Abstract
In 1910, the Russian biologist Konstantin Sergejewitch Mereschkowsky (Константин Сергеевич Мережковский, in standard transliterations also written as Konstantin Sergeevič Merežkovskij and Konstantin Sergeevich Merezhkovsky) published a notable synthesis of observations and inferences concerning the origin of life and the origin of nucleated cells. His theory was based on physiology and leaned heavily upon the premise that thermophilic autotrophs were ancient. The ancestors of plants and animals were inferred as ancestrally mesophilic anucleate heterotrophs (Monera) that became complex and diverse through endosymbiosis. He placed a phylogenetic root in the tree of life among anaerobic autotrophic bacteria that lack chlorophyll. His higher level classification of all microbes and macrobes in the living world was based upon the presence or absence of past endosymbiotic events. The paper's primary aim was to demonstrate that all life forms descend from two fundamentally distinct organismal lineages, called mykoplasma and amoeboplasma, whose very nature was so different that, in his view, they could only have arisen independently of one another and at different times during Earth history. The mykoplasma arose at a time when the young Earth was still hot, it later gave rise to cyanobacteria, which in turn gave rise to plastids. The product of the second origin of life, the amoeboplasma, arose after the Earth had cooled and autotrophs had generated substrates for heterotrophic growth. Lineage diversification of that second plasma brought forth, via serial endosymbioses, animals (one symbiosis) and then plants (two symbioses, the second being the plastid). The paper was published in German, rendering it inaccessible to many interested scholars. Here we translate the 1910 paper in full and briefly provide some context.
Keywords: Endosymbiosis, Symbiogenesis, Symbioses, Mereschkowsky, Origin of eukaryotes, Origin of the nucleus
Background. The primary split among living things that Mereschkowsky (1910) suggested corresponds to an almost clean divide of what we now call prokaryotes (mykoplasma) from what we now call eukaryotes (amoeboplasma), names that would not enter the literature until 1925 and would not come into common use until the 1960s (Katscher, 2004). Because Mereschkowsky grouped the fungi together with the bacteria, he missed the prokaryote eukaryote dichotomy we now recognize. The fungi have always been problematic: “Fungorum ordo in opprobrium artis etiamnum Chaos est, nescientibus Botanicis in his, quid Species, quid Varietas sit.” (The order of the fungi is still a disgrace to the discipline [of classification], as botanists have yet to ascertain what is a species and what is a variety. Linnaeus, 1751).
The traits Mereschkowsky used for classification are physiological, emergent from a set of dichotomies that distinguish different kinds of cells with regard to:
-
•
Oxygen respiration: anaerobes ancient, aerobes derived;
-
•
Temperature: thermophiles ancient, mesophiles derived;
-
•
Nitrogen requirement: assimilation of inorganic N ancient, organic N derived;
-
•
Cytoplasmic movement: non-streaming cytoplasm ancient, streaming cytoplasm derived;
-
•
Chemical composition: high P ancient, low P derived; high N ancient, low N derived;
-
•
Tolerance of cytotoxins and harsh environments: extremophiles ancient, others derived;
-
•
CO2 assimilation: autotrophs ancient, heterotrophs derived; plus a few other traits (high Fe ancient, low Fe derived), cell wall (nitrogenous cell wall ancient, cellulose cell wall or no cell wall derived), ability to form true tissues (absence ancient, presence derived), and chromatin (presence ancient, absence derived).
The last criterion, absence of chromatin being derived, seems odd, but for Mereschkowsky the amoeboplasma was the “pure” cytosol of plant and animal cells, cytosol without organelles. Plant and animal cytosol lacks demonstrable chromatin. Chromatin in the nucleus was, in Mereschkowsky's view, the result of bacterial intruders, endosymbionts that “… assembled in the cell's center and finally surrounding themselves by a membrane, thereby formed the cell nucleus. The cell nucleus opened up completely new possibilities with regard to the further evolution of the Monera. Without this symbiosis the anuclear Monera would have been condemned for ever to remain the same lowly life form that they originally were.” (from the full translation in this paper). In that passage, it sounds like Mereschkowsky was suggesting that symbiosis was the key hurdle to eukaryotic complexity. Yes, that is exactly what he was saying.
Mereschkowsky had to invoke all manner of convergence to explain the origin of traits among the fungi that conflicted with their grouping with bacteria. We have flagged some of those passages in the text. For example, he saw respiration in fungi as analogous, not homologous, hence convergent to that in plants and animals. He interpreted the nucleus of fungi as convergent to that in plants and animals, not as the product of symbiosis, and the cytoplasmic streaming of fungi as analogous, not as homologous, to cytoplasmic streaming in plants. He attributed the diversity of form among plants and animals to the diversity of their enzymes, which in his view were synthesized by the nucleus because of the exceptional protein synthetic ability of the bacterial endosymbionts from which it stemmed. That concept, namely that increased protein synthesis in nucleated cells was a consequence of the first endosymbiotic event in eukaryote evolution, is now a widely recognized component of endosymbiotic theory, although it took 100 years to resurface (Lane and Martin, 2010). The first demonstrable endosymbiosis in eukaryote history involved mitochondria, organelles that Mereschkowsky ignored, not bacteria that congregate in the center of the cell to surround themselves by a membrane and thereby form a nucleus.
One wonders why Mereschkowsky did not adhere more closely to Occams's razor by placing fungi among the amoeboplasma so as to define eukaryotes in a modern sense and avoid complicated explanations involving convergence for fungal respiration and nuclei. The text provides clear clues as to why he grouped fungi and bacteria together as the mykoid kingdom. In the passages on tolerance to harsh conditions, he emphasizes the robustness of fungi towards extreme environments as a strong character linking them to bacteria. On chemical composition, the presence of N in the cell wall is also interpreted as a strong character linking fungi to bacteria. But one character in particular stands out in this regard, namely his reliance upon a small number of papers that reported the growth of fungi in the presence of N2 as the sole nitrogen source. He viewed the ability to fix N2 as extremely ancient, just as the ability to fix CO2 was ancient in his view, and the first organisms he inferred (micrococci) were able to do both without the help of chlorophyll. Today we would call that chemolithoautotrophic origins as it relates to the origin of life (Preiner et al., 2020), an idea that was well ahead of its time. As it pertained to his classification scheme, he had the right interpretation (N2 fixation is ancient), but the observation was erroneous (diazotrophic fungal growth), leading him to place fungi within the mykoid kingdom, closer to Clostridia than to animals. By weighting the tendency of fungi to tolerate extreme conditions and their ability to assimilate inorganic nitrogen sources (erroneously including N2) more heavily than respiration or the presence of a nucleus, he put them on the wrong side of what became the prokaryote eukaryote divide. Mereschkowsky failed to incorporate anaerobic eukaryotes into his scheme, although he was aware of them, mentioning the anaerobic ciliates, including Nyctotherus (Boxma et al., 2005), which possesses hydrogenosomes (Müller, 1993) in footnote 5. Because of fungi, he ultimately named the entire realm of bacteria as mykoids (Greek mukēs, fungi) derived from mykoplasma, rather than bacteria derived from bacterioplasma.
Using physiological traits, the 1910 paper fleshes out the foundation for his initial exposé (Mereschkowsky, 1905) of what we today call endosymbiotic theory, or symbiogenesis, to use the original term. In the 1905 paper he made a very strong case for the endosymbiotic origin of plastids. In the 1910 paper he (rightly) considered the plastid pillar of the theory to be so obviously correct that it needed neither further evidence nor argumentation. As such, plastids themselves play only a minor role in the 1910 paper.
In order to better understand the title and the main message of the paper — “zwei Plasmaarten” — which translates literally to “two species of plasma”, we have to consider the mindset of biologists in 1910. Physics already had the Planck constant and relativity, chemists were already celebrating decades of colorful diazo dyes and the first plastics (Bakelite), while biologists did not have much more than the educated guess as to what was going on in cells. For example, Mereschkowsky cited work by Pflüger in which it was suggested, in some detail, that the CO2 exhaled during respiration did not derive from ingested food but instead was emitted from the chemical backbone of proteins through a myriad of tiny high temperature explosions. Otto Warburg's work had not yet transformed the field, his first papers appearing in 1905 (Krebs, 1972; Höxtermann, 2007). It would be 1929 before Lohmann discovered ATP (Langen and Hucho, 2008). Given that biologists had no energetic or chemical basis to understand what cells are or how they work, what did Mereschkowsky mean with the term “plasma”? He meant protoplasm.
The concept of protoplasm, Protoplasma in the abundant German literature of the 1800s, was omnipresent in the biological sciences in 1910 and roughly as mainstream as it gets. It was still in wide use up until about 1960. Protoplasm is a concept with its own interesting history (Liu, 2017), the term tracing to the Czech and German physiologists Jan E. Purkinje and Hugo von Mohl. It became linked with various concepts, inter alia that a special life energy, vital force, or vis vitalis is associated with living substance. Strong proponents of that view were called vitalists, their opponents mechanists (Geison, 1969). In the absence of a chemical understanding of the life process within cells, protoplasm represented a special kind or organization of matter that bestows the property of life and distinguishes living from non-living things. Literally it is the first plasm (protos, Greek first) and represented a continuous lineage via cell cytoplasm that is the thread of continuity in life across countless generations from origins to the present, and that irreversibly dissolves at death. In his book The Protoplasmic Theory of Life, Drysdale (1874, p. 5) described protoplasm like this “… the elements are in a state of combination not to be called chemical at all in the ordinary sense, but one which is utterly sui generis. That, in fact, no albumin, fibrin, myosin, protagon, or fats exist at all in the living matter, but that the sum of the elements of all these is united into a compound, for which we have no chemical name, and the complex mode in which the atoms are combined we can form no idea; and it is only at the moment of death that those chemical compounds, with which we are familiar, take their origin. […] Vitality is thus a property inherent in each particle of the living matter, and all the parts of a complex organism differ in function, each part has a specific kind of vitality peculiar to itself.” Such was the nature of protoplasm.
Among other things, the concept of protoplasm conveniently displaced the burden of understanding how the life process inside the cell actually works into the inaccessible realm of understanding how life arose at its origins. The papers in volume 30 of Biologisches Centralblatt in which Mereschkowsky's paper appeared were replete with the term protoplasm. Mereschkowsky used it dozens of times, and we can be certain that different authors meant different things when they used it. As the origin of life was seen in 1910 as a singular event in the primordial phases of Earth's history, the origin of protoplasm and the origin of life were, to many biologists of the time, the same thing. It was not until about 1920 when biological chemists started getting a handle on enzymes that convert small molecular weight compounds during metabolism, such that the notion of protoplasm having special properties fell quietly out of favor.
Mereschkowsky, however, was convinced that he had identified two kinds of (proto)plasm that were so different in nature that they only could have arisen independently from one another, as opposed to one being derived from the other via direct filiation. The consequence of that, in his view, could only mean one thing: life arose twice. He had already mentioned this in the closing passages of his 1905 paper. In the 1910 paper we are given the underlying observations plus the fuller reasoning that led him to that conclusion. According to Mereschkowsky, the first kind of (proto)plasm to arise was robust in nature, corresponded to autotrophic bacteria that had not yet evolved chlorophyll, and appeared shortly after Earth's formation at a time while the Earth was still hot (prokaryotes). The second kind (Art) of (proto)plasm arose later, after autotrophs had generated organic substrates to support their heterotrophic lifestyle and was more fragile, less thermophilic and less able to tolerate extremes in its overall nature (eukaryotes). In the final pages of the paper, Mereschkowsky makes that case explicitly, using comparative cytology and physiology in a rationally staged early Earth history context.
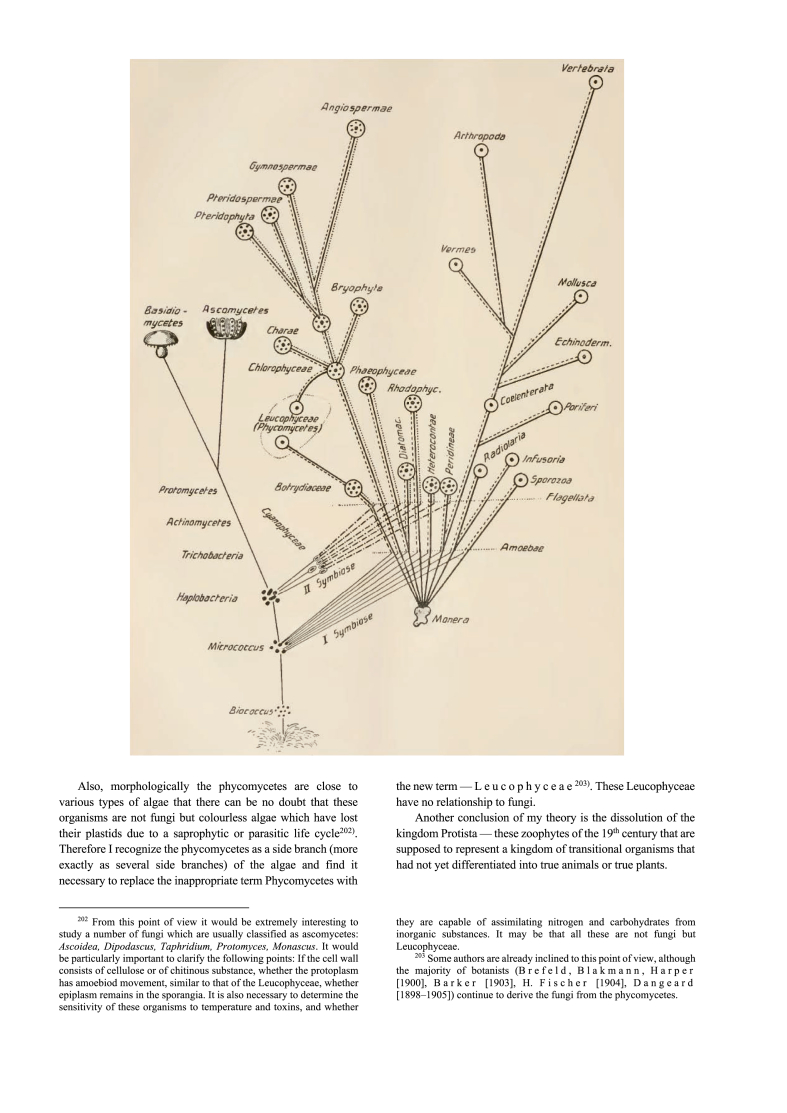
In that sense, there is a case to be made for translating the 1910 title as “two origins of life”, which is what he argues in the paper, but not what he wrote in the title. Rather the title focusses on two kinds of protoplasm whose differences explained the deepest and most fundamental spilt in the living world, notwithstanding a few corollary convergences among fungi. The two kinds of plasma furthermore retained their ancestral properties even in the wake of ancient symbiotic associations within the same cell. Plastids for example, as the seat of autotrophy in plants, remained recognizable as descendants of cyanobacteria (mykoplasma) living in an amoeboplasma cell. Thus, Mereschkowsky was thinking in terms of two independently arisen plasma lineages that united to form complex cells. Moreover, the unification of those lineages with persistence of their properties, together with occasional endosymbiont loss, form the basis of life's highest level classification. Any questions as to whether Mereschkowsky was thinking in terms of lineages and lineage diversification are answered by the lone figure at the end of the paper.
For those reasons, we translate Art (kind, type, species) in the title “Theorie der zwei Plasmaarten …” as lineage, “The theory of two plasma lineages …”, because it was not just the fundamental differentness of the plasmas but also a distinctive immiscibility of their properties that persisted despite ancient symbiotic associations, one in the animal lineage and two in the plant lineage. That persistence allowed the endosymbionts and their host to be recognized as independent lineages (organellar and cytosolic) even within modern plant and animal cells, as his figure unmistakably depicts. Mereschkowsky could have easily entitled his paper “zwei Protoplasmaarten” and it would have been synonymous with the title he selected.
For today's microbiologists, the excitement that extremophiles have always harbored as providing windows into ancient life and origins will seem very familiar in Mereschkowsky’s 1910 paper. We have not hyped up any passages, taking every effort to convey the emphasis and level of conviction of the author, also in those passages where he was clearly getting it all wrong. We have, however, cut some of the very long and complicated sentences into two, sometimes three, shorter and simpler sentences.
Many terms in the paper such as Monera, mykoids, amoeboids, infusoria, protoplasm, sarcode and others are no longer in use today. Instead, we are familiar with the terms describing a cell as being either prokaryotic or eukaryotic and cytoplasm an aqueous protein solution (cytoplasm is about 400 mg/ml protein), a product of gene expression, not a kind of matter comprised of molecules that are themselves endowed with special innate properties lacking in other organic material. Some terms that he used have changed meaning over the years, for example Zellmembran (cell membrane) was used to designate the bacterial cell wall up until the 1950s, Mereschkowsky used Zellmembran to designate cell walls in bacteria in some cases. Infusoria could mean several things from ciliates to diverse pond water protists and it is not always clear which meaning he intended, hence we just stuck with infusoria.
Several of Mereschkowsky's ideas were afloat in various manifestations at his time. The concept of the Monera, uptake of organisms from different phyla, incorporation into the host cell, endosymbionts living in subordination to the cytoplasm while being transformed into new organs of the new organism of higher rank, had been mentioned occasionally in the American, German and Russian literature around the turn of the 19th to the 20th century. Famintzyn (1907), for example, was an early proponent for symbiosis as a mechanism generating new forms, in particular lichens. But for perspective, Famintzyn (1907) wrote “The equivalence  of plastids and cyanobacteria is pulled out of thin air, as is the claim by the author (p. 601): ‘that plastids are cyanobacteria that invaded the cytoplasm.’” Famintzyn criticized both Mereschkowsky for not knowing the literature and August Weismann for his “strange”
of plastids and cyanobacteria is pulled out of thin air, as is the claim by the author (p. 601): ‘that plastids are cyanobacteria that invaded the cytoplasm.’” Famintzyn criticized both Mereschkowsky for not knowing the literature and August Weismann for his “strange”  theory of evolution involving a germline.
theory of evolution involving a germline.
Mereschkowsky's intuition allowed him to incorporate a fairly vast spectrum of observations into a new theory, the theory of two (proto)plasma lineages. Remarkably, he interpreted all plant and animal cells as still harboring both kinds of plasma in a form that had not undergone hybridization or homogenization of their properties. That reflects the strength of his conviction that the main physiological properties that separate plants from animals reside within plastids, which he saw as irrefutably descended from cyanobacterial endosymbionts. The scientific historical context in which Mereschkowsky found himself, as well as accounts of his troubled personal life are given in Höxtermann (1998), Sapp et al. (2002) and in chapters of the volume by Geus and Höxtermann (2007). The 1910 paper was published during his time of employment at the University of Kazan 1902–1914. Mereschkowsky had politically influential adversaries who drove him out of Kazan in 1914 (Höxtermann, 1998).
The concept of secondary and tertiary endosymbioses with eukaryotic algae as endosymbionts was unknown to Mereschkowsky and only proposed much later by Sarah Gibbs (1978), well after electron microscopy had revealed the number of membranes surrounding the plastids in different groups. Based mainly on pigmentation, Mereschkowsky thought that the plastids of red algae, green algae, brown algae, diatoms etc., resulted from seven independent symbioses involving different cyanobacterial progenitors. This idea of polyphyletic plastid origins was discussed well into the 1980s, yet the evolutionary hurdle of inventing a protein import machinery favored a single origin (discussed in Cavalier-Smith, 1982). Plastid genomes resolved the issue though, as they left no doubt that the DNA in different plastid lineages was descended from a single successful primary event involving one cyanobacterium as endosymbiont taken up by a heterotroph (Kowallik, 1989). From that symbiosis, the primary plastids of glaucocystophytes, red algae, and green algae emerged, the latter two subsequently giving rise to secondary symbioses among the green and red lineages (Kowallik, 1993). Though he missed secondary symbiosis, Mereschkowsky did point out that lichens are the result of a threefold (dreifache) symbiosis.
Endosymbiosis in evolution is, however, a Pandora's box, because once one has accepted the principle that symbiotic associations can give rise to novel organelles (mitochondria and plastids), and taxa at the highest ranks (eukaryotes and algae), what constraints tell us where to stop invoking additional symbiotic events to explain various aspects of cells? That problem has always plagued endosymbiotic theory since its inception. The creative nature of line drawings to represent lipid bilayers was an advance of 1960s electron microscopy. It formed the basis of Lynn Margulis' proposition that eukaryotic flagella arose from symbiotic spirochaetes (Margulis et al., 2006). Line drawings have also been used to suggest a symbiotic origin of peroxisomes and even the endoplasmic reticulum (discussed in Martin, 1999). Line drawings also underlie modern incarnations of Mereschkowsky’s 1910 proposal that the nucleus arose from an endosymbiotic intruder within an anucleate host (López-García and Moreira, 2020). In a modern context, that theory (López-García and Moreira, 2020) predicts that the cytosolic ribosomes of eukaryotes should be of bacterial rather than of archaeal origin. But the observations soundly reject that idea. There are no bacterial ribosomes in the eukaryotic cytosol that would betray a spirochaete origin of flagella, and there are no bacterial ribosomes in the eukaryotic cytosol that would betray a δ-proteobacterial host for an archaeal nucleus. The only bacterial ribosomes in eukaryotes are in mitochondria and plastids, those in the eukaryotic cytosol are archaeal, indicating that the host for mitochondria was an archaeon (Martin et al., 2015; Imachi et al., 2020).
In the course of publishing this paper, two readers asked “What about Lynn Margulis and the origin of mitochondria?” We and others have explained in earlier writings that the priority for the symbiotic origin of mitochondria does not go to Margulis (Sagan 1967), nor does it go to Altmann (1890), whose bioblasts were not mitochondria despite many claims to the contrary. Priority might go to Portier (1918) in French, but in our view should probably go to the American cell biologist Ivan Wallin, who had the basic idea so right that he even predicted gene transfer from organelles to the nucleus (Wallin, 1925, 1927). Margulis (Sagan) wrote on the second page of her 1967 paper “… these ideas are not new …”, mentioning Mereschkowsky and Wallin but not saying a word about what they had written on symbiogenesis.
Margulis learned about endosymbiotic theory at the University of Wisconsin in her undergraduate genetics class held by Hans Ris, who wrote in 1962: “With the demonstration of “nucleoplasm” in chloroplasts, the similarity in ultrastructural organization of a chloroplast and a blue-green algal cell becomes indeed striking. Both are enveloped in a double membrane. Both contain the photosynthetic apparatus in membrane systems of similar organization […]. Both contain particles which look like ribosomes in the electron microscope. Whether they are in fact ribosomes remains to be established by isolation and biochemical analysis. Both contain DNA in the form of a nucleoplasm; i.e., areas of low density which contain fibrils about 25 Å thick. We suggest that this similarity in organization is not fortuitous but shows some historical relationship and lends support to the old hypothesis of Famintzyn (1907) and Mereschkowsky (1905) that chloroplasts originate from endosymbiotic blue-green algae” (Ris and Plaut, 1962, p. 388). How do we know that she heard about endosymbiosis in that class? We know that because Jonathan Gressel (pers. comm. to WM) at the Weizmann Institute, sat next to Margulis in Hans Ris’ genetics class and told us about it. Margulis popularized endosymbiotic theory but did not rediscover it, she was taught it.
In the old days, biologists were taught Occam's razor, that explanations of unknowns are first to be sought in the terms of known quantities. More so than any other evolutionary mechanism, endosymbiotic theory requires restraint. It should only be used in explanatory emergencies, as a last resort when all other evolutionary mechanisms fail, as in the origin of mitochondria and photosynthetic eukaryotes. Endosymbiotic theory also works best when founded in physiology, rather than in line drawings that purport to represent the evolution of thin sections as viewed through the electron microscope. If one asks: What membrane systems in cells might we explain as the result of endosymbioses, many possibilities come to mind: The nucleus? Flagella? Peroxisomes? The ER? The problem with endosymbiosis is that it is so interesting as an evolutionary mechanism that it opens the floodgates to overuse — for each eukaryotic membrane we see, we can just add one more endosymbiosis. But where to stop? When is enough? If we stick to the physiological foundations of endosymbiosis and ask "What physiological or thermodynamic conditions favor symbiotic associations" (Imachi et al., 2020) we obtain welcome constraints on the number of cellular partners that a symbiosis can support. Endosymbiosis should only be invoked when standard evolutionary mechanisms fall short.
What is so special about endosymbiosis? Endosymbiosis creates a unique physical relationship between cells, one within the other, that alters the fate of genes and membrane vesicles that are naturally released by the endosymbiont. The release of genes to the host is the source of the lineage transforming power of symbiosis that generates new taxa at the highest level via cell combination during evolution. Yet symbioses involving prokaryotes (the origin of mitochondria and plastids) are extremely rare, having occurred only once each in the last four billion years, that is, at the same rate as the origin of life. The origin of eukaryote complexity, which is founded in the eukaryote endomembrane system, occurred at the same rate as the origin of mitochondria (Lane and Martin, 2010).
There are two views concerning the origin of the eukaryotic endomembrane system. In the traditional view, the endomembrane system stems from invaginations of the plasma membrane before the origin of mitochondria. A newer, alternative view has it that the release of outer membrane vesicles from the mitochondrial symbiont to the host precipitated the origin of the endomembrane system from which the nucleus is derived during the cell cycle as well as the origin of bacterial lipids in eukaryotes (discussed in Gould et al., 2016). It is not pure coincidence that the only organelles of eukaryotic cells that we know with certainty to have arisen via endosymbiosis, mitochondria and the plastid family, are bioenergetic organelles. The nucleus, by contrast, is derived from the endoplasmic reticulum (ER), it is not a bioenergetic compartment. The ER is, in turn, derived from vesicles of bacterial-type lipids that stem inter alia from the mitochondrion (McBride, 2018). The ER is eukaryote specific because eukaryotes have mitochondria. Some prokaryotes have bacterial endosymbionts, but they do not have bioenergetic organelles (Lane and Martin, 2010). That is the most sensible reason why prokaryotes remained simple while eukaryotes became complex.
Mereschkowsky did not classify the Saprolegniales as members of the fungal kingdom. Instead, he identified these siphonaceous filaments as plants that have lost their plastids and classified the Oomycota among heterokont algae to which they exhibit the closest affinities. This statement poses the question, however, as to whether diatom plastids can be lost and, more generally, the degree to which symbiosis is a process of evolutionary addition and subtraction. Indeed, the origin of higher taxa via combinatorial processes is nowhere better summarized than the passage from the 1910 paper:
“From this we may set up the following equation:
Diatoms – Plastids = Animals
and from that
Animals + Plastids = Plants.”
The second equation is generally correct, the first is problematic as formulated with regard to the diatoms. Mereschkowsky confirmed observations from other scientists that diatoms may lose their photosynthetic pigments as soon as they are cultivated in media containing organic food. But he erroneously concluded that the diatoms lose their plastids altogether. It was only ultrathin sections using diamond knives and transmission electron microscopy that allowed Schnepf (1969) to demonstrate proplastids in permanently apochlorotic diatoms, first in Nitzschia alba. Those techniques also uncovered relict plastids among the malaria parasites, which are surrounded by four membranes and harbour a highly reduced plastid genome (McFadden et al., 1996).
For clarity, we have replaced the term Cyanophyceae with cyanobacteria and the term chromatophores with plastids. Our translators’ notes and comments are in  , all footnote numbers correspond to the original, as does the use of italics and any emphasis conveyed by increased character spacing. We have indicated the page breaks of the original in brackets, the many footnotes also generate correspondence to the German text. We have corrected minor typographical and bibliographical errors in the footnotes, but have made no corrections to the text. The original is inconsistent with regard to the spelling of mykoid and mykoplasm, sometimes written with c, sometimes with k; the original Greek term μύκης (fungus) is written with κ, which transcribes as k, the convention we have used here. We have rendered species names in lower case throughout, though many are capitalized in the original.
, all footnote numbers correspond to the original, as does the use of italics and any emphasis conveyed by increased character spacing. We have indicated the page breaks of the original in brackets, the many footnotes also generate correspondence to the German text. We have corrected minor typographical and bibliographical errors in the footnotes, but have made no corrections to the text. The original is inconsistent with regard to the spelling of mykoid and mykoplasm, sometimes written with c, sometimes with k; the original Greek term μύκης (fungus) is written with κ, which transcribes as k, the convention we have used here. We have rendered species names in lower case throughout, though many are capitalized in the original.
One aspect of Mereschkowsky's lone 1910 figure has gone unnoticed. He depicted the origin of some eukaryotes, namely animals and plants, as involving a physiologically argued serial endosymbiotic mechanism (symbiogenesis), with no gradual intermediates. In the very same figure, however, he depicted the origin of other eukaryotes, the fungi, through a series of stepwise transitions from the first bacteria via haplobacteria (simple forms), trichobacteria (filaments), actinobacteria (endspore forming filaments), and then protomycetes, a hypothetical missing link in a continuous evolutionary grade connecting actinobacteria to the true fungi — a perfectly traditional gradualist transition. That is, not only did he present both sides of a century old debate on symbiogenesis versus gradualism for the origin of eukaryotes (Martin, 2017) in the same paper, he summarized both the case for a symbiogenic origin of eukaryotes and the case for a gradualist origin of eukaryotes in the same figure. Ironic would be an understatement.
Today, the host for the first endosymbiosis in eukaryote evolution looks much more like the micrococci in Mereschkowsky's figure than the amoeboid Moneran. According to current data, the host was an archaeon (Imachi et al., 2020), not an amoeboid Moneran. It was a typical archaeon, small and lacking any trace of eukaryotic complexity (Lane, 2020). Though many evolutionary biologists still believe that there was a gradual transition from archaea to Monera like cells of the type Mereschkowsky drew on the left side of his 1910 figure leading to fungi, the data in 2020 has it that the archaeon that is most closely related to the host (Imachi et al., 2020) was a garden variety archaeon, making the prokaryote eukaryote transition steeper than ever before (Gould et al., 2016; Lane, 2020; Speijer, 2020). That brings us to the last words of Mereschkowsky’s 1910 paper, which appear in a footnote: “Either the symbiosis is present, and they are lichens, or the symbiosis is not present, and they are fungi; there are no transitional forms nor can they exist.” Such is the nature of symbiogenesis.
Declaration of competing interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We thank Johanna Meeh, Julia Brueckner, and Rebecca Gerhards for help with the formatting, tables and footnotes, and Josip Skejo for the Linnaeus quote. We extend our particular thanks to Dan Graur and Ekkehard Höxtermann for their very helpful comments. We thank the ERC (666053), the VW Foundation (93 046) and the Moore Simons initiative on the origin of the eukaryotic cell (9743) for funding. This paper is dedicated to Miklós Müller, biochemist and historian of science, on the occasion of his 90th birthday.
Contributor Information
Klaus V. Kowallik, Email: klaus.kowallik@hhu.de.
William F. Martin, Email: bill@hhu.de.
Appendix.
References
- Altmann R. Verlag von Veit & Comp.; Leipzig: 1890. Die Elementarorganismen und ihre Beziehungen zu den Zellen. [Google Scholar]
- Boxma B. An anaerobic mitochondrion that produces hydrogen. Nature. 2005;434:74–79. doi: 10.1038/nature03343. [DOI] [PubMed] [Google Scholar]
- Cavalier Smith T. The origins of plastids. Biol. J. Linn. Soc. 1982;17:289–306. [Google Scholar]
- Drysdale J. Baillière, Tindall and Cox; London: 1874. The Protoplasmic Theory of Life. [Google Scholar]
- Famintzyn A. Die Symbiose als Mittel zur Synthese der Organismen. Biol. Centralbl. 1907;27:353–364. [Google Scholar]
- Geison G.L. The protoplasmic theory of life and the vitalist-mechanist debate. Isis. 1969;60:273–292. doi: 10.1086/350498. [DOI] [PubMed] [Google Scholar]
- Geus A., Höxtermann A., editors. Evolution durch Kooperation und Integration. Basilisken-Presse; Marburg an der Lahn: 2007. p. 751. [Google Scholar]
- Gibbs S.P. The chloroplast of Euglena may have evolved from symbiotic green algae. Can. J. Bot. 1978;56:2883–2889. [Google Scholar]
- Gould S.B., Garg S.G., Martin W.F. Bacterial vesicle secretion and the evolutionary origin of the eukaryotic endomembrane system. Trends Microbiol. 2016;24:525–534. doi: 10.1016/j.tim.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Höxtermann E. Konstantin S. Merezkovskij und die Symbiogenesetheorie der Zellevolution. In: Geus A., editor. Bakterienlicht und Wurzelpilz. Basiliken-Presse, Marburg/Lahn; 1998. pp. 11–29. [Google Scholar]
- Höxtermann E. A comment on Warburg's early understanding of biocatalysis. Photosynth. Res. 2007;92:121–127. doi: 10.1007/s11120-007-9164-2. [DOI] [PubMed] [Google Scholar]
- Imachi H. Isolation of an archaeon at the prokaryote-eukaryote interface. Nature. 2020;577:519–525. doi: 10.1038/s41586-019-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katscher F. The history of the terms prokaryotes and eukaryotes. Protist. 2004;155:257–263. doi: 10.1078/143446104774199637. [DOI] [PubMed] [Google Scholar]
- Kowallik K.V. Molecular aspects and phylogenetic implications of plastid genomes in certain chromophytes. In: Green J.C., Leadbeater B.S.C., Diver W.L., editors. The Chromophyte Algae: Problems and Perspectives. Clarendon Press; Oxford: 1989. pp. 101–124. [Google Scholar]
- Kowallik K.V. From endosymbionts to chloroplasts: evidence for a single prokaryotic/eukaryotic endocytobiosis. Endocytobiosis Cell Res. 1993;10:137–149. [Google Scholar]
- Krebs H.A. Otto Heinrich Warburg. 1883–1970. Biogr. Mem. Fellows R. Soc. 1972;18:628–699. [PubMed] [Google Scholar]
- Lane N. How energy flow shapes cell evolution. Curr. Biol. 2020;30:R471–R476. doi: 10.1016/j.cub.2020.03.055. [DOI] [PubMed] [Google Scholar]
- Lane N., Martin W.F. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- Langen P., Hucho F. Karl Lohmann and the discovery of ATP. Angew. Chem. Int. Ed. 2008;47:1824–1827. doi: 10.1002/anie.200702929. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. Aulae Typographi & Bibliopolae; Viennae: 1751. Philosophia Botanica: in qua explicantur fundamenta Botanica cum definitionibus partium, exemplis terminorum, observationibus rariorum, adiectis figuris aeneis. Ioannis Thomae Trattner, Caef. Reg; p. 241. [Google Scholar]
- Liu D. The cell and protoplasm as container, object, and substance, 1835–1861. J. Hist. Biol. 2017;50:889–925. doi: 10.1007/s10739-016-9460-9. [DOI] [PubMed] [Google Scholar]
- López-García P., Moreira D. The syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 2020;5:655–667. doi: 10.1038/s41564-020-0710-4. [DOI] [PubMed] [Google Scholar]
- Margulis L., Chapman M., Guerrero R., Hall J. The last eukaryotic common ancestor (LECA): acquisition of cytoskeletal motility from aerotolerant spirochetes in the Proterozoic Eon. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13080–13085. doi: 10.1073/pnas.0604985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.F. A briefly argued case that mitochondria and plastids are descendants of endosymbionts, but that the nuclear compartment is not. Proc. Roy. Soc. Lond. B. 1999;266:1387–1395. [Google Scholar]
- Martin W.F. Symbiogenesis, gradualism and mitochondrial energy in eukaryote evolution. Period. Biol. 2017;119:141–158. [Google Scholar]
- Martin W.F., Garg S., Zimorski V. Endosymbiotic theories for eukaryote origin. Phil. Trans. Roy. Soc. Lond. B. 2015;370:20140330. doi: 10.1098/rstb.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M. Mitochondria and endomembrane origins. Curr. Biol. 2018;28:R367–R372. doi: 10.1016/j.cub.2018.03.052. [DOI] [PubMed] [Google Scholar]
- McFadden G.I., Reith M.E., Munholland J., Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- Mereschkowsky C. Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biol. Centralblatt. 1905;25:593–604. (English translation in Martin W, Kowallik K. 1999 Annotated English translation of Mereschkowsky's 1905 paper "Über Natur und Ursprung der Chromatophoren im Pflanzenreiche." Eur. J. Phycol. 34, 287–295.) [Google Scholar]
- Mereschkowsky C. Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen. Biol. Centralbl. 1910;30:278–288. 289–303; 321–347; 353–367. [Google Scholar]
- Müller M. The hydrogenosome. J. Gen. Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- Portier P. Masson; Paris, France: 1918. Les Symbiotes. [Google Scholar]
- Preiner M. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020;4:534–542. doi: 10.1038/s41559-020-1125-6. [DOI] [PubMed] [Google Scholar]
- Ris H., Plaut W. Ultrastructure of DNA-containing areas in the chloroplasts of Chlamydomonas. J. Cell Biol. 1962;12:383–391. doi: 10.1083/jcb.13.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagan L. On the origin of mitosing cells. J. Theor. Biol. 1967;14:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- Sapp J., Carrapiço F., Zolotonosov M. Symbiogenesis: the hidden face of Constantin Merezhkowsky. Hist. Philos. Life Sci. 2002;24:413–440. doi: 10.1080/03919710210001714493. [DOI] [PubMed] [Google Scholar]
- Schnepf E. Leukoplasten bei Nitzschia alba. Osterreichische Botanische Zeitschrift. 1969;116:65–69. [Google Scholar]
- Speijer D. Debating eukaryogenesis – Part 2: how anachronistic reasoning can lure us into inventing intermediates. BioEssays. 2020;42:1900153. doi: 10.1002/bies.201900153. [DOI] [PubMed] [Google Scholar]
- Wallin I.E. On the nature of mitochondria. IX. Demonstration of the bacterial nature of mitochondria. Am. J. Anat. 1925;36:131–139. [Google Scholar]
- Wallin I.E. Bailliere, Tindall and Cox; London: 1927. Symbionticism and the Origin of Species. [Google Scholar]