Abstract
Background
Natural plants and plant-derived formulations have been used by mankind from the ancient period of time. For the past few years, many investigations elaborated the therapeutic potential of various secondary chemicals present in the plants. Literature revealed that the various secondary metabolites, viz. phenolics and flavonoids, are responsible for a variety of therapeutic action in humans.
Main body
In the present review, an attempt has been made to compile the exploration of natural phenolic compounds with major emphasis on flavonoids and their therapeutic potential too. Interestingly, long-term intake of many dietary foods (rich in phenolics) proved to be protective against the development and management of diabetes, cancer, osteoporosis, cardiovascular diseases and neurodegenerative diseases, etc.
Conclusion
This review presents an overview of flavonoid compounds to use them as a potential therapeutic alternative in various diseases and disorders. In addition, the present understanding of phenolics and flavonoids will serve as the basis for the next scientific studies.
Keywords: Phenolics, Flavonoids, Secondary metabolites, Therapeutic action
Background
Polyphenols is one of the major classes of naturally occurring compounds having at least one phenol group in their structure, present in the plants, including vegetables, fruits, cereals and dry legumes [1–3]. As these compounds show multiple physiological effects, when consumed as a component or dietary supplement, polyphenolics become a subject of interest in a scientific fraternity [4]. Polyphenols are secondary metabolites consisting of polyhydroxy phytochemicals of the plant kingdom and effective defense against pathogenic aggression and ultraviolet radiation [5]. These secondary compounds are biosynthesized through shikimic acid and phenylpropanoid pathways and believed to be participative in adapting the plants in a stressed situation due to environmental changes [6]. From this extensive class of polyphenolic compounds, more than 8000 have been already isolated, identified and described in detail [7].
In food, initially, polyphenols are used to manipulate astringency, bitterness, flavor, color, odor and oxidative stability. Throughout evolution in various plant lines, the ability to synthesize phenolic compounds was selected when these compounds met unique needs, allowing plants to cope with continuously evolving environmental conditions over evolutionary time [8]. Afterward, various epidemiological research activities and accompanying meta-analyses intensely recommended protection offered against the development of diabetes, cancer, osteoporosis, cardiovascular diseases and neurodegenerative diseases by long-term intake of plant polyphenols in our daily diet [9, 10]. These compounds, based on their chemical structures, are divided into various subclasses like phenolic acids, flavonoids, tannins, coumarins, lignans, quinones, stilbenes and curcuminoids [1].
Flavonoids are ubiquitously occurring polyphenolic compounds and comprise the broad class of natural products. To date, it has documented over 8000 different flavonoids and most of them are present in the cells or surfaces of various plant tissue organs [11]. A large variety of edible plant species contains flavonoids, which are considered to be important human dietary constituents [12, 13]. Flavonoids are among the most abundant and widespread secondary metabolites groups, which is extremely valuable to mankind, not merely because of their contribution to imparting plant colors, but also due to its several physiologically active members too [14]. The confirmation of flavonoid’s reliable positive benefits like cancer prevention has produced a considerable interest in research, including serving foods containing flavonoids [15].
Main body
Polyphenols and their therapeutic role
Epidemiological studies revealed an inverse relationship between the intake of polyphenolic rich food and threat of chronic human ailments [2, 9]. The generation of phenoxyl radicals via an acceptance of an electron by the phenolic groups of polyphenols creates the favorable disturbance in oxidative chain reactions inside cells [16]. Study revealed that polyphenols present in food and beverages exhibited an increase plasma antioxidant activity owing to accumulation of these reducing polyphenols, along with endogenous antioxidants in plasma, which, in turn, assist in absorption of iron like a pro-oxidative food component [2].
Consumption of polyphenol-rich diet protects lymphocytic deoxyribonucleic acid (DNA) from oxidative damage and work as antioxidants. A similar protective effect was evident for the beverages rich in polyphenols [17]. Polyphenols not only protect the cell and cellular components from oxidative damage but also reduce the risk of oxidative stress linked to different degenerative diseases [18–20].
Antidiabetic effect
Diabetes mellitus is a condition of physiological imbalance due to the alteration of various physiological parameters due to the impairment of the metabolism of glucose which resulted in hyperglycemia. Type 1 and type 2 are the two main categories of diabetes [21]. As diabetes is chronic, it leads to retinopathy, nephropathy and neuropathy, which may further result in blindness, kidney failure and amputations, respectively. Neuropathy may further create complications associated with sexual dysfunctions.
Scientific studies have revealed that polyphenols play an important role as an antidiabetic agent. Various catechin compounds from tea show antidiabetic action [22]. The potential of these secondary compounds as antidiabetic agents may be due to its inhibitory action in the gut for glucose absorption or by its peripheral tissue uptake. Diacetylated anthocyanins showed the antidiabetic effect at a dose of 1 mg/kg given with maltose as a source of glucose. This effect was not observed when it is given with glucose or sucrose [23]. Inhibition of α-glucosidase found in gut mucosa is responsible for such type of effect. Along with this, polyphenols have been investigated for their glucose transporter and intestinal glycosidase inhibitory activity [24].
A variety of polyphenols like isoflavones from soybeans, (−) epicatechin, epicatechin gallate, (+) catechin, (−) epigallocatechin, tannic acid, saponins, chlorogenic acid and compounds like glycyrrhizin from the liquorice root reduces S-Glut-1 mediated transport of glucose from the intestine. Owing to saponins, the transfer of the glucose from the stomach to the small intestine is delayed [25]. Stilbene compounds like resveratrol improvise whole-body glucose homeostasis and sensitivity to insulin in diabetic rats [26]. It also improves the status of diabetic nephropathy, dysfunction in the kidney and oxidative stress of diabetic rats. As resveratrol inhibits K+ adenosine triphosphate (ATP) and K+ (V) channel in beta cells considered to be a possible mechanism due to which effects like delaying of the onset of insulin resistance and decrease in insulin secretion [27]. Polyphenolic compound quercetin protects the lipid peroxidation and oxidative stress, which, in turn, helps in antidiabetic activity [28]. The antidiabetic potential of quercetin has been associated with its glucose uptake inhibitory phenomenon and modulation of mitogen-activated protein kinase pathway [29, 30]. One more study performed on Hibiscus sabdariffa extract proved that polyphenols (flavonoids, polyphenolic acids, protocatechuic acid and anthocyanins) lessen oxidative markers in the kidney, serum lipid profile and diabetic neuropathy [31]. Maize bran and vegetable contains ferulic acid, one of the polyphenolic compounds that helps in lowering lood glucose with an increase in insulin in plasma responsible for its strong antidiabetic activity [32]. Myricetin also showed strong glycemic control via insulin resistance amelioration and human pancreatic α-amylase inhibition [33]. In vivo study performed on diabetic rats elicits antidiabetic potential of resveratrol via intracellular glucose uptake stimulation and modulation of sirtuin-1 activity [34, 35]. The antidiabetic action of hispolon has been attributed with α-glucosidase and aldose reductase inhibitory action [36]. Gallic acid and p-coumaric acid also exhibited antidiabetic action via reduction in serum glucose level and rise in insulin level in diabetic rats [37]. The antidiabetic activity of cinnamic acid and caffeic acid was studied using mice and found effective through increase in glucose uptake and insulin sensitivity which results in reduction in glucose level [38, 39].
Anticancer effect
Various in vitro and in vivo studies of polyphenols were performed using human cancer cell lines. These studies concluded that polyphenols are protective and responsible for lowering tumor growth [40]. This type of beneficial effect was observed for various cancer sites, including the mammary glands, skin, lung and liver, and some sites of the digestive tract like the intestine, stomach and mouth. Despite the different mechanisms of action of different polyphenols, they show protective anticancer potential in few anticancer study models. Some of them are flavanones, isoflavones, catechins, ellagic acid, resveratrol, curcumin and red wine polyphenols [41].
Polyphenolic compounds show chemoprevention by several identified mechanisms like oxidation prevention, antiproliferation, detoxification of enzymes, initiation of apoptosis or cell cycle arrest, host immune system regulation, estrogenic/antiestrogenic activity and anti-inflammatory activity by producing alterations in cellular signaling [42]. They inhibit the expression of cytochrome p450 enzymes, which are involved in the process of activation of carcinogens. An increase in expression of phase II conjugating enzymes is facilitated by their excretion. Overexpression of phase II enzymes is associated with the toxicity of polyphenols [2].
Polyphenols affect pro-carcinogen metabolism by modifying the manifestation of cytochrome p450 enzymes involved in their stimulation to carcinogens. Carcinogen excretion may also be facilitated using these polyphenols by enhancing the phase II conjugating enzyme expression. Along with this, the stimulation of stage II enzymes can be caused by polyphenol toxicity [2]. The substrate of these enzymes is possible due to the formation of potentially toxic quinones. Improvisation of the body’s defense against xenobiotics is induced due to the self-detoxification of these enzymes by the intake of polyphenols [43]. Tea catechins proved its efficiency in cancer as it inhibits the alteration into cancer from high-grade prostate intraepithelial neoplasia lesions in men when given in the capsular form [44].
Along with this, polyphenols present in black tea (theaflavins and thearubigins) have good anticancer potential as they inhibit increase and proliferation in Du 145 prostate cancer cells [45]. The free radical scavenging potential of quercetin assists to show anticancer activity in lung cancer in mice induced by benzo(a)pyrene [46]. With this, inhibition of mutant p53 expression and apoptosis induction of treated cells is another potential of quercetin [47]. Resveratrol, a stilbene polyphenol, substantially proved for its anticancer potential via different in vitro and in vivo studies. Some of them are hepatocyte growth factor targeting and induction of apoptosis in human hepatocellular carcinoma (HCC), induction of cell death via the mediation of the epidermal growth factor receptor (EGFR) signaling pathway, regulation of AMP-activated protein kinase (AMPK) and increment in cell apoptosis induced by cisplatin [48–50]. Similarly, the antioxidant activity of resveratrol is helpful for its anticancer activity by modulation of various pathways like apoptosis, cell growth and inflammation [51]. Epigallocatechin gallate (EGCG), a major biologically active phenolic compound from green tea, also stated for its anticancer potential through different signal pathways [52]. Amyloid precursor protein (APP) acetylation and induction of apoptosis in human neuroblastoma, modulation of β-catenin activity and inhibition of head and neck cancer cell proliferation, rise in level of reactive oxygen species (ROS) with activation of caspase-3 and lowering the expression of vascular endothelial growth factor (VEGF) in esophageal squamous cells are some of the pathways for EECG’s anticancer action [53–55]. Curcumin, a major polyphenolic curcuminoid from turmeric rhizomes, exerts it anticancer potential via p53 pathway targeting in human osteosarcoma [56, 57]. Along with this, downregulation of Yes-associated protein expression in pancreatic cancer and extrinsic and intrinsic pathway triggering are other mechanisms for its anticancer action [58, 59]. In vivo studies of flavonoids like apigenin and chrysin and luteolin found in honey control the proliferation of pancreatic, glioma and aortic vascular smooth muscle cells in rats respectively [60–62].
Antiosteoporotic activity
Bone loss due to a deficiency of estrogen in menopausal women is considered to be a major cause of osteoporosis. Polyphenolic compounds like isoflavones show weak estrogenic action when observed in estrogen deficiency-induced rats or mice by ovariectomy. Loss of trabecular volume and bone density due to ovariectomy can be prevented by several weeks of dietary supplementation of daidzein, genistein and their glycosides [63–65]. Supplementation of soy proteins with reduced isoflavones shows antiosteoporotic activity in ovariectomized rats [66]. Inhibition of osteoclast cell differentiation, elevation in mineralization of bone, increase in alkaline phosphate action of osteoblast cells and decline in calcium stone formation induced by oxidative stress are the probable mechanisms for the antiosteoporotic activity of EGCG in rats [67–69]. Oleuropein, a polyphenol compound present in the olive leaf, acts via reduction of inflammatory biomarkers which may result in a decrease in bone loss in rats [70]. In vivo study of flavonoid fisetin showed the prevention of inflammation and bone loss in mice [71]. Resveratrol modulates SIRT1 (Sirtuin 1) activation and is responsible of its antiosteoporotic action [72]. Age-associated bone loss may be minimized by dietary intake of anthocyanin-rich berries due to their antioxidant potential via free radical scavenging [73]. In vivo antiosteoporotic study performed in female albino rats showed that ovarian hormone deficiency-induced bone loss has been prevented by aqueous black tea extract [74].
Cardioprotective effect
Numerous studies validated that the intake of polyphenols minimizes the risk of coronary heart diseases [75–77]. Atherosclerotic lesions developed in arteries remain clinically silent and then become active after decades and are responsible for the development of unstable angina, myocardial infarction or unexpected death [78]. Oxidation of low-density lipoprotein (LDL) is found to be the main mechanism in atherosclerosis development in the arteries, which is inhibited by the use of polyphenols [79]. Improvement in endothelial function, antiplatelet action, high-density lipoprotein (HDL), anti-inflammatory effects and antioxidant activity may be other mechanisms contributing to the protective effect of polyphenols in cardiovascular diseases. Quercetin found in an onion cause interruption in the formation of atherosclerotic plaques and inhibit the metalloproteinase I enzyme to reduce the mortality in patients with coronary heart diseases [42]. Various epidemiological investigations concluded that the risk of cardiovascular diseases like myocardial infarction is reduced due to the consumption of food enriched with polyphenols [80]. Polyphenols present in grape juice and red wine inhibit platelet aggregation, reduce bleeding time and exert antithrombotic effects [81]. Soy protein and green tea isolated from cocoa were found clinically effective for the decline in incidences of coronary heart disease and associated mortality via lowering of LDL and induction of nitric oxide-dependent vasodilation, respectively [82–84]. Clinical study of resveratrol revealed that 100 mg oral consumption for 12 weeks may support in the prevention of cardiovascular diseases and atherosclerosis via stimulation of endothelial function [85]. With this, it also modulates NO metabolism and helps for the improvement in vascular function in hypertensive and dyslipidemic patients [86].
Neuroprotective effects
Various neurodegenerative diseases, including Alzheimer’s disease, consist of damage to cellular components like DNA, lipids and proteins. In these conditions, oxidative stress is considered as a regulatory key factor. Intake of polyphenols may be responsible for the safeguarding of neurological diseases due to their strong antioxidant potential [87]. The onset of Alzheimer’s disease can be delayed by the intake of vegetables and fruit juices rich in polyphenols when taken three times per week [88]. The vital potential of vegetable and fruit polyphenols in neuroprotection plays an important role in influencing and modulating various cellular processes like proliferation, signaling, apoptosis and redox balance [89]. The risk of the development of Parkinson’s disease is reduced by the consumption of polyphenols in the form of green tea. These nutritional studies also revealed the protective role of polyphenols in Parkinson’s disease [90]. Tea consumption and incidence of neurodegenerative diseases show an inverse relationship due to its polyphenolic compounds including EGCG [91]. Curcumin found in turmeric showed its neuroprotective potential via reduction in Alzheimer’s disease pathogenesis [92]. Reduction in age-related cognitive impairment has been reduced by dietary intake of resveratrol in mice [93]. Additionally, resveratrol was also found efficacious in the prevention of blood-brain barrier impairment [94]. Quercetin has been reported for its protection in pheochromocytoma cell neurodegeneration induced by hydrogen peroxide [95]. In another study, mitochondria-targeted activities of quercetin were found to be a mechanism in protection against neurodegenerative diseases [96].
Antioxidant effects
Polyphenols are studied and recognized for their potential as natural antioxidant compounds for human health by combating and avoiding oxidative damage due to free radicals [97]. The hydroxycinnamic acid derivatives like caffeic acid and p-coumaric acid showed effective antioxidant activity against LDL peroxidation [98]. Ferulic acid which is a phenolic acid mostly found in oats, wheat and barley demonstrated prominent antioxidant activity and has been protective on human skin against UV rays [99, 100]. Quercetin, one of the important flavonoids, showed prominent antioxidant potential and is found as an effective, strong free radical scavenger in various in vitro research studies [101–103]. At an optimal dose of 1000 mg/day, rutin is a profound concentration-dependent free radical scavenger. Along with this, it is used in the management of hypertension, cancer and hypercholesterolemia [104–106]. Tea catechins including epigallocatechin gallate were found to be in vitro free radical scavengers. With this, these compounds were also found effective in decreasing protein carbonylation and lipid peroxidation in animal studies [107–109].
Others
Despite various health benefits of polyphenols mentioned above, they can be used in a few other health ailments too. Polyphenols are used in obstructive lung diseases like asthma [110, 111] as improvement in lung function in asthma patients was observed due to increased intake of genistein [112]. Polyphenols of tea minimize sunlight-induced skin damage, lipid peroxidation and erythema when given orally or applied topically studied in an animal study [113]. Theaflavins of black tea possesses antiviral action and shows anti-human immunodeficiency virus-1 (anti-HIV-1) activity. Theaflavin 3' gallate and theaflavin 3 3' digallate showed antiviral activity on the corona virus by inhibiting chymotrypsin-like protease [45].
Classes of polyphenols
Polyphenolics is considered as one of the major classes of secondary metabolites consisting of more than 8000 polyphenolic compounds found in different plants. These phenolic compounds consist of shikimic acid as a close precursor and phenylalanine as a common intermediate. Conjugated forms of polyphenolic compounds were primarily found in which sugar residues are either linked with hydroxyl groups or directly to the aromatic carbon. These compounds also form conjugation with amines, organic acids, carboxylic acids, lipids and other phenolic compounds [114]. These compounds show profound protection against the development and worsening of several long-lasting pathological illnesses like aging, diabetes, cancer and cardiovascular problems (Table 1).
Table 1.
Classes of polyphenols
| Class | Main structure | Compound | Effects | Reference |
|---|---|---|---|---|
| Phenolic acids | Hydroxy-benzoic acid |
p-Hydroxy-benzoic acid Gallic acid |
Hypoglycemic Antimicrobial Antihypertensive Antihyperglycaemic |
[115] [116] [117] [118] |
| Cinnamic acid |
Rosmarinic acid Ferulic acid Caffeic acid Chlorogenic acid |
Hepatoprotective Nephroprotective Antihypertensive Antihyperglycaemic Neuroprotective Anti-inflammatory Antidiabetic Anti-inflammatory |
[119] [120] [121] [122] [123] [124] [125] [126] |
|
| Flavonoids | Flavones |
Chrysin Luteolin |
Neuroprotective Cytotoxic Antiallergic Apoptotic |
[127] [128] [129] [130] |
| Flavanones |
Naringenin Hesperetin Eriodictyol |
Anti-inflammatory Antidiabetic Antiplatelet Apoptotic Hepatoprotective Anticancer |
[131] [132] [133] [134] [135] [136] |
|
| Flavonols |
Quercetin Kaempferol Fisetin |
Neuroprotective Antihypertensive Apoptotic Anti-inflammatory Cardioprotective Anti-inflammatory |
[137] [138] [139] [140] [141] [142] |
|
| Flavanols |
Catechin Epicatechin |
Neuroprotective Antioxidant Antidiabetic Nephroprotective |
[91] [143] [144] [145] |
|
| Stilbenoids | – |
Piceatannol Resveratrol |
Antimutagenic Anticancer Apoptotic Immunomodulatory |
[146] [147] [148] [149] |
| Lignans | – |
Isotaxiresinol Secoisolariciresinol |
Anti-osteoporotic Hepatoprotective Hepatoprotective Antioxidant |
[150] [151] [151] [152] |
Polyphenolic compounds subdivided into subclasses like phenolic acids, phenolic alcohols, flavonoids, stilbenoids and lignans are given in Fig. 1 [3]. Out of these, most of the isolated, identified compounds are from the class of flavonoids.
Fig. 1.
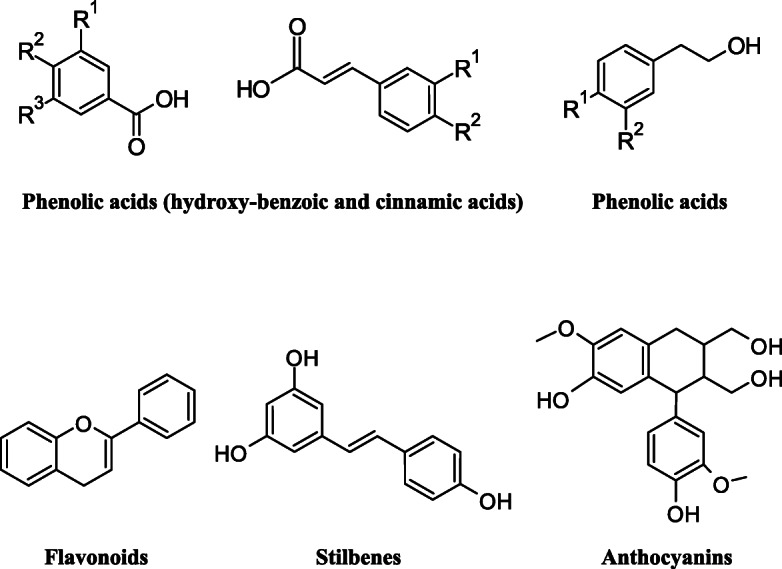
Chemical structure of the different classes of polyphenols
Flavonoids
As earlier mentioned, flavonoids comprise the most isolated, identified and diversified class of polyphenolic compounds. Flavonoids are secondary plant metabolites that are responsible for the flower’s color and fragrance. Flavonoids are attributed to a wide range of health-promoting properties and are an integral part of many pharmaceutical nutraceuticals and medicinal and cosmetic formulations. They possess various pharmacological actions like antioxidant, antiviral, antibacterial, anti-inflammatory and anti-allergic potentials [153–155]. Flavonoids interact with several signal transduction pathways in the process of carcinogenesis, thereby reducing proliferation, angiogenesis and metastasis and increasing apoptosis [156]. To date, more than 6000 different flavonoids have been identified in plants and the list is continuously increasing [157]. They consist of a common diphenyl propane carbon skeleton along with two benzene rings A and B linked through the linear three-carbon chain (C6–C3–C6). Closed pyran ring C is formed by this central carbon chain. Variation of heterocycle involved, flavonoids may be classified into subclasses like flavones, flavanones, flavanols, flavonols (catechins and proanthocyanidins), anthocyanidins and isoflavonoids (Fig. 2 and Table 2). This classification depends on the existence or nonexistence of a double bond on the position 4 of the C ring and a double bond between C2 and C3 and the hydroxyl groups in the ring B.
Fig. 2.
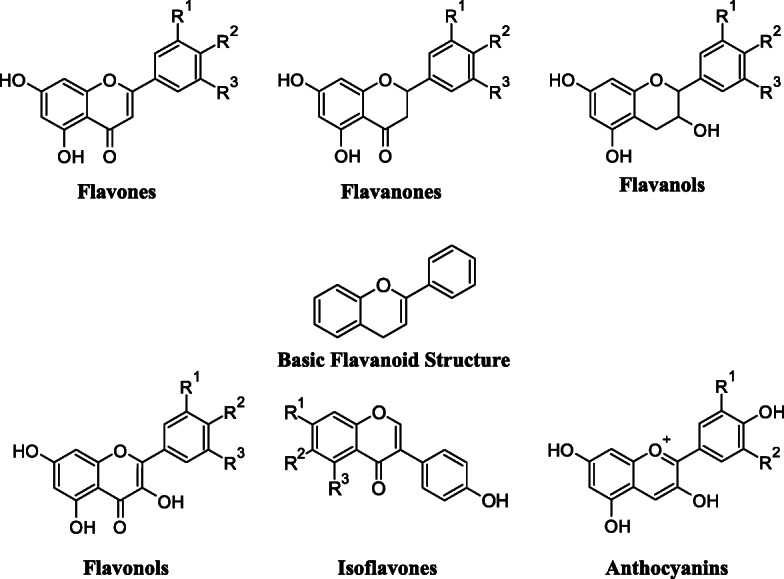
Chemical structure of classes of flavonoids
Table 2.
Classes of flavonoids
| Flavonoid class | Examples | Source | Reference |
|---|---|---|---|
| Flavones | Chrysin | Honey, blue passion flower | [158] |
| Luteolin | Common balm, parsley | [159, 160] | |
| Flavanones | Hesperidin | Lemon, sweet orange | [161] |
| Naringenin | Lemon, grapefruit | [162] | |
| Flavonols | Quercetin | Apple, onion | [163] |
| Kaempferol | Apple, onion | [163] | |
| Flavanols | (+) Catechin | Green tea | [164] |
| Epigallocatechin | Green tea | [164] | |
| Anthocyanins | Cyanidin | Berries, grapes | [165] |
| Delphinidin | Berries, grapes | [165] | |
| Isoflavonoids | Daidzein | Soybeans | [166] |
| Genistein | Soybeans | [166] |
Flavone is a class of less common flavonoids consisting of a double bond between C2 and C3 in the heterocyclic ring of the flavan skeleton. A few of the important sources of flavones are celery and parsley. The skin of mandarin fruit also contains a large amount of polymethoxylated flavones.
Flavones
One of the flavonoids, flavones, are with a non-saturated 3-C chain and a double bond between C-2 and C-3, similar to flavonols, with which they vary in the lack of 3-position of the hydroxyl group. Flavones are commonly dispersed in the form of aglycones or glycosides among the higher plants. The distinction in composition between flavones and flavonols appears to have very significant implications in the roles of biogenesis, physiology and pharmacology and the phylogenetic and chemotaxonomic implications of these compounds [167]. Flavones are widespread as O-glycosides in biodiversity [168]. Several flavones such as chrysin, tangeretin and apigenin were researched for the therapy of experimental colitis; 30-day mice-supplemented apigenin feeding reduced dextran sulfate sodium (DSS)-induced colitis macroscopic and microscopic impairment [169]. In preclinical models, several flavones were studied for neuroprotection. In the streptozotocin-induced Alzheimer’s disease (AD) rat model, luteolin, a flavonoid discovered in celery, rosemary and parsley, has proved a definite neuroprotective impact that improves memory impairment and spatial learning. Apigenin, another prevalent flavone, has demonstrated comparable neuroinflammatory prevention activity. Apigenin-treated mice enhanced memory and learning capabilities by decreasing amyloid fibrillary deposits through modulation of beta-secretase 1. Luteolin in the liposomal form in olive fruit extract enhanced attention in kids with autism spectrum illnesses and brain fog in patients with mild cognitive consequences. Chrysin, a flavone found in multiple vegetables, fruits and mushrooms, has been suggested as a neurotrophic for nervous cells, anti-inflammatory and anti-amyloidogenic [170]. In addition, it also reduces the signs of DSS-induced colitis in mice by considerably diminishing colonic myeloperoxidase activity and decreasing proinflammatory cytokine, prostaglandin E2 (PGE2) and nitric oxide (NO) output [171].
Flavanones
Flavanones are usually glycosylated compounds with the disaccharide at C7 position and consisting of saturation between C2 and C3 and the presence of oxygen atom at the C4 position. Along with tomatoes and aromatic plants like mint, these compounds are abundantly present in citrus fruits. The aglycone flavanones naringenin, hesperetin and eriodictyol are present in grapefruit, oranges and lemons, respectively [172]. Compared to the glass of orange juice, whole citrus fruit contains five times more flavanone content as it is mostly accumulated in the spongy white portion and the segment separating the membranes of these fruits.
Flavonols
Flavonol is the most diverse class of flavonoids present in a food which is represented by a double bond between C2 and C2 with C3 position linked with a hydroxyl group. The representative compound from this class is quercetin. Flavonols are abundantly present in broccoli, onions, leeks and blueberries along with red wine and tea. It was observed that flavonol concentration differs among fruits or between different sides of the same fruit grown on the same tree. This type of effect was evident as the biosynthesis of flavonols is stimulated by sunlight. Probably due to sameness, flavonols are accumulated in the aerial and outer tissue of the fruits [173].
Flavanols
Flavanols are generally not available in the glycosylated form in foods consisting of the hydroxyl group at C3 position with saturation between C2 and C3. They occur in both the monomer (catechins) and the polymer (proanthocyanidins) forms. Epicatechin and catechin are the representative flavanols in fruit, while tea contains gallocatechin, epigallocatechin and epigallocatechingallate [174].
Cherry and apricots are the sources of catechins, while chocolate and green tea are the rich ones. Dimers, oligomers and polymers of catechins are also called condensed tannins or proanthocyanidins with varied structures and molecular weights. The range of degree of polymerization from 4 to 11 in cider apples is one representative example of the same [175]. The astringency of some fruits (berries, grapes) and beverages (beer, wine) and the bitter taste of chocolate is due to the proanthocyanidin content of the same [176].
Anthocyanins
Anthocyanins are abundantly and widely present in fruit skins, vegetables and cereals and are responsible for the different colors of fruits, flowers and vegetables [177]. The anthocyanin content of the fruit is generally proportional to the color intensity and ripening. Red wine is one of the sources in which these water-soluble pigments are present (up to 350 mg anthocyanins/L) which show the structural transformation on aging [178, 179]. Anthocyanins primarily occurs as glycosides which are called anthocyanidins formed from their respective aglycones. In this, the position of sugar moiety attachment is generally either at position 3 of C ring or 5, 7 position of A ring [180].
Isoflavones
Isoflavones are almost exclusively found in leguminous plants having a structural resemblance to estrogens. Glycitein, genistein and daidzein are isoflavone aglycones found in the soya plant which more often form conjugation with glucose [181, 182]. During storage and industrial processing, they often hydrolyze and form glycosides as they show sensitivity to heat [182].
Conclusion
Polyphenols are naturally occurring secondary metabolites which is one of the most established categories of bioactive compounds. They constitute a broad repository of natural chemical diversity that includes a vast array of phytochemicals and enzymes. In humans, various scientific studies on consumable foods rich with these compounds revealed their potential health outcomes. They are found to be effective in the management of various chronic conditions like diabetes, cancer, cardiovascular diseases etc. Being a major subclass of polyphenol compounds and due to their widespread dietary distribution, flavonoids are believed to be non-toxic with little or no toxicity. This property is notable as numbers of phytochemicals in this class are available as a medicine in different dosage forms. So based on our literature review, we can conclude that the present understanding of phenolics and flavonoids will serve as the basis for the next scientific studies.
Acknowledgements
The authors are thankful to the principal of H. R. Patel Institute of Pharmaceutical Education and Research Shirpur, Dist: Dhule (MS) 425 405, for providing the necessary library facilities.
Abbreviations
- AD
Alzheimer’s disease
- AMPK
Adenosine monophosphate-activated protein kinase
- ATP
Adenosine triphosphate
- DNA
Deoxyribonucleic acid
- DSS
Dextran sulphate sodium
- EGFR
Epidermal growth factor receptor
- HCC
Human hepatocellular carcinoma
- HDL
High density lipoprotein
- LDL
Low density lipoprotein
- NO
Nitric oxide
- PGE2
Prostaglandin E2
- ROS
Reactive oxygen species
- SIRT1
Sirtuin 1
- VEGF
Vascular endothelial growth factor
Authors’ contributions
REM carried out the literature review on the role of flavonoids in therapeutics. AUT organized a preliminary draft of the article. SJS contributed to the writing style and proofreading. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Data and materials are available upon request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rakesh E. Mutha, Email: rakeshmutha123@yahoo.co.in
Anilkumar U. Tatiya, Email: aniltatiya12171@gmail.com
Sanjay J. Surana, Email: sjsurana@yahoo.com
References
- 1.Gan RY, Chan CL, Yang QQ, Li H Bin, Zhang D, Ge YY, Gunaratne A, Ge J, Corke H (2018) Bioactive compounds and beneficial functions of sprouted grains. Sprouted grains: nutritional value, production, and applications, AACC International pp 191–246. 10.1016/B978-0-12-811525-1.00009-9
- 2.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 3.Spencer JPE, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 4.Hu M. Commentary: Bioavailability of flavonoids and polyphenols: call to arms. Mol Pharm. 2007;6:803–806. doi: 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman CH. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol Mol Plant Pathol. 2000;57:101–110. doi: 10.1006/pmpp.2000.0287. [DOI] [Google Scholar]
- 6.Zhao H (2015) Effects of processing stages on the profile of phenolic compounds in beer. Processing and Impact on Active Components in Food. 10.1016/B978-0-12-404699-3.00064-0
- 7.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Lattanzio V. Phenolic compounds: introduction. In: Ramawat K, Mérillon JM, editors. Natural Products. Berlin: Springer; 2013. [Google Scholar]
- 9.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 10.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8:281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 11.Jan S, Abbas N (2018) Chemistry of Himalayan phytochemicals. In: Himalayan Phytochemicals. 10.1016/b978-0-08-102227-6.00004-8
- 12.Verri WA, Vicentini FTMC, Baracat MM, Georgetti SR, Cardoso RDR, Cunha TM, Ferreira SH, Cunha FQ, Fonseca Maria JV, Casagrande R. Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms. In: Studies in Natural Products Chemistry, Elsevier; 2012. pp. 297–330. [Google Scholar]
- 13.Patil VM, Masand N. Anticancer potential of flavonoids: chemistry, biological activities, and future perspectives. In: Studies in Natural Products Chemistry, Elsevier; 2018. pp. 401–430. [Google Scholar]
- 14.Muhaisen HMH. Introduction and interpretation of flavonoids. Adv Sci Eng Med. 2015;6:1235–1250. doi: 10.1166/asem.2014.1630. [DOI] [Google Scholar]
- 15.Khajuria R, Singh S, Bahl A. Tuli H Current Aspects of Flavonoids: Their Role in Cancer Treatment. Singapore: Springer; 2019. General introduction and sources of flavonoids; pp. 1–7. [Google Scholar]
- 16.Clifford MN (2000) Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric 80:1033-1043. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1033::AID-JSFA595>3.0.C O;2-T
- 17.Vitrac X, Monti JP, Vercauteren J, Deffieux G, Meérillon JM. Direct liquid chromatographic analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection. Anal Chim Acta. 2002;458:103–110. doi: 10.1016/S0003-2670(01)01498-2. [DOI] [Google Scholar]
- 18.Luqman S, Rizvi SI. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phyther Res. 2006;20:303–306. doi: 10.1002/ptr.1861. [DOI] [PubMed] [Google Scholar]
- 19.Pandey KB, Mishra N, Rizvi SI. Protective role of myricetin on markers of oxidative stress in human erythrocytes subjected to oxidative stress. Nat Prod Commun. 2009;4:221–226. [PubMed] [Google Scholar]
- 20.Pandey KB, Rizvi SI. Protective effect of resveratrol on markers of oxidative stress in human erythrocytes subjected to in vitro oxidative insult. Phyther Res. 2010;24:S11–S14. doi: 10.1002/ptr.2853. [DOI] [PubMed] [Google Scholar]
- 21.Rizvi SI, Zaid MA. Intracellular reduced glutathione content in normal and type 2 diabetic erythrocytes: effect of insulin and (−) epicatechin. J Physiol Pharmacol. 2001;52:483–488. [PubMed] [Google Scholar]
- 22.Ibrahim Rizvi S, Abu Zaid M. Impairment of sodium pump and Na/H exchanger in erythrocytes from non-insulin dependent diabetes mellitus patients: effect of tea catechins. Clin Chim Acta. 2005;354:59–67. doi: 10.1016/j.cccn.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N, Matsumoto K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J Agric Food Chem. 2002;50:7244–7248. doi: 10.1021/jf025913m. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 2. α-Glucosidase inhibition by isolated acylated anthocyanins. J Agric Food Chem. 2001;49:1952–1956. doi: 10.1021/jf0012502. [DOI] [PubMed] [Google Scholar]
- 25.Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H. Antioxidant phytochemicals against type 2 diabetes. Br J Nutr. 2008;99:109–117. doi: 10.1017/S000711450896579X. [DOI] [PubMed] [Google Scholar]
- 26.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–277. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi SI, Mishra N. Anti-oxidant effect of Quercetin on type 2 diabetic erythrocytes. J Food Biochem. 2009;33:404–415. doi: 10.1111/j.1745-4514.2009.00228.x. [DOI] [Google Scholar]
- 29.Pandey KB. Rizvi SI (2010) Protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress. Med Chem Res. 2010;19:186–192. doi: 10.1007/s00044-009-9183-y. [DOI] [Google Scholar]
- 30.Aguirre L, Arias N, Macarulla MT, Gracia A, Portillo M. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2014;4:189–198. [Google Scholar]
- 31.Lee WC, Wang CJ, Chen YH, Hsu JD, Cheng SY, Chen HC, Lee HJ. Polyphenol extracts from Hibiscus sabdariffa Linnaeus attenuate nephropathy in experimental type 1 diabetes. J Agric Food Chem. 2009;57:2206–2210. doi: 10.1021/jf802993s. [DOI] [PubMed] [Google Scholar]
- 32.Eun HJ, Sung RK, In KH, Tae YH. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 33.Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007;81:1479–1488. doi: 10.1016/j.lfs.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D, Maulik N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol Med. 2008;12:2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi TC, Chen WP, Chi TL, Kuo TF, Lee SS, Cheng JT, Su MJ. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007;80:1713–1720. doi: 10.1016/j.lfs.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Huang GJ, Hsieh WT, Chang HY, Huang SS, Lin YC, Kuo YH. α-Glucosidase and aldose reductase inhibitory activities from the fruiting body of Phellinus merrillii. J Agric Food Chem. 2011;59:5702–5706. doi: 10.1021/jf2003943. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Moneim A, Yousef AI, Abd El-Twab SM, Abdel Reheim ES, Ashour MB. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab Brain Dis. 2017;32:1279–1286. doi: 10.1007/s11011-017-0039-8. [DOI] [PubMed] [Google Scholar]
- 38.Huang DW, Shen SC, Wu JS. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J Agric Food Chem. 2009;57:7687–7692. doi: 10.1021/jf901376x. [DOI] [PubMed] [Google Scholar]
- 39.Jung UJ, Lee MK, Park YB, Jeon SM, Choi MS. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006;318:476–483. doi: 10.1124/jpet.106.105163. [DOI] [PubMed] [Google Scholar]
- 40.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 41.Johnson IT, Williamson G, Musk SRR. Anticarcinogenic factors in plant foods: a new class of nutrients? Nutr Res Rev. 1994;7:175–204. doi: 10.1079/NRR19940011. [DOI] [PubMed] [Google Scholar]
- 42.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 43.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma V, Rao LJM. A thought on the biological activities of black tea. Crit Rev Food Sci Nutr. 2009;49:379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- 46.Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol Pharm Bull. 2007;30:2268–2273. doi: 10.1248/bpb.30.2268. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Wang JJ, Chen XL, Du L, Li F. Quercetin-loaded freeze-dried nanomicelles: improving absorption and anti-glioma efficiency in vitro and in vivo. J Control Rel. 2016;235:276–290. doi: 10.1016/j.jconrel.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 48.Gao F, Deng G, Liu W, Zhou K, Li M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol Rep. 2017;37:1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 49.Jin Z, Feng W, Ji Y, Jin L. Resveratrol mediates cell cycle arrest and cell death in human esophageal squamous cell carcinoma by directly targeting the EGFR signaling pathway. Oncol Lett. 2017;13:347–355. doi: 10.3892/ol.2016.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM, Tsao JW, Juan YN, Chiu HY, Yang JS, Wang CC. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol. 2017;50:873–882. doi: 10.3892/ijo.2017.3866. [DOI] [PubMed] [Google Scholar]
- 51.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung JE, Tan S, Gao SJ, Yongvongsoontorn N, Kim SH, Lee JH, Choi HS, Yano H, Zhuo L, Kurisawa M, Ying JY. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nature Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Hou L, Gu S, Zuo X, Meng D, Luo M, Zhang X, Huang S, Zhao X. Molecular mechanism of epigallocatechin-3-gallate in human esophageal squamous cell carcinoma in vitro and in vivo. Oncol Rep. 2015;33:297–303. doi: 10.3892/or.2014.3555. [DOI] [PubMed] [Google Scholar]
- 54.Shin YS, Kang SU, Park JK, Kim YE, Kim YS, Baek SJ, Lee SH, Kim CH. Anti-cancer effect of (-)-epigallocatechin-3-gallate (EGCG) in head and neck cancer through repression of transactivation and enhanced degradation of β-catenin. Phytomedicine. 2016;23:1344–1355. doi: 10.1016/j.phymed.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Hu Q, Chang X, Yan R, Rong C, Yang C, Cheng S, Gu X, Yao H, Hou X, Mo Y, Zhao L, Chen Y, Dinlin X, Wang Q, Fang S (2015) (-)-Epigallocatechin-3-gallate induces cancer cell apoptosis via acetylation of amyloid precursor protein. Med Oncol 32:390. 10.1007/s12032-014-0390-0 [DOI] [PubMed]
- 56.Momtazi AA, Shahabipour F, Khatibi S, Johnston TP, Pirro M, Sahebkar A. Curcumin as a MicroRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol. 2016;171:1–38. doi: 10.1007/112_2016_3. [DOI] [PubMed] [Google Scholar]
- 57.Jun W, Peng C, Wen J, Ming-zhi G. Experimental study on curcumin inhibiting proliferation and invasion of human osteosarcoma cells. Biomed Res-Tokyo. 2017;28:4396–4401. [Google Scholar]
- 58.Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, Wang Z. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7:79062–79074. doi: 10.18632/oncotarget.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q, Zhai YL. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm Biol. 2016;54:1303–1311. doi: 10.3109/13880209.2015.1060508. [DOI] [PubMed] [Google Scholar]
- 60.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 61.Milner JA. Reducing the risk of cancer. In: Israel G, editor. Functional Foods. New York: Van Nostrand Reinhold; 1994. pp. 39–70. [Google Scholar]
- 62.Pyrzynska K, Biesaga M. Analysis of phenolic acids and flavonoids in honey. TrAC, Trends Anal Chem. 2009;28:893–902. doi: 10.1016/j.trac.2009.03.015. [DOI] [Google Scholar]
- 63.Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129:399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]
- 64.Sturgeon C. Practice guidelines for tumour marker use in the clinic. Clin Chem. 2007;48:1151–1159. doi: 10.1093/clinchem/48.8.1151. [DOI] [PubMed] [Google Scholar]
- 65.Yoshizumi M, Tsuchiya K, Suzaki Y, Kirima K, Kyaw M, Moon JH, Terao J, Tamaki T. Quercetin glucuronide prevents VSMC hypertrophy by angiotensin II via the inhibition of JNK and AP-1 signaling pathway. Biochem Biophys Res Commun. 2002;293:1458–1465. doi: 10.1016/S0006-291X(02)00407-2. [DOI] [PubMed] [Google Scholar]
- 66.Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000;60:4112–4121. [PubMed] [Google Scholar]
- 67.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-κB. Biochem Biophys Res Commun. 2009;379:1033–1037. doi: 10.1016/j.bbrc.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, Yamada S, Inagaki K, Oguchi K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. 2012;118:55–64. doi: 10.1254/jphs.11082FP. [DOI] [PubMed] [Google Scholar]
- 69.Shen CL, Yeh JK, Stoecker BJ, Chyu MC, Wang JS. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone. 2009;44:684–690. doi: 10.1016/j.bone.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Puel C, Quintin A, Mathey J, Obled C, Davicco MJ, Lebecque P, Kati-Coulibaly S, Horcajada MN, Coxam V. Prevention of bone loss by phloridzin, an apple polyphenol, in ovariectomized rats under inflammation conditions. Calcif Tissue Int. 2005;77:311–318. doi: 10.1007/s00223-005-0060-5. [DOI] [PubMed] [Google Scholar]
- 71.Leotoing L, Wauquier F, Guicheux J, Miot-Noirault E, Wittrant Y, Coxam V. The polyphenol fisetin protects bone by repressing NF-κB and MKP-1-dependent signalling pathways in osteoclasts. PloS One. 2013;8:e68388. doi: 10.1371/journal.pone.0068388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kondo A, Otsuka T, Kuroyanagi G, Yamamoto N, Matsushima-Nishiwaki R, Mizutani J, Kozawa O, Tokuda H. Resveratrol inhibits BMP-4-stimulated VEGF synthesis in osteoblasts: suppression of S6 kinase. Int J Mol Med. 2014;33:1013–1018. doi: 10.3892/ijmm.2014.1626. [DOI] [PubMed] [Google Scholar]
- 73.Hubert PA, Lee SG, Lee SK, Chun OK. Dietary polyphenols, berries, and age-related bone loss: a review based on human, animal, and cell studies. Antioxidants. 2014;3:144–158. doi: 10.3390/antiox3010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das AS, Mukherjee M, Mitra C. Evidence for a prospective anti-osteoporosis effect of black tea (Camellia sinensis) extract in a bilaterally ovariectomized rat model. Asia Pac J Clin Nutr. 2004;13:210–216. [PubMed] [Google Scholar]
- 75.Renaud S, de Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339:1523-6 10.1016/0140-6736(92)91277-F [DOI] [PubMed]
- 76.Dubick MA, Omaye ST. Evidence for grape, wine and tea polyphenols as modulators of atherosclerosis and ischemic heart disease in humans. J Nutraceuticals, Funct Med Foods. 2001;3:67–93. doi: 10.1300/J133v03n03_04. [DOI] [Google Scholar]
- 77.Nardini M, Natella F, Scaccini C. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets. 2007;18:224–243. doi: 10.1080/09537100601078083. [DOI] [PubMed] [Google Scholar]
- 78.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81:292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 79.Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 80.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 81.Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.CIR.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 82.Gould AL, Davies GM, Alemao E, Yin DD, Cook JR. Cholesterol reduction yields clinical benefits: meta-analysis including recent trials. Clin Ther. 2007;29:778–794. doi: 10.1016/j.clinthera.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from the national high blood pressure education program. J Am Med Assoc. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 84.F Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK (2003) Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21:2281–2286. 10.1097/00004872-200312000-00016 [DOI] [PubMed]
- 85.Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int Heart J. 2017;58:577–583. doi: 10.1536/ihj.16-373. [DOI] [PubMed] [Google Scholar]
- 86.Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V, Vecchione C. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- 87.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 88.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer’s disease: the kame project. Am J Med. 2006;119:751–759. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- 90.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33:2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 91.Pervin M, Unno K, Ohishi T, Tanabe H, Miyoshi N, Nakamura Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules. 2018;23:1297. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nam SM, Choi JH, Yoo DY, Kim W, Jung HY, Kim JW, Yoo M, Lee S, Kim CJ, Yoon YS, Hwang IK. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food. 2014;17:641–649. doi: 10.1089/jmf.2013.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegri C, Sanfeliu C, Camins A, Pallas M, del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordr) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omar SH. Biophenols pharmacology against the amyloidogenic activity in Alzheimer’s disease. Biomed Pharmacother. 2017;89:396–413. doi: 10.1016/j.biopha.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 95.Heo HJ, Lee CY. Protective effects of quercetin and vitamin c against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52:7514–7517. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 96.Dajas F, Abin-Carriquiry JA, Arredondo F, Blasina F, Echeverry C, Martínez M, Rivera F, Vaamonde L. Quercetin in brain diseases: potential and limits. Neurochem Int. 2015;89:140–148. doi: 10.1016/j.neuint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and their benefits: a review. Int J Food Prop. 2017;20:1700–1741. [Google Scholar]
- 98.Cheng JC, Dai F, Zhou B, Liu ZL. Antioxidant activity of hydroxycinnamic acid derivatives in human low-density lipoprotein: mechanism and structure–activity relationship. Food Chem. 2007;104:132–139. doi: 10.1016/j.foodchem.2006.11.012. [DOI] [Google Scholar]
- 99.Calinoiu LF, Vodnar DC. Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability. Nutrients. 2018;10:1615–1646. doi: 10.3390/nu10111615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zdunska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol. 2018;31:332–336. doi: 10.1159/000491755. [DOI] [PubMed] [Google Scholar]
- 101.Amic A, Lucic B, Stepanic V, Markovic Z, Markovic S, Dimitric Markovic JM, Amic D. Free radical scavenging potency of quercetin catecholic colonic metabolites: thermodynamics of 2H+/2e− processes. Food Chem. 2017;218:144–151. doi: 10.1016/j.foodchem.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 102.Chen X, Zou L, Liu W, McClements DJ. Potential of excipient emulsions for improving quercetin bioaccessibility and antioxidant activity: an in vitro study. J Agric Food Chem. 2016;64:3653–3660. doi: 10.1021/acs.jafc.6b01056. [DOI] [PubMed] [Google Scholar]
- 103.Andarwulan N, Batari R, Sandrasari DA, Bolling B, Wijaya H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010;121:1231–1235. doi: 10.1016/j.foodchem.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle JA, Collins AR. Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr. 2000;54:774–782. doi: 10.1038/sj.ejcn.1601090. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs. 2013;22:1063–1079. doi: 10.1517/13543784.2013.805744. [DOI] [PubMed] [Google Scholar]
- 106.Kessler M, Ubeaud G, Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003;55:131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- 107.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 108.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 109.Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, Kalaiselvi P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate. Int J Dev Neurosci. 2008;26:217–223. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Tabak C, Arts ICW, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the morgen study. Am J Respir Crit Care Med. 2001;164:61–64. doi: 10.1164/ajrccm.164.1.2010025. [DOI] [PubMed] [Google Scholar]
- 111.Woods RK, Walters EH, Raven JM, Wolfe R, Ireland PD, Thien FCK, Abramson MJ. Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr. 2003;78:414–421. doi: 10.1093/ajcn/78.3.414. [DOI] [PubMed] [Google Scholar]
- 112.Smith LJ, Holbrook JT, Wise R, Blumenthal M, Dozor AJ, Mastronarde J, Williams L. Dietary intake of soy genistein is associated with lung function in patients with asthma. J Asthma. 2004;41:833–843. doi: 10.1081/JAS-200038447. [DOI] [PubMed] [Google Scholar]
- 113.Nakajima D, Kim CS, Oh TW, Yang CY, Naka T, Igawa S, Ohta F. Suppressive effects of genistein dosage and resistance exercise on bone loss in ovariectomized rats. J Physiol Anthropol Appl Human Sci. 2001;20(5):285–291. doi: 10.2114/jpa.20.285. [DOI] [PubMed] [Google Scholar]
- 114.Kondratyuk TP, Pezzuto JM. Natural product polyphenols of relevance to human health. Pharm Biol. 2004;42:46–63. doi: 10.3109/13880200490893519. [DOI] [Google Scholar]
- 115.Peungvicha P, Thirawarapan SS, Watanabe H. Hypoglycemic effect of 4-hydroxybenzoic acid, a constituent of Pandanus odorus root. Jpn J Pharmacol. 1998;78:395–398. doi: 10.1254/jjp.78.395. [DOI] [PubMed] [Google Scholar]
- 116.Merkl R, Hradkova I, Filip V, Smidrkal J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci. 2010;28:275–279. doi: 10.17221/132/2010-CJFS. [DOI] [Google Scholar]
- 117.Jin L, Lin MQ, Piao ZH, Cho JY, Kim GR, Choi SY, Ryua Y, Suna S, Keea HJ, Jeong MH. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-argininemethyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J Hypertens. 2017;35:1–11. doi: 10.1097/HJH.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 118.Punithavathi VR, Prince PSM, Kumar R, Selvakumari J (2011) Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. 650:465–471 10.1016/j.ejphar.2010.08.059 [DOI] [PubMed]
- 119.Elufioyea TO, Habtemariam S. Hepatoprotective effects of rosmarinic acid: insight into its mechanisms of action. Biomed Pharmacother. 2019;112:108600. doi: 10.1016/j.biopha.2019.108600. [DOI] [PubMed] [Google Scholar]
- 120.Domitrovic R, Potocnjak I, Crncevic-Orlic Z, Skoda M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem Toxicol. 2014;66:321–328. doi: 10.1016/j.fct.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Alam MA. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front Nutr. 2019;6:121. doi: 10.3389/fnut.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohnishi M, Matuo T, Tsuno T, Hosoda A, Nomura E, Taniguchi H, Sasaki H, Morishita H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors. 2004;21:315–319. doi: 10.1002/biof.552210161. [DOI] [PubMed] [Google Scholar]
- 123.Pereira P, de Oliveira PA, Patricia A, Rotta L, Henriques JAP, Picada JN. Neuropharmacological analysis of caffeic acid in rats. Basic Clin Pharmacol Toxicol. 2006;99:374–378. doi: 10.1111/j.1742-7843.2006.pto_533.x. [DOI] [PubMed] [Google Scholar]
- 124.Chao P, Hsu C, Yin M. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab. 2009;6:33. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meng S, Cao J, Feng Q, Peng J, Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complementary Altern Med. 2013;2013:801457. doi: 10.1155/2013/801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.dos Santos MD, Almeida MC, Lopes NP, de Souza GE. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29:2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 127.Aishwarya V, Sumathi T. Chrysin, a natural flavonoid attenuates cognitive dysfunction and neuronal loss associated with amyloid β(25-35)-induced oxidative stress: an experimental model of Alzheimer’s disease. Int J Pharmacogn Phytochem. 2015;7:224–236. [Google Scholar]
- 128.Samarghandian S, Azimi-Nezhad M, Borji A, Hasanzadeh M, Jabbari F, Farkhondeh T, Samini M. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn Mag. 2020;2:436–440. doi: 10.4103/0973-1296.191453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato A, Tamura H. High antiallergic activity of 5,6,4′-trihydroxy-7,8,3′-trimethoxyflavone and 5,6-dihydroxy-7,8,3′,4′-tetramethoxyflavone from eau de cologne mint (Mentha x piperita citrata) Fitoterapia. 2015;102:74–83. doi: 10.1016/j.fitote.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ, Hyun JW. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int J Oncol. 2017;51:1169–1178. doi: 10.3892/ijo.2017.4091. [DOI] [PubMed] [Google Scholar]
- 131.Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, Verri WA., Jr Naringenin reduces inflammatory pain in mice. Neuropharmacology. 2016;105:508–519. doi: 10.1016/j.neuropharm.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 132.Priscilla DH, Roy D, Suresh A, Kumar V, Thirumurugan K. Naringenin inhibits α-glucosidase activity: a promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem Biol Interact. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 133.Jin Y, Han X, Zhang Y, Lee J, Lim Y, Chung J, Yun Y. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-γ2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis. 2007;194:144–152. doi: 10.1016/j.atherosclerosis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 134.Alshatwi AA, Ramesh E, Periasamy VS, Subash-Babu P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam Clin Pharmacol. 2013;27:581–592. doi: 10.1111/j.1472-8206.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 135.Zhu GF, Guo HJ, Huang Y, Wu CT, Zhang XF. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp Ther Med. 2015;10:2259–2266. doi: 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y, Zhang R, Ni H. Eriodictyol exerts potent anticancer activity against A549 human lung cancer cell line by inducing mitochondrial-mediated apoptosis, G2/M cell cycle arrest. Arch Med Sci. 2020;16:446–452. doi: 10.5114/aoms.2019.85152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Costa LG, Garrick JM, Roque PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Perez-Vizcaino F, Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen TTT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 140.Alam W, Khan H, Shah MA, Cauli O, Saso L. Kaempferol as a dietary anti-inflammatory agent: current therapeutic standing. Molecules. 2020;25:4073. doi: 10.3390/molecules25184073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodius S, de Klein N, Jeanty C, Sanchez-Iranzo H, Crespo I, Ibberson M, Xenarios I, Dittmar G, Mercader N, Niclou SP, Azuaje F. Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Sci Rep. 2020;10:2896. doi: 10.1038/s41598-020-59894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu SH, Lin CH, Hung SK, Chou JH, Chi CW, Fu SL. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J Agric Food Chem. 2010;58:10831–10839. doi: 10.1021/jf1017093. [DOI] [PubMed] [Google Scholar]
- 143.Ahmadi SM, Farhoosh R, Sharif A, Rezaie M. Structure-antioxidant activity relationships of luteolin and catechin. J Food Sci. 2020;85:298–305. doi: 10.1111/1750-3841.14994. [DOI] [PubMed] [Google Scholar]
- 144.Abdulkhaleq LA, Assi MA, Noor MHM, Abdullah R, Saad MZ, Taufiq-Yap YH. Therapeutic uses of epicatechin in diabetes and cancer. Vet World. 2017;10:869–872. doi: 10.14202/vetworld.2017.869-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kpemissi M, Eklu-Gadegbeku K, Veerapur VP, Negru M, Taulescu M, Chandramohan V, Hiriyan J, Banakar SM, Nv T, Suhas DS, Puneeth TA, Vijayakumar S, Metowogo K, Aklikokou K. Nephroprotective effect of Combretum micranthum G. Don in nicotinamide-streptozotocin induced diabetic nephropathy in rats: in-vivo and in-silico experiments. Biomed Pharmacother. 2019;116:108961. doi: 10.1016/j.biopha.2019.108961. [DOI] [PubMed] [Google Scholar]
- 146.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res Rev Mutat Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 147.Banik K, Ranaware A, Chaudhary H, Thakur N, Girisa S, Deshpande V, Fan L, Nalawade SP, Sethi G, Kunnumakkara AB. Piceatannol: a natural stilbene for the prevention and treatment of cancer. Pharmacol Res. 2020;153:104635. doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- 148.Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 149.Chen M, Fu Q, Song X, Muhammad A, Jia R, Zou Y, Yin L, Li L, He C, Ye G, Lv C, Liang X, Huang J, Cui M, Yin Z. Pharmaceutical biology preparation of resveratrol dry suspension and its immunomodulatory and anti-inflammatory activity in mice. Pharm Biol. 2020;58:8–15. doi: 10.1080/13880209.2019.1699123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yin J, Tezuka Y, Subehan SL, Nobukawa M, Nobukawa T, Kadota S. In vivo anti-osteoporotic activity of isotaxiresinol, a lignan from wood of Taxus yunnanensis. Phytomedicine. 2006;13:37–42. doi: 10.1016/j.phymed.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 151.Banskota AH, Nguyen NT, Tezuka Y, Le Tran Q, Nobukawa T, Kurashige Y, Sasahara M, Kadota S. Secoisolariciresinol and isotaxiresinol inhibit tumor necrosis factor-α-dependent hepatic apoptosis in mice. Life Sci. 2004;74:2781–2792. doi: 10.1016/j.lfs.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 152.Kezimana P, Dmitriev AA, Kudryavtseva AV, Romanova EV, Melnikova NV. Secoisolariciresinol diglucoside of flaxseed and its metabolites: biosynthesis and potential for nutraceuticals. Front Genet. 2018;9:641. doi: 10.3389/fgene.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:1–15. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3. Biotech. 2013;3:439–459. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tapas A, Sakarkar D, Kakde R. Flavonoids as nutraceuticals: a review. Trop J Pharm Res. 2008;7:1089–1099. doi: 10.4314/tjpr.v7i3.14693. [DOI] [Google Scholar]
- 156.Ravishankar D, Rajora AK, Greco F, Osborn HMI. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 157.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 158.Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 159.Mencherini T, Picerno P, Scesa C, Aquino R. Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. J Nat Prod. 2007;70:1889–1894. doi: 10.1021/np070351s. [DOI] [PubMed] [Google Scholar]
- 160.Manzoor MF, Ahmad N, Manzoor A, Kalsoom A. Food based phytochemical luteolin their derivatives, sources and medicinal benefits. IJALS. 2017;3:195–207. [Google Scholar]
- 161.Breccia JD, Mazzaferro LS. Quantification of hesperidin in citrus-based foods using a fungal diglycosidase. Food Chem. 2012;134:2338–2344. doi: 10.1016/j.foodchem.2012.03.107. [DOI] [PubMed] [Google Scholar]
- 162.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bae J, Kim N, Shin Y, Kim S-Y, Kim Y-J. Activity of catechins and their applications. Biomed Dermatol. 2020;4:8. doi: 10.1186/s41702-020-0057-8. [DOI] [Google Scholar]
- 165.Eng Khoo H, Azlan A, Teng Tang S, Meng Lim S. Food & nutrition research anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orhan I, Ozcelik B, Kartal M, Aslan S, Sener B, Ozguven M. Quantification of daidzein, genistein and fatty acids in soybeans and soy sprouts, and some bioactivity studies. Acta Biol Crac Ser. 2007;Bot 49:61–68. [Google Scholar]
- 167.Murkovic M. Phenolic compounds. In: Caballero B, Finglas P, Toldra F, editors. Encyclopedia of Food Sciences and Nutrition. 2. Cambridge: Academic Press; 2003. pp. 4507–4514. [Google Scholar]
- 168.Prasain JK, Barnes S, Wyss JM. Analyzing ingredients in dietary supplements and their metabolites. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols: Mechanisms of Action in Human Health and Disease. 2. Cambridge: Academic Press; 2018. pp. 337–346. [Google Scholar]
- 169.Asakura H, Kitahora T. Antioxidants and polyphenols in inflammatory bowel disease: ulcerative colitis and crohn disease. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols: Prevention and Treatment of Human Disease. 2. Cambridge: Academic Press; 2018. pp. 279–292. [Google Scholar]
- 170.Bonetti F, Brombo G, Zuliani G. Nootropics, functional foods, and dietary patterns for prevention of cognitive decline. In: Watson RR, editor. Nutrition and Functional Foods for Healthy Aging. Cambridge: Academic Press; 2017. pp. 211–232. [Google Scholar]
- 171.Shin EK, Kwon HS, Kim YH, Shin HK, Kim JK. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun. 2009;381:502–507. doi: 10.1016/j.bbrc.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 172.Leuzzi U, Caristi C, Panzera V, Licandro G. Flavonoids in pigmented orange juice and second-pressure extracts. J Agric Food Chem. 2000;8:5501–5506. doi: 10.1021/jf000538o. [DOI] [PubMed] [Google Scholar]
- 173.Cortell JM, Kennedy JA. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot noir fruit and extraction in a model system. J Agric Food Chem. 2006;54:8510–8520. doi: 10.1021/jf0616560. [DOI] [PubMed] [Google Scholar]
- 174.Arts ICW, Van De Putte B, Hollman PCH. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J Agric Food Chem. 2000;48:1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 175.Guyot S, Marnet N, Drilleau JF. Thiolysis—HPLC characterization of apple procyanidins covering a large range of polymerization states. J Agric Food Chem. 2001;49:14–20. doi: 10.1021/jf000814z. [DOI] [PubMed] [Google Scholar]
- 176.Rasmussen SE, Frederiksen H, Krogholm KS, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutr Food Res. 2005;9:159–174. doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 177.Mazza G, Cacace JE, Kay CD. Methods of analysis for anthocyanins in plants and biological fluids. J AOAC Int. 2004;87:129–145. doi: 10.1093/jaoac/87.1.129. [DOI] [PubMed] [Google Scholar]
- 178.Brouillard R, George F, Fougerousse A. Polyphenols produced during red wine ageing. BioFactors. 1997;6:403–410. doi: 10.1002/biof.5520060406. [DOI] [PubMed] [Google Scholar]
- 179.Es-Safi NE, Cheynier V, Moutounet M. Interactions between cyanidin 3-O-glucoside and furfural derivatives and their impact on food color changes. J Agric Food Chem. 2002;50:5586–5595. doi: 10.1021/jf025504q. [DOI] [PubMed] [Google Scholar]
- 180.Mazza G. Anthocyanins in fruits, vegetables, and grains. 1. Boca Raton: CRC Press; 1993. [Google Scholar]
- 181.Reinli K, Block G. Phytoestrogen content of foods—a compendium of literature values. Nutr Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 182.Eisen B, Ungar Y, Shimoni E. Stability of isoflavones in soy milk stored at elevated and ambient temperatures. J Agric Food Chem. 2003;51:2212–2215. doi: 10.1021/jf025783h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available upon request.


