Abstract
N-Acylphosphatidylethanolamine phospholipase D (NAPE-PLD) is regarded as the main enzyme responsible for the biosynthesis of N-acylethanolamines (NAEs), a family of bioactive lipid mediators. Previously, we reported N-(cyclopropylmethyl)-6-((S)-3-hydroxypyrrolidin-1-yl)-2-((S)-3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (1, LEI-401) as the first potent and selective NAPE-PLD inhibitor that decreased NAEs in the brains of freely moving mice and modulated emotional behavior [Mocket al. Nat Chem. Biol., 2020, 16, 667−675]. Here, we describe the structure–activity relationship (SAR) of a library of pyrimidine-4-carboxamides as inhibitors of NAPE-PLD that led to the identification of LEI-401. A high-throughput screening hit was modified at three different substituents to optimize its potency and lipophilicity. Conformational restriction of an N-methylphenethylamine group by replacement with an (S)-3-phenylpiperidine increased the inhibitory potency 3-fold. Exchange of a morpholine substituent for an (S)-3-hydroxypyrrolidine reduced the lipophilicity and further increased activity by 10-fold, affording LEI-401 as a nanomolar potent inhibitor with drug-like properties. LEI-401 is a suitable pharmacological tool compound to investigate NAPE-PLD function in vitro and in vivo.
Introduction
N-Acylphosphatidylethanolamine phospholipase D (NAPE-PLD) is considered to be the principal enzyme that produces N-acylethanolamines (NAEs), a family of signaling lipids.2 NAPE-PLD catalyzes the hydrolysis of N-acylphosphatidylethanolamines (NAPEs) to NAEs, which includes the endocannabinoid anandamide (N-arachidonoylethanolamine, AEA).3 The NAE lipids exert their biological activity through the activation of various G-protein-coupled receptors (cannabinoid receptors CB1 and CB2, GPR55, GPR110, and GPR119), ion channels (transient receptor potential vanilloid 1, TRPV1), and nuclear receptors (peroxisome proliferator-activated receptor α, PPAR-α).4 Accordingly, NAEs are involved in numerous physiological processes such as appetite, satiety, pain, inflammation, fertility, stress, and anxiety.5 Furthermore, aberrant NAE levels are associated with metabolic syndrome and non-alcoholic steatohepatitis (NASH).6−8 Thus, there is a need for pharmacological tools that can inhibit NAPE-PLD to study its role in cellular and animal models to further our understanding of NAE biology.
To date, NAPE-PLD has been characterized in several biochemical studies.3,9,10 It was found to have a wide distribution among murine organs with higher abundance in brain, kidney, and testis tissues.3 A crystal structure of NAPE-PLD confirmed that the enzyme has a metallo-β-lactamase fold with two Zn2+ ions in its active site.11 NAPE-PLD was shown to be membrane-associated and activated by phosphatidylethanolamine (PE), suggesting that the enzyme is constitutively active.10 Interestingly, these structural studies also revealed that, in vitro, NAPE-PLD can form a homodimer, which binds to specific bile acids in an allosteric site that promote dimerization and increase enzymatic activity.11,12 Also, polyamines have been reported to enhance NAPE-PLD activity in vitro.13 Whether bile acids and polyamines play an active role in regulating NAPE-PLD dimerization and activity in vivo has yet to be established, in particular, in the CNS.
Several inhibitors for NAPE-PLD have been reported (Figure 1). Out of a small library of NAPE substrate mimics, phosphoramidate AHP-71B was described as an inhibitor with micromolar potency (half-maximum inhibitory concentration IC50 ≈ 10 μM).14 Other reported active compounds were the β-lactamase substrate nitrocefin,14 desketoraloxifene analogue 17b(15) that also targeted phospholipase D1 (PLD1), endogenous bile acid lithocholic acid,12 and sulfonamide ARN19874.16 All compounds showed poor to moderate potency for NAPE-PLD in vitro. Of note, ARN19874 was able to increase NAPE levels in HEK293 cells but did not affect most NAE levels.16 Recently, the disinfectant hexachlorophene was reported as a NAPE-PLD inhibitor with low micromolar activity.17 However, this compound has neurotoxic effects and is therefore not suited for in vivo use.18 Thus, to study the biological functions of NAEs in cellular and animal models, new and more potent chemotypes are needed that can inhibit the enzymatic activity of NAPE-PLD.
Figure 1.

Structures of reported NAPE-PLD inhibitors.
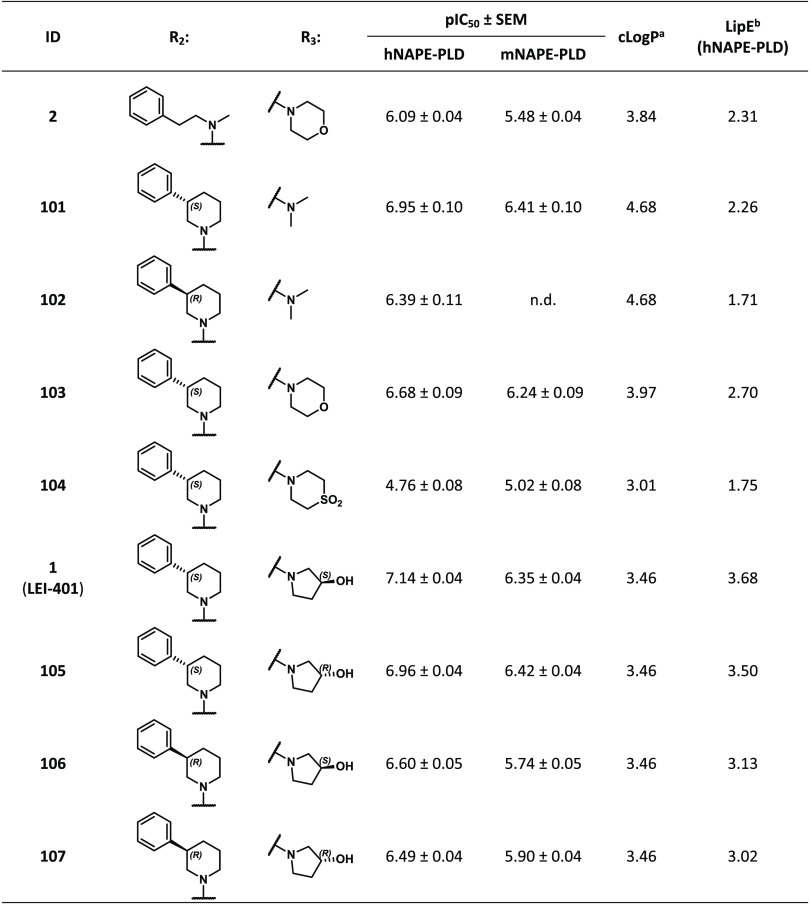
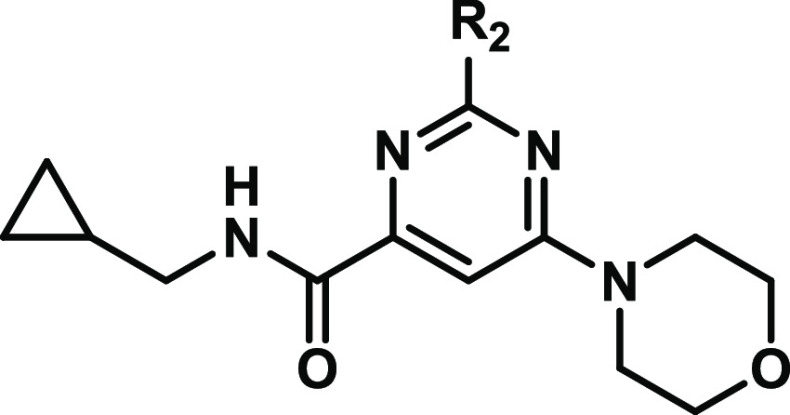
Previously, we performed a high-throughput screening of a library of ∼350,000 compounds, which identified pyrimidine-4-carboxamide 2 as an inhibitor of NAPE-PLD with sub-micromolar potency (pIC50 = 6.09 ± 0.04, Figure 2).1 Generation of a small library of close analogues of 2 afforded the optimized NAPE-PLD inhibitor 1 (LEI-401), which exhibited nanomolar potency (pIC50 = 7.14 ± 0.04 μM, Figure 2). LEI-401 reduced NAE levels including anandamide in Neuro-2a cells as well as in the brains of freely moving mice. In addition, LEI-401 elicited a marked effect on emotional behavior in mice by activating the hypothalamus-pituitary–adrenal (HPA) axis and reducing fear extinction of an aversive memory. Here, we describe the structure–activity relationship (SAR) of a library of NAPE-PLD inhibitors that afforded LEI-401.
Figure 2.
Structures of in vivo active NAPE-PLD inhibitor 1 (LEI-401), HTS-hit 2, and the core pyrimidine-4-carboxamide scaffold.
Results and Discussion
Chemistry
To study the SAR of hit 2, different synthetic routes were employed that allowed systematic variation of the pyrimidine scaffold, the R1 amide, or R2 and R3 substituents (Figure 2). This led to the synthesis of compounds 1 and 3–107 with modified core scaffolds (compounds 3–6) or modifications at R1 (7–30), R2 (31–70), and R3 (71–100) or combinations thereof (1 and 101–107). First, the influence of the nitrogen atoms in the pyrimidyl ring was investigated with the synthesis of pyridyl analogues 3 and 4 (Scheme 1). For compound 3, this commenced with the regioselective nucleophilic aromatic substitution (SNAr) of dichloride 108 with N-methylphenethylamine generating 109. Subsequent ester hydrolysis and amide coupling afforded 111, which was converted to 3 with morpholine using Buchwald–Hartwig amination conditions.19 Isomer 4 was synthesized in four steps from symmetric dichloride 112: SNAr with morpholine, ester hydrolysis, and amide coupling giving 115 followed by similar Pd-catalyzed amination with N-methylphenethylamine.
Scheme 1. Synthesis of Pyridyl Analogues 3 and 4.
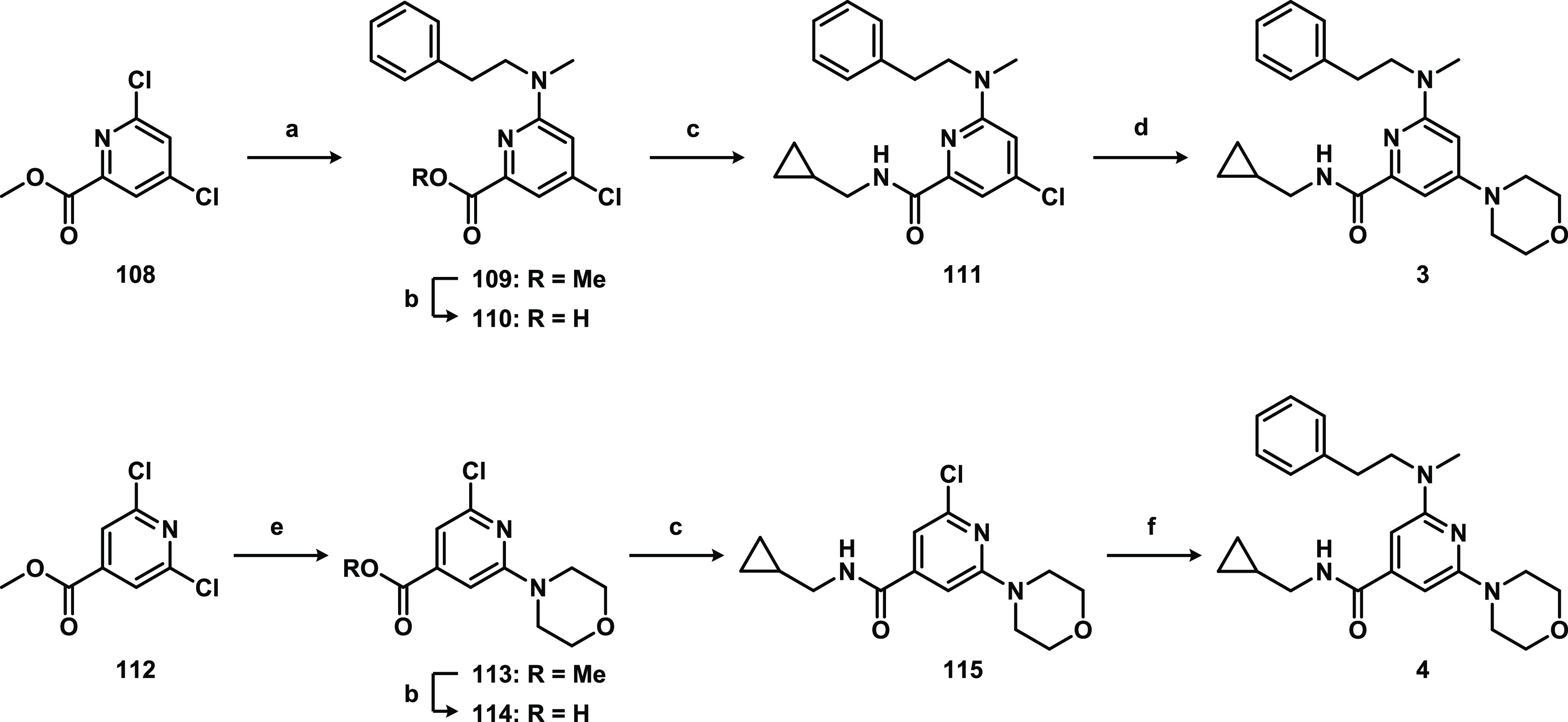
Reagents and conditions: (a) N-methylphenethylamine, DiPEA, MeOH, rt, 41%; (b) NaOH, THF, H2O, rt; for 110: 89% and for 114: 99%; (c) cyclopropylmethanamine, EDC·HCl, HOBt, DCM, rt; for 111: 24% and for 115: 80%; (d) morpholine, RuPhos-Pd-G3, NaOtBu, THF, toluene, 110 °C, 37%; (e) morpholine, K2CO3, CH3CN, reflux, 66%; (f) N-methylphenethylamine, RuPhos-Pd-G3, NaOtBu, THF, toluene, 110 °C, 41%.
Next, a systematic synthesis of analogues of 2 with varying R1, R2, and R3 substituents was performed. R1 amide derivatives were generated via two general four-step sequences, which either introduced the amide in the second (compounds 10, 11, 13–17, 27, and 28, Scheme 2A) or final step (8, 9, 12, 18, 23, 25, and 26, Scheme 2B). This shortened the synthetic sequence from three to only one reaction for each R1 amide derivative, respectively. The route depicted in Scheme 2A was also used to synthesize R2 amine analogues (33–67 and 69) and R3 amine (71–76 and 80–96), heteroaromatic rings (97 and 98), or phenol derivatives (99 and 100). The synthesis started with orotic acid (116), which was converted to acyl chloride 117 using phosphorous oxychloride. Cold addition (−78 to 0 °C) of various primary amines gave amides 118a–k. The more electrophilic 4-chloro substituent of the dichloropyrimidine was regioselectively substituted with various amine, heteroaromatic, or phenolic nucleophiles to afford 119a–af. Finally, high temperature and/or microwave irradiation was used to couple different R3 amines to the 2-chloropyrimidine scaffold, which provided the desired products. Non-commercially available N-methylphenethylamines that were used as R2 amines were synthesized from benzylic halides 120a and 120b, which were converted to their corresponding nitriles (121a and 121b) followed by hydrogenation, affording their primary amines (122a and 122b). Mono-N-methylation was achieved by carbamoylation and subsequent LiAlH4 reduction, giving the N-methylphenethylamines 123a–o. Alternatively, phenethylamine was converted to the N-phenyl analogue 124 via Chan–Lam coupling20 or to N-alkyl derivatives 125a–e by reductive amination with aldehydes or ketones. The secondary route for introduction of the R1 amide in the final step consisted of regioselective substitution of dichloropyrimidine 126 to give 127 (Scheme 2B). Then, ester hydrolysis followed by coupling with N-methylphenethylamine gave carboxylic acid 19, which was condensed with various amines. Molecules not listed in Schemes 1 or 2 (compounds 5–7, 20–22, 24, 29, 30, 32, 68, 69, 77–79, and 92) were synthesized according to the routes described in Schemes S1–S10.
Scheme 2. (A) General Synthetic Route for Analogues of Compound 2; (B) Alternative Synthetic Route for Amide Analogues.

Reagents and conditions: (a) POCl3, DMF, reflux, 60%; (b) R1NH2, Et3N, DCM, −78 °C to 0 °C, 78–99%; (c) (cyclo)alkylNH, DiPEA, MeOH, 0 °C, 32–99% or (hetero)arylOH or heteroarylNH, K2CO3, DMF, rt, 51–76%; (d) 123a–o or 124 or 125a–e, DiPEA, n-BuOH, μW, 160 °C or oil bath, 120 °C, 21% – 97%; (e) KCN, EtOH, dioxane, H2O, reflux, 84–99%; (f) H2, Pd/C, HCl, EtOH, rt, 98–99%; (g) methyl chloroformate, DiPEA, DCM, 0 °C to rt; (h) LiAlH4, THF, 0 °C to reflux, 40–94% over 2 steps; (i) phenylboronic acid, Cu(OAc)2·H2O, 4 Å MS, O2, DCE, rt, 32%; (j) aldehyde or ketone, NaB(OAc)3H, AcOH, DCM, rt, 18–63%. (k) NaOH, THF, MeOH, H2O, rt, 99%; (l) N-methylphenethylamine, DiPEA, n-BuOH, 120 °C, 51%; (m) R1NH2, PyBOP, DiPEA, DMF, 0 °C to rt, 43–55%.
Biology
A biochemical NAPE-PLD activity assay1 was performed to measure the inhibitory potency of compounds 1–107 using membrane lysates of HEK293T cells overexpressing human NAPE-PLD. The assay uses the fluorescence-quenched substrate PED6 (Figure S1), which can report on various phospholipase activities including PLA2 and PLD. In the case of NAPE-PLD, hydrolysis of the PED6 phosphodiester results in the release of the quencher from the BODIPY fluorophore, providing a direct read-out of enzyme activity. The data are reported in Tables 1–7 as pIC50 ± SEM (N = 2, n = 2; the mean of two independent experiments with two biological replicates). First, to identify the essential nitrogen atom contributions of the scaffold, pyridyl analogues 3 and 4, pyrimidyl regioisomer 5, and triazine 6 were evaluated (Table 1). The removal of the X2-nitrogen (compound 3) but not X1 (compound 4) resulted in a 10-fold drop in potency. This suggested that the X2-nitrogen may form an important H-bond interaction with the protein, while the electron-withdrawing effect seems less important. A significant decrease in potency was also observed for regioisomer 5, while triazine 6 was completely inactive. This indicated that the pyrimidine scaffold of the hit 2 was optimal.
Table 1. Activity Data for Hit 2 and Scaffold Analogues 3–6.
| ID | X1 | X2 | X3 | pIC50 ± SEM | cLogPa |
|---|---|---|---|---|---|
| 2 | N | N | CH | 6.09 ± 0.04 | 3.84 |
| 3 | N | CH | CH | 4.98 ± 0.03 | 4.25 |
| 4 | CH | N | CH | 5.84 ± 0.03 | 3.90 |
| 5 | CH | N | N | 5.39 ± 0.11 | 3.84 |
| 6 | N | N | N | <4.3 | 3.09 |
cLogP was calculated using Chemdraw 15.
Table 7. Structure–Activity Relationship (SAR)-Analysis of Optimized Analogues 101–107.

cLogP was calculated using Chemdraw 15.
Lipophilic efficiency (LipE) = pIC50 – cLogP.
Next, the influence of the amide R1 substituent was investigated. Methylation of the amide in compound 2 resulted in complete loss of potency (compound 7, Table 2), suggesting that the amide may form another hydrogen bond or, alternatively, that the methyl group has a steric clash with the enzyme. Removal of the methylene group (8) reduced the activity, whereas linear alkylamides 9–14 showed optimal inhibition with a propyl chain. Branching of the alkyl substituent and introduction of heteroatoms or larger aromatic groups were less favorable (compounds 15, 16, 18, and 26–28). The 10-fold drop in potency for isobutylamide 15 may be attributed to the increased size of the isobutyl group or lack of π character compared to the cyclopropyl moiety.21 Of note, propargylamide 17 was equally active compared to the hit. Substituting the lipophilic amide for more polar analogues did not result in increased activities (compounds 19–25), although glycine methyl ester 21 showed to be equipotent to 2. Lastly, the amide bioisostere imidazole 29 displayed a substantial decrease in potency, while the amide isomer 30 was 10-fold less active than 2. In conclusion, the cyclopropylmethylamide of 2 is the optimal R1 substituent of the tested series, which suggests that it occupies a small lipophilic pocket.
Table 2. Structure–Activity Relationship Analysis of R1 Amide Analogues 7–30.

cLogP was calculated using Chemdraw 15.
To assess the influence of the R2 substituent on the inhibitory activity, a large number of structural analogues (31–70) were evaluated (Tables 3–5). Analogues 31–53 demonstrated that the N-methylphenethylamine is important for inhibitory activity as its complete removal resulted in inactive compounds (31 and 32) (Table 3). N-Methyl was found to be preferred over the hydrogen of 33. A similar trend was apparent for benzylic amines 34 and 35. Reducing (34) or increasing the alkyl chain length (36 and 37) decreased the potency, indicating that an ethylene linker is optimal. Various large substituents (e.g., phenyl) on the phenyl group were tolerated, but only at the ortho position (compounds 38–49), suggesting that there is space in the binding pocket. Both electron-donating (methyl (41) and methoxy (43)) and withdrawing (chloro (38) and CF3 (45)) substituents at the para position reduced the activity. Replacing the phenyl for a pyridyl ring was not favorable (50–52), while the thiophene isostere 53 displayed similar potency compared to 2. N-Alkyl analogues 54–59 demonstrated that larger groups than methyl are allowed (Table 4). In particular, isopropyl derivative 55 displayed a 2-fold increase in activity, albeit with a significant lipophilicity penalty. Next, several cyclic phenethylamine derivatives were evaluated (compounds 60–70) to study the effect of conformational restriction by reducing the number of rotatable bonds (Table 5). A 2-fold activity improvement was observed for both 3-phenylpiperidine 62 and 2-benzylpyrrolidine 63. Introduction of heteroatoms in the piperidine ring was not favored as witnessed by morpholine 67 and piperazine 68, but the activity could be recovered by introducing a N-benzyl group in the piperazine analogue 69.
Table 3. Structure–Activity Relationship (SAR) Analysis of R2 Analogues 31–53.


cLogP was calculated using Chemdraw 15.
Table 5. Structure–Activity Relationship (SAR) Analysis of R2 Analogues 60–70.

cLogP was calculated using Chemdraw 15.
Table 4. Structure–Activity Relationship (SAR) Analysis of R2 Analogues 54–59.
cLogP was calculated using Chemdraw 15.
To study the SAR of the R3 substituent, inhibitors 71–100 were evaluated (Table 6). Substitution of the morpholine for a more hydrophobic piperidine (71) was allowed, while the 3,3-difluoropiperidine 72 increased the potency 2-fold. The 4-position of the morpholine ring was less favorable for substitution (compounds 73–80). Replacing the morpholine with a dimethylamine 81 increased the activity 2-fold, suggesting that the morpholine 1 is too polar or may experience steric hindrance in the pocket. Several other small alkylamines were tested (82–87). Pyrrolidine 87 was the most effective with almost a 4-fold increase in potency. Substitutions on the pyrrolidine ring were investigated (compounds 88–94), revealing that hydroxylation on the 3-position (89) resulted in similar potency to pyrrolidine 87 while decreasing the cLogP with more than one log unit. Both enantiomers of the 3-hydroxypyrrolidine (90 and 91) were equally active. Of note, introduction of aromatic substituents was allowed (94–100) but did not improve the potency of the inhibitors.
Table 6. Structure–Activity Relationship (SAR) Analysis of R3 Analogues 71–100.

cLogP was calculated using Chemdraw 15.
Combination of the optimal R1 (cyclopropylmethylamide), R2 ((R/S)-3-phenylpiperidine), and various R3 substituents (dimethylamine, morpholine, or (R/S)-3-hydroxypyrrolidine) resulted in compounds 1 and 101–107 (Table 7). It was found that the combination of (S)-3-phenylpiperidine with (S)-3-hydroxypyrrolidine afforded the most potent compound (1, pIC50 = 7.14 ± 0.04, IC50 = 72 nM; 95% confidence interval: 0.061–0.086 nM), having a 10-fold increase in activity compared to 2. Importantly, 1 completely blocked the turnover of PED6 at a dose of 10 μM, indicating full efficacy (Figure S2). Interestingly, the (R,R) enantiomer of 1 (compound 107) showed 3-fold-reduced activity. The substantial cLogP reduction for 1 resulted in the highest lipophilic efficiency of this series (LipE = 3.68). In view of the inhibitory activity and optimal LipE, compound 1 (termed LEI-401) was selected as the lead compound for further biological profiling.
Our attempts to dock LEI-401 in the active site of the reported NAPE-PLD crystal structure (PDB ID: 4QN911), did not provide binding poses that confidently recapitulated the SAR as described in this work. This may be attributed to the large hydrophobic binding cavity of the endogenous NAPE substrate, which facilitates a large number of possible poses for LEI-401. Alternatively, LEI-401 may bind in an unidentified allosteric pocket. Future co-crystallization studies are needed to identify the binding pocket of LEI-401 in NAPE-PLD.
Because the biological profiling of NAPE-PLD inhibitors is mostly performed in mouse models, it was assessed whether LEI-401 showed any species difference using recombinant mouse NAPE-PLD expressed in HEK293T cells. Despite high homology between human and mouse NAPE-PLD (89%), it was found that LEI-401 showed somewhat lower potency (pIC50 = 6.35 ± 0.04) for mouse NAPE-PLD, although optimal activity compared to other inhibitors was retained (Table 7).
Lastly, the NAPE-PLD PED6 activity assay was used to compare the potency of LEI-401 to three reported inhibitors: lithocholic acid, ARN19874, and hexachlorophene (Figure S2, Table S1). ARN19874 and hexachlorophene were active (IC50 of 54 μM and 11 μM, respectively) in a similar order of magnitude as previously reported,16,17 whereas lithocholic acid was not active.
Conclusions
We have described the optimization of a library of pyrimidine-4-carboxamide derivatives as inhibitors of the NAE-producing enzyme NAPE-PLD. Our primary focus was to increase the potency of hit compound 2 and to improve its physicochemical properties to allow in vivo use. The main findings of the SAR of 2 are depicted in Figure 3. No improvement in inhibitory activity could be achieved by changing the substituent at R1, which suggests that it may bind in a shallow lipophilic pocket. Conformational restriction of the N-methylphenethylamine substituent at R2 by introduction of an (S)-3-phenylpiperidine afforded a 3-fold potency increase. Exchange of the morpholine group at R3 for the smaller and more polar (S)-3-hydroxypyrrolidine gave a 10-fold increase in activity when combined with the optimal R2 substituent. This provided the most potent NAPE-PLD inhibitor so far, termed LEI-401 (pIC50 ± SEM = 7.14 ± 0.04; Ki = 27 nM), with favorable drug-like properties. Previously, we have shown target engagement of LEI-401 with NAPE-PLD in live cell photoaffinity labeling experiments.1 Furthermore, LEI-401 decreased anandamide levels in neuronal cells and in mouse brain at a dose of 30 mg/kg (intraperitoneal injection). At this dose, LEI-401 displayed profound effects on mouse emotional behavior. We anticipate that LEI-401, by blocking the biosynthesis of NAEs, will provide new opportunities to study the biological role of NAPE-PLD and its enzymatic products in health and disease.
Figure 3.
Structure–activity map for the pyrimidine-4-carboxamide NAPE-PLD inhibitor library.
Experimental Section
Biological Procedures
Cloning of Plasmid DNA
Full length human cDNA of human or mouse NAPE-PLD (obtained from Natsuo Ueda) was cloned into mammalian expression vector pcDNA3.1, containing a C-terminal FLAG-tag and genes for ampicillin and neomycin resistance. All plasmids were grown in XL-10 Z-competent cells and prepped (Maxi Prep, Qiagen). Constructs were verified by Sanger sequencing (Macrogen).
Cell Culture
HEK293T cells (ATCC) were cultured at 37 °C and 7% CO2 in DMEM (Sigma Aldrich, D6546) with GlutaMax (2 mM), penicillin (100 μg/mL, Duchefa), streptomycin (100 μg/mL, Duchefa), and 10% (v/v) newborn calf serum (Seradigm). Cells were passaged twice a week to appropriate confluence by thorough pipetting.
Transient Transfection
One day before transfection 107 cells were seeded on a 15 cm dish. Two hours before transfection, the medium was refreshed with 13 mL of the medium. Transfection was performed with polyethyleneimine (PEI, 60 μg/dish) in a ratio of 3:1 with plasmid DNA (20 μg per dish). The PEI and plasmid DNA were incubated in serum-free medium (2 mL/dish) at rt for 15 min followed by dropwise addition to the cells. Transfection with the empty pcDNA3.1 vector was used to generate control (mock) samples. The medium was refreshed after 24 h and cells were harvested after 48 or 72 h in cold PBS. Cells were centrifuged (10 min, 200g, 4 °C), and the supernatant was removed. The cell pellets were flash-frozen in liquid N2 and stored at −80 °C.
Cell Lysate Preparation
Cell pellets were resuspended in cold lysis buffer (20 mM HEPES, 2 mM DTT, 0.25 M sucrose, 1 mM MgCl2, and 2.5 U/mL benzonase) and incubated 30 min in ice. The cytosolic fraction (supernatant) was separated from the membranes by ultracentrifugation (30 min, 100,000g, 4 °C). The pellet was resuspended in storage buffer: 20 mM HEPES and 2 mM DTT (membrane fraction). All samples were stored at −80 °C. Protein concentrations were determined using a Bradford assay (Bio-Rad).
NAPE-PLD Surrogate Substrate Activity Assay
The NAPE-PLD activity assay was performed as previously described.1 The membrane fraction from transient overexpression of human or mouse NAPE-PLD in HEK293T cells was diluted to 0.4 μg/μL in assay buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.02% v/v Triton X-100). The substrate PED6 (Invitrogen, D23739) 1 mM stock in DMSO was consecutively diluted in DMSO (10×) and in assay buffer (10×) to make a 10 μM working solution. Relevant concentrations of compounds (100× working solution) were prepared in DMSO. The assay was performed in a black Greiner 96-well plate (flat bottom), final volume of 100 μL. The compound or DMSO was incubated with membrane protein lysate (final concentration of 0.04 μg/μL) for 30 min at 37 °C. Then, substrate PED6 was added (final concentration of 1 μM) and the measurement was started immediately on a TECAN infinite M1000 pro at 37 °C (excitation 485 nm, emission 535 nm, gain = 100), scanning every 2 min for 1 h. Mock membrane lysate was used for background subtraction. The slope of t = 4 min to t = 14 min was used as the enzymatic rate (RFU/min), which was normalized to DMSO as 100%. IC50 curves were generated using Graphpad Prism v6 (log(inhibitor) vs normalized response with variable slope). Ki values were calculated from the Cheng–Prusoff equation Ki = IC50/(1 + ([S]/KM)) where KM = 0.59 μM for both mouse and human NAPE-PLD. All measurements were performed in N = 2 and n = 2 or N = 2 and n = 4 for controls and with Z′ ≥ 0.6.
Synthetic Procedures
General
All chemicals (Sigma-Aldrich, Fluka, Acros, Merck, Combi-Blocks, Fluorochem, TCI) were used as received. All solvents used for reactions were of analytical grade. THF, Et2O, DMF, CH3CN, and DCM were dried over activated 4 Å molecular sieves; MeOH over 3 Å molecular sieves. Flash chromatography was performed on silica gel (Screening Devices BV, 40–63 μm, 60 Å). The eluent EtOAc was of technical grade and distilled before use. Reactions were monitored by thin-layer chromatography (TLC) analysis using Merck aluminum sheets (silica gel 60, F254). Compounds were visualized by UV absorption (254 nm) and spraying for general compounds: KMnO4 (20 g/L) and K2CO3 (10 g/L) in water or, for amines, ninhydrin (0.75 g/L) and acetic acid (12.5 mL/L) in ethanol followed by charring at ∼150 °C. 1H and 13C NMR experiments were recorded on a Bruker AV-300 (300/75 MHz), Bruker AV-400 (400/101 MHz), Bruker DMX-400 (400/101 MHz), Bruker AV-500 (500/126 MHz), and Bruker AV-600 (600/151 MHz). Chemical shifts are given in parts per million (δ) relative to tetramethylsilane or CDCl3 as internal standards. Multiplicity: s = singlet, br s = broad singlet, d = doublet, dd = doublet of doublet, t = triplet, q = quartet, p = pentet, m = multiplet. Coupling constants (J) are given in hertz. LC–MS measurements were performed on a Thermo Finnigan LCQ Advantage MAX ion-trap mass spectrometer (ESI+) coupled to a Surveyor HPLC system (Thermo Finnigan) equipped with a standard C18 (Gemini, 4.6 mm D × 50 mm L, 5 μm particle size, Phenomenex) analytical column and buffers A: H2O, B: CH3CN, and C: 0.1% aq. TFA. High-resolution mass spectra were recorded on an LTQ Orbitrap (Thermo Finnigan) mass spectrometer or a Synapt G2-Si high-definition mass spectrometer (Waters) equipped with an electrospray ion source in the positive mode (source voltage of 3.5 kV, sheath gas flow of 10 mL/min, and capillary temperature of 250 °C) with resolution R = 60,000 at m/z 400 (mass range m/z = 150–2000) and dioctylphthalate (m/z = 391.28428) as a lock mass. Preparative HPLC was performed on a Waters Acquity Ultra Performance LC with a C18 column (Gemini, 150 x 21.2 mm, Phenomenex). All final compounds were determined to be >95% pure by integrating UV intensity recorded via HPLC.
General Procedure A
A microwave tube with a magnetic stir bar was charged with the appropriate 2-chloropyrimidine (1 equiv), n-BuOH (0.2 M), the appropriate amine (1.5 equiv), and DiPEA (3–4 equiv). The tube was capped, flushed with N2, and heated to 160 °C in a microwave reactor (75 W) for 4–36 h or heated to 120 °C in an oil bath for 1–6 days. When the reaction was completed as judged by LC–MS, it was transferred to a round-bottom flask, concentrated under reduced pressure, and coevaporated with toluene (2×). The residue was purified by silica gel column chromatography, affording the product, or alternatively by HPLC–MS purification, yielding the TFA salt. The free base was generated by dissolving the TFA salt in EtOAc followed by washing with sat. aq. NaHCO3 (2×). The organic layer was dried (Na2SO4), filtered, and concentrated under reduced pressure, affording the pure product.
General Procedure B
A round-bottom flask was charged with carboxylic acid (1 equiv) and dissolved in dry DMF (0.2 M). PyBOP (1.2–1.5 equiv), DiPEA (3–5 equiv), and the appropriate amine (1.2–5 equiv) were added, and the mixture was stirred overnight at rt. Work-up involved dilution with EtOAc, washing with H2O (1×) and brine (2×), drying (Na2SO4), filtering, and concentrating under reduced pressure. The residue was purified by silica gel column chromatography, affording the pure product.
General Procedure C
A microwave vial was charged with dichloropyrimidine (1 equiv) and dry MeOH (0.1 M) and cooled to 0 °C. DiPEA (1.5–2.5 equiv) and the appropriate amine (1.05 equiv) were added, and the mixture was stirred for 1–2 h at 0 °C. The solvents were evaporated under reduced pressure. The vial was charged with n-BuOH (0.2 M), N-methylphenethylamine (1.5 equiv), and DiPEA (3–4 equiv). The tube was capped, flushed with N2, and heated to 160 °C in a microwave reactor (75 W) for 4 h. When the reaction was completed as judged by LC–MS, it was transferred to a round-bottom flask, concentrated under reduced pressure, and co-evaporated with toluene (2×). The residue was purified by silica gel column chromatography, affording the product, or alternatively by HPLC–MS purification, yielding the TFA salt. The free base was generated by dissolving the TFA salt in EtOAc followed by washing with sat. aq. NaHCO3 (2x). The organic layer was dried (Na2SO4), filtered, and concentrated under reduced pressure, affording the pure product.
General Procedure D
A round-bottom flask with dry DCM (0.1 M) was charged via syringe with 2,6-dichloropyrimidine-4-carbonyl chloride (1 equiv) and cooled to −78 °C. Et3N (1.3–2.3 equiv) and the appropriate amine (1.025 equiv) were added, and the mixture was stirred, while letting the acetone bath warm up to 0 °C (3–4 h). The mixture was transferred to a separatory funnel, and the organic layer was washed with H2O (2×) and brine (1×), dried (Na2SO4), filtered, and concentrated under reduced pressure. Silica gel column chromatography afforded the pure amide.
General Procedure E
A round-bottom flask was charged with the dichloropyrimidine (1 equiv) and dry MeOH (0.1 M) and cooled to 0 °C. DiPEA (1.5–2.5 equiv) and the appropriate amine (1.05 equiv) were added, and the mixture was stirred for 1–2 h at 0 °C. The solvents were evaporated under reduced pressure, and the crude material was purified by silica gel column chromatography, affording the pure product.
General Procedure F
A round-bottom flask was charged with the dichloropyrimidine (1 equiv) and dry DMF (0.1 M). K2CO3 (1.5 equiv) and the appropriate phenol or heteroaryl (1.05 equiv) were added, and the mixture was stirred overnight at rt. H2O was added, and the mixture was extracted with EtOAc (3×). The organic layers were combined and washed with brine (2×), dried (Na2SO4), and concentrated under reduced pressure. The residue was purified by silica gel column chromatography, affording the pure product.
General Procedure G
Carbamoylation
a round-bottom flask was charged with the primary amine (1 equiv) and dry DCM (0.2 M). The solution was cooled to 0 °C and DiPEA (2 equiv) and methylchloroformate (1.5 equiv) were added. The reaction was stirred and allowed to warm up to room temperature over 1–2 h. Then, the mixture was diluted with DCM and washed with sat. aq. NaHCO3 (2×), brine (1×), dried (MgSO4), filtered, and concentrated under reduced pressure. The resulting crude material was purified by silica gel column chromatography, affording the methyl carbamate.
Carbamate Reduction
A round-bottom flask was charged with the methyl carbamate (1 equiv) and dry THF (0.15 M). The solution was cooled to 0 °C, and LiAlH4 (2 M in THF solution, 1.6 equiv) was added dropwise. The reaction was then stirred at reflux for 1–2 h. The Fieser workup involved dilution of the reaction mixture with Et2O (3×) and cooling to 0 °C followed by the sequential addition of water (1 μL for every 1 mg of LiAlH4), NaOH (aq) 15% (1 μL for every 1 mg of LiAlH4), and water (3 μL for every 1 mg of LiAlH4). The mixture was allowed to warm to room temperature and stirred for 15 min. Then, it was dried (MgSO4), filtered, and concentrated under reduced pressure to afford the product as a clear oil, which was used without further purification or purified by silica gel chromatography.
N-(Cyclopropylmethyl)-6-((S)-3-hydroxypyrrolidin-1-yl)-2-((S)-3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (1, LEI-401)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119aa (37 mg, 0.12 mmol, 1 equiv), DiPEA (65 μL, 0.37 mmol, 3 equiv), and (S)-3-phenylpiperidine (26 mg, 0.16 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 100% EtOAc/pentane) afforded the product (26 mg, 62 μmol, 51%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.40–7.20 (m, 5H), 6.53 (s, 1H), 4.84 (t, J = 14.3 Hz, 2H), 4.57 (s, 1H), 3.91–3.37 (m, 4H), 3.36–3.18 (m, 2H), 2.96–2.81 (m, 2H), 2.81–2.70 (m, 1H), 2.17–1.94 (m, 3H), 1.93–1.50 (m, 4H), 1.11–0.97 (m, 1H), 0.59–0.43 (m, 2H), 0.26 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.97, 162.17, 160.90, 155.63, 144.41, 128.62, 127.31, 126.58, 91.75, 71.02, 70.38, 54.95, 51.36, 44.68, 44.39, 44.05, 42.53, 32.19, 25.59, 10.91, 3.50. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2555 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-morpholino-pyrimidine-4-carboxamide (2)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (59 mg, 0.20 mmol, 1 equiv), N-methylphenethylamine HBr salt (66 mg, 0.30 mmol, 1.5 equiv), and DiPEA (140 μL, 0.80 mmol, 4 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (40 mg, 0.10 mmol, 52%). TLC: Rf = 0.3 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.34–7.25 (m, 2H), 7.25–7.12 (m, 3H), 6.72 (s, 1H), 3.88–3.72 (m, 6H), 3.72–3.55 (m, 4H), 3.30 (t, J = 6.5 Hz, 2H), 3.13 (s, 3H), 2.90 (t, J = 7.7 Hz, 2H), 1.14–0.99 (m, 1H), 0.64–0.44 (m, 2H), 0.38–0.19 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.66, 163.97, 160.86, 156.78, 139.92, 128.95, 128.58, 126.29, 90.08, 66.74, 51.68, 44.50, 44.11, 35.70, 33.93, 10.88, 3.48. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2387 found.
N-(Cyclopropylmethyl)-6-(methyl(phenethyl)amino)-4-morpholino-picolinamide (3)
A microwave vial with a magnetic stir bar under N2 was charged with 4-chloropyridine 111 (30 mg, 87 μmol, 1 equiv), morpholine (9 μL, 0.1 mmol, 1.2 equiv), and dry toluene (87 μL). The vial was capped, and the solution was purged with N2. This was followed by the addition of RuPhosPd G3 (0.01 M THF solution, 237 μL, 2.37 μmol, 0.027 equiv) and NaOtBu (2 M THF solution, 97 μL, 0.19 mmol, 2.2 equiv), and the mixture was purged again with N2 and stirred in a preheated oil bath at 110 °C for 44 h. The mixture was filtered through a plug of Celite, and the filtrate was concentrated under reduced pressure. The crude material was purified by silica gel column chromatography (30% → 60% EtOAc/pentane), affording the product (5 mg, 13 μmol, 15%). TLC: Rf = 0.2 (30% EtOAc/pentane) and recovered starting material (11 mg, 32 μmol, 37%). 1H NMR (500 MHz, CDCl3) δ 8.03 (t, J = 5.7 Hz, 1H), 7.29 (t, J = 7.3 Hz, 2H), 7.24–7.13 (m, 4H), 5.77 (d, J = 2.0 Hz, 1H), 3.91–3.81 (m, 4H), 3.63 (t, J = 7.4 Hz, 2H), 3.51–3.40 (m, 4H), 3.34–3.29 (m, 2H), 2.92–2.83 (m, 5H), 1.11–1.05 (m, 1H), 0.58–0.50 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 165.54, 160.20, 156.01, 148.69, 139.16, 129.00, 128.80, 126.61, 99.04, 90.28, 66.98, 54.09, 46.37, 44.16, 38.55, 33.55, 11.08, 3.57. HRMS [C23H30N4O2 + H]+: 395.2442 calculated, 395.2438 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-morpholino-isonicotinamide (4)
A microwave vial with a magnetic stir bar under N2 was charged with 2-chloropyridine 115 (31 mg, 0.1 mmol, 1 equiv), N-methylphenethylamine HBr salt (28 mg, 0.13 mmol, 1.3 equiv), and dry toluene (0.1 mL). The vial was capped, and the solution purged with N2. This was followed by the addition of RuPhosPd G3 (0.01 M THF solution, 100 μL, 1 μmol, 0.01 equiv) and NaOtBu (2 M THF solution, 120 μL, 0.24 mmol, 2.4 equiv), and the mixture was purged again with N2 and stirred in a preheated oil bath at 110 °C. After 24 h, the reaction was complete as judged by LC–MS. The mixture was filtered through a plug of Celite, and the filtrate was concentrated under reduced pressure to provide the crude material. Purification by HPLC (C18 reverse phase, 45% → 55% CH3CN/H2O + 0.2% TFA, RT, 12.3 min) afforded the product (16 mg, 40 μmol, 41%). TLC: Rf = 0.5 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.35–7.24 (m, 2H), 7.25–7.14 (m, 3H), 6.24–6.13 (m, 2H), 6.10 (s, 1H), 3.87–3.78 (m, 4H), 3.78–3.67 (m, 2H), 3.59–3.47 (m, 4H), 3.28 (dd, J = 7.2, 5.4 Hz, 2H), 2.98 (s, 3H), 2.93–2.82 (m, 2H), 1.15–0.95 (m, 1H), 0.64–0.48 (m, 2H), 0.35–0.19 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 168.07, 159.06, 157.76, 145.86, 140.05, 128.98, 128.60, 126.26, 92.90, 91.48, 66.94, 52.51, 45.80, 44.97, 36.87, 33.92, 10.82, 3.68. HRMS [C23H30N4O2 + H]+: 395.2442 calculated, 395.2434 found.
N-(Cyclopropylmethyl)-6-(methyl(phenethyl)amino)-2-morpholinopyrimidine-4-carboxamide (5)
The title compound was prepared according to General Procedure A using 4-chloropyrimidine 129 (21 mg, 70 μmol, 1.0 equiv), N-methylphenethylamine HBr salt (16 mg, 70 μmol, 1 equiv), and DiPEA (36.6 μL, 0.21 mmol, 3 equiv) in MeOH. Total heating time: 6 h at 70 °C. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (20 mg, 50 μmol, 71%). TLC: Rf = 0.4 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.44 (br s, 1H), 7.33–7.27 (m, 2H), 7.25–7.13 (m, 3H), 6.84 (s, 1H), 3.79 (br s, 10H), 3.38–3.22 (m, 2H), 3.02 (s, 3H), 2.90 (t, J = 7.4 Hz, 2H), 1.14–1.00 (m, 1H), 0.62–0.46 (m, 2H), 0.36–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 162.97, 159.05, 128.91, 128.82, 126.75, 93.22, 66.81, 44.92, 44.69, 10.71, 3.65. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2385 found.
N-(Cyclopropylmethyl)-4-(methyl(phenethyl)amino)-6-morpholino-1,3,5-triazine-2-carboxamide (6)
A round-bottom flask was charged with carboxylic acid 134 (17 mg, 50 μmol, 1 equiv), PyBOP (39 mg, 75 μmol, 1.5 equiv), DiPEA (35 μL, 0.2 mmol, 4.0 equiv), cyclopropylmethanamine (5.2 μL, 60 μmol, 1.2 equiv), and DMF (0.25 mL). The solution was stirred for 70 h, diluted with water (20 mL), and extracted with EtOAc (20 mL). The organic layer was washed with brine (2× 20 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (40%→ 80% EtOAc/pentane), affording the product (13 mg, 34 μmol, 67%). 1Η NMR analysis showed two rotamers in a ratio of 1:1 (CDCl3, 295 K), which was confirmed by high temperature 1H NMR experiments. 1H NMR (500 MHz, CDCl3, T = 295 K) δ 7.93–7.80 (m, 1H), 7.29 (t, J = 7.5 Hz, 2H), 7.25–7.15 (m, 3H), 3.99–3.77 (m, 4H), 3.89–3.76 (m, 2H), 3.77–3.69 (m, 4H), 3.31–3.26 (m, 2H), 3.13 (d, J = 38.5 Hz, 3H), 2.90 (q, J = 8.1 Hz, 2H), 1.12–1.00 (m, 1H), 0.59–0.50 (m, 2H), 0.32–0.25 (m, 2H). 13C NMR (126 MHz, CDCl3, T = 295 K) δ 165.20 (d, J = 34.3 Hz), 164.96 (d, J = 6.8 Hz), 164.32 (d, J = 20.0 Hz), 163.21 (d, J = 2.9 Hz), 139.27 (d, J = 5.3 Hz), 128.88, 128.67, 126.53 (d, J = 4.6 Hz), 66.89, 51.01 (d, J = 10.9 Hz), 44.47, 43.87, 35.22 (d, J = 30.2 Hz), 33.85 (d, J = 90.2 Hz), 10.84 (d, J = 2.3 Hz), 3.54. 1H NMR (500 MHz, CDCl3, T = 332 K) δ 7.78 (br s, 1H), 7.29–7.24 (m, 2H), 7.23–7.13 (m, 3H), 3.85 (br s, 6H), 3.74–3.69 (m, 4H), 3.31–3.26 (m, 2H), 3.12 (d, J = 31.4 Hz, 3H), 2.91 (t, J = 7.5 Hz, 2H), 1.10–1.00 (m, 1H), 0.57–0.49 (m, 2H), 0.30–0.24 (m, 2H). 13C NMR (126 MHz, CDCl3, T = 332 K) δ 165.39, 165.39, 164.71, 163.32, 139.44, 128.93, 128.72, 126.57, 66.95, 51.06, 44.49, 44.12, 35.19, 33.73, 10.93, 3.50. HRMS [C21H28N6O2 + H]+: 397.2347 calculated, 397.2343 found.
N-(Cyclopropylmethyl)-N-methyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (7)
A round-bottom flask was charged with amide 2 (36 mg, 90 μmol, 1 equiv), dry DMF (1.5 mL), and cooled to 0 °C. NaH (60% in mineral oil, 4 mg, 0.10 mmol, 1.1 equiv) was added, and the mixture was stirred for 30 min followed by addition of methyl iodide (11 μL, 0.18 mmol, 2 equiv). The reaction was allowed to warm to rt while stirring overnight. The reaction was quenched with H2O (20 mL) followed by extraction with EtOAc (3× 20 mL). The combined organic layers were washed with brine (1× 50 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (50 → 80% EtOAc/pentane) affording the product (18 mg, 40 μmol, 48%). TLC: Rf = 0.3 (60% EtOAc/pentane). 1Η NMR analysis showed two rotamers in a ratio of 6:4 (CDCl3, 298 K), which was confirmed by high-temperature 1H NMR experiments. 1H NMR (400 MHz, CDCl3) δ 7.32–7.24 (m, 2H), 7.23–7.16 (m, 3H), 6.07–6.02 (m, 1H), 3.83–3.71 (m, 6H), 3.62–3.55 (m, 4H), 3.43–3.23 (m, 2H), 3.16–3.10 (m, 3H), 3.10–3.02 (m, 3H), 2.92–2.83 (m, 2H), 1.14–1.01 (m, 1H), 0.60–0.43 (m, 2H), 0.36–0.12 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 163.34, 162.35, 160.82, 156.31, 140.04, 128.91, 128.52, 126.20, 90.43, 66.76, 55.26, 51.72, 51.57, 51.44, 44.33, 36.75, 35.80, 35.69, 33.94, 33.10, 10.33, 9.26, 3.66. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2545 found.
N-Cyclopropyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (8)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (34 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), PyBOP (78 mg, 0.12 mmol, 1.2 equiv), and cyclopropylamine (8.3 μL, 0.12 mmol, 1.2 equiv). Column chromatography (50% → 80% EtOAc/pentane) afforded the product (8 mg, 44 μmol, 66%). TLC: Rf = 0.3 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.92 (br s, 1H), 7.34–7.27 (m, 2H), 7.25–7.15 (m, 3H), 6.70 (s, 1H), 3.84–3.72 (m, 6H), 3.66 (br s, 4H), 3.09 (s, 3H), 2.94–2.82 (m, 3H), 1.36–1.21 (m, 1H), 0.92–0.82 (m, 2H), 0.67–0.58 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 166.25, 163.97, 160.84, 156.51, 139.98, 128.84, 128.64, 126.34, 89.86, 66.78, 51.67, 44.52, 35.72, 33.98, 22.53, 6.78. HRMS [C21H27N5O2 + H]+: 382.2238 calculated, 382.2241 found.
2-(Methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (9)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (27 mg, 79 μmol, 1 equiv), DiPEA (56 μL, 0.32 mmol, 4 equiv), PyBOP (62 mg, 0.12 mmol, 1.5 equiv), HOBt (16 mg, 0.12 mmol, 1.5 equiv), and ammonium chloride (15 mg, 0.32 mmol, 3.5 equiv). Column chromatography (80% → 100% EtOAc/pentane) afforded the product (20 mg, 59 μmol, 74%). TLC: Rf = 0.5 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.74 (br s, 1H), 7.34–7.25 (m, 2H), 7.25–7.16 (m, 3H), 6.71 (br s, 1H), 5.83 (s, 1H), 3.86–3.72 (m, 6H), 3.66 (br s, 4H), 3.09 (s, 3H), 2.90 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 167.46, 163.96, 161.04, 156.27, 139.93, 128.86, 128.62, 126.31, 90.17, 66.74, 51.60, 44.50, 35.77, 33.97. HRMS [C18H23N5O2 + H]+: 342.1925 calculated, 342.1934 found.
N-Methyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide TFA Salt (10)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119a (8.5:1 mixture of regioisomers) (51 mg, 0.20 mmol, 1 equiv), DiPEA (139 μL, 0.80 mmol, 4 equiv), and N-methylphenethylamine HBr salt (65 mg, 0.30 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Purification by preparative HPLC (C18 reverse phase, 25% to 35% CH3CN/H2O + 0.2% TFA, RT = 8.77) afforded the product as the TFA salt (83 mg, 0.18 mmol, 88%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, MeOD) δ 7.30–7.12 (m, 5H), 6.90 (s, 1H), 3.92 (t, J = 7.0 Hz, 2H), 3.85–3.69 (m, 8H), 3.18 (s, 3H), 3.00–2.88 (m, 5H). 13C NMR (101 MHz, MeOD) δ 162.45, 162.16 (q, J = 35.8 Hz), 154.95, 147.48, 139.88, 129.99, 129.67, 127.62, 117.79 (q, J = 291.3 Hz), 94.22, 67.37, 53.01, 46.62, 36.22, 34.27, 26.90. HRMS [C19H25N5O2 + H]+: 356.2081 calculated, 356.2079 found.
N-Ethyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (11)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119b (54 mg, 0.20 mmol, 1 equiv), DiPEA (139 μL, 0.80 mmol, 4 equiv), and N-methylphenethylamine HBr salt (65 mg, 0.30 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (50% → 70% EtOAc/pentane) afforded the product (64 mg, 0.17 mmol, 86%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.88 (s, 1H), 7.32–7.26 (m, 2H), 7.24–7.16 (m, 3H), 6.71 (s, 1H), 3.84–3.77 (m, 2H), 3.77–3.73 (m, 4H), 3.69–3.62 (m, 4H), 3.50–3.42 (m, 2H), 3.11 (s, 3H), 2.94–2.84 (m, 2H), 1.25 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 164.65, 163.98, 160.88, 156.82, 139.99, 128.83, 128.59, 126.29, 90.02, 66.74, 51.63, 44.51, 35.71, 34.26, 33.98, 14.94. HRMS [C20H27N5O2 + H]+: 370.2238 calculated, 370.2236 found.
N-Propyl-2-(methyl(phenethyl)amino)-6-morpholino-pyrimidine-4-carboxamide (12)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (23 mg, 67 μmol, 1 equiv), DiPEA (60 μL, 0.34 mmol, 3 equiv), PyBOP (52 mg, 0.10 mmol, 1.5 equiv), and propylamine HCl salt (8 mg, 80 μmol, 1.2 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (17 mg, 44 μmol, 66%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.97 (br s, 1H), 7.34–7.25 (m, 2H), 7.25–7.16 (m, 3H), 6.72 (s, 1H), 3.87–3.72 (m, 6H), 3.66 (br s, 4H), 3.39 (q, J = 6.7 Hz, 2H), 3.10 (s, 3H), 2.89 (t, J = 7.5 Hz, 2H), 1.68–1.60 (m, 2H), 0.99 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.80, 164.01, 160.86, 156.82, 139.98, 128.85, 128.61, 126.31, 90.06, 66.77, 51.65, 44.52, 41.08, 35.73, 33.98, 23.01, 11.61. HRMS [C21H29N5O2 + H]+: 384.2394 calculated, 384.2394 found.
N-Butyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (13)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119c (30 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 45 h at 120 °C. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (29 mg, 73 μmol, 73%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.94 (br s, 1H), 7.33–7.25 (m, 2H), 7.24–7.16 (m, 3H), 6.72 (s, 1H), 3.84–3.72 (m, 6H), 3.66 (br s, 4H), 3.42 (q, J = 6.6 Hz, 2H), 3.11 (s, 3H), 2.95–2.84 (m, 2H), 1.60 (p, J = 7.1 Hz, 2H), 1.42 (h, J = 7.3 Hz, 2H), 0.96 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.74, 164.00, 160.86, 156.83, 139.97, 128.84, 128.60, 126.31, 90.05, 66.76, 51.64, 44.52, 39.12, 35.72, 33.97, 31.81, 20.30, 13.94. HRMS [C22H31N5O2 + H]+: 398.2551 calculated, 398.2560 found.
N-Hexyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (14)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119d (33 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 3 days at 120 °C. Column chromatography (40% → 50% EtOAc/ pentane) afforded the product (36 mg, 85 μmol, 85%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.33–7.25 (m, 2H), 7.25–7.17 (m, 3H), 6.72 (s, 1H), 3.83–3.73 (m, 6H), 3.71–3.62 (m, 4H), 3.41 (q, J = 6.9 Hz, 2H), 3.10 (s, 3H), 2.95–2.85 (m, 2H), 1.61 (p, J = 7.6, 7.2 Hz, 2H), 1.44–1.27 (m, 6H), 0.94–0.84 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 164.71, 164.01, 160.86, 156.85, 139.97, 128.84, 128.60, 126.30, 90.05, 66.76, 51.65, 44.53, 39.43, 35.73, 33.98, 31.63, 29.68, 26.78, 22.67, 14.14. HRMS [C24H35N5O2 + H]+: 426.2864 calculated, 426.2857 found.
N-Isobutyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (15)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119e (30 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 3 days at 120 °C. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (29 mg, 73 μmol, 73%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (br s, 1H), 7.33–7.25 (m, 2H), 7.25–7.16 (m, 3H), 6.72 (s, 1H), 3.88–3.73 (m, 6H), 3.73–3.62 (m, 4H), 3.26 (t, J = 6.5 Hz, 2H), 3.10 (s, 3H), 2.96–2.85 (m, 2H), 1.97–1.82 (m, 1H), 0.98 (d, J = 6.7 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 164.77, 164.01, 160.84, 156.84, 139.94, 128.84, 128.58, 126.29, 90.07, 66.75, 51.63, 46.65, 44.52, 35.72, 33.98, 28.80, 20.28. HRMS [C22H31N5O2 + H]+: 398.2551 calculated, 398.2552 found.
N-Neopentyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (16)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119f (31 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 3 days at 120 °C. Column chromatography (20% → 50% EtOAc/pentane) afforded the product (30 mg, 73 μmol, 73%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.14 (br s, 1H), 7.32–7.25 (m, 2H), 7.24–7.17 (m, 3H), 6.73 (s, 1H), 3.85–3.78 (m, 2H), 3.78–3.73 (m, 4H), 3.71–3.61 (m, 4H), 3.23 (d, J = 6.6 Hz, 2H), 3.10 (s, 3H), 2.95–2.86 (m, 2H), 0.97 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 164.82, 164.03, 160.82, 156.82, 139.89, 128.85, 128.59, 126.30, 90.15, 66.76, 51.62, 50.57, 44.53, 35.72, 33.99, 32.28, 27.38. HRMS [C23H33N5O2 + H]+: 412.2707 calculated, 412.2710 found.
2-(Methyl(phenethyl)amino)-6-morpholino-N-(prop-2-yn-1-yl)pyrimidine-4-carboxamide (17)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119g (42 mg, 0.15 mmol, 1 equiv), DiPEA (105 μL, 0.60 mmol, 4 equiv), and N-methylphenethylamine HBr salt (49 mg, 0.225 mmol, 1.5 equiv). Total heating time: 45 h at 120 °C. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (43 mg, 0.11 mmol, 76%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.33–7.25 (m, 2H), 7.25–7.17 (m, 3H), 6.69 (s, 1H), 4.22 (dd, J = 5.6, 2.5 Hz, 2H), 3.86–3.72 (m, 6H), 3.72–3.60 (m, 4H), 3.11 (s, 3H), 2.96–2.85 (m, 2H), 2.29 (t, J = 2.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 164.60, 163.89, 160.88, 156.03, 139.91, 128.90, 128.60, 126.30, 90.15, 79.57, 71.67, 66.72, 51.70, 44.50, 35.75, 33.98, 29.21. HRMS [C21H25N5O2 + H]+: 380.2081 calculated, 380.2089 found.
N-(2,2,2-Trifluoroethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (18)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (25 mg, 73 μmol, 1 equiv), DiPEA (51 μL, 0.29 mmol, 4 equiv), PyBOP (57 mg, 0.11 mmol, 1.5 equiv), and 2,2,2-trifluoroethylamine HCl salt (12 mg, 88 μmol, 1.2 equiv). Column chromatography (30% → 40% EtOAc/pentane) afforded the product (17 mg, 40 μmol, 55%). TLC: Rf = 0.8 (50% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.20 (br s, 1H), 7.32–7.26 (m, 2H), 7.23–7.17 (m, 3H), 6.70 (s, 1H), 4.12–4.02 (m, 2H), 3.83–3.78 (m, 2H), 3.78–3.74 (m, 4H), 3.71–3.59 (m, 4H), 3.11 (s, 3H), 2.92–2.86 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 165.31, 163.88, 160.92, 155.42, 139.85, 128.86, 128.65, 126.37, 124.31 (q, J = 278.4 Hz), 90.45, 66.75, 51.70, 44.58, 40.89 (q, J = 34.8 Hz), 35.77, 34.04. HRMS [C20H24F3N5O2 + H]+: 424.1955 calculated, 424.1958 found.
2-(Methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxylic acid (19)
Ester Hydrolysis
A round-bottom flask was charged with methyl ester 127 (680 mg, 2.64 mmol, 1 equiv) in 12.5 mL of THF/MeOH (4:1). A 1.5 M aqueous NaOH solution (1.76 mL, 2.64 mmol, 1 equiv) was added together with 0.75 mL of H2O. The reaction was stirred overnight at rt after which the solvents were evaporated yielding the product as the Na+ salt (128), which was used without further purification (779 mg, 2.64 mmol, quant.).
SNAr Reaction
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 128 (244 mg, 1.0 mmol, 1 equiv), DiPEA (0.52 mL, 3.0 mmol, 3 equiv), and N-methylphenethylamine (189 μL, 1.3 mmol, 1.3 equiv). Total heating time: 6 days at 120 °C. Column chromatography (2.5% → 15% MeOH/DCM) afforded the product (175 mg, 0.51 mmol, 51%). TLC: Rf = 0.5 (100% EtOAc with 3 drops of AcOH). 1H NMR (400 MHz, MeOD + CDCl3) δ 7.35–7.26 (m, 2H), 7.26–7.15 (m, 3H), 6.87 (s, 1H), 3.91 (t, J = 7.0 Hz, 2H), 3.81 (br s, 8H), 3.17 (s, 3H), 2.97 (t, J = 7.0 Hz, 2H). 13C NMR (101 MHz, MeOD + CDCl3) δ 161.70, 152.17, 148.40, 137.57, 133.96, 128.50, 128.38, 126.57, 93.89, 66.02, 51.86, 45.10, 33.13. HRMS [C18H22N4O3 + H]+: 343.1765 calculated, 343.1772 found.
(2-(Methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carbonyl)glycine (20)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 135 (150 mg, 0.50 mmol, 1 equiv), DiPEA (0.43 mL, 2.5 mmol, 5 equiv), and N-methylphenethylamine HBr salt (162 mg, 0.76 mmol, 1.5 equiv). Total heating time: 6 h at 160 °C with μW irradiation. Purification by HPLC (C18 reverse phase, 10% to 70% CH3CN/H2O + 50 mM NH4HCO3) afforded the product (40 mg, 0.10 mmol, 20%). TLC: Rf = 0.3 (5% MeOH/DCM). 1H NMR (500 MHz, MeOD + CDCl3) δ 7.32–7.14 (m, 5H), 6.65 (s, 1H), 4.01 (s, 2H), 3.83 (t, J = 7.3 Hz, 2H), 3.80–3.75 (m, 4H), 3.73–3.60 (m, 4H), 3.11 (s, 3H), 2.91 (t, J = 7.4 Hz, 2H). 13C NMR (126 MHz, MeOD + CDCl3) δ 173.63, 164.96, 163.55, 160.59, 155.89, 139.54, 128.50, 128.00, 125.67, 89.33, 66.26, 51.10, 49.06, 43.96, 42.64, 39.58, 35.05, 33.47, 20.71. HRMS [C20H25N5O4 + H]+: 400.1979 calculated, 400.1984 found.
Methyl (2-(Methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carbonyl) Glycinate (21)
A round-bottom flask was charged with carboxylic acid 20 (28 mg, 70 μmol, 1 equiv) in dry DCM (1.5 mL). This was followed by addition of HOBt (15 mg, 0.11 mmol, 1.5 equiv) and EDC·HCl (20 mg, 0.11 mmol, 1.5 equiv). The reaction was stirred for 1 h at rt, and after which, MeOH (11 μL, 0.28 mmol, 4 equiv) was added and then stirred overnight at rt. The reaction was diluted with EtOAc (25 mL), washed with sat. aq. NaHCO3 (2× 25 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified using silica gel column chromatography (60% → 80% EtOAc/pentane) affording the product (18 mg, 44 μmol, 62%). TLC: Rf = 0.3 (70% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.40 (br s, 1H), 7.39–7.13 (m, 5H), 6.69 (s, 1H), 4.22 (d, J = 5.5 Hz, 2H), 3.86–3.71 (m, 9H), 3.71–3.59 (m, 4H), 3.11 (s, 3H), 2.96–2.85 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 170.25, 165.14, 163.91, 160.92, 155.99, 139.96, 128.98, 128.58, 126.27, 90.13, 66.75, 52.52, 51.68, 44.51, 41.37, 35.79, 34.01. HRMS [C21H27N5O4 + H]+: 414.2136 calculated, 414.2144 found.
N-(2-(Methylamino)-2-oxoethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (22)
The title compound was prepared according to General Procedure B using carboxylic acid 20 (12 mg, 30 μmol, 1 equiv), DiPEA (21 μL, 120 μmol, 4 equiv), PyBOP (19 mg, 45 μmol, 1.5 equiv), and methylamine HCl salt (3 mg, 36 μmol, 1.2 equiv). Column chromatography (2.5% → 10% MeOH/DCM) afforded the product (6 mg, 15 μmol, 48%). TLC: Rf = 0.4 (5% MeOH/DCM). 1H NMR (500 MHz, CDCl3) δ 8.43 (br s, 1H), 7.32–7.26 (m, 2H), 7.23–7.16 (m, 3H), 6.69 (br s, 1H), 6.22 (br s, 1H), 4.08 (d, J = 6.1 Hz, 2H), 3.86–3.79 (m, 2H), 3.79–3.73 (m, 4H), 3.67 (br s, 4H), 3.10 (s, 3H), 2.93–2.86 (m, 2H), 2.84 (d, J = 4.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 169.58, 165.74, 163.84, 160.90, 155.66, 139.91, 128.93, 128.65, 126.36, 90.21, 66.75, 51.65, 44.60, 43.84, 35.86, 34.06, 26.41. HRMS [C21H28N6O3 + H]+: 413.2296 calculated, 413.2294 found.
N-(2-Hydroxyethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (23)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (39 mg, 0.11 mmol, 1 equiv), DiPEA (60 μL, 0.34 mmol, 3 equiv), PyBOP (89 mg, 0.17 mmol, 1.5 equiv), and ethanolamine (34 μL, 0.57 mmol, 5 equiv). Column chromatography (70% → 100% EtOAc/pentane to 5% MeOH/EtOAc) afforded the product (25 mg, 65 μmol, 59%). TLC: Rf = 0.3 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.29 (br s, 1H), 7.33–7.27 (m, 2H), 7.24–7.16 (m, 3H), 6.70 (s, 1H), 3.87–3.78 (m, 4H), 3.78–3.74 (m, 4H), 3.70–3.62 (m, 4H), 3.62–3.55 (m, 2H), 3.10 (s, 3H), 2.98–2.80 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 166.19, 163.93, 160.87, 156.29, 139.96, 128.89, 128.61, 126.32, 90.14, 66.74, 62.71, 51.61, 44.51, 42.71, 35.77, 33.98. HRMS [C20H27N5O3 + H]+: 386.2187 calculated, 386.2191 found.
N-(2-Methoxyethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (24)
A round-bottom flask was charged with alcohol 23 (17 mg, 44 μmol, 1 equiv) in dry DMF (1 mL) and cooled to 0 °C. NaOtBu (2 M in THF, 33 μL, 66 μmol, 1.5 equiv) and methyl iodide (3.1 μL, 49 μmol, 1.1 equiv) were added. The reaction was allowed to warm to rt while stirring overnight. EtOAc (25 mL) was added followed by washing with H2O (1× 25 mL) and brine (2× 25 mL), drying (Na2SO4), filtering, and concentrating under reduced pressure. The residue was purified by silica gel column chromatography (70 → 80% EtOAc/pentane), affording the product (5 mg, 13 μmol, 28%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.26 (br s, 1H), 7.33–7.27 (m, 2H), 7.25–7.16 (m, 3H), 6.71 (s, 1H), 3.85–3.71 (m, 6H), 3.72–3.59 (m, 6H), 3.59–3.51 (m, 2H), 3.38 (s, 3H), 3.11 (s, 3H), 2.95–2.83 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.96, 163.98, 160.89, 156.67, 139.95, 128.93, 128.62, 126.29, 90.08, 71.38, 66.78, 59.02, 51.72, 44.53, 39.25, 35.77, 33.96. HRMS [C21H29N5O3 + H]+: 400.2343 calculated, 400.2345 found.
N-(Cyanomethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (25)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (21 mg, 61 μmol, 1 equiv), DiPEA (53 μL, 0.31 mmol, 5 equiv), PyBOP (48 mg, 92 μmol, 1.5 equiv), and aminoacetonitrile bisulfate (11 mg, 73 μmol, 1.2 equiv). Column chromatography (50% - > 70% EtOAc/pentane) afforded the product (10 mg, 26 μmol, 43%). TLC: Rf = 0.6 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.12 (br s, 1H), 7.35–7.27 (m, 2H), 7.25–7.14 (m, 3H), 6.67 (s, 1H), 4.34 (d, J = 6.1 Hz, 2H), 3.86–3.79 (m, 2H), 3.79–3.74 (m, 4H), 3.66 (br s, 4H), 3.10 (s, 3H), 2.95–2.84 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 165.12, 163.72, 160.92, 154.96, 139.94, 128.88, 128.68, 126.41, 115.95, 90.37, 66.71, 51.58, 44.50, 35.86, 34.05, 27.52. HRMS [C20H24N6O2 + H]+: 381.2034 calculated, 381.2042 found.
N-(Thiazol-2-ylmethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (26)
The title compound was prepared according to General Procedure B using carboxylic acid 19 (27 mg, 79 μmol, 1 equiv), DiPEA (82 μL, 0.47 mmol, 6 equiv), PyBOP (62 mg, 0.12 mmol, 1.5 equiv), and 2-aminomethylthiazole double HCl salt (19 mg, 0.10 mmol, 1.3 equiv). Purification by preparative HPLC (C18 reverse phase, 34% to 37% CH3CN/H2O + 0.2% TFA) afforded the product (11 mg, 25 μmol, 32%). TLC: Rf = 0.3 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.62 (br s, 1H), 7.74 (d, J = 3.3 Hz, 1H), 7.30 (d, J = 3.3 Hz, 1H), 7.30–7.21 (m, 2H), 7.23–7.15 (m, 3H), 6.75 (s, 1H), 4.95 (d, J = 6.2 Hz, 2H), 3.86–3.72 (m, 6H), 3.72–3.60 (m, 4H), 3.10 (s, 3H), 2.94–2.82 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 167.49, 165.05, 163.87, 160.86, 155.95, 142.63, 139.91, 128.91, 128.59, 126.29, 119.73, 90.33, 66.75, 51.65, 44.53, 41.06, 35.81, 33.99. HRMS [C22H26N6O2S + H]+: 439.1911 calculated, 439.1913 found.
N-Benzyl-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (27)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119i (67 mg, 0.20 mmol, 1 equiv), DiPEA (139 μL, 0.80 mmol, 4 equiv), and N-methylphenethylamine HBr salt (65 mg, 0.30 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (75 mg, 0.17 mmol, 87%). TLC: Rf = 0.8 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.25 (br s, 1H), 7.39–7.33 (m, 4H), 7.33–7.26 (m, 1H), 7.24–7.14 (m, 3H), 7.14–7.04 (m, 2H), 6.76 (s, 1H), 4.63 (d, J = 6.1 Hz, 2H), 3.80–3.71 (m, 6H), 3.71–3.62 (m, 4H), 3.08 (s, 3H), 2.89–2.80 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.83, 163.90, 160.82, 156.52, 139.79, 138.35, 128.80, 128.77, 128.54, 127.69, 127.51, 126.20, 90.17, 66.70, 51.63, 44.46, 43.37, 35.68, 33.88. HRMS [C25H29N5O2 + H]+: 432.2394 calculated, 432.2390 found.
N-([1,1′-Biphenyl]-4-ylmethyl)-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (28)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119j (41 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40%→ 60% EtOAc/pentane) afforded the product (40 mg, 79 μmol, 79%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.29 (br s, 1H), 7.57 (d, J = 7.9 Hz, 4H), 7.47–7.39 (m, 4H), 7.38–7.30 (m, 1H), 7.23–7.07 (m, 5H), 6.77 (s, 1H), 4.68 (d, J = 6.1 Hz, 2H), 3.81–3.72 (m, 6H), 3.71–3.61 (m, 4H), 3.08 (s, 3H), 2.90–2.81 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.92, 163.94, 160.86, 156.54, 140.84, 140.51, 139.82, 137.43, 128.88, 128.79, 128.56, 128.17, 127.56, 127.42, 127.17, 126.24, 90.22, 66.73, 51.65, 44.50, 43.12, 35.73, 33.91. HRMS [C31H33N5O2 + H]+: 508.2707 calculated, 508.2704 found.
4-(5-Cyclopropyl-1H-imidazol-2-yl)-N-methyl-6-morpholino-N-phenethylpyrimidin-2-amine (29)
Acyloxymethylketone Synthesis
A round-bottom flask was charged with carboxylic acid 19 (53 mg, 0.15 mmol, 1 equiv) in dry DMF (1 mL). Cs2CO3 (91 mg, 0.28 mmol, 1.8 equiv) and 2-bromocyclopropylethanone (16 μL, 0.16 mmol, 1.05 equiv) were added, and the mixture was stirred for 1.5 h. The reaction was diluted with EtOAc (25 mL), and the mixture was washed with H2O (1× 25 mL) and brine (2× 25 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (30% → 50% EtOAc/pentane), affording the acyloxymethylketone 136 (34 mg, 80 μmol, 53%). 1H NMR (400 MHz, CDCl3) δ 7.36–7.12 (m, 5H), 6.63 (s, 1H), 5.04 (s, 2H), 3.87–3.78 (m, 2H), 3.78–3.72 (m, 4H), 3.71–3.53 (m, 4H), 3.14 (s, 3H), 2.97–2.80 (m, 2H), 2.16–2.00 (m, 1H), 1.22–1.08 (m, 2H), 1.04–0.88 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 203.64, 165.50, 163.61, 161.85, 154.67, 139.97, 128.93, 128.50, 126.17, 93.17, 69.31, 66.69, 51.54, 44.41, 35.64, 33.77, 17.38, 11.70.
Imidazole Synthesis
A microwave vial was charged with acyloxymethylketone 136 (34 mg, 80 μmol, 1 equiv) and NH4OAc (31 mg, 0.40 mmol, 5 equiv) in xylene (0.7 mL). The vial was capped and stirred at 140 °C for 2 h. Purification by preparative HPLC (C18 reverse phase, 35% to 40% CH3CN/H2O + 0.2% TFA) afforded the product (2 mg, 5 μmol, 6%). TLC: Rf = 0.7 (40% EtOAc/pentane). 1H NMR (850 MHz, CDCl3) δ 9.89 (br s, 1H), 7.33–7.27 (m, 2H), 7.24–7.16 (m, 3H), 6.98–6.49 (m, 2H), 3.84 (s, 2H), 3.80–3.73 (m, 4H), 3.68 (br s, 4H), 3.14 (s, 3H), 2.97–2.83 (m, 2H), 1.91 (br s, 1H), 0.92 (br s, 2H), 0.74 (br s, 2H). 13C NMR (214 MHz, CDCl3) δ 163.48, 161.12, 154.40, 145.32, 140.18, 128.91, 128.66, 126.35, 111.74, 87.19, 66.84, 51.62, 44.66, 35.84, 34.08, 9.44, 7.18, 6.01. HRMS [C23H28N5O + H]+: 405.2397 calculated, 405.2403 found.
2-Cyclopropyl-N-(2-(methyl(phenethyl)amino)-6-morpholinopyrimidin-4-yl)acetamide (30)
A round-bottom flask was charged with 2-cyclopropylacetic acid (10.5 μL, 0.12 mmol, 1.2 equiv) and dry DCM-d2 (0.5 mL). Oxalyl chloride (21 μL, 0.24 mmol, 2.4 equiv) was added, and the solution was stirred for 3 h. When the reaction was completed as judged by NMR, it was concentrated under reduced pressure followed by the addition of aminopyrimidine 139 (31 mg, 0.1 mmol, 1 equiv) in dry DCM (0.5 mL). The reaction was stirred for 3 h and then concentrated under reduced pressure. The residue was purified by flash column chromatography (0% → 3% MeOH/DCM) affording the product (14 mg, 34 μmol, 34%). 1H NMR (400 MHz, CDCl3) δ 7.85 (br s, 1H), 7.33–7.27 (m, 2H), 7.24–7.17 (m, 3H), 6.86 (s, 1H), 3.79–3.70 (m, 6H), 3.64–3.56 (m, 4H), 3.04 (s, 3H), 2.91–2.84 (m, 2H), 2.29 (d, J = 7.1 Hz, 2H), 1.15–1.03 (m, 1H), 0.72–0.65 (m, 2H), 0.30–0.25 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 171.84, 164.73, 160.88, 157.66, 140.19, 128.92, 128.54, 126.20, 80.09, 66.85, 51.30, 44.70, 42.92, 35.44, 33.92, 7.16, 4.75. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2390 found.
2-Chloro-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (31)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (1.7 g, 7.07 mmol, 1 equiv), DiPEA (1.85 mL, 10.6 mmol, 1.5 equiv), and morpholine (0.64 mL, 7.42 mmol, 1.05 equiv). Column chromatography (30% → 60% EtOAc/pentane) afforded the product (1.7 g, 6.3 mmol, 89%). TLC: Rf = 0.5 in 40% EtOAc/pentane. 1H NMR (400 MHz, CDCl3) δ 7.94 (t, J = 5.9 Hz, 1H), 7.28 (s, 1H), 3.87–3.63 (m, 8H), 3.33–3.25 (m, 2H), 1.12–1.00 (m, 1H), 0.61–0.52 (m, 2H), 0.32–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 163.83, 162.21, 159.84, 157.90, 99.36, 66.35, 44.45, 10.65, 3.64. Regioselectivity was confirmed by 1H-NOESY NMR analysis. HRMS [C13H17ClN4O2 + H]+: 297.1113 calculated, 297.1116 found. Regioisomer 6-chloro-N-(cyclopropylmethyl)-2-morpholinopyrimidine-4-carboxamide (129) was also obtained (99 mg, 0.33 mmol, 5%). Rf = 0.6 in 40% EtOAc/pentane. 1H NMR (400 MHz, CDCl3) δ 7.80–7.65 (m, 1H), 7.32 (s, 1H), 3.90–3.72 (m, 8H), 3.30 (t, J = 6.5 Hz, 2H), 1.14–0.98 (m, 1H), 0.69–0.46 (m, 2H), 0.39–0.17 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 163.48, 162.31, 160.68, 158.86, 107.51, 66.64, 44.42, 44.30, 10.80, 3.55.
N-(Cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (32)
A round-bottom flask was charged with 2-chloropyrimidine 31 (24 mg, 80 μmol, 1 equiv), NaHCO3 (8 mg, 0.10 mmol, 1.2 equiv), and MeOH (0.5 mL). The solution was purged with N2 followed by addition of Pd/C (10% w/w, 40 mg, 50 μmol, 5 mol %), purged again with N2 and then stirred overnight under a H2 atmosphere (balloon). The mixture was filtered through a plug of Celite, which was washed with MeOH and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (40% → 70% EtOAc/pentane) to afford the product (20 mg, 76 μmol, 95%). TLC: Rf = 0.2 (40% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.57 (s, 1H), 8.09 (t, J = 5.7 Hz, 1H), 7.36 (s, 1H), 3.80–3.77 (m, 4H), 3.76–3.67 (m, 4H), 3.33–3.28 (m, 2H), 1.10–1.02 (m, 1H), 0.59–0.54 (m, 2H), 0.32–0.26 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 163.56, 163.02, 157.44, 155.78, 100.60, 66.59, 44.44, 44.37, 10.79, 3.65. HRMS [C13H18N4O2 + H]+: 263.1503 calculated, 263.1502 found.
N-(Cyclopropylmethyl)-6-morpholino-2-(phenethylamino)pyrimidine-4-carboxamide (33)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (59 mg, 0.20 mmol, 1 equiv), 2-phenethylamine (30 μL, 0.24 mmol, 1.2 equiv) and DiPEA (70 μL, 0.40 mmol, 2 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (2% → 5% MeOH/DCM) afforded the product (40 mg, 0.10 mmol, 50%). TLC: Rf = 0.4 (4% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.02 (br s, 1H), 7.39–7.28 (m, 2H), 7.28–7.15 (m, 3H), 6.78 (s, 1H), 4.96 (br s, 1H), 3.84–3.71 (m, 4H), 3.71–3.49 (m, 6H), 3.27 (t, J = 6.4 Hz, 2H), 2.92 (t, J = 7.2 Hz, 2H), 1.13–0.95 (m, 1H), 0.64–0.41 (m, 2H), 0.37–0.18 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.46, 163.98, 161.46, 156.84, 139.40, 128.89, 128.71, 126.53, 91.72, 66.68, 44.50, 43.00, 36.13, 10.79, 3.63. HRMS [C21H27N5O2 + H]+: 382.2238 calculated, 382.2241 found.
2-(Benzyl(methyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (34)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (59 mg, 0.20 mmol, 1 equiv), N-methylbenzylamine (38 μL, 0.30 mmol, 1.5 equiv), and DiPEA (140 μL, 0.80 mmol, 4 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (2% → 5% MeOH/DCM) afforded the product (40 mg, 0.10 mmol, 50%). TLC: Rf = 0.5 (4% MeOH/DCM). 1H NMR (500 MHz, CDCl3) δ 7.96 (br s, 1H), 7.34–7.28 (m, 2H), 7.28–7.21 (m, 3H), 6.75 (s, 1H), 4.85 (s, 2H), 3.82–3.69 (m, 4H), 3.69–3.54 (m, 4H), 3.26 (t, J = 6.5 Hz, 2H), 3.15 (s, 3H), 1.10–0.95 (m, 1H), 0.56–0.44 (m, 2H), 0.29–0.17 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.58, 163.97, 161.35, 156.85, 139.11, 128.56, 127.34, 127.03, 90.41, 66.68, 52.76, 44.47, 44.00, 35.18, 10.85, 3.43. HRMS [C21H27N5O2 + H]+: 382.2238 calculated, 382.2241 found.
N-(Cyclopropylmethyl)-2-(benzylamino)-6-morpholinopyrimidine-4-carboxamide (35)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (59 mg, 0.20 mmol, 1 equiv), DiPEA (0.14 mL, 0.80 mmol, 4 equiv), and benzylamine (33 μL, 0.30 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (2% → 4% MeOH/DCM) afforded the product (19 mg, 50 μmol, 25%). TLC: Rf = 0.5 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.94 (br s, 1H), 7.41–7.19 (m, 5H), 6.78 (s, 1H), 5.24 (br s, 1H), 4.60 (d, J = 5.9 Hz, 2H), 3.76–3.69 (m, 4H), 3.69–3.60 (m, 4H), 3.25 (t, J = 7.1, 5.8 Hz, 2H), 1.09–0.95 (m, 1H), 0.57–0.48 (m, 2H), 0.29–0.22 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 164.20, 164.03, 161.41, 156.91, 139.73, 128.65, 127.53, 127.27, 91.86, 66.67, 45.79, 44.48, 44.25, 10.78, 3.59. HRMS [C20H25N5O2 + H]+: 368.2081 calculated, 368.2081 found.
N-(Cyclopropylmethyl)-2-(methyl(3-phenylpropyl)amino)-6-morpholinopyrimidine-4-carboxamide (36)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (39 mg, 0.13 mmol, 1 equiv), amine 123b (30 mg, 0.20 mmol, 1.5 equiv), and DiPEA (87 μL, 0.53 mmol, 4 equiv). Total heating time: 48 h at 120 °C. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (38 mg, 93 μmol, 71%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (br s, 1H), 7.32–7.22 (m, 2H), 7.22–7.14 (m, 3H), 6.69 (s, 1H), 3.76–3.68 (m, 4H), 3.65–3.59 (m, 2H), 3.59–3.51 (m, 4H), 3.28 (t, J = 7.0, 5.9 Hz, 2H), 3.14 (s, 3H), 2.66 (t, J = 7.5 Hz, 2H), 1.95 (p, J = 9.0, 7.5 Hz, 2H), 1.10–0.98 (m, 1H), 0.57–0.49 (m, 2H), 0.30–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.69, 163.93, 161.04, 156.75, 142.00, 128.49, 128.43, 125.93, 89.96, 66.73, 48.99, 44.42, 43.97, 35.31, 33.52, 28.92, 10.93, 3.43. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2548 found.
N-(Cyclopropylmethyl)-2-(methyl(4-phenylbutyl)amino)-6-morpholinopyrimidine-4-carboxamide (37)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123c (23 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 24 h at 120 °C. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (40 mg, 94 μmol, 94%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (t, J = 5.8 Hz, 1H), 7.31–7.22 (m, 2H), 7.21–7.12 (m, 3H), 6.70 (s, 1H), 3.77–3.70 (m, 4H), 3.67–3.58 (m, 6H), 3.31–3.24 (m, 2H), 3.11 (s, 3H), 2.73–2.59 (m, 2H), 1.76–1.56 (m, J = 3.5, 2.9 Hz, 4H), 1.11–0.95 (m, 1H), 0.56–0.44 (m, 2H), 0.28–0.18 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.70, 163.96, 161.10, 156.74, 142.44, 128.42, 128.40, 125.87, 89.89, 66.72, 49.12, 44.47, 44.00, 35.88, 35.32, 29.03, 27.20, 10.86, 3.39. HRMS [C24H33N5O2 + H]+: 424.2707 calculated, 424.2706 found.
2-((4-Chlorophenethyl)(methyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (38)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123d (34 mg, 0.20 mmol, 2 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 25 h at 120 °C. Column chromatography (30% → 70% EtOAc/pentane) afforded the product (10 mg, 30 μmol, 30%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (br s, 1H), 7.28–7.23 (m, 2H), 7.17–7.09 (m, 2H), 6.73 (s, 1H), 3.82–3.72 (m, 6H), 3.65 (t, J = 4.8 Hz, 4H), 3.35–3.24 (m, 2H), 3.09 (s, 3H), 2.94–2.82 (m, 2H), 1.12–1.01 (m, 1H), 0.60–0.49 (m, 2H), 0.36–0.23 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.64, 163.98, 156.81, 138.45, 132.12, 130.22, 128.72, 90.28, 66.76, 51.50, 44.53, 44.15, 35.86, 33.39, 10.91, 3.51. HRMS [C22H28ClN5O2 + H]+: 430.2004 calculated, 430.2004 found.
2-((3-Chlorophenethyl)(methyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (39)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (20 mg, 70 μmol, 1 equiv), amine 123e (20 mg, 0.12 mmol, 1.5 equiv) and DiPEA (49 μL, 0.28 mmol, 4 equiv). Total heating time: 5 days at 120 °C. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (26 mg, 62 μmol, 89%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.00 (t, J = 5.9 Hz, 1H), 7.25–7.14 (m, 3H), 7.14–7.02 (m, 1H), 6.73 (s, 1H), 3.86–3.72 (m, 6H), 3.66 (t, J = 4.9 Hz, 4H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 3.12 (s, 3H), 2.93–2.83 (m, 2H), 1.12–1.01 (m, 1H), 0.60–0.50 (m, 2H), 0.33–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.62, 163.98, 160.86, 156.78, 141.98, 134.32, 129.82, 128.99, 127.06, 126.50, 90.27, 66.74, 51.34, 44.51, 44.12, 35.72, 33.68, 10.90, 3.48. HRMS [C22H28ClN5O2 + H]+: 430.2004 calculated, 430.2004 found.
2-((2-Chlorophenethyl)(methyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (40)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123f (28 mg, 0.17 mmol, 1.7 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 3 days at 120 °C. Purification by HPLC (C18 reverse phase, 35% → 45% CH3CN/H2O + 0.2% TFA, RT 10.8 min) afforded the product (31 mg, 70 μmol, 70%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.06 (br s, 1H), 7.37–7.32 (m, 1H), 7.21–7.12 (m, 3H), 6.70 (s, 1H), 3.89–3.83 (m, 2H), 3.78–3.74 (m, 4H), 3.69–3.62 (m, 4H), 3.29 (dd, J = 7.0, 5.9 Hz, 2H), 3.14 (s, 3H), 3.06–3.02 (m, 2H), 1.13–1.01 (m, 1H), 0.59–0.51 (m, 2H), 0.31–0.27 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.70, 163.95, 160.97, 156.79, 137.46, 134.14, 131.17, 129.60, 127.93, 127.01, 90.11, 66.78, 49.36, 44.51, 44.14, 35.52, 31.93, 10.97, 3.59. HRMS [C22H28ClN5O2 + H]+: 430.2004 calculated, 430.2003 found.
N-(Cyclopropylmethyl)-2-(methyl(4-methylphenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (41)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123g (23 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 3 days at 120 °C. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (16 mg, 40 μmol, 40%). TLC: Rf = 0.8 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (t, J = 5.8 Hz, 1H), 7.11 (s, 4H), 6.72 (s, 1H), 3.82–3.72 (m, 6H), 3.66 (t, J = 4.8 Hz, 4H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 3.13 (s, 3H), 2.90–2.82 (m, 2H), 2.33 (s, 3H), 1.14–0.99 (m, 1H), 0.61–0.49 (m, 2H), 0.33–0.25 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.72, 164.01, 160.89, 156.79, 136.81, 135.80, 129.28, 128.72, 90.06, 66.76, 51.81, 44.52, 44.12, 35.69, 33.46, 21.16, 10.90, 3.49. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2549 found.
N-(Cyclopropylmethyl)-2-(methyl(2-methylphenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (42)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123h (23 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 4 days at 120 °C. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (39 mg, 95 μmol, 95%). TLC: Rf = 0.4 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (t, J = 5.9 Hz, 1H), 7.23–7.06 (m, 4H), 6.73 (s, 1H), 3.85–3.71 (m, 6H), 3.66 (t, J = 4.8 Hz, 4H), 3.29 (dd, J = 7.1, 5.9 Hz, 2H), 3.15 (s, 3H), 2.97–2.83 (m, 2H), 2.39 (s, 3H), 1.15–0.98 (m, 1H), 0.62–0.47 (m, 2H), 0.34–0.22 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.69, 163.99, 160.91, 156.84, 137.97, 136.13, 130.38, 129.54, 126.51, 126.22, 90.12, 66.74, 50.23, 44.50, 44.12, 35.58, 31.31, 19.46, 10.91, 3.52. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2546 found.
N-(Cyclopropylmethyl)-2-((4-methoxyphenethyl)(methyl)amino)-6-morpholinopyrimidine-4-carboxamide (43)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123i (36 mg, 0.20 mmol, 2 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 7 days at 120 °C. Column chromatography (30% → 70% EtOAc/pentane) afforded the product (38 mg, 90 μmol, 90%). TLC: Rf = 0.4 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (t, J = 5.6 Hz, 1H), 7.18–7.07 (m, 2H), 6.87–6.80 (m, 2H), 6.72 (s, 1H), 3.79 (s, 3H), 3.79–3.72 (m, 6H), 3.66 (t, J = 4.8 Hz, 4H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 3.11 (s, 3H), 2.88–2.81 (m, 2H), 1.11–1.02 (m, 1H), 0.58–0.52 (m, 2H), 0.32–0.27 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.71, 164.00, 160.88, 158.16, 156.79, 131.97, 129.76, 114.01, 90.06, 66.76, 55.39, 51.86, 44.51, 44.11, 35.75, 33.01, 10.90, 3.49. HRMS [C23H31N5O3 + H]+: 426.2500 calculated, 426.2497 found.
N-(Cyclopropylmethyl)-2-((2-methoxyphenethyl)(methyl)amino)-6-morpholinopyrimidine-4-carboxamide (43)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123j (24 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (30% → 70% EtOAc/pentane) afforded the product (42 mg, 0.10 mmol, 99%). TLC: Rf = 0.3 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.08 (t, J = 6.0 Hz, 1H), 7.20 (td, J = 7.8, 1.8 Hz, 1H), 7.13 (dd, J = 7.3, 1.7 Hz, 1H), 6.94–6.82 (m, 2H), 6.71 (s, 1H), 3.83 (s, 3H), 3.81–3.72 (m, 6H), 3.66 (t, J = 4.8 Hz, 4H), 3.30 (dd, J = 7.0, 5.9 Hz, 2H), 3.12 (s, 3H), 3.03–2.84 (m, 2H), 1.13–0.97 (m, 1H), 0.65–0.44 (m, 2H), 0.35–0.20 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.81, 163.99, 160.95, 157.81, 156.77, 130.55, 128.37, 127.64, 120.64, 110.48, 89.88, 66.79, 55.47, 49.93, 44.49, 44.04, 35.65, 28.70, 10.94, 3.47. HRMS [C23H31N5O3 + H]+: 426.2500 calculated, 426.2496 found.
N-(Cyclopropylmethyl)-2-(methyl(4-(trifluoromethyl)phenethyl)amino)-6-morpholino-pyrimidine-4-carboxamide (45)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (22 mg, 73 μmol, 1 equiv), amine 123k (29 mg, 0.10 mmol, 1.5 equiv), and DiPEA (51 μL, 0.29 mmol, 4 equiv). Total heating time: 25 h at 120 °C. Purification by HPLC (C18 reverse phase, 47% → 55% CH3CN/H2O + 0.2% TFA, RT 12 min) afforded the product (9 mg, 20 μmol, 28%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (br s, 1H), 7.54 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 7.9 Hz, 2H), 6.73 (s, 1H), 3.87–3.79 (m, 2H), 3.79–3.71 (m, 4H), 3.65 (t, J = 4.8 Hz, 4H), 3.35–3.24 (m, 2H), 3.10 (s, 3H), 3.02–2.93 (m, 2H), 1.12–0.99 (m, 1H), 0.65–0.48 (m, 2H), 0.39–0.22 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.61, 163.98, 160.88, 156.81, 144.18 (q, J = 1.4 Hz), 129.21, 128.89, 128.57, 125.73, 125.51 (q, J = 3.7 Hz), 123.03, 90.37, 66.75, 51.30, 44.51, 44.13, 35.87, 33.91, 10.92, 3.50. HRMS [C23H28F3N5O2 + H]+: 464.2268 calculated, 464.2267 found.
N-(Cyclopropylmethyl)-2-(methyl(4-phenoxyphenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (46)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 123l (34 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 48 h at 120 °C. Column chromatography (30% → 70% EtOAc/pentane) afforded the product (26 mg, 50 μmol, 50%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (600 MHz, CDCl3) δ 8.00 (br s, 1H), 7.35–7.28 (m, 2H), 7.19–7.13 (m, 2H), 7.10–7.06 (m, 1H), 6.99–6.89 (m, 4H), 6.72 (s, 1H), 3.84–3.78 (m, 2H), 3.78–3.73 (m, 4H), 3.69–3.63 (m, 4H), 3.31–3.26 (m, 2H), 3.13 (s, 3H), 2.93–2.86 (m, 2H), 1.11–0.98 (m, 1H), 0.56–0.51 (m, 2H), 0.29–0.25 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 164.67, 164.00, 160.92, 157.63, 156.80, 155.60, 134.93, 130.09, 129.83, 123.17, 119.27, 118.63, 90.12, 66.75, 51.67, 44.54, 44.12, 35.73, 33.25, 10.90, 3.49. HRMS [C28H33N5O3 + H]+: 488.2656 calculated, 488.2656 found.
N-(Cyclopropylmethyl)-2-(methyl(3-phenoxyphenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (47)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (25 mg, 84 μmol, 1 equiv), amine 123m (28 mg, 0.12 mmol, 1.5 equiv), and DiPEA (60 μL, 0.34 mmol, 4 equiv). Total heating time: 5 days at 120 °C. Purification by HPLC (C18 reverse phase, 40% → 50% CH3CN/H2O + 0.2% TFA, RT 11.2 min) afforded the product (14 mg, 29 μmol, 34%). TLC: Rf = 0.5 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.00 (br s, 1H), 7.35–7.28 (m, 2H), 7.29–7.20 (m, 1H), 7.13–7.06 (m, 1H), 6.99–6.94 (m, 3H), 6.92–6.87 (m, 1H), 6.86–6.83 (m, 1H), 6.71 (s, 1H), 3.84–3.77 (m, 2H), 3.76–3.70 (m, 4H), 3.67–3.61 (m, 4H), 3.30–3.24 (m, 2H), 3.12 (s, 3H), 2.93–2.83 (m, 2H), 1.11–0.98 (m, 1H), 0.57–0.49 (m, 2H), 0.30–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.67, 163.98, 160.89, 157.41, 157.32, 156.81, 142.03, 129.86, 123.91, 123.26, 119.57, 118.70, 116.91, 90.19, 66.75, 51.48, 44.52, 44.14, 35.69, 33.87, 10.90, 3.51. HRMS [C28H33N5O3 + H]+: 488.2656 calculated, 488.2653 found.
N-(Cyclopropylmethyl)-2-(methyl(2-phenoxyphenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (48)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (34 mg, 0.10 mmol, 1 equiv), amine 123n (37 mg, 0.16 mmol, 1.6 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 3 days at 120 °C. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (45 mg, 90 μmol, 90%). TLC: Rf = 0.5 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.06 (t, J = 5.4 Hz, 1H), 7.34–7.28 (m, 2H), 7.26 (dd, J = 7.4, 1.5 Hz, 1H), 7.17 (td, J = 7.8, 1.7 Hz, 1H), 7.12–7.00 (m, 2H), 6.93 (dd, J = 8.6, 0.9 Hz, 2H), 6.86 (d, J = 8.0 Hz, 1H), 6.69 (s, 1H), 3.90–3.79 (m, 2H), 3.76–3.54 (m, 8H), 3.27 (t, J = 6.4 Hz, 2H), 3.07 (s, 3H), 2.99–2.88 (m, 2H), 1.11–0.98 (m, 1H), 0.60–0.44 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.74, 163.94, 160.90, 157.74, 156.72, 155.09, 131.31, 129.89, 127.84, 123.92, 122.99, 119.36, 118.07, 89.98, 66.70, 50.17, 44.44, 44.03, 35.57, 28.69, 10.92, 3.49. HRMS [C28H33N5O3 + H]+: 488.2656 calculated, 488.2653 found.
2-((2-([1,1′-Biphenyl]-2-yl)ethyl)(methyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (48)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (29 mg, 96 μmol, 1 equiv), amine 123o (31 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 24 h at 160 °C with μW irradiation. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (43 mg, 91 μmol, 95%). TLC: Rf = 0.4 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.97 (t, J = 5.9 Hz, 1H), 7.41–7.19 (m, 9H), 6.68 (s, 1H), 3.77–3.70 (m, 4H), 3.66–3.57 (m, 6H), 3.28 (dd, J = 7.0, 5.8 Hz, 2H), 2.94–2.89 (m, 2H), 2.77 (s, 3H), 1.10–1.00 (m, 1H), 0.59–0.49 (m, 2H), 0.31–0.25 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.69, 163.87, 160.70, 156.65, 142.44, 141.63, 137.33, 130.26, 129.83, 129.28, 128.15, 127.56, 126.94, 126.29, 89.99, 66.72, 51.00, 44.44, 44.04, 35.31, 30.93, 10.90, 3.47. HRMS [C28H33N5O2 + H]+: 472.2707 calculated, 472.2703 found.
N-(Cyclopropylmethyl)-2-(methyl(2-(pyridin-4-yl)ethyl)amino)-6-morpholinopyrimidine-4-carboxamide (50)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), N-methyl-2-(pyridin-4-yl)ethan-1-amine (21 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 17 h at 120 °C. Column chromatography (2% - > 6% MeOH/DCM) afforded the product (8 mg, 21 μmol, 21%). TLC: Rf = 0.2 (4% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 5.7 Hz, 2H), 7.96 (br s, 1H), 7.15 (d, J = 5.7 Hz, 2H), 6.74 (s, 1H), 3.90–3.80 (m, 2H), 3.80–3.72 (m, 4H), 3.72–3.53 (m, 4H), 3.36–3.24 (m, 2H), 3.11 (s, 3H), 2.98–2.85 (m, 2H), 1.12–0.99 (m, 1H), 0.64–0.47 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.54, 163.96, 160.83, 156.80, 149.96, 148.99, 124.32, 90.46, 66.74, 50.59, 44.50, 44.13, 35.87, 33.44, 10.91, 3.51. HRMS [C21H28N6O2 + H]+: 397.2347 calculated, 397.2345 found.
N-(Cyclopropylmethyl)-2-(methyl(2-(pyridin-3-yl)ethyl)amino)-6-morpholinopyrimidine-4-carboxamide (51)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), N-methyl-2-(pyridin-3-yl)ethan-1-amine (21 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 17 h at 120 °C. Column chromatography (2% → 6% MeOH/DCM) afforded the product (11 mg, 29 μmol, 29%). TLC: Rf = 0.15 (4% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.65–8.28 (m, 2H), 7.95 (br s, 1H), 7.51 (d, J = 7.9 Hz, 1H), 7.21 (dd, J = 7.9, 4.8 Hz, 1H), 6.72 (s, 1H), 3.89–3.69 (m, 6H), 3.69–3.53 (m, 4H), 3.29 (t, J = 6.4 Hz, 2H), 3.11 (s, 3H), 2.92 (t, J = 7.4 Hz, 2H), 1.12–0.99 (m, 1H), 0.66–0.45 (m, 2H), 0.36–0.19 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.57, 163.95, 160.86, 156.77, 150.27, 147.86, 136.33, 135.30, 123.50, 90.36, 66.74, 51.19, 44.49, 44.15, 35.84, 31.23, 10.91, 3.51. HRMS [C21H28N6O2 + H]+: 397.2347 calculated, 397.2345 found.
N-(Cyclopropylmethyl)-2-(methyl(2-(pyridin-2-yl)ethyl)amino)-6-morpholinopyrimidine-4-carboxamide (52)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), N-methyl-2-(pyridin-2-yl)ethan-1-amine (21 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (3% → 4% MeOH/DCM) afforded the product (26 mg, 66 μmol, 66%). TLC: Rf = 0.3 (3% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.66–8.44 (m, 1H), 8.18 (br s, 1H), 7.58 (td, J = 7.6, 1.7 Hz, 1H), 7.21–7.03 (m, 2H), 6.72 (s, 1H), 3.99 (t, J = 7.2 Hz, 2H), 3.88–3.71 (m, 4H), 3.71–3.54 (m, 4H), 3.31 (t, J = 6.4 Hz, 2H), 3.21–3.03 (m, 5H), 1.16–1.00 (m, 1H), 0.62–0.43 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.62, 163.91, 160.79, 159.86, 156.60, 149.21, 136.68, 123.60, 121.48, 90.12, 66.72, 49.98, 44.48, 44.01, 36.15, 35.62, 10.94, 3.45. HRMS [C21H28N6O2 + H]+: 397.2347 calculated, 397.2345 found.
N-(Cyclopropylmethyl)-2-(methyl(2-(thiophen-2-yl)ethyl)amino)-6-morpholinopyrimidine-4-carboxamide (53)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methyl-2-thiopheneethylamine (18 μL, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (16 mg, 40 μmol, 40%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (t, J = 5.3 Hz, 1H), 7.15 (dd, J = 5.1, 1.1 Hz, 1H), 6.94 (dd, J = 5.1, 3.4 Hz, 1H), 6.83 (d, J = 2.9 Hz, 1H), 6.73 (s, 1H), 3.88–3.80 (m, 2H), 3.80–3.72 (m, 4H), 3.71–3.62 (m, 4H), 3.33–3.26 (m, 2H), 3.20–3.08 (m, 5H), 1.13–0.99 (m, 1H), 0.60–0.48 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.62, 163.97, 160.84, 156.81, 142.18, 127.06, 125.03, 123.68, 90.26, 66.75, 51.90, 44.51, 44.13, 35.75, 27.94, 10.91, 3.55. HRMS [C20H27N5O2S + H]+: 402.1958 calculated, 402.1956 found.
N-(Cyclopropylmethyl)-2-(ethyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (54)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (28 mg, 93 μmol, 1 equiv), amine 125a (21 mg, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 12 h at 160 °C with μW irradiation. Column chromatography (20% → 50% EtOAc/pentane) afforded the product (15 mg, 40 μmol, 43%). TLC: Rf = 0.3 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (t, J = 5.9 Hz, 1H), 7.35–7.27 (m, 2H), 7.25–7.20 (m, 3H), 6.72 (s, 1H), 3.80–3.73 (m, 6H), 3.70–3.63 (m, 4H), 3.58 (q, J = 7.0 Hz, 2H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 2.97–2.89 (m, 2H), 1.18 (t, J = 7.0 Hz, 3H), 1.11–1.01 (m, 1H), 0.59–0.51 (m, 2H), 0.31–0.25 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.74, 164.12, 160.29, 156.86, 140.06, 128.83, 128.62, 126.32, 90.06, 66.78, 49.83, 44.53, 44.09, 43.04, 34.76, 13.24, 10.90, 3.44. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2549 found.
N-(Cyclopropylmethyl)-2-(isopropyl(phenethyl)amino)-6-morpholinopyrimidine-4-carboxamide (55)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (59 mg, 0.20 mmol, 1 equiv), amine 125b (50 mg, 0.30 mmol, 1.5 equiv), and DiPEA (140 μL, 0.80 mmol, 4 equiv). Total heating time: 12 h at 160 °C with μW irradiation. Column chromatography (50% → 60% EtOAc/pentane) afforded the product (19 mg, 40 μmol, 22%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.05 (br s, 1H), 7.37–7.29 (m, 2H), 7.29–7.20 (m, 3H), 6.75 (s, 1H), 4.96 (hept, J = 6.8 Hz, 1H), 3.82–3.74 (m, 4H), 3.72–3.64 (m, 4H), 3.64–3.55 (m, 2H), 3.35–3.26 (m, 2H), 3.01–2.88 (m, 2H), 1.24 (d, J = 6.8 Hz, 6H), 1.11–1.00 (m, 1H), 0.60–0.49 (m, 2H), 0.33–0.23 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.79, 164.06, 160.40, 156.81, 140.36, 128.68, 128.65, 126.35, 90.26, 66.77, 46.38, 44.57, 44.48, 44.17, 36.29, 20.65, 10.90, 3.51. HRMS [C24H33N5O2 + H]+: 424.2707 calculated, 424.2705 found.
2-(Cyclopropyl(phenethyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (56)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 125c (25 mg, 0.16 mmol, 1.6 equiv), and DiPEA (115 μL, 0.60 mmol, 6 equiv). Total heating time: 36 h at 160 °C with μW irradiation. Purification by HPLC (C18 reverse phase, 37% → 47% CH3CN/H2O + 0.2% TFA) afforded the product (7 mg, 20 μmol, 20%). TLC: Rf = 0.4 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.34–7.24 (m, 2H), 7.25–7.16 (m, 3H), 6.79 (s, 1H), 3.87–3.73 (m, 6H), 3.69 (t, J = 4.7 Hz, 4H), 3.30 (dd, J = 7.1, 5.7 Hz, 2H), 2.98–2.85 (m, 2H), 2.73–2.64 (m, 1H), 1.14–0.98 (m, 1H), 0.90–0.77 (m, 2H), 0.68–0.59 (m, 2H), 0.59–0.49 (m, 2H), 0.35–0.22 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.62, 163.99, 162.12, 156.64, 140.18, 128.88, 128.59, 126.31, 91.08, 66.78, 50.52, 44.57, 44.11, 34.58, 30.41, 10.86, 8.67, 3.42. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2549 found.
N-(Cyclopropylmethyl)-6-morpholino-2-(phenethyl(phenyl)amino)pyrimidine-4-carboxamide (57)
A microwave vial with a magnetic stir bar under N2 was charged with 2-chloropyrimidine 31 (29 mg, 98 μmol, 1 equiv), amine 124 (24 mg, 0.12 mmol, 1.2 equiv), and dry toluene (0.1 mL). The vial was capped, and the solution purged with N2. This was followed by the addition of RuPhosPd G3 (0.01 M THF solution, 100 μL, 1 μmol, 0.01 equiv) and NaOtBu (2 M THF solution, 60 μL, 0.12 mmol, 1.2 equiv), and the mixture was purged again with N2 and stirred in a preheated oil bath at 110 °C. After 24 h, the reaction was complete as judged by LC–MS. The mixture was filtered through a plug of Celite, and the filtrate was concentrated under reduced pressure to provide the crude material. Purification by HPLC (C18 reverse phase, 5% → 50% CH3CN/H2O + 0.2% TFA, RT 12.0 min) afforded the product (12 mg, 26 μmol, 27%). TLC: Rf = 0.4 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.79 (t, J = 4.9 Hz, 1H), 7.43–7.33 (m, 2H), 7.32–7.17 (m, 8H), 6.79 (s, 1H), 4.28–4.11 (m, 2H), 3.79–3.67 (m, 4H), 3.60 (t, J = 4.8 Hz, 4H), 3.20 (dd, J = 7.1, 5.7 Hz, 2H), 3.09–2.96 (m, 2H), 1.02–0.89 (m, 1H), 0.55–0.41 (m, 2H), 0.27–0.12 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.24, 163.97, 160.50, 156.64, 144.67, 139.63, 128.84, 128.62, 127.72, 126.40, 125.73, 91.42, 66.71, 52.33, 44.51, 43.96, 34.64, 10.70, 3.34. HRMS [C27H31N5O2 + H]+: 458.2551 calculated, 458.2547 found.
2-(Benzyl(phenethyl)amino)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (58)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (26 mg, 88 μmol, 1 equiv), amine 125d (28 mg, 0.13 mmol, 1.5 equiv), and DiPEA (44 μL, 0.25 mmol, 3 equiv). Total heating time: 7 days at 120 °C. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (20 mg, 40 μmol, 48%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (400 MHz, MeOD + CDCl3) δ 7.40–7.08 (m, 10H), 6.71 (s, 1H), 4.78 (s, 2H), 3.95–3.54 (m, 10H), 3.23 (br s, 2H), 2.98–2.83 (m, 2H), 1.02 (br s, 1H), 0.54 (br s, 2H), 0.25 (br s, 2H). 13C NMR (101 MHz, CDCl3) δ 164.60, 164.09, 160.81, 156.93, 139.90, 139.50, 128.82, 128.62, 128.57, 127.31, 127.04, 126.34, 90.66, 66.72, 51.39, 49.88, 44.56, 44.13, 34.22, 10.84, 3.47. HRMS [C28H33N5O2 + H]+: 472.2707 calculated, 472.2704 found.
N-(Cyclopropylmethyl)-2-(diphenethylamino)-6-morpholinopyrimidine-4-carboxamide (59)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 125e (37 mg, 0.16 mmol, 1.6 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 12 h at 160 °C with μW irradiation. Column chromatography (20% → 50% EtOAc/pentane) afforded the product (24 mg, 50 μmol, 50%). TLC: Rf = 0.5 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (t, J = 5.8 Hz, 1H), 7.34–7.26 (m, 4H), 7.24–7.15 (m, 6H), 6.75 (s, 1H), 3.83–3.60 (m, 12H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 2.97–2.82 (m, 4H), 1.12–1.00 (m, 1H), 0.63–0.49 (m, 2H), 0.35–0.23 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.66, 164.08, 160.34, 156.84, 139.91, 128.82, 128.62, 126.35, 90.34, 66.75, 50.69, 44.57, 44.23, 34.58, 10.86, 3.52. HRMS [C29H35N5O2 + H]+: 486.2864 calculated, 486.2861 found.
N-(Cyclopropylmethyl)-2-(3,4-dihydroisoquinolin-2(1H)-yl)-6-morpholinopyrimidine-4-carboxamide (60)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (29 mg, 97 μmol, 1 equiv), 1,2,3,4-tetrahydroisoquinoline (19 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 18 h at 120 °C. Column chromatography (20% → 60% EtOAc/pentane) afforded the product (34 mg, 90 μmol, 93%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (t, J = 6.0 Hz, 1H), 7.25–7.13 (m, 4H), 6.76 (s, 1H), 4.90 (s, 2H), 4.04 (t, J = 5.9 Hz, 2H), 3.81–3.62 (m, 8H), 3.36–3.28 (m, 2H), 2.93 (t, J = 5.8 Hz, 2H), 1.16–1.01 (m, 1H), 0.61–0.51 (m, 2H), 0.31 (dt, J = 6.1, 4.6 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.61, 163.99, 160.78, 156.88, 135.40, 134.49, 128.77, 126.58, 126.40, 126.23, 90.80, 66.76, 46.46, 44.54, 44.10, 41.63, 29.14, 10.99, 3.54. HRMS [C22H27N5O2 + H]+: 394.2238 calculated, 394.2231 found.
(±)-N-(Cyclopropylmethyl)-6-morpholino-2-(3-phenylpyrrolidin-1-yl)pyrimidine-4-carboxamide (61)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), (±)-3-phenylpyrrolidine (22 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (30% → 70% EtOAc/pentane) afforded the product (39 mg, 95 μmol, 95%). TLC: Rf = 0.4 (40% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.09 (br s, 1H), 7.40–7.29 (m, 4H), 7.29–7.24 (m, 1H), 6.75 (s, 1H), 4.18–4.03 (m, 1H), 3.88 (t, J = 9.5 Hz, 1H), 3.79–3.71 (m, 4H), 3.71–3.59 (m, 5H), 3.58–3.51 (m, 1H), 3.51–3.43 (m, 1H), 3.35–3.20 (m, 2H), 2.43–2.33 (m, 1H), 2.18–2.06 (m, 1H), 1.13–0.97 (m, 1H), 0.61–0.44 (m, 2H), 0.34–0.20 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.65, 163.89, 159.58, 156.77, 142.22, 128.70, 127.29, 126.84, 90.15, 66.73, 53.27, 46.49, 44.42, 44.19, 44.02, 33.28, 10.90, 3.49. HRMS [C23H29N5O2 + H]+: 408.2394 calculated, 408.2391 found.
(±)-N-(Cyclopropylmethyl)-6-morpholino-2-(3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (62)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), (±)-3-phenylpiperidine (24 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 70% EtOAc/pentane) afforded the product (37 mg, 88 μmol, 88%). TLC: Rf = 0.3 (40% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.97 (t, J = 5.9 Hz, 1H), 7.35 (t, J = 7.5 Hz, 2H), 7.32–7.22 (m, 3H), 6.73 (s, 1H), 4.89–4.70 (m, 2H), 3.74 (t, J = 4.8 Hz, 4H), 3.69–3.56 (m, 4H), 3.37–3.16 (m, 2H), 2.97–2.83 (m, 2H), 2.76 (tt, J = 11.5, 3.7 Hz, 1H), 2.12–2.02 (m, 1H), 1.91–1.83 (m, 1H), 1.82–1.72 (m, 1H), 1.72–1.57 (m, 1H), 1.11–0.99 (m, 1H), 0.61–0.43 (m, 2H), 0.27 (q, J = 5.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.60, 164.07, 160.94, 156.93, 144.28, 128.65, 127.30, 126.66, 90.57, 66.71, 51.24, 44.67, 44.48, 44.04, 42.63, 32.27, 25.56, 10.94, 3.50, 3.48. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2548 found.
(±)-2-(2-Benzylpyrrolidin-1-yl)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (63)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), (±)-2-benzylpyrrolidine (24 μL, 0.15 mmol, 1.5 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 3 days at 120 °C. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (42 mg, 0.10 mmol, 99%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.11 (t, J = 5.8 Hz, 1H), 7.35–7.25 (m, 2H), 7.26–7.18 (m, 3H), 6.76 (s, 1H), 4.48–4.34 (m, 1H), 3.80–3.73 (m, 4H), 3.73–3.65 (m, 4H), 3.65–3.59 (m, 1H), 3.59–3.50 (m, 1H), 3.37–3.24 (m, 3H), 2.59 (dd, J = 13.1, 9.7 Hz, 1H), 1.91–1.82 (m, 4H), 1.14–0.98 (m, 1H), 0.65–0.46 (m, 2H), 0.37–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.71, 164.02, 159.56, 156.78, 139.83, 129.39, 128.49, 126.24, 90.22, 66.76, 59.06, 47.38, 44.54, 44.18, 39.30, 29.42, 23.16, 10.87, 3.51. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2549 found.
(±)-2-(2-Benzylpiperidin-1-yl)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (64)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), (±)-2-benzylpiperidine hydrochloride (52 mg, 0.24 mmol, 2.4 equiv), and DiPEA (104 μL, 0.60 mmol, 6 equiv). Total heating time: 6 days at 120 °C. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (12 mg, 27 μmol, 27%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (600 MHz, CDCl3) δ 7.98 (t, J = 5.2 Hz, 1H), 7.26 (t, J = 7.4 Hz, 2H), 7.24–7.16 (m, 3H), 6.70 (s, 1H), 5.13–4.97 (m, 1H), 4.77–4.60 (m, 1H), 3.82–3.73 (m, 4H), 3.66 (br s, 4H), 3.40–3.21 (m, 2H), 3.07–2.98 (m, 1H), 2.95 (dd, J = 13.1, 10.0 Hz, 1H), 2.81 (dd, J = 13.1, 5.1 Hz, 1H), 1.85–1.73 (m, 2H), 1.73–1.67 (m, 2H), 1.57–1.46 (m, 2H), 1.13–1.04 (m, 1H), 0.63–0.53 (m, 2H), 0.31 (q, J = 4.7 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 164.75, 163.97, 160.84, 156.81, 140.16, 129.26, 128.47, 126.16, 90.34, 66.78, 52.27, 44.53, 44.24, 39.47, 35.32, 26.29, 25.82, 19.30, 10.95, 3.65, 3.61. HRMS [C25H33N5O2 + H]+: 436.2707 calculated, 436.2706 found.
(±)-N-(Cyclopropylmethyl)-2-(2-(cyclohexylmethyl)piperidin-1-yl)-6-morpholinopyrimidine-4-carboxamide (65)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (24 mg, 80 μmol, 1 equiv), DiPEA (70 μL, 0.4 mmol, 5 equiv), and (±)-2-(cyclohexylmethyl)piperidine (22 mg, 0.12 mmol, 1.5 equiv). Total heating time: 24 h at 160 °C with μW irradiation. Column chromatography (10% → 60% EtOAc/pentane) afforded the product (6 mg, 14 μmol, 17%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.98 (s, 1H), 6.67 (s, 1H), 5.07–4.94 (m, 1H), 4.61 (dd, J = 13.8, 4.4 Hz, 1H), 3.81–3.70 (m, 4H), 3.70–3.56 (m, 4H), 3.37–3.17 (m, 2H), 2.90 (td, J = 13.1, 2.5 Hz, 1H), 1.80 (dd, J = 25.9, 12.9 Hz, 2H), 1.73–1.59 (m, 8H), 1.56–1.38 (m, 3H), 1.24–1.10 (m, 4H), 1.10–1.00 (m, 1H), 0.99–0.86 (m, 2H), 0.62–0.47 (m, 2H), 0.27 (q, J = 4.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 164.86, 164.07, 160.88, 156.90, 89.87, 66.82, 47.48, 44.55, 44.22, 38.83, 36.74, 34.55, 34.22, 33.47, 29.85, 28.23, 26.76, 26.48, 26.42, 25.94, 19.46, 10.90, 3.57, 3.54. HRMS [C25H39N5O2 + H]+: 442.3177 calculated, 442.3174 found.
(±)-N-(Cyclopropylmethyl)-2-(2-(4-methoxybenzyl)piperidin-1-yl)-6-morpholinopyrimidine-4-carboxamide (66)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), (±)-2-(4-methoxybenzyl)piperidine (46 mg, 0.22 mmol, 2.2 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 28 h at 160 °C with μW irradiation. Purification by HPLC (C18 reverse phase, 5% → 90% CH3CN/H2O + 0.2% TFA, RT 9.3 min) afforded the product (13 mg, 29 μmol, 29%). TLC: Rf = 0.3 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (t, J = 5.8 Hz, 1H), 7.16–7.10 (m, 2H), 6.85–6.75 (m, 2H), 6.69 (s, 1H), 5.00 (dt, J = 10.4, 4.9 Hz, 1H), 4.67 (dd, J = 13.5, 3.7 Hz, 1H), 3.84–3.72 (m, 7H), 3.65 (t, J = 4.8 Hz, 4H), 3.40–3.21 (m, 2H), 3.00 (td, J = 13.2, 2.8 Hz, 1H), 2.90 (dd, J = 13.2, 10.0 Hz, 1H), 2.74 (dd, J = 13.2, 5.1 Hz, 1H), 1.85–1.71 (m, 2H), 1.65 (s, 2H), 1.59–1.42 (m, 2H), 1.15–1.01 (m, 1H), 0.64–0.50 (m, 2H), 0.36–0.25 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.78, 164.00, 160.88, 158.03, 156.84, 132.18, 130.15, 113.89, 90.31, 66.79, 55.40, 52.38, 44.54, 44.23, 39.47, 34.37, 26.18, 25.83, 19.29, 10.97, 3.65, 3.61. HRMS [C26H35N5O3 + H]+: 466.2813 calculated, 466.2809 found.
(±)-N-(Cyclopropylmethyl)-6-morpholino-2-(2-phenylmorpholino)pyrimidine-4-carboxamide (67)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and (±)-2-phenylmorpholine (21 μL, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (37 mg, 87 μmol, 87%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.92 (br s, 1H), 7.50–7.31 (m, 5H), 6.80 (s, 1H), 4.66 (d, J = 13.3 Hz, 1H), 4.59–4.48 (m, 2H), 4.20–4.13 (m, 1H), 3.80 (td, J = 11.8, 2.8 Hz, 1H), 3.77–3.70 (m, 4H), 3.70–3.59 (m, 4H), 3.36–3.22 (m, 2H), 3.22–3.12 (m, 1H), 2.97 (dd, J = 13.3, 10.6 Hz, 1H), 1.12–0.99 (m, 1H), 0.60–0.46 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.34, 163.92, 160.97, 156.85, 139.91, 128.62, 128.23, 126.51, 91.50, 78.26, 67.02, 66.67, 50.63, 44.47, 44.11, 43.99, 10.94, 3.53. HRMS [C23H29N5O3 + H]+: 424.2343 calculated, 424.2340 found.
(±)-N-(Cyclopropylmethyl)-6-morpholino-2-(3-phenylpiperazin-1-yl)pyrimidine-4-carboxamide (68)
A round-bottom flask was charged with Cbz-protected amine 70 (56 mg, 0.10 mmol, 1 equiv) and MeOH (0.5 mL). The solution was purged with N2 and Pd/C (10% w/w, 50 mg, 50 μmol, 0.5 equiv) was added. The mixture was purged with N2 and then with H2 and stirred for 2 h under a H2 atmosphere (balloon). The mixture was filtered through a plug of Celite and washed with MeOH, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (1% → 4% MeOH/DCM) to afford the product (38 mg, 90 μmol, 90%). TLC: Rf = 0.3 (2% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.94 (t, J = 5.9 Hz, 1H), 7.51–7.44 (m, 2H), 7.43–7.30 (m, 3H), 6.76 (s, 1H), 4.69 (d, J = 12.5 Hz, 2H), 3.88–3.78 (m, 1H), 3.78–3.71 (m, 4H), 3.68–3.61 (m, 4H), 3.32–3.23 (m, 2H), 3.21 (d, J = 10.6 Hz, 1H), 3.13–2.94 (m, 2H), 2.90 (t, J = 11.7 Hz, 1H), 2.11 (br s, 1H), 1.09–1.00 (m, 1H), 0.57–0.47 (m, 2H), 0.30–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.51, 163.99, 161.01, 156.89, 142.01, 128.73, 127.97, 127.32, 91.05, 66.72, 60.57, 51.48, 46.27, 44.49, 44.26, 44.10, 10.97, 3.55. HRMS [C23H30N6O2 + H]+: 423.2503 calculated, 423.2501 found.
(±)-2-(4-Benzyl-3-phenylpiperazin-1-yl)-N-(cyclopropylmethyl)-6-morpholinopyrimidine-4-carboxamide (69)
A round-bottom flask was charged with amine 68 (19 mg, 45 μmol, 1 equiv) in dry CH3CN (0.5 mL). This was followed by DiPEA (16 μL, 90 μmol, 2 equiv) and benzyl bromide (6.4 μL, 54 μmol, 1.2 equiv). The reaction was stirred for 4 h at rt after which the solvents were concentrated under reduced pressure. The residue was purified by silica gel column chromatography (1 → 5% MeOH/DCM) affording the product (17 mg, 33 μmol, 74%). TLC: Rf = 0.5 (2% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.90 (t, J = 5.7 Hz, 1H), 7.57 (d, J = 7.2 Hz, 2H), 7.41 (t, J = 7.4 Hz, 2H), 7.36–7.27 (m, 5H), 7.25–7.18 (m, 1H), 6.74 (s, 1H), 4.70–4.54 (m, 2H), 3.83 (d, J = 13.4 Hz, 1H), 3.77–3.67 (m, 4H), 3.66–3.54 (m, 4H), 3.35 (dd, J = 10.6, 3.1 Hz, 1H), 3.32–3.18 (m, 2H), 3.08 (td, J = 12.7, 2.7 Hz, 1H), 3.03–2.92 (m, 2H), 2.87 (d, J = 13.4 Hz, 1H), 2.17 (td, J = 11.8, 3.0 Hz, 1H), 1.09–0.95 (m, 1H), 0.57–0.44 (m, 2H), 0.25 (q, J = 4.6 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.48, 164.02, 160.74, 156.91, 141.70, 138.93, 128.92, 128.87, 128.29, 128.20, 127.87, 127.00, 90.97, 67.34, 66.71, 59.23, 51.90, 51.73, 44.47, 44.36, 44.08, 10.92, 3.53, 3.51. HRMS [C30H36N6O2 + H]+: 513.2973 calculated, 513.2973 found.
(±)-Benzyl 4-(4-((Cyclopropylmethyl)carbamoyl)-6-morpholinopyrimidin-2-yl)-2-phenylpiperazine-1-carboxylate (70)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), amine 141 (45 mg, 0.12 mmol, 1.2 equiv), and DiPEA (70 μL, 0.40 mmol, 4 equiv). Total heating time: 41 h at 120 °C. Column chromatography (40% → 70% EtOAc/pentane) afforded the product (56 mg, 0.1 mmol, 99%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.90 (t, J = 5.9 Hz, 1H), 7.45–7.19 (m, 10H), 6.77 (s, 1H), 5.47 (br s, 1H), 5.31–5.12 (m, 2H), 5.04 (d, J = 13.8 Hz, 1H), 4.52–4.31 (m, 1H), 4.17 (d, J = 9.4 Hz, 1H), 3.75 (t, J = 4.8 Hz, 4H), 3.71–3.60 (m, 4H), 3.53 (d, J = 13.4 Hz, 1H), 3.29 (t, J = 6.5 Hz, 2H), 3.17 (d, J = 9.2 Hz, 2H), 1.13–0.97 (m, 1H), 0.61–0.46 (m, 2H), 0.34–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.36, 163.88, 160.76, 156.82, 155.79, 139.31, 136.59, 128.68, 128.65, 128.23, 128.03, 127.44, 127.04, 91.44, 67.65, 66.70, 45.36, 44.54, 44.13, 43.82, 39.89, 10.93, 3.53. HRMS [C31H36N6O4 + H]+: 557.2871 calculated, 557.2869 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(piperidin-1-yl)pyrimidine-4-carboxamide (71)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119k (44 mg, 0.15 mmol, 1 equiv), DiPEA (105 μL, 0.60 mmol, 4 equiv), and N-methylphenethylamine HBr salt (49 mg, 0.23 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 50% EtOAc/pentane) afforded the product (37 mg, 94 μmol, 63%). TLC: Rf = 0.4 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.07 (t, J = 4.8 Hz, 1H), 7.35–7.16 (m, 5H), 6.76 (s, 1H), 3.84–3.75 (m, 2H), 3.67 (br s, 4H), 3.34–3.25 (m, 2H), 3.13 (s, 3H), 2.95–2.87 (m, 2H), 1.74–1.65 (m, 2H), 1.65–1.55 (m, 4H), 1.13–1.00 (m, 1H), 0.59–0.50 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 165.02, 163.41, 161.01, 156.35, 140.10, 128.88, 128.56, 126.22, 90.36, 51.80, 45.37, 44.07, 35.66, 33.94, 25.77, 24.95, 10.91, 3.48. HRMS [C23H31N5O + H]+: 394.2601 calculated, 394.2592 found.
N-(Cyclopropylmethyl)-6-(3,3-difluoropiperidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (72)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119l (50 mg, 0.15 mmol, 1 equiv), DiPEA (78 μL, 0.45 mmol, 3 equiv), and N-methylphenethylamine (33 μL, 0.27 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (10% → 30% EtOAc/pentane) afforded the product (10 mg, 23 μmol, 15%). TLC: Rf = 0.5 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (br s, 1H), 7.34–7.27 (m, 2H), 7.26–7.18 (m, 3H), 6.78 (s, 1H), 4.01 (t, J = 11.7 Hz, 2H), 3.86–3.73 (m, 2H), 3.71–3.58 (m, 2H), 3.35–3.25 (m, 2H), 3.13 (s, 3H), 2.95–2.86 (m, 2H), 2.17–2.03 (m, 2H), 1.88–1.78 (m, 2H), 1.12–1.01 (m, 1H), 0.61–0.50 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.61, 163.70, 160.80, 157.10, 139.92, 128.91, 128.63, 126.32, 119.79 (t, J = 244.2 Hz), 90.16, 51.89, 49.40 (t, J = 32.8 Hz), 44.14, 43.73, 35.80, 33.92, 33.03 (t, J = 23.5 Hz), 22.07 (t, J = 4.4 Hz), 10.91, 3.50. HRMS [C23H29F2N5O + H]+: 430.2413 calculated, 430.2419 found.
N-(Cyclopropylmethyl)-6-(4,4-difluoropiperidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (72)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119m (45 mg, 0.14 mmol, 1 equiv), DiPEA (73 μL, 0.42 mmol, 3 equiv), and N-methylphenethylamine (31 μL, 0.21 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (5% → 30% EtOAc/pentane) afforded the product (27 mg, 63 μmol, 45%). TLC: Rf = 0.5 (15% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (t, J = 5.2 Hz, 1H), 7.33–7.27 (m, 2H), 7.25–7.18 (m, 3H), 6.79 (s, 1H), 3.88–3.74 (m, 6H), 3.36–3.25 (m, 2H), 3.13 (s, 3H), 2.95–2.85 (m, 2H), 2.06–1.92 (m, 4H), 1.13–0.99 (m, 1H), 0.63–0.50 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.58, 163.23, 160.99, 157.16, 139.85, 128.83, 128.62, 126.35, 122.10 (t, J = 242.0 Hz), 90.06, 51.73, 44.13, 41.27, 35.74, 33.95, 33.84 (t, J = 23.0 Hz), 10.89, 3.49. HRMS [C23H29F2N5O + H]+: 430.2413 calculated, 430.2422 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-thiomorpholinopyrimidine-4-carboxamide (74)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119n (41 mg, 0.13 mmol, 1 equiv), DiPEA (91 μL, 0.52 mmol, 4 equiv), and N-methylphenethylamine HBr salt (43 mg, 0.20 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (10% → 40% EtOAc/pentane) afforded the product (51 mg, 0.12 mmol, 95%). TLC: Rf = 0.4 (25% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.05 (br s, 1H), 7.33–7.27 (m, 2H), 7.25–7.18 (m, 3H), 6.73 (s, 1H), 4.03 (br s, 4H), 3.84–3.74 (m, 2H), 3.35–3.26 (m, 2H), 3.13 (s, 3H), 2.96–2.85 (m, 2H), 2.70–2.58 (m, 4H), 1.13–0.99 (m, 1H), 0.55 (q, J = 5.7 Hz, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.67, 163.05, 156.85, 139.87, 128.80, 128.58, 126.29, 90.34, 51.74, 47.24, 44.08, 35.71, 33.90, 26.68, 10.88, 3.47. HRMS [C22H29N5OS + H]+: 412.2166 calculated, 412.2159 found.
N-(Cyclopropylmethyl)-6-(1,1-dioxidothiomorpholino)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (75)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119o (4:1 mixture of regioisomers) (35 mg, 0.10 mmol, 1 equiv), DiPEA (70 μL, 0.40 mmol, 4 equiv), and N-methylphenethylamine HBr salt (32 mg, 0.15 mmol, 1.5 equiv). Total heating time: 2 days at 120 °C. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (35 mg, 79 μmol, 79%). TLC: Rf = 0.6 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.99 (br s, 1H), 7.36–7.26 (m, 2H), 7.26–7.16 (m, 3H), 6.81 (s, 1H), 4.20 (br s, 4H), 3.83 (t, J = 7.5 Hz, 2H), 3.30 (t, J = 6.4 Hz, 2H), 3.13 (s, 3H), 3.04 (br s, 4H), 2.91 (t, J = 7.4 Hz, 2H), 1.14–1.00 (m, 1H), 0.66–0.49 (m, 2H), 0.37–0.24 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.04, 162.66, 160.94, 157.93, 139.56, 128.76, 128.68, 126.49, 89.88, 51.67, 51.54, 44.19, 43.04, 35.82, 33.95, 10.86, 3.50. HRMS [C22H29N5O3S + H]+: 444.2064 calculated, 444.2074 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(4-methylpiperazin-1-yl)pyrimidine-4-carboxamide (76)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119p (42 mg, 0.14 mmol, 1 equiv), DiPEA (98 μL, 0.56 mmol, 4 equiv), and N-methylphenethylamine HBr salt (44 mg, 0.20 mmol, 1.5 equiv). Total heating time: 45 h at 120 °C. Purification by preparative HPLC (C18 reverse phase, 25% to 35% CH3CN/H2O + 0.2% TFA, RT 8.98 min) afforded the product (21 mg, 51 μmol, 37%). TLC: Rf = 0.3 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.03 (t, J = 5.4 Hz, 1H), 7.33–7.26 (m, 2H), 7.25–7.18 (m, 3H), 6.74 (s, 1H), 3.87–3.73 (m, 6H), 3.34–3.25 (m, 2H), 3.13 (s, 3H), 2.94–2.86 (m, 2H), 2.64–2.51 (m, 4H), 2.42 (s, 3H), 1.12–1.00 (m, 1H), 0.60–0.50 (m, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.70, 163.60, 160.91, 156.82, 139.95, 128.85, 128.60, 126.30, 90.22, 54.58, 51.72, 45.88, 44.12, 43.58, 35.71, 33.94, 10.89, 3.49. HRMS [C23H32N6O + H]+: 409.2710 calculated, 409.2708 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(piperazin-1-yl)pyrimidine-4-carboxamide (77)
A round-bottom flask was charged with Cbz-protected amine 80 (175 mg, 0.33 mmol, 1 equiv), dry MeOH (3 mL), and AcOH (0.3 mL). The flask was purged with N2 followed by addition of Pd/C (10% w/w, 18 mg, 0.02 mmol, 5 mol %) and then purging with H2 (balloon). The reaction was stirred for 2 days then filtered over a cellulose filter (Whatman), which was washed with MeOH. The filtrate was concentrated under reduced pressure, and the residue was purified using silica gel column chromatography (2.5% → 5% MeOH/DCM with 5% Et3N) affording the product (82 mg, 0.21 mmol, 63%). 1H NMR (400 MHz, CDCl3) δ 8.05 (t, J = 5.5 Hz, 1H), 7.35–7.26 (m, 2H), 7.26–7.17 (m, 3H), 6.74 (s, 1H), 3.84–3.76 (m, 2H), 3.75–3.58 (m, 4H), 3.30 (t, J = 6.5 Hz, 2H), 3.13 (s, 3H), 2.96–2.85 (m, 6H), 1.96 (s, 1H), 1.13–1.00 (m, 1H), 0.61–0.47 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.78, 163.74, 160.85, 156.51, 139.95, 128.80, 128.52, 126.20, 90.16, 51.66, 45.94, 45.26, 44.02, 35.62, 33.88, 10.84, 3.42. HRMS [C22H30N6O + H]+: 395.2554 calculated, 395.2558 found.
6-(4-Acetylpiperazin-1-yl)-N-(cyclopropylmethyl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (78)
A round-bottom flask was charged with amine 77 (22 mg, 56 μmol, 1 equiv) in dry DCM (1.5 mL). This was followed by addition of DiPEA (49 μL, 0.28 mmol, 5 equiv) and Ac2O (10.5 μL, 0.11 mmol, 2 equiv). The reaction was stirred for 3 h at rt, and after which, it was diluted with EtOAc (25 mL). The organic layer was washed with sat. aq. NaHCO3 (1× 25 mL) and brine (1× 25 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (2.5% → 10% MeOH/DCM) affording the product (19 mg, 44 μmol, 78%). TLC: Rf = 0.8 (10% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.02 (t, J = 5.3 Hz, 1H), 7.34–7.26 (m, 2H), 7.26–7.17 (m, 3H), 6.74 (s, 1H), 3.87–3.77 (m, 2H), 3.77–3.65 (m, 6H), 3.59–3.50 (m, 2H), 3.34–3.26 (m, 2H), 3.13 (s, 3H), 2.95–2.86 (m, 2H), 2.15 (s, 3H), 1.14–0.99 (m, 1H), 0.61–0.50 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 169.32, 164.57, 163.63, 160.85, 156.97, 139.84, 128.82, 128.61, 126.33, 90.13, 51.70, 45.94, 44.12, 43.82, 41.12, 35.73, 33.92, 21.57, 10.87, 3.48. HRMS [C24H32N6O2 + H]+: 437.2660 calculated, 437.2661 found.
6-(4-Benzoylpiperazin-1-yl)-N-(cyclopropylmethyl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (79)
A round-bottom flask was charged with amine 77 (22 mg, 56 μmol, 1 equiv) in dry DCM (1.5 mL). This was followed by Et3N (16 μL, 0.11 mmol, 2 equiv) and benzoyl chloride (8 μL, 67 μmol, 1.2 equiv). The reaction was stirred for 3 h at rt, and after which, it was diluted with EtOAc (25 mL). The organic layer was washed with sat. aq. NaHCO3 (1× 25 mL) and brine (1× 25 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (60% → 80% EtOAc/pentane) affording the product (20 mg, 40 μmol, 72%). TLC: Rf = 0.3 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.49–7.40 (m, 5H), 7.32–7.25 (m, 2H), 7.25–7.17 (m, 3H), 6.75 (s, 1H), 3.95–3.74 (m, 6H), 3.66 (br s, 2H), 3.52 (br s, 2H), 3.36–3.25 (m, 2H), 3.12 (s, 3H), 2.94–2.82 (m, 2H), 1.14–0.99 (m, 1H), 0.63–0.48 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 170.77, 164.61, 163.71, 160.87, 156.99, 139.85, 135.48, 130.14, 128.83, 128.76, 128.62, 128.49, 127.23, 126.34, 90.21, 51.70, 47.55, 44.16, 42.05, 35.75, 33.94, 10.88, 3.50. HRMS [C29H34N6O2 + H]+: 499.2816 calculated, 499.2825 found.
Benzyl 4-(6-((Cyclopropylmethyl)carbamoyl)-2-(methyl(phenethyl)amino)pyrimidin-4-yl)piperazine-1-carboxylate (80)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119q (202 mg, 0.47 mmol, 1 equiv), DiPEA (0.40 mL, 2.28 mmol, 5 equiv), and N-methylphenethylamine HBr salt (161 mg, 0.74 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (30% → 60% EtOAc/pentane) afforded the product (199 mg, 0.38 mmol, 80%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (t, J = 5.4 Hz, 1H), 7.42–7.25 (m, 7H), 7.24–7.17 (m, 3H), 6.73 (s, 1H), 5.17 (s, 2H), 3.86–3.77 (m, 2H), 3.69 (br s, 4H), 3.62–3.53 (m, 4H), 3.34–3.26 (m, 2H), 3.12 (s, 3H), 2.94–2.85 (m, 2H), 1.12–1.00 (m, 1H), 0.61–0.50 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.58, 163.64, 160.85, 156.90, 155.32, 139.84, 136.56, 128.79, 128.62, 128.56, 128.23, 128.08, 126.27, 90.16, 67.46, 51.66, 44.07, 43.86, 43.53, 35.67, 33.91, 10.85, 3.44. HRMS [C30H36N6O3 + H]+: 529.2922 calculated, 529.2933 found.
N-(Cyclopropylmethyl)-6-(dimethylamino)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (81)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119r (27 mg, 0.11 mmol, 1 equiv), N-methylphenethylamine HBr salt (35 mg, 0.16 mmol, 1.6 equiv), and DiPEA (92 μL, 0.53 mmol, 5 equiv). Total heating time: 8 h at 160 °C with μW irradiation. Column chromatography (20% → 50% EtOAc/pentane) afforded the product (32 mg, 90 μmol, 90%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.08 (t, J = 5.8 Hz, 1H), 7.34–7.27 (m, 2H), 7.26–7.17 (m, 3H), 6.69 (s, 1H), 3.92–3.70 (m, 2H), 3.30 (dd, J = 7.1, 5.8 Hz, 2H), 3.23–3.01 (m, 9H), 3.00–2.80 (m, 2H), 1.17–0.98 (m, 1H), 0.64–0.46 (m, 2H), 0.36–0.22 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.89, 164.01, 160.68, 155.88, 140.01, 128.79, 128.47, 126.12, 90.10, 51.66, 43.98, 37.16, 35.52, 33.84, 10.82, 3.39. HRMS [C20H27N5O + H]+: 354.2288 calculated, 354.2290 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(methylamino)pyrimidine-4-carboxamide (82)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119s (25 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (50% → 70% EtOAc/pentane) afforded the product (26 mg, 77 μmol, 77%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.33–7.27 (m, 2H), 7.26–7.18 (m, 3H), 6.54 (s, 1H), 5.13–4.72 (m, 1H), 3.93–3.66 (m, 2H), 3.34–3.24 (m, 2H), 3.13 (s, 3H), 2.98 (d, J = 4.9 Hz, 3H), 2.94–2.87 (m, 2H), 1.12–1.00 (m, 1H), 0.61–0.50 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.97, 164.71, 161.06, 156.00, 140.05, 128.89, 128.55, 126.24, 92.54, 51.66, 44.06, 35.62, 33.93, 28.23, 10.89, 3.48. HRMS [C19H25N5O + H]+: 340.2132 calculated, 340.2138 found.
N-(Cyclopropylmethyl)-6-((2-hydroxyethyl)(methyl)amino)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (83)
The title compound was prepared according to General Procedure C using dichloropyrimidine 118a (39 mg, 0.16 mmol, 1 equiv), DiPEA (43 μL, 0.24 mmol, 1.5 equiv), and N-methylethanolamine (13 μL, 0.16 mmol, 1.0 equiv) in MeOH (1.6 mL) followed by concentration and addition of DiPEA (84 μL, 0.48 mmol, 3 equiv), N-methylphenethylamine (35 μL, 0.24 mmol, 1.5 equiv), and n-BuOH (0.75 mL). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 40% EtOAc/pentane) afforded the product (15 mg, 33 μmol, 21%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (br s, 1H), 7.33–7.27 (m, 2H), 7.26–7.18 (m, 3H), 6.68 (s, 1H), 4.12–3.52 (m, 7H), 3.33–3.26 (m, 2H), 3.14 (s, 3H), 3.11 (s, 3H), 2.93–2.86 (m, 2H), 1.12–1.00 (m, 1H), 0.60–0.51 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.77, 164.63, 160.52, 156.34, 139.80, 128.86, 128.60, 126.32, 90.28, 62.14, 52.62, 51.83, 44.12, 37.11, 35.91, 33.97, 10.86, 3.48. HRMS [C21H29N5O2 + H]+: 384.2394 calculated, 384.2399 found.
N-(Cyclopropylmethyl)-6-(diethylamino)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (84)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119t (37 mg, 0.13 mmol, 1 equiv), DiPEA (68 μL, 0.39 mmol, 3 equiv), and N-methylphenethylamine (28 μL, 0.20 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 40% EtOAc/pentane) afforded the product (30 mg, 79 μmol, 60%). TLC: Rf = 0.8 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (br s, 1H), 7.40–7.14 (m, 5H), 6.65 (s, 1H), 3.92–3.71 (m, 2H), 3.54 (br s, 4H), 3.30 (t, J = 6.3 Hz, 2H), 3.14 (s, 3H), 3.02–2.85 (m, 2H), 1.20 (t, J = 6.9 Hz, 6H), 1.13–0.99 (m, 1H), 0.64–0.46 (m, 2H), 0.37–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 165.05, 162.59, 160.96, 155.94, 140.12, 128.87, 128.55, 126.21, 90.19, 51.79, 44.03, 42.44, 35.60, 34.04, 13.26, 10.92, 3.46. HRMS [C22H31N5O + H]+: 382.2601 calculated, 382.2599 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(azetidin-1-yl)pyrimidine-4-carboxamide (85)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119u (27 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (25 mg, 68 μmol, 68%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.04 (br s, 1H), 7.33–7.27 (m, 2H), 7.26–7.16 (m, 3H), 6.38 (s, 1H), 4.11 (t, J = 7.5 Hz, 4H), 3.85–3.71 (m, 2H), 3.35–3.26 (m, 2H), 3.13 (s, 3H), 2.94–2.84 (m, 2H), 2.40 (p, J = 7.5 Hz, 2H), 1.14–1.00 (m, 1H), 0.60–0.48 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.78, 164.75, 160.96, 155.60, 140.10, 128.90, 128.55, 126.22, 89.42, 51.73, 49.83, 44.07, 35.54, 33.88, 16.75, 10.90, 3.49. HRMS [C21H27N5O + H]+: 366.2288 calculated, 366.2296 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(2-oxa-6-azaspiro[3.3]heptan-6-yl)pyrimidine-4-carboxamide (86)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119v (31 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 90% EtOAc/pentane) afforded the product (7 mg, 17 μmol, 17%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.00 (br s, 1H), 7.35–7.27 (m, 2H), 7.26–7.19 (m, 3H), 6.39 (s, 1H), 4.85 (s, 4H), 4.24 (s, 4H), 3.84–3.71 (m, 2H), 3.36–3.22 (m, 2H), 3.12 (s, 3H), 2.94–2.82 (m, 2H), 1.13–0.98 (m, 1H), 0.60–0.48 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.68, 164.52, 160.91, 156.07, 139.97, 128.89, 128.62, 126.32, 89.61, 81.23, 59.40, 51.71, 44.12, 39.24, 35.61, 33.89, 10.90, 3.51. HRMS [C23H29N5O2 + H]+: 408.2394 calculated, 408.2396 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(pyrrolidin-1-yl)pyrimidine-4-carboxamide (87)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119w (28 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (26 mg, 69 μmol, 69%). TLC: Rf = 0.7 (40% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.09 (t, J = 5.3 Hz, 1H), 7.33–7.27 (m, 2H), 7.27–7.18 (m, 3H), 6.54 (s, 1H), 3.89–3.71 (m, 2H), 3.62 (br s, 2H), 3.53–3.34 (m, 2H), 3.30 (dd, J = 6.9, 5.9 Hz, 2H), 3.14 (s, 3H), 2.97–2.85 (m, 2H), 2.15–1.85 (m, 4H), 1.12–1.01 (m, 1H), 0.63–0.47 (m, 2H), 0.35–0.22 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 165.03, 161.92, 160.90, 155.49, 140.19, 128.89, 128.54, 126.18, 91.37, 51.73, 46.43, 44.05, 35.55, 33.95, 25.78, 25.01, 10.91, 3.47. HRMS [C22H29N5O + H]+: 380.2445 calculated, 380.2452 found.
N-(Cyclopropylmethyl)-6-(3,3-difluoropyrrolidin-1-yl)-2-(methyl(phenethyl) amino)pyrimidine-4-carboxamide (88)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119x (32 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 40% EtOAc/pentane) afforded the product (34 mg, 82 μmol, 82%). TLC: Rf = 0.8 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (br s, 1H), 7.34–7.27 (m, 2H), 7.25–7.18 (m, 3H), 6.52 (br s, 1H), 3.89 (br s, 2H), 3.84–3.78 (m, 2H), 3.74 (br s, 2H), 3.37–3.22 (m, 2H), 3.13 (s, 3H), 2.97–2.83 (m, 2H), 2.56–2.37 (m, 2H), 1.14–1.00 (m, 1H), 0.64–0.47 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.50, 162.05, 160.76, 156.36, 139.91, 128.86, 128.61, 127.48 (t, J = 247.3 Hz), 126.31, 90.39, 53.35 (t, J = 32.2 Hz), 51.68, 44.12, 43.95, 35.66, 33.94 (t, J = 22.8 Hz), 10.88, 3.50. HRMS [C22H27F2N5O + H]+: 416.2256 calculated, 416.2260 found.
(±)-N-(Cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (89)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119y (65 mg, 0.22 mmol, 1 equiv), DiPEA (115 μL, 0.66 mmol, 3 equiv), and N-methylphenethylamine (48 μL, 0.33 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 100% EtOAc/pentane) afforded the product (60 mg, 0.15 mmol, 69%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (t, J = 5.2 Hz, 1H), 7.32–7.26 (m, 2H), 7.25–7.17 (m, 3H), 6.50 (s, 1H), 4.58 (br s, 1H), 3.98–3.33 (m, 6H), 3.31–3.23 (m, 2H), 3.11 (s, 3H), 3.02–2.62 (m, 3H), 2.08 (br s, 2H), 1.10–0.98 (m, 1H), 0.63–0.45 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 165.08, 162.07, 160.78, 155.35, 140.10, 128.87, 128.54, 126.19, 91.26, 54.89, 51.73, 44.36, 44.10, 35.57, 33.91, 10.83, 3.48. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2400 found.
(R)-N-(Cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (90)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119z (38 mg, 0.13 mmol, 1 equiv), DiPEA (67 μL, 0.38 mmol, 3 equiv), and N-methylphenethylamine (28 μL, 0.19 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (80% → 100% EtOAc/pentane) afforded the product (37 mg, 94 μmol, 78%). ee: >99% (as determined by chiral HPLC using 75:25 hexane/ethanol, Chiralcell OD). TLC: Rf = 0.3 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.09 (br s, 1H), 7.33–7.27 (m, 2H), 7.26–7.17 (m, 3H), 6.52 (s, 1H), 4.59 (br s, 1H), 3.79 (dd, J = 8.7, 5.8 Hz, 2H), 3.74–3.37 (m, 4H), 3.33–3.22 (m, 2H), 3.12 (s, 3H), 2.96–2.82 (m, 2H), 2.70–2.21 (m, 1H), 2.09 (br s, 2H), 1.12–0.99 (m, 1H), 0.61–0.49 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 165.04, 162.12, 160.82, 155.49, 140.13, 128.90, 128.58, 126.22, 91.28, 70.52, 54.92, 51.75, 44.34, 44.12, 35.60, 33.93, 10.87, 3.50. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2394 found.
(S)-N-(Cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (91)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119aa (36 mg, 0.12 mmol, 1 equiv), DiPEA (63 μL, 0.36 mmol, 3 equiv), and N-methylphenethylamine (27 μL, 0.18 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (80% → 100% EtOAc/pentane) afforded the product (36 mg, 91 μmol, 70%). ee: 97% (as determined by chiral HPLC using 75:25 hexane/ethanol, Chiralcell OD). TLC: Rf = 0.3 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (t, J = 5.1 Hz, 1H), 7.35–7.27 (m, 2H), 7.25–7.15 (m, 3H), 6.51 (s, 1H), 4.58 (br s, 1H), 3.82–3.74 (m, 2H), 3.74–3.35 (m, 4H), 3.32–3.22 (m, 2H), 3.12 (s, 3H), 2.94–2.84 (m, 2H), 2.80–2.48 (m, 1H), 2.08 (br s, 2H), 1.12–0.98 (m, 1H), 0.60–0.48 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 165.07, 162.07, 160.77, 155.36, 140.10, 128.88, 128.55, 126.20, 91.26, 70.88, 70.32, 54.89, 51.74, 44.35, 44.11, 35.58, 33.91, 10.83, 3.48. HRMS [C22H29N5O2 + H]+: 396.2394 calculated, 396.2398 found.
(±)-N-(Cyclopropylmethyl)-6-(3-methoxypyrrolidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (92)
A round-bottom flask was charged with alcohol 89 (35 mg, 88 μmol, 1 equiv) in dry DMF (0.5 mL) and cooled to 0 °C. NaH (60% in mineral oil, 4 mg, 106 μmol, 1.2 equiv) was added, and the mixture was stirred for 15 min followed by addition of methyl iodide (6.0 μL, 97 μmol, 1.1 equiv). The reaction was allowed to warm to rt while stirring overnight. The reaction was quenched with H2O (20 mL) followed by extraction with DCM (3× 20 mL), drying (Na2SO4), filtering, and concentrating under reduced pressure. The residue was purified by silica gel column chromatography (60 → 70% EtOAc/pentane), affording the product (16 mg, 39 μmol, 44%). TLC: Rf = 0.4 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.07 (br s, 1H), 7.33–7.27 (m, 2H), 7.26–7.17 (m, 3H), 6.54 (s, 1H), 4.07 (br s, 1H), 3.88–3.72 (m, 3H), 3.72–3.45 (m, 3H), 3.37 (s, 3H), 3.33–3.23 (m, 2H), 3.13 (s, 3H), 2.98–2.84 (m, 2H), 2.29–1.96 (m, 2H), 1.14–0.99 (m, 1H), 0.62–0.48 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.96, 162.10, 160.86, 155.62, 140.16, 128.91, 128.57, 126.22, 91.25, 79.95, 79.15, 56.76, 51.75, 51.42, 44.42, 44.09, 35.62, 33.94, 30.45, 10.91, 3.50. HRMS [C23H31N5O2 + H]+: 410.2551 calculated, 410.2549 found.
(±)-N-(Cyclopropylmethyl)-6-(3-(dimethylamino)pyrrolidin-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (93)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119ab (50 mg, 0.15 mmol, 1 equiv), DiPEA (78 μL, 0.60 mmol, 4 equiv), and N-methylphenethylamine (33 μL, 0.23 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (2.5% → 10% MeOH/DCM) afforded the product (3 mg, 7 μmol, 5%). TLC: Rf = 0.5 (5% MeOH/DCM). 1H NMR (600 MHz, CDCl3) δ 8.10 (br s, 1H), 7.31–7.27 (m, 2H), 7.24–7.18 (m, 3H), 6.69 (s, 1H), 3.98 (dd, J = 10.6, 7.2 Hz, 1H), 3.92–3.64 (m, 3H), 3.58–3.50 (m, 1H), 3.42 (br s, 1H), 3.35–3.25 (m, 2H), 2.99 (br s, 3H), 2.89 (t, J = 7.7 Hz, 2H), 2.41 (br s, 6H), 2.24 (br s, 1H), 2.06–1.89 (m, 1H), 1.80–1.51 (m, 1H), 1.12–1.02 (m, 1H), 0.59–0.49 (m, 2H), 0.34–0.24 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 164.89, 163.37, 159.54, 156.17, 128.99, 128.68, 126.44, 90.62, 65.56, 51.98, 45.61, 44.02, 33.80, 28.71, 11.00, 3.52. HRMS [C24H34N6O + H]+: 423.2867 calculated, 423.2868 found.
(±)-N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(3-phenylpyrrolidin-1-yl)pyrimidine-4-carboxamide (94)
The title compound was prepared according to General Procedure C using dichloropyrimidine 118a (40 mg, 0.16 mmol, 1 equiv), DiPEA (43 μL, 0.24 mmol, 1.5 equiv), and (±)-3-phenylpyrrolidine (24 μL, 0.16 mmol, 1.0 equiv) in MeOH (1.6 mL) followed by concentration and addition of DiPEA (84 μL, 0.48 mmol, 3 equiv), N-methylphenethylamine (35 μL, 0.24 mmol, 1.5 equiv), and n-BuOH (0.75 mL). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 90% EtOAc/pentane) afforded the product (35 mg, 91 μmol, 57%). TLC: Rf = 0.4 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.08 (br s, 1H), 7.39–7.32 (m, 2H), 7.32–7.17 (m, 8H), 6.57 (s, 1H), 4.19 (br s, 1H), 3.94 (br s, 1H), 3.81 (br s, 2H), 3.74–3.38 (m, 3H), 3.30 (t, J = 6.4 Hz, 2H), 3.14 (s, 3H), 2.92 (br s, 2H), 2.42 (br s, 1H), 2.16 (br s, 1H), 1.14–1.00 (m, 1H), 0.62–0.46 (m, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.96, 161.90, 160.92, 155.72, 141.79, 140.15, 128.92, 128.80, 128.57, 127.19, 126.97, 126.23, 91.13, 52.89, 51.76, 46.36, 44.10, 43.39, 35.62, 33.98, 33.31, 10.93, 3.51. HRMS [C28H33N5O + H]+: 456.2758 calculated, 456.2757 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(benzyl(methyl)amino)pyrimidine-4-carboxamide (95)
The title compound was prepared according to General Procedure C using dichloropyrimidine 118a (37 mg, 0.15 mmol, 1 equiv), DiPEA (39 μL, 0.23 mmol, 1.5 equiv), and N-methylbenzylamine (19 μL, 0.15 mmol, 1.0 equiv) in MeOH (1.5 mL) followed by concentration, and addition of DiPEA (78 μL, 0.45 mmol, 3 equiv), N-methylphenethylamine (33 μL, 0.23 mmol, 1.5 equiv), and n-BuOH (0.75 mL). Total heating time: 4 h at 160 °C with μW irradiation. Purification by HPLC (C18 reverse phase, 43% to 49% CH3CN/H2O + 0.2% TFA) afforded the product (47 mg, 0.11 mmol, 73%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.08 (br s, 1H), 7.36–7.28 (m, 2H), 7.28–7.03 (m, 8H), 6.75 (br s, 1H), 4.86 (br s, 2H), 3.77 (br s, 2H), 3.39–3.22 (m, 2H), 3.13 (s, 3H), 3.08 (br s, 2H), 2.86 (br s, 2H), 1.14–0.98 (m, 1H), 0.62–0.48 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.88, 164.13, 160.83, 156.51, 139.96, 128.89, 128.74, 128.57, 127.24, 126.24, 90.07, 52.48, 51.85, 44.14, 35.75, 33.95, 10.94, 3.52. HRMS [C26H31N5O + H]+: 430.2601 calculated, 430.2604 found.
N-(Cyclopropylmethyl)-2,6-bis(methyl(phenethyl)amino)pyrimidine-4-carboxamide (96)
The title compound was prepared according to General Procedure A using dichloropyrimidine 118a (28 mg, 0.11 mmol, 1 equiv), DiPEA (79 μL, 0.46 mmol, 4 equiv), and N-methylphenethylamine (41 μL, 0.28 mmol, 2.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 40% EtOAc/pentane) afforded the product (30 mg, 68 μmol, 62%). TLC: Rf = 0.6 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.09 (t, J = 5.2 Hz, 1H), 7.32–7.24 (m, 4H), 7.24–7.12 (m, 6H), 6.68 (br s, 1H), 4.02–3.56 (m, 4H), 3.34–3.26 (m, 2H), 3.15 (s, 3H), 2.99 (br s, 3H), 2.96–2.87 (m, 4H), 1.15–1.01 (m, 1H), 0.60–0.48 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.96, 163.42, 160.86, 156.14, 140.03, 128.97, 128.90, 128.68, 128.60, 126.43, 126.25, 90.24, 51.72, 44.09, 35.71, 34.01, 33.85, 10.93, 3.50. HRMS [C27H33N5O + H]+: 444.2758 calculated, 444.2765 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(1H-pyrazol-1-yl)pyrimidine-4-carboxamide (97)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119ac (28 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and N-methylphenethylamine (22 μL, 0.15 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (20% → 40% EtOAc/pentane) afforded the product (30 mg, 80 μmol, 80%). TLC: Rf = 0.5 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.51 (s, 1H), 7.98–7.81 (m, 2H), 7.81–7.73 (m, 1H), 7.33–7.27 (m, 2H), 7.26–7.18 (m, 3H), 6.56–6.39 (m, 1H), 3.97–3.83 (m, 2H), 3.33 (dd, J = 6.9, 6.0 Hz, 2H), 3.21 (s, 3H), 3.01–2.91 (m, 2H), 1.18–1.00 (m, 1H), 0.57 (q, J = 5.4 Hz, 2H), 0.30 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 163.17, 160.57, 159.73, 159.48, 143.40, 139.40, 128.84, 128.69, 127.30, 126.52, 108.54, 94.69, 51.83, 44.24, 36.01, 33.79, 10.87, 3.52. HRMS [C21H24N6O + H]+: 377.2084 calculated, 377.2088 found.
N-(Cyclopropylmethyl)-6-(1H-imidazol-1-yl)-2-(methyl(phenethyl)amino)pyrimidine-4-carboxamide (98)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119ad (42 mg, 0.15 mmol, 1 equiv), DiPEA (78 μL, 0.45 mmol, 3 equiv), and N-methylphenethylamine (33 μL, 0.23 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Purification by preparative HPLC (C18 reverse phase, 35% to 45% CH3CN/H2O + 0.2% TFA, RT 8.87 min) afforded the product (27 mg, 72 μmol, 48%). TLC: Rf = 0.5 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.46 (s, 1H), 7.99–7.72 (m, 1H), 7.68 (s, 1H), 7.33–7.27 (m, 3H), 7.25–7.17 (m, 4H), 4.00–3.81 (m, 2H), 3.38–3.29 (m, 2H), 3.22 (s, 3H), 3.02–2.90 (m, 2H), 1.16–1.03 (m, 1H), 0.69–0.51 (m, 2H), 0.32 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 162.88, 160.89, 160.19, 157.03, 139.14, 135.37, 131.10, 128.82, 128.73, 126.61, 116.00, 93.80, 51.89, 44.36, 36.06, 33.78, 10.84, 3.56. HRMS [C21H24N6O + H]+: 377.2084 calculated, 377.2087 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-phenoxypyrimidine-4-carboxamide (99)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119ae (49 mg, 0.16 mmol, 1 equiv), DiPEA (84 μL, 0.48 mmol, 3 equiv), and N-methylphenethylamine (35 μL, 0.24 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (5% → 25% EtOAc/pentane) afforded the product (40 mg, 99 μmol, 62%). TLC: Rf = 0.5 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.91 (br s, 1H), 7.46–7.36 (m, 2H), 7.35–7.05 (m, 7H), 7.04–6.68 (m, 2H), 4.00–3.39 (m, 2H), 3.30 (t, J = 6.4 Hz, 2H), 3.10 (br s, 3H), 2.97–2.53 (m, 2H), 1.13–1.01 (m, 1H), 0.56 (q, J = 5.6 Hz, 2H), 0.35–0.23 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 171.62, 163.46, 160.96, 159.59, 152.85, 139.32, 129.64, 128.89, 128.50, 126.33, 125.47, 122.04, 51.85, 44.18, 35.78, 33.63, 10.88, 3.51. HRMS [C24H26N4O2 + H]+: 403.2129 calculated, 403.2137 found.
N-(Cyclopropylmethyl)-2-(methyl(phenethyl)amino)-6-(pyridin-3-yloxy) Pyrimidine-4-carboxamide (100)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119af (38 mg, 0.12 mmol, 1 equiv), DiPEA (62 μL, 0.36 mmol, 3 equiv), and N-methylphenethylamine (26 μL, 0.18 mmol, 1.5 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (60% → 100% EtOAc/pentane) afforded the product (26 mg, 64 μmol, 54%). TLC: Rf = 0.5 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.66–8.42 (m, 2H), 7.90 (br s, 1H), 7.65–7.44 (m, 1H), 7.41–7.32 (m, 1H), 7.31–7.12 (m, 4H), 7.07–6.71 (m, 2H), 3.82 (br s, 1H), 3.51 (br s, 1H), 3.38–3.26 (m, 2H), 3.13 (br s, 2H), 2.93 (br s, 2H), 2.65 (br s, 1H), 1.15–1.02 (m, 1H), 0.57 (q, J = 5.1 Hz, 2H), 0.30 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 170.88, 163.22, 160.69, 160.01, 149.45, 146.41, 144.26, 129.68, 128.80, 128.56, 126.44, 123.96, 51.74, 44.23, 35.78, 33.59, 10.88, 3.53. HRMS [C23H25N5O2 + H]+: 404.2081 calculated, 404.2088 found.
(S)-N-(Cyclopropylmethyl)-6-(dimethylamino)-2-(3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (101)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119r (25 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and (S)-3-phenylpiperidine (21 mg, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (28 mg, 74 μmol, 74%). ee: >99% (as determined by chiral HPLC using 70:30 hexane/isopropanol, Chiralcell OD). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (br s, 1H), 7.43–7.16 (m, 5H), 6.70 (s, 1H), 4.84 (t, J = 14.1 Hz, 2H), 3.39–3.19 (m, 2H), 3.10 (s, 6H), 2.89 (t, J = 12.1 Hz, 2H), 2.77 (t, J = 10.2 Hz, 1H), 2.07 (d, J = 13.1 Hz, 1H), 1.88–1.60 (m, 3H), 1.14–0.98 (m, 1H), 0.63–0.44 (m, 2H), 0.36–0.20 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.91, 164.18, 160.89, 156.13, 144.43, 128.61, 127.31, 126.58, 90.71, 51.38, 44.70, 44.02, 42.56, 37.30, 32.21, 25.58, 10.96, 3.51, 3.49. HRMS [C22H29N5O + H]+: 380.2445 calculated, 380.2452 found.
(R)-N-(Cyclopropylmethyl)-6-(dimethylamino)-2-(3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (102)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119r (25 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv) and (R)-3-phenylpiperidine (21 mg, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (30% → 50% EtOAc/pentane) afforded the product (32 mg, 84 μmol, 84%). ee: >97% (as determined by chiral HPLC using 70:30 hexane/isopropanol, Chiralcell OD). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.01 (t, J = 5.4 Hz, 1H), 7.39–7.28 (m, 4H), 7.28–7.21 (m, 1H), 6.70 (s, 1H), 4.95–4.74 (m, 2H), 3.40–3.18 (m, 2H), 3.10 (s, 6H), 2.96–2.83 (m, 2H), 2.83–2.70 (m, 1H), 2.07 (d, J = 13.9 Hz, 1H), 1.90–1.81 (m, 1H), 1.81–1.62 (m, 2H), 1.11–0.99 (m, 1H), 0.59–0.46 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.91, 164.18, 160.90, 156.12, 144.43, 128.61, 127.31, 126.58, 90.71, 51.38, 44.70, 44.02, 42.56, 37.30, 32.21, 25.58, 10.96, 3.51, 3.49. HRMS [C22H29N5O + H]+: 380.2445 calculated, 380.2452 found.
(S)-N-(Cyclopropylmethyl)-6-morpholino-2-(3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (103)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 31 (30 mg, 0.10 mmol, 1 equiv), DiPEA (52 μL, 0.30 mmol, 3 equiv), and (S)-3-phenylpiperidine (21 mg, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (40% → 60% EtOAc/pentane) afforded the product (41 mg, 97 μmol, 97%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.97 (br s, 1H), 7.40–7.32 (m, 2H), 7.32–7.21 (m, 3H), 6.73 (s, 1H), 4.90–4.70 (m, 2H), 3.83–3.70 (m, 4H), 3.69–3.56 (m, 4H), 3.36–3.19 (m, 2H), 2.97–2.83 (m, 2H), 2.76 (tt, J = 11.5, 3.6 Hz, 1H), 2.13–2.02 (m, 1H), 1.90–1.72 (m, 2H), 1.72–1.59 (m, 1H), 1.12–0.98 (m, 1H), 0.59–0.45 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 164.63, 164.11, 160.97, 156.96, 144.30, 128.66, 127.30, 126.67, 90.60, 66.73, 51.27, 44.71, 44.52, 44.06, 42.64, 32.28, 25.57, 10.94, 3.50, 3.48. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2549 found.
(S)-N-(Cyclopropylmethyl)-6-(1,1-dioxidothiomorpholino)-2-(3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (104)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119o (4:1 mixture of regioisomers) (35 mg, 0.10 mmol, 1 equiv), DiPEA (53 μL, 0.30 mmol, 3 equiv), and (S)-3-phenylpiperidine (21 mg, 0.13 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Purification by preparative HPLC (C18 reverse phase, 45% to 55% CH3CN/H2O + 0.2% TFA, RT 12.52 min) afforded the product (27 mg, 57 μmol, 56%). TLC: Rf = 0.5 (60% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.94 (br s, 1H), 7.43–7.32 (m, 2H), 7.32–7.27 (m, 3H), 6.83 (s, 1H), 4.78 (t, J = 11.0 Hz, 2H), 4.19 (br s, 4H), 3.38–3.19 (m, 2H), 3.12–2.99 (m, 4H), 2.94 (t, J = 12.2 Hz, 2H), 2.82–2.68 (m, 1H), 2.10 (dd, J = 17.2, 4.5 Hz, 1H), 1.88 (d, J = 13.1 Hz, 1H), 1.85–1.72 (m, 1H), 1.72–1.57 (m, 1H), 1.11–0.96 (m, 1H), 0.54 (q, J = 5.3 Hz, 2H), 0.28 (q, J = 4.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 164.07, 162.85, 160.95, 158.16, 143.95, 128.77, 127.24, 126.85, 90.31, 51.54, 51.28, 44.77, 44.14, 43.08, 42.71, 32.18, 25.50, 10.92, 3.52, 3.50. HRMS [C24H31N5O3S + H]+: 470.2220 calculated, 470.2223 found.
N-(Cyclopropylmethyl)-6-((R)-3-hydroxypyrrolidin-1-yl)-2-((S)-3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (105)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119z (24 mg, 81 μmol, 1 equiv), DiPEA (42 μL, 0.24 mmol, 3 equiv), and (S)-3-phenylpiperidine (17 mg, 0.11 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 90% EtOAc/pentane) afforded the product (23 mg, 55 μmol, 67%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.02 (br s, 1H), 7.39–7.22 (m, 5H), 6.54 (s, 1H), 4.84 (t, J = 14.0 Hz, 2H), 4.57 (s, 1H), 3.86–3.37 (m, 4H), 3.37–3.17 (m, 2H), 2.87 (t, J = 12.0 Hz, 2H), 2.81–2.70 (m, 1H), 2.16–1.93 (m, 3H), 1.90–1.54 (m, 4H), 1.11–0.97 (m, 1H), 0.59–0.45 (m, 2H), 0.27 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.93, 162.20, 160.91, 155.71, 144.42, 128.64, 127.33, 126.60, 91.76, 54.96, 51.30, 44.67, 44.38, 44.07, 42.59, 32.24, 25.59, 10.93, 3.52. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2551 found.
N-(Cyclopropylmethyl)-6-((S)-3-hydroxypyrrolidin-1-yl)-2-((R)-3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (106)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119aa (32 mg, 0.11 mmol, 1 equiv), DiPEA (56 μL, 0.32 mmol, 3 equiv), and (R)-3-phenylpiperidine (23 mg, 0.14 mmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 100% EtOAc/pentane) afforded the product (26 mg, 62 μmol, 56%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (t, J = 5.7 Hz, 1H), 7.40–7.19 (m, 5H), 6.53 (s, 1H), 4.84 (t, J = 14.3 Hz, 2H), 4.57 (s, 1H), 3.90–3.37 (m, 4H), 3.37–3.17 (m, 2H), 2.94–2.81 (m, 2H), 2.81–2.69 (m, 1H), 2.16–1.96 (m, 3H), 1.89–1.57 (m, 4H), 1.10–0.98 (m, 1H), 0.60–0.42 (m, 2H), 0.26 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.84, 162.10, 160.81, 155.58, 144.32, 128.52, 127.22, 126.48, 91.64, 70.84, 70.16, 54.84, 51.19, 44.55, 44.27, 43.95, 42.47, 32.13, 25.48, 10.82, 3.40. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2552 found.
N-(Cyclopropylmethyl)-6-((R)-3-hydroxypyrrolidin-1-yl)-2-((R)-3-phenylpiperidin-1-yl)pyrimidine-4-carboxamide (107)
The title compound was prepared according to General Procedure A using 2-chloropyrimidine 119z (22 mg, 74 μmol, 1 equiv), DiPEA (39 μL, 0.22 mmol, 3 equiv), and (R)-3-phenylpiperidine (16 mg, 96 μmol, 1.3 equiv). Total heating time: 4 h at 160 °C with μW irradiation. Column chromatography (70% → 90% EtOAc/pentane) afforded the product (23 mg, 55 μmol, 74%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.03 (t, J = 5.7 Hz, 1H), 7.40–7.19 (m, 5H), 6.53 (s, 1H), 4.84 (t, J = 14.3 Hz, 2H), 4.57 (s, 1H), 3.90–3.37 (m, 4H), 3.37–3.17 (m, 2H), 2.94–2.81 (m, 2H), 2.81–2.69 (m, 1H), 2.56 (s, 1H), 2.07 (d, J = 12.7 Hz, 3H), 1.89–1.57 (m, 4H), 1.10–0.98 (m, 1H), 0.60–0.42 (m, 2H), 0.26 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.96, 162.19, 160.91, 155.67, 144.43, 128.62, 127.32, 126.59, 91.75, 70.36, 54.95, 51.36, 44.68, 44.38, 44.06, 42.54, 32.20, 25.60, 10.92, 3.51. HRMS [C24H31N5O2 + H]+: 422.2551 calculated, 422.2552 found.
Methyl 4-Chloro-6-(methyl(phenethyl)amino)picolinate (109)
A round-bottom flask was charged with methyl 4,6-dichloropicolinate (108) (206 mg, 0.99 mmol, 1 equiv), N-methylphenethylamine HBr salt (218 mg, 1.01 mmol, 1.02 equiv), DiPEA (436 μL, 2.5 mmol, 2.5 equiv), and dry MeOH (2 mL). The solution was stirred at rt for 3 days and then refluxed for 24 h. The reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (5% → 35% EtOAc/pentane), affording the product (122 mg, 0.40 mmol, 41%). TLC: Rf = 0.2 (5% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.35–7.26 (m, 3H), 7.23 (t, J = 7.3 Hz, 1H), 7.16 (d, J = 7.0 Hz, 2H), 6.56 (d, J = 2.3 Hz, 1H), 3.96 (s, 3H), 3.63 (t, J = 7.3 Hz, 2H), 2.93–2.81 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 165.68, 155.45, 152.41, 147.85, 138.19, 128.84, 128.80, 126.84, 107.83, 107.79, 53.84, 53.05, 38.50, 33.14. Regioselectivity was confirmed by 1H and 13C-HMBC and 1H-NOESY 2D NMR. HRMS [C16H17ClN2O2 + H]+: 305.1051 calculated, 305.1054 found.
4-Chloro-6-(methyl(phenethyl)amino)picolinic Acid (110)
A round-bottom flask was charged with methyl ester 109 (122 mg, 0.4 mmol, 1 equiv) and THF (2 mL). An aqueous 1.5 M NaOH solution (0.53 mL, 0.8 mmol, 2 equiv) was added dropwise, and the reaction was stirred for 1.5 h at rt. The mixture was cooled to 0 °C and acidified to pH 1 by dropwise addition of 37% w/w aq. HCl. The mixture was then extracted with DCM (3× 5 mL), and the combined organic layers were washed with brine (1× 15 mL), dried (MgSO4), filtered, and concentrated under reduced pressure to afford the product (104 mg, 0.36 mmol, 89%). TLC Rf = 0.1 (5% MeOH/DCM with 3 drops of AcOH). 1H NMR (400 MHz, CDCl3) δ 8.92 (br s, 1H), 7.37–7.20 (m, 4H), 7.20–7.12 (m, 2H), 6.57 (d, J = 2.4 Hz, 1H), 3.66 (t, J = 7.2 Hz, 2H), 2.96–2.81 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 164.25, 156.33, 150.90, 146.25, 138.01, 128.96, 128.86, 127.01, 108.27, 105.87, 54.04, 38.78, 33.21.
4-Chloro-N-(cyclopropylmethyl)-6-(methyl(phenethyl)amino)picolinamide (111)
A round-bottom flask was charged with carboxylic acid 110 (104 mg, 0.36 mmol, 1 equiv), HOBt (73 mg, 0.47 mmol, 1.3 equiv), EDC hydrochloride (102 mg, 0.53 mmol, 1.5 equiv), and dry DCM (1.8 mL). The suspension was stirred for 1 h at rt followed by the addition of cyclopropylmethanamine (37 μL, 0.43 mmol, 1.2 equiv). After stirring for 20 h, the solvent was removed under reduced pressure and the residue was dissolved in EtOAc (10 mL) and washed with 1 M aq. HCl (1× 10 mL), sat. aq. NaHCO3 (1× 10 mL), and brine (1× 10 mL). The organic layer was dried (MgSO4), filtered, and concentrated under reduced pressure. The crude material was purified by silica gel column chromatography (isocratic, 30% EtOAc/pentane), affording the product (30 mg, 87 μmol, 9%), Rf = 0.35 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.99 (t, J = 5.9 Hz, 1H), 7.43 (d, J = 2.4 Hz, 1H), 7.35–7.26 (m, 2H), 7.28–7.18 (m, 1H), 7.20–7.15 (m, 2H), 6.50 (d, J = 2.4 Hz, 1H), 3.64 (t, J = 7.3 Hz, 2H), 3.30 (dd, J = 7.1, 5.9 Hz, 2H), 2.91–2.84 (m, 5H), 1.13–1.01 (m, 1H), 0.58–0.52 (m, 2H), 0.31–0.26 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 163.90, 155.94, 151.08, 150.38, 138.41, 128.93, 128.90, 126.86, 106.84, 104.87, 54.02, 44.51, 38.71, 33.25, 10.92, 3.76.
Methyl 2-Chloro-6-morpholinoisonicotinate (113)
A round-bottom flask was charged with methyl-2,6-dichloroisonicotinate (112) (410 mg, 1.99 mmol, 1 equiv), K2CO3 (549 mg, 3.97 mmol, 2 equiv), morpholine (259 μL, 3.00 mmol, 1.5 equiv), and dry CH3CN (10 mL). The mixture was heated to reflux. After 45 h, the reaction was complete as judged by TLC and cooled to room temperature. The mixture was filtered, and the filtrate was concentrated under reduced pressure. The crude material was purified by silica gel column chromatography (10% → 30% EtOAc/pentane), affording the product (323 mg, 1.26 mmol, 66%). TLC: Rf = 0.6 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.13 (d, J = 0.9 Hz, 1H), 7.07 (d, J = 1.0 Hz, 1H), 3.92 (s, 3H), 3.86–3.76 (m, 4H), 3.64–3.52 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 165.16, 159.40, 150.36, 141.33, 111.77, 104.69, 66.55, 52.83, 45.26. HRMS [C11H13ClN2O3 + H]+: 257.0688 calculated, 257.0690 found.
2-Chloro-6-morpholinoisonicotinic Acid (114)
A round-bottom flask was charged with methyl ester 113 (323 mg, 1.26 mmol, 1 equiv) and THF (5 mL). A 1 M aqueous solution of NaOH (2.52 mL, 2.52 mmol, 2 equiv) was added dropwise. After stirring for 20 min at room temperature, the reaction mixture was acidified carefully with 37% w/w HCl to pH 1, and THF was removed under reduced pressure. The mixture was extracted with DCM (3× 10 mL), the combined organic layers were washed with brine (1× 15 mL), dried (MgSO4), filtered, and concentrated under reduced pressure affording the product (325 mg, 1.26 mmol, 99%). TLC: Rf = 0.2 (30% EtOAc/pentane with 3 drops of AcOH). 1H NMR (400 MHz, MeOD + CDCl3) δ 7.12 (s, 1H), 7.09 (s, 1H), 3.78 (t, J = 4.9 Hz, 4H), 3.54 (t, J = 4.9 Hz, 4H). 13C NMR (101 MHz, MeOD + CDCl3) δ 167.10, 160.29, 150.79, 143.21, 112.56, 105.74, 67.18, 45.94. HRMS [C10H11ClN2O3 + H]+: 243.0531 calculated, 243.0533 found.
2-Chloro-N-(cyclopropylmethyl)-6-morpholinoisonicotinamide (115)
A round-bottom flask was charged with carboxylic acid 114 (325 mg, 1.26 mmol, 1 equiv), EDC hydrochloride (302 mg, 1.95 mmol, 1.5 equiv), and HOBt (298 mg, 1.95 mmol, 1.5 equiv) and dry DCM (7 mL). The suspension was stirred for 1 h at room temperature followed by addition of cyclopropylmethanamine (139 μL, 1.6 mmol, 1.2 equiv). After 20 h, DCM was removed under reduced pressure and the residue was dissolved in EtOAc (15 mL) and sequentially washed with 1 M HCl (aq) (2× 15 mL), sat. aq. NaHCO3 (2× 15 mL), and brine (1× 20 mL). The organic layer was dried (MgSO4), filtered, and concentrated under reduced pressure. The resulting crude material was purified by silica gel column chromatography (30% → 60% EtOAc/pentane) affording the product (296 mg, 1 mmol, 80%). TLC: Rf = 0.3 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 6.91 (s, 1H), 6.81 (s, 1H), 6.45–6.31 (m, 1H), 3.84–3.73 (m, 4H), 3.66–3.51 (m, 4H), 3.28 (dd, J = 7.2, 5.4 Hz, 2H), 1.12–0.98 (m, 1H), 0.64–0.52 (m, 2H), 0.38–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 165.28, 159.52, 150.25, 146.27, 109.10, 103.25, 66.61, 45.27, 45.25, 10.68, 3.73. HRMS [C14H18ClN3O2 + H]+: 296.1160 calculated, 296.1158 found.
2,6-Dichloropyrimidine-4-carbonyl chloride (117)
In a 500 mL round-bottom flask, orotic acid (116) (15.6 g, 100 mmol, 1 equiv) was dissolved in phosphorous oxychloride (46 mL, 500 mmol, 5 equiv.) and 10 drops of DMF were added. The mixture was heated to reflux and stirred for 19 h. n-Hexane (250 mL) was added, and the mixture was stirred vigorously for 10 min and then transferred to a separatory funnel containing 100 mL of H2O. The flask was washed with 50 mL of hexane. After shaking, the aqueous layer was removed, and the organic layer was washed with brine (1× 100 mL), dried (MgSO4), and concentrated under reduced pressure to yield the product (12.8 g, 60.4 mmol, 60%). 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 167.17, 165.27, 161.56, 158.19, 119.70.
2,6-Dichloro-N-(cyclopropylmethyl)pyrimidine-4-carboxamide (118a)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.63 mL, 5 mmol, 1 equiv), Et3N (0.91 mL, 6.5 mmol, 1.3 equiv), and cyclopropylmethanamine (444 μL, 5.13 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.99 g, 4.0 mmol, 81%). TLC: Rf = 0.8 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.11 (s, 1H), 7.96 (br s, 1H), 3.44–3.27 (m, 2H), 1.20–1.03 (m, 1H), 0.68–0.52 (m, 2H), 0.32 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.74, 160.44, 160.04, 159.77, 118.22, 44.72, 10.57, 3.67. HRMS [C9H9Cl2N3O + H]+: 246.0195 calculated, 246.0196 found.
2,6-Dichloro-N-methylpyrimidine-4-carboxamide (118b)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.63 mL, 5.0 mmol, 1 equiv), Et3N (1.6 mL, 11.5 mmol, 2.3 equiv), and methylamine HCl salt (0.35 g, 5.13 mmol, 1.025 equiv). Column chromatography (10% → 30% EtOAc/pentane) afforded the product (0.97 g, 4.7 mmol, 94%). TLC: Rf = 0.3 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.90 (br s, 1H), 3.08 (d, J = 5.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.84, 160.85, 160.25, 159.82, 118.11, 26.51. HRMS [C6H5Cl2N3O + H]+: 205.9882 calculated, 205.9884 found.
2,6-Dichloro-N-ethylpyrimidine-4-carboxamide (118c)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.63 mL, 5 mmol, 1 equiv), Et3N (1.6 mL, 11.5 mmol, 2.3 equiv), and ethylamine HCl salt (0.42 g, 5.13 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.88 g, 4.0 mmol, 80%). TLC: Rf = 0.6 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.83 (br s, 1H), 3.65–3.38 (m, 2H), 1.30 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.84, 160.47, 160.06, 159.83, 118.18, 34.86, 14.63. HRMS [C7H7Cl2N3O + H]+: 220.0039 calculated, 220.0040 found.
2,6-Dichloro-N-butyl-pyrimidine-4-carboxamide (118d)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.25 mL, 2.0 mmol, 1 equiv), Et3N (0.36 mL, 2.60 mmol, 1.3 equiv), and n-butylamine (0.20 mL, 2.05 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.50 g, 2.0 mmol, 99%). TLC: Rf = 0.7 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.85 (br s, 1H), 3.49 (q, J = 7.0 Hz, 2H), 1.74–1.56 (m, 2H), 1.53–1.35 (m, 2H), 0.97 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 164.78, 160.46, 160.13, 159.78, 118.18, 39.64, 31.40, 20.09, 13.72. HRMS [C9H11Cl2N3O + H]+: 248.0352 calculated, 248.0354 found.
2,6-Dichloro-N-hexyl-pyrimidine-4-carboxamide (118e)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.25 mL, 2.0 mmol, 1 equiv), Et3N (0.36 mL, 2.6 mmol, 1.3 equiv), and n-hexylamine (0.27 mL, 2.05 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.58 g, 2.0 mmol, 99%). TLC: Rf = 0.6 (10% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.89 (br s, 1H), 3.48 (q, J = 7.0 Hz, 2H), 1.66 (p, J = 7.8, 7.4 Hz, 2H), 1.46–1.23 (m, 6H), 0.98–0.83 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 164.71, 160.46, 160.06, 159.73, 118.14, 39.91, 31.38, 29.30, 26.55, 22.48, 13.96. HRMS [C11H15Cl2N3O + H]+: 276.0665 calculated, 276.0668 found.
2,6-Dichloro-N-isobutylpyrimidine-4-carboxamide (118f)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.25 mL, 2.0 mmol, 1 equiv), Et3N (0.36 mL, 2.60 mmol, 1.3 equiv), and isobutylamine (0.20 mL, 2.05 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.50 g, 2.0 mmol, 99%). TLC: Rf = 0.8 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.11 (s, 1H), 7.94 (br s, 1H), 3.33 (t, J = 6.6 Hz, 2H), 2.07–1.87 (m, 1H), 1.00 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 164.68, 160.41, 160.15, 159.70, 118.17, 47.10, 28.52, 20.07. HRMS [C9H11Cl2N3O + H]+: 248.0352 calculated, 248.0354 found.
2,6-Dichloro-N-neopentylpyrimidine-4-carboxamide (118g)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.25 mL, 2.0 mmol, 1 equiv), Et3N (0.36 mL, 2.6 mmol, 1.3 equiv), and neopentylamine (0.24 mL, 2.05 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.58 g, 2.0 mmol, 99%). TLC: Rf = 0.9 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.11 (s, 1H), 7.91 (br s, 1H), 3.30 (d, J = 6.7 Hz, 2H), 1.01 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 164.69, 160.38, 160.22, 159.71, 118.24, 50.89, 32.35, 27.20. HRMS [C10H13Cl2N3O + H]+: 262.0508 calculated, 262.0510 found.
2,6-Dichloro-N-(prop-2-yn-1-yl)pyrimidine-4-carboxamide (118h)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.25 mL, 2.0 mmol, 1 equiv), Et3N (0.36 mL, 2.60 mmol, 1.3 equiv), and propargylamine (0.13 mL, 2.05 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.44 g, 1.91 mmol, 96%). TLC: Rf = 0.6 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.24–8.00 (m, 2H), 4.30 (dd, J = 5.7, 2.6 Hz, 2H), 2.36 (t, J = 2.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 164.92, 160.08, 159.94, 159.63, 118.35, 78.30, 72.42, 29.52. HRMS [C8H5Cl2N3O + H]+: 229.9882 calculated, 229.9884 found.
Methyl (2,6-Dichloropyrimidine-4-carbonyl)glycinate (118i)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.32 mL, 2.5 mmol, 1 equiv), Et3N (0.80 mL, 5.75 mmol, 2.3 equiv), and glycine methylester HCl salt (0.32 g, 2.56 mmol, 1.025 equiv). Column chromatography (5% → 20% EtOAc/pentane) afforded the product (0.51 g, 1.95 mmol, 78%). TLC: Rf = 0.5 (30% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 8.31 (br s, 1H), 8.09 (s, 1H), 4.28 (d, J = 5.8 Hz, 2H), 3.81 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.30, 164.89, 160.65, 160.02, 159.55, 118.31, 52.66, 41.35. HRMS [C8H7Cl2N3O3 + H]+: 263.9937 calculated, 263.9939 found.
2,6-Dichloropyrimidine-N-benzyl-4-carboxamide (118j)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.63 mL, 5.0 mmol, 1 equiv), Et3N (0.91 mL, 6.5 mmol, 1.3 equiv), and benzylamine (0.56 mL, 5.13 mmol, 1.025 equiv). Column chromatography (10% → 25% EtOAc/pentane) afforded the product (1.38 g, 4.9 mmol, 98%). TLC: Rf = 0.7 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.18 (br s, 1H), 8.08 (s, 1H), 7.39–7.23 (m, 5H), 4.63 (d, J = 6.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.81, 160.20, 160.17, 159.81, 137.00, 128.81, 127.90, 127.86, 118.35, 43.82. HRMS [C12H9Cl2N3O + H]+: 282.0195 calculated, 282.0197 found.
2,6-Dichloropyrimidine-N-([1,1′-biphenyl]-4-ylmethyl)-4-carboxamide (118k)
The title compound was prepared according to General Procedure D using 2,6-dichloropyrimidine-4-carbonyl chloride 117 (0.63 mL, 5.0 mmol, 1 equiv), Et3N (0.91 mL, 6.5 mmol, 1.3 equiv), and 4-phenylbenzylamine (0.94 g, 5.13 mmol, 1.025 equiv). Column chromatography (10% → 30% EtOAc/pentane) afforded the product (1.46 g, 4.1 mmol, 81%). TLC: Rf = 0.8 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.16 (t, J = 5.7 Hz, 1H), 8.07 (s, 1H), 7.54 (d, J = 7.9 Hz, 4H), 7.46–7.29 (m, 5H), 4.65 (d, J = 6.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.86, 160.26, 160.14, 159.86, 140.82, 140.40, 136.01, 128.84, 128.43, 127.52, 127.49, 127.03, 118.37, 43.57. HRMS [C18H13Cl2N3O + H]+: 358.0508 calculated, 358.0508 found.
2-Chloro-N-methyl-6-morpholinopyrimidine-4-carboxamide (119a)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118b (0.31 g, 1.5 mmol, 1 equiv), DiPEA (0.39 mL, 2.25 mmol, 1.5 equiv), and morpholine (0.14 mL, 1.58 mmol, 1.05 equiv). Column chromatography (40% → 80% EtOAc/pentane) afforded the product as an 8.5:1 mixture of regioisomers (0.39 g, 1.5 mmol, 99%). TLC: Rf = 0.4 (100% EtOAc/pentane). Major regioisomer: 1H NMR (400 MHz, CDCl3) δ 7.93–7.77 (m, 1H), 7.26 (s, 1H), 3.83–3.61 (m, 8H), 3.00 (d, J = 5.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.82, 162.92, 159.79, 157.70, 99.16, 66.34, 44.60, 44.38, 26.19. HRMS [C10H13ClN4O2 + H]+: 257.0800 calculated, 257.0800 found.
2-Chloro-N-ethyl-6-morpholinopyrimidine-4-carboxamide (119b)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118c (0.42 g, 1.89 mmol, 1 equiv), DiPEA (0.49 mL, 2.84 mmol, 1.5 equiv), and morpholine (0.17 mL, 1.98 mmol, 1.05 equiv). Column chromatography (50% → 70% EtOAc/pentane) afforded the product (0.48 g, 1.77 mmol, 94%). TLC: Rf = 0.2 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.82 (br s, 1H), 7.27 (s, 1H), 3.99–3.55 (m, 8H), 3.54–3.37 (m, 2H), 1.25 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.86, 162.17, 159.85, 157.93, 99.27, 66.39, 44.63, 34.53, 14.71. HRMS [C11H15ClN4O2 + H]+: 271.0956 calculated, 271.0957 found.
2-Chloro-N-butyl-6-morpholinopyrimidine-4-carboxamide (119c)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118d (193 mg, 0.78 mmol, 1 equiv), DiPEA (203 μL, 1.17 mmol, 1.5 equiv), and morpholine (71 μL, 0.82 mmol, 1.05 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (212 mg, 0.71 mmol, 91%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.84 (br s, 1H), 7.27 (s, 1H), 3.91–3.61 (m, 8H), 3.43 (q, J = 7.1 Hz, 2H), 1.70–1.51 (m, 2H), 1.48–1.34 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.79, 162.19, 159.75, 157.88, 99.21, 66.31, 44.57, 39.29, 31.48, 20.07, 13.72. HRMS [C13H19ClN4O2 + H]+: 299.1269 calculated, 299.1269 found.
2-Chloro-N-hexyl-6-morpholinopyrimidine-4-carboxamide (119d)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118e (0.28 g, 1.02 mmol, 1 equiv), DiPEA (0.27 mL, 1.54 mmol, 1.5 equiv), and morpholine (94 μL, 1.08 mmol, 1.05 equiv). Column chromatography (40% → 70% EtOAc/pentane) afforded the product (0.31 g, 0.95 mmol, 93%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.87 (t, J = 5.8 Hz, 1H), 7.28 (s, 1H), 4.01–3.53 (m, 8H), 3.42 (q, J = 6.9 Hz, 2H), 1.61 (p, J = 7.7, 7.3 Hz, 2H), 1.46–1.22 (m, 6H), 0.99–0.80 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 163.71, 162.11, 159.68, 157.81, 99.17, 66.25, 44.55, 39.56, 31.37, 29.34, 26.53, 22.45, 13.97. HRMS [C15H23ClN4O2 + H]+: 327.1582 calculated, 327.1582 found.
2-Chloro-N-isobutyl-6-morpholinopyrimidine-4-carboxamide (119e)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118f (275 mg, 1.11 mmol, 1 equiv), DiPEA (290 μL, 1.66 mmol, 1.5 equiv), and morpholine (101 μL, 1.16 mmol, 1.05 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (330 mg, 1.11 mmol, 99%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.91 (t, J = 5.8 Hz, 1H), 7.28 (s, 1H), 4.01–3.52 (m, 8H), 3.26 (t, J = 6.7 Hz, 2H), 2.00–1.81 (m, 1H), 0.97 (d, J = 6.7 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 163.75, 162.25, 159.71, 157.83, 99.23, 66.26, 46.82, 44.56, 28.58, 20.11. HRMS [C13H19ClN4O2 + H]+: 299.1269 calculated, 299.1270 found.
2-Chloro-6-morpholino-N-neopentylpyrimidine-4-carboxamide (119f)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118g (0.25 g, 0.96 mmol, 1 equiv), DiPEA (0.25 mL, 1.44 mmol, 1.5 equiv), and morpholine (88 μL, 1.01 mmol, 1.05 equiv). Column chromatography (30% → 60% EtOAc/pentane) afforded the product (0.30 g, 0.96 mmol, 99%). TLC: Rf = 0.5 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.92 (br s, 1H), 7.28 (s, 1H), 3.96–3.50 (m, 8H), 3.24 (d, J = 6.7 Hz, 2H), 0.98 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 163.77, 162.36, 159.74, 157.82, 99.30, 66.27, 50.64, 44.60, 32.34, 27.23. HRMS [C14H21ClN4O2 + H]+: 313.1426 calculated, 313.1424 found.
2-Chloro-6-morpholino-N-(prop-2-yn-1-yl)pyrimidine-4-carboxamide (119g)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118h (221 mg, 0.96 mmol, 1 equiv), DiPEA (251 μL, 1.44 mmol, 1.5 equiv), and morpholine (88 μL, 1.01 mmol, 1.05 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (249 mg, 0.89 mmol, 92%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.00 (br s, 1H), 7.26 (s, 1H), 4.22 (dd, J = 5.7, 2.6 Hz, 2H), 3.88–3.59 (m, 8H), 2.29 (t, J = 2.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 163.84, 162.23, 160.04, 157.20, 99.53, 78.84, 72.05, 66.41, 29.32. HRMS [C12H13ClN4O2 + H]+: 281.0800 calculated, 281.0800 found.
Methyl (2-Chloro-6-morpholinopyrimidine-4-carbonyl)glycinate (119h)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118i (396 mg, 1.50 mmol, 1 equiv), DiPEA (392 μL, 2.25 mmol, 1.5 equiv), and morpholine (137 μL, 1.58 mmol, 1.05 equiv). Column chromatography (60% → 80% EtOAc/pentane) afforded the product (298 mg, 0.95 mmol, 63%). TLC: Rf = 0.5 (70% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.25 (t, J = 5.4 Hz, 1H), 7.25 (s, 1H), 4.22 (d, J = 5.9 Hz, 2H), 3.90–3.61 (m, 11H). 13C NMR (101 MHz, CDCl3) δ 169.59, 163.83, 162.85, 160.06, 157.09, 99.50, 66.39, 52.51, 44.69, 41.29. HRMS [C12H15ClN4O4 + H]+: 315.0855 calculated, 315.0851 found.
2-Chloro-N-benzyl-6-morpholinopyrimidine-4-carboxamide (119i)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118j (0.71 g, 2.50 mmol, 1 equiv), DiPEA (0.65 mL, 3.75 mmol, 1.5 equiv), and morpholine (0.23 mL, 2.63 mmol, 1.05 equiv). Column chromatography (30% → 60% EtOAc/pentane) afforded the product (0.68 g, 2.03 mmol, 81%). TLC: Rf = 0.6 (60% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.18 (br s, 1H), 7.39–7.24 (m, 6H), 4.61 (d, J = 6.2 Hz, 2H), 3.90–3.54 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 163.79, 162.37, 159.88, 157.63, 137.59, 128.77, 127.92, 127.67, 99.52, 66.37, 44.47, 43.60. HRMS [C16H17ClN4O2 + H]+: 333.1113 calculated, 333.1112 found.
2-Chloro-N-([1,1’-biphenyl]-4-ylmethyl)-6-morpholinopyrimidine-4-carboxamide (119j)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118k (0.31 g, 1.0 mmol, 1 equiv), DiPEA (0.26 mL, 1.5 mmol, 1.5 equiv), and morpholine (91 μL, 1.05 mmol, 1.05 equiv). Column chromatography (20% → 50% EtOAc/pentane) afforded the product (0.38 g, 0.94 mmol, 94%). TLC: Rf = 0.4 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.24 (t, J = 6.0 Hz, 1H), 7.61–7.52 (m, 4H), 7.47–7.31 (m, 5H), 7.28 (s, 1H), 4.64 (d, J = 6.2 Hz, 2H), 3.91–3.45 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 163.79, 162.44, 159.91, 157.63, 140.62, 136.62, 128.86, 128.43, 127.48, 127.11, 99.53, 66.37, 44.65, 43.36. HRMS [C22H21ClN4O2 + H]+: 409.1426 calculated, 409.1421 found.
2-Chloro-N-(cyclopropylmethyl)-6-(piperidin-1-yl)pyrimidine-4-carboxamide (119k)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (258 mg, 1.05 mmol, 1 equiv), DiPEA (274 μL, 1.58 mmol, 1.5 equiv), and piperidine (109 μL, 1.10 mmol, 1.05 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (293 mg, 0.99 mmol, 95%). TLC: Rf = 0.2 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.95 (br s, 1H), 7.27 (s, 1H), 3.71 (br s, 4H), 3.29 (t, J = 6.5 Hz, 2H), 1.85–1.52 (m, 6H), 1.15–0.98 (m, 1H), 0.55 (q, J = 5.4 Hz, 2H), 0.28 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 163.12, 162.45, 159.77, 157.35, 99.29, 44.31, 25.55, 24.31, 10.61, 3.58. HRMS [C14H19ClN4O + H]+: 295.1320 calculated, 295.1321 found.
2-Chloro-N-(cyclopropylmethyl)-6-(3,3-difluoropiperidin-1-yl)pyrimidine-4-carboxamide (119l)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (0.10 g, 0.41 mmol, 1 equiv), DiPEA (100 μL, 0.57 mmol, 2.4 equiv), and 3,3-difluoropiperidine HCl salt (68 mg, 0.43 mmol, 1.05 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (88 mg, 0.26 mmol, 63%). TLC: Rf = 0.4 (20% EtOAc/pentane). 1H NMR (300 MHz, CDCl3) δ 7.91 (br s, 1H), 7.35 (s, 1H), 3.99 (br s, 2H), 3.74 (br s, 2H), 3.29 (t, J = 6.5 Hz, 2H), 2.13 (tt, J = 13.3, 6.3 Hz, 2H), 2.02–1.74 (m, 2H), 1.17–0.95 (m, 1H), 0.67–0.44 (m, 2H), 0.28 (q, J = 5.1 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 164.08, 162.15, 159.87, 158.33, 119.17 (t, J = 244.8 Hz), 99.62, 44.54, 44.05, 32.63 (t, J = 23.5 Hz), 29.76, 21.70 (t, J = 4.5 Hz), 10.72, 3.71. HRMS [C14H17ClF2N4O + H]+: 331.1132 calculated, 331.1127 found.
2-Chloro-N-(cyclopropylmethyl)-6-(4,4-difluoropiperidin-1-yl)pyrimidine-4-carboxamide (119m)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (0.12 g, 0.49 mmol, 1 equiv), DiPEA (0.21 mL, 1.23 mmol, 2.5 equiv), and 4,4-difluoropiperidine HCl salt (86 mg, 0.52 mmol, 1.05 equiv). Column chromatography (5% → 25% EtOAc/pentane) afforded the product (90 mg, 0.27 mmol, 55%). TLC: Rf = 0.4 (15% EtOAc/pentane). 1H NMR (300 MHz, CDCl3) δ 7.91 (br s, 1H), 7.35 (s, 1H), 3.89 (br s, 4H), 3.38–3.20 (m, 2H), 2.06 (tt, J = 13.2, 5.9 Hz, 4H), 1.13–0.98 (m, 1H), 0.64–0.48 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 163.47, 162.18, 160.08, 158.40, 121.29 (t, J = 242.4 Hz), 99.44, 44.55, 41.49, 33.82 (t, J = 23.7 Hz), 10.72, 3.72. HRMS [C14H17ClF2N4O + H]+: 331.1132 calculated, 331.1127 found.
2-Chloro-N-(cyclopropylmethyl)-6-thiomorpholinopyrimidine-4-carboxamide (119n)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (117 mg, 0.48 mmol, 1 equiv), DiPEA (124 μL, 0.71 mmol, 1.5 equiv), and thiomorpholine (51 μL, 0.50 mmol, 1.05 equiv). Column chromatography (20% → 50% EtOAc/pentane) afforded the product (48 mg, 0.15 mmol, 32%). TLC: Rf = 0.2 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.91 (br s, 1H), 7.28 (s, 1H), 4.07 (br s, 4H), 3.36–3.23 (m, 2H), 2.74–2.63 (m, 4H), 1.12–0.99 (m, 1H), 0.65–0.48 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 163.33, 162.30, 160.04, 158.07, 99.66, 44.55, 27.05, 10.73, 3.74. HRMS [C13H17ClN4OS + H]+: 313.0884 calculated, 313.0884 found.
2-Chloro-N-(cyclopropylmethyl)-6-(1,1-dioxidothiomorpholino)pyrimidine-4-carboxamide (119o)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (92 mg, 0.37 mmol, 1 equiv), DiPEA (98 μL, 0.56 mmol, 1.5 equiv), and thiomorpholine-1,1-dioxide (53 mg, 0.39 mmol, 1.05 equiv). Column chromatography (60% → 70% EtOAc/pentane) afforded the product as a 4:1 mixture of regioisomers (69 mg, 0.20 mmol, 54%). TLC: Rf = 0.5 (60% EtOAc/pentane). Major regioisomer: 1H NMR (500 MHz, CDCl3 + MeOD) δ 7.41 (s, 1H), 4.26 (br s, 4H), 3.24 (d, J = 7.1 Hz, 2H), 3.18–3.12 (m, 4H), 1.10–0.98 (m, 1H), 0.59–0.49 (m, 2H), 0.32–0.21 (m, 2H). 13C NMR (126 MHz, CDCl3 + MeOD) δ 163.16, 162.11, 159.92, 158.47, 99.62, 51.14, 44.23, 42.86, 42.43, 10.18, 3.19. HRMS [C13H17ClN4O3S + H]+: 345.0783 calculated, 345.0782 found.
2-Chloro-N-(cyclopropylmethyl)-6-(4-methylpiperazin-1-yl)pyrimidine-4-carboxamide (119p)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (96 mg, 0.39 mmol, 1 equiv), DiPEA (102 μL, 0.59 mmol, 1.5 equiv), and 1-methylpiperazine (45 μL, 0.41 mmol, 1.05 equiv). Column chromatography (5% → 10% MeOH/DCM) afforded the product (77 mg, 0.25 mmol, 64%). TLC: Rf = 0.4 (5% MeOH/DCM). 1H NMR (500 MHz, CDCl3) δ 7.99–7.84 (m, 1H), 7.28 (s, 1H), 3.96–3.58 (m, 4H), 3.35–3.24 (m, 2H), 2.54–2.44 (m, 4H), 2.34 (s, 3H), 1.12–0.99 (m, 1H), 0.64–0.50 (m, 2H), 0.28 (q, J = 4.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 163.61, 162.39, 159.88, 157.74, 99.52, 54.50, 46.06, 44.49, 43.44, 10.70, 3.71. HRMS [C14H20ClN5O + H]+: 310.1429 calculated, 310.1429 found.
Benzyl-4-(2-chloro-6-((cyclopropylmethyl)carbamoyl)pyrimidin-4-yl) piperazine-1-carboxylate (119q)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (181 mg, 0.74 mmol, 1 equiv), DiPEA (193 μL, 1.11 mmol, 1.5 equiv), and 1-Cbz-piperazine (149 μL, 0.77 mmol, 1.05 equiv). Column chromatography (30% → 60% EtOAc/pentane) afforded the product (290 mg, 0.67 mmol, 91%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.92 (t, J = 5.7 Hz, 1H), 7.40–7.30 (m, 5H), 7.28 (s, 1H), 5.17 (s, 2H), 3.74 (br s, 4H), 3.65–3.55 (m, 4H), 3.34–3.22 (m, 2H), 1.12–0.98 (m, 1H), 0.63–0.46 (m, 2H), 0.36–0.19 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 163.64, 162.10, 159.81, 158.01, 155.01, 136.26, 128.54, 128.20, 128.00, 99.44, 67.50, 44.40, 43.17, 10.63, 3.61.
2-Chloro-N-(cyclopropylmethyl)-6-(dimethylamino)pyrimidine-4-carboxamide (119r)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (246 mg, 1.0 mmol, 1 equiv), DiPEA (261 μL, 1.5 mmol, 1.5 equiv), and dimethylamine (2 M in THF, 0.53 mL, 1.05 mmol, 1.05 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (225 mg, 0.88 mmol, 88%). TLC: Rf = 0.5 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.97 (br s, 1H), 7.19 (s, 1H), 3.33–3.27 (m, 2H), 3.27–3.06 (m, 6H), 1.13–0.99 (m, 1H), 0.62–0.49 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 164.03, 162.29, 159.36, 156.93, 99.19, 44.26, 37.76, 37.29, 10.56, 3.53. HRMS [C11H15ClN4O + H]+: 255.1007 calculated, 255.1005 found.
2-Chloro-N-(cyclopropylmethyl)-6-(methylamino)pyrimidine-4-carboxamide (119s)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (123 mg, 0.50 mmol, 1 equiv), DiPEA (218 μL, 1.25 mmol, 2.5 equiv), and methylamine HCl salt (35 mg, 0.53 mmol, 1.05 equiv). Column chromatography (50% → 70% EtOAc/pentane) afforded the product (77 mg, 0.32 mmol, 64%). TLC: Rf = 0.4 (60% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.93 (br s, 1H), 7.40–7.04 (m, 1H), 6.62–6.20 (m, 1H), 3.29 (br s, 2H), 3.04 (br s, 3H), 1.13–1.00 (m, 1H), 0.65–0.50 (m, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 175.67, 166.23, 165.44, 162.63, 162.22, 160.34, 158.43, 155.80, 103.55, 98.02, 44.55, 29.81, 29.06, 28.22, 3.76. HRMS [C10H13ClN4O + H]+: 241.0851 calculated, 241.0849 found.
2-Chloro-N-(cyclopropylmethyl)-6-(diethylamino)pyrimidine-4-carboxamide (119t)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (123 mg, 0.50 mmol, 1 equiv), DiPEA (131 μL, 0.75 mmol, 1.5 equiv), and diethylamine (55 μL, 0.53 mmol, 1.05 equiv). Column chromatography (20% → 50% EtOAc/pentane) afforded the product (94 mg, 0.33 mmol, 66%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.94 (br s, 1H), 7.16 (s, 1H), 3.77–3.37 (m, 4H), 3.34–3.24 (m, 2H), 1.22 (t, J = 7.0 Hz, 6H), 1.12–1.00 (m, 1H), 0.61–0.51 (m, 2H), 0.29 (q, J = 4.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 162.96, 162.57, 159.79, 157.11, 99.29, 44.41, 42.96, 42.73, 12.64, 10.69, 3.67. HRMS [C13H19ClN4O + H]+: 283.1320 calculated, 283.1319 found.
2-Chloro-N-(cyclopropylmethyl)-6-(azetidin-1-yl)pyrimidine-4-carboxamide (119u)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and azetidine HCl salt (39 mg, 0.42 mmol, 1.05 equiv). Column chromatography (30% → 50% EtOAc/pentane) afforded the product (79 mg, 0.30 mmol, 74%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.90 (br s, 1H), 6.88 (s, 1H), 4.44–4.07 (m, 4H), 3.28 (dd, J = 7.0, 6.0 Hz, 2H), 2.49 (p, J = 7.6 Hz, 2H), 1.14–0.99 (m, 1H), 0.62–0.51 (m, 2H), 0.28 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 163.91, 162.30, 159.95, 156.54, 98.54, 50.20, 44.44, 16.51, 10.69, 3.69. HRMS [C12H15ClN4O + H]+: 267.1007 calculated, 267.1005 found.
2-Chloro-N-(cyclopropylmethyl)-6-(2-oxa-6-azaspiro[3.3]heptan-6-yl)pyrimidine-4-carboxamide (119v)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and 2-oxa-6-azaspiro[3.3]heptane hemioxalate salt (61 mg, 0.42 mmol, 1.05 equiv). Column chromatography (40% → 100% EtOAc/pentane) afforded the product (96 mg, 0.31 mmol, 78%). TLC: Rf = 0.2 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.88 (br s, 1H), 6.92 (s, 1H), 4.86 (br s, 4H), 4.35 (s, 4H), 3.28 (dd, J = 7.0, 6.0 Hz, 2H), 1.11–0.98 (m, 1H), 0.63–0.50 (m, 2H), 0.34–0.23 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.01, 162.11, 160.06, 157.10, 98.96, 80.71, 59.73, 44.56, 39.16, 10.72, 3.76. HRMS [C14H17ClN4O2 + H]+: 309.1113 calculated, 309.1110 found.
2-Chloro-N-(cyclopropylmethyl)-6-(pyrrolidin-1-yl)pyrimidine-4-carboxamide (119w)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (123 mg, 0.50 mmol, 1 equiv), DiPEA (131 μL, 0.75 mmol, 1.5 equiv), and pyrrolidine (43 μL, 0.53 mmol, 1.05 equiv). Column chromatography (30% → 60% EtOAc/pentane) afforded the product (150 mg, 0.40 mmol, 79%). TLC: Rf = 0.5 (40% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.93 (br s, 1H), 7.05 (s, 1H), 3.65 (t, J = 6.6 Hz, 2H), 3.46 (t, J = 6.7 Hz, 2H), 3.29 (t, J = 6.5 Hz, 2H), 2.09 (p, J = 6.3 Hz, 2H), 2.00 (p, J = 6.3 Hz, 2H), 1.13–0.99 (m, 1H), 0.56 (q, J = 5.2 Hz, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 162.52, 161.97, 159.61, 156.52, 100.53, 47.23, 46.98, 44.43, 25.52, 24.82, 10.69, 3.69. HRMS [C13H17ClN4O + H]+: 281.1164 calculated, 281.1160 found.
2-Chloro-N-(cyclopropylmethyl)-6-(3,3-difluoropyrrolidin-1-yl)pyrimidine-4-carboxamide (119x)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and 3,3-difluoropyrrolidine HCl salt (60 mg, 0.42 mmol, 1.05 equiv). Column chromatography (20% → 40% EtOAc/pentane) afforded the product (108 mg, 0.34 mmol, 85%). TLC: Rf = 0.6 (40% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.90 (br s, 1H), 7.22–6.87 (m, 1H), 4.19–3.59 (m, 4H), 3.36–3.22 (m, 2H), 2.55 (br s, 2H), 1.14–0.97 (m, 1H), 0.63–0.50 (m, 2H), 0.37–0.18 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 162.52, 162.11, 159.90, 157.70, 100.24, 99.83, 53.66 (t, J = 32.9 Hz), 44.62, 33.91, 3.78. HRMS [C13H15ClF2N4O + H]+: 317.0975 calculated, 317.0974 found.
(±)-2-Chloro-N-(cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)pyrimidine-4-carboxamide (119y)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and (±)-3-hydroxypyrrolidine HCl salt (52 mg, 0.42 mmol, 1.05 equiv). Column chromatography (70% → 100% EtOAc/pentane) afforded the product (89 mg, 0.30 mmol, 75%). TLC: Rf = 0.2 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (t, J = 5.3 Hz, 1H), 7.08–6.89 (m, 1H), 4.73–4.55 (m, 1H), 3.87–3.45 (m, 4H), 3.33–3.20 (m, 2H), 2.25–2.07 (m, 2H), 1.12–0.98 (m, 1H), 0.63–0.50 (m, 2H), 0.29 (q, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 162.63, 162.32, 162.16, 159.59, 156.38, 100.55, 100.49, 70.46, 69.78, 55.58, 55.35, 45.26, 45.02, 44.58, 33.87, 33.29, 10.62, 3.75. HRMS [C13H17ClN4O2 + H]+: 297.1113 calculated, 297.1110 found.
(R)-2-Chloro-N-(cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)pyrimidine-4-carboxamide (119z)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and (R)-3-hydroxypyrrolidine HCl salt (52 mg, 0.42 mmol, 1.05 equiv). Column chromatography (70% → 100% EtOAc/pentane) afforded the product (105 mg, 0.35 mmol, 88%). TLC: Rf = 0.2 (80% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.98 (t, J = 5.4 Hz, 1H), 7.08–6.91 (m, 1H), 4.74–4.54 (m, 1H), 3.90–3.39 (m, 5H), 3.34–3.20 (m, 2H), 2.24–2.07 (m, 2H), 1.13–0.98 (m, 1H), 0.63–0.48 (m, 2H), 0.36–0.17 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 162.64, 162.34, 162.18, 159.73, 159.60, 156.40, 100.57, 100.52, 70.50, 69.81, 55.59, 55.36, 45.27, 45.03, 44.59, 33.87, 33.32, 10.63, 3.76. HRMS [C13H17ClN4O2 + H]+: 297.1113 calculated, 297.1110 found.
(S)-2-Chloro-N-(cyclopropylmethyl)-6-(3-hydroxypyrrolidin-1-yl)pyrimidine-4-carboxamide (119aa)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (174 μL, 1.0 mmol, 2.5 equiv), and (S)-3-hydroxypyrrolidine HCl salt (52 mg, 0.42 mmol, 1.05 equiv). Column chromatography (70% → 100% EtOAc/pentane) afforded the product (97 mg, 0.33 mmol, 82%). TLC: Rf = 0.2 (80% EtOAc/pentane). 1H NMR (300 MHz, CDCl3) δ 7.98 (t, J = 5.2 Hz, 1H), 7.11–6.86 (m, 1H), 4.81–4.47 (m, 1H), 3.93–3.35 (m, 5H), 3.33–3.21 (m, 2H), 2.25–2.06 (m, 2H), 1.16–0.96 (m, 1H), 0.57 (q, J = 5.5 Hz, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 162.65, 162.34, 162.19, 159.73, 159.61, 156.41, 100.55, 100.51, 70.48, 69.79, 55.58, 55.35, 45.27, 45.03, 44.58, 33.87, 33.31, 10.62, 3.74. HRMS [C13H17ClN4O2 + H]+: 297.1113 calculated, 297.1110 found.
(±)-2-Chloro-N-(cyclopropylmethyl)-6-(3-(dimethylamino)pyrrolidin-1-yl)pyrimidine-4-carboxamide (119ab)
The title compound was prepared according to General Procedure E using dichloropyrimidine 118a (98 mg, 0.40 mmol, 1 equiv), DiPEA (244 μL, 1.4 mmol, 3.5 equiv), and (±)-3-(dimethylamino)pyrrolidine double HCl salt (79 mg, 0.42 mmol, 1.05 equiv). Column chromatography (2.5% → 10% MeOH/DCM) afforded the product (50 mg, 0.15 mmol, 39%). TLC: Rf = 0.4 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.10–7.77 (m, 1H), 7.06 (s, 1H), 4.06–3.91 (m, 2H), 3.90–3.60 (m, 1H), 3.59–3.32 (m, 2H), 3.32–3.25 (m, 2H), 2.95–2.70 (m, 1H), 2.40–2.30 (m, 6H), 2.30–2.17 (m, 1H), 2.08–1.78 (m, 1H), 1.13–0.99 (m, 1H), 0.66–0.45 (m, 2H), 0.35–0.21 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 171.69, 163.73, 162.56, 162.47, 162.15, 161.99, 159.76, 159.69, 159.50, 158.70, 158.27, 156.79, 106.57, 100.46, 100.22, 93.93, 65.41, 64.76, 53.71, 51.47, 51.33, 50.76, 46.42, 46.22, 45.79, 44.54, 44.51, 44.42, 44.28, 44.22, 44.02, 30.41, 29.81, 10.90, 10.85, 10.74, 3.76, 3.51, 3.47. HRMS [C15H22ClN5O + H]+: 324.1586 calculated, 324.1583 found.
2-Chloro-N-(cyclopropylmethyl)-6-(1H-pyrazol-1-yl)pyrimidine-4-carboxamide (119ac)
The title compound was prepared according to General Procedure F using dichloropyrimidine 118a (123 mg, 0.50 mmol, 1 equiv), K2CO3 (104 mg, 0.75 mmol, 1.5 equiv), and pyrazole (36 mg, 0.53 mmol, 1.05 equiv). Column chromatography (10% → 30% EtOAc/pentane) afforded the product (76 mg, 0.27 mmol, 55%). TLC: Rf = 0.8 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 8.61 (s, 1H), 8.56 (d, J = 2.7 Hz, 1H), 7.92 (br s, 1H), 7.85 (d, J = 1.3 Hz, 1H), 6.55 (dd, J = 2.8, 1.6 Hz, 1H), 3.36 (dd, J = 7.1, 5.9 Hz, 2H), 1.19–1.00 (m, 1H), 0.71–0.49 (m, 2H), 0.32 (q, J = 4.7 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 161.28, 160.88, 160.48, 159.64, 145.18, 128.34, 110.22, 105.51, 44.73, 3.76. HRMS [C12H12ClN5O + H]+: 278.0803 calculated, 278.0802 found.
2-Chloro-N-(cyclopropylmethyl)-6-(1H-imidazol-1-yl)pyrimidine-4-carboxamide (119ad)
The title compound was prepared according to General Procedure F using dichloropyrimidine 118a (123 mg, 0.50 mmol, 1 equiv), K2CO3 (104 mg, 0.75 mmol, 1.5 equiv), and imidazole (36 mg, 0.53 mmol, 1.05 equiv). Column chromatography (60% → 80% EtOAc/pentane) afforded the product as an inseparable mixture of regioisomers (2.5:1, 6-imidazolyl: 2-imidazolyl), which was used for the following step without further purification (76 mg, 0.27 mmol, 55%). TLC: Rf = 0.4 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 8.10 (s, 1H), 7.98 (br s, 1H), 7.77 (t, J = 1.4 Hz, 1H), 7.26 (s, 1H), 3.47–3.26 (m, 2H), 1.18–1.03 (m, 1H), 0.68–0.53 (m, 2H), 0.41–0.27 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 161.93, 160.55, 159.94, 158.14, 135.73, 132.38, 116.02, 104.68, 44.84, 10.66, 3.76. HRMS [C12H12ClN5O + H]+: 278.0803 calculated, 278.0800 found.
2-Chloro-N-(cyclopropylmethyl)-6-phenoxypyrimidine-4-carboxamide (119ae)
The title compound was prepared according to General Procedure F using dichloropyrimidine 118a (100 mg, 0.41 mmol, 1 equiv), K2CO3 (86 mg, 0.62 mmol, 1.5 equiv), and phenol (44 mg, 0.43 mmol, 1.05 equiv). Column chromatography (5% → 30% EtOAc/pentane) afforded the product (100 mg, 0.33 mmol, 80%). TLC: Rf = 0.4 (20% EtOAc/pentane). 1H NMR (300 MHz, CDCl3) δ 7.90 (br s, 1H), 7.54 (s, 1H), 7.50–7.39 (m, 2H), 7.36–7.28 (m, 1H), 7.20–7.08 (m, 2H), 3.41–3.17 (m, 2H), 1.15–0.96 (m, 1H), 0.66–0.47 (m, 2H), 0.29 (q, J = 4.7 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 172.25, 161.17, 161.02, 159.73, 151.84, 130.19, 126.62, 121.27, 104.58, 44.68, 10.70, 3.75. HRMS [C15H14ClN3O2 + H]+: 304.0847 calculated, 304.0845 found.
2-Chloro-N-(cyclopropylmethyl)-6-(pyridin-3-yloxy)pyrimidine-4-carboxamide (119af)
The title compound was prepared according to General Procedure F using dichloropyrimidine 118a (0.11 g, 0.45 mmol, 1 equiv), K2CO3 (93 mg, 0.68 mmol, 1.5 equiv), and pyridine-3-ol (45 mg, 0.47 mmol, 1.05 equiv). Column chromatography (60% → 100% EtOAc/pentane) afforded the product (70 mg, 0.23 mmol, 51%). TLC: Rf = 0.4 (80% EtOAc/pentane). 1H NMR (300 MHz, CDCl3) δ 8.56 (dd, J = 9.3, 3.2 Hz, 2H), 7.94 (br s, 1H), 7.69 (s, 1H), 7.63–7.50 (m, 1H), 7.43 (dd, J = 8.3, 4.7 Hz, 1H), 3.46–3.19 (m, 2H), 1.17–0.99 (m, 1H), 0.66–0.51 (m, 2H), 0.31 (q, J = 4.8 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 171.32, 161.44, 160.74, 159.43, 148.48, 147.52, 143.41, 129.09, 124.31, 105.26, 44.66, 10.66, 3.71. HRMS [C14H13ClN4O2 + H]+: 305.0800 calculated, 305.0796 found.
2-(3-Phenoxyphenyl)acetonitrile (121a)
A round-bottom flask was charged with 1-(chloromethyl)-3-phenoxybenzene (120a) (0.37 mL, 2 mmol, 1 equiv) in dioxane/EtOH/H2O (2:2:1, 6 mL) and KCN (260 mg, 4 mmol, 2 equiv). The reaction mixture was stirred overnight at reflux. The mixture was diluted with water and extracted with EtOAc (3×). The organic layer was washed with brine, dried (Na2SO4), filtered, and concentrated under reduced pressure. The crude material was purified by silica gel column chromatography (5% → 7% EtOAc/pentane) to provide the product (352 mg, 1.68 mmol, 84%). TLC: Rf = 0.6 (10% EtOAc/pentane). 1H NMR (500 MHz, CDCl3) δ 7.37–7.19 (m, 3H), 7.15–7.03 (m, 1H), 7.04–6.94 (m, 3H), 6.95–6.84 (m, 2H), 3.56 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 157.73, 156.32, 131.81, 130.23, 129.71, 123.58, 122.34, 118.97, 117.96, 117.78, 117.51, 23.00.
2-([1,1′-Biphenyl]-2-yl)acetonitrile (121b)
A round-bottom flask was charged with 2-(bromomethyl)-1,1′-biphenyl (120b) (0.37 mL, 2 mmol, 1 equiv), solvent mixture dioxane/EtOH/H2O (2:2:1, 6 mL), and KCN (260 mg, 4 mmol, 2 equiv). The reaction mixture was stirred overnight at reflux. The mixture was diluted with water and extracted with EtOAc (3×). The organic layer was washed with brine, dried (Na2SO4), filtered, and concentrated under reduced pressure. The crude material was purified by silica gel column chromatography (0.5% → 5% EtOAc/pentane) to provide the product (400 mg, 2.0 mmol, 99%). TLC: Rf = 0.6 (3% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.59–7.12 (m, 9H), 3.57 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 141.72, 139.78, 130.35, 128.86, 128.84, 128.59, 128.12, 127.68, 118.23, 21.92.
N-Methylphenethylamine HBr Salt (123a)
A round-bottom flask was charged with methylamine (33 wt % in ethanol, 8.83 mL, 73.0 mmol, 10 equiv) and cooled to 0 οC. (2-Bromoethyl)benzene (1.00 mL, 7.3 mmol, 1 equiv) was added, and the reaction was stirred and allowed to warm up to room temperature. After 40 h, the reaction showed complete conversion on TLC and the solvents were concentrated under reduced pressure. The product was obtained as a mixture with the di-substituted by product N-methyl-N-phenethyl-2-phenylethan-1-amine (9:1) and used without further purification (1.4 g, 6.6 mmol, 90%). TLC: Rf = 0.35 (6% MeOH/DCM). 1H NMR (400 MHz, MeOD) δ 7.52–6.99 (m, 5H), 3.42–3.10 (m, 2H), 3.10–2.85 (m, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, MeOD) δ 137.75, 129.86, 129.78, 128.10, 51.50, 33.92, 33.31.
N-Methyl-3-phenylpropan-1-amine (123b)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 3-phenylpropan-1-amine (71 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (20% - > 50% EtOAc/pentane) afforded the product (91 mg, 0.47 mmol, 94%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.33–7.22 (m, 2H), 7.22–7.13 (m, 3H), 4.82 (br s, 1H), 3.65 (s, 3H), 3.26–3.07 (m, 2H), 2.69–2.57 (m, 2H), 1.82 (p, J = 7.3 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.18, 141.47, 128.49, 128.40, 126.02, 52.08, 40.68, 33.06, 31.68. HRMS [C11H15NO2 + H]+: 194.1176 calculated, 194.1175 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (91 mg, 0.47 mmol, 1 equiv), LiAlH4 (2 M THF solution, 0.75 mL, 1.54 mmol, 3.3 equiv), and was used without further purification (39 mg, 0.13 mmol, 27%). TLC: Rf = 0.1 (5% MeOH/DCM). 1H NMR (500 MHz, CDCl3) δ 7.31–7.13 (m, 5H), 2.68–2.63 (m, 2H), 2.63–2.58 (m, 2H), 2.42 (s, 3H), 1.90–1.76 (m, 2H), 1.71–1.58 (m, 1H). HRMS [C10H15N + H]+: 150.1277 calculated, 150.1278 found.
N-Methyl-4-phenylbutan-1-amine (123c)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 4-phenylbutan-1-amine (79 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (97 mg, 0.47 mmol, 94%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.26 (t, J = 7.5 Hz, 2H), 7.17 (t, J = 7.9 Hz, 3H), 4.80 (br s, 1H), 3.64 (s, 3H), 3.29–3.00 (m, 2H), 2.61 (t, J = 7.6 Hz, 2H), 1.69–1.57 (m, 2H), 1.51 (p, J = 6.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.15, 142.15, 128.42, 128.37, 125.84, 52.01, 40.93, 35.54, 29.65, 28.55. HRMS [C12H17NO2 + H]+: 208.1332 calculated, 208.1333 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (97 mg, 0.47 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.38 mL, 0.75 mmol, 1.6 equiv) and was used without further purification (64 mg, 0.39 mmol, 83%). TLC: Rf = 0.1 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.31–7.21 (m, 2H), 7.20–7.11 (m, 3H), 2.62 (t, J = 7.6 Hz, 2H), 2.59–2.54 (m, 2H), 2.40 (s, 3H), 1.77–1.57 (m, 3H), 1.57–1.46 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 142.51, 128.45, 128.31, 125.73, 52.05, 36.57, 35.90, 29.61, 29.24. HRMS [C11H17N + H]+: 164.1434 calculated, 164.1433 found.
2-(4-Chlorophenyl)-N-methylethan-1-amine (123d)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(4-chlorophenyl)ethan-1-amine (70 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (10% → 50% EtOAc/pentane) afforded the product (104 mg, 0.50 mmol, 99%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.35–7.21 (m, 2H), 7.16–7.05 (m, 2H), 5.01–4.39 (m, 1H), 3.65 (s, 3H), 3.41 (q, J = 6.7 Hz, 2H), 2.78 (t, J = 7.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.04, 137.32, 132.39, 130.22, 128.80, 52.20, 42.17, 35.62. HRMS [C10H12ClNO2 + H]+: 214.0629 calculated, 214.0631 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (104 mg, 0.48 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.39 mL, 0.77 mmol, 1.6 equiv). Column chromatography (isocratic, 5% MeOH/DCM + 0.5% Et3N) afforded the product (36 mg, 0.21 mmol, 44%). TLC: Rf = 0.1 (2% MeOH/DCM with 3 drops of Et3N). 1H NMR (500 MHz, CDCl3) δ 7.28–7.20 (m, 2H), 7.19–7.10 (m, 2H), 2.92–2.74 (m, 4H), 2.66–2.52 (m, 1H), 2.45 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 138.30, 132.03, 130.14, 128.66, 52.87, 36.12, 35.26. HRMS [C9H12ClN + H]+: 170.0731 calculated, 170.0732 found.
2-(3-Chlorophenyl)-N-methylethan-1-amine (123e)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(3-chlorophenyl)ethan-1-amine (70 μL, 0.40 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 2.0 mmol, 2 equiv). Column chromatography (20% → 40% EtOAc/pentane) afforded the product (67 mg, 0.31 mmol, 77%). TLC: Rf = 0.7 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.25–7.15 (m, 3H), 7.11–7.03 (m, 1H), 4.89 (br s, 1H), 3.65 (s, 3H), 3.41 (q, J = 6.8 Hz, 2H), 2.78 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.05, 140.94, 134.37, 129.90, 128.93, 127.04, 126.75, 52.15, 42.04, 35.90. HRMS [C10H12ClNO2 + H]+: 214.0629 calculated, 214.0630 found.
Carbamate Reduction:
The title compound was prepared according to General Procedure G using the methyl carbamate (67 mg, 0.31 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.250 mL, 0.50 mmol, 1.6 equiv) and was used without further purification (33 mg, 0.14 mmol, 63%). Rf = 0.15 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.26–7.16 (m, 3H), 7.13–7.04 (m, 1H), 2.92–2.76 (m, 4H), 2.47 (s, 3H), 2.45 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 141.75, 134.40, 129.90, 128.92, 127.06, 126.63, 52.69, 36.06, 35.51. HRMS [C9H12ClN + H]+: 170.0731 calculated, 170.0730 found.
2-(2-Chlorophenyl)-N-methylethan-1-amine (123f)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(2-chlorophenyl)ethan-1-amine (141 μL, 1.0 mmol, 1 equiv), methylchloroformate (116 μL, 1.5 mmol, 1.5 equiv), and DiPEA (348 μL, 2.0 mmol, 2 equiv). Column chromatography (30% → 50% EtOAc/pentane) afforded the product (183 mg, 0.85 mmol, 86%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.39–7.30 (m, 1H), 7.25–7.12 (m, 3H), 4.94 (br s, 1H), 3.65 (s, 3H), 3.44 (q, J = 6.7 Hz, 2H), 2.95 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.10, 136.50, 134.17, 131.06, 129.65, 128.06, 126.97, 52.10, 40.67, 33.93. HRMS [C10H12ClNO2 + H]+: 214.0629 calculated, 214.0630 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (183 mg, 0.85 mmol, 1 equiv), LiAlH4 (2 M THF solution, 0.68 mL, 1.36 mmol, 1.6 equiv) and was used without further purification (130 mg, 0.77 mmol, 90%). TLC: Rf = 0.1 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.38–7.26 (m, 1H), 7.26–7.08 (m, 3H), 3.02–2.73 (m, 4H), 2.50–2.26 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 137.55, 134.08, 130.82, 129.58, 127.67, 126.81, 51.32, 36.18, 33.82. HRMS [C9H12ClN + H]+: 170.0731 calculated, 170.0732 found.
N-Methyl-2-(p-tolyl)ethan-1-amine (123g)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(p-tolyl)ethan-1-amine (145 μL, 1.0 mmol, 1 equiv), methylchloroformate (116 μL, 1.5 mmol, 1.5 equiv), and DiPEA (348 μL, 2.0 mmol, 2 equiv). Column chromatography (30% → 50% EtOAc/pentane) afforded the product (198 mg, 1.0 mmol, 99%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.18–6.91 (m, 4H), 5.02 (br s, 1H), 3.61 (s, 3H), 3.38 (q, J = 6.8 Hz, 2H), 2.74 (t, J = 7.2 Hz, 2H), 2.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.00, 135.82, 135.66, 129.18, 128.55, 51.85, 42.26, 35.60, 20.91. HRMS [C11H15NO2 + H]+: 194.1176 calculated, 194.1176 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (198 mg, 1.0 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.80 mL, 1.0 mmol, 1.6 equiv) and was used without further purification (130 mg, 0.87 mmol, 87%). TLC: Rf = 0.2 (5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.11–7.07 (m, 4H), 2.83–2.72 (m, 4H), 2.41 (s, 3H), 2.33–2.28 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 136.87, 135.59, 129.17, 128.60, 80.66, 53.26, 36.28, 35.65, 21.02. HRMS [C10H15N + H]+: 150.1277 calculated, 150.1277 found.
N-Methyl-2-(o-tolyl)ethan-1-amine (123h)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(o-tolyl)ethan-1-amine (70 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (30% → 50% EtOAc/pentane) afforded the product (102 mg, 0.50 mmol, 99%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.19–7.07 (m, 4H), 5.00–4.79 (m, 1H), 3.65 (s, 3H), 3.37 (q, J = 6.9 Hz, 2H), 2.81 (t, J = 7.4 Hz, 2H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.12, 136.93, 136.37, 130.44, 129.34, 126.62, 126.11, 52.03, 41.16, 33.59, 19.29. HRMS [C11H15NO2 + H]+: 194.1176 calculated, 194.1176 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (102 mg, 0.5 mmol, 1 equiv) and LiAlH4 (1 M THF solution, 0.85 mL, 0.85 mmol, 1.6 equiv) and was used without further purification (74 mg, 0.50 mmol, 94%). TLC: Rf = 0.1 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.21–7.02 (m, 4H), 2.80 (s, 4H), 2.45 (s, 3H), 2.32 (s, 3H), 1.71 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 138.15, 136.21, 130.34, 129.25, 126.29, 126.01, 52.11, 36.44, 33.56, 19.42. HRMS [C10H15N + H]+: 150.1277 calculated, 150.1277 found.
2-(4-Methoxyphenyl)-N-methylethan-1-amine (123i)
Carbamoylation
the methyl carbamate was prepared according to General Procedure G using 2-(4-methoxyphenyl)ethan-1-amine (73 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (108 mg, 0.50 mmol, 99%). TLC: Rf = 0.6 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.09 (d, J = 8.5 Hz, 2H), 6.83 (d, 2H), 4.93 (br s, 1H), 3.77 (s, 3H), 3.63 (s, 3H), 3.38 (q, J = 6.8 Hz, 2H), 2.73 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 158.21, 157.04, 130.80, 129.69, 113.99, 55.21, 51.96, 42.42, 35.21. HRMS [C11H15NO3 + H]+: 210.1125 calculated, 210.1125 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (105 mg, 0.50 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.40 mL, 0.80 mmol, 1.6 equiv) and was used without further purification (78 mg, 0.47 mmol, 93%). TLC: Rf = 0.1 (5% MeOH/DCM with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.17–7.05 (m, 2H), 6.87–6.77 (m, 2H), 3.77 (s, 3H), 2.87–2.65 (m, 4H), 2.49–2.19 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 158.08, 132.00, 129.68, 113.96, 55.30, 53.37, 36.29, 35.18. HRMS [C10H15NO + H]+: 166.1226 calculated, 166.1226 found.
2-(2-Methoxyphenyl)-N-methylethan-1-amine (123j)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(2-methoxyphenyl)ethan-1-amine (145 μL, 1.0 mmol, 1 equiv), methylchloroformate (116 μL, 1.5 mmol, 1.5 equiv), and DiPEA (348 μL, 2.0 mmol, 2 equiv). Column chromatography (20% → 40% EtOAc/pentane) afforded the product (230 mg, 1.0 mmol, 99%). TLC: Rf = 0.5 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.19 (td, J = 8.0, 1.7 Hz, 1H), 7.11 (d, J = 6.9 Hz, 1H), 6.94–6.78 (m, 2H), 5.19–4.71 (m, 1H), 3.79 (s, 3H), 3.61 (s, 3H), 3.39 (q, J = 6.6 Hz, 2H), 2.81 (t, J = 6.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.48, 157.04, 130.51, 127.75, 127.20, 120.51, 110.27, 55.14, 51.81, 41.08, 30.65. HRMS [C11H15NO3 + H]+: 210.1125 calculated, 210.1125 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (230 mg, 1.0 mmol, 1 equiv) and LiAlH4 (1 M THF solution, 1.6 mL, 1.6 mmol, 1.6 equiv) and was used without further purification (160 mg, 0.97 mmol, 88%). TLC: Rf = 0.3 (2% MeOH/DCM with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.24–7.07 (m, 2H), 6.95–6.74 (m, 2H), 3.80 (s, 3H), 2.90–2.70 (m, 4H), 2.43 (s, 3H), 1.26 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 157.59, 130.33, 128.44, 127.36, 120.39, 110.30, 55.20, 51.85, 36.37, 30.70. HRMS [C10H15NO + H]+: 166.1226 calculated, 166.1225 found.
N-Methyl-2-(4-(trifluoromethyl)phenyl)ethan-1-amine (123k)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(4-(trifluoromethyl)phenyl)ethan-1-amine (80 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (5% → 40% EtOAc/pentane) afforded the product (113 mg, 0.45 mmol, 91%). TLC: Rf = 0.7 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 8.0 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 4.89–4.64 (m, 1H), 3.66 (s, 3H), 3.45 (q, J = 6.7 Hz, 2H), 2.88 (t, J = 7.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.06, 143.06, 129.25, 128.82, 128.37, 125.62 (q, J = 3.8 Hz), 122.97, 52.25, 42.03, 36.14. HRMS [C11H12F3NO2 + H]+: 248.0893 calculated, 248.0896 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (113 mg, 0.45 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.36 mL, 0.72 mmol, 1.6 equiv). Column chromatography (isocratic, 5% MeOH/DCM + 0.5% Et3N) afforded the product (40 mg, 0.20 mmol, 44%). TLC: Rf = 0.3 (2% MeOH/DCM with 3 drops of Et3N). 1H NMR (500 MHz, CDCl3) δ 7.55 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 7.9 Hz, 2H), 3.80 (br s, 1H), 2.99–2.91 (m, 4H), 2.51 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 143.51, 129.13, 125.57 (q, J = 3.7 Hz), 125.41, 123.25, 52.36, 35.73, 35.29. HRMS [C10H12F3N + H]+: 204.0995 calculated, 204.0996 found.
N-Methyl-2-(4-phenoxyphenyl)ethan-1-amine (123l)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(4-phenoxyphenyl)ethan-1-amine TFA salt (211 mg, 0.64 mmol, 1 equiv), methylchloroformate (74 μL, 0.96 mmol, 1.5 equiv), and DiPEA (245 μL, 1.41 mmol, 2.2 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (145 mg, 0.53 mmol, 83%). TLC: Rf = 0.8 (100% EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.40–7.27 (m, 2H), 7.22–7.04 (m, 3H), 7.03–6.89 (m, 4H), 4.96–4.65 (m, 1H), 3.65 (s, 3H), 3.51–3.29 (m, 2H), 2.77 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.36, 157.05, 155.85, 133.69, 130.06, 129.77, 123.20, 119.12, 118.76, 52.08, 42.36, 35.48. HRMS [C16H17NO3 + H]+: 272.1281 calculated, 272.1281 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (144 mg, 0.53 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.42 mL, 0.85 mmol, 1.6 equiv). Column chromatography (isocratic, 5% MeOH/DCM + 0.5% Et3N) afforded the product (65 mg, 0.29 mmol, 55%). TLC: Rf = 0.1 (5% MeOH/DCM with 3 drops of Et3N). 1H NMR (500 MHz, CDCl3) δ 7.35–7.27 (m, 2H), 7.20–7.14 (m, 2H), 7.12–7.03 (m, 1H), 7.02–6.96 (m, 2H), 6.96–6.91 (m, 2H), 3.08 (br s, 1H), 2.92–2.80 (m, 4H), 2.48 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 157.46, 155.65, 134.48, 130.01, 129.77, 123.15, 119.13, 118.73, 53.02, 36.00, 35.00. HRMS [C15H17NO + H]+: 228.1383 calculated, 228.1385 found.
N-Methyl-2-(3-phenoxyphenyl)ethan-1-amine (123m)
Nitrile Reduction
A round-bottom flask was charged with nitrile 121a (211 mg, 1 mmol, 1 equiv), EtOH (5 mL), and 37% w/w aqueous HCl (0.16 mL, 2 mmol, 2 equiv). N2 was bubbled through the solution for 5 min, and Pd/C (10% w/w, 52 mg, 50 μmol, 5 mol %) was added. The mixture was purged with N2 and then with H2 and was kept under a H2 atmosphere (balloon). After 50 h, the reaction was complete as judged by TLC. The mixture was filtered over a Whatman filter, which was washed with EtOH, and the filtrate was concentrated under reduced pressure. This afforded the primary amine (122a) as the HCl salt, which was used without further purification (246 mg, 0.98 mmol, 98%). TLC: Rf = 0.05 (6% MeOH/DCM).
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using the primary amine 122a (246 mg, 0.98 mmol, 1 equiv), methylchloroformate (115 μL, 1.48 mmol, 1.5 equiv), and DiPEA (517 μL, 3.0 mmol, 3 equiv). Column chromatography (5% → 40% EtOAc/pentane) afforded the product (176 mg, 0.65 mmol, 66%). TLC: Rf = 0.3 (10% EtOAc/pentane).
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (170 mg, 0.62 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.52 mL, 1.04 mmol, 1.6 equiv) and was used without further purification (125 mg, 0.55 mmol, 88%). TLC: Rf = 0.1 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.35–7.28 (m, 2H), 7.25–7.20 (m, 1H), 7.12–7.05 (m, 1H), 7.03–6.97 (m, 2H), 6.95–6.91 (m, 1H), 6.89–6.81 (m, 2H), 2.91–2.67 (m, 5H), 2.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.45, 157.19, 142.10, 129.77, 129.74, 123.65, 123.26, 119.12, 118.94, 116.55, 52.94, 36.27, 35.99. HRMS [C15H17NO + H]+: 228.1383 calculated, 228.1384 found.
N-Methyl-2-(2-phenoxyphenyl)ethan-1-amine (123n)
Carbamoylation
The methyl carbamate was prepared according to General Procedure G using 2-(2-phenoxyphenyl)ethan-1-amine (99 μL, 0.50 mmol, 1 equiv), methylchloroformate (58 μL, 0.75 mmol, 1.5 equiv), and DiPEA (174 μL, 1.0 mmol, 2 equiv). Column chromatography (20% → 40% EtOAc/pentane) afforded the product (115 mg, 0.42 mmol, 84%). TLC: Rf = 0.7 (30% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.36–7.22 (m, 3H), 7.21–7.15 (m, 1H), 7.10–7.04 (m, 2H), 6.96–6.91 (m, 2H), 6.89–6.83 (m, 1H), 4.89 (br s, 1H), 3.62 (s, 3H), 3.44 (q, J = 6.6 Hz, 2H), 2.85 (t, J = 6.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 157.49, 157.08, 154.97, 131.27, 130.31, 129.85, 128.11, 123.97, 123.02, 119.26, 118.07, 52.05, 41.35, 30.73. HRMS [C16H17NO3 + H]+: 272.1281 calculated, 272.1281 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (115 mg, 0.42 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.34 mL, 0.67 mmol, 1.6 equiv). Column chromatography (4% → 7% MeOH/DCM + 0.5% Et3N) afforded the product (72 mg, 0.32 mmol, 76%). TLC: Rf = 0.3 (4% MeOH/DCM with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.34–7.23 (m, 3H), 7.18 (td, J = 7.8, 1.7 Hz, 1H), 7.11–7.01 (m, 2H), 6.97–6.83 (m, 3H), 3.04 (br s, 1H), 2.87 (s, 4H), 2.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.79, 154.81, 131.17, 131.12, 129.80, 127.88, 123.99, 122.82, 119.58, 117.88, 51.55, 35.80, 30.23. HRMS [C15H17NO + H]+: 228.1383 calculated, 228.1383 found.
2-([1,1′-Biphenyl]-2-yl)-N-methylethan-1-amine (123o)
Nitrile Reduction
A round-bottom flask was charged with nitrile 121b (195 mg, 1 mmol, 1 equiv), EtOH (5 mL), and 37% w/w aqueous HCl (0.16 mL, 2 mmol, 2 equiv). N2 was bubbled through the solution for 5 min and Pd/C (10% w/w, 52 mg, 50 μmol, 5 mol %) was added. The mixture was purged with N2 and then with H2 and was kept under a H2 atmosphere (balloon). After 3.5 days, the reaction was complete as judged by TLC. The mixture was filtered over a Whatman filter, and the filtrate was concentrated under reduced pressure to afford the primary amine (122b) as the HCl salt, which was used without further purification (235 mg, 1.0 mmol, 99%). TLC: Rf = 0.15 (6% MeOH/DCM).
Carbamoylation
the methyl carbamate was prepared according to General Procedure G using the amine HCl salt 122b (235 mg, 1.0 mmol, 1 equiv), methylchloroformate (116 μL, 1.5 mmol, 1.5 equiv), and DiPEA (522 μL, 3.0 mmol, 3 equiv). Column chromatography (10% → 40% EtOAc/pentane) afforded the product (210 mg, 0.82 mmol, 82%). TLC: Rf = 0.5 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.48–7.16 (m, 9H), 4.86–4.47 (m, 1H), 3.56 (s, 3H), 3.28–3.08 (m, 2H), 2.79 (t, J = 7.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 156.89, 142.40, 141.50, 136.16, 130.32, 129.68, 129.19, 128.26, 127.64, 127.05, 126.46, 51.98, 41.83, 33.26. HRMS [C16H17NO2 + H]+: 256.1332 calculated, 256.1333 found.
Carbamate Reduction
The title compound was prepared according to General Procedure G using the methyl carbamate (210 mg, 0.82 mmol, 1 equiv) and LiAlH4 (2 M THF solution, 0.66 mL, 1.32 mmol, 1.6 equiv) and was used without further purification (157 mg, 0.74 mmol, 90%). TLC: Rf = 0.3 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.42–7.36 (m, 2H), 7.35–7.19 (m, 7H), 2.83–2.73 (m, 2H), 2.70–2.58 (m, 2H), 2.26 (s, 3H), 2.11 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 142.24, 141.70, 137.29, 130.25, 129.56, 129.22, 128.16, 127.50, 126.93, 126.13, 52.77, 36.06, 33.16. HRMS [C15H17N + H]+: 212.1434 calculated, 212.1434 found.
N-Phenethylaniline (124)
A round-bottom flask under a N2 atmosphere was charged with phenylboronic acid (488 mg, 4 mmol, 2 equiv), Cu(OAc)2·H2O (40 mg, 0.2 mmol, 0.1 equiv), powdered 4 Å molecular sieves (1.5 g), and dry DCE (16 mL). The suspension was stirred for 5 min at room temperature followed by addition of 2-phenethylamine (252 μL, 2 mmol, 1 equiv). The blue mixture was purged using a balloon of O2 causing a color shift to purple and stirred under an Ο2 atmosphere for 26 h. The mixture was then filtered through a plug of Celite and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (2% → 8% EtOAc/pentane) affording the product (127 mg, 0.64 mmol, 32%). TLC: Rf = 0.6 (5% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.34–7.25 (m, 2H), 7.24–7.12 (m, 5H), 6.74–6.66 (m, 1H), 6.61–6.54 (m, 2H), 3.63 (br s, 1H), 3.36 (t, J = 7.1 Hz, 2H), 2.87 (t, J = 7.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 148.06, 139.38, 129.35, 128.86, 128.67, 126.48, 117.50, 113.04, 45.08, 35.55. HRMS [C14H15N + H]+: 198.1277 calculated, 198.1275 found.
N-Ethyl-2-phenylethan-1-amine (125a)
A round-bottom flask was charged with acetaldehyde (56 μL, 1 mmol, 1 equiv), 2-phenethylamine (189 μL, 1.5 mmol, 1.5 equiv), and dry DCM (10 mL). The solution was stirred at room temperature for 10 min, and then NaHB(OAc)3 (424 mg, 2 mmol, 2 equiv) was added. After 16 h, the reaction was complete as judged by TLC. The mixture was diluted with DCM (20 mL), washed with sat. aq. NaHCO3 (1× 30 mL), brine (1× 30 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (5% → 7% MeOH/DCM with 0.5% Et3N) affording the product (27 mg, 0.18 mmol, 18%). TLC: Rf = 0.3 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.34–7.24 (m, 2H), 7.24–7.16 (m, 3H), 2.96–2.77 (m, 4H), 2.68 (q, J = 7.1 Hz, 2H), 1.10 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 140.14, 128.83, 128.59, 126.27, 51.11, 46.98, 44.09, 15.24. HRMS [C10H15N + H]+: 150.1277 calculated, 150.1277 found.
N-Phenethylpropan-2-amine (125b)
A round-bottom flask was charged with NaBH4 (218 mg, 5.76 mmol, 1.9 equiv) and dry DCM (5 mL). The solution was stirred in an ice bath for 3 min, and glacial acetic acid (1 mL, 14.4 mmol, 5.8 equiv) was added. The mixture was stirred for 1 h at 0 °C and 30 min at room temperature. Separately, a solution of 2-phenethylamine (377 μL, 3 mmol, 1 equiv) was prepared in dry DCM (2.5 mL) and acetone (198 μL, 2.7 mmol, 0.9 equiv). This solution was added dropwise to the NaBH(OAc)3 mixture and was stirred at rt overnight. After 20 h, the reaction mixture was acidified to pH 2 with 0.1 M HCl (aq.) and washed with DCM (2× 30 mL) to remove by-products. Then, the solution was basified with 1 M NaOH (aq.) until pH 10 and extracted with DCM (3× 50 mL). The combined organic layers were dried (Na2SO4), filtered, and concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (isocratic, 0.5% Et3N/EtOAc) to provide the product (277 mg, 1.7 mmol, 63%). TLC: Rf = 0.3 (100% EtOAc with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.30–7.23 (m, 2H), 7.22–7.14 (m, 3H), 2.88–2.82 (m, 2H), 2.81–2.74 (m, 3H), 1.08 (br s, 1H), 1.03 (d, J = 6.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 139.98, 128.52, 128.28, 125.95, 48.70, 48.38, 36.48, 22.80. HRMS [C11H17N + H]+: 164.1434 calculated, 164.1434 found.
N-Phenethylcyclopropanamine (125c)
A round-bottom flask was charged with phenylacetaldehyde (125 μL, 1 mmol, 1 equiv), cyclopropanamine (139 μL, 2 mmol, 2 equiv), and dry DCM (5 mL). The solution was stirred at room temperature for 10 min, and then NaHB(OAc)3 (425 mg, 2 mmol, 2 equiv) and glacial AcOH (114 μL, 2 mmol, 2 equiv) were added. After 21 h, the reaction mixture was diluted with DCM (10 mL), washed with sat. aq. NaHCO3 (1× 15 mL), brine (1× 15 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (30% → 50% EtOAc/pentane with 0.5% Et3N) affording the product (85 mg, 0.53 mmol, 53%), Rf = 0.1 (6% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.42–7.07 (m, 5H), 3.01–2.93 (m, 2H), 2.80 (t, J = 7.2 Hz, 2H), 2.26–2.02 (m, 2H), 0.47–0.40 (m, 2H), 0.37–0.31 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 140.23, 128.78, 128.54, 126.19, 50.84, 36.37, 30.24, 6.33. HRMS [C11H15N + H]+: 162.1277 calculated, 162.1277 found.
N-Benzyl-2-phenylethan-1-amine (125d)
A round-bottom flask was charged with phenylacetaldehyde (125 μL, 1 mmol, 1 equiv), benzylamine (218 μL, 2 mmol, 2 equiv), and DCM (10 mL). The solution was stirred at room temperature for 3 min, and then NaHB(OAc)3 (424 mg, 2 mmol, 2 equiv) and glacial AcOH (114 μL, 2 mmol, 2 equiv) were added. After 18 h, the reaction mixture was diluted with DCM (20 mL), washed with sat. aq. NaHCO3 (1× 30 mL) and brine (1× 30 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with (4% → 5% MeOH/DCM with 0.5% Et3N) affording the product (125 mg, 0.59 mmol, 59%). TLC: Rf = 0.4 (5% MeOH/DCM with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.31–7.21 (m, 7H), 7.21–7.17 (m, 3H), 3.77 (s, 2H), 2.91–2.85 (m, 2H), 2.84–2.78 (m, 2H), 1.97 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 140.21, 140.04, 128.77, 128.51, 128.44, 128.15, 126.98, 126.20, 53.88, 50.57, 36.36.
Diphenethylamine (125e)
A round-bottom flask was charged with phenylacetaldehyde (125 μL, 1 mmol, 1 equiv), 2-phenethylamine (251 μL, 2 mmol, 2 equiv), and dry DCM (5 mL). The solution was stirred for 10 min, and then NaHB(OAc)3 (425 mg, 2 mmol, 2 equiv) and glacial AcOH (114 μL, 2 mmol, 2 equiv) were added. After 19 h, the reaction mixture was diluted with DCM (5 mL), washed with sat. aq. NaHCO3 (1× 10 mL), brine (1× 10 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (isocratic, 5% MeOH/DCM with 0.5% Et3N), affording the product (128 mg, 0.57 mmol, 57%). TLC: Rf = 0.3 (6% MeOH/DCM with 3 drops of Et3N). 1H NMR (400 MHz, CDCl3) δ 7.73–6.67 (m, 10H), 3.05–2.61 (m, 8H), 1.96 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 139.91, 128.67, 128.45, 126.12, 51.00, 36.25. HRMS [C16H19N + H]+: 226.1590 calculated, 226.1591 found.
Methyl 2-Chloro-6-morpholinopyrimidine-4-carboxylate (127)
The title compound was prepared according to General Procedure E using methyl 2,6-dichloropyrimidine-4-carboxylate (126) (0.62 g, 3.0 mmol, 1 equiv), DiPEA (0.78 mL, 4.5 mmol, 1.5 equiv), and morpholine (0.27 mL, 3.15 mmol, 1.05 equiv). Column chromatography (40% → 60% EtOAc/pentane) afforded the product (684 mg, 2.65 mmol, 88%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H), 3.96 (s, 3H), 3.91–3.55 (m, 8H). 13C NMR (101 MHz,) δ 163.80, 163.09, 160.65, 155.10, 101.89, 65.83, 52.84, 44.18. HRMS [C10H12ClN3O3 + H]+: 258.0640 calculated, 258.0638 found.
2-4-Dichloro-6-morpholino-1,3,5-triazine (131)
A round-bottom flask was charged with cyanuric chloride (0.92 g, 5.0 mmol, 1 equiv) and DCM (16 mL) and cooled to 0 °C. A mixture of morpholine (0.44 mL, 5.0 mmol, 1 equiv) and DiPEA (0.96 mL, 5.5 mmol, 1.1 equiv) in DCM (4 mL) was added dropwise. The solution was stirred for 2 h followed by the addition of 1 M HCl (25 mL). The organic layer was separated, and the water layer was extracted with DCM (25 mL). The combined organic layers were washed with water (25 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (5% → 60% Et2O/pentane) affording the product (1.18 g, 3.6 mmol, 72%). 1H NMR (400 MHz, CDCl3) δ 3.92–3.87 (m, 4H), 3.78–3.73 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 170.56, 164.21, 66.51, 44.58. HRMS [C7H8Cl2N4O + H]+: 235.0148 calculated, 235.0145 found.
2-Chloro-4-(methyl(phenethyl)amino)-6-morpholino-1,3,5-triazine (132)
A round-bottom flask was charged with 2–4-dichloro-1,3,5-triazine 131 (0.59 g, 2.5 mmol, 1 equiv), K2CO3 (1.38 g, 10.0 mmol, 4 equiv), and acetone (10 mL). N-Methylphenethylamine (0.36 mL, 2.5 mmol, 1 equiv) in acetone (2.5 mL) was added dropwise. The solution was stirred for 3 h followed by the addition of 1 M HCl (25 mL). The organic layer was separated, and the water layer was extracted with DCM (25 mL). The combined organic layers were washed with water (25 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (0% - > 20% Et2O/pentane) affording the product (0.79 g, 2.4 mmol, 95%). 1Η NMR analysis showed two rotamers in a ratio of 1:1 (CDCl3, 295 K). 1H NMR (400 MHz, CDCl3) δ 7.33–7.26 (m, 2H), 7.25–7.14 (m, 3H), 3.85–3.64 (m, 10H), 3.03 (d, J = 35.3 Hz, 3H), 2.93–2.84 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 169.44 (d, J = 3.7 Hz), 164.84 (d, J = 7.5 Hz), 164.50 (d, J = 16.3 Hz), 139.11, 128.92 (d, J = 17.3 Hz), 128.63 (d, J = 9.6 Hz), 126.49 (d, J = 8.9 Hz), 66.77, 50.97 (d, J = 27.9 Hz), 43.89, 35.36 (d, J = 2.7 Hz), 33.78 (d, J = 60.1 Hz). HRMS [C16H20ClN5O + H]+: 334.1429 calculated, 334.1425 found.
2-Cyano-4-(methyl(phenethyl)amino)-6-morpholino-1,3,5-triazine (133)
A round-bottom flask was charged with 2-chloro-1,3,5-triazine 132 (0.33 g, 1.0 mmol, 1 equiv), DABCO (22 mg, 0.2 mmol, 0.2 equiv), KCN (72 mg, 1.1 mmol, 1.1 equiv), and DMF (5 mL). The solution was stirred for 68 h followed by the addition of KCN (72 mg, 1.1 mmol, 1.1 equiv). After 3 h, the solution was diluted with sat. aq. NaHCO3 (25 mL) and extracted with DCM (2× 25 mL). The combined organic layers were washed with brine (25 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (0% → 25% Et2O/pentane), affording the product (0.17 g, 0.53 mmol, 53%). 1Η NMR analysis showed two rotamers in a ratio of 1:1 (CDCl3, 295 K). 1H NMR (500 MHz, CDCl3) δ 7.32–7.27 (m, 2H), 7.25–7.20 (m, 2H), 7.17–7.14 (m, 1H), 3.83–3.68 (m, 10H), 3.04 (d, J = 37.5 Hz, 3H), 2.87 (td, J = 7.7, 1.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 163.98 (d, J = 4.8 Hz), 163.57 (d, J = 17.7 Hz), 151.91 (d, J = 14.0 Hz), 138.90 (d, J = 15.8 Hz), 128.93 (d, J = 24.0 Hz), 128.70 (d, J = 9.3 Hz), 126.63 (d, J = 7.4 Hz), 115.77 (d, J = 12.1 Hz), 66.74 (d, J = 30.0 Hz), 50.93 (d, J = 25.3 Hz), 43.77 (d, J = 61.7 Hz), 35.28 (d, J = 26.0 Hz), 33.73 (d, J = 94.9 Hz). HRMS [C17H20N6O + H]+: 325.1771 calculated, 325.1768 found.
4-(Methyl(phenethyl)amino)-6-morpholino-1,3,5-triazine-2-carboxylic Acid (134)
A round-bottom flask was charged with 2-cyano-1,3,5-triazine 133 (97 mg, 0.3 mmol, 1 equiv) and 1 M NaOH (1.5 mL). The solution was stirred at 60 °C for 2.5 h followed by the addition of THF (1.5 mL). The solution was stirred at 60 °C for 5 days, quenched with 1 M HCl (20 mL), and extracted with DCM (3× 20 mL). The combined organic layers were washed with brine (30 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (0% → 10% MeOH/DCM), affording the product (88 mg, 0.26 mmol, 85%). 1Η NMR analysis showed two rotamers in a ratio of 1:1 (CDCl3, 295 K). 1H NMR (400 MHz, MeOD) δ 7.32–7.14 (m, 5H), 3.95–3.78 (m, 6H), 3.74 (t, J = 4.6 Hz, 4H), 3.10 (d, J = 43.6 Hz, 3H), 2.90 (t, J = 7.3 Hz, 2H). 13C NMR (101 MHz, MeOD) δ 167.17, 164.97, 164.94, 164.78, 139.35, 129.11 (d, J = 12.6 Hz), 128.78 (d, J = 6.3 Hz), 126.65 (d, J = 4.1 Hz), 67.00, 51.11 (d, J = 33.1 Hz), 49.86, 44.10, 35.36 (d, J = 14.3 Hz), 33.92 (d, J = 77.5 Hz). HRMS [C17H21N5O3 + H]+: 344.1717 calculated, 344.1714 found.
Sodium (2-Chloro-6-morpholinopyrimidine-4-carbonyl) Glycinate (135)
A round-bottom flask was charged with methyl ester 119h (267 mg, 0.85 mmol, 1 equiv) in 5 mL of THF/MeOH (4:1, v/v). A 1.5 M aqueous NaOH solution (0.57 mL, 0.85 mmol, 1 equiv) was added together with 0.43 mL of H2O. The reaction was stirred overnight at rt, and after which, the solvents were evaporated, yielding the product, which was used without further purification (270 mg, 0.85 mmol, quant.). TLC: Rf = 0.2 (5% MeOH/DCM). 1H NMR (400 MHz, MeOD) δ 7.30 (s, 1H), 3.95–3.86 (m, 2H), 3.81–3.65 (m, 8H). 13C NMR (101 MHz, MeOD) δ 180.70, 159.60, 159.40, 158.60, 152.43, 99.06, 65.99, 43.24. HRMS [C11H13ClN4O4 + H]+: 301.0698 calculated, 301.0696 found.
4-Amino-6-chloro-2-(methyl(phenethyl)amino)pyrimidine (138)
A round-bottom flask was charged with 4-amino-2,6-dichloropyrimidine (1.0 g, 6.1 mmol, 1 equiv), N-methylphenethylamine (0.66 mL, 6.7 mmol, 1.1 equiv), DiPEA (3.2 mL, 18.3 mmol, 3 equiv), and dry 2-PrOH (6.1 mL). The solution was refluxed for 16 h followed by the addition of water (30 mL), and the resulting mixture was extracted with EtOAc (30 mL). The organic layer was washed with water (2× 30 mL). The combined water layers were back-extracted with ethyl acetate (30 mL), and the combined organic layers were washed with brine (30 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (10% → 40% EtOAc/pentane), affording the product (1.0 g, 3.9 mmol, 64%). 1H NMR (300 MHz, MeOD) δ 7.30–7.08 (m, 5H), 5.77 (s, 1H), 4.60 (d, J = 4.8 Hz, 2H), 3.72 (dd, J = 8.3, 6.8 Hz, 2H), 2.95 (s, 3H), 2.91–2.77 (m, 2H). 13C NMR (75 MHz, MeOD) δ 164.52, 161.00, 159.03, 139.25, 128.44, 127.91, 125.67, 91.72, 50.97, 35.18, 33.27. HRMS [C13H15ClN4 + H]+: 263.1058 calculated, 263.1056 found.
4-Amino-2-(methyl(phenethyl)amino)-6-morpholinopyrimidine (139)
A microwave vial was charged with 6-chloropyrimidine 138 (0.13 g, 0.5 mmol, 1 equiv), morpholine (65 μL, 0.75 mmol, 1.5 equiv), DiPEA (0.26 mL, 1.5 mmol, 3 equiv), and n-BuOH (2.5 mL). The vial was capped and heated to 180 °C in a microwave reactor (75 W) for 10 h. The solution was concentrated under reduced pressure and coevaporated with toluene (2×). The residue was purified by flash column chromatography (0% → 4% MeOH/DCM) affording the product (0.14 g, 0.44 mmol, 89%). Regioselectivity was confirmed by 1H-NOESY NMR analysis and is in accordance with previous reports.221H NMR (500 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 7.24–7.17 (m, 3H), 5.05 (s, 1H), 4.45 (s, 2H), 3.78–3.72 (m, 6H), 3.53–3.48 (m, 4H), 3.05 (s, 3H), 2.91–2.85 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 164.51, 140.37, 128.98, 128.52, 126.12, 73.73, 66.87, 51.47, 44.76, 35.57, 34.01. HRMS [C17H23N5O + H]+: 314.1975 calculated, 314.1973 found.
(±)-Benzyl 2-Phenylpiperazine-1-carboxylate Hydrochloride (141)
Cbz Protection
A round-bottom flask was charged with tert-butyl 3-phenylpiperazine-1-carboxylate (0.29 g, 1.1 mmol, 1 equiv), NaHCO3 (0.46 g, 5.5 mmol, 5 equiv) in THF/H2O (8 mL, 1:1). The mixture was cooled to 0 °C and CbzCl (0.19 mL, 1.3 mmol, 1.2 equiv) was added dropwise. The reaction mixture was allowed to warm to room temperature and stirred for 1.5 h. The mixture was extracted with EtOAc (3× 20 mL), and the combined organic layers were washed with brine (50 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (5% → 40% EtOAc/pentane), affording the product (0.40 g, 1.0 mmol, 90%). TLC: Rf = 0.4 (20% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 7.55–7.04 (m, 10H), 5.36 (br s, 1H), 5.27–5.12 (m, 2H), 4.50 (d, J = 13.9 Hz, 1H), 4.13–3.68 (m, 2H), 3.33 (dd, J = 14.0, 4.3 Hz, 1H), 3.19–2.79 (m, 2H), 1.42 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 155.77, 154.43, 138.40, 136.48, 128.66, 128.61, 128.21, 128.00, 127.39, 126.88, 80.32, 67.64, 54.15, 46.04, 42.81, 39.80, 28.42. HRMS [C23H28N2O4 + Na]+: 419.1941 calculated, 419.1934 found.
Boc Deprotection
A round-bottom flask was charged with the double-protected piperazine (103 mg, 0.26 mmol, 1 equiv) and HCl (4 M solution in 1,4-dioxane, 1 mL, 4 mmol, 15.5 equiv) and stirred at rt. After 1 h, the reaction was concentrated under reduced pressure to afford the product as the hydrochloride salt (87 mg, 0.26 mmol, 99%). TLC: Rf = 0.3 (50% EtOAc/pentane). 1H NMR (400 MHz, CDCl3) δ 10.24 (br s, 1H), 9.33–8.80 (m, 1H), 7.51–7.02 (m, 10H), 5.55 (br s, 1H), 5.29–4.95 (m, 2H), 4.22 (d, J = 14.1 Hz, 1H), 3.99–3.77 (m, 1H), 3.49–3.22 (m, 3H), 3.06 (br s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.26, 135.78, 135.54, 129.31, 128.64, 128.42, 128.28, 128.11, 126.34, 68.19, 51.77, 44.38, 42.59, 36.98.2
Acknowledgments
M.v.d.S. was supported by a VICI grant from the Netherlands Organization for Scientific Research (NWO) and funding from Oncode Institute. Leiden University Faculty of Science “Profiling Programme: Endocannabinoids” is also acknowledged for financial support to E.D.M. and M.v.d.S. Hans van den Elst and Sebastian Grimm are kindly acknowledged for HPLC purifications, Rian van den Nieuwendijk for optical rotation measurements, and Karthick Sai Sankar Gupta and Fons Lefeber for helping with NMR experiments.
Glossary
Abbreviations
- NAPE-PLD
N-acylphosphatidylethanolamine phospholipase D
- NAPE
N-acylphosphatidylethanolamine
- NAE
N-acylethanolamine
- PE
phosphatidylethanolamine
- PA
phosphatidic acid
- ECS
endocannabinoid system
- AEA
N-arachidonoylethanolamine
- CB1/2
cannabinoid receptor 1/2
- TRPV1
transient receptor potential vanilloid 1
- PPAR-α
peroxisome proliferator-activated receptor α
- PLD1
phospholipase D1
- SAR
structure–activity relationship
- NASH
non-alcoholic steatohepatitis
- HPA
hypothalamus-pituitary–adrenal
- LipE
Lipophilic efficiency
- DiPEA
N,N-diisopropylethylamine
- EDC
N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide
- HOBt
1-hydroxybenzotriazole
- PyBOP
(benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01441.
The authors declare the following competing financial interest(s): E.D.M., I.K., C.A.A.v.B. and M.v.d.S. are listed as inventors on patent application WO 2019/229250A1 filed by Leiden University in which inhibitors of NAPE-PLD are disclosed.
Supplementary Material
References
- Mock E. D.; Mustafa M.; Gunduz-Cinar O.; Cinar R.; Petrie G. N.; Kantae V.; Di X.; Ogasawara D.; Varga Z. V.; Paloczi J.; Miliano C.; Donvito G.; van Esbroeck A. C. M.; van der Gracht A. M. F.; Kotsogianni I.; Park J. K.; Martella A.; van der Wel T.; Soethoudt M.; Jiang M.; Wendel T. J.; Janssen A. P. A.; Bakker A. T.; Donovan C. M.; Castillo L. I.; Florea B. I.; Wat J.; van den Hurk H.; Wittwer M.; Grether U.; Holmes A.; van Boeckel C. A. A.; Hankemeier T.; Cravatt B. F.; Buczynski M. W.; Hill M. N.; Pacher P.; Lichtman A. H.; van der Stelt M. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat. Chem. Biol. 2020, 16, 667–675. 10.1038/s41589-020-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z.; Uyama T.; Tsuboi K.; Ueda N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta 1862, 2017, 1546–1561. [DOI] [PubMed] [Google Scholar]
- Okamoto Y.; Morishita J.; Tsuboi K.; Tonai T.; Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Tsuboi K.; Uyama T.; Okamoto Y.; Ueda N. Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflammation Regener. 2018, 38, 28. 10.1186/s41232-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M. Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front. Mol. Neurosci. 2017, 10, 166. 10.3389/fnmol.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazier W.; Saucisse N.; Gatta-Cherifi B.; Cota D. The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol. Metab. 2015, 26, 524–537. 10.1016/j.tem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Kimberly W. T.; O’Sullivan J. F.; Nath A. K.; Keyes M.; Shi X.; Larson M. G.; Yang Q.; Long M. T.; Vasan R.; Peterson R. T.; Wang T. J.; Corey K. E.; Gerszten R. E. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017, 2, e92989 10.1172/jci.insight.92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F.; Mezzullo M.; Repaci A.; Belluomo I.; Ibarra Gasparini D.; Di Dalmazi G.; Mastroroberto M.; Vicennati V.; Gambineri A.; Morselli-Labate A. M.; Pasquali R.; Pagotto U. Profiling plasma N-acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol. Metab. 2018, 14, 82–94. 10.1016/j.molmet.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Okamoto Y.; Morishita J.; Tsuboi K.; Miyatake A.; Ueda N. Functional analysis of the purified anandamide-generating phospholipase D as a member of the metallo-β-lactamase family. J. Biol. Chem. 2006, 281, 12325–12335. 10.1074/jbc.M512359200. [DOI] [PubMed] [Google Scholar]
- Wang J.; Okamoto Y.; Tsuboi K.; Ueda N. The stimulatory effect of phosphatidylethanolamine on N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). Neuropharmacology 2008, 54, 8–15. 10.1016/j.neuropharm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Magotti P.; Bauer I.; Igarashi M.; Babagoli M.; Marotta R.; Piomelli D.; Garau G. Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids. Structure 2015, 23, 598–604. 10.1016/j.str.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margheritis E.; Castellani B.; Magotti P.; Peruzzi S.; Romeo E.; Natali F.; Mostarda S.; Gioiello A.; Piomelli D.; Garau G. Bile acid recognition by NAPE-PLD. ACS Chem. Biol. 2016, 11, 2908–2914. 10.1021/acschembio.6b00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Tonai T.; Ueda N. Activation of N-acylethanolamine-releasing phospholipase D by polyamines. Chem. Phys. Lipids 2002, 115, 77–84. 10.1016/s0009-3084(02)00015-4. [DOI] [PubMed] [Google Scholar]
- Petersen G.; Pedersen A. H.; Pickering D. S.; Begtrup M.; Hansen H. S. Effect of synthetic and natural phospholipids on N-acylphosphatidylethanolamine-hydrolyzing phospholipase D activity. Chem. Phys. Lipids 2009, 162, 53–61. 10.1016/j.chemphyslip.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Scott S. A.; Spencer C. T.; O’Reilly M. C.; Brown K. A.; Lavieri R. R.; Cho C.-H.; Jung D.-I.; Larock R. C.; Brown H. A.; Lindsley C. W. Discovery of desketoraloxifene analogues as inhibitors of mammalian, Pseudomonas aeruginosa, and NAPE phospholipase D enzymes. ACS Chem. Biol. 2015, 10, 421–432. 10.1021/cb500828m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani B.; Diamanti E.; Pizzirani D.; Tardia P.; Maccesi M.; Realini N.; Magotti P.; Garau G.; Bakkum T.; Rivara S.; Mor M.; Piomelli D. Synthesis and characterization of the first inhibitor of N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD). Chem. Commun. 2017, 53, 12814–12817. 10.1039/C7CC07582K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal G.; Zarrow J. E.; Mashhadi Z.; Flynn C. R.; Vinson P.; Weaver C. D.; Davies S. S. Symmetrically substituted dichlorophenes inhibit N-acyl-phosphatidylethanolamine phospholipase D. J. Biol. Chem. 2020, 295, 7289–7300. 10.1074/jbc.RA120.013362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman R. M.; Leech R. W.; Alvord E. C. Jr. Neurotoxicity of hexachlorophene in humans: II. a clinicopathological study of 46 premature infants. Arch. Neurol. 1975, 32, 320–325. 10.1001/archneur.1975.00490470064009. [DOI] [PubMed] [Google Scholar]
- Bruno N. C.; Tudge M. T.; Buchwald S. L. Design and preparation of new palladium precatalysts for C–C and C–N cross-coupling reactions. Chem. Sci. 2013, 4, 916–920. 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach T. D.; Batey R. A. Ligand- and base-free copper(II)-catalyzed C–N bond formation: cross-coupling reactions of organoboron compounds with aliphatic amines and anilines. Org. Lett. 2003, 5, 4397–4400. 10.1021/ol035681s. [DOI] [PubMed] [Google Scholar]
- Talele T. T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]
- Li C.; Rosenau A. A practical strategy for the synthesis of 2-dialkylamino-4-arylamino-6-aminopyrimidines. Tetrahedron Lett. 2009, 50, 5888–5893. 10.1016/j.tetlet.2009.07.154. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.