Abstract
The innate immune system responds in a rapid and non‐specific manner against immunologic threats; inflammation is part of this response. This is followed by a slower but targeted and specific response termed the adaptive or acquired immune response. There is emerging evidence that dietary components, including yeast‐derived β‐glucans, can aid host defense against pathogens by modulating inflammatory and antimicrobial activity of neutrophils and macrophages. Innate immune training refers to a newly recognized phenomenon wherein compounds may “train” innate immune cells, such that monocyte and macrophage precursor biology is altered to mount a more effective immunological response. Although various human studies have been carried out, much uncertainty still exists and further studies are required to fully elucidate the relationship between β‐glucan supplementation and human immune function. This review offers an up‐to‐date report on yeast‐derived β‐glucans as immunomodulators, including a brief overview of the current paradigm regarding the interaction of β‐glucans with the immune system. The recent pre‐clinical work that has partly decrypted mode of action and the newest evidence from human trials are also reviewed. According to pre‐clinical studies, β‐1,3/1,6‐glucan derived from baker's yeast may offer increased immuno‐surveillance, although the human evidence is weaker than that gained from pre‐clinical studies.
Keywords: diet and inflammation, innate immunity, metabolic‐inflammation, trained immunity, yeast β‐glucan
Emerging evidence indicates that dietary components, like β‐glucans, can aid host defense by modulating the inflammatory and antimicrobial activity of neutrophils and macrophages. This attribute is traced to innate immune training, wherein the first encounter between classical innate immune cells and a pathogen‐associated molecular pattern triggers intracellular functional changes that expedite future defense toward the same or an unrelated pathogen.
1. Introduction to β‐Glucans and Immunity
1.1. Structural Diversity with Varied Health Impacts
β‐Glucans are naturally occurring polysaccharides of d‐glucose monomers linked by β‐glycosidic bonds. They serve as energy stores and structural components of plant, algal, fungal, and bacterial cell walls.[ 1 ] β‐glucans show a very defined structure–activity relationship: research to date demonstrates that varied source and structure lead to varied biological activity.[ 2 , 3 , 4 ] For example, although oat‐derived β‐glucans act as effective dietary fibers that improve metabolic health parameters, including dyslipidemia and insulin resistance[ 4 ]; yeast‐derived β‐glucans behave as immunomodulators, specifically targeting the innate immune response.[ 5 ] The chemistry of β‐glucans is well characterized; they all share a β‐1,3‐glucan backbone; however, among β‐glucans, there is variation regarding source as well as extraction and purification methods used in their commercial preparation,[ 6 , 7 , 8 ] which gives rise to great diversity in branching pattern, insertions, and impurities. This yields a wide range of molecular structures within the β‐glucan family to ultimately determine a range of health effects[ 1 ] (Figure 1 ). Long side chains along the β‐1,3 backbone confer insolubility in the case of some yeast and fungal β‐glucans.[ 9 , 10 ] The formation of purely linear β‐1,3‐glucans in fungi and yeast is highly uncommon; however, these are present in soluble extracts of S. cerevisiae (S. cerevisiae).[ 11 ] Interestingly, only β‐glucans with a high molecular weight and degree of branching, like those from fungi and yeast, have been reported to exert an immunomodulatory action.[ 3 , 12 , 13 , 14 , 15 ] In fact, it was the preliminary work of Pillemer and Ecker[ 16 ] on Fleischmann's Yeast (S. cerevisiae) that emerged during the 1940s which first suggested the connection between β‐glucans and immunity. Several decades later, evidence has accumulated relating to the immunomodulatory activity of both research‐exclusive and commercially available forms of β‐glucans derived from various sources, and by various extraction and purification methods[ 5 , 8 , 17 ]; however, the translation of any nutritional immunomodulatory agent from cellular and/or pre‐clinical models to use in humans is a great challenge. This specifically applies to β‐glucans, since aside from their wide structural variability leading to diverse biological activities, there are differences between β‐glucan receptors in humans and mice,[ 18 , 19 ] immune health markers are ambiguous, and variation in administration route, dose, populations studied, and time points between studies are likely to contribute to inconsistent results relating to the health impact of β‐glucans. As such, the first aim of this review is to collate and interpret the existing pre‐clinical research on β‐1,3/1,6‐glucan with regard to immunity in order to clarify its molecular mechanism of immunomodulatory action. This will be achieved by considering its binding to immune receptors and the downstream signaling events leading to trained immunity and cytotoxic activity. The second aim of this review is to collate and evaluate the literature in order to provide a comprehensive overview of the human studies assessing the effect of supplementation with high quality, well‐characterized β‐1,3/1,6‐glucan from commercially available sources (presented in Table 1 ) on immunity across multiple populations. For this, inclusion criteria consist of randomized, double‐blind, placebo‐controlled human studies that investigated the efficacy of orally administered β‐glucan with a purity of over 75% (obtained by stripping β‐glucan from the external mannoprotein layer, the interlinked chitin in the cell wall, and the components inside the cell membrane).[ 8 , 20 , 21 ] Exclusion criteria include β‐glucan formulations that lack human studies or are used in combination with other active ingredients (e.g., β‐glucan and monoclonal antibodies in cancer research). Overall, no adverse events were detected, and no major safety concerns were presented in response to any of the selected intervention studies. Statistical significance was set at p < 0.05, and all results included are by default significant unless otherwise stated. All β‐glucans covered herein are by nature β‐1,3/1,6‐glucans, and the term β‐glucan will refer to β‐1,3/1,6‐glucan unless otherwise specified.
Figure 1.
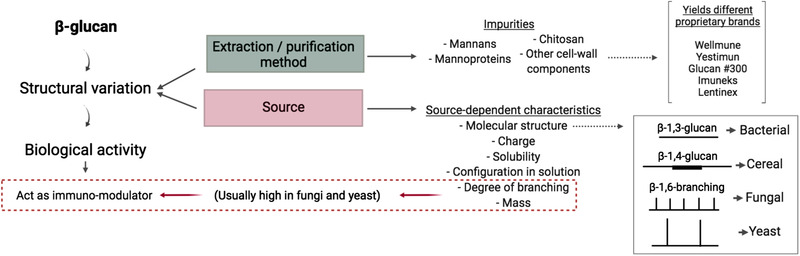
β‐Glucan structure–activity relationship. Variability in β‐glucan structure is due to differences in source and extraction and/or purification methods, which likely explains the divergent functionalities that exist among β‐glucans. Depending on the source, differences arise such as the nature of molecular linkages and the degree of branching, together with variability in mass, charge, solubility, and configuration in solution (single helix, triple helix, or random coil), as well as in impurity levels and content. These variabilities will result in different interactions with the host. Bacterial β‐glucans represent the most basic form of the polysaccharide with a linear β‐1,3 structure; cereal β‐glucans follow the same pattern with dominant β‐1,4 stretches; fungal (e.g., mushroom) and yeast (i.e., single cell fungi) β‐glucans have frequent β‐1,3‐d‐glucose side chains at β‐1,6 branching points that are short and spaced in fungal species (e.g., mushroom) and longer in yeast species. Further variations to these general structures are common. Only highly purified β‐1,3‐1,6‐glucans with a high degree of branching along the β‐1,3‐glucan backbone and a high molecular weight are able to exert immunomodulatory properties.
Table 1.
Proprietary β‐glucans examined in this review
| Proprietary name | Source | Structure | Soluble |
|---|---|---|---|
| Wellmune | S. cerevisiae (yeast) | β‐1,3/1,6‐glucan | No |
| Yestimun | S. cerevisiae (yeast) | β‐1,3/1,6‐glucan | No |
| Glucan #300 | S. cerevisiae (yeast) | β‐1,3/1,6‐glucan | No |
| Imuneks | S. cerevisiae (yeast) | β‐1,3/1,6‐glucan | Yes |
| Lentinex | Lentinula edodes (mushroom) | β‐1,3/1,6‐glucan | Yes |
1.2. The Immune Response to Pathogen Invasion
The immune response is composed of innate and adaptive components. The initial responder to pathogens is the innate component, comprised mainly of phagocytic cells (e.g., neutrophils, monocytes, macrophages, and dendritic cells) able to engulf and kill pathogens. After phagocytosing and digesting pathogens and then processing pathogen‐derived antigens, innate immune cells release inflammatory mediators and cytokines, and present the processed antigen to T helper cells and B lymphocytes, leading to the development of the more specific adaptive immune response.[ 22 , 23 ] The T cells and B cells are responsible for the antigen‐specific recognition and destruction of pathogens.[ 24 , 25 ] Traditionally, the classical adaptive response was attributed to T and B cells able to express different receptors for maximal specificity based on the antigen being presented, while the innate response was understood to rely on receptors only able to recognize highly conserved microbial structures. More recently, it is evident that innate cells have the ability to modify responses by pattern recognition,[ 23 ] leading to the concept of innate immune memory or innate immune training.
The main challenge for the innate immune system is to differentiate self or benign from pathogenic antigens. Evolution's ingenious solution has been to identify microbe‐ or pathogen‐associated molecular patterns (MAMPs or PAMPs), which are different motifs present in invading microorganisms that are lacking in higher eukaryotes.[ 26 , 27 ] During the early phase of the immune response, MAMPs are recognized by pattern recognition receptors (PRRs) and complement binding (e.g., dectin‐1 and complement receptor 3 for β‐glucan signaling), which prompt a rapid detection and control of pathogen invasion via initiation of an innate immune response.[ 5 , 28 ] PRRs are expressed on dendritic cells, the classical antigen presenting cells (APCs), and play an essential role in recognizing endogenous (host‐derived) and exogenous (environmental) ligands prior to phagocytosis. APCs are specialized phagocyte like dendritic cells and macrophages distributed across the host. Following pathogen internalization and phagosome formation, a key aspect of the antimicrobial component of the innate immune response is the production of reactive oxygen species (ROS) against pathogens, a phenomenon known as oxidative burst.[ 29 ] This response has been reported against β‐glucans from fungal and yeast cell walls, and results in the phosphorylation of the NADPH oxidase complex and ultimately production of ROS (e.g., superoxide anion)[ 30 ] (Figure 2 ).
Figure 2.
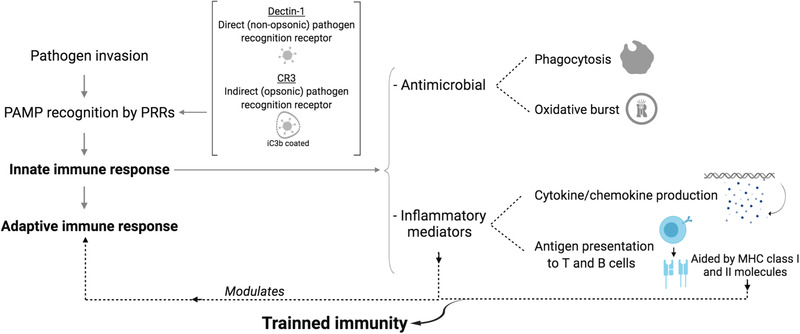
Immune response overview. The immune system has the ability to recognize and eliminate pathogens by first activating the innate response, which, without prior antigen exposure, acts in a fast and un‐targeted manner to phagocytose and kill the invader. APCs use PRRs to recognize pathogens directly (e.g., β‐glucan recognition by dectin‐1) or indirectly (e.g., β‐glucan recognition by CR3 by binding to iC3b opsonized β‐glucan). After PRR‐pathogen binding, APCs proceed with an antimicrobial response followed by an inflammatory response with a complex intracellular signaling cascade that culminates in the activation of transcription factors to produce inflammatory mediators and antigen presentation aided by MHC Class I and II molecules.
1.3. β‐Glucans as Trainers of Innate Immunity
Initial understanding suggested that the rapid onset and non‐specific innate immune response which lacks memory was followed by an adaptive response that is antigen‐specific and has immunological memory. However, it was recognized that some organisms that lack adaptive immune components show immunological memory.[ 31 ] The paradox whereby a first encounter between classical innate immune cells and a pathogen triggers intracellular functional changes that expedite future defense toward the same or an unrelated pathogen has been labeled “trained immunity or innate immune memory.”[ 32 ] It is now well established that PRRs expressed in innate immune cells show discrete pathogen recognition.[ 33 ] Trained immunity has been traced to epigenetic changes that result from reprogramming of chromatin marks within the progenitors of innate immune cells. This offers a mechanism whereby β‐glucan is able to prime innate immune cells. This idea was strengthened by the evidence of a more robust innate response from monocytes and macrophages to various pathogens after β‐glucan exposure, with enhanced antimicrobial and inflammatory properties derived from dectin‐1/toll‐like receptor (TLR) activation.[ 34 ] However, compared to the classical acquired immunity offered by antigen receptors as part of the adaptive immune response, PRRs show less specificity and duration of memory.
2. β‐Glucans as Immunomodulators: Mechanism of Action
β‐Glucans are highly conserved structural components that make up the majority of the cell walls of yeast and fungi. Since β‐glucans are not produced by mammalian cells, they act as biological response modifiers and are recognized as MAMPs by PRRs present on the surface of innate immune cells.[ 11 , 35 , 36 ] β‐Glucans are phagocytosed and processed by monocytes, macrophages and dendritic cells found in the upper intestinal lymphatic tissue to later be shuttled toward different immune organs like the spleen, where fragmented soluble β‐1,3‐glucan particles are released and prime immune cells for a more efficient antimicrobial and inflammatory response to pathogenic challenges.
2.1. β‐Glucan Internalization
Following oral β‐glucan administration, PRRs expressed on the cell surface of macrophages from the microfolds of Peyer's patches (M cells) bind and internalize β‐glucan upon arrival in the small intestinal lumen, as seen by fluorescence microscopy in murine spleen and bone marrow tissues.[ 22 , 37 , 38 ] Once internalized, β‐glucan‐containing innate immune cells travel to the different organs of the immune system where smaller and more soluble β‐1,3‐glucan fragments are released over several days to interact with and modulate the functional capacity of the innate immune response.[ 3 , 37 , 39 ] Whether β‐glucan‐containing macrophages are transported to the lymphoid organs via lymphatic or systemic circulation in humans is still unclear,[ 3 , 40 ] although rat and mouse models show intact β‐glucan being shuttled from the gastrointestinal tract to the different immune organs through the systemic circulation.[ 38 , 41 ] Subsequent fragmentation into smaller biologically active β‐1,3‐glucan particles occurs on days 3–5 after ingestion, β‐1,3‐glucan fragments are released extracellularly from days 5–10 and diminish after 14–21 days.[ 37 ] β‐glucan receptors include dectin‐1,[ 23 , 26 , 42 , 43 , 44 , 45 , 46 ] CR3,[ 26 , 47 , 48 , 49 ] lactosylceramide,[ 23 , 50 ] TLR 2, 4, and 6,[ 28 , 51 , 52 , 53 ] cluster of differentiation 36 (CD36),[ 54 ] and scavenger receptors like CD5.[ 55 ] Dectin‐1 and CR3 have been extensively characterized for their direct interaction with β‐glucans and their ability to subsequently alter the immune response, whereas the other receptors mainly become involved later on during the intracellular signaling cascade.[ 17 ] While CR3 is highly expressed on neutrophils, monocytes, natural killer (NK) cells, and to a lesser extent on macrophages, dectin‐1 is mainly expressed on macrophages, followed by granulocytes, and is absent from NK cells. Consequently, the main receptor mediating yeast phagocytosis in macrophages is dectin‐1, but CR3 is the predominant receptor in granulocytes.[ 40 ]
2.2. Dectin‐1 Structure and Signaling
Murine dectin‐1 and its human equivalent (also known as β‐glucan receptor) are type II C lectin‐like membrane receptors from the PRR family. Although some differences in expression pattern and regulation exist between dectin‐1 and its human homologue (e.g., N‐linked glycosylation is present in the C‐type lectin domain of murine dectin‐1 receptors and only in the full‐length stalks of β‐glucan receptor), they both seem to serve the same function as reviewed by Willment et al.[ 56 ] They share 60% sequence identity, and 71% sequence homology,[ 57 ] and tend to preferentially express the stalkless isoform of the receptor.[ 19 ]
Dectin‐1 is expressed on the surface of non‐specific myeloid immune cells (monocytes, macrophages, dendritic cells, and neutrophils), for example, in M cells (in gut‐associated lymphoid tissue). Extensive work done by Goodridge et al.[ 17 ] on RAW264.7 macrophages, primary mouse bone marrow‐derived macrophages, and dendritic cells as well as on human monocytes, shows that β‐glucans successfully bind to the C‐type lectin‐like carbohydrate recognition domain of dectin‐1; however, insoluble whole glucan particles (WGPs) but not soluble glucans (SGs) are able to successfully elicit dectin‐1 signaling.[ 17 , 19 , 42 ] Even when both WGP and SG are derived from the same (micro)organism (most studied is S. cerevisiae) they differ based on their structure, molecular weight, spatial conformation, solubility, etc. WGPs are insoluble β‐glucan particles that maintain the 3D yeast cellular structure with a hollow cytoplasm (i.e., only the cell wall remains) and are recognized by dectin‐1 as an immobilized form of β‐glucan, that is, on the surface of a phagocytosed particle such as yeast cells. SGs like PGG glucan, a β‐1,3/1,6‐glucan triple helix molecule of pharmaceutical grade, or smaller fragments of soluble β‐glucan released by macrophages after WGP internalization are recognized as “free floating or unattached” particles. PGG glucan is commonly used to test this mechanism in human peripheral blood mononuclear cells (PBMCs) and neutrophils.[ 58 ] One explanation for dectin‐1 only activating upon interaction with WGPs and not SGs is the “phagocytic‐synapse” mechanistic model, whereby dectin‐1 exclusively initiates its signaling cascade to immobilized ligands as opposed to soluble “floating” substances shed from their parent particle.[ 17 ] “Phagocytic synapse” is required for dectin‐1 hemITAM signaling regulated by CD45, and its related membrane tyrosine phosphatase CD148.[ 19 ] CD45 and CD148 are membrane proteins with a large extracellular domain and intrinsic tyrosine phosphatase activity. Both are required for surface molecule rearrangement following macrophage‐WGP binding, and to assist with the removal of inhibitory phosphatases to allow Src activation; however, to permit productive signaling, CD45 and CD148 are then isolated from the contact site of dectin‐1 with WGP in the forming phagosome (Figure 3 ). SGs seem to fail to sequester CD45 and CD148, and therefore they are incapable of continuing the signaling cascade. Consequently, dectin‐1, unlike other PRRs, plays a key role within the innate immune response by distinguishing between direct binding to microbes and binding to substances shed from microbes.
Figure 3.
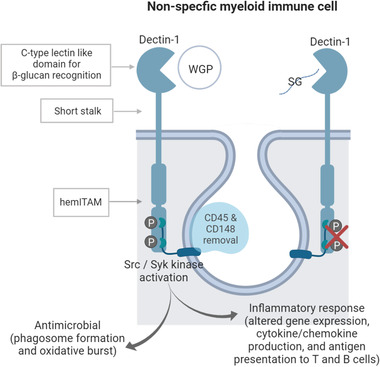
Dectin‐1 structure and signaling. Murine and human dectin‐1 consist of an extracellular C‐type lectin domain (which recognizes β‐1,3‐glucans) joined to a short stalk that continues into a single transmembrane domain followed by an intracellular ITAM‐like motif signaling domain (hemITAM). Upon whole glucan particle (WGP) attachment and Src phosphorylation, CD45 and CD148 are isolated from the contact site of dectin‐1‐WGP to allow hemITAM signaling. The downstream responses include 1) a rapid antimicrobial response lead by ligand uptake, phagocytosis, and oxidative burst, and 2) a consequent pro‐inflammatory response that is mediated by the production of cytokines and chemokines as a result of gene transcription modulation, together with antigen presentation to T and B cells to further continue with adaptive cell differentiation to the specific antigen. The binding to both dectin stalks is necessary to induce downstream Src activation and Syk recruiting.
In vitro studies of murine and human dectin‐1 clones highlights a new dectin‐1 signaling model, which proposes the existence of two parallel similar structures of the entire dectin‐1 unit, where the bridging of the twin dectin‐1 monomers is necessary to allow dual Src and spleen tyrosine kinase (Syk) activation and recruitment, and consequent NFAT‐and NF‐κB‐induced transcription for inflammatory regulation to drive the production of cytokines and chemokines.[ 19 , 59 , 60 ] Although the oligomerization of dectin‐1 has been questioned by Brown,[ 23 ] agreement exists regarding the concept of the ability of dectin‐1 to recognize carbohydrates, a function unusual in the classical C‐type lectin family of receptors. For the antimicrobial aspect of the response however, the phosphorylation of a unique Src domain appears sufficient for stimulation of phagocytosis and subsequent oxidative burst, as shown in murine bone marrow‐derived macrophages and the RAW macrophage lines.[ 30 ] In macrophages, phagocytosis is Syk independent, whilst in dendritic cells, it is Syk dependent.[ 43 ] This may present another cue for innate immune memory, as it is only activated in a subspecialized population of macrophages primed for ROS production.[ 30 ] Another sign that suggests a resemblance between innate and adaptive response via regulation of transcription is the dectin‐1 synergy with TLR pathways, in particular TLR2, TLR4, and TLR6, to enhance cytokine production from monocytes and macrophages against fungal pathogens.[ 23 ] As reviewed by Goodridge et al.,[ 19 ] this synergy is mediated via caspase recruitment domain containing protein 9 (CARD9) found in the sub‐population of murine bone marrow‐derived dendritic cells and macrophages destined for cytokine production upon β‐glucan binding. TLR2‐dectin‐1 synergy leads to TNF‐α and IL‐12 secretion and promotes IL‐2 and IL‐10 production via Syk activation.[ 61 , 62 ] Multiple other components like CD36 are involved in the complex interaction between dectin‐1 and TLRs.[ 23 ] Moreover, both IL‐10 and IL‐2 are involved in the development of regulatory T cells during fungal infection, pointing to the innate–adaptive immune interaction.[ 63 , 64 ] Lastly, the most novel role for dectin‐1 involving trained immunity has been described for the regulation of cell death in murine macrophages and dendritic cells, where CARD9 has the dual role of enhancing TLR signaling through MAP kinase activation and directly promoting NF‐κB activation.[ 19 , 65 , 66 , 67 ]
2.3. Complement Receptor 3 Structure and Signaling
The second pertinent receptor for β‐glucan interaction with the innate immune system is complement receptor3 (CR3), a β2 integrin protein that serves as an adhesion and recognition receptor with an exceptionally broad pathogen‐recognition capability.[ 68 ] CR3, while under the PRR umbrella, belongs to the complement system. The complement system is composed of proinflammatory serum proteins that “complement” inflammation and circulate as sedentary precursors until activated to cause cell lysis or opsonization.[ 23 , 69 ] Complement receptors are expressed on several leukocytes and are key mediators for yeast, fungal, and bacterial infection; hence β‐glucan recognition is essential, as it is a main component of the cell wall of these (micro)organisms.[ 70 ] CR3 triggers phagocytosis of complement opsonized particles by binding to complement activation molecule iC3 as shown in murine models,[ 71 , 72 , 73 , 74 ] and human PBMCs and neutrophils,[ 48 , 75 , 76 ] but it also works by direct pathogen binding.[ 77 , 78 ] The CR3‐β‐glucan signaling cascade is yet to be fully decoded[ 79 , 80 ]; however, it is thought that phosphorylation of tyrosine kinase Syk, PI3K, and p38MAPK are involved in the response, as reflected in murine[ 37 ] and human immune cells.[ 81 , 82 ] Fluorescence microscopy of mouse macrophages reveals how ingested β‐glucans are rapidly taken up in a CR3‐independent manner (dectin‐1 supported) and are shuttled to the bone marrow, spleen, and lymph nodes to slowly release soluble β‐1,3‐glucan active moiety fragments.[ 3 , 37 ] As evident in human neutrophils, it is these smaller fragments, and not the parent polysaccharide, that are responsible for CR3‐dependent signaling. CR3 is unique among integrins as it consists of a β‐subunit (CD18) and an α‐chain (CD11b).[ 83 , 84 ] As shown in Figure 4 , CD11b hosts two binding sites, one at the I‐domain site near the N‐terminus for ligands such as iC3b, and another one at the lectin site near the C‐terminus for short carbohydrates like β‐1,3‐glucan. The soluble smaller β‐glucan fragments prime phagocytes by binding to the lectin site of CD11b in mouse and human cells.[ 37 , 48 , 73 ] Signaling of CR3 requires dual ligation of CD11b, since the activation of the lectin site triggers configurational changes that expose the antigen binding site to ultimately mediate chemotaxis and phagocytosis toward the iC3b opsonized pathogenic cell. Subsequently, β‐glucan primed circulating phagocytes more avidly cause cytotoxicity of iC3b opsonized particles as shown in human neutrophils.[ 81 ] Upon iC3b opsonization, the chemotactic factors C3a and C5a are produced. C3a mainly recruits eosinophils (also basophils and mast cells), whereas C5a predominantly attracts neutrophils. In murine tumor models, the major cytotoxic activity of the β‐glucan C3‐dependent immune response has been attributed to the C5a chemokine cascade.[ 85 ]
Figure 4.
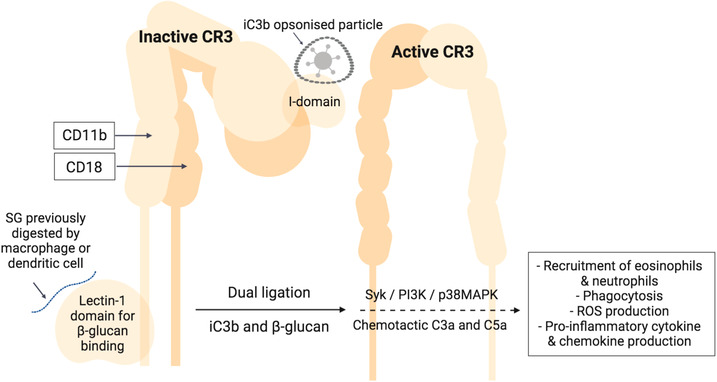
CR3 signaling. Dual ligation of the α‐chain (CD11b) by complement iC3b and soluble glucan (SG) is needed to activate CR3. iC3b coats the antigen and attaches to the I‐domain, while SG, derived from the fragmentation of its parent β‐glucan by phagocytes, attaches to the lectin‐1 domain.
2.4. Enhanced Oxidative Burst of Primed Innate Immune Cells
Following dectin‐1‐dependent WGP phagocytosis and CR3‐dependent priming, an oxidative burst response within the phagolysosome is activated in PBMCs.[ 18 ] Research with human PBMCs revealed that the WGP‐induced oxidative burst is dectin‐1 mediated and activates Src and PI3K/AKT signaling pathways. On the other hand, SG‐induced oxidative burst is CR3 mediated and activates actin polymerization and focal adhesion, followed by Src, Syk, PI3K/AKT, p38 MAPK, and PLC/PKC pathways.[ 18 ] Both signaling cascades (WGP via dectin‐1, and SG via CR3) ultimately activate the NADPH oxidase system to generate O2 − from O2 molecules. In the absence of phagocytosis, ROS are released into the environment via the signaling pathway that activates NADPH oxidase in response to focal adhered β‐glucan; this involves recognition by CR3 and signaling through p38 MAPK pathway in a process called “frustrated phagocytosis,” observed in mouse macrophages[ 86 ] and human neutrophils[ 82 ] (Figure 5 ). Furthermore, the priming of PBMCs with WGP significantly upregulates chemotaxis toward C5a and suppresses that toward IL‐8, which reduces the handicap of traveling through endogenous chemotactic gradients to reach final chemoattractant C5a.[ 87 ]
Figure 5.
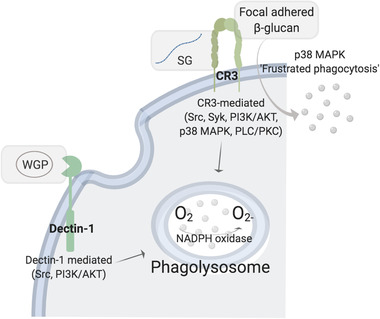
Oxidative burst. β‐glucan primed immune cells produce reactive oxygen species within the lysosome by activating NADPH oxidase; however, the activation of NADPH oxidase is achieved via different molecular signaling according to the form of β‐glucan, that is, WGP, SG, or focal adhered β‐glucan.
3. Translating β‐Glucan Immune Training to Humans
Many studies have explored biological response modifiers of the innate immune system to increase host resistance to pathogens, with β‐glucans ranking as one of the most widely researched. In conjunction with other standard immunomodulatory treatments, this strategy may offer a non‐specific protection potentially becoming a method of prevention and treatment for infections and enhancing the immune resistance of immuno‐compromised populations including the very young and older, frail people, and athletes. However, it must be acknowledged that, as the majority of the human studies available use primarily questionnaire‐based methodologies (addressing incidence, duration, and symptoms of infection) to draw conclusions, much uncertainty still exists about the evidence‐based relationship between β‐glucan supplementation in humans and consequent immunologic changes. This review organizes the existing human studies according to population, highlighting demographic, intervention, and outcome details for each study presented, according to the pre‐specified inclusion criteria indicated above, and with a focus on upper respiratory tract infection (URTI). The identity of all commercial preparations used is detailed in Table 1.
3.1. Upper Respiratory Tract Infection in Different Age Groups and in Athletes
Due to the large body of work focusing on the potential impact of β‐glucans to alleviate URTI, this condition and its prevalence will be described briefly. URTI, usually referred to as the common cold, is the most frequent infectious disease in humans, with an average prevalence of two to four episodes per year in adults and six to ten episodes per year in children.[ 88 , 89 , 90 ] Symptoms include nasal congestion, sore throat, and headaches; and although usually self‐limiting, they carry an increased risk for secondary bacterial infection and further co‐morbidities following inadequate recovery. Due to the heterogenic etiology of URTI, there is no reliable intervention to reduce the frequency of infection, making it the primary cause for antibiotic prescription in the United Kingdom with a total disease‐associated cost of €40 billion per year nationwide.[ 89 , 91 ] Hence, any alternative treatments showing a moderate alleviation of URTI incidence and severity would be vastly beneficial for population health and for the health system.
Respiratory infections are highly common in the first 5 years of life due to children's narrower airways, and the immature lungs and immune system.[ 92 ] In fact, respiratory infections followed by diarrhea are the two main causes of morbidity and mortality in children younger than 5 years old worldwide.[ 93 ] The Department of Pediatrics, ChangPing Women and Children Health Care Hospital in China reported that Wellmune supplementation (35 mg day−1 for 12 weeks) kept children healthier during the cold and flu season by significantly decreasing the incidence and duration of the common cold by 66% compared to placebo.[ 94 ] No significant difference in efficacy or safety parameters between the Wellmune groups (35 and 75 mg day−1) was found, suggesting that 35 mg of Wellmune daily is sufficient to achieve optimal benefit during the cold and flu season in children aged 1–4 years. On an immuno‐cellular level, Glucan #300 is the most studied β‐glucan in older children and teens and has been shown to improve mucosal innate immunity. Three studies evaluated mucosal innate immunity in children aged 8–12 years with recurrent respiratory problems treated with 100 mg of Glucan #300 daily.[ 95 , 96 , 97 ] One further study examined mucosal innate immunity of children aged 6–16 years[ 98 ] who were treated with 100 mg of Glucan#300 daily. Mucosal immunity represents the first line of defense against respiratory viruses and bacteria, and is mainly composed of salivary IgA and antimicrobial proteins. A reduction in IgA secretion levels has been linked to an increased incidence of URTI; thus, IgA is used as a proxy measure for mucosal immune function.[ 99 ] The first two studies measured salivary albumin, lysozyme, C‐reactive protein (CRP), and calprotectin levels, and reported a significant increase in lysozyme and CRP, compared to placebo.[ 95 , 96 ] The third study assessed salivary antibody levels (IgA, IgM, and IgG), showing a significant increase in all tested antibodies, compared to a reduction in the placebo group.[ 97 ] Finally, the fourth study explored not only the effects of β‐glucan on immune status using salivary IgA and salivary eNOS, but also on physical activity using the 6‐min walking test.[ 98 ] There were positive effects of β‐glucan supplementation in the two age groups, 6–10 and 11–16 years, via a significant stabilization of salivary IgA compared to a decrease in the placebo counterpart, and higher physical activity scores. Taken together, these results suggest that β‐glucan can improve mucosal innate immunity to reduce URTI incidence and symptom severity in children and teens.
Young children are especially susceptible to inadequate nutritional intake with a cascade effect on immunity and increased risk for infection and allergic reactions.[ 100 , 101 , 102 ] Mead Johnson Nutrition conducted two studies with children in day‐care settings in China[ 103 ] and Brazil,[ 104 ] in which the incidence of acute respiratory infection (ARI) was evaluated. Children in the experimental group consumed follow‐up formula fortified with vitamins A (380 vs 630 IU, control and treatment, respectively) and D (31 vs 119 IU, control and treatment, respectively), 25 mg docosahexaenoic acid (DHA), 1.2 g of polydextrose/galacto‐oligosaccharides, and 8.7 mg of Wellmune three times a day for 28 weeks. In the first study, children who consumed the fortified formula showed a significant decrease in the incidence and duration of ARI, antibiotic use, and missed day‐care hours, together with higher serum concentrations of the anti‐inflammatory cytokine IL‐10, and higher blood leukocyte levels, compared with children who were given an iso‐caloric unfortified, cow's milk‐based beverage.[ 103 ] The second study reported a significant reduction in allergic manifestations in the skin and respiratory tract between groups, although no significant difference was reported for ARI nor diarrheal disease.[ 104 ] This may be due to an increase in serum/plasma/cellular IL‐10 and its ability to inhibit Th2 mediators, which are usually elevated during allergic inflammation. These results cannot be attributed solely to β‐glucan, since vitamins, prebiotics, and polyunsaturated fatty acids were present in the experimental group's beverage but not in the control drink (Table 2 ).
Table 2.
Studies evaluating the effect of β‐glucans on immune and inflammatory biomarkers and infection in children
| Reference | Population | Intervention | Outcome vs placebo (*p < 0.05) |
|---|---|---|---|
| Meng et al. 2016 [ 94 ] | 154 children aged 1–4 years | 35 mg, 75 mg Wellmune or placebo per day during cold and flu season (12 weeks) |
*Decrease in the incidence and duration of the common cold by 66% No significant difference in efficacy or safety parameters between dose groups |
| Vetvicka et al. 2013 [ 95 ] | 40 children aged 8–12 years with recurrent respiratory problems | 100 mg Glucan #300 or placebo per day (4 weeks) |
*Increase in salivary lysozyme and CRP concentrations Non‐significant changes in salivary albumin |
| Richter et al. 2014 [ 96 ] | 56 children aged 8–12 years with recurrent respiratory problems | 100 mg Glucan #300 or placebo per day (4 weeks) |
*Increase in salivary lysozyme and CRP concentrations Non‐significant changes in salivary albumin and calprotectin concentrations |
| Vaclav et al. 2013 [ 97 ] | 40 children aged 8–12 years with recurrent respiratory problems | 100 mg Glucan #300 or placebo per day (4 weeks) | *Increase in salivary IgA, IgM, and IgG concentrations |
| Richter et al. 2015 [ 98 ] | 77 children aged 6–10 and 11–16 years with recurrent respiratory problems | 100 mg Glucan #300 or placebo per day (4 weeks) |
*Stabilization of salivary IgA and higher physical activity scores Non‐significant changes in salivary eNOS level |
| Li et al. 2014 [ 103 ] | 250 children aged 3–4 years in day‐care settings | Follow‐up formula fortified with vitamins A and D, 25 mg DHA, 1.2 g of polydextrose/galacto‐oligosaccharides, and 8.7 mg of Wellmune or iso‐caloric unfortified drink three times a day (28 weeks) |
*Fewer episodes and shorter duration of acute respiratory infection *Less antibiotic use and missed days of day care due to illness *Higher IL‐10 and white blood cell count Non‐significant changes in salivary albumin or calprotectin concentrations |
| Pontes et al. 2016 [ 104 ] | 256 children aged 1–4 years in day‐care settings | Follow‐up formula fortified with vitamins A and D, 25 mg DHA, 1.2 g of polydextrose/galacto‐oligosaccharides, and 8.7 mg of Wellmune or iso‐caloric unfortified drink three times a day (28 weeks) |
*Fewer episodes of allergic manifestations (includes rhinitis or conjunctivitis and wheezing, allergic cough, eczema, and urticaria) Non‐significant changes in the incidence of respiratory infections and diarrheal disease |
Immuno‐senescence refers to the age‐related decline of the immune system, which is characterized by a reduced ability to protect against and combat infections.[ 105 , 106 , 107 ] A study evaluating whether 250 mg day−1 of Wellmune enhanced immune function during the winter months in healthy older adults, reported a reduction in symptomatic days, which approached but was not significant, compared to the placebo group.[ 108 ] Wellmune supplementation significantly increased LPS‐stimulated IFN‐γ production, suggesting priming of innate immunity. However, Wellmune supplementation did not change symptom severity, plasma cytokine or chemokine levels, or salivary IgA concentration. A cross‐over study investigated the humoral immune and inflammatory response in participants aged 65 years or older after consuming 2.5 mg of Lentinex each day for 6 weeks,[ 109 ] and showed a significant increase in circulating B cell number. Other immunological and inflammatory markers, such as immunoglobulins, complement proteins, and cytokines, were not significantly altered. Taken together, these results suggest that there may be an association between β‐glucan supplementation and defense against infection in the elderly. Nonetheless, it is important to note that the mechanisms behind putative health effects summarized in Table 3 require greater clarity and definition.
Table 3.
Studies evaluating the effects of β‐glucans on immune and inflammatory biomarkers and infection in older adults
| Reference | Population | Intervention | Outcome vs placebo (*p < 0.05) |
|---|---|---|---|
| Fuller et al. 2017 [ 108 ] | 49 participants, mean age 56 years | 250 mg Wellmune or placebo per day (13 weeks) |
*Significant increase in LPS‐stimulated IFN‐γ production Reduction in symptomatic days (p = 0.067); Non‐significant changes in symptom severity, plasma cytokine, chemokine, and salivary IgA concentrations |
| Gaullier et al. 2016 [ 109 ] | 42 participants aged 65 years and over | 2.5 mg Lentinex or placebo per day (6 weeks) |
*Increased number of circulating B cells and prevention of decline in T cells (CD3+) Non‐significant changes in other immunological and inflammatory markers such as immunoglobulins, complement proteins, and cytokines |
Athletes often experience a transient state of immuno‐suppression after highly demanding physical effort leading to an “open window” for opportunistic infections. This is reflected in the greater incidence of URTI among ultra‐marathoners and marathoners.[ 110 , 111 , 112 ] A “J” shaped model has been suggested to reflect the relationship between risk of URTI and amount and intensity of exercise; where sedentarism shows average infection risk, moderate‐regular exercise confers maximal infection resistance, and extremely heavy acute or chronic exercise decreases resistance to infection.[ 113 ] It should be highlighted that fitness level highly impacts how the body reacts to exercise‐induced stress.[ 114 ] Physical stress is similar to other stressors, such as psychological stress, which can lead to a weakened immune system.[ 115 ] Psychological stress may also arise from prolonged training and competition, which is also posited to play a role in the “open window.” Likewise, elite athletes show deterioration in their mood during intense exercise periods, such as marathon training.[ 116 , 117 ] The mechanisms explaining this immuno‐suppression have not been firmly established; however it may be caused by impaired cell‐mediated immunological responses and psychological factors including stress and anxiety.[ 118 ] An impaired cell‐mediated immunological response during the “open window” is characterized by an overall reduction in the concentration of circulating immune cells, blunted TLR expression,[ 119 ] together with a concurrent state of inflammation,[ 120 ] all leading to a net suppression of cellular immunity and consequent increased risk of infection. In particular, monocyte and T cell numbers increase during and right after a bout of intense exercise to later decrease below pre‐exercise levels over 2–6 h, slowly recovering to baseline status within 24 h. This, together with a suppression of LPS‐stimulated cytokines,[ 121 ] explains why mucosal immunity is subjected to a temporary disruption during the 24 h window after strenuous exercise.[ 99 ]
The effect of Wellmune supplementation (250 mg day−1 for 10 days) was measured after a high heat and humidity bout of aerobic exercise in two clinical trials.[ 122 , 123 ] Wellmune significantly prevented the drop of T cells and monocytes commonly seen after an intense exercise session, and improved the LPS‐challenged response in monocytes from recreational athletes[ 122 ] and individuals of average fitness level.[ 123 ] In a third study, Wellmune prevented post‐exercise suppression of mucosal immunity as measured by a 32% increase in salivary IgA 2 h post‐exercise compared to the placebo group.[ 99 ] The plasma cytokine profile seen in the β‐glucan supplemented group is linked to lymphocyte proliferation (IL‐7), neutrophil‐related increased chemotaxis (IL‐8), anti‐inflammatory action (IL‐10),[ 122 ] reduced drop of circulating monocyte levels, and favorable alteration in their cell‐surface receptors. The last refers to receptors CD38, CD86, and TLR4, which confer improved T cell stimulation capacity, increased T cell concentration and activity (IL‐2), leukocyte priming (IFN‐γ), and other markers that may suggest increased immuno‐surveillance (CD8+ TCM cells).[ 123 ] The LPS‐stimulated cytokine profile showed a significant increase in IL‐2, IL‐4, IL‐7, and IFN‐γ compared to the placebo counterpart, which also suggests enhanced immuno‐surveillance by the priming of monocytes, reflected in the higher Th1 cytokine production and increased TLR4 expression.[ 123 , 124 ]
Aside from artificially‐taxing physical conditions like heat and humidity, Wellmune supplementation was evaluated in athletes during a naturally challenging scenario, a pre‐ and post‐marathon period. Participants were randomized to incorporate dispersible Wellmune (250 mg Wellmune per day) or placebo in a dairy‐based beverage 45 days prior to, on the day of, and 45 days post‐marathon.[ 125 ] In a second study, participants were randomized to receive a soluble Wellmune formulation, a dispersible Wellmune formulation or placebo for 28 days post‐marathon.[ 99 ] Results showed a significant decrease in post‐marathon URTI symptomatic days,[ 99 , 125 ] severity (especially nasal discharge and sore throat), and average missed workout days[ 125 ] in comparison to the placebo groups, while no significant difference was observed for average duration or incidence of URTI in either study. Another study supplementing Wellmune for 4 weeks of post‐marathon training (250 or 500 mg Wellmune per day) also evaluated URTI symptoms as well as overall health and mood using a questionnaire style health log and the profile of mood state (POMS) questionnaire, respectively.[ 126 ] The authors reported significantly lower URTI symptoms and better overall health and mood profiles in both supplement‐dose groups compared to placebo.
Overall, Wellmune has been shown to reduce the duration of the “open window” by priming granulocytes for faster activation and cytokine production upon pathogenic challenges, increasing salivary IgA, improving circulating monocyte and T cell count, and altering the balance of T helper cytokines (Th1/Th2). This helped to reduce URTI severity and improve overall health and mood in physically induced stress independent of fitness level, as was evident in trained[ 99 , 125 , 126 ] and recreational athletes[ 122 ] as well as individuals of average fitness level[ 123 ] (Table 4 ). This set of studies provides a good immuno‐cellular insight into the β‐glucan interactions with the immune system in regard to physically induced stress. Previous research has outlined that monocyte adhesion molecule expression, such as that determined by CD38 gene expression, affects leukocyte migration capacity into damaged tissue. CD38 plays a role in the transport and adhesion mechanisms of white blood cells,[ 127 ] and its downregulation on classical monocytes upon Wellmune supplementation is in part regulated by changes in IFN‐γ and may explain the observed increased levels of circulating monocytes. Additionally, Wellmune supplementation caused CD86 upregulation in non‐classic monocytes, in turn resulting in T cell co‐stimulation for rapid response upon antigen invasion.[ 123 ] Another sign of amplified immuno‐surveillance is the upregulation of TLR4 and TLR2 on non‐classic monocytes upon supplementation, which has been previously linked to the regulation of resistance exercise‐induced inflammation.[ 123 , 128 ] T cells follow a similar pattern as monocytes during exercise and the recovery period, a slight increase during exercise followed by a drop below baseline levels post‐exercise[ 129 ]; however, Wellmune supplementation triggered an overall increase in circulating T cells prior to and after exercise.[ 123 ] One mechanism of action that may underlie these events is the priming of neutrophils, which increases MAMP recognition, and the priming of monocytes, which increases Th1 production capacity. The enhanced monocyte response from TLR4 upregulation supports this hypothesis.[ 40 ]
Table 4.
Studies evaluating the effect of β‐glucans on immune and inflammatory biomarkers and infection in athletes
| Reference | Population | Intervention | Outcome vs placebo (*p < 0.05) |
|---|---|---|---|
| McFarlin et al. 2013 Study 2 [ 99 ] | 60 men and women physically active for > 6 months. Average age 22 years | 250 mg Wellmune or placebo per day (10 days). Exercise protocol: 50‐min strenuous cycling at high heat and humidity |
*Increase in salivary IgA at 2‐h post‐exercise |
| Carpenter et al. 2013 [ 122 ] | 60 recreationally active men and women. Average age 23 years |
250 mg Wellmune or placebo per day (10 days) using a cross‐over design with a 7‐day washout period Exercise protocol: 60‐min strenuous cycling at high heat and humidity |
*Increase in total (CD14⁺) and pro‐inflammatory (CD14⁺/CD16⁺) monocytes at 2‐h post‐exercise *Increased LPS‐stimulated production of IL‐2, IL‐4, IL‐5, and IFN‐γ pre‐ and post‐exercise *Increased plasma IL‐4, *IL‐5, and IFN‐γ (p = 0.053) concentrations at 2‐h post‐exercise |
| McFarlin et al. 2017 [ 123 ] | 109 participants of average fitness level. Average age 22 years | 250 mg Wellmune or placebo per day (10 days). Exercise protocol: 90‐min exercise session at high heat and humidity |
*Increase in total and classic monocytes and decreased expression of CD38 on classic monocytes *Increased CD4+ and CD8+ T cells *Increased response of CCR7+/CD45RA− central memory (TCM) cells to exercise *Increased serum IFN‐γ and IL‐2, and LPS‐stimulated IFN‐γ, IL‐2, IL‐4, and IL‐7 |
| Mah et al. 2018 [ 125 ] |
202 marathon participants. Average age 36 years |
Dairy‐based beverages (250 mL day−1) containing 250 mg of dispersible Wellmune or a macronutrient‐ and calorie‐matched control per day (91 days): 45 days pre‐marathon, the day of, and 45 days post‐marathon |
*Fewer URTI symptomatic days and decreased total URTI severity (nasal discharge and sore throat) *Fewer missed post‐marathon workout days due to URTI Non‐significant differences in average duration and number of URTI episodes |
| McFarlin et al. 2013 Study 1 [ 99 ] | 182 men and women physically active for >6 months. Average age 34 years | 250 mg Wellmune or placebo per day (28 days post‐marathon) |
*Reduction in the number of cold/flu symptom days |
| Talbott et al. 2009 [ 126 ] | 75 marathon participants. Average age 36 years | 250 mg, 500 mg Wellmune or placebo per day (4 weeks post‐marathon) |
*Fewer URTI symptoms and better overall health *Decreased confusion, fatigue, tension, and anger and increased vigor |
3.2. Studies Regarding Overall Health
Allergic rhinitis (AR, commonly termed hay fever), is a leading cause of seasonal allergy symptoms and affects more than one‐fifth of the adult population in Western Europe.[ 130 ] Typical symptoms include nasal congestion, sneezing, itchy eyes, and difficulty breathing. AR is an IgE‐mediated inflammatory allergic reaction of the nasal mucosa that is in part driven by the overproduction of Th2 cytokines (IL‐4, IL‐5, IL‐13), and subsequent stimulation of eosinophil generation and chemotaxis. These can be suppressed by Th1 cytokines like IL‐12 and IFN‐γ,[ 131 , 132 ] where β‐glucans are thought to play a role. The available standard allergen immuno‐therapy for hay fever includes high doses of allergen administration with the view of desensitization[ 133 ]; however, results are variable and there is still a need for safe, effective, and convenient therapy.[ 134 ] Thus, Kirmaz et al.[ 135 ] evaluated the effect of Imuneks on AR with the aim of assessing whether 12 weeks of β‐glucan (10 mg Imuneks twice daily) could assist Th1‐mediated immune response in Olea europaea sensitive patients with AR. For this, they challenged the participants with a nasal provocation test of O. europaea followed by a nasal lavage and measured IL‐4, IL‐5, IL‐12, IFN‐γ, and eosinophil count in the nasal fluid as well as peripheral blood eosinophil levels. Results from the nasal lavage showed a significant decrease in Th2 cytokines (IL‐4 and IL‐5), and an increase in Th1 cytokines (IL‐12) together with a reduction in eosinophils compared to placebo; however, IFN‐γ and peripheral blood eosinophil levels did not show a significant difference between groups.[ 135 ] Altogether, β‐glucan could be considered as an adjunct to standard AR treatment due to its robust alleviation of the exaggerated Th2 activity in AR patients.
With a focus on symptom severity, Wellmune has been studied for improving ragweed allergy symptoms in adults supplemented with 250 mg Wellmune per day for 4 weeks.[ 136 ] The results showed, compared to placebo, significant reduction in total allergy symptoms and symptom severity, overall increased POMS score and an improvement in global mood state, physical function, and all Rhinoconjunctivitis Quality of Life Questionnaire ratings including sleep problems, nasal and non‐nasal symptoms, eye symptoms, activities, emotion and quality of life; although non‐nasal symptoms and emotion were also significantly improved in the placebo group. Nonetheless, even though allergic rhinitis is considered to result from an IgE‐mediated allergic response, no significant difference was found for serum IgE levels between groups. In addition, Yestimun, another proprietary highly purified baker's yeast β‐1,3/1,6‐glucan was evaluated in three studies regarding effects on incidence and symptoms of the common cold in adults with recurring infections.[ 137 , 138 , 139 ] In the first study, participants took 450 mg of Yestimun twice a day for 26 weeks, whereas the second and third studies supplemented with 900 mg of Yestimun once a day for 16 weeks. All studies showed an overall prophylactic effect, with a significant reduction of common cold incidence during the infection season; however, whilst the first and third study showed significant symptom reduction, the second study did not reach significance for this parameter, but showed significant reduction in sleep disturbances caused by infections and better efficacy rating by participants and physicians. Furthermore, a randomized phase II clinical trial was conducted to evaluate immune support during the cold and flu season by assessing symptoms and overall well‐being, where participants took 500 mg of Wellmune or placebo per day for 12 weeks.[ 140 ] While the treatment group experienced a significant increase in quality of life as rated by the SF‐36V2‐QOL physical component summary score and a decrease in missed days of work/school, there was no significant difference in the incidence of symptomatic respiratory infections between the groups. Lastly, with a molecular emphasis, Glucan #300 was used to evaluate β‐glucan's effects on innate immune response in adults.[ 141 ] For this, serum β‐glucan levels, ex vivo stimulation of leukocytes, and microbicidal activity in blood were analyzed in a randomized open‐label intervention pilot study with 15 healthy male adults receiving 1 g of Glucan #300 daily for 7 days. At all points, β‐glucan was barely detectable in serum, and leukocyte cytokine production together with microbicidal activity were unaltered compared to the placebo group. Taken together, there seems to be some evidence to indicate that individuals suffering from allergies or at increased risk of respiratory infection may benefit from β‐glucan supplementation to reduce symptom severity and overall quality of life during infection season. However, aside from AR, where β‐glucan's effect on suppressing Th2 cytokine response was evident, how β‐glucan affects immunity at a cellular level to achieve such benefits is not clear (Table 5 ).
Table 5.
Studies evaluating the effect of β‐glucans on immune and inflammatory biomarkers and health outcomes
| Reference | Population | Intervention | Outcome vs placebo (*p < 0.05) |
|---|---|---|---|
| Kirmaz et al. 2005 [ 135 ] | 24 Olea europaea mono‐sensitized patients with allergic rhinitis | 10 mg Imuneks or placebo twice a day (12 weeks). A nasal provocation test with O. europaea was performed at the beginning and end of treatment followed by nasal lavage (NL) |
*NL fluid decrease in IL‐4, IL‐5, and eosinophils and increase in IL‐12 Non‐significant differences in NL fluid IFN‐γ and peripheral blood percentage eosinophil levels |
| Talbott et al. 2013 [ 136 ] | 48 participants with self‐reported moderate ragweed allergy. Average age 36 years | 250 mg Wellmune or placebo per day (4 weeks) |
*Decrease in total allergy symptoms and allergy symptom severity ratings *Increase in vigor, physical health, energy, and emotional well‐being *Decrease in tension, depression, anger, fatigue, confusion, sleep problems, nasal symptoms, eye symptoms, and non‐nasal symptoms *Improved quality of life and global mood state Non‐significant difference in serum IgE levels |
| Graubaum et al. 2012 [ 137 ] | 100 participants. Average age 46 years | 450 mg Yestimun or placebo per day (26 weeks) |
*Reduced overall incidence of common cold episodes *Reduced infections during the most intense infection season (first 13 weeks) and fewer cold symptoms including sore throat, difficulty swallowing, hoarseness, cough runny nose |
| Auinger et al. 2013 [ 138 ] | 162 participants with recurring infections. Average age 43 years | 900 mg Yestimun or placebo per day (16 weeks) |
*Reduction in the number of symptomatic common cold infections, mean symptom score, and sleep difficulties caused by infection *Higher physician rated efficacy of treatment |
| Dharsono et al. 2019 [ 139 ] | 299 participants reporting at least three URTIs during the previous year. Average age 38 years | 900 mg Yestimun or placebo per day (16 weeks) |
*Lower symptom severity and systolic and diastolic blood pressure *Increase in the joy subscore |
| Feldman et al. 2009 [ 140 ] | 27 participants. Average age 33 years | 500 mg Wellmune or placebo per day (13 weeks) |
*Reduction in missed days of work/school and fevers score *Improvement in quality of life Non‐significant difference in incidence of symptomatic respiratory infections |
| Leentjen et al. 2014.[ 141 ] | 15 male participants. Average age 20 years | 1000 mg Glucan #300 or placebo per day (1 week) in an open‐label intervention pilot study |
β‐glucan barely detectable in serum at all time points Non‐significant changes in cytokine production and microbicidal activity of leukocytes |
4. Dietary Regulations and Emerging Areas of Research
There is great interest in the application of β‐glucans in the food and beverage section worldwide. Food Marketing Industry data showed that the global β‐glucan market was worth $307.8 million in 2016 with a prediction by Markets and Markets to reach $476.5 million in 2022.[ 142 ] Yestimun is the only β‐glucan that has attempted to hold a European Food Safety Authority (EFSA) health claim in regard to immunity; however, it received a negative response when applying for the health claim “initiation of appropriate innate and adaptive immune responses in adults” in 2009 at a dose of 0.45 g twice daily. The EFSA Panel on Dietetic Products, Nutrition, and Allergies concluded that cause and effect relationship could not be established between the consumption of Yestimun and the initiation of appropriate innate and adaptive immune responses due to the existence of contradicting results in the literature.[ 143 ] On the other hand, Wellmune successfully holds a claim as a novel food ingredient granted in 2011 by the EFSA Panel on Dietetic Products, Nutrition, and Allergies. This application refers to both the insoluble and soluble formulations of Wellmune at dose levels up to 375 mg day−1 in food supplements and up to 600 mg day−1 in foods for particular nutritional uses (PARNUTS, also referred to as functional or “smart” food), but not for infant or follow‐up formulas.[ 20 ]
5. Gaps in Knowledge and Perspectives for the Future, Uncertain Immunity Biomarkers
Hard primary endpoints of innate immune functionality that are sensitive to nutritional interventions in man are lacking. In the pharma space, inflammatory biomarkers, such as CRP and other acute‐phase proteins, are sometimes used as endpoints. However, it is recognized that there are significant challenges regarding biomarkers of immunity and inflammation as highlighted by an International Life Sciences Institute Europe (ILSI Europe) workshop focused on “Low‐grade inflammation, a high‐grade challenge: biomarkers and modulation by dietary strategies.”[ 144 ] Thus, to more effectively capture innate immune changes in response to nutritional contexts in man, it was proposed that an integrated high‐dimensional data approach fusing epigenetic, transcriptomic, and proteomic signatures might be appropriate.[ 145 , 146 ] Furthermore, given the close inter‐relationship between metabolism and the immune response, there is no doubt that in time metabolic signatures may act as surrogate biomarkers of innate immune functionality. Such approaches will allow us to better dissect the dynamic inter‐relationship between nutritional/metabolic status, metabolism, and innate immunity, in order to more robustly define potential food‐derived nutrient and/or non‐nutrient sensitive immune health versus disease associations. It is only with the hard‐mechanistic evidence that the field can truly advance in terms of understanding the real ability of dietary components, including β‐glucans, to positively modulate metabolism and innate immune cell functionality. Additionally, there is no doubt that the impact of nutritional interventions is highly variable between individuals. This common phenomenon of high variability between subjects is particularly pertinent within the context of nutritional modulation of the immune system. Simple determinants of variability include age, sex, body mass index, or medications. In addition, the magnitude of response between individuals to a similar dose of a nutrient can be highly variable.[ 147 ] These additional challenges associated with personalized responses need to be factored into the research evidence underpinning any new product, including β‐glucans, in association with putative health effects.
Overall the balance of evidence suggests that β‐1,3/1,6‐glucan supplementation from baker's yeast (S. cerevisiae) shows immune‐enhancing and immune‐modulatory effects across different populations. Pre‐clinical studies have revealed major insights in relation to β‐1,3/1,6‐glucan signaling pathways and toxicological parameters; however, further research involving large randomized controlled trials with a clear immunological target need to be undertaken before the association of β‐glucan and immuno‐stimulation in humans can be more clearly understood. Studies into the cellular and molecular mechanisms of action via fluorescence microscopy show that orally administered β‐1,3/1,6‐glucans are engulfed and processed by macrophages and dendritic cells that later travel to the different immune organs and release fragmented soluble β‐1,3‐glucan particles. This results in the priming of leukocytes via dectin‐1, CR3 and other collaborating receptors to enhance immuno‐surveillance and counter pathogen attack by increasing the function of the complement system and innate immune cells, leading to improved antimicrobial and inflammatory responses. β‐1,3/1,6‐glucans have been examined in several randomized, double‐blind, placebo‐controlled human studies using different proprietary β‐1,3/1,6‐glucan formulations. Overall, these indicate that oral β‐glucan supplementation may improve symptom severity and duration in populations prone to respiratory infections and allergies. The most studied supplement is Wellmune, which has been evaluated across the widest population range to reveal significant alleviation of URTI, allergic episodes, and exercise‐ and age‐related immuno‐suppression. Other proprietary β‐1,3/1,6‐glucans have also shown promise; Glucan #300 improved mucosal innate immunity in older children and teens, Lentinex was shown to alleviate immuno‐senescence through an increase in immune cell count, Yestimun had a prophylactic effect in adults with recurring infections, and Imuneks reduced the exaggerated Th2 response in allergic rhinitis. However, for many of these studies, non‐significant differences between placebo and supplement groups indicate no reduction in infection incidence, cytokine production or microbial activity. Thus, further well‐designed human intervention trials are required to deeper evaluate immunity biomarkers that could potentially indicate the mechanisms of action behind the effect of β‐glucans on immune function in humans.
Conflict of Interest
P.C.C. has received funding as part of trials investigating Wellmune. E.D.M.C. is in receipt of a PhD studentship co‐funded by the Irish Research Council and Kerry.
Author Contributions
E.D.M.C. drafted the manuscript with advice from H.M.R.; H.M.R. and P.C.C. provided critical revisions. All authors approved the final version of the manuscript.
Acknowledgements
E.D.M.C. was supported by the Irish Research Council Employment Based PhD Scholarship Scheme. P.C.C. has received funding as part of trials investigating Wellmune. Work pertaining to this review and H.M.R. were supported by the Irish Department of Agriculture, Food and Marine “ImmunoMet” Programme (Grant number:14/F/828).
Biographies
Elena de Marco is a PhD researcher in Helen Roche's nutrigenomics lab at the Conway Institute, UCD, supported by the IRC Employment‐Based Scheme in collaboration with Kerry Foods. She is also part of the Diabetes Complications Research Centre, UCD. Her research focuses on the role of pre‐ and probiotics in modulating the microbiome in vivo and its putative effects on a diabetic mice model. She is also the lead researcher in a pilot nutritional‐intervention human study that aims to assess the rates of muscle protein synthesis and changes in aminoacidemia in the elderly population in response to a plant‐based diet.

Philip C. Calder is a professor of nutritional immunology within the School of Medicine at the University of Southampton. His work aims to understand how nutrition affects the functioning of the human body. A better understanding is key to developing strategies to improve human health and well‐being, lower disease risk, and treat nutrition‐related illnesses. He has broad research interests in nutritional modulation of immunity, inflammation, and cardiometabolic disease risk, and much of his work has been devoted to exploring the metabolism and functionality of fatty acids with an emphasis on the roles of omega‐3 fatty acids.

Helen M. Roche is a professor of nutrition and nutrigenomics in the School of Public Health and leads the Nutrigenomics Research Group at Conway Institute and Institute of Food & Health, UCD. Her research focuses on personalized nutrition. Nutrigenomics uses stat‐of‐the‐art ‘omics’ technologies to gain a greater understanding of the molecular effects of nutrition on health. Helen's team has a specific interest in personalized nutrition by exploring the molecular interactions between food‐related, nutritional stressors, metabolism, and inflammation within the context of obesity, insulin resistance, and T2DM. She leads/is co‐PI within several (inter)national research programs.

De Marco E. Castro, Calder P. C., Roche H. M., β‐1,3/1,6‐Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2020, 65, 1901071 10.1002/mnfr.201901071
References
- 1. Estrada A., Yun C.‐H., Kessel A. V., Li B., Hauta S., Laarveld B., Microbiol. Immunol. 1997, 41, 991. [DOI] [PubMed] [Google Scholar]
- 2. Rondanelli M., Opizzi A., Monteferrario F., Minerva Med. 2009, 100, 237. [PubMed] [Google Scholar]
- 3. Volman J. J., Ramakers J. D., Plat J., Physiol. Behav. 2008, 94, 276. [DOI] [PubMed] [Google Scholar]
- 4. El Khoury D., Cuda C., Luhovyy B., Anderson G., J. Nutr. Metab. 2011, 2012, 851362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novak M., Vetvicka V., J. Immunotoxicol. 2008, 5, 47. [DOI] [PubMed] [Google Scholar]
- 6. Talbott S. M., Talbott J. A., Talbott T. L., Dingler E., Food Sci. Nut. 2013, 1, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim H. S., Hong J. T., Kim Y., Han S.‐B., Immune Netw. 2011, 11, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stier H., Ebbeskotte V., Gruenwald J., Nutr. J. 2014, 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeković D. B., Kwiatkowski S., Vrvić M. M., Jakovljević D., Moran C. A., Crit. Rev. Biotechnol. 2005, 25, 205. [DOI] [PubMed] [Google Scholar]
- 10. Hewawasam R., Udawatte C., Weliwegamage S., Sotheeswaran S., Rajapakse S., Ferment. Technol. 2015, 4, 2. [Google Scholar]
- 11. Ruiz‐Herrera J., Ortiz‐Castellanos L., Cell Surf. 2019, 5, 100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demleitner S., Kraus J., Franz G., Carbohydr. Res. 1992, 226, 247. [DOI] [PubMed] [Google Scholar]
- 13. Driscoll M., Hansen R., Ding C., Cramer D. E., Yan J., Cancer Biol. Therapy 2009, 8, 218. [DOI] [PubMed] [Google Scholar]
- 14. Bohn J. A., BeMiller J. N., Carbohydr. Polym. 1995, 28, 3. [Google Scholar]
- 15. Wang Q., Sheng X., Shi A., Hu H., Yang Y., Liu L., Fei L., Liu H., Molecules 2017, 22, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pillemer L., Ecker E. E., J. Biol. Chem. 1941, 137, 139. [Google Scholar]
- 17. Goodridge H. S., Reyes C. N., Becker C. A., Katsumoto T. R., Ma J., Wolf A. J., Bose N., Chan A. S., Magee A. S., Danielson M. E., Nature 2011, 472, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bose N., Wurst L. R., Chan A. S., Dudney C. M., LeRoux M. L., Danielson M. E., Will P. M., Nodland S. E., Patchen M. L., Dalle Lucca J. J., Lebeda F. J., Glycobiology 2014, 24, 379. [DOI] [PubMed] [Google Scholar]
- 19. Goodridge H. S., Wolf A. J., Underhill D. M., Immunol. Rev. 2009, 230, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. EFSA Panel on Dietetic Products, Nutrition and Allergies , EFSA J. 2011, 9, 2137. [Google Scholar]
- 21. Babíček K., Čechová I., Simon R., Harwood M., Cox D., Food Chem. Toxicol. 2007, 45, 1719. [DOI] [PubMed] [Google Scholar]
- 22. Hong F., Hansen R. D., Yan J., Allendorf D. J., Baran J. T., Ostroff G. R., Ross G. D., Cancer Res. 2003, 63, 9023. [PubMed] [Google Scholar]
- 23. Brown G. D., Nat. Rev. Immunol. 2006, 6, 33. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann J. A., Kafatos F. C., Janeway C. A., Ezekowitz R., Science 1999, 284, 1313. [DOI] [PubMed] [Google Scholar]
- 25. Aderem A., Underhill D. M., Annu. Rev. Immunol. 1999, 17, 593. [DOI] [PubMed] [Google Scholar]
- 26. Brown G. D., Taylor P. R, Reid D. M, Willment J. A, Williams D. L, Martinez‐Pomares L., Wong S. Y, Gordon S, J. Exp. Med. 2002, 196, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fesel P. H., Zuccaro A., Fungal Genet. Biol. 2016, 90, 53. [DOI] [PubMed] [Google Scholar]
- 28. Aderem A., Ulevitch R. J., Nature 2000, 406, 782. [DOI] [PubMed] [Google Scholar]
- 29. Nathan C., Nat. Rev. Immunol. 2006, 6, 173. [DOI] [PubMed] [Google Scholar]
- 30. Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M., Blood 2005, 106, 2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowdish D. M. E., Loffredo M. S., Mukhopadhyay S., Mantovani A., Gordon S., Microbes Infect. 2007, 9, 1680. [DOI] [PubMed] [Google Scholar]
- 32. Netea M. G., Joosten L. A., Latz E., Mills K. H., Natoli G., Stunnenberg H. G., O'Neill L. A., Xavier R. J., Science 2016, 352, aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Netea M. G., Quintin J., van der Meer J. W., Cell Host Microbe 2011, 9, 355. [DOI] [PubMed] [Google Scholar]
- 34. Quintin J., Saeed S., Martens J. H., Giamarellos‐Bourboulis E. J., Ifrim D. C., Logie C., Jacobs L., Jansen T., Kullberg B.‐J., Wijmenga C., Cell Host Microbe 2012, 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCann F., Carmona E., Puri V., Pagano R. E., Limper A. H., Infect. Immun. 2005, 73, 6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowman S. M., Free S. J., BioEssays 2006, 28, 799. [DOI] [PubMed] [Google Scholar]
- 37. Li B., Allendorf D. J., Hansen R., Marroquin J., Ding C., Cramer D. E., Yan J., J. Immunol. 2006, 177, 1661. [DOI] [PubMed] [Google Scholar]
- 38. Rice P. J., Adams E. L., Ozment‐Skelton T., Gonzalez A. J., Goldman M. P., Lockhart B. E., Barker L. A., Breuel K. F., DePonti W. K., Kalbfleisch J. H., J. Pharmacol. Exp. Ther. 2005, 314, 1079. [DOI] [PubMed] [Google Scholar]
- 39. Ganda Mall J.‐P., Casado‐Bedmar M., Winberg M. E., Brummer R. J., Schoultz I., Keita Å. V., Inflamm. Bowel Dis. 2018, 24, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong F., Yan J., Baran J. T., Allendorf D. J., Hansen R. D., Ostroff G. R., Xing P. X., Cheung N.‐K. V., Ross G. D., J. Immunol. 2004, 173, 797. [DOI] [PubMed] [Google Scholar]
- 41. Sandvik A., Wang Y., Morton H., Aasen A., Wang J., Johansen F. E., Clin. Exp. Immunol. 2007, 148, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown G. D., Gordon S., Nature 2001, 413, 36. [DOI] [PubMed] [Google Scholar]
- 43. Tsoni S. V., Brown G. D., Ann. N. Y. Acad. Sci. 2008, 1143, 45. [DOI] [PubMed] [Google Scholar]
- 44. Taylor P. R., Brown G. D., Reid D. M., Willment J. A., Martinez‐Pomares L., Gordon S., Wong S. Y., J. Immunol. 2002, 169, 3876. [DOI] [PubMed] [Google Scholar]
- 45. Brown G. D., Herre J., Williams D. L., Willment J. A., Marshall A. S., Gordon S., J. Exp. Med. 2003, 197, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steele C., Rapaka R. R., Metz A., Pop S. M., Williams D. L., Gordon S., Kolls J. K., Brown G. D., PLoS Pathog. 2005, 1, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ross G. D., Cain J. A., Myones B. L., Newman S. L., Lachmann P. J., Complement 1987, 4, 61. [DOI] [PubMed] [Google Scholar]
- 48. Thornton B. P., Vĕtvicka V., Pitman M., Goldman R. C., Ross G. D., J. Immunol. 1996, 156, 1235. [PubMed] [Google Scholar]
- 49. Czop J. K., Austen K. F., Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zimmerman J. W., Lindermuth J., Fish P. A., Palace G. P., Stevenson T. T., DeMong D. E., J. Biol. Chem. 1998, 273, 22014. [DOI] [PubMed] [Google Scholar]
- 51. Luther K., Torosantucci A., Brakhage A. A., Heesemann J., Ebel F., Cell. Microbiol. 2007, 9, 368. [DOI] [PubMed] [Google Scholar]
- 52. Chan G. C.‐F., Chan W. K., Sze D. M.‐Y., J. Hematol. Oncol. 2009, 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meier A., Kirschning C., Nikolaus T., Wagner H., Heesemann J., Ebel F., Cell. Microbiol. 2003, 5, 561. [DOI] [PubMed] [Google Scholar]
- 54. Li X., Zhang X., Pang L., Yao L., ShangGuan Z., Pan Y., Nat. Prod. Res. 2019, 1. [DOI] [PubMed] [Google Scholar]
- 55. Rice P. J., Kelley J. L., Kogan G., Ensley H. E., Kalbfleisch J. H., Browder I. W., Williams D. L., J. Leukocyte Biol. 2002, 72, 140. [PubMed] [Google Scholar]
- 56. Willment J. A., Marshall A. S. J., Reid D. M., Williams D. L., Wong S. Y. C., Gordon S., Brown G. D., Eur. J. Immunol. 2005, 35, 1539. [DOI] [PubMed] [Google Scholar]
- 57. Hernanz‐Falcon P., Arce I., Roda‐Navarro P., Fernandez‐Ruiz E., Immunogenetics 2001, 53, 288. [DOI] [PubMed] [Google Scholar]
- 58. Bose N., Chan A. S., Guerrero F., Maristany C., Walsh R., Ertelt K., Jonas A., Gorden K., Dudney C., Wurst L., Danielson M., Elmasry N., Magee A., Patchen M., Qiu X., Vasilakos J., Front. Immunol. 2013, 4, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kerrigan A. M., Brown G. D., Trends Immunol. 2011, 32, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kerrigan A. M., Brown G. D., Immunol. Rev. 2010, 234, 335. [DOI] [PubMed] [Google Scholar]
- 61. Ferwerda G., Meyer‐Wentrup F., Kullberg B. J., Netea M. G., Adema G. J., Cell. Microbiol. 2008, 10, 2058. [DOI] [PubMed] [Google Scholar]
- 62. Goodridge H. S., Underhill D. M., Handb. Exp. Pharmacol. 2008, 183, 87. [DOI] [PubMed] [Google Scholar]
- 63. Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., R. e Sousa C., Immunity 2005, 22, 507. [DOI] [PubMed] [Google Scholar]
- 64. Osorio F., LeibundGut‐Landmann S., Lochner M., Lahl K., Sparwasser T., Eberl G., R. e Sousa C., Eur. J. Immunol. 2008, 38, 3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Inoue M., Okinaga T., Usui M., Kawano A., Thongsiri C., Nakashima K., Ariyoshi W., Nishihara T., FEMS Microbiol. Lett. 2019, 366, fnz093. [DOI] [PubMed] [Google Scholar]
- 66. Li X., Luo H., Ye Y., Chen X., Zou Y., Duan J., Xiang D., Int. J. Oncol. 2019, 54, 271. [DOI] [PubMed] [Google Scholar]
- 67. Ding J., Ning Y., Bai Y., Xu X., Sun X., Qi C., Med. Microbiol. Immunol. 2019, 208, 39. [DOI] [PubMed] [Google Scholar]
- 68. Schittenhelm L., Hilkens C. M., Morrison V. L., Front. Immunol. 2017, 8, 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moticka E. J., A Historical Perspective on Evidence‐Based Immunology, Newnes, Oxford: 2015. [Google Scholar]
- 70. Cramer D. E., Allendorf D. J., Baran J. T., Hansen R., Marroquin J., Li B., Ratajczak J., Ratajczak M. Z., Yan J., Blood 2006, 107, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu H., Smith C. W., Perrard J., Bullard D., Tang L., Shappell S. B., Entman M. L., Beaudet A. L., Ballantyne C. M., J. Clin. Invest. 1997, 99, 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., Arnaout M. A., Mayadas T. N., Immunity 1996, 5, 653. [DOI] [PubMed] [Google Scholar]
- 73. Vetvicka V., Thornton B. P., Ross G. D., J. Clin. Invest. 1996, 98, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cain J. A., Newman S. L., Ross G. D., Complement 1987, 4, 75. [DOI] [PubMed] [Google Scholar]
- 75. Ross G., Cain J., Lachmann P., J. Immunol. 1985, 134, 3307. [PubMed] [Google Scholar]
- 76. Krych M., Atkinson J. P., Holers V. M., Curr. Opin. Immunol. 1992, 4, 8. [DOI] [PubMed] [Google Scholar]
- 77. Ross G. D., Cain J. A., Myones B. L., Newman S. L., Lachmann P. J., Complement 1987, 4, 61. [DOI] [PubMed] [Google Scholar]
- 78. Ross G. D., Crit. Rev. Immunol. 2000, 20, 197. [PubMed] [Google Scholar]
- 79. Berton G., Lowell C. A., Cell. Signalling 1999, 11, 621. [DOI] [PubMed] [Google Scholar]
- 80. Mocsai A., Abram C. L., Jakus Z., Hu Y., Lanier L. L., Lowell C. A., Nat. Immunol. 2006, 7, 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. O'Brien X. M., Heflin K. E., Lavigne L. M., Yu K., Kim M., Salomon A. R., Reichner J. S., J. Biol. Chem. 2012, 287, 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lavigne L. M., Albina J. E., Reichner J. S., J. Immunol. 2006, 177, 8667. [DOI] [PubMed] [Google Scholar]
- 83. Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D., J. Cell Biol. 1989, 109, 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ehlers M. R., Microbes Infect. 2000, 2, 289. [DOI] [PubMed] [Google Scholar]
- 85. Allendorf D. J., Yan J., Ross G. D., Hansen R. D., Baran J. T., Subbarao K., Wang L., Haribabu B., J. Immunol. 2005, 174, 7050. [DOI] [PubMed] [Google Scholar]
- 86. Kruskal B. A., Maxfield F. R., J. Cell Biol. 1987, 105, 2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tsikitis V. L., Albina J. E., Reichner J. S., Surgery 2004, 136, 384. [DOI] [PubMed] [Google Scholar]
- 88. Monto A. S., Am. J. Med. 2002, 112, 4.11812400 [Google Scholar]
- 89. Heikkinen T., Järvinen A., Lancet 2003, 361, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burt C. W., McCaig L. F., Rechtsteiner E. A., Adv. Data 2007, 388, 1. [PubMed] [Google Scholar]
- 91. Bramley T. J., Lerner D., Sarnes M., J. Occup. Environ. Med. 2002, 44, 822. [DOI] [PubMed] [Google Scholar]
- 92. Darrow L. A., Klein M., Flanders W. D., Mulholland J. A., Tolbert P. E., Strickland M. J., Am. J. Epidemiol. 2014, 180, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J. E., Cousens S., Mathers C., Black R. E., Lancet 2015, 385, 430. [DOI] [PubMed] [Google Scholar]
- 94. Meng F., J. Nutr. Food Sci. 2016, 6, 2. [Google Scholar]
- 95. Vetvicka V., Richter J., Svozil V., Dobiášová R., L. K., Ann. Transl. Med. 2013, 1, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Richter J., Svozil V., Kral V., R. Dobiasova L., Stiborova I., Vetvicka V., Ann. Transl. Med. 2014, 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vaclav V., Josef R., Vladimir S., Lucie R. D., Vlastimil K., Am. J. Immunol. 2013, 9, 43. [Google Scholar]
- 98. Richter J., Svozil V., Král V., R. Dobiášová L., Vetvicka V., Ann. Transl. Med. 2015, 3, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McFarlin B. K., Carpenter K. C., Davidson T., McFarlin M. A., J. Diet. Suppl. 2013, 10, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Briefel R., Reidy K., Karwe V., Devaney B., J. Am. Diet. Assoc. 2004, 104, s31. [DOI] [PubMed] [Google Scholar]
- 101. Calder P. C., Proc. Nutr. Soc. 2013, 72, 299. [DOI] [PubMed] [Google Scholar]
- 102. Smithers L. G., Golley R. K., Brazionis L., Lynch J. W., Nutr. Rev. 2011, 69, 449. [DOI] [PubMed] [Google Scholar]
- 103. Li F., Jin X., Liu B., Zhuang W., Scalabrin D., Pediatrics 2014, 133, e1533. [DOI] [PubMed] [Google Scholar]
- 104. Pontes M. V., Ribeiro T. C. M., Ribeiro H., de Mattos A. P., Almeida I. R., Leal V. M., Cabral G. N., Stolz S., Zhuang W., Scalabrin D. M. F., Nutr. J. 2016, 15, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ventura M. T., Casciaro M., Gangemi S., Buquicchio R., Clin. Mol. Allergy 2017, 15, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Aw D., Silva A. B., Palmer D. B., Immunology 2007, 120, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pawelec G., Exp. Gerontol. 2018, 105, 4. [DOI] [PubMed] [Google Scholar]
- 108. Fuller R., Moore M. V., Lewith G., Stuart B. L., Ormiston R. V., Fisk H. L., Noakes P. S., Calder P. C., Nutrition 2017, 39–40, 30. [DOI] [PubMed] [Google Scholar]
- 109. Gaullier J.‐M., Sleboda J., Ofjord E. S., Ulvestad E., Nurminiemi M., Moe C., Albrektsen T., Gudmundsen O., Int. J. Med. Mushrooms 2011, 13, 319. [DOI] [PubMed] [Google Scholar]
- 110. M. Peters E., D. Bateman E., S. Afr. Med. J. 1983, 64, 582. [PubMed] [Google Scholar]
- 111. Nieman D. C., Johanssen L. M., Lee J. W., Arabatzis K., J. Sports Med. Phys. Fit. 1990, 30, 316. [PubMed] [Google Scholar]
- 112. Robson‐Ansley P., Howatson G., Tallent J., Mitcheson K., Walshe I., Toms C., Toit D. U. G., Smith M., Ansley L., Med. Sci. Sports Exercise 2012, 44, 999. [DOI] [PubMed] [Google Scholar]
- 113. Nieman D. C., Int. J. Sports Med. 1994, 15, S131. [DOI] [PubMed] [Google Scholar]
- 114. Walsh N. P., Gleeson M., Shephard R. J., Gleeson M., Woods J. A., Bishop N. C., Fleshner M., Green C., Pedersen B. K., Hoffman‐Goetz L., Rogers C. J., Northoff H., Abbasi A., Simon P., Exerc. Immunol. Rev. 2011, 17, 6. [PubMed] [Google Scholar]
- 115. Mackinnon L. T., Int. J. Sports Med. 1997, 18, S62. [DOI] [PubMed] [Google Scholar]
- 116. Achten J., Halson S. L., Moseley L., Rayson M. P., Casey A., Jeukendrup A. E., J. Appl. Physiol. 2004, 96, 1331. [DOI] [PubMed] [Google Scholar]
- 117. Hassmen P., Blomstrand E., Scand. J. Psychol. 1991, 32, 225. [DOI] [PubMed] [Google Scholar]
- 118. Campbell J. P., Turner J. E., Front. Immunol. 2018, 9, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gleeson M., Bishop N. C., Exercise Sport Sci. Rev. 2013, 41, 148. [DOI] [PubMed] [Google Scholar]
- 120. Nieman D. C., Henson D. A., Smith L. L., Utter A. C., Vinci D. M., Davis J. M., Kaminsky D. E., Shute M., J. Appl. Physiol. 2001, 91, 109. [DOI] [PubMed] [Google Scholar]
- 121. Weinstock C., Konig D., Harnischmacher R., Keul J., Berg A., Northoff H., Med. Sci. Sports Exercise 1997, 29, 345. [DOI] [PubMed] [Google Scholar]
- 122. Carpenter K. C., Breslin W. L., Davidson T., Adams A., McFarlin B. K., Br. J. Nutr. 2013, 109, 478. [DOI] [PubMed] [Google Scholar]
- 123. McFarlin B. K., Venable A. S., Carpenter K. C., Henning A. L., Ogenstad S., Front. Physiol. 2017, 8, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Iijima N., Mattei L. M., Iwasaki A., Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mah E., Kaden V. N., Kelley K. M., Liska D. J., J. Diet. Suppl. 2018, 17, 200. [DOI] [PubMed] [Google Scholar]
- 126. Talbott S., Talbott J., J. Sports Sci. Med. 2009, 8, 509. [PMC free article] [PubMed] [Google Scholar]
- 127. Musso T., Deaglio S., Franco L., Calosso L., Badolato R., Garbarino G., Dianzani U., Malavasi F., J. Leukoc. Biol. 2001, 69, 605. [PubMed] [Google Scholar]
- 128. McFarlin B. K., Flynn M. G., Campbell W. W., Stewart L. K., Timmerman K. L., Med. Sci. Sports Exerc. 2004, 36, 1876. [DOI] [PubMed] [Google Scholar]
- 129. Kruger K., Mooren F. C., Exerc. Immunol. Rev. 2007, 13, 37. [PubMed] [Google Scholar]
- 130. Bauchau V., Durham S., Eur. Respir. J. 2004, 24, 758. [DOI] [PubMed] [Google Scholar]
- 131. Bousquet J., Khaltaev N., Cruz A. A., Denburg J., Fokkens W., Togias A., Zuberbier T., Baena‐Cagnani C., Canonica G., Van Weel C., Allergy 2008, 63, 8. [DOI] [PubMed] [Google Scholar]
- 132. Abbas A. K., Murphy K. M., Sher A., Nature 1996, 383, 787. [DOI] [PubMed] [Google Scholar]
- 133. Bousquet J., Dhivert H., Michel F. B., Allergy 1994, 49, 31. [DOI] [PubMed] [Google Scholar]
- 134. Kim H. S., Hong J. T., Kim Y., Han S. B., Immune Netw. 2011, 11, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kirmaz C., Bayrak P., Yilmaz O., Yuksel H., Eur. Cytokine Network 2005, 16, 128. [PubMed] [Google Scholar]
- 136. Talbott S. M., Talbott J. A., Talbott T. L., Dingler E., Food Sci. Nutr. 2013, 1, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Graubaum H.‐J., Busch R., Stier H., Gruenwald J., Food Nutr. Sci. 2012, 3, 738. [Google Scholar]
- 138. Auinger A., Riede L., Bothe G., Busch R., Gruenwald J., Eur. J. Nutr. 2013, 52, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dharsono T., Rudnicka K., Wilhelm M., Schoen C., J. Am. Coll. Nutr. 2019, 38, 40. [DOI] [PubMed] [Google Scholar]
- 140. Feldman S., Schwartz H. I., Kalman D. S., Mayers A., Kohrman H. M., Clemens R., Krieger D. R., J. Appl. Res. 2009, 9, 30. [Google Scholar]
- 141. Leentjens J., Quintin J., Gerretsen J., Kox M., Pickkers P., Netea M. G., PLoS One 2014, 9, e108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bai J., Ren Y., Li Y., Fan M., Qian H., Wang L., Wu G., Zhang H., Qi X., Xu M., Trends Food Sci. Technol. 2019, 88, 57. [Google Scholar]
- 143. EFSA Panel on Dietetic Products, Nutrition and Allergies , EFSA J. 2016, 14, 4369. [Google Scholar]
- 144. Minihane A. M., Vinoy S., Russell W. R., Baka A., Roche H. M., Tuohy K. M., Teeling J. L., Blaak E. E., Fenech M., Vauzour D., McArdle H. J., Kremer B. H., Sterkman L., Vafeiadou K., Benedetti M. M., Williams C. M., Calder P. C., Br. J. Nutr. 2015, 114, 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Furman D., Davis M. M., Vaccine 2015, 33, 5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Manzoni C., Kia D. A., Vandrovcova J., Hardy J., Wood N. W., Lewis P. A., Ferrari R., Brief. Bioinform. 2018, 19, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben‐Yacov O., Lador D., Avnit‐Sagi T., Lotan‐Pompan M., Suez J., Mahdi J. A., Matot E., Malka G., Kosower N., Rein M., Zilberman‐Schapira G., Dohnalova L., Pevsner‐Fischer M., Bikovsky R., Halpern Z., Elinav E., Segal E., Cell 2015, 163, 1079. [DOI] [PubMed] [Google Scholar]


