Abstract
Introduction
Therapeutic drug monitoring (TDM) for personalized dosing of fluoroquinolones has been recommended to optimize efficacy and reduce acquired drug resistance in the treatment of MDR TB. Therefore, the aim of this study was to develop a simple, low-cost, robust assay for TDM using mobile UV/visible light (UV/VIS) spectrophotometry to quantify levofloxacin in human saliva at the point of care for TB endemic settings.
Methods
All experiments were performed on a mobile UV/VIS spectrophotometer. The levofloxacin concentration was quantified by using the amplitude of the second-order spectrum between 300 and 400 nm of seven calibrators. The concentration of spiked samples was calculated from the spectrum amplitude using linear regression. The method was validated for selectivity, specificity, linearity, accuracy and precision. Drugs frequently co-administered were tested for interference.
Results
The calibration curve was linear over a range of 2.5–50.0 mg/L for levofloxacin, with a correlation coefficient of 0.997. Calculated accuracy ranged from –5.2% to 2.4%. Overall precision ranged from 2.1% to 16.1%. Application of the Savitsky–Golay method reduced the effect of interferents on the quantitation of levofloxacin. Although rifampicin and pyrazinamide showed analytical interference at the lower limit of quantitation of levofloxacin concentrations, this interference had no implication on decisions regarding the levofloxacin dose.
Conclusions
A simple UV/VIS spectrophotometric method to quantify levofloxacin in saliva using a mobile nanophotometer has been validated. This method can be evaluated in programmatic settings to identify patients with low levofloxacin drug exposure to trigger personalized dose adjustment.
Introduction
TB remains one of the major infectious diseases worldwide, with an estimated number of 10.0 million new cases in 2018, and is the leading killer from a single pathogen.1 Driving that mortality is rifampicin-resistant (RR)/MDR-TB, with an estimated 484 000 new patients in 2018.1 The multidrug regimen required to treat RR/MDR-TB is less efficacious than that used for drug-susceptible TB. Furthermore, the duration is extended from 9 to as long as 20 months, which represents a burden to both patients and the staff and systems within programmes delivering MDR-TB care.2
Moxifloxacin and levofloxacin, the two fluoroquinolones listed as Group A drugs in the WHO consolidated guideline for the treatment of MDR-TB, are the drugs of first choice in combination with bedaquiline and linezolid.2 The role of fluoroquinolones is important to prevent acquired resistance in bedaquiline-based shorter all-oral MDR-TB regimens.3 Despite being very active drugs, low fluoroquinolone drug exposure is associated with a lower treatment response and acquired drug resistance.4 In a large prospective cohort of 832 patients without baseline fluoroquinolone resistance, 11.2% acquired resistance to fluoroquinolones despite good adherence.5 Suboptimal moxifloxacin pharmacokinetics may be of particular concern, as only 40% of patients given recommended doses achieve drug concentrations that suppress drug resistance.6,7 Similarly, MDR-TB regimens that give higher doses of fluoroquinolones have been associated with improved outcomes.8 Furthermore, pharmacokinetic/pharmacodynamic (PK/PD) studies of moxifloxacin and levofloxacin in pre-clinical models, such as the hollow fibre infection model, have generated clinically achievable PK/PD serum targets that predict bactericidal activity and prevention of acquired resistance.9
Considering that PK/PD targets exist for levofloxacin and moxifloxacin, PK variability has been substantial in multiple clinical studies of people being treated for TB,10,11 and higher dosages have been explored to increase drug exposure to improve outcomes;12,13 we therefore argue that fluoroquinolones represent an ideal drug class for therapeutic drug monitoring (TDM) and personalized dose adjustment to optimize the MDR-TB regimen.2,14,15
Currently, TDM by LC–MS/MS has become the analytical method of choice for quantitation of analytes in biological matrices,16 but the use of TDM has been restricted to low TB burden regions with access to personnel, sample shipment procedures and equipment necessary to quantify serum drug exposure.14,17,18
Although TDM for TB treatment has been recommended for almost two decades,19 the financial and logistical challenges of TDM implementation have limited its widespread use.14,20 We and others have previously argued that TDM represents a critical tool in the ‘End TB’ strategies,18 especially to limit the amplification and transmission of drug resistance. Treatment should be personalized, and person-centred care can be provided by measuring drug exposure and subsequently individualizing the dose.21 While different approaches to implementation may be needed in different settings, the ability to have a semi-quantitative screening test for key drugs such as fluoroquinolones at the community level could then free resources for quantitative measurement of key drugs in selected patients at a regional or central level.22Semi-quantitative screening of levofloxacin in saliva to detect patients with unacceptably low or high concentrations seems feasible based on a study comparing plasma and saliva concentrations.23
Two alternative matrixes have been explored for the semi-quantitative measurement of drug exposure; oral fluid (saliva) and urine. Although these techniques have their limitations as penetration in oral fluid or renal excretion are prerequisites for these tests to be potentially useful, a major advantage is non-invasive sample collection.23–29 As most of the anti-TB drugs including fluoroquinolones have a UV spectrum and are present in the mg/L range, mobile microvolume UV/visible light (VIS) spectrophotometers may be suitable for measuring drug concentrations in saliva and in urine. These devices tend to be user friendly and require a minimum of laboratory skills, which could deliver TDM to a large group of patients that otherwise would not have benefited from traditional TDM programmes.30 The aim of this study was therefore to develop a simple, low-cost, robust assay using mobile spectrophotometry to quantify levofloxacin in human saliva that would be applicable for TDM in TB endemic settings.
Materials and methods
Materials
Acetaminophen, amoxicillin·3H2O, azithromycin, diclofenac sodium, ethambutol diHCl, fluconazole, isoniazid, levofloxacin, linezolid, metformin, sulfamethoxazole and trimethoprim were purchased from Sigma–Aldrich (St Louis, MO, USA). Bedaquiline, ciprofloxacin, dolutegravir, efavirenz and rifampicin were purchased from Alsachim (Illkirch, France). Clofazimine, d-cycloserine, ethionamide and prothionamide were obtained from Toronto Research Chemicals (Ontario, Canada). Pyrazinamide was acquired from Honeywell Fluka (Bucharest, Romania). All reference materials were of ≥98% purity. Ultrapure water (resistivity >15 MΩ·cm at 25°C) was obtained from a Milli-Q Advantage A10 system (Millipore Corporation, Billerica, MA, USA). Absolute methanol of UPLC–MS grade was acquired from Biosolve BV (Valkenswaard, the Netherlands).
Separate stock solutions were used for the preparation of the calibration standards and the quality control (QC) samples. For all experiments, the total volume of (diluted) stock solutions added to filtered drug-free saliva never exceeded 5% (v/v). Calibration standards and QC samples were portioned into vials and stored at −20°C. Vials were discarded after a day of use.
Equipment and assay procedure
All experiments were performed on a mobile NP80 NanoPhotometer (Implen, München, Germany). The NP80 is a mobile UV/VIS nano spectrophotometer with a scan range of 200–900 nm, a scan time of 2.5–4 s and a bandwidth of <1.8 nm with a sample volume of 0.3–2 μL. Samples of healthy volunteers were collected using a Salivette® (Sarstedt, Nümbrecht, Germany).31 Samples were filtered through a Millex-GP (polyethersulphone) of 0.22 μm pore size (Tullagreen, Carrigtwohill, Ireland) using a syringe.32 A small drop (≥3 μL) of saliva was placed on the sample surface, with the use of a disposable Pasteur pipette. The path length was set at 0.67 mm and a UV/VIS spectrum was scanned in the 200–900 nm range. The smoothing function was turned off. After each measurement, the sample surface was cleaned, disinfected and dried using lint-free tissues, deionized water and 70% ethanol.
Method development
According to Lambert–Beer’s law, the light absorbance is directly proportional to the concentration of the absorbing components of the sample.33 In our case, this applies to our drug of interest (levofloxacin), but also to all other potentially interfering substances. Finding the wavelength that is most specific for levofloxacin does not make the method impervious to interferences of co-medication or endogenous compounds. Therefore, we developed a strategy to strengthen the selectivity and specificity of spectrophotometry using derivative spectroscopy.34 Derivative spectroscopy increases spectral resolution and decreases baseline shifts. Relative broad absorbance bands, caused by light scattering from large molecules (e.g. proteins), are suppressed relative to the sharp absorbance bands of smaller molecules such as levofloxacin. These characteristics allow for detection and quantification of analytes in the presence of a strongly absorbing matrix.34,35
In our described method, the concentration of levofloxacin was evaluated by use of the second-order derivative of the UV/VIS spectrum. As the correlation between the concentration and absorbance of a zero-order spectrum follows Lambert–Beer’s law, we also expect the amplitude of a second-order derivative of the spectrum to exhibit a similar linear function.
where A is absorbance, λ is wavelength, ε is extinction coefficient, b is sample path length and c is sample concentration.
The levofloxacin concentration was quantified by using the amplitude of the second-order spectrum between 300 and 400 nm of seven calibrators. Sample concentrations were calculated from the spectrum amplitude using linear regression. The second-order derivative spectra were calculated by polynomial fitting of the spectral scan, using the Savitsky–Golay method.36 Polynomial coefficients were calculated as a vector, using the following matrix equation:37
where k is polynomial order and n is wavelength interval.
The second-order derivative of the polynomial was expressed by:
The wavelength interval and polynomial order of the polynomial fitting were optimized for deconvolution and signal-to-noise ratio, by minimization of the bias and precision of calculated levofloxacin concentration in the presence of various potential interferents. All calculations were done by importing all raw data into a proprietary Excel spreadsheet (Microsoft, Redmond, WA, USA).
Method validation
Method validation was performed according to FDA and EMA guidelines for selectivity, specificity, linearity, accuracy and precision.
The levofloxacin calibration curve consisted of seven points at the concentrations of 2.50, 5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 mg/L, which is suitable for clinical practice as levofloxacin peak concentration ranges from 8 to 40 mg/L.38 The lower limit of quantitation (LLOQ), low, medium and high QC concentrations were at 2.50, 5.00, 25.0 and 40.0 mg/L, respectively. For specificity, six human drug-free saliva samples, each obtained from separate healthy volunteers, were tested for interference. Measurements of these drug-free samples ideally result in a levofloxacin concentration less than the LLOQ. For selectivity, these drug-free samples were spiked with levofloxacin at the LLOQ concentration. Measurements of the spiked samples ideally result in a bias <20%. Interpatient variance was assessed by spiking separate drug-free saliva samples from six different healthy volunteers at low and high concentrations. Bias and precision should be <15% at all concentrations. To assess the effect of exogenous components (e.g. other medicines), a pool of single donor, drug-free saliva was spiked at the LLOQ and high levofloxacin concentrations. The unspiked drug-free saliva and the spiked saliva were additionally spiked with medicines likely to be present in our patient population. The drug-free saliva was spiked at the expected maximum concentration (Cmax) of these drugs in saliva retrieved from the literature. If a Cmax value in saliva could not be retrieved from the literature, Cmax in plasma was used instead.19,24 All spiked samples were analysed in five replicates on a single day. Unspiked blank saliva ideally result in responses less than the LLOQ. Drug-free saliva samples spiked with levofloxacin ideally result in a bias <20% at the LLOQ and a bias <15% at high concentration. Accuracy and precision were determined by measuring the LLOQ, low, medium and high QC samples in replicates of five over three separate days. The samples were quantified using a single seven-point calibration curve that was measured on that same day. Within-day, between-day and overall precision were calculated with the use of a one-way ANOVA. The acceptance criterion for bias and precision was <20% at the LLOQ and <15% at the low, medium and high concentrations.
Results
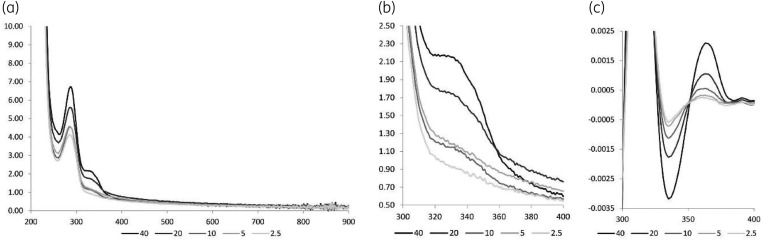
Absorbance scans of saliva samples showed clear baseline shifts. Figure 1(a and b) shows scans of five concentrations of levofloxacin spiked to the same drug-free saliva, doubling the levofloxacin concentration at every successive concentration. Theoretically the absorbance at 285 nm and 320 nm can be used to quantify levofloxacin, according to Lambert–Beer’s law. However, the baseline shifts, from sample to sample, resulted in a lack of correlation between the levofloxacin concentration and the absorbance. Figure 1c shows that the amplitudes of the second-order derivative of the same spectra do correlate with the levofloxacin concentration. In effect, the concavity of the inflection point of the zero-order absorbance band is used to quantify levofloxacin in the saliva sample.
Figure 1.
Spectra of levofloxacin in saliva. (a) Full zero-order spectra of levofloxacin in saliva at 2.5, 5, 10, 20 and 40 mg/L, (b) detail of the zero-order spectra and (c) detail of second-order spectra [S-G(8,61)].
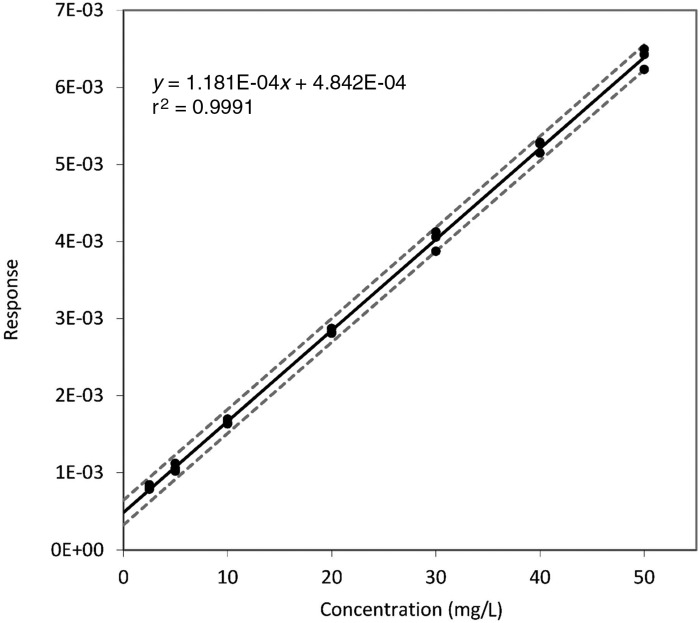
Specificity and selectivity were assessed by analysing six separate drug-free samples. All six drug-free samples resulted in responses below the response of the LLOQ. Biases ranged from 87% to 115% at the LLOQ level, from 93% to 113% at the low concentration and from 94% to 102% at the high concentration. Precision was 10.4%, 7.1% and 2.9%, respectively. Linearity was assessed using a seven-point calibration curve (n = 3). The linear range was proven to be 2.5–50 mg/L, with a weighting factor of 1 (r2=0.9991, n = 3, Figure 2). The accuracy, within-day precision, between-day precision and overall precision were assessed at four concentrations. The results are shown in Table 1.
Figure 2.
Calibration curve in drug-free saliva (n = 3) with 95% CI.
Table 1.
Accuracy and precision
| Value at different concentrations |
||||
|---|---|---|---|---|
| Criterion | LLOQ | Low | Medium | High |
| Nominal concentration (mg/L) | 2.50 | 5.00 | 25.0 | 40.0 |
| Accuracy [bias (%)] | −5.2 | 0.4 | 1.9 | 2.4 |
| Within-day precision [CV (%)] | 11.4 | 4.4 | 1.0 | 0.7 |
| Between-day precision [CV (%)] | 11.4 | 7.8 | 1.9 | 2.0 |
| Overall precision [CV (%)] | 16.1 | 9.0 | 2.1 | 2.1 |
CV=coefficient of variation calculated as (SD/mean) × 100%.
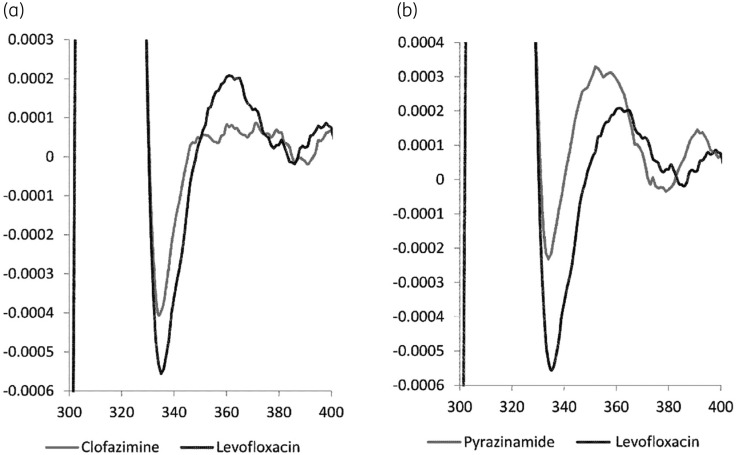
The effect of co-medication on the quantitation of levofloxacin was minimized by optimizing the Savitsky–Golay method. Figures S1 and S2 (available as Supplementary data at JAC Online) show how the calculation of the second-order derivative spectrum at the LLOQ, by the Savitsky–Golay method, is affected by changes in the wavelength interval and order of the polynomial fit. Of all tested combinations of polynomial order and wavelength interval, a polynomial of the eighth order fitted to a 61 nm interval [S-G(8,61)] gave the best overall results. Figure 3 shows the differences in second-order derivative spectra between drug-free saliva spiked with 0.4 mg/L clofazimine, drug-free saliva spiked with 42 mg/L pyrazinamide and drug-free saliva spiked with levofloxacin at the LLOQ. All drug-free saliva samples spiked with potential co-medication (Table 2) gave responses of <2.5 mg/L levofloxacin in the absence of levofloxacin, with the exception of rifampicin and pyrazinamide. Rifampicin and pyrazinamide resulted in a positive bias of 171.7% and 27.3% of levofloxacin at the LLOQ concentration, respectively. This means that a levofloxacin concentration is reported as 3.2 mg/L instead of 2.5 mg/L in the presence of a pyrazinamide concentration of 42 mg/L. This difference will not affect clinical decision making as the absolute level is very low as levofloxacin peak concentrations typically range from 8 to 40 mg/L.38
Figure 3.
Differences in second-order derivative spectra of different co-administered drugs. (a) Clofazimine at a concentration of 0.4 mg/L gives a lower response than the response of the levofloxacin LLOQ and (b) pyrazinamide at a concentration of 42 mg/L gives a higher response than the response of the levofloxacin LLOQ.
Table 2.
Effect of co-medication and anti-TB drugs on levofloxacin results
| Drug | Tested concentration (mg/L) | Bias (CV) of LLOQ (2.5 mg/L) (%) | Bias (CV) of high (40 mg/L) (%) |
|---|---|---|---|
| Acetaminophen | 12.0 | 2.1 (4.7) | 1.9 (0.7) |
| Amoxicillin | 6.5 | 6.0 (6.4) | 6.9 (0.9) |
| Azithromycin | 0.6 | 8.1 (6.3) | 5.0 (0.9) |
| Ciprofloxacin | 0.4 | 9.0 (3.6) | 6.4 (0.6) |
| Diclofenac | 1.5 | 4.8 (6.1) | 1.0 (1.3) |
| Dolutegravir | 1.0 | −11.1 (2.5) | −2.1 (2.4) |
| Efavirenz | 1.0 | −1.6 (3.9) | −1.3 (1.6) |
| Fluconazole | 10.0 | 7.7 (4.6) | 0.1 (0.5) |
| Metformin | 2.0 | −9.4 (8.0) | −3.2 (1.8) |
| Sulfamethoxazole | 9.0 | 6.5 (3.4) | 0.7 (0.7) |
| Trimethoprim | 4.5 | 4.2 (3.7) | 0.6 (0.8) |
| Bedaquiline | 3.5 | 11.8 (5.7) | 2.3 (0.6) |
| Clofazimine | 0.4 | 6.3 (6.0) | 4.1 (0.6) |
| Cycloserine | 19.5 | 0.3 (2.9) | 4.1 (0.8) |
| Ethambutol | 1.3 | 8.6 (10.5) | 1.6 (0.7) |
| Ethionamide | 2.5 | 7.6 (6.8) | 8.4 (0.8) |
| Isoniazid | 7.5 | 12.2 (4.1) | 2.4 (1.2) |
| Linezolid | 10.0 | 8.7 (7.8) | 3.0 (1.5) |
| Prothionamide | 5.0 | 1.9 (4.8) | 3.7 (0.8) |
| Pyrazinamide | 42.0 | 27.3 (2.3) | 9.3 (0.8) |
| Rifampicin | 12.0 | 171.7 (2.0) | 21.0 (0.6) |
CV=coefficient of variation calculated as (SD/mean) × 100%.
Discussion
We developed an accurate and precise analytical method suitable for the measurement of levofloxacin in human saliva using a mobile microvolume UV/VIS spectrophotometer. The main challenge during the development of this method was ensuring acceptable selectivity, specificity and robustness in the presence of co-medication. Because we aimed at an easy-to-use assay under field conditions, it was decided that extensive sample clean-up was not acceptable. After exploring different strategies for isolating the response of levofloxacin from various background signals, such as the subtraction of a drug-free saliva spectrum or standard addition per sample, it became apparent that derivative spectroscopy was the most viable option for routine use.
Derivative spectroscopy requires complex mathematics to generate reproducible results. As such, considerable effort was dedicated to the development of pre-specified calculations to make concentration determination virtually effortless during routine use. Ideally, the Savitsky–Golay method should be integrated into the firmware of the mobile UV/VIS spectrophotometer. The Savitsky–Golay method ensures optimal robustness in the presence of co-medication when a 61 nm range was used to fit an eighth-order polynomial. Nevertheless, it must be commented that the presence of rifampicin and pyrazinamide can affect the measurement of levofloxacin at the LLOQ. Given that levofloxacin is used primarily as a core agent against MDR-TB, which is by definition resistant to rifampicin, it will be highly unlikely that rifampicin will be present in our intended patient group. The updated WHO guidelines for MDR-TB advises a regimen with at least five effective anti-TB drugs during the intensive phase.2 Currently, pyrazinamide is listed as a Group C drug and only to be counted as an effective drug in cases where susceptibility has been proven by drug susceptibility testing.2 Therefore, the use of pyrazinamide in our intended patient group is possible, but becoming less common in current global MDR-TB strategies. Moreover, our validation showed that the level of pyrazinamide interference is negligible at higher levofloxacin concentrations. Furthermore, as samples are collected after the absorption phase to capture the peak concentration, the interference of pyrazinamide is not expected to have clinical implications. Developed limited sampling strategies have shown that single or multiple samples collected after drug administration can be used to quantify levofloxacin exposure, which mitigates the risk of interference.39
To demonstrate the usefulness of this method, as a next step, we will perform a clinical validation in an MDR-TB endemic setting among people being treated with levofloxacin and pyrazinamide utilizing paired saliva and plasma collection. Saliva samples will be measured not only using the UV/VIS spectrophotometer but also using LC–MS/MS31 to show if other factors potentially impact the results obtained with the nanophotometer. In our opinion, the use of the mobile nanophotometer has the potential to comply with most criteria defined for diagnostics tests in low resource settings [ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable to end-users)].40 Compared with traditional chromatographic methods for TDM, the nanophotometer is more affordable. At least for levofloxacin, we have shown that the assay is sensitive and specific for its purpose. The simple sample preparation required for the assay ensures user-friendliness and a high degree of acceptance with end-users. UV/VIS spectrometry is fast and robust, but requires equipment. Fortunately, the equipment can be used in field conditions, which means that samples of patients do not have to be transported to a laboratory and results are immediately available for the end-users. For implementation in routine care, we envisage that the levofloxacin saliva AUC can be adequately estimated using a limited sampling strategy in combination with linear regression Bayesian dose selection39 and converted into a plasma AUC based on the saliva/plasma penetration ratio. Subsequently, the required dose to target the appropriate AUC to achieve an AUC/MIC ratio associated with optimal kill9 can be calculated. The new dose can be selected on available tablet size rounded up to the closest whole tablet up to a maximum of 25 mg/kg daily9 while adequately monitoring patient safety.41
To conclude, we have developed and validated a UV/VIS spectrophotometric assay for measurement of levofloxacin concentration in saliva. After clinical validation, this assay will greatly expand access to personalized dosing strategies for people with MDR-TB at a community level.
Funding
This project was financially support by the Bill & Melinda Gates Foundation, Grant Challenges programme (grant number OPP1191221).
Transparency declarations
None to declare.
Supplementary data
Figures S1 and S2 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1.WHO. Global Tuberculosis Report 2019. 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1.
- 2.WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva, 2019. https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1. [PubMed]
- 3.WHO. Rapid Communication: Key Changes to the Treatment of Drug-Resistant Tuberculosis. 2019. https://www.who.int/tb/publications/2019/WHO_RapidCommunicationMDR_TB2019.pdf?ua=1.
- 4. Davies Forsman L, Bruchfeld J, Alffenaar J.. Therapeutic drug monitoring to prevent acquired drug resistance of fluoroquinolones in the treatment of tuberculosis. Eur Respir J 2017; 49: 1700173. [DOI] [PubMed] [Google Scholar]
- 5. Cegielski J, Dalton T, Yagui M. et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2014; 59: 1049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gumbo T, Louie A, Deziel MR. et al. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190: 1642–51. [DOI] [PubMed] [Google Scholar]
- 7. Pranger AD, Van Altena R, Aarnoutse RE. et al. Evaluation of moxifloxacin for the treatment of tuberculosis: 3 years of experience. Eur Respir J 2011; 38: 888–94. [DOI] [PubMed] [Google Scholar]
- 8. Aung KJM, Van Deun A, Declercq E. et al. Successful ‘9-month Bangladesh regimen’ for multidrug resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014; 18: 1180–7. [DOI] [PubMed] [Google Scholar]
- 9. Deshpande D, Pasipanodya JG, Mpagama SG. et al. Levofloxacin pharmacokinetics/pharmacodynamics, dosing, susceptibility breakpoints, and artificial intelligence in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67: S293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van’t Boveneind-Vrubleuskaya N, Seuruk T, Van Hateren K. et al. Pharmacokinetics of levofloxacin in multidrug- and extensively drug-resistant tuberculosis patients. Antimicrob Agents Chemother 2017; 61: pii: e00343–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghimire S, Maharjan B, Jongedijk EM. et al. Levofloxacin pharmacokinetics, pharmacodynamics and outcome in multidrug-resistant tuberculosis patients. Eur Respir J 2019; 53: 1802107. [DOI] [PubMed] [Google Scholar]
- 12. Peloquin CA, Phillips PPJ, Mitnick CD. et al. Increased doses lead to higher drug exposures of levofloxacin for treatment of tuberculosis. Antimicrob Agents Chemother 2018; 62: e00770–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Shaer MH, Alghamdi WA, Alsultan A. et al. Fluoroquinolones in drug-resistant tuberculosis: culture conversion and pharmacokinetic/pharmacodynamic target attainment to guide dose selection. Antimicrob Agents Chemother 2019; 63: e00279–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nahid P, Dorman SE, Alipanah N. et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2017; 63: e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nahid P, Mase SR, Migliori GB. et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e93–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veringa A, Sturkenboom MGG, Dekkers BGJ. et al. LC-MS/MS for therapeutic drug monitoring of anti-infective drugs. Trends Anal Chem 2016; 84: 34–40. [Google Scholar]
- 17. van der Burgt EP, Sturkenboom MGG, Bolhuis MS. et al. End TB with precision treatment! Eur Respir J 2016; 47: 680–2. [DOI] [PubMed] [Google Scholar]
- 18. Ghimire S, Bolhuis MS, Sturkenboom MGG. et al. Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur Respir J 2016; 47: 1867–9. [DOI] [PubMed] [Google Scholar]
- 19. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002; 62: 2169–83. [DOI] [PubMed] [Google Scholar]
- 20. Kim HY, Heysell SK, Mpagama S. et al. Challenging the management of drug-resistant tuberculosis. Lancet 2020; 395: 783. [DOI] [PubMed] [Google Scholar]
- 21. Alffenaar JWC. Therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Infect Dis 2017; 64: 104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alffenaar JWC, Heysell SK, Mpagama SG.. Therapeutic drug monitoring: the need for practical guidance. Clin Infect Dis 2019; 68: 1065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghimire S, Maharjan B, Jongedijk EM. et al. Evaluation of saliva as a potential alternative sampling matrix for therapeutic drug monitoring of levofloxacin in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2019; 63: e02379–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Den Elsen SHJ, Oostenbrink LM, Heysell SK. et al. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther Drug Monit 2018; 40: 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zentner I, Modongo C, Zetola NM. et al. Urine colorimetry for therapeutic drug monitoring of pyrazinamide during tuberculosis treatment. Int J Infect Dis 2018; 68: 18–23. [DOI] [PubMed] [Google Scholar]
- 26. Szipszky C, Van Aartsen D, Criddle S. et al. Determination of rifampin concentrations by urine colorimetry and mobile phone readout for personalized dosing in tuberculosis treatment. J Pediatric Infect Dis Soc 2020; doi:10.1093/jpids/piaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Elsen SHJ, Akkerman OW, Huisman JR. et al. Lack of penetration of amikacin into saliva of tuberculosis patients. Eur Respir J 2018; 51: 1702024. [DOI] [PubMed] [Google Scholar]
- 28. Van Den Elsen SHJ, Akkerman OW, Jongedijk EM. et al. Therapeutic drug monitoring using saliva as matrix: an opportunity for linezolid, but challenge for moxifloxacin. Eur Respir J 2020; 55: 1901903. [DOI] [PubMed] [Google Scholar]
- 29. van den Elsen SHJ, Akkerman OW, Wessels M. et al. Dose optimisation of first-line tuberculosis drugs using therapeutic drug monitoring in saliva: feasible for rifampicin, not for isoniazid. Eur Respir J 2020: 2000803. [DOI] [PubMed] [Google Scholar]
- 30. Alffenaar J-WC, Gumbo T, Dooley KE. et al. Integrating pharmacokinetics and pharmacodynamics in operational research to end tuberculosis. Clin Infect Dis 2019; 70: 1774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghimire S, Jongedijk E, van den Elsen S. et al. Cross validation of liquid chromatography tandem mass spectrometry method for quantification of levofloxacin in saliva. J Appl Bioanal 2020; 6: 68–70. [Google Scholar]
- 32. Van Den Elsen SHJ, Van Der Laan T, Akkerman OW. et al. Membrane filtration is suitable for reliable elimination of Mycobacterium tuberculosis from saliva for therapeutic drug monitoring. J Clin Microbiol 2017; 55: 3292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atkins P, de Paula J, Keeler J.. Molecular Spectroscopy. Atkins’ Physical Chemistry. Oxford: Oxford University Press, 2018; 417–86. [Google Scholar]
- 34. Giese AT, French CS.. The analysis of overlapping spectral absorption bands by derivative spectrophotometry. Appl Spectrosc 1955; 9: 78–96. [Google Scholar]
- 35. Owen A. Uses of Derivative Spectroscopy, UV-Visible Spectroscopy. Agilent Technologies, 1995. [Google Scholar]
- 36. Savitzky A, Golay MJE.. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 1964; 36: 1627–39. [Google Scholar]
- 37. Kleinbaum DG, Kupper LL, Nizam A. et al. Applied Regression Analysis and Other Multivariable Methods. Cengage Learning, 2013. [Google Scholar]
- 38. Ghimire S, van’t Boveneind-Vrubleuskaya N, Akkerman OW. et al. Pharmacokinetic/pharmacodynamic-based optimization of levofloxacin administration in the treatment of MDR-TB. J Antimicrob Chemother 2016; 71: 2691–703. [DOI] [PubMed] [Google Scholar]
- 39. Van den Elsen SHJ, Sturkenboom MGG, Van’t Boveneind-Vrubleuskaya N. et al. Population pharmacokinetic model and limited sampling strategies for personalized dosing of levofloxacin in tuberculosis patients. Antimicrob Agents Chemother 2018; 62: e01092–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mabey D, Peeling RW, Ustianowski A. et al. Diagnostics for the developing world. Nat Rev Microbiol 2004; 2: 231–40. [DOI] [PubMed] [Google Scholar]
- 41. Borisov S, Danila E, Maryandyshev A. et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J 2019; 54: 1901522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.