Highlights
-
•
The effects of air pollution on the placental iodine load are scarcely studied.
-
•
PPM2.5 in the third trimester of pregnancy was inversely associated with the placental iodine load.
-
•
DLNMs estimated the week-specific associations between PM2.5 and the placental iodine load.
-
•
Iodine mediates the effect of gestational PM2.5 exposure on cord blood FT4.
Keywords: Particulate matter, Air pollution, Placenta, Iodine, Thyroxine, Dlnm
Abbreviations: DE, Direct effect; DLNM, Distributed lag nonlinear model; ENVIRONAGE, Environmental influence on early ageing; FT4, Free thyroxine; IE, Indirect effect; NO2, Nitrogen dioxide; PM2.5, Particulate matter with a diameter less than 2.5 µm; T4, Thyroxine; T3, Triiodothyronine; WHO, World Health Organization
Abstract
Background
Adequate intake of iodine is required for the production of thyroid hormones and contributes in pregnant women to a healthy brain development and growth in their offspring. To date, some evidence exists that fine particulate air pollution is linked with the fetal thyroid hormone homeostasis. However, possible effects of air pollutants on the placental iodine storage have not been investigated so far.
Objectives
We investigated the association between air pollution exposure to particulate matter with a diameter less than 2.5 µm (PM2.5), NO2, and black carbon and the placental iodine load.
Methods
The current study is part of the ENVIRONAGE birth cohort and included 470 mother-newborn pairs. Iodine concentrations were measured in placental tissue. A high-resolution air pollution model was used to estimate the daily exposure to PM2.5, NO2, and black carbon over the entire pregnancy based on the maternal residential addresses. Distributed lag nonlinear models (DLNMs) were used to estimate gestational week-specific associations between placental iodine concentrations and the air pollutants to understand the impact of specific exposure windows.
Results
PM2.5 showed a positive association with placental iodine concentration between the 16th and 22nd week of gestation. In contrast, a significant inverse association between PM2.5 and placental iodine concentration was observed in gestational weeks 29–35. The effect estimate, for a 5 µg/m3 increment in PM2.5 concentration, was the strongest at week 32 (β −0.11 µg/kg; 95%CI: −0.18 to −0.03). No associations were observed between placental iodine concentrations and NO2 or black carbon. Assuming causality, we estimated that placental iodine mediated 26% (−0.33 pmol/L; 95%CI: −0.70 to 0.04 pmol/L) of the estimated effect of a 5 µg/m3 increment in PM2.5 exposure on cord blood free thyroxine (FT4) concentrations.
Conclusion
In utero exposure to particulate matter during the third trimester of pregnancy is linked with a lower placental iodine load. Furthermore, the effect of air pollution on cord blood FT4 levels was partially mediated by the placental iodine load.
1. Introduction
An adequate intake of iodine, an essential trace element, is required for the production of thyroid hormones and contributes in pregnant women to a healthy brain development and growth of their offspring (Andersson et al., 2012). During gestation, the placenta acts as a storage organ for iodine in a concentration-dependent manner and therefore represents a biomarker for the long-term iodine load (Burns et al., 2011, Neven et al., 2020b). This storage property allows for an adaptive mechanism to protect the foetus from (short-term) inadequacy of maternal iodine intake. The World Health Organization (WHO) recommends a minimal daily iodine intake of 150 µg in adults, and 250 µg for pregnant and lactating women (WHO - UNICEF - ICCIDD 2007). However, it is estimated that approximately 38% of the global population is affected by iodine deficiency (Leung et al., 2011), representing a major culprit of preventable cognitive impairment in children around the world (WHO - UNICEF - ICCIDD 2007).
Iodine is used by the thyroid gland to produce thyroxine (T4), and triiodothyronine (T3). Over 99% of these hormones are bound to plasma proteins, which carry them throughout the body and can cross the placenta to reach the fetal circulation (Calvo et al., 2002). During the first trimester of gestation the fetal thyroid gland develops (Burrow et al., 1994), and afterwards, the fetus is capable of producing its supply of T4 (Contempre et al., 1993).
Air pollution is a major contributor to the global burden of disease (Cohen et al., 2017). In Europe, a citizen loses approximately nine months of healthy life due to ambient air pollution (ECA 2018). As the world’s population grows and ages, the prevalence of major disabling neurological disorders increases (GBD Neurology Collaborators 2019, 2016). To some extent, air pollution adversely influences (1) normal fetal development, as indicated by a lower birth weight (Dadvand et al., 2013, Liang et al., 2019) or risk at cardiovascular diseases (Madhloum et al., 2019, Yang et al., 2020); (2) the cognitive performance, as indicated by worse verbal and math tests in adults (Zhang et al., 2018), accelerated cognitive decline in adults (Chen et al., 2017), and slower rates of cognitive development in children (Kicinski et al., 2015, Saenen et al., 2016a, Sunyer et al., 2015). Part of the air pollution-induced health effects can probably be traced down to the gestational period, as fine particulate matter with a diameter of less than 2.5 µm (PM2.5) can cross the placental barrier (Bové et al., 2019) and has been linked with low birth weight (Pedersen et al., 2013, Smith et al., 2017) and a reduced head circumference at birth (Pedersen et al., 2013). Further biochemical and molecular evidence of these health outcomes can be found in the dysregulation of the thyroid hormones associated with ambient air pollution throughout the gestation (Ghassabian et al., 2019, Janssen et al., 2017b).
While over a third of the world’s population is considered iodine deficient and ambient air pollution is omnipresent, it is surprising that, to our knowledge, no epidemiological studies have investigated the association between common air pollutants (such as PM2.5, nitrogen dioxide [NO2], or black carbon) and the iodine concentrations in the human placenta. We hypothesize that gestational exposure to these residential air pollutants is associated with alterations in the placental iodine load. Moreover, previous findings of our cohort provides evidence of an inverse association between air pollution and FT4 concentrations in the third trimester of pregnancy. We additionally aim to investigate a potential mediating role of iodine in that association in a secondary analysis.
2. Methods
Study design and sample collection – As part of the prospective birth cohort ENVIRonmental influences ON early AGEing (ENVIRONAGE), we recruited 799 mother-newborn pairs between March 1st,2013 and April 1st, 2017 according to our study procedures, detailed previously (Janssen et al., 2017a). Funding allowed us to determined the concentration of placental iodine for 500 pairs, and finally, 469 mother-newborn pairs were included in the current study based on the following exclusion criteria: samples with iodine concentrations below the LOQ (n = 2), mothers with preeclampsia (n = 8), hyperthyroidism (n = 4), hypothyroidism (n = 12), a gestational duration less than 37 weeks (n = 2), and missing air pollution data (n = 3). We were able to measure the thyroid hormone levels for 454 mother-newborn pairs. The study protocol was approved by both the Ethics Committee of Hasselt University and the East-Limburg Hospital and was carried out following the Declaration of Helsinki. We obtained written informed consent from all participating mothers.
Maternal pre-pregnancy BMI was recorded at the first antenatal visit at gestational weeks 7–9. The date of the last menstrual period and the first ultrasound exam were used to estimate the date of conception. Mothers filled out a questionnaire, which gave us detailed information on household smoking status, maternal alcohol consumption, maternal education, multivitamin use, residential address, parity, season of delivery, and the neonates’ ethnicity. The hospital’s medical records provided us with information regarding the pre-pregnancy BMI, gestational weight gain, gestational age, and date at delivery. Mothers were categorized as “exposed to indoor second-hand smoke (SHS)” if anyone in the household smoked except the mother, and “not exposed to indoor SHS” if no one smoked in the household. Mothers who consumed any alcoholic beverage during pregnancy were classified as “yes”, and otherwise they were classified as “no”. Consumption of multivitamins during pregnancy was also classified as “yes” or “no” if the mothers consumed any (multi-)vitamins or none at all respectively. Maternal smoking behavior during pregnancy was coded as “non-smoker”, “cessation before pregnancy”, or “current smoker”, and maternal education was classified as “low” for no diploma or primary school, “middle” for high school, or “high” for college or university degree. Season of delivery was categorized based on the date at delivery as “winter” (December 21st – March 20th), “spring” (March 21st – June 20th), “summer” (June 21st – September 20th), and “autumn” (September 21st – December 20th).
Placental sample collection and iodine analysis – The full protocol, including quality control and performance characteristics, has been published previously (Neven et al., 2020b). In short, placentas were collected within 10 min after birth and frozen at −20 °C. Placentas were minimally thawed and biopsies were taken at three standardized locations, 2 cm from the umbilical cord. The membranes were curtailed, and any excess blood was eliminated by rubbing it against a Grade 54 filter paper (GE Healthcare, Chicago, United States). Tissues were stored in metal-free containers (sterile propylene tubes; VWR, Pennsylvania, United States) and bio-banked at −20 °C until iodine determination. For each placenta, biopsies were pooled from each of the three sampling locations to obtain 500 mg of tissue that was placed in a 0.5% tetramethylammonium hydroxide (TMAH) solution, and heated to 90 °C for three hours in a DigiPREP block digestion system (SCP SCIENCE, Quebec, Canada). Iodine was measured via ICP-MS on an Elan DRC II (Perkin Elmer, Massachusetts, United States) on mass 127I, with 125Te as an internal standard.
Cord blood collection and thyroid hormone measurements – Immediately after birth, 8 mL of umbilical cord blood was collected in plastic BD Vacutainer® Lithium Heparin Tubes (BD, NJ, United States). Samples were centrifuged at 2,500 g for 15 min to obtain plasma, which was immediately stored at −80 °C. The clinical laboratory of East-Limburg Hospital determined the plasma levels of FT4 (pmol/L), FT3 (pmol/L), and TSH (mIU/L) with an electro-chemiluminescence immunoassay using the Modular E170 automatic analyzer (Roche, Basel, Switzerland). The laboratory reference values range from 4.0 to 6.8 pmol/L for FT3, from 12.0 to 21.9 pmol/L for FT4, and 0.3 to 4.2 mIU/L for TSH. Thyroid hormones in cord blood plasma could be determined in 454 subjects.
Prenatal air pollution exposure assessment – We used a high-resolution spatial-temporal interpolation to determine the outdoor PM2.5, NO2, and black carbon concentrations (in µg/m3) based on the maternal residential address (Janssen et al., 2008). Pollution data, provided by the official Belgian fixed monitoring network upon simple request, is interpolated with the use of land cover data from satellite images from the CORINE land cover dataset, in combination with a dispersion model (Lefebvre et al., 2011, Lefebvre et al., 2013). This model chain provides interpolated air pollution values from the Belgian telemetric air quality networks, ‘point’ sources like industries, and ‘line’ sources like highways, on a dense, irregular receptor point grid. This grid has a maximum cell size of 25 m by 25 m. The performance of the overall model was assessed by a leave-one-out cross-validation, which included 34 monitoring stations for PM2.5, 44 for NO2, and 14 for black carbon. The validation statistics of our interpolation tool explained more than 80% of the spatial-temporal variability in our study area for PM2.5, 78% for NO2, and 74% for black carbon (Lefebvre et al., 2011, Maiheu et al., 2013). The model was additionally validated by linking the modelled residential PM2.5 and black carbon levels with the biomarkers of internal exposure to nanosized black carbon particles in urine (Saenen et al., 2017) and placental tissue (Bové et al., 2019). Daily air pollution levels for the total pregnancy period were modelled at each mother’s home address. Besides the weekly average of the air pollutant values, we also calculated the exposures for specific time windows during the pregnancy: the first trimester (i.e., date of conception until 13 weeks of pregnancy), the second trimester (i.e., 14 weeks until 26 weeks of pregnancy), and the third trimester (i.e., 26 weeks of pregnancy until delivery). Address changes of mothers during the pregnancy (n = 51) were taken into account for these calculations.
Statistical methods – First, we used the averaged trimester-specific residential air pollution exposures and evaluated the association between the exposure to each pollutant and placental iodine concentration in a generalized linear model. Next, to model a more detailed association (with higher temporal resolution), we applied the distributed lag nonlinear models (DLNMs) (Gasparrini 2011) to model the gestational week-specific association between placental iodine concentrations and the air pollutants PM2.5, NO2, and black carbon. DLNMs are flexible in modelling the exposure levels while adjusting for lagged exposures so that both the exposure-response and the lag-response relationships are accounted for in a single model. This was achieved by constructing a cross-basis combing two basis functions, one for modelling the exposure structure and the other for the lag structure (Gasparrini 2014). We assumed the former to be linear and for the latter, we specified a natural cubic spline with three equally spaced inner knots (i.e. 5 degrees of freedom) between gestational weeks 1 and 40. In all models, we accounted for a priori selected variables including the known determinants of placental iodine concentrations, i.e. maternal pre-pregnancy BMI, gestational weight gain, maternal alcohol consumption, multivitamin use, gestational age, date of delivery, and season of delivery, and those variables that might have a possible link with air pollution and placental iodine such as indoor SHS exposure, maternal smoking behavior during pregnancy, maternal education, maternal age at delivery, newborns’ sex, parity, and ethnicity.
We reported week-specific estimates with the 95% confidence intervals (95%CI) of DLNM-based associations as the changes of placental iodine concentration for an increment of 5 µg/m3 in PM2.5, 10 µg/m3 in NO2, or 1 µg/m3 in black carbon. The cumulative associations per trimester of pregnancy were calculated and expressed similarly. The significance level used throughout this study was 0.05.
In a secondary analysis, we performed a mediation analysis on the subset of individuals for whom thyroid hormones could be measured (n = 454) to investigate potential associations that may exist between the air pollutants (i.e. exposure variables), placental iodine (i.e. mediator of the effect) and cord blood plasma thyroid hormones (i.e. outcome variables) (Valeri and Vanderweele 2013). This model decomposes the total effect into a direct effect (DE; effect of the exposure on the outcome at a fixed level of the mediator) and an indirect effect (IE; effect of the exposure on the outcome that acts through the mediator). This analysis requires several assumptions with the four-step approach (Baron and Kenny, 1986, Valeri and Vanderweele, 2013): (1) The causal variable has to be correlated with the outcome; (2) The causal variable has to be correlated with the mediator; (3) the mediator needs to affect the outcome variable; and (4) the mediator needs to affect the outcome variable (whilst controlling for the causal variable).
Sensitivity analyses were performed for the total population after excluding the mothers with hypertension (n = 27), excluding the current smokers (n = 45), and including weekly averaged ambient temperature. Furthermore, we reran the DLNM models for the subset wherein cord blood thyroid hormones were determined (n = 453). To take into account potential co-pollution effects, we reanalyzed the significant air pollutants as the main model, and added the other air pollutants individually to the main model.
Role of the funding source – The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Population characteristics – The general characteristics of the study population (n = 470) are provided in Table 1. Gestation lasted on average, 39.9 weeks (SD 1.0) and the newborns weighed 3464 g (423) and measured 50.3 cm (1.9). Over half of all newborns were boys (51.6%), and most of the newborns were of European descent (87.2%). Maternal age at the moment of delivery was 29.4 years (4.4). The average pre-pregnancy BMI was 24.6 kg/m2 (4.8) and the mother gained, on average, 14.0 kg (5.8). A minority of the mothers were exposed to indoor SHS (8.3%) or consumed a maximum of two glasses of alcoholic beverages per week (13.6%). Placental iodine concentrations averaged 26.1 µg/kg (4.3).
Table 1.
Clinical and sociodemographic characteristics of the mother-newborn pairs.
| Characteristics | Total (n = 469) |
|---|---|
| Mother | |
| Age, years | 29.4 (4.4) |
| Pre-pregnancy BMI, kg/m2 | 24.6 (4.8) |
| Net weight gain, kg | 14.0 (5.8) |
| Hypertension | |
| No | 442 (94.2%) |
| Yes | 27 (5.8%) |
| Exposure to indoor second-hand smoke | |
| None | 429 (91.7%) |
| Yes | 39 (8.3%) |
| Maternal smoking behavior | |
| Never smoked | 303 (64.6%) |
| Cessation before pregnancy | 121 (25.8%) |
| Current smoker | 45 (9.6%) |
| Alcohol consumption | |
| None | 405 (86.4%) |
| Yesa | 64 (13.6%) |
| Maternal educationb | |
| Low | 61 (13.0%) |
| Middle | 156 (33.3%) |
| High | 252 (53.7%) |
| Multivitamin use | |
| None | 209 (44.6%) |
| Yes | 260 (55.4%) |
| Newborn | |
| Gestational age, weeks | 39.9 (1.0) |
| Birth weight, g | 3464 (423) |
| Birth length, cmd | 50.3 (1.9) |
| Sex | |
| Male | 242 (51.6%) |
| Ethnicityc | |
| European | 409 (87.2%) |
| Non-European | 60 (12.8%) |
| Parity | |
| 1 | 248 (52.9%) |
| 2 | 158 (33.7%) |
| ≥ 3 | 63 (13.4%) |
| Season at delivery | |
| Winter (Dec 21 to March 20) | 109 (23.2%) |
| Spring (March 21 to June 20) | 112 (23.9%) |
| Summer (June 21 to Sept 22) | 135 (28.8%) |
| Autumn (Sept 23 to Dec 20) | 113 (2413%) |
| Cord blood thyroid hormonese | |
| Free thyroxine (FT4), pmol/L | 16.5 (2.1) |
| Free triiodothyronine (FT3), pmol/L | 2.3 (5.8) |
| Thyroid-stimulating hormone (TSH), mIU/L | 11.1 (8.3) |
| Placental iodine concentration, µg/kg | 26.1 (4.3) |
Data are mean (SD) or n (%).
Mothers who consumed a maximum of two glasses of alcoholic beverages per week.
Maternal education was coded as low (no diploma or primary school), middle (high school), and high (college or university degree).
Classification of ethnicity is based on the native country of the neonates' grandparents as either European (at least two grandparents were European) or non-European (at least three grandparents were of non-European origin).
Data available for 467 participants.
Data available for 454 participants.
Table 2 shows the residential prenatal exposure concentrations of air pollutants by the gestational time window. The median (5th to 95th percentile) ambient PM2.5, NO2, and black carbon concentrations over the entire pregnancy was 11.9 µg/m3 (8.4–16.0 µg/m3), 15.9 µg/m3 (11.1–24.2 µg/m3), and 1.1 µg/m3 (0.8–1.7 µg/m3) respectively.
Table 2.
Exposure to PM2.5, NO2, and black carbon by gestational time window.
| Median (5th – 95th percentile) | |
|---|---|
| PM2.5 exposure (µg/m3) | |
| Trimester 1 (1–13 weeks) | 11.6 (7.6–19.9) |
| Trimester 2 (14–26 weeks) | 12.5 (7.0–20.8) |
| Trimester 3 (27 weeks – delivery) | 10.4 (6.8–19.3) |
| Entire pregnancy | 11.9 (8.4–16.0) |
| NO2 exposure (µg/m3) | |
| Trimester 1 (1–13 weeks) | 16.9 (9.8–26.0) |
| Trimester 2 (14–26 weeks) | 16.2 (9.2–25.8) |
| Trimester 3 (27 weeks – delivery) | 15.1 (8.5–24.6) |
| Entire pregnancy | 15.9 (11.1–24.2) |
| Black carbon exposure (µg/m3) | |
| Trimester 1 (1–13 weeks) | 1.2 (0.6–1.9) |
| Trimester 2 (14–26 weeks) | 1.1 (0.6–1.8) |
| Trimester 3 (27 weeks–delivery) | 1.0 (0.6–1.7) |
| Entire pregnancy | 1.1 (0.8–1.7) |
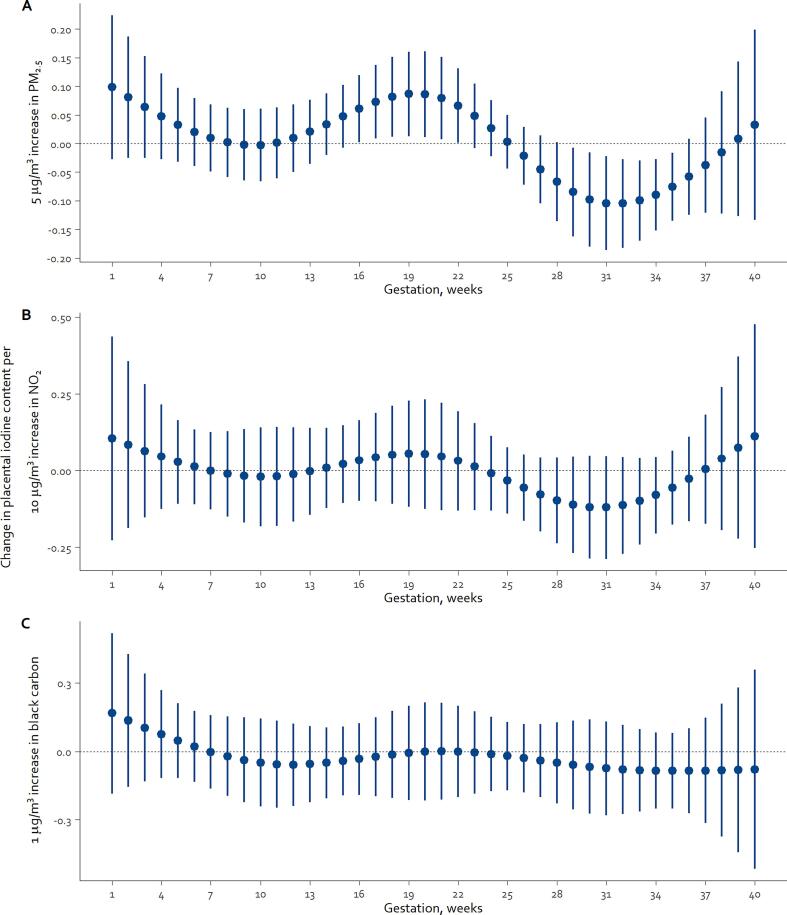
Associations between air pollutants and placental iodine concentration – In the first analysis, using the averaged trimester-specific residential air pollution exposures, we observed that 5 µg/m3 increment in PM2.5 was associated with a 0.70 µg/kg increase in placental iodine during the second trimester (95%CI: 0.00–1.39; p = 0.049), while in the third trimester a decrease of 0.74 µg/kg (95%CI: −1.45 to −0.03; p = 0.04) occurred. To obtain a more detailed association analysis, the DLNM model was applied. Fig. 1 shows for the entire pregnancy period the course of the weekly associations between the changes in placental iodine concentration and exposure for each pollutant-specific increment in concentration. As to PM2.5, a 5 µg/m3 increment was associated with a significant increase in placental iodine content from gestational week 16–22, and a decrease in placental iodine concentration from gestational week 29–35 (Fig. 1A). In the second trimester, week 19 had the largest effect estimate (β 0.09 µg/kg; 95%CI: 0.01–0.16) and week 16 the smallest (β 0.06 µg/kg; 95%CI: 0.003–0.12). For the third trimester, the effect estimate was the strongest at week 32 (β −0.10 µg/kg; 95%CI: −0.18 to −0.03), and the weakest at week 29 (β −0.08; 95%CI: −0.16 to −0.007) of gestation. The cumulative estimates of the second and third trimester of pregnancy (week 14 until week 26 and week 27 until delivery, respectively) showed that an increment of 5 µg/m3 in ambient PM2.5 exposure was associated with an increase of 0.67 µg/kg in placental iodine concentration (95%CI: 0.01–1.3) and decrease of 0.84 µg/kg (95%CI: −1.54 to −0.13), respectively. For NO2 and black carbon exposure, similar temporal patterns as with PM2.5 were observed, however, none of the associations reached the level of statistical significance (Fig. 1B and Fig. 1C).
Fig. 1.
Change in placental iodine concentrations (in µg/kg) in association with week-specific prenatal exposure to PM2.5 (A), NO2 (B), and black carbon (C) during pregnancy. Week-specific estimates of changes are given for the air pollutant specific increment in concentration: 5, 10, and 1 µg/m3 for PM2.5, NO2, and black carbon, respectively. Models were adjusted for maternal pre-pregnancy BMI, gestational weight gain, indoor SHS exposure, maternal alcohol consumption, multivitamin use, maternal smoking behavior during pregnancy, maternal education, maternal age at delivery, newborns’ sex, gestational age, date of delivery, and season of delivery.
In a sensitivity analysis, we excluded mothers with hypertension or current smokers. These exclusions did not significantly alter the estimated associations between placental iodine concentrations and exposure to PM2.5 (Supplemental Fig. S1). Furthermore, the results from the co-pollutant analysis showed that adding NO2, black carbon or temperature to the main model of PM2.5 did not alter the reported associations (Supplemental Fig. S2).
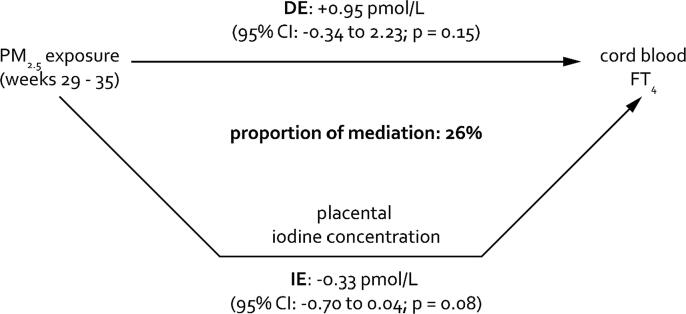
Air pollution and thyroid hormones – Since we observed an association between PM2.5 and FT4 (Janssen et al., 2017b) in addition to an association between placental iodine and FT4, we performed a mediation analysis to estimate the proportion of the PM2.5 exposure effect on the cord blood thyroid hormones as mediated by placental iodine concentrations. For PM2.5, the concentrations of the significant gestational weeks suggested by the DLNM model (weeks 29 until 35) were averaged. In the first step of the four-step approach (Baron and Kenny 1986), no significant effect was observed between PM2.5 and FT4 (p = 0.39), FT3 (p = 0.35), or TSH (p = 0.67). However, there is consensus among statisticians that the exposure and outcome association does not need to be statistically significant when considering a mediator (MacKinnon et al., 2007, Valeri and Vanderweele, 2013). For the second step, placental iodine was significantly associated with PM2.5 (p = 0.046). In the third step, only FT4 was significantly associated with the placental iodine concentrations (p < 0.0001). For the fourth step, PM2.5 was added to the model and showed that only placental iodine remained significant (p = 0.027) while PM2.5 was not significant (p = 0.57). These findings support a full mediation effect. Assuming causality, the adjusted estimates of the proportion of mediation showed that placental iodine concentrations explained 26% with borderline significance (IE: −0.33; 95%CI: −0.70–0.04; p = 0.08) of the association between weeks 29–35 of PM2.5 exposure and cord blood FT4 levels (Fig. 2). We did not perform mediation analysis for NO2 or black carbon or for FT3 or TSH because the assumptions of mediation were not met (i.e. no significant association between the mediator and outcome).
Fig. 2.
Estimated proportion of the association between PM2.5 exposure from weeks 29 to 35 of pregnancy and cord blood free thyroxine (FT4 in pmol/L) levels, mediated by placental iodine concentrations. The proportion of mediation is calculated based on the estimate of the direct effect (DE) and the indirect effect (IE) via the following formula: IE/(IE + DE). Estimates are presented for a 5 µg/m3 increment in PM2.5 exposure during week 29–35 of pregnancy. The model was adjusted for maternal pre-pregnancy BMI, gestational weight gain, indoor SHS exposure, maternal alcohol consumption, multivitamin use, maternal smoking behavior during pregnancy, maternal education, maternal age at delivery, newborns’ sex, gestational age, date of delivery, and season of delivery.
4. Discussion
Iodine is an essential nutrient and its placental load might reflect the long-term iodine availability to the fetus. This is the first study to show an association between prenatal ambient air pollution, especially PM2.5, and placental iodine load. In 470 newborns, a 5 µg/m3 increment in PM2.5 exposure during the second trimester of pregnancy was associated with 0.67 µg/kg higher placental iodine concentration, while the third trimester of pregnancy was associated with a 0.84 µg/kg lower concentration. Furthermore, the mediation analysis showed that placental iodine partially mediates the association between pregnancy weeks 29–35 of PM2.5 air pollution exposure and FT4 hormone in cord blood. In short, our findings emphasize the hazardousness of in utero exposure to particulate matter for gestational iodine concentrations in the placenta and thyroid hormones. Furthermore, the results were robust as they remained unaltered in multiple sensitivity analyses.
Iodine deficiency during gestation is considered one of the main culprits of cognitive impairment in children around the world (WHO - UNICEF - ICCIDD 2007). Despite the implementation of numerous iodine supplementation programs over the past decades in several countries (Public Health Committee of the American Thyroid Association et al., 2006, WHO - UNICEF - ICCIDD, 2007), some studies have shown a steady decrease of iodine status in adults (Caldwell et al., 2003, Caldwell et al., 2011, Leung et al., 2011). Even pregnant women from the prospective ENVIRONAGE birth cohort are considered iodine deficient with a median urinary iodine concentration of 67.8 µg/g creatinine (Neven et al., 2020b). Moreover, findings from NHANES (National Health and Nutrition Examination Survey) showed that pregnant women in the United States were marginally iodine sufficient (median urinary iodine concentration was 153 µg/L) (Perrine et al., 2010), which is in line with findings from a Belgian nation-wide study (Vandevijvere et al., 2013). This trend of iodine insufficiency, even mild, during a vulnerable period in early life, can have detrimental consequences in later life. Children, aged 7–9 years, whose mothers had untreated mild hypothyroidism during gestation had on average 7 IQ points less than the offspring of matched euthyroid control mothers (Haddow et al., 1999). Moreover, children from iodine-deficient pregnancies had low verbal IQ scores and impaired reading accuracy and comprehension compared to iodine replete pregnancies (Bath et al., 2013). Our findings indicate that ambient levels of PM2.5 air pollution could have a disadvantageous effect during gestation on the iodine load in the placenta, even at PM2.5 concentrations well below the recommended European Air Quality Standards (a maximum annual PM2.5 concentration of 25 µg/m3) (European Parliament, 2015).
The third trimester of pregnancy, more specifically from week 29 to 35, was found to be the critical time window for in utero PM2.5 exposure and decremental placental iodine concentrations. To date, no studies were found that associate ambient air pollution during pregnancy with either urinary or placental iodine concentrations. However, in line with our observations, a recent study about household air pollution in pregnant Nigerian women found that cord blood levels of iodine, and other essential elements, were lower in women who cooked with kerosene compared to those who cooked with liquefied natural gas (Arinola et al., 2018). Furthermore, our findings are consistent with those of Ghassabian and colleagues, who investigated five cohorts, located in Europe and the United States (Ghassabian et al., 2019). They observed that exposure to PM2.5 was associated with mild thyroid dysfunction throughout pregnancy, while neither NO2 nor PM2.5 absorbance (i.e. a measure of black carbon) was associated. These findings reinforce the idea that the exposure to the composition of PM2.5 might be more harmful to the iodine status and the homeostasis of thyroid hormones during gestation than traffic-related pollution like NO2 and black carbon (Ghassabian et al., 2019). For example, it is well known that components like waterborne perchlorate, thiocyanate from cigarette smoke, and nitrates present in PM pose a threat to the iodine uptake as they are potent inhibitors of the sodium/iodide symporter (De Groef et al., 2006, Miller and Rayalam, 2017, Ozpinar et al., 2014). Studies in rats showed a decrease in T4 and T3 after a single PM exposure event. However, the atmospheric contribution of these compounds on humans has yet to be investigated.
Recently, we described a significant association between placental iodine load and FT4 levels in maternal and cord blood from the same cohort (Neven et al., 2020a). So, we investigated the placental iodine concentrations as a mediator of PM2.5 on the cord blood FT4 concentrations. The estimated mediated proportion was 26% (on average −0.33 pmol/L per 5 µg/m3 increment in PM2.5), albeit borderline significant. We have to note though that, given the observational study design, we are unable to verify causal conclusions between each pair of factors in the mediation analysis. Still, we identified the third trimester as the significant gestational time window in which the PM2.5 exposure is inversely associated with placental iodine concentrations, and subsequently the cord blood FT4 levels. In line with previous observations (Ghassabian et al., 2019, Janssen et al., 2017b), our results provide evidence that air pollution adversely affects the placental iodine load, which in turn might lead to a lower thyroid hormone concentration, because of a positive association between iodine and FT4 (Neven et al., 2020a).
In contrast to the third trimester of pregnancy, week 16–22 was positively associated with ambient PM2.5 exposure. Although significant, we should note that the effect size in the second trimester is smaller than in the third, and was borderline significant. These findings are similar to those observed by Howe and colleagues who observed a positive association in the second trimester of pregnancy between PM2.5 and neonatal T4 concentrations (Howe et al., 2018). The authors indicated that the positive association could be attributed to the increased production of maternal hormones, which are transported to the foetus. Nevertheless, it should be noted that higher levels of FT4 throughout pregnancy could lead to a lower birth weight (Derakhshan et al., 2020), which in turn can have a wide range of adverse health outcomes (Belbasis et al., 2016). As air pollution can induce oxidative and nitrosative stress (Gangwar et al., 2020, Saenen et al., 2016b) during gestation, antioxidants are required to cope with this additional stress. Iodine has been found to have potential antioxidant properties (Kupper et al., 2008, Soriguer et al., 2011, Winkler et al., 2000). We may thus propose that iodine could be conserved during the second trimester of pregnancy because of two reasons: neurodevelopmental iodine requirements (i.e. neuronal migration and differentiation) and antioxidant requirements due to air pollution exposure. Therefore, the iodine storage needs to be replenished and iodine retention is required and facilitated by iodotyrosine deiodinases (Zicker and Schoenherr 2012) and the sodium/iodide symporter.
Strengths and limitations – Our study has several strengths. First of all, we can generalize our findings to the Belgian population, because our study population is representative of the general population in the gestational segment of life (Supplemental Table S1) (Cox et al., 2013). Second, the use of placental iodine concentrations as a marker of long-term gestational iodine accumulation is more reliable and accurate compared to urinary iodine concentrations, which is a short-term marker of iodine intake (Neven et al., 2020b). Third, the utilization of a high-resolution exposure model allowed us to integrate the daily exposure concentrations of air pollutants on the home address of the mothers into weekly mean exposure estimates during the entire pregnancy. Furthermore, the air pollutant concentrations in our study are similar to most of the 22 cohorts in the ESCAPE project (Beelen et al., 2014) and were validated with a biomarker in urine (Saenen et al., 2017) and placenta (Bové et al., 2019).
Despite these strengths, we acknowledge some potential limitations of this study. Firstly, the air pollution levels were modelled solely on the maternal residential address. Therefore, we recognize the possibility of exposure misclassification because we were unable to account for other sources that would contribute to personal exposure which can occur during commuting, at work, and elsewhere. Nevertheless, Saenen and colleagues showed that modelled estimates of residential ambient PM2.5 and black carbon are linked with internal black carbon load (Saenen et al., 2017). It is thus plausible that our models reflect well the individual ambient exposures. Secondly, the placental iodine concentrations could only be determined after delivery and therefore a time-dependent iodine dynamic during gestation could not be established. Thirdly, we did not include any statistical corrections for dietary habits as we only had such data for 97 individuals (Neven et al., 2020a). Although nutrition is the main source of iodine, nutrition is unlikely to be a confounder as it is reasonable to assume that prenatal exposure to air pollution does not relate to nutritional variability. Moreover, we reported a series of determinants of placental iodine previously (Neven et al., 2020a). We postulated that socio-economic status in our statistical model could be a better proxy of a possible confounding structure between the nutritional iodine intake and air pollution exposure (Mtumwa et al., 2017).
5. Conclusion
The current study provides insights in the alteration of placental iodine load in association with prenatal exposure to PM2.5 concentrations below the European Union air pollution guidelines. Our findings are in line with previous reports on the impact of air pollution on thyroid hormones, and suggest a possible mechanism wherein placental iodine could be a mediator of the effects observed on the neonatal thyroid hormone levels. Nevertheless, further experimental research is warranted to investigate functional alterations of the thyroid pathway by particulate matter air pollution.
CRediT authorship contribution statement
Kristof Y. Neven: Conceptualization, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization. Congrong Wang: Methodology, Software, Formal analysis. Bram G. Janssen: Methodology, Resources, Data curation, Writing - review & editing. Harry A. Roels: Writing - review & editing. Charlotte Vanpoucke: Methodology, Software, Resources. Ann Ruttens: Conceptualization, Methodology, Validation, Investigation, Writing - review & editing, Supervision, Project administration. Tim S. Nawrot: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The ENVIRONAGE birth cohort is supported by grants from the European Research Council (ERC-2012-StG 310898) and Flemish Research Council (FWO G073315N). Bram G. Janssen is a postdoctoral fellow of the FWO (FWO 12W3218N). We thank R. Machiels for his skillful technical assistance in relation to the iodine determinations in the Sciensano lab.
Handling Editor: Shoji Nakayama
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106334.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Andersson M., Karumbunathan V., Zimmermann M.B. Global iodine status in 2011 and trends over the past decade. J. Nutr. 2012;142:744–750. doi: 10.3945/jn.111.149393. [DOI] [PubMed] [Google Scholar]
- Arinola G.O., Dutta A., Oluwole O., Olopade C.O. Household air pollution, levels of micronutrients and heavy metals in cord and maternal blood, and pregnancy outcomes. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15122891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bath S.C., Steer C.D., Golding J., Emmett P., Rayman M.P. Effect of inadequate iodine status in uk pregnant women on cognitive outcomes in their children: Results from the avon longitudinal study of parents and children (alspac) Lancet. 2013;382:331–337. doi: 10.1016/S0140-6736(13)60436-5. [DOI] [PubMed] [Google Scholar]
- Beelen R., Raaschou-Nielsen O., Stafoggia M., Andersen Z.J., Weinmayr G., Hoffmann B. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 european cohorts within the multicentre escape project. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Belbasis L., Savvidou M.D., Kanu C., Evangelou E., Tzoulaki I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14:147. doi: 10.1186/s12916-016-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bové H., Bongaerts E., Slenders E., Bijnens E.M., Saenen N.D., Gyselaers W. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019;10:3866. doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R., Azizi F., Hedayati M., Mirmiran P., O'herlihy C., Smyth P.P. Is placental iodine content related to dietary iodine intake? Clin. Endocrinol. (Oxf.) 2011;75:261–264. doi: 10.1111/j.1365-2265.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- Burrow G.N., Fisher D.A., Larsen P.R. Maternal and fetal thyroid function. N. Engl. J. Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Caldwell K.L., Maxwell C.B., Makhmudov A., Pino S., Braverman L.E., Jones R.L. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in nhanes 2000: Comparison with previous method. Clin. Chem. 2003;49:1019–1021. doi: 10.1373/49.6.1019. [DOI] [PubMed] [Google Scholar]
- Caldwell K.L., Makhmudov A., Ely E., Jones R.L., Wang R.Y. Iodine status of the u.S. Population, national health and nutrition examination survey, 2005–2006 and 2007–2008. Thyroid. 2011;21:419–427. doi: 10.1089/thy.2010.0077. [DOI] [PubMed] [Google Scholar]
- Calvo R.M., Jauniaux E., Gulbis B., Asuncion M., Gervy C., Contempre B. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J. Clin. Endocrinol. Metab. 2002;87:1768–1777. doi: 10.1210/jcem.87.4.8434. [DOI] [PubMed] [Google Scholar]
- Chen H., Kwong J.C., Copes R., Tu K., Villeneuve P.J., van Donkelaar A. Living near major roads and the incidence of dementia, parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017;389:718–726. doi: 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contempre B., Jauniaux E., Calvo R., Jurkovic D., Campbell S., de Escobar G.M. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 1993;77:1719–1722. doi: 10.1210/jcem.77.6.8263162. [DOI] [PubMed] [Google Scholar]
- Cox B., Martens E., Nemery B., Vangronsveld J., Nawrot T.S. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: Analysis of routinely collected birth data. BMJ. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P., Parker J., Bell M.L., Bonzini M., Brauer M., Darrow L.A. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ. Health Perspect. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groef B., Decallonne B.R., Van der Geyten S., Darras V.M., Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: Potential thyroid-related health effects. Eur. J. Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Derakhshan A., Peeters R.P., Taylor P.N., Bliddal S., Carty D.M., Meems M. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2020;8:501–510. doi: 10.1016/S2213-8587(20)30061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECA, 2018. Air pollution: Our health still insufficiently protected. European Court of Auditors.
- European Parliament, 2015. Directive 2008/50/ec of the european parliament and of the council of 21 may 2008 on ambient air quality and cleaner air for europe. Document 02008L0050-20150918.European Parliament.
- Gangwar R.S., Bevan G.H., Palanivel R., Das L., Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: recent insights. Redox Biol. 2020;34:101545. doi: 10.1016/j.redox.2020.101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in r: The package dlnm. J. Stat. Softw. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2014;33:881–899. doi: 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Neurology Collaborators 2019 Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2016;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A., Pierotti L., Basterrechea M., Chatzi L., Estarlich M., Fernandez-Somoano A. Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open. 2019;2:e1912902. doi: 10.1001/jamanetworkopen.2019.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow J.E., Palomaki G.E., Allan W.C., Williams J.R., Knight G.J., Gagnon J. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Howe C.G., Eckel S.P., Habre R., Girguis M.S., Gao L., Lurmann F.W. Association of prenatal exposure to ambient and traffic-related air pollution with newborn thyroid function: findings from the children's health study. JAMA Netw Open. 2018;1:e182172. doi: 10.1001/jamanetworkopen.2018.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B.G., Madhloum N., Gyselaers W., Bijnens E., Clemente D.B., Cox B. Cohort profile: the environmental influence on early ageing (environage): a birth cohort study. Int. J. Epidemiol. 2017;46:1386–1387m. doi: 10.1093/ije/dyw269. [DOI] [PubMed] [Google Scholar]
- Janssen B.G., Saenen N.D., Roels H.A., Madhloum N., Gyselaers W., Lefebvre W. Fetal thyroid function, birth weight, and in utero exposure to fine particle air pollution: a birth cohort study. Environ. Health Perspect. 2017;125:699–705. doi: 10.1289/EHP508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S., Dumont G., Fierens F., Mensink C. Spatial interpolation of air pollution measurements using corine land cover data. Atmos. Environ. 2008;42:4884–4903. [Google Scholar]
- Kicinski M., Vermeir G., Van Larebeke N., Den Hond E., Schoeters G., Bruckers L. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ. Int. 2015;75:136–143. doi: 10.1016/j.envint.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Kupper F.C., Carpenter L.J., McFiggans G.B., Palmer C.J., Waite T.J., Boneberg E.M. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6954–6958. doi: 10.1073/pnas.0709959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre W., Vercauteren J., Schrooten L., Janssen S., Degraeuwe B., Maenhaut W. Validation of the mimosa-aurora-ifdm model chain for policy support: modeling concentrations of elemental carbon in flanders. Atmos. Environ. 2011;45:6705–6713. [Google Scholar]
- Lefebvre W., Degraeuwe B., Beckx C., Vanhulsel M., Kochan B., Bellemans T. Presentation and evaluation of an integrated model chain to respond to traffic- and health-related policy questions. Environ. Modell. Softw. 2013;40:160–170. [Google Scholar]
- Leung A.M., Pearce E.N., Braverman L.E. Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. North Am. 2011;40:765–777. doi: 10.1016/j.ecl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Yang Y., Qian Z., Ruan Z., Chang J., Vaughn M.G. Ambient pm2.5 and birth outcomes: Estimating the association and attributable risk using a birth cohort study in nine chinese cities. Environ. Int. 2019;126:329–335. doi: 10.1016/j.envint.2019.02.017. [DOI] [PubMed] [Google Scholar]
- MacKinnon D.P., Fairchild A.J., Fritz M.S. Mediation analysis. Annu. Rev. Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhloum N., Nawrot T.S., Gyselaers W., Roels H.A., Bijnens E., Vanpoucke C. Neonatal blood pressure in association with prenatal air pollution exposure, traffic, and land use indicators: an environage birth cohort study. Environ. Int. 2019;130:104853. doi: 10.1016/j.envint.2019.05.047. [DOI] [PubMed] [Google Scholar]
- Maiheu, B., Veldeman, N., Viaene, P., De Ridder, K., Lauwaet, D., Smeets, N., et al., 2013. Identifying the best available large-scale concentration maps for air quality in belgium. (Study Commissioned by the Flemish Environment, MIRA [in Dutch] Flemish Institute for Technological Research (VITO)). Mol, Belgium:VITO.
- Miller C.N., Rayalam S. The role of micronutrients in the response to ambient air pollutants: potential mechanisms and suggestions for research design. J. Toxicol. Environ. Health Part B. 2017;20:38–53. doi: 10.1080/10937404.2016.1261746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtumwa A.H., Ntwenya J.E., Paul E., Huang M., Vuai S. Socio-economic and spatial correlates of subclinical iodine deficiency among pregnant women age 15–49 years in tanzania. BMC Nutr. 2017;3:47. doi: 10.1186/s40795-017-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven K.Y., Cox B., Vrijens K., Plusquin M., Roels H.A., Ruttens A. Determinants of placental iodine concentrations in a mild-to-moderate iodine-deficient population: an environage cohort study. J. Transl. Med. 2020;18:426. doi: 10.1186/s12967-020-02601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven K.Y., Marien C.B.D., Janssen B.G., Roels H.A., Waegeneers N., Nawrot T.S. Variability of iodine concentrations in the human placenta. Sci. Rep. 2020;10:161. doi: 10.1038/s41598-019-56775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpinar A., Kelestimur F., Songur Y., Can O., Valentin L., Caldwell K. Iodine status in turkish populations and exposure to iodide uptake inhibitors. PLoS ONE. 2014;9:e88206. doi: 10.1371/journal.pone.0088206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M., Giorgis-Allemand L., Bernard C., Aguilera I., Andersen A.M., Ballester F. Ambient air pollution and low birthweight: a european cohort study (escape) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- Perrine C.G., Herrick K., Serdula M.K., Sullivan K.M. Some subgroups of reproductive age women in the united states may be at risk for iodine deficiency. J. Nutr. 2010;140:1489–1494. doi: 10.3945/jn.109.120147. [DOI] [PubMed] [Google Scholar]
- Public Health Committee of the American Thyroid Association, Becker D.V., Braverman L.E., Delange F., Dunn J.T., Franklyn J.A. Iodine supplementation for pregnancy and lactation-united states and Canada: recommendations of the american thyroid association. Thyroid. 2006;16:949–951. doi: 10.1089/thy.2006.16.949. [DOI] [PubMed] [Google Scholar]
- Saenen N.D., Provost E.B., Viaene M.K., Vanpoucke C., Lefebvre W., Vrijens K. Recent versus chronic exposure to particulate matter air pollution in association with neurobehavioral performance in a panel study of primary schoolchildren. Environ. Int. 2016;95:112–119. doi: 10.1016/j.envint.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Saenen N.D., Vrijens K., Janssen B.G., Madhloum N., Peusens M., Gyselaers W. Placental nitrosative stress and exposure to ambient air pollution during gestation: a population study. Am. J. Epidemiol. 2016;184:442–449. doi: 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- Saenen N.D., Bove H., Steuwe C., Roeffaers M.B.J., Provost E.B., Lefebvre W. Children's urinary environmental carbon load. A novel marker reflecting residential ambient air pollution exposure? Am. J. Respir. Crit. Care Med. 2017;196:873–881. doi: 10.1164/rccm.201704-0797OC. [DOI] [PubMed] [Google Scholar]
- Smith R.B., Fecht D., Gulliver J., Beevers S.D., Dajnak D., Blangiardo M. Impact of london's road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ. 2017;359:j5299. doi: 10.1136/bmj.j5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriguer F., Gutierrez-Repiso C., Rubio-Martin E., Linares F., Cardona I., Lopez-Ojeda J. Iodine intakes of 100–300 mug/d do not modify thyroid function and have modest anti-inflammatory effects. Br. J. Nutr. 2011;105:1783–1790. doi: 10.1017/S0007114510005568. [DOI] [PubMed] [Google Scholar]
- Sunyer J., Esnaola M., Alvarez-Pedrerol M., Forns J., Rivas I., Lopez-Vicente M. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 2015;12:e1001792. doi: 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with sas and spss macros. Psychol. Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevijvere S., Amsalkhir S., Mourri A.B., Van Oyen H., Moreno-Reyes R. Iodine deficiency among belgian pregnant women not fully corrected by iodine-containing multivitamins: a national cross-sectional survey. Br. J. Nutr. 2013;109:2276–2284. doi: 10.1017/S0007114512004473. [DOI] [PubMed] [Google Scholar]
- WHO - UNICEF – ICCIDD, 2007. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. Switzerland:World Health Organization.
- Winkler R., Griebenow S., Wonisch W. Effect of iodide on total antioxidant status of human serum. Cell Biochem. Funct. 2000;18:143–146. doi: 10.1002/(SICI)1099-0844(200006)18:2<143::AID-CBF857>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lin Q., Liang Y., Ruan Z., Acharya B.K., Zhang S. Maternal air pollution exposure associated with risk of congenital heart defect in pre-pregnancy overweighted women. Sci. Total Environ. 2020;712:136470. doi: 10.1016/j.scitotenv.2019.136470. [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen X., Zhang X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. U.S.A. 2018;115:9193–9197. doi: 10.1073/pnas.1809474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicker S., Schoenherr B. Focus on nutrition: the role of iodine in nutrition and metabolism. Compend. Contin. Educ. Vet. 2012;34:E1–E4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.