Abstract
An influential paradigm in coral reef ecology is that fishing causes trophic cascades through reef fish assemblages, resulting in reduced herbivory and thus benthic phase shifts from coral to algal dominance. Few long‐term field tests exist of how fishing affects the trophic structure of coral reef fish assemblages, and how such changes affect the benthos. Alternatively, benthic change itself may drive the trophic structure of reef fish assemblages. Reef fish trophic structure and benthic cover were quantified almost annually from 1983 to 2014 at two small Philippine islands (Apo, Sumilon). At each island a No‐Take Marine Reserve (NTMR) site and a site open to subsistence reef fishing were monitored. Thirteen trophic groups were identified. Large planktivores often accounted for >50% of assemblage biomass. Significant NTMR effects were detected at each island for total fish biomass, but for only 2 of 13 trophic components: generalist large predators and large planktivores. Fishing‐induced changes in biomass of these components had no effect on live hard coral (HC) cover. In contrast, HC cover affected biomass of 11 of 13 trophic components significantly. Positive associations with HC cover were detected for total fish biomass, generalist large predators, piscivores, obligate coral feeders, large planktivores, and small planktivores. Negative associations with HC cover were detected for large benthic foragers, detritivores, excavators, scrapers, and sand feeders. These associations of fish biomass to HC cover were most clear when environmental disturbances (e.g., coral bleaching, typhoons) reduced HC cover, often quickly (1–2 yr), and when HC recovered, often slowly (5–10 yr). As HC cover changed, the biomass of 11 trophic components of the fish assemblage changed. Benthic and fish assemblages were distinct at all sites from the outset, remaining so for 31 yr, despite differences in fishing pressure and disturbance history. HC cover alone explained ~30% of the variability in reef fish trophic structure, whereas fishing alone explained 24%. Furthermore, HC cover affected more trophic groups more strongly than fishing. Management of coral reefs must include measures to maintain coral reef habitats, not just measures to reduce fishing by NTMRs.
Keywords: coral cover, coral reef fish, environmental disturbances, fishing effects, no‐take marine reserves, Philippines, trophic biomass
Introduction
One of the first attempts to define what we today call ecology was made by Haeckel: “…the total relations of the animal both to its inorganic and its organic environment” (Haeckel 1870, cited in Andrewartha and Birch 1954:13). While not a definition that would endear itself to too many botanists or microbiologists, the ecological point is clear, if one replaces the word “animal” with “organism” in this definition. The initial focus of “ecology” was thus on the environmental conditions, both physical and biological, that determined the distribution and abundance of organisms. Pianka (1974) noted that such definitions should correctly include also the effects of organisms on the environmental conditions. Andrewartha and Birch (1954) spoke of “…two sorts of ecology.” The first was studies of the environmental conditions that determined the distribution and abundance of populations or species, often species of importance to humans such as harmful insects and useful fishes, birds and mammals. The second was studies of the environmental conditions that determined the structure (e.g., species composition, species richness) of assemblages or communities of species. A definition of “environment” is essential to the understanding of these “sorts” of ecology. Andrewartha and Birch (1954) identified four components of environment that they felt determined the organisms’ chances to survive and breed, and thus the distribution and abundance of their populations: weather, food, other animals and organisms causing harm, and a place in which to live. This definition of environment, and that implied by Haeckel (1870), had a strong emphasis on physical conditions and habitats suitable for survival and reproduction, as well as biological factors like food resources, mates, and predation. Andrewartha and Birch (1954) maintained that competition is often difficult to detect and can be dampened or eliminated by physical disturbance and predation, a position that brought them into disagreement with the view that competition was often an important cause of “population regulation” (Nicholson 1933). Andrewartha and Birch (1954:Chapter 14) presented models of how a complex abiotic and biotic environment, coupled with spatial complexity of population structure reminiscent of what modern ecologists now call “meta‐population structure,” can determine the numbers in animal populations. The views of Andrewartha and Birch (1954) were no doubt influenced strongly by the fact that they specialized in the study of insect populations in the often harsh and variable climatic conditions of Australia. They thus often stressed the important roles of changing weather conditions and environmental disturbances to habitats as major determinants of distribution and abundance.
As the subject of ecology transitioned from a focus on lower (organism, population) to higher (assemblages, communities, ecosystems) levels of organization, a subtle shift in emphasis of what environmental conditions were important determinants of survival and reproduction seems to have occurred. Modern (1960s onward), often experimental, ecology developed a far greater emphasis on biological (predation, competition) than physical environmental factors, particularly in community ecology (Hairston et al. 1960, Connell 1961, Paine 1966, Dayton 1971, Sale 1977). To be fair, some of these authors did stress the important role of environmental disturbances, often to habitats, and their interactions with predation and competition (Dayton 1971, Menge and Sutherland 1976, Connell 1978) in maintaining assemblage/community structure.
As ecology shifted its focus to higher levels of organization, emphasis shifted from physical to biological environmental drivers. A novel way of looking at ecological populations, assemblages, and communities was proposed by Hairston et al. (1960). The concepts of top‐down and bottom‐up regulation of population numbers and community structure derive from food web theory (Hairston et al. 1960, Hunter and Price 1992, Menge 2000). Regulation is assumed to be by predators (top‐down) or resources (bottom‐up). Resources are usually trophic (food), often driven by availability of nutrients and light. There is consensus that the top‐down/bottom‐up dichotomy is somewhat artificial, with both likely operating in species‐specific ways at different times/places (Hunter and Price 1992). Environmental variations (e.g., productivity gradients or environmental “stresses”) modulate the relative strengths of top‐down/bottom‐up drivers on a case by case basis (Hunter and Price 1992).
A strong emphasis of top‐down control of populations and communities emerged, particularly in marine ecology (Paine 1966, Dayton 1971, Estes et al. 2011). This strong influence of top‐down control of communities flowed into coral reef ecology in the early 1990’s with the publication of several studies that suggested that overfishing, particularly of herbivorous coral reef fish, caused reductions in grazing and shifts in dominance of benthos from coral to algal‐dominated states (Done 1992, Hughes 1994, McClanahan and Mutere 1994). The concept of a strong, top‐down driver, fishing (a proxy for predation; e.g., Hixon 1991), causing “trophic cascades” through assemblages of coral reef fish that resulted in “benthic phase shifts” became a dominant concept in coral reef ecology that persists to this day (Houk et al. 2018, Lefcheck et al. 2019). In the past 25 yr, it has become almost paradigmatic in the coral reef literature that fishing‐induced reductions in abundance of herbivorous coral reef fish cause benthic phase shifts from coral to macroalgal dominance (Hughes 1994, Bellwood et al. 2004, Mumby et al. 2006; but see Aronson and Precht 2006, Bruno et al. 2009). Mumby and Steneck (2008) highlight reduced herbivory on coral reefs, mostly caused by fishing, as a rapidly evolving ecological paradigm. The classic example of this paradigm comes from Jamaica where overfishing of herbivorous fish, declines of herbivorous urchins due to a disease, and the effects of a hurricane, led to a benthic phase shift from coral to macroalgal dominance (Hughes 1994; but see Côté et al. 2013). This strong top‐down control of communities by fishing has been generalized to marine fisheries and to many marine ecosystems (Pauly et al. 1998, Jackson et al. 2001, Estes et al. 2011).
Top‐down control of assemblages of reef fish and benthos by fishing was such a strong influence in coral reef ecology from the 1990s onward, that coral reef ecologists seemed to place less focus on the basic components of environment stressed by Haeckel (1870) and Andrewartha and Birch (1954). That is, they tended to lose sight of the fact that a vitally important part of the determinants of distribution and abundance of organisms, and the structure of communities, are physical environmental conditions, habitats, and the effects of disturbances to those conditions and habitats. Contemporary coral reef ecologists lost touch somewhat with their historical roots, a process that Graham and Dayton (2002) warned against. A brief review of papers on the stressors that cause major degradation of coral reefs, and how to manage those stressors, published in the journals Science and Nature over the past 26 yr (1994–2019; n = 64 papers, see Appendix S1: Fig. S1) supports this supposition. During the 13 yr from 1994 to 2006 (n = 23 papers), 39% of papers focused on overfishing as the causal and management emphasis of coral reef degradation, with 30% focusing on climate change (particularly coral bleaching), with the remaining papers focusing on multiple stressors (e.g., water quality, coral predators, coral diseases, hurricanes). This pattern shifted substantially in the next 13 yr (2007–2019, n = 41 papers) with 76% of papers focusing on climate change as the causal and management emphasis of coral reef degradation, 12% focusing on overfishing, with the remainder dealing with multiple stressors. To be fair, many scientists have pointed out for a long time that multiple stressors threaten coral reefs, including overfishing, climate change, water quality, coral predators, and coral diseases (Aronson et al. 2003, Hughes et al. 2003, Aronson and Precht 2006, 2016). Few coral reef scientists would disagree, however, that emphasis on overfishing, both as a short‐term (Mumby et al. 2006) and a long‐term (Jackson et al. 2001, Pandolfi et al. 2003) stressor, was a dominant theme in the literature on the degradation of coral reefs from 1994 to about 2006 (Appendix S1: Fig. S1). One could even suggest that when it came to fishing as a major determinant of benthos on coral reefs was concerned, many coral reef ecologists tended toward what Graham and Dayton (2002) described as “…restrictive gravitation toward favored models and pet theories.” Aronson et al. (2003), commenting on the Pandolfi et al. (2003) paper put it even more plainly: “The hypothesis that overfishing caused corals to decline is argued by default, and no cogent mechanistic explanation is offered.”
A common potential solution advocated to address this overfishing issue was implementation of no‐take marine reserves (Dayton et al. 2000, Sale et al. 2005, Mumby et al. 2006) and networks of such reserves at appropriate spatial scales (Sale 2002, Sale et al. 2005). Emphasis has clearly shifted to climate change as the dominant driver of coral reef degradation in the past 13 yr (Aronson and Precht 2006, 2016, Hoegh‐Guldberg et al. 2007, Hughes et al. 2017, 2018). Thus, the focus on overfishing as the key driver of coral reef degradation has been replaced by a focus on the substantial physical and chemical disturbances that coral reefs have been subjected to globally, particularly in the past decade, of mass coral mortality due to bleaching caused by excessive water temperatures and reduced pH levels related to climate change (Hoegh‐Guldberg 1999, Hoegh‐Guldberg et al. 2007, Hughes et al. 2017, 2018). These major physical disturbances to coral reefs also have strong effects on assemblages of fishes associated with coral reefs (Jones et al. 2004, Pratchett et al. 2008, Wilson et al. 2008, Graham et al. 2015).
From the viewpoint of reef fish assemblages, emphasis on the stressors causing major coral reef degradation shifted from a top‐down driver (fishing) to a driver involving environmental disturbance directly to the coral habitat of reef fish, the latter sometimes referred to as “side‐in” impacts (Precht and Aronson 2006). Ecosystems globally are now described as having entered the “Anthropocene” (Hughes et al. 2017, 2018), an age of human‐induced disturbances to climate on an unprecedented spatial scale, with coral reefs metaphorically considered the “canary in the coal mine” due to their particular sensitivity to changes in physical and chemical environmental conditions. Hixon (2011), “With a touch of nostalgia…” divided a 60‐yr history of coral reef fish ecology (1950–2010) into six decades. If he were today to add the decade from 2010 to 2020, we suspect he would have named the recent history as “the Climate Change Decade.” Such effects of climate change are not only predicted to increase levels of coral mortality directly through coral bleaching (Hoegh‐Guldberg 1999, Hughes et al. 2017, 2018), but have also been related to potential increases in the frequency and intensity of coral disease outbreaks and tropical storms (Harvell et al. 1999, Knutson et al. 2010).
Philippine coral reefs are heavily fished (Alcala and Russ 2002) and have also been subjected to major coral bleaching and typhoon events causing substantial coral loss in recent decades (Licuanan et al. 2017). Environmental disturbances that cause loss of coral cover and habitat complexity affect density, species richness, and assemblage structure of coral reef fishes significantly and usually negatively (Pratchett et al. 2008, Wilson et al. 2008, Graham et al. 2015). Thus, Philippine coral reefs are excellent candidates for testing the relative importance of top‐down effects of coral reef fish on benthos and bottom‐up effects of benthos on coral reef fish.
This study investigates whether fishing or benthic habitat are more likely to affect the assemblage structure (measured as biomass of different trophic groups) of coral reef fishes at two small Philippine Islands, each island with a No‐Take Marine Reserve (NTMR) site and a fished site, monitored almost annually for 31 yr (1983–2014). We express these potential effects in terms of two alternative models:
Model 1: Fishing modifies trophic structure of reef fish assemblages, which modifies the benthos.
Model 2: Environmental disturbances modify benthos, which modifies trophic structure of reef fish assemblages.
We predict that direct effects of environmental disturbances to the benthos (Model 2) will have a much greater effect on trophic structure of the reef fish assemblages than direct effects of fishing that then lead to indirect effects on the fish assemblage and, subsequently, the benthos (Model 1). The prediction of Model 2 is more simple and parsimonious than that of Model 1. Furthermore, each model implies a different suite of management objectives and methods to address the coral reef degradation.
The specific questions addressed in this study are (1) What is the trophic structure, in terms of biomass, of the coral reef fish assemblages? (2) Which components of this trophic structure are most affected by fishing? (3) Is trophic structure modified by fishing to the extent that it can alter the benthos? (4) Which trophic components are most affected by benthic change caused by environmental disturbances? (5) What accounts for more of the variability in trophic structure of the reef fish assemblage, live hard coral cover or fishing?
Methods
Study sites, history of NTMRs, history of environmental disturbances
This study was conducted at four sites, located on two different islands in the central Philippines: Apo and Sumilon (Fig. 1). Fish assemblages and benthic cover were monitored almost annually for 31 yr from 1983 to 2014 at one NTMR site and one “control” fished site on each island. However, both the fishing status and the sequence of environmental disturbance events at each site varied greatly (Appendix S1: Fig. S2; Russ et al. 2015a, 2015b, 2015c).
Fig. 1.
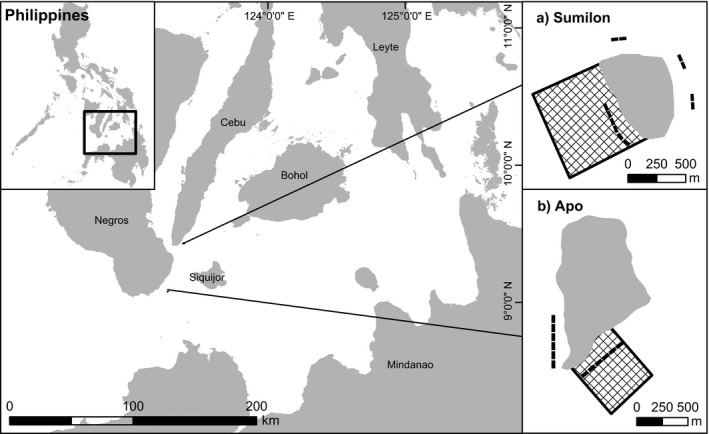
Location of the study sites in the central Philippines. Inset (a) Sumilon Island. Inset (b) Apo Island. Cross‐hatch indicates marine reserve area and black rectangles indicate approximate positions of permanent 50 × 20 m replicate transects for fish and benthic surveys.
Apo island is a small (74 ha) volcanic island located off the major island of Negros (Fig. 1). It has a very small (~25 ha) NTMR on its southeastern side that was implemented in 1982 and has been maintained successfully since then (Appendix S1: Fig. S2; Alcala and Russ 2006). The use of destructive fishing techniques has been banned around the entire island since 1986 (Alcala and Russ 2006). Both the reserve and fished study sites at this island were impacted by the 1998 bleaching event (Russ et al. 2015c ). The west‐facing reserve site was impacted by a tropical storm in early December 2010, by Severe Tropical Storm Washi in late December 2011 and by Super Typhoon Bopha in early December 2012 (Russ et al. 2015c ).
Sumilon island is a very small (23 ha) coral island located off the major island of Cebu (Fig. 1). It has a small (~40 ha) NTMR on its western side that was implemented in 1974, but was subject to non‐compliance, including the use of highly destructive fishing techniques such as explosives and drive nets (muro ami) in 1984 (Appendix S1: Fig. S2; Russ and Alcala 1998). Unrestricted fishing, but mostly using techniques non‐destructive to the benthos, took place inside the reserve area between 1992 and 1994, and hook and line fishing was permitted in the reserve area from 1995 to 1998 (Alcala and Russ 2006) and from 2008 onward (Appendix S1: Fig. S2; R. A. Abesamis and G. R. Russ, personal observation). The reserve was affected by the 1998 coral bleaching event and a crown‐of‐thorns (COTS) outbreak, possibly beginning as early as 1997 (Russ et al. 2015c ). The effects of the 1998 bleaching/COTS event were limited to the reserve site and did not appear to affect the fished site, potentially due to lower baseline levels of live hard coral cover at the fished site. The fished site was closed to all fishing between 1987 and 1991, and has been a hook and line fishing only site since 2009 (Appendix S1: Fig. S2; Russ et al. 2015b ). It was impacted by Super Typhoon Bopha in December 2012 (Russ et al. 2015c ).
Fishing at Apo and Sumilon islands
Apo and Sumilon islands are two of the best studied coral reefs in the world in terms of long‐term catch composition and fishery yields, dating back to 1974 (Alcala et al. 2005, Abesamis et al. 2006). The coral reefs of Apo and Sumilon are fished by subsistence fishers using traps, gillnets, spear and hook and line. Fishery yields averaging ~10–20 Mg·km−2·yr−1, some of the highest fishery yields recorded from coral reefs globally, have been reported consistently at these two islands for over 30 yr (Russ 1991, Alcala et al. 2005, Abesamis et al. 2006). A key reason for these exceptionally high fishery yields is that the catch at both islands is dominated by planktivores, principally fusiliers and planktivorous surgeonfish (Russ 1991, Russ and Alcala 1998, Abesamis et al. 2006). Russ and Alcala (1998) also showed that almost all reef fish families/trophic groups were caught in similar proportion to their relative biomass on the reef. Russ and Alcala (1998), based on a decade‐long study (1983–1993), concluded that this relatively non‐selective fishing at Apo and Sumilon was unlikely to modify trophic structure of the reef fish assemblage greatly.
Reef fish surveys
A total of 185 species in 13 trophic groups (Appendix S1: Table S1) were counted in underwater visual censuses (UVC) performed on SCUBA by the same observer (G. R. Russ). Fish surveys were made in six 1,000 m2 (50 × 20 m) replicates on the reef slope (3–17 m in reserves, 9–17 m at fished sites) at each of the four sites. The positions of the replicates were the same every year, and surveys were performed at the same time every year (November/December) almost annually between 1983 and 2014 (Appendix S1: Fig. S2). Juveniles (<5 cm total length [TL]) were not counted. Actual counts (number/1,000 m2) were made for the duration of the study for four trophic groups: generalist large predators, piscivores, large benthic foragers, and obligate corallivores.
To account for the high density of some small‐bodied reef fish, log4 abundance categories (category 1. 1 fish; category 2, 2–4 fish; category 3, 5–16 fish; category 4, 17–64 fish; category 5, 65–256 fish; category 6, 257–1,024 fish; category 7, 1,025–4,096 fish; category 8, 4,097–16,384 fish) were used to estimate density of two trophic groups: omnivorous pomacentrids (damselfish) and small planktivores. Estimates of density for the other seven trophic groups were made using a mixture of actual counts and abundance categories. Actual counts were made for the duration of the study for croppers and detritivores (except some species of small surgeonfish from 1983 to 1998), sand feeders (except parrotfish within this trophic group from 1983 to 1998), small benthic foragers (except small wrasses from 1983 to 1998), and large planktivores (except fusiliers from 1983 to 1998). Actual counts were made for excavators and scrapers (except for 1983–1998). Those groups not counted directly from 1983 to 1998 (small surgeonfish, parrotfish, small wrasses, fusiliers) had density estimated in log4 abundance categories. From 1999 to 2014, all of these groups were counted directly. When the log4 abundance categories (1–8) were used to estimate density, they were converted to “best estimates” of actual density in different ways. For species with actual count data from 1999 to 2014, these counts were placed into a Log4 abundance category. The mode of the frequency distribution of actual counts within an abundance category for the period 1999 to 2014 was used as the best estimate of actual density in a Log4 abundance category for the period 1983–1998. For omnivorous pomacentrids and small planktivores, “best estimates” of density within an abundance category were either the mid‐point of the category (Categories 1–6 = 1, 3, 10, 40, 160, and 640 fish respectively) or the minimum within that category (Categories 7 and 8 = 1,025 and 4,097 fish, respectively).
Length estimates (to ±5 cm TL) were made during UVC throughout the study for generalist predators, piscivores, large benthic foragers (except goatfish), and sand feeders (except parrotfish within this group), the Naso (within the croppers), the Acanthurus (within the detritivores), and the Acanthurus, Naso, and Aphareus (within the large planktivores). Length estimates were not made for species in the remaining six trophic groups. For species lacking length estimates, a modal length was assigned, based on extensive field observations. All fish lengths (estimated directly or based on a modal length for the species) were converted to fish biomass using published length–mass relationships (Kulbicki et al. 2005). Numbers of fish and their individual weights were converted to biomass per replicate at each site and year of sampling. Each fish was then assigned to one of the 13 trophic groups (Appendix S1: Table S1), to produce an estimate of biomass per trophic group per replicate, site, and sampling year.
Benthic surveys
Benthic surveys were conducted in the same reef slope areas as the fish surveys, and immediately following the fish surveys. Between 1983 and 1998, benthos was recorded using the point‐intercept technique every 20 cm along a 50‐m transect tape. Between six and nine replicate benthic transects were conducted at each site in each year. From 1999 to 2014, the 1,000 m2 (50 × 20 m) of each replicate fish transect was subdivided into 10 10 × 10 m quadrats, and the cover of major benthic components within each quadrat was estimated by eye to the nearest 5%, while a structural complexity index (SCI) was estimated on a relative scale from 0 to 4. Benthic cover and SCI were then averaged across the 10 quadrats to produce the percent cover and SCI for each 50 × 20 m replicate. The benthic categories reported in this paper were consistent throughout the entire study period (1983–2014): branching hard corals (CB), massive hard corals (CM), and encrusting hard corals (CE), soft coral (SC), hard dead substratum (HDS), rubble (R), sand (S), algal assemblage (AA), and other. CB, CM, and CE cover were combined into one category for live hard coral (HC) for this study. HDS, S, and R cover were pooled into a single category, dead substratum (DS) for this study. Values for the AA and “other” benthic categories were extremely low (Russ et al. 2015c ) and are not reported further here.
Graphical presentations
Temporal trends in fish biomass and benthic cover are presented graphically. Polynomials were fitted to fish biomass and live hard coral (HC) cover to emphasize trends over time at each site, in particular to highlight where major changes to HC cover led to major changes in fish biomass.
Data analysis
Collinearity between pairwise comparisons of benthic variables (i.e., live hard coral [HC] and dead substratum [DS]) was assessed prior to including each term in any regression‐based statistical analyses, as high levels of collinearity will artificially cause one of the other collinear variables to appear strongly significant, while the other appears non‐significant in explanatory models. When high levels of collinearity (r ≥ |0.60|) existed between HC and DS, one of the variables was omitted from further analyses (Zuur et al. 2007, 2013). Under these criteria, DS was removed from analyses due to collinearity with HC cover at both Apo (−0.78) and Sumilon Island (−0.77). Structural complexity (SCI) was measured on a spatial scale of 100 m2. At this scale SCI not only accounted for benthic complexity caused by HC cover, but also by larger structural features of reef slopes, like caves and overhangs, that rarely change in structure. For this reason we included only cover of HC in our analyses, as SCI was much less sensitive to environmental disturbances.
Linear mixed‐effects models (LMEs) were used to assess the effects of NTMR protection from fishing and benthic composition (i.e., live hard coral cover, HC) on reef fish biomass at both islands (Apo and Sumilon). Fixed explanatory variables in each model were NTMR protection status, time (duration of protection), the interaction of NTMR protection status and time, and live hard coral cover (HC). Replicate transects for each year were included as a random factor to account for spatial dependency. LMEs were conducted for each trophic group and total reef fish biomass at each island separately because of the strongly differing management and disturbance histories at each island (Appendix S1: Fig. S2). Additional LMEs were performed to assess whether NTMR protection status, time, or the interaction of the two had an influence on live hard coral cover (HC). Due to the complex management history at Sumilon Island (Appendix S1: Fig. S2), with alternating periods of protection and fishing of the “reserve” site, as well as some intermittent protection of the “fished” site, duration of protection of Sumilon reserve did not correspond to chronological time. Thus, for data analysis, each time protection of the reserve site was re‐implemented, it was treated as having duration of protection = 0, and each time fishing activities were re‐implemented, it was treated as having duration of (non) protection = 0. All LMEs were built and fitted in R (R Core Team 2017) using the nlme package (Pinheiro et al. 2017). Model fit was assessed using residual plots and where necessary data were square‐root transformed.
Spatial and temporal patterns in the biomass of reef fish trophic groups were explored using non‐metric multidimensional scaling (NMDS) based on Bray‐Curtis dissimilarities in which data were square‐root transformed to down‐weigh higher biomass of certain groups. Similarly, spatial and temporal patterns in HC and DS cover, along with duration of NTMR protection, were explored using NMDS based on Euclidean distances; data were natural‐log‐transformed and normalized to improve the spread of the data. Each NMDS was conducted on mean fish biomass, percent cover, or duration of NTMR protection at each site within each year (i.e., all replicate surveys were averaged within each site within each year). NMDS analyses were performed in PRIMER v7 (Clarke et al. 2014).
Relationships between the trophic structure of the reef fish assemblage, benthic data, and duration of protection were assessed via the BEST (Bio‐Env) routine in PRIMER. Using a Spearman rank correlation, benthic habitat and duration of NTMR protection and fish assemblage matrices were compared, allowing identification of the variable(s) that explained the greatest variance in trophic structure of the reef fish assemblage (Clarke and Warwick 2001) with significance tested with 999 permutations. Three variables were considered: (1) duration of NTMR protection (years), (2) live hard coral cover (HC), and (3) dead substratum (DS) cover.
Results
Effects of no‐take marine reserve protection and fishing on total reef fish biomass and live hard coral cover
No‐take marine reserve protection is an experimental manipulation of fishing at the two study islands. Detection of an NTMR effect requires a significant NTMR status × time interaction in the LMEs (Table 1) coupled with a clear visual divergence of the fish biomass (or coral cover) trajectories between the NTMR and fished site over time (Figs. 2 and 3). A direct NTMR effect would be an increase in the fish biomass (or coral cover) in the NTMR relative to the fished site over time. An indirect NTMR effect on fish biomass would be a decrease in the fish biomass in the NTMR relative to the fished site over time, possibly caused, for example, by the increase in biomass in the NTMR of a predator or competitor of the fish group in question. An indirect NTMR effect on coral cover could be due to a direct or indirect NTMR effect on biomass of benthic feeding fish that precedes a positive or negative change in coral cover. Note that lack of direct NTMR effects reduces the chances of indirect NTMR effects. By these criteria, direct NTMR effects were detected at both islands for total fish biomass but not for coral cover (Fig. 2). At Apo Island, the direct NTMR effects on total fish biomass were likely caused by long‐term decline at the fished site relative to the NTMR site (Fig. 2a). The direct NTMR effects on total fish biomass were much clearer at Apo than at Sumilon Island (Fig. 2a). A clear divergence of the NTMR and fished trajectories for total fish biomass was not detected until 13–14 yr of protection at Sumilon, after which biomass in both NTMR and fished sites declined from 15 to 20 yr of protection (Fig. 2a).
Table 1.
Parameter estimates for the linear mixed‐effects (LME) models.
| Response variable and effect | Apo | Sumilon | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | df | t | P | Estimate | SE | df | t | P | |
| Generalist large predators | ||||||||||
| Intercept | 0.383 | 0.153 | 222 | 2.506 | 0.013 | 0.459 | 0.14 | 238 | 3.273 | 0.001 |
| Status | −0.030 | 0.273 | 222 | −0.111 | 0.912 | 0.308 | 0.136 | 238 | 2.266 | 0.024 |
| Time | −0.008 | 0.008 | 222 | −1.061 | 0.290 | 0.02 | 0.008 | 238 | 2.318 | 0.021 |
| Status × Time | 0.025 | 0.012 | 222 | 2.199 | 0.029 | 0.029 | 0.011 | 238 | 2.614 | 0.01 |
| HC | 0.016 | 0.002 | 222 | 8.134 | <0.001 | −0.004 | 0.003 | 238 | −1.036 | 0.301 |
| Piscivores | ||||||||||
| Intercept | 0.254 | 0.143 | 222 | 1.468 | 0.144 | 0.484 | 0.109 | 238 | 4.447 | <0.001 |
| Status | 0.016 | 0.301 | 222 | 0.054 | 0.957 | 0.271 | 0.111 | 238 | 2.435 | 0.016 |
| Time | 0.004 | 0.009 | 222 | 0.049 | 0.621 | 0.032 | 0.007 | 238 | 4.529 | <0.001 |
| Status × Time | 0.017 | 0.013 | 222 | 1.346 | 0.179 | 0.013 | 0.009 | 238 | 1.446 | 0.15 |
| HC | 0.009 | 0.002 | 222 | 4.518 | <0.001 | −0.001 | 0.002 | 238 | −0.231 | 0.818 |
| Large benthic foragers | ||||||||||
| Intercept | 0.417 | 0.092 | 222 | 4.513 | <0.001 | 0.669 | 0.036 | 238 | 18.762 | <0.001 |
| Status | 0.414 | 0.169 | 222 | 2.453 | 0.015 | −0.092 | 0.039 | 238 | −2.375 | 0.018 |
| Time | 0.004 | 0.004 | 222 | 0.917 | 0.36 | −0.002 | 0.002 | 238 | −0.637 | 0.525 |
| Status × Time | −0.013 | 0.007 | 222 | −1.747 | 0.082 | 0.004 | 0.003 | 238 | 1.238 | 0.217 |
| HC | −0.006 | 0.001 | 222 | −4.956 | <0.001 | −0.0001 | 0.001 | 238 | −0.133 | 0.895 |
| Croppers | ||||||||||
| Intercept | 0.569 | 0.057 | 222 | 10.032 | <0.001 | 0.473 | 0.046 | 238 | 10.275 | <0.001 |
| Status | 0.169 | 0.105 | 222 | 1.621 | 0.106 | 0.15 | 0.05 | 238 | 3.002 | 0.003 |
| Time | 0.002 | 0.003 | 222 | 0.782 | 0.435 | −0.002 | 0.003 | 238 | −0.766 | 0.444 |
| Status × Time | −0.007 | 0.004 | 222 | −1.674 | 0.096 | 0.006 | 0.004 | 238 | 1.511 | 0.132 |
| HC | −0.001 | 0.001 | 222 | −1.305 | 0.193 | −0.003 | 0.001 | 238 | −2.294 | 0.023 |
| Detritivores | ||||||||||
| Intercept | 6.132 | 0.285 | 222 | 21.539 | <0.001 | 0.964 | 0.222 | 238 | 4.343 | <0.001 |
| Status | −3.848 | 0.514 | 222 | −7.488 | <0.001 | 1.451 | 0.24 | 238 | 6.046 | <0.001 |
| Time | −0.113 | 0.015 | 222 | −7.57 | <0.001 | 0.058 | 0.015 | 238 | 3.835 | <0.001 |
| Status × Time | 0.105 | 0.022 | 222 | 4.845 | <0.001 | −0.101 | 0.02 | 238 | −5.112 | <0.001 |
| HC | −0.017 | 0.003 | 222 | −4.505 | <0.001 | −0.004 | 0.006 | 238 | −0.693 | 0.489 |
| Excavators | ||||||||||
| Intercept | 1.097 | 0.088 | 222 | 12.45 | <0.001 | 1.332 | 0.111 | 238 | 12.021 | <0.001 |
| Status | 0.666 | 0.161 | 222 | 4.147 | <0.001 | 0.739 | 0.12 | 238 | 6.171 | <0.001 |
| Time | 0.013 | 0.005 | 222 | 2.733 | 0.007 | 0.009 | 0.007 | 238 | 1.167 | 0.244 |
| Status × Time | −0.021 | 0.007 | 222 | −3.074 | 0.002 | −0.031 | 0.01 | 238 | −3.163 | 0.002 |
| HC | −0.008 | 0.001 | 222 | −6.792 | <0.001 | −0.008 | 0.003 | 238 | −2.773 | 0.006 |
| Scrapers | ||||||||||
| Intercept | 1.132 | 0.085 | 222 | 13.276 | <0.001 | 1.296 | 0.066 | 238 | 19.497 | <0.001 |
| Status | 0.549 | 0.157 | 222 | 3.499 | 0.001 | 0.266 | 0.072 | 238 | 3.698 | <0.001 |
| Time | 0.015 | 0.005 | 222 | 3.281 | 0.001 | −0.005 | 0.004 | 238 | −0.997 | 0.32 |
| Status × Time | −0.024 | 0.007 | 222 | −3.649 | <0.001 | −0.01 | 0.005 | 238 | −1.711 | 0.088 |
| HC | −0.005 | 0.001 | 222 | −4.445 | <0.001 | −0.001 | 0.002 | 238 | −0.481 | 0.631 |
| Sand feeders | ||||||||||
| Intercept | 0.606 | 0.125 | 222 | 4.857 | <0.001 | 0.872 | 0.084 | 238 | 10.388 | <0.001 |
| Status | −0.058 | 0.229 | 222 | −0.252 | 0.801 | −0.287 | 0.089 | 238 | −3.229 | 0.001 |
| Time | 0.010 | 0.007 | 222 | 1.442 | 0.151 | 0.016 | 0.006 | 238 | 2.813 | 0.005 |
| Status × Time | 0.006 | 0.01 | 222 | 0.611 | 0.542 | −0.028 | 0.007 | 238 | −3.89 | <0.001 |
| HC | −0.010 | 0.002 | 222 | −6.195 | <0.001 | −0.003 | 0.002 | 238 | −1.434 | 0.153 |
| Small benthic foragers | ||||||||||
| Intercept | 0.338 | 0.024 | 222 | 14.065 | <0.001 | 0.784 | 0.027 | 238 | 29.298 | <0.001 |
| Status | −0.061 | 0.043 | 222 | −1.399 | 0.163 | −0.279 | 0.028 | 238 | −9.938 | <0.001 |
| Time | −0.005 | 0.001 | 222 | −3.939 | <0.001 | −0.013 | 0.002 | 238 | −7.503 | <0.001 |
| Status × Time | 0.004 | 0.001 | 222 | 2.309 | 0.022 | 0.009 | 0.002 | 238 | 3.804 | <0.001 |
| HC | 0.0001 | 0.0003 | 222 | 0.336 | 0.738 | −0.001 | 0.001 | 238 | −1.549 | 0.123 |
| Obligate corallivores | ||||||||||
| Intercept | 0.047 | 0.031 | 222 | 1.504 | 0.134 | 0.328 | 0.023 | 238 | 14.186 | <0.001 |
| Status | −0.168 | 0.057 | 222 | −2.943 | 0.004 | −0.098 | 0.022 | 238 | −4.466 | <0.001 |
| Time | 0.006 | 0.002 | 222 | 3.605 | <0.001 | −0.005 | 0.001 | 238 | −3.805 | <0.001 |
| Status × Time | 0.002 | 0.002 | 222 | 0.817 | 0.415 | 0.01 | 0.001 | 238 | 4.878 | <0.001 |
| HC | 0.003 | 0.0004 | 222 | 6.358 | <0.001 | 0.001 | 0.001 | 238 | 1.415 | 0.159 |
| Omnivorous pomacentrids | ||||||||||
| Intercept | 0.930 | 0.031 | 222 | 29.92 | <0.001 | 1.135 | 0.057 | 238 | 19.999 | <0.001 |
| Status | 0.113 | 0.056 | 222 | 2.018 | 0.045 | −0.313 | 0.061 | 238 | −5.094 | <0.001 |
| Time | −0.003 | 0.001 | 222 | −1.783 | 0.076 | −0.02 | 0.004 | 238 | −5.146 | <0.001 |
| Status × Time | −0.009 | 0.002 | 222 | −3.927 | <0.001 | 0.015 | 0.005 | 238 | 2.985 | 0.003 |
| HC | −0.001 | 0.0004 | 222 | −1.425 | 0.156 | 0.006 | 0.001 | 238 | 4.114 | <0.001 |
| Large planktivores | ||||||||||
| Intercept | 3.267 | 0.296 | 222 | 11.022 | <0.001 | 4.712 | 0.315 | 238 | 14.946 | <0.001 |
| Status | −0.276 | 0.485 | 222 | −0.57 | 0.569 | −0.756 | 0.324 | 238 | −2.333 | 0.021 |
| Time | −0.067 | 0.014 | 222 | −4.795 | <0.001 | −0.074 | 0.02 | 238 | −3.651 | <0.001 |
| Status × Time | 0.076 | 0.021 | 222 | 3.709 | <0.001 | 0.086 | 0.027 | 238 | 3.217 | 0.002 |
| HC | 0.015 | 0.004 | 222 | 4.307 | <0.001 | −0.002 | 0.008 | 238 | −0.273 | 0.785 |
| Small planktivores | ||||||||||
| Intercept | 1.453 | 0.082 | 222 | 17.742 | <0.001 | 1.187 | 0.059 | 238 | 20.282 | <0.001 |
| Status | 0.19 | 0.15 | 222 | 1.262 | 0.208 | 0.727 | 0.063 | 238 | 11.606 | <0.001 |
| Time | −0.014 | 0.004 | 222 | −3.157 | 0.002 | −0.009 | 0.004 | 238 | −2.209 | 0.028 |
| Status × Time | −0.004 | 0.006 | 222 | −0.638 | 0.524 | −0.019 | 0.005 | 238 | −3.726 | <0.001 |
| HC | 0.009 | 0.001 | 222 | 8.635 | <0.001 | 0.001 | 0.002 | 238 | 0.804 | 0.422 |
| Total biomass | ||||||||||
| Intercept | 5.117 | 0.222 | 222 | 23.013 | <0.001 | 5.804 | 0.267 | 238 | 21.737 | <0.001 |
| Status | −0.382 | 0.362 | 222 | −1.057 | 0.292 | −0.009 | 0.272 | 238 | −0.035 | 0.972 |
| Time | −0.061 | 0.011 | 222 | −5.784 | <0.001 | −0.048 | 0.017 | 238 | −2.801 | 0.006 |
| Status × Time | 0.065 | 0.015 | 222 | 4.222 | <0.001 | 0.049 | 0.022 | 238 | 2.209 | 0.028 |
| HC | 0.013 | 0.003 | 222 | 4.84 | <0.001 | −0.006 | 0.007 | 238 | −0.819 | 0.414 |
| Hard coral | ||||||||||
| Intercept | 0.978 | 0.467 | 223 | 2.087 | 0.038 | 20.677 | 2.078 | 239 | 9.95 | <0.001 |
| Status | 0.942 | 0.646 | 223 | 13.838 | <0.001 | 11.566 | 2.421 | 239 | 4.778 | <0.001 |
| Time | 0.219 | 0.021 | 223 | 10.536 | <0.001 | −0.413 | 0.157 | 239 | −2.635 | 0.009 |
| Status × Time | −0.377 | 0.029 | 223 | −13.15 | <0.001 | 0.47 | 0.206 | 239 | 2.284 | 0.023 |
Shown are effect size estimates, standard errors, degrees of freedom (df), t values, and associated P values for predictors of biomass of each individual trophic group, total biomass, and hard coral cover at Apo and Sumilon Islands. Status effects are relative to fished areas. P values in boldface type are significant at the 0.05 level.
Fig. 2.
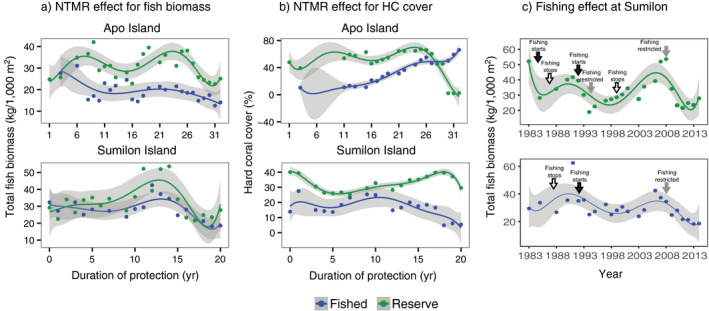
Long‐term (1983–2014) temporal trends in (a) total reef fish biomass and (b) live hard coral cover (HC) vs. years of protection by no‐take marine reserve (NTMR) status at Apo Island (top panels of a and b) and Sumilon Island (bottom panels of a and b). (c) Total reef fish biomass vs. chronological time (1983–2014) at Sumilon NTMR (top panel) and Sumilon fished site (bottom panel). Arrows in c indicate times when fishing starts (black), stops (white), or was restricted to hook and line fishing only (gray). Data points are means, trend lines are sixth order polynomials, and shading represents 95% confidence intervals. NTMR sites are shown in green and fished sites in blue. Note that the y‐axis for hard coral is percent cover, not biomass. Note y‐axis ranges differ among panels.
Fig. 3.
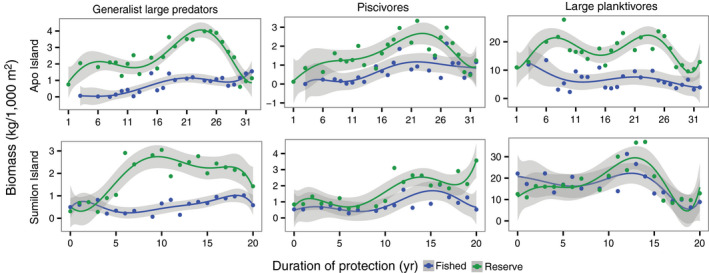
Long‐term (1983–2014) temporal trends in biomass of three trophic groups vs. years of protection by no‐take marine reserve (NTMR) status at Apo (upper panels) and Sumilon (lower panels) Islands. These three trophic groups had the clearest evidence of direct NTMR effects. Data points are means, trend lines are sixth‐order polynomials, and shading represents 95% confidence intervals. NTMR sites shown in green and fished sites in blue. Note y‐axis ranges differ among panels.
There was no visual evidence that NTMR protection or fishing had a direct or indirect effect on live hard coral cover (HC) (Fig. 2b), despite significant NTMR status × time interactions at both islands (Table 1). At Apo Island, HC increased slightly during the first 15 yr of NTMR protection (1983–1997), before declining sharply after 26 yr of NTMR protection (Fig. 2b). At the Apo fished site, HC cover was low (~10–15%) and stable from 1983 to 1997, then increased steadily for the next 15 yr (1999–2014) (Fig. 2b). At Sumilon Island, HC cover was higher in the NTMR than in the fished site from the outset, with the largest divergence of the HC trajectories from 15 to 20 yr of NTMR protection (Fig. 2b).
Sumilon NTMR had a very dynamic history of protection from fishing (Appendix S1: Fig. S2). NTMR protection was interrupted by two bouts of fishing, each of three years duration (1984–1986 and 1992–1994; black arrows in Fig. 2c, upper panel). After almost a decade of effective protection from fishing (1974–1983, see Appendix S1: Fig. S2) Sumilon NTMR was opened to fishing and fished intensively with explosives, drive nets, traps, hook and line, spears, and gill nets between 1983 and 1985 (Fig. 2c, upper panel). Total reef fish biomass almost halved in just two years (1983–1985) due to this intensive fishing that used techniques (explosives, drive nets) destructive to the benthos (Fig. 2c, upper panel). Fishing was again banned in Sumilon NTMR from 1987 to 1991, and total reef fish biomass increased markedly (Fig. 2c, upper panel), before declining again when fishing occurred from 1992 to 1994 (Fig. 2c, upper panel). Only line fishing was permitted in Sumilon NTMR from 1995 to 1998, after which all fishing was banned from 1999 to 2008 (Fig. 2c, upper panel). From 1995 to 2008 total reef fish biomass increased markedly to levels seen in 1983 (after almost a decade of protection from fishing), before declining again from 2009 to 2014, likely associated with the re‐introduction of hook and line fishing from 2008 onward (R. A. Abesamis and G. R. Russ, personal observation; gray “fishing restricted” arrow in 2008 in Fig. 2c, upper panel; Appendix S1: Fig. S2). The Sumilon fished site also had changes to fishing regulations during the study (Appendix S1: Fig. S2; Fig. 2c, lower panel). All fishing was banned at this site from 1987 to 1991, leading to a strong increase in total fish biomass during this 5‐yr period (Fig. 2c, lower panel). When fishing reopened at this site in 1992 (black arrow in Fig. 2c, lower panel), reef fish biomass declined (Fig. 2c, lower panel). Fishing was restricted to hook and line only at the Sumilon fished site in 2009 (Fig. 2c, lower panel), but this had little effect on total fish biomass, which actually declined to the lowest levels seen in 31 yr (Fig. 2c, lower panel).
Direct effects of NTMR protection on trophic components of the reef fish biomass
Direct NTMR effects were detected at both islands for only two of the 13 trophic components of reef fish biomass, large generalist predators, and large planktivores (Fig. 3, Table 1; see Appendix S1: Figs. S3, S4 for NTMR effects for all 13 trophic components). A third trophic group, piscivores, displayed visual evidence of increase in biomass in the NTMR relative to the fished site over time, but no significant NTMR status × time interaction at both islands (Fig. 3, Table 1). Large generalist predators, and piscivores, increased in biomass in the NTMRs relative to the fished sites over time at both islands (Fig. 3). Large planktivores (mostly fusiliers and surgeonfish), usually dominated total reef fish biomass, with this trophic group often accounting for more than 50% of reef fish biomass at most sites and times (Appendix S1: Fig. S5; compare y‐axis scales for large planktivores in Fig. 3 with total fish biomass in Fig. 2a). At Apo Island, in contrast to generalist large predators and piscivores, the direct NTMR effect on large planktivores appears to have been due to long‐term decline at the fished site relative to the NTMR site (Fig. 3). The direct NTMR effects on large planktivores were much clearer at Apo than at Sumilon Island (Fig. 3). A divergence of the NTMR and fished trajectories of large planktivores was not clear until 13–14 yr of protection at Sumilon, after which biomass in both the NTMR and fished site declined at 15–20 yr of protection (Fig. 3).
Indirect effects of NTMR protection on trophic components of reef fish biomass
Just 3 of 13 trophic groups showed limited evidence of possible indirect NTMR effects (long‐term decline in biomass in NTMR relative to fished site, associated with long‐term increase in predators in NTMR; significant NTMR status × time interaction), omnivorous pomacentrids at Apo (Table 1; Appendix S1: Figs. S3, S4), and small planktivores and excavators at Sumilon (Table 1; Appendix S1: Figs. S3, S4). Patterns of change of biomass in the NTMR relative to the fished site for these three groups at these particular islands seem consistent with expectations of a true indirect NTMR effect, that is, green and blue lines converge, rather than diverge, over time and there is a significant NTMR status × time interaction. It is possible that all three of these trophic groups are common prey of large generalist predators and piscivores that increased in biomass over time in both NTMRs (Fig. 3).
Brief history of environmental disturbances to the benthos
Throughout the 31‐yr study, both Apo and Sumilon NTMRs and fished sites were affected by a number of environmental disturbances (Appendix S1: Fig. S2; red brackets under x‐axis in Fig. 4). Between 1983 and 1985, a breakdown in protection at the Sumilon NTMR led to a use of destructive fishing techniques (explosives, drive nets) that reduced live hard coral cover (HC) by 50%, and substantially increased cover of dead substratum (sand, rubble, and hard dead substratum; Fig. 4). Cover of HC recovered after this event in Sumilon NTMR from 1988 to 1995 (Fig. 4). Coral bleaching in 1998 reduced HC and increased cover of dead substratum substantially at two of the four study sites (Apo NTMR, Sumilon NTMR) (Fig. 4). The reduction of HC cover at Sumilon NTMR in 1998 was also associated with a crown‐of‐thorns starfish outbreak. HC cover increased substantially for ≈10 yr in both NTMRs following the 1998 bleaching event (Fig. 4). At the Apo fished site, the 1998 coral bleaching event killed soft corals, which had been the dominant benthic cover for the previous 15 yr (Russ and Leahy 2017). This bleaching event caused a marked shift at the Apo fished site from a soft coral‐dominated benthos to one dominated by hard corals (mostly Acropora), with HC increasing from 15% in 1997 to 70% cover in 2014 (Fig. 4). HC cover again declined substantially within the Apo NTMR when a storm (2010) and two typhoons (2011, 2012) affected the benthos (Fig. 4). These major environmental disturbance events resulted in HC cover dropping in the Apo NTMR from ≈60% to ~1% from 2009 to 2012–2014 (Fig. 4). After the storm and typhoons, the reef slope of the Apo NTMR was dominated by dead substratum (coral rubble and sand). The Sumilon fished site was also impacted by the same typhoon in 2012, reducing HC cover by more than 70% (from 18% to <5%; Fig. 4). Four of six replicates at this site became almost 100% covered with sand that cascaded down the reef slope from the shallows, smothering live corals.
Fig. 4.
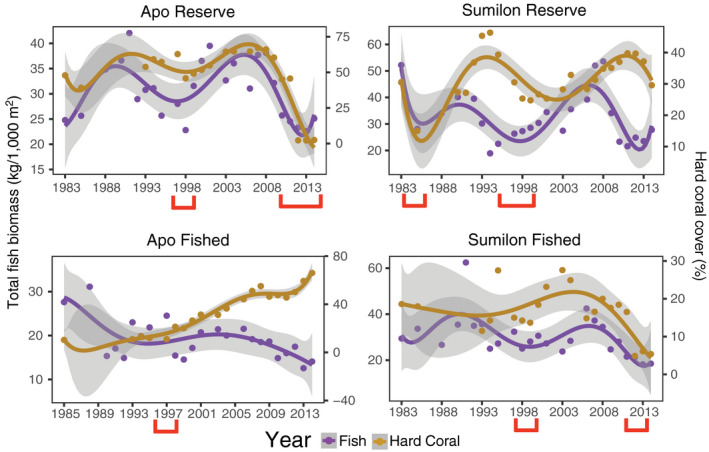
Long‐term (1983–2014) temporal trends in total reef fish biomass (left y‐axis) and hard coral cover (HC) (right y‐axis) at Apo NTMR and Apo fished sites (left column) and Sumilon NTMR and fished site (right column). Data points are means, trend lines are sixth‐order polynomials, and shading represents 95% confidence intervals. Fish biomass is purple and live hard coral is brown. Note y‐axis ranges differ among panels. Environmental disturbances (red brackets) are coral bleaching (1998) at all four sites, local storm (2010) and back‐to‐back typhoons (2011–2012) at Apo NTMR, destructive fishing (1984) at Sumilon NTMR, and typhoon (2012) at Sumilon fished site.
Relationships between total reef fish biomass and live hard coral cover over time
Strong evidence for effects of cover of live hard corals (HC) on reef fish biomass was taken to be visual changes in fish biomass following change in cover of HC (Figs. 4, 5, 6) and a significant HC effect in the LMEs (Table 1). The latter statistical test, integrated over the entire 31 yr of study, was, however, sometimes insensitive to some very clear short‐term and long‐term changes in HC cover that led to subsequent changes in fish biomass, particularly at Sumilon Island. Thus, we also used clear visual changes in fish biomass following change in HC cover, but no significant effect of HC in the LMEs, as a medium level of evidence for effects of HC cover on fish biomass.
Fig. 5.
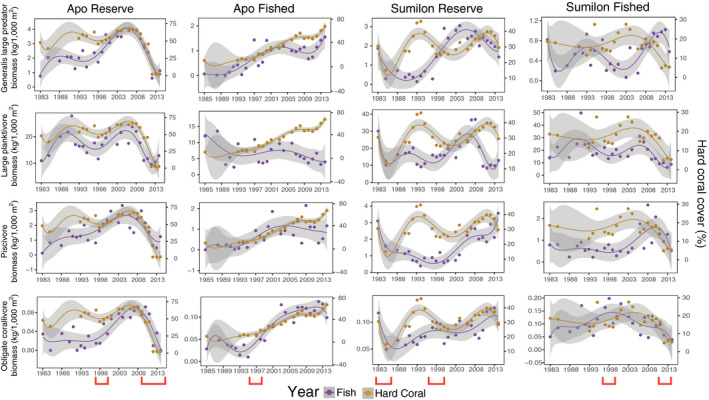
Long‐term (1983–2014) temporal trends in biomass (left y‐axis) of four trophic groups of reef fish and live hard coral cover (HC; right y‐axis) at Apo NTMR (first column), Apo fished site (second column), Sumilon NTMR (third column), and Sumilon fished site (fourth column). These are examples of trophic groups that had a generally positive relationship with HC. Data points are means, trend lines are sixth‐order polynomials, and shading represents 95% confidence intervals. Fish biomass is purple and live hard coral is brown. Note y‐axis ranges differ among panels. Environmental disturbances (red brackets) are coral bleaching (1998) at all four sites, local storm (2010) and back‐to‐back typhoons (2011–2012) at Apo NTMR, destructive fishing (1984) at Sumilon NTMR, and typhoon (2012) at Sumilon fished site.
Fig. 6.
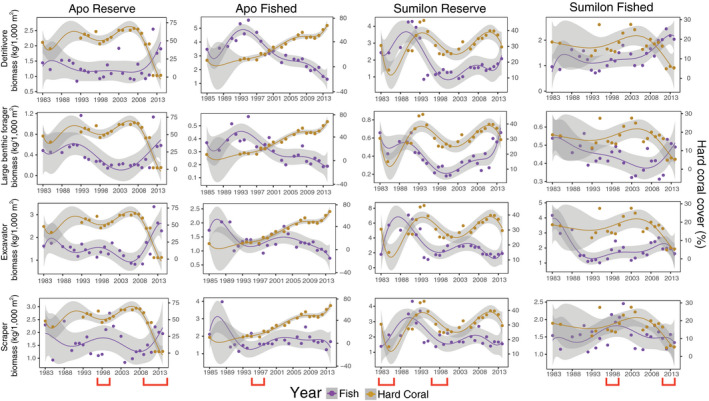
Long‐term (1983–2014) temporal trends in biomass (left y‐axis) of four trophic groups of reef fish and live hard coral cover (HC) (right y‐axis) at Apo NTMR (first column), Apo fished site (second column), Sumilon NTMR (third column), and Sumilon fished site (fourth column). These are examples of trophic groups that had a generally negative relationship with HC. Data points are means, trend lines are sixth‐order polynomials, and shading represents 95% confidence intervals. Fish biomass is purple and live hard coral is brown. Note y‐axis ranges differ among panels. Environmental disturbances (red brackets) are coral bleaching (1998) at all four sites, local storm (2010) and back‐to‐back typhoons (2011–2012) at Apo NTMR, destructive fishing (1984) at Sumilon NTMR, and typhoon (2012) at Sumilon fished site.
At the Apo NTMR site, total reef fish biomass declined due to coral bleaching in 1998 (Fig. 4), recovered for the next decade (1999–2009), and then declined sharply from 2010 onward due to a storm (2010) and two typhoons (2011–2012) that reduced HC from ~60% to ~1% cover (Fig. 4). The Apo fished site had relatively low HC cover from 1983 to 1997, but a clear monotonic increase in HC cover from 1998 to 2014 from ~15% to almost 70% (Fig. 4). Total fish biomass was positively and significantly related to HC cover at Apo Island (Table 1), but at the Apo fished site the total fish biomass was stable to declining as HC increased from 1998 to 2014 (Fig. 4, Table 1). At Sumilon Island, total fish biomass was not significantly related to HC cover statistically (Table 1). Total fish biomass at Sumilon NTMR had a sharp decline from 1983 to 1985, likely caused by the direct effect of destructive fishing confounded with reduction in HC cover (Fig. 4). Total fish biomass at Sumilon NTMR recovered somewhat from 1985 to 1994 as HC recovered (Fig. 4). Total fish biomass at Sumilon NTMR declined when HC cover declined due to the coral bleaching/COTS event in 1998 (Fig. 4), recovered as HC recovered during the period 1999–2013 (Fig. 4), before declining again late in the study (Fig. 4). At the Sumilon fished site, just one of the two major disturbance events had a clear effect on total biomass of reef fish, the typhoon in 2012 (Fig. 4). This typhoon reduced HC cover substantially and led to a decrease in total biomass in 2012 (Fig. 4).
Relationships between trophic components of the reef fish biomass and live hard coral cover over time
Six of 13 trophic groups of reef fish had largely positive relationships with live hard coral (HC) cover: generalist large predators, large planktivores, piscivores, and obligate corallivores (all shown in Fig. 5), and small planktivores and omnivorous pomacentrids (both shown in Appendix S1: Figs. S6–S9). HC cover at Apo Island affected biomass of all six of these trophic groups significantly and positively (Table 1). Only omnivorous pomacentrids had a statistically significant positive association with HC cover at Sumilon Island (Table 1).
At the Apo NTMR site, biomass of generalist large predators and large planktivores declined as HC cover declined due to coral bleaching in 1998 (Fig. 5). Biomass of generalist large predators, large planktivores, piscivores and obligate corallivores all increased as HC recovered for the next decade (1999–2009) at the Apo NTMR, and then declined sharply from 2010 onward due to a storm (2010) and two typhoons (2011–2012) that reduced HC from ~60% to ~1% cover (Fig. 5). Small planktivores also declined sharply in biomass due to the storm and typhoons (2010–2012) at Apo NTMR (Appendix S1: Fig. S6).
The Apo fished site had relatively low HC cover from 1983 to 1997, but a clear, monotonic increase in HC cover from 1998 to 2014 from ~15% to almost 70% (Fig. 5). Generalist large predators, piscivores and obligate corallivores all showed long‐term (15 yr), significant increases in biomass from 1998 to 2014 (Fig. 5, Table 1). Biomass of large planktivores was positively and significantly related to HC cover at Apo Island (Table 1), but at the Apo fished site the biomass of these fish was stable to declining as HC cover increased from 1998 to 2014 (Fig. 5, Table 1).
At Sumilon NTMR, biomass of large generalist large predators, large planktivores, piscivores, and obligate corallivores (all shown in Fig. 5) and small planktivores, croppers, and omnivorous pomacentrids (all shown in Appendix S1: Fig. S8) all declined sharply from 1983 to 1985 due to the destructive fishing event. Biomass of large planktivores (Fig. 5), obligate corallivores (Fig. 5), and omnivorous pomacentrids (Appendix S1: Fig. S8) all recovered in biomass somewhat from 1985 to 1994 as HC recovered. In contrast, the biomass of generalist large predators and piscivores did not recover until HC had recovered during the period 1999–2013 (Fig. 5). Piscivores, large planktivores, obligate corallivores (all Fig. 5), and croppers and omnivorous pomacentrids (both Appendix S1: Fig. S8) had small but detectable drops, or an asymptote, in biomass when HC declined due to the coral bleaching/COTS event in 1998 (Fig. 5). Most of these trophic groups then recovered biomass as HC recovered during the period 1999–2013 (Fig. 5).
At the Sumilon fished site, just one of the two major disturbance events had a clear effect on biomass of reef fish: the typhoon in 2012 (Fig. 5). This severe environmental disturbance to the benthos decreased biomass of generalist large predators, large planktivores, piscivores, and obligate corallivores (all shown in Fig. 5) and small planktivores, omnivorous pomacentrids, and small benthic foragers (all shown in Appendix S1: Fig. S9).
Five of 13 trophic groups of reef fish had largely negative relationships with live hard coral cover (HC): detritivores, large benthic foragers, excavators, scrapers (all shown in Fig. 6), and sand feeders (Appendix S1: Figs. S6–S9). All of these groups had statistically significant negative relationships with HC cover at Apo Island (Table 1). Only excavators had a significantly negative relationship with HC cover at Sumilon Island (Table 1).
At Apo NTMR, excavators and scrapers (both parrotfish) increased in biomass following the coral bleaching (1998) and storms/typhoons (2010–2014), and decreased in biomass as HC cover increased (1983–1997 and 1999–2009; Fig. 6, Table 1). Large benthic foragers (Fig. 6), detritivores (Fig. 6), and sand feeders (Appendix S1: Fig. S6) all increased sharply in biomass following the reduction in HC due to storms and typhoons (2010–2014; Fig. 6, Table 1).
At the Apo fished site, detritivores, large benthic foragers and excavators (all shown in Fig. 6 and Table 1), and sand feeders (Appendix S1: Fig. S7), all showed long‐term, significant declines in biomass as HC cover increased, particularly from 1999 to 2014 (Fig. 6, Table 1). Scrapers had a significant negative association with HC at Apo Island (Table 1) but at best maintained biomass as HC cover increased from 1999 to 2014 at the Apo fished site (Fig. 6).
At Sumilon NTMR, detritivores, excavators, and scrapers increased in biomass almost immediately, or a few years after, the destructive fishing event in 1984, likely due to the reduction in HC cover (and subsequent increase in cover of dead substrata; Fig. 6). Detritivores, large benthic foragers, excavators, scrapers (all shown in Fig. 6), and sand feeders (Appendix S1: Fig. S8) all decreased in biomass as HC recovered in Sumilon NTMR from 1988 to 1994. The similar temporal oscillations in HC cover and biomass of detritivores, excavators, scrapers (all shown in Fig. 6), and sand feeders (Appendix S1: Fig. S8) at Sumilon NTMR are interpreted as delayed responses of fish groups to changes in HC cover. Increases in biomass of these groups often occurred several years after decline in HC cover, and decreases in biomass often occurred gradually as HC recovered (Fig. 6).
At the Sumilon fished site, just one of the two major disturbance events had a clear effect on biomass of reef fish, the typhoon in 2012 (Fig. 6). Detritivores, large benthic foragers (both shown in Fig. 6), and sand feeders (Appendix S1: Fig. S9) increased in biomass in response to this disturbance. It is noteworthy that parrotfish (scrapers, excavators) did not increase in biomass in response to the typhoon in 2012 (Fig. 6), since the benthos became dominated by a substratum not generally favorable to feeding by these groups: sand.
Assemblage structure of benthos and reef fish
The benthic and fish assemblages were distinct at all sites from the outset, and remained so for most of the 31 yr, despite large differences among sites in fishing pressure and disturbance history (Fig. 7). In the first 15 yr of the study (1983–1998), the two NTMRs had higher cover of live hard coral (HC) than their paired fished sites, which tended to have higher cover of dead substrata (DS; Fig. 7a). Substantial shifts in HC cover occurred at three sites during the study. At the Apo fished site, HC cover went from ~15% in 1997 to ~70% in 2014 (Fig. 4) following the 1998 coral bleaching event that killed most of the dominant benthos (soft corals), allowing branching Acropora corals to become dominant over the next 15 yr (Russ and Leahy 2017). HC cover at both the Apo NTMR and the Sumilon fished site declined very sharply due to the effects of typhoons in 2012 (circled outliers in Figs. 4, 7).
Fig. 7.
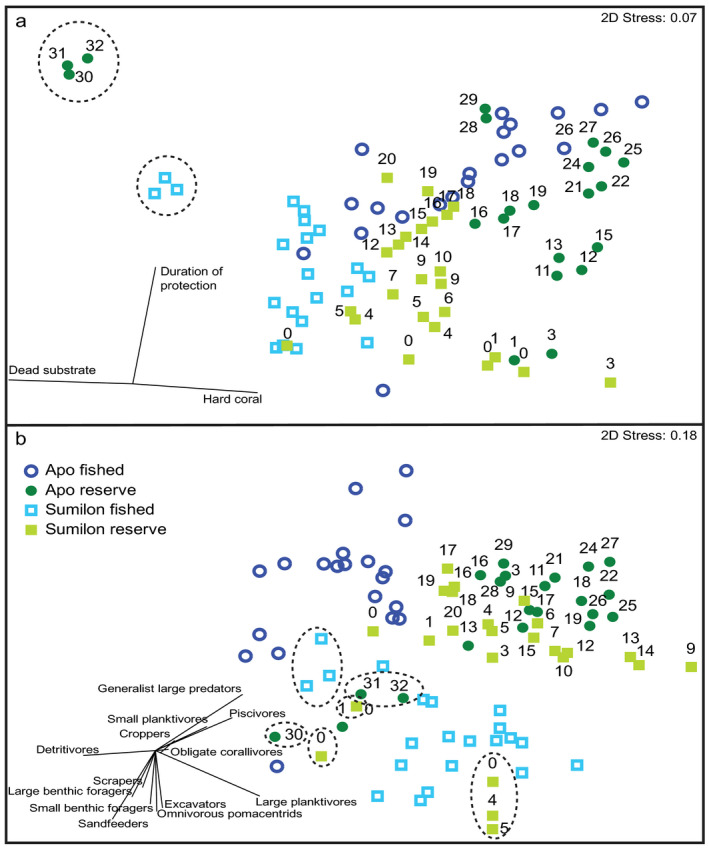
Nonmetric multidimensional scaling (NMDS) analysis performed on distance matrices for (a) benthic cover of hard coral and dead substrate and duration of NTMR protection (yr) and (b) the biomass of reef fish trophic groups. Apo fished sites are dark blue, Apo NTMR sites are dark green, Sumilon fished sites are light blue, and Sumilon NTMR sites are light green. Dotted circles (benthos) and ellipses (fish) represent years when typhoons hit Apo NTMR and the Sumilon fished site, and when Sumilon NTMR was opened to fishing in 1984 and 1992. Numbers adjacent to NTMR sites (dark and light green) indicate the duration of NTMR protection in years. Vectors represent Pearson correlations between the original variables ([a] hard coral, dead substrate, duration of NTMR protection; [b] biomass of reef fish trophic groups). Lengths of the vectors are proportional to the squared multiple correlation coefficient.
As was the case for benthos, the trophic structure of the fish assemblages remained distinct at each site except when typhoons affected the Apo NTMR and the Sumilon fished site (ellipses in Fig. 7b). The fish assemblage at the Sumilon NTMR shifted following the opening of the NTMR to fishing in 1984 (cluster of three outliers in Fig. 7b) and 1992 (cluster of two outliers in Fig. 7b). In general, NTMRs were characterized by biomass of generalist large predators, piscivores, obligate corallivores, large and small planktivores, and croppers (Fig. 7b), whereas fished sites were characterized by biomass of detritivores, scrapers, excavators, small and large benthic foragers, sand feeders, and omnivorous pomacentrids (Fig. 7b). The division of the fish assemblages between NTMRs and fished sites largely reflected the fact that NTMRs had high HC cover and fished sites high DS cover (compare Fig. 7a, b). The BEST BIO‐ENV routine identified the benthic habitat (HC or DS), or duration of protection factor(s), that explained the greatest variation in trophic structure of the reef fish assemblage (Table 2). Duration of protection from fishing and live hard coral (HC) cover combined explained 35.9% of the variance in the fish assemblage structure (ρ = 0.359, P = 0.01; Table 2). Models that included only one of the three variables (fishing, HC, DS) indicated that HC cover alone accounted for 29.8% of the variance in fish assemblage structure, DS cover alone accounted for 26.8%, and duration of protection from fishing alone just 24.1% (Table 2).
Table 2.
Variance explained (%) of fish assemblage structure by the BEST BIO‐ENV routine for biomass of reef fish trophic groups.
| Variables | No. variables | Variance explained (%) |
|---|---|---|
| Years protected + HC | 2 | 35.9 |
| Years protected + HC + DS | 3 | 35.7 |
| Years protected + DS | 2 | 32.9 |
| HC + DS | 2 | 30.6 |
| HC | 1 | 29.8 |
| DS | 1 | 26.8 |
| Years protected | 1 | 24.1 |
DS, dead substrate; HC, hard coral. All models are shown with the top model in boldface type.
Discussion
The biomass of only 2 of 13 trophic groups of reef fish was affected significantly and clearly by fishing in this study (Table 3). The generalist large predators and large planktivores both had clear direct effects of no‐take marine reserve (NTMR) protection at both islands. A third trophic group, the piscivores, appeared to build up inside the NTMRs over time (10–15 yr) at each island relative to the fished areas, but this result was not supported statistically. In contrast, the biomass of 11 of 13 trophic groups showed clear evidence of either positive (n = 6) or negative (n = 5) association with cover of live hard coral (HC) (Table 3). These results are consistent with findings from these Philippine islands that the density of some types of reef fish have a strong positive association with HC cover (butterflyfish, damselfish, snappers, emperors, and fusiliers; Russ et al. 2015b , 2017a , Russ and Leahy 2017), while density of other types of reef fish have a strong negative association with HC cover (goatfish, parrotfish, and some species of wrasses and surgeonfish; Russ et al. 2015a , c , 2017b , 2018). These associations of fish with benthos were most clear following environmental disturbances (e.g., coral bleaching, typhoons) that reduced HC cover, often quickly (1–2 yr), or following gradual (5–10 yr) recovery of HC. The trophic structure of the reef fish assemblage was more strongly affected by HC cover than by protection from fishing by NTMRs. These results suggest that HC cover was a greater short‐term ecological driver of biomass of the various trophic groups of reef fish than fishing.
Table 3.
Trophic groups whose biomass was affected significantly by No‐Take Marine Reserve (NTMR) protection and/or cover of live hard coral (HC).
| Trophic group |
Direct positive NTMR effect (no. islands with : without NTMR effect) |
Live HC effect (no. sites with + : − : neutral HC effect) |
|---|---|---|
| Generalist large predators | 2:0 | 4:0:0 |
| Large planktivores | 2:0 | 3:0:1 |
| Piscivores | 0:2 † | 4:0:0 |
| Obligate corallivores | 0:2 | 4:0:0 |
| Small planktivores | 0:2 | 4:0:0 |
| Omnivorous pomacentrids | 0:2 | 3:0:1 |
| Small benthic foragers | 0:2 | 2:2:0 |
| Croppers | 0:2 | 1:1:2 |
| Detritivores | 0:2 | 0:4:0 |
| Sand feeders | 0:2 | 0:4:0 |
| Large benthic foragers | 0:2 | 0:4:0 |
| Excavators | 0:2 | 0:3:1 |
| Scrapers | 0:2 | 0:3:1 |
| Total biomass | 2:0 | 3:0:1 |
| Proportion of clear effects | 2 of 13 trophic groups | 11 of 13 trophic groups |
Ratios in column two show the number of islands (Sumilon, Apo) with : without a significant, direct NTMR effect on biomass of the trophic group. Ratios in column three show the number of sites (Sumilon NTMR, Sumilon Fished, Apo NTMR, Apo Fished) with a positive : negative : neutral effect of live hard coral cover on the biomass of the trophic group.
An effect that was statistically not significant but was visually clear.
A caveat is required that benthic cover was a stronger driver of biomass of many trophic groups of fish than was fishing. The study likely underestimates the effects of fishing for several reasons. First, the coral reefs of the Philippines have been fished heavily for a very long time. Thus, some trophic groups, including upper‐level predators like sharks and large groupers, were likely depleted before the study began. Second, only one of two NTMRs, Apo, was fully no‐take for the entire study (Appendix S1: Fig. S2). Thus, the study may well have underestimated the strength of the top‐down effects of fishing and natural levels of predators on reef fish assemblages. On the other hand, the dynamic history of fishing being switched on and off in the Sumilon NTMR and the Sumilon Fished site (Appendix S1: Fig. S2) during the study showed clearly how fishing can affect total reef fish biomass (Fig. 2c; Appendix S1: Fig. S5), and to a much lesser extent trophic structure of the reef fish assemblage (Fig. 7; Appendix S1: Fig. S5).
Estimates of coral reef fish biomass (Fig. 2; Appendix S1: Fig. S5) at the four sites over the three decades of study compare well with those reported for 45 coral reef reserves globally (MacNeil et al. 2015). This meta‐analysis suggested that median coral reef fish biomass ranged from ~15 kg/1,000 m2 for 0 years of NTMR protection, up to ~60 kg/1,000 m2 for 30 yr of NTMR protection (Fig. 1b of MacNeil et al. 2015). In the present study, minima at the two fished sites were 12–18 kg/1,000 m2, with maxima around 40–50 kg/1,000 m2 for 15–20 yr of NTMR protection at Sumilon and Apo Islands (Fig. 2). A maximum of ~60 kg/1,000 m2 was recorded at the Sumilon fished site in 1991 after just 5 yr of protection from fishing (Appendix S1: Fig. S5). Thus, the estimates of coral reef fish biomass in this study are representative of those reported globally. Furthermore, at conservative estimates of published mean annual catch of ~10 (Apo) and ~15 (Sumilon) Mg·km−2·yr−1 (Russ 1991, Alcala et al. 2005, Abesamis et al. 2006), approximate harvest rates of the entire reef fish assemblage, based on estimates of fish biomass in Appendix S1: Fig. S5 in the fished sites of this study, are 52% at Apo and 50% at Sumilon. Most fishery scientists would consider such harvest rates of a multispecies assemblage high, or on the limits of sustainability (Worm et al. 2009).
High fishery yields over long periods have been achieved at Sumilon and Apo Islands, likely because the majority of the reef fish biomass and catch are planktivores (Appendix S1: Fig. S5; Russ 1991; Russ and Alcala 1998). Many species of planktivorous coral reef fish have high rates of growth, natural mortality, and recruitment, breed early in life, and are thus capable of sustaining intense fisheries (Polunin 1996, Russ et al. 2017a ). The high biomass and yield suggest a major role of planktivory in the trophodynamics of these coral reefs. Planktivory has likely been underestimated as a trophodynamic process on many coral reefs, particularly on reef slopes (Hobson and Chess 1978, Russ 1991, Polunin 1996, Gove et al. 2016). The “classic” food chain thought to be the major basis of secondary production of coral reef fishes (benthic algae to herbivores to carnivores; e.g., Odum and Odum 1955, Hatcher 1988) may not apply strictly to a large number of coral reefs globally. The traditional explanation for “Darwin’s Paradox” of 1842, how can such small standing biomass of food support so many animals on coral reefs? (Darwin 1842, Steneck et al. 2017), is very high production/biomass ratios of turf algae (Hatcher 1988) and the existence of symbiotic zooxanthellae in corals (Stanley and Swart 1995). An additional part of the explanation may be planktivory, supported by external inputs of nutrients and plankton (Russ 1991, Polunin 1996, Gove et al. 2016).
The dominance of the reef fish biomass and fishery catch by planktivores at Sumilon and Apo Islands contrasts with the small biomass and catch of true herbivores (Russ and Alcala 1998, Abesamis et al. 2006). True herbivores in this study are the croppers, which consist of some species of Acanthurus, Naso, Siganus, and Zebrasoma (Appendix S1: Table S1). Note that parrotfish (scrapers, excavators, and some sand feeders in this study) have recently been identified as microphagous feeders that target epilithic and endolithic cyanobacteria and other protein‐rich autotrophic microorganisms (Clements et al. 2017), and thus are not true herbivores in the sense that they target their feeding on multicellular algae. The dominance of planktivores, and the modest biomass of true herbivores in the reef fish assemblage, combined with the relatively non‐selective nature of the reef fishery at Sumilon and Apo islands (Russ and Alcala 1998), suggests that fishing, even at relatively high harvest rates (~50%), is unlikely to cause shifts in the relative abundance of trophic groups within the reef fish assemblage, and thus unlikely to cause “trophic cascades” that could subsequently reduce herbivory and thus affect the benthos. The evidence that protection from fishing in NTMRs at Sumilon and Apo Islands affected benthic cover (hard coral cover in this study; hard coral and macroalgal cover in Russ et al. 2015c ) on decadal scales was virtually nonexistent. In fact, the long‐term experimental evidence that NTMRs can alter abundance of “herbivorous” reef fish such as parrotfish, and that this subsequently reverses benthic phase shifts from macroalgal to coral dominance in NTMRs, is surprisingly equivocal (Aronson and Precht 2006, 2016, Bruno et al. 2009, 2019).
The effects of fishing can act at the level of populations and communities. Furthermore, effects of fishing can operate directly on populations and communities by removal of individuals, or indirectly if fishing techniques modify fish habitats or predatory and competitive interactions (Russ 1991, Jennings and Polunin 1996). NTMRs are proposed as a potential mechanism to reverse these direct and indirect effects of fishing on populations and communities of fish (Dayton et al. 2000, Sale et al. 2005). The focus of this paper is on the effects of fishing on the trophic structure of coral reef fish communities (assemblages) and if such effects result in “trophic cascades” and subsequent benthic change. Effects of fishing on the trophic structure of coral reef fish assemblages will depend on at least four main factors: degree of targeting, harvest rate, and turnover rate of each trophic group, and the strength of connections among trophic groups. Most of the early reviews of this subject (Russ 1991, Jennings and Polunin 1996) suggested high targeting and harvest rates of a relatively slow turnover trophic group: the predators. The effect such fishing had on lower trophic groups, particularly herbivorous reef fish, was suggested to be potentially high (Jennings and Polunin 1996), that is, a strong connection between predators and herbivores was hypothesized. Some empirical and modeling studies support the hypothesis that fishing of top predators can alter trophic structure of coral reef fish assemblages (Graham et al. 2017, Houk et al. 2018). However, empirical evidence for strong effects of fishing on top predators that results in measurable changes to the trophic structure of coral reef fish assemblages remains surprisingly equivocal (Jennings and Polunin 1996, Russ and Alcala 1998, Planes et al. 2005, Emslie et al. 2015, Rizzari et al. 2015, Ruppert et al. 2017). This is despite predation being known to be an important process affecting the structure of coral reef fish assemblages (Hixon 1991).
Reducing biomass of predators by fishing should reduce predation on herbivores, resulting in greater biomass of herbivores, and thus increased herbivory. A related idea, derived from global fisheries, was that fishing first depleted upper trophic levels and then shifted to target lower and lower trophic levels over time (“fishing down marine food webs”; Pauly et al. 1998). The evidence that fishing alters the trophic structure of coral reef fish assemblages significantly, to the extent that it can indirectly alter the benthos, remains an open question. Recent literature now emphasizes that human activities can affect trophic structure of coral reef fish assemblages from both “top‐down” (e.g., fishing) and “bottom‐up” (degradation of coral reef benthos, with benthos serving as shelter and food for reef fish) processes (Graham et al. 2015, 2020, Russ et al. 2015c , Ruppert et al. 2017).
A major shift in the perceived significance of effects of fishing on the trophic structure of coral reef fish assemblages occurred in the early 1990s, when intense fishing directly for herbivorous reef fish (Done 1992, Hughes 1994), or for herbivorous and urchin‐feeding reef fish (Hughes 1994, McClanahan and Mutere 1994), was implicated in reductions in grazing pressure by herbivores and thus semipermanent changes of benthos from coral to algal dominance (benthic “phase shifts”). Thus, the necessary condition for fishing to affect benthos required that fishing, having initially reduced predators, then switched to targeting herbivores (Hughes 1994). Fishing of herbivorous coral reef fish, resulting in “trophic cascades” and “benthic phase shifts” from coral to algal dominance, became a dominant paradigm in coral reef ecology (Hughes 1994, McClanahan and Mutere 1994, Jackson et al. 2001, 2014, Bellwood et al. 2004, Mumby et al. 2006, Mumby and Steneck 2008). The idea that fishing of herbivorous coral reef fish caused benthic change became so deeply ingrained in the literature that few authors pointed out that, not just targeting of herbivores, but very high harvest rates on herbivores, would likely be required to reduce grazing rates to a level required to cause benthic phase shifts. A trophic decimation of herbivores, as opposed to a trophic cascade, would likely be required to lead to benthic phase shifts. Such ideas led to suggestions that thresholds or tipping points of reef fish biomass, particularly biomass of herbivorous coral reef fish, existed below which benthic phase shifts from coral to algal dominance were likely (e.g., McClanahan et al. 2011 [Indian Ocean], Karr et al. 2015 [Caribbean], Holbrook et al. 2016 [Indo‐Pacific]). Much of the evidence that fishing of herbivorous reef fish causes benthic phase shifts is restricted to the Caribbean, and is not as common on Indo‐Pacific coral reefs (Aronson and Precht 2006, Bruno et al. 2009, 2019, Graham et al. 2015, Russ et al. 2015c , Ruppert et al. 2017). However, such evidence from the Caribbean now seems far more equivocal, with some recent studies questioning the role of fishing of herbivores in driving changes from coral to algal dominance (Côté et al. 2013, Toth et al. 2014, Suchley et al. 2016, Cox et al. 2017).
Nine of the 13 trophic groups were targeted by fishing in this study (Russ and Alcala 1998). Those not targeted were small planktivores, omnivorous pomacentrids, obligate corallivores, and small benthic foragers. The nine trophic groups targeted by fishing were often harvested at Sumilon and Apo Islands roughly in proportion to their biomass in the trophic assemblage (Russ and Alcala 1998). Such harvesting clearly did not alter the relative abundance of trophic groups in the assemblage much, if at all (Appendix S1: Fig. S5; Russ and Alcala 1998). There was little convincing evidence that targeting generalist large predators or piscivores resulted in expansion of other trophic groups, such as croppers, scrapers, or excavators, the latter two often classified in many studies as “herbivores” (Appendix S1: Fig. S5). On the contrary, Russ et al. (2015b ) argued that abundance of generalist large predators at these two islands was positively correlated with abundance of their pomacentrid prey (small planktivores, omnivorous pomacentrids). The fact that fish assemblages were distinct at all sites from the outset, and remained so for 31 yr, despite large differences among sites in fishing pressure and disturbance history (Fig. 7) supports the suggestion of a weak effect of fishing on the trophic structure of the coral reef fish assemblage in this study, despite high harvest rates.
Habitats play a critical role in determining the distribution and abundance of organisms (Andrewartha and Birch 1954, MacArthur et al. 1962, MacArthur and Connell 1966). MacArthur et al. (1962) showed that species diversity of birds could be predicted from a knowledge of habitat. Similarly, a quick swim around a coral reef shows that presence and abundance of different species of reef fish are strongly associated with types of benthic habitat. However, such static observations of association do not establish a causal link between habitat and distribution and abundance of reef fish. If one manipulates the habitat, one can predict changes in the local distribution and abundance of organisms following the manipulation, and during recovery from the manipulation. MacArthur and Connell (1966) showed that species composition of birds changed during secondary succession as terrestrial vegetation recovered on abandoned farmland in the southeastern United States. The number of species of birds increased as vegetation transitioned from grassland to hardwood forest on time scales of 100+ yr (see Fig. 7.16 of Pianka 1974).
Similarly, on coral reefs, environmental disturbances, and recovery from those disturbances, cause benthic habitats to change (Connell et al. 1997), with subsequent change in the reef fish assemblages associated with those habitats (Jones et al. 2004, Pratchett et al. 2008, Wilson et al. 2008, Alvarez‐Filip et al. 2011a , 2015, Emslie et al. 2015, Graham et al. 2015, 2020, Russ et al. 2015c ). Considerable research has demonstrated the important role that benthic habitat composition plays in determining the distribution and abundance of many coral reef fish species (reviewed by Pratchett et al. 2008, Wilson et al. 2008). Most coral reef fish rely on certain benthic habitats for food, shelter, or recruitment (Jones et al. 2004, Graham et al. 2015). Live hard coral typically fulfils at least one of these requirements for most coral reef fishes (Pratchett et al. 2008, Wilson et al. 2008, Graham et al. 2015). For example, up to 65% of reef fish species use live coral as a recruitment habitat (Jones et al. 2004). Alternatively, a subset of coral reef fishes (e.g., goatfish, parrotfish, some species of wrasses, and detritivorous surgeonfish) depend on other types of benthic habitat (e.g., rubble, sand, dead hard substrata; Russ et al. 2015a , c , 2017b , 2018 Graham et al. 2020). The types of benthic substratum on which species rely heavily influences how each species reacts to benthic disturbance and subsequent recovery (Pratchett et al. 2008, Wilson et al. 2008, Russ et al. 2015a , b , c , 2017b , 2018, Graham et al. 2020). There is general agreement that NTMRs, designed to stop or reduce fishing pressure, cannot prevent effects of environmental disturbances to coral reef benthos such as coral bleaching or typhoons, and thus to coral reef fish assemblages (Jones et al. 2004, Huntington et al. 2010, Toth et al. 2014, Aronson and Precht 2016, Cox et al. 2017, Graham et al. 2017, Ruppert et al. 2017, Bruno et al. 2019, Graham et al. 2020).
Some studies of environmental disturbances to coral reef benthos stress that not just coral cover, but also the related attribute of structural complexity, are important attributes of the benthos for reef fish assemblages (Graham et al. 2007, Alvarez‐Filip et al. 2011b , Emslie et al. 2014). Some types of disturbance can reduce cover of live corals, but not, at least in the short‐term, structural complexity of the benthos, e.g., coral bleaching, outbreaks of coral predators (Graham et al. 2007, Emslie et al. 2014). In the present study, structural complexity (SCI) was measured on a spatial scale (100 m2) much larger than measured in many other similar studies. Thus SCI in the present study accounted for not just structural complexity of the benthos caused by live coral cover, but also that accounted for by larger structural features of reef slopes, like caves and overhangs, that rarely change in structure, even when subjected to environmental disturbances. Thus, we included only cover of live hard coral (HC) in our analyses, as SCI was considered much less sensitive to environmental disturbances.
Clearly, NTMR protection from fishing and benthic habitat can interact to affect biomass of trophic groups of reef fish (Appendix S1: Fig. S5). The strong emphasis on fishing as a driver of the trophic biomass of reef fish over the past few decades resulted in a literature on NTMRs that often downplayed this interaction (Miller and Russ 2014). A recent review of methods to partition NTMR and benthic habitat effects on reef fish abundance revealed a surprising lack of rigor in correcting for the effects of benthic habitat over the past three decades (Miller and Russ 2014). The most disturbing result of this review was that over half of the studies (54.3%) made no statistical attempt to account for benthic habitat effects on fish abundance (Miller and Russ 2014). Given the results of the present study, it suggests that far better account of the effects of benthic habitat on reef fish abundance needs to be made in future NTMR studies.
If, as this study suggests, benthos is often a stronger driver of the trophic biomass of reef fish than fishing, this means that any environmental disturbances that affect the benthos directly will have major effects on reef fish assemblages (Pratchett et al. 2008, Wilson et al. 2008, Emslie et al. 2015) and the trophic structure of exploitable biomass and reef fish catch (Bruno et al. 2019, Robinson et al. 2019, Graham et al. 2020). For almost 20 yr, coral reef ecology emphasized the dominant role fishing had on the trophic structure of reef fish and subsequent effects on benthos (Done 1992, Hughes 1994, Jackson et al. 2001, 2014, Mumby et al. 2006, Mumby and Steneck 2008; Appendix S1: Fig. S1). NTMRs, either singularly or in networks, were seen as a small spatial scale solution to effects of fishing on biomass and trophic structure of reef fish assemblages and on benthic composition (Dayton et al. 2000, Sale et al. 2005, Mumby et al. 2006). This management emphasis has changed relatively recently with the advent of the effects of climate change (Hoegh‐Guldberg et al. 2007, Hughes et al. 2017, 2018) on coral reef benthos, causing extensive coral bleaching (Hoegh‐Guldberg et al. 2007, Hughes et al. 2017, 2018) or increased frequency or intensity of environmental disturbances such as typhoons (Knutson et al. 2010). Such environmental disturbances will have far more direct effects on reef fish (Jones et al. 2004, Pratchett et al. 2008, Wilson et al. 2008, Emslie et al. 2015, Graham et al. 2020) and on coral reef benthos (Graham et al. 2015, Hughes et al. 2017, 2018) than fishing. This contention is supported in recent literature on what environmental drivers are important in determining coral cover relative to that of macroalgal cover (Appendix S1: Fig. S1). Graham et al. (2015) found that depth and structural complexity in the Seychelles were key predictors of whether the reef trajectory trended toward coral‐dominated or macroalgal‐dominated states. Hughes et al. (2017, 2018) have documented coral mortality due to bleaching on a massive spatial scale on Australia’s Great Barrier Reef (GBR) caused by unusually high water temperatures.
Clearly, management strategies to address coral reef degradation have to move from a focus on solely controlling fishing by NTMRs to more holistic management, aimed at also ameliorating the effects of major environmental disturbances to coral reef habitats (e.g., coral bleaching, typhoons), with many of these disturbances related to global climate change (Hughes et al. 2017, 2018). Most single NTMRs, and even networks of NTMRs, are small spatial scale measures that might exclude fishing, but will generally not exclude warmer and more acidic waters nor strong winds and waves (Jones et al. 2004). Opinion is divided on whether NTMRs might (Roberts et al. 2017) or might not (Aronson and Precht 2016, Bruno et al. 2019) ameliorate damage caused to coral reefs by climate change. Recent research has shown that NTMRs can maintain a higher biomass of coral reef fish than in fished areas, even when reefs are affected by typhoons in the Philippines (McClure et al. 2020). NTMRs can not only maintain higher biomass of target reef fish than fished areas, despite coral bleaching and floods on Australia’s Great Barrier Reef, they can be important sources of future recovery of fish biomass (Williamson et al. 2014). Nevertheless, a more holistic management strategy that addresses climate change, coastal pollution, fishing, and other stressors is essential to prevent further degradation of coral reefs.
Andrewartha and Birch (1954) emphasized that the bigger drivers of distribution and abundance of individual organisms are often large‐scale physical, climatic, and environmental ones, especially those that affect habitats. For coral reefs, within these large‐scale, physical environmental constraints, biological mechanisms like predation (fishing) and competition are likely to drive more local‐scale patterns of distribution and abundance of individual reef fish (Aronson and Precht 2016).
Conclusion
Just as early definitions of ecology overlooked plants and microorganisms (Haeckel 1870), modern‐day coral reef ecologists for almost two decades partially under‐emphasized the role of physical factors, habitats, and environmental disturbances to those habitats in favor of biotic factors such as fishing, predation, and competition as major drivers of structure in coral reef assemblages. Climate change and mass coral bleaching have, in effect, forced a correction of this under‐emphasis (Appendix S1: Fig. S1). In the present study, both benthic habitat and fishing are important drivers of assemblage structure of coral reef fish. For almost two decades, an accepted paradigm in coral reef ecology was that fishing causes trophic cascades through reef fish assemblages, which eventually result in reduced “herbivory”, and that this indirectly caused benthic phase shifts from coral to algal dominance. The present, long‐term study demonstrates that direct change to coral reef benthos by environmental disturbances is often a far greater threat to the trophic structure of reef fish assemblages than is fishing. We conclude that direct effects of environmental disturbances to the benthos (Model 2 in Introduction) had a much greater effect on trophic structure of the reef fish assemblages than direct effects of fishing that then lead to indirect effects on the fish assemblage and, subsequently, the benthos (Model 1 in Introduction). The aim here is to reduce the strong focus on the “fishing causes trophic cascades/benthic phase shift paradigm” that has occurred in the past, and thus re‐balance coral reef ecology, by acknowledging that both physical disturbances to the benthos and fishing are important drivers of reef fish trophic structure (see also Aronson and Precht 2016, Ruppert et al. 2017, Graham et al. 2020), and that such drivers also need to be managed effectively. In doing so, we hope to remind coral reef ecologists of the basics of ecology and environment laid down by Haeckel (1870) and Andrewartha and Birch (1954): that in addition to biological factors like fishing (a proxy for predation; Hixon 1991) and competition, physical conditions, habitats, and environmental disturbances to those conditions and habitats, are important drivers of the distribution and abundance of organisms and of the structure of communities.
Supporting information
Appendix S1
Acknowledgments
Financial support to G. R. Russ and A. C. Alcala was provided by the United Nations Environment Program‐Natural Resources Management Center (UNEP‐NRMC) Philippines (1983), the Great Barrier Reef Marine Park Authority (1985), a Pew Fellowship (1999–2000), an Australian Research Council (ARC) Discovery grant (2002–2004), and funding from the ARC Centre of Excellence for Coral Reef Studies (2006‐2014). J. R. Rizzari was supported by the College of Science and Engineering at James Cook University. We thank Tata Duran for long‐term assistance in the field.
Russ, G. R. , Rizzari J. R., Abesamis R. A., and Alcala A. C..2021. Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecological Applications 31(1):e02224 10.1002/eap.2224
Corresponding Editor: Ilsa B. Kuffner.
Data Availability
Data are available from the James Cook University data archive (Russ 2020): https://doi.org/10.25903/5efad601a0795
Literature Cited
- Abesamis, R. A. , Alcala A. C., and Russ G. R.. 2006. How much does the fishery at Apo Island benefit from spillover? Fisheries Bulletin 104:360–375. [Google Scholar]
- Alcala, A. C. , and Russ G. R.. 2002. Status of Philippine coral reef fisheries. Asian Fisheries Science 15:177–192. [Google Scholar]
- Alcala, A. C. , and Russ G. R.. 2006. No‐take marine reserves and reef fisheries management in the Philippines: a new people power revolution. Ambio 35:245–254. [DOI] [PubMed] [Google Scholar]
- Alcala, A. C. , Russ G. R., Maypa A. P., and Calumpong H. P.. 2005. A long‐term, spatially replicated experimental test of the effect of marine reserves on local fish yields. Canadian Journal of Fisheries and Aquatic Science 62:98–108. [Google Scholar]
- Alvarez‐Filip, L. , Gill J. A., and Dulvy N. K.. 2011a. Complex reef architecture supports more small‐bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2:1–17. [Google Scholar]
- Alvarez‐Filip, L. , Côté I. M., Gill J. A., Watkinson A. R., and Dulvy N. K.. 2011b. Region‐wide temporal and spatial variation in Caribbean reef architecture: Is coral cover the whole story? Global Change Biology 17:2470–2477. [Google Scholar]
- Alvarez‐Filip, L. , Paddack M. J., Collen B., Robertson D. R., and Côté I. M.. 2015. Simplification of Caribbean reef‐fish assemblages over decades of coral reef degradation. PLoS ONE 10:e0126004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrewartha, H. G. , and Birch L. C.. 1954. The distribution and abundance of animals. University of Chicago Press, London, UK. [Google Scholar]
- Aronson, R. B. , et al. 2003. Causes of coral reef degradation. Science 302:1502. [DOI] [PubMed] [Google Scholar]
- Aronson, R. B. , and Precht W. F.. 2006. Conservation, precaution, and Caribbean coral reefs. Coral Reefs 25:441–450. [Google Scholar]
- Aronson, R. B. , and Precht W. F.. 2016. Physical and biological drivers of coral‐reef dynamics Pages 261–275 in Hubbard D. K., et al. Coral reefs at the crossroads. Coral Reefs of the World 6. Springer, Dordrecht, Netherlands: 10.1007/978-94-017-7567-0_11 [DOI] [Google Scholar]
- Bellwood, D. R. , et al. 2004. Confronting the coral reef crisis. Nature 429:827–833. [DOI] [PubMed] [Google Scholar]
- Bruno, J. F. , Cote I. M., and Toth L. T.. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annual Review of Marine Science 11:307–334. [DOI] [PubMed] [Google Scholar]
- Bruno, J. F. , Sweatman H., Precht W. F., Selig E. R., and Schutte V. G.. 2009. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–1484. [DOI] [PubMed] [Google Scholar]
- Clarke, K. , Gorley R. P., Somerfield P., and Warwick R.. 2014. Change in marine communities: an approach to statistical analysis and interpretation. PRIMER‐E, Plymouth, UK. [Google Scholar]
- Clarke, K. , and Warwick R.. 2001. Changes in marine communities: an approach to statistical analysis and interpretation. PRIMER‐E, Plymouth, UK. [Google Scholar]
- Clements, K. D. , German D. P., Piché J., Tribollet A., and Choat J. H.. 2017. Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biological Journal of the Linnaean Society 120:729–751. [Google Scholar]
- Connell, J. H. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus . Ecology 42:710–723. [Google Scholar]
- Connell, J. H. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. [DOI] [PubMed] [Google Scholar]
- Connell, J. H. , Hughes T. P., and Wallace C. C.. 1997. A 30‐year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecological Monographs 67:461–488. [Google Scholar]
- Côté, I. M. , Precht W. F., Aronson R. B., and Gardner T. A.. 2013. Is Jamaica a good model for understanding Caribbean coral reef dynamics? Marine Pollution Bulletin 76:28–31. [DOI] [PubMed] [Google Scholar]
- Cox, C. , Valdivia A., McField M., Castillo K., and Bruno J. F.. 2017. Establishment of marine protected areas alone does not restore coral reef communities in Belize. Marine Ecology Progress Series 563:65–79. [Google Scholar]
- Darwin, C. R. 1842. The structure and distribution of coral reefs. Being the first part of the geology of the voyage of the Beagle, under the command of Capt. Fitzroy, R.N. during the years 1832 to 1836. Smith Elder and Co., London, UK. [Google Scholar]
- Dayton, P. K. 1971. Competition, disturbance, and community organization: The provision and subsequent utilization of space in a rocky intertidal community. Ecological Monographs 41:351–389. [Google Scholar]
- Dayton, P. K. , Sala E., Tegner M. J., and Thrush S.. 2000. Marine reserves: Parks, baselines, and fishery enhancement. Bulletin of Marine Science 66:617–634. [Google Scholar]
- Done, T. J. 1992. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132. [Google Scholar]
- Emslie, M. J. , et al. 2015. Expectations and outcomes of reserve network performance following re‐zoning of the Great Barrier Reef Marine Park. Current Biology 25:983–992. [DOI] [PubMed] [Google Scholar]
- Emslie, M. J. , Cheal A. J., and Johns K. A.. 2014. Retention of habitat complexity minimizes disassembly of reef fish communities following disturbance: A large‐scale natural experiment. PLoS ONE 9:e105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes, J. A. , et al. 2011. Trophic downgrading of planet earth. Science 333:301–306. [DOI] [PubMed] [Google Scholar]
- Gove, J. M. , et al. 2016. Near‐island biological hotspots in barren ocean basins. Nature Communications 7:10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, M. H. , and Dayton P. K.. 2002. On the evolution of ecological ideas: paradigms and scientific progress. Ecology 83:1481–1489. [Google Scholar]
- Graham, N. A. J. , Jennings S., MacNeil M. A., Mouillot D., and Wilson S. K.. 2015. Predicting climate‐driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97. [DOI] [PubMed] [Google Scholar]
- Graham, N. A. J. , McClanahan T. R., MacNeil M. A., Wilson S. K., Cinner J. E., Huchery C., and Holmes T. H.. 2017. Human disruption of coral reef trophic structure. Current Biology 27:231–236. [DOI] [PubMed] [Google Scholar]
- Graham, N. A. J. , Robinson J. P. W., Smith S. E., Govinden R., Gendron G., and Wilson S. K.. 2020. Changing role of coral reef marine reserves in a warming climate. Nature Communications 11:2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, N. A. J. , Wilson S. K., Jennings S., Polunin N. V. C., Robinson J., Bijoux J. P., and Daw T. M.. 2007. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries and ecosystems. Conservation Biology 21:1291–1300. [DOI] [PubMed] [Google Scholar]
- Haeckel, E. 1870. Uber entwickelungsgang u. Aufgabe der Zoologie. Jenaische Zeitschrift für Naturwissenschaft 5:353–370. [Google Scholar]
- Hairston, N. G. , Smith F. E., and Slobodkin L. B.. 1960. Community structure, population control, and competition. American Naturalist 94:421–425. [Google Scholar]
- Harvell, C. D. , et al. 1999. Emerging marine diseases – climate links and anthropogenic factors. Science 285:1505–1510. [DOI] [PubMed] [Google Scholar]
- Hatcher, B. G. 1988. Coral reef primary productivity: A beggar’s banquet. Trends in Ecology and Evolution 3:106–111. [DOI] [PubMed] [Google Scholar]
- Hixon, M. A. 1991. Predation as a process structuring coral reef fish communities Chapter 17. Pages 475–508 in Sale P. F., editor. The ecology of fishes on coral reefs. Academic Press, San Diego, California, USA. [Google Scholar]
- Hixon, M. A. 2011. 60 years of coral reef fish ecology: past, present, future. Bulletin of Marine Science 87:727–765. [Google Scholar]
- Hobson, E. S. , and Chess J. R.. 1978. Trophic relationships among fishes and plankton in the lagoon at Enewetak Atoll, Marshall Islands. Fisheries Bulletin 76:133–153. [Google Scholar]
- Hoegh‐Guldberg, O. 1999. Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50:839–866. [Google Scholar]
- Hoegh‐Guldberg, O. , et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742. [DOI] [PubMed] [Google Scholar]
- Holbrook, S. J. , Schmitt R. J., Adam T. C., and Brooks A. J.. 2016. Coral reef resilience, tipping points and the strength of herbivory. Scientific Reports 6:35817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk, P. , Cuetos‐Bueno J., Kerr A., and McCann K.. 2018. Linking fishing pressure with ecosystem thresholds and food web stability on coral reefs. Ecological Monographs 88:109–119. [Google Scholar]
- Hughes, T. P. 1994. Catastrophes, phase shifts, and large‐scale degradation of a Caribbean coral reef. Science 265:1547–1551. [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933. [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543:373–377. [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83. [DOI] [PubMed] [Google Scholar]
- Hunter, M. D. , and Price P. W.. 1992. Playing chutes and ladders: heterogeneity and the relative roles of bottom‐up and top‐down forces in natural communities. Ecology 73:724–732. [Google Scholar]
- Huntington, B. E. , Karnauskas M., Babcock E. A., and Lirman D.. 2010. Untangling natural seascape variation from marine reserve effects using a landscape approach. PLoS ONE 5:e12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, J. B. C. , et al. 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638. [DOI] [PubMed] [Google Scholar]
- Jackson, J. B. C. , Donovan M. K., Cramer K. L., and Lam V. V., editors. 2014. Status and trends of Caribbean coral reefs: 1970–2012. Global Coral Reef Monitoring Network, IUCN, Gland, Switzerland. [Google Scholar]
- Jennings, S. , and Polunin N. V. C.. 1996. Impacts of fishing on tropical reef ecosystems. Ambio 25:44–49. [Google Scholar]
- Jones, G. P. , McCormick M. I., Srinivasan M., and Eagle J. V.. 2004. Coral decline threatens fish biodiversity in marine reserves. Proceedings of the National Academy of Sciences USA 101:8251–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr, K. A. , Fujita R., Halpern B. S., Kappel C. V., Crowder L., Selkoe K. A., Alcolado P. M., and Rader D.. 2015. Thresholds in Caribbean coral reefs: implications for ecosystem‐based fishery management. Journal of Applied Ecology 52:402–412. [Google Scholar]
- Knutson, T. R. , et al. 2010. Tropical cyclones and climate change. Nature Geoscience 3:157–163. [Google Scholar]
- Kulbicki, M. , Guillemot N., and Amand M.. 2005. A general approach to length‐weight relationships for New Caledonian lagoon fishes. Cybium 29:235–252. [Google Scholar]
- Lefcheck, J. S. , Innes‐Gold A. A., Brandl S. J., Steneck R. S., Torres R. E., and Rasher D. B.. 2019. Tropical fish diversity enhances coral reef functioning across multiple scales. Science Advances 5:eaav6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licuanan, A. M. , Reyes M. Z., Luzon K. S., Chan M. A. A., and Licuanan W. Y.. 2017. Initial findings of the nationwide assessment of Philippine coral reefs. Philippine Journal of Science 146:177–185. [Google Scholar]
- MacArthur, R. H. , and Connell J. H.. 1966. The biology of populations. Wiley, New York, New York, USA. [Google Scholar]
- MacArthur, R. H. , MacArthur J. W., and Preer J.. 1962. On bird species diversity. II. Prediction of bird census from habitat measurements. American Naturalist 96:167–174. [Google Scholar]
- MacNeil, M. A. , et al. 2015. Recovery potential of the world's coral reef fishes. Nature 520:341–344. [DOI] [PubMed] [Google Scholar]
- McClanahan, T. R. , Graham N. A. J., MacNeil M. A., Muthiga M. A., Cinner J. E., Bruggerman J. H., and Wilson S. K.. 2011. Critical thresholds and tangible targets for ecosystem‐based management of coral reef fisheries. Proceedings of the National Academy of Sciences USA 108:17230–17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClanahan, T. R. , and Mutere J. C.. 1994. Coral and sea urchin assemblage structure and interrelationships in Kenyan reef lagoons. Hydrobiologia 286:109–124. [Google Scholar]
- McClure, E. A. , Sievers K. T., Abesamis R. A., Hoey A. S., Alcala A. C., and Russ G. R.. 2020. Higher fish biomass inside than outside marine protected areas despite typhoon impacts in a complex reefscape. Biological Conservation 241:108354. [Google Scholar]
- Menge, B. A. 2000. Top‐down and bottom‐up community regulation in marine rocky intertidal habitats. Journal of Experimental Marine Biology and Ecology 250:257–289. [DOI] [PubMed] [Google Scholar]
- Menge, B. A. , and Sutherland J. P.. 1976. Species diversity gradients: synthesis of the roles of predation, competition and temporal heterogeneity. American Naturalist 110:351–369. [Google Scholar]
- Miller, K. I. , and Russ G. R.. 2014. Studies of no‐take marine reserves: methods for differentiating reserve and habitat effects. Ocean and Coastal Management 96:51–60. [Google Scholar]
- Mumby, P. J. , et al. 2006. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101. [DOI] [PubMed] [Google Scholar]
- Mumby, P. J. , and Steneck R. S.. 2008. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends in Ecology and Evolution 23:555–563. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. J. 1933. The balance of animal populations. Journal of Animal Ecology 2:132–178. [Google Scholar]
- Odum, H. T. , and Odum E. P.. 1955. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecological Monographs 25:291–320. [Google Scholar]
- Paine, R. T. 1966. Food web complexity and species diversity. American Naturalist 100:65–76. [Google Scholar]
- Pandolfi, J. M. , et al. 2003. Global trajectories of the long‐term decline of coral reef ecosystems. Science 301:955–958. [DOI] [PubMed] [Google Scholar]
- Pauly, D. , Christensen V., Dalsgaard J., Froese R., and Torres F.. 1998. Fishing down food webs. Science 279:860–863. [DOI] [PubMed] [Google Scholar]
- Pianka, E. R. 1974. Evolutionary ecology. Harper and Row Publishers, New York, New York, USA. [Google Scholar]
- Pinheiro, J. , Bates D., DebRoy S., Sarkar D., and R Core Team . 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1‐131. https://CRAN.R‐project.org/package=nlme
- Planes, S. , Galzin R., Bablet J. P., and Sale P. F.. 2005. Stability of coral reef fish assemblages impacted by nuclear tests. Ecology 86:2578–2585. [Google Scholar]
- Polunin, N. V. C. 1996. Trophodynamics of reef fisheries production. Chapter 5 Pages 113–135 in Polunin N. V. C. and Roberts C. M., editors. Reef fisheries. Chapman and Hall, London, UK. [Google Scholar]
- Pratchett, M. S. , et al. 2008. Effects of climate‐induced coral bleaching on coral‐reef fishes. Ecological and economic consequences. Oceanography and Marine Biology Annual Review 46:251–296. [Google Scholar]
- Precht, W. F. , and Aronson R. B.. 2006. Death and resurrection of Caribbean coral reefs: a palaeoecological approach Pages 40–77 in Côté I. M. and Reynolds J. D., editors. Coral reef conservation. Cambridge University Press, Cambridge, UK. [Google Scholar]
- R Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R‐project.org [Google Scholar]
- Rizzari, J. R. , Bergseth B. J., and Frisch A. J.. 2015. Impact of conservation areas on trophic interactions between apex predators and herbivores on coral reefs. Conservation Biology 29:418–429. [DOI] [PubMed] [Google Scholar]
- Roberts, C. M. , et al. 2017. Marine reserves can mitigate and promote adaptation to climate change. Proceedings of the National Academy of Sciences USA 114:6167–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. P. W. , Wilson S. K., Robinson J., Gerry C., Lucas J., Assan C., Govinden R., Jennings S., and Graham N. A. J.. 2019. Productive instability of coral reef fisheries after climate‐driven regime shifts. Nature Ecology and Evolution 3:183–190. [DOI] [PubMed] [Google Scholar]
- Ruppert, J. L. W. , Vigliola L., Kulbicki M., Labrosse P., Fortin M., and Meekan M. G.. 2017. Human activities as a driver of spatial variation in the trophic structure of fish communities on Pacific coral reefs. Global Change Biology 24:e67–e79. [DOI] [PubMed] [Google Scholar]
- Russ, G. R. 1991. Coral reef fisheries: effects and yields. Chapter 19 Pages 601–635 in Sale P. F., editor. The ecology of coral reef fishes. Academic Press, San Diego, California, USA. [Google Scholar]
- Russ, G. 2020. Data from: Russ GR et al “Coral cover a stronger driver of reef fish trophic biomass than fishing”. Ecological Applications. James Cook University. Data set. 10.25903/5efad601a0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ, G. R. , and Alcala A. C.. 1998. Natural fishing experiments in marine reserves 1983–1993: community and trophic responses. Coral Reefs 17:383–397. [Google Scholar]
- Russ, G. R. , Aller‐Rojas O. D., Rizzari J. R., and Alcala A. C.. 2017a. Off‐reef planktivorous reef fishes respond positively to decadal‐scale no‐take marine reserve protection and negatively to benthic habitat change. Marine Ecology 38:e12442. [Google Scholar]
- Russ, G. R. , Bergseth B. J., Rizzari J. R., and Alcala A. C.. 2015a. Decadal‐scale effects of benthic habitat and marine reserve protection on Philippine goatfish (F. Mullidae). Coral Reefs 34:773–787. [Google Scholar]
- Russ, G. R. , and Leahy S. M.. 2017. Rapid decline and decadal‐scale recovery of corals and Chaetodon butterflyfish on Philippine coral reefs. Marine Biology 164:29. [Google Scholar]
- Russ, G. R. , Lowe J. R., Rizzari J. R., Bergseth B. J., and Alcala A. C.. 2017b. Partitioning no‐take marine reserve (NTMR) and benthic habitat effects on density of small and large‐bodied tropical wrasses. PLoS One 12:e0188515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ, G. R. , Miller K. I., Rizzari J. R., and Alcala A. C.. 2015b. Long‐term no‐take marine reserve and benthic habitat effects on coral reef fishes. Marine Ecology Progress Series 29:233–248. [Google Scholar]
- Russ, G. R. , Payne C. S., Bergseth B. J., Rizzari J. R., Abesamis R. A., and Alcala A. C.. 2018. Decadal‐scale response of detritivorous surgeonfish to no‐take marine reserve protection and change in benthic habitat. Journal of Fish Biology 93:887–900. [DOI] [PubMed] [Google Scholar]
- Russ, G. R. , Questel S.‐L.‐A., Rizzari J. R., and Alcala A. C.. 2015c. The parrotfish–coral relationship: refuting the ubiquity of a prevailing paradigm. Marine Biology 162:2029–2045. [Google Scholar]
- Sale, P. F. 1977. Maintenance of high diversity in coral reef fish communities. American Naturalist 111:337–359. [Google Scholar]
- Sale, P. F. 2002. The science we need to develop for more effective management. Chapter 16 Pages 361–376 in Sale P. F., editor. Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic Press, San Diego, California, USA. [Google Scholar]
- Sale, P. F. , et al. 2005. Critical science gaps impede use of no‐take fishery reserves. Trends in Ecology and Evolution 20:74–80. [DOI] [PubMed] [Google Scholar]
- Stanley, G. D. , and Swart P.. 1995. Evolution of the coral‐zooxanthellae symbiosis during the Triassic: a geochemical approach. Paleobiology 21:179–199. [Google Scholar]
- Steneck, R. S. , Bellwood D. R., and Hay M. E.. 2017. Herbivory in the marine realm. Current Biology 27:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchley, A. , McField M. D., and Alvarez‐Filip L.. 2016. Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ 4:e2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, L. T. , Van Woesik R., Murdoch T. J. T., Smith S. R., Ogden J. C., Precht W. F., and Aronson R. B.. 2014. Do no‐take reserves benefit Florida’s corals? 14 years of change and stasis in the Florida Keys National Marine Sanctuary. Coral Reefs 33:565–577. [Google Scholar]
- Williamson, D. H. , Ceccarelli D. M., Evans R. D., Jones G. P., and Russ G. R.. 2014. Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecology and Evolution 4:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. K. , et al. 2008. Exploitation and habitat degradation as agents of change within coral reef fish communities. Global Change Biology 14:2796–2809. [Google Scholar]
- Worm, B. , et al. 2009. Rebuilding global fisheries. Science 325:578–585. [DOI] [PubMed] [Google Scholar]
- Zuur, A. F. , Ieno E. N., and Smith G. M.. 2007. Analysing ecological data. Springer, New York, New York, USA. [Google Scholar]
- Zuur, A. F. , Hilbe J. M., and Ieno E. N.. 2013. A beginner’s guide to GLM and GLMM with R: a frequentist and Bayesian perspective for ecologists. Highland Statistics Newburgh, Newburgh, UK. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data are available from the James Cook University data archive (Russ 2020): https://doi.org/10.25903/5efad601a0795


