Abstract
Background
Persistent apnea despite an adequate rise in arterial pressure of CO2 is an essential component of the criteria for brain death (BD) determination. Current guidelines vary regarding the utility of arterial pH changes during the apnea test (AT). We aimed to study the effect of incorporating an arterial pH target < 7.30 during the AT (in addition to the existing PaCO2 threshold) on brain death declarations.
Methods
We performed retrospective analysis of consecutive adult patients who were diagnosed with BD and underwent AT at the Cleveland Clinic over the last 10 years. Data regarding baseline and post-AT blood gas analyses were collected and analyzed.
Results
Ninety-eight patients underwent AT in the study period, which was positive in 89 (91%) and inconclusive in 9 (9%) patients. The mean age was 50 years old (standard deviation [SD] 16) and 54 (55%) were female. The most common etiology BD was hypoxic ischemic brain injury (HIBI) due to cardiac arrest (42%). Compared to those with positive AT, patients with inconclusive AT had a higher post-AT pH (7.24 vs 7.17, p = 0.01), lower PaO2 (47 vs 145, p < 0.01), and a lower PaCO2 (55 vs 73, p = 0.01). Among patients with a positive AT using PaCO2 threshold alone, the frequency of patients with post-AT pH < 7.30 was 95% (83/87).
Conclusion
Implementing a BD criteria requiring both arterial pH and PaCO2 thresholds reduced the total number of positive ATs; these inconclusive cases would have required longer duration of AT to reach both targets, repeated ATs, or ancillary studies to confirm BD. The impact of this on the overall number BD declarations requires further research.
Keywords: Apnea test, Brain death, pH, Respiratory drive
Introduction
Brain death (BD), or death by neurological criteria (DNC), is defined by the American Academy of Neurology (AAN) as the cessation of all functions of the brain, including the brainstem [1]. The clinical determination of BD as guided by the AAN has to fulfill three core criteria in the absence of confounders: an irreversible coma of a known cause, a clinical examination consistent with loss of all brain function (including the brainstem), and apnea [1]. The apnea test (AT), usually the last step in BD determination, is diagnostic of BD if a rise of partial pressure arterial of CO2 (PaCO2) more than 60 mm Hg (or 20 mm Hg above baseline) fails to stimulate the respiratory drive. The rise of PaCO2 is most commonly induced via disconnecting the patient from the ventilator while maintaining adequate oxygenation by inserting an O2 cannula in the endotracheal tube (oxygen diffusion method) or by other methods [1].
There exists a significant variability regarding BD determination in general, particularly when it comes to the details of the AT [2]. For example, although following arterial pH is recommended before and after the AT, guidelines in the USA and the UK depend solely on a PaCO2 threshold, with no specific recommendations regarding changes in arterial pH [3]. On the other hand, Canadian and Australian/New Zealand guidelines require certain pH thresholds (pH ≤ 7.28 in Canada and < 7.3 in Australia/New Zealand) at the end of the AT in order to confirm BD, in addition to the PaCO2 threshold [4, 5]. Most recently, an international expert panel formulated multiple consensus statements on the recommendations on determination of BD and suggested that the targets during the AT be pH less than 7.30 and PaCO2 of at least 60 mm Hg [6].
In this study, we retrospectively studied brain dead patients who underwent AT in order to investigate the effect of incorporating an arterial pH target < 7.30 during the AT for BD determinations by answering 2 key questions: (1) How often is the pH < 7.30 at the conclusion of a positive AT? and (2) how often is the pH < 7.30 at the end of an inconclusive AT with inadequate CO2 rise? We secondarily aimed to study the frequency of AT complications and use of ancillary studies.
Methods
Study design and setting
This retrospective observational study was approved by the Cleveland Clinic local institutional review board (IRB 20-335). Written informed consent was waived given the retrospective nature of the study. Using International Classification of Diseases codes for BD, we obtained medical records of consecutive adult patients (≥ 18 years old) who were declared brain dead and underwent the AT at the Cleveland Clinic Health System (CCHS) over a 10-year period (January 2010 to January 2020).
At our institution, determination of BD follows the 2010 AAN guidelines and requires the clinical determination of irreversible neurological injury, an examination consistent with coma and absent brainstem reflexes, and a positive AT. Confounders such as severe hypotension, hypothermia, hypoxia, severe acid-base abnormalities, and other severe metabolic or endocrine abnormalities have to be excluded and corrected prior to BD declaration. All physicians who participate in the declaration of DNC must complete competency training (usually via an online training course) prior to evaluating patients for DNC, and competency must be certified at least every 5 years. The institutional policy requires two consistent examinations performed by two DNC-trained physicians or one trained physician doing two exams separated in time, with no defined time mandated between the two examinations. The AT is performed using the oxygen diffusion technique. Patients are preoxygenated on the ventilator 100% FiO2 for 10 min after which a baseline arterial blood gas (ABG) is drawn and the patient is disconnected from the ventilator. Oxygen is then supplied through a tracheal cannula connected to 4–6 L per minute (Lpm) oxygen source, and the patient is carefully monitored for respiratory effort and hemodynamic stability for 5–10 min, after which another ABG is drawn. Repeat ABGs can be done every 5 min after that until goal PaCO2 is reached if the patient remains hemodynamically stable. If PaCO2 rises to above 60 mm Hg (or 20 mm Hg above baseline) and the patient displays no spontaneous respiratory effort during the AT, the AT is considered positive and the patient declared brain dead. Of note, an arterial pH target is not required at our institution. Ancillary studies are mandated only if complete clinical examination (including AT) cannot be performed or interpreted with complete certainty. If ancillary testing is required, the policy does not mandate a specific number of ancillary studies as one confirmatory study is generally considered acceptable according to AAN guidelines; however, more than one study could be performed at the physician’s discretion.
Study population
Included patients were ≥ 18 years old, had a documented clinical examination consistent with BD along with a known irreversible cause of coma, underwent the AT, and declared brain-dead via a positive AT, ancillary studies, or both. Formal BD assessment had to be clearly documented for inclusion. Patients were excluded if age < 18, BD declared using clinical exam alone, or if BD was declared using ancillary studies without undergoing the AT. Patients were included if they completed an inconclusive or complicated AT only if they underwent ancillary studies confirming BD. After obtaining patients’ charts, we screened all patients for the above and exclusion criteria. Each patient’s chart, including imaging studies, were reviewed independently by two investigators (I.M. and M.A.) prior to inclusion. Disagreements were resolved through consensus.
Data collection and reporting
Data were gathered on patients’ demographics (age at time of BD, sex), precipitant and primary etiologies of BD, timing of BD declaration, details of clinical examination, presence of confounders, details of AT including ABG changes, use and results of ancillary studies. If performed, we reviewed radiologic reports and imagines of brain computed tomography (CT), magnetic resonance imaging and angiography (MRI, MRA), cerebral digital subtraction angiography (DSA), transcranial Doppler ultrasounds (TCDs), and single photon emission computed tomography (SPECT). Additionally, reports of electroencephalogram (EEG) were reviewed.
Positive AT is defined according to the AAN guidelines as persistent absence of breathing drive despite an increase of PaCO2 to > 60 mm Hg or a 20 mm Hg above baseline in the absence of confounders. The AT is considered inconclusive due to the presence of significant confounders, inadequate CO2 rise or development of hypoxia, hypotension, or arrhythmia precluding the completion of the AT. A negative AT constitutes a return of spontaneous respirations during the AT [1]. Ancillary studies were defined as tests of cerebral blood flow or bioelectrical activity supporting the diagnosis of BD as recommended by AAN guidelines [1]. An ancillary study was considered positive if the findings were supportive of brain death, negative if findings are not supportive of brain death, and inconclusive if reliable interpretation of the test was not possible, commonly due to technical reasons (such as artifact on EEG or hyperostosis for TCD). A positive EEG for BD demonstrates electrocerebral silence with no non-artifactual electrical activity > 2 microvolts during a 30-min recording; a positive TCD demonstrates small systolic peaks with absent diastolic flow or a reverberating flow pattern in anterior and posterior circulation bilaterally (if no flow is seen on TCD, the test is considered inconclusive); a positive DSA demonstrates absence of blood flow at the level of entry of the carotid and vertebral arteries to the skull; a positive SPECT demonstrates no cerebral isotope uptake on both anterior and lateral views, including the brainstem; a positive CTA similarly demonstrates no opacification of intracranial vessels.
Data synthesis and outcomes
The primary objective of this study was to investigate the effect of incorporating an arterial pH target < 7.30 during the AT for BD determinations by answer the following two key questions: (1) how often is the pH < 7.30 at the conclusion of a positive AT (defined as PaCO2 > 60 or > 20 mm Hg above baseline)? and (2) how often is the pH < 7.30 at the end of an inconclusive AT due to inadequate CO2 rise? In order to answer the first question, we divided the number of brain-dead patients with a post-AT pH < 7.30 by the total number of patients who underwent a positive AT according to current AAN guidelines (post-AT PaCO2 > 60 mm Hg or > 20 above baseline). To answer the second question, we the divided the number of patients with post-AT pH < 7.30 by the number of patients with an inconclusive AT due inadequate PaCO2 rise
We secondarily aimed to report the frequency of AT complications and use of ancillary studies.
Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD) as the data were normally distributed. Categorical data were expressed as number and percentage. For comparative analysis, we used Student t test for continuous parametric data, Mann-Whitney U or Wilcoxon tests for continuous nonparametric data, and Fisher’s exact or Pearson’s chi-square for categorical data as appropriate. A two-sided p value < 0.05 was considered statistically significant.
Results
Patients’ characteristics
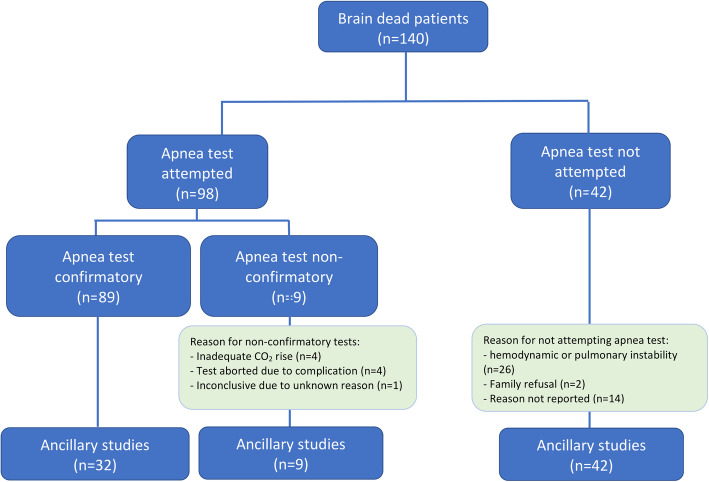
Of the 140 patients who were declared brain dead at the Cleveland Clinic Health System between 2010 and 2020, 98 (70%) underwent the AT (meeting our inclusion criteria) and the rest (n = 42, 30%) were declared brain-dead using ancillary tests (Fig. 1). The AT was deemed positive in 89 patients (91%) and inconclusive in 9 patients (9%) (Table 1). None of the patients had a negative AT. Among all patients who underwent the AT, the mean age was 50 years old (± 16) and 54 (55%) were female. The primary etiology of BD was hypoxic ischemic brain injury (HIBI) due to cardiac arrest in 41 patients (42%), nontraumatic intracranial hemorrhage in 29 patients (30%), acute ischemic stroke in 11 patients (11%), traumatic brain injury in 9 patients (9%), and cerebral edema due to other causes in 5 patients (5%; 2 due to intracranial malignancy, 1 due to hemodialysis, 1 due to status epilepticus, and 1 due to lupus cerebritis). Three patients (3%) developed cardiac arrest and were found to have evidence of intracranial hemorrhage and later developed HIBI. All patients had two separate clinical examinations confirming coma and absent brainstem reflexes; none of the patients had conflicting clinical exams. The mean interval time between the two exams was 7 h (± 9), which was longer among patients with inconclusive AT compared to those with positive AT (6 vs. 18 h, p = 0.03). Reasons for inconclusive AT were inadequate CO2 rise in 4 patients (4% of all ATs), inability to complete the AT due to hemodynamic complications in 4 patients (4% of all AT%) and inconclusive results due to unknown reason in 1 patient (1%). Among the 4 patients with inadequate CO2 rise, 3 did not tolerate the AT longer than 8–10 min because of hypotension and desaturation; thus, continuing the AT to allow PaCO2 to reach target was not attempted; the fourth patient did not achieve adequate PaCO2 rise (41 mm Hg to 51) despite performing the AT for 12 min and a subsequent drop of the pH from 7.32 to 7.23. In the majority of patients, the duration of the AT was documented to be between 8 and 10 min and to follow institutional guidelines, though there was significant difficulty elucidating the actual duration from the charts.
Fig. 1.
Flow chart of selected patients
Table 1.
Characteristics and blood gas changes in 98 patients undergoing apnea test
| Variable | All patients (n = 98) | Positive AT (n = 89) | Inconclusive AT (n = 9) | P value |
|---|---|---|---|---|
| Age (years), mean (±SD) | 50 (± 16) | 50 (± 16) | 52 (± 18) | 0.80 |
| Female, n (%) | 54 (55) | 47 (53) | 7 (89) | 0.18 |
| Primary etiology of BD, n (%) | ||||
| HIBI | 41 (42) | 35 (40) | 6 (67) | 0.31 |
| Nontraumatic ICH | 29 (30) | 29 (33) | 1 (11) | 0.26 |
| HIBI + Nontraumatic ICH | 3 (3) | 3 (3) | 0 | 1 |
| AIS | 11 (11) | 9 (10) | 2 (22) | 0.27 |
| TBI | 9 (9) | 9 (10) | 0 | 1 |
| Other cerebral edema | 5 (5) | 4 (4) | 1 (11) | 0.39 |
| Malignancy | 0 | 2 | 0 | |
| Status epilepticus | 0 | 1 | 0 | |
| Lupus cerebritis | 0 | 0 | 1 | |
| Hemodialysis | 0 | 1 | 0 | |
| Duration between clinical exams (hours), mean (±SD) | 7 (± 9) | 6 (± 9) | 18 (± 11) | 0.03 |
| Mean BP at the beginning of AT (mm Hg), mean (±SD) | ||||
| SBP | 127 (± 28) | 128 (± 28) | 113 (± 31) | 0.49 |
| DBP | 72 (± 16) | 73 (± 16) | 65 (± 17) | 0.50 |
| MAP | 90 ± 18 | 91 (± 18) | 81 (± 21) | 0.50 |
| Temperature (°C), mean (±SD) | 36.9 ± 0.6 | 36.9 (± 0.7) | 36.9 (± 0.3) | 0.79 |
| Vasopressor support, n (%) | 78 (80) | 71 (80) | 7 (78) | 1 |
| Pre-AT blood gas (mm Hg), mean (±SD) | ||||
| pH | 7.38 ± 0.06 | 7.38 ± 0.06 | 7.37 ± 0.04 | 0.66 |
| PaO2 | 242 ± 130 | 246 ± 130 | 187 ± 127 | 0.37 |
| PaCO2 | 39 ± 5 | 39 ± 5 | 37 ± 5 | 0.37 |
| Post-AT blood gas (mm Hg), mean (±SD) | ||||
| pH | 7.17 ± 0.07 | 7.17 ± 0.07 | 7.24 ± 0.04 | 0.01 |
| PaO2 | 234 ± 125 | 145 ± 120 | 47 ± 17 | < 0.01 |
| PaCO2 | 72 ± 13 | 73 ± 12 | 55 ± 9 | 0.01 |
| Reasons for inconclusive test, n (%) | ||||
| Inadequate CO2 rise | 4 (4) | NA | 4 (44) | |
| Inability to complete the test due to complication | 4 (4) | NA | 4 (44) | |
| Unclear | 1 (1) | NA | 1 (11) | |
| Ancillary studies, total n (%) | 49 | 38 | 11 | < 0.01 |
| TCD | 18 (37) | 16 (42) | 2 (18) | 0.18 |
| SPECT | 14 (29) | 11 (29) | 3 (27) | 1 |
| EEG | 15 (30) | 10 (26) | 5 (45) | 0.28 |
| CTA | 2 (4) | 1 (3) | 1 (9) | 0.40 |
AT apnea test, SD standard deviation, BD brain death, HIBI hypoxic ischemic brain injury, ICH intracranial hemorrhage, AIS acute ischemic stroke, TBI traumatic brain injury, BP blood pressure, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, PaO2 partial pressure of arterial O2, PaCO2 partial pressure of arterial CO2, TCD transcranial Doppler, SPECT single photon emission computed tomography, EEG electroencephalogram, CTA computed tomography angiography
Physiological changes during the AT
Blood pressure values at the beginning of AT were available in 74 patients (75%); mean SBP, DBP, and MAP (mmHg) were 127 (± 28), 72 (± 16), and 90 (± 18), respectively. Body temperature at the time of AT was available in 87 patients (89%) and the mean temperature was 36.9 °C (± 0.6). Seventy-eight patients (80%) were on vasopressor at the time of AT. There was no significant difference in mean SBP, DBP, MAP, temperature, or vasopressor support between patients with positive and inconclusive tests (Table 1).
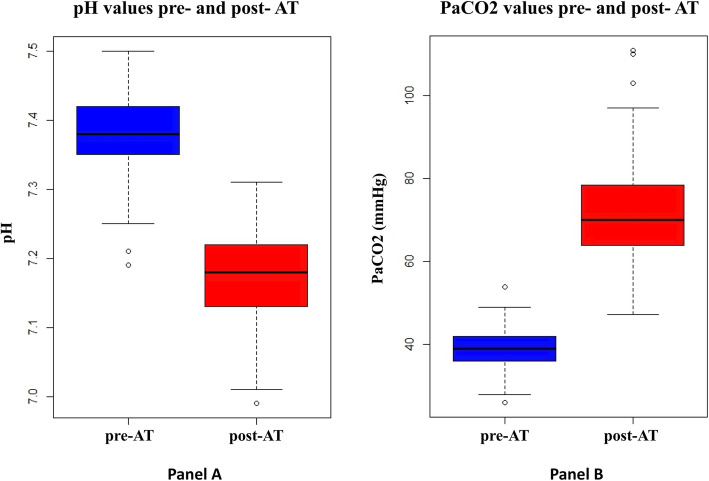
Pre- and post-AT ABGs were available for 91/98 patients (93%). In the remaining 7 patients, the outcome of the AT (2 positive, 4 complicated and 1 inconclusive due to unknown reason) was documented in the chart but not all ABG values were available for review. The mean pre-AT pH, PaO2, and PaCO2 for all patients were 7.38 (± 0.06), 243 mm Hg (± 130) and 44 mm Hg (± 6) respectively. The mean post-AT pH, PaO2, and PaCO2 among all patients were 7.17 (± 0.07), 234 mm Hg (± 125), and 72 mm Hg (± 13), respectively (Fig. 2). Compared to those with positive AT, patients with inconclusive AT had a higher post-AT pH (7.24 vs 7.17, p = 0.01), lower PaO2 (47 vs 145, p < 0.01), and a lower PaCO2 (55 vs 73, p = 0.01) (Table 1).
Fig. 2.
Box plots of PaCO2 and pH values before and after the apnea test. a pH values pre-AT (median = 7.38, IQR = 0.07) and post-AT (median = 7.18, IQR = 0.09). b PaCO2 values pre-AT (median = 39.0, IQR = 5.8) and post-AT (median = 70.0, IQR = 14.3). Abbreviations: PaCO2, partial pressure of arterial CO2; AT, apnea test; IQR, interquartile range
Outcomes
Among patients with a positive AT using PaCO2 threshold according to current AAN guidelines, the frequency of patients with post-AT pH < 7.30 was 95% (83/87) (Table 2). Among those with an inconclusive test due to inadequate CO2 rise, the frequency of patients with post-AT pH < 7.30 was 75% (3/4).
Table 2.
Relationship between arterial pH and PaCO2 at the conclusion of apnea test
| Value at end of AT | PaCO2 > 60 or > 20 mm Hg above baseline (positive AT) ‡, n | PaCO2 < 60 or < 20 mm Hg above baseline (inconclusive AT) ‡, n |
|---|---|---|
| pH < 7.30 | 83 | 3 |
| pH ≥ 7.30 | 4 | 1 |
| Total | 87 | 4 |
AT apnea test, BD brain death
‡Seven patients were excluded from analysis (4 due aborted AT due to complication, and 3 due unavailable pH data). Patients with inconclusive AT underwent ancillary studies to confirm BD
Collectively, complications of the AT due to hypoxia, hypotension, or arrhythmia occurred in 9 patients (9%), 4 of which (4% of all ATs) resulted in early termination of AT. Among patients with a positive AT, 32/89 patients (36%) underwent a total of 38 ancillary studies (16 TCD, 11 SPECT, 10 EEG, 1 CTA; 4 patients underwent two ancillary tests). All 9 patients (100%) with inconclusive AT underwent a total of 11 ancillary studies (5 EEG, 3 SPECT, 2 TCD and 1 CTA; 2 patients underwent two ancillary studies).
Discussion
In this retrospective study involving a large number of patients who underwent AT and eventually diagnosed with BD in compliance with the 2010 AAN guidelines, arterial pH < 7.30 in 95% of those with a positive AT. In other words, implementing new BD guidelines that require both an arterial pH threshold of < 7.30 in addition to PaCO2 > 60 or > 20 mm Hg above baseline (as recommended by the World Brain Death Project Consensus Statement) [6] would mean that 5% of patients who were declared BD using AT relying on PaCO2 thresholds alone would not immediately be declared BD using clinical criteria if both pH and PaCO2 threshold were to be used, and their AT would either need to be repeated, prolonged, or an ancillary study to be used. In other words, if such a criteria had been in place, it is possible that clinicians would perform the AT for a longer period of time until reaching target pH and PaCO2, repeat the AT, or confirm BD with ancillary studies. This may not cause any change in the total number of BD declarations, since those patients may still be diagnosed with BD using a repeated AT or ancillary studies. Interestingly, among 4 patients who were persistently apneic during AT despite an inadequate PaCO2 rise, 3 had pH < 7.30, raising the question whether low arterial pH alone can be relied upon to assess respiratory drive; however, the small number patients with this ABG profile limits any overreaching conclusions.
The ABG results in our cohort is consistent with recent studies of ABG changes during AT. In a recent analysis of 114 brain dead patients who underwent the AT at the Mayo Clinic, the pre-AT median arterial pH was 7.38 (interquartile range [IQR] 1.0) the post-AT median pH of 7.18 (IQR 0.09) [7]. An earlier analysis of 63 patients who underwent the apnea showed a pre-AT median arterial pH of 7.36 (IQR 1.0) and post-AT pH of 7.19 (IQR 1.0) [8]. However, these studies have not examined the use of post-AT arterial pH as threshold for confirming apnea. Although the most recent AAN guidelines (in 2010) did not incorporate arterial pH into the criteria for a positive AT, as discussed above, Canadian and Australian/New Zealand guidelines require certain pH thresholds (pH ≤ 7.28 in Canada and < 7.30 in Australia/New Zealand) at the end of the AT in order to confirm BD, in addition to the PaCO2 threshold [4, 5]. There have been recent calls for a worldwide standardization of BD determination, including incorporating an arterial pH threshold during the AT for during BD determinations [6].
The utility of arterial pH during AT has a strong physiological basis. The goal of the AT is to stimulate the respiratory drive by increasing PaCO2; however, in addition to PaCO2, pH has an essential role in stimulating the respiratory drive. First, peripheral chemoreceptors in the carotid and aortic bodies function to detect the PaO2 in the blood. Once stimulated by hypoxia, neuronal signals are sent to medullary nuclei to stimulate the respiratory drive and improve oxygenation in a pH-dependent manner (i.e., the sensitivity of peripheral chemoreceptors to low PaO2 is increased by acidosis) [9]. Second, the central chemoreceptors in the lower brainstem (medulla) are activated primarily in response to direct effects of local H+ on pH-sensing proteins, which changes in response to changes in PaCO2 and arterial pH [10]. Due to the enzyme carbonic anhydrase, CO2 is in equilibrium with H+, and the effect of hypercapnia on the breathing drive is largely mediated through changes in local pH around these pH-sensing neurons [10]. In addition to the effect of hypercapnic acidosis on the respiratory drive, several in vitro studies showed that local “isocapnic acidosis” (low pH in the setting of normal pressure of CO2) can result in a similar magnitude of respiratory drive stimulation [11, 12], and in vivo animal studies utilizing microinjections of acetazolamide (a carbonic anhydrase inhibitor inducing local acidosis) into different areas of the brainstem increases ventilation [13, 14]. Similarly, changes in cerebrospinal fluid (CSF) pH from 7.30 to 7.25 were shown to double the rate of alveolar ventilation [15]. One could argue that changes in arterial pH are not necessarily reflected in CSF pH and thus may not directly stimulate the respiratory drive; however, several human studies showed a predictable change in CSF pH compared to arterial pH, resulting in CSF pH ranging between 0.02 and 0.10 units below that of the arterial blood [16]. This concept is the primary mechanism behind the respiratory compensation (manifesting as hyperventilation) in response to metabolic acidosis. However, there are several unanswered questions regarding the use of pH in AT; the lowest pH at which a person has the potential to breathe has not been clearly defined; and changes in arterial pH alone may not reflect fast enough on CSF pH to stimulate the respiratory drive within the duration of the AT. Whether there are clinically brain-dead patients who are apneic at PaCO2 > 60 but start breathing when pH is lower than 7.30 (or other suggested targets) has not been well-studied. Additionally, the effect of a pH criterion on AT outcomes in patients on extracorporeal membrane oxygenation remains to be investigated [17].
This study is motivated by a strong physiological basis for using arterial pH to aid in clinical determination of BD, as discussed above. Another strength of the study is the large number of patients who underwent AT, which improves our understanding of ABG changes. The major limitation of our study is its retrospective nature, which renders it prone to recall and misclassification bias although the electronic charts were carefully assessed to ensure BD was diagnosed according to AAN guidelines in order to limit the possibility of bias. The impact of incorporating pH targets during the AT is better assessed prospectively. Additionally, there is a significant variability of BD practice worldwide [6], which limits the generalizability of findings from this single-center study.
Conclusion
During AT for BD determination, requiring both arterial pH and PaCO2 thresholds resulted in 5% decrease in the frequency of patients with positive AT; these cases would have required either longer period of time until reaching target pH and PaCO2, repetition of the AT, or confirmation of BD with ancillary studies. The impact of this on the overall number of BD declarations requires further study.
Acknowledgements
Not applicable
Abbreviations
- BD
Brain death
- DNC
Death by neurological criteria
- AAN
American Academy of Neurology
- AT
Apnea test
- CCHS
Cleveland Clinic Health System
- ABG
Arterial blood gas
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- MRA
Magnetic resonance angiography
- DSA
Digital subtraction angiography
- TCD
Transcranial Doppler
- SPECT
Single photon emission computed tomography
- EEG
Electroencephalogram
- SD
Standard deviation
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- MAP
Mean arterial pressure
- IQR
Interquartile range
- CSF
Cerebral spinal fluid
Authors’ contributions
IM designed and conceptualized the study, contributed to the data acquisition, analyzed the data, interpreted the data, and drafted the manuscript for intellectual content; MA contributed to the data acquisition, interpreted the data, and revised the manuscript for intellectual content; AS contributed to the data acquisition and revised the manuscript for intellectual content; CH interpreted the data and revised the manuscript for intellectual content; SMC interpreted the data and revised the manuscript for intellectual content; PG interpreted the data and revised the manuscript for intellectual content; ARG conceptualized study, interpreted the data, and revised the manuscript for intellectual content. The authors read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
The data supporting the conclusions of this article are available upon request from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This retrospective observational study was approved by the Cleveland Clinic local institutional review board (IRB 20-335). Written informed consent was waived given the retrospective nature of the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wijdicks EFM, Varelas PN, Gronseth GS, Greer DM, American Academy of Neurology Evidence-based guideline update: determining brain death in adults: report of the quality standards Subcommittee of the American Academy of neurology. Neurology. 2010;74:1911–1918. doi: 10.1212/WNL.0b013e3181e242a8. [DOI] [PubMed] [Google Scholar]
- 2.Greer DM, Wang HH, Robinson JD, et al. Variability of brain death policies in the United States. JAMA Neurol. 2016;73:213–218. doi: 10.1001/jamaneurol.2015.3943. [DOI] [PubMed] [Google Scholar]
- 3.Academy of Medical royal Colleges. A code of practice for the diagnosis and confirmation of death 2 a code of practice for the diagnosis and confirmation of death a code of practice for the diagnosis and confirmation of death contents. (2008). Available at: aomrc.org.uk/wp-content/uploads/2016/04/Code_Practice_Confirmation_Diagnosis_Death_1008-4.pdf. (Accessed: 10th May 2020).
- 4.Shemie SD, Doig C, Dickens B, et al. Brain arrest: the neurological determination of death and organ donor management in Canada. Can Med Assoc J. 2006;174:1–12. doi: 10.1503/cmaj.045142. [DOI] [Google Scholar]
- 5.Australian and New Zealand Intensive Care Society (ANZICS) The ANZICS statement on death and organ donation. (2013). Available at: https://csds.qld.edu.au/sdc/Provectus/ELI/Module 2 - Organ donation after brain death/files/ANZICS Statement on Death and Organ Donation Edition 3.2 (3).pdf. (Accessed: 10th May 2020).
- 6.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the world brain death project. JAMA. 2020. 10.1001/jama.2020.11586. [DOI] [PubMed]
- 7.Daneshmand A, Rabinstein AA, Wijdicks EFM. The apnea test in brain death determination using oxygen diffusion method remains safe. Neurology. 2019;92:386–387. doi: 10.1212/WNL.0000000000006963. [DOI] [PubMed] [Google Scholar]
- 8.Datar S, Fugate J, Rabinstein A, Couillard P, Wijdicks EFM. Completing the apnea test: decline in complications. Neurocrit Care. 2014;21:392–396. doi: 10.1007/s12028-014-9958-y. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron. 2015;87:946–961. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and PCO2. J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphé. J Appl Physiol. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/S0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 15.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol. 2012;2:221–254. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siesjo BK. The regulation of cerebrospinal fluid pH. Kidney Int. 1972;1. [DOI] [PubMed]
- 17.Migdady I, Stephens RS, Price C, et al. The use of apnea test and brain death determination in patients on extracorporeal membrane oxygenation: a systematic review. J Thorac Cardiovasc Surg. 2020. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are available upon request from the corresponding author upon reasonable request.