Abstract
Communication signals serve crucial survival and reproductive functions. In Gabon, the widely distributed mormyrid fish Paramormyrops kingsleyae emits an electric organ discharge (EOD) signal with a dual role in communication and electrolocation that exhibits remarkable variation: populations of P. kingsleyae have either biphasic or triphasic EODs, a feature which characterizes interspecific signal diversity among the Paramormyrops genus. We quantified variation in EODs of 327 P. kingsleyae from 9 populations and compared it to genetic variation estimated from microsatellite loci. We found no correlation between electric signal and genetic distances, suggesting that EOD divergence cannot be explained by drift alone. An alternative hypothesis is that EOD differences are used for mate discrimination, which would require P. kingsleyae be capable of differentiating between divergent EOD waveforms. Using a habituation-dishabituation assay, we found that P. kingsleyae can discriminate between biphasic and triphasic EOD types. Nonetheless, patterns of genetic and electric organ morphology divergence provide evidence for hybridization between these signal types. Although reproductive isolation with respect to signal type is incomplete, our results suggest that EOD variation in P. kingsleaye could be a cue for assortative mating.
Keywords: animal communication, electric organ, electrocytes, genetic drift, signal evolution, weakly electric fish
Introduction
An overall goal in speciation research is to identify the evolutionary forces that generate divergence in populations, the targets of those evolutionary forces, and the relative order of the interactions that lead to speciation (Coyne and Orr, 2004). Rapidly speciating lineages are often characterized by highly diverse courtship signals (Diamond 1986; Allender et al. 2003; Mendelson and Shaw 2005; Boul et al. 2006; Mullen et al. 2007; Arnegard et al. 2010b). This has impressed on numerous evolutionary biologists the importance of behavioral isolation as a driving force in speciation, perhaps best summarized by the perspective of Mayr: “if we were to rank the various isolating mechanisms of animals according to their importance, we would have to place behavioral isolation far ahead of all others” (Mayr, 1963). Thus, it is widely appreciated that differences in courtship signals are crucial in the maintenance of reproductive isolation between species. What is less clear is the relative importance of these differences in initiating speciation, and which evolutionary forces may act to produce these differences.
Communication signal differences can evolve due to selection or drift acting directly on signals or preferences for those signals, or alternatively, by selection to avoid maladaptive hybridization between populations divergent in other traits (Kirkpatrick and Ryan 1991). Contemporary patterns of phenotypic divergence within species radiations are most likely a combination of all these evolutionary mechanisms acting either separately, simultaneously, or sequentially (Glaubrecht 2010). It is a fundamentally difficult problem to tease apart the contributions of the evolutionary forces that act on communication signals because of two important limitations. First is the ability in many systems to link contemporary forces acting in populations and the historical forces that have led to species diversity. Second, numerous communication system modalities are characterized by difficulty linking the anatomical and physiological substrates of signal generation and perception.
The first limitation can be circumvented in systems where intraspecific phenotypic variation parallels interspecific differences. This has been found in numerous adaptive radiations, such as in East African and Central American cichlids (Liem and Kaufmann 1984; West-Eberhard 1986; Meyer 1987; Maan et al. 2004), Darwin’s finches (Smith 1987; Werner and Sherry 1987), Heliconius butterflies (Brower 1994), sticklebacks (Schluter and McPhail 1992) or coral reef hamlet fish (Picq et al. 2019). Intraspecific polymorphisms in traits that are characteristic of the inter-species diversity provide unique opportunities to decipher how evolutionary forces act in the early stages of divergence. The second limitation may be addressed by the study of model systems with comparatively simple physiological and anatomical substrates for signal generation and perception.
Electrocommunication in weakly electric fish offers an excellent opportunity to study the evolution of communication signals as electric signals are diverse, and easily measured and quantified. Most importantly, owing partly to the uniqueness of this sensory modality and the fact that weakly electric fish have served as a model system in neuroscience for more than 50 years (Lissmann 1958; Heiligenberg 1977), the anatomical and physiological basis of both perception and production of electric signals is well characterized and understood (Bennett and Grundfest 1961; Arnegard et al. 2010a; Gallant et al. 2011; Carlson and Gallant 2013). Weakly electric fish generate electric organ discharges (EODs) for orientation, navigation, and communication, and can sense perturbations in these self-generated signals through an array of electroreceptors distributed over their skin. In the species-rich African weakly electric fish (Mormyridae), a radiation within the Paramormyrops genus has given rise to more than 20 species over the last 0.5–2 million years in drainages of West-Central Africa (Sullivan et al. 2002; Sullivan et al. 2004; Lavoué et al. 2008). Species within this genus exhibit highly divergent EODs, which vary primarily in duration (0.5–8 ms) and in the number of phases present (Fig. 1A; Sullivan et al. 2000). P0-present EODs have three phases (triphasic) and are hypothesized to be the ancestral condition for Paramormyrops (Sullivan et al. 2004). They are characterized by the presence of a small, head negative prepotential (P0), that is absent in other species (termed P0-absent EODs, or biphasic EODs). Despite the subtle difference in EOD signals, the anatomical substrate for P0-present and P0-absent signals requires a considerable structural reorganization of the cells that comprise the electric organ (electrocytes): individuals with triphasic EODs possess electrocytes with penetrating stalks and anterior innervation, whereas in biphasic EODs, electrocytes have non-penetrating stalks with posterior innervation.
Figure 1.
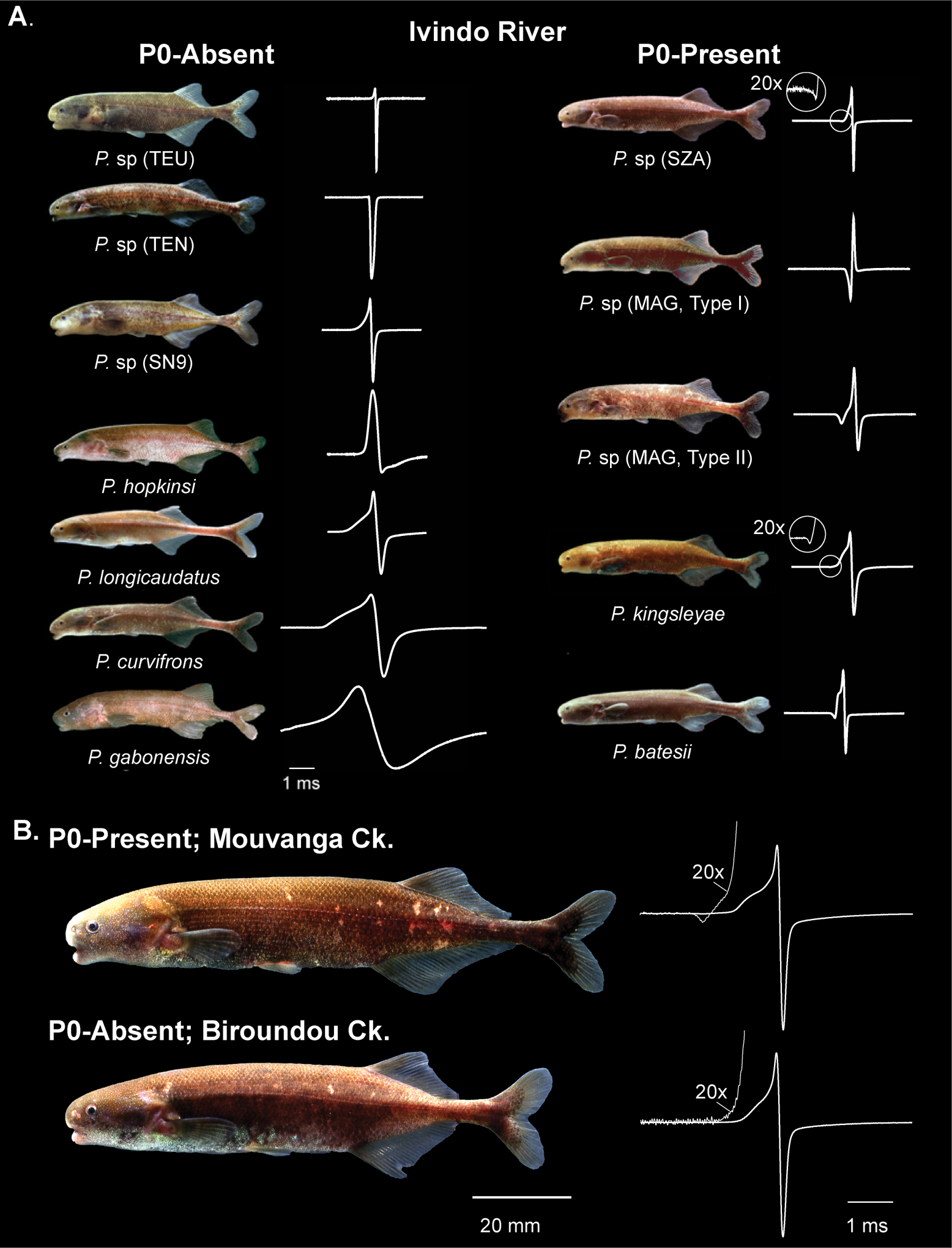
Paramormyrops kingsleyae EOD variation is a microcosm of EOD variation in Paramormyrops. A. 12 sympatric Paramormyrops specimens captured from the same locality in the Ivindo River illustrate EOD variation within the Paramormyrops genus in terms of duration and polarity, as well as in the number of phases. All Paramormyrops on the left have biphasic (P0‐absent EOD) waveforms and all Paramormyrops on the right have triphasic (P0‐present) EOD waveforms. B. P. kingsleyae EODs are variable in the presence of a small head negative phase (P0), see Gallant et al. (2011).
Within the watersheds of Gabon, we recently described an intraspecific EOD polymorphism in the geographically widespread species Paramormyrops kingsleyae, whereby the majority of populations have triphasic EODs and a few have biphasic EODs (Fig. 2; Gallant et al. 2011). P. kingsleyae thus reflects macroevolutionary patterns of EOD diversity across the Paramormyrops genus (Fig. 1B, Gallant et al. 2011), representing a “microcosm” of signal diversity and providing a great opportunity to study the evolutionary forces that initiate signal divergence. Among Paramormyrops species, playback experiments have revealed strong mating preferences for species-specific EODs (Hopkins and Bass 1981; Arnegard et al. 2006). In addition, Knollenorgan receptors, which mediate electrical communication in mormyrids, have been found to exhibit distinctive responses to different EOD waveforms (Arnegard et al. 2006). These findings indicate that individuals in the Paramormyrops species radiation are capable of detecting interspecific differences in EODs: is it also the case in the intraspecific polymorphism of P. kingsleyae?
Figure 2.

Map of study populations. Top map shows relationship between populations across Gabon. Box in the south indicates the location of the bottom, higher detail map, which shows the relationship of Southern populations near the confluence of the Louétsi and Ngounié rivers. For all maps, populations are indicated by pie charts, as reported in Gallant et al. (2011). Orange indicates proportion of individuals with triphasic (P0‐present) EODs and blue indicates proportion of individuals with biphasic (P0‐absent) EODs. Bongolo Falls is indicated in the map of Southern populations, and a grey region bounded by a dotted line indicates the small streams and creeks that drain into the Louétsi river above the Bongolo Falls.
Here, we assess evidence for neutral vs. selective divergence of EOD properties in populations of Paramormyrops kingsleyae, in an ongoing effort to determine the evolutionary mechanisms that shape early divergence in EODs, the key variable trait within the Paramormyrops radiation as well as between mormyrids. This intraspecific polymorphism allows us to connect population-level processes to macroevolutionary patterns of diversity.
Gallant et al. (2011) considered drift as the most likely force to explain the observed variation in P. kingsleyae EODs based on three major findings: (1) evidence of substantial genetic partitioning between allopatric populations of P. kingsleyae (Arnegard et al. (2005)); (2) clinal variation patterns in EOD duration and P0 magnitude across Gabon; (3) the discovery of morphologically ‘intermediate’ individuals in Apassa and Bambomo Creek, where both biphasic and triphasic signal types co-occur, which were putatively considered as evidence of hybridization, suggesting that mating may not be fully assortative with respect to signal type. Taken together, these results suggest that signal divergence may increase with geographic distance, that mating may be random with respect to signal type in sympatric populations, and that allopatric P. kingsleyae populations are genetically isolated, patterns that are expected when drift is important in driving signal divergence (Wilkins et al. 2013). Whether genetic differentiation is accompanied by proportional levels of signal variation in this species is still unknown.
The first goal of this study was to therefore explicitly test the hypothesis that genetic drift is responsible for the biogeographic distribution of EOD signals in P. kingsleaye. If this were the case, we would expect signal divergence to increase linearly with genetic divergence (Wilkins et al. 2013). Alternatively, if adaptive forces were the main determinants of signal diversity, we would expect no correlation between signal divergence and genetic divergence.
A prominent alternative hypothesis for the distribution of signals is that mormyrid species may mate assortatively on the basis of EOD differences (Arnegard et al. 2005; Feulner et al. 2009a, b; Arnegard et al. 2010b), which would imply that EOD divergence is under sexual selection. For this to be a valid explanation for the present distribution of signal types within P. kingsleyae, it would require that P. kingsleyae have the perceptual ability to discriminate EOD differences. We tested this alternative hypothesis directly, using a dishabituation behavioral assay to determine whether P. kingsleyae from a sympatric location are able to discriminate between EOD types. We also assessed this hypothesis indirectly by examining patterns of genotypic differentiation and electric organ morphology to determine whether there was any evidence of assortative mating among P. kingsleyae EOD types.
Materials & Methods
Field collections
Collections of Paramormyrops kingsleyae specimens were made during field trips to Gabon, West Central Africa in 1999, 2001 and 2009 from 9 locations summarized in Table 1 and Figure 2. A catalog of individual specimens used in this analysis is provided as Table S1, which provides Cornell Museum of Vertebrates accession numbers for all specimens used in this paper, along with associated metadata. We sampled two locations, Mouvanga and Bambomo Creeks, twice: once in 1999–2001 and again in 2009. Samples collected in different years at these locations were kept separate for all analyses, resulting in a total of 11 sampled populations.
Table 1.
Summary of locations and years of population samples, and analyses performed on each population.
| Apassa | 2009 | 2° 12’ 42” S | 11° 27’ 50” E | 28 | X | X | X (4) | |
| Bale | 2001 | 0° 31’ 9” S | 12° 47’ 58” E | 8 | X | X | ||
| Bambomo | 2009 | 2° 9’ 49” S | 11° 27’ 42” E | 106 | X | X | X (7) | X (17)* |
| Bambomo | 1999 | 2° 9’ 49” S | 11° 27’ 42” E | 27 | X | X | ||
| Bavavela | 2009 | 2°14’ 33”S | 11° 33’ 22” E | 24 | X | X | ||
| Bikagala | 2009 | 2° 11’ 43” S | 11° 33’ 40” E | 28 | X | X | ||
| Mouvanga | 2009 | 2° 19’ 23” S | 11° 41’ 18” E | 32 | X | X | X (6) | X (10)* |
| Mouvanga | 1999 | 2° 19’ 23” S | 11° 41’ 18” E | 19 | X | X | ||
| Nyame Pende | 2001 | 0° 30’ 16” S | 12° 47’ 48” E | 9 | X | X | ||
| Okano | 2001 | 0° 48’ 57” S | 11° 39’ 1” E | 37 | X | X | ||
| Songou | 2009 | 2° 16’ 42” S | 11° 36’ 41” E | 9 | X | X | X (4) |
Indicates additional individuals were sampled from these populations to perform these analyses. Numbers in parentheses indicate sample sizes, if no sample size is reported, the entire N reported was analyzed. Column EO indicates population utilized in histological analysis of electric organs (see methods). Column Behavior indicates population utilized in EOD discrimination task (see methods). Table S1 contains the full metadata for all specimens, including Cornell Museum of Vertebrates accession numbers for vouchers.
Fish were collected using a variety of methods, including fish traps baited with worms, hand nets combined with electric fish detectors, and by light rotenone applications. Following any application of rotenone, we immediately transferred the fish to fresh, aerated water, where they recovered completely.
We georeferenced sampling locations and calculated pairwise geographic distances between all study populations using digitized topographic maps, which were superimposed over satellite imagery provided in Google Earth software (Google, Inc. v.6.0.1). For each pair of populations, the distance between any two populations was assumed to be the shortest river path between the populations and was calculated by tracing currently mapped rivers between these populations. We note that additional, shorter paths connecting populations could potentially be created by seasonal flooding events (see Arnegard et al. 2006).
EOD recordings
P. kingsleyae exhibit a sex-difference in their signals during the rainy season breeding period, with sexually mature males showing a 2–3 fold elongation of their EODs compared to female or non-breeding males. Given the under-representation of breeding male recordings in our collections, the current analysis of electric signal variation is restricted to the species-typical female-like EODs exhibited by adult females and males outside the breeding season.
EODs of each specimen were originally recorded within 24 hours of capture in 1- to 5 liter plastic boxes filled with water from the collection site. Signals were recorded with bipolar chloride-coated silver wire electrodes and amplified (bandwidth =0.0001–50 kHz) with a differential bioamplifier (CWE, Inc: Ardmore, PA), and digitized at a 100 kHz-1 MHz sampling rate, with head-positivity plotted upward using a Daqbook or WaveBook (IOTECH: Cleveland, OH), or a USB-powered A-D Converter (National Instruments: Austin, TX). All EOD recordings were made at a vertical resolution of 16 bits per sample. After recording their EODs, we euthanized individual specimens with an overdose of MS-222. We removed one or more paired fins from specimens and preserved these tissues in 95% ethanol. Each specimen was given a unique specimen identification tag, and fixed free-floating in 10% formalin (phosphate-buffered; pH 7.2) for at least 2 weeks. Specimens were then transferred to 70% ethanol and deposited in the Cornell University Museum of Vertebrates. All methods conform to protocols approved by Cornell University’s Center for Research Animal Resources and Education.
Analysis of electric signal variation
Following the methods described by Gallant et al. (2011) and by Arnegard et al. (2003), we made 21 measurements from each recorded EOD waveform using a custom written program in MATLAB (Mathworks, Inc.: Natick, MA). For each waveform, we calculated amplitudes, times, and slopes at nine landmarks defined by peaks, zero crossings, first derivative peaks, and threshold crossings (Fig. 3; Table S2). In addition, we calculated a power spectrum for each EOD waveform using the MATLAB Fast Fourier transform function to determine the peak frequency and frequency values with power 3 dB below the peak frequency for each EOD recording. We quantified patterns of EOD variation among all P. kingsleyae individuals by performing a principal components analysis (PCA) on the set of all 21 measurements using the function princomp in R version 3.4.3 (R Core Team 2017). The measurements were normalized with the function scale. Electric signal distance between populations was calculated as the Euclidean distance between group centroids for the first two principal components using the R package vegan (Oksanen et al. 2018). To test the null hypothesis of no differentiation between EOD waveforms from different populations, we also computed the Euclidean distance between all pairs of individuals and performed a permutational MANOVA (PERMANOVA) directly on this pairwise signal distance matrix using 1000 permutations to determine probability values, using the adonis function from vegan.
Figure 3.

Example EOD Waveforms and Landmarks Measured. EOD landmarks and measurements used in the PCA were identical to those described in Gallant et al. (2011) and are listed for the reader’s convenience in Table S2. We indicate two example EOD waveforms: biphasic (P0‐absent) EOD. A. and triphasic (P0‐present) EOD. B. to illustrate the locations of these landmarks on EOD waveforms and their associated power spectra. Voltages shown are relative to a normalized P1‐P2 voltage set equal to 1.0 volts, as described in methods.
Landmark-based signal processing methods as described above focus only on pre-selected signal features that do not encompass a comparison of the entire waveforms and may thus be discriminatory. Therefore, we complemented our PCA analysis by performing cross-correlation analysis of all 327 waveforms, as described in Carlson et al. (2011) and performed in Picq et al. (2016). EOD waveforms that were sampled at 150, 200, or 250kHz (n = 57) were first down-sampled to 100kHz using the downsample and resample functions in MATLAB. We then used the maximum of the absolute values of the cross-correlation function as a measure of pairwise waveform similarity, resulting in a matrix of pairwise similarities ranging from 0 (dissimilar waveforms) to 1 (identical waveforms). Multidimensional scaling (MDS) was then applied to this cross-correlation matrix using the “mdscale” function in Matlab with Kruskal’s normalized stress 1 criterion (Kruskal and Wish 1978). Electric signal distance between populations was calculated as the Euclidean distance between group centroids in the MDS space using vegan. Electric signal distances between all pairs of individuals were also estimated in the same way. As for the PCA, a PERMANOVA analysis was also performed on the individual pairwise signal matrix to test the null hypothesis of no differentiation between EOD waveforms from different localities.
Recording temperature is known to affect EOD duration (Kramer and Westby 1985). Gallant et al. (2011) showed that applying a Q10 temperature correction on 491 P. kingsleyae EODs recorded across Gabon at temperatures ranging from 21 to 26.7°C did not result in a significantly different PCA scores of signal variation. As the range of recording temperatures in the current study is narrower (20.5 to 25.1°C), we considered the effect of temperature on our PCA configuration of signal variation as negligible. Nevertheless, we still investigated the effect of temperature on signal variation quantified through multidimensional scaling of signal cross correlations. The full methodology and results of this analysis are included in Supplementary File 1. We concluded from this analysis that recording temperature was not a significant source of variation in our study, and therefore present our analysis on the full dataset without temperature correction for both PCA-derived and MDS-derived signal analyses.
Microsatellite genotyping
We extracted DNA from the ethanol-preserved fin clips using DNeasy Tissue Kits (Qiagen, Inc.) for the 1998–1999 samples, or AgenCourt DNAadvance kits (Beckman-Coulter, Inc) for the 2009 samples. The 1998–1999 DNA samples originally genotyped by Arnegard et al. (2005) were re-genotyped for this study so that genotypes could be scored using identical criteria. We amplified DNA at each of five microsatellite loci (NBB001-NBB005) originally identified by Arnegard et al. (2005) using the Qiagen Type-It multiplex PCR System (Qiagen, Inc.). Reaction volumes were 15μl, consisting of 1μl template DNA, 7.5μl Type-it Multiplex Master Mix (containing HotStarTaq Plus DNA Polymerase and PCR buffer with 6μM MgCl2), and 2 pmol of each primer (5’ primers labeled with Applied Biosystems fluorescent dyes FAM, HEX or NED). Thermal cycling (under mineral oil) was 5 min at 95°C (initial activation) followed by 28 cycles of 95° for 30 sec, 60°C for 90 sec, and 72°C for 30 sec. Each individual reaction (containing PCR products for all 5 loci) was resolved by electrophoresis on an ABI 3100 automated sequencer (Applied Biosystems). Under these thermal conditions, reactions for locus NBB004 failed for the Bambomo, Nyamé-Pendé, and Balé Creek populations. For these populations, an additional PCR reaction was successfully performed as above using primers only for the NBB004 locus, with thermal cycling (under mineral oil) for 5 min at 95°C (initial activation) followed by 35 cycles of 95° for 30 sec, 50°C for 90 sec and 72°C for 30 sec. Following genotyping, individual fragment lengths were analyzed and binned according to size by visual inspection, using Genemapper 4.1 software (Applied Biosystems).
Genotyping data analysis
For each microsatellite locus (NBB001-NBB005), we examined possible deviations from expected Hardy-Weinberg equilibrium within populations using the two-tailed exact test (Weir 1990) as implemented by GENEPOP v4.1 (Rousset 2008). Next, we also performed exact tests of linkage disequilibrium between all pairs of loci (within and between populations) in GENEPOP, to test the independent assortment of loci. Statistical significance in both sets of tests was evaluated using Markov chain methods (10,000 dememorization steps; 1,000 batches; 5,000 iterations per batch). We additionally calculated observed and expected heterozygosity under Hardy-Weinberg equilibrium for each locus in every population using the software Arlequin 3.5 (Excoffier and Lischer 2010). The significance of each was evaluated at both the p=0.05 α probability threshold and the Bonferroni corrected threshold of p=0.001.
Population structure
We quantified genetic differentiation between populations using Fst (Weir, Cockerham, 1984) with Arlequin v 3.5 (Excoffier and Lischer 2010). This standardized measure of population genetic structure is equivalent to the variance of allele frequencies between populations (i.e., subpopulations, or s) divided by the variance of allele frequencies in the total population consisting of both subpopulations combined (t). Using the same software, we evaluated the statistical significance of the Fst estimates (at both the uncorrected and Bonferroni-corrected thresholds) by permuting genotypes between populations 50,000 times.
Because only five microsatellite loci were scored, we performed power analysis simulations using powsim version 4.1 (Ryman and Palm 2006) to determine if our sample sizes, number of microsatellite loci, and allele diversity were sufficient to detect genetic differentiation (see full methodology and results in Supplementary File 2). These analyses showed that our sample sizes and specific genetic markers were adequate for detecting levels of genetic differentiation as low as Fst = 0.003 with a high probability (>0.9) (Fig. A2 in Supplementary File 2). We can therefore consider our genetic markers as sufficiently powerful for the purpose of this study, namely to give a reliable estimate of genetic differentiation between P. kingsleyae allopatric populations.
Isolation by distance
Genetic isolation by distance (IBD) was explored following Rousset (1997), i.e. using a linear regression between genetic distance (Fst/(1-Fst)) versus geographic distance for all pairs of populations. The correlation was tested with a Mantel test (Mantel 1967), which accounts for non-independence of points in a distance matrix, using 1000 permutations in the R package vegan. The IBD analyses were performed on the whole dataset and repeated on Southern populations separated by short distances (<120 km) where sampling was high (including Bambomo, Apassa, Bikagala, Bavavela, Songou, and Mouvanga, Fig.2 lower panel). When considering the entire dataset, it is important to note that populations included in this study represent two distant and separate zones along the species’ otherwise continuous distribution across Gabon (Gallant et al. 2011). To control for this gap distribution in our sampling efforts and for the associated potential confounding effect of hierarchical structure (i.e. of regional-level effects between discontinuous Southern and Northern populations) on relationships between distance matrices, we ran a partial Mantel test, including a model matrix of regional membership that identified which populations were included in each pairwise comparison (e.g. North-North, South-South, South-North; (Smouse et al. 1986; Meirmans 2012). When only considering the subset of Southern populations, we additionally tested for the effect of Bongolo Falls as a barrier to gene flow between upstream (Bavavela and Bikagala) and downstream (Bambomo, Apassa, Songou, Mouvanga) populations by running a Mantel test between genetic distance and a model matrix coding for populations separated (1) or not separated (0) by this putative barrier.
Comparison of signal and genetic divergence
To investigate whether electric signals evolve at a rate consistent with neutral evolution, we tested the correlation between signal and genetic distance for all pairs of populations using Mantel tests. This was performed for the whole dataset with a partial Mantel test (accounting for potential regional-level effects between discontinuous Northern and Southern populations) and repeated on Southern populations. We also tested whether signal differences between populations could be the product of isolation by distance by testing the correlation between signal and geographic distances, again using a partial Mantel test for the whole dataset and a Mantel test for the Southern populations. The potential effect of Bongolo Falls in driving signal divergence in Southern populations was tested using the same rationale as for the genetic data: a Mantel test was performed between signal distance and a model matrix coding for populations separated or not by the falls. All analyses were performed on both PCA and MDS derived signal distances.
Evaluating discrimination ability: behavioral playback experiments
We performed two sets of electrical playback experiments to assess the ability of P. kingsleyae to discriminate P0-present and P0-absent waveforms. As for previous analyses, these experiments were restricted to adult specimens exhibiting non-breeding EODs. For all experiments, we assessed behavioral discrimination of EOD waveforms using a dishabituation paradigm described in detail by Carlson et al. (2011). Specimens were placed in a rectangular PVC enclosure containing both chlorided silver wire stimulus electrodes (Ag/AgCl) and Ag/AgCl recording electrodes, with the two pairs of electrodes oriented orthogonally with respect to one another. We delivered stimulus trains consisting of 10 bursts of 10 EOD pulses each, with an intra-burst interval of 30 ms, and inter-burst interval of 10 s, with a peak-to-peak intensity of 145 mV/cm. We constructed negative control trains in which all 10 bursts of EOD pulses were identical. We also constructed positive controls known to exhibit reliable responses, using 8 bursts of identical EODs (background), with the 9th burst consisting of a novel waveform (a 90 degree phase-shifted EOD used during the first 8 bursts), followed by a 10th burst of the original background waveform. Positive control novel EOD stimuli were constructed by phase-shifting the background EOD waveform by 90°, which is maximally divergent in the time domain while preserving the frequency-spectrum. Phase shifting of EODs was accomplished by performing an FFT on the EOD waveform, followed by adding 90 ° to the phase angle at each positive frequency, and subtracting 90° from each the phase angle at each negative frequency. This was followed, in turn, by inverse FFT, which yielded a reconstructed EOD characterized by an altered (i.e., 90° shifted) phase spectrum with an unaltered power spectrum (Heiligenberg and Altes 1978; Hopkins and Bass 1981; Carlson and Arnegard 2011). P. kingsleyae have been shown to respond to this specific phase-shifted experimental stimulus by increasing their electrical discharge rate (Carlson et al. 2011). This trial was thus included in our experiments to demonstrate that the fish are capable of exhibiting electrical responses when they are presented with electrical stimuli that they can discriminate. The experimental stimuli consisted of presenting eight bursts of the same P. kingsleyae EOD with the 9th burst consisting of a different P. kingsleyae EOD. We computed the EOD rate of the subject fish by converting the EOD times of occurrence into a series of a delta functions, and convolving these a 300-ms Gaussian waveform (Carlson and Hopkins 2004). The specimen’s response was then recorded as the maximum EOD rate within a 2 sec window following each burst of EODs (Carlson and Arnegard 2011). The magnitude of the specimen’s response declined over repeated presentation of the background bursts (habituation). Therefore, we measured each specimen’s ‘novelty response’ as the change in EOD discharge rate (dishabituation) following the 9th (novel) burst, relative to the EOD discharge rate following the 8th presentation of the background burst. Statistical significance in signal discrimination was assessed using Dunnett’s multiple comparison test with control (Dunnett 1955), whereby we compared novelty responses towards each experimental stimulus (and toward the positive control) with the negative control. Significantly different novelty responses to experimental stimuli vs. negative control were considered evidence for discrimination between background and novel waveforms.
Some trials included ‘hybrid’ waveforms generated artificially from two normalized natural EOD waveforms: one P0 absent waveform from Bambomo and one P0 present waveform from Mouvanga. We centered these waveforms using the midpoint between peaks P1 and P2 of the waveform, and generated hybrids of varying P0 character by taking different weighted averages of the two waveforms (Fig. S1).
One set of experiments was performed within 6 hours of capture on P0-absent P. kingsleyae specimens from Bambomo creek (n=10), where both signal types are known to co-occur, as well as on P0-present P. kingsleyae individuals from Mouvanga creek (n=10), where only P0-present P. kingsleyae individuals occur but other Paramormyrops species are found. A second set of experiments was performed on additional P0-absent individuals from Bambomo Creek, which were captured as juveniles and transported to the Hopkins laboratory at Cornell University (Ithaca, NY), and reared to adulthood for further study (n=13). These fish were housed in community tanks with other individuals captured from their home stream. In addition, the fish were fed live blackworms daily, and they were maintained on a 12-hour light/dark cycle, with water temperatures maintained between 25–27°C, pH between 6.5–7.0, and water conductivity between 200–400μS.
Electric organ histology & analysis
Serial sections of electric organs from selected individuals were made for light-microscopy analysis following the methods described by Gallant et al. (2011). Briefly, electric organs were removed from fixed specimens, decalcified overnight, dehydrated in a graded alcohol series, and infiltrated in glycol methacrylate resin (JB-4 resin; Polysciences, Inc). Serial parasagittal sections, each 6 μm thick, were cut from lateral to medial with a tungsten carbide microtome knife, mounted on glass slides, and stained with 0.5% Toluidine blue for 30 sec. For each specimen, we reconstructed one of four columns of electrocytes from these serial sections. Because each column of electrocytes surrounds the spinal cord, we began our reconstruction at the lateral edge of the electric organ, and stopped the reconstruction when the spinal cord was clearly visible (approximately 234–648 μm depending on the size of the individual). For each section, the number of stalk penetrations through each electrocyte was counted in, and averaged across, 50–70 electrocytes per section. An electrocyte was scored with a penetration whenever a stalk was observed to pass through either or both faces of the electrocyte. For our analysis, we considered each 6 μm section to have an independent number of penetrations from all other sections to minimize the probability of underestimating the total number of penetrations.
Results
Patterns of EOD variation
We assessed variation in EOD signals using both Principal Components Analysis (PCA; Fig. 4) and multidimensional scaling of signal cross correlations (MDS; Fig. S2) on each of the 327 individual EODs in this study.
Figure 4.

Principal component analysis of EOD waveform variation in 327 P. kingsleyae individuals from 9 populations in Gabon. A. Variation in waveforms was quantified using Principal Components Analysis (see Gallant et al. (2011) and text for further details). Variables related primarily to EOD duration loaded most strongly on principal component 1, whereas variables related to the magnitude of P0 loaded most strongly on principal component 2 (Table S3). Polygons enclose EOD waveforms from each recording locality. Polygon centroids are represented with black dots. Asterisks in the legend represent two populations with mixed signal types (Apassa is mostly composed of P0‐present individuals, whereas Bambomo is mostly composed of P0‐absent individuals). B. Overlay diagram of EOD waveforms from five different localities.
The first principal component of our PCA related strongly to duration and explained 43.71% of the variation between individuals, while the second factor related strongly to the magnitude of P0 and comprised 15.94% of the variation between electric signals. Factor loadings for these two principal components are summarized in Table S3. In our MDS analysis, the number of dimensions was set to N = 2, which resulted in a stress of 0.0526, considered to give a good ordination representation of the cross-correlation matrix with low probability of misinterpretation (Clarke 1993).
Both MDS and PCA identified significant variation among recording localities (PERMANOVA on PCA signal distances: F10,326 = 58.62, p<0.001, PERMANOVA on MDS signal distances: F10,326 = 45.44, p<0.001). Mantel tests showed a strong and significant correlation between signal distances estimated from PCA and MDS (Fig. S3, Mantel test, R = 0.677, p = 0.001), which is also evident from comparison of Fig. 4 and Fig. S2. Interestingly, inter-population distances between Southern populations and Bale as well as between Southern populations and Nyame Pendé were larger with PCA-derived methods than with MDS-derived methods (Fig. S3). This is likely due to the fact that the PCA analysis included three variables pertaining to the frequency content of the EODs (peak frequency, low and high frequency with 3dB below the peak frequency) in addition to variables pertaining to the temporal content of the EODs. The cross-correlation analysis that the MDS was applied to, on the other hand, involves the progressive sliding of one waveform past the other, and thus indicates EOD similarity essentially in temporal content. Although a change in frequency content will be associated with a change in temporal content, the MDS analysis likely attributed less weight to specific frequency content differences. Regarding the two populations that were sampled in 1999 and again in 2009, we note that the overlap between population polygons corresponding to different sampling years is more extensive for Mouvanga than for Bambomo in both PCA and MDS, suggesting more extensive EOD divergence in Bambomo than in Mouvanga within a period of 10 years.
Pattern of genetic variation
We were able to amplify fragments without any failed reactions (i.e. possible null homozyogotes) for all 327 individuals genotyped in this study. Total number of alleles detected at each of 5 loci over all populations ranged from 2–21 (Table 2). For each population, locus-specific expected heterozygosities ranged from 0.05–0.92 (Table 2). Exact tests produced no evidence of linked loci across all populations (p > 0.17). Of 55 unique locus-by-population combinations, only a few cases exhibited evidence of a deviation of observed heterozygosity from the expectation under Hardy-Weinberg equilibrium (at uncorrected p=0.05, see Table 2). After Bonferroni adjustment for multiple comparisons, only the NBB004 samples for Bambomo 1999, Bambomo 2009, and Nyamé-Pendé remain significant.
Table 2.
Summary of loci attributes.
| p | p | p | p | p | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apassa | 6 | 0.71 | 0.67 | 0.955 | 4 | 0.79 | 0.71 | 0.393 | 6 | 0.75 | 0.68 | 0.212 | 4 | 0.64 | 0.75 | 0.276 | 17 | 0.75 | 0.92 | 0.184 |
| Bale | 4 | 0.50 | 0.53 | 0.655 | 8 | 0.75 | 0.89 | 0.432 | 7 | 0.75 | 0.85 | 0.762 | 3 | 0.50 | 0.49 | 0.283 | 9 | 0.75 | 0.92 | 0.241 |
| Bambomo 09 | 6 | 0.37 | 0.35 | 0.953 | 9 | 0.82 | 0.73 | 0.135 | 7 | 0.68 | 0.68 | 0.259 | 10 | 0.38 | 0.62 | 0.000 | 21 | 0.72 | 0.79 | 0.183 |
| Bavavela | 6 | 0.67 | 0.60 | 0.693 | 4 | 0.58 | 0.59 | 0.184 | 6 | 0.83 | 0.81 | 0.546 | 4 | 0.83 | 0.73 | 0.059 | 6 | 0.71 | 0.71 | 0.584 |
| Bambomo 99 | 4 | 0.41 | 0.38 | 0.537 | 7 | 0.67 | 0.77 | 0.251 | 6 | 0.44 | 0.64 | 0.006 | 8 | 0.22 | 0.69 | 0.000 | 13 | 0.78 | 0.81 | 0.455 |
| Bikagala | 5 | 0.68 | 0.64 | 0.485 | 4 | 0.50 | 0.50 | 0.04 | 4 | 0.50 | 0.59 | 0.437 | 4 | 0.34 | 0.33 | 0.373 | 4 | 0.57 | 0.54 | 0.053 |
| Mouvanga 09 | 2 | 0.09 | 0.09 | 1.000 | 3 | 0.16 | 0.15 | 1.000 | 4 | 0.25 | 0.23 | 1.000 | 6 | 0.44 | 0.43 | 0.445 | 3 | 0.44 | 0.51 | 0.576 |
| Mouvanga 99 | 2 | 0.05 | 0.05 | 1.000 | 3 | 0.16 | 0.15 | 1.000 | 5 | 0.26 | 0.41 | 0.023 | 6 | 0.37 | 0.48 | 0.228 | 4 | 0.63 | 0.56 | 0.644 |
| Nyamé Pendé | 2 | 0.11 | 0.11 | 1.000 | 10 | 0.67 | 0.91 | 0.046 | 7 | 1.00 | 0.81 | 0.691 | 2 | 0.00 | 0.47 | 0.004 | 10 | 0.89 | 0.88 | 0.993 |
| Okano | 7 | 0.53 | 0.55 | 0.271 | 2 | 0.24 | 0.25 | 0.569 | 7 | 0.42 | 0.37 | 1.000 | 8 | 0.45 | 0.43 | 0.489 | 16 | 0.82 | 0.90 | 0.197 |
| Songou | 3 | 0.22 | 0.57 | 0.014 | 2 | 0.11 | 0.11 | 1.000 | 4 | 0.33 | 0.65 | 0.12 | 2 | 0.22 | 0.52 | 0.172 | 2 | 0.22 | 0.21 | 1.000 |
For each locus and population, the number of alleles (k) at each locus, the observed (Ho) and expected heterozygosity (He) are reported. Probabilities of deviation from HWE are reported as p
The allele frequency histograms at each of 5 loci for each of the 327 individuals in the full dataset are shown in Figure 5. For the two populations that were sampled in 1999 and again in 2009 (Mouvanga and Bambomo), allele frequencies appeared to change little over this ten year period between sampling. Between populations, there are apparent differences between the distribution of alleles; of particular note are the presence of several alleles (NBB002, ~250 bp, NBB005 several alleles between 300–400 bp), which are present in Bambomo and Apassa creeks but absent in other populations.
Figure 5.

Allele frequency histograms for each population and microsatellite locus. Microsatellite loci were designated as NBB001‐NBB005 by Arnegard et al. (2005). For each population, sample sizes are reported as number of individuals genotyped, so the plots include twice as many allele copies.
Fst values of all pairwise comparisons between the P. kingsleyae populations surveyed are shown in Figure 6. All populations were significantly differentiated from one another at the p=0.05 and the Bonferroni-corrected threshold (p=0.001), with the exceptional pairwise comparisons of Bambomo Creek 1999 vs. 2009, Mouvanga Creek 1999 vs. 2009, and Nyamé-Pendé vs. Bale Creek. Among all pairs of populations, the magnitude of genetic differentiation between populations varied from Fst = 0.055 to Fst = 0.65. We note that Mouvanga was highly differentiated from all other populations, with Fst values ranging from 0.377 (Mouvanga99-Nyame Pende) to 0.65 (Mouvanga09-Songou). Most importantly, the populations of Bambomo (predominantly biphasic individuals) and Apassa (predominantly triphasic individuals), which represent a potential hybrid zone, exhibit the lowest Fst values of our dataset (Fst<0.06, Fig. 5), despite being phenotypically distinct with respect to P0-presence/absence.
Figure 6.

FST values for each pairwise comparison of populations. The lower half of the matrix codes FST value by color and the upper half of the matrix reports the individual FST value. All pairwise comparisons of FST values were significant at Bonferroni‐corrected thresholds, with the exception of Mouvanga Creek (1999 vs. 2009), Bambomo Creek (1999 vs. 2009), and Balé Creek vs. Nyamé Pendé Creek. Underlined sites represent Southern populations. Localities in bold represent sites with triphasic (P0‐present) EODs, whereas non‐bolded localities indicate sites with biphasic (P0‐absent) EODs.
Isolation by distance
We investigated the relationship between genetic differentiation and geographic distance, measured as shortest river distance between populations (Fig.7A). Among all population pairs in Gabon, we found no significant relationship between genetic and geographic distance (Mantel test, R=−0.042, p=0.568), even after correcting for potential regional-level effects (partial Mantel test R=0.099, p=0.278). These results were consistent across different genetic distance measures (Table S4) or when samples collected in different years in Bambomo and Mouvanga were treated as one instead of separate populations (Table S5&S6).
Figure 7.
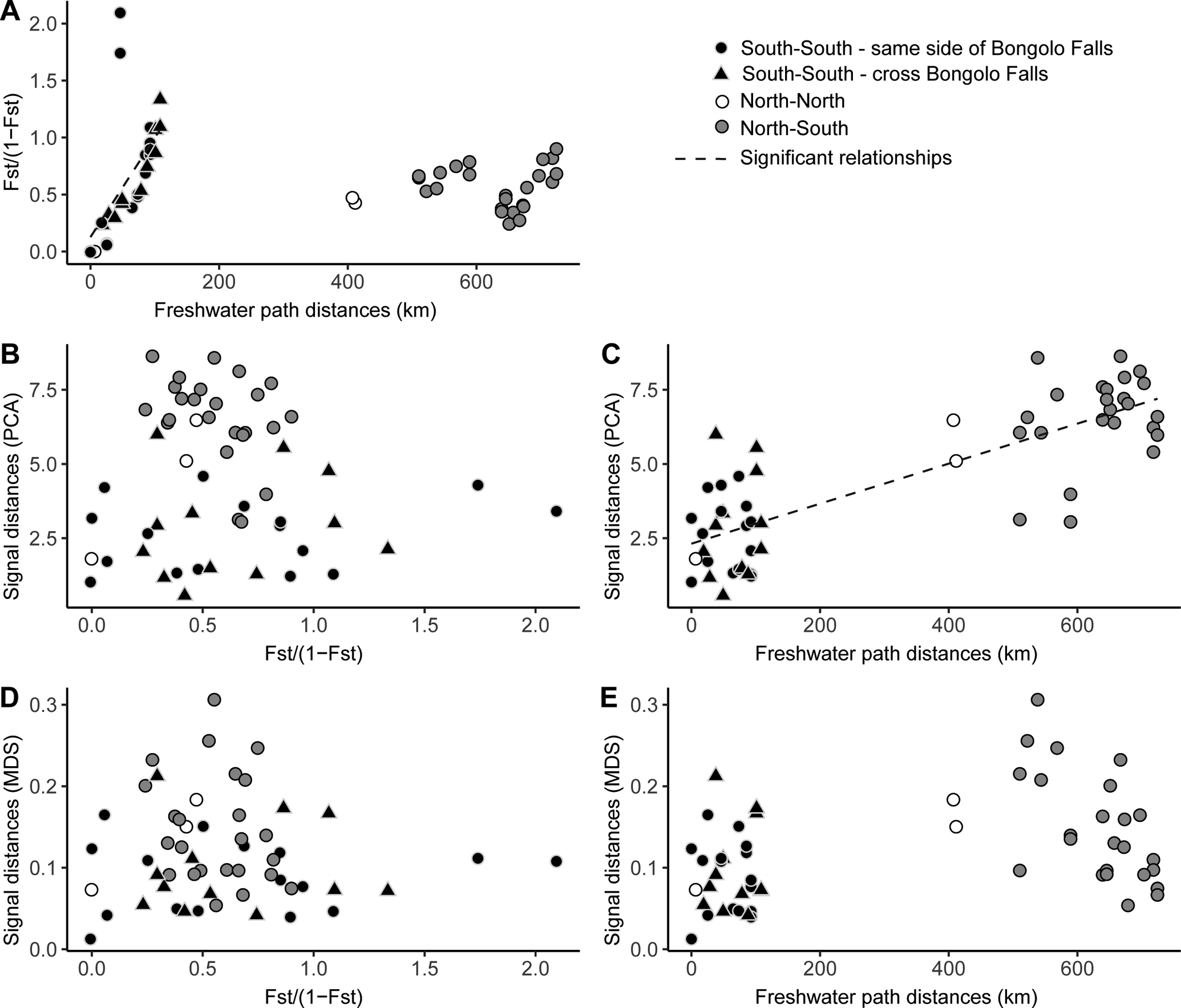
Correlation plots between genetic, signal and geographic distances for all populations (n = 11populations, all symbols) and within Southern populations only (n = 8 populations, black symbols). A. Population pairwise genetic distance in relation to geographic distance. B. Correlation between PCA‐derived signal and genetic distances. C. Correlation between PCA‐derived signal and geographic distances. D. Correlation between MDS‐derived signal and genetic distances. E. Correlation between MDS‐derived signal and genetic distances. Significant relationships identified by Mantel tests are shown with a black dashed fit line (see Table 3).
In contrast, among population pairs restricted to the South of Gabon, genetic distance was strongly related to geographic distance (Fig. 7A; R= 0.576, p = 0.02), regardless of the genetic distance metric used (Table S4) or whether Bambomo and Mouvanga samples from different years were treated as independent populations or not (Table S5&S6). However, we found that genetic distances between populations separated by Bongolo falls were on average no greater than those between populations on the same side of the Falls (Fig. S4). This was confirmed by the lack of correlation between genetic distance and the Bongolo model matrix (Table 3).
Table 3.
Results of standard and partial Mantel tests between genetic distances (Fst/(1-Fst)), signal distances (obtained through PCA (left) and MDS (right)), geographic distances (km), and presence/absence of Bongolo Falls (Southern populations) at two spatial scales.
| PCA-derived signal distances | MDS-derived signal distances | ||||
|---|---|---|---|---|---|
| Mantel’s r | p | Mantel’s r | p | ||
| Total range | Correlation | ||||
| (n=11 populations) | genetic - geographic | −0.042 | 0.568 | −0.042 | 0.568 |
| signal - genetic | −0.041 | 0.554 | −0.051 | 0.559 | |
| signal - geographic | 0.813 | 0.003 | 0.369 | 0.037 | |
| Partial correlation | |||||
| genetic - geographic (regional clusters as covariate) | 0.099 | 0.278 | 0.099 | 0.278 | |
| signal - genetic (regional clusters as covariate) | 0.025 | 0.438 | −0.023 | 0.502 | |
| signal - geographic (regional clusters as covariate) | 0.421 | 0.030 | −0.063 | 0.603 | |
| Southern populations | Correlation | ||||
| (n=8 populations) | genetic - geographic | 0.576 | 0.021 | 0.576 | 0.021 |
| genetic - Bongolo Falls | −0.042 | 0.550 | −0.042 | 0.550 | |
| signal - genetic | 0.181 | 0.175 | 0.062 | 0.303 | |
| signal - geographic | 0.069 | 0.324 | 0.018 | 0.365 | |
| signal - Bongolo Falls | 0.081 | 0.314 | 0.110 | 0.272 | |
Significance at the 0.05 level or below is marked in bold.
Correlation between signal, genetic, and geographic distances
We investigated the relationship between signal, genetic and geographic distance (Fig. 7B,C,D,E) at two spatial scales (for correlation plots focusing only on Southern population, see Fig. S5 A,B,C,D). When considering the entire dataset, signal distances were not correlated with genetic distances (Fig.7 B&D, Table 3), even after correcting for potential regional-level effects (partial Mantel test R=0.025 p=0.438). These results were consistent regardless of how signal or genetic distances were estimated (Table 3, Table S4) and of whether samples collected from different years in Bambomo and Mouvanga were considered as one instead of separate populations (Table S5&S6).
On the other hand, we found a strong and significant relationship between signal and geographic distance using PCA-derived signal distances (Fig. 7C, Table3), even after correcting for potential regional-level effects (partial Mantel test R=0.421 p=0.03) and after treating samples collected from different years as one instead of separate populations (TableS5&S6). Using MDS-derived signal distances, the signal-geography correlation was weaker yet significant (Fig. 7E, Table 3); however, it disappeared when we controlled for regional-level effects (partial Mantel test=−0.063, p=0.603). The same results were found regardless of how Bambomo and Mouvanga samples were grouped (Table S5,S6).
When considering only Southern populations, none of the variables tested (genetic distance, geographic distance, and Bongolo Falls model matrix) were found to correlate with signal distances (Fig. S5A,B,C,D, Table 3, Table S4,S5,S6).
P. kingsleyae can detect intraspecific EOD waveform variation
We utilized a habituation-dishabituation paradigm (Carlson et al. 2011) to determine whether individual P. kingsleyae were capable of discriminating between sympatric and allopatric P. kingsleyae EOD waveforms that were either P0-absent or P0-present (Fig. 8A,B). We performed two sets of experiments to assess this: the first set of experiments was performed with single stimulus presentations and recordings of responses across 20 field-captured individuals (Fig. 8C,D), whereas the second was performed with 5 repetitions of randomly interleaved stimulus trains to laboratory maintained individuals, which enabled us to average responses and thereby reduce variability (Fig8E–H; see Methods).
Figure 8.

Results of dishabituation playback experiments performed on P. kingsleyae. A. Stimuli used: 10 trains of 10 EODs each are presented in each trial. 8 trains were comprised of one repeated EOD waveform type (background) followed by one train of 10 EODs (novel) followed by one train of 10 background EODs. B. Example of subject response. Subject’s EOD discharges were continuously monitored (black dots), and discharge frequency was fitted with a spike density function (red line). The maximum and starting discharge rate of the fish was calculated over the 2sec interval from the onset of each train presentation. The difference between these values defined the change in frequency (ΔF) for that train. Novelty response was defined as the difference in ΔF between the 8th and 9th (novel) train presentation. See methods for details. C‐D. Experiments performed on field captured specimens. E‐H. Experiments performed in the laboratory. Localities and sample sizes are described above each plot. Bars show mean novelty responses averaged for all specimens ± standard error of the mean. Subtext indicates the localities where background and novel waveforms were recorded, if applicable. Stars reflect significance as compared to negative control (Dunnett’s Test w/Control: NS: not significant, *p=0.05; **p<0.01; ***p<0.001).
In the first field experiment (Fig. 8C), P0-absent P. kingsleyae from Bambomo creek showed behavioral evidence of discriminating phase-shifted EODs from P0-absent EODs (p<0.001, as compared to negative control), but did not show evidence of discriminating between P0-absent EODs (background) and Mouvanga P0-present EODs or Bambomo P0-present EODs (novel). In the second field experiment (Fig. 8D), P0-present P. kingsleyae from Mouvanga creek showed greater dishabituation to phase shifted EODs than test EODs, but this difference was not significant (p=0.1751). There was no significant difference in response between Mouvanga P0-present EODs (background) and Bambomo P0-absent EODs or Bambomo P0-present EODs (novel). Taken together, these field experiments performed on P. kingsleyae from Bambomo Creek and Mouvanga creek did not support the hypothesis that P. kingsleyae are able to discriminate between the subtly different P0-absent and P0-present waveforms. We therefore performed laboratory experiments that were designed to be more sensitive at detecting small differences in response through averaging.
In the first laboratory experiment (Fig. 8E), we tested the hypothesis that P. kingsleyae from Bambomo Creek are able to discriminate allopatric P0-present EODs from sympatric P0-absent EODs. We were able to determine a statistically significant response to the positive control (novel=90° phase shifted EOD; p<0.001). In addition, we detected statically significant changes in novelty responses to stimulus trains where both Mouvanga P0-present EODs were novel (p<0.01), and where Bambomo P0-absent EODs were novel (p<0.001). This evidence is consistent with the hypothesis that P. kingsleyae are able to discriminate between P0-present and P0-absent waveforms, but is confounded by the fact that allopatric populations, and even individuals, can differ slightly in EOD waveform (Gallant et al. 2011). Thus, there may be cues other than P0 that mediated this discrimination.
To rule out the possibility of individual EOD discrimination on the basis of characters other than P0-presence/absence, we tested P. kingsleyae captured in Bambomo Creek with individual sympatric EOD waveforms where P0 was not available as a cue (Fig. 8F). Though subjects responded significantly to positive controls (+90° and Mouvanga which have large P0-absent waveforms; p<0.001), subjects did not exhibit a significant novelty response when presented background and novel stimuli that were all P0-absent (p=0. 3478), or when background and novel stimuli were all P0-present (p=0. 3869). In a third, corollary laboratory experiment, we tested the hypothesis that P. kingsleyae from Bambomo Creek are able to discriminate between sympatrically occurring EOD variants (Fig. 8G) where P0-presence/absence was available as a cue. Subjects responded significantly to positive controls (p<0.001), and to P0-present waveforms from Bambomo in a background of P0-absent waveforms from Bambomo (p=0.02), though not to P0-present waveforms from Ivindo (which have very large P0s; p=0. 7189) from a background of P0-absent waveforms. Together, these three laboratory experiments support the hypothesis that P. kingsleyae can differentiate between sympatric EOD waveforms when P0 is available as a cue.
Finally, in the fourth laboratory experiment (Fig. 8H), we examined the ability of P. kingsleyae to discriminate between artificial EOD waveforms that had very small P0 peaks present (1:1 P0-absent/P0-present hybrid; see methods), and natural EOD waveforms that had no P0-present. We determined a statistically significant response to positive controls (p<0.01), and to EODs that represented 1:3 ratios of P0-absent to P0-present waveforms (p<0.01), however, subjects did not elicit a statistically significant response to 3:1 (p=0.873) or 1:1 ratios (p=1.0) of P0-absent to P0-present EODs.
The results of the field and laboratory experiments are consistent with the hypothesis that P. kingsleyae can differentiate between P0-absent and P0-present waveforms.
Morphological analysis of electric organs identifies additional specimens with mixed anatomy in Apassa creek
Gallant et al (2011) showed that P0-absent EODs are produced by fish with NPp electrocyte anatomy (posterior innervation with non-penetrating stalks), while P0-present EODs were produced by fish with Pa electrocytes in the electric organ (anterior innervation with penetrating stalks). In addition, Gallant et al (2011) presented evidence for electric organs with mixed morphology, wherein some electrocytes had penetrating stalks while other electrocytes from the same organ had non-penetrating stalks. We confirmed this observation with an additional analysis of 21 electric organs collected in 2009. Our present analysis supports the existence of these morphological types. In Fig. 9 we show four examples of electric organs surveyed in Bambomo, Apassa, Mouvanga, and Songou Creeks, and summarize our analysis of all 21 individuals in Table 7. In Apassa and Mouvanga all individuals were of the Pa morphology type, with one exceptional individual in Apassa exhibiting mixed morphology (NPp + Pa morphology in the same individual). In Bambomo Creek and Songou creek, we detected individuals that had entirely NPp type morphology, with one exceptional individual in Bambomo creek that had entirely Pa type morphology.
Figure 9.

Summary of histological survey of electric organs, which are restricted to the caudal peduncle in mormyrids. A. Individual P. kingsleyae were sectioned sagittally from lateral to medial, following one of the four column of electrocytes that comprise the electric organ. Example histology showing the two basic types of anatomical configuration in the electric organ: Pa‐type (stalk‐penetrated with anterior innervation) and NPp–type (non‐penetrated with posterior innervation). Stalks (S) can be seen clearly passing through the electrocyte penetrations (P) in Pa but not in NPp type electrocytes. Microstalklets (M) can be observed on the posterior face in both cases, and connective tissue boundaries (C) bounding each electrocyte are indicated. Innervation (N) can be observed on the anterior or posterior side of the electrocyte accordingly. Pa‐type electrocytes result in EOD signals that have a small head negative phase P0, which is absent in individuals with electrocytes that have NPp electrocyte anatomy (see Gallant et al. (2011)). B. Examples of histological analysis of four electric organs from each population. Each pixel represents an individual electrocyte in an individual section that was scored for presence of penetrations (black). In Apassa creek, most individuals have Pa‐type observed in each electrocyte from anterior to posterior, whereas one individual had NPp‐type and Pa‐type electrocytes (patches of black in mostly grey background). In Bambomo, most individuals were NPp with one individual exhibiting all Pa type electrocytes. Songou was entirely comprised of individuals with NPp‐type electrocytes, and Mouvanga was comprised of individuals with entirely Pa‐type electrocytes. Full analysis is summarized in Table 4.
Discussion
This study combined signal, genetic, and morphological analyses with behavioral experiments to investigate the evolutionary forces driving the intraspecific polymorphism of biphasic and triphasic signals in P. kingsleyae. This variation provides a rare window into the early stages of divergence in a key character that consistently varies between species in the Paramormyrops radiation and can thus inform us on the evolutionary processes that triggered this explosive signal diversification.
A key prediction of the drift hypothesis is that signal variation should be highly correlated with variation in neutral genetic markers. We found that signal distances were not related to genetic distances at any scale of our study, implying that while genetic drift is observable between populations in our dataset, it is insufficient to explain the evolution of diversity in the waveforms of this species. On the other hand, our behavioral experiments demonstrated that P. kingsleyae possess the perceptual ability to discriminate between biphasic and triphasic EODs, suggesting that EOD divergence in this species has the potential to be used as a cue for assortative mating with respect to signal type. This finding is consistent with previous studies that reported species-specific signal preferences among Paramormyrops species (Arnegard et al. 2006, where Brienomyrus sp. CAB refers to P. kingsleyae) as well as among the other major mormyrid radiation of Campylomormyrus species (Feulner et al. 2009a, b; Nagel et al. 2018a; Nagel et al. 2018b).
The Relationship between Genetic, Geographic, and Phenotypic Distance
Since the biogeographic distributions of tropical freshwater fishes are mainly constrained by landscape and ecological features such as basin geomorphology and river capture dynamics, differentiation patterns in these taxa at large spatial scales are often purely the result of vicariance and drift (Albert and Reis 2011). It is therefore unsurprising that we found a significant relationship between genetic and geographic distances in Southern Gabon, as would be predicted with an isolation-by-distance model (Wright 1984). Despite this, we draw the reader’s attention to a few key observations regarding the patterns of genetic differentiation between populations. First, we demonstrate that the presence of Bongolo Falls did not explain any of the genetic structure in this region (Table 2, Table S3,S4,S5), implying that populations separated by this 15m waterfall are not necessarily genetically more diverged than populations not separated by it, which may be caused by occasional downstream migration. Second, we were unable to find a significant relationship between genetic and geographic distance that extended over large geographic scales. The observed pattern in P.kingsleyae’s genetic structure in our dataset across Gabon is likely related to the balance between mutational processes within populations and gene flow between them (Hutchison and Templeton 1999), whereby gene flow and drift influence regional population structure differently depending on scale. As microsatellites have very high mutation rates and unique mutational processes (Selkoe and Toonen 2006), it is likely that the signal of gene flow between Southern and Northern populations is overwhelmed by the influence of genetic drift and mutation, thus reducing Fst estimates. Future studies, with more numerous markers (i.e. single nucleotide polymorphisms) will be necessary to fully understand P. kingsleyae’s genetic structure and colonization history over the Gabonese landscape. Third, we note that in Bambomo and Mouvanga Creeks, allele frequencies were remarkably stable over a 10 year period, suggesting large effective population sizes, at least in these locations (Waples 1989).
At a small geographic scale (i.e. southern populations) where migration is much more likely between populations, the combination of strong genetic-geographic correlation with the total lack of correlation of EOD divergence with any variable (genetic or geographic) strongly implies that differences in selective pressures between populations are a likely important determinant of phenotypic divergence. At larger geographic scales, where migration is highly unlikely, the relationships between genetic, geographic, and phenotypic distances must be interpreted with more care for two reasons. First, microsatellite marker loci may underestimate genetic distances between geographically distant populations (as mentioned above). Second, the gap distribution in our sampling efforts between Southern and Northern populations may introduce potential confounding regional-level effects on EODs. Given these limitations, at this scale, our data identified that only PCA-derived signal and geographic distances were significantly correlated. This relationship disappeared when using MDS-derived signal distances and correcting for regional-level effects (Table 3). These results may be indicative of a potential clinal EOD variation pattern along geographic degrees of isolation such as reported in Gallant et al. (2011). However, the disparity between signal measures calls for caution and suggests that P. kingsleyae’s pattern of geographical EOD variation most likely does not simply result from classic isolation-by distance processes (i.e. limited migration and gradual genetic drift). It also supports the likelihood of regional-level effects actively acting on phenotypic divergence, possibly in the form of different regional selective pressures or different regional colonization histories (e.g. founder effects.) At these geographical scales, our results can speak to a relatively low likelihood of drift acting alone on signals. Nevertheless, studies should undoubtedly focus on a larger number of markers and a more continuous sampling scheme to corroborate these findings.
Potential Selective Pressures on EOD Diversification
Ecological Selection -
Previous studies have considered ecological selection as an unlikely driver of EOD waveform divergence in the Paramormyrops radiation (Arnegard et al. 2010; Gallant et al. 2011). However, studies on a second radiation of mormyrids in the Campylomormyrus genus have implicated ecological selection in the evolution of divergent snout morphologies and EOD types (Feulner et al. 2009b). Low-capacitance objects such as small invertebrate larvae, which seem to be a major part of mormyrid diets (Blake 1977; Hyslop 1986; Nwani et al. 2006), attenuate higher-frequencies more readily (Meyer 1982; von der Emde and Ringer 1992; Crampton 1998). Recent work on mormyrid electrolocation has revealed that animals, plants, and invertebrates all create unique amplitude and waveform modulations referred to “electric colors” that allow these animals to reliably identify and differentiate these items (Gottwald et al. 2018), indicating that different EOD waveforms could therefore differ in their range of ‘electric color’ detections.
In our study, of the three frequency-content variables measured, we found significant differences only in the high frequency content of EOD signals (expressed by the variable ffthi, frequency above the peak power frequency at −3dB) between triphasic and biphasic individuals (mean ffthi for triphasic individuals = 2200.3 Hz, mean ffthi for biphasic individuals= 2854.6 Hz, t-test t=8.32, p<0.001). It is therefore conceivable that biphasic vs. triphasic EODs in P. kingsleyae may confer differential electrolocation capacities, particularly for prey detection, and should be an avenue for future studies in this system.
Assortative Mating -
A communication context where EODs constitute the primary communication channel for mormyrids is sexual signaling, where males and females actively interact with one another (Bratton and Kramer 1989; Werneyer and Kramer 2005; Wong and Hopkins 2007). More specifically, many experimental studies have shown that both males and females exhibit mating preferences for specific EOD waveform features (Hopkins and Bass 1981; Arnegard et al. 2006;Machnik and Kramer 2008;Feulner et al. 2009a; Markowski et al. 2008; Machnik et al. 2010; Nagel et al. 2018a; Nagel et al. 2018b). Among Paramormyrops species, field and laboratory playback experiments have also revealed strong preferences for species-specific EOD waveforms during courtship (Hopkins and Bass, 1981), indicating that EODs are most likely involved in maintaining pre-zygotic isolation between closely related species.
Our data is consistent with some degree of assortative mating between biphasic and triphasic P. kingsleyae EOD types. However, it is likely incomplete given numerous lines of evidence for hybridization. First, we found weak yet significant genetic differentiation between Apassa and Bambomo Creeks (Fst<0.07), despite the fact that these populations are nearly fixed for alternate electric organ anatomies and signal types. Another line of evidence is that both populations exhibit a so-called “rare alleles” phenomenon (Fig. 5): both have additional alleles that are absent from all other populations genotyped, which has been demonstrated as a signature of hybrid zones in other taxa (Golding 1983; Barton 1985; Hoffman and Brown 1995). A third line of evidence is the existence of mixed electric organ morphology individuals in both Apassa and Bambomo (Fig. 9 and Table 4 in addition to those already discovered in Gallant et al. (2011)). We note that the only other documented case of mormyrids with mixed-morphology electrocytes was described in artificially created crosses between the biphasic mormyrid Campylomormyrus tshokwe and triphasic Campylomormyrus tamandua (Kirschbaum et al. 2016).
Table 4.
Summary of electric organ histological survey, performed on 21 specimens of Paramormyrops kingsleyae.
| Morphology | |||||||
|---|---|---|---|---|---|---|---|
| Apassa | 6681 | 420 | 70 | 60 | 15 | 45 | Mixed |
| Apassa | 6675 | 648 | 108 | 64 | 50 | 14 | Mixed |
| Apassa | 6676 | 366 | 61 | 69 | 58 | 11 | Pa |
| Apassa | 6637 | 258 | 43 | 70 | 65 | 5 | Pa |
| Bambomo | 6489 | 240 | 40 | 60 | 10 | 50 | NPp |
| Bambomo | 6494 | 384 | 64 | 61 | 1 | 60 | NPp |
| Bambomo | 6497 | 276 | 46 | 67 | 0 | 67 | NPp |
| Bambomo | 6500 | 270 | 45 | 59 | 1 | 58 | NPp |
| Bambomo | 6547 | 378 | 63 | 52 | 1 | 51 | NPp |
| Bambomo | 6549 | 186 | 31 | 60 | 5 | 55 | NPp |
| Bambomo | 6597 | 438 | 73 | 58 | 43 | 15 | Mixed |
| Mouvanga | 6789 | 516 | 86 | 58 | 53 | 5 | Pa |
| Mouvanga | 6811 | 342 | 57 | 62 | 55 | 7 | Pa |
| Mouvanga | 6807 | 582 | 97 | 50 | 49 | 1 | Pa |
| Mouvanga | 6810 | 390 | 65 | 66 | 61 | 5 | Pa |
| Mouvanga | 6804 | 570 | 95 | 65 | 65 | 0 | Pa |
| Mouvanga | 6802 | 570 | 95 | 57 | 57 | 0 | Pa |
| Songou | 6611 | 252 | 42 | 59 | 0 | 59 | NPp |
| Songou | 6612 | 564 | 94 | 70 | 4 | 66 | NPp |
| Songou | 6613 | 234 | 39 | 64 | 5 | 59 | NPp |
| Songou | 6616 | 480 | 80 | 57 | 2 | 55 | NPp |
For each individual, the collection locality and specimen number are provided. For each specimen, one column of the entire electric organ was surveyed for the specified depth (μm) from lateral to medial (see Fig. 9). Total number of electrocytes, and the number of those exhibiting Pa type morphology and NPp type morphology are provided. Finally, the assessment of the overall EO morphology is specified as either Pa (>75% of electrocytes Pa) NPp (>75% electrocytes NPp) or mixed (<75% of electrocytes of one type). Note that some individuals determined to have Pa anatomy did not have penetrations in the rostral or caudal portion of the organ (See Fig. 9). In these cases, electrocytes could not be fully surveyed because of the orientation of tissue during sectioning.
Our behavioral experiments provide evidence that P. kingsleyae from the putative hybrid zone are capable of discriminating between P0-present and P0-absent EODs, a finding that is consistent with several studies that have characterized the neural encoding of EOD waveforms (Amagai et al. 1998; Friedman and Hopkins 1998; Xu-Friedman and Hopkins 1999; Carlson 2009; Carlson and Arnegard 2011; Baker et al. 2013; Lyons-Warren et al. 2013). These results indicate that in zones of signal sympatry, the observed EOD variation has the potential to be meaningful to P. kingsleyae receivers and could be a basis for assortative mating in this species. It is possible that hybridization is facilitated by the considerable variation in discrimination ability between individuals that we found in our field and laboratory experiments, despite the overall evidence of discrimination (Fig. 8). Variation in discrimination and perceptual biases among choosers in the context of mate choice has been demonstrated extensively (Rodríguez and Andrew Snedden 2004; Ryan and Cummings 2013) and can have important implications for hybridization and evolutionary diversification. This variation may accurately reflect that not all P. kingsleyae are equally good at discriminating EOD waveforms, which may be due to differences in reproductive state, age, condition and sensory acuity of the individuals (Rosenthal 2017). In the same way, a lack of novelty response in our experiments does not necessarily equate a lack of discrimination, as some fish may be able to distinguish signals and still not show a behavioral response.
We can conceive of two alternative scenarios that may explain the divergence of EOD signals in P. kingsleyae. First, P. kingsleyae populations could evolve divergence in EODs and concordant divergence in mate preferences due to sexual selection, which has been found to facilitate the rapid evolution of mating signals in many anurans (e.g. Pröhl et al. 2006; Boul et al. 2007; Pröhl et al. 2007; Lemmon 2009) and even to drive speciation in some of these cases (Masta and Maddison 2002; Boul et al. 2007). Second, variation in P. kingsleyae signals may be due to reinforcing selection against maladaptive hybridization between populations that initially diverged due to natural selection on ecological traits (Dobzhansky 1927).
Under both scenarios, we would expect P. kingsleyae to exhibit preferences for local vs. foreign EODs in P. kingsleyae, which is an important future study. Second, the likelihood of these alternative scenarios is largely contingent on assessing the relative fitness and frequency of hybrid individuals. The small proportion of intermediate morphology individuals did not show obvious signs of morphological or behavioral abnormalities. However, further studies that characterize the relative fitness of these individuals compared to phenotypically normal individuals is another important area of future research. Plausibly, if differences in EOD are adaptations to capturing different prey items (see preceding section), hybrid EOD signals could perform relatively poorly in prey detection. This would suggest that the presence or absence of P0 might act similarly to a “magic trait” (Gavrilets 2004; Feulner et al. 2009b), i.e. a trait resulting from divergent ecological selection that also contributes to assortative mating.
Concluding Remarks
Our study demonstrates that drift is insufficient to explain EOD diversity within P. kingsleyae and supports an important role of selection in the evolution of EODs, even on very small geographic scales within a single species. While drift has been found to play an important role in the divergence of communication signals in several taxa including South American electric fish (Picq et al. 2016), Neotropical singing mice (Campbell et al. 2010), Amazonian and Microhylid frogs (Amézquita et al. 2009; Lee et al. 2016), and greenish warblers (Irwin et al. 2008), it is important to note that most studies testing the contribution of drift in communication signals rarely report evidence for neutral signal evolution (Soha et al. 2003; Nicholls et al. 2006; Pröhl et al. 2006; Ruegg et al. 2006; Pröhl et al. 2007; Rudh et al. 2007; Dingle et al. 2008; Huttunen et al. 2008; Tobias et al. 2010; Cadena et al. 2011; Lin et al. 2015; Sathyan et al. 2017). Lastly, signal evolution is rarely explained by a single force; drift has been implicated to work in conjunction with selection in driving signal divergence both theoretically (Uyeda et al. 2009) and empirically in many signaling modalities including echolocation calls (Jacobs and Mutumi 2018), bird songs (Irwin et al. 2008), mice songs (Campbell et al. 2010), and electric fish signals (Picq et al. 2016).
The intraspecific polymorphism in the signals of P. kingsleyae is recurrent between Paramoryrops species, a common pattern in species radiations, which gives the impression that certain traits “blink on and off” during evolution (West-Eberhard 2003). These parallelisms inform us on how key traits, in this case EODs, can initially diverge and diversify. Our results suggest that divergent selection operating over small geographic scales promotes EOD signal divergence for a widely variable character in mormyrid electric fish. This phenomenon is consistent with other species radiations characterized by intra- and interspecific parallelisms in key traits, where either sexual selection (e.g. in East African cichlids (Maan et al. 2004) and in coral reef hamlet fish (Puebla et al. 2012)) or natural selection (e.g. in Darwin’s finches (Werner and Sherry 1987)) have been identified as the main drivers of diversification. The framework provided by this study presents a clear set of testable hypotheses, such that future studies might explicitly identify the source of selection acting on EOD signals in P. kingsleaye. These may be the same forces that contributed to the rapid radiation of the Paramormyrops genus, and of other mormyrid genera.
Supplementary Material
Acknowledgments
Permits to collect fishes in Gabon and export them for this study were granted by l’Institut de Recherche en Ecologie Tropicale, l’Institut de Recherches Agronomiques et Forestières and the Centre National de la Recherche Scientifique et Technologique. We are grateful for the valuable assistance and logistical support we received from J. D. Mbega and students working in these institutions. All techniques used are in accordance with protocols approved by Cornell University’s Center for Research Animal Resources and Education (CARE). Additionally, we thank C.D. Hopkins (CDH), M. Arnegard, A. McCune and K. Shaw, J. Fetcho, D. Deitcher, S. Mullen as well as two anonymous reviewers for comments on earlier versions of this manuscript. This work was supported by NIMH TG T32 MH015793, NIH TG 2T32GM007469, and NSF 1455405 to JRG, NIH RO1-DC6206, NSF 0818305 to CDH, and NSF 0818390 as well as NSF 1255396 to BAC.
Footnotes
Data Availability
Source code for this analysis is available on GitHub (DOI: 10.5281/zenodo.3691905) and all original data are archived in Dryad (DOI: 10.5061/dryad.2z34tmphj).
References
- Albert J and Reis R. 2011. Introduction to Neotropical Freshwaters Pp. 3–19 in Albert JS, and Reis R, eds. Historical biogeography of Neotropical freshwater fishes. University of California Press, Los Angeles. [Google Scholar]
- Allender CJ, Seehausen O, Knight ME, Turner GF, and Maclean N. 2003. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. PNAS 100:14074–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai S, Friedman M, and Hopkins C. 1998. Time coding in the midbrain of mormyrid electric fish. I. Physiology and anatomy of cells in the nucleus exterolateralis pars anterior. JOURNAL OF COMPARATIVE PHYSIOLOGY A-NEUROETHOLOGY SENSORY NEURAL AND BEHAVIORAL PHYSIOLOGY 182:115–130. [DOI] [PubMed] [Google Scholar]
- Amézquita A, Lima AP, Jelhe R, Castellanos L, Ramos Ó, Crawford AJ, Gasser H, and Hödl W. 2009. Calls, colours, shape, and genes: a multi-trait approach to the study of geigraphic variaiton in the Amazonian frog Allobates femoralis. Biology Journa of the Linnean Society 98:826–838. [Google Scholar]
- Arnegard M, Zwickl D, and Lu Y. 2010a. Old gene duplication facilitates origin and diversification of an innovative communication system—twice. Proceedings of the …. [DOI] [PMC free article] [PubMed]
- Arnegard ME, Bogdanowicz SM, and Hopkins CD. 2005. Multiple cases of striking genetic similarity between alternate electric fish signal morphs in sympatry. Evolution 59:324–343. [PubMed] [Google Scholar]
- Arnegard ME and Hopkins CD. 2003. Multivariate analysis of electric signal variation among seven blunt-snouted Brienomyrus species (Teleostei: Mormyridae) from Gabon. Environmental Biol. of Fishes 67:321–339. [Google Scholar]
- Arnegard ME, Jackson BS, and Hopkins CD. 2006. Time-domain signal divergence and discrimination without receptor modification in sympatric morphs of electric fishes. J Exp Biol 209:2182–2198. [DOI] [PubMed] [Google Scholar]
- Arnegard ME, McIntyre PB, Harmon LJ, Zelditch ML, Crampton WGR, Davis JK, Sullivan JP, Lavoué S, and Hopkins CD. 2010b. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Evolution Int J Org Evolution 176:335–356. [DOI] [PubMed] [Google Scholar]
- Baker C, Kohashi T, Lyons-Warren A, Ma X, and Carlson B. 2013. Multiplexed temporal coding of electric communication signals in mormyrid fishes. J Exp Biol 216:2365–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N 1985. Analysis of hybrid zones. Annual Review of Ecology and Systematics. [Google Scholar]
- Bennett MVL and Grundfest H. 1961. Studies on morpology and electrophysiology of electric organs III. Electrophysiology of electric organs in mormyrids. Bioelectrogenesis (Chagas C and Paes de Carvalho A, Eds.):113–135. [Google Scholar]
- Blake BF 1977. Food and feeding of the mormyrid fishes of Lake Kainji, Nigeria, with special reference to seasonal variation and interspecific differences. Journal of Fish Biology 11:315–328. [Google Scholar]
- Boul KE, Chris Funk W, Darst CR, Cannatella DC, and Ryan MJ. 2006. Sexual selection drives speciation in an Amazonian frog. Proceedings of the Royal Society B: Biological Sciences 274:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boul KE, Funk WC, Darst CR, Cannatella DC, and Ryan MJ. 2007. Sexual selection drives speciation in an Amazonian frog. Proceedings of the Royal Society B: Biological Sciences 274:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton BO and Kramer B. 1989. Patterns of the electric organ discharge during courtship and spawning in the momryrid fish, Pollimyrus isidori. Behavioral Ecology and Sociobiology 24:349–368. [Google Scholar]
- Brower AVZ 1994. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proceedings of the National Academy of Sciences 91:6491–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena CD, Cheviron ZA, and Funk WC. 2011. Testing the molecular and evolutionary causes of a ‘leapfrog’ pattern of geographical variation in coloration. Journal of Evolutionary Biology 24:402–414. [DOI] [PubMed] [Google Scholar]
- Campbell P, Pasch B, Pino JL, Crino OL, Phillips M, and Phelps SM. 2010. Geographic variation in the songs of neotropical singing mice: testing the relative importance of drift and local adaptation. Evolution 64:1955–1972. [DOI] [PubMed] [Google Scholar]
- Carlson BA 2009. Temporal-Pattern Recognition by Single Neurons in a Sensory Pathway Devoted to Social Communication Behavior. Journal of Neuroscience 29:9417–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BA and Arnegard ME. 2011. Neural innovations and the diversification of African weakly electric fishes. Communicative & Integrative Biology 4:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BA and Gallant JR. 2013. From Sequence to Spike to Spark: Evo-devo-neuroethology of Electric Communication in Mormyrid Fishes. Journal of Neurogenetics 27:106–129. [DOI] [PubMed] [Google Scholar]
- Carlson BA and Hopkins CD. 2004. Stereotyped temporal patterns in electrical communication. Anim Behav 68:867–878. [Google Scholar]
- Crampton WGR 1998. Electric signal desin and habitat preferences in a species rich assemblage of fymnotiform fishes from the Upper Amazon Basin. Anais-Academia Brasileira de Ciencias 70:805–848. [Google Scholar]
- Diamond J 1986. Biology of Birds of Paradise and Bowerbirds. Annual Reviews in Ecology and Systematics 17:17:37. [Google Scholar]
- Dingle C, Halfwerk W, and Slabbekoorn H. 2008. Habitat-dependent song divergence at subspecies level in the grey-breasted wood-wren. Journal of Evolutionary Biology 21:1079–1089. [DOI] [PubMed] [Google Scholar]
- Dunnett CW 1955. A multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association 50:1096–1121. [Google Scholar]
- Excoffier L and Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10:564–567. [DOI] [PubMed] [Google Scholar]
- Feulner PG, Plath M, Engelmann J, Kirschbaum F, and Tiedemann R. 2009a. Electrifying love: electric fish use species-specific discharge for mate recognition. Biology Letters 5:225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feulner PG, Plath M, Engelmann J, Kirschbaum F, and Tiedemann R. 2009b. Magic trait electric organ discharge (EOD). Communicative and Integrative Biology 2:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MA and Hopkins CD. 1998. Neural substrates for species recognition in the time-coding electrosensory pathway of mormyrid electric fish. The Journal of neuroscience : the official journal of the Society for Neuroscience 18:1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant Jason et al. (2020), Genetic drift does not sufficiently explain patterns of electric signal variation among populations of the mormyrid electric fish Paramormyrops kingsleyae, Dryad, Dataset, 10.5061/dryad.2z34tmphj [DOI] [PMC free article] [PubMed]
- Gallant Jason et al. (2020) Github Code Respository DOI: 10.5281/zenodo.3691905. [DOI]
- Gallant JR, Arnegard ME, Sullivan JP, Carlson BA, and Hopkins CD. 2011. Signal variation and its morphological correlates in Paramormyrops kingsleyae provide insight into evolution of electrogenic signal diversity in mormyrid fish. Journal of Comparative Physiology A. [DOI] [PubMed] [Google Scholar]
- Gavrilets S 2004. Fitness Landscapes and the Origin of Species. Princeton University Press, Princeton, NJ. [Google Scholar]
- Glaubrecht M 2010. Evolution in action: case studies in adaptive radiation, speciation and the origin of biodiversity. Springer, New York, NY, USA. [Google Scholar]
- Golding G 1983. Increased number of alleles found in hybrid populations due to intragenic recombination. Evolution; international journal of organic evolution. [DOI] [PubMed] [Google Scholar]
- Gottwald M, Singh N, Haubrich AN, Regett S, and von der Emde G. 2018. Electric-Color Sensing in Weakly Electric Fish Suggests Color Perception as a Sensory Concept beyond Vision. Curr Biol 28:3648–3653. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W 1977. Principles of electrolocation and jamming avoidance in electric fish: a neuroethological approach. Springer, New York, NY, USA. [Google Scholar]
- Heiligenberg W and Altes RA. 1978. Phase sensitivity in electroreception. Science 199:1001–1004. [DOI] [PubMed] [Google Scholar]
- Hoffman SMG and Brown WM. 1995. The molecular mechanism underlying the “rare allele phenomenon” in a subspecific hybrid zone of the California field mouse, Peromyscus californicus. Journal of Molecular Evolution 41. [DOI] [PubMed] [Google Scholar]
- Hopkins CD and Bass A. 1981. Temporal coding of species recognition signals in an electric fish. Science 212:85–87. [DOI] [PubMed] [Google Scholar]
- Hutchison D and Templeton A. 1999. Correlation of pairwise genetic and geographic distance measures: inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution:1898–1914. [DOI] [PubMed] [Google Scholar]
- Huttunen S, Aspi J, Schlotterer C, Routtu J, and Hoikkala A. 2008. Variation in male courtship song traits in Drosophila virilis: the effects of selection and drift on song divergence at the intraspecific level. Behav Genet 38:82–92. [DOI] [PubMed] [Google Scholar]
- Hyslop EJ 1986. The food habits of four small-sized species of Mormyridae from the floodplain pools of the Sokoto-Rima river basin, Nigeria. Journal of Fish Biology 28:147–151. [Google Scholar]
- Irwin DE, Thimgan MP, and Irwin JH. 2008. Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): a strong role for stochasticity in signal evolution? Journal of Evolutionary Biology 21:435–448. [DOI] [PubMed] [Google Scholar]
- Jacobs DS and Mutumi GL. 2018. The Relative Roles of Selection and Drift in Phenotypic Variation: Some Like It Hot, Some Like It Wet. Pp. 215–237. Origin and Evolution of Biodiversity. [Google Scholar]
- Kirkpatrick M and Ryan MJ. 1991. The evolution of mating preferences and the paradox of the lek. Nature 350:33–38. [Google Scholar]
- Kirschbaum F, Nguyen L, Baumgartner S, Chi HWL, Wolfart R, Elarbani K, Eppenstein H, Korniienko Y, Guido-Bohm L, Mamonekene V, Vater M, and Tiedemann R. 2016. Intragenus (Campylomormyrus) and intergenus hybrids in mormyrid fish: Physiological and histological investigations of the electric organ ontogeny. Journal of Physiology - Paris 110:281–301. [DOI] [PubMed] [Google Scholar]
- Kramer B and Westby GWM. 1985. No sex difference in the waveform of the pulse type electric fish, Gnathonemus petersii (Mormyridae). Experientia 41:1530–1531. [Google Scholar]
- Kruskal J and Wish M. 1978. Multidimensional scaling, Sage, Beverly Hills, CA. [Google Scholar]
- Lavoué S, Sullivan JP, Arnegard ME, and Hopkins CD. 2008. Differentiation of morphology, genetics and electric signals in a region of sympatry between sister species of African electric fish (Mormyridae). Journal of Evolutionary biology 21:1030–1045. [DOI] [PubMed] [Google Scholar]
- Lee KH, Shaner PJ, Lin YP, and Lin SM. 2016. Geographic variation in advertisement calls of a Microhylid frog - testing the role of drift and ecology. Ecology and Evolution 6:3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon EM 2009. Diversification of conspecific signals in sympatry: geographic overlap drives multidimensional reproductive character displacement in frogs. Evolution 63:1155–1170. [DOI] [PubMed] [Google Scholar]
- Liem KF and Kaufmann LS. 1984. Intraspecific macroevolution: functional biology of the polymorphic cichlid species Cichlasoma minckleyi Pp. 203–215 in Echelle AA, and Kornfield I, eds. Evolution of Fish Species Flocks. University of Maine Press, Orono. [Google Scholar]
- Lin A, Jiang T, Kanwal JS, Lu G, Luo J, Wei X, Luo B, and Feng J. 2015. Geographical variation in echolocation vocalizations of the Himalayan leaf-nosed bat: contribution of morphological variation and cultural drift. Oikos 124:364–371. [Google Scholar]
- Lissmann HW 1958. On the function and evolution of electric organs in fish. Journal of Experimental Biology 35:156–191. [Google Scholar]
- Lyons-Warren A, Kohashi T, Mennerick S, and Carlson B. 2013. Detection of submillisecond spike timing differences based on delay-line anti-coincidence detection. Journal of Neurophysiology 110:2295–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan ME, Seehausen O, Soderberg L, Johnson L, Ripmeester EA, Mrosso HD, Taylor MI, van Dooren TJ, and van Alphen JJ. 2004. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc Biol Sci 271:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnik P and Kramer B. 2008. Female choice by electric pulse duration: attractiveness of the males’ communication signal assessed by female bulldog fish, Marcusenius pongolensis (Mormyridae, Teleostei). The Journal of Experimental Biology 211:1969–1977. [DOI] [PubMed] [Google Scholar]
- Machnik P, Markowski B, and Kramer B. 2010. Intra- versus inter-sexual selection in the dimorphic electric organ discharges of the snoutfish Marcusenius altisambesi (Mormyriformes, Teleostei). Behaviour 147:677–704. [Google Scholar]
- Markowski B, Baier B, and Kramer B. 2008. Differentiation in electrical pulse waveforms in a pair of sibling dwarf stonebashers, Pollimyrus castelnaui and P. marianne: possible mechanisms and functions (Mormyridae, Teleostei). Behaviour 145:115–135. [Google Scholar]
- Masta SE and Maddison WP. 2002. Sexual selection driving diversification in jumping spiders. Proceedings of the National Academy of Sciences 99:4442–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirmans PG 2012. The trouble with isolation by distance. Molecular Ecology 21: 2839–2846. [DOI] [PubMed] [Google Scholar]
- Mendelson TC and Shaw KL. 2005. Rapid speciation in an arthropod. Nature 433:375. [DOI] [PubMed] [Google Scholar]
- Meyer A 1987. Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces,Ccichlidae) and their implications for speciation in cichlid fishes. Evolution 4:1357–1369. [DOI] [PubMed] [Google Scholar]
- Meyer H 1982. Behavioral responses of weakly electric fish to complex impedances. Journal of Comparative Physiology 145:459–470. [Google Scholar]
- Mullen SP, Mendelson TC, Schal C, and Shaw KL. 2007. Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse hawaiian crickets (gryllidae: trigonidiinae: laupala). Evolution; international journal of organic evolution 61:223–231. [DOI] [PubMed] [Google Scholar]
- Nagel R, Kirschbaum F, Engelmann J, Hofmann V, Pawelzik F, and Tiedemann R. 2018a. Male-mediated species recognition among African weakly electric fishes. Royal Society Open Science 5:170443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R, Kirschbaum F, Hofmann V, Engelmann J, and Tiedemann R. 2018b. Electric pulse characteristics can enable species recognition in African weakly electric fish species. Scientific Reports 8:10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JA, Austin C, Moritz C, and Goldizen AW. 2006. Genetic population structure and call variation in a passerine bird, the satin bowerbird, Ptilonorhynchus violaceus. Evolution 60:1279–1290. [PubMed] [Google Scholar]
- Nwani CD, Eyo JE, and Udeh EF. 2006. Food and feeding habits of Campylomormyrus tamandua in Anambra river, Nigeria. Animal Research International 3:410–414. [Google Scholar]
- Oksanen J, Blanchet FG, M. F, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, and Wagner H. 2018. vegan: Community Ecology Package. R package version 2.4–6.
- Picq S, Alda F, Bermingham E, and Krahe R. 2016. Drift-driven evolution of electric signals in a Neotropical knifefish. Evolution 70:2134–2144. [DOI] [PubMed] [Google Scholar]
- Picq S, Scotti M, and Puebla O. 2019. Behavioural syndromes as a link between ecology and mate choice: a field study in a reef fish population. Animal Behaviour 150:219–237. [Google Scholar]
- Pröhl H, Hagemann S, Karsch J, and Höbel G. 2007. Geographic variation in male sexual signals in strawberry poison frogs (Dendrobates pumilio). Ethology 113:825–837. [Google Scholar]
- Pröhl H, Koshy RA, Mueller U, Rand MJ, and Ryan MJ. 2006. Geographic variation of genetic and behavioral traits in Northern and Southern túngara frogs. Evolution 60:1669–1679. [PubMed] [Google Scholar]
- Puebla O, Bermingham E, and Guichard F. 2012. Pairing dynamics and the origin of species. Proceedings of the Royal Society B: Biological Sciences 279:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2017. R: A language and environment for statisticsl computing. R Founation for Statistical Computing., Vienna, Austria. [Google Scholar]
- Rodríguez RL and Andrew Snedden W. 2004. On the functional design of mate preferences and receiver biases. Animal Behaviour 68:427–432. [Google Scholar]
- Rosenthal GG 2017. Mate choice: the evolution of sexual decision making from microbes to humans. Princeton University Press. [Google Scholar]
- Rousset F 2008. GENEPOP’007: a complete re-implmentation of the GENEPOP software for windows and linux. Molecular Ecology Resources 8:103–106. [DOI] [PubMed] [Google Scholar]
- Rudh A, Rogell B, and Hoglund J. 2007. Non-gradual variation in colour morphs of the strawberry poison frog Dendrobates pumilio: genetic and geographical isolation suggest a role for selection in maintaining polymorphism. Molecular Ecology 16:4284–4294. [DOI] [PubMed] [Google Scholar]
- Ruegg K, Slabbekoorn H, Clegg S, and Smith TB. 2006. Divergence in mating signals correlates with ecological variation in the migratory songbird, Swainson’s thrush (Catharus ustulatus). Molecular Ecology 15:3147–3156. [DOI] [PubMed] [Google Scholar]
- Ryan MJ and Cummings ME. 2013. Perceptual Biases and Mate Choice. Annual Review of Ecology, Evolution, and Systematics 44:437–459. [Google Scholar]
- Ryman N and Palm S. 2006. POWSIM: a computer program for assessing statistical power when testing for genetic differentiation. Molecular Ecology 6:600–602. [DOI] [PubMed] [Google Scholar]
- Sathyan R, Engelbrecht A, and Couldridge VCK. 2017. Morphological, acoustic and genetic divergence in the bladder grasshopper Bullacris unicolor. Ethology Ecology & Evolution 29:552–573. [Google Scholar]
- Schluter D and McPhail JD. 1992. Ecological character displacement and speciation in sticklebacks. The American Naturalist 140:85–108. [DOI] [PubMed] [Google Scholar]
- Selkoe KA and Toonen RJ. 2006. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Letters 9:615–629. [DOI] [PubMed] [Google Scholar]
- Smith TB 1987. Bill size polymorphism and intraspecfic niche utilization in an African finch. Nature 329:717–719. [Google Scholar]
- Smouse PE, Long JC, and Sokal RR. 1986. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology 35:627–632. [Google Scholar]
- Soha JA, Nelson DA, and Parker PG. 2003. Genetic analysis of song dialect populations in Puget Sound white-crowned sparrows. Behavioral Ecology 15:636–646. [Google Scholar]
- Sullivan JP, Lavoué S, Arnegard ME, and Hopkins CD. 2004. AFLPs Resolve Phylogeny and Reveal Mitochondrial Introgression within a Species Flock of African Electric Fish (Mormyroidea: Teleostei). Evolution 58:824–841. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Lavoue S, and Hopkins CD. 2000. Molecular systematics of the African electric fishes (Mormyroidea: teleostei) and a model for the evolution of their electric organs. The Journal of experimental biology 203:665–683. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Lavoué S, and Hopkins CD. 2002. Discovery and phylogenetic analysis of a riverine species flock of African electric fishes (Mormyridae: Teleostei). Evolution 56:597–616. [DOI] [PubMed] [Google Scholar]
- Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, and Seddon N. 2010. Song divergence by sensory drive in Amazonian birds. Evolution 64:2820–3289. [DOI] [PubMed] [Google Scholar]
- Uyeda JC, Arnold SJ, Hohenlohe PA, and Mead LS. 2009. Drift promotes speciation by sexual selection. Evolution 63:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Emde G and Ringer T. 1992. Electrolocation of capacitive objects in four species of pulse-type weakly electric fish I. Discrimination Performance. Ethology 91:326–338. [Google Scholar]
- Waples RS 1989. A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BS 1990. Genetic data analysis: methods for discrete population genetic data. Sinauer, Sunderland, MA. [DOI] [PubMed] [Google Scholar]
- Werner TK and Sherry TW. 1987. Behavioral feeding specialization in Pinaroloxias inornata, the “Darwin’s Finch” of Cocos Island, Costa Rica. Proceedings of the National Academy of Sciences 84:5506–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneyer M and Kramer B. 2005. Electric signalling and reproductive behaviour in a mormyrid fish, the bulldog Marcusenius macrolepidotus (South African form). Journal of Ethology 23:113–125. [Google Scholar]
- West-Eberhard MJ 1986. Alternative adaptations, speciation, and phylogeny (A review). Proceedings of the National Academy of Sciences 83:1388–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RY and Hopkins CD. 2007. Electrical and behavioral courtship displays in the mormyrid fish Brienomyrus brachyistius. The Journal of Experimental Biology 210:2244–2252. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman M and Hopkins C. 1999. Central mechanisms of temporal analysis in the knollenorgan pathway of mormyrid electric fish. Journal of Experimental Biology 202:1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


