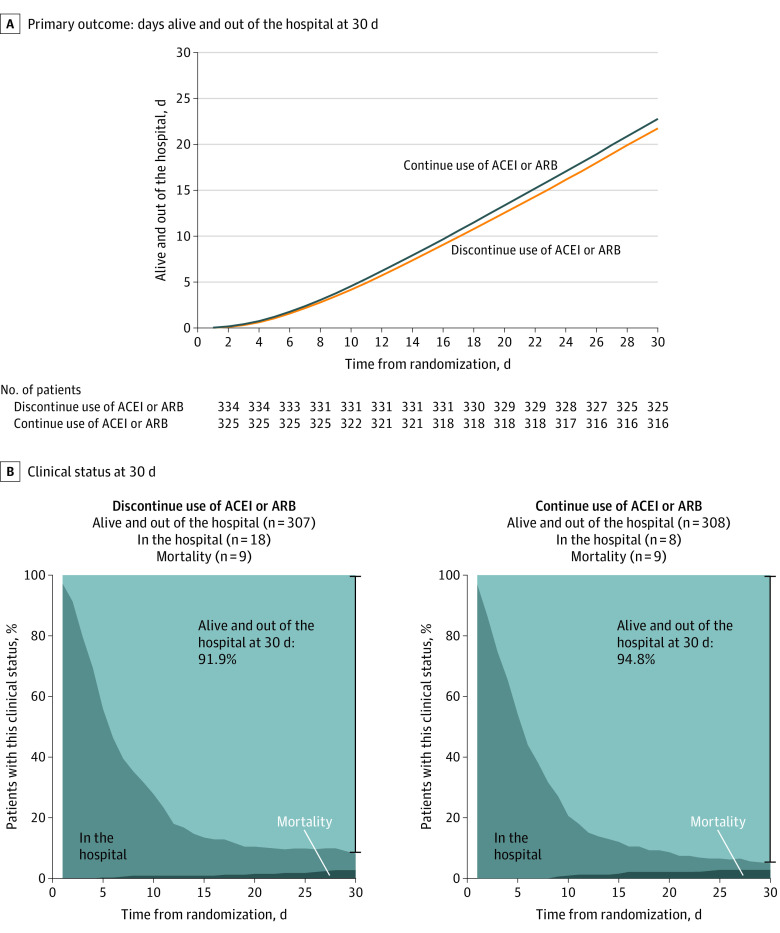
The mean number of days alive and out of the hospital for patients randomized to discontinue use of ACEIs or ARBs was 21.9 days (SD, 8.0 days) vs 22.9 days (SD, 7.1 days) for those randomized to continue use of ACEIs or ARBs (Figure 2A). For days alive and out of the hospital, the between-group mean ratio was 0.95 (95% CI, 0.90 to 1.01; P = .09) and the between-group mean difference was −1.10 days (95% CI, −2.30 to 0.13 days). The median number of days alive and out of the hospital at 30 days was 25 days for both groups and the between-group median difference was 0 days (95% CI, −1 to 1 days). The proportion of patients alive and out of the hospital at 30 days was 91.9% in the discontinuation group and 94.8% in the continuation group (Figure 2B).
Figure 2. Primary Outcome and Clinical Status at 30 Days.
For the primary outcome, the mean number of days alive and out of the hospital was 21.9 days (SD, 8.0 days) in the discontinue use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) group vs 22.9 days (SD, 7.1 days) in the continue use of ACEI or ARB group (median, 25.0 days [interquartile range, 20.0-27.0 days] vs 25.0 days [interquartile range, 21.0-27.0 days], respectively) and the between-group mean ratio was 0.95 (95% CI, 0.90-1.01; P = .09).
From the overall population, 7.5% of patients in the discontinuation group had 0 days alive and out of the hospital compared with 4.6% in the continuation group (eFigure 3 in Supplement 2). The mortality rate at 30 days for patients discontinuing ACEIs or ARBs was 2.7% vs 2.8% for patients continuing ACEI or ARBs (OR, 0.97 [95% CI, 0.38-2.52]; eFigure 4 in Supplement 2).
In a sensitivity analysis in which patients who died received 0 days alive and out of the hospital (regardless of when death occurred), there was no significant between-group difference for the primary outcome (mean ratio, 0.95 [95% CI, 0.89-1.01]). When site was used as a random effect, the between-group mean ratio of days alive and out of the hospital was 0.94 (95% CI, 0.89-0.997; P = .04).
In the on-treatment analysis, 9.7% of patients in the discontinuation group had 0 days alive and out of the hospital compared with 3.0% in the continuation group; the between-group mean ratio was 0.91 (95% CI, 0.84 to 0.96).
Secondary Outcomes
The secondary outcomes appear in Table 2 and eTable 3 in Supplement 2. For the number of days in the hospital, the between-group mean ratio was 1.21 (95% CI, 1.02 to 1.42). No other significant between-group differences were seen for cardiovascular events, biomarkers, or COVID-19 progression. The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinue ACEIs or ARBs group vs 7.7% in the continue ACEIs or ARBs group), shock requiring vasopressors (8.4% vs 7.1%, respectively), acute myocardial infarction (7.5% vs 4.6%), new or worsening heart failure (4.2% vs 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs 2.8%). The secondary outcomes according to the on-treatment analysis appear in eTable 4 in Supplement 2.
Table 2. Primary and Secondary Outcomes at 30 Daysa.
| ACEI or ARB | Absolute difference (95% CI) |
Effect size (95% CI)b | ||
|---|---|---|---|---|
| Discontinue use (n = 334) |
Continue use (n = 325) |
|||
| Primary outcome | ||||
| Days alive and out of the hospital | ||||
| Mean (SD) | 21.9 (8.0) | 22.9 (7.1) | −1.10 (−2.30 to 0.13) | MR, 0.95 (0.90 to 1.01) |
| Median (IQR) | 25.0 (20.0 to 27.0) | 25.0 (21.0 to 27.0) | ||
| Secondary outcomes | ||||
| Length of hospitalization, d | ||||
| Mean (SD) | 7.8 (7.4) | 6.7 (6.3) | 1.46 (0.12 to 2.67) | MR, 1.21 (1.02 to 1.42) |
| Median (IQR) | 5.0 (3.0 to 10.0) | 5.0 (3.0 to 9.0) | ||
| Death at 30 d, No. (%) | 9 (2.7) | 9 (2.8) | −0.07 (−2.56 to 2.41) | OR, 0.97 (0.38 to 2.52) |
| In-hospital death, No. (%) | 9 (2.7) | 7 (2.2) | 0.54 (−1.81 to 2.89) | OR, 1.26 (0.46 to 3.56) |
| Cardiovascular death, No. (%) | 2 (0.6) | 1 (0.3) | 0.29 (−0.73 to 1.31) | OR, 1.95 (0.19 to 42.12) |
| COVID-19 progression, No. (%)c | 128 (38.3) | 105 (32.3) | 6.02 (−1.27 to 13.30) | OR, 1.30 (0.95 to 1.80) |
| Respiratory failure requiring invasive mechanical ventilation, No. (%)d | 32 (9.6) | 25 (7.7) | 1.89 (−2.40 to 6.17) | RR, 1.25 (0.76 to 2.07) |
| Shock requiring vasopressors, No. (%) | 28 (8.4) | 23 (7.1) | 1.31 (−2.77 to 5.38) | RR, 1.19 (0.70 to 2.03) |
| Cardiovascular outcomes, No. (%) | ||||
| Acute myocardial infarction | 25 (7.5) | 15 (4.6) | 2.87 (−0.76 to 6.50) | RR, 1.62 (0.88 to 3.09) |
| New or worsening heart failure | 14 (4.2) | 16 (4.9) | −0.73 (−3.92 to 2.45) | RR, 0.85 (0.42 to 1.72) |
| Other outcomes, No. (%) | ||||
| Acute kidney failure requiring hemodialysis | 11 (3.3) | 9 (2.8) | 0.52 (−2.09 to 3.14) | RR, 1.19 (0.50 to 2.91) |
| Thromboembolic events | 6 (1.8) | 4 (1.2) | 0.57 (−1.30 to 2.43) | RR, 1.46 (0.42 to 5.67) |
| Stroke or TIA | 3 (0.9) | 3 (0.9) | −0.02 (−1.48 to 1.43) | RR, 0.97 (0.18 to 5.23) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COVID-19, coronavirus disease 2019; IQR, interquartile range; MR, mean ratio; OR, odds ratio; RR, relative risk; TIA, transient ischemic attack.
Additional information appears in eTable 3 and eTable 4 in Supplement 2.
Comparisons were made using generalized additive models for the primary outcome and log binomial models for the secondary outcomes.
Defined as change in clinical severity status during hospitalization. Mild defined as blood oxygen saturation of 94% or greater and lung infiltrates less than or equal to 50%; moderate, blood oxygen saturation less than 94%, or lung infiltrates greater than 50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen less than 300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
The decision for intubation was based on clinical judgment.
Subgroup Analysis
Overall, the effect on the primary outcome was consistent across major predefined subgroups, including age, obesity, type of RAAS inhibitor (ACEI or ARB), days of symptoms to randomization, and opacities on the chest computed tomographic scan (Figure 3). However, there was a significant interaction among the treatment effect, oxygen saturation, and COVID-19 clinical severity at hospital admission, with the results slightly favoring the group continuing ACEI or ARB therapy among patients with lower oxygen saturation and greater disease severity at presentation.
Figure 3. Subgroup Analysis for the Primary Outcome.
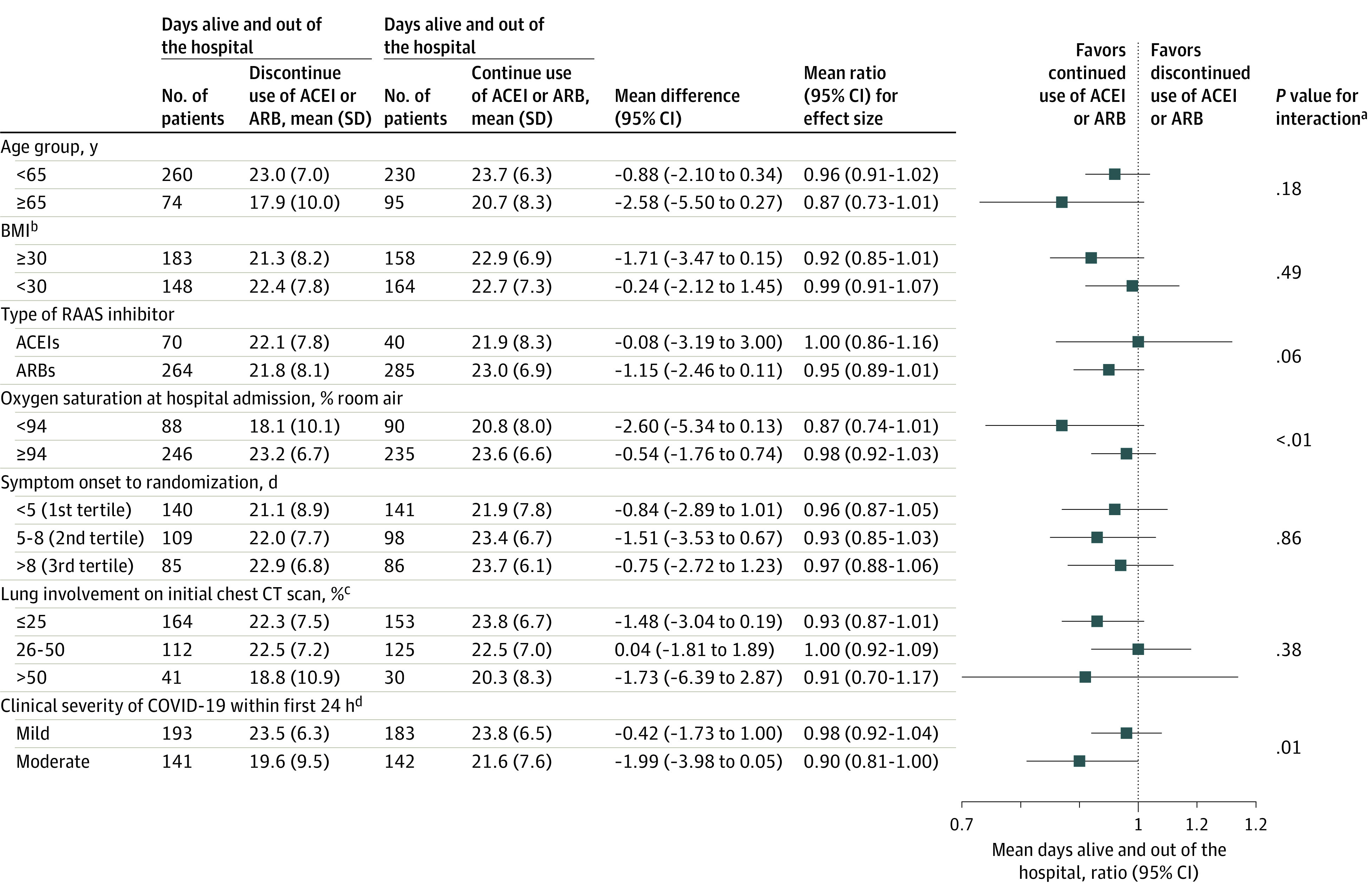
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CT, computed tomographic; RAAS, renin-angiotensin-aldosterone system.
aThe subgroup analyses were performed using the same generalized additive model of location, scale, and shape with beta binomial distribution inflated at zero that was used for the primary outcome, including interaction terms between each subgroup and study treatments.
bCalculated as weight in kilograms divided by height in meters squared.
cEstimated by visual assessment performed by a radiologist.
dDefined as change in clinical severity status during hospitalization. Mild defined as blood oxygen saturation of 94% or greater and lung infiltrates less than or equal to 50%; moderate, blood oxygen saturation less than 94%, or lung infiltrates greater than 50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen less than 300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Discussion
In this pragmatic, registry-based randomized clinical trial, discontinuing ACEI or ARB therapy for 30 days did not affect the number of days alive and out of the hospital in patients hospitalized with mild to moderate COVID-19. These results were generally consistent across major subgroups. There were no significant between-group differences in death, cardiovascular outcomes, or COVID-19 progression.
The interplay between SARS-CoV-2 and the RAAS has led to competing speculation about the effect of RAAS inhibitors on the course of COVID-19.16 Because animal models found that ACEIs and ARBs upregulated expression of ACE2, a receptor involved in the SARS-CoV-2 infection of host target cells, it was theorized that these medications could enhance viral binding and cell entry.2 Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.16 Animal data suggest an inherent protective effect of ARBs against COVID-19 pneumonia by limiting lung damage in mice infected with SARS-CoV, a close viral relative of SARS-CoV-2.17 Uncertainty around the role of RAAS inhibitors in patients with COVID-19 has intensified due to observational data and a systematic review.18,19,20,21,22,23,24,25,26,27,28,29,30,31 Scientific societies have recommended that patients should not discontinue ACEI or ARB therapy during the COVID-19 pandemic.32,33 To date, randomized clinical trials designed to determine whether ACEIs or ARBs are beneficial, harmful, or neutral with respect to clinical outcomes in patients with COVID-19 have been lacking.
Hypertension is an important comorbidity in patients with COVID-19. Recent data have postulated that immune dysfunction may contribute to poor outcomes in patients with COVID-19 and hypertension.28 Considering the importance of treating hypertension, it has been shown that when use of long-term medications is discontinued during hospitalization, the medications often are not restarted due to clinical inertia, thereby worsening long-term outcomes.34 The results of this trial support the continued use of ACEIs or ARBs in patients hospitalized with COVID-19. In this study, all patients had hypertension and more than 50% were obese, both comorbidities that increase the risk of poor outcomes with COVID-19.35,36
In addition, in multicenter trials, site effect may play an important role in study outcomes. The sensitivity analysis that treated site as a random effect found a statistically significant result favoring the group that continued ACEIs or ARBs. These results were similar in the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission, in which continuing ACEIs or ARBs may be beneficial. The primary analyses with the null results but wide 95% CIs suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs.
In this study, 14.7% of patients were aged 70 years or older. Other studies12,37,38,39 that included hospitalized patients with COVID-19 had median or mean ages very similar to the population in this trial (median, 58.0 years [IQR, 49.0-68.0 years]37; median, 59.8 years [IQR, 50.6-70.1 years]38; median, 52 years [IQR, 32-62 years]39; and mean, 50.3 years [SD, 14.6 years]).12 The relatively younger age of the population in COVID-19 trials compared with hypertension or heart failure trials may be a specific characteristic of this infection.
Limitations
This study has several limitations. First, this was an open-label study; therefore, some inherent bias in the treatment of patients could have occurred.
Second, the in-hospital study setting with randomization a median of 2 days after hospitalization may limit the generalizability of these results to patients with COVID-19 in other settings and with earlier modification of the therapy. However, the intervention in the study (discontinuing or continuing ACEIs or ARBs) was continued from randomization through 30 days, and most participants adhered to the study intervention through 30 days.
Third, the relatively small number of patients taking ACEIs with a diagnosis of heart failure might limit the extension of these results to a broader population. Nonetheless, prescription of ARBs has increased worldwide, and the results presented reflect this trend.40
Fourth, the effect of ACEIs or ARBs on the susceptibility to COVID-19 was not studied because the focus was only on clinical outcomes in patients already infected.
Fifth, data on race, ethnicity, chronic obstructive pulmonary disease, immunosuppression, and use of mineralocorticoid receptor antagonists were not systematically collected.
Conclusions
Among patients hospitalized with mild to moderate COVID-19 and who were taking ACEIs or ARBs before hospital admission, there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue vs continue these medications. These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment.
Trial protocol
eMethods. Inclusion and exclusion criteria; secondary endpoint definitions; and additional analysis of the primary outcome
eTable 1. Characteristics of the 29 enrolling hospitals
eTable 2. Patient characteristics
eTable 3. Secondary outcomes
eTable 4. On-treatment analysis of primary and secondary outcomes
eFigure 1. Details of the primary outcome model diagnosis
eFigure 2. Number of randomized patients and percentage of occupancy with COVID-19 at peak per individual site
eFigure 3. Primary outcome analysis using GAMLSS
eFigure 4. All-cause mortality at 30 days
eLists. Executive committee, steering committee, data safety monitoring board, coordinating centers, clinical endpoints committee, CEC reviewers, and enrolling centers and site principal investigators
eReference
Statistical analysis plan
Data sharing statement
References
- 1.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2. Circ Res. 2020;126(10):1456-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soler MJ, Barrios C, Oliva R, et al. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10(5):410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varagic J, Ahmad S, Nagata S, et al. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16(3):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung MK, Karnik S, Saef J, et al. SARS-CoV-2 and ACE2. EBioMedicine. 2020;58:102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. JAMA. 2020;323(18):1769-1770. [DOI] [PubMed] [Google Scholar]
- 6.Jarcho JA, Ingelfinger JR, Hamel MB, et al. Inhibitors of the renin–angiotensin–aldosterone system and Covid-19. N Engl J Med. 2020;382(25):2462-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanaroff AC, Califf RM, Harrington RA, et al. Randomized trials versus common sense and clinical observation. J Am Coll Cardiol. 2020;76(5):580-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellenberg SS, Keusch GT, Babiker AG, et al. Rigorous clinical trial design in public health emergencies is essential. Clin Infect Dis. 2018;66(9):1467-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanaroff AC, Califf RM, Lopes RD. New approaches to conducting randomized controlled trials. J Am Coll Cardiol. 2020;75(5):556-559. [DOI] [PubMed] [Google Scholar]
- 10.Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Am Heart J. 2020;226:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanaroff AC, Cyr D, Neely ML, et al. Days alive and out of hospital. Circ Cardiovasc Qual Outcomes. 2018;11(12):e004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19. N Engl J Med. 2020;383(19):1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J R Stat Soc Ser C Appl Stat. 2005;54(3):507-554. doi: 10.1111/j.1467-9876.2005.00510.x [DOI] [Google Scholar]
- 15.R: A Language and Environment for Statistical Computing v. 3.5. 1. R Foundation for Statistical Computing; 2019. [Google Scholar]
- 16.Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancia G, Rea F, Ludergnani M, et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671-1681. Published correction appears in Circ Res. 2020;127(6):e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Wang X, Chen J, et al. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One. 2020;15(6):e0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8(7):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera R, Clark C, Lu Y, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease-19. medRxiv. 2020:2020.05.17.20104943. doi: 10.1101/2020.05.17.20104943. [DOI] [PMC free article] [PubMed]
- 26.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital. Lancet. 2020;395(10238):1705-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1020-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W, Zhang J, Wang M, et al. Clinical features of COVID-19 in patients with essential hypertension and the impacts of renin-angiotensin-aldosterone system inhibitors on the prognosis of COVID-19 patients. Hypertension. 2020;76(3):732-741. [DOI] [PubMed] [Google Scholar]
- 29.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elijovich F, Laffer CL. What kind of evidence is needed to dictate practice regarding inhibitors of the renin-angiotensin system in COVID-19? Hypertension. 2020;76(3):665-669. [DOI] [PubMed] [Google Scholar]
- 31.Bean DM, Kraljevic Z, Searle T, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. 2020;22(6):967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Society of Cardiology Council on Hypertension Position statement on ACE-inhibitors and angiotensin receptor blockers. Published March 13, 2020. Accessed October 27, 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 33.Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA statement addresses concerns. J Card Fail. 2020;26(5):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;52(3):190-199. [DOI] [PubMed] [Google Scholar]
- 35.Drager LF, Pio-Abreu A, Lopes RD, et al. Is hypertension a real risk factor for poor prognosis in the COVID-19 pandemic? Curr Hypertens Rep. 2020;22(6):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection. Circulation. 2020;142(1):4-6. [DOI] [PubMed] [Google Scholar]
- 37.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II). Lancet. 2020;396(10256):959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19. Lancet. 2020;395(10238):1695-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ah Y-M, Lee J-Y, Choi Y-J, et al. Persistence with antihypertensive medications in uncomplicated treatment-naïve patients. J Korean Med Sci. 2015;30(12):1800-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Inclusion and exclusion criteria; secondary endpoint definitions; and additional analysis of the primary outcome
eTable 1. Characteristics of the 29 enrolling hospitals
eTable 2. Patient characteristics
eTable 3. Secondary outcomes
eTable 4. On-treatment analysis of primary and secondary outcomes
eFigure 1. Details of the primary outcome model diagnosis
eFigure 2. Number of randomized patients and percentage of occupancy with COVID-19 at peak per individual site
eFigure 3. Primary outcome analysis using GAMLSS
eFigure 4. All-cause mortality at 30 days
eLists. Executive committee, steering committee, data safety monitoring board, coordinating centers, clinical endpoints committee, CEC reviewers, and enrolling centers and site principal investigators
eReference
Statistical analysis plan
Data sharing statement