This comparative effectiveness research reports the radiation exposure, adverse events, and health expenditures associated with the varying intensities of lung nodule detection.
Key Points
Question
Is the intensity of the diagnostic evaluation of incidentally detected lung nodules associated with patient outcomes and health expenditures?
Findings
In this comparative effectiveness research study of 5057 individuals with an incidentally detected lung nodule, evidence of an association between evaluation intensity and lung cancer stage distribution was inconclusive. Compared with guideline-concordant evaluations, less intensive evaluations were associated with less radiation exposure, fewer procedure-related adverse events, and lower health expenditures, whereas more intensive evaluations were associated with greater radiation exposure, more procedure-related adverse events, and higher health expenditures.
Meaning
Findings from this study underscore the need to increase the level of evidence that supports current guideline recommendations and to decrease unnecessarily intensive diagnostic evaluations of lung nodules.
Abstract
Importance
Whether guideline-concordant lung nodule evaluations lead to better outcomes remains unknown.
Objective
To examine the association between the intensity of lung nodule diagnostic evaluations and outcomes, safety, and health expenditures.
Design, Setting, and Participants
This comparative effectiveness research study analyzed health plan enrollees at Kaiser Permanente Washington in Seattle, Washington, and Marshfield Clinic in Marshfield, Wisconsin, with an incidental lung nodule detected between January 1, 2005, and December 31, 2015. Included patients were 35 years or older, had no high suspicion of infection, had no history of malignant neoplasm, and had no evidence of advanced lung cancer on nodule detection. Data analysis was conducted from January 7 to August 19, 2020.
Exposures
With the 2005 Fleischner Society guidelines (selected for their applicability to the time frame under investigation) as the comparator, 2 other intensities of lung nodule evaluation were defined. Guideline-concordant evaluation followed the guidelines. Less intensive evaluation was the absence of recommended testing, longer-than-recommended surveillance intervals, or less invasive testing than recommended. More intensive evaluation consisted of testing when the guidelines recommended no further testing, shorter-than-recommended surveillance intervals, or more invasive testing than recommended.
Main Outcomes and Measures
The main outcome was the proportion of patients with lung cancer who had stage III or IV disease, radiation exposure, procedure-related adverse events, and health expenditures 2 years after nodule detection.
Results
Among the 5057 individuals included in this comparative effectiveness research study, 1925 (38%) received guideline-concordant, 1863 (37%) less intensive, and 1269 (25%) more intensive diagnostic evaluations. The entire cohort comprised 2786 female patients (55%), and the mean (SD) age was 67 (13) years. Adjusted analyses showed that compared with guideline-concordant evaluations, less intensive evaluations were associated with fewer procedure-related adverse events (risk difference [RD], −5.9%; 95% CI, −7.2% to −4.6%), lower mean radiation exposure (−9.5 milliSieverts [mSv]; 95% CI, −10.3 mSv to −8.7 mSv), and lower mean health expenditures (−$10 916; 95% CI, −$16 112 to −$5719); no difference in stage III or IV disease was found among patients diagnosed with lung cancer (RD, 4.6%; 95% CI, −22% to +31%). More intensive evaluations were associated with more procedure-related adverse events (RD, +8.1%; 95% CI, +5.6% to +11%), higher mean radiation exposure (+6.8 mSv; 95% CI, +5.8 mSv to +7.8 mSv), and higher mean health expenditures ($20 132; 95% CI, +$14 398 to +$25 868); no difference in stage III or IV disease was observed (RD, −0.5%; 95% CI, −28% to +27%).
Conclusions and Relevance
This study found inconclusive evidence of an association between less intensive diagnostic evaluations and more advanced stage at lung cancer diagnosis compared with guideline-concordant care; higher intensities of diagnostic evaluations were associated with greater procedural complications, radiation exposure, and expenditures. These findings underscore the need for more evidence on better ways to evaluate lung nodules and to avoid unnecessarily intensive diagnostic evaluations of lung nodules.
Introduction
Each year in the United States, the incidental detection of a lung nodule by computed tomography (CT) occurs in approximately 1.6 million people.1 Lung cancer is the main concern in such detections,2,3 but only 5% to 10% of individuals with nodules have cancer.1,4 Clinicians must balance the benefits of prompt lung cancer identification with the risks and costs of diagnostic testing.
Practice guidelines recommend using varying intensities of diagnostic evaluation for lung nodules that are based on an individual’s underlying lung cancer risk factors (eg, smoking) and nodule characteristics (eg, size).5,6 Rates of guideline-concordant evaluations vary from 39% to 67% across different practice environments.4,7,8 Barriers to adopting guideline-concordant nodule care exist at the patient, clinician, and health system levels, leading to calls for multilevel interventions that increase guideline-concordant practices.2,4,9,10,11,12 The benefits of such interventions are unclear because of the low levels of evidence supporting guideline recommendations and because the association between guideline-concordant care and outcomes has not been demonstrated.5,6,10
In this comparative effectiveness research study, we examined the observed patterns of care that reflect the association between the intensity of lung nodule diagnostic evaluations and outcomes, safety, and health expenditures. We hypothesized that, compared with guideline-concordant care, (1) less intensive evaluations would be associated with a higher risk of stage III or IV disease among patients with lung cancer and higher health expenditures (because costs are higher in advanced-stage cancers13,14) and (2) more intensive evaluations would be associated with greater radiation exposure, more procedure-related adverse events (AEs), and higher health expenditures without a difference in stage III or IV disease.
Methods
We conducted a retrospective comparative effectiveness research study of health plan enrollees at Kaiser Permanente Washington in Seattle, Washington, and Marshfield Clinic in Marshfield, Wisconsin, who had an incidentally detected lung nodule between January 1, 2005, and December 31, 2015. Institutional review boards at the collaborating institutions ceded oversight to Kaiser Permanente Washington, which approved the study and waived individual informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (eTable 2 in the Supplement).15
To be included in the study, individuals needed to be enrolled in the health plan for at least 2 years after nodule detection or enrolled until death, whichever occurred first. The data source was a collaborative model implemented in health systems across the United States that provides harmonized information on demographic characteristics, smoking status, health care utilization, cancer characteristics, enrollment status, and vital status as well as access to an electronic health record.16 To identify individuals with a lung nodule, we used (1) International Classification of Diseases, Ninth Revision (ICD-9), International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), and Current Procedural Terminology (CPT) codes to ascertain which individuals underwent chest CT; (2) natural language processing to find individuals whose incidentally detected lung nodule was documented in free-text radiology reports1,17; and (3) trained medical record abstractors to confirm the documentation of the nodule in the free-text radiology report.18
We used the 2005 Fleischner Society guidelines for managing small pulmonary nodules that were detected on CT scans5 to define guideline concordance because these guidelines were applicable to the time frame under investigation in this study. These 2005 guidelines recommended up to 2 years of periodic follow-up for individuals who were 35 years or older, had no high suspicion of infection, had no history of malignant neoplasm, and had no evidence of advanced lung cancer on nodule detection (exclusions are shown in eTable 1 in the Supplement).5
Intensity of Nodule Evaluation and Lung Cancer Diagnosis
We defined the intensities of nodule diagnostic evaluation using the first diagnostic step recommended by the 2005 Fleischner Society guidelines5 (eTable 3 in the Supplement) as the comparator. Such guidelines stratify individuals on the basis of nodule size and smoking history followed by strata-specific diagnostic recommendations, including no further follow-up, chest CT at established time intervals, positron emission tomography (PET), or biopsy. We defined less intensive evaluation as the absence of recommended testing, a longer-than-recommended surveillance interval (delays in testing), or less invasive testing than recommended (use of chest CT instead of PET or biopsy). More intensive evaluation was defined as testing when the guidelines recommended no further testing, a shorter-than-recommended surveillance interval for chest CT, or more invasive testing than recommended (use of PET or biopsy instead of CT) (eMethods in the Supplement). To provide some allowance for scheduling preferences, we considered deviations from recommended testing intervals of up to 1 month to be guideline concordant.
To identify individuals who were diagnosed with lung cancer within 2 years after nodule detection, we used validated tumor registry data (eMethods in the Supplement). Because cancer registries assign a diagnosis date on the basis of first evidence of disease, which in some cases could be the lung nodule detection date, we used the date of pathological confirmation in the medical records to ensure a consistent date of diagnosis across patients, sites, and time. For cases without pathological specimens, we reviewed the electronic health record to establish the first date that a clinician documented findings suspicious for lung cancer with associated recommendations for treatment or best supportive care. Because more intensive evaluations may lead to overdiagnosis, we examined the associations between the intensity of nodule evaluation and the proportion of patients with lung cancer who had stage III or IV disease at diagnosis.
Radiation Exposure, Procedure-Related AEs, and Health Expenditures
We used ICD-9, ICD-10, and CPT codes to ascertain the use of CT and PET after nodule detection through the end of follow-up or until lung cancer diagnosis, whichever occurred first and independent of the evaluation intensity. We estimated radiation exposure for each patient by multiplying the number of CTs and PETs within the 2 years after nodule detection by modality-specific mean doses for these examinations (eTable 5 in the Supplement).19
We used ICD-9, ICD-10, and CPT codes to identify procedure-related AEs after nodule detection and through the end of follow-up or until lung cancer diagnosis, whichever occurred first and independent of the evaluation intensity. Because lung cancer guidelines recommend investigation of nonpulmonary findings suspicious for cancer spread (eg, nodal, pericardial, pleural, or extrathoracic metastases) before lung biopsy when possible,20,21,22,23 we used a broad set of percutaneous, endoscopic, and surgical procedures to define biopsies (eMethods in the Supplement). We measured procedure-related AEs against previously reported definitions.24,25,26,27,28,29,30
With a payer perspective, we estimated total health expenditures for each individual using a previously validated algorithm that measured health care utilization.31 The time horizon was 2 years or until death regardless of lung cancer diagnosis. We adjusted expenditures for inflation using the Consumer Price Index for medical care in 2017.32,33
Covariates
We selected, a priori, 13 potential confounders on the basis of their known or suspected associations with the intensity of nodule evaluation and/or outcomes.4,34 Information was readily available on age, sex, race and ethnicity, smoking status, health system, and nodule detection year. Enrollees self-reported their race using a minimum of 5 categories and their ethnicity using at least 2 categories, as recommended by the National Institutes of Health. These variables were included in the risk-adjustment model to account for the potential confounding effects of racial and ethnic inequality in the United States. We used information during the year before nodule detection to calculate the Klabunde-modified comorbidity index.35 In addition, we used information 6 months before nodule detection to measure the probability of frailty with a validated algorithm, and we dichotomized the variable using a previously defined threshold probability.36,37 Trained medical record abstractors recorded the size of the dominant nodule, number of nodules, and whether the radiologist suspected an infectious cause or recommended follow-up as documented in the free-text radiology reports.
Statistical Analysis
We described patient characteristics and outcomes using univariate descriptive statistics that estimated 95% CIs with the Wald method in R package DescTools, MultinomCI function (R Foundation for Statistical Computing). Next, anticipating few events relative to the number of covariates, we performed propensity score–adjusted regression analyses to address confounding.38 We developed 2 propensity score models using all 13 potential confounders, with 1 model estimating less intensive vs guideline-concordant nodule evaluations and the other estimating more intensive vs guideline-concordant nodule evaluations. We created outcome-specific regression models with 2 classes of independent variables: (1) an indicator for the intensity of nodule evaluation and (2) the fitted propensity score using a natural spline with knots identified by observed quartiles. This approach results in a desirable balance between low bias and power.39 We used logistic regression for comparisons of cancer stage and procedure-related AEs. To obtain adjusted risk-differences (RDs) for binary outcomes, we used marginal standardization.40 The primary analysis that compared cancer stage across exposure groups was restricted to patients diagnosed with lung cancer.
Recognizing that individuals with lung nodules can experience several different outcomes after nodule detection, we conducted a planned sensitivity analysis using multinomial regression for the entire cohort. We used linear regression for radiation exposure and health expenditures.41 Analyses were case complete because of the low frequency of missing data (3.4% for covariates, 0.1% for exposure, and 0% for outcomes and costs). The sample size was fixed as a consequence of the study design.
We used R statistical software, version 3.5.0 (R Foundation for Statistical Computing) for all analyses.42 Data analysis was conducted from January 7 to August 19, 2020.
Results
Among 5057 individuals with an incidentally detected lung nodule, 1925 (38%) received guideline-concordant, 1863 (37%) received less intensive, and 1269 (25%) received more intensive diagnostic evaluations. Overall, the entire cohort comprised 2786 female (55%) and 2271 male (45%) patients, with a mean (SD) age of 67 (13) years (Table 1). Baseline characteristics varied across groups, although absolute differences tended to be less than 5% for most variables (Table 1). The less intensive evaluation group had the highest frequency of individuals who were 85 years or older (n = 201 [11%]), frail (n = 393 [21%]), or smokers (n = 1254 [67%]). Mean (SD) use of imaging (0.5 [0.9] vs 2.0 [1.5]) and invasive diagnostic procedures (0.03 [0.3] vs 0.4 [1.6]) was lowest for the less intensive evaluation group and was highest for the more intensive evaluation group (eTable 4 in the Supplement).
Table 1. Characteristics of Individuals by Intensity of Lung Nodule Evaluation.
| Characteristic | Nodule evaluation, No. (%) | |||
|---|---|---|---|---|
| All patients (n = 5057) | Less intensive (n = 1863) | Guideline concordant (n = 1925) | More intensive (n = 1269) | |
| Age, y | ||||
| Median (IQR) | 67 (19) | 68 (20) | 68 (18) | 66 (18) |
| Mean (SD) | 67 (13) | 67 (14) | 67 (13) | 66 (13) |
| 35-44 | 284 (6) | 100 (5) | 106 (6) | 78 (6) |
| 45-54 | 693 (14) | 276 (15) | 222 (12) | 195 (15) |
| 55-64 | 1199 (24) | 418 (22) | 470 (24) | 311 (25) |
| 65-74 | 1427 (28) | 494 (27) | 574 (30) | 359 (28) |
| 75-84 | 1037 (21) | 374 (20) | 412 (21) | 251 (20) |
| ≥85 | 417 (8) | 201 (11) | 141 (7) | 75 (6) |
| Sex | ||||
| Female | 2786 (55) | 973 (52) | 1081 (56) | 732 (58) |
| Male | 2271 (45) | 890 (48) | 844 (44) | 537 (42) |
| Race/ethnicity | ||||
| White | 4597 (91) | 1692 (91) | 1764 (92) | 1141 (90) |
| Black | 110 (2) | 40 (2) | 42 (2) | 28 (2) |
| Asian | 203 (4) | 74 (4) | 66 (3) | 63 (5) |
| American Indian/Alaska Native | 23 (1) | 9 (1) | 7 (0.4) | 7 (1) |
| Native Hawaiian/Pacific Islander | 15 (0.3) | 7 (0.4) | 5 (0.3) | 3 (0.2) |
| Othera | 52 (1) | 22 (1) | 15 (1) | 15 (1) |
| Multiracial | 57 (1) | 19 (1) | 26 (1) | 12 (1) |
| Hispanic | ||||
| Yes | 126 (3) | 44 (2) | 51 (3) | 31 (2) |
| No | 4931 (98) | 1819 (98) | 1874 (97) | 1238 (98) |
| Smoking status | ||||
| Never | 2149 (42) | 609 (33) | 865 (45) | 675 (53) |
| Ever | 2908 (58) | 1254 (67) | 1060 (55) | 594 (47) |
| Comorbidity Index35 | ||||
| 0 | 2288 (45) | 830 (45) | 849 (44) | 609 (48) |
| 1 | 1334 (26) | 453 (24) | 548 (28) | 333 (26) |
| 2 | 635 (13) | 248 (13) | 249 (13) | 138 (11) |
| ≥3 | 800 (16) | 332 (18) | 279 (14) | 189 (15) |
| Frailty index36,37 | ||||
| Probability of frailty | ||||
| Median (IQR) | 0.06 (0.13) | 0.07 (0.15) | 0.07 (0.12) | 0.06 (0.11) |
| Mean (SD) | 0.12 (0.13) | 0.13 (0.15) | 0.11 (0.12) | 0.11 (0.13) |
| Frail | ||||
| Yes | 916 (18) | 393 (21) | 329 (17) | 194 (15) |
| No | 4141 (82) | 1470 (79) | 1596 (83) | 1075 (85) |
| Size of dominant nodule, mm | ||||
| Median (IQR) | 5.9 (5.0) | 6.0 (5.0) | 7.0 (8.0) | 4.5 (2.6) |
| Mean (SD) | 7.4 (5.4) | 7.5 (5.1) | 8.8 (6.4) | 5.0 (2.7) |
| ≤4 | 1694 (33) | 502 (27) | 568 (30) | 624 (49) |
| >4-6 | 1334 (26) | 555 (30) | 334 (17) | 445 (35) |
| >6-8 | 693 (14) | 264 (14) | 286 (15) | 143 (11) |
| >8 | 1336 (26) | 542 (29) | 737 (38) | 57 (5) |
| Multiple nodules | ||||
| Yes | 2766 (55) | 1001 (54) | 1101 (57) | 664 (52) |
| No | 2291 (45) | 862 (46) | 824 (43) | 605 (48) |
| Radiologist-recommended follow-up | ||||
| Yes | 3708 (73) | 1336 (72) | 1408 (73) | 964 (76) |
| No | 1349 (27) | 527 (28) | 517 (27) | 305 (24) |
| Infection suspected as a possible cause | ||||
| Yes | 125 (3) | 43 (2) | 58 (3) | 24 (2) |
| No | 4932 (98) | 1820 (98) | 1867 (97) | 1245 (98) |
| Health system | ||||
| A | 3220 (64) | 1106 (59) | 1286 (67) | 828 (65) |
| B | 1837 (36) | 757 (41) | 639 (33) | 441 (35) |
| Year of nodule detection | ||||
| 2005 | 251 (5) | 72 (4) | 95 (5) | 84 (7) |
| 2006 | 359 (7) | 110 (6) | 119 (6) | 130 (10) |
| 2007 | 388 (8) | 149 (8) | 121 (6) | 118 (9) |
| 2008 | 433 (9) | 167 (9) | 162 (8) | 104 (8) |
| 2009 | 441 (9) | 167 (9) | 176 (9) | 98 (8) |
| 2010 | 477 (9) | 194 (10) | 179 (9) | 104 (8) |
| 2011 | 476 (9) | 192 (10) | 195 (10) | 89 (7) |
| 2012 | 522 (10) | 201 (11) | 206 (11) | 115 (9) |
| 2013 | 522 (10) | 173 (9) | 200 (10) | 149 (12) |
| 2014 | 582 (12) | 204 (11) | 233 (12) | 145 (11) |
| 2015 | 606 (12) | 234 (13) | 239 (12) | 133 (10) |
| Fleischner Society guideline risk strata | ||||
| Low risk, mm nodule | ||||
| ≤4 | 779 (15) | 0 | 470 (24) | 309 (24) |
| >4-6 | 595 (12) | 276 (15) | 69 (4) | 250 (20) |
| >6-8 | 286 (6) | 108 (6) | 83 (4) | 95 (8) |
| >8 | 489 (10) | 225 (12) | 243 (13) | 21 (2) |
| High risk, mm nodule | ||||
| ≤4 | 915 (18) | 502 (27) | 98 (5) | 315 (25) |
| >4-6 | 739 (15) | 279 (15) | 265 (14) | 195 (15) |
| >6-8 | 407 (8) | 156 (8) | 203 (11) | 48 (4) |
| >8 | 847 (17) | 317 (17) | 494 (26) | 36 (3) |
Abbreviation: IQR, interquartile range.
Race/ethnicity was self-reported by patients; those who selected other did not indicate a specific race/ethnicity.
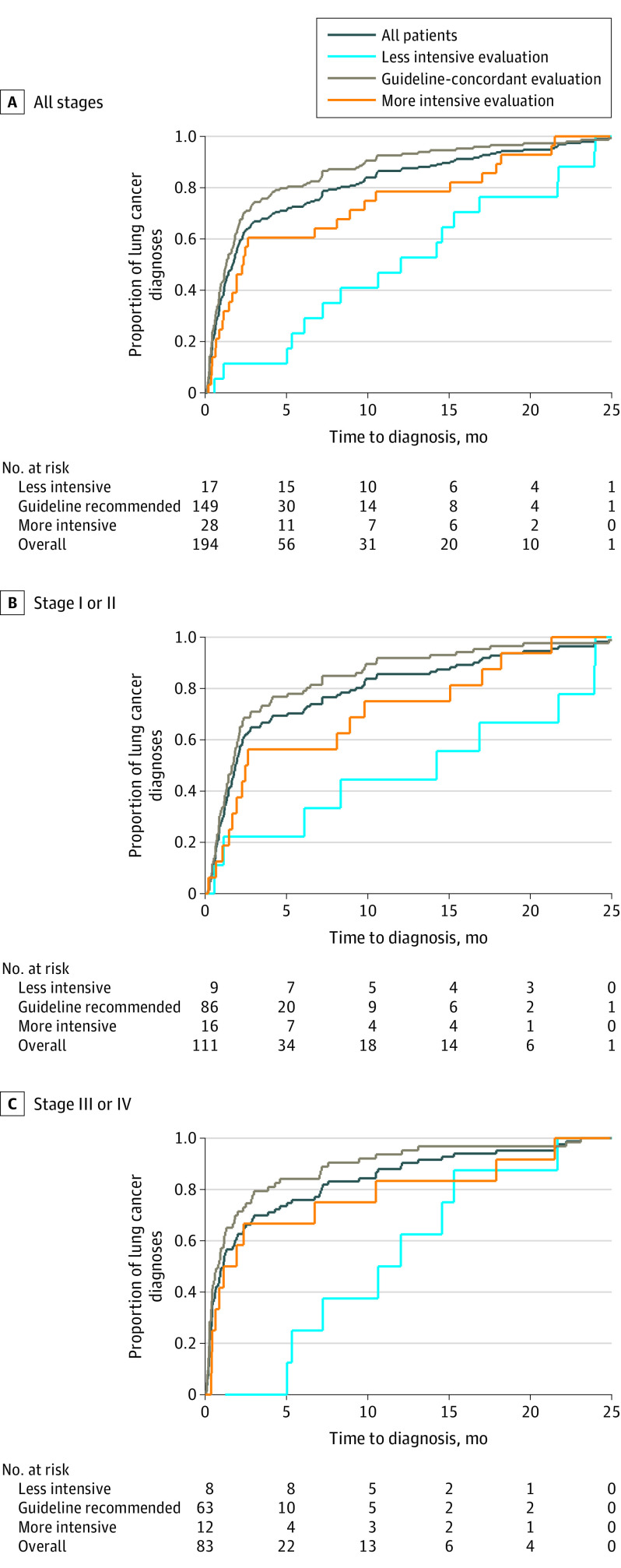
The proportion of individuals diagnosed with lung cancer according to the intensity of diagnostic evaluation was 8% (95% CI, 7%-9%) for the guideline-concordant, 1% (95% CI, 1%-1%) for the less intensive evaluation, and 2% (95% CI, 1%-3%) for the more intensive evaluation groups (Table 2). Median time to diagnosis of lung cancer by intensity of diagnostic evaluation was 1.3 months for the guideline-concordant, 12 months for the less intensive evaluation, and 2.3 months for the more intensive evaluation groups (Figure, A and eTable 6 in the Supplement). Time-to-diagnosis patterns were similar in subgroups defined by lung cancer stage (Figure, B and C), with a longer time to diagnosis among patients with less intensive evaluations.
Table 2. Unadjusted 2-Year Outcomes and Health Expenditures by Intensity of Lung Nodule Evaluation.
| Variable | Nodule evaluation | |||
|---|---|---|---|---|
| All patients (n = 5057) | Less intensive (n = 1863) | Guideline concordant (n = 1925) | More intensive (n = 1269) | |
| Outcomes, No. (%) [95% CI] | ||||
| No lung cancer and alive | 4487 (89) [88-90] | 1691 (91) [89-92] | 1667 (87) [85-88] | 1129 (89) [87-91] |
| No lung cancer and died | 376 (7) [7-8] | 155 (8) [7-10] | 109 (6) [5-7] | 112 (9) [7-10] |
| Lung cancer | 194 (4) [3-4] | 17 (1) [1-1] | 149 (8) [7-9] | 28 (2) [1-3] |
| Lung cancer stage, No. (%) [95% CI]a | ||||
| I or II | 111 (57) [50-64] | 9 (53) [29-77] | 86 (58) [50-66] | 16 (57) [39-75] |
| III or IV | 83 (43) [36-50] | 8 (47) [23-71] | 63 (42) [34-50] | 12 (43) [25-61] |
| Radiation exposure, mSv | ||||
| Median (IQR) | 7 (14) | 0 (7) | 7 (21) | 14 (14) |
| Mean (SD) | 10 (13) | 3 (7) | 13 (15) | 16 (14) |
| Frequency, No. (%) [95% CI] | ||||
| ≥10 mSv | 1816 (36) [35-37] | 192 (10) [9-12] | 923 (48) [46-50] | 701 (55) [53-58] |
| ≥20 mSv | 1034 (20) [19-22] | 87 (5) [4-6] | 577 (30) [28-32] | 370 (29) [27-32] |
| Procedure-related adverse events | ||||
| Frequency, No. (%) [95% CI] | ||||
| 0 | 4763 (94) [94-95] | 1837 (99) [98-99] | 1767 (92) [91-93] | 1159 (91) [90-93] |
| 1 | 106 (2) [2-3] | 10 (1) [0-1] | 61 (3) [2-4] | 35 (3) [2-4] |
| 2 | 77 (2) [1-2] | 5 (0) [0-1] | 38 (2) [1-3] | 34 (3) [2-4] |
| ≥3 | 111 (2) [2-3] | 11 (1) [0-1] | 59 (3) [2-4] | 41 (3) [2-4] |
| Two-year total costs of care, US $ | ||||
| Median (IQR) | 26 727 (46 976) | 22 736 (36 947) | 27 004 (52 084) | 32 933 (56 516) |
| Mean (SD) | 49 843 (75 705) | 41 815 (78 727) | 51 231 (69 089) | 59 521 (79 480) |
Abbreviations: IQR, interquartile range; mSv, milliSievert.
Proportions calculated among patients diagnosed with lung cancer.
Figure. Time to Lung Cancer Diagnosis by Intensity of Lung Nodule Evaluation for the Entire Cohort and Subgroups of Patients .
Among patients with lung cancer, the frequency of stage III or IV disease by intensity of diagnostic evaluation was 42% (95% CI, 34%-50%; n = 63) for guideline-concordant, 47% (95% CI, 23%-71%; n = 8) for less intensive evaluation, and 43% (95% CI, 25%-61%; n = 12) for more intensive evaluation groups. The adjusted absolute difference in stage III or IV disease between the less intensive evaluation and guideline-concordant groups was not statistically significant (RD, 4.6%; 95% CI, −22% to +31%) (Table 3). No meaningful differences in the risk of stage III or IV disease were observed between the more intensive evaluation and guideline-concordant groups (RD, −0.5%; 95% CI, −28% to +27%). A sensitivity analysis using propensity score–adjusted multinomial models that accounted for all possible outcomes experienced by the entire cohort (with stage I or II lung cancer, with stage III or IV lung cancer, without lung cancer at 2 years, or died within 2 years without lung cancer) revealed no statistically significant difference in the relative risk (RR) of stage III or IV vs stage I or II disease in the comparison between the less intensive and guideline-concordant groups (RR, 1.21; 95% CI, 0.44-3.34) or between the more intensive evaluation and guideline-concordant groups (RR, 1.01; 95% CI, 0.40-2.52).
Table 3. Adjusted 2-Year Outcome Comparisons by Intensity of Nodule Evaluation.
| Outcome | Less intensive vs guideline concordant | More intensive vs guideline concordant | ||
|---|---|---|---|---|
| Relative risk (95% CI) | Risk difference (95% CI) | Relative risk (95% CI) | Risk difference (95% CI) | |
| Stage III or IV vs I or II lung cancer diagnosisa | 1.11 (0.47 to 1.75) | +4.6 (−22 to +31) | 0.99 (0.33 to 1.64) | −0.5 (−28 to +27) |
| Any procedure-related adverse event | 0.21 (0.12 to 0.29) | −5.9 (−7.2 to −4.6) | 2.28 (1.79 to 2.77) | +8.1 (+5.6 to +11) |
Estimated among individuals diagnosed with lung cancer.
Unadjusted radiation exposure, rates of procedure-related AEs, and health care expenditures are shown in Table 2 by intensity of diagnostic evaluation. After adjustment (Table 3 and Table 4) and compared with guideline-concordant evaluation, less intensive evaluations were associated with significantly fewer procedure-related AEs (RD, −5.9%; 95% CI, −7.2% to −4.6%), lower mean radiation exposure (−9.5 milliSieverts [mSv]; 95% CI, −10.3 mSv to −8.7 mSv), and lower mean health expenditures (−$10 916; 95% CI, −$16 112 to −$5719). Meanwhile, more intensive evaluations were associated with more procedure-related AEs (RD, +8.1%; 95% CI, +5.6% to +11%), higher mean radiation exposure (+6.8 mSv; 95% CI, +5.8 mSv to +7.8 mSv), and higher mean health expenditures ($20 132; 95% CI, +$14 398 to +$25 868). A post hoc analysis of patients who experienced procedure-related AEs revealed that most patients (72%; 95% CI, 67%-77%) did not have a lung cancer diagnosis 2 years after nodule detection (eTable 7 in the Supplement).
Table 4. Adjusted 2-Year Health Expenditure Comparisons by Intensity of Nodule Evaluation.
| Outcome | Less intensive vs guideline concordant | More intensive vs guideline concordant | ||
|---|---|---|---|---|
| Ratio of geometric means (95% CI) | Mean difference (95% CI) | Ratio of geometric means (95% CI) | Mean difference (95% CI) | |
| Radiation exposure, mSv | 0.29 (0.27 to 0.32) | −9.5 (−10.3 to −8.7) | 2.70 (2.49 to 2.94) | +6.8 (+5.8 to +7.8) |
| Health expenditures, 2017 US $ | 0.72 (0.67 to 0.79) | −10 916 (−16 112 to −5719) | 1.56 (1.43 to 1.70) | +20 132 (+14 398 to +25 868) |
Abbreviation: mSv, milliSievert.
Discussion
We assessed the association between varying intensities of diagnostic evaluations and 2-year patient outcomes and health expenditures for patients with incidentally detected lung nodules. We found inconclusive evidence of an association between the intensity of diagnostic evaluations and advanced stage of lung cancer at diagnosis. Higher-intensity diagnostic evaluations were associated with greater radiation exposure, more procedure-related AEs, and higher health care expenditures.
We designed this comparative effectiveness research study under the assumption that the proportion of lung cancer diagnoses would be largely invariant across different groups of diagnostic evaluation intensity. The reason for this assumption was that the intensity of nodule evaluation generally impacts the cancer stage at diagnosis but not the frequency of lung cancer diagnoses. Unlike after screening, at the time of nodule detection the patient either has or does not have lung cancer (ie, the nodule represents cancer or a benign condition). To the extent that overdiagnoses occur, we recognized the likelihood of an excess of stage I lung cancers in the guideline-concordant and more intensive evaluation groups. For this reason, we compared the proportion of stage III or IV lung cancers across intensity groups. However, we unexpectedly observed a substantially higher proportion of lung cancer diagnoses in the guideline-concordant group than in the less intensive evaluation and more intensive evaluation groups—a pattern that was highly suggestive of confounding. A recently published study showed a similar pattern of variation in the proportion of lung cancer diagnoses by diagnostic evaluation intensity (0 for less intensive evaluation, 42% for guideline-concordant, and 15% for more intensive evaluation groups).8 That previous study showed that specialists deviated from guidelines if they thought the probability of lung cancer was low on the basis of radiographic characteristics (eg, edge characteristics or density), the patient had an indolent form of lung cancer on the basis of radiographic features of the nodule (eg, ground-glass opacity), and/or the patient had an equal or more likely alternative diagnosis for the nodule (eg, sarcoid).8 Although we were able to adjust for factors, such as age, comorbidity, frailty, smoking status, and nodule size, that might have changed the intensity of nodule evaluation, we were unable to measure other factors that may have altered clinicians’ estimate of the probability of lung cancer (eg, nodule density). We believe that confounding by indication likely explains the variable proportion of lung cancer diagnoses across intensity groups.
To address this confounding, we restricted the primary analysis to the subgroup of patients with lung cancer. As a consequence of a substantially smaller sample size, we likely had insufficient power to detect small but clinically important differences in the frequency of stage III or IV lung cancer across intensity groups. Despite these null results, we observed a median time to diagnosis of 12 months among patients in the less intensive evaluation group who were ultimately diagnosed with stage III or IV disease. This finding, along with a modestly higher proportion of stage III or IV lung cancer diagnoses in the less intensive evaluation group (if not owing to chance), supported the hypothesis that less intensive evaluations would be associated with delays in diagnoses and thus a higher stage of disease at the time of diagnosis.
We found relatively higher-intensity diagnostic evaluations to be associated with clinically meaningful differences in safety and health expenditures. The magnitude of differences in mean radiation exposure over 2 years for both comparisons in this study was on par with the mean radiation dose for 1 diagnostic chest CT (approximately 8 mSv). This amount of radiation was expected to lead to approximately 1 radiation-related cancer diagnosis for every 1500 persons who had imaging.43 Although any decrease in procedure-related AEs is clinically important from a patient perspective, the differences in procedure-related AEs across groups were arguably large. The magnitude of differences in procedure-related AEs for both comparisons was similar to the overall rate of procedure-related AEs for the entire cohort (approximately 6%). Similarly, the magnitude of differences in health care expenditures (approximately $11 000 for the less intensive evaluation group vs approximately $20 000 for the more intensive evaluation group) represent approximately 20% to 40% of the overall mean 2-year health expenditures for this cohort. We may have overestimated the differences in safety and expenditures because we were unable to attribute subsequent testing to lung nodule evaluation. Adjustments for patient demographic characteristics, comorbidity, and frailty may have decreased the misattribution errors across groups. We also may have overestimated the risk of procedure-related AEs because of the inaccuracies of administrative diagnostic codes (ICD-9 and ICD-10) in characterizing postprocedural complications,44,45,46 although we have no reason to believe that these errors were distributed unequally across groups defined by diagnostic intensity.
We believe that the findings in this study will complement the forthcoming results of the noninferiority Watch the Spot Trial.47,48 The Watch the Spot Trial compares 2 different guideline-concordant nodule evaluation strategies, whereas the present study compared more extreme deviations from guideline-concordant care that can only be observed in actual clinical practice. This study also underscored the need for more evidence on better ways to evaluate lung nodules, and its findings will have implications for future investigations and quality improvement efforts. In addition, we believe that the magnitude of harm associated with more intensive nodule evaluations demonstrated in this study will motivate and justify quality improvement initiatives that curb unnecessarily intensive diagnostic evaluations. For instance, efforts to decrease radiation exposure by approximately 8 milliSieverts may prevent more than 267 radiation-related cancers among the estimated 400 000 people with an incidentally detected lung nodule each year who received more intensive nodule evaluations.1,43
Limitations
This study has several limitations. First, the proportion of lung cancer in this cohort was more than 50% lower than we had anticipated owing to a 2014 study of US veterans with incidental nodules who had a 9% prevalence of lung cancer.4 This finding limited our ability to detect small but potentially clinically important differences in stage III or IV lung cancer diagnoses across exposure groups. Since the publication of that 2014 veteran study, a large population-based study from Kaiser Permanente Southern California reported a 5.2% prevalence of lung cancer.1 These past findings suggested that we inadvertently underpowered the present study and that its results might be more generalizable than those of the study in the Veterans Affairs population. Second, the intensity of nodule evaluation was potentially misclassified because the approach that we used to categorize this variable was based on the first diagnostic step recommended by the 2005 Fleischner Society guidelines. We took this approach to avoid misattributing subsequent testing performed for other reasons (eg, dyspnea) as part of the nodule evaluation. Consequently, however, we may have misclassified patients as having received a guideline-concordant evaluation if they received only the first and not the subsequent diagnostic tests as recommended by guidelines. However, we believe the impact of such bias was small because most lung cancer diagnoses occurred within 4 months of nodule detection. Third, the 2005 Fleischner Society guidelines are no longer applicable to contemporary practice (new guidelines were issued in 201749); however, there remains an opportunity to gain knowledge about the implications (if any) of guideline deviations for patients and health systems.
Conclusions
This comparative effectiveness research study found inconclusive evidence of an association between evaluation intensity and lung cancer stage distribution. Compared with guideline-concordant evaluations, less intensive evaluations were associated with less radiation exposure, fewer procedure-related adverse events, and lower health expenditures, whereas more intensive evaluations were associated with greater radiation exposure, more procedure-related adverse events, and higher health expenditures. Findings from this study underscore the need to increase the level of evidence that supports current guideline recommendations and to decrease unnecessarily intensive diagnostic evaluations of lung nodules.
eTable 1. Sequential Exclusions
eTable 2. Strobe Guidelines Checklist
eTable 3. Summary of the 2005 Fleischner Society Guidelines
eTable 4. Use of Diagnostic Tests by Intensity of Lung Nodule Evaluation
eTable 5. Modality-Specific Average Radiation Exposure Estimates
eTable 6. Time to Lung Cancer Diagnosis by Intensity of Lung Nodule Evaluation and Stratified by Lung Cancer Stage
eTable 7. Lung Cancer Diagnosis by Intensity of Nodule Evaluation Among Patients Who Experienced a Procedure-Related Adverse Event
eMethods.
References
- 1.Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. doi: 10.1164/rccm.201505-0990OC [DOI] [PubMed] [Google Scholar]
- 2.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: a qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143(3):672-677. doi: 10.1378/chest.12-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slatore CG, Golden SE, Ganzini L, Wiener RS, Au DH. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules: a cohort study. Ann Am Thorac Soc. 2015;12(2):184-192. doi: 10.1513/AnnalsATS.201406-283OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener RS, Gould MK, Slatore CG, Fincke BG, Schwartz LM, Woloshin S. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med. 2014;174(6):871-880. doi: 10.1001/jamainternmed.2014.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacMahon H, Austin JH, Gamsu G, et al. ; Fleischner Society . Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887 [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e93S-e120S. doi: 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414. doi: 10.1378/chest.15-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdial FC, Madtes DK, Cheng GS, et al. Multidisciplinary team-based management of incidentally detected lung nodules. Chest. 2020;157(4):985-993. doi: 10.1016/j.chest.2019.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiener RS, Slatore CG, Gillespie C, Clark JA. Pulmonologists’ reported use of guidelines and shared decision-making in evaluation of pulmonary nodules: a qualitative study. Chest. 2015;148(6):1415-1421. doi: 10.1378/chest.14-2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slatore CG, Horeweg N, Jett JR, et al. ; ATS Ad Hoc Committee on Setting a Research Framework for Pulmonary Nodule Evaluation . An official American Thoracic Society research statement: a research framework for pulmonary nodule evaluation and management. Am J Respir Crit Care Med. 2015;192(4):500-514. doi: 10.1164/rccm.201506-1082ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons J, Gould MK, Iaccarino J, Slatore CG, Wiener RS. Systems-level resources for pulmonary nodule evaluation in the United States: a national survey. Am J Respir Crit Care Med. 2016;193(9):1063-1065. doi: 10.1164/rccm.201511-2163LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden SE, Wiener RS, Sullivan D, Ganzini L, Slatore CG. Primary care providers and a system problem: a qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148(6):1422-1429. doi: 10.1378/chest.14-2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banegas MP, Yabroff KR, O’Keefe Rosetti M, et al. Long-term medical care costs of breast, prostate, lung and colorectal cancer for HMO members. J Patient Cent Res Rev. 2015;2:80. doi: 10.17294/2330-0698.1061 [DOI] [Google Scholar]
- 14.Cipriano LE, Romanus D, Earle CC, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health. 2011;14(1):41-52. doi: 10.1016/j.jval.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross TR, Ng D, Brown JS, et al. The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Wash DC). 2014;2(1):1049. doi: 10.13063/2327-9214.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262. doi: 10.1097/JTO.0b013e31825bd9f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. EGEMS (Wash DC). 2016;4(1):1254. doi: 10.13063/2327-9214.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. doi: 10.1001/jama.2012.5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvestri GA, Gould MK, Margolis ML, et al. ; American College of Chest Physicians . Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 suppl):178S-201S. doi: 10.1378/chest.07-1360 [DOI] [PubMed] [Google Scholar]
- 21.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA; American College of Chest Physicians . Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 suppl):202S-220S. doi: 10.1378/chest.07-1362 [DOI] [PubMed] [Google Scholar]
- 22.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e211S-e250S. doi: 10.1378/chest.12-2355 [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network . Non-small cell lung cancer. Accessed September 2, 2020.. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 24.Huo J, Xu Y, Sheu T, Volk RJ, Shih YT. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179(3):324-332. doi: 10.1001/jamainternmed.2018.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tukey MH, Wiener RS. Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med. 2012;106(11):1559-1565. doi: 10.1016/j.rmed.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137-144. doi: 10.7326/0003-4819-155-3-201108020-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdial FC, Berfield KS, Wood DE, et al. Safety and costs of endobronchial ultrasound-guided nodal aspiration and mediastinoscopy. Chest. 2020;157(3):686-693. doi: 10.1016/j.chest.2019.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang MC, Fan D, Sung SH, et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care. 2017;55(12):e137-e143. doi: 10.1097/MLR.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison S, Tangel V, Wu X, et al. Are minimum volume standards appropriate for lung and esophageal surgery? J Thorac Cardiovasc Surg. 2018;155(6):2683-2694.e1. doi: 10.1016/j.jtcvs.2017.11.073 [DOI] [PubMed] [Google Scholar]
- 30.Francis DO, Pearce EC, Ni S, Garrett CG, Penson DF. Epidemiology of vocal fold paralyses after total thyroidectomy for well-differentiated thyroid cancer in a Medicare population. Otolaryngol Head Neck Surg. 2014;150(4):548-557. doi: 10.1177/0194599814521381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Keeffe-Rosetti MC, Hornbrook MC, Fishman PA, et al. A standardized relative resource cost model for medical care: application to cancer control programs. J Natl Cancer Inst Monogr. 2013;2013(46):106-116. doi: 10.1093/jncimonographs/lgt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53(1):175-196. doi: 10.1111/1475-6773.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Bureau of Labor and Statistics . Consumer Price Index. Accessed September 2, 2020. https://www.bls.gov/cpi/data.htm
- 34.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 35.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584-590. doi: 10.1016/j.annepidem.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 36.Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims-based frailty index in the National Health And Aging Trends Study cohort. Am J Epidemiol. 2017;186(6):745-747. doi: 10.1093/aje/kwx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716-722. doi: 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 39.Shi X, Wellman R, Heagerty PJ, Nelson JC, Cook AJ. Safety surveillance and the estimation of risk in select populations: flexible methods to control for confounding while targeting marginal comparisons via standardization. Stat Med. 2020;39(4):369-386. doi: 10.1002/sim.8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962-970. doi: 10.1093/ije/dyu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151-169. doi: 10.1146/annurev.publhealth.23.100901.140546 [DOI] [PubMed] [Google Scholar]
- 42.R Foundation for Statistical Computing. The R project for statistical computing. Accessed August 19, 2020. http://www.R-project.org/
- 43.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086. doi: 10.1001/archinternmed.2009.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82(2):243-248. doi: 10.2105/AJPH.82.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano PS, Chan BK, Schembri ME, Rainwater JA. Can administrative data be used to compare postoperative complication rates across hospitals? Med Care. 2002;40(10):856-867. doi: 10.1097/00005650-200210000-00004 [DOI] [PubMed] [Google Scholar]
- 46.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f [DOI] [PubMed] [Google Scholar]
- 47.Gould MK, Smith-Bindman R, Kelly K, et al. Methods for the Watch the Spot Trial: a pragmatic trial of more - versus less - intensive strategies for active surveillance of small pulmonary nodules. Ann Am Thorac Soc. 2019;16(12):1567-1576. doi: 10.1513/AnnalsATS.201903-268SD [DOI] [PubMed] [Google Scholar]
- 48.The Watch the Spot Trial (WTS) . ClinicalTrials.gov identifier NCT02623712. Updated October 28, 2019. Accessed July 25, 2020. https://clinicaltrials.gov/ct2/show/NCT02623712
- 49.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi: 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sequential Exclusions
eTable 2. Strobe Guidelines Checklist
eTable 3. Summary of the 2005 Fleischner Society Guidelines
eTable 4. Use of Diagnostic Tests by Intensity of Lung Nodule Evaluation
eTable 5. Modality-Specific Average Radiation Exposure Estimates
eTable 6. Time to Lung Cancer Diagnosis by Intensity of Lung Nodule Evaluation and Stratified by Lung Cancer Stage
eTable 7. Lung Cancer Diagnosis by Intensity of Nodule Evaluation Among Patients Who Experienced a Procedure-Related Adverse Event
eMethods.