Abstract
Psoriasis is a chronic, recurrent skin disease requiring long‐term management. Agents that repair the skin's barrier function are invaluable additives in topical treatments of psoriasis. This multicenter, randomized, controlled trial evaluated the efficacy and safety of a linoleic acid‐ceramide‐containing moisturizer (LA‐Cer) for mild‐to‐moderate psoriasis vulgaris. We randomized 178 patients from both northern and southern regions of China into two groups: 81 patients in the control group received mometasone furoate (MF, 0.1%) cream, while MF in combination with LA‐Cer was administered to 86 patients in the treatment group for 4 weeks. The LA‐Cer‐MF group maintained the use of moisturizer after topical glucocorticoid administration. The primary endpoint, Psoriasis Area and Severity Index 50 (PASI 50) response, revealed the superiority of LA‐Cer‐MF with lower relapse rates at week 8. The use of the LA‐Cer‐containing moisturizer as maintenance therapy resulted in a continuous improvement in the clinical state in terms of body surface area, PASI, investigators' assessment of skin dryness and desquamation, and Physician Global Assessment of Psoriasis score, and in the patients' quality of life. Thus, the LA‐Cer‐containing moisturizer is a promising agent to prevent and treat psoriasis as it enhances the therapeutic effect induced by topical glucocorticoids and delays relapse.
Keywords: ceramide, linoleic acid, moisturizer, psoriasis, relapse, skin barrier
1. INTRODUCTION
Psoriasis is a chronic inflammatory and proliferative skin disorder characterized by erythrosquamous plaques, skin dryness, and various degrees of pruritus. 1 With its intermittent episodes of exacerbations and remissions, psoriasis has a substantial impact on both physical health and quality of life (QoL) of the affected individuals, which requires lifelong management. 2 Furthermore, 20% to 30% of the affected individuals require continuous, long‐term, systemic therapy to achieve effective symptom control. 3
Conventional treatments include topical treatment, phototherapy, and systemic treatment, which have different degrees of therapeutic efficacy and adverse reactions. Despite the good response to biological agents, relapse is inevitable after withdrawal of therapy. Improvement of epidermal function with topical emollients can mitigate and/or prevent the relapse of psoriasis. 4 Therefore, long‐term skin care could be an effective and economical way to prolong the therapeutic effects of topical or systemic treatments and delaying relapse.
Ceramides (Cers), which are the major constituents of the free extractable lipids present in the stratum corneum (SC), play an essential role in structuring and maintaining the permeability barrier function of the skin. 5 Besides their structural role in the SC, they also serve as intracellular signaling molecules mediating several biological processes such as proliferation, differentiation, apoptosis, and immune responses. 6 Low levels of serine palmitoyltransferase (SPT), a critical enzyme for Cer biosynthesis, have been detected in the lesional skin of psoriasis. Additionally, the decrease in Cer levels was observed to be proportional to the psoriasis area and severity index (PASI) scores. 7 The fact that lipid changes are observed in lesions, whereas no significant difference is detected in uninvolved skin implies the involvement of factors such as inflammation or changes in the process of differentiation in the observed changes in the lipid properties of SC. 8 Safflower seed oil, with a high linoleic acid (LA) content, increases the synthesis of Cers and exerts positive effects on the skin barrier. 9 Therefore, formulations containing Cers and LA have the potential to normalize lipid composition and organization and improve the condition of the skin in psoriasis. LA can also serve as an peroxisome proliferator‐activated receptor‐alpha (PPAR‐α)agonist, 10 while oxidized LA is a PPAR‐γ activator. 11 The activation of PPAR, a nuclear receptor expressed by keratinocytes, is responsible for regulating keratinocyte proliferation, inflammation, and skin barrier homeostasis by increasing epidermal lipid metabolism and synthesis. 12 , 13
Topical glucocorticoids (GCs) are the first‐line treatment for mild‐to‐moderate psoriasis (<10% body surface area involvement). 14 However, they possess several adverse effects (AEs) including cutaneous atrophy and skin barrier impairment, 15 , 16 and their influence on the SC and barrier integrity has received much attention. A broad (15%‐37%) reduction in SC lipid synthesis was observed in cultured human keratinocytes treated with GCs. 17 GC treatment resulted in abnormalities in permeability barrier homeostasis, which were shown to be partly normalized by topical treatment with a triple‐lipid mixture of SC lipids. 18 Hence, a barrier‐repairing product, particularly one that contains Cers and a PPAR‐α activator to normalize hyperproliferation and differentiation and to exert anti‐inflammatory effects 19 could be an alternative therapy for psoriasis.
Therefore, in this study, we aimed to examine the therapeutic and preventive benefits of an LA‐Cer‐containing moisturizer for psoriasis patients, during and after topical GC use.
2. MATERIALS AND METHODS
2.1. Study overview
A multicenter, parallel, randomized, controlled trial (ChiCTR‐IOR‐15007138) was conducted from March 2013 to July 2014 at 6 medical centers in China in accordance to the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Boards at all sites. Informed consent forms were signed by all participants.
2.2. Patient eligibility criteria
Patients were enrolled from six multiple dermatology centers in China. Eligible subjects were at least 12 years old, were classified as having mild‐to‐moderate psoriasis vulgaris, defined as lesions covering ≤10% of their body surface area (BSA) and a PASI score ≤ 10.
Exclusion criteria included history of severe systemic disease(s) and/or other severe skin diseases; pregnancy or lactation; known allergic history to the ingredients in the drug formulation or moisturizer under study; discrepancy with the definition of patients with mild‐to‐moderate psoriasis vulgaris; frequent use of herbal, hypnotics, sedatives, mood stabilizers, or other addictive medications; current alcohol abuse; and psychiatric or any other medical conditions, which in the investigator's opinion rendered the subject unsuitable for the trial. Patients having received systemic therapy (including traditional Chinese medicines) or phototherapy in the past one month for psoriasis were also excluded, as were those who had received any topical medications or very potent topical GCs in the past 2 weeks.
2.3. Study design
Patients eligible for inclusion in the study were randomly assigned to either treatment group or control group (1:1 ratio). The randomization sequence was created using SAS 9.2 statistical software for windows (Cary, NC)and was stratified by center using a block size of 2. All patients were enrolled and assigned to the interventions by researchers at each center.
During the 4‐week treatment period, participants in the control group were treated topically with 500 mg of moderate GC cream (mometasone furoate 0.1% cream, 0.1% MF, Elocon, Merck Sharp & Dohme, China) once daily per 2% of psoriatic lesional body surface area and were referred to as the MF group. In addition to the MF cream, patients in the treatment group received 15 mL of LA‐Cer‐containing moisturizer (YuZe Skin Barrier Recovery Body Lotion, developed by Ruijin Hospital and produced by Shanghai Jahwa United Company, China) once daily as an adjunctive therapy on the entire body after showering. The main constituents of the moisturizer are safflower seed oil and rice bran oil. The ingredients of the moisturizer are listed in Table S1.
Participants entered an 8‐week maintenance period after the end of topical GC use, during which patients in the treatment group maintained the use of the moisturizer as a preventive therapy while the control group discontinued the MF treatment. Patients in the control group reapplied 0.1% MF when there was a relapse, which was defined by loss of at least 50% improvement in PASI achieved between baseline and week 12 in patients who had achieved a clinically meaningful response. 20 The trial was ended after 12 weeks of observation and was stopped when exacerbation (125% of PASI baseline) occurred. The patient with exacerbation was asked to withdraw and received other effective treatment. The trial was terminated with participants requesting for withdrawal or loss to follow‐up.
2.4. Efficacy and safety assessments
The proportion of patients showing improvement of ≥50% in the PASI score from baseline (PASI 50 response) at week 8 was used as the primary endpoint. The secondary outcomes included affected BSA, PASI score, Physician Global Assessment of Psoriasis (PGA) score, Dermatology QoL Index (DLQI) score, and the assessment of skin dryness and desquamation using a 10‐cm visual analogue scale (VAS) at weeks 2, 4, 8, and 12. All the assessments were performed by an investigator who was blinded to the treatment allocation of the patients. All assessments were compared between both groups and with the baseline. Clinical relapse and exacerbation rates at weeks 8 and 12 were compared between both groups. The secondary outcomes also included the patients' reported outcomes. Participants were asked to rate the degree of skin dryness and pruritus as follows: 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Safety was assessed at every treatment visit by the operator. AEs included atypical or unusual worsening of the disease and/or the occurrence of new psoriasis morphologies during treatment. Patients were asked to report any AEs and concomitant medication(s) since their previous visit. Additional safety evaluations included PASI improvement rates at weeks 4, 8, and 12.
2.5. Analysis set
All data analysis was performed for the intention‐to‐treat population, defined as all randomized patients who had taken at least one dose of the study treatment. Based on this principle, the full‐analysis set (FAS) was mainly used to compare the treatment effect for the primary endpoint, with the statistical results from the per‐protocol set (PPS) being used as supplementary analysis (Table S2). The safety analysis dataset was used to perform AE/adverse reaction analysis.
2.6. Statistical analysis and sample size
Statistical analyses were independently performed by two statisticians using SAS 9.4 (SAS Institute Inc., Cary, NC). Using a one‐sided significance level of .025 and a power of 90%, a sample size of 180 patients was determined to be able to detect a difference between PASI 50 response rates of 0.16 and 0.58 in the control and LA‐Cer‐MF groups, respectively, at week 8. A drop‐out rate of 20% was added. The comparison of data between groups was performed with the Student's t‐test under the condition of homogeneity of variance. The Welch's test was used for unequal variance. PASI 50 and PASI 75 were compared between both groups using a center‐adjusted Cochran‐Mantel‐Haenszel (CMH) approach with the statistic, Q CMH. Inter‐group comparison of relapse and exacerbation rates was performed using the Chi‐Square test. Fisher's exact probability was used when the chi‐square test could not be used. Wilcoxon signed‐rank test was used for inter‐group comparison of hierarchical data with Z‐value. The data are presented as the mean ± SD for clarity. For all tests, P < .05 was considered significant.
3. RESULTS
3.1. Patient characteristics
Of 167 randomized patients, 137 (82.04%) completed week 12 (LA‐Cer‐MF, n = 74 [86.04%]; MF, n = 63 [77.78%]). Among the patients who did not complete week 12, 23 (13.77%) were discontinued (LA‐Cer‐MF, n = 8 [9.30%]; MF, n = 15 [18.52%]) and 7 (4.19%) were eliminated (LA‐Cer‐MF, n = 4 [4.65%]; MF, n = 3 [3.70%]) (Figure 1). Patient demographics and clinical characteristics at baseline were similar across the treatment groups, except that the treatment group had higher PASI scores than the control MF group (Table 1).
FIGURE 1.
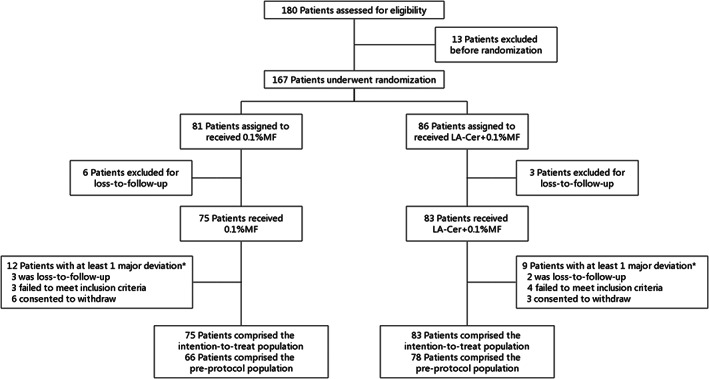
Flow diagram of the RCT. 167 patients underwent randomization to MF or La‐Cer‐MF with a 1:1 ratio. *Patients could have one or several major deviation(s)
TABLE 1.
Patient demographics and clinic characteristics at baseline
| Characteristic | LA‐Cer‐MF (N = 83) | MF (N = 75) |
|---|---|---|
| Male, n (%) | 53 (63.86) | 46 (61.33) |
| Age, mean (SD) [years] | 39.36 (12.41) | 41.54 (15.42) |
| Previous use of antipsoriatic in the past month a | 0 (0.00%) | 2 (2.67%) |
| Medical history b | 8 (9.64%) | 13 (17.33%) |
| Duration of psoriasis, mean (SD) [years] | 95.22 (91.77) | 95.00 (119.15) |
| VAS dryness and desquamation score | 4.39 (1.98) | 4.32 (1.86) |
| % affected BSA, mean (SD) | 4.88 (2.42) | 4.69 (2.87) |
| PGA score, mean (SD) | 2.08 (0.81) | 2.01 (0.83) |
| PASI score, mean (SD) | 4.58 (2.16) | 3.89 (2.18) |
| DLQI score, mean (SD) | 8.28 (5.04) | 7.95 (4.83) |
| Objective skin dryness index, n (%) | ||
| None | 2 (2.41) | 4 (5.33) |
| Mild | 28 (33.73) | 27 (36.00) |
| Moderate | 41 (49.40) | 35 (46.67) |
| Severe | 12 (14.46) | 9 (12.00) |
| Objective skin pruritus index, n (%) | ||
| None | 8 (9.64%) | 4 (5.33%) |
| Mild | 39 (46.99%) | 42 (56.00%) |
| Moderate | 26 (31.33%) | 27 (36.00%) |
| Severe | 10 (12.05%) | 2 (2.67%) |
Includes topical, conventional, biologics, phototherapy (PUVA or non‐PUVA) or traditional Chinese medicine.
Involves the cardiovascular, respiratory, digestive, endocrine, nervous and genitourinary systems, as well as the disease affected eye, ear, throat and nose.
3.2. Efficacy endpoints
The proportion of patients achieving PASI 50 response signifying treatment efficacy at week 8 was 65.06% in the LA‐Cer‐MF group and 40.00% in the control group (Figure 2C, both P < .05). The FAS showed significant difference in improvement in PASI between both treatment groups at weeks 8 and 12 (Figure 2A, both P < .05), as did the PPS (data not shown, both P < .05). PASI 75 was achieved by 28.92% of patients in the LA‐Cer‐MF group compared with 13.33% of the control group after 8 weeks of therapy (Figure 2D, both P < .05).
FIGURE 2.
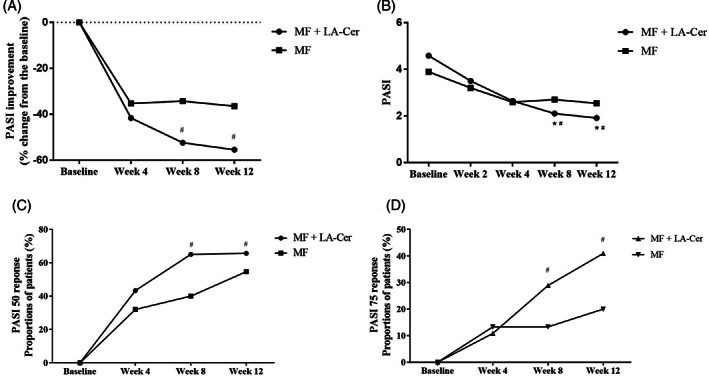
Comparison of PASI improvement between patients treated with or without LA‐Cer moisturizer Graph A, and B, represent PASI improvement and PASI score. Observed clinical response rate was shown as the percentage of patients achieving PASI 50, C, and PASI 75, D, in FAS (Full analysis set). Results are expressed as mean ± SD. n = 75 for MF group, n = 83 for LA‐Cer‐MF group. *Represent the significance differences (P < .05) compared with Week 4; #Represent the significance differences (P < .05) between two groups
At the end of the 4‐week topical corticotherapy, both LA‐Cer‐MF and MF groups achieved a significant decrease in affected BSA (from baseline at 4.88 ± 2.42 to 3.52 ± 2.37, from baseline at 4.69 ± 2.87 to 3.57 ± 2.44, respectively, Figure 3A, P < .05); PASI (from baseline at 4.58 ± 2.16 to 2.64 ± 1.69, from baseline at 3.89 ± 2.18 to 2.59 ± 1.87, respectively, Figure 2B, P < .05); and PGA (from baseline at 2.08 ± 0.81 to 1.13 ± 0.78, from baseline at 2.01 ± 0.83 to 1.37 ± 0.8, respectively, Figure 3B, P < .05),and was associated with a significant decrease in the skin dryness and desquamation (from baseline at 4.39 ± 1.98 to 1.9 ± 1.59, from baseline at 4.32 ± 1.86 to 3.65 ± 2.24, respectively, Figure 3C, P < .05). These changes resulted in significantly lower DLQI scores, 4.51 ± 4.26 in the treatment group and 6.00 ± 4.51 in the control group (Figure 3D, both P < .05). However, significant differences between the two groups were merely observed in PGA, DLQI, and VAS dryness and desquamation scores (P < .05). The amelioration in the patients' disease perception with the initial application of the LA‐Cer moisturizer during topical GC use could result from its activity in establishing the normal skin permeability barrier and consequent remission of symptoms such as scaling, itching, and skin dryness.
FIGURE 3.
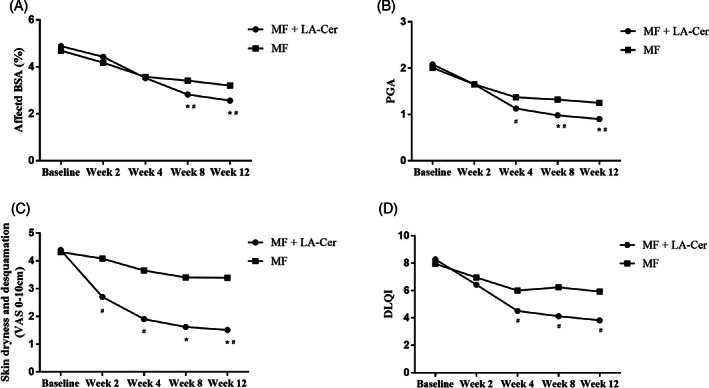
Comparison of clinical assessment between patients treated with or without LA‐Cer moisturizer. Graphs, A‐D, represent BSA, PGA, VAS dryness and desquamation and DLQI, respectively. n = 75 for MF group, n = 83 for LA‐Cer + MF group. Results are expressed as mean ± SD. *Represent the significance differences (P < .05) compared with Week 4; #Represent the significance differences (P < .05) between two groups
After the end of the corticotherapy, continued amelioration with the maintenance therapy with the LA‐Cer moisturizer was achieved in BSA (2.82 ± 2.17 at week 8 vs 2.56 ± 2.25 at week 12), PASI (2.10 ± 1.89 at week 8 vs 1.91 ± 1.87 at week 12), and PGA (0.98 ± 0.81 at week 8 vs 0.90 ± 0.82 at week 12) compared to baseline and week 4 (P < .05). A significant decrease was observed in VAS dryness and desquamation scores at weeks 8 and 12 (P < .05). These improvements in disease severity and lesional area were accompanied by a decrease (improvement) in the DLQI scores (4.13 ± 3.98 at week 8 vs 3.83 ± 3.98 at week 12). Significant differences between the two groups were observed in all the clinician assessments at weeks 8 and 12, reflecting an improved clinical state and QoL (P < .05) with the treatment using the LA‐Cer moisturizer.
Among the patients' reported outcomes, participants in both groups experienced relief in skin dryness and pruritus, with a significant improvement with the addition of the LA‐Cer moisturizer.
Furthermore, the relapse rate was significantly different between both groups at week 8 (34.94% in the LA‐Cer‐MF group vs 60.00% in the MF group) and week 12 (44.58% vs 64.00%, respectively) (Table S3). Thus, relapse was significantly slowed down and reduced in the group using the LA‐Cer moisturizer throughout the study duration. There was no significant difference in the exacerbation rates of the two groups (Table S4).
3.3. Adverse events
Trial medications were well tolerated, and no serious AEs were observed during the trial. Side effects of the therapy included 3 patients who reported exacerbation, 1 case of pigmentation and skin atrophy, 1 case of erythema around the skin lesion, 1 case of skin dryness, 1 case of subcutaneous hemorrhage, and 1 case of peripheral folliculitis. Except for 1 case of exacerbation in the LA‐Cer‐MF group, the remaining were in the MF group. All of the AEs occurred within 2 cm of the site of application. Except for folliculitis that was considered to be irrelevant to the treatments, the other AEs were considered to be relevant.
4. DISCUSSION
As demonstrated in our previous study, this multicenter randomized, controlled trial (RCT) specifically addressed the benefits of application of a barrier‐repairing moisturizer in the management of mild‐to‐moderate psoriasis vulgaris with a high level of evidence. The results of this multicenter, evaluator‐blinded RCT demonstrated the superiority of the LA‐Cer‐containing moisturizer in continuous improvement of PASI 50 response after topical GC use over the control group. PASI 75, which is a well‐accepted, clinically meaningful endpoint, was achieved by more patients maintaining the use of LA‐Cer after 8 and 12 weeks of treatment with lower relapse rates and fewer AEs. Additionally, patients using adjunctive LA‐Cer showed greater improvement in QoL during the treatment in comparison with the control group.
Although there are various treatments for the temporary control of skin lesions, no curative or preventive therapy is available for psoriasis, partly due to epidermal dysfunction. The mechanisms whereby this moisturizer exerts benefits in psoriasis by reducing the incidence of AEs and preventing relapse are likely due to the improvement of the epidermal permeability barrier. Human skin forms an impermeable barrier to prevent transepidermal water loss (TEWL) and the penetration of pathogens, allergens, as well as irritants. Many reports suggest that Cers are required for the functioning of the normal skin permeability barrier. 21 Adjunctive LA‐Cer has been shown to induce higher skin capacitance and earlier reduction of TEWL and maintain the stability of GC‐induced effects after 8‐week topical corticotherapy. 22 The replenishment of Cers results in the recovery of the permeability barrier by also increasing skin hydration.
A keratinocyte‐specific SPT‐cKO mouse model with decreased Cer levels and increased numbers of IL‐17‐producing γδT cells in skin lesions revealed the interactions between barrier dysfunction and immunological alterations that lead to the psoriasis phenotype. 23 Abnormal permeability barrier function can increase the epidermal production of pro‐inflammatory cytokines, the majority of which further weaken barrier function, leading to a vicious circle that can exacerbate disease manifestations. 24 However, this vicious circle can be interdicted by the anti‐inflammatory effects of Cer replenishment.
Activation of PPARs could be another mechanism by which the LA‐Cer containing moisturizer improves psoriasis. 22 PPAR‐α activators used to stimulate lipid synthesis inhibit cutaneous 25 and even systemic inflammation involved in the pathogenesis of cardiovascular comorbidities in psoriasis, 26 indicating the systemic benefit of topical treatment.
Topical GCs remain a cornerstone treatment of psoriasis vulgaris owing to their potent antiproliferative and anti‐inflammatory effects. However, the long‐term use of topical GCs is associated with numerous AEs and recurrence of psoriasis after discontinuation of treatment. Our study proved the greater efficacy of the LA‐Cer moisturizer in the prevention and attenuation of the development of psoriasis by prolonging the remission and delaying the relapse. AEs were limited in this study, the majority of which were related to the topical application of MF. The efficacy of LA‐Cer in the prevention of rapid relapse and AEs is likely due to the adjunctive steroid‐sparing action. 9 Importantly, the activation of PPAR‐γ has been found to attenuate GC‐induced functional abnormalities in differentiation and proliferation of epidermal cells. 27
This trial has some limitations. Instead of an open‐label trial, the outcomes of the study have been evaluated in an assessor‐blinded fashion to increase the internal validity of the results. Furthermore, we could not rule out any interaction of both treatments because factorial design is ethically unfeasible.
5. CONCLUSIONS
This study clearly demonstrates that the topical LA‐Cer moisturizer alleviated psoriasis by maintaining and prolonging the improvement achieved by topical GC treatment both in the clinical state and in QoL and preventing relapses and AEs after the end of corticotherapy. The results of this trial suggest that the LA‐Cer‐containing moisturizer, used during and after topical GC use, is a promising therapeutic agent for the management and prevention of psoriasis, providing an inexpensive alternative to improve the skin's barrier function.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Shanghai Jiaotong University Medical School Affiliated Ruijin Hospital and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Supporting information
Table S1. Ingredients of the moisturizer.
Table S2. Set analysis.
Table S3. Comparisons of relapse rates between patients treated with or without LA‐Cer moisturizer after 8 weeks and 12 weeks (FAS).
Table S4. Comparisons of exacerbation rates between patients treated with or without LA‐Cer moisturizer after 8 weeks and 12 weeks (FAS).
ACKNOWLEDGMENT
This work was funded by National Natural Science Foundation of China (81673056 and 81830095).
Li X, Yang Q, Zheng J, et al. Efficacy and safety of a topical moisturizer containing linoleic acid and ceramide for mild‐to‐moderate psoriasis vulgaris: A multicenter randomized controlled trial. Dermatologic Therapy. 2020;33:e14263 10.1111/dth.14263
Xia Li and Qi Yang contributed equally to this study and are co‐first authors.
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81673056, 81830095
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496‐509. [DOI] [PubMed] [Google Scholar]
- 2. Boehncke W‐H, Schön MP. Psoriasis. The Lancet. 2015;386(9997):983‐994. [DOI] [PubMed] [Google Scholar]
- 3. Lecluse LLA, Naldi L, Stern RS, Spuls PI. National Registries of Systemic Treatment for Psoriasis and the European ‘Psonet’ Initiative. Dermatology. 2009;218(4):347‐356. [DOI] [PubMed] [Google Scholar]
- 4. Man M‐Q, Ye L, Hu L, Jeong S, Elias PM, Lv C. Improvements in epidermal function prevent relapse of psoriasis: a self‐controlled study. Clin Exp Dermatol. 2019;44(6):654‐657. [DOI] [PubMed] [Google Scholar]
- 5. Coderch L, López O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003;4(2):107‐129. [DOI] [PubMed] [Google Scholar]
- 6. Gangoiti P, Camacho L, Arana L, et al. Control of metabolism and signaling of simple bioactive sphingolipids: Implications in disease. Prog Lipid Res. 2010;49(4):316‐334. [DOI] [PubMed] [Google Scholar]
- 7. Hong K‐K, Cho H‐R, Ju W‐C, Cho Y, Kim N‐I. A Study on Altered Expression of Serine Palmitoyltransferase and Ceramidase in Psoriatic Skin Lesion. J Korean Med Sci. 2007;22(5):862‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta BBA—Mol Cell Biol Lipids. 2014;1841(3):295‐313. [DOI] [PubMed] [Google Scholar]
- 9. Eichenfield LF, McCollum A, Msika P. The Benefits of Sunflower Oleodistillate (SOD) in Pediatric Dermatology. Pediatr Dermatol. 2009;26(6):669‐675. [DOI] [PubMed] [Google Scholar]
- 10. Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator‐activated receptors. Proc Natl Acad Sci U S A. 1994;91(15):7355‐7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bull AW, Steffensen KR, Leers J, Rafter JJ. Activation of PPAR γ in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR γ. Carcinogenesis. 2003;24(11):1717‐1722. [DOI] [PubMed] [Google Scholar]
- 12. Kömüves LG, Hanley K, Lefebvre AM, et al. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115(3):353‐360. [DOI] [PubMed] [Google Scholar]
- 13. Man M‐Q, Choi E‐H, Schmuth M, et al. Basis for Improved Permeability Barrier Homeostasis Induced by PPAR and LXR Activators: Liposensors Stimulate Lipid Synthesis, Lamellar Body Secretion, and Post‐Secretory Lipid Processing. J Invest Dermatol. 2006;126(2):386‐392. [DOI] [PubMed] [Google Scholar]
- 14. Bailey J, Whitehair B. Topical Treatments for Chronic Plaque Psoriasis. Am Fam Physician. 2010;81(5):596. [PubMed] [Google Scholar]
- 15. Barnes L, Kaya G, Rollason V. Topical Corticosteroid‐Induced Skin Atrophy: A Comprehensive Review. Drug Saf. 2015;38(5):493‐509. [DOI] [PubMed] [Google Scholar]
- 16. Røpke MA, Alonso C, Jung S, et al. Effects of glucocorticoids on stratum corneum lipids and function in human skin—a detailed lipidomic analysis. J Dermatol Sci. 2017;88(3):330‐338. [DOI] [PubMed] [Google Scholar]
- 17. Kolbe L, Kligman AM, Schreiner V, Stoudemayer T. Corticosteroid‐induced atrophy and barrier impairment measured by non‐invasive methods in human skin. Skin Res Technol. 2001;7(2):73‐77. [DOI] [PubMed] [Google Scholar]
- 18. Kao JS, Fluhr JW, Man M‐Q, et al. Short‐term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120(3):456‐464. [DOI] [PubMed] [Google Scholar]
- 19. Chon S‐H, Tannahill R, Yao X, Southall MD, Pappas A. Keratinocyte differentiation and upregulation of ceramide synthesis induced by an oat lipid extract via the activation of PPAR pathways. Exp Dermatol. 2015;24(4):290‐295. [DOI] [PubMed] [Google Scholar]
- 20. Carey W, Glazer S, Gottlieb AB, et al. Relapse, rebound, and psoriasis adverse events: An advisory group report. J Am Acad Dermatol. 2006;54(4, Supplement):S171‐S181. [DOI] [PubMed] [Google Scholar]
- 21. Masukawa Y, Narita H, Shimizu E, et al. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49(7):1466‐1476. [DOI] [PubMed] [Google Scholar]
- 22. Liu M, Li X, Chen X‐Y, Xue F, Zheng J. Topical application of a linoleic acid‐ceramide containing moisturizer exhibit therapeutic and preventive benefits for psoriasis vulgaris: a randomized controlled trial. Dermatol Ther. 2015;28(6):373‐382. [DOI] [PubMed] [Google Scholar]
- 23. Nakajima K, Terao M, Takaishi M, et al. Barrier abnormality due to ceramide deficiency leads to psoriasiform inflammation in a mouse model. J Invest Dermatol. 2013;133(11):2555‐2565. [DOI] [PubMed] [Google Scholar]
- 24. Hänel KH, Cornelissen C, Lüscher B, Baron JM. Cytokines and the Skin Barrier. Int J Mol Sci. 2013;14(4):6720‐6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheu MY, Fowler AJ, Kao J, et al. Topical peroxisome proliferator activated receptor‐α activators reduce inflammation in irritant and allergic contact dermatitis models1. J Invest Dermatol. 2002;118(1):94‐101. [DOI] [PubMed] [Google Scholar]
- 26. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non‐alcoholic fatty liver disease. J Hepatol. 2015;62(3):720‐733. [DOI] [PubMed] [Google Scholar]
- 27. Demerjian M, Choi E‐H, Man M‐Q, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009;18(7):643‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Ingredients of the moisturizer.
Table S2. Set analysis.
Table S3. Comparisons of relapse rates between patients treated with or without LA‐Cer moisturizer after 8 weeks and 12 weeks (FAS).
Table S4. Comparisons of exacerbation rates between patients treated with or without LA‐Cer moisturizer after 8 weeks and 12 weeks (FAS).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


